Abstract
Drug-drug interactions (DDIs) with oral anticoagulants may lead to under-anticoagulation and increased risk of thromboembolism. While warfarin is susceptible to numerous DDIs, few studies have examined DDIs resulting in thromboembolism or those involving direct-acting oral anticoagulants (DOACs). We aimed to identify medications that increase the rate of hospitalization for thromboembolic events when taken concomitantly with oral anticoagulants. We conducted a high-throughput pharmacoepidemiologic screening study using Optum Clinformatics Data Mart, 2000–2016. We performed self-controlled case series studies among adult users of oral anticoagulants (warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban) with at least one hospitalization for a thromboembolic event. Among eligible patients, we identified all oral medications frequently co-prescribed with oral anticoagulants as potential interacting precipitants. Conditional Poisson regression was used to estimate rate ratios comparing precipitant exposed vs. unexposed time for each anticoagulant-precipitant pair. To minimize within-person confounding by indication for the precipitant, we used pravastatin as a negative control object drug. Multiple estimation was adjusted using semi-Bayes shrinkage. We screened 1,622 oral anticoagulant-precipitant drug pairs and identified 226 (14%) drug pairs associated with statistically significantly elevated risk of thromboembolism. Using pravastatin as the negative control object drug, this list was reduced to 69 potential DDI signals for thromboembolism, 33 (48%) of which were not documented in the DDI knowledge databases Lexicomp and/or Micromedex. There were more DDI signals associated with warfarin than DOACs. This study reproduced several previously documented oral anticoagulant DDIs and identified potential DDI signals that deserve to be examined in future etiologic studies.
Keywords: Pharmacoepidemiology, drug-drug interactions, coagulation, thrombosis
INTRODUCTION
Oral anticoagulants are used for multiple thromboembolic disorders, including the prevention of stroke and systemic embolism in patients with atrial fibrillation, the treatment and prevention of venous thromboembolism, and thrombo-prophylaxis in patients undergoing total hip and/or knee replacement. Polypharmacy—taking multiple medications at the same time—is very common among oral anticoagulant users. More than half of oral anticoagulant users take more than 5 medications at the same time and are therefore susceptible to adverse drug events due to drug-drug interactions (DDIs). 1–3 DDIs between oral anticoagulants and other concomitantly administered medications can result in either enhanced anticoagulant effects and an increased risk of bleeding, or in reduced anticoagulant effects and an increased risk of thromboembolic events.
Warfarin, the mainstay oral anticoagulant for many decades, is susceptible to numerous DDIs. Mechanisms through which warfarin DDIs can occur include the inhibition of cytochrome P450 (CYP) metabolizing enzymes, changes in vitamin K levels, displacement of plasma protein binding or concurrent use of medications that affect hemorrhagic risk.4,5 Direct-acting oral anticoagulants (DOACs), also known as non-vitamin K oral anticoagulants, were recently introduced as alternatives of warfarin to address its limitations.6–10 Unlike warfarin, DOACs can be administered in fixed doses and do not require routine laboratory monitoring. However, DOACs are substrates of P-glycoprotein and some DOACs (rivaroxaban and apixaban) are extensively metabolized by CYP enzymes.6–14 DOACs therefore may potentially interact with inhibitors or inducers of P-glycoproteins and CYP enzymes. In addition to these known DDI mechanisms, other as-yet unidentified mechanisms may exist. The clinical effect of the potential DDIs between DOACs and other medications on the risk of thromboembolism has not been examined.
To facilitate systematic identification of signals of potential DDIs that lead to thromboembolic events among patients receiving oral anticoagulants, we used real-world clinical data to conduct high-throughput pharmacoepidemiologic screening studies with the self-controlled case series (SCCS) design to identify which potentially interacting drugs may affect the rate of hospitalization of thromboembolism when taken together with oral anticoagulants.
METHODS
Data
We conducted retrospective bidirectional SCCS studies (with sensitivity analyses using a unidirectional design, described below) using Optum Clinformatics Data Mart, a large healthcare claim database in the United States. The database records administrative claims from a large national United States commercial insurance plan including approximately 71 million commercially insured and Medicare Advantage enrollees. Information on patient demographics, enrollment, pharmacy claims, medical claims, and laboratory data are recorded. Only individuals with both medical and prescription coverage are included in the database. The study was exempted from institutional board review by the University of Pennsylvania’s Office of Regulatory Affairs.
Study Population and Observation Time
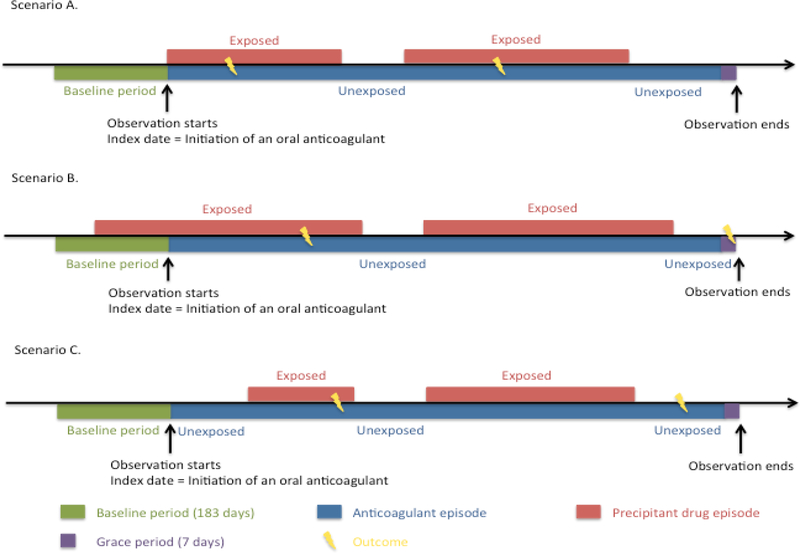
We identified all individuals aged 18 years and above with at least one dispensed prescription of an oral anticoagulant (i.e., object drugs: warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban) from April 1, 2000, to December 31, 2016. Each patient was observed starting from the date of the first prescription of an oral anticoagulant dispensed (i.e., the index date) until the earliest of the following: a prescription of an oral anticoagulant that was different from the one initially received, disenrollment from the plan, the end of the data available (i.e., December 31, 2016), and/or the last day of supply of the oral anticoagulant supply plus 7 days, which was the grace period to account for any non-adherence to the anticoagulant (Figure 1). We counted each day within the observation period as a unit of observation. To be eligible for the study, individuals needed to have continuous enrollment and no prescription of that oral anticoagulant dispensed during the 183 days prior to the index date (i.e., the baseline period). This was implemented to ensure that we observed the incident concomitant drug use. This would reduce the potential to underestimate the frequency of events occurring at the beginning of concomitancy and potential confounding introduced by the inclusion of prevalent users in observational studies.15 To control for within-person confounding by indication for the potential interacting precipitant drug and to distinguish an interaction with an oral anticoagulant from an inherent effect of the precipitant on thromboembolism risk, we used pravastatin as a negative control object drug.16 We selected pravastatin because it is not believed to be metabolized extensively by CYPs.17 Eligible pravastatin users were identified using the same criteria for oral anticoagulant users, except that observation days during which they were exposed to an oral anticoagulant were excluded.
Figure 1.
Study design.
The patient may enter the cohort in three different scenarios. In all three scenarios, observation started on the date of initiation of an oral anticoagulant (i.e., the index date). In scenario A, an oral anticoagulant and the precipitant drug were initiated on the same date (i.e., the concomitancy was combination-triggered). In scenario B, the patient initiated an oral anticoagulant when he/she was already on the precipitant drug (i.e., object-triggered). In scenario C, the patient initiated the precipitant drug after he/she initiated an oral anticoagulant (i.e., precipitant-triggered). All three scenarios were included in the analyses.
Outcome
The outcome of interest was thromboembolism, defined as a composite outcome of stroke (ischemic stroke and transient ischemic attack) and venous thromboembolism (venous thrombosis and pulmonary embolism). All outcomes were identified using algorithms based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes from 2000 to 2016, and International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes from 2015 to 2016. The ICD-9-CM algorithms have been validated in prior studies with positive predictive values of 73–90% (Table S1).18,19 The ICD-10-CM algorithms were translated from ICD-9-CM using General Equivalence Mappings (GEMs) published by the Centers for Medicare and Medicaid Services and Centers for Disease Control and Prevention using Forward Backward Mapping method.20–22 We identified all eligible anticoagulant and pravastatin users with at least one thromboembolic event during the time of an active prescription for that object drug. Any days of hospitalization were excluded because medication use during hospitalization is not recorded in the data.
Exposure – Potential Interacting Precipitant Drugs
The potential interacting precipitants were identified as all oral medications (by active ingredient) that were co-dispensed with each oral anticoagulant and with pravastatin among eligible patients. For each observation day of each patient, exposure indicators were generated to indicate if the patient was exposed (i.e., on the oral anticoagulant and the precipitant drug) or unexposed (i.e., on the oral anticoagulant without the precipitant drug) to the potential precipitant drug. To simplify the high-throughput screening of hundreds of precipitant drugs and to maximize the strength of resulting signals, no grace period was added following the precipitant days’ supply.
Covariates
The SCCS design intrinsically controls for fixed multiplicative covariates such as sex and genetic factors. We controlled for two time-varying covariates: the use of nonsteroidal anti-inflammatory drug (NSAIDs: celecoxib, diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic acid, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, and tolmetin) and the use of antiplatelet agents (abciximab, anagrelide, aspirin, cilostazol, clopidogrel, dipyridamole, eptifibatide, prasugrel, ticagrelor, ticlopidine, and tirofiban) in the past 30 days, each calculated as a binary indicator for each observation day. In the model analyzing a NSAID or an antiplatelet agent as the precipitant drug, we only controlled for the use of antiplatelet agent in the past 30 days or the use of NSAIDs in the past 30 days, respectively.
Statistical analysis
We used conditional Poisson regression to estimate rate ratios (RRs) and 95% confidence intervals (CI) comparing precipitant exposed time vs. precipitant unexposed time for each object-precipitant drug pair. An overall RR was estimated for each object-precipitant pair for each outcome over the entire observation period. To ensure statistically stable estimates of the model, we excluded the drug pair if there were fewer than 5 cases who were ever exposed to that precipitant drug or if the variance of the estimated beta for the parameter of interest was larger than 10. To examine the duration-response relationships, we divided the exposure time into 5 mutually exclusive risk windows: 0–15, 16–30, 31–60, and 61–120 and 120+ days since the initiation of concomitancy. Separate RRs were estimated for each risk window. To ensure stable estimates, if there was no event in a risk window for a given object-precipitant pair, we excluded the exposed and unexposed time of that risk window.
We adjusted for examination of multiple precipitant drugs using semi-Bayes shrinkage.23,24 We pre-specified the variance around the true RRs as 0.25 for the overall RRs and risk window-specific RRs, which corresponds to the assumption that 95% of the true RRs falls within an unspecified 7-fold range of each other. In the secondary analysis, we assumed a larger variance (0.67, corresponding to a 25-fold range).25 For precipitants identified in both cohorts, we calculated the ratio of RRanticoagulant + precipitant vs. anticoagulant to RRpravastatin + precipitant vs. pravastatin. The variance of the ratio of RRs was calculated using the delta method.26
Four prespecified sensitivity analyses were performed. First, we restricted the analysis to patients who only had one event over the observation period to assess the effect of a potential violation of the independent events assumption underlying the SCCS design. Second, we excluded the observation days after September 30, 2015, when ICD-10 was mandatorily implemented in the US to assess the effect of the uncertain validity of ICD-10 algorithms. Lastly, we performed left-censored (excluding the observation time before the first exposed episode) and right-censored (excluding the observation time after the first exposed episode) unidirectional analyses to assess the effect for potential reverse-causality bias and immortal time bias introduced by the bidirectional design.27 However, these unidirectional analyses may be susceptible to exposure-trend bias. 27,28
Cohorts were constructed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina, United States) and SCCS analyses were performed using Stata version 15.0 (StataCorp LP, College Station, Texas, United States). RRs and ratios of RRs were depicted graphically in heat maps plotted using Python Seaborn package.29
RESULTS
We identified 12 to 45,719 anticoagulant and/or pravastatin users who had experienced at least one thromboembolic event during the observation period (i.e., cases). The baseline characteristics of eligible patients are presented in Table 1. As expected, there were many more warfarin users than DOAC users. There were only 12 edoxaban users because edoxaban only became available in the US in January 2015 and has been infrequently used. The mean age was above 70 years for dabigatran, apixaban, edoxaban and pravastatin users, and 66 years for warfarin and rivaroxaban users. The majority (60–79%) of dabigatran and apixaban users had a prior diagnosis of atrial fibrillation, while a lower percentage (30–42%) of warfarin, rivaroxaban and edoxaban users had atrial fibrillation. Approximately 45–50% of dabigatran and apixaban users had a prior thromboembolic event, while the percentage was smaller (~30%) among warfarin, rivaroxaban, edoxaban, and pravastatin users. Approximately 4–7% of oral anticoagulant and pravastatin users had a prior prescription of NSAIDs within 30 days before oral anticoagulant or pravastatin initiation. A higher percentage (15–16%) of edoxaban and pravastatin users had a prior prescription of antiplatelet agents compared to 5–7% among the other four oral anticoagulants (warfarin, dabigatran, rivaroxaban, and apixaban) users.
Table 1.
Baseline characteristics of patients who experienced at least one thromboembolic event (i.e. cases).
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Pravastatin | |
|---|---|---|---|---|---|---|
| Number of cases | 45,719 | 1,394 | 4,924 | 1,993 | 12 | 14,342 |
| Observation days (Person-days) | 30,300,150 | 743,696 | 1,435,539 | 548,364 | 3,147 | 12,271,985 |
| Number of events | 70,979 | 2,238 | 7,056 | 3,047 | 14 | 22,130 |
| Female (%) | 23,626 (51.7) | 693 (49.7) | 2,581 (52.4) | 1,072 (53.8) | 7 (58.3) | 7,762 (54.1) |
| Mean Age (SD) | 66.39 (14.3) | 72.44 (11.4) | 66.12 (15.4) | 72.07 (13.8) | 73.02 (11.7) | 70.51 (10.8) |
| Age groups (%) | ||||||
| 18–44 | 4,494 (9.8) | 35 (2.5) | 514 (10.4) | 103 (5.2) | --- | 310 (2.2) |
| 45–64 | 13,034 (28.5) | 280 (20.1) | 1,547 (31.4) | 406 (20.4) | 3 (25.0) | 3,473 (24.2) |
| 65–74 | 12,161 (26.6) | 405 (29.1) | 1,219 (24.8) | 484 (24.3) | 3 (25.0) | 4,923 (34.3) |
| 75–84 | 14,777 (32.3) | 594 (42.6) | 1,151 (23.4) | 625 (31.4) | 4 (33.3) | 5,051 (35.2) |
| 85–90 | 1,253 (2.7) | 80 (5.7) | 493 (10.0) | 374 (18.8) | 2 (16.7) | 585 (4.1) |
| Atrial fibrillation | 13,484 (29.5) | 1,095 (78.6) | 1,654 (33.6) | 1,187 (59.6) | 5 (41.7) | 2,102 (14.7) |
| Stroke (%) | 13,459 (29.4) | 707 (50.7) | 1,457 (29.6) | 897 (45.0) | 4 (33.3) | 4,825 (33.6) |
| Serious bleeding (%) | 8,466 (18.5) | 346 (24.8) | 1,144 (23.2) | 586 (29.4) | 3 (25.0) | 2,634 (18.4) |
| Cirrhosis (%) | 445 (1.0) | 11 (0.8) | 59 (1.2) | 32 (1.6) | 1 (8.3) | 124 (0.9) |
| Chronic kidney disease (%) | 6,614 (14.5) | 282 (20.2) | 894 (18.2) | 582 (29.2) | 5 (41.7) | 2,451 (17.1) |
| Hypertension (%) | 33,227 (72.7) | 1,270 (91.1) | 3,855 (78.3) | 1,774 (89.0) | 11 (91.7) | 12,212 (85.2) |
| Use of NSAIDs within past 30 days (%)* | 2,842 (6.2) | 51 (3.7) | 366 (7.4) | 97 (4.9) | 0 (0.0) | 994 (6.9) |
| Use of antiplatelet agents within past 30 days (%) † | 2,950 (6.5) | 97 (7.0) | 262 (5.3) | 141 (7.1) | 2 (16.7) | 2,116 (14.8) |
NSAIDs – Non steroidal anti-inflammatory drugs, RR – risk ratio, SD – Standard deviation
Antiplatelet agents include abciximab, anagrelide, aspirin, cilostazol, clopidogrel, dipyridamole, eptifibatide, prasugrel, ticagrelor, ticlopidine, and tirofiban.
NSAIDs include celecoxib, diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic acid, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, and tolmetin.
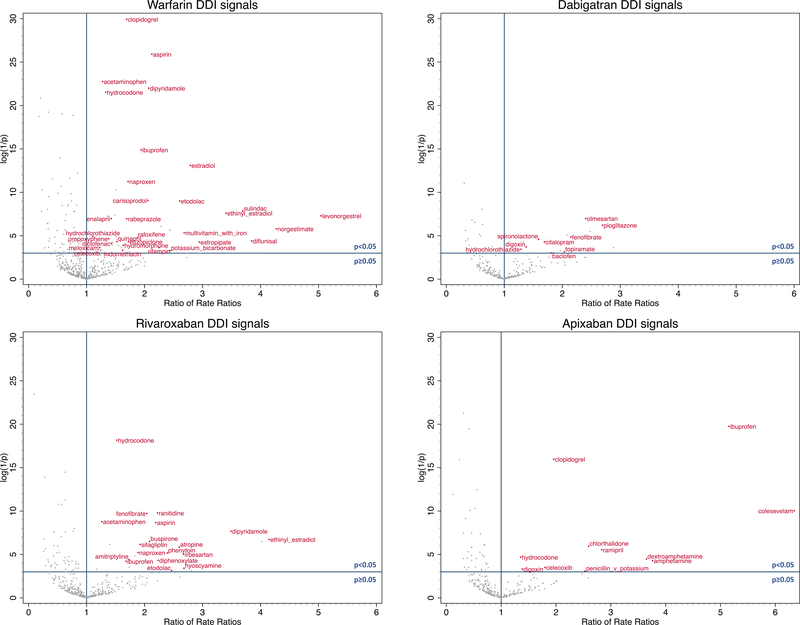
Among 2,779 object-precipitant drug pairs identified among all eligible cases (Table 2), we estimated the overall RRs and risk window specific RRs for 2,163 drug pairs. Excluding 130 unstable estimates, we estimated semi-Bayes adjusted overall RRs for 2,033 object-precipitant drug pairs, which ranged from 0.1–15.1. There were 129, 24, 54, 19, 0 and 84 precipitants associated with statistically significantly elevated overall semi-Bayes adjusted RRs for warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, and pravastatin, respectively. Using pravastatin as the negative control object drug, we calculated overall ratios of RRs for 1,379 oral anticoagulant-precipitant drug pairs with values ranging from 0.1 to 6.3. There were 47, 17, 24, 11, and 0 precipitants associated with statistically significantly elevated overall ratios of RRs for warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban, respectively. Excluding precipitants that seemed less likely to be DDIs (i.e., precipitants that were not associated with a statistically increased risk of thromboembolism in oral anticoagulant users, and with a statistically reduced risk in pravastatin users), we identified 31 potential DDI signals among warfarin users, 9 among dabigatran users, 18 among rivaroxaban users, 11 among apixaban users and none for edoxaban. Among these DDI signals, 33 (48%) were not documented in either of two major DDI references: Lexicomp30 and Micromedex.31 Summaries of the results are presented in Table 2. The list of potential DDI signals are presented in Table 3 and depicted in Figure 2, in which the ratio of rate ratios is plotted on the horizontal axis and the log of the inverse of the p-value is plotted on the vertical axis, so that small p-values (which are affected both by strength of association and sample size) are higher on the vertical axis. The semi-Bayes adjusted overall RRs and risk window specific RRs for each object-precipitant drug pair are presented in Figure S1. The semi-Bayes adjusted overall and risk window specific ratios of RRs for each object-precipitant drug pair are presented in Figure S2. The secondary analysis which assumed a larger variation of true RRs had similar findings (Table S2, and Figures S3 and S4).
Table 2.
Summary of results and potential drug-drug interaction signals identified among oral anticoagulant users.
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Pravastatin | |
|---|---|---|---|---|---|---|
| Number of precipitants identified | 736 | 424 | 519 | 424 | 38 | 638 |
| Number of precipitants with a RR estimate | 655 | 278 | 389 | 296 | 4 | 541 |
| Semi-Bayes adjusted RRs | ||||||
| Number of precipitants included | 626 | 256 | 374 | 280 | 4 | 493 |
| Range | 0.3–15.1 | 0.2–2.7 | 0.3–14.2 | 0.1–5.4 | 1.2–1.4 | 0.4–6.2 |
| Number of statistically significantly elevated semi-Bayes adjusted RR | 129 | 24 | 54 | 19 | 0 | 84 |
| Ratio of RRs | ||||||
| Number of precipitants examined | 493 | 249 | 360 | 273 | 4 | N/A |
| Range | 0.2–5.0 | 0.3–2.9 | 0.1–4.1 | 0.1–6.3 | 1.0–1.5 | N/A |
| Number of statistically significantly elevated ratio of RRs | 47 | 17 | 24 | 11 | 0 | N/A |
| Potential DDI signals * | 31 | 9 | 18 | 11 | 0 | N/A |
DDI – Drug-drug interactions, N/A – Not applicable, RR – Rate ratio.
Number of precipitants with statistically elevated ratio of RRs, excluding the ones not associated with statistically elevated RR among oral anticoagulant users, and associated with statistically reduced RR among pravastatin users.
Table 3.
Potential drug-drug interaction (DDI) signals.
| Precipitant drug used concomitantly with an oral anticoagulant | Ratio of RRs (95% Confidence interval) | Possible mechanism of DDIs on the risk of thromboembolism46–49 |
|---|---|---|
| Warfarin | ||
| levonorgestrel | 5.04 (1.98, 12.83) | Unknown |
| norgestimate | 4.27 (1.64, 11.16) | Induction of CYP3A4 |
| diflunisal | 3.85 (1.34, 11.03) | Effect on hemostasis |
| sulindac | 3.70 (1.79, 7.62) | Effect on hemostasis |
| ethinyl_estradiol | 3.41 (1.71, 6.79) | Estrogen affects hemostasis |
| estropipate | 2.94 (1.24, 6.97) | Estrogen affects hemostasis |
| estradiol | 2.79 (1.83, 4.26) | Estrogen affects hemostasis |
| multivitamin_with_iron | 2.68 (1.35, 5.33) | Vitamin K, or possibly folic acid |
| etodolac | 2.61 (1.60, 4.25) | Effect on hemostasis |
| potassium_bicarbonate* | 2.44 (1.05, 5.65) | Bicarbonate may interfere with blood coagulation |
| rifampin | 2.25 (1.10, 4.61) | Induction of CYP1A2, 2A6, 2B6, 2C19, 2C8, 2E1, 3A4, 3A5, 3A7 |
| aspirin | 2.13 (1.72, 2.64) | Induction of CYP2C19 |
| dipyridamole | 2.07 (1.65, 2.60) | Unknown |
| carisoprodol* | 2.06 (1.43, 2.97) | Unknown |
| ibuprofen | 1.94 (1.50, 2.50) | Effect on hemostasis |
| raloxifene | 1.88 (1.18, 3.00) | Protein binding interaction |
| naproxen | 1.72 (1.35, 2.19) | Effect on hemostasis |
| eszopiclone* | 1.72 (1.12, 2.65) | Unknown |
| clopidogrel | 1.69 (1.56, 1.84) | Unknown |
| rabeprazole | 1.69 (1.24, 2.31) | Induction of CYP1A2 |
| hydromorphone* | 1.63 (1.08, 2.47) | Unknown |
| indomethacin | 1.62 (1.03, 2.55) | Effect on hemostasis |
| quinapril* | 1.53 (1.09, 2.13) | Unknown |
| enalapril* | 1.43 (1.16, 1.76) | Unknown |
| diclofenac | 1.43 (1.07, 1.92) | Effect on hemostasis |
| propoxyphene | 1.38 (1.08, 1.77) | Unknown |
| hydrocodone* | 1.33 (1.22, 1.46) | Unknown |
| acetaminophen | 1.28 (1.18, 1.38) | Unknown |
| celecoxib | 1.24 (1.02, 1.53) | Effect on hemostasis |
| meloxicam | 1.23 (1.02, 1.47) | Effect on hemostasis |
| hydrochlorothiazide* | 1.11 (1.03, 1.20) | Hemoconcentration of clotting factors |
| Dabigatran | ||
| pioglitazone* | 2.69 (1.44, 5.05) | Unknown |
| olmesartan* | 2.41 (1.43, 4.04) | Unknown |
| fenofibrate | 2.15 (1.22, 3.78) | Unknown |
| topiramate* | 2.04 (1.02, 4.05) | Induction of CYP3A4, substrate of P-glycoprotein |
| baclofen* | 1.81 (1.00, 3.26) | Unknown |
| citalopram | 1.69 (1.11, 2.57) | Unknown |
| spironolactone* | 1.59 (1.12, 2.27) | Induction of P-glycoprotein, hemoconcentration of clotting factors |
| digoxin* | 1.38 (1.04, 1.82) | Substrate of P-glycoprotein |
| hydrochlorothiazide* | 1.29 (1.02, 1.62) | Hemoconcentration of clotting factors |
| Rivaroxaban | ||
| ethinyl_estradiol | 4.15 (1.75, 9.81) | Estrogen affects hemostasis |
| dipyridamole | 3.49 (1.08, 6.64) | Effect on hemostasis |
| hyoscyamine* | 2.68 (1.31, 5.46) | Unknown |
| irbesartan* | 2.68 (1.39, 4.88) | Unknown |
| atropine* | 2.60 (1.03, 5.92) | Unknown |
| etodolac | 2.47 (1.18, 4.22) | Effect on hemostasis |
| phenytoin | 2.39 (1.33, 3.29) | Induction of CYP1A2, 2B6, 2C8, 2C19, 2C9, 3A4, 3A5, 3A7 |
| diphenoxylate* | 2.23 (1.44, 2.89) | Unknown |
| ranitidine* | 2.22 (1.27, 2.92) | Unknown |
| aspirin | 2.19 (1.21, 2.95) | Induction of CYP2C19 and P-glycoprotein |
| buspirone* | 2.09 (1.13, 2.66) | Unknown |
| fenofibrate | 2.04 (1.11, 2.55) | Unknown |
| sitagliptin* | 1.92 (1.32, 1.76) | Substrate of CYP3A4 and P-glycoprotein |
| naproxen | 1.89 (1.12, 1.43) | Effect on hemostasis |
| amitriptyline* | 1.73 (1.73, 7.03) | Unknown |
| ibuprofen | 1.68 (1.29, 4.44) | Effect on hemostasis |
| hydrocodone* | 1.52 (1.51, 3.28) | Unknown |
| acetaminophen* | 1.26 (1.46, 3.30) | Unknown |
| Apixaban | ||
| colesevelam* | 6.35 (2.61, 15.40) | Unknown |
| ibuprofen | 5.16 (3.00, 8.85) | Effect on hemostasis |
| amphetamine* | 3.76 (1.31, 10.80) | Unknown |
| dextroamphetamine* | 3.65 (1.34, 9.96) | Unknown |
| ramipril* | 2.84 (1.40, 5.76) | Unknown |
| chlorthalidone* | 2.60 (1.39, 4.84) | Unknown |
| penicillin_v_potassium* | 2.53 (1.02, 6.30) | Unknown |
| clopidogrel | 1.96 (1.53, 2.51) | Unknown |
| celecoxib | 1.80 (1.06, 3.06) | Effect on hemostasis |
| digoxin* | 1.39 (1.02, 1.88) | Substrate of P-glycoprotein |
| hydrocodone* | 1.36 (1.08, 1.71) | Unknown |
DDI – Drug-drug interaction, CYP – Cytochrome P450
Drug-drug interactions not documented in Lexicomp and/or Micromedex.
Figure 2.
Potential oral anticoagulant drug-drug interaction (DDI) signals for thromboembolism.
The x-axis represents ratio of semi-Bayes adjusted rate ratios (RR) anticoagulant + precipitant vs. anticoagulant to RRpravastatin + precipitant vs. pravastatin. The y-axis represents log (1/semi-Bayes adjusted p-value). The data points in the upper right corner are the precipitant drugs with statistically elevated ratio of RRs for thromboembolism. The potential DDI signals labeled in the figure are precipitant drugs with statistically elevated ratio of RRs for thromboembolism, excluding the ones not associated with statistically elevated RR among oral anticoagulant users, and associated with statistically reduced RR among pravastatin users.
The results from four sensitivity analyses are summarized in Table S3–S6. The semi-Bayes adjusted RRs and ratios of RRs estimated over the entire observation period from the four sensitivity analyses are depicted in Figure S5 and S6, respectively. In the analysis excluding observation days after September 30, 2015, few RRs changed direction or statistical significance (Table S4). There were variations in the RRs depending on the specific object-precipitant drug pairs in the sensitivity analyses that restricted to patients with one event and the unidirectional analyses that excluded observation time before or after the first exposed period (Tables S3, S5 and S6).
DISCUSSION
To our knowledge, this is the first pharmacoepidemiologic study to systematically evaluate the association between DDIs and the risk of thromboembolism among patients receiving oral anticoagulants. Using the SCCS design, we identified 226 oral anticoagulant-precipitant drug pairs associated with an elevated overall rate of thromboembolic events following the precipitant initiation. Using pravastatin as the quantitative negative control object drug, we found 99 oral anticoagulant-precipitant drug pairs associated with an increased overall rate of thromboembolic events. Among these pairs, 69 (70%) seem likely to be potential DDI signals. More DDI signals were identified involving warfarin than DOACs.
We replicated several previously documented warfarin DDIs, which supports the validity of our approach. Concomitant use of raloxifene with warfarin was associated with an increased rate of thromboembolism (RR=1.50, 95% CI: 1.20–1.88, ratio of RRs=1.88, 95% CI: 1.18–3.00). Although the clinical significance of this interaction was not previously examined, single doses of concurrent administration of raloxifene and warfarin were shown to reduce the prothrombin time by 10% within two weeks.32 We also found a positive association between rifampin and the rate of thromboembolism (RR=2.35, 95% CI:1.82–3.01, ratio of RRs=2.25, 95% CI:1.10–4.61). This interaction between warfarin and rifampin, a strong CYP inducer,33 to reduce the anticoagulant effect of warfarin has been documented in many case reports.34–37
On the other hand, some previously documented DDIs were not associated with statistically significantly elevated RR or ratio of RRs in our study, for example, carbamazepine (RR=1.07, 95% CI: 0.88–1.30, ratio of RRs=0.82, 95% CI: 0.56–1.21), phenytoin (RR=0.90, 95% CI: 0.80–1.00, ratio of RRs=1.19, 95% CI: 0.90–1.58), and phenobarbital (RR=0.82, 95% CI: 0.63–1.08, ratio of RRs=1.37, 95% CI: 0.69–2.75).38,39 This may be due to lack of dose adjustment as patients may be told to skip doses when they initiated these precipitant drugs, potential misclassification for days on warfarin, and/or insufficient power. Our approach was designed to screen a large number of potentially interacting precipitant drugs in a high-throughput fashion and generate new DDI hypotheses. Future large-scale etiological studies are needed to examine the clinical relevance and the magnitude of the observed DDI signals in independent populations.
We identified eight NSAIDs that were associated with an increased rate of thromboembolism when administered concurrently with warfarin, including celecoxib (RR=1.07, 95% CI: 0.98–1.17, ratio of RRs=1.24, 95% CI: 1.02–1.53), diclofenac (RR=1.49, 95% CI: 1.26–1.78, ratio of RRs=1.43, 95% CI: 1.07–1.92), diflunisal (RR=4.59, 95% CI: 2.47–8.53, ratio of RRs=3.85, 95% CI: 1.34–11.03), etodolac (RR=2.31, 95% CI:1.73–3.08, ratio of RRs=2.61, 95% CI: 1.60–4.25), ibuprofen (RR=2.05, 95% CI:1.81–2.32, ratio of RRs=1.94, 95% CI: 1.50–2.50), indomethacin (RR=1.62, 95% CI: 1.32–1.99, ratio of RRs=1.62, 95% CI: 1.03, 2.55), meloxicam (RR=1.29, 95% CI: 1.14–1.44, ratio of RRs=1.23, 95% CI: 1.02–1.47), and naproxen (RR=1.65, 95% CI: 1.44–1.89, ratio of RRs=1.72, 95% CI: 1.35–2.19). Although NSAIDs have an intrinsic effect on bleeding risk40 and the concomitant use of oral anticoagulants and NSAIDs is associated with an increased risk of bleeding among atrial fibrillation patients,41 NSAIDs use has also been found to be associated with thromboembolism in healthy people and high-risk patients.42,43 A large cohort study of Danish atrial fibrillation patients on vitamin K antagonists (warfarin or phenprocoumon) found that short-term NSAID use was associated with a hazard ratio (HR) of 1.67 (95% CI: 1.41–1.98) for thromboembolism.41 A post-hoc analysis of patients enrolled in the RE-LY (Randomized Evaluation of Long Term Anticoagulant Therapy) trial also found that concomitant use of NSAIDs with warfarin or dabigatran was associated with an increased risk of stroke and systemic embolism (HR=1.50, 95% CI: 1.12–2.01).44 Our findings of elevated RRs and ratios of RRs support this association between NSAIDs and thromboembolism in warfarin users. However, we could not exclude the possibility that patients were told to hold doses of their anticoagulant while initiating NSAIDs to avoid potential bleeding, which could lead to under-anticoagulation. Furthermore, we did not have information on over-the-counter (OTC) NSAIDs and used prescribed NSAIDs as a proxy for NSAIDs exposure. In a cohort of asymptomatic patients age 45–84 years, OTC NSAIDs (aspirin and ibuprofen) were more frequently used than prescribed NSAIDs.45 Comparing OTC NSAIDs and prescribed NSAIDs users, differences in patient characteristics, including ethnicity, intentional exercises and social-economic status were observed. This may contribute to differential misclassification of NSAIDs exposure and thus biased estimates towards either direction.
One DDI signal involving DOACs and P-glycoprotein and/or CYP3A4 inducers was found (rivaroxaban-phenytoin: RR=1.80, 95% CI: 1.03–3.16, ratio of RRs=2.39, 95% CI: 1.29–4.44) in the primary analysis. Two additional signals were identified in the secondary analysis which assumed a larger variation of true RRs including dabigatran-carbamazepine (RR=2.75, 95% CI: 1.27–5.95, ratio of RRs=2.10, 95% CI: 0.91–4.88; secondary analysis: RR=4.77, 95% CI: 1.84–12.39, ratio of RRs=3.61, 95% CI: 1.31–9.96) and rivaroxaban-phenobarbital (RR=1.57, 95% CI: 0.63–3.89, ratio of RRs=2.61, 95% CI: 0.86–7.95; secondary analysis: RR=2.40, 95% CI: 0.63–9.06, ratio of RRs=5.02, 95% CI: 1.09–23.04). DOACs are substrates of P-glycoprotein.6–9 Rivaroxaban and apixaban are also metabolized by CYP enzymes.7,8,12,13 The US labeling of dabigatran recommends avoiding the concomitant use of P-glycoprotein inducers with dabigatran.6 The US labeling of rivaroxaban and apixaban warns to avoid concomitant use of rivaroxaban and apixaban with strong dual P-glycoprotein and CYP3A4 inducers such as carbamazepine and phenytoin.7,8 Our findings suggest the existence of possible clinically significant DDIs among these drugs. We did not observe elevated RRs and/or ratio of RRs associated with many P-glycoprotein inducers or CYP3A4 inducers, for instance, bosentan, oxarbazepine, or primidone. This may suggest either that these DDIs are not clinical relevant, or that we had insufficient sample size to detect such a signal.
Our study has several notable strengths. First, the SCCS design controls for fixed multiplicative covariates by design. Second, to minimize confounding by indication by the precipitant drug, we examined pravastatin as a quantitative negative control object drug. Third, four sensitivity analyses were performed to assess the effect of the potential violation of assumptions underlying SCCS design and the uncertain validity of ICD-10 algorithms. Finally, we systematically evaluated 1,622 oral anticoagulant-precipitant drug pairs that had been frequently co-dispensed in clinical practice.
Our study has several limitations. First, like all studies using administrative data, we did not have data on over-the-counter medications. This may contribute to insufficient adjustment of self-administered NSAIDs and/or potential misclassification of exposure to precipitants that are available over-the-counter. If the misclassification is nondifferential with regard to outcome, the results would be biased toward the null. If the misclassification is differential, the results could be biased in both directions and should be interpreted with caution. Second, we lacked adherence data and assumed full adherence to precipitant drugs. Third, we did not evaluate dose-response relationships and did not adjust for anticoagulant dose, in part because the dispensed warfarin dose may not be a good measure of the warfarin dose actually taken. Fourth, there may be misclassification of days on anticoagulant. It was particularly challenging to assess days on warfarin, which is dose-titrated. We used days’ supply as a proxy for ingestion. If the patients were told to hold doses, this would not be reflected in pharmacy claims data. Fifth, we did not have data on the rationale for ending therapy. In the traditional bi-directional design of SCCS, because observation time before and after the first exposed period are both included in the analysis, one underlying assumption is that the occurrence of an event should not substantially affect subsequent exposure and censoring. If the event leads to subsequent termination of the precipitant drug, there may be reverse causality bias. This limitation was mitigated by the conduct of the unidirectional analyses that are not subject to this bias. Our findings of sensitivity analyses suggested that any effect of violation of the event-independent censoring assumption is likely to depend on the individual drug pair of interest.
Using the SCCS design, we identified 69 potential oral anticoagulant DDIs associated with elevated rates of thromboembolism. Thirty-three of these signals have not been currently documented in existing DDI references. Future studies are needed to confirm or refute these observed associations and to examine potential DDI mechanisms. Precipitant drugs that are more frequently used among anticoagulant users may be prioritized in future etiologic studies.
Supplementary Material
Table S1. International Classification of Diseases codes to identify thromboembolic events.
Table S2. Summary of results and potential drug-drug interaction signals identified among oral anticoagulant users.
Table S3. Summary of results from sensitivity analyses that restricted to patients with only one event.
Table S4. Summary of results from sensitivity analyses that excluded the observation time after October 2010.
Table S5. Summary of results from the left-censored unidirectional analyses.
Table S6. Summary of results from the right-censored unidirectional analyses.
Figure S1. Heatmaps presenting the rate ratio comparing precipitant exposed time vs. precipitant unexposed time.
Figure S2. Heatmaps presenting ratio of rate ratios comparing precipitant exposed time vs. precipitant unexposed time.
Figure S3. Heatmaps presenting ratio of rate ratios comparing precipitant exposed time vs. precipitant unexposed time in the secondary analysis.
Figure S4. Heatmaps presenting rate ratios comparing precipitant exposed time vs. precipitant unexposed time in the secondary analysis.
Figure S5. Heatmaps presenting the overall rate ratio comparing precipitant exposed time vs. precipitant unexposed time from the sensitivity analyses.
Figure S6. Heatmaps presenting the overall ratio of rate ratio comparing precipitant exposed time vs. precipitant unexposed time from the sensitivity analyses.
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Drug-drug interactions (DDIs) with oral anticoagulants may lead to under-anticoagulation and increased risk of thromboembolism. While warfarin is susceptible to numerous DDIs, few studies have examined DDIs resulting in thromboembolism or those involving direct-acting oral anticoagulants.
What question did this study address?
Which medications taken concomitantly with oral anticoagulants would increase the rate of hospitalization for thromboembolic events?
What does this study add to our knowledge?
We screened 1,622 anticoagulant-precipitant drug pairs and identified 69 DDI signals. Thirty-three of these were not documented in two major DDI knowledge databases. Careful management of patients on warfarin and direct-acting oral anticoagulants may be warranted as we identified novel DDIs that are not documented in major DDI databases.
How might this change clinical pharmacology or translational science?
Large scale pharmacoepidemiologic studies in independent population are needed to confirm or refute the clinical importance of these potentially, but previously unidentified DDIs for thromboembolic events in patients receiving oral anticoagulants. Future research evaluating the potential mechanisms for these potential DDI signals may contribute to pharmacologic knowledge of these drugs.
ACKNOWLEDGEMENTS
The authors thank Min Du and Qing Liu from the University of Pennsylvania for their computer programming support.
Funding
This work was supported by grants R01AG025152 and R01AG060975 from the United States Department of Health and Human Services’ National Institute on Aging.
Conflict of Interest Statement
Drs. Kimmel and Hennessy have served as consultants for several pharmaceutical companies, all unrelated to the contents of this study. Dr. Hennessy is the Director of Center for Pharmacoepidemiology Research and Training (CPeRT) at the University of Pennsylvania, and Dr. Leonard serves on the Executive Committee of CPeRT. The center receives funding for educational purposes from Pfizer and Sanofi. Dr. Zhou was affiliated with University of Pennsylvania at the time of this study. She is currently affiliated with Johnson and Johnson. All other authors declared no competing interests for this work.
REFENENCES
- 1.Skov J, Bladbjerg EM, Sidelmann J, Vamosi M & Jespersen J Plenty of pills: polypharmacy prevails in patients of a Danish anticoagulant clinic. Eur J Clin Pharmacol 2011. November;67(11):1169–74. doi: 10.1007/s00228-011-1045-0. Epub 2011 May 12. [DOI] [PubMed] [Google Scholar]
- 2.Piccini JP et al. Polypharmacy and the Efficacy and Safety of Rivaroxaban Versus Warfarin in the Prevention of Stroke in Patients With Nonvalvular Atrial Fibrillation. Circulation. 2016. January 26;133(4):352–60. doi: 10.1161/CIRCULATIONAHA.115.018544. Epub 2015 Dec 16. [DOI] [PubMed] [Google Scholar]
- 3.Jaspers Focks J et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: post hoc analysis of the ARISTOTLE trial. BMJ. 2016. June 15;353:i2868. doi: 10.1136/bmj.i2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Minno A et al. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev 2017. July;31(4):193–203. doi: 10.1016/j.blre.2017.02.001. Epub 2017 Feb 5. [DOI] [PubMed] [Google Scholar]
- 5.Bungard TJ, Yakiwchuk E, Foisy M & Brocklebank C Drug Interactions Involving Warfarin: Practice Tool and Practical Management Tips. Can Pharm J 144, 21–25.e9 (2011). [Google Scholar]
- 6.Ingelheim Boehringer. Pradaxa® (Dabigatran exetitate mesylate) prescribing information. <https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf> (2019). Assessed 21 February 2019.
- 7.Janssen. Xarelto® (Rivaroxaban) prescribing information. < https://www.xarelto-us.com/shared/product/xarelto/prescribing-information.pdf> (2019). Assessed 21 February 2019.
- 8.Squibb Bristol-Meyers. Eliquis® (Apixaban) prescribing information. < http://packageinserts.bms.com/pi/pi_eliquis.pdf> (2019). Assessed 21 February 2019.
- 9.Daiichi-Sankyo. Savaysa® (Edoxaban) prescribing information. <https://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true> (2019). Assessed 21 February 2019. [Google Scholar]
- 10.Portola Phharmaceuticals, Inc. Bevyxxa® (Betrixaban) prescribing information. <http://www.bevyxxa.com/docs/Bevyxxa-Full-Prescribing-Information.pdf> (2019). Assessed 21 February 2019.
- 11.Stangier J & Clemens A Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009. Sep-Oct;15 Suppl 1:9S–16S. doi: 10.1177/1076029609343004. Epub 2009 Aug 19. [DOI] [PubMed] [Google Scholar]
- 12.Kreutz R Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012. February;26(1):27–32. doi: 10.1111/j.1472-8206.2011.00981.x. Epub 2011 Aug 16. [DOI] [PubMed] [Google Scholar]
- 13.Cada DJ, Levien TL & Baker DE Apixaban. Hosp Pharm 2013. June;48(6):494–509. doi: 10.1310/hpj4806-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stacy ZA, Call WB, Hartmann AP, Peters GL & Richter SK Edoxaban: A Comprehensive Review of the Pharmacology and Clinical Data for the Management of Atrial Fibrillation and Venous Thromboembolism. Cardiol Ther 2016. June;5(1):1–18. doi: 10.1007/s40119-016-0058-2. Epub 2016 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray WA Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003. November 1;158(9):915–20. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy S et al. Pharmacoepidemiologic Methods for Studying the Health Effects of Drug-Drug Interactions. Clin Pharmacol Ther 2016. January;99(1):92–100. doi: 10.1002/cpt.277. Epub 2015 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatanaka T Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet 2000. December;39(6):397–412. [DOI] [PubMed] [Google Scholar]
- 18.Tirschwell DL & Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002. October;33(10):2465–70. [DOI] [PubMed] [Google Scholar]
- 19.White RH et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res 2010. July;126(1):61–7. doi: 10.1016/j.thromres.2010.03.009. Epub 2010 Apr 28. [DOI] [PubMed] [Google Scholar]
- 20.Center for Medicare & Medicaid Services. 2018 ICD-10-CM and GEMs. < https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs> (2019). Assessed 21 February 2019.
- 21.Fung KW et al. Preparing for the ICD-10-CM Transition: Automated Methods for Translating ICD Codes in Clinical Phenotype Definitions. EGEMS (Wash DC). 2016. April 12;4(1):1211. doi: 10.13063/2327-9214.1211. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panozzo CA et al. Early impact of the ICD-10-CM transition on selected health outcomes in 13 electronic health care databases in the United States. Pharmacoepidemiol Drug Saf 2018. August;27(8):839–847. doi: 10.1002/pds.4563. Epub 2018 Jun 26. [DOI] [PubMed] [Google Scholar]
- 23.Greenland SA semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat Med 1992. January 30;11(2):219–30. [DOI] [PubMed] [Google Scholar]
- 24.Momoli F, Abrahamowicz M, Parent ME, Krewski D & Siemiatycki J Analysis of multiple exposures: an empirical comparison of results from conventional and semi-bayes modeling strategies. Epidemiology 2010. January;21(1):144–51. doi: 10.1097/EDE.0b013e3181c297c7. [DOI] [PubMed] [Google Scholar]
- 25.Leonard CE et al. Clopidogrel Drug Interactions and Serious Bleeding: Generating Real-World Evidence via Automated High-Throughput Pharmacoepidemiologic Screening. Clin Pharmacol Ther 2019. November;106(5):1067–1075. doi: 10.1002/cpt.1507. Epub 2019 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieler GS & Williams RL Ratio estimates, the delta method, and quantal response tests for increased carcinogenicity. Biometrics. 1993. September;49(3):793–801. [PubMed] [Google Scholar]
- 27.Farrington P, Whitaker H & Weidenbach YG Self-controlled case series studies: Amodelling guide with R. (Chapman and Hall/CRC, 2018). [Google Scholar]
- 28.Zhou M, Leonard CE, Bilker WB & Hennessy S The Self-Controlled Case Series Design as a Viable Alternative to Studying Clinically Relevant Drug Interactions. Clin Pharmacol Ther 2020. February;107(2):321–322. doi: 10.1002/cpt.1631. Epub 2019 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waskom M et al. (2018, July 16). mwaskom/seaborn: v0.9.0 (July 2018) (Version v0.9.0). Zenodo. 10.5281/zenodo.1313201 [DOI] [Google Scholar]
- 30.Lexicomp Online: Interactions. Wolters Kluwer Clinical Drug Information, Inc. 2019. Assessed 15 August 2019. [Google Scholar]
- 31.IBM Watson Health. IBM Micromedex Solutions – Drug Interactions. IBM Watson Health, Inc. 2019. Assessed 15 August 2019. [Google Scholar]
- 32.Miller JW, Skerjanec A, Knadler MP, Ghosh A & Allerheiligen SR Divergent effects of raloxifene HCI on the pharmacokinetics and pharmacodynamics of warfarin. Pharm Res 2001. July;18(7):1024–8. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesan K Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokinet 1992. January;22(1):47–65. [DOI] [PubMed] [Google Scholar]
- 34.Casner PR Inability to attain oral anticoagulation: warfarin-rifampin interaction revisited. South Med J 1996. December;89(12):1200–3. [DOI] [PubMed] [Google Scholar]
- 35.Kim KY, Epplen K, Foruhari F & Alexandropoulos H Update on the interaction of rifampin and warfarin. Prog Cardiovasc Nurs 2007. Spring;22(2):97–100. [DOI] [PubMed] [Google Scholar]
- 36.Lee CR & Thrasher KA Difficulties in anticoagulation management during coadministration of warfarin and rifampin. Pharmacotherapy 2001. October;21(10):1240–6. [DOI] [PubMed] [Google Scholar]
- 37.Romankiewicz JA & Ehrman M Rifampin and warfarin: a drug interaction. Ann Intern Med 1975. February;82(2):224–5. [DOI] [PubMed] [Google Scholar]
- 38.Mannheimer B, Andersson ML, Järnbert-Pettersson H & Lindh JD The effect of carbamazepine on warfarin anticoagulation: a register-based nationwide cohort study involving the Swedish population. J Thromb Haemost 2016. April;14(4):765–71. doi: 10.1111/jth.13268. Epub 2016 Mar 8. [DOI] [PubMed] [Google Scholar]
- 39.Clark NP, Hoang K, Delate T, Horn JR & Witt DM Warfarin Interaction With Hepatic Cytochrome P-450 Enzyme-Inducing Anticonvulsants. Clin Appl Thromb Hemost 2018. January; 24(1): 172–178. Published online 2017 Jan 25. doi: 10.1177/1076029616687849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafer AI Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol 1995. March;35(3):209–19. [DOI] [PubMed] [Google Scholar]
- 41.Lamberts M et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med 2014. November 18;161(10):690–8. doi: 10.7326/M13-1581. [DOI] [PubMed] [Google Scholar]
- 42.Chang CH, Shau WY, Kuo CW, Chen ST & Lai MS Increased risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study. Stroke. 2010. September;41(9):1884–90. doi: 10.1161/STROKEAHA.110.585828. Epub 2010 Jul 29. [DOI] [PubMed] [Google Scholar]
- 43.Fosbøl EL et al. Use of nonsteroidal anti-inflammatory drugs among healthy people and specific cerebrovascular safety. Int J Stroke. 2014. October;9(7):943–5. doi: 10.1111/j.1747-4949.2012.00863.x. Epub 2012 Oct 23. [DOI] [PubMed] [Google Scholar]
- 44.Kent AP et al. Concomitant Oral Anticoagulant and Nonsteroidal Anti-Inflammatory Drug Therapy in Patients With Atrial Fibrillation. J Am Coll Cardiol 2018. July 17;72(3):255–267. doi: 10.1016/j.jacc.2018.04.063. Epub 2018 Jul 9. [DOI] [PubMed] [Google Scholar]
- 45.Delaney JA, Biggs ML, Kronmal RA, Psaty BM Demographic, medical, and behavioral characteristics associated with over the counter non-steroidal anti-inflammatory drug use in a population-based cohort: results from the Multi-Ethnic Study of Atherosclerosis. Pharmacoepidemiol Drug Saf 2011. January;20(1):83–9. doi: 10.1002/pds.2065. Epub 2010 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wishart DS et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 2017. November 8. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong DW, Mishkin FS & Tanaka TT The effects of bicarbonate on blood coagulantion. JAMA. 1980. July 4;244(1):61–2. [PubMed] [Google Scholar]
- 48.Scott JA, Da Camara CC, Early JE Raloxifene: A selective estrogen receptor modulator. Am Fam Physician 1999. September 15; 60 (4): 1131–1138. [PubMed] [Google Scholar]
- 49.Stangier J, Stähle H, Rathgen K, Roth W, Reseski K & Körnicke T Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, with coadministration of digoxin. J Clin Pharmacol 2012. February;52(2):243–50. doi: 10.1177/0091270010393342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases codes to identify thromboembolic events.
Table S2. Summary of results and potential drug-drug interaction signals identified among oral anticoagulant users.
Table S3. Summary of results from sensitivity analyses that restricted to patients with only one event.
Table S4. Summary of results from sensitivity analyses that excluded the observation time after October 2010.
Table S5. Summary of results from the left-censored unidirectional analyses.
Table S6. Summary of results from the right-censored unidirectional analyses.
Figure S1. Heatmaps presenting the rate ratio comparing precipitant exposed time vs. precipitant unexposed time.
Figure S2. Heatmaps presenting ratio of rate ratios comparing precipitant exposed time vs. precipitant unexposed time.
Figure S3. Heatmaps presenting ratio of rate ratios comparing precipitant exposed time vs. precipitant unexposed time in the secondary analysis.
Figure S4. Heatmaps presenting rate ratios comparing precipitant exposed time vs. precipitant unexposed time in the secondary analysis.
Figure S5. Heatmaps presenting the overall rate ratio comparing precipitant exposed time vs. precipitant unexposed time from the sensitivity analyses.
Figure S6. Heatmaps presenting the overall ratio of rate ratio comparing precipitant exposed time vs. precipitant unexposed time from the sensitivity analyses.