Abstract
Background: Pediatric epileptic encephalopathy and severe neurological disorders comprise a group of heterogenous diseases. We used whole-exome sequencing (WES) to identify genetic defects in pediatric patients. Methods: Patients with refractory seizures using ≥2 antiepileptic drugs (AEDs) receiving one AED and having neurodevelopmental regression or having severe neurological or neuromuscular disorders with unidentified causes were enrolled, of which 54 patients fulfilled the inclusion criteria, were enrolled, and underwent WES. Results: Genetic diagnoses were confirmed in 24 patients. In the seizure group, KCNQ2, SCN1A, TBCID 24, GRIN1, IRF2BPL, MECP2, OSGEP, PACS1, PIGA, PPP1CB, SMARCA4, SUOX, SZT2, UBE3A, 16p13.11 microdeletion, [4p16.3p16.1(68,345–7,739,782)X1, 17q25.1q25.3(73,608,322–81,041,938)X3], and LAMA2 were identified. In the nonseizure group, SCN2A, SPTBN2, DMD, and FBN1 were identified. Ten novel mutations were identified. The recurrent genes included SCN1A, KCNQ2, and TBCID24. Male pediatric patients had a significantly higher (57% vs. 29%; p < 0.05, odds ratio = 3.18) yield than their female counterparts. Seventeen genes were identified from the seizure groups, of which 82% were rare genetic etiologies for childhood seizure and did not appear recurrently in the case series. Conclusions: Wide genetic variation was identified for severe childhood seizures by WES. WES had a high yield, particularly in male infantile patients.
Keywords: WES, infantile epilepsy, genetic variation, personal therapy
1. Introduction
In children, genetic disorders cause severe neurological disease, congenital malformation, inborn errors of metabolism, and developmental epileptic encephalopathy (DEE). DEEs refer to a group of ictal and interictal epileptiform anomalies (clinical and encephalographic) associated with severe cognitive and behavioral impairments according to the classification and terminology criteria of the International League against Epilepsy (ILAE) [1,2]. DEEs are age-specific and of diverse etiologies. Increasing evidence suggests that genetics play a pivotal role in pediatric DEEs and severe neurological disorders [3,4,5]. Although the incidence of each disease is low, the combined incidence is not adequately estimated and unknown. DEEs are highly heterogeneous genetically, but genetic etiologies have been identified in only half of the cases, typically in the form of de novo dominant mutations [6]. Diagnosing severe childhood neurological disorders is important but challenging.
DEEs are characterized by (a) early-onset seizures that are often intractable, (b) electroencephalographic abnormalities, (c) developmental delay or regression, and (d) early death in some patients [2,7,8]. In infants with KCNQ2 or STXBP1 encephalopathy, seizures may be controlled early after onset or may cease spontaneously after a few years. However, the developmental consequences are profound [3].
Despite the recent advances in molecular diagnostics, the genetic causes of only approximately 50% of patients have been identified [3,9]. The identification of the genetic etiology of DEE has greatly improved our understanding of disease pathophysiology at the molecular level. However, understanding the genotype–phenotype correlation remains a challenge. Some epileptic syndromes, such as Dravet syndrome (DS), are attributed to SCN1A mutations in approximately 80% of patients. Otahara syndrome is attributed to mutations in KCNQ2, STXBP1, and ARX. The outcomes are varied and may range from self-limited and drug-responsive to severe debilitating syndromes [9].
The aim of this study was to determine the diagnostic utility of whole-exome sequencing (WES) for a heterogeneous group of childhood DEEs and to develop a viable protocol for identifying severe neurological disease. We performed WES in 54 pediatric patients with suspected severe neurological or neuromuscular disorders, which remained etiologically undiagnosed despite comprehensive clinical, neuroimaging, and biochemical studies. Patients were enrolled from two medical centers in central Taiwan. The identified genetic etiologies are rare. The case series and diagnostic yield are discussed.
2. Materials and Methods
2.1. Patients
We enrolled patients from 2017 to 2020 that matched at least 1 of the following criteria: (1) Epileptic encephalopathy defined by ILAE, with the use of ≥2 AEDs, (2) epileptic seizures with the use of 1 AED but with symptoms of neurological regression, (3) nonepileptic patients with symptoms of neurological regression. We collected samples from 54 patients from 2 medical centers in central Taiwan, including the Chung Shan Medical University Hospital and the Children’s Hospital of China Medical University from 2017–2020. This study was approved by the institutional research ethics board (CMUH105-REC3-123) and Chung Shan Medical University Hospital’s Internal Review Board (IRB #: CS13036). Informed consent was obtained from the parents.
2.2. Extracting DNA from Peripheral Blood
A genomic DNA purification kit (Gentra Puregene Buccal Cell Kit; Qiagen Taiwan, Taipei City, Taiwan) was used to extract genomic DNA from peripheral blood. Three volumes of RBC lysis buffers were added to blood sample and mixed by inverting 30 times, incubated for 10 min at room temperature, and centrifuged 3000 rpm for 5 min. The supernatant was discarded. Next, we added 3 volumes of cell lysis buffers (3 mL) to the pellet and vortexed vigorously for 10 s to lyse the cell and centrifuged 3000 rpm for 5 min, followed by transferring the supernatant into a new 15 mL tube. Then, we added 3 mL isopropanol into a 15 mL tube, mixed by inverting 50 times, and centrifuged 3000 rpm for 5 min. The supernatant was carefully discarded and the pellet was air dried for 10 to 20 min. The dried pellet was resuspended in 300 μL nuclease-free water and frozen at −20 °C or −80 °C for storage. DNA were also extracted from their parents.
2.3. WES
We used the SureSelect XT HS2 DNA reagent kit (Agilent Technologies, Inc., Santa Clara, CA, USA) protocol for the Illumina Hiseq paired-end sequencing library (catalog# G9611A), and raw reads were mapped to the human genome assembly GRCh37 (also known as hg19) using Burrows-Wheeler Aligner software (version 0.6.1; Intel Corporation, Santa Clara, CA, United States). The SureSelectXT Human All Exon Version 6 (51 Mb) probe (Agilent Technologies, Inc., Santa Clara, CA, USA) set was used. For Library Preparation, 50 ng genomic DNA was used. The adapter-ligated DNA sample was purified using Agencourt Ampure Xp Pcr Purification Beads and analyzed using an Agilent DNA Kit. From the purified sample, the hybridization between DNA libraries (750 ng) and baits was carried out and purified using Agencourt Ampure Xp Pcr Purification Beads. The Agilent protocol was used for adding tags by Post-Hybridization Amplification. Finally, the sample was sequenced on Illumina NextSeq500 using the generated reads of 2 × 150 bp. Every analyte passed all the quality control requirements, and 99% of targeted nucleotides were covered at more than 20×.
2.4. Data Analysis and Interpretation
Variant calling was performed using the recommended best practices of GATK version1.0.5506 (Broad Institute). Variant annotation and prioritization were performed using a well-developed pipeline called wANNOVAR [10], which is used for functional annotations, including various gene annotations, alternative allele frequency in the 1000 Genomes Project, conserved element annotation, dbSNP annotation, deleteriousness prediction scores for nonsynonymous variants, ClinVar variant annotation, and genome-wide association study (GWAS) variant annotation [11].
We implemented a variant-reduction pipeline based on commonly used filters and disease models to select nonsynonymous variants and splice variants, rare or novel variants in the 1000 Genomes Project database, and predicted deleterious variants. Thus, synonymous variants, variants with variant frequency <10%, and variants with allele frequency >1% were removed. The remaining variants were annotated with reference to ClinVar, a freely accessible human variation and phenotype database hosted by the National Center for Biotechnology Information (NCBI) [12]. Pathogenicity prediction programs, including PolyPhen2 [13], SIFT [14], and Combined Annotation Dependent Depletion (CADD) [15], were used. All the variants identified were further confirmed by Sanger sequencing. The corresponding gene contexts were evaluated according to Online Mendelian Inheritance in Man® [16] with the individual phenotypes (clinical, laboratory, and imaging data). Segregation analysis was performed to select de novo or compound heterozygous variants. The identified variants were classified “pathogenic,” “likely pathogenic,” or “of uncertain significance” according to the American College of Medical Genetics (ACMG) standards and guidelines [17].
3. Results
3.1. Demographic Data of the Enrolled Patients
A total of 54 patients were enrolled, of which 45 had a history of seizure, including 10 patients with seizure onset before 1 month of age, 31 patients with seizure onset between 1 month and 2 years of age, and 4 patients with seizure onset after 2 years of age. Of the patients, 10 received 1 drug and 35 received at least ≥2 AEDs. Nine patients had no history of seizures and were suspected to have severe neurological or neuromuscular disease with regression of symptoms. The demographic data are shown in Table 1. The detailed clinical information including antiepileptic drugs, magnetic resonance imaging (MRI) findings, and outcomes are demonstrated in Table 2.
Table 1.
The demographic data in identified genetic group and nonidentified genetic group by whole-exome sequencing (WES) are demonstrated.
| Clinical Data (N) | Identified Genetic Group (N = 24) |
Nonidentified Genetic Group (N = 30) |
|
|---|---|---|---|
| Symptoms | NS | ||
| Seizures (45) | 20 (44%) | 25 (56%) | |
| No seizure (9) | 4 (44%) | 5 (56%) | |
| Age at first seizure onset | NS | ||
| 0–1 month (10) | 5 (50%) | 5 (50%) | |
| 1 month–2 years (31) | 13 (42%) | 18 (58%) | |
| >2 years (4) | 2 (50%) | 2 (50%) | |
| No seizure (8) | 4 (44%) | 5 (56%) | |
| Sex | p < 0.05 ※ | ||
| Male (30) | 17 (57%) | 13 (43%) | |
| Female (24) | 7 (29%) | 17 (71%) | |
| Number of antiepileptic drugs at the time of study | NS | ||
| 0 (8) | 4 (44%) | 5 (56%) | |
| 1 (10) | 2 (20%) | 8 (80%) | |
| 2 (11) | 5 (49%) | 6 (51%) | |
| >3 or =3 (25) | 13 (54%) | 11 (46%) |
NS indicates not significant. ※ Odds ratio = 3.18 (95% confidence interval = 1.04 to 9.70).
Table 2.
The genetic and clinical features are summarized in those patients with identified mutations by WES.
| Gene Name | Genotype | Inheritance Pattern |
Phenotype | Age of Diagnosis/ Age of First Seizure |
Sex | NCBI ClinVar | Seizure Types |
Neurodevelopmental Outcomes |
|
|---|---|---|---|---|---|---|---|---|---|
| P 1 |
PACS1 (NM_018026.4) |
c.607C > T p.(Arg203Trp) |
AD, de novo |
DEE, spinal tethered cord | 12 years/ Newborn |
F | Pathogenic | Focal seizures |
Walk by ambulance, cognition delay at 14 years |
| P 2 |
TBCID 24 (NM_001199 107.1) |
c.1499C > T (p.Ala500Val)/c.229_240del (p.77_80del) | AR | DEE | 2 years /10 months |
M | Pathogenic/Pathogenic | Multiple focal, general, nonconvulsive status epilepticus | Attention deficit. cognition delay |
| P 3 |
FBN1 (NM_000138. 4) |
c.794_795insGTAT (p.Val266Tyr fs * 6) | AD | Marfan syndrome | 16 years /- |
F | Pathogenic | No seizure | Normal |
| P 4 |
KCNQ2 (NM_172107.3) |
c.740C > T (p.Ser247Leu) |
AD, de novo |
DEE | 10 months /Day 3 |
M | Pathogenic | General tonic, apnea | No language, hypotonia at 3 years |
| P 5 |
KCNQ2 (NM_172107.3) |
c.853C > A (p.Pro285Thr) |
AD, de novo |
DEE | 2 months /Day 3 |
F | Novel | General tonic | No language, hypotonia at 1 year |
| P 6 |
OSGEP (NM_017807.4) |
c.740G > A (p.Arg247Gln)/ c.740G > A (p.Arg247Gln) |
AR | Lissencephaly, seizures |
3 months /3 weeks |
M | Pathogenic/Pathogenic | Focal | Severe, died at 6 months |
| P 7 |
SCN2A (NM_021007.2) |
c.4958delT (p.Leu1653Ter) | AD, de novo |
Autism | 4 years/- | M | Pathogenic | No seizure | Severe autism, no language at 4 years |
| P 8 | 16p13.11 microdeletion | AD | Frequent seizures | 12 years/10 years | F | Pathogenic | General tonic | Attention deficit | |
| P 9 ※ | 4p16.3p16.1(68,345–7,739,782)X1, 17q25.1q25.3(73,608,322–81,041,938)X3 | AD, de novo |
DEE | 1 year/ 6 months |
M | Pathogenic | General | Global DD | |
| P 10 |
IRF2BPL (NM_024496.3) |
c.562C > T (p.Arg188Ter) |
AD, de novo |
DEE and regressive encephalopathy | 12 years /11 years |
F | Pathogenic | General | Profound ID |
| P 11 |
PIGA: (NM.002641) |
c.356G > A (p.Arg119Gln) |
X-linked | DEE | 0.5 month /Day 3 |
M | Pathogenic | Apnea, cyanosis, absence, atonic, spasms | Severe global DD |
| P 12 | PPP1CB | c.548A > C (p.Glu183Ala) |
AD, de novo |
DEE, dysmorphism | 4 months/ 2 months |
M | Pathogenic | Apnea, eye gazed deviation, myoclonic | Severe global DD |
| P 13 |
TBCID 24 (NM_001199 107.1) |
c.119G > A (p.Arg40His)/ c.1499C > T (p.Ala500Val) |
AR | DEE | 8 months /6 months |
M | Pathogenic /Pathogenic |
Multifocal, myoclonus | Global DD |
| P 14 |
UBE3A (NM_130838) |
c.C219A (p.Thr73Ter) |
AD, maternal imprinting | Regressive encephalopathy |
14 months /11 months |
M | Novel | General tonic | Profound ID |
| P 15 |
SCN1A (NM_001165963) |
c.362delC (p.Ala121fs) |
AD, de novo |
DEE | 5 months /4 months |
M | Novel | Focal clonic, general tonic | Profound ID |
| P 16 |
SCN1A (NM_001165963) |
c.3918 + 1 G > - | AD, de novo |
DEE | 8 months /5 months |
F | Novel | Focal clonic, general tonic | Profound ID |
| P 17 $ |
MECP2 (NM_004992.3) |
rsa Xq28 MECP2 (exons3-4) x1 | X-linked | Regressive encephalopathy |
14 months /8 months |
F | Pathogenic | General tonic | Profound ID |
| P 18 |
DMD (NM_004006.2) |
c.C5287T (p.Arg1763Ter) | X-linked | Muscle dystrophy | 3 years /- |
M | Pathogenic | No seizure | Motor delay |
| P 19 | SMARCA4 (NM_001128849) | c.3595G > A (p.Val1199Met) | AD, de novo |
Seizures, encephalopathy |
3 months /2 months |
M | Novel | General tonic | Severe global DD |
| P 20 |
GRIN1 (NM_007327.3) |
c.C2414T (p.Pro805Leu) |
AD, de novo |
DEE | 4 months /2 months |
M | Pathogenic | Infantile spasms | Global DD |
| P 21 |
SZT2 (NM_015284) |
c.1496 + 2T > C/c.9055T > C (p.Arg3019Ter) | AR | DEE | 3 years /6 months |
M | Novel/Novel | Focal motor | Severe hypotonia, gastrostomy |
| P 22 |
SUOX (NM_001032386.2) |
c.650G > A (p.Arg217Gln)/ c.258dupT (p.Lys87Ter) |
AR | Leigh-like, regression | 8 months /4 months |
M | Pathogenic /novel |
Apnea, cyanosis, opisthotonos | Severe DD, hypertonia |
| P 23 |
SPTBN2 (NM_006946. 2) |
c.5515C > A (p.Gln1839Lys) |
AD | Ataxia, vertigo | 14 years /- |
M | Novel | No seizure | Frequent ataxia |
| P 24 | LAMA2 NM_000426.3 | c.1583dupA (p.Ser529GlufsTer19) /c.6931A > T (p.Lys2311Ter) |
AR | Hypotonia, seizures | 2 years /1.5 months |
M | Novel/Novel | Focal | Severe global DD |
※ Diagnosed by WES and 750K microarray chip (Affymetrix CytoScan 750K Array); $ Diagnosed by WES and multiplex ligation-dependent probe amplification (MLPA). P, patient; DEE, developmental epileptic encephalopathy; F, female; M, male; AD, autosomal dominant; AR, autosomal recessive; DEE, developmental epileptic encephalopathy; DD, developmental delay; ID, intelligence disability; NA, not available; NCBI ClinVar, National Center for Biotechnology Information, clinical variability and predictability [12]. The sequence data of each patient were checked against the GenBank reference sequence and version number of genes.
3.2. Diagnostic Yield
The overall diagnostic rate was 44.4% (24/54). We compared patients with identified and nonidentified genetic etiologies through WES, which indicated a significant difference (p < 0.05) in sex between the two groups. In the identified genetic group (N = 24), the sex ratio was 18 males to 6 females. The inheritance patterns were 3 X-linked, 6 autosomal recessive, and 15 autosomal dominant. Male pediatric patients had a significantly higher yield than their female counterparts (57% vs. 29%, odds ratio = 3.18 (95% confidence interval = 1.04 to 9.70)). We found no difference in the number of epileptic drugs taken and the time of seizure onset between the identified genetic and nonidentified genetic groups (Table 1). Among 45 patients with seizures, the yield was 44% (20/45). Considerable genetic variability was found in the seizure group, as recurrent genetic etiologies included KCNQ2, TBCID24, and SCN1A in the cases series, with two patients carrying each gene. Other genes were nonrecurrent for each case in the case series. Seventeen genetic etiologies were identified in the seizure group, of which 82% (14/17) were rare and nonrecurrent.
3.3. Genotype
The clinical genotype and phenotype in genetically positive cases are shown in Table 2. Clinical and molecular data for 24 patients are listed. In 54 patients, 10 novel mutations were identified (Table 3). Among 24 patients with identified genetic etiologies, there were 13 with DEE, 6 patients had seizures with neurodevelopmental regressive symptoms, 2 patients with muscle disease (1 patient with muscle dystrophy carrying DMD gene and 1 patient with congenital muscle dystrophy with seizures), 1 with autism with SCN2A mutation, 1 with spinocerebellar ataxia, and 1 patient with Marfan syndrome. Eleven patients had a single autosomal-dominant disorder and all showed de novo variants. Of these, 10 mutations were novel (Table 3). The diagnosis in four patients was changed after WES, including one with migraine changed to spinocerebellar ataxia, one with Leigh-like syndrome changed to sulfite oxidase deficiency, and two which remained undiagnosed but were suspected of carrying copy number variation after read depth was computed from WES. Thereafter, a final diagnosis was given through multiplex ligation-dependent probe amplification (MLPA) or microarray-based comparative genomic hybridization (aCGH) (patients 9 and 17). The 10 novel mutations include 2 missense, 2 splicing, 3 nonsense, 2 indel, and 1 frameshift mutations. They were considered pathogenic or likely pathogenic mutations by ACMG guidelines (Table 3).
Table 3.
Ten novel mutations in the cases-series.
| Gene Name | Genotype (N) | Phenotype | NCBI ClinVar | Critical Functional | Global | East Asia | Taiwan Biobank. | CADD | Poly | SIFT | ACMG | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | MAF | MAF | Predict | Phen2 | Score | ||||||||
| Patient 15 |
SCN1A (NM_001165963) |
c.362delC (p.Ala121fs) |
Frameshift | DEE | Novel | 0 | 0 | 0 | D | D | D | AD, de novo, (PVS1, PM2, PP3) |
|
| Patient 14 |
UBE3A (NM_130838) |
c.C219A (p.Thr73Ter) |
Nonsense | Encephalopathy and regression | Novel | 0 | 0 | 0 | D | D | D | AD from mother (PVS1, PM2, PP3) |
|
| Patient 16 |
SCN1A NM_001165963) |
c.3918 + 1 G > - | Splicing | DEE | Novel | 0 | 0 | 0 | D | D | D | AD, de novo (PVS1, PM2, PP3) |
|
| Patient 19 | SMARCA4 (NM_001128849) | c.3595G > A (p.Val1199Met) |
Missense | Seizure, severe global delay | Novel | Helicase C-terminal’ | 0 | 0 | 0 | D | D | D | AD, de novo (PS2, PM1, PM2, PP3) |
| Patient 21 |
SZT2 (NM_015284) |
c.1496 + 2T > C | Splicing | DEE | Novel | 0 | 0 | 0 | D | D | D | AR (PVS1, PM2, PP3) |
|
| Patient 21 |
SZT2 (NM_015284) |
c.9055T > C (p.Arg3019Ter) |
Nonsense | DEE | Novel | 0 | 0 | 0 | D | D | D | AR (PVS1, PM2, PP3) |
|
| Patient 22 | SUOX (NM_001032386.2) | c.258dupT (p.Lys87Ter) |
Indel | Leigh-like, regression | Novel | 0 | 0 | 0 | D | D | D | AR (PVS1, PM2, PP3) |
|
| Patient 23 |
SPTBN2 (NM_006946. 2) |
c.5515C > A (p.Gln1839Lys) |
Missense | Spinocerebellar ataxia 5 | Novel | Ankyrin binding domain | 0 | 0 | 0 | D | D | T | AD from mother (PM1 + PM2 + PP1 + PP3 + PP4) |
| Patient 24 | LAMA2 NM_000426.3 | c.1583dupA(p.Ser529GlufsTer19) | Indel | CMD, seizures | Novel | 0 | 0 | 0 | D | D | D | AR (PVS1, PM2, PP3) | |
| Patient 24 | LAMA2 NM_000426.3 | /c.6931A > T (p.Lys2311Ter) | Nonsense | CMD, seizures | Novel | 0 | 0 | 0 | D | D | D | AR (PVS1, PM2, PP3) |
DEE, developmental epileptic encephalopathy; CMD, Congenital muscular dystrophies; NCBI ClinVar, National Center for Biotechnology Information, clinical variability and predictability [12]; Global MAF, Global mutation allele frequency in EXAC browser; East Asia MAF, East Asia mutation allele frequency in EXAC browser; CADD predict, Combined Annotation Dependent Depletion prediction; D, damage; T, tolerant. ACMG, American College of Medical Genetics and Genomics and the Association for Molecular Pathology. The sequence data of each patient were checked against the GenBank reference sequence and version number of genes.
3.4. Refractory Seizure Cases
Of the 45 patients with seizure, a definitive genetic etiology was identified in 20 (44%). Of the 10 patients with seizure onset before the first month, etiology was confirmed through WES in 5 (50%) patients. These included two patients with KCNQ2 and one patient each with PACS1, OSGEP, and PIGA (Table 2). All patients had poor neurodevelopmental outcomes. One patient with homozygous OSGEP mutations died before 4 months of age due to severe pleural effusions. One patient with PACS1 has survived and is 15 years old.
Of the 31 patients with seizure onset between 1 month and 2 years of age, 13 (42%) received a definitive diagnosis, including 2 patients each with TBCID24 and SCN1A and 1 patient each with PPP1CB, UBE3A, MECP2 duplication syndrome (MECP2:rsa Xq28), SZT2 (Figure 1), SMARCA4 (Figure 2), GRIN1, [4p16.3p16.1(68,345–7,739,782)X1 17q25.1q25.3(73,608,322–81,041,938)X3], SUOX, and LAMA2 SZT2 (Figure 3). Although the 13 patients were initially diagnosed with DEE, their phenotypes are heterogeneous.
Figure 1.
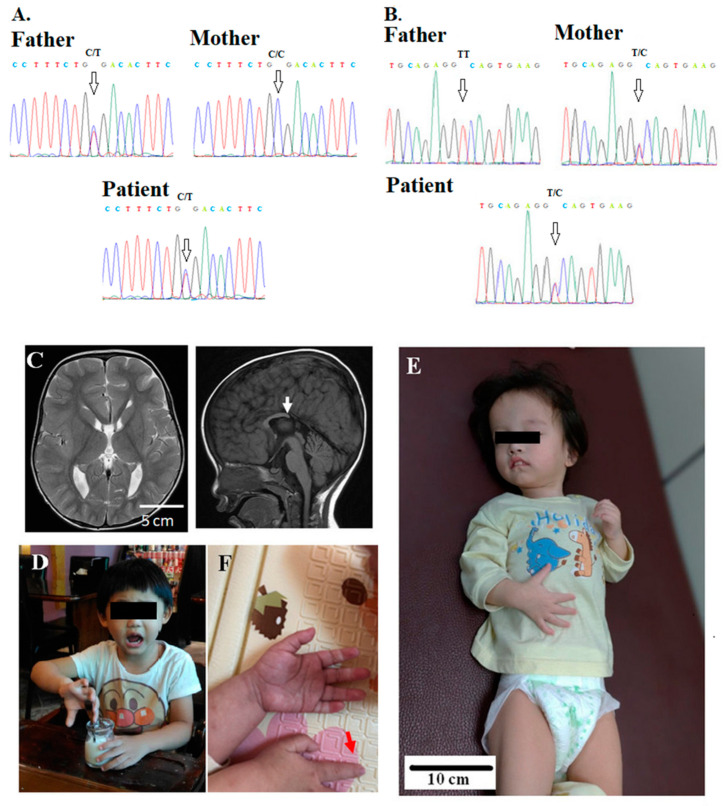
Patient 21 has compound heterozygous mutations of SZT2, (A) c.C9055T (p.Arg3019Ter) (arrows) and (B) c.1496 + 2T > C (arrows), from the asymptomatic father and mother, respectively. Seizure onset occurred at 6 months old. His electroencephalogram shows developmental epileptic encephalopathy with multifocal epileptiform discharges. (C) The MRI shows a short and thick corpus callosum with missing of selenium (arrow). (D) Normal child with the same age demonstrates for comparison with patient. (E) Her examination was notable for macrocephaly, dysmorphic features, frontal bossing, hypertelorism, microphthalmia, depressed nasal bridge, long-tapered fingers, and hyperextensible joints. (F) Long-tapered fingers in the patient (red arrow).
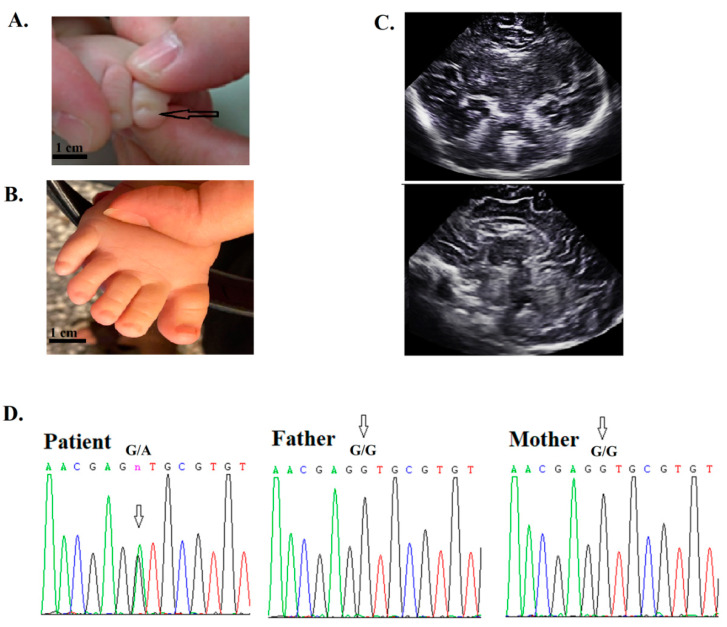
Figure 2.
Patient 3 had facial dysmorphic feature, hypotonia, and orogastric tube dependency after birth. (A) His toenails were hypoplastic (arrow). Seizure onset occurred at 3 months old. (B) Normal toenails in a healthy infant. (C) The image of the head ultrasound was unremarkable. (D) The WES and sequencing results indicated a novel de novo (no mutation in both parents) mutation in the SMARCA4 gene. The arrow indicates a G-to-A substitution at nucleotide 3595 (c.3595G > A, p.Val1199Met) in the patient (arrow), father, and mother. At 2 years, the patient was not able to sit without support.
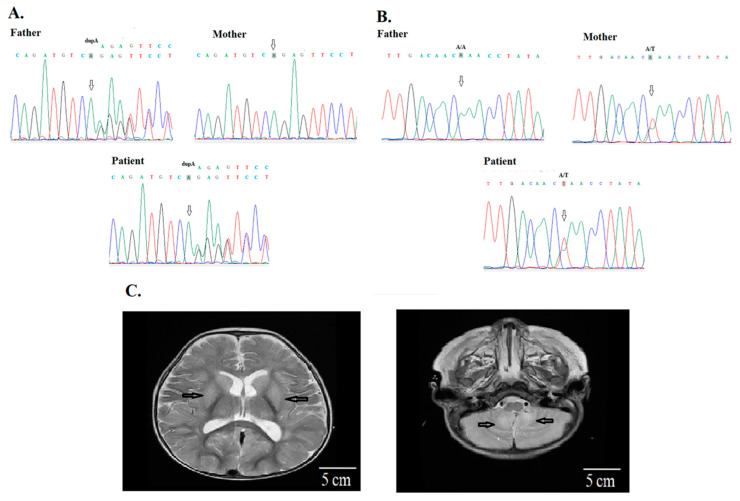
Figure 3.
Patient 24 has compound heterozygous mutations of LAMA2, (A) c.1583dupA (p.Ser529GlufsTer19), and (B) c.6931A > T (p.Lys2311Ter) from the asymptomatic mother and father, respectively (arrows). (C) His MRI showed basal ganglion, thalamus, white matter and cerebellar vermis signal abnormalities. The finding indicated a dysmyelinating process. A creatine phosphokinase was 548 IU/L (reference values, 40-397 IU/L). The genetic study for spinal muscle atrophy was unremarkable. He had developmental delay, recurrent sepsis, and pneumonia with lung atelectasis, and needed respiratory supportive care at home since birth. He died at 1 year and 2 months old due to respiratory failure.
A wide range of genetic disorders were identified. The genes involved in seizures included KCNQ2, SCN1A, TBCID 24, GRIN1, IRF2BPL, MECP2, OSGEP, PACS1, PIGA, PPP1CB, SMARCA4, SUOX, SZT2, UBE3A, 16p13.11 microdeletion, [4p16.3p16.1(68,345–7,739,782)X1, 17q25.1q25.3(73,608,322–81,041,938)X3], and LAMA2. Most patients had poor neurological outcomes and refractory seizures controlled by AEDs.
3.5. Recurrent Mutations
The recurrent mutations involved SCN1A, KCNQ2, and TBC1D24. Six cases were attributed to these genes, with seizure onset before 2 years of age. The recurrence of these mutations in the case series indicates that these genes are more commonly involved in childhood DEE, particularly in cases where seizure onset occurs before 2 years of age. Two patients with mutations in KCNQ2 had severe neurological outcomes, and they could not speak at 3 years of age or walk. Two patients with mutations in TBCID24 had persistent refractory seizures with an attention deficit. Two patients with mutations in SCN1A had severe neurological outcomes and refractory seizures.
3.6. Genes Accounting for Epilepsy Demonstrated by the Age of Seizure Onset
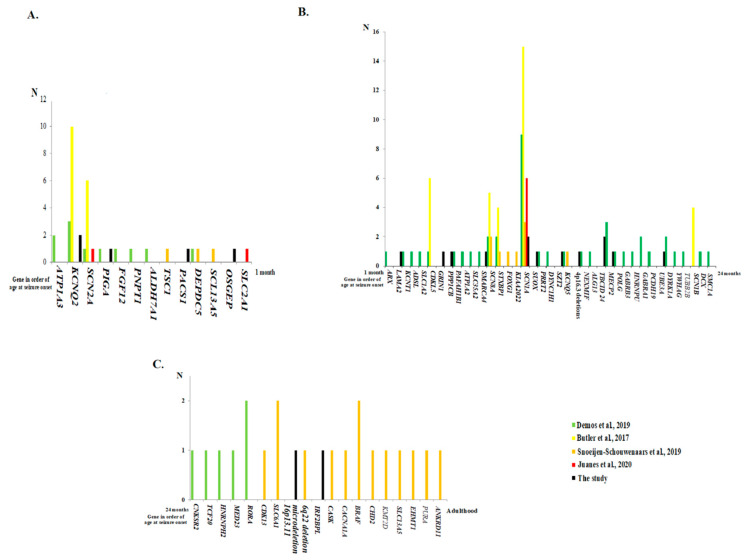
The genes accounting for epileptic patients in the study and in a literature review [18,19,20,21,22,23] (Table 4) are demonstrated in the order of age of seizure onset from birth to 1 month old (Figure 4A), 1 month to 24 months old (Figure 4B), and 24 months to adulthood (Figure 4C).
Table 4.
Diagnostic yield from different methods to identify genetic causes.
| Reference | The Study | [18] | [19] | [20] | [21] | [22] | [23] |
|---|---|---|---|---|---|---|---|
| Method | WES | WES | WES | 110-gene panel | 47-gene panel | WGS | WES |
| Cases (N) | 54 | 360 | 100 | 339 | 17 | 14 | 78 |
| Ethnicity or region | Taiwan | Canada | Caucasian (2 patients had African ethnicity), Netherlands | United States | Argentinean | United States | United States |
| Age | 43.7 ± 62.0 months 41 (76%) seizure onset <2 years |
Seizure onset was 18 months (range 0.03–60 months) | 24.1 ± 16.2 years (ranged 2.8–67.6 years) |
Age ranged 2.5 months to 74 years |
Average age 4 months (range: 0–10 months) | Seizure onset before first month |
8.6 ± 5.8 years (ranged 1.6–26.3 years) |
| Inclusion criteria | 45 epilepsy (35 with AEDs ≥2; 10 with 1 AED and regression) + 9 nonepileptic with regressive symptoms | Seizure onset at ≤5 years |
Unexplained epilepsy and borderline intelligence disability | Epileptic patients | EE with age of onset under 12 months | EIEE | Neurodevelopmental disabilities |
| Male/Female | 30/24 | 154/206 | 55/45 | About equal | 8/9 | 5/9 | 41/37 |
| Yield | 44% | 33% | 25% | 18% | ∼50% | 100% | 41% |
|
Male/Female in identified
genetic group |
17 (57%)/ 7 (29%) |
23/154 (15%)/ 36/206 (19%); In 53 epilepsy diagnosis < 6 months, 12/27 (44.4%) in males and 9/26 (35%) in females |
12 (22%)/ 13 (29%) |
NA | 4 (50%)/ 4 (44%) |
5 (100%)/ 9 (100%) |
NA |
WES, whole-exome sequencing; WGS, whole-genome sequencing; EE, epileptic encephalopathy; AED, antiepileptic drug; NA, not available from the reference.
Figure 4.
The genes accounting for epilepsy are demonstrated in the order of age of seizure onset from birth to 1 month old (A), from 1 month to 24 months old (B), and from 24 months to adulthood (C) from the study and from a literature review.
3.7. Nonseizure Group
In the nonseizure group, the yield was 44% (4/9), including one with SCN2A with autism, one FBN1 with Marfan syndrome, one DMD with muscle dystrophy, and one SPTBN2 with spinocerebellar ataxia.
4. Discussion
Of 54 patients with severe neurological disorders, we identified variants corresponding with the disease in 24 patients. In neonatal infants with seizures, genetic etiologies varied. SCN1A, KCNQ2, and TBCID24 had recurrent mutations in the case series. Mutations in other genes were sporadic and not recurrent. Seventeen genetic etiologies were identified in the seizure group. Of those, 82% (14/17) responsible for childhood seizure were rare and nonrecurrent, indicating that the genetic spectrum for neonatal infantile seizures is wide. Thus, the genes selected for the epileptic panel may not represent all etiologies.
The diagnostic yield of WES was 44%, which is not significantly different from that in other studies, which varied from 30% to 70% [19]. However, results vary greatly and depend on the disease groups selected. Studies have reported diagnostic rates of 19% [24], 41% [23], and 49.1% [25]. Neonatal or infantile DEE was the most commonly found syndrome in this study. Studies have used comprehensive epilepsy gene panel analysis, with a success rate ranging from 10% to 40% [20,21] depending on gene selection and number of cases. However, previous studies have not performed gene panel analysis, which explains why WES is more effective in diagnosing genetic etiologies.
The finding that the WES yield was significant in male pediatric patients provides a viable treatment strategy. The finding needs further explanation: One factor is X-linked inheritance. The sex ratio was 18 males to 6 females. However, only 3 were X-linked. The second factor is that the majority (76%) of 54 patients were aged below 2 years old. In the identified genetic group, seizures onset before 1 year old were found in 18 (75%) patients. Demos et al. [18] found that 12 (44.4%) of 27 males and 9 (35%) of 26 females with epileptic diagnosis <6 months had a genetic diagnosis by WES in 360 epileptic cases with an average seizure onset at ≤5 years (Table 4). That indicated a trend of higher yield in male newborn infants with a younger age of seizure onset. Genetic counseling to prevent next offspring was carried out in all patients, even in the autosomal dominant patients with de novo mutation. In our cohort, 12 patients with autosomal dominant inheritance had de novo mutations. The next offspring were explained to prevent unnecessary concern. In addition, we changed antiepileptic drug selection after detecting a causative KCNQ2 variant in patients 4 and 5, which resulted in more effective seizure control. A patient was identified with a mutation in KCNMA1, which encodes the α-subunit of the large conductance calcium-sensitive potassium channel. Mutations in KCNMA1 increase Ca2+ sensitivity of the channel by three- to five-fold, resulting in generalized epilepsy and paroxysmal dyskinesia [24]. We chose to administer levetiracetam (LEV; 20 mg/kg/day) because LEV can limit epileptogenesis by inhibiting Ca2+ elevation following seizures, thus exerting neuroprotective and antiepileptogenic effects [26,27]. The administration of LEV resulted in no episodes of seizure and low frequency of paroxysmal dyskinesia in the patient. Thus, WES data can support precision therapy and provide information for managing pediatric patients with epilepsy.
SCN1A is studied in the context of genotype–phenotype correlations in the GEFS+ spectrum and DS [20,28,29]. Pathological mutations in structural and functional proteins correlate with specific clinical presentations. Modifier genes also contribute to modulating the phenotype. Most phenotypes that cause severe disability are a result of de novo mutations [20,30]. However, the hypothesis does not account for mosaicism [31]. Some studies have proposed that epigenetic factors contribute to determining phenotypes [20,30,31]. In SCN1A channelopathy, DS is the most severe phenotype. Approximately 70–80% of patients with DS [20,30] have SCN1A mutations, which are mostly de novo, as in our patients. Most patients with DS have mutations in the “ion pore” of SCN1A [32,33,34,35]. However, one of our patients with a mutation in the ion pore region had an identical unaffected twin sister. Although studies have reported that patients with pathogenic SCN1A mutations may remain unaffected, the mechanism is unknown. In a large family with familial pathogenic SCN1A missense mutations, clustering of three unaffected carriers was observed in the same generation [35]. Genetic or epigenetic changes were proposed as the causative factors but not established, because the effects of other confounding factors could not be excluded. KCNQ2 encodes a voltage-gated potassium channel that is expressed in the brain and is involved in the etiology of epileptic encephalopathy, early infantile (EIEE7, phenotype MIM# 613720), and benign familial neonatal seizures-1 (BFNS1, phenotype MIM#121200) [36,37]. Two (patients 4 and 5) de novo heterozygous mutations in KCNQ2, namely c.740C > T (p.Ser247Leu) and c.740C > T (p.Pro285Thr), are highly pathogenic and located in the critical pore domain [38]. This finding helps us select suitable AEDs, such as oxcarbazepine, to control seizures.
The diagnostic methods focusing on the genotype–phenotype correlation followed by specialized biochemical tests and Sanger sequencing for suspected genes are time-consuming and costly [39]. Moreover, only “typical” diseases can be diagnosed, and even when the same causative mutations are present, atypical diseases can be overlooked. In summary, we provide a perspective for diagnosing severe neurological disorders in children using WES. The study presents important findings on the genetic causes of severe neurological disorders in childhood, particularly in epilepsy and neurodegenerative diseases.
In the clinical setting, if the clinical course is thought to be benign, including benign epileptic syndromes [40] in childhood, WES is not performed. However, if, after treatment with AEDs, the response is unfavorable and seizures are refractory, to obtain the best time of seizure control and to avoid sacrificing the moment of children’s brain development, as in our case with UBE3A mutation whose seizure was far preceded to her developmental regression, WES should be performed. Therefore, we recommend WES for assessing all childhood seizures with poor response to AEDs without definitive etiology to save time in selecting appropriate AEDs.
5. Conclusions
The findings on the genetic causes of severe childhood neurological disorders are significant. A scientific and efficient WES analysis is particularly important and should be emphasized because the affected gene may not be present in the panel. This findings of this study highlight the diagnostic relevance of WES for childhood patients with epilepsy in clinical practice.
Acknowledgments
We thank Lee-Jun Wong in Baylor Medical School, USA and everyone who participated in the present project.
Author Contributions
Conceptualization, S.-Y.H. and I.-C.L.; methodology, S.-Y.H. and I.-C.L.; validation, S.-Y.H. and I.-C.L.; formal analysis, S.-Y.H. and I.-C.L.; investigation, S.-Y.H. and I.-C.L.; data curation, I.-C.L.; writing—original draft preparation, S.-Y.H. and I.-C.L.; writing—review and editing, J.-J.Y. and S.-Y.L.; supervision, I.-C.L.; project administration, I.-C.L.; funding acquisition, I.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant MOST 107-2314-B-040-021 from the Taiwan Ministry of Science and Technology and CSH-2020-C-030 Chung Shan Medical University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Engel J., Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Kosseifi C., Cornet M.C., Cilio M.R. Neonatal Developmental and Epileptic Encephalopathies. Semin. Pediatr. Neurol. 2019;32:100770. doi: 10.1016/j.spen.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Howell K.B., Harvey A.S., Archer J.S. Epileptic encephalopathy: Use and misuse of a clinically and conceptually important concept. Epilepsia. 2016;57:343–347. doi: 10.1111/epi.13306. [DOI] [PubMed] [Google Scholar]
- 5.McTague A., Howell K.B., Cross J.H., Kurian M.A., Scheffer I.E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15:304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- 6.Nashabat M., Al Qahtani X.S., Almakdob S., Altwaijri W., Ba-Armah D.M., Hundallah K., Al Hashem A., Al Tala S., Maddirevula S., Alkuraya F.S., et al. The landscape of early infantile epileptic encephalopathy in a consanguineous population. Seizure. 2019;69:154–172. doi: 10.1016/j.seizure.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Nieh S.E., Sherr E.H. Epileptic encephalopathies: New genes and new pathways. Neurotherapeutics. 2014;11:796–806. doi: 10.1007/s13311-014-0301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S., Al Baradie R. Epileptic encephalopathies: An overview. Epilepsy Res. Treat. 2012;2012:403592. doi: 10.1155/2012/403592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noh G.J., Asher Y.J.T., Graham J.M., Jr. Clinical review of genetic epileptic encephalopathies. Eur. J. Med. Genet. 2012;55:281–298. doi: 10.1016/j.ejmg.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang X., Wang K. wANNOVAR: Annotating genetic variants for personal genomes via the web. J. Med. Genet. 2012;49:433–436. doi: 10.1136/jmedgenet-2012-100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 2015;10:1556–1566. doi: 10.1038/nprot.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landrum M.J., Lee J.M., Riley G.R., Jang W., Rubinstein W.S., Church D.M., Maglott D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic. Acids. Res. 2014;42 (Database issue):D980–D985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim N.L., Kumar P., Hu J., Henikoff S., Schneider G., Ng P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amberger J.S., Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic. Acids. Res. 2015;43 (Database issue):D789–D798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical. Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demos M., Guella I., DeGuzman C., McKenzie M.B., Buerki S.E., Evans D.M., Toyota E.B., Boelman C., Huh L.L., Datta A., et al. Diagnostic Yield and Treatment Impact of Targeted Exome Sequencing in Early-Onset Epilepsy. Front Neurol. 2019;10:434. doi: 10.3389/fneur.2019.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snoeijen-Schouwenaars F.M., van Ool J.S., Verhoeven J.S., van Mierlo P., Braakman H.M., Smeets E.E., Nicolai J., Schoots J., Teunissen M.W., Rouhl R.P., et al. Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia. 2019;60:155–164. doi: 10.1111/epi.14618. [DOI] [PubMed] [Google Scholar]
- 20.Butler K.M., da Silva C., Alexander J.J., Hegde M., Escayg A. Diagnostic Yield from 339 Epilepsy Patients Screened on a Clinical Gene Panel. Pediatric Neurol. 2017;77:61–66. doi: 10.1016/j.pediatrneurol.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juanes M., Veneruzzo G., Loos M., Reyes G., Araoz H.V., Garcia F.M., Gomez G., Alonso C.N., Chertkoff L.P., Caraballo R. Molecular diagnosis of epileptic encephalopathy of the first year of life applying a customized gene panel in a group of Argentinean patients. Epilepsy Behav. 2020;111:107322. doi: 10.1016/j.yebeh.2020.107322. [DOI] [PubMed] [Google Scholar]
- 22.Ostrander B.E., Butterfield R.J., Pedersen B.S., Farrell A.J., Layer R.M., Ward A., Miller C., DiSera T., Filloux F.M., Candee M.S., et al. Whole-genome analysis for effective clinical diagnosis and gene discovery in early infantile epileptic encephalopathy. NPJ Genom. Med. 2018;3:22. doi: 10.1038/s41525-018-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava S., Cohen J.S., Vernon H., Barañano K., McClellan R., Jamal L., Naidu S., Fatemi A. Clinical whole exome sequencing in child neurology practice. Ann Neurol. 2014;76:473–483. doi: 10.1002/ana.24251. [DOI] [PubMed] [Google Scholar]
- 24.Dixon-Salazar T.J., Silhavy J.L., Udpa N., Schroth J., Bielas S., Schaffer A.E., Olvera J., Bafna V., Zaki M.S., Abdel-Salam G.H., et al. Exome sequencing can improve diagnosis and alter patient management. Sci. Transl. Med. 2012;4:138ra178. doi: 10.1126/scitranslmed.3003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuperberg M., Lev D., Blumkin L., Zerem A., Ginsberg M., Linder I., Carmi N., Kivity S., Lerman-Sagie T., Leshinsky-Silver E. Utility of Whole Exome Sequencing for Genetic Diagnosis of Previously Undiagnosed Pediatric Neurology Patients. J. Child Neurol. 2016;31:1534–1539. doi: 10.1177/0883073816664836. [DOI] [PubMed] [Google Scholar]
- 26.Du W., Bautista J.F., Yang H., Diez-Sampedro A., You S.A., Wang L., Kotagal P., Lüders H.O., Shi J., Cui J. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 27.Lee I.C., Hong S.Y. Treatment of intractable seizure in Wolf-Hirschhorn syndrome with bromide. Brain Dev. 2017;39:633. doi: 10.1016/j.braindev.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Marini C., Scheffer I.E., Nabbout R., Mei D., Cox K., Dibbens L.M., McMahon J.M., Iona X., Carpintero R.S., Elia M., et al. SCN1A duplications and deletions detected in Dravet syndrome: Implications for molecular diagnosis. Epilepsia. 2009;50:1670–1678. doi: 10.1111/j.1528-1167.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin W.D., Chang K.P., Wang C.H., Chen S.J., Fan P.C., Weng W.C., Lin W.C., Tsai Y., Tsai C.H., Chou I.C., et al. Molecular aspects of Dravet syndrome patients in Taiwan. Clin. Chim. Acta. 2013;421:34–40. doi: 10.1016/j.cca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Claes L., Del-Favero J., Ceulemans B., Lagae L., Van Broeckhoven C., De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am. J. Hum. Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadlamudi L., Dibbens L.M., Lawrence K.M., Iona X., McMahon J.M., Murrell W., Mackay-Sim A., Scheffer I.E., Berkovic S.F. Timing of de novo mutagenesis—A twin study of sodium-channel mutations. N. Engl. J. Med. 2010;363:1335–1340. doi: 10.1056/NEJMoa0910752. [DOI] [PubMed] [Google Scholar]
- 32.Kanai K., Hirose S., Oguni H., Fukuma G., Shirasaka Y., Miyajima T., Wada K., Iwasa H., Yasumoto S., Matsuo M., et al. Effect of localization of missense mutations in SCN1A on epilepsy phenotype severity. Neurology. 2004;63:329–334. doi: 10.1212/01.WNL.0000129829.31179.5B. [DOI] [PubMed] [Google Scholar]
- 33.Ceulemans B.P., Claes L.R., Lagae L.G. Clinical correlations of mutations in the SCN1A gene: From febrile seizures to severe myoclonic epilepsy in infancy. Pediatric Neurol. 2004;30:236–243. doi: 10.1016/j.pediatrneurol.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Lim B.C., Hwang H., Chae J.H., Choi J.E., Hwang Y.S., Kang S.H., Ki C.S., Kim K.J. SCN1A mutational analysis in Korean patients with Dravet syndrome. Seizure. 2011;20:789–794. doi: 10.1016/j.seizure.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg-Stern H., Aharoni S., Afawi Z., Bennett O., Appenzeller S., Pendziwiat M., Kuhlenbäumer G., Basel-Vanagaite L., Shuper A., Korczyn A.D., et al. Broad phenotypic heterogeneity due to a novel SCN1A mutation in a family with genetic epilepsy with febrile seizures plus. J. Child Neurol. 2014;29:221–226. doi: 10.1177/0883073813509016. [DOI] [PubMed] [Google Scholar]
- 36.Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., Dixon J.E., McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 37.Weckhuysen S., Mandelstam S., Suls A., Audenaert D., Deconinck T., Claes L.R., Deprez L., Smets K., Hristova D., Yordanova I., et al. KCNQ2 encephalopathy: Emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 38.Dedek K., Fusco L., Teloy N., Steinlein O.K. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res. 2003;54:21–27. doi: 10.1016/S0920-1211(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 39.Shashi V., McConkie-Rosell A., Rosell B., Schoch K., Vellore K., McDonald M., Jiang Y.H., Xie P., Need A., Goldstein D.B. The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders. Genet. Med. 2014;16:176–182. doi: 10.1038/gim.2013.99. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar K. Differential operator in seizure detection. Comput. Biol. Med. 2012;42:70–74. doi: 10.1016/j.compbiomed.2011.10.010. [DOI] [PubMed] [Google Scholar]