Abstract
Background
Mucormycosis involves life-threatening rapidly progressive angioinvasion with infiltration across tissue planes, resulting in necrosis and thrombosis, most commonly seen in the setting of immunocompromised states. We describe 2 cases of isolated cerebral mucormycosis in immunocompetent adults and describe this syndrome in detail in the context of a systemic literature review.
Methods
Using the criteria (1) isolated cerebral disease, (2) mucormycosis (by polymerase chain reaction, culture, or pathology), and (3) affected an immunocompetent individual, we identified 53 additional cases from 1969 to 2020.
Results
Of these 55 cases, ~60% occurred in men, >70% were in patients under age 35, 92% were associated with intravenous drug use, and >85% had infection centered in the basal ganglia. Many presented with cranial nerve deficits, headache, focal weakness, or altered mental status.
Conclusions
No patient survived without amphotericin, and steroid administration was associated with worse outcomes. Given the current opioid crisis, this syndrome may be seen more frequently.
Keywords: basal ganglia abscess, isolated cerebral mucormycosis, IVDU
Rhizopus, Rhizomucor, Mucor, and Absidia belong to the Mucorales order of environmental fungi and are identified by their characteristic broad-based aseptate hyphae. Although Mucorales can often be cultured from the oropharynx and stool of healthy individuals, they can also cause rapidly progressive angioinvasion with infiltration across tissue planes that results in necrosis and thrombosis and is life-threatening [1].
Mucormycosis most commonly occurs in the context of acute myeloid leukemia (or other hematologic malignancies), severe neutropenia, graft-vs-host disease, diabetes, or end-stage renal disease on dialysis and use of iron chelators (eg, deferoxamine). The various mucormycosis syndromes (disseminated, rhinocerebral, pulmonary, cutaneous, gastrointestinal, and isolated cerebral disease) differ in predisposing conditions and prognosis.
We define isolated cerebral disease in immunocompetent adults as a syndrome fulfilling 3 criteria: (1) isolated cerebral localization (infection in cerebellum, cerebral hemispheres, or brainstem, without direct extension to sinuses or cranial bones and without involvement of other organ systems); (2) Mucorales identified as pathogen by positive culture, polymerase chain reaction (PCR), or characteristic broad-based aseptate hyphae on pathology from brain biopsy or autopsy sample; and (3) host is immunocompetent without hematologic malignancy or rheumatologic disease, diabetics in DKA, or receiving immunosuppressing medications, including chronic steroids.
In individuals with diabetes, particularly in the presence of ketoacidosis, disease due to mucormycosis is most commonly the rhinocerebral form, due to direct invasion of the central nervous system (CNS) through the sinuses [2]. Individuals with no underlying condition are most likely to present with cutaneous mucormycosis, often following trauma, but can present with cerebral disease, including rhinocerebral, sinus/sino-orbital, or isolated cerebral disease [2]. Isolated cerebral disease, with a focus most commonly in the basal ganglia, is due to hematogenous seeding following intravenous inoculation and, among immunocompetent individuals, is an important presentation. The vast majority of the published cases of isolated cerebral mucormycosis are associated with injection drug use, making this a critical component of the social history, particularly given the current opioid crisis.
We describe 2 cases of isolated cerebral mucormycosis in immunocompetent patients and perform a systematic review, identifying 53 cases in the published literature. We present a thorough synopsis of this syndrome, focusing on associated risk factors, presentation, diagnosis, microbiology, treatment, and outcomes.
CASE REPORTS
Case 1
A 53-year-old man with a long history of intermittent intravenous drug use who had been treated for poorly differentiated squamous cell carcinoma of the neck presented with acute mental status alteration, confusion, agitation, and tonic-clonic movements, without headaches.
The patient was febrile to 38.5°C and hemodynamically stable. He was alert but unable to follow commands and had left-sided ptosis and intermittent involuntary movements of his left arm. Initial laboratory studies showed a mild leukocytosis and normal serum creatinine, electrolytes, and liver transaminases, and negative HIV test (Table 1). Toxicology was positive for cocaine, opiates, and benzodiazepines. Brain imaging showed an infiltrative mass involving the right basal ganglia (Figure 1A, B). Cerebral spinal fluid (CSF) examination showed 83 nucleated cells (reference range, 0–5/μL) with 31% neutrophils, 47% lymphocytes, and 22% mononuclear cells, normal glucose, and elevated protein (81 mg/dL; reference range, 5–55 mg/dL). CSF nucleic acid–based testing for herpes simplex virus 1 and 2 and other viruses was negative.
Table 1.
Initial Laboratory Values
| Initial Lab Investigations | Case 1 | Case 2 |
|---|---|---|
| White blood cell count (ref range, 4.5–11.0 K/μL), K/μL | 15.51 | 15.44 |
| % neutrophils | 91.7 | 87.6 |
| Hematocrit (ref range, 41.0–53.0%), % | 35.8 | 22.5 |
| Platelets (ref range, 150–400 K/μL), K/μL | 389 | 403 |
| Serum sodium (ref range, 136–145 mmol/L), mmol/L | 133 | 140 |
| Serum bicarbonate (ref range, 20–31 mmol/L), mmol/L | 26 | 10 |
| Serum creatinine (ref range, 0.50–1.30 mg/dL), mg/dL | 0.60 | 15.04 |
| Venous pH (ref range, 7.32–7.42) | N/A | 7.14 |
| HIV 1/2 Ab/Ag | Negative | Negative |
| CSF RBC count (ref range, 0–5 /μL), /μL | 4 | 61 |
| CSF WBC count and differential (ref range, 0–5/μL), /μL | 83 (31% PMNs, 47% lymphs) | 1 (61% lymphs) |
| CSF glucose (ref range, 50–75 mg/dL), mg/dL | 50 | 71 |
| CSF protein (ref range, 5–55 mg/dL), mg/dL | 81 | 30 |
| CSF HSV 1/2 PCR | Negative | Negative |
| CSF VZV PCR | Negative | Negative |
| CSF adenovirus PCR | Negative | Negative |
| CSF enterovirus PCR | Negative | Negative |
| CSF culture | Negative | Negative |
Abbreviations: Ab, antibody; Ag, antigen; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HSV, herpes simplex virus; PCR, polymerase chain reaction; RBC, red blood cell; WBC, white blood cell; VZV, varicella zoster virus.
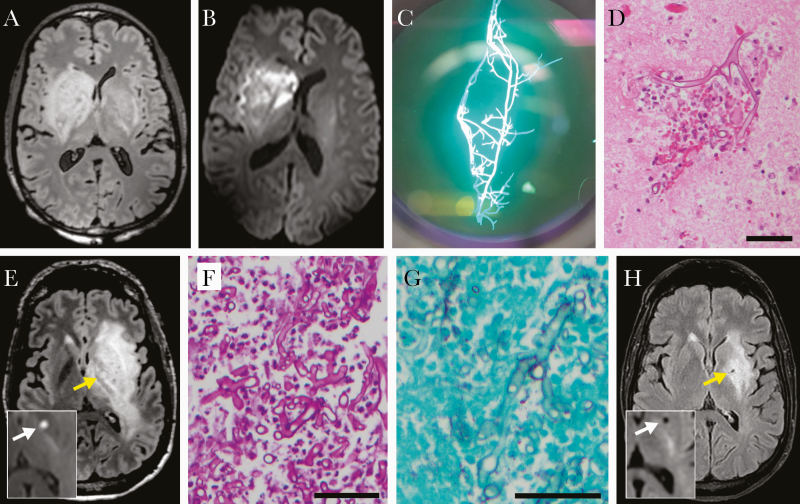
Figure 1.
Diagnostic data. A–D, Case 1. Day 2 brain MRI axial FLAIR (A) and diffusion-weighted (B) images. Fungal forms seen on media by calcofluor-white stain (C) and hematoxylin and eosin–stained histology (D) from brain biopsy. E–H, Case 2. Day 3 (E) or 1 month later (H) brain MRI axial FLAIR images; insets, diffusion-weighted images; arrows, micro-abscess. Fungal forms on periodic acid-Schiff-stained (F) and silver-stained (G) histology from brain biopsy. Abbreviations: MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery. Size bars, 50 µm.
Given a high concern for metastasis of the prior cancer, dexamethasone was started. Simultaneously, out of concern for an infectious process, acyclovir, ceftriaxone, metronidazole, and vancomycin were also initiated.
Over the next few days, the patient’s mental status declined, and he required intubation for airway protection. New bradycardia raised concern for increasing intracranial pressure, and mannitol was provided. Interval imaging showed progression of the basal ganglia lesion now involving the contralateral side (Figure 1A, B). A stereotactic brain biopsy revealed aseptate hyphal organisms consistent with Mucorales spp. by calcofluor and histopathology (Figure 1C, D). Liposomal amphotericin B (LAmB) was started; however, given worsening episodes of hypertension and bradycardia, the family transitioned the patient to comfort measures, and the patient passed quickly. Culture of the brain biopsy subsequently grew Rhizopus species.
Case 2
A 37-year-old man with a history of intravenous fentanyl use presented with 1 week of right-sided hemiparesis, dysarthria, and right facial droop, without headaches or fevers. He was initially afebrile and hemodynamically stable. His labs showed a mild leukocytosis, with markedly elevated BUN (120 mg/dL; reference range, 9–23 mg/dL) and serum creatinine (15.04 mg/dL; reference range, 0.50–1.30 mg/dL), a markedly low serum bicarbonate (10 mmol/L; reference range, 20–31 mmol/L), and normal liver function tests (Table 1). A venous blood gas showed a pH of 7.14, confirming acidemia. Urine analysis showed nephrotic range proteinuria.
Brain imaging showed a left basal ganglia mass with restricted diffusion and surrounding edema. CSF examination revealed 61 red blood cells/mL, 1 nucleated cell (61% lymphs), and normal glucose and protein (Table 1). Initial treatment was vancomycin, cefepime, and metronidazole for presumed bacterial brain abscess and a bicarbonate drip for acidemia. On day 3, repeat brain magnetic resonance imaging (MRI) showed features of probable microhemorrhage within the lesion (Figure 1E), raising concern for mucormycosis. The patient developed progressive word-finding difficulty, lethargy, dysarthria, and seizures. Antifungal agents (LAmB, micafungin, and posaconazole) were added to the regimen, and stereotactic brain biopsy revealed broad-based aseptate hyphae consistent with Mucorales spp. (Figure 1F, G). Fungal cultures did not isolate fungi, but PCR-based analysis of the biopsy (University of Washington) was positive for Rhizopus oryzae.
A kidney biopsy showed systemic amyloid A amyloidosis, thought to be related to longstanding injection drug use [3].
The patient completed 4 weeks of 3-drug therapy with posaconazole, LAmB, and micafungin, followed by 3 weeks of 2-drug therapy with posaconazole and intravenous (IV) LAmB then de-escalation to posaconazole alone, which he continues currently, 15 months after diagnosis. Serial brain MRIs have shown gradual improvement over time, with decreased T2 intensity and reduced edema and mass effect (Figure 1H). Residual neurologic deficits are a slight right facial droop and mild right-hand weakness.
METHODS
A systematic review the literature was performed using key words “isolated cerebral mucormycosis,” “brain mucor,” “isolated cerebral phycomycosis,” “basal ganglia zygomycosis,” “cerebral phycomycosis heroin,” “cerebral mucormycosis IV drug use,” and a conditional search of [(“mucormycosis” or “zygomycosis” or “Mucor” or “Absidia” or “Rhizopus” or “Rhizomucor”) and either (“primary” or “isolated”)] in PubMed. Additional studies were identified by reviewing the reference lists of the papers identified in PubMed.
Cases were defined as adults meeting all 3 of the following criteria: (1) isolated cerebral disease, (2) mucormycosis, and (3) affecting an immunocompetent host. Patients were considered to have “isolated cerebral” disease when the infection only involved the brain tissue (cerebellum, cerebral hemispheres, or brainstem), without direct extension from the sinuses or other cranial bones or related to trauma or prior cranial surgery and without infection of other body sites (lungs, kidneys, skin, etc.). Mucormycosis was defined as a culture, molecular, or histopathologic diagnosis identifying an organism from the Mucorales order of fungi. Molecular diagnosis included PCR and next-generation sequencing. Histopathology that showed characteristic broad-based aseptate hyphae was also considered diagnostic.
Patients were considered immunocompetent if they were not on immunosuppressing medications (including long-term steroids), did not have diabetes, and did not have a history of hematologic malignancy or rheumatologic disease. Accordingly, patients with these conditions or who were on immunosuppressive medications, including long-term steroids, were excluded from our analysis even if they were included in other reports. Patients with HIV were included regardless of CD4 cell count, as HIV infection is not considered a risk factor for mucormycosis [4]. One small series of potential cases was excluded due to insufficient detail on the presentation, diagnosis, and treatment course of the patients. Papers not written in English were also excluded.
Details about each of the cases that met the inclusion criteria were collected and tabulated (Tables 2–4). Specifically, we sought to describe the presenting syndrome including the duration and type of symptoms and whether cranial nerve deficits were present. We detail how the diagnosis was made and whether the patient had HIV infection, and we list the treatments the patients received, including antifungal agents, steroids, and surgical procedures. Finally, we describe the clinical outcomes and, where possible, the duration of survival and the long-term functional status of the survivors at the time of the publication using language from the reports.
Table 2.
Clinical Presentation
| Case No. | Age/Sex | Presenting Symptoms | Time From Onset of Symptoms to Diagnosis | Cranial Neuropathy | Source/Year |
|---|---|---|---|---|---|
| 1 | 37/F | Alerted mental status, headache, fever | Several weeks | None reported | [5]/1980 |
| 2 | 32/M | Positional vertigo, nystagmus, vomiting | Days | 12th nerve | [6]/1984 |
| 3 | 24/F | Fever, chills, slurred speech | Diagnosed on hospital day 24 | 6th and 3rd nerves | [7]/1986 |
| 4 | 20/F | Headache, fever | Days | None reported | [8]/1988 |
| 5 | 24/M | Parkinsonian symptoms | >3 wk of symptoms | Dysarthria/dysphagia | [9]/1989 |
| 6 | 28/M | Headache, right-sided weakness | 2 wk | Multiple | [10]/1992 |
| 7 | 28/M | Right-sided weakness, slurred speech, headache, unsteady gait | 2 wk | 7th nerve | [11]/1994 |
| 8 | 30/F | Dysarthria, left-sided weakness | Days | 7th nerve | [12]/1996 |
| 9 | 24/F | Alerted mental status, fever | Days | None reported | [13]/2007 |
| 10 | 42/F | Headache, fever, left-sided weakness | >2 wk | None reported | [14]/2008 |
| 11 | 43/M | Worsening balance | Possibly 9–12 mo | 7th nerve | [15]c/2010 |
| 12 | 40/M | Unresponsive | Diagnosed >4 wk after admission | None reported | [16]/2017 |
| 13 | 23/F | Headache, confusion | Days | None | [17]/2019 |
| 14 | 37/M | Right-sided weakness and facial droop, dysarthria | Days | Dysarthria + 7th nerve | (This study)/2020 |
| 15 | 44/M | Altered mental status, fever, flu-like illness | Days | None reported | [18]/1989 |
| 16 | 26/M | Headache, vomiting, fever | Days | None reported | [19]/1990 |
| 17 | 31/F | Headache, speech change | 1 wk | 7th and 12th nerves | [20]/1990 |
| 18 | 34/M | Ataxia, fever | Unknown (disoriented on admission) | None | [21]d/1991 |
| 19 | 53/F | Confusion, right-sided weakness | Diagnosed >3 mo after admission | 7th nerve | [22]/1992 |
| 20 | 29/F | Fever, headache, confusion | Days | None reported | [23]/1994 |
| 21 | 28/M | Lethargy, severe headache, vomiting | Hours | None reported | [24]/1982 |
| 22 | 21/M | Fever, disorientation | Unclear prodrome as patient altered on arrival | None reported | [25]/1988 |
| 23 | 23/F | Unconscious, fever | Unclear prodrome as comatose on arrival | None reported | [25]/1988 |
| 24 | 25/M | “Comatose” | Days | None reported | [26]e/1988 |
| 25 | 33/F | Right-sided weakness, lethargy, headache, fever | Acute onset | None | [27]/2000 |
| 26 | “Young”/M | Headache, fever, chills | 1 day | None | [28]/2005 |
| 27 | 24/M | Headache, confusion, left-sided weakness | Days | None reported | [29]/2007 |
| 28 | 22/M | Severe headache, difficulty walking | Hours | 7th nerve | [30]/2011 |
| 29 | 43/M | Altered mental status | Last seen normal 4 days before brought in altered | None reported | [31]/2016 |
| 30 | 27/M | Fever, headache, nausea, vomiting | Days | 7th nerve | [32]/2020 |
| 31 | 26/F | Fever, headache, nausea, vomiting | Days | 6th and 7th nerves | [33]/1985 |
| 32 | 27/F | Tonic-clonic movements, headache, vomiting | Days | 7th nerve | [34]/1994 |
| 33 | 37/M | Aches, fever, chills, headache | Hours | 7th nerve | [34]/1994 |
| 34 | 32/F | Headache, right-sided weakness, dysarthria | Days | Multiple | [34]/1994 |
| 35 | 49/M | Tonic-clonic movements, fever | Days | None | [35]/2006 |
| 36 | 22/F | “Fever and neurologic deficits” | Prodrome unclear based on information in report | N/A | [36]/2007 |
| 37 | 24/M | Left-sided weakness, headache | “New onset” | Facial droop | [37]/2018 |
| 38 | 24/M | Dead on arrival | Unclear prodrome as dead on arrival | Deceased on arrival | [38]/1969 |
| 39 | 32/M | Altered mental status, unable to swallow, fever | 1 day | Multiple | [39]/1970 |
| 40 | 27/M | Sudden left-sided weakness and headache | Days | Multiple | [40]/1982 |
| 41 | 27/M | Lethargy, severe headache, left-sided weakness | Days | Dysarthria | [24]f/1982 |
| 42 | 23/F | Headache | 1 wk | 12th nerve | [6]/1984 |
| 43 | 28/M | Fever, headache, vomiting, seizure | 1 wk | Facial weakness | [41]/1987 |
| 44 | 40/M | Fever, headache, right-sided weakness | Days | Dysarthria | [41]/1987 |
| 45 | 25/M | “Acute neurologic deficit” | Acute | Not assessed | [36]/2007 |
| 46 | 28/M | Lethargy, confusion, fever | Days | None reported | [42]/2013 |
| 47 | 50/M | Garbled speech, confusion, right arm and facial weakness | Acute | Dysarthria | [43]/2015 |
| 48 | 38/F | Fever, lethargy, dysphagia, right-sided weakness | 2 wk | Dysarthria | [44]/1985 |
| 49 | 30/M | Lethargy, aphasia, right-sided weakness, fever | Days | Multiple | [44]/1985 |
| 50 | 22/F | Seizures, vomiting, headache | 3 mo | Diplopia | [45]/1985 |
| 51 | 47/F | Severe headache | 3 mo | None | [46]g/1986 |
| 52 | 41/M | Right-sided weakness, headache, fever | 2 day | None reported | [47]/1987 |
| 53 | 25/M | Confusion, left-sided weakness, headache, chills | 2 wk | None reported | [48]/1988 |
| 54 | 31/F | “Acute neurologic deficit” | Acute | N/A | [36]/2007 |
| 55 | 53/M | Altered mental status, tonic-clonic movements, fever | Days | Dysarthria | (This study)/2020 |
Abbreviations: F, female; M, male; wk, week(s); mo, month(s).
Table 4.
Treatment and Outcomes
| Case No. | Age/Sex | Outcome | Antifungal Therapy & Durationa of Treatment (if Survived) | Steroidsb | Survival Duration at Time Case Reported | Functional Status After Treatment | Source |
|---|---|---|---|---|---|---|---|
| 1 | 37/F | Survived | AmB,c duration not stated | No | Not stated | “Patient returned to normal health” | [5] |
| 2 | 32/M | Survived | AmB for “weeks” | No | At least months | “Now aphasic, spastic and has only the ability to follow simple commands” | [6] |
| 3 | 24/F | Survived | AmB for “over a four-month period” | No | >5 mo | “Functioning normally” | [7] |
| 4 | 20/F | Survived | AmB for weeks to months | No | >4 mo | “Only minimal weakness in the right extremities” | [8] |
| 5 | 24/M | Survived | AmB for “8 weeks” | No | Months | “Enjoying gradual but steady improvement” | [9] |
| 6 | 28/M | Survived | AmB for “12 days” | No | Unclear | “Symptoms improved” | [10] |
| 7 | 28/M | Survived | AmB for weeks to months | No | At least months | “Returned to employment”; symptoms resolved | [11] |
| 8 | 30/F | Survived | AmB for 6 mo | No | At least months | “Recovered from her left hemiparesis and regained ability to walk” independently | [12] |
| 9 | 24/F | Survived | AmB, duration not stated | No | Not specifically stated | “Alert and able to follow commands, but she remained mute” | [13] |
| 10 | 42/F | Survived | LAmBd for 6 mo with 80 day of IT AmB and some debridement | No | >3 years | “Completely recovered from left leg but only partially from left arm paralysis” | [14] |
| 11 | 43/M | Survived | AmB for 2 wk following surgical resection (AmB stopped due to renal failure) | No | Months | “Continues to be ambulatory” | [15] |
| 12 | 40/M | Survived | LAmB followed by isavuconazole after acute renal injury, duration not stated, some debridement | No | At least months | Clinical improvement “initially with his speech, followed by improved wakefulness and awareness” | [16] |
| 13 | 23/F | Survived | LAmB and posaconazole for 45 d followed by posaconazole suppression | No | >25 mo | “Able to ambulate without assistance” and “severely dysarthric” and “mild spasticity and discoordination” | [17] |
| 14 | 37/M | Survived | LAmB, posaconazole, and micafungin for 4 wk, followed by LAmB and posaconazole for 3 wk, followed by >1 y of ongoing posaconazole suppression | No | >6 mo | “Remarkable neurologic recovery with only a faint right facial droop and mild residual right-hand weakness” | (This study) |
| 15 | 44/M | Survived | AmB for 10 wk | Yes | Not specifically stated, at least months | “Able to ambulate without assistance, can verbally communicate, and is oriented to person, place, and time” | [18] |
| 16 | 26/M | Survived | AmB, including intrathecal AmB for several months | Yes | >20 mo | “Continued disability in the form of partial weakness of his left leg and complete weakness of his left arm” | [19] |
| 17 | 31/F | Survived | AmB for months | Yes | >5.5 mo | “Alive and well, with minimal neurologic deficits” | [20] |
| 18 | 34/M | Survived | AmB for months | Yes | >6 mo | “Able to ambulate with assistance” | [21] |
| 19 | 53/F | Survived | AmB, including intrathecal AmB, for months | Yes | >7 mo | “Mental status and hemiparesis improved”; “alive and well” after completion of AmB | [22] |
| 20 | 29/F | Survived | AmB for months plus intrathecal AmB for 18 d | Yes | At least 3 mo | “Alert and able to follow simple commands, but she had a persistent dense left hemiplegia” | [23] |
| 21 | 28/M | Deceased | AmB | No | — | — | [24] |
| 22 | 21/M | Deceased | AmB | No | — | — | [25] |
| 23 | 23/F | Deceased | AmB | No | — | — | [25] |
| 24 | 25/M | Deceased | AmB | No | — | — | [26] |
| 25 | 33/F | Deceased | AmB | No | — | — | [27] |
| 26 | “Young”/M | Deceased | LAmB | No | — | — | [28] |
| 27 | 24/M | Deceased | AmB | No | — | — | [29] |
| 28 | 22/M | Deceased | AmB | No | — | — | [30] |
| 29 | 43/M | Deceased | IV and IT AmB and posaconazole | No | — | — | [31] |
| 30 | 27/M | Deceased | AmB and posaconazole | No | — | — | [32] |
| 31 | 26/F | Deceased | AmB | Yes | — | — | [33] |
| 32 | 27/F | Deceased | AmB | Yes | — | — | [34] |
| 33 | 37/M | Deceased | AmB | Yes | — | — | [34] |
| 34 | 32/F | Deceased | AmB | Yes | — | — | [34] |
| 35 | 49/M | Deceased | AmB | Yes | — | — | [35] |
| 36 | 22/F | Deceased | AmB | Yes | — | — | [36] |
| 37 | 24/M | Deceased | IV LAmB, IT AmB, isavuconazole | Yes | — | — | [37] |
| 38 | 24/M | Deceased | None | No | — | — | [38] |
| 39 | 32/M | Deceased | None | No | — | — | [39] |
| 40 | 27/M | Deceased | None | No | — | — | [40] |
| 41 | 27/M | Deceased | None | No | — | — | [24] |
| 42 | 23/F | Deceased | None | No | — | — | [6] |
| 43 | 28/M | Deceased | None | No | — | — | [41] |
| 44 | 40/M | Deceased | None | No | — | — | [41] |
| 45 | 25/M | Deceased | None | No | — | — | [36] |
| 46 | 28/M | Deceased | None | No | — | — | [42] |
| 47 | 50/M | Deceased | None | No | — | — | [43] |
| 48 | 38/F | Deceased | None | Yes | — | — | [44] |
| 49 | 30/M | Deceased | None | Yes | — | — | [44] |
| 50 | 22/F | Deceased | None | Yes | — | — | [45] |
| 51 | 47/F | Deceased | None | Yes | — | — | [46] |
| 52 | 41/M | Deceased | None | Yes | — | — | [47] |
| 53 | 25/M | Deceased | None | Yes | — | — | [48] |
| 54 | 31/F | Deceased | None | Yes | — | — | [36] |
| 55 | 53/M | Deceased | Nonee | Yes | — | — | (This study) |
Abbreviations: AmB, amphotericin B; F, female, IT, intrathecal; IV, intravenous; M, male; mo, month(s); wk, week(s).
aDuration reported directly when described in case report. In some cases, duration calculated from total dose of AmB reported.
bIf not mentioned as administered in the case report, steroids were presumed not given.
cAmB, amphotericin B, formulation not specified.
dLAmB, liposomal amphotericin B.
eThis patient received only 1 dose of LAmB within hours of dying.
RESULTS
We identified and reviewed 790 publications. The most common reasons for exclusion from this review were (1) cases did not represent isolated cerebral disease (384); (2) duplicate studies (102); (3) a host that did not meet the study criteria, either immunosuppressed including due to diabetes or animal studies (101); or (4) not written in English [39]. Some studies were excluded for multiple reasons. Six studies were excluded because the subjects were children. One study was excluded because it lacked sufficient detail about the clinical course, treatment, or diagnosis. Initially included were 32 papers; after reviewing the reference lists of these 32 papers, an additional 11 papers were identified. From these 43 papers, 53 cases of isolated cerebral mucormycosis in immunocompetent adults were identified. With the 2 cases presented here, a total of 55 distinct cases of isolated cerebral mucormycosis in immunocompetent adults were analyzed.
Among the 55 individuals, 34 (61.8%) were men (Table 2). Age ranged from 20 to 53 (mean, 31.1) years old, with 38 of 54 (70.4%) under age 35 (note 1 individual was listed as “young” without an age indicated). The most commonly reported presenting symptoms were headache (29/55, 52.7%), fever (26/55, 47.3%), focal weakness (18/55, 32.7%), and altered mental status (14/55, 25.5%). Cranial nerve deficits were present in at least 28/55 (50.9%) individuals.
Injection drug use was common, reported in 92% of cases where drug use was queried (46/50 cases for which IV drug use history was assessed) (Table 3). Five cases were in individuals who were HIV positive, although 12 cases were reported before 1986, the year testing became widely available.
Table 3.
Risk Factors and Diagnosis
| Case No. | Age/Sex | IV Drug Type | Localization of Infection | HIV Ab | Diagnosis (Method)/Manner Obtained | Source |
|---|---|---|---|---|---|---|
| 1 | 37/F | Drug use history not stated | Ventricular infection causing obstructive hydrocephalus | N/Aa | Aseptate hyphae/CSF exam after drain placed | [5] |
| 2 | 32/M | Heroin and cocaine | Right basal ganglia | N/A | Rhizopus/Absidia (immunostains)/biopsy | [6] |
| 3 | 24/F | Pentazocine and tripelennamine | Brain stem | N/A | Aseptate hyphae/brain biopsy | [7] |
| 4 | 20/F | Drug use history not stated | Left basal ganglia | N/A | Aseptate hyphae/brain biopsy | [8] |
| 5 | 24/M | Heroin and cocaine | Bilateral basal ganglia | Neg | Aseptate hyphae/biopsy | [9] |
| 6 | 28/M | IVDU, not further specified | Left basal ganglia | N/A | Rhizopus spp. (culture)/stereotactic biopsy | [10] |
| 7 | 28/M | IVDU, not further specified | Left basal ganglia | Neg | Aseptate hyphae/stereotactic brain biopsy | [11] |
| 8 | 30/F | IVDU, not further specified | Left basal ganglia | Pos | Aseptate hyphae/stereotactic brain biopsy | [12] |
| 9 | 24/F | Heroin | Right basal ganglia | Neg | Rhizopus arrhizus (PCR)/stereotactic brain biopsy | [13] |
| 10 | 42/F | IVDU, not further specified | Right basal ganglia | Neg | Aseptate hyphae/stereotactic biopsy | [14] |
| 11 | 43/M | IVDU, not further specified | Left cerebellar hemisphere | Neg | Aseptate hyphae/open biopsy | [15] |
| 12 | 40/M | IVDU, not further specified | Left basal ganglia | Neg | Rhizopus oryzae (PCR)/stereotactic brain biopsy | [16] |
| 13 | 23/F | Methamphetamine | Bilateral basal ganglia | Neg | Rhizopus spp. (culture)/brain biopsy | [17] |
| 14 | 37/M | Fentanyl | Left basal ganglia | Neg | Rhizopus oryzae (PCR)/stereotactic brain biopsy | (This study) |
| 15 | 44/M | Cocaine and opioids | Bilateral basal ganglia | Neg | Aseptate hyphae/stereotactic biopsy | [18] |
| 16 | 26/M | Amphetamines | Right basal ganglia | Neg | Rhizopus oryzae (culture)/aspiration | [19] |
| 17 | 31/F | Cocaine | Left basal ganglia | Pos | Aseptate hyphae/stereotactic biopsy | [20] |
| 18 | 34/M | Heroin | Left basal ganglia | Neg | Rhizopus arrhizus (culture)/biopsy | [21] |
| 19 | 53/F | Heroin and cocaine | Multiple abscesses including in left basal ganglia | Neg | Aseptate hyphae/brain biopsy | [22] |
| 20 | 29/F | Opioids | Left basal ganglia | Neg | Aseptate hyphae/biopsy | [23] |
| 21 | 28/M | IVDU, not further specified | Left basal ganglia | N/A | Rhizopus oryzae (culture)/open brain biopsy | [24] |
| 22 | 21/M | Heroin | Bilateral basal ganglia | Pos | Aseptate hyphae/brain biopsy | [25] |
| 23 | 23/F | IVDU, not further specified | Bilateral basal ganglia | Pos | Aseptate hyphae/brain biopsy | [25] |
| 24 | 25/M | IVDU, not further specified | Right temporal lobe | Neg | Rhizopus arrhizus (culture)/aspiration | [26] |
| 25 | 33/F | IVDU, not further specified | Left basal ganglia | Neg | Ventriculostomy culture with aseptate branching hyphae | [27] |
| 26 | “Young”/M | Heroin | Bilateral basal ganglia | Neg | Fungi with aseptate hyphae/autopsy | [28] |
| 27 | 24/M | Drug use history not stated | Bilateral basal ganglia | Neg | Rhizomucor (culture)/biopsy | [29] |
| 28 | 22/M | Heroin and cocaine | Right basal ganglia | Neg | Hyphal elements from autopsy | [30] |
| 29 | 43/M | IVDU presumed but not confirmed | Ventricular disease with a left basal ganglia mass | Neg | Aseptate hyphae/ventricular biopsy | [31] |
| 30 | 27/M | Drug use history not stated | Right basal ganglia | Neg | Rhizopus spp. (next-generation sequencing)/CSF exam from time of drain placement | [32]/2020 |
| 31 | 26/F | Drug use history not stated | Bilateral basal ganglia | N/A | Aseptate hyphae/brain biopsy | [33] |
| 32 | 27/F | Cocaine and opioids | Right basal ganglia | Neg | Aseptate hyphae/stereotactic brain biopsy | [34] |
| 33 | 37/M | Heroin and cocaine | Left basal ganglia | Neg | Aseptate hyphae/autopsy | [34] |
| 34 | 32/F | Heroin and cocaine | Left basal ganglia | Neg | Aseptate hyphae/autopsy | [34] |
| 35 | 49/M | No history of IVDU | Left frontal lesion progressed to involve right frontal and right basal ganglia | Neg | Aseptate hyphae/autopsy | [35] |
| 36 | 22/F | IVDU, not further specified | Bilateral basal ganglia | Neg | Aseptate hyphae/stereotactic brain biopsy | [36] |
| 37 | 24/M | IVDU, not further specified | Bilateral basal ganglia | N/A | Aseptate hyphae from culture from ventriculostomy | [37] |
| 38 | 24/M | Heroin | Right basal ganglia | N/A | Aseptate hyphae/autopsy | [38] |
| 39 | 32/M | Heroin | Left basal ganglia | N/A | Aseptate hyphae/autopsy | [39] |
| 40 | 27/M | Heroin | Bilateral basal ganglia | N/A | Aseptate hyphae/autopsy | [40] |
| 41 | 27/M | IVDU, not further specified | Multiple bilateral cerebral abscesses | N/A | Aseptate hyphae/autopsy | [24] |
| 42 | 23/F | IVDU, not further specified | Right basal ganglia | N/A | Aseptate hyphae/autopsy | [6] |
| 43 | 28/M | Heroin | Ventricular infection causing obstructive hydrocephalus | N/A | Aspirate during craniotomy with aseptate hyphae | [41] |
| 44 | 40/M | Heroin | Left basal ganglia | N/A | Autopsy with aseptate hyphae | [41] |
| 45 | 25/M | IVDU, not further specified | Right basal ganglia | Neg | Not stated | [36] |
| 46 | 28/M | Heroin | Bilateral basal ganglia | Neg | Aseptate hyphae/autopsy | [42] |
| 47 | 50/M | Heroin and cocaine | Bilateral basal ganglia | Neg | Aseptate hyphae/autopsy | [43] |
| 48 | 38/F | Amphetamines | Left basal ganglia | N/A | Mucor spp. (culture)/autopsy | [44] |
| 49 | 30/M | Cocaine and amphetamines | Bilateral basal ganglia | N/A | Mucor spp. (culture)/autopsy | [44] |
| 50 | 22/F | No history of IVDU | Ventricular infection causing obstructive hydrocephalus and basilar meningitis | N/A | Autopsy with aseptate hyphae | [45] |
| 51 | 47/F | No history of IVDU | Right frontal lobe | N/A | Culture from abscess drainage | [46] |
| 52 | 41/M | Heroin and amphetamines | Left basal ganglia | N/A | Aseptate hyphae seen at autopsy | [47] |
| 53 | 25/M | IVDU, not further specified | Right basal ganglia | Neg | Rhizopus oryzae (culture)/autopsy | [48] |
| 54 | 31/F | IVDU, not further specified | Bilateral basal ganglia | Pos | Not stated | [36] |
| 55 | 53/M | Heroin and cocaine | Bilateral basal ganglia | Neg | Rhizopus spp. (culture)/stereotactic brain biopsy | (This study) |
Abbreviations: Ab, antibody; CSF, cerebrospinal fluid; F, female, M, male; IV, intravenous; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; IVDU, intravenous drug use; spp, species.
aN/A, HIV status not explicitly stated.
In 47 cases (85.5%), basal ganglia involvement was explicitly stated, and 4 cases (7.3%) involved ventricular infection, generally with obstructing hydrocephalus. In 31 cases (56.4%), diagnosis was made by biopsy, most often stereotactic; in 4 cases, diagnosis was made on samples obtained at the time of ventricular drain placement; in 18 cases, diagnosis was made at autopsy. In 37 cases (67.3%), diagnosis was made by histopathological finding of aseptate hyphae. In several cases, culture, PCR, and next-generation sequencing were used.
The survival rate for patients receiving AmB was 54.1% (20/37), and for patients not receiving AmB it was 0% (0/18) (Table 4). The survival rate for those receiving AmB and steroids was 46.2% (6/13), and for those receiving AmB without steroids it was 58.3% (14/24). The clinical course in those who died tended to be more fulminant, with shorter presentation-to-diagnosis rates. Among those who died, 29/35 presented with less than a week of symptoms, whereas among those who survived, approximately half had symptoms for a week or longer before diagnosis.
Although excluded for the purposes of this review, it should be noted that similar presentations were seen in other hosts, including individuals with diabetes [49]. One case was reported in an individual with poorly controlled diabetes and severe Crohn’s disease who was receiving infliximab and injecting drugs [50]. One additional case in the literature that deserves mention describes a strikingly similar clinical syndrome due to Acremonium alabamensis (a non-Mucorales fungus) [6].
DISCUSSION
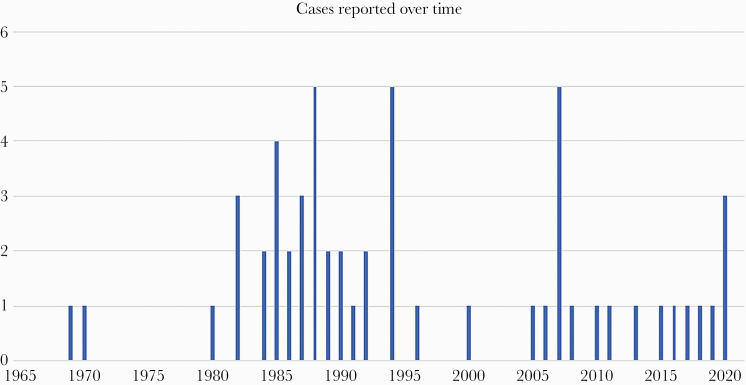
Isolated cerebral mucormycosis in immunocompetent adults, defined as infection with Mucorales species (demonstrated by pathology, culture, or molecular testing), isolated from the brain only, in an immunocompetent host without diabetes, is a rare entity. In our review of 53 previously reported cases in the literature and 2 new cases described here, injection drug use was a prominent feature, present in 92% of cases for which it was assessed. The strong association with injection drug use is almost certainly because it facilitates hematogenous introduction of the mold. The increasing prevalence of people who inject drugs is associated with significantly higher rates of hospitalizations for infections related to injection drug use [51–53]. In the context of the current opioid epidemic, we anticipate that many more cases of cerebral mucormycosis will be recognized in the years ahead. An epidemic curve showing the reported cases plotted over time (Figure 2) suggests increased numbers of cases between 1980 and 1995 and again since 2005, although reporting bias limits conclusions that can be drawn from this.
Figure 2.
Epidemic curve of reported cases since 1965, plotted as a function of year of presentation.
The disease appears to be one primarily of young adults, with >70% of individuals reviewed here under age 35 at the time of diagnosis. Headache, fever, focal weakness, and altered mental status were all commonly reported. More than half had cranial nerve deficits reported at the time of presentation.
That more than 85% had infection centered in the basal ganglia is quite notable. Basal ganglia brain abscesses are unusual; in a meta-analysis of nearly 10 000 cases of brain abscesses, basal ganglia localization occurred in only 3% [54]. Moreover, fungal etiologies account for only 1% of all brain abscesses [54]. The observed association of Mucorales spp. with the basal ganglia may result from the predilection of the organism for the brain; as early as 48 hours after injection of Absidia ramosa spores into the tail vein of mice, hyphae were seen in the brain and kidney [55]. This predilection for the basal ganglia has been hypothesized to be due to the high iron content of this tissue, as germination of Rhizopus spp. spores requires free iron [50, 56]. Brain abscesses due to Listeria monocytogenes, another organism that requires iron for virulence, are also commonly found in the basal ganglia [57, 58]. In addition to the basal ganglia, localization of Mucorales infection to the ventricles, with associated obstructive hydrocephalus, was reported in several cases. Whereas the differential diagnosis for inflammatory lesions of the basal ganglia is broad, aggressive Mucorales spp. infection should be considered.
Approximately half of those who survived presented subacutely, reporting symptoms for at least a week or more, whereas among those who died, the vast majority (>80%) presented with less than a week of symptoms, most reporting hours to days of symptoms. The observed differences in duration of symptoms could be explained by an inoculum effect, wherein individuals whose brains were seeded with small numbers of Mucorales spores of vegetative cells have a more indolent course than individuals whose brains were seeded with greater numbers of Mucorales spores. Alternatively, more effective host immune function, including neutrophil activity, might allow better control of the infection, slowing the angioinvasive and necrotizing processes. Also possible is that another initial inciting event that predisposes to secondary superinfection by Mucorales, such as focal ischemia, may modulate the duration of symptoms. Further study of this syndrome in an animal model could help shed light on these aspects of the syndrome.
Diagnosis requires tissue, which should be sent for culture and pathology to look for characteristic broad-based aseptate hyphae (Figure 1). Even when visible on histopathology, the organisms do not always grow in culture, potentially because they are nonviable. This is particularly likely if antifungals are administered before sample collection, highlighting the utility of PCR-based testing. The imaging modality of choice is brain MRI with special sequences, including gradient echo and susceptibility weighting, to assess for microhemorrhage, which suggests a potentially invasive process.
There are several important treatment considerations for this syndrome. Amphotericin is essential to treatment, as no patients survived without amphotericin B (AmB). In patients with underlying hematologic malignancy, survival from mucormycosis is significantly increased with early treatment with AmB [59]. These findings are consistent with our retrospective observations herein, together strongly suggesting that early diagnosis and early initiation of AmB improve survival in immunocompetent patients with mucormycosis. Whether to add additional agents for combination therapy is controversial, with no benefit shown for combination therapy in the context of hematologic malignancy [60]. However, some providers may prefer adding a triazole with anti-Mucorales activity, such as posaconazole or isavuconazole, to initial treatment, as was done in Case 2 in this series [61, 62]. Because of potentially better CNS penetration [63], isavuconazole is preferred to posaconazole by some clinicians, but both triazoles demonstrate good clinical outcomes in CNS infections [64–67]. Echinocandins show in vitro synergy with AmB against Rhizopus oryzae but display poor CNS penetration [68]. In addition, treatment failures with echinocandins when used as monotherapy for susceptible fungal brain abscesses are documented [69].
We found that steroid use was associated with worse outcomes, raising the possibility that they are harmful in this setting. As steroids are frequently administered for the treatment of edema associated with brain lesions, early diagnosis is particularly important in isolated cerebral mucormycosis, so that inappropriate steroid administration can be avoided. Additionally, as acidemia promotes Mucorales growth and binding to endothelial cells, thereby accelerating angioinvasion, control of hyperglycemia and acidemia are critical [70].
CONCLUSIONS
Isolated cerebral mucormycosis in immunocompetent adults is a rare and possibly underrecognized syndrome that requires a high index of suspicion for diagnosis and prompt treatment. Many patients present with headaches and cranial nerve deficits, and some with fevers or altered mental status. The vast majority of cases involve the basal ganglia. Tissue from a biopsy is important for establishing the diagnosis. Treatment should include amphotericin B. Good outcomes are possible. Given the current opioid crisis, the frequency of cases may increase.
Acknowledgments
Patient consent. This study does not include factors necessitating patient consent.
Financial support. This research was supported by institutional funds from Massachusetts General Hospital to M.B.G.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sugar AM. Mucormycosis. Clin Infect Dis 1992; 14:S126–9. [DOI] [PubMed] [Google Scholar]
- 2. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634–53. [DOI] [PubMed] [Google Scholar]
- 3. Berns JS, Rapalino O, Fenves AZ, et al. Case 11-2020: a 37-year-old man with facial droop, dysarthria, and kidney failure. N Engl J Med 2020; 382:1457–66. [DOI] [PubMed] [Google Scholar]
- 4. Moreira J, Varon A, Galhardo MC, et al. The burden of mucormycosis in HIV-infected patients: a systematic review. J Infect 2016; 73:181–8. [DOI] [PubMed] [Google Scholar]
- 5. Sweeney PJ, Hahn JF, McHenry MC, Mitsumoto H. Mucormycosis presenting as positional nystagmus and hydrocephalus. Case report. J Neurosurg 1980; 52:270–2. [DOI] [PubMed] [Google Scholar]
- 6. Wetli CV, Weiss SD, Cleary TJ, Gyori E. Fungal cerebritis from intravenous drug abuse. J Forensic Sci 1984; 29:260–8. [PubMed] [Google Scholar]
- 7. Woods KF, Hanna BJ. Brain stem mucormycosis in a narcotic addict with eventual recovery. Am J Med 1986; 80:126–8. [DOI] [PubMed] [Google Scholar]
- 8. Tang LM, Ryu SJ, Chen TJ, Cheng SY. Intracranial phycomycosis: case reports. Neurosurgery 1988; 23:108–11. [DOI] [PubMed] [Google Scholar]
- 9. Adler CH, Stem MB, Brooks TL. Parkinsonism secondary to bilateral striatal fungal abscesses. Mov Disord 1989; 4:333–7. [DOI] [PubMed] [Google Scholar]
- 10. Terk MR, Underwood DJ, Zee CS, Colletti PM. MR imaging in rhinocerebral and intracranial mucormycosis with CT and pathologic correlation. Magn Reson Imaging 1992; 10:81–7. [DOI] [PubMed] [Google Scholar]
- 11. Gollard R, Rabb C, Larsen R, Chandrasoma P. Isolated cerebral mucormycosis: case report and therapeutic considerations. Neurosurgery 1994; 34:174–7. [DOI] [PubMed] [Google Scholar]
- 12. Blázquez R, Pinedo A, Cosín J, et al. Nonsurgical cure of isolated cerebral mucormycosis in an intravenous drug user. Eur J Clin Microbiol Infect Dis 1996; 15:598–9. [DOI] [PubMed] [Google Scholar]
- 13. Mackowiak PA, Carpenter M, Polk C, et al. Encephalitis of the basal ganglia in an injection drug user. Clin Infect Dis 2007; 45:1522–4. [DOI] [PubMed] [Google Scholar]
- 14. Metellus P, Laghmari M, Fuentes S, et al. Successful treatment of a giant isolated cerebral mucormycotic (zygomycotic) abscess using endoscopic debridement: case report and therapeutic considerations. Surg Neurol 2008; 69:510–5; discussion 515. [DOI] [PubMed] [Google Scholar]
- 15. Air EL, Vagal AA, Kendler A, McPherson CM. Isolated cerebellar mucormycosis, slowly progressive over 1 year in an immunocompetent patient. Surg Neurol Int 2010; 1:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hazama A, Galgano M, Fullmer J, et al. Affinity of mucormycosis for basal ganglia in intravenous drug users: case illustration and review of literature. World Neurosurg 2017; 98:872.e1–3. [DOI] [PubMed] [Google Scholar]
- 17. Kerezoudis P, Watts CR, Bydon M, et al. Diagnosis and treatment of isolated cerebral mucormycosis: patient-level data meta-analysis and Mayo Clinic experience. World Neurosurg 2019; 123:425–34.e5. [DOI] [PubMed] [Google Scholar]
- 18. Stave GM, Heimberger T, Kerkering TM. Zygomycosis of the basal ganglia in intravenous drug users. Am J Med 1989; 86:115–7. [DOI] [PubMed] [Google Scholar]
- 19. Fong KM, Seneviratne EM, McCormack JG. Mucor cerebral abscess associated with intravenous drug abuse. Aust N Z J Med 1990; 20:74–7. [DOI] [PubMed] [Google Scholar]
- 20. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 52–1990. A 31-year-old HIV-seropositive woman with a cerebral lesion seven years after treatment of carcinoma of the cervix. N Engl J Med 1990; 323:1823–33. [DOI] [PubMed] [Google Scholar]
- 21. Riefler J 3rd, Batbouta J, Uphoff DF. Case reports Rhizopus brain abscess: report of a case and review of the literature. Mil Med 1991; 156:497–9. [PubMed] [Google Scholar]
- 22. Gaing AA, Corbalan F, Weinberger J. Phycomycosis (mucormycosis) in differential-diagnosis of cerebral mass lesions in intravenous-drug-users. Mt Sinai J Med 1992; 59:69–71. [PubMed] [Google Scholar]
- 23. Siddiqi US, Freedman DJ. Isolated central nervous system mucormycosis. Southern Med J 1994; 87:997–1000. [DOI] [PubMed] [Google Scholar]
- 24. Pierce PF Jr, Solomon SL, Kaufman L, et al. Zygomycetes brain abscesses in narcotic addicts with serological diagnosis. JAMA 1982; 248:2881–2. [PubMed] [Google Scholar]
- 25. Cuadrado LM, Guerrero A, Garcia Asenjo JA, et al. Cerebral mucormycosis in two cases of acquired immunodeficiency syndrome. Arch Neurol 1988; 45:109–11. [DOI] [PubMed] [Google Scholar]
- 26. Oliveri S, Cammarata E, Augello G, et al. Rhizopus arrhizus in Italy as the causative agent of primary cerebral zygomycosis in a drug addict. Eur J Epidemiol 1988; 4:284–8. [DOI] [PubMed] [Google Scholar]
- 27. Jaitly ER, Dhaduk EN, Jensen EM, et al. Primary cerebral mucormycosis: a case report and literature review. Neurologist 2000; 6:232–7. [Google Scholar]
- 28. Greene S, Richard S, Causby N, et al. Fever and headache in an intravenous drug user. J La State Med Soc 2005; 157:135–9; quiz 140. [PubMed] [Google Scholar]
- 29. Pandian JD, McCarthy JS, Goldschlager T, et al. Rhizomycosis infection in the basal ganglia. Arch Neurol 2007; 64:134–5. [DOI] [PubMed] [Google Scholar]
- 30. Clark D, Al Mohajer M, Broderick J. Pearls & Oy-sters: isolated cerebral zygomycosis in an intravenous drug user. Neurology 2011; 76:e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terry AR, Kahle KT, Larvie M, et al. Case records of the Massachusetts General Hospital. Case 5-2016. A 43-year-old man with altered mental status and a history of alcohol use. N Engl J Med 2016; 374:671–80. [DOI] [PubMed] [Google Scholar]
- 32. Zhang GJ, Zhang SK, Wang Z, et al. Fatal and rapid progressive isolated cerebral mucormycosis involving the bilateral basal ganglia: a case report. Front Neurol 2020; 11:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watson DF, Stern BJ, Levin ML, Dutta D. Isolated cerebral phycomycosis presenting as focal encephalitis. Arch Neurol 1985; 42:922–3. [DOI] [PubMed] [Google Scholar]
- 34. Hopkins RJ, Rothman M, Fiore A, Goldblum SE. Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect Dis 1994; 19:1133–7. [DOI] [PubMed] [Google Scholar]
- 35. Verma A, Brozman B, Petito CK. Isolated cerebral mucormycosis: report of a case and review of the literature. J Neurol Sci 2006; 240:65–9. [DOI] [PubMed] [Google Scholar]
- 36. Cuellar H, Riascos R, Palacios E, et al. Imaging of isolated cerebral mucormycosis. A report of three cases. Neuroradiol J 2007; 20:525–30. [DOI] [PubMed] [Google Scholar]
- 37. Dewitt J, Buschur P, Zilioli G. 624: a case of mucor cerebral abscess. Crit Care Med 2018; 46:298. [Google Scholar]
- 38. Adelman LS, Aronson SM. The neuropathologic complications of narcotics addiction. Bull N Y Acad Med 1969; 45:225–34. [PMC free article] [PubMed] [Google Scholar]
- 39. Hameroff BS, Eckholdt WJ, Lindenberg WR. Cerebral phycomycosis in a heroin addict. Neurology 1970; 20:261–5. [DOI] [PubMed] [Google Scholar]
- 40. Masucci EF, Fabara JA, Saini N, Kurtzke JF. Cerebral mucormycosis (phycomycosis) in a heroin addict. JAMA Neurol 1982; 39:304–6. [DOI] [PubMed] [Google Scholar]
- 41. Kasantikul V, Shuangshoti S, Taecholarn C. Primary phycomycosis of the brain in heroin addicts. Surg Neurol 1987; 28:468–72. [DOI] [PubMed] [Google Scholar]
- 42. Lin TP, Thompson R, Coull B. A 28-year-old i.v. drug user with bilateral basal ganglia and brainstem lesions. Neurology 2013; 80:e73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dhakar MB, Rayes M, Kupsky W, et al. A cryptic case: isolated cerebral mucormycosis. Am J Med 2015; 128:1296–9. [DOI] [PubMed] [Google Scholar]
- 44. Micozzi MS, Wetli CV. Intravenous amphetamine abuse, primary cerebral mucormycosis, and acquired immunodeficiency. J Forensic Sci 1985; 30:504–10. [PubMed] [Google Scholar]
- 45. Bichile LS, Abhyankar SC, Hase NK. Chronic mucormycosis manifesting as hydrocephalus. J Neurol Neurosurg Psychiatry 1985; 48:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tay AG, Tan CT. Mucormycosis and chronic brain abscess. Med J Aust 1986; 144:725–6. [DOI] [PubMed] [Google Scholar]
- 47. Ginsberg F, Peyster RG, Hoover ED, Finkelstein SD. Isolated cerebral mucormycosis: case report with CT and pathologic correlation. Am J Neuroradiol 1987; 8:558–60. [PMC free article] [PubMed] [Google Scholar]
- 48. Miller NS, Nance MA, Brummitt CF, et al. Fungal infection associated with intravenous drug abuse: a case of localized cerebral phycomycosis. J Clin Psychiatry 1988; 49:320–2. [PubMed] [Google Scholar]
- 49. Han SR, Choi CY, Joo M, Whang CJ. Isolated cerebral mucormycosis. J Korean Neurosurg Soc 2007; 42:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malik AN, Bi WL, McCray B, et al. Isolated cerebral mucormycosis of the basal ganglia. Clin Neurol Neurosurg 2014; 124:102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jacka B, Larney S, Degenhardt L, et al. Prevalence of injecting drug use and coverage of interventions to prevent HIV and hepatitis C virus infection among people who inject drugs in Canada. Am J Public Health 2020; 110:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chatterjee S, Tempalski B, Pouget ER, et al. Changes in the prevalence of injection drug use among adolescents and young adults in large U.S. metropolitan areas. AIDS Behav 2011; 15:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barocas JA, Eftekhari Yazdi G, Savinkina A, et al. Long-term infective endocarditis mortality associated with injection opioid use in the United States: a modeling study. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brouwer CM, Coutinho MJ, Van De Beek MD. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 2014; 82:806–13. [DOI] [PubMed] [Google Scholar]
- 55. Smith JM, Jones RH. Localization and fate of Absidia ramosa spores after intravenous inoculation of mice. J Comp Pathol 1973; 83:49–55. [DOI] [PubMed] [Google Scholar]
- 56. Kousser C, Clark C, Sherrington S, et al. Pseudomonas aeruginosa inhibits Rhizopus microsporus germination through sequestration of free environmental iron. Sci Rep 2019; 9:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McLaughlin HP, Hill C, Gahan CG. The impact of iron on Listeria monocytogenes; inside and outside the host. Curr Opin Biotechnol 2011; 22:194–9. [DOI] [PubMed] [Google Scholar]
- 58. Tiri B, Priante G, Saraca LM, et al. Listeria monocytogenes brain abscess: controversial issues for the treatment-two cases and literature review. Case Rep Infect Dis 2018; 2018:6549496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008; 47:503–9. [DOI] [PubMed] [Google Scholar]
- 60. Kyvernitakis A, Torres HA, Jiang Y, et al. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 2016; 22:811.e1–8. [DOI] [PubMed] [Google Scholar]
- 61. Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16:828–37. [DOI] [PubMed] [Google Scholar]
- 62. van Burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis 2006; 42:e61–5. [DOI] [PubMed] [Google Scholar]
- 63. Rouzaud C, Jullien V, Herbrecht A, et al. Isavuconazole diffusion in infected human brain. Antimicrob Agents Chemother 2019; 63:e02474–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schwartz S, Cornely OA, Hamed K, et al. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol 2020; 58:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol 2007; 3:573–81. [DOI] [PubMed] [Google Scholar]
- 66. Elzein F, Kalam K, Mohammed N, et al. Treatment of cerebral mucormycosis with drug therapy alone: a case report. Med Mycol Case Rep 2019; 23:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pitisuttithum P, Negroni R, Graybill JR, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother 2005; 56:745–55. [DOI] [PubMed] [Google Scholar]
- 68. Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin North Am 2016; 30:143–63. [DOI] [PubMed] [Google Scholar]
- 69. Prabhu RM, Orenstein R. Failure of caspofungin to treat brain abscesses secondary to Candida albicans prosthetic valve endocarditis. Clin Infect Dis 2004; 39:1253–4. [DOI] [PubMed] [Google Scholar]
- 70. Liu M, Spellberg B, Phan QT, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest 2010; 120:1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]