Abstract
The cyclooxygenase-2 (COX-2)–prostaglandin E2 (PGE2) pathway has been implicated in carcinogenesis, with BRAF mutation shown to promote PGE2 synthesis. This study was conducted to evaluate COX-2 expression in a large cohort of Middle Eastern papillary thyroid carcinoma (PTC), and further evaluate the prognostic significance of COX-2 expression in strata of BRAF mutation status. BRAF mutation analysis was performed using Sanger sequencing, and COX-2 expression was evaluated immunohistochemically using tissue microarray (TMA). COX-2 overexpression, noted in 43.2% (567/1314) of cases, was significantly associated with poor prognostic markers such as extra-thyroidal extension, lymph-node metastasis, and higher tumor stage. COX-2 was also an independent predictor of poor disease-free survival (DFS). Most notably, the association of COX-2 expression with DFS differed by BRAF mutation status. COX-2 overexpression was associated with poor DFS in BRAF-mutant but not BRAF wild-type PTCs, with a multivariate-adjusted hazard ratio of 2.10 (95% CI = 1.52–2.92; p < 0.0001) for COX-2 overexpressed tumors in BRAF-mutant PTC. In conclusion, the current study shows that COX-2 plays a key role in prognosis of PTC patients, especially in BRAF-mutated tumors. Our data suggest the potential therapeutic role of COX-2 inhibition in patients with BRAF-mutated PTC.
Keywords: cyclooxygenase-2, BRAF mutation, papillary thyroid carcinoma, disease-free survival
1. Introduction
Thyroid cancer is the most common endocrine malignancy. Its incidence is steadily rising worldwide [1,2], with the most common histology being papillary thyroid carcinoma (PTC) [3]. The incidence of PTC in Saudi Arabia is high—it is the second most common cancer affecting Saudi women, after breast cancer [4]. Although PTC is an indolent and slow-growing malignancy that can be successfully treated, there are patients that progress to more aggressive disease, with recurrence and metastasis [5,6,7]. It is critical to identify this subset of patients who might benefit from more aggressive therapy. Therefore, identification of a molecular marker that can be useful for prognostication of PTC patients is critically needed.
Tumor-promoting inflammation is a promising target for cancer therapy [8] due to its role in promoting cancer progression by increasing several growth, angiogenic, and immunosuppressive factors [9]. One of the important processes causing cancer inflammation is the cyclooxygenase-2 (COX-2) pathway. COX is a group of enzymes that are required for prostaglandin E2 (PGE2) synthesis [10]. In normal physiological status, COX-2 and PGE2 are upregulated and act as pro-inflammatory factors [11]. Although COX-2 expression is usually undetectable in normal tissue, it has been observed to be overexpressed in several human cancers [12,13,14,15]. Many studies have described the mechanisms by which COX-2 can promote carcinogenesis, including inhibition of apoptosis, increase in cell proliferation, stimulation of angiogenesis, and development of a tumor-promoting inflammatory microenvironment [9,16,17,18,19,20].
COX-2 overexpression in different thyroid cancer histotypes has also been investigated in several studies [21,22,23], the majority of which have been aimed at evaluation of its prognostic relevance by correlating the expression levels with patients’ clinicopathological features. Other studies have attempted to estimate the prognostic value of COX-2 expression in thyroid cancer [15,24]. Those studies found that COX-2 overexpression in thyroid cancer is associated with aggressive clinical behavior and tumor recurrence [15,25].
Another characteristic of PTC is that a single nucleotide mutation in the BRAF gene, V600E, is detected in a majority of PTC patients [26,27], often associated with aggressive disease [7,28]. BRAFV600E mutations have been shown to induce oncogenic cellular proliferation by constitutively activating the mitogen-activated protein (MAP) kinase pathway [29]. A recent study by Kosumin et al. [30] showed the interactive role of BRAF mutation status and COX-2 overexpression in the prognostication of colorectal cancer patients, suggesting that upregulation ofMAP kinase pathway mediates overexpression of COX-2 in BRAFV600E tumor cells.
Therefore, we conducted this study to evaluate COX-2 overexpression in a large cohort (n = 1335) of Middle Eastern PTC, and to analyze its correlation with clinicopathological parameters. Then we further evaluated the prognostic significance of COX-2 expression in strata of BRAF mutation status.
2. Results
2.1. Patient Characteristics
The mean age of the study population was 40.4 years (SD = ±16.1 years), with a male-to-female ratio of 1:3. A majority of the cases were of classical variant (67.1%) and Stage I tumors (83.4%). A nearly equal proportion of tumors exhibited presence and absence of extra-thyroidal extension, as well as multifocality (Table 1). Patient characteristics stratified by BRAF mutation status is presented in Table 1.
Table 1.
Patient characteristics according to BRAF mutation status.
| Total | BRAF Wild-Type | BRAF Mutant | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Total | 1335 | 584 | 43.7 | 751 | 56.3 | |
| Age at surgery (years) | ||||||
| Mean ± SD | 40.4 ± 16.1 | 36.6 ± 15.5 | 43.3 ± 15.9 | |||
| <55 | 1087 | 81.4 | 509 | 87.2 | 578 | 77.0 |
| ≥55 | 248 | 18.6 | 75 | 12.8 | 173 | 23.0 |
| Sex | ||||||
| Male | 330 | 24.7 | 138 | 23.6 | 192 | 25.6 |
| Female | 1005 | 75.3 | 446 | 76.4 | 559 | 74.4 |
| Histologic subtype | ||||||
| Classical variant | 895 | 67.1 | 314 | 53.7 | 581 | 77.4 |
| Follicular variant | 232 | 17.4 | 185 | 31.7 | 47 | 6.3 |
| Tall cell variant | 118 | 8.8 | 21 | 3.6 | 97 | 12.9 |
| Other variants | 90 | 6.7 | 64 | 11.0 | 26 | 3.5 |
| Extrathyroidal extension | ||||||
| Present | 590 | 44.2 | 178 | 30.5 | 412 | 54.9 |
| Absent | 745 | 55.8 | 406 | 69.5 | 339 | 45.1 |
| Multifocality | ||||||
| Yes | 670 | 50.2 | 259 | 44.4 | 411 | 54.7 |
| No | 665 | 49.8 | 325 | 55.6 | 340 | 45.3 |
| pT | ||||||
| T1 | 369 | 28.5 | 176 | 31.3 | 193 | 26.4 |
| T2 | 266 | 20.6 | 138 | 24.6 | 128 | 17.5 |
| T3 | 549 | 42.4 | 212 | 37.7 | 337 | 46.0 |
| T4 | 110 | 8.5 | 36 | 6.4 | 74 | 10.1 |
| pN | ||||||
| N0 | 527 | 39.5 | 280 | 48.0 | 247 | 32.9 |
| N1 | 683 | 51.1 | 245 | 41.9 | 438 | 58.3 |
| Nx | 125 | 9.4 | 59 | 10.1 | 66 | 8.8 |
| pM | ||||||
| M0 | 1205 | 95.4 | 518 | 93.7 | 687 | 96.8 |
| M1 | 58 | 4.6 | 35 | 6.3 | 23 | 3.2 |
| TNM Stage | ||||||
| I | 1084 | 83.4 | 499 | 87.5 | 585 | 80.1 |
| II | 148 | 11.4 | 51 | 8.9 | 97 | 13.3 |
| III | 21 | 1.6 | 4 | 0.7 | 17 | 2.3 |
| IV-A | 17 | 1.3 | 2 | 0.4 | 15 | 2.1 |
| IV-B | 30 | 2.3 | 14 | 2.5 | 16 | 2.2 |
| NRAS mutation | ||||||
| Present | 85 | 6.4 | 83 | 14.3 | 2 | 0.3 |
| Absent | 1244 | 93.6 | 498 | 85.7 | 746 | 99.7 |
| HRAS mutation | ||||||
| Present | 30 | 2.3 | 28 | 4.2 | 2 | 0.3 |
| Absent | 1298 | 97.7 | 553 | 95.2 | 745 | 99.7 |
2.2. Clinicopathological Associations of COX-2 Expression in PTC
We performed immunohistochemical analysis to look for COX-2 protein expression in our cohort of 1335 PTC cases. Immunohistochemistry data were interpretable in 1314 cases, and cytoplasmic staining was noted (Figure 1). COX-2 overexpression, noted in 43.2% (567/1314) of cases, was significantly associated with older age (p < 0.0001), extra-thyroidal extension (p < 0.0001), T4 tumors (p = 0.0094), lymph-node metastasis (p = 0.0003), and advanced stage (p < 0.0001) (Table 2). A significant association was also observed between COX-2 overexpression and poor disease-free survival (DFS; p < 0.0001) (Table 2; Figure 2A). Multivariate analysis using the Cox regression model, after adjusting for possible confounding factors of survival, showed that COX-2 overexpression was an independent predictor of poor DFS (HR = 1.57; 95% CI = 1.22–2.03; p = 0.0004) (Table 3).
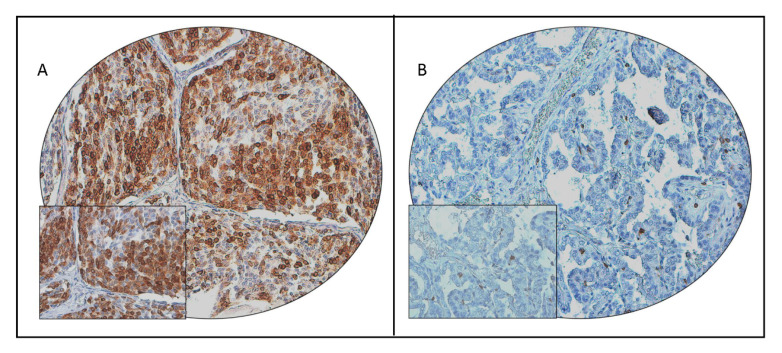
Figure 1.
Cyclooxygenase-2 (COX-2) immunohistochemical staining in papillary thyroid carcinoma (PTC) tissue microarray (TMA). Representative examples of tumors showing (A) high expression and (B) low expression of COX-2 (20×/0.70 objective on an Olympus BX 51 microscope (Olympus America Inc., Center Valley, PA, USA), with the inset showing a 40×/0.85 aperture magnified view of the same TMA spot).
Table 2.
Association of clinicopathological characteristics with Cox-2 expression in PTC.
| Total | Cox-2 Positive | Cox-2 Negative | p Value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total | 1314 | 567 | 43.2 | 747 | 56.8 | ||
| Age at surgery (years) | |||||||
| <55 | 1072 | 81.6 | 418 | 39.0 | 654 | 61.0 | <0.0001 |
| ≥55 | 242 | 18.4 | 149 | 61.6 | 93 | 38.4 | |
| Sex | |||||||
| Male | 320 | 24.4 | 146 | 45.6 | 174 | 54.4 | 0.3049 |
| Female | 994 | 75.6 | 421 | 42.4 | 174 | 57.6 | |
| Histologic subtype | |||||||
| Classical variant | 879 | 66.9 | 394 | 44.8 | 485 | 55.2 | 0.1542 |
| Follicular variant | 229 | 17.4 | 84 | 36.7 | 145 | 63.3 | |
| Tall cell variant | 116 | 8.8 | 52 | 44.8 | 64 | 55.2 | |
| Other variants | 90 | 6.9 | 37 | 41.1 | 53 | 58.9 | |
| Extrathyroidal extension | |||||||
| Present | 579 | 44.1 | 291 | 50.3 | 288 | 49.7 | <0.0001 |
| Absent | 735 | 55.9 | 276 | 37.6 | 459 | 62.4 | |
| Multifocality | |||||||
| Yes | 658 | 50.1 | 295 | 44.8 | 363 | 55.2 | 0.2175 |
| No | 656 | 49.9 | 272 | 41.5 | 384 | 58.5 | |
| pT | |||||||
| T1 | 365 | 28.7 | 140 | 38.4 | 225 | 61.6 | 0.0094 |
| T2 | 265 | 20.8 | 101 | 38.1 | 164 | 61.9 | |
| T3 | 538 | 42.2 | 253 | 47.0 | 285 | 53.0 | |
| T4 | 106 | 8.3 | 53 | 50.0 | 53 | 50.0 | |
| pN | |||||||
| N0 | 516 | 43.3 | 194 | 37.6 | 322 | 62.4 | 0.0003 |
| N1 | 675 | 56.7 | 325 | 48.2 | 350 | 51.8 | |
| pM | |||||||
| M0 | 1186 | 95.4 | 509 | 42.9 | 677 | 57.1 | 0.3575 |
| M1 | 57 | 4.6 | 28 | 49.1 | 29 | 50.9 | |
| TNM Stage | |||||||
| I | 1069 | 83.4 | 415 | 38.8 | 654 | 61.2 | <0.0001 |
| II | 146 | 11.4 | 86 | 58.9 | 60 | 41.1 | |
| III | 20 | 1.6 | 17 | 85.0 | 3 | 15.0 | |
| IV-A | 17 | 1.3 | 10 | 58.8 | 7 | 41.2 | |
| IV-B | 29 | 2.3 | 21 | 72.4 | 8 | 27.6 | |
| BRAF mutation | |||||||
| Present | 740 | 56.3 | 344 | 46.5 | 396 | 53.5 | 0.0055 |
| Absent | 574 | 43.7 | 223 | 38.9 | 351 | 61.1 | |
| 5-year disease-free survival | 69.5 | 81.7 | <0.0001 | ||||
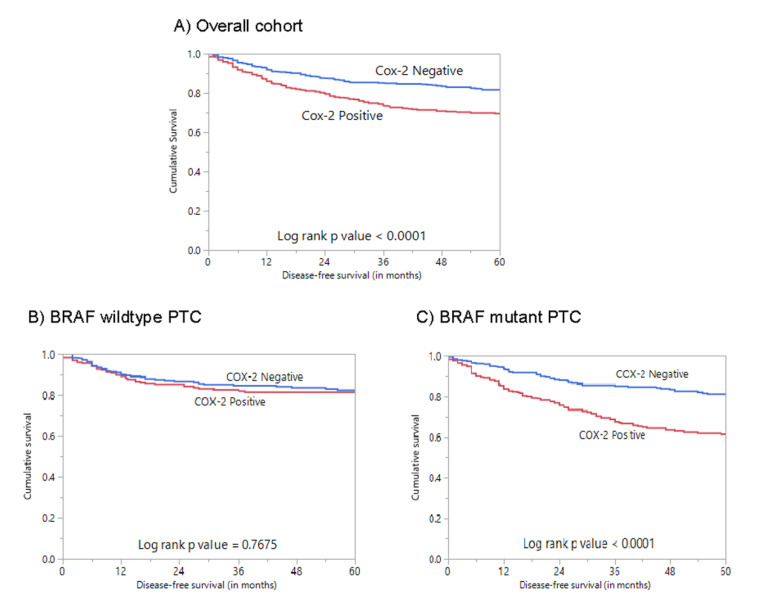
Figure 2.
Survival analyses of COX-2 protein expression in the overall cohort and based on BRAF mutation status. (A) Kaplan–Meier survival plot showing statistically significant poor disease-free survival in COX-2 high-expression cases compared to COX-2 low-expression cases (p < 0.0001); (B) Kaplan–Meier survival plot showing no statistically significant difference in disease-free survival for COX-2 expression in BRAF wild-type PTC (p = 0.7675); (C) Kaplan–Meier survival plot showing statistically significant poor disease-free survival for cases with COX-2 overexpression in BRAF-mutant PTC (p < 0.0001).
Table 3.
Univariate and multivariate analysis of COX-2 expression using the Cox proportional-hazards model.
| Clinical Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age | ||||
| ≥55 years (vs. <55 years) | 2.82 (2.21–3.57) | <0.0001 | 2.04 (1.51–2.76) | <0.0001 |
| Sex | ||||
| Male (vs. Female) | 0.59 (0.47–0.74) | <0.0001 | 0.66 (0.51–0.85) | 0.0016 |
| Extrathyroidal extension | ||||
| Present (vs. Absent) | 2.89 (2.24–3.77) | <0.0001 | 2.26 (1.72–2.99) | <0.0001 |
| pM | ||||
| M1 (vs. M0) | 4.52 (3.12–6.34) | <0.0001 | 2.84 (1.74–4.63) | <0.0001 |
| Stage | ||||
| IV (vs. I–III) | 4.40 (2.84–6.52) | <0.0001 | 0.83 (0.45–1.54) | 0.5560 |
| COX-2 IHC | ||||
| Overexpression (vs. low expression) | 1.66 (1.32–2.08) | <0.0001 | 1.57 (1.22–2.03) | 0.0004 |
2.3. COX-2 Expression and BRAF Mutation in PTC
We found a significant association between COX-2 overexpression and BRAF mutation (p = 0.0055) in our cohort. Next, we examined the prognostic association of COX-2 expression in the strata of BRAF mutation status. Using Kaplan–Meier survival analyses, we found COX-2 expression was associated with a significantly shorter DFS in BRAF-mutant PTC, but not in BRAF wild-type cases (Figure 2B,C). Multivariate analysis showed COX-2 overexpression was associated with shorter DFS in BRAF-mutant PTC cases (HR = 2.10; 95% CI = 1.52–2.92; p < 0.0001) (Table 4).
Table 4.
Univariate and multivariate analysis of COX-2 expression in BRAF-mutant PTC using the Cox proportional-hazards model.
| Clinical Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | p-Value | Risk Ratio (95% CI) | p-Value | |
| Age | ||||
| ≥55 years (vs. <55 years) | 2.45 (1.84–3.25) | <0.0001 | 1.75 (1.22–2.48) | 0.0020 |
| Sex | ||||
| Male (vs. Female) | 0.55 (0.42–0.74) | <0.0001 | 0.52 (0.39–0.72) | <0.0001 |
| Extrathyroidal extension | ||||
| Present (vs. Absent) | 2.77 (1.96–4.01) | <0.0001 | 2.11 (1.46–3.10) | <0.0001 |
| pM | ||||
| M1 (vs. M0) | 4.69 (2.64–7.72) | <0.0001 | 4.44 (1.61–11.71) | 0.0036 |
| Stage | ||||
| IV (vs. I–III) | 3.15 (1.77–5.16) | <0.0001 | 0.49 (0.17–1.25) | 0.1656 |
| COX-2 IHC | ||||
| Overexpression (vs. low expression) | 2.10 (1.59–2.79) | <0.0001 | 2.10 (1.52–2.92) | <0.0001 |
3. Discussion
We conducted this study using a large cohort of more than 1300 Middle Eastern PTC tumors, and found a frequency of COX-2 expression of 43.2%. There was a strong association of COX-2 expression with adverse markers of PTC prognosis, most notably extra-thyroidal extension, lymph-node metastasis, and higher tumor stage. A recent study by Fu et al. [15] demonstrated a similar association of COX-2 expression with aggressive clinicopathological features such as extra-thyroidal extension and multifocality in 252 PTC samples. Several previous studies have explored the usefulness of COX-2 as a biomarker of thyroid malignancies and its potential role in PTC carcinogenesis [15,31,32,33], but the prognostic role of COX-2 in PTC is still controversial [34,35]. Our current study shows that elevated COX-2 expression is an independent predictor of poor DFS in patients with PTC.
COX-2 mainly generates prostaglandin E2 (PGE2). The COX-2–PGE2 pathway is known to play an important role in tumor progression, and its oncogenic role has been shown in several tumor types [36,37,38,39]. However, recent evidence shows that PGE2 is important in modifying the tumor microenvironment to induce immune tolerance [40,41,42]. PGE2 induces alterations in cytokine balance and causes suppression of lymphocyte proliferation following mitogen stimulation and inhibition of dendritic cells [43,44,45]. Given the known function of PGE2 in modulating the tumor microenvironment to suppress an immune response [46], our data clearly show the strong relationship among cancer progression, aggressiveness, lymph-node metastasis, and COX-2 expression.
We observed a differential prognostic association of COX-2 expression according to BRAF mutation status in our study. The prognostic association of COX-2 expression was significantly pronounced in BRAF-mutated PTC compared to BRAF wild-type PTC. A similar observation has been documented recently in CRC [30]. This further supports the role of BRAF mutation in the acceleration of the production of PGE2 via COX-2 [41,47,48], and highlights how a subset of PTC might use increased COX-2 activity as a possible pathway that affects the survival of patients with the BRAF mutation.
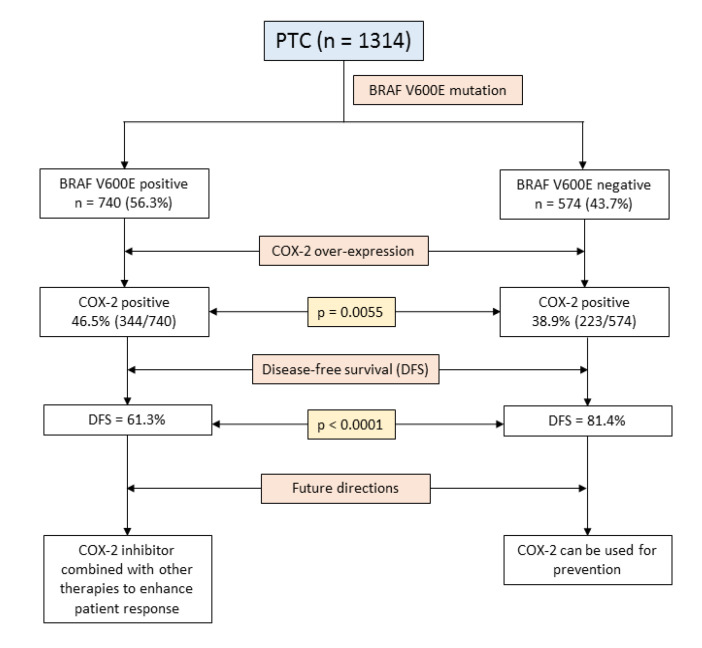
Our study reveals the necessity for additional prognostic biomarkers for PTC, especially markers of tumor-associated inflammation, which could be used to tailor therapeutic approaches and improve patient survival. Although our findings suggest that inhibition of COX-2 in BRAF-mutated PTC might be a good therapeutic strategy, clinical trials and further clinical research is needed to explore the therapeutic advantage of adding COX-2 inhibitors to the current iodine therapy in BRAF-mutated PTC patients. The findings of our study are summarized in Figure 3.
Figure 3.
Schematic presentation of the study findings and future directions.
4. Materials and Methods
4.1. Sample Selection
The study included 1335 PTC patients diagnosed between 1989 and 2018 at King Faisal Specialist Hospital and Research Center (Riyadh, Saudi Arabia) with available archival tissue samples. Clinicopathological data were collected from case records (Table 1). The hospital’s institutional review board approved the collection of archival samples. For this study, since only archived paraffin tissue blocks were used, a waiver of consent was obtained from the Research Advisory Council (RAC) under project RAC# 2110 031.
4.2. DNA Isolation
DNA was extracted from PTC formalin-fixed and paraffin-embedded (FFPE) tumor tissues using the Gentra DNA isolation kit (Gentra, Minneapolis, MN, USA) according to the manufacturer’s protocols as elaborated in previous studies [49].
4.3. Sanger Sequencing Analysis
The sequencing of entire coding and splicing regions of exon 15 in BRAF genes, and exon 2 and 3 in HRAS and NRAS genes, in the 1335 PTC samples was carried out using Sanger sequencing technology. Primer 3 online software was used to design the primers (available upon request). PCR and Sanger sequencing analysis were conducted as described previously [50]. The reference sequence was downloaded from NCBI GenBank. The sequencing results were compared with the reference sequence by Mutation Surveyor V4.04 (Soft Genetics, LLC, State College, PA, USA).
4.4. Tissue Microarray (TMA) Construction and Immunohistochemistry (IHC) Analysis
The tissue microarray (TMA) format was used for immunohistochemical analysis of the PTC samples. TMA was constructed as previously described [51]. A modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI, USA) was used to punch tissue cylinders with a diameter of 0.6 mm from a representative tumor area of the donor tissue block into the recipient paraffin block. Two 0.6 mm cores of PTC were arrayed from each case.
Tissue microarray slides were processed and stained manually as described previously [52], using a primary antibody against COX-2 (ab15191, 1:1000 dilution, pH 6.0, Abcam, Cambridge, UK). The Dako Envision Plus System kit was used as the secondary detection system with 3,30-diaminobenzidine as chromogen. All slides were counterstained with hematoxylin, dehydrated, cleared, and mounted. The negative controls included omission of the primary antibody. Normal tissues of different organ system were also included in the TMA to serve as controls. Only fresh-cut slides were stained simultaneously to minimize the influence of slide aging and maximize reproducibility of the experiment.
A cytoplasmic staining was observed and scored. COX-2 was scored as described previously [53] using the H score. The intensity of staining was scored from 0–3 (0: absent, 1+: weak, 2+: moderate, 3+: strong), and the proportion of tumor-cell staining for a particular intensity was recorded in 5% increments in the range of 0–100. A final H score was assigned using the following formula: H score = (1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)). This H score ranged from 0–300. Two scores per tumor were analyzed to minimize the number of missing/uninterpretable spots. However, the higher of the two scores was used as the final score. X-tile plots were constructed for the assessment of biomarkers and the optimization of cut-off points based on an outcome described earlier [54]. Based on X-tile plots, PTC cases were classified into two subgroups: those with an H score of 0, defined as negative expression of COX-2, and those with an H score > 0, defined as overexpression.
4.5. Statistical Analysis
The associations between clinico-pathological variables and protein expression were derived using contingency table analysis and Chi square tests. The Mantel–Cox log-rank test was used to evaluate disease-free survival. Survival curves were generated using the Kaplan–Meier method. The Cox proportional-hazards regression model was used for multivariate analysis. Two-sided tests were used for statistical analyses, with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP11.0 (SAS Institute, Inc., Cary, NC, USA) software package.
5. Conclusions
Recently, the possibility of combining different therapies, including biological therapies and kinase inhibitors, has contributed to tailoring the treatment of cancer patients and improving their survival. Our data show that COX-2 correlates with PTC disease-free survival in BRAF-mutated tumors, representing a useful prognostic marker for risk stratification of thyroid cancer patients. These findings have clinical relevance because they provide a rationale to test COX-2 inhibition as a potential treatment to prevent PTC progression and enhance the antitumor activity of other cancer therapies to treat patients with aggressive PTC and BRAF mutations.
Acknowledgments
The authors would like to thank Felisa DeVera for technical assistance.
Abbreviations
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| PTC | Papillary thyroid carcinoma |
| DFS | Disease-free survival |
| FFPE | Formalin-fixed and paraffin-embedded |
| PCR | Polymerase chain reaction |
| TMA | Tissue microarray |
| IHC | Immunohistochemistry |
| SD | Standard deviation |
| HR | Hazard ratio |
| CI | Confidence interval |
Author Contributions
Conceptualization, K.S.A.-K.; Methodology, S.K.P. and A.K.S.; Software, S.K.P.; Validation, S.K.P. and P.A.; Formal analysis, S.K.P., A.K.S. and P.A.; Investigation, S.K.P., P.A., S.S.A.-S. and F.A.-D.; Resources, S.K.P., S.S.A.-S. and F.A.-D.; Data curation, S.K.P., A.K.S. and P.A.; Writing—Original draft preparation, K.S.A.-K.; Writing—Review and editing, S.K.P., A.K.S. and K.S.A.-K.; Visualization, A.K.S. and S.K.P.; Supervision, K.S.A.-K. and A.K.S.; Project administration, K.S.A.-K. and A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C., Malvezzi M., Bosetti C., Garavello W., Bertuccio P., Levi F., Negri E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Lim H., Devesa S.S., Sosa J.A., Check D., Kitahara C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317:1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alrawaji A., Alshahrani Z., Alzahrani W., Alomran F., Almadouj A., Alshehri S., Alzahrani A., Bazarbashi S., Alhashmi H., Almutlaq H., et al. Saudi Cancer Registry. Saudi Health Council; Riyadh, Saudi Arabia: 2018. Cancer Incidence Report Saudi Arabia 2015. [Google Scholar]
- 5.Ritter A., Mizrachi A., Bachar G., Vainer I., Shimon I., Hirsch D., Diker-Cohen T., Duskin-Bitan H., Robenshtok E. Detecting recurrence following lobectomy for thyroid cancer: Role of thyroglobulin and thyroglobulin antibodies. J. Clin. Endocrinol. Metab. 2020;105:dgaa152. doi: 10.1210/clinem/dgaa152. [DOI] [PubMed] [Google Scholar]
- 6.Tumino D., Frasca F., Newbold K. Updates on the management of advanced, metastatic, and radioiodine refractory differentiated thyroid cancer. Front. Endocrinol. 2017;8:312. doi: 10.3389/fendo.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing M., Alzahrani A.S., Carson K.A., Shong Y.K., Kim T.Y., Viola D., Elisei R., Bendlová B., Yip L., Mian C. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015;33:42. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Wang D., DuBois R.N. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 11.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. doi: 10.1096/fasebj.12.12.1063. [DOI] [PubMed] [Google Scholar]
- 12.Petkova D., Clelland C., Ronan J., Pang L., Coulson J., Lewis S., Knox A. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004;98:164–172. doi: 10.1016/j.rmed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal U., Kumari N., Vasudeva P., Mohanty N.K., Saxena S. Overexpression of COX2 indicates poor survival in urothelial bladder cancer. Ann. Diagn. Pathol. 2018;34:50–55. doi: 10.1016/j.anndiagpath.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Ogino S., Kirkner G.J., Nosho K., Irahara N., Kure S., Shima K., Hazra A., Chan A.T., Dehari R., Giovannucci E.L. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin. Cancer Res. 2008;14:8221–8227. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X., Zhang H., Chen Z., Yang Z., Shi D., Liu T., Chen W., Yao F., Su X., Deng W. TFAP2B overexpression contributes to tumor growth and progression of thyroid cancer through the COX-2 signaling pathway. Cell Death Dis. 2019;10:1–13. doi: 10.1038/s41419-019-1600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Y., Teng Y., Zhang R., Luo L. Antitumor effect of the selective COX-2 inhibitor celecoxib on endometrial adenocarcinoma in vitro and in vivo. Oncol. Lett. 2012;4:1219–1224. doi: 10.3892/ol.2012.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L., Stevens J., Hilton M.B., Seaman S., Conrads T.P., Veenstra T.D., Logsdon D., Morris H., Swing D.A., Patel N.L. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci. Transl. Med. 2014;6:242ra84. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Wang Y., Yang J., Zhang Z., Zhang Y., Du H. Celecoxib induces apoptosis but up-regulates VEGF via endoplasmic reticulum stress in human colorectal cancer in vitro and in vivo. Cancer Chemother. Pharmacol. 2016;77:797–806. doi: 10.1007/s00280-016-2996-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu B., Qu L., Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015;15:106. doi: 10.1186/s12935-015-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhough A., Smartt H.J., Moore A.E., Roberts H.R., Williams A.C., Paraskeva C., Kaidi A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 21.Krawczyk-Rusiecka K., Lewiński A. Cyclooxygenase-2 expression and its association with thyroid lesions. Arch. Med. Sci. 2010;6:653. doi: 10.5114/aoms.2010.17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawczyk-Rusiecka K., Wojciechowska-Durczynska K., Cyniak-Magierska A., Zygmunt A., Lewinski A. Assessment of cyclooxygenase-1 and 2 gene expression levels in chronic autoimmune thyroiditis, papillary thyroid carcinoma and nontoxic nodular goitre. Thyroid Res. 2014;7:10. doi: 10.1186/s13044-014-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynik D.M., Prayson R.A. Immunohistochemical expression of cyclooxygenase 2 in follicular carcinomas of the thyroid. Arch. Pathol. Lab. Med. 2005;129:736–741. doi: 10.5858/2005-129-736-IEOCIF. [DOI] [PubMed] [Google Scholar]
- 24.Perisa M.M., Sarcevic B., Troselj K.G., Grsic K., Sitic S., Seiwerth S. Expression of nm23-H1 and COX-2 in thyroid papillary carcinoma and microcarcinoma. Oncol. Lett. 2017;13:3547–3555. doi: 10.3892/ol.2017.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji B., Liu Y., Zhang P., Wang Y., Wang G. COX-2 expression and tumor angiogenesis in thyroid carcinoma patients among northeast Chinese population-result of a single-center study. Int. J. Med. Sci. 2012;9:237. doi: 10.7150/ijms.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge J., Wang J., Wang H., Jiang X., Liao Q., Gong Q., Mo Y., Li X., Li G., Xiong W. The BRAF V600E mutation is a predictor of the effect of radioiodine therapy in papillary thyroid cancer. J. Cancer. 2020;11:932. doi: 10.7150/jca.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C., Huang M., Li X., Wang T., Ling R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr. Connect. 2019;8:988–996. doi: 10.1530/EC-19-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan X., Shen F., Deng X., Feng J., Lu J., Cai W., Peng L., Zheng W., Wang W., Huang P. Prognostic implications of the BRAF-V600E mutation in papillary thyroid carcinoma based on a new cut-off age stratification. Oncol. Lett. 2020;19:631–640. doi: 10.3892/ol.2019.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan P.T., Garnett M.J., Roe S.M., Lee S., Niculescu-Duvaz D., Good V.M., Project C.G., Jones C.M., Marshall C.J., Springer C.J. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 30.Kosumi K., Hamada T., Zhang S., Liu L., da Silva A., Koh H., Twombly T.S., Mima K., Morikawa T., Song M. Prognostic association of PTGS2 (COX-2) over-expression according to BRAF mutation status in colorectal cancer: Results from two prospective cohorts and CALGB 89803 (Alliance) trial. Eur. J. Cancer. 2019;111:82–93. doi: 10.1016/j.ejca.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng X.-Y., Zhang Q., Li Q., Lin S., Li J. Immunohistochemical levels of cyclo-oxygenase-2, matrix metalloproteinase-9 and vascular endothelial growth factor in papillary thyroid carcinoma and their clinicopathological correlations. J. Int. Med. Res. 2014;42:619–627. doi: 10.1177/0300060513505485. [DOI] [PubMed] [Google Scholar]
- 32.Erdem H., Gündogdu C., Şipal S. Correlation of E-cadherin, VEGF, COX-2 expression to prognostic parameters in papillary thyroid carcinoma. Exp. Mol. Pathol. 2011;90:312–317. doi: 10.1016/j.yexmp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Giaginis C., Alexandrou P., Delladetsima I., Karavokyros I., Danas E., Giagini A., Patsouris E., Theocharis S. Clinical significance of Hu-antigen receptor (HuR) and cyclooxygenase-2 (COX-2) expression in human malignant and benign thyroid lesions. Pathol. Oncol. Res. 2016;22:189–196. doi: 10.1007/s12253-015-9997-5. [DOI] [PubMed] [Google Scholar]
- 34.Cunha L.L., Marcello M.A., Nonogaki S., Morari E.C., Soares F.A., Vassallo J., Ward L.S. CD 8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clin. Endocrinol. 2015;83:246–253. doi: 10.1111/cen.12586. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.R., Si Y., Lee Y.S., Kim J.S., Jeon H.M., Park W.C. The Clinical Significance of Expression of Cyclooxygenase-2 in the Prognosis of the Papillary Thyroid Cancer. Korean J. Endocr. Surg. 2007;7:75–79. doi: 10.16956/kjes.2007.7.2.75. [DOI] [Google Scholar]
- 36.Wang D., Fu L., Sun H., Guo L., DuBois R.N. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–1895.e4. doi: 10.1053/j.gastro.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Sun H., Hu M., Zhang Y., Chen S., Tighe S., Zhu Y. The role of cyclooxygenase-2 in colorectal carcinogenesis. Clin. Colorectal Cancer. 2017;16:165–172. doi: 10.1016/j.clcc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Xie C., Xu X., Wang X., Wei S., Shao L., Chen J., Cai J., Jia L. Cyclooxygenase-2 induces angiogenesis in pancreatic cancer mediated by prostaglandin E2. Oncol. Lett. 2018;16:940–948. doi: 10.3892/ol.2018.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashemi Goradel N., Najafi M., Salehi E., Farhood B., Mortezaee K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno R., Kawada K., Sakai Y. Prostaglandin E2/EP Signaling in the Tumor Microenvironment of Colorectal Cancer. Int. J. Mol. Sci. 2019;20:6254. doi: 10.3390/ijms20246254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelenay S., Van Der Veen A.G., Böttcher J.P., Snelgrove K.J., Rogers N., Acton S.E., Chakravarty P., Girotti M.R., Marais R., Quezada S.A. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller A.J., Scherle P.A. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat. Rev. Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Zhu B., Li Y. Resolution of cancer-promoting inflammation: A new approach for anticancer therapy. Front. Immunol. 2017;8:71. doi: 10.3389/fimmu.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Göbel C., Breitenbuecher F., Kalkavan H., Hähnel P., Kasper S., Hoffarth S., Merches K., Schild H., Lang K., Schuler M. Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis. 2014;5:e1568. doi: 10.1038/cddis.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu A.A., Drake V., Huang H.-S., Chiu S., Zheng L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. doi: 10.1080/2162402X.2015.1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M., Matsumura N., Mandai M., Li K., Yagi H., Baba T., Suzuki A., Hamanishi J., Fukuhara K., Konishi I. Classification using hierarchical clustering of tumor-infiltrating immune cells identifies poor prognostic ovarian cancers with high levels of COX expression. Mod. Pathol. 2009;22:373–384. doi: 10.1038/modpathol.2008.187. [DOI] [PubMed] [Google Scholar]
- 47.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 48.Shi C., Yang Y., Xia Y., Okugawa Y., Yang J., Liang Y., Chen H., Zhang P., Wang F., Han H. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65:1470–1481. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 49.Abubaker J., Jehan Z., Bavi P., Sultana M., Al-Harbi S., Ibrahim M., Al-Nuaim A., Ahmed M., Amin T., Al-Fehaily M. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J. Clin. Endocrinol. Metab. 2008;93:611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 50.Bu R., Siraj A.K., Al-Obaisi K.A., Beg S., Al Hazmi M., Ajarim D., Tulbah A., Al-Dayel F., Al-Kuraya K.S. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int. J. Cancer. 2016;139:1091–1097. doi: 10.1002/ijc.30143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siraj A., Bavi P., Abubaker J., Jehan Z., Sultana M., Al-Dayel F., Al-Nuaim A., Alzahrani A., Ahmed M., Al-Sanea O. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J. Pathol. 2007;213:190–199. doi: 10.1002/path.2215. [DOI] [PubMed] [Google Scholar]
- 52.Bavi P., Jehan Z., Atizado V., Al-Dossari H., Al-Dayel F., Tulbah A., Amr S.S., Sheikh S.S., Ezzat A., El-Solh H. Prevalence of fragile histidine triad expression in tumors from Saudi Arabia: A tissue microarray analysis. Cancer Epidemiol. Prev. Biomark. 2006;15:1708–1718. doi: 10.1158/1055-9965.EPI-05-0972. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed M., Hussain A.R., Siraj A.K., Uddin S., Al-Sanea N., Al-Dayel F., Al-Assiri M., Beg S., Al-Kuraya K.S. Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol. Cancer. 2015;14:1–14. doi: 10.1186/s12943-015-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camp R.L., Dolled-Filhart M., Rimm D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]