Abstract
Background:
Elevations in inflammatory biomarkers, including neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR), are reportedly associated with decreased overall survival (OS) or recurrence-free survival (RFS) in patients with numerous cancers. A large multicenter sarcoma data set was used to determine if elevated NLR or PLR was associated with worse survival and can guide treatment selection.
Materials and methods:
A total of 409 patients with a primary retroperitoneal sarcoma (n = 268) or truncal (n = 141) sarcoma from 2000 to 2015 were analyzed using the US Sarcoma Collaboration database. Binary NLR and PLR values were developed using receiver operating characteristic curves. Kaplan-Meier model and Cox proportional hazards model identified predictors of decreased OS and RFS. Point biserial analyses were used to correlate binary and continuous data.
Results:
Neither elevated NLR nor PLR was predictive of decreased OS or RFS. These findings persisted despite exclusion of comorbid inflammatory conditions. Further, NLR and PLR were not correlated with tumor grade. In multivariate models, decreased RFS was associated with tumor factors (e.g., positive margins, tumor grade, tumor size, necrosis, positive nodes); decreased OS was associated with histologic subtype, male gender, and nodal involvement.
Conclusions:
Although several small studies have suggested that elevated NLR and PLR are associated with decreased survival in patients with abdominal or truncal sarcoma, this large multicenter study demonstrates no association with decreased OS, decreased RFS, or tumor grade. Rather, survival outcomes are best predicted using previously established tumoral factors.
Keywords: Sarcoma, Biomarker, Retroperitoneal, Truncal, Survival
Introduction
Soft tissue sarcomas are a rare tumor type, accounting for less than 1% of all adult malignancies.1 Derived from mesenchymal origins, there are more than 50 distinct tumoral histiotypes.2 Soft tissue sarcomas are found in the retroperitoneal and truncal locations (16% and 10%, respectively).3 Liposarcoma (20%), leiomyosarcoma (14%), and undifferentiated pleomorphic sarcoma (UPS; 14%) comprise the most common tumoral histiotypes.4 Because of the relative rarity of these tumors, and the vast number of histiotypes, there has been a paucity of high-quality evidence to guide the management of patients with these tumors.
Much previous effort has focused on identifying patient and tumoral factors that are predictive of worse recurrence-free survival (RFS) and overall survival (OS), including tumor grade and specific tumor histiotype.5,6 Although a few histiotypes (e.g., well-differentiated liposarcoma) can be effectively determined by preoperative imaging, much of the tumoral-based information prognostic of long-term outcomes is not entirely known until after definitive pathology returns.7 Recent research has focused on identification of preoperative factors that can help in the prognostication of soft-tissue sarcomas. One such potential avenue is serum biomarkers.
Prior research has demonstrated that inflammation and its severity in certain cancers are negatively correlated with survival.8 Elevations in acute phase reactants, such as Creactive protein or platelet count, have been shown to be associated with worse survival in pancreatic, lung, colon cancers, and pancreatic neuroendocrine tumors among others.9–12 Similarly, some have suggested that the serum neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) may be a reasonable surrogate for inflammation within the tumor microenvironment.8,13 Elevated NLR and PLR have been recently reported, in several smaller studies, to be independently predictive of shorter RFS and OS in soft tissue sarcomas.13–15 However, heterogeneity in the association of these biomarkers with survival outcomes has created uncertainty.
The aim of this study was to use a contemporary multi-institutional data set to determine if elevated NLR and PLR were predictive of decreased RFS or OS in a large cohort of patients undergoing curative intent resection for primary retroperitoneal sarcoma (RPS) and truncal sarcomas (TS). We also sought to validate if grade, a known strong prognostic factor in soft tissue sarcomas, could be correlated with NLR and PLR. We hypothesized that preoperative elevations in NLR and PLR would both be independently predictive of decreased RFS and OS and positively correlated with tumor grade.
Methods
Patient population
This retrospective cohort was derived from the US Sarcoma Collaboration database, created from the following tertiary centers: University of Wisconsin, Emory University, Stanford University, Medical College of Wisconsin, Wake Forest University, The Ohio State University, University of Chicago Medicine, and Washington University. A total of 409 patients, including 141 TS and 268 RPSs, from January 2000 to February 2016 were analyzed. Soft tissue extremity tumors were unavailable for analysis and therefore excluded. All recurrent tumors were excluded. All patients analyzed underwent resection with oncologic follow-up recorded in months. Histologic diagnosis was confirmed, and grade was assigned as either low grade (Federation Nationale des Centers de Lutte Contre le Cancer [FNCLCC] = G1), high grade (FNCLCC = G2 or G3), or unable to be assessed (FNCLCC = GX) according to the FNCLCC or TNM classification of malignant tumors two-tier grading schema.2,16
Outcomes of interest
Independent variables of interest included gender, age, race, history of diabetes mellitus (DM), history of coronary artery disease (CAD), smoking status, obesity (body mass index >35), history of prior radiation exposure, identified genetic syndrome, sepsis at the time of surgery, multifocal disease, pathologic margin status, histology identified on surgical pathology, the presence of tumor necrosis in pathologic specimen, tumor grade, the presence of positive nodes, tumor size, neoadjuvant or adjuvant therapy, and preoperative NLR and PLR.
Biomarker definition
The NLR and PLR were derived from preoperative absolute neutrophil, platelet, and lymphocyte counts. Continuous NLR and PLR values were converted to binary outcomes for both RFS and OS through receiver operating characteristic (ROC) curve analysis. Cutoffs were chosen by maximizing the sensitivity and specificity by using the Youden index.17 Through this method, the point at which sensitivity and 1 - specificity is highest is chosen. This graphically corresponds to the top-leftmost point on an ROC curve (Fig. 1). These values are presented with their associated area under the curve (AUC) for the ROC, sensitivity, and specificity. This was separately performed for both RFS and OS for RPS, TS, and the entire cohort.
Fig. 1 -.

Example of ROC curves for NLR against RFS and OS. The red line denotes the midpoint (0.5). Information regarding sensitivity, specificity, and the associated AUC analysis is shown below.
Statistical analysis
All continuous variables were reported as a median value with associated interquartile range (IQR). All categorical variables were reported as a percentage of the total. Univariate Kaplan-Meier analysis was conducted using log-rank analysis testing. Subsequent multivariate testing was conducted using the Cox proportional hazards ratios technique. After univariate analysis, a P value of <0.05 was used for inclusion in the multivariate analysis. All factors with P < 0.05 were considered statistically significant on multivariate analysis and reported with associated hazard ratio and 95% confidence interval (95% CI). Notably, histologic subtype was analyzed in reference to UPS. All data were stored using Excel (Microsoft Corporation, Redmond, WA), and all statistical analyses were performed using SPSS, version 24 (IBM Corporation, Armonk, NY). All institutions obtained institutional review board approval with an approved waiver of consent before beginning any research efforts.
Various sensitivity analyses were then performed to validate our findings. Prior data have shown that inflammatory comorbidities may influence certain inflammatory biomarker levels.18 To control for this, data were analyzed with and without inclusion of inflammatory conditions, including sepsis, DM, CAD, tobacco abuse, radiation exposure, neoadjuvant/adjuvant therapy, tumor necrosis, and obesity. Because the ROC curve analysis showed poor sensitivity and specificity with low AUC for both NLR and PLR, an empiric approach was also examined. We used a cutoff of five, derived from a systematic review cohort of nearly 34,000 patients, including a combined 483 with soft tissue sarcoma, and a multivariate analysis of NLR was conducted to compare outcomes in an a priori fashion, as previously described.19,20 Point biserial analysis compared continuous NLR and PLR values against a categorical grade to determine correlation as defined by Pearson correlation coefficient (r).
Results
Patient and tumoral demographics
A total of 409 patients were analyzed, including 268 RPSs and 141 TSs. The study cohort was 55% females and predominately Caucasian (75%) (Table 1). The median age of the cohort was 56 (IQR, 44.9–68.9) with a median body mass index of 27 (IQR, 23.5–32.2). A small fraction, 2%, of the patients harbored a known genetic mutation, including Li-Fraumeni, neurofibromatosis types 1 and 2, or familial adenomatous polyposis syndrome. Few patients were treated with neoadjuvant (17%) or adjuvant (17%) chemotherapy, radiotherapy, or both.
Table 1 -.
Patient and tumoral characteristics.
| Patient characteristics | N (%) |
|---|---|
| Tumor location | |
| RPS | 268 (66) |
| Truncal sarcoma | 141 (44) |
| Gender | |
| Male | 185 (45) |
| Female | 224 (55) |
| Age | |
| 65 and older | 131 (32) |
| Younger than 65 | 278 (68) |
| Race | |
| Caucasian | 305 (75) |
| African American | 40 (10) |
| Other | 64 (15) |
| Body mass index | |
| ≥35 | 109 (27) |
| <35 | 207 (50) |
| DM | |
| Yes | 63 (15) |
| CAD | |
| Yes | 43 (11) |
| Tobacco abuse | |
| Yes | 56 (14) |
| Prior radiation exposure | |
| Yes | 31 (8) |
| Genetic syndrome | |
| Yes | 8 (2) |
| Sepsis | |
| Yes | 7 (2) |
| Neoadjuvant therapy | |
| Yes | 71 (17) |
| Adjuvant therapy | |
| Yes | 71 (17) |
| Tumoral characteristics | |
| Tumor size >20 cm | |
| Yes | 80 (20) |
| Margin | |
| R0 | 236 (58) |
| R1 | 137 (33) |
| R2 | 32 (8) |
| Grade | |
| Low grade | 78 (19) |
| High grade | 235 (58) |
| Tumor necrosis | |
| Yes | 171 (42) |
| Nodal status | |
| N0 | 391 (96) |
| N1 | 18 (4) |
| Multifocal disease | |
| Yes | 35 (9) |
| Histiotype | |
| Pleomorphic undifferentiated sarcoma | 42 (10) |
| Chondrosarcoma | 7 (2) |
| Leiomyosarcoma | 85 (21) |
| Liposarcoma | 122 (30) |
| Myxofibrosarcoma | 4 (1) |
| MPNST | 10 (2) |
| Primitive neuroectodermal tumor | 5 (2) |
| Rhabdomyosarcoma | 3 (1) |
| Angiosarcoma | 13 (3) |
| Sarcoma not otherwise specified | 17 (4) |
| Small round cell tumor | 3 (1) |
| Synovial sarcoma | 19 (5) |
The most common overall tumor histiotypes included liposarcoma (30%), leiomyosarcoma (21%), and UPS (10%) (Table 1). Median tumor size was 10.5 cm (IQR, 6.2–18) with 20% of the tumors being >20 cm. Most patients had high-grade (58%) tumors as defined by the FNLCC or TNM two-tier grading system with 42% of patients having necrosis on tumor pathology. A small proportion of patients had either nodal positive (4%) or multifocal (9%) disease. Most resections were R0 (58%); however, R1 and R2 resections occurred in 33% and 8% of cases, respectively.
Median follow-up for the cohort was 30.6 mo (IQR, 11.2–63.7). Median RFS as determined by Kaplan-Meier analysis was 29.9 mo (95% CI, 19.4–40.5) (Fig. 2). Median OS as determined by Kaplan-Meier analysis was 60.6 mo (95% CI, 50.2–71.0) (Fig. 2).
Fig. 2 -.
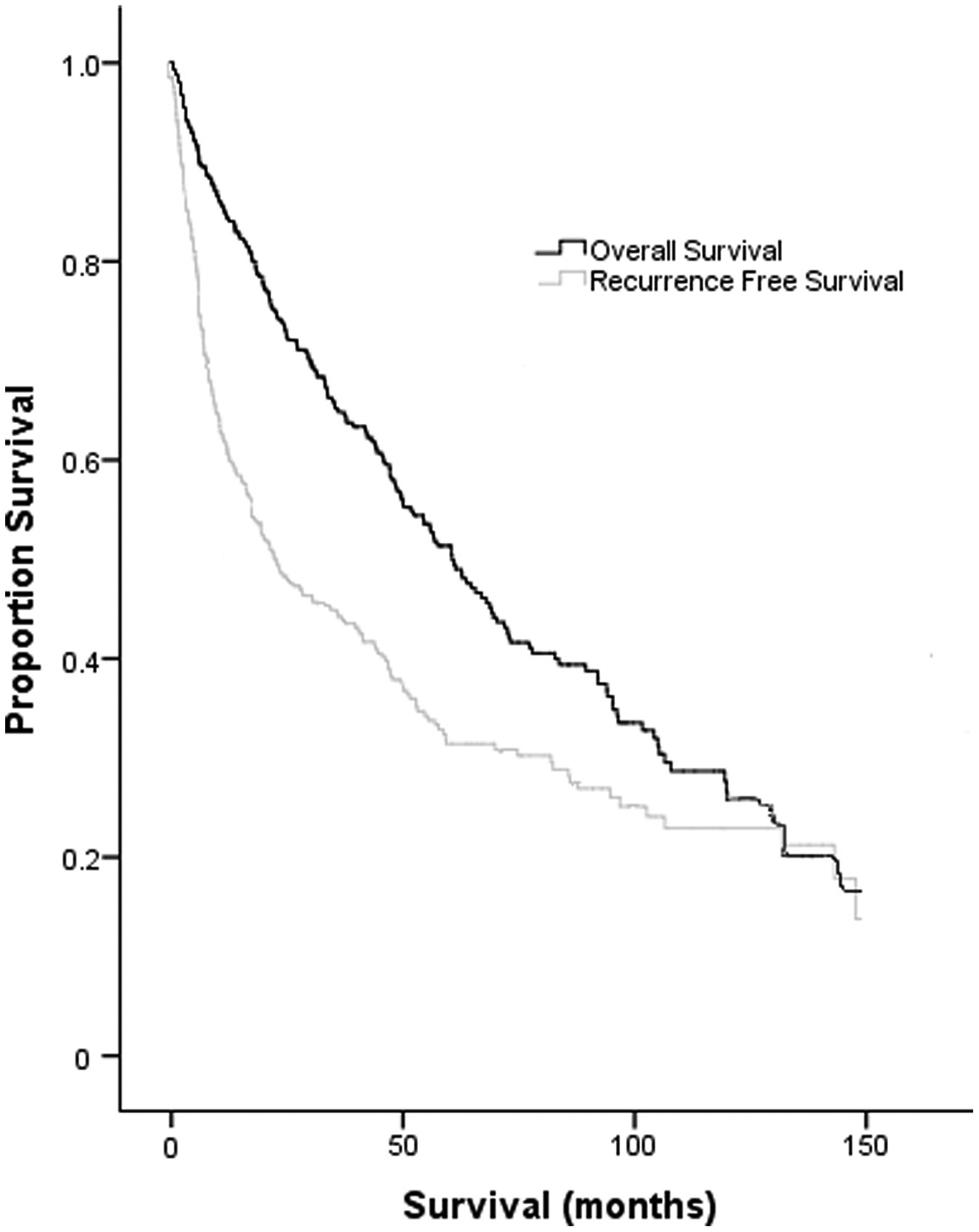
Kaplan-Meier survival curves demonstrating the RFS and OS for the cohort.
Inflammatory biomarker ROC analysis
Binary values for the NLR and PLR ratios were created from continuous data using ROC curve analysis using the Youden index. The analysis was performed for both RFS and OS. Examples of ROC curves for NLR and all tumors are shown in Figure 1. The AUC, sensitivity, specificity, and binary cutoff values are found in Table 2.
Table 2 -.
ROC analysis for RPS, TS, and all patients in the cohort for both NLR and PLR.
| Biomarker | Tumor location | Outcome | Binary cutoff* | AUC | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| NLR | All | RFS | 0.97 | 0.58 | 58 | 54 |
| OS | 1.08 | 0.47 | 49 | 50 | ||
| RPS | RFS | 1.24 | 0.55 | 54 | 53 | |
| OS | 1.31 | 0.55 | 55 | 55 | ||
| TS | RFS | 0.81 | 0.60 | 56 | 57 | |
| OS | 0.84 | 0.37 | 41 | 41 | ||
| PLR | All | RFS | 50.9 | 0.59 | 64 | 51 |
| OS | 65.5 | 0.49 | 54 | 46 | ||
| RPS | RFS | 67.5 | 0.56 | 56 | 54 | |
| OS | 74.2 | 0.56 | 56 | 56 | ||
| TS | RFS | 44.2 | 0.60 | 58 | 57 | |
| OS | 44.5 | 0.41 | 46 | 45 |
Determined by Youden index.
Analysis of predictors--RFS
For all tumors in the cohort, the univariate predictors for RFS on log-rank Kaplan-Meier analysis included male gender, older age, DM, sepsis, multifocal tumors, positive margin status, tumor histiotype, high-grade tumors, tumor necrosis, nodal positivity, adjuvant therapy, large (>20 cm) tumors, and elevated NLR and PLR (Table 3). Multivariate predictors of decreased RFS for all tumors included older age, male gender, sepsis, R1 and R2 margins (as compared with R0), malignant peripheral nerve sheath tumor (MPNST) and angiosarcoma histiotype (as compared with UPS), high-grade tumors, tumor necrosis, and nodal positive disease. Multivariate predictors of longer RFS included elevated NLR.
Table 3 -.
Univariate Kaplan-Meier log-rank analysis and multivariate Cox proportional hazards models for RFS for RPS, TS, and all patients within cohort.
| RFS | RPS | Multivariate HR (95% CI); P | TS | Multivariate HR (95% CI); P | All | Multivariate HR (95% CI); P |
|---|---|---|---|---|---|---|
| Factor | Univariate | Univariate | Univariate | |||
| Female gender | P < 0.01 | 0.59 (0.42–0.84); P < 0.01 | P = 0.76 | P < 0.01 | 0.67 (0.50–0.89); P < 0.01 | |
| Age older than 65 y | P = 0.04 | 1.43 (1.01–2.02); P = 0.04 | P = 0.73 | P < 0.01 | 1.42 (1.05–7.42); P = 0.02 | |
| Race | P = 0.73 | P = 0.58 | P = 0.29 | |||
| DM | P = 0.11 | P = 0.37 | P = 0.03 | 1.11 (0.76–1.63); P = 0.58 | ||
| CAD | P = 0.29 | P = 0.22 | P = 0.36 | |||
| Smoking history | P = 0.66 | P = 0.01 | 1.68 (0.88–3.18); P = 0.12 | P = 0.26 | ||
| Prior radiation | P = 0.28 | P < 0.01 | 0.89 (0.39–2.00); P = 0.77 | P = 0.07 | ||
| Known genetic syndrome | P = 0.94 | P = 0.18 | P = 0.08 | |||
| Sepsis | P < 0.01 | 3.05 (1.24–7.46); P = 0.02 | P = 0.87 | P < 0.01 | 3.28 (1.45–7.42); P < 0.01 | |
| Multifocal | P = 0.04 | P = 0.36 | P < 0.01 | 1.25 (0.81–1.93); P = 0.31 | ||
| Margin | P < 0.01 | P = 0.83 | P < 0.01 | |||
| R0 | Reference | Reference | Reference | |||
| R1 | 1.81 (1.23–2.67); P < 0.01 | 1.85 (1.34–2.55); P < 0.01 | ||||
| R2 | 1.99 (1.05–3.75); P = 0.03 | 2.41 (1.39–4.17); P < 0.01 | ||||
| Histology | P < 0.01 | P < 0.01 | P < 0.01 | |||
| UPS | Reference | Reference | Reference | |||
| Chondrosarcoma | 3.77 (0.80–17.65); P = 0.09 | 0.26 (0.03–2.00); P = 0.20 | 1.48 (0.43–5.15); P = 0.54 | |||
| Leiomyosarcoma | 0.97 (0.51–1.85); P = 0.92 | 0.65 (0.22–1.90); P = 0.43 | 0.92 (0.56–1.50); P = 0.74 | |||
| Liposarcoma | 0.66 (0.344–1.25); P = 0.20 | 0.35 (0.11–1.10); P = 0.07 | 0.73 (0.45–1.19); P = 0.21 | |||
| Myxofibrosarcoma | 0.00 (0.00–>100); P = 0.96 | <0.01 (<0.01–>100); P = 0.97 | 0.00 (0.00–>100); P = 0.95 | |||
| MPNST | 2.10 (0.22–19.95); P = 0.52 | 2.41 (1.00–5.77); P = 0.05 | 3.55 (1.34–9.41): P = 0.01 | |||
| PNET | 0.41 (0.05–3.39); P = 0.41 | 0.45 (0.06–3.42); P = 0.44 | 0.43 (0.10–1.87); P = 0.26 | |||
| Rhabdomyosarcoma | 2.10 (0.24–19.77); P = 0.52 | 1.15 (0.14–9.30); P = 0.90 | 1.21 (0.23–6.40); P = 0.83 | |||
| Angiosarcoma | 12.76 (1.40–>100); P = 0.2 | 1.23 (0.49–3.10); P = 0.67 | 3.28 (1.34–8.06); P = 0.01 | |||
| Sarcoma not otherwise specified | 1.20 (9.46–3.18); P = 0.71 | 0.73 (0.23–2.39); P = 0.62 | 1.21 (0.57–2.59); P = 0.62 | |||
| Small round cell tumors | 8.39 (0.82–85.6); P = 0.07 | 0.71 (0.09–5.42); P = 0.74 | 1.27 (0.27–6.00); P = 0.77 | |||
| Synovial sarcoma | 7.99 (2.86–22.33); P < 0.01 | 0.67 (0.27–1.67); P = 0.39 | 1.20 (0.60–2.40); P = 0.60 | |||
| Other | 0.54 (0.23–1.29); P = 0.17 | 0.24 (0.10–0.58); P < 0.01 | 0.45 (0.23–0.88); P = 0.02 | |||
| Grade––low versus high grade | P < 0.01 | 2.19 (1.39–3.47); P < 0.01 | P = 0.22 | P < 0.01 | 2.27 (1.45–3.57); P < 0.01 | |
| Tumor necrosis | P < 0.01 | 1.36 (0.89–2.09); P = 0.16 | P = 0.62 | P < 0.01 | 1.68 (1.13–2.51); P = 0.01 | |
| Nodal status | P = 0.13 | P = 0.16 | P = 0.01 | 2.18 (1.17–4.06); P = 0.01 | ||
| Neoadjuvant | P = 0.38 | P = 0.61 | P = 0.12 | |||
| Adjuvant | P = 0.67 | P = 0.88 | P < 0.01 | 0.92 (0.63–1.33); P = 0.64 | ||
| Obesity | P = 0.92 | P = 0.09 | P = 0.46 | |||
| Size >20 cm | P = 0.95 | P = 0.06 | P < 0.01 | 1.40 (0.91–2.16); P = 0.12 | ||
| Elevated NLR | P = 0.20 | P = 0.05 | P = 0.02 | 0.64 (0.43–0.97); P = 0.03 | ||
| Elevated PLR | P = 0.06 | P = 0.03 | 1.06 (0.59–1.93); P = 0.84 | P < 0.01 | 1.49 (0.98–2.27); P = 0.06 |
HR = hazard ratio; PNET = primitive neuroectodermal tumor
For RPSs, significant univariate predictors on log-rank Kaplan-Meier analysis for RFS were male gender, older age, sepsis, positive margin status, tumor histiotype, high-tumor grade, and tumor necrosis (Table 3). Significant predictors of decreased RFS on multivariate Cox proportional hazards analysis were older age, male gender, sepsis, R1 and R2 margins (as compared with R0), angiosarcoma and synovial sarcoma histiotype (as compared with UPS), and high-grade tumors.
For TSs, significant univariate predictors for RFS on log-rank Kaplan-Meier analysis included smoking history, history of prior radiation, tumor histiotype, and PLR (Table 3). Significant predictors of decreased RFS for TS on multivariate Cox proportional hazards analysis included only MPNSTs (as compared with UPS).
Analysis of predictors--OS
For all tumors, the univariate predictors for OS on log-rank Kaplan-Meier analysis included older age, DM, prior radiation exposure, the presence of a known genetic syndrome, sepsis, positive margin status, tumor histiotype, high-grade tumor, tumor necrosis, and nodal positivity (Table 4). Multivariate predictors of shorter OS for all tumors included DM, sepsis, R1 and R2 margins (as compared with R0), chondrosarcoma, MPNST, angiosarcoma, and small round cell tumor histiotype (as compared with UPS), high-grade tumors, and nodal positivity.
Table 4 -.
Univariate Kaplan-Meier log-rank analysis and multivariate Cox proportional hazards models for OS for RPS, TS, and all patients within cohort.
| OS | RPS | Multivariate HR (95% CI); P | TS | Multivariate HR (95% CI); P | All | Multivariate HR (95% CI); P |
|---|---|---|---|---|---|---|
| Factor | Univariate | Univariate | Univariate | |||
| Female gender | P = 0.11 | P = 0.27 | P = 0.09 | |||
| Age | P < 0.01 | 1.52 (1.04–2.22); P = 0.03 | P < 0.01 | 0.65 (0.29–1.45); P = 0.29 | P = 0.01 | 1.19 (0.86–1.65); P = 0.29 |
| Race | P = 0.81 | P = 0.27 | P = 0.73 | |||
| DM | P < 0.01 | 1.85 (1.14–2.99); P = 0.01 | P = 0.42 | P < 0.01 | 1.73 (1.56–2.58); P = 0.01 | |
| CAD | P = 0.10 | P = 0.48 | P = 0.08 | |||
| Smoking history | P = 0.40 | P = 0.01 | 2.65 (1.34–5.23); P < 0.01 | P = 0.52 | ||
| Prior radiation | P = 0.33 | P = 0.12 | P = 0.02 | 1.36 (0.79–2.35); P = 0.27 | ||
| Known genetic syndrome | P = 0.12 | P < 0.01 | 0.00 (0.00–>100); P = 0.96 | P = 0.04 | 0.00 (0.00–>100); P = 0.93 | |
| Sepsis | P < 0.01 | 4.99 (1.90–13.1); P < 0.01 | P < 0.01 | 0.00 (0.00–>100); P = 1.00 | P < 0.01 | 5.78 (2.23–14.89); P < 0.01 |
| Multifocal | P = 0.07 | P = 0.21 | P = 0.06 | |||
| Margin | P < 0.01 | P < 0.01 | P = 0.03 | |||
| R0 | Reference | Reference | Reference | |||
| R1 | 1.55 (1.00–2.41); P = 0.05 | 1.31 (0.71–2.41); P = 0.39 | 1.49 (1.06–2.09); P = 0.02 | |||
| R2 | 2.67 (1.45–4.94); P < 0.01 | 2.05 (0.2–21.04); P = 0.55 | 1.90 (1.04–3.50); P = 0.04 | |||
| Histology | P = 0.08 | P < 0.01 | P = 0.03 | |||
| UPS | Reference | Reference | Reference | |||
| Chondrosarcoma | 0.51 (0.14–1.88); P = 0.31 | 4.00 (1.41–11.32); P < 0.01 | ||||
| Leiomyosarcoma | 1.10 (0.37–3.32); P = 0.86 | 1.06 (0.61–1.83); P = 0.85 | ||||
| Liposarcoma | 0.72 (0.29–1.82); P = 0.49 | 0.77 (0.45–1.33); P = 0.35 | ||||
| Myxofibrosarcoma | 2.47 (0.62–9.77); P = 0.20 | 2.61 (0.76–9.01); P = 0.13 | ||||
| MPNST | 3.25 (0.90–11.81); P = 0.07 | 5.01 (1.48–17.00); P = 0.01 | ||||
| PNET | 0.94 (0.20–4.39); P = 0.94 | 0.93 (0.21–4.08); P = 0.93 | ||||
| Rhabdomyosarcoma | 0.00 (0.00–>100); P = 0.99 | 1.15 (0.14–9.60); P = 0.90 | ||||
| Angiosarcoma | 1.82 (0.60–5.54); P = 0.29 | 3.60 (1.30–9.95); P = 0.01 | ||||
| Sarcoma not otherwise specified | 0.78 (0.23–2.64); P = 0.69 | 1.89 (0.84–4.23); P = 0.13 | ||||
| Small round cell tumors | 1.54 (0.26–9.03); P = 0.63 | 4.39 (1.17–16.50); P = 0.03 | ||||
| Synovial sarcoma | 0.61 (0.24–1.55); P = 0.30 | 1.27 (0.61–2.66); P = 0.52 | ||||
| Other | 0.35 (0.15–0.72); P = 0.01 | 1.11 (0.57–2.17); P = 0.76 | ||||
| Grade—low versus high grade | P < 0.01 | 3.25 (1.75–6.05); P < 0.01 | P = 0.22 | P < 0.01 | 2.47 (1.55–3.95); P < 0.01 | |
| Tumor necrosis | P < 0.01 | 1.96 (1.12–3.43); P = 0.02 | P < 0.01 | 1.04 (0.15–0.72); P = 0.90 | P = 0.02 | 1.30 (0.88–1.91); P = 0.18 |
| Nodal status | P < 0.01 | 1.98 (1.01–3.89); P = 0.05 | P = 0.02 | 6.59 (0.82–52.99); P = 0.08 | P = 0.01 | 1.95 (1.03–3.68); P = 0.04 |
| Neoadjuvant | P = 0.89 | P = 0.06 | P = 0.78 | |||
| Adjuvant | P = 0.95 | P < 0.01 | 1.23 (0.54–2.79); P = 0.57 | P = 0.83 | ||
| Obesity | P = 0.58 | P < 0.01 | 0.99 (0.57–1.73); P = 0.62 | P = 0.51 | ||
| Size >20 cm | P = 0.41 | P = 0.89 | P = 0.65 | |||
| Elevated NLR | P = 0.04 | 1.06 (0.61–1.84); P = 0.83 | P = 0.58 | P = 0.23 | ||
| Elevated PLR | P = 0.02 | 1.07 (0.61–1.87); P = 0.82 | P = 0.46 | P = 0.09 |
HR = hazard ratio; PNET = primitive neuroectodermal tumor
For RPSs, significant univariate predictors on log-rank Kaplan-Meier analysis for OS were older age, DM, sepsis, positive margin status, high-tumor grade, tumor necrosis, nodal positivity, NLR, and PLR (Table 4). Significant predictors of decreased OS on multivariate Cox proportional hazards analysis were older age, DM, sepsis, R1 and R2 margins (as compared with R0), high-grade tumors, tumor necrosis, and nodal positivity.
For TSs, significant univariate predictors for OS on log-rank Kaplan-Meier analysis included older age, smoking history, the presence of a known genetic syndrome, sepsis, positive margin status, tumor histiotype, tumor necrosis, nodal positivity, adjuvant therapy, and obesity (Table 4). Significant predictors of decreased OS for TS on multivariate Cox proportional hazards analysis included only smoking history.
Inflammatory subanalysis
Previous data have suggested that NLR and PLR could be accounted for, in part, by inflammatory states (e.g., sepsis, CAD, and others), and not controlling for these conditions could confound the analysis.18 To determine if inflammatory conditions and tumoral properties had any association with NLR and PLR, any statistically significant inflammatory univariate factor was withheld from the subsequent Cox proportional hazards multivariate analysis. Overall, we found that no major change occurred, but for the analysis of all tumors for RFS, we found that where elevated PLR was not independently predictive of decreased RFS, that it was now independently predictive with a hazard ratio of 1.61 (P = 0.02; 95% CI, 1.07–2.43).
A priori subanalysis
Previous data have suggested that a cutoff for NLR of greater than five had better predictive value for both decreased RFS and OS than the ROC curve analysis herein suggested.19 Kaplan-Meier log-rank analysis was therefore applied to this population using this a priori cutoff to determine if this would reveal a stronger association. No significant values were found using log-rank analysis for neither RFS for RPS (P = 0.54), TS (P = 0.34), or all tumors (P = 0.15) nor OS for RPS (P = 0.64), TS (P = 0.75), or all tumors (P = 0.84).
Grade-biomarker correlational analysis
Continuous NLR and PLR values were compared against the categorical tumoral grade. No significant correlations were found between tumoral grade and NLR for RPS (P = 0.07; r = −0.11), TS (P = 0.71; r = 0.03), or all tumors (P = 0.08; r = −0.09). Likewise, no significant correlations were found between tumoral grade and PLR for RPS (P = 0.15; r = − 0.09), TS (P = 0.18; r = 0.11), or all tumors (P = 0.24; r = − 0.06).
Discussion
Recently, a small number of studies have suggested an association between survival outcomes and inflammatory biomarkers, including the NLR and PLR. In this context, this multi-institutional study of 409 RPS and TS patients demonstrated that elevated NLR and PLR were not independently predictive of decreased survival outcomes. Furthermore, no correlation was found between tumoral grade and either inflammatory biomarker.
There are a number of explanations why differences exist between this study and others, including differences in the study population, methodologic considerations, and inclusion of inflammatory comorbid conditions in the multivariate model. For example, in a study by Vasquez et al.13 with 100 children, elevated NLR but not PLR was shown to be independently predictive of decreased OS in both osteosarcoma and rhabdomyosarcoma but not Ewing’s sarcoma. In a study by Kim et al.,21 the entire studied cohort analyzed was women with uterine sarcomas, including carcinosarcoma, leiomyo-sarcoma, and endometrial stromal sarcoma. Finally, in a study by Liang et al.,22 most patients had soft tissue extremity tumors (38%), and neither NLR nor PLR by themselves were predictive of survival outcomes. In this study cohort, most (66%) had RPS, and the histiotype was primarily liposarcoma (30%). Previous research has suggested that tumoral factors, such as tumor grade and histiotype, are the primary drivers of decreased RFS.5,6 These cohort differences may therefore portend a difference in the inflammatory response.
Methodologic differences have varied in the literature on inflammatory biomarkers, and these variations may account for some differences in statistical outcomes. Prior research on NLR, using population data, suggested an optimal binary cutoff of five.19 This was extrapolated to various tumor types in the literature despite only 482 of the 40,559 patients (1.2%) having a soft tissue sarcoma.20,21,23,24,25 When applied in an a priori fashion to our own study, this cutoff was still not predictive of survival outcomes. This may be indicative of the heterogeneity of sarcomas seen between studies.
Prior research has suggested that inflammatory biomarkers can be influenced by comorbid conditions included in multivariate analyses.18,26 The granularity of the data set for this study allowed for inclusion of a number of comorbid conditions. For example, elevated PLR in this study was independently predictive of decreased RFS when inflammatory comorbid conditions were not included in the multivariate model. This effect was eliminated through inclusion of the comorbid inflammatory conditions, including sepsis, CAD, and tobacco abuse among others. This would suggest that the absence of these conditions in other studies may be cause for falsely elevated inflammatory biomarkers.
This study has a number of important limitations to consider. First, the binary values derived from the ROC curves for PLR and NLR showed substantially poor sensitivity and specificity. This may be a product of this specific cohort, however, because prior data sets have shown better predictive value of both NLR and PLR for survival in soft tissue sarcomas.13,27 This limitation was mitigated by also using an a priori cutoff value derived from another retrospective cohort.19 Second, the values used for NLR and PLR were derived from the most recent complete blood count with associated differential before surgical resection. These values can be influenced by a number of different factors, including infection, steroids, and others features not characterized in large data sets. Despite attempts to control for these comorbid conditions, it is likely some patients could have had different values on different days; although, many of these values were most likely routine preoperative laboratory samples. A more standardized cohort of patients would result if a standardized approach to the preoperative timing and patient condition was used. Finally, the retrospective nature of the study lends itself to selection bias. To partially overcome this, all sequential patients who underwent primary resection of their sarcoma were included.
In summary, contrary to the published evidence, neither elevated NLR nor PLR were found to be prognostic for worse survival outcomes in a large multi-institutional cohort of patients from the US Sarcoma Collaboration database comprising both RPS and TS. The use of neither NLR nor PLR is supported by this study for soft tissue sarcomas in these locations. Rather, factors typically regarded as prognostic, such as negative margins and tumoral factors, including low-grade tumors and favorable tumor histology, are more important for prognostication. Future study is needed to determine the role, if any, that NLR and PLR should have in guiding management of patients with soft tissue sarcomas.
Acknowledgment
The authors thank Dr Bret Hanlon, PhD, for his assistance with methodology. This work was supported through the following National Institutes of Health T32 training grant: T32 ES007015.
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
This research was presented as an oral presentation at the American College of Surgeons annual meeting, October 24, 2018.
REFERENCES
- 1.Kneisl JS, Coleman MM, Raut CP. Outcomes in the management of adult soft tissue sarcomas. J Surg Oncol. 2014;110:527–538. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD. WHO classification on tumors. Lyon: IARC Press; 20:10–11. [Google Scholar]
- 3.Brennan MF. Lessons learned from the study of soft tissue sarcoma. Int J Surg. 2013;11(suppl 1):S8–S10. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–421 [discussion 421–422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremjit PJ, Jones RL, Chai X, et al. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann Surg Oncol. 2014;21:2150–2158. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morosi C, Stacchiotti S, Marchiano A, et al. Correlation between radiological assessment and histopathological diagnosis in retroperitoneal tumors: analysis of 291 consecutive patients at a tertiary reference sarcoma center. Eur J Surg Oncol. 2014;40:1662–1670. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez I, Crippa S, Thayer SP, et al. Preoperative platelet count and survival prognosis in resected pancreatic ductal adenocarcinoma. World J Surg. 2008;32:1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez Barcala FJ, Garcia Prim JM, Moldes Rodriguez M, et al. Platelet count: association with prognosis in lung cancer. Med Oncol. 2010;27:357–362. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen M, Kersten C, Sorbye H, et al. Interleukin-6 and Creactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget. 2016;7:75013–75022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panni RZ, Lopez-Aguiar AG, Liu J, et al. Association of preoperative monocyte-to-lymphocyte and neutrophil-to-lymphocyte ratio with recurrence-free and overall survival after resection of pancreatic neuroendocrine tumors (USNETSG). J Surg Oncol. 2019;120:632–638. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez L, Leon E, Beltran B, Maza I, Oscanoa M, Geronimo J. Pretreatment neutrophil-to-lymphocyte ratio and lymphocyte recovery: independent prognostic factors for survival in pediatric sarcomas. J Pediatr Hematol Oncol. 2017;39:538–546. [DOI] [PubMed] [Google Scholar]
- 14.Szkandera J, Absenger G, Liegl-Atzwanger B, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer. 2013;108:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135:362–370. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Liu T. Does the derived neutrophil/lymphocyte ratio predict clinical outcome in soft tissue sarcoma patients? Am J Surg. 2015;210:962. [DOI] [PubMed] [Google Scholar]
- 19.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 20.Idowu OK, Ding Q, Taktak AF, Chandrasekar CR, Yin Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers. 2012;17:539–544. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Han KH, Chung HH, et al. Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. Eur J Surg Oncol. 2010;36:691–698. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Wang W, Li J, et al. Combined use of the neutrophill-ymphocyte and platelet-lymphocyte ratios as a prognostic predictor in patients with operable soft tissue sarcoma. J Cancer. 2018;9:2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. [DOI] [PubMed] [Google Scholar]
- 24.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. [DOI] [PubMed] [Google Scholar]
- 25.Idowu OK, Ding Q, Taktak AF, Chandrasekar CR, Yin Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers. 2012;17:539–544. [DOI] [PubMed] [Google Scholar]
- 26.Balta S, Demirkol S, Sarlak H, Kurt O. Comment on ‘Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients’: neutrophil to lymphocyte ratio may be predictor of mortality in patients with soft-tissue sarcoma. Br J Cancer. 2013;108:2625–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Que Y, Qiu H, Li Y, et al. Preoperative platelet- lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer. 2015;15:648. [DOI] [PMC free article] [PubMed] [Google Scholar]


