Abstract
Background:
Motoric cognitive risk (MCR) syndrome is a cognitive-motor syndrome associated with increased risk of transition to dementia. The clinical phenotype of MCR is not yet established.
Objective:
To systematically assess clinical gait abnormalities in older adults with MCR.
Methods:
Of the 522 community-dwelling non-demented adults aged 65 and older enrolled in the Central Control of Mobility in Aging study, 43 were diagnosed with MCR (47% women) based on presence of cognitive complaints and slow gait velocity (MCRv). Four additional subtypes of MCR were defined by substituting slow gait with short stride length (MCRsl, n = 41), slow swing time (MCRsw, n = 21), high stride length variability (MCRslv, n = 24), and high swing time variability (MCRswv, n = 25). The prevalence of clinical gait abnormalities (neurological or non-neurological) in MCR overall (n = 81) and subtypes was studied. We also examined if gait abnormalities predicted further cognitive and functional decline in MCR cases.
Results:
Most clinical gait abnormalities were mild (walked without assistance) in the five MCR subtypes (44 to 61%). Neurological (range 24 to 46%) and non-neurological gait abnormalities (33 to 61%) were common in all MCR subtypes. Neurological gaits were most frequent in MCRsl (46%) and non-neurological gaits in MCRv (61%). Over a median 3.02 years of follow-up, presence of gait abnormality in MCR cases at baseline predicted worsening disability scores (estimate 0.17, p-value = 0.033) but not decline on cognitive scores (p-value = 0.056).
Conclusion:
Clinical gait abnormalities are common in MCR syndrome and its subtypes, and are associated with accelerated functional decline.
Keywords: Cognition, gait, motoric cognitive risk syndrome
INTRODUCTION
Motoric cognitive risk (MCR) syndrome is a pre-dementia syndrome characterized by the presence of subjective cognitive complaints and slow gait velocity [1, 2]. Individuals with MCR are at high risk for transitioning to dementia; both Alzheimer’s disease and vascular dementia [2, 3]. In a 17-country study with over 26,000 older adults, MCR was shown to be common with a prevalence of 9.7% [3] and incidence of 65/1000 person-years [1]. In addition to dementia, studies have shown that MCR increases risk for a number of other adverse outcomes in aging including cognitive decline [3], falls [4], disability [5], and mortality [6].
The gait criterion in MCR was based on extensive research that has shown that slowing of gait occurs early in the course of dementia [7–10] and predicts progression to dementia in cognitively normal adults as well as those with mild cognitive impairment (MCI) [9]. The MCR diagnostic procedure is independent of the MCI diagnosis as well as results of complex cognitive tests or assays, increasing its clinical utility. Gait velocity can be easily measured in clinics and enhances the accessibility of MCR as a dementia risk assessment tool. But MCR subtypes have also been defined using gait parameters other than velocity [11], and were shown to have commonalities and differences in cognitive profiles and risk factors [11]. This concept is similar to that of amnestic and non-amnestic MCI subtypes, which are characterized by impairments in different domains of cognitive function, and have been shown to have different risk profiles [12, 13] and be predictive of Alzheimer’s and non-Alzheimer’s dementias, respectively [14].
The clinical phenotype of MCR is not yet established. While slow gait is associated with clinical gait abnormalities [15], the relationship is not absolute. In a community-based sample of 380 older adults, we reported that of the 59 individuals with slow gait, 44 (74%) had either neurological or non-neurological gait abnormalities, and the rest (26%) were not diagnosed with a gait abnormality as assessed by the study clinician [15]. Based on previous evidence showing an association between clinically defined gait abnormalities and objectively measured slow gait [15], we hypothesized that patterns of neurological and non-neurological clinical gait abnormalities would differ by MCR subtypes. As clinical gait abnormalities and MCR have been shown to predict outcomes such as dementia and disability [5, 16], as a secondary objective, we also examined the influence of clinical gait abnormalities on cognitive and functional decline in these individuals. Determining gait phenotypes in MCR cases may provide insights into underlying etiologies and improve prognostication.
METHODS
Study population
We studied community-residing adults age 65 and older enrolled in the “Central Control of Mobility in Aging” (CCMA) study [17]. The objective of CCMA is to determine cognitive processes and brain substrates controlling mobility [17–20]. CCMA procedures have been reported [17, 21, 22]. In brief, research assistants interviewed potential participants living in lower Westchester County by telephone to assess eligibility, and to rule out dementia using cognitive screeners [17–20]. Individuals who passed the telephone interview and expressed interest in participating in the study were invited to our center where they underwent detailed demographic and medical history ascertainment, clinical assessments, and neuropsychological testing [17–20]. Dementia diagnoses were assigned at consensus case conferences after review of all clinical and neuropsychological data [23]. Exclusion criteria for CCMA were presence of dementia, inability to walk (even with assistance), active neurological or psychiatric disorders severe enough to interfere with study assessments, presence of major visual or hearing loss, and recent or planned surgical procedures restricting walking. All participants provided written informed consents. The Einstein institutional review board approved the study protocol.
Clinical gait
Study clinicians determined whether gaits were normal or abnormal following visual inspection of gait patterns and turns while subjects walked up and down a well-lit hallway at their normal pace, as previously described [16, 24]. Study clinicians further determined whether abnormal gaits were due to neurological or non-neurological causes. Neurological gaits were subtyped as unsteady, ataxic, frontal, Parkinsonian, neuropathic, hemiparetic, and spastic as previously described (see references [16, 24] for detailed description and web links to videos of neurological gait subtypes [16]). Non-neurological gaits were subtyped into causes such as arthritis, cardiac disease, chronic lung disease, and peripheral vascular disease. In cases, where an individual was diagnosed with more than one neurological or non-neurological gait subtype, the subtype that contributed most to the clinical presentation in the judgment of the study clinician was reported (Table 2). If abnormal gaits in the judgment of the clinician resulted from both neurological and non-neurological causes, they were classified as “combined.” As it is likely that the neurological or non-neurological diseases resulting in these combined gait subtypes would both contribute to the outcomes, we included these cases in both neurological and non-neurological models. Gait abnormalities were graded as mild (walks unassisted), moderate (uses walking aids), or severe (wheelchair-bound) [25]. We reported 89% agreement on classifying gait as neurological or non-neurological on evaluations one year apart in 189 participants in the Bronx Aging Study [24]. Inter-rater reliability between clinicians using this gait protocol was found to be very good (kappa 0.8) [24].
Table 2.
Clinical gait abnormalities by MCR status
| Gait Abnormalities | Any MCR Subtype (n = 81) | MCRv (n = 43) | MCRsl (n = 41) | MCRsw (n = 21) | MCRslv (n = 24) | MCRswv (n = 25) |
|---|---|---|---|---|---|---|
| Any abnormality (n = 52) | 52 (64.2) | 34 (79.1) | 33 (80.5) | 11 (52.4) | 14 (58.3) | 14 (56.0) |
| Neurological gait (n = 28) | 28 (34.6) | 19 (44.2) | 19 (46.3) | 5 (23.8) | 9 (37.5) | 6 (24.0) |
| Non-Neurological gait (n = 39) | 39 (48.1) | 26 (60.5) | 24 (58.5) | 11 (52.4) | 8 (33.3) | 10 (40.0) |
| Severity of gait abnormality | ||||||
| Mild (n = 44) | 44 (54.3) | 26 (60.5) | 25 (61.0) | 10 (47.6) | 11 (45.8) | 11 (44.0) |
| Moderate (n = 8) | 8 (9.9) | 8 (18.6) | 8 (19.5) | 1 (4.8) | 3 (12.5) | 3 (12.0) |
| Neurological gait subtype | ||||||
| Parkinsonian (n = 1) | 1 (1.2) | 1 (2.3) | 1 (2.4) | 0 | 1 (4.2) | 0 |
| Ataxic (n = 2) | 2 (2.5) | 1 (2.3) | 2 (4.9) | 1 (4.8) | 0 | 0 |
| Unsteady (n = 6) | 6 (7.4) | 3 (7.0) | 4 (9.8) | 1 (4.8) | 4 (16.7) | 1 (4.0) |
| Spastic/stiff (n = 2) | 2 (2.5) | 1 (2.3) | 1 (2.4) | 0 | 1 (4.2) | 0 |
| Neuropathic (n = 17) | 17 (21.0) | 13 (30.2) | 11 (26.8) | 3 (14.3) | 3 (12.5) | 5 (20.0) |
| Non-neurological gait subtype | ||||||
| Arthritis (n = 25) | 25 (30.9) | 18 (41.9) | 16 (39.0) | 7 (33.3) | 7 (29.2) | 5 (20.0) |
| Joint deformity (n = 5) | 5 (6.2) | 2 (4.7) | 2 (4.9) | 2 (9.5) | 0 | 2 (8.0) |
| Respiratory (n = 1) | 1 (1.2) | 1 (2.3) | 1 (2.4) | 0 | 0 | 0 |
| Other (n = 8) | 4 (9.9) | 5 (11.6) | 5 (12.2) | 2 (9.5) | 1 (4.2) | 3 (12.0) |
MCR, motoric cognitive risk; MCRv, MCR velocity; MCRsl, MCR stride length; MCRsw, MCR swing time; MCRslv, MCR stride length variability; MCRswv, MCR swing time variability.
Quantitative gait
Research assistants conducted quantitative gait studies, independent of clinicians’ evaluations of gait, using a computerized walkway (180×35.5×0.25 inches) with embedded pressure sensors (GAITRite, CIR systems) [8]. Subjects were asked to walk on the mat at their ‘normal pace’ for two trials in a quiet well-lit hallway wearing comfortable footwear and without any attached monitoring devices. Start and stop points were marked by white lines on the floor, and included three feet from the walkway edge for initial acceleration and terminal deceleration. From footfalls recorded on the walkway, the software automatically computes gait parameters as the mean of two trials. The GAITRite system is widely used in clinical and research settings, and has excellent reliability [8].
Motoric cognitive risk syndrome and subtypes
MCR syndrome was diagnosed in participants based on established criteria [1–3]. MCR is defined as presence of subjective cognitive complaints and slow gait velocity in older individuals without dementia or mobility disability [1–3]. MCR builds on definitions of MCI; substituting the objective cognitive impairment criterion with slow gait. Cognitive complaints were assessed by one or more of the following: cognitive symptoms noted by the study clinician [26], a score of ≥1 on the AD8-dementia screener [27], or a ‘yes’ response the memory item on the Geriatric Depression Scale [28]. Gait speed and parameters were measured as described above. Slow gait was defined as walking speed one SD or more below age and sex specific means, as previously described [1–3]. Though gait dysfunction is multifactorial in nature, previous studies have shown that slow gait predicts cognitive decline irrespective of the underlying etiology [8].
For the purpose of the current study, we defined MCR based on the operational definition (described earlier) of presence of cognitive complaints and slow gait velocity (cm/s) as MCR velocity (MCRv). We adapted this definition to define four additional subtypes of MCR, as previously described [11], by substituting the slow gait criterion with abnormalities in other quantitative gait variables. These variables were selected as they had the highest loading on three previously described domains of gait (pace, rhythm and variability) [8, 29] and include stride length (cm), swing time (s), stride length variability (standard deviation (SD)) and swing time variability (SD). For the additional subtypes, we substituted slow gait with abnormalities in the other quantitative gait variables: MCR stride length (MCRsl), MCR swing time (MCRsw), MCR stride length variability (MCRslv), and MCR swing time variability (MCRswv). Short stride length and slow swing time were defined as one SD or more below age and sex-appropriate mean values in our cohort. High stride length variability and high swing time variability was one SD or more above age and sex-appropriate mean values. The remaining three MCR criteria (cognitive complaints, no dementia, and no mobility disability) remained the same. These MCR subtypes were not mutually exclusive.
Cognition
An extensive neuropsychological test battery was administered at all visits. We examined performance on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), a widely used omnibus test of general cognition as well as various cognitive domains [30]. It is a valid and reliable tool for detecting cognitive deficits across different age levels and diagnostic groups, and consists of 12 subtests making up five indices: immediate memory, delayed memory visuospatial/constructional, language, and attention [30]. Index scores as well as total scores were calculated; scores range from 62 to 138 with higher scores reflecting better performance. For the purpose of this study, we examined baseline and annual change in continuous RBANS total scores over follow-up.
Functional status
Seven activities of daily living were assessed by a disability scale developed for use in community-based cohorts [31]: bathing, dressing, grooming, feeding, toileting, walking around home, and getting up from a chair. Subjects were asked by the research assistant if they were able to perform an activity unassisted (0 points), unassisted but with difficulty (1 point), or whether they required assistance or were unable to do the activity (2 points). A summary disability score was then computed (range 0 to 14, higher worse) [7, 31]. The scale was administered at baseline and annual study visits [7].
Covariates
Participants were interviewed at each visit about socio-demographic variables and medical illnesses. A summary comorbidity index (range 0–10) was calculated as described previously [8] by summing the following self-reported physician diagnosed diseases: diabetes, chronic heart failure, arthritis, hypertension, major depression, stroke, Parkinson’s disease, chronic obstructive lung disease, angina, and myocardial infarction.
Data analysis
Demographic characteristics and variables of interest were tabulated per diagnostic group and compared using ANOVA and Chi-square tests [32]. Prevalence of clinical gait abnormalities was compared across MCR subtypes. We compared cognitive test performance at baseline in MCR overall (all subtypes combined) by clinical gait abnormality status (overall, neurological, and non-neurological). We then examined if clinical gait abnormality status (overall, neurological, and non-neurological) in individuals diagnosed with MCR at baseline would predict subsequent decline on total RBANS and increasing disability scores cases over follow-up using linear mixed effects models. The ‘time’ term in the models (summarized in Table 3) represents average rate of change in performance on the cognitive and functional assessments over follow-up. The two-way interaction term represents the longitudinal effect of baseline measure of gait abnormality on the annual rate of decline on the RBANS or disability scores. “Time” was calculated in years from baseline to each study visit. An interaction between individual clinical gait abnormality diagnosis (overall, neurological, and non-neurological) and time was included to model the effect of presence of a clinical gait abnormality on rate of change in cognitive and functional assessments. Results are reported as parameter estimates with 95% confidence intervals (CI). The linear mixed effects model can accommodate unbalanced data resulting from missing data points, unequal numbers of follow-up visits, and unequal intervals between visits. A random intercept was included in the model to allow entry point to vary across individuals. Analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC) statistical software.
Table 3.
Results of Linear mixed effect models of gait abnormalities and their association with cognitive decline in 81 individuals with any MCR subtype. See explanation of Model in Methods
| Variables | Cognitive decline* Adjusted estimate, (95% CI), p-value | Functional decline* Adjusted estimate, (95% CI), p-value |
|---|---|---|
| Any gait abnormality | 1.03 (−6.85–4.79), 0.728 | 0.84 (0.21–1.47), 0.010 |
| Time | −0.67 (−1.42–0.07), 0.076 | 0.00 (−0.12–0.12), 0.951 |
| Any gait abnormality × Time | 0.95 (−0.03–1.93), 0.056 | 0.17 (0.01–0.33), 0.033 |
| Neurological gaits | −2.47 (−8.14–3.20), 0.391 | 0.18 (−0.51–0.87), 0.611 |
| Time | −0.26 (−0.87–0.34), 0.386 | 0.12 (0.02–0.22), 0.015 |
| Neurological gaits × Time | 0.51 (−0.65–1.67), 0.386 | −0.10 (−0.29–0.09), 0.302 |
| Non-Neurological gaits | −0.68 (−6.20–4.83), 0.806 | 0.85 (0.26–1.44), 0.005 |
| Time | −0.27 (−0.96–0.43), 0.448 | 0.00 (−0.10–0.11), 0.938 |
| Non-neurological gaits × Time | 0.33 (−0.72–1.37), 0.534 | 0.22 (0.06–0.38), 0.008 |
Adjusted for age, sex, education years, ethnicity and comorbidity index.
RESULTS
Study population
Between June 2011 and August 2018, 522 (90.5%) out of 589 CCMA participants received detailed clinical and gait assessments. Reasons for not obtaining assessments included clinician or tester unavailability (17), incomplete study visit (32), dementia diagnosed at consensus case conferences (9), or refusal (9). Subjects who did and did not receive the study assessments were similar in terms of age, sex and education, but had significantly worse performance on the RBANS total score (p = 0.026) at enrollment.
Of the 522 eligible participants, 81 met MCR criteria (any subtype): 43 MCRv, 41 MCRsl, 21 MCRsw, 24 MCRslv, and 26 MCRswv. Baseline demographic and medical characteristics are presented in Table 1. MCR subtypes were not mutually exclusive: 44 participants (54.3%) met criteria for two or more of the five subtypes. MCR diagnosis was independent of MCI diagnosis or cognitive testing. Of the 81 participants who met criteria for any MCR subtype, 27 also had a MCI diagnosis at baseline (4 amnestic, 9 non-amnestic, and 14 combined). Over a median follow-up of 3.0 years, 9 (11.1%) of the participants with any subtype of MCR converted to dementia.
Table 1.
Baseline characteristics by MCR overall and MCR subtype status
| Any MCR Subtype (n = 81) | MCRv (n = 43) | MCRsl (n = 41) | MCRsw (n = 21) | MCRslv (n = 24) | MCRswv (n = 26) | |
|---|---|---|---|---|---|---|
| Age (y) | 77.73 ± 7.48 | 79.36 ± 7.90* | 78.80 ± 7.89 | 78.28 ± 7.92 | 77.63 ± 8.38 | 77.05 ± 7.32 |
| Female, n (%) | 42 (51.9) | 20 (46.5) | 20 (48.8) | 13 (61.2) | 13 (54.2) | 13 (52.0) |
| Ethnicity, n (% White) | 61 (75.3) | 32 (74.4) | 31 (75.6) | 19 (90.5) | 21 (87.5) | 18 (72.0) |
| Education (y) | 13.56 ± 2.92 | 13.67 ± 3.18 | 13.61 ± 3.22 | 14.76 ± 2.19* | 13.29 ± 3.33 | 13.68 ± 3.76 |
| Comorbidity index | 1.95 ± 1.07 | 1.81 ± 1.16 | 2.02 ± 1.11 | 2.00 ± 0.95 | 2.04 ± 1.20 | 2.08 ± 1.00 |
| RBANS Score | 87.20 ± 11.31 | 83.81 ± 10.64* | 83.61 ± 10.92* | 90.71 ± 13.13 | 90.33 ± 8.53 | 84.56 ± 10.89 |
| Disability Score | 1.65 ± 1.50 | 2.19 ± 1.31* | 2.20 ± 1.31* | 1.62 ± 1.40 | 1.58 ± 1.50 | 1.88 ± 1.86 |
| Gait | ||||||
| Velocity (cm/s) | 80.93 ± 21.97 | 65.23 ± 11.56* | 66.57 ± 14.02* | 96.61 ± 29.34* | 77.25 ± 23.38 | 76.38 ± 19.48 |
| Stride length (cm) | 100.08 ± 18.04 | 89.40 ± 16.01* | 87.67 ± 14.53* | 102.53 ± 24.67 | 98.81 ± 23.49 | 98.31 ± 21.87 |
| Stride length SD (cm) | 4.23 ± 2.17 | 4.20 ± 1.74 | 4.31 ± 1.72 | 3.15 ± 1.92* | 6.72 ± 2.00* | 4.55 ± 1.86 |
| Swing time (s) | 0.40 ± 0.06 | 0.42 ± 0.06* | 0.40 ± 0.05 | 0.33 ± 0.03* | 0.41 ± 0.06 | 0.40 ± 0.06 |
| Swing time SD (s) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.02* | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.04 ± 0.01* |
MCR subtypes are not mutually exclusive;
Difference with non-MCR group (p < 0.05). MCR, motoric cognitive risk; MCRv, MCR velocity; MCRsl, MCR stride length; MCRsw, MCR swing time; MCRslv, MCR stride length variability; MCRswv, MCR swing time variability; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
Clinical gait abnormalities
Table 2 presents clinical gait abnormalities in MCR overall (all subtypes combined) and by different MCR subtypes. Of the 81 participants with MCR overall (any subtype), 52 (64.2%) had clinical gait abnormalities as diagnosed by the study clinician. Neurological gaits were seen in 28 (34.6%) of the MCR overall group, and non-neurological gaits in 39 (48.1%). Of the 81 participants in the sample, 15 (18.5%) were diagnosed with combined neurological and non-neurological gait abnormalities. Figure 1 presents the pattern of clinical gait abnormalities overall and by MCR subtype. Most clinical gait abnormalities were mild (walked without assistance or walking aid) in MCR overall (54.3%) and in MCR subtypes (44.0 to 61.0%). Neurological (range 23.8 to 46.3%) and non-neurological gait abnormalities (33.3 to 60.5%) were common in all MCR subtypes. Neurological gaits were most frequent in MCRsl and non-neurological gaits in MCRv. Neuropathic and unsteady gait subtypes accounted for 28.4% of the neurological gait abnormalities in MCR overall. Arthritis accounted for 30.9% of non-neurological gait abnormalities in MCR overall. Longitudinal follow-up time did not differ between groups with and without clinical gait abnormalities (2.5 versus 3.2 years, p = 0.154), with and without neurological gait abnormalities (2.3 versus 3.0 years, p = 0.109), or with and without non-neurological gait abnormalities (2.6 versus 2.9 years, p = 0.623).
Fig. 1.
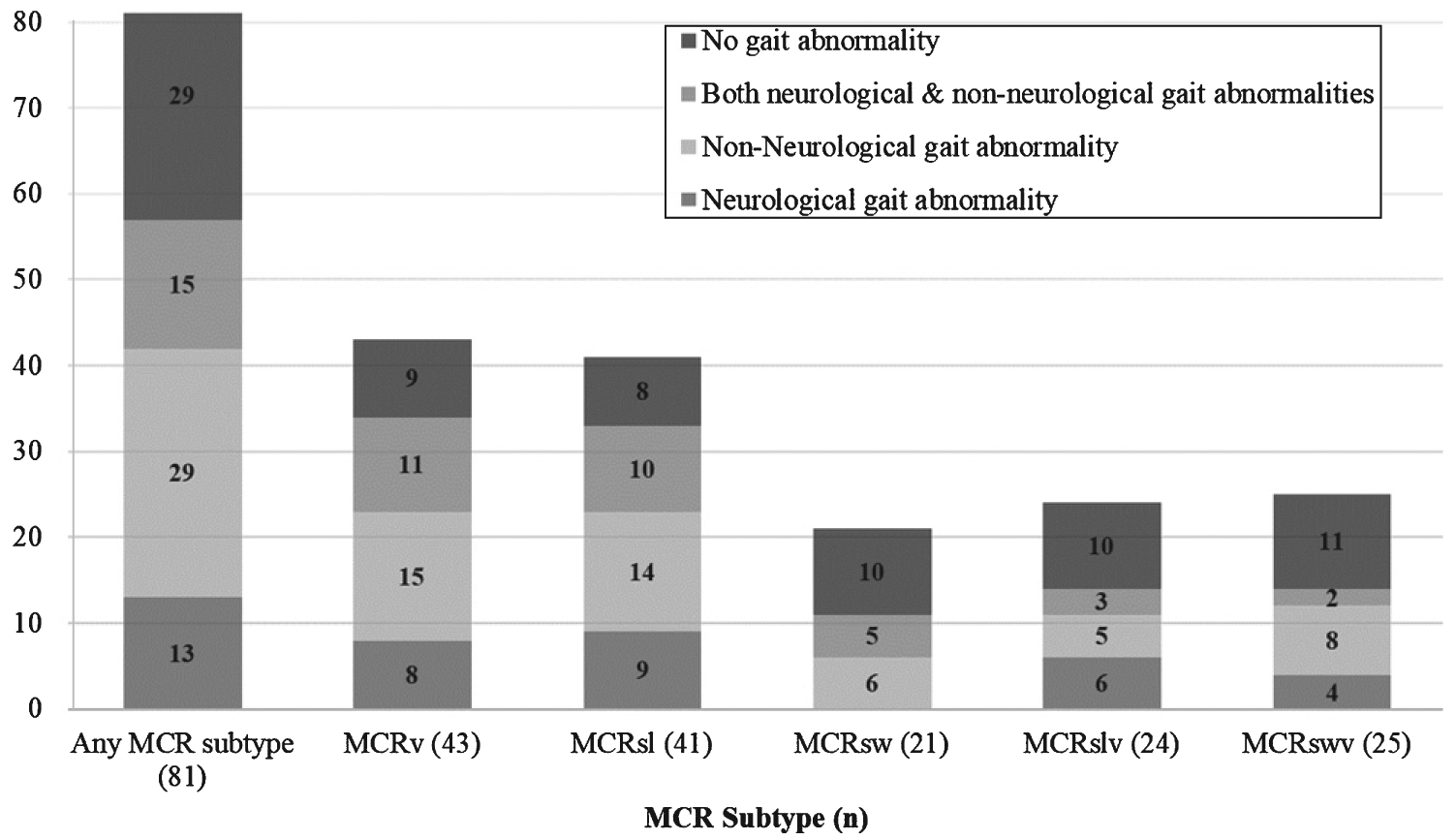
Gait abnormalities by MCR status. MCR, motoric cognitive risk; MCRv, MCR velocity; MCRsl, MCR stride length; MCRsw, MCR swing time; MCRslv, MCR stride length variability; MCRswv, MCR swing time variability.
Cognition
Table 3 shows that after controlling for age, sex, education years, ethnicity and comorbidity index, participants with MCR overall and abnormal gait did not perform different on the RBANS from MCR cases without a gait abnormality at baseline (p = 0.728). Average rate of change in cognitive scores was not significant for either group. Presence of gait abnormalities in MCR overall was not a predictor of longitudinal change in RBANS scores. Neurological or non-neurological abnormalities also did not predict decline in RBANS.
Disability
Table 3 shows that after controlling for age, sex, education years, ethnicity and comorbidity index, participants with MCR overall and abnormal gait had worse (higher) disability scores at baseline compared to MCR cases without gait abnormalities (group difference 0.84 points, p = 0.010). Participants with non-neurological gaits had worse disability scores at baseline compared to those without non-neurological gait (group difference 0.85 points, p = 0.005). There was no difference in disability scores for those with and without a neurological gait abnormality at baseline. Of the seven activities of daily living assessed at baseline, the most common activities that participants reported difficulty with were bathing (43.2%), getting up from a chair (35.8%), using the bathroom (35.8%) and walking inside their home (28.4%). Four participants (4.9%) reported that they required assistance to get up from a chair and one participant also reported needing assistance to bathe, groom, and eat.
Presence of gait abnormalities in participants with MCR overall (Table 3) predicted longitudinal worsening in disability scores (0.17 points/year, p = 0.033). Results remained significant after additional adjustment for depressive symptoms and presence of MCI (estimate 0.18, 95% CI 0.02–0.34, p = 0.032). After additionally adjusting for baseline disability scores, gait abnormalities remained a significant predictor of longitudinal change in disability scores (estimate 0.17, 95% CI 0.01–0.34, p = 0.040). Non-neurological gaits in participants with MCR predicted longitudinal change in disability scores (0.22 points/year, p = 0.008) over follow-up.
In fully adjusted models, participants with MCRv and abnormal gait scored 1.05 points higher (worse) on the disability scale compared to those with MCRv and no gait abnormality (p = 0.025) at baseline. Presence of gait abnormalities in MCRv predicted longitudinal change in disability scores (estimate 0.36 points/year, 95% CI 0.19–0.53, p < 0.001).
In sensitivity analyses excluding the 15 individuals with combined neurological and non-neurological gaits from the analysis, the association of non-neurological gaits with functional decline did not change (estimate 0.29, 95% CI 0.13–0.44, p < 0.001). The association of non-neurological gaits with cognitive decline and neurological gaits with both cognitive and functional decline still remained non-significant in models excluding these individuals. The non-significant association between gait abnormalities and cognitive decline was unchanged when 9 individuals who developed dementia over follow-up were excluded.
DISCUSSION
Our findings show that clinical gait abnormalities are common in older adults diagnosed with MCR in a large well-characterized community-based cohort. Gait abnormalities were most common in MCRv, and were strongly predictive of functional decline.
Presence and type of clinical gait abnormalities differed by MCR subtype. Non-neurological gait abnormalities were more common than neurological in MCR overall, and neurological gaits were most frequent in MCRsl. Our previous findings showing different cognitive and risk factor profiles in the different MCR subtypes in the same cohort [11] likely accounts for the differing clinical gait profiles observed in this study. Caveats include the relatively small samples and overlap in individuals between MCR subgroups.
While, to our knowledge, this is the first report on clinical gait abnormalities in MCR, the prevalence of gait abnormalities has been reported to be high in other pre-dementia syndromes such as MCI [33]. In the Einstein Aging Study, neurological gaits were present in 31% of participants with amnestic subtype of MCI and in 19% of participants with non-amnestic MCI [33]. The prevalence of neurological (34%) and non-neurological gaits (48%) was, as may be expected for a pre-dementia syndrome defined based on gait impairment, higher in MCR than MCI. Neuropathic and unsteady gait accounted for the majority of neurological gait abnormalities. Parkinsonian gaits were rare in this sample; reflecting the older age group. Cerebrovascular lesions, which may result in specific neurological gaits [16], is common in MCR [1–3]. Cerebrovascular disease has also been linked to quantitative gait measures in older adults [34]. Hence, vascular risk factors such as hypertension and diabetes could be the common etiological link between these neurological gait subtypes and MCR [1, 3, 16].
Slow gait is associated with gait abnormalities in older adults, and is predictive of cognitive decline regardless of underlying etiology [8]. Gait abnormalities, especially neurological, predict dementia in healthy older adults [16]. But the presence of gait abnormalities in MCR patients was not associated with worse cognitive function at cross-section or longitudinally in this analysis. Our findings suggest that once individuals meet criteria for slow gait and MCR, then presence of gait abnormalities does not add to prognostication. This hypothesis is supported by preliminary analysis from our group that shows that gait velocity at the time of MCR diagnosis does not predict future transition from MCR to dementia [35]. This does not mean that neurological gaits in individuals with MCR are not reflective of underlying brain pathology [36, 37]. Neurological gaits in MCR could be a marker of brain pathologies that result in gait impairment in their earliest stages. The progression to dementia in participants with MCR may reflect the worsening and spread of dementia related pathology into areas of the brain responsible for other non-motor behaviors and cognitive impairments associated with dementia [36, 37]. Furthermore, clinical gait abnormalities are examiner dependent, and subtle or early abnormalities may be missed by even experienced clinicians. More research, including clinicopathological investigations, is needed to build on these findings. Alternatively, our negative findings may be due to the short follow-up time and low sample size. A previous study by Allali et al. [11], examining MCR subtypes in the same cohort, indicated a distinct pattern of cognitive profiles for each subtype. For example, only MCRv and MCRsl predicted global cognitive decline, whereas MCRswv predicted decline in memory and visuospatial domains. Hence, our analysis combining the subtypes may have weakened the effect of the subtypes in predicting global cognitive decline as measured by RBANS. The smaller samples of MCR subtypes precluded us from examining decline in specific cognitive domains, and should be explored in larger samples.
Our findings are consistent with prior studies in our and other cohorts reporting an association between cognitive disorders and functional decline [5, 38]. For instance, participants with MCRv in a Japanese cohort had an increased risk of developing disability (hazard ratio 1.7) over a short period of time (mean 29 months) compared to those without MCRv. Our results corroborate those findings, and provide further evidence that clinical gait impairments, particularly those related to a non-neurological conditions increase risk for functional decline in participants with MCR.
Strengths of this study include our large sample size with systematic gait assessments, validated diagnostic procedures, and application of MCR criteria independent of clinical gait assessments. Limitations include the relatively small sample size of MCR cases and the relatively short follow-up time. Gait abnormalities are dependent on clinicians and may be missed if only subtle or early signs are present. Furthermore, our study was not designed to address whether slow gait preceded appearance of clinical gait abnormalities, and this temporal association will be addressed in future longitudinal analyses.
Our findings show that clinical gait abnormalities are common in MCR and its subtypes, and are associated with accelerated functional decline in this high-risk population. Longer follow-up time and larger samples are needed to explore the role of clinical gait abnormalities in participants with MCR on cognitive decline. Future studies are also needed to explore the biological pathways of MCR overall as well as within the individual subtypes. A recent neuroimaging study of MCR conducted in the same cohort found gray matter atrophy associated with MCR in the motor, insular, and prefrontal cortex regions, indicating that MCR is associated with atrophy in brain regions linked to the control aspects of gait [36]. These regions have also been linked to cognitive functions of attention and memory awareness providing further evidence of MCR as a cognitive syndrome [39, 40].
ACKNOWLEDGMENTS
This study was supported by funds from the National Institute on Aging (R56AG057548). The Central Control of Mobility in Aging is supported by National Institute on Aging grants (R01AG044007-01A1: J Verghese and R01AG036921-01A1: R Holtzer).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-1227r1).
REFERENCES
- [1].Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, Katz MJ, Lipton RB, Wang C (2014) Motoric cognitive risk syndrome: Multicenter incidence study. Neu- rology 83, 2278–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Verghese J, Wang C, Lipton RB, Holtzer R (2013) Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 68, 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, Bridenbaugh SA, Buchman AS, Callisaya ML, Camicioli R, Capistrant B, Chatterji S, De Cock AM, Ferrucci L, Giladi N, Guralnik JM, Hausdorff JM, Holtzer R, Kim KW, Kowal P, Kressig RW, Lim JY, Lord S, Meguro K, Montero-Odasso M, Muir-Hunter SW, Noone ML, Rochester L, Srikanth V, Wang C (2014) Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology 83, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Callisaya ML, Ayers E, Barzilai N, Ferrucci L, Guralnik JM, Lipton RB, Otahal P, Srikanth VK, Verghese J (2016) Motoric cognitive risk syndrome and falls risk: A multi-center study. J Alzheimers Dis 53, 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T (2017) Motoric cognitive risk syndrome: Association with incident dementia and disability. J Alzheimers Dis 59, 77–84. [DOI] [PubMed] [Google Scholar]
- [6].Ayers E, Verghese J (2016) Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement 12, 556–564. [DOI] [PubMed] [Google Scholar]
- [7].Verghese J, Holtzer R, Lipton RB, Wang C (2012) Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc 60, 1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verghese J, Wang C, Lipton RB, Holtzer R, Xue X (2007) Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 78, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J (2010) The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 67, 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ayers E, Verghese J (2014) Locomotion, cognition and influences of nutrition in ageing. Proc Nutr Soc 73, 302–308. [DOI] [PubMed] [Google Scholar]
- [11].Allali G, Ayers EI, Verghese J (2016) Motoric cognitive risk syndrome subtypes and cognitive profiles. J Gerontol A Biol Sci Med Sci 71, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA, Petersen RC (2012) The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology 78, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Kang K, Lux O, Mather KA, Brodaty H (2012) Risk profiles of subtypes of mild cognitive impairment: The sydney memory and ageing study. J Am Geriatr Soc 60, 24–33. [DOI] [PubMed] [Google Scholar]
- [14].Petersen RC (2011) Clinical practice. Mild cognitive impairment. N Engl J Med 364, 2227–2234. [DOI] [PubMed] [Google Scholar]
- [15].Allali G, Ayers EI, Verghese J (2015) Multiple modes of assessment of gait are better than one to predict incident falls. Arch Gerontol Geriatr 60, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H (2002) Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 347, 1761–1768. [DOI] [PubMed] [Google Scholar]
- [17].Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J (2015) Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 112, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].England SE, Verghese J, Mahoney JR, Trantzas C, Holtzer R (2015) Three-level rating of turns while walking. Gait Posture 41, 300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan J, Blumen HM, Verghese J, Holtzer R (2015) Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: A resting-state fMRI study. Hum Brain Mapp 36, 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holtzer R, Wang C, Verghese J (2014) Performance variance on walking while talking tasks: Theory, findings, and clinical implications. Age (Dordr) 36, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR (2016) Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr 29, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB (2008) Within-person across-neuropsychological test variability and incident dementia. JAMA 300, 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verghese J, Ambrose AF, Lipton RB, Wang C (2010) Neurological gait abnormalities and risk of falls in older adults. J Neurol 257, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB (2006) Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc 54, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [27].Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC (2005) The AD8: A brief informant interview to detect dementia. Neurology 65, 559–564. [DOI] [PubMed] [Google Scholar]
- [28].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [29].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L (2013) Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci 68, 820–827. [DOI] [PubMed] [Google Scholar]
- [30].Randolph C, Tierney MC, Mohr E, Chase TN (1998) The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol 20, 310–319. [DOI] [PubMed] [Google Scholar]
- [31].Hardy SE, Gill TM (2004) Recovery from disability among community-dwelling older persons. JAMA 291, 1596–1602. [DOI] [PubMed] [Google Scholar]
- [32].Fleiss JL (2011) Design and analysis of clinical experi- ments, John Wiley & Sons. [Google Scholar]
- [33].Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, Lipton RB (2008) Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 56, 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rosano C, Brach J, Longstreth WT Jr, Newman AB (2006) Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 26, 52–60. [DOI] [PubMed] [Google Scholar]
- [35].Ayers E, Lipton R, Verghese J (2018) Motoric cognitive risk syndrome: A gait or cognitive syndrome? Alzheimers Dementia 14, P972–P973. [Google Scholar]
- [36].Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J (2018) A gray matter volume covariance network associated with the motoric cognitive risk syndrome: A multi-cohort MRI study. J Gerontol A Biol Sci Med Sci, doi: 10.1093/gerona/gly158 [DOI] [PubMed] [Google Scholar]
- [37].Blumen HM, Brown LL, Habeck C, Allali G, Ayers E, Beauchet O, Callisaya M, Lipton RB, Mathuranath PS, Phan TG, Pradeep Kumar VG, Srikanth V, Verghese J (2018) Gray matter volume covariance patterns associated with gait speed in older adults: A multi-cohort MRI study. Brain Imaging Behav, doi: 10.1007/s11682-018-9871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pettersson AF, Olsson E, Wahlund LO (2005) Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord 19, 299–304. [DOI] [PubMed] [Google Scholar]
- [39].Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, Shaked D, Huey ED, Briner T, Stern Y (2015) The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia 75, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Menon V, Uddin LQ (2010) Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]


