Abstract
Background: coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome—coronavirus-2 (SARS-CoV-2)—is an ongoing pandemic with high morbidity and mortality rates. Preliminary evidence suggests that acute kidney injury (AKI) is uncommon in patients with COVID-19 and associated with poor outcomes. Study aims and design: we performed a systematic review of the literature with a meta-analysis of clinical studies to evaluate the frequency of AKI and dialysis requirement in patients who underwent hospitalization due to COVID-19. The incidence of AKI according to the death risk was calculated in these patients. The random-effects model of DerSimonian and Laird was adopted, with heterogeneity and stratified analyses. Results: thirty-nine clinical studies (n = 25,566 unique patients) were retrieved. The pooled incidence of AKI was 0.154 (95% CI, 0.107; 0.201; p < 0.0001) across the studies. Significant heterogeneity was found (p = 0.0001). The overall frequency of COVID-19-positive patients who underwent renal replacement therapy (RRT) was 0.043 (95% CI, 0.031; 0.055; p < 0.0001); no publication bias was found (Egger’s test, p = 0.11). The pooled estimate of AKI incidence in patients with severe COVID-19 was 0.53 (95% CI, 0.427; 0.633) and heterogeneity occurred (Q = 621.08, I2 = 97.26, p = 0.0001). According to our meta-regression, age (p < 0.007) and arterial hypertension (p < 0.001) were associated with AKI occurrence in hospitalized COVID-19 positive patients. The odds ratio (OR) for the incidence of AKI in deceased COVID-19 positive patients was greater than among survivors, 15.4 (95% CI, 20.99; 11.4; p < 0.001). Conclusions: AKI is a common complication in hospitalized COVID-19 positive patients. Additional studies are under way to assess the risk of AKI in COVID-19 patients and to deepen the mechanisms of kidney injury.
Keywords: acute kidney injury, renal replacement therapy, COVID-19, mortality, SARS-CoV-2, severe disease
1. Introduction
The newly discovered Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2, previously named as 2019 novel coronavirus or 2019-nCoV) has been identified as the pathogen of Coronavirus Disease 2019 (COVID-19). The novel virus identified in Wuhan, China, in December 2019 and has spread rapidly all over the world. The World Health Organization (WHO) declared COVID-19 a pandemic in March 2020 [1]. Typical patient clinical manifestations included fever, unproductive cough, dyspnea, fatigue, normal or lowered white blood cell count, and imaging evidence of pneumonia. The clinical course of infection by SARS-CoV-2 is widely unpredictable and variable, ranging from asymptomatic infection to multi-organ systemic failure and death.
SARS-CoV-2 belongs to the large family of viruses named coronaviruses. Other coronaviruses are capable of causing illnesses including human severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV). It appears that SARS-CoV-2 has a lower fatality incidence but a higher infection rate than SARS-CoV and MERS-CoV which caused previous epidemics [2].
Several epidemiological studies have shown that patients with comorbidities, such as diabetes, arterial hypertension, metabolic syndrome, and cardiovascular disease as well as older individuals are more susceptible to exhibit symptoms with SARS-CoV-2 infection and carry an increased risk of progression to severe disease [3]. The death rate, in addition, is high when accompanied by organ dysfunction such as in the lungs or kidneys [3]. A close association between acute kidney injury (AKI) and coronavirus infection has been recorded in SARS-CoV and MERS-CoV epidemics. It has been shown that AKI developed in 5% to 15% cases and gave a high death rate (70% to 90%) in SARS and MERS-CoV infections [4,5].
There is limited information regarding the development of AKI in patients with COVID-19. We performed a systematic review with a meta-analysis of clinical studies to assess the incidence of AKI in hospitalized COVID-19 population. We evaluated the association of AKI with the outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
2. Materials and Methods
2.1. Search Strategy and Data Extraction
The study was made according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations and criteria for the reporting of meta-analysis guidelines [6]. National Library of Medicine MEDLINE and manual searches were combined, as it had been previously demonstrated that a MEDLINE search alone may not be sensitive enough [7]. The following key words were adopted: (‘COVID-19’ OR ‘Severe Acute Respiratory Syndrome Coronavirus 2’ OR ‘SARS-CoV-2’ OR ‘2019-nCoV’ OR ‘novel coronavirus’) AND (‘Acute Kidney Injury’ OR ‘Acute Renal Impairment’ OR ‘Acute Renal Failure’ OR ‘Renal Replacement Therapy’) AND (‘Mortality’ OR ‘Severe Disease’ OR ‘Death Rate’). General reviews, references from published clinical trials, letters to pharmacological companies, and current contents were also used. All articles were retrieved by a search from 1 December 2019 to 30 June 2020. Data extraction was performed independently by two investigators (F. F., and R. C.), and consensus was obtained for all data. Studies were compared to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. Eligibility and exclusion criteria were pre-specified.
2.2. Criteria for Inclusion
The included studies had to meet the following criteria: (I) clinical trials including cohort or case-control or descriptive studies; (II) human studies involving the identification of COVID-19 patients; (III) studies providing evidence on the clinical features of patients with COVID-19. Patients who were admitted to ICU and non-ICU were also categorized into a severe and non-severe subgroup. Studies which were preprint were also included.
2.3. Ineligible Studies
Studies from which data extraction was not possible were excluded. Studies focusing on pregnant patients or other coronaviruses, such as MERS or SARS, were excluded. We excluded studies which did not give information on grouping between severe and non-severe patients if unable to obtain data from the investigators. Studies that were only published as abstracts, letters, case reports or interim reports were excluded; review articles were not evaluated for the current review.
2.4. End-Points of Interest
Primary outcomes of interest were the pooled incidence of AKI and the requirement of renal replacement therapy (RRT), in hospitalized patients with COVID-19. An additional end-point was the AKI occurrence in hospitalized patients with severe COVID-19. The impact of AKI on the death risk of COVID-19 patients was addressed by calculating the summary estimate for unadjusted or adjusted death risk. As detailed below, the adjusted death risk was generated by multivariate analysis in a subset of reports. The adjusted relative risks (aRR) of all-cause mortality was calculated in each study.
2.5. Statistical Methods
The summary estimate of the incidence of AKI and the need of RRT was calculated. We computed fixed and random effect estimates and the random-effects model of Der Simonian and Laird was adopted if moderate to severe heterogeneity occurred [8]. To assess the between-study heterogeneity, we used Cochran’s Q test (p > 0.10 for statistical significance) and I2 test [9]. To further explore the origin of heterogeneity, we restricted the analysis to subgroups of studies defined by study characteristics such as the country of origin (China, United States of America), and study design (retrospective or not), among others. We made a funnel plot to detect a publication bias in the relation exposure at hand; publication bias was calculated by Egger’s test. Meta-regression was carried out to assess the independent effect of continuous covariates on the incidence of AKI in hospitalized patients with COVID-19. We adopted the odds ratio (OR) with 95% CI for the dichotomous outcomes. In a subset of reports, a summary estimate of the adjusted RR of all-cause mortality among hospitalized COVID-19 patients who developed AKI compared with those who did not was generated by weighting the study-specific RR’s (by the inverse of the variance). The aRR was calculated by multivariate analysis (i.e., after adjustment for potential confounders such as comorbidities and complications). Ri (the proportion of total variance due to between studies variance) was adopted to take into account the heterogeneity. All the statistical analyses were performed using Rev Man (Review Manager) 5.0, The Cochrane Collaboration (2020), Comprehensive meta-analysis (CMA 1.0), and HEpiMA, version 2.1.3 [10]. The 5% significance level was adopted for alpha risk. Every estimate was recorded with 95% confidence intervals (CIs).
3. Results
3.1. Literature Review
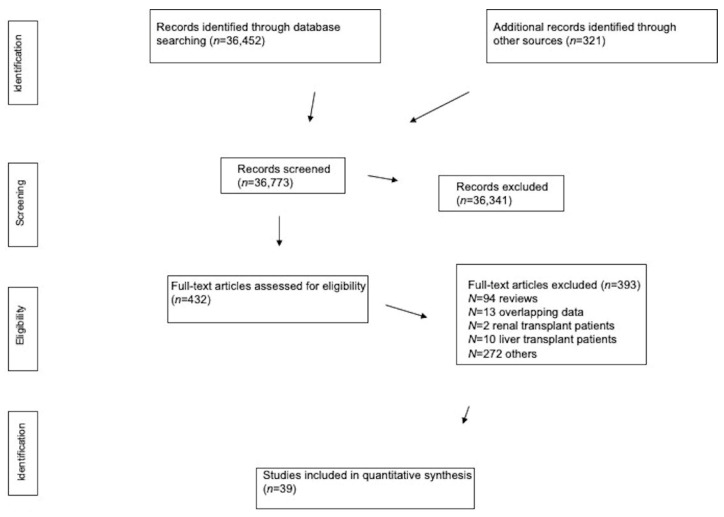
Our electronic and manual searches identified 432 full-text articles that were considered potentially relevant and selected for full-text review. A complete list of the 432 full-text articles reviewed is available from the authors on request. We excluded 393 full-text articles, as detailed in Figure 1.
Figure 1.
Flow diagram of the study selection.
We included a total of 39 reports giving information on 25,566 patients who had been admitted to tertiary hospitals all over the world and were diagnosed with COVID-19 (Figure 1). There was a 100% concordance between reviewers with respect to the final inclusion and exclusion of studies based on the predefined exclusion criteria.
3.2. Patient Characteristics
According to the design of the study, two sets of reports were identified. The first set was constituted by studies listed in Table 1 and Table 2 and Supplementary Table S1 (n = 22; n = 8792 patients) which evaluated the frequency of AKI based on the severity of COVID-19 [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The frequency of chronic obstructive pulmonary disease (COPD) ranged between 1.1 and 13%, and the chronic liver disease (CLD) rate between 0.9% and 11.8%.
Table 1.
Acute kidney injury (AKI) according to severe COVID-19: observational studies.
| Authors (Ref.) | Country | Publication Date | Patients, n |
|---|---|---|---|
| HUANG Chaolin et al. [11] | Jin Yin-tan Hospital (Wuhan, China) |
24 January 2020 | 41 |
| WANG Dawei et al. [12] | Zhongnan Hospital (Wuhan, China) |
7 February 2020 | 138 |
| XU Yonghao et al. [13] | Seven Hospitals (Guangdong, China) |
6 March 2020 | 45 |
| WAN Suxin et al. [14] | Three Gorges Central Hospital (Chongqing, China) |
21 March 2020 | 135 |
| LI Qiang et al. [15] | Shanghai Hospital (Shangai, China) |
24 March 2020 | 325 |
| XU Shen et al. [16] | Two Hospitals (Hubei and Anhui, China) |
26 March 2020 | 355 |
| LI Zhen et al. [17] | Four Hospitals (Wuhan, Huangshi, Chongqing, China) |
27 March 2020 | 193 |
| ZHAO Wen et al. [18] | YouAn Hospital (Beijing, China) |
30 March 2020 | 77 |
| WANG Luwen et al. [19] | Remnin Hospital (Wuhan, China) |
31 March 2020 | 116 |
| ZHANG Guqin et al. [20] | Zhongnan Hospital (Wuhan, China) |
9 April 2020 | 221 |
| LI Xiaochen et al. [21] | Tongji Hospital (Wuhan, China) |
12 April 2020 | 548 |
| JIANG Xiufeng et al. [22] | Wuxi Fifth People’s Hospital (Jiangsu, China) |
14 April 2020 | 55 |
| HONG Kyung Soo et al. [23] | Yeungnam Hospital (Daegu, Korea) |
24 April 2020 | 98 |
| PEI Guangchang et al. [24] | Tongji Hospital (Wuhan, China) |
28 April 2020 | 333 |
| AGGARWAL Saraubah et al. [25] | UnityPoint Clinic (Des Moines, USA) |
29 April 2020 | 16 |
| GUAN Wej et al. [26] | 552 hospitals (Mainland, China) | 30 April 2020 | 1099 |
| HU Ling et al. [27] | Tianyou Hospital (Wuhan, China) |
3 May 2020 | 323 |
| CHAN Lili et al. [28] | 5 hospitals (New York City, USA) | 8 May 2020 | 3235 |
| ZHENG Yi et al. [29] | First Hospital (Hangzhou, China) |
20 May 2020 | 34 |
| YANG Luhuan et al. [30] | Yichang Hospital, China | 26 May 2020 | 200 |
| ARGENZIANO Michael et al. [31] | NYP/CUIMC (New York Presbyterian/Columbia University Irving Medical Center) (New York City, USA) |
29 May 2020 | 850 |
| SULEYMAN Geehan et al. [32] | HFHS (Henry Ford Health System) (Detroit, USA) | 16 June 2020 | 355 |
Table 2.
AKI according to severe COVID-19: patient characteristics.
| Authors | Males, n | Age, Years | Chronic Kidney Disease (CKD) |
|---|---|---|---|
| HUANG Chaolin et al. | 30 (73%) | 49 (41–58) | NA |
| WANG Dawei et al. | 75 (54.3%) | 56 (42–68) | 4 (2.9%) |
| XU Yonghao et al. | 29 (64.4%) | 56.7 ± 15.4 | NA |
| WAN Suxin et al. | 72 (53.3%) | 47 (36–55) | NA |
| LI Qiang et al. | 167 (51.4%) | 51 (36–64) | 4 (1.2%) |
| XU Shen et al. | 193 (54.3%) | NA | NA |
| LI Zhen et al. | 95 (49%) | 57 (46–67) | NA |
| ZHAO Wen et al. | 34 (44.2%) | 52 ± 20 | 5 (6.5%) |
| WANG Luwen et al. | 67 (57.8%) | 54 (38–69) | 5 (4.3% |
| ZHANG Guqin et al. | 108 (48.9%) | 55 (39–66) | 6 (2.7%) |
| LI Xiaochen et al. | 279 (50.9%) | 60 (48–69) | 10 (1.8%) |
| JIANG Xiufeng et al. | 27 (49.1%) | 45 (27–60) | 1 (1.8%) |
| HONG Kyung et al. | 38 (38.8%) | 55.4 ± 17.1 | NA |
| PEI Guangchang et al. | 182 (54.7%) | 56.3 ± 13.4 | NA |
| GUAN Wej et al. | 637 (58.1%) | 47 (35–58) | 8 (0.8%) |
| AGGARWAL Saraubah et al. | 12 (75%) | 67 (38–95) | 6 (37.5%) |
| HU Ling et al. | 166 (51.4%) | 61 (23–91) | 7 (2.2%) |
| CHAN Lili et al. | 1868 (57.7%) | 66.5 (55–78) | 323 (10%) |
| ZHENG Yi et al. | 23 (67.6%) | 66 (58–76) | 2 (5.9%) |
| YANG Luhuan et al. | 98 (49%) | 55 ± 17.1 | 3 (1.5%) |
| ARGENZIANO Michael et al. | 511 (60%) | 63 (50–75) | NA |
| SULEYMAN Geehan et al. | 165 (46.5%) | 61.8 ± 15.3 | 161 (45.3%) |
The second set included reports shown in Table 3 and Table 4 and Supplementary Table S2 (n = 20, n = 16,774 patients) which assessed the impact of AKI development upon the outcomes (death rate) of COVID-19 patients [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Some (n = 3) studies gave information on both relationships [16,24,28]. The frequency of COPD varied between 1.9 and 12%, and the rate of CLD between 0.52% and 6.3%.
Table 3.
AKI and death risk in COVID-19: observational studies.
| Authors | Country | Publication Date | Patients, n |
|---|---|---|---|
| YANG Xiaobo et al. [33] | Jin Yin-tan Hospital (Wuhan, China) | 21 February 2020 | 52 |
| LU Zhibing et al. [34] | Zhongnan and Seventh Hospitals (Wuhan, China) |
3 March 2020 | 123 |
| ZHOU Fei et al. [35] | Jin Yin-tan and Pulmonary Hospitals (Wuhan, China) |
9 March 2020 | 191 |
| DENG Yan et al. [36] | Tongji Hospital (Wuhan, China) | 20 March 2020 | 225 |
| CHENG Yichun et al. [37] | Tongji Hospital, (Wuhan, China) | 20 March 2020 | 701 |
| LUO Xiaomin et al. [38] | Renmin Hospital (Wuhan, China) | 23 March 2020 | 403 |
| CAO Wen et al. [39] | Jinyintan Hospital (Wuhan, China) |
24 March 2020 | 61 |
| XU Shen et al. [16] | Huazhong (Hubei) and Fuyang (Anhui) Hospitals (China) |
26 March 2020 | 355 |
| CHEN Tao et al. [40] | Tongji Hospital (Wuhan, China) | 26 March 2020 | 274 |
| WANG Lang et al. [41] | Renmin Hospital (Wuhan, China) | 30 March 2020 | 339 |
| RICHARDSON Safiya et al. [42] | New York City (USA) | 22 April 2020 | 5700 |
| PEI Guangchang et al. [24] | Tongji Hospital (Wuhan, China) | 28 April 2020 | 333 |
| CHAN Lili et al. [28] | NYC, USA | 8 May 2020 | 3235 |
| WANG Dawei and Yin D. et al. [43] | Zhongnan and Xishui Hospitals (Wuhan, China) |
30 April 2020 | 107 |
| SHI Qiao et al. [44] | Renmin and Zhongnan Hospitals (Wuhan, China) |
14 May 2020 | 1561 |
| HIRSCH Jamie et al. [45] | NYC, (USA) | 16 May 2020 | 5449 |
| YANG Kunyu et al. [46] | Local Hospitals (n=9) (Hubei, China) | 29 May 2020 | 205 |
| LIM, Jeong-Hoon et al. [47] | Kyungpook University Hospital (Daegu, Korea) |
3 June 2020 | 160 |
| ZHAO Mengmeng et al. [48] | Remnin Hospital (Wuhan, China) |
4 June 2020 | 1000 |
| PELAYO J. et al. [49] | Einstein Medical College (Philadelphia, USA) |
18 June 2020 | 223 |
Table 4.
AKI and death risk in COVID-19: patient characteristics.
| Authors | Male, n | Age, Years | CKD |
|---|---|---|---|
| YANG Xiaobo et al. | 35 (67%) | 59.7 ± 13.3 | NA |
| LU Zhibing et al. | 61 (49.5%) | 57.8 ± 12.7 | 7 (5.7%) |
| ZHOU Fei et al. | 119 (62%) | 56 (46–67) | 2 (1.0%) |
| DENG Yan et al. | 124 (55.1%) | 54 (33–74) | NA |
| CHENG Yichun et al. | 367 (52.4%) | 63 (50–71) | 14 (2.0%) |
| LUO Xiaomin et al. | 193 (47.9%) | 56 (39–68) | 7 (1.7%) |
| CAO Wen et al. | 36 (59%) | 61 (48–70) | NA |
| XU Shen et al. | NA | NA | NA |
| CHEN Tao et al. | 171 (62.4%) | 62 (44–70) | 4 (1.4%) |
| WANG Lang et al. | 166 (48.9%) | 69 (65–76) | 13 (3.8%) |
| RICHARDSON Safiya et al. | 3437 (60.3%) | 63 (52–75) | 268 (4.7%) |
| PEI Guangchang et al. | 182 (54.7%) | 56.3 ± 13.4 | NA |
| CHAN Lili et al. | 1868 (52.8%) | 66.5 (55–78) | 323 (10%) |
| WANG Dawei and Yin D. et al. | 57 (53.3%) | 51 (36–65) | 3 (2.8%) |
| SHI Qiao et al. | 150 (49%) | 64.5 (56–72) | 12 (3.9%) |
| HIRSCH Jamie et al. | 3317 (60.9%) | 64 (52–75) | NA |
| YANG Kunyu et al. | 96 (47%) | 63 (56–70) | 4 (1.9%) |
| LIM, Jeong-Hoon et al. | 86 (53%) | 68.5 (24–98) | NA |
| ZHAO Mengmeng et al. | 466 (46.6%) | 61 (46, 70) | 24 (2.4%) |
| PELAYO Jerald et al. | 108 (48.4%) | 65.9 | 39 (17.8%) |
Table 1 and Table 3 report the list of studies evaluated, the countries where they were carried out, the reference year and some demographic data. All studies were conducted between January and June 2020. As listed in Table 1, Table 2, Table 3 and Table 4, the majority of reports were from China (n = 30), and the others from the USA (n = 7) and South Korea (n = 2), respectively. The frequency of male patients ranged from 38.8% to 75%, and the mean age from 47 to 69 years. Comorbidities (arterial hypertension, diabetes mellitus, chronic kidney disease (CKD), cardiovascular disease) have been recorded in Table 2 and Table 4 and Supplementary Tables S1 and S2. The majority of the studies adopted the definition of AKI according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines, where AKI is defined as any of the following: increase in serum creatinine by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h; or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or < urine volume 0.5 mL/kg/h for 6 h [50]. In some papers, the definition of AKI was not mentioned [16,36,38,40,46].
3.3. AKI Incidence: Primary and Stratified Analysis
Table 5 shows that the summary estimate for the occurrence of AKI across the identified trials was 0.154 (95% CI, 0.107, 0.201). Significant heterogeneity was found (Table 5) (p = 0.0001). The Egger’s regression intercept shows that there was publication bias (p = 0.025) (Figure 2).
Table 5.
Summary estimates for unadjusted point estimates of AKI in hospitalized COVID-19 patients.
| Study, n | Point Estimate (Random Effects Model) (95% CI) | p-Value (By Q Test) | I 2 | |
|---|---|---|---|---|
| All studies | 39 | 0.154 (0.107; 0.201) | 0.0001 (6929,1) | 99.4% |
| Studies (China) | 30 | 0.094 (0.075; 0.114) | 0.0001 (567.09) | 94.8% |
| US studies | 7 | 0.353 (0.286; 0.42) | 0.0001 (374.9) | 98.4% |
| Studies (Korea) | 2 | 0.139 (0.044; 0.233) | 0.023 (5.168) | 80.6% |
| Retrospective studies | 28 | 0.17 (0.109; 0.23) | 0.0001 (2433.1) | 98.8% |
| Population-based studies | 4 | 0.268 (0.04; 0.496) | 0.0001 (6049.3) | 99.9% |
| Small studies * | 10 | 0.17 (0.098; 0.243) | 0.0001 (108.4) | 92.6% |
* small studies (studies with size < 100 pts).
Figure 2.
The pooled incidence of AKI in COVID-19 positive patients during their hospital stay was 0.154 (see text); the funnel plot asymmetry suggests the occurrence of publication bias (this is confirmed by Egger’s test, p = 0.025).
Stratified analyses were undertaken to explain the heterogeneity across studies (Table 5). The analysis by the fixed-effects model yielded very similar findings to the random-effects model (data not shown).
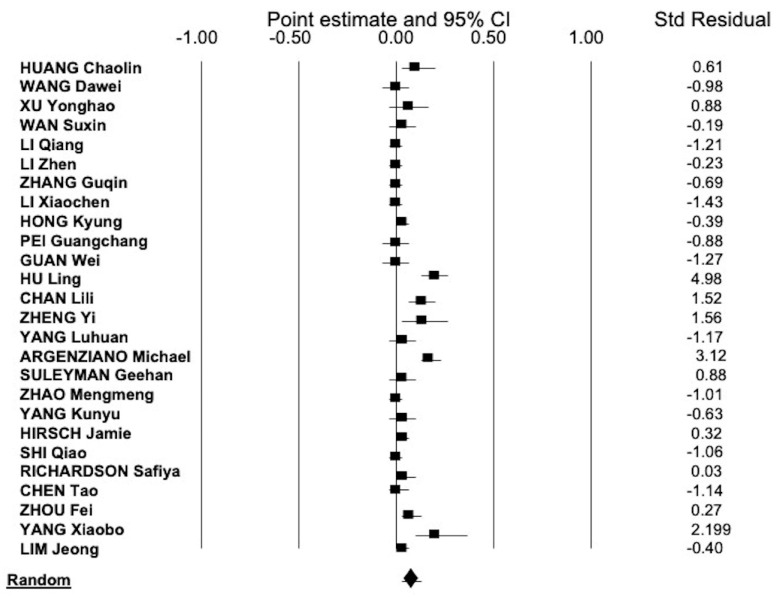
The overall estimate for the frequency of COVID-19 positive patients who had AKI and underwent RRT during their hospital stay was 0.043 (95% CI, 0.031; 0.055) (Figure 3). Heterogeneity occurred, Q = 596,8 d(f) = 25, I2 = 95.8 (p = 0.0001). No publication bias was found (Egger’s test, p = 0.11).
Figure 3.
The summary estimate of the frequency of renal replacement therapy (RRT)-dependent AKI in hospitalized patients with COVID-19 was 0.043 (95% CI, 0.031; 0.055) (random-effects model).
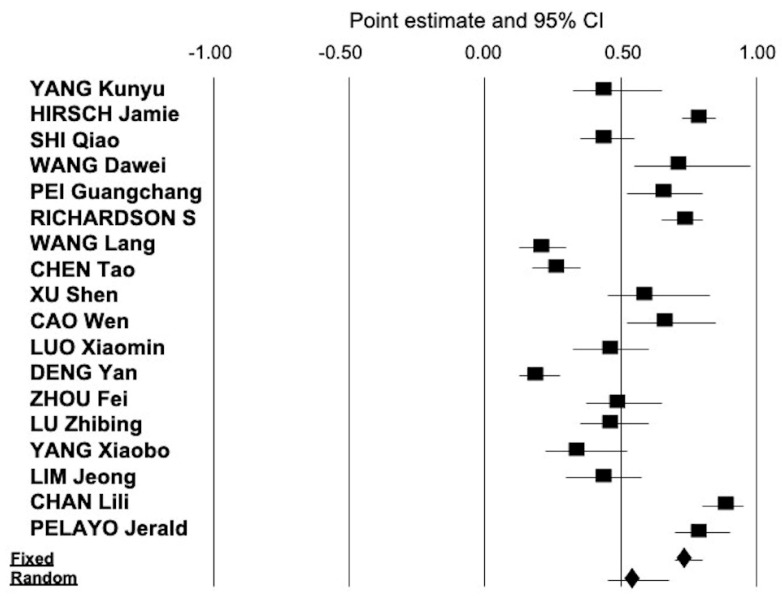
3.4. AKI Incidence and Severe Disease in COVID-19-Positive Patients
As reported in Figure 4, the pooled estimate of frequency of AKI in patients with severe COVID-19 was 0.53 (95% CI, 0.427; 0.633). There was consistent heterogeneity (Q = 621.08, I2 = 97.26, p = 0.0001).
Figure 4.
The summary estimate of the frequency of AKI in hospitalized patients with severe COVID-19 was 0.53 (95% CI, 0.427; 0.633) (random-effects model).
3.5. AKI Incidence and Death Rate in COVID-19 Positive Patients
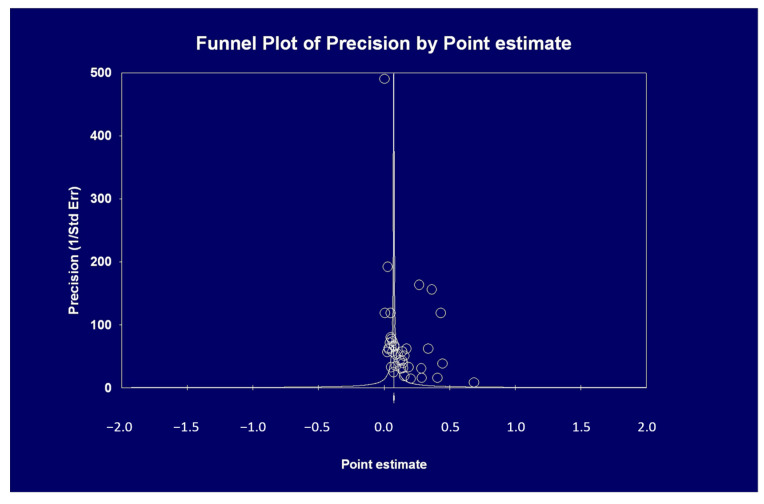
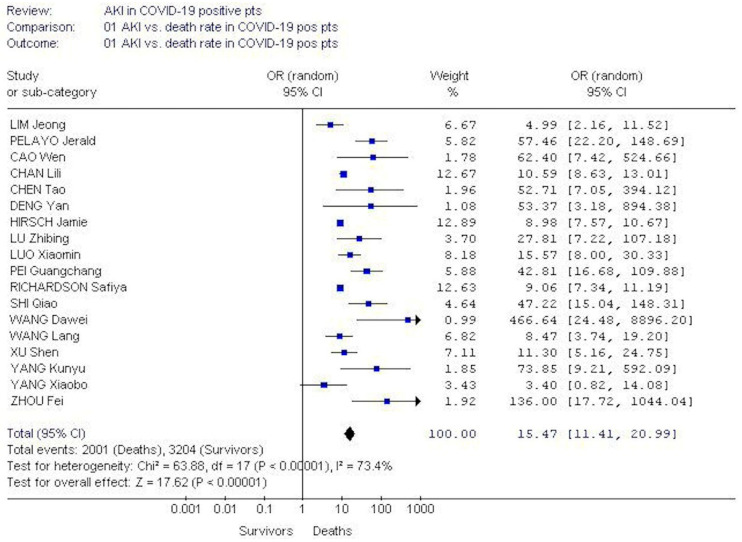
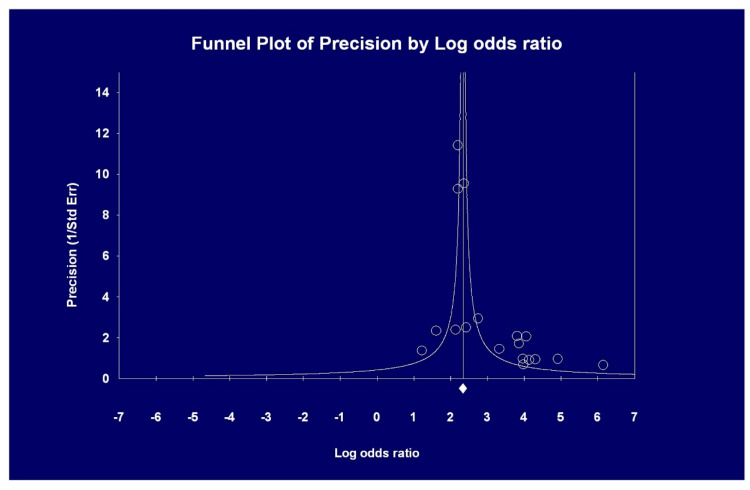
The pooled OR of AKI incidence among deceased COVID-19 positive patients was greater than among survivors, 15.4 (95% CI, 20.99; 11.4). Test for heterogeneity was significant (p = 0.00001) (Figure 5). Publication bias occurred (Egger’s regression, p = 0.0016) (Figure 6).
Figure 5.
The pooled OR of AKI incidence was greater in deceased COVID-19-positive patients than among survivors during their hospital stay, 15.4 (95% CI, 20.99; 11.4) (random-effects model).
Figure 6.
Pooled odds ratio (OR) of AKI in deceased vs. survivor patients with COVID 19 (see text); the asymmetry of the funnel plot indicates the possibility of publication bias and this is confirmed by the Egger ‘s test (p = 0.0016).
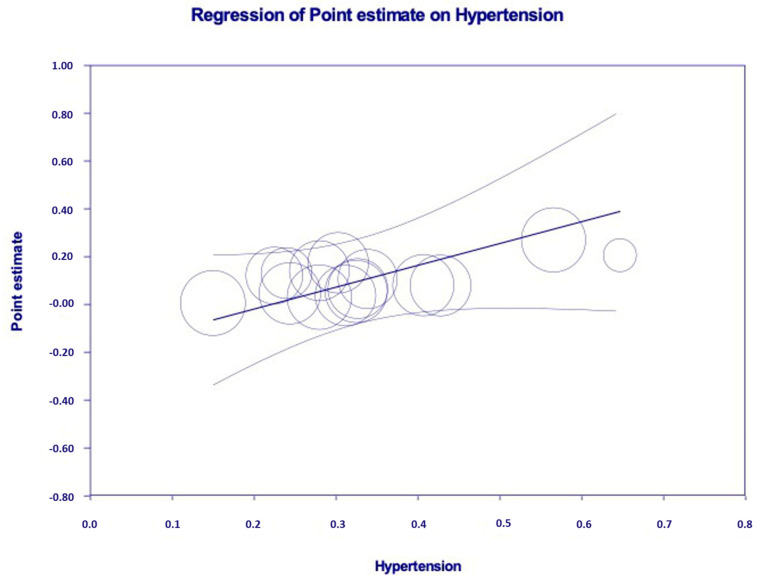
Some authors (n = 5, n = 5435 unique patients) evaluated the association between AKI and death risk by multivariate analysis. As shown in Table 6, the link between AKI and death risk remained significant in many comparisons. The results of meta-regression are reported in Table 7. Age (p = 0.007) and arterial hypertension (p = 0.001) are significantly associated with the frequency of AKI. The independent and significant relationship between the frequency of AKI and arterial hypertension according to meta-regression is shown in Figure 7.
Table 6.
Summary estimates for adjusted relative risks (aRR, adjusted relative risk by Cox proportional hazard model) of all-cause mortality among hospitalized patients with COVID-19.
| Authors | Study, n | Fixed Effects aRR (95% CI) |
Random Effects aRR (95% CI) |
Ri |
p-Value (by Q-Test) |
|---|---|---|---|---|---|
| All studies | 5 | 5.24 (4.31; 6.38) | 2.80 (0.96, 8.13) | 0.97 | 0.00001 |
| Studies (China) | 3 | 1.62 (1.13; 2.32) | 1.66 (0.56; 4.91) | 0.89 | 0.00001 |
| Studies (others from China) | 2 | 8.67 (6.86; 10.96) | 6.27 (2.43; 16.19) | 0.93 | 0.0127 |
Chan L. et al. [28]: aRR adjusted for age, gender, comorbidities including hypertension, congestive heart failure, diabetes mellitus, liver disease, peripheral vascular disease, chronic kidney disease, laboratory values including white blood cell count, lymphocyte percentage, hemoglobin, platelets, sodium, potassium, chloride, bicarbonate, urea, creatinine, aspartate aminostransferase, alanine aminotransferase, alkaline phosphatase, albumin, and vitals (including systolic blood pressure, diastolic BP, heart rate, respiratory rate, oxygen saturation). Cheng Y. et al. [37]: aRR adjusted for age, gender, disease severity, any comorbidity (CKD, chronic obstructive pulmonary disease (COPD), hypertension, diabetes, and tumor), and lymphocyte count. Wang L. et al. [41]: aRR adjusted for age, acute cardiac injury, arrhythmia, acute respiratory distress syndrome (ARDS), cardiac insufficiency, bacterial infection). Lim J. et al. [47]: aRR adjusted for age, gender, hypertension, diabetes. Zhao M. et al. [48]: aRR adjusted for age, shock, acute cardiac injury, acute liver injury, and number of complications (1, 2 or more).
Table 7.
Meta-regression: impact of continuous covariates on the outcome of interest (AKI rate among hospitalized patients with COVID-19).
| Covariate | Coefficient | Standard Error | 95% CI | Z-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 0.616 | 0.330 | −0.031; 1.265 | 1.86 | 0.062 |
| Diabetes mellitus | −0.179 | 0.185 | −0.5426; 0.184 | −0.97 | 0.333 |
| Male | −0.110 | 0.330 | −0.758; 0.5379 | −0.33 | 0.739 |
| Age | −0.012 | 0.004 | −0.021; −0.003 | −2.7 | 0.007 |
| Hypertension | 0.913 | 0.286 | 0.352; 1.475 | 3.19 | 0.001 |
| cardiovascular disease | 0.160 | 0.099 | −0.034; 0.356 | 1.61 | 0.107 |
| CKD | −1.352 | 1.041 | −3.393; 0.687 | −1.3 | 0.193 |
| COPD | 1.297 | 0.908 | −0.482; 3.076 | 1.43 | 0.153 |
| chronic liver disease | −0.523 | 0.607 | −1.714; 0.666 | −0.86 | 0.388 |
Figure 7.
Independent and significant relationship between arterial hypertension and the frequency of AKI (p = 0.001) in COVID-19 positive patients who underwent hospitalization (meta-regression analysis).
4. Discussion
Controversy exists about kidney involvement in COVID-19-positive patients. Preliminary evidence indicated that the frequency of kidney disease in the COVID-19 population was negligible and limited interest has been given to the incidence of AKI in patients with COVID-19 [3]. Additional studies have highlighted the frequency of kidney abnormalities in patients with COVID-19 [51]. The current systematic review of the scientific literature with a meta-analysis of clinical studies indicated that the incidence of AKI in patients with COVID-19 during their hospital stay was common (around 15%). The frequency of AKI among patients with severe COVID-19 was much greater (around 50%). We noted important heterogeneity that could be explained by numerous factors such as patient characteristics, severity of illness, differences in daily clinical practice (regarding fluid management, ventilation options and medications, among others).
According to our meta-regression analysis, some comorbidities (age and arterial hypertension) were significantly related to AKI occurrence and this is in keeping with the evidence on the development of AKI in patients without COVID-19.
The pathophysiological mechanisms which are responsible for COVID-19-related AKI are yet to be discovered [51]. Unspecific mechanisms exist including comorbidities (diabetes mellitus, arterial hypertension, and others) which confer vulnerability to kidneys, nephrotoxic drugs or contrast media, hypovolemic conditions and subsequent pre-renal AKI. Multiorgan involvement is common in patients with COVID-19 including damage to kidneys, heart, and gastrointestinal tract; this mirrors the presence of the ACE2 receptors in various organs which serve as an entrance door for SARS-CoV-2. It has been hypothesized that the development of AKI in COVID-19 patients include viral cytopathic activity, hypoperfusion, cytokine storm, and microvascular thrombosis [51]. Alternatively, patients with severe SARS-CoV-2 infection frequently show acute respiratory distress syndrome (ARDS); severe hypoxemia or high intra-thoracic pressures have been linked to AKI in the ARDS population [52]. COVID-19-specific mechanisms include the entry of SARS-CoV-2 into the kidneys and the binding of SARS-CoV-2 with the ACE2 receptor on the cell membrane of the host cells; in the kidneys, the ACE2 receptor is expressed in the apical brush borders of the proximal tubules as well as podocytes [53]. In addition, COVID-19 promotes an imbalanced activation of the renin–angiotensin–aldosterone system (RAAS), which induces the downregulation of the membrane-bound ACE2 receptor that promotes the accumulation of angiotensin II by lowering its degradation into angiotensin 1–7. Imbalanced RAAS activation leads to inflammation, vasoconstriction and fibrosis at the kidney level [54]. Some studies have suggested that ACE inhibitors and angiotensin receptor blockers (ARBs) may improve ACE2 expression and therefore increase the susceptibility of patients to SARS-CoV-2 infection [55]. High levels of inflammatory cytokines have been noted in severe COVID-19 patients and may participate to AKI in these patients [35].
The current meta-analysis is flawed by numerous issues. Most studies included in this study had retrospective design; there were no RCTs. It is well known that prospective studies having data at baseline and over follow-up provide better evidence. Second, individual data from each study (e.g., ‘patient-level data’) were not available; thus, it was impossible to make our own adjustments. An additional limitation is given by the occurrence of publication bias: negative studies are less likely to be published. In addition, an enormous body of data is rapidly accumulating on COVID-19 patients, including those with kidney disease, and this clearly makes difficult the retrieval of the whole evidence on the subject. We have not adopted the studies published as abstracts or letters as information presented in this format as these are not of high quality.
In conclusion, this meta-analysis of clinical studies shows that AKI is common in COVID-19-positive patients during their hospital stay. The frequency of AKI was much greater in patients with severe disease. There is a consistent relationship between the development of AKI and unsatisfactory outcomes (death rate) in hospitalized patients with COVID-19.
Abbreviations
| ACE | Angiotensin converting enzyme |
| AKI | Acute kidney injury |
| ARB | Angiotensin receptor blocker |
| ARDS | Acute respiratory distress syndrome |
| CI | Confidence intervals |
| CLD | Chronic liver disease |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CKD | Chronic kidney disease |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End-stage renal disease |
| ICU | Intensive care unit |
| HD | Hemodialysis |
| MERS-COV | Middle East respiratory syndrome coronavirus |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RAAS | Renin–angiotensin–aldosterone system |
| RR | Relative risk |
| RRT | Renal replacement therapy |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/12/1052/s1, Table S1: AKI according to severe COVID-19: Comorbidities, Table S2: AKI and death risk in COVID-19: Comorbidities.
Funding
This paper was not funded.
Conflicts of Interest
All the authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Director-General’s Remarks at the Media Briefing on COVID-19 on 11 March 2020. [(accessed on 11 March 2020)]; Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19.
- 2.Boldog P., Tekeli T., Vizi Z., Dénes A., Bartha F.A., Röst G. Risk Assessment of Novel Coronavirus COVID-19 Outbreaks Outside China. J. Clin. Med. 2020;9:571. doi: 10.3390/jcm9020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naicker S., Yang C.-W., Hwang S.-J., Liu B.-C., Chen J.-H., Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W., et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung M.-L., Yao Y., Jia L., Chan J.F.W., Chan K.-H., Cheung K.-F., Chen H., Poon V.K.M., Tsang A.K.L., To K.K., et al. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat. Microbiol. 2016;1:16004. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., Atkins D., Barbour V., Barrowman N., Berlin J.A., et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poynard T., Conn H. The retrieval of randomised clinical trials in liver diseases from medical literature: A comparison of MEDLARS and manual methods. Control. Clin. Trials. 1985;6:271–279. doi: 10.1016/0197-2456(85)90103-5. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Petitti D.B. Approaches to heterogeneity in meta-analysis. Stat. Med. 2001;20:3625–3633. doi: 10.1002/sim.1091. [DOI] [PubMed] [Google Scholar]
- 10.Costa J., Takkouche B., Cadarso-Suárez C., Spiegelman D. HEpiMA: Software for the identification of heterogeneity in meta-analysis. Comput. Methods Programs Biomed. 2001;64:101–107. doi: 10.1016/S0169-2607(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Fan G., Xu J., Gu X., Cheng Z., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Xu Z., Liu X., Cai L., Zheng H., Huang Y., Zhou L., Huang L., Lin Y., Deng L., et al. Clinical findings in critically ill patients infected with SARS-CoV-2 in Guangdong Province, China: A multi-center, retrospective, observational study. MedRxiv. 2020 doi: 10.1101/2020.03.20030668. [DOI] [Google Scholar]
- 14.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lamg C., Huang D., Sun Q., Xiong Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Ling Y., Zhang J., Li W., Zhang X., Jin Y., Li L., Fu Q., Huang Y., Lu H., et al. Clinical characteristics of SARS-CoV-2 infections involving 325 hospitalized patients outside Wuhan. BMC Inf. Dis. 2020 doi: 10.21203/rs.3.rs-18699/v1. [DOI] [Google Scholar]
- 16.Xu S., Fu L., Fei J., Xiang H., Xiang Y., Tan Z., Li M., Liu F., Li Y., Han M., et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: A hospital-based retrospective analysis. medRixv. 2020 doi: 10.1101/2020.03.24.20042408. [DOI] [Google Scholar]
- 17.Li Z., Wu M., Yao J., Guo J., Liao X., Song S., Li J., Duan G., Zhou Y., Wu X., et al. Caution on kidney dysfunctions of COVID-10 patients. medRxiv. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 18.Zhao W., Yu S., Zha X., Wang N., Pang Q., Li D., Li A. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.03.13.20035436. [DOI] [Google Scholar]
- 19.Wang L., Li X., Chen H., Yan S., Li D., Li Y., Gong Z. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am. J. Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., Peng Z., Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Tao J., Wu H., Wang Y., Zhao W., Zhou M., Huang J., You Q., Meng H., Zhu F., et al. Clinical features and management of severe COVID-19: A retrospective study in Wuxi, Jiangsu Province, China. medRxix. 2020 doi: 10.1101/2020.04.10.20060335. [DOI] [Google Scholar]
- 23.Hong K., Lee K., Chung J., Shih K., Choi E., Jin H., Jang J., Lee W., Ahn J. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: A brief descriptive study. Yonsei Med. J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C., Ma Z., Huang Y., Liu W., Yao Y., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis. 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 26.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L., Chen S., Fu Y., Gao Z., Long H., Ren H.-W., Zuo Y., Wang J., Li H., Xu Q.-B., et al. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clin. Infect. Dis. 2020;71:2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gameiro J., Fonseca J.A., Oliveira J., Marques F., Bernardo J., Costa C., Carreiro C., Braz S., Alvoeiro L., Lopes J.A. Acute kidney injury in hospitalized patients with COVID-19. medRxiv. 2020 doi: 10.21203/rs.3.rs-39131/v1. [DOI] [Google Scholar]
- 29.Zheng Y., Sun L.-J., Xu M., Pan J., Zhang Y.-T., Fang X.-L., Fang Q., Cai H.-L. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J. Zhejiang Univ. Sci. B. 2020;21:378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Liu J., Zhnag R., Li M., Li Z., Zhou X., Hu C., Tian F., Zhou F., Lei Y. Epidemiological and clinical features of 200 hospitalized patients with coronavirus disease 2019 outside Wuhan, China: A descriptive study. J. Clin. Virol. 2020;129:104475. doi: 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., Chang B.P., Chau K.H., Choi J.J., Gavin N., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: A retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., Demertzis Z., Hanna Z., Failla A., Dagher C., et al. Clinical Characteristics and Morbidity Associated with Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open. 2020;3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Z., Chen M., Fan Y., Wu X., Zhang L., Guo T., Deng K., Cao J., Luo H., He T., et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan. Lancet. 2020 doi: 10.2139/ssrn.3546069. [DOI] [Google Scholar]
- 35.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y., Liu W., Liu K., Fang Y., Shang J., Zhou L., Wang K., Leng F., Wei S., Chen L., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: A retrospective study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo X., Xia H., Yang W., Wang B., Guo T., Xiong J., Jiang Z., Liu Y., Yan X., Zhou W., et al. Characteristics of patients with COVID-19 during epidemic outbreak in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 39.Wen C., Yali Q., Zirui G., Shuo L., Chaoyang L., Wenjuan X., Qian Z., Ning H., Ruijun G. Prevalence of Acute Kidney Injury in Severe and Critical COVID-19 Patients in Wuhan, China. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3555223. [DOI] [Google Scholar]
- 40.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., The Northwell COVID-19 Research Consortium Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., Jian M., Xu H., Prowle J.R., Hu B., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., Feng J., Yan S., Guan Y., Xu D., et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch J., Ng J., Ross D., Sharma P., Shah H., Barnett R., Hazzan A., Fishbane S., Jhaveri K., the Northwell COVID-9 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., Lu H., Liu J., Yang J., Dong Y., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim J.-H., Park S.-H., Jeon Y., Cho J.-H., Jung H.-Y., Choi J.-Y., Kim C.-D., Lee Y.-H., Seo H., Lee J., et al. Fatal Outcomes of COVID-19 in Patients with Severe Acute Kidney Injury. J. Clin. Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M., Wang M., Zhang J., Gu J., Zhang P., Xu Y., Ye J., Wang Z., Ye D., Pan W., et al. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12:10070–10086. doi: 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelayo J., Lo K.B., Bhargav R., Gul F., Peterson E., Iii R.D., Salacup G.F., Albano J., Gopalakrishnan A., Azmaiparashvili Z., et al. Clinical Characteristics and Outcomes of Community- and Hospital-Acquired Acute Kidney Injury with COVID-19 in a US Inner City Hospital System. Cardiorenal Med. 2020;10:223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L., Herzog C.A., Joannidis M., Kribben A., Levey A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- 51.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensiv. Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joannidis M., Forni L.G., Klein S.J., Honore P.M., Kashani K., Ostermann M., Prowle J., Bagshaw S.M., Cantaluppi V., Darmon M., et al. Lung–kidney interactions in critically ill patients: Consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensiv. Care Med. 2020;46:654–672. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuiri S. ACE and ACE2 in kidney disease. World J. Nephrol. 2015;4:74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaduganathan M., Vardeny O., Michel T., McMurray J., Pfeffer M., Solomon S. Renin-Angiotensin Aldosterone system inhibitors in patients with COVID-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.