Abstract
This pilot study investigated the impact of freezing of gait, objectively measured with three inertial sensors, on mobility function during seven days of community-living monitoring in people with Parkinson’s disease.
Twenty-four subjects with PD, of which 14 experiencing freezing of gait, were recruited in this study. Subjects wore three inertial sensors (Opals, APDM) attached to both feet and the lumbar region for a week of continuous monitoring. Walking bouts, of at least 10s, were first identified, and then features of freezing, quantity and quality of mobility were extracted and averaged across the seven days.
Results showed significant impairments in freezing and quality of mobility in the freezers group compared to the non-freezers. Our measures of average and variability of time spent freezing was associated to the subjects’ perception of freezing, assessed with the New Freezing of Gait Questionnaire. These preliminary results are introducing promising measures of mobility impairments measured during community-living in PD.
I. Introduction
Gait disturbances in Parkinson’s disease (PD) such as reduced gait speed, shorter stride length, increased time of double support and slow turns, are a major cause for functional dependence and the largest risk factor for falls, institutionalization and death in PD [1, 2]. In addition, over 80% of people with PD eventually develop freezing of gait (FoG), an intermittent failure to initiate or maintain locomotion, especially while turning [3, 4]. FoG and slow walking are the most significant factors affecting the quality of life in people with PD and are associated with increased risk of falls [5].
The assessment of patient mobility in the clinic or laboratory is not an accurate representation of typical mobility function in daily life. Increased attention, alertness, and effort to impress the examiner during testing may enhance motor performance. This is particularly true for FoG because freezing is often difficult to observe during a clinical visit or in the laboratory [6–8] when patients are attending to their walking, which is primarily assessed on a straight path.
Turning is generally difficult for people with PD and known to trigger FoG [9], with links to balance confidence implicated [5, 10]. Freezing during turning may be even more common in the home than in the clinic or laboratory because freezing is triggered in specific conditions, such as multi-tasking in crowded environments. However, a recent study [11], measuring turning during community-living activities in a large sample of PD with and without freezing, showed that quantity of turning was similar among freezers and non-freezers during 72 hours of monitoring with one wearable sensor on the belt.
The majority of activity monitors reflect the quantity but not the quality of mobility or the patterns of activity that emerge from continuous monitoring [12, 13]. Activity monitors measure total daily movements as reflected by accelerations and/or the percent of the day a subject is standing, walking or sitting/lying. Common measures include total activity duration, total number of steps taken, and the time spent in each activity. Whilst informative, these measures tend to not characterize specific gait impairments, such as freezing, quality of turning performance, or quality of walking, such as stride length or variability aspect of gait. Characterization of mobility impairments during daily life of people with PD will help inform effects of treatment, disease progression, characterize fall risk and explain differences in activity.
Recently, a few studies [12–15] have focused on quality of mobility in PD during daily life, using wearable, light-weight inertial sensors placed on different places on the body. Novel measures calculated from both accelerometers and gyroscopes enables a detailed analysis of gait bouts and turning over a week of continuous recording, as well as analysis of patterns of accumulated activity. Recent studies suggest that quantity of walking and turning at home is similar among freezers and non-freezers [11, 12]. However, the quality of walking and turning, such as variability and consistency may be different in freezers versus non-freezers with PD.
A recent review [16] pointed out that while the studies detecting freezing of gait in a laboratory environment are well validated at this point, studies focused on detecting freezing of gait during community-living activities are not. In addition, the true impact of FoG on balance perception and mobility disturbances have not yet been investigated. Moreover, although a growing number of successful studies are focusing on freezing episode detection during daily activities [16–18], surprisingly the impact or percentage of freezing during daily life have not yet been reported.
Here, we aimed to introduce a novel, objective measure of time spent freezing at home and investigate its association with perception of freezing severity (clinical validity), balance confidence and mobility function during community-living in a group of PD with and without freezing of gait.
II. Methods
A. Participants
Twenty-four subjects with PD (67±7 years old) participated in this study. Fourteen subjects, out of the 24, were classified as freezers, according to the New Freezing of Gait Questionnaire, NFOG-Q (≥1 the Questionnaire [19]). Inclusion criteria for all subjects were: diagnosis of idiopathic Parkinson’s disease with sensitivity to levodopa; exclusion criteria were other factors affecting gait (hip replacement, musculoskeletal disorder, uncorrected vision or vestibular problem), or an inability to stand or walk for 2 minutes at a time. All participants provided informed consent approved by the Oregon Health & Science University Institutional Review Board.
B. Procedure
A research assistant met subjects at their homes the first morning to set up the sensors, instruct subjects how to wear them and charge them at the end of the day, and conduct clinical tests. Then, participants were monitored with the wearable sensors throughout the day, for a week. Subjects wore 3 Opal inertial sensors (APDM, Inc., Portland, OR): on their lower back (lumbar region), right and left foot (on the shoes) for 7 days and docked them to recharge each night. The sensors were easily placed in the body location with a Velcro belt and straps. Each Opal records tri-axial acceleration (± 2g or 6g), rotation (±1500 deg/s), and magnetic field strength (±6 gauss) at a sample rate of 128 Hz. Each sensor is wirelessly synchronized with each other (≤10 μs) and can store up to 8Gb of data (enough for over 30 days of continuous monitoring). Researchers returned to their homes after 7 days to pick up equipment, conduct a structured interview about feasibility and perform clinical tests. Severity of disease was rated based on the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS, Part III [20]), and balance confidence was assessed with the Activity Specific Balance confidence scale (ABC, [21]).
In addition, a subset of the subjects (n=20) performed two 2-minute walk tests in their home. The research assistant along with the subject determined a stretch (> 4.5 m) in the home that allowed unobstructed back and forth walking. Beginning and end of the stretch were temporarily marked off with tape. Subjects were provided with an interval timer (Gymboss) that was programmed to give an acoustical signal after two minutes, indicating the end of a trial. Subjects with PD completed the first walking test before intake of their levodopa medication and the second test one hour after intake of the first dose of levodopa the day.
C. Data Analysis
Freezing Ratio and Turning features
From the data collected over 7 days, first the periods of walking were detected, from the 3D angular velocity and 3D acceleration of the lumbar sensor, in windows of 30 minutes [11, 22–24]. Walking bouts of 10s and longer were then used for further analysis.
Freezing proxy:
the FoG identification algorithm used the antero-posterior acceleration of the sensors worn on the feet during each identified gait bout. It has been shown that freezing is accompanied by high-frequency components in the 3–8 Hz band of the leg movement. Recently a ‘Freezing Ratio’ was defined as the power in the “freeze band” (3–8 Hz) divided by the power in the “locomotor band” (0.5–3Hz) with larger ratios indicating more freezing [25, 26]. Such ‘high-frequency’ components of gait have been associated with the ‘trembling’ observed during freezing episodes. The power spectral densities (PSD) were calculated for both right and left foot using a 4-s Hanning window with 50% overlap (using the Welch method). The total power was then normalized to the area under the PSD and frequency Ratio was calculated as the square of the total power in the 3–8 Hz band, divided by the square of the total power in the 0.5–3 Hz band. Lastly, for each gait bout the percentage of time in which the ratio (for either right or left foot) was higher than 1 was calculated, see Figure 1. Our primary outcome measure for freezing was the cumulative sum of such percentage normalized to the total hours of recording (average time spent freezing per hour). In addition, the variability of the percentage of time spent freezing was reported.
Figure 1.
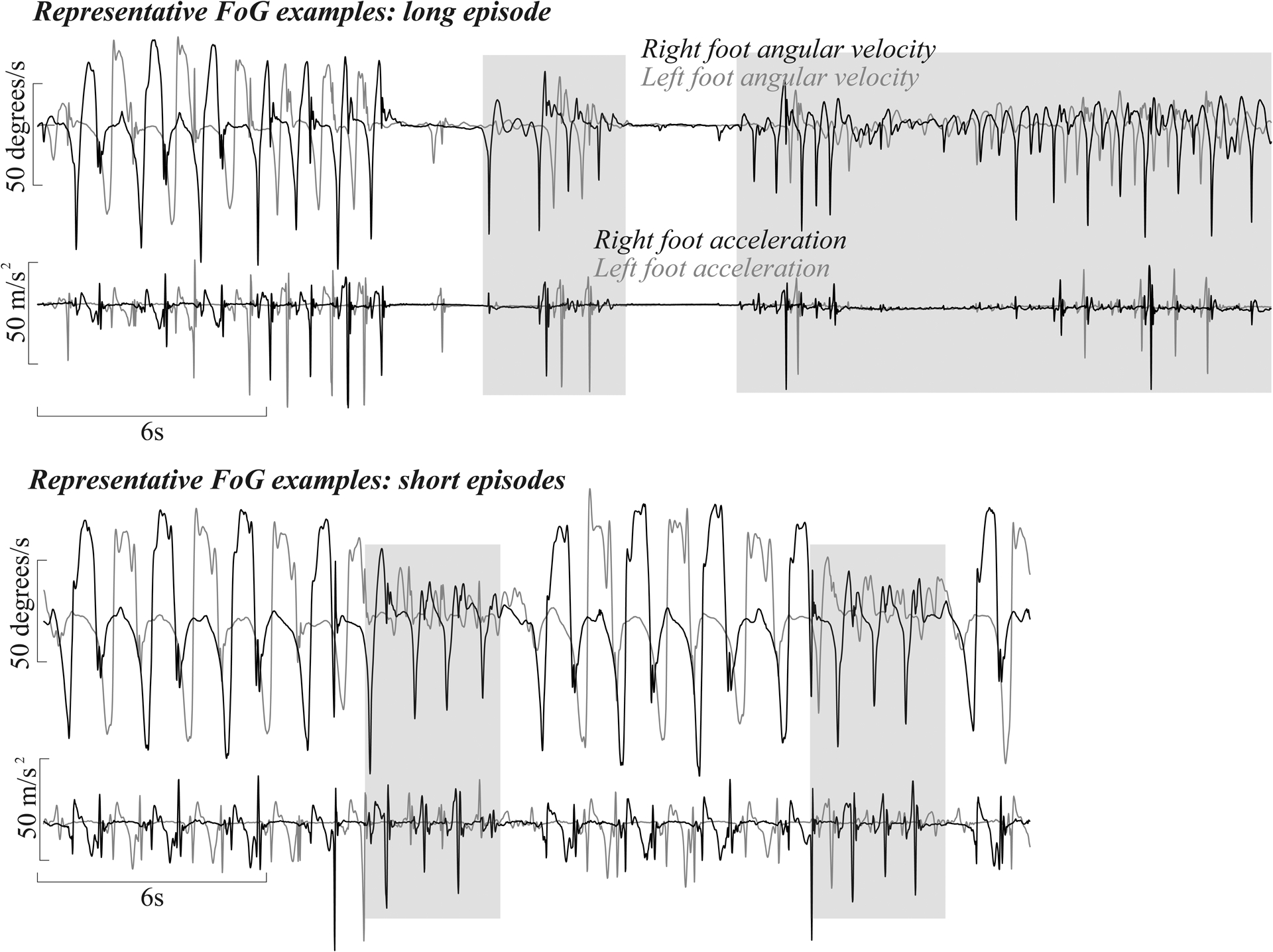
Representative examples of Freezing episodes (grey areas)
Turn detection:
the algorithm used the horizontal rotational rate (yaw) of the lumbar sensor during each identified gait bout. Details are described elsewhere. Briefly, a turn was defined as a trunk rotation around the vertical plane with a minimum of 40 degrees [22]. Turning angle was then obtained integrating the angular rate of the lumbar sensor around the vertical axis. Quantity of turning is reported as the mean numbers of turns per 30 minutes and quality of turning is reported as average turning angle (degrees). Both outcomes were averaged across the hours of recording over the 7 days.
Walking features
A separate algorithm, using Unscented Kalman Filter to fuse information from the accelerometers, gyroscopes, and magnetometers to estimate the orientation and position trajectory of the sensors on the feet, was used to quantify quality of walking. Gait was defined as walking bouts of at least 3 consecutive strides, a minimum duration of 3 seconds and intermittent breaks of no longer than 6 seconds.
The selected outcomes for walking were gait speed (m/s), and the pitch angle of the foot at initial contact (degrees) selected as an indicator of shuffling. Both outcomes were averaged across the hours of recording. Steps that occurred during turning were excluded.
C. Statistical Analysis
Pearson’s correlation was used to evaluate the association between subjects’ perception of FoG and the objective measures of freezing (clinical validity). Pearson’s correlation was also used to determine the association among the extracted features of mobility and balance confidence for all the subjects with PD.
Independent t-tests were used to determine differences in mobility between the Freezer and Non-freezer groups (p<0.05). The Levene’s test for equality of variance was carried out in advance for all the selected features to determine if the variance among the two groups was equal and in case of rejected hypothesis, equality of variance was not assumed for the t-test of that feature. The statistical analyses were performed using SPSS Software v.24.
III. Results
The objective measures of freezing in the home, average time spent freezing and its variability, were significantly related to scores on the NFOG-Q questionnaire (r=0.56 and r=0.62, p<0.05) demonstrating moderate clinical (face) validity. Balance perception, measured by the ABC clinical scale, was also significantly associated with the time spent freezing and its variability across the hours (r=−0.44 and r=−0.44, p<0.05).
In addition, the objective measures of freezing (time and variability) were significantly associated with the quality of mobility at home, but not with the quantity of disease severity (measured by the MDS-UPDRS Part III), Table I. Specifically, a higher average time spent freezing and higher variability of time spent freezing were associated with lower average pitch angle at initial contact, higher variability of pitch angle at initial contact, and smaller average turning angle. Interestingly, no significant associations were found between measures of freezing with severity of disease, gait speed or number of turns. Similarly, only the quality, and not quantity of mobility at home differed between freezers and non-freezers, Table II.
TABLE I.
Associations between the average time spent freezing and variability of time spent freezing with disease severity, balance confidence, and mobility
| Time spent freezing | CV time spent freezing | |||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Clinical | ||||
| MDS-UPDRS Part III | 0.36 | 0.12 | 0.27 | 0.25 |
| ABC | −0.44 | 0.05 | −0.44 | 0.05 |
| Quantity of Mobility | ||||
| Average Turns # | −0.26 | 0.24 | −0.31 | 0.16 |
| Average Bouts # | −0.26 | 0.24 | −0.28 | 0.21 |
| Average Bout Duration | −0.01 | 0.97 | −0.06 | 0.79 |
| Quality of Mobility | ||||
| Average Pitch angle | 0.54 | 0.01 | 0.56 | 0.01 |
| CV Pitch Angle | −0.71 | 0.00 | −0.79 | 0.00 |
| Average Gait Speed | −0.34 | 0.14 | −0.31 | 0.19 |
| Average Turn Angle | −0.48 | 0.02 | −0.45 | 0.04 |
TABLE II.
Means and sem of the clinical and objective measures of mobility.
| Non-freezers | Freezers | ||||
|---|---|---|---|---|---|
| Clinical | Mean and SEM | Mean and SEM | p-value | ||
| MDS-UPDRS Part III | 35.6 | 3.9 | 36.1 | 4.0 | 0.927 |
| ABC | 87.0 | 3.6 | 71.6 | 5.2 | 0.037 |
| Quantity of Mobility | |||||
| Average Turns # | 28.38 | 5.80 | 30.33 | 6.94 | 0.846 |
| Average Bouts # | 8.19 | 0.98 | 8.83 | 0.75 | 0.608 |
| Average Bouts Duration (s) | 5178.31 | 548.94 | 4708.45 | 334.31 | 0.446 |
| Quality of Mobility | |||||
| Average Pitch angle (degrees) | −18.20 | 1.92 | −12.12 | 2.09 | 0.052 |
| CV Pitch Angle | −0.515 | 0.049 | −0.949 | 0.151 | 0.017 |
| Average Gait Speed (m/s) | 0.85 | 0.04 | 0.77 | 0.06 | 0.305 |
| Average Turn Angle (degrees) | 97.26 | 1.90 | 90.17 | 1.25 | 0.004 |
| Time spent freezing (%) | 5.09 | 1.31 | 10.94 | 2.33 | 0.041 |
| CV time spent freezing | 5.94 | 0.97 | 10.48 | 1.48 | 0.019 |
IV. Conclusion and Future Work
Our novel, objective measures of freezing from inertial sensors on the feet during community-living monitoring for 7 days differentiated subjects with PD who reported freezing of gait and those who did not report freezing. In addition, both average time spent freezing and variability of time spent freezing were significantly associated with patient freezing severity perception (measured by total NFOG-Q score) and balance perception (measured by the ABC).
Quality of turning and gait were significantly altered in freezers compared to non-freezers. Specifically, the average turning angle was smaller in freezers compared to non-freezers, as previously reported in a larger cohort [11]; and such difference could potentially be attributed to the fact that freezers may avoid larger turning angles, known to elicit more freezing. Consistent with previous works [11, 12], quantity of mobility, as assessed by average number of turns, number of hours recorded, and number of gait bouts was similar among freezers and non-freezers.
The average pitch angle at initial foot contact with the ground was significantly smaller in freezers compared to non-freezers, consistent with more shuffling gait in freezers than non-freezers. The large variability of the pitch angle at initial contact and the high variability of the time spent freezing could potentially reflect fluctuations in number of freezing episodes due to periodic medication intake throughout the day.
These findings, although promising, should be cautiously taken, as the sample size of this pilot study is small. Future work will increase the number of subjects and determine the validity of our objective freezing measures in daily life. Specifically, we plan to use a mini-camera pointed at the feet as a gold standard comparison for home recording of gait and turning, for comparison with the inertial sensor data.
A valid, objective measure of freezing with wearable technology has tremendous value to assess the efficacy of interventions such as medications and rehabilitation on quality of mobility and frequency of FoG during community-living.
Figure 2.
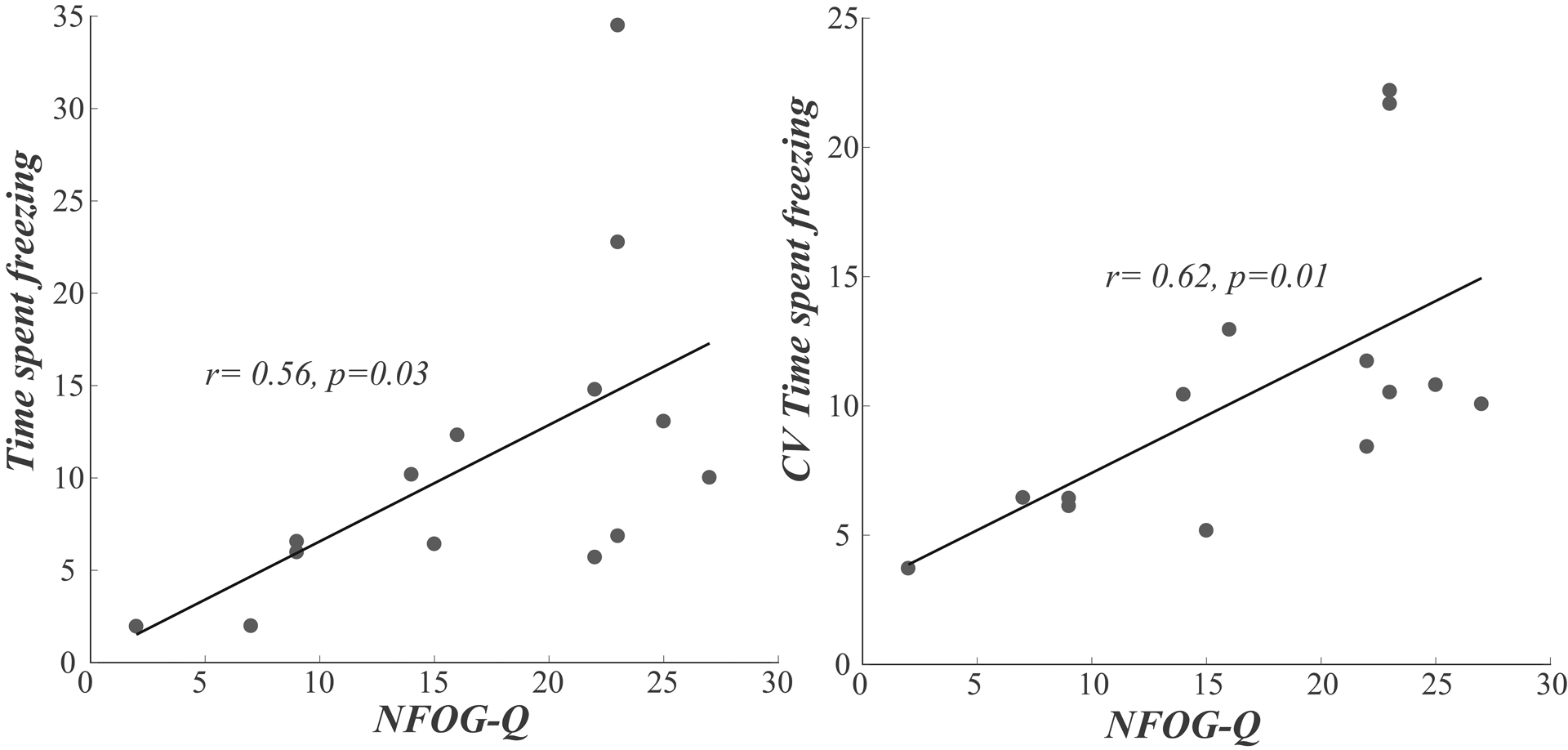
Associations between with NFOG-Q and time spent freezing (average and CV)
Acknowledgments
The authors gratefully acknowledge support from the National Institutes of Health and the participants for donating their time. Drs. Horak, El-Gohary, and McNames have significant financial interests in APDM, a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Oversight Council.
Research supported by NIH, R00 HD07849201, R43 AG044863-02, and R41NS076088-02.
References
- [1].Morris ME, Huxham FE, McGinley J, and Iansek R, “Gait disorders and gait rehabilitation in Parkinson’s disease,” Advances in neurology, vol. 87, pp. 347–61, 2001. [PubMed] [Google Scholar]
- [2].Morris ME, Iansek R, and Galna B, “Gait festination and freezing in Parkinson’s disease: pathogenesis and rehabilitation,” Movement disorders : official journal of the Movement Disorder Society, vol. 23 Suppl 2, pp. S451–60, 2008. [DOI] [PubMed] [Google Scholar]
- [3].Giladi N and Nieuwboer A, “Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage,” Movement disorders : official journal of the Movement Disorder Society, vol. 23 Suppl 2, pp. S423–5, 2008. [DOI] [PubMed] [Google Scholar]
- [4].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, and Nieuwboer A, “Freezing of gait: moving forward on a mysterious clinical phenomenon,” Lancet Neurol, vol. 10, no. 8, pp. 734–44, August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moore O, Peretz C, and Giladi N, “Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait,” Movement disorders : official journal of the Movement Disorder Society, vol. 22, no. 15, pp. 2192–5, November 15 2007. [DOI] [PubMed] [Google Scholar]
- [6].Heremans E, Nieuwboer A, and Vercruysse S, “Freezing of gait in Parkinson’s disease: where are we now?,” Curr Neurol Neurosci Rep, vol. 13, no. 6, p. 350, June 2013. [DOI] [PubMed] [Google Scholar]
- [7].Snijders AH, Haaxma CA, Hagen YJ, Munneke M, and Bloem BR, “Freezer or non-freezer: clinical assessment of freezing of gait,” Parkinsonism Relat Disord, vol. 18, no. 2, pp. 149–54, February 2012. [DOI] [PubMed] [Google Scholar]
- [8].Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, and Bloem BR, “Clinimetrics of freezing of gait,” Mov Disord, vol. 23 Suppl 2, pp. S468–74, 2008. [DOI] [PubMed] [Google Scholar]
- [9].Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, and Nieuwboer A, “Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning,” Movement disorders : official journal of the Movement Disorder Society, vol. 25, no. 15, pp. 2563–70, November 15 2010. [DOI] [PubMed] [Google Scholar]
- [10].Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, and Horak FB, “Objective Gait and Balance Impairments Relate to Balance Confidence and Perceived Mobility in People With Parkinson Disease,” Phys Ther, May 5 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mancini M, Weiss A, Herman T, and Hausdorff JM, “Turn Around Freezing: Community-Living Turning Behavior in People with Parkinson’s Disease,” Frontiers in Neurology, vol. 9, no. 18, 2018-January-26 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weiss A, Herman T, Giladi N, and Hausdorff JM, “New evidence for gait abnormalities among Parkinson’s disease patients who suffer from freezing of gait: insights using a body-fixed sensor worn for 3 days,” J Neural Transm (Vienna), vol. 122, no. 3, pp. 403–10, March 2015. [DOI] [PubMed] [Google Scholar]
- [13].Del Din S, Godfrey A, Mazza C, Lord S, and Rochester L, “Free-living monitoring of Parkinson’s disease: Lessons from the field,” Mov Disord, vol. 31, no. 9, pp. 1293–313, September 2016. [DOI] [PubMed] [Google Scholar]
- [14].Weiss A, Herman T, Giladi N, and Hausdorff JM, “Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days,” PLoS One, vol. 9, no. 5, p. e96675, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weiss A, Sharifi S, Plotnik M, van Vugt JP, Giladi N, and Hausdorff JM, “Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer,” Neurorehabil Neural Repair, vol. 25, no. 9, pp. 810–8, Nov-Dec 2011. [DOI] [PubMed] [Google Scholar]
- [16].Silva de Lima AL et al. , “Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review,” J Neurol, vol. 264, no. 8, pp. 1642–1654, August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Palmerini L, Rocchi L, Mazilu S, Gazit E, Hausdorff JM, and Chiari L, “Identification of Characteristic Motor Patterns Preceding Freezing of Gait in Parkinson’s Disease Using Wearable Sensors,” Front Neurol, vol. 8, p. 394, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mazilu S, Calatroni A, Gazit E, Mirelman A, Hausdorff JM, and Troster G, “Prediction of Freezing of Gait in Parkinson’s From Physiological Wearables: An Exploratory Study,” IEEE J Biomed Health Inform, vol. 19, no. 6, pp. 1843–54, November 2015. [DOI] [PubMed] [Google Scholar]
- [19].Nieuwboer A et al. , “Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers,” Gait Posture, vol. 30, no. 4, pp. 459–63, November 2009. [DOI] [PubMed] [Google Scholar]
- [20].Goetz CG, “[Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease],” Rev Neurol (Paris), vol. 166, no. 1, pp. 1–4, January 2010. [DOI] [PubMed] [Google Scholar]
- [21].Powell LE and Myers AM, “The Activities-specific Balance Confidence (ABC) Scale,” J Gerontol A Biol Sci Med Sci, vol. 50A, no. 1, pp. M28–34, January 1995. [DOI] [PubMed] [Google Scholar]
- [22].El-Gohary M et al. , “Continuous monitoring of turning in patients with movement disability,” Sensors (Basel), vol. 14, no. 1, pp. 356–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mancini M et al. , “Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential,” Neurorehabilitation vol. 37, no. 1, pp. 3–10, 22 August 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mancini M et al. , “Continuous Monitoring of Turning Mobility and Its Association to Falls and Cognitive Function: A Pilot Study,” J Gerontol A Biol Sci Med Sci, vol. 71, no. 8, pp. 1102–8, August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moore ST et al. , “Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental accelerometry,” J Neuroeng Rehabil, vol. 10, p. 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mancini M, Smulders K, Cohen RG, Horak FB, Giladi N, and Nutt JG, “The clinical significance of freezing while turning in Parkinson’s disease,” Neuroscience, vol. 343, pp. 222–228, February 20 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


