Abstract
Simple Summary
Nowadays, the number of malignancies diagnosed during pregnancy is increasing. Despite the fact that diagnosis is occurring on a global scale, their number is still too limited to prepare proper standards of treatment. These problems appear specifically in the least developed countries. The aim of our review is to bring ovarian cancer (OC) as a complication of pregnancy to the attention of doctors and other medical professionals who have to cope with these rare cases. We noted that a variety of malignancies can be included under the heading “ovarian cancer”, and we describe obstetric and patient outcomes, which depend on the histopathology of the tumor. We focus on the current recommendations for diagnostics and treatment, and present future possibilities for the management of OC.
Abstract
The frequency of concomitant adnexal tumors in pregnancy is reported to be at 0.15–5.7%, while ovarian cancer complicates 1 in 15,000 to 1 in 32,000 pregnancies, being the second most common gynecologic cancer diagnosed during pregnancy. The aim of this review is to discuss the problem of ovarian cancer complicating pregnancy and the current recommendations for diagnostics and treatment, with an emphasis on the risk to the fetus. A detailed analysis of the literature found in the PubMed and MEDLINE databases using the keywords “ovarian cancer”, “ovarian malignancy”, “adnexal masses”, “ovarian tumor” and “pregnancy” was performed. There were no studies on a large series of pregnant women treated for ovarian malignancies and the management has not been well established. The diagnostics and therapeutic procedures need to be individualized with respect to the histopathology of the tumor, its progression, the gestational age at the time of diagnosis and the mother’s decisions regarding pregnancy preservation. The multidisciplinary cooperation of specialists in perinatal medicine, gynecological oncology, chemotherapy, neonatology and psychology seems crucial in order to obtain the best possible maternal and neonatal outcomes.
Keywords: adnexal masses, management in pregnancy, ovarian cancer, ovarian malignancy, ovarian tumor, pregnancy
1. Introduction
Because of the delay due to planning pregnancy at a later reproductive age and the fact that the frequency of the incidence of numerous neoplasms increases in the fourth decade of life, the number of pregnant women affected by cancer is rising [1,2]. This challenging problem complicates 1 in 1000 pregnancies and is becoming more common nowadays [3,4,5]. The frequency of adnexal tumors in pregnancy is reported to be 0.15–5.7%, with most being benign [6]. The transformation of benign ovarian tumors such as dermoid cysts into ovarian cancer (OC) is very rare and just four cases have been reported in the literature devoted to this subject [7,8,9,10]. Ovarian cancer is the second most common gynecologic cancer diagnosed during pregnancy, complicating 1 in 15,000 to 1 in 32,000 pregnancies [6,11,12]. OC represents 3–6% of neoplasms and 49–75% of ovarian malignancies in pregnancy [1,13,14]. According to the statistics, ovarian cancer takes the fifth position among the most common malignancies affecting pregnant women, following breast cancer, thyroid cancer, cervical cancer and Hodgkin lymphoma [15]. However, one of the reviews reported that in the Asian population, ovarian cancer takes the sixth position [16].
The morbidity and mortality rates of ovarian cancer have fallen in recent years due to an increased reliance on oral contraceptives, but it still takes seventh place among the most common malignancies in women worldwide and third place for the most common gynecological cancer [17]. In 2018, 240,000 new cases were diagnosed, resulting in a rate of incidence of 10–15 per 100,000, with the highest mortality rate among female cancers. It is the fifth leading cause of death in women worldwide [18]. The 5-year survival rates depend on the advancement of cancer and vary from 92% in Stage I to 30% in Stage IV. The term “ovarian cancer” refers to a neoplasm that can originate in the ovary, fallopian tube, peritoneum and other tissues of the pelvis, where the site cannot be assigned to one of the aforementioned ones [19]. A variety of malignancies such as epithelial ovarian cancers (EOCs), including borderline tumors, germ cell tumors and sex cord tumors, might also be included under the umbrella of “ovarian cancer”.
EOCs encompass the vast majority of ovarian cancers of non-pregnant women (approximately 90%), but it is responsible for fewer cases of OC during pregnancy [20] (Table 1). Much more common are borderline, germ cell and sex cord tumors, which appear to be chiefly responsible for the increased prevalence during reproductive age [21]. Borderline tumors are neoplasms of low malignant potential, but in pregnant women, they present a higher incidence of more aggressive histologic features, such as microinvasion [22]. The two most common germ cell tumors in pregnancy are dysgerminoma and yolk-sac tumor [21]. Five subtypes of epithelial ovarian cancers can be distinguished: high-grade serous (70%), low-grade serous (<5%), endometrioid (10%), clear cell (10%) and mucinous (3%) ovarian cancer. An alternative classification of EOC categorizes it into two main groups: Type I and Type II, with Type I being less common (30%) and diagnosed in earlier stages [20]. Most ovarian cancers during pregnancy present a good prognosis, due to early diagnosis in the first stage of the disease [23]. The 5-year survival rate for ovarian tumors complicating pregnancy is estimated to be 72–90% [24].
Table 1.
Frequency of ovarian cancer in pregnancy by tumor histology in the literature.
| Author and Year | |||||
|---|---|---|---|---|---|
| Histopathology | Copeland, 1996 [25] | Zanotti, 2000 [26] | Zhao, 2006 [24] | Behtash, 2008 [27] | Morikawa, 2014 [25] |
| EOC | 37.5% | 33–34% | 50% | 39.1% | 81% |
| Invasive EOC | - | - | 22.7% | 21.7% | 20% |
| LMP | - | - | 27.3% | 17.4% | 61% |
| Germ cell tumor | 45% | 30–33% | 40.9% | 47.8% | 17% |
| SCT | 10% | 17–20% | 9.1% | 13% | 2% |
| Others | 7.5% | 12–13% | 0 | 0 | 0 |
EOC, epithelial ovarian cancer; LMP, low malignant potential tumor; SCT, sex cord stromal tumor.
Aim
The aim of this review was to present the problem of ovarian cancer complicating pregnancy and the current recommendations for diagnostics and treatment, with an emphasis on the risk to the fetus. This review also presents future possibilities for OC management.
2. Material and Methods
A literature search in the electronic databases PubMed and MEDLINE was performed. We focused on pregnancy complicated by ovarian cancer. A detailed analysis of eligible publications in the literature using MESH terms such as “ovarian cancer”, “ovarian malignancy”, “adnexal masses”, “ovarian tumor” and “pregnancy” as keywords was conducted. Only publications in English were considered. The references included in these selected publications were also taken into account with the aim of finding additional relevant articles. We analyzed the following types of articles: case reports, clinical trials, population-based studies, reviews, systematic reviews and meta-analyses, due to the rarity of the discussed problem.
3. Results
A computerized literature search in the electronic databases PubMed and MEDLINE was performed to identify articles on ovarian cancer and pregnancy using the keywords “ovarian cancer”, “ovarian malignancy”, “adnexal masse”, “ovarian tumor” and “pregnancy”. Only publications in English were considered. Since the discussed problem is extremely rare, the following types of articles were analyzed: case reports, clinical trials, population-based studies, reviews, systematic reviews and meta-analyses. We initially identified a total of 1319 papers. Next, the duplicates were removed and 551 articles were left for our assessment, of which 414 articles were excluded based on the titles and abstracts, leaving 137. After reading the full-text articles, 81 papers were excluded from the analysis because of incomplete case reporting or the inability to assign individual diagnostics, clinicopathological data, treatment regimens or outcomes. We also excluded articles devoted to only the technical aspects of surgical management and detailed histopathological analyses. The final results of the review of 56 articles are presented in five sections: diagnostics, management, obstetric outcomes, patient outcomes and perspectives.
3.1. Diagnostics
Diagnostics start with anamnesis and clinical examination, which might be much more difficult during pregnancy but can still be performed without any limitations. The presenting symptoms of ovarian cancer in pregnant women are the same as in non-pregnant women but can be overlooked as being related to ovarian cancer because they overlap and are therefore attributed as being symptoms that result from the physiological changes during pregnancy. Fatigue, anemia, nausea, vomiting, increased abdominal circumference, constipation, shortness of breath or urinary symptoms can be confounded with pregnancy, which results in delayed diagnosis [28,29]. However, during pregnancy, emergency abdominal events such as torsion of an ovarian mass, rupture of an ovarian mass or intraperitoneal hemorrhage are common [30]. In some pregnant women, a suspicious adnexal mass, a cul-de-sac mass or a nodularity can be found during an antenatal physical examination.
Although blood examinations are not limited, some of them should be considered with caution, such as those regarding tumor markers, the levels of which could be elevated during gestation. It is particularly important to take into account the fact that levels of cancer antigen 125 (CA 125) and human chorionic gonadotropin (hCG) in blood are typically increased during gestation, especially in the first trimester. Increased levels of inhibin B, lactate dehydrogenase (LDH), human epididymis protein 4 (HE4), cancer antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA) and anti-Müllerian hormone (AMH) can be useful in the diagnosis of ovarian cancer as a pregnancy complication, since their levels are expected to be normal during gestation [23,29,31]. Unexplained high levels of alpha-fetoprotein (AFP) and inhibin A during antenatal screening can be confused with indications of neural tube defects or Down’s syndrome and may also be the first signs of the presence of adnexal masses [29].
Different types of imaging tests during pregnancy may be considered, e.g., ultrasonography (USG), magnetic resonance imaging (MRI) and computed tomography (CT). USG and MRI do not include ionizing radiation and are not harmful to the embryo or fetus. Prenatal USG as a routine procedure contributes to the increased number of diagnosed asymptomatic ovarian masses [12]. The most common model used to evaluate pelvic masses in non-pregnant women is the International Ovarian Tumor Analysis (IOTA) Group Simple Rules [32] (Table 2), though the usefulness of this scoring system has not yet been recognized in pregnant women. When the ovarian mass is too big to access or the ultrasonography diagnosis is inconclusive, or there exists an increased risk of malignancy, further imaging examination is needed. The most optimal second-line imaging in pregnant women is MRI, which is highly accurate in examining the characteristics of complex or indeterminate ovarian masses, or in presurgical evaluation of the extent of disease, peritoneal dissemination and nodal metastases [33,34]. The Assessment of Different NEoplasias in the adneXa magnetic resonance (ADNEX MR) scoring system allows the detection of cancer with an overall accuracy higher than 80% [35] (Table 3). Pineapple juice can be used as a negative contrast for MRI; indeed, it is useful in the investigation of peritoneal/intra-abdominal lesions and adhesions, especially in evaluating ovarian cancer. Additionally, pineapple juice is safe for the mother and child, which is crucial for pregnant women [36]. Computed tomography (CT) is not recommended during pregnancy because it could be related to potential teratogenic effects. Despite the fact that the radiation dose absorbed by the fetus is lower than the dose needed to damage the baby, the scholastic effect of ionizing radiation may occur in intensely dividing fetal cells, which can lead to the development of a neoplasm in childhood [37,38].
Table 2.
Adnexal masses in pregnancy: benign versus malignant features (International Ovarian Tumor Analysis (IOTA) Group Simple Rules) [32].
| B-Features | M-Features |
|---|---|
| B1- Unilocular | M1- Irregular solid tumor |
| B2- Presence of solid components with a largest diameter of <7 mm | M2- Presence of ascites |
| B3- Presence of acoustic shadows | M3- At least 4 papillary structures |
| B4- Smooth multilocular tumor with a largest diameter of <100 mm | M4- Irregular multilocular-solid tumor with a largest diameter of ≥100 mm |
| B5- No blood flow (color score 1) | M5- Very strong blood flow (color score 4) |
Table 3.
ADNEX MR scoring system [39].
| Score 1 | No mass visible in MRI |
| Score 2 | Purely cystic ovarian mass Purely endometriotic ovarian mass Purely fatty ovarian mass Ovarian mass without wall enhancement Low b = 1000 sec/mm2 –weighted and low T2-weighted signal intensity within solid tissue |
| Score 3 | Mass without solid tissue Curve type 1 within solid tissue |
| Score 4 | Curve type 2 within solid tissue |
| Score 5 | Peritoneal implants Curve type 3 within solid tissue |
| Score 1 to 3- benign or probably benign; Score 4- indeterminate Score 5- probably malignant | |
3.2. Management
The current management of ovarian cancer involves surgery, chemotherapy and radiotherapy. There are no definitive guidelines in the literature regarding the management of ovarian cancer in pregnancy. Earlier publications recommended that pregnant women with ovarian cancer should be treated in the same way as non-pregnant women with immediate laparotomy, regardless of the duration of pregnancy and the condition of the mother and child [40,41,42,43,44,45]. Nowadays, the management depends on a few factors such as the duration of pregnancy, the general condition of the mother and fetus, and the mother’s desire to continue the pregnancy.
Surgery seems to be the least controversial type of oncologic management in pregnancy, as there have been no adverse effects reported for surgical treatment for non-oncological cases. Non- obstetrical surgery during pregnancy is performed in 1–4 out of 200 cases. Ovarian cancer surgery during gestation requires special attention, as for abdominal approaches. Two things seem important: optimal surgical outcomes and the safety of the mother and fetus [46]. A very careful decision must be made with regard to performing an operation in adequate time, obviously not too early (because of the risk of loss of luteal function by the ovary before the fourth month of gestation and miscarriage) and not too late (progression of malignancy, preterm labor, ovarian torsion, rupture or bleeding). An adnexal tumor found incidentally during cesarean section should be removed [47,48]. The general ovarian tumor consensus management is that surgery is needed when the adnexal mass is:
Larger than 10 cm in diameter;
Persists into the second trimester; or
Presents solid or mixed cystic and solid, highly suspicious characteristics during ultrasound [11,47,49,50,51].
The optimal time for surgical treatment during pregnancy is early in the second trimester (between 16 and 20 weeks of gestation). There are numerous reasons for this:
Organogenesis is complete, which minimizes the risk of teratogenesis induced by medications;
The placenta replaces the hormonal function of the corpus luteum and resection does not affect progesterone concentration;
Low risk of pregnancy loss related to second trimester surgery;
By this time, almost all functional cysts will have been resolved;
Spontaneous miscarriages connected with fetal abnormalities are likely to have already occurred and will not be mistakenly attributed to the surgical treatment [52].
If surgery in a pregnant woman is required before 14 weeks of gestation, when the placenta becomes capable of producing hormones in sufficient levels, progesterone should be administered in doses of 60–120 mg i.m./day or 300–600 mg vag. or p.o./day [53].
Laparoscopy is feasible during pregnancy but depends on the following rules:
The operator has proper experience in performing laparoscopy during pregnancy;
The optimal time for laparoscopy, which is 16–20 weeks of gestation;
Trocars’ localization, which depends on gestational age; the first should be inserted at least 3–4 cm above the fundus of the uterus;
Must be no longer than 90–120 min;
Low abdominal pressure: 10 and 13 mm Hg;
The preferred technique is open introduction without a Veress needle [54,55,56,57].
A comparison between laparoscopy and laparotomy in pregnant women has recently been published. It was found that laparoscopy is associated with shorter hospital stays, shorter operative times and fewer adverse effects for the fetus [58]. In addition to this, pregnant patients undergoing laparotomy for an adnexal tumor have more frequent preterm contractions than patients undergoing laparoscopic surgery [59]. We have to bear in mind that laparoscopy may cause perforation of the uterus, hypercapnia and reduced blood flow due to increased abdominal pressure and use of carbon dioxide [55,56,57].
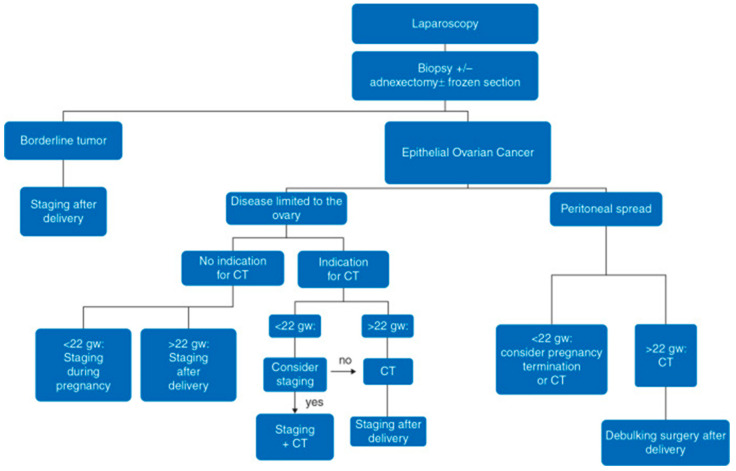
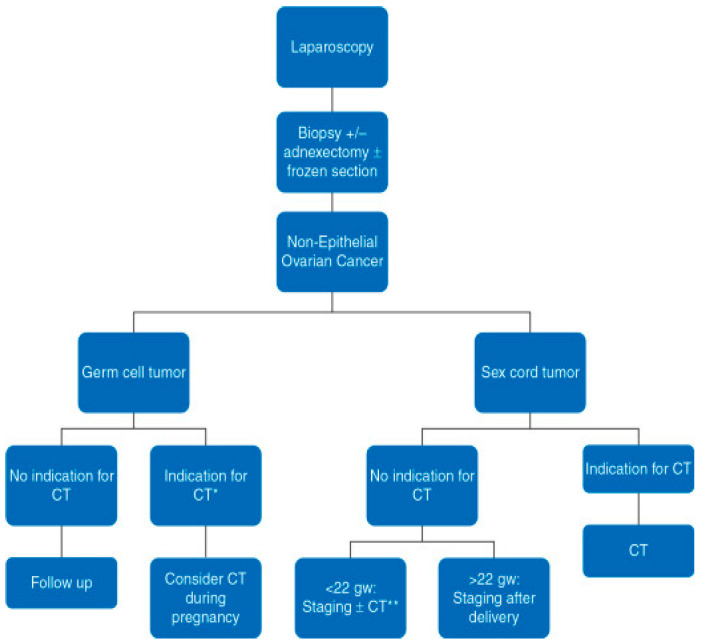
Complete surgical resection of the neoplasm is preferred over aspiration and cytologic evaluation. In most cases, there will be an inappropriate incision for surgical staging, so a frozen section during operation is needed to decide about the scope of the operation and later management [53,60] (Figure 1 and Figure 2). The staging procedure during pregnancy can include appendectomy, infracolic omentectomy, pelvic peritoneal biopsies or lymph node dissection. There is a general recommendation that restaging should be planned postpartum if the pouch of Douglas and the pelvic peritoneum cannot be reliably examined during the operation in pregnant women. Based on the opinion of experts, a proper gynecological surgical assessment may be proposed at approximately 22 weeks of gestation. If a low progression to invasive cancer is diagnosed during the second or third trimester in an adnexal mass with a low malignant potential, the surgery can be postponed until postpartum. If the diagnosis of advanced epithelial ovarian cancer is made in the first part of pregnancy, termination of the pregnancy should be considered and discussed with the patient. If the pregnant woman decides on the preservation of pregnancy, a biopsy or adnexectomy with subsequent platinum-based chemotherapy should be recommended. In cases such as this, debulking (cytoreductive) surgery should be planned after delivery, since this type of surgery cannot be performed during pregnancy [53] (Figure 1 and Figure 2).
Figure 1.
Scheme for the management of epithelial ovarian cancer tumors. According to ESMO guidelines. Staging refers to surgical staging. CT, chemotherapy; gw, gestational weeks [53].
Figure 2.
Scheme for the management of nonepithelial ovarian cancer tumors. Staging refers to surgical staging. CT, chemotherapy; gw, gestational weeks. * According to ESMO guidelines; ** CT administered according to restaging surgery findings (Reprinted with permission from Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. Copyright Elsevier 2020) [53].
Chemotherapy (CT) is the basis for adjuvant and neoadjuvant therapy of OC but it is linked with a number of problems that are not observed in non-pregnant women, such as spontaneous abortion, congenital abnormalities, or hypotrophy [6]. The highest risk (10–20%) for congenital malformations is noted between 4 and 10 weeks of gestation, so chemotherapy is recommended from the second trimester onward, when fetal organogenesis is complete [61,62]. Beyond 35 weeks of gestation, CT is not recommended, as the three-week window between the last course of chemotherapy and delivery is needed for the recovery of both fetal and maternal bone marrow, which should decrease the possibility of bleeding, infection or anemia with avoidance drug accumulation in the fetus. This is especially important for preterm babies, who do not have the enzymes to metabolize CT adequately. Most chemotherapeutics involve relatively small molecules that might cross the placenta [63]. The administration of chemotherapy is dependent on the histological examination. In the case of borderline tumors, there are no indications for adjuvant therapy [33,53]. Chemotherapy is also not recommended in epithelial OC Stage IAG1, dysgerminoma Stage I, immature teratoma Stage IG1, or folliculoma Stages IA and IB with no adverse risk factors [54,64]. In epithelial ovarian cancer, chemotherapy consists of the administration of paclitaxel or docetaxel and carboplatin or cisplatin. Germ cell tumors and stromal cell tumors are treated with paclitaxel and carboplatin (first-line treatment) or with a bleomycin, viblastine and cisplatin (BVP) or cisplatin, etoposide, bleomycin (PEB) regimen (second-line treatment). First-line treatment with paclitaxel and carboplatin is preferred, as it has the most favorable safety prolife for the fetus and these drugs have proven effectiveness [65,66] (Table 4).
Table 4.
| Drug | Fetal Abnormalities | Comments |
|---|---|---|
| Cisplatin | Impaired development, hypoacusia, neutropenia, ventriculomegaly, hair loss | - |
| Carboplatin | None | - |
| Paclitaxel | Myelosuppression, pyrolic stenosis | Single cases |
| Etoposide | Pancytopenia, hypoacusia, secondary leukemias | Particular fear of secondary tumors |
| Bleomycin | Syndactyly | - |
| Vinblastine | Syndactyly, plagiocephaly | Safer than etoposide |
Targeted therapy, which is used in OC cases in non-pregnant women, should also be discussed; the data are limited to case reports and case series in humans [67,68]. Two main questions need to be answered: (1) How might the targeted therapies influence fetal development? (2) How do we cope with such a great number of different drugs with different pharmacological properties? Small molecules such as poly(ADP-ribose), polymerase (PARP) inhibitors, or tyrosine kinase inhibitors (TKIs) have the capacity to cross through the placenta throughout the entire pregnancy. Large molecules (for example, monoclonal antibodies, which require active transport through the placenta) can reach the fetus after 14 weeks of gestation [53]. Research on animals demonstrated their potential embryotoxicity and risk of adverse fetal outcomes [69]. Angiogenesis inhibitors such as bevacizumab, a humanized anti-vascular endothelial growth factor (VEGF) antibody, are teratogenic and induce intrauterine growth restriction (IUGR), pregnancy loss and skeletal malformation in animal models (mice and rats). As is well known, angiogenesis is crucial for the development of the fetus and the placenta, so bevacizumab and other antiangiogenic drugs are definitely contraindicated in pregnancy. There are no cases of the systemic administration of PARP inhibitors, other targeted agents or immunotherapy during pregnancy in humans with ovarian cancer or other gynecological malignancies. Due to limited evidence for the clinical use and safety of targeted therapies, it is recommended to postpone their use until after delivery [53].
Radiotherapy (RT) denotes the use of high-energy radiation from X-rays, gamma rays, neutrons, protons, and other sources to kill cancer cells and to restrict tumors. The influence of RT on pregnancy is generally recognized and might involve fetal death, fetal abnormalities and growth disturbances, and may lead to carcinogenesis during pregnancy or in childhood. These effects depend on the gestational age during exposure and radiation dose [70,71]. There is no place for radiotherapy during pregnancy for gynecologic cancers, unless embryonic or fetal death is considered unavoidable [37,72].
3.3. Obstetric Outcomes
All pregnant women with ovarian cancer have high-risk pregnancies and need a specially dedicated and well-equipped perinatal and oncological center. At the same time, the standard screening, diagnostics for chromosomal and structural defects or other pregnancy complications cannot be neglected. Ultrasound fetal examination with the assessment of growing intervals, amniotic fluid index (AFI), cervical length and Doppler exam of blood flows in the middle cerebral artery (MCA) and umbilical artery (UA) seems obligatory [73]. The assessment of the peak systolic velocity (PSV) in the MCA is of great clinical value in the care of high-risk pregnancies for the diagnosis and management of fetal anemia and intrauterine growth restriction (IUGR) [74]. Supplementation of folic acid and nutritional counseling is also essential to optimize maternal and fetal status. Pregnant patients with surgical treatment have better obstetric outcomes than women with CT or surgery + CT. There are fewer cases of admission to neonatal intensive care units (NICUs), IUGR, preterm premature rupture of the membranes (pPROM), preterm contractions or neonates being small for their gestational age [73]. If possible, the patient should not be delivered before 37 weeks, which can help to avoid neonatal morbidities related to prematurity. When preterm delivery is inevitable, the administration of steroids should be considered [53]. After delivery, the placenta should also be examined histopathologically for metastases; some gynecological cancers are exceptionally related to metastases in the child and placenta [75,76,77,78].
3.4. Patient Outcomes
In a descriptive cohort study, 1170 pregnant women with cancer were reported by de Haan et al. with results from over 20 years of research; 37 centers from 16 countries participated in the International Network on Cancer, Infertility and Pregnancy (INCIP). The researchers registered and analyzed data on obstetric, maternal, neonatal and oncological outcomes. In most cases (893 patients), the diagnosed cancer was in local or regional advancement. In this report, ovarian cancer was diagnosed in 7% of cases (88 patients), which corresponds to the fourth position among all types of cancer concomitant with pregnancy. The increased frequency of OC can be observed with respect to previous statistics. No women in that study died due to ovarian cancer during pregnancy [73]. Lee et al. analyzed the most common malignancies associated with pregnancy in Australia. The researchers defined them as cancers with an initial diagnosis made during pregnancy or in a period of 12 months after delivery. These data were collected in New South Wales between 1994 and 2008, and cancer was diagnosed in 1798 patients (499 during pregnancy and 1299 postpartum) being, in most cases, in local or regional advancement. The most common malignancy was melanoma (33.3%) and ovarian cancer took the seventh position (2.6% of all cases). The researchers did not specify information about OC [79]. Similar results were presented by Parazzini et al. The researchers diagnosed 45 ovarian cancer cases among 1475 of all cancers cases in 1,200,263 pregnancies between 2001 and 2012 (eighth position in the ranking of pregnancy-associated cancers), resulting in a risk of pregnancy-associated cancer of 3.7 per 100,000 pregnancies [80]. Smith et al. analyzed 4,846,505 deliveries between 1991 and 1999 in California; they identified 4539 malignancies with 253 cases of ovarian cancer. The highest mortality rate was when the diagnosis of cancer was made between 0 and 3 months before delivery [3]. A clinicopathological analysis of 23 cases of ovarian cancer associated with pregnancy was performed by Behtash and colleagues, in which the patients were treated between 1991 and 2002 at Vali-Asr Hospital. Seventeen pregnant women were diagnosed in Stage I; chemotherapy was administered to 44% of these cases; five patients relapsed and died [27]. In another study, Morikawa et al. analyzed 41 cases of malignant ovarian tumors during pregnancy between 1985 and 2010 in a retrospective study; the researchers focused on pathology-oriented treatment. Thirty-eight pregnant women were diagnosed in Stage I and 12 patients underwent chemotherapy; one of them died due to ovarian cancer [81]. Zhao et al. summarized patients’ experiences of ovarian cancer diagnosed in pregnancy between 1985 and 2003 at Peking Union Medical College Hospital. Twenty-two patients with ovarian malignancy were treated; 16 pregnant women were in Stage I of OC and achieved complete remission, while four of the five patients in an advanced stage died [24]. Only data from these and a few other studies, which are presented in Table 5, satisfactorily described patient outcomes.
Table 5.
Ovarian cancer in pregnancy in the literature and patient outcomes.
| Study | Year of Publication | Country | Years | Number of Cases of Ovarian Malignancy | Type of Malignancy (Number) | Stage of Malignancy (Number of Cases) | Relapses (Number) | Deaths (Number) |
|---|---|---|---|---|---|---|---|---|
| Cottreau et al. [82] | 2019 | US, 5 states | 2001–2013 | 44 | ovarian cancer | no data | no data | incomplete data |
| de Haan et al. [73] | 2018 | Europe, 16 countries | 1996–2016 | 88 | ovarian cancer | stage I—66 cases stage II—4 cases stage III—7 cases stage IV—2 cases unknown—9 cases |
no data | 0 during pregnancy |
| Parazzini et al. [80] | 2017 | Italy, Lombardia | 2001–2012 | 45 | ovarian cancer | no data | no data | incomplete data |
| Shim et al. [16] | 2016 | South Korea | 1995–2013 | 5 | ovarian cancer: EOC (4), dysgerminoma (1) | stage I—5 cases | 0 | 1 |
| Zhao et al. [24] | 2016 | China | 1985–2003 | 22 | ovarian cancer: germ cell tumor (9), EOC (5), sex cord stromal tumor (2) + LMP tumor (5) | stage I—16 cases, stage II—1 case, stage III—3 cases, stage IV—2 cases | 4 | 4 |
| Andersson et al. [83] | 2015 | Sweden | 1963–2007 | 175 | ovarian cancer | no data | no data | incomplete data |
| Nazer et al. [84] | 2015 | US | 2003–2011 | 180 | ovarian cancer (93) + low malignant potential tumor (87) | incomplete data | no data | no data |
| Morikawa et al. [81] | 2014 | Japan | 1985–2010 | 41 | ovarian cancer: borderline tumor (25), EOC (8), germ cell tumor (7), sex cord stromal tumor (1) | stage I—38 cases, stage II—1 case, stage III—1 case, stage IV—1 case | incomplete data | incomplete data |
| Eibye et al. [85] | 2013 | Denmark | 1977–2006 | 74 | ovarian cancer | incomplete data | no data | no data |
| Lee et al. [79] | 2012 | Australia, New South Wales | 1994–2008 | 47 | ovarian cancer | incomplete data | no data | incomplete data |
| Fauvet et al. [22] | 2011 | France, 6 centers | 1997–2009 | 40 | ovarian cancer: borderline tumor (40) | stage I—35 cases, stage II—2 cases, stage III—2 cases, staging not available—1 case | 3 | 0 |
| Kwon et al. [86] | 2010 | South Korea | 1996–2006 | 27 | ovarian cancer: borderline tumor (15), EOC (7), germ cell tumor (5) | no data | 1 | 0 |
| Van Calsteren et al. [1] | 2010 | Europe: Belgium, The Netherlands, Czech Republic | 1998–2008 | 4 | ovarian cancer | no data | incomplete data | no data |
| Stensheim et al. [87] | 2009 | Norway | 1967–2002 | 53 | ovarian cancer | incomplete data | no data | 11 |
| Behtash et al. [27] | 2008 | Iran | 1991–2002 | 23 | ovarian cancer: EOC (5), germ cell tumor (11), sex cord stromal tumor (3)+ LMP (5) | stage I—17 cases, stage II—1 case, stage III—3 cases, stage IV—1 case | 5 | 5 |
| Leiserowitz et al. [11] | 2006 | US, California | 1991–1999 | 202 | ovarian cancer: EOC (52), germ cell tumor (34), sex cord stromal tumor (1) + low malignant potential tumor (115) | incomplete data | no data | no data |
| Smith et al. [3] | 2003 | US, California | 1991–1999 | 253 | ovarian cancer | no data | no data | incomplete data |
| Matsuyama et al. [41] |
1989 | Japan | 1978–1986 | 6 | ovarian cancer: EOC (4), immature teratoma (1), metastatic cancer of colon origin (1) | stage I—4 cases, stage III 1 case, stage IV—1 case | 1 | 1 |
| Haas [68] | 1984 | GDR | 1970–1979 | 20 | ovarian cancer | incomplete data | no data | no data |
EOC- epithelial ovarian cancer; GDR- German Democratic Republic; US- United States.
3.5. Perspectives
Hallum et al. conducted a prospective case–cohort study in which 700 women from the Danish Diet, Cancer and Health cohort participated. The researchers looked for a correlation between microchimerism and ovarian cancer by analyzing women’s blood samples and their responses to questionnaires. In the blood samples, they looked for the presence of a Y chromosome as a marker of male microchimerism. Samples were positive in 65.9% of the control group and in 46% of the ovarian cancer group. The results of this study suggest that male microchimerism reduces the hazard rate of ovarian cancer (HR = 0.44, 95% CI: 0.29–0.68), but the underlying mechanisms are currently unknown [88].
A novel idea for OC treatment is viral-based cancer therapy with ofranergene obadenovec (VB-111). VB-111 I is an adenoviral vector which carries the tumor necrosis factor receptor 1-Fas cell surface (TNFR1-FAS), a chimeric death receptor transgene with an altered pre-endothelin 1 promoter, which is activated in angiogenic endothelial cells. It is postulated to restrict tumor angiogenesis through TNF-induced, TNFR1-FAS-mediated endothelial cell apoptosis. The death of the associated tumor cells and antigen release, along with the immune effect of the virus itself may result in enhanced antitumor immunity [89].
Xi et al. reported the high expression of bone morphogenic protein endothelial cell precursor-derived regulator (BMPER) in ovarian cancer, which was detected by immunohistochemistry. This upregulation of BMPER was also related to increased metastasis to lymph nodes and a lower survival rate. It is postulated that the overexpression of BMPER is an independent risk factor of poor prognosis in patients. The suppression of BMPER inhibits the migration, invasion and proliferation of OC, and promotes apoptosis. In that study, the researchers considered that BMPER may become a potential prognostic marker and that modification of this pathway may change the role of BMPER in promoting the malignant biological behavior of ovarian cancer cells [90].
Ferreira et al., in their systematic review with meta-analysis, examined the prognostic role of microRNAs (miRNAs) in epithelial OC. They identified 12 miRNAs that may be useful in diagnosis, prognosis and chemotherapy sensitivity in epithelial ovarian cancer management, but further investigations involving prospective randomized trials are needed to validate these data [91].
Lin et al. analyzed the role of the long non-coding RNA (lncRNA) AOC4P in the suppression of metastasis in epithelial ovarian cancer through regulation of the epithelial–mesenchymal transition. It has been found that the expression of AOC4P is decreased in epithelial OC tissues and cell lines. Additionally, this underexpression is positively correlated with lymph node metastasis and the clinico-pathological stage of epithelial ovarian cancer. This anti-metastatic role was also verified in vivo by tumor dissection and bioluminescence imaging. AOC5P may become a novel and probably effective target for anti-metastatic clinical management of epithelial ovarian cancer [92]. The same idea of using lncRNA was presented by Salamini-Montemurri et al. in their review, which analyzed the challenges and opportunities of using lncRNA in clinical practice. In their opinion, lncRNA may create a novel generation of tools in the diagnostics, prognosis and treatment resistance of OC [93].
The diversity of ovarian cancer and different risk factors for its development need to be emphasized. OC is related to a specific lifestyle, geographic location or age. Blake et al., in their secondary analysis of a previously prepared systematic review on ovarian cancer diagnosed during pregnancy, noticed the fact that teenage women (≤20 years old) whose pregnancies were complicated by ovarian cancer might be at increased risk of poor survival from OC (adjusted HR = 5.51; 95% CI = 1.29–21.8; p = 0.021) [94]. The reason for this observation is still unclear, so new research and analytical insights are needed to solve this problem.
These different data presented in the articles demonstrate that ovarian cancer concomitant with pregnancy appears to be a very rare occurrence, and only through thorough analysis of all of these cases can we understand the problem and find proper solutions (improved diagnostics and treatment), which can be introduced worldwide. Many logistic and geographical barriers may impede patient access to multidisciplinary tumor boards in referral hospitals, which results in an astounding number of women that get suboptimal care. In order to improve upon this, the “Advisory Board on Cancer in Pregnancy” was created in the Netherlands and France. The two email-based tumor advisory boards consist of highly integrated teams of specialized physicians that remotely discuss clinical cases of cancer in pregnancy and provide advice to other physicians who need expertise on how to manage these female patients [53].
4. Conclusions
The basic principles of the management of ovarian cancer during pregnancy are summarized in Table 6. To conclude, pregnancy complicated by ovarian cancer remains a challenge for physicians. Due to a low prevalence of OC in pregnancy and the absence of large randomized trials and major patient cohorts, universal standards of treatment have not yet been proposed. A link between chemotherapy and its influence on the fetus and its later development has been under close examination. The key factor in this situation is the relationship between the doctor’s conscience and the patient’s trust. Decisions concerning the choice of the best management of ovarian cancer are very complex and difficult because of the conflict between the mother’s and the fetus’s wellbeing; thus, a multidisciplinary team consisting of an obstetrician, oncologist, pathologist, anesthesiologist, neonatologist and psychologist is mandatory.
Table 6.
Ovarian cancer during pregnancy: basic principles of management.
|
The aim for pregnant patients is the same as for non-pregnant women regarding malignancy, namely, to survive free of neoplasm for as long as possible. Fortunately, the recurrence-free and overall survival rates for pregnant women are very similar to those reported for non-pregnant patients.
Authors Contributions
Conceptualization and methodology, B.L.-G. and D.F.D.; formal analysis, D.F.D.; data curation, D.F.D., R.M. and E.P.-C.; writing—original draft preparation, D.F.D., R.M. and E.P.-C.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Calsteren K., Heyns L., De Smet F., Van Eycken L., Gziri M.M., Van Gemert W., Halaska M., Vergote I., Ottevanger N., Amant F. Cancer during pregnancy: Analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J. Clin. Oncol. 2010;28:683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 2.Voulgaris E., Pentheroudakis G., Pavlidis N. Cancer and pregnancy: A comprehensive review. Surg. Oncol. 2011;20:e175–e185. doi: 10.1016/j.suronc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Smith L.H., Danielsen B., Allen M.E., Cress R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am. J. Obstet. Gynecol. 2003;189:1128–1135. doi: 10.1067/S0002-9378(03)00537-4. [DOI] [PubMed] [Google Scholar]
- 4.Korenaga T.K., Tewari K.S. Gynecologic cancer in pregnancy. Gynecol. Oncol. 2020;157:799–809. doi: 10.1016/j.ygyno.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonelli N.M., Dotters D.J., Katz V.L., Kuler J.A. Cancer in pregnancy: A review of literature. Part 1. Obstet. Gynecol. Surv. 1996;51:125–134. doi: 10.1097/00006254-199602000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A., Shinde A., Naik R. Ovarian cysts and cancer in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016;33:58–72. doi: 10.1016/j.bpobgyn.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Mekaru K., Kamiyama S., Masamoto H., Yagi C., Hirakawa M., Inamine M., Nagai Y., Sakumoto K., Aoki Y. Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy: A case report. Arch. Gynecol. Obstet. 2008;278:287–290. doi: 10.1007/s00404-008-0573-z. [DOI] [PubMed] [Google Scholar]
- 8.Budiman H.D., Burges A., Friese K., Hasbargen U. Squamous cell carcinoma arising in dermoid cyst of the ovary in pregnancy. Arch. Gynecol. Obstet. 2010;281:535–537. doi: 10.1007/s00404-009-1193-y. [DOI] [PubMed] [Google Scholar]
- 9.Mierzyński R., Dłuski D.F., Gogacz M., Golubka I., Leszczyńska-Gorzelak B. Pregnancy complicated by ovaria planoepithelial carcinoma arising immature cystic teratoma. J. Obstet. Gynaecol. 2019;39:408–409. doi: 10.1080/01443615.2018.1465899. [DOI] [PubMed] [Google Scholar]
- 10.Feng X., Xu L. Rare case of Squamous cell carcinoma arising in a recurrent ovarian mature cystic teramota of a young woman. A case report and review of the literature. Medicine. 2018;97:e10802. doi: 10.1097/MD.0000000000010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leiserowitz G.S., Xing G., Cress R., Brahmbhatt B., Dalrymple J.L., Smith L.L. Adnexal masses in pregnancy: How often are they malignant? Gynecol. Oncol. 2006;101:315–321. doi: 10.1016/j.ygyno.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Boussios S., Moschetta M., Tatsi K., Tsiouris A.K., Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors. J. Adv. Res. 2018;12:1–9. doi: 10.1016/j.jare.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuntoli R.L., Vang R.S., Bristow R.E. Evaluation and management of adnexal masses during pregnancy. Clin. Obstet. Gynecol. 2006;49:492–505. doi: 10.1097/00003081-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Oehler M.K., Wain G.V., Brand A. Gynaecological malignancies in pregnancy: A review. Aust. N. Z. J. Obstet. Gynaecol. 2003;43:414–420. doi: 10.1046/j.0004-8666.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 15.Grimm D., Woelber L., Trillsch F., Keller-v.Amsberg G., Mahner S. Clinical management of epithelial ovarian cancer during pregnancy. Eur. J. Cancer. 2014;50:963–971. doi: 10.1016/j.ejca.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Shim M.H., Mok C.W., Chang K.H., Sung J.H., Choi S.J., Oh S.J., Roh C.R., Kim J.H. Clinical characteristics and outcome of cancer diagnosed during pregnancy. Obstet. Gynecol. Sci. 2016;59:1–8. doi: 10.5468/ogs.2016.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruegl A.S., Joshi S., Batman S., Weisenberger M., Munro E., Becker T. Gynecologic cancer incidence and mortality among American Indian/Alaska Native women in the Pacific Northwest, 1996–2016. Gynecol. Oncol. 2020 doi: 10.1016/j.ygyno.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Ovarian Cancer. [(accessed on 11 October 2020)]; Available online: https://seer.cancer.gov/statfacts/html/ovary.html.
- 19.Zeppernick F., Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch. Gynecol. Obstet. 2014;290:839–842. doi: 10.1007/s00404-014-3364-8. [DOI] [PubMed] [Google Scholar]
- 20.Prat J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows. Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 21.Yacobozzi M., Nguyen D., Rakita D. Adnexal masses in pregnancy. Semin Ultrasound CT. MR. 2012;33:55–64. doi: 10.1053/j.sult.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Fauvet R., Brzakowski M., Morice P., Resch B., Marret H., Graesslin O., Daraï E. Borderline ovarian tumors diagnosed during pregnancy exhibit a high incidence of aggressive features: Results of a French multicenter study. Ann. Oncol. 2012;33:55–64. doi: 10.1093/annonc/mdr452. [DOI] [PubMed] [Google Scholar]
- 23.Botha M., Rajaram S., Karunaratne K. Figo cancer report 2018. Cancer in pregnancy. Int. J. Gynecol. Obstet. 2018;143:137–142. doi: 10.1002/ijgo.12621. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X.Y., Huang H.F., Lian L.J., Lang J.H. Ovarian cancer in pregnancy: A clinicopathologic analysis of 22 cases and review of the literature. Int. J. Gynecol. Cancer. 2006;16:8–15. doi: 10.1111/j.1525-1438.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 25.Copeland L.J., Landon M.B. Malignant disease in pregnancy. In: Gabbe S.G., Niebyl J.R., Simpson J.L., editors. Obstetrics, Normal and Problem Pregnancies. 3rd ed. Churchill Livingstone; New York, NY, USA: 1996. pp. 1155–1181. [Google Scholar]
- 26.Zanotti K.S., Belinson J.L., Kennedy A.W. Treatment of gynecologic cancers in pregnancy. Semin. Oncol. 2000;27:686–698. [PubMed] [Google Scholar]
- 27.Behtash N., Zarchi M.K., Gilani M.M., Ghaemmaghami F., Mousavi A., Ghotbizadeh F. Ovarian carcinoma associated with pregnancy: A clinicopathological analysis of 23 cases and review of literature. BMC Pregnancy. Childbirth. 2008;8:3. doi: 10.1186/1471-2393-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salani R., Billingsley C., Crafton S. Cancer and pregnancy: An overview for obstetricians and gynecologists. Am. J. Obstet. Gynecol. 2014;211:7–14. doi: 10.1016/j.ajog.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Sarandakou A., Protonotariou E., Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit. Rev. Clin. Lab. Sci. 2007;44:151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]
- 30.Morice P., Uzan C., Gouy S., Verschraegen C., Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012;379:558–569. doi: 10.1016/S0140-6736(11)60829-5. [DOI] [PubMed] [Google Scholar]
- 31.Han S.N., Lotgerink A., Gziri M.M., Van Calsteren K., Hanssees M., Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: A systematic review. BMC Med. 2012;10:86. doi: 10.1186/1741-7015-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmerman D., Ameye L., Fischerova D., Epstein E., Melis G.B., Guerriero S., Van Holsbeke C., Savelli L., Fruscio R., Lissoni A.A., et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ. 2010;341:c6839. doi: 10.1136/bmj.c6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fruscio R., de Haan J., Van Calsteren K., Verheecke M., Mhallem M., Amant F. Ovarian cancer in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2017;41:108–117. doi: 10.1016/j.bpobgyn.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Forstner R., Thomassin-Naggara I., Cunha T.M., Kinkel K., Masselli G., Kubik-Huch R., Spencer J.A., Rockall A. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: An update. Eur. Radiol. 2017;27:2248–2257. doi: 10.1007/s00330-016-4600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomassin-Naggara I., Fedida B., Sadowski R., Chervier M.-C., Chabbert-Buffet N., Ballester M., TAvolaro S., Darai E. Complex US adnexal masses during pregnancy. Is pelvic MR imaging accurate for characterization? Eur. J. Radiol. 2017;93:200–208. doi: 10.1016/j.ejrad.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Frisch A., Walter T.C., Hamm B., Denecke T. Efficacy of oral contrast agents for upper geastrointestinal signal suppression in MRCP: A systematic review of the literature. Acta Radiol. Open. 2017;6:1–7. doi: 10.1177/2058460117727315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kal H.B., Struikmans H. Radiotherapy during pregnancy: Facts and fiction. Lancet Oncol. 2005;6:328–333. doi: 10.1016/S1470-2045(05)70169-8. [DOI] [PubMed] [Google Scholar]
- 38.Doll R., Wakeford R. Risk of childhood cancer from fetal irradiation. Br. J. Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 39.Thomassin-Naggara I., Aubert E., Rockall A., Jalaguier-Coudray A., Rouzier R., Daraï E., Bazot M. Adnexal masses development and preliminary validation of an MR imaging scoring system. Radiology. 2013;267:432–443. doi: 10.1148/radiol.13121161. [DOI] [PubMed] [Google Scholar]
- 40.Jubb E.D. Primary ovarian cancer in pregnancy. Am. J. Obstet. Gynecol. 1963;85:345–354. doi: 10.1016/S0002-9378(16)35444-8. [DOI] [PubMed] [Google Scholar]
- 41.Matsuyama T., Tsukamoto N., Matsukuma K., Kamura T., Kaku T., Saito T. Maligant ovarian tumors associated with pregnancy: Report of six cases. Int. J. Gynaecol. Obstet. 1989;28:61–66. doi: 10.1016/0020-7292(89)90545-6. [DOI] [PubMed] [Google Scholar]
- 42.Betson J.R., Golden M.L. Primary carcinoma, of the ovary coexisting with pregnancy. Obstet. Gynecol. 1958;12:589–595. [PubMed] [Google Scholar]
- 43.Altaras M., Rosen D., Shapira J., Cohen I., Bernheim J., Ravid M. Advanced primary ovarian carcinoma in pregnancy. Am. J. Obstet. Gynecol. 1989;160:1210–1211. doi: 10.1016/0002-9378(89)90193-2. [DOI] [PubMed] [Google Scholar]
- 44.Tabata T., Nishiura K., Tanida K., Kondo E., Okugawa T., Sagawa N. Carboplatin chemotherapy in a pregnant patient with undifferentiated ovarian carcinoma: Case report and review of the literature. Int. J. Gynecol. Cancer. 2008;18:181–184. doi: 10.1111/j.1525-1438.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 45.Creasman W.T., Rutledge F., Smith J.P. Carcinoma of the ovary associated with pregnancy. Obstet. Gynecol. 1971;38:111–116. [PubMed] [Google Scholar]
- 46.Pearl J., Price R., Richardson W., Fanelli R. Society of American Gastrointestinal Endoscopic Surgeons. Guideline for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy. Surg. Endosc. 2011;25:3479–3492. doi: 10.1007/s00464-011-1927-3. [DOI] [PubMed] [Google Scholar]
- 47.Leiserowitz G.S. Managing ovarian masses during pregnancy. Obstet. Gynecol. Surv. 2006;61:463–470. doi: 10.1097/01.ogx.0000224614.51356.b7. [DOI] [PubMed] [Google Scholar]
- 48.Glanc P., Salem S., Farine D. Adnexal masses in pregnant patient: A diagnostic and management challenge. Ultrasound. Q. 2008;24:225–240. doi: 10.1097/RUQ.0b013e31819032f. [DOI] [PubMed] [Google Scholar]
- 49.Schmeler K.M., Mayo-Smith W.W., Peipert J.F., Weitzen S., Manuel M.D., Gordinier M.E. Adnexal masses in pregnancy: Surgery compared with observation. Obstet. Gynecol. 2005;105:1098–1103. doi: 10.1097/01.AOG.0000157465.99639.e5. [DOI] [PubMed] [Google Scholar]
- 50.Wang P.H., Chao H.T., Yuan C.C., Lee W.L., Chao K.C., Ng H.T. Ovarian tumours complicating pregnancy. Emergency and elective surgery. J. Reprod. Med. 1999;44:279–287. [PubMed] [Google Scholar]
- 51.Lee G.S., Hur S.Y., Shin J.C., Kim S.P., Kim S.J. Elective vs. conservative management of ovarian tumors in pregnancy. Int. J. Gynaecol. Obstet. 2004;85:250–254. doi: 10.1016/j.ijgo.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Runowicz C.D., Brewer M. Management of Ovarian Cancer in Pregnant Women. [(accessed on 11 October 2020)];2012 Available online: http://www.uptodate.com/contents/management-of-ovarian-cancer-in-pregnant-women.
- 53.Amant F., Berveiller P., Boere I.A., Cardonick E., Fruscio R., Fumagalli M., Halaska M.J., Hasenburg A., Johansson A.L.V., Lambertini M., et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019;30:1601–1612. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- 54.Amant F., Halaska M.J., Fumagalli M., Steffensen K.D., Lok C., Van Calsteren K., Han S.N., Mir O., Fruscio R., Uzan C., et al. ESGO task force “Cancer in Pregnancy”. Gynecologic cancers in pregnancy: Guidelines of second international consensus meeting. Int. J. Gynecol. Cancer. 2014;24:394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 55.Estadella J., Español P., Grandal B., Gine M., Parra J. Laparoscopy during pregnancy: Case report and key points to improve laparoscopic management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;217:83–88. doi: 10.1016/j.ejogrb.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Jackson H., Granger S., Price R., Rollins M., Earle D., Richardson W., Fanelli R. Diagnosis and laparoscopic treatment of surgical diseases during pregnancy: An evidence-based review. Surg. Endosc. 2008;22:1917–1927. doi: 10.1007/s00464-008-9989-6. [DOI] [PubMed] [Google Scholar]
- 57.Ye P., Zhao N., Shu J., Shen H., Wang Y., Chen L., Yan X. Laparoscopy versus open surgery for adnexal masses in pregnancy: A meta-analytic review. Arch. Gynecol. Obstet. 2019;299:625–634. doi: 10.1007/s00404-018-05039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shigemi D., Aso S., Matsui H., Fushimi K., Yasunaga H. Safety of laparoscopic surgery for benign diseases during pregnancy: A nationwide retrospective cohort study. J. Minim. Invasive Gynecol. 2019;26:501–506. doi: 10.1016/j.jmig.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Webb K., Sakhel K., Chauhan S., Abuhamad A. Adnexal mass during pregnancy: A review. Am. J. Perinatol. 2015;32:1010–1016. doi: 10.1055/s-0035-1549216. [DOI] [PubMed] [Google Scholar]
- 60.Dede M., Yeren M.C., Yilmaz A., Goktolga U., Baser I. Treatment of incidental adnexal masses at cesarean section: A retrospective study. Int. J. Gynecol. Cancer. 2007;17:339–341. doi: 10.1111/j.1525-1438.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 61.Ebert U., Löffler H., Kirch W. Cytotoxic therapy and pregnancy. Pharmacol. Ther. 1997;74:207–220. doi: 10.1016/S0163-7258(97)82004-9. [DOI] [PubMed] [Google Scholar]
- 62.Weisz B., Meirow D., Schiff E., Lishner M. Impact and treatment of cancer during pregnancy. Expert. Rev. Anticancer Ther. 2004;117:179–188. doi: 10.1586/14737140.4.5.889. [DOI] [PubMed] [Google Scholar]
- 63.Sadler N.C., Nandhikonda P., Webb-Robertson B.-J., Ansong C., Anderson L.N., Smith J.N., Corley R.A., Wright A.T. Hepatic cytochrome, P450 activity, abundance, and expression throughout human development. Drug Metab. Dispos. 2016;44:984–991. doi: 10.1124/dmd.115.068593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams S., Blessing J.A., Liao S.Y., Ball H., Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: A trial of the Gynecologic Oncology Group. J. Clin. Oncol. 1994;12:701–706. doi: 10.1200/JCO.1994.12.4.701. [DOI] [PubMed] [Google Scholar]
- 65.Ngu S.F., Ngan H.Y.S. Chemotherapy in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016;33:86–101. doi: 10.1016/j.bpobgyn.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Van Calsteren K., Verbesselt R., Ottevanger N., Halaska M., Heyns L., Van Bree R., de Bruijn E., Chai D., Delforge M., Noens L., et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: A preclinical and clinical study. Acta Obstet. Gynecol. Scand. 2010;89:1338–1345. doi: 10.3109/00016349.2010.512070. [DOI] [PubMed] [Google Scholar]
- 67.Hannuna K.Y., Putignani L., Silvestri E., Pisa R., Angioli R., Signore F. Incidental endometrial adenocarcinoma in early pregnancy. Int. J. Gynecol. Cancer. 2009;19:1580–1584. doi: 10.1111/IGC.0b013e3181a841a7. [DOI] [PubMed] [Google Scholar]
- 68.Haas J.F. Pregnancy in association with a newly diagnosed cancer: A population-based epidemiologic assessment. Int. J. Cancer. 1984;34:229–235. doi: 10.1002/ijc.2910340214. [DOI] [PubMed] [Google Scholar]
- 69.Matsuo K., Whitman S.A., Blake E.A., Conturie C.L., Ciccone M.A., Jung C.E., Takuchi T., Nishimura M. Feto-maternal outcome of pregnancy complicated by vulvar cancer: A systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;179:216–223. doi: 10.1016/j.ejogrb.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 70.Valentin J. Biological effects after prenatal irradiation (embryo and fetus) Ann. ICRP. 2003;33:1–206. doi: 10.1016/S0146-6453(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 71.ICRP Pregnancy and medical radiation. Ann. ICRP. 2000;84:1–43. doi: 10.1016/s0146-6453(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 72.Vandenbroucke T., Verheecke M., Fumagalli M. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc. Health. 2017;1:302–310. doi: 10.1016/S2352-4642(17)30091-3. [DOI] [PubMed] [Google Scholar]
- 73.De Haan J., Verheecke M., Van Calsteren K., Van Calster B., Shmakov R.G., Gziri M.M., Halaska M.J., Fruscio R., Lok C.A.R., Boere I.A., et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 74.Halaska M.J., Komar M., Vlk R., Tomek V., Skultety J., Robova H., Rob L. A pilot study on peak systolic velocity monitoring of fetal anemia after administrator of chemotherapy during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;174:76–79. doi: 10.1016/j.ejogrb.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 75.Can N.T.T., Robertson P., Zaloudek C.J., Gill R.M. Cervical squamous cell carcinoma metastatic to placenta. Int. J. Gynecol. Pathol. 2013;32:516–519. doi: 10.1097/PGP.0b013e3182763178. [DOI] [PubMed] [Google Scholar]
- 76.Alexander A., Samlowski W.E., Grossman D., Bruggers C.S., Harris R.M., Zone J.J., Noyes R.D., Bowen G.M., Leachman S.A. Metastatic melanoma in pregnancy: Risk of transplacental metastates in the infant. J. Clin. Oncol. 2003;21:2179–2186. doi: 10.1200/JCO.2003.12.149. [DOI] [PubMed] [Google Scholar]
- 77.Orr J.W., Grizzle W.E., Huddleston J.F. Squamous cell carcinoma metastatic to placenta and ovary. Obstet. Gynecol. 1982;59:81S–83S. [PubMed] [Google Scholar]
- 78.Greaves M., Hughes W. Cancer cell transmission via placenta. Evol. Med. Public Health. 2018;1:106–115. doi: 10.1093/emph/eoy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Y.Y., Roberts C.L., Dobbins T., Stavrou E., Black K., Morris J., Young J. Incidence and outcomes of pregnancy- associated cancer in Australia, 1994-2008: A population-based linkage study. BJOG. 2012;119:1572–1582. doi: 10.1111/j.1471-0528.2012.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parazzini F., Franchi M., Tavani A., Negri E., Peccatori F.A. Frequency of pregnancy related cancer: A popularion based linkage study in Lombardy, Italy. Int. J. Gynecol. Cancer. 2017;27:613–619. doi: 10.1097/IGC.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 81.Morikawa A., Ueda K., Takahashi K., Fukunaga M., Iwashita M., Kobayashi Y., Takechi K., Umezawa S., Terauchi F., Kiguchi K., et al. Pathology-oriented treatment strategy of malignant ovarian tumor in pregnant women: Analysis of 41 cases in Japan. Int. J. Clin. Oncol. 2014;19:1074–1079. doi: 10.1007/s10147-014-0669-3. [DOI] [PubMed] [Google Scholar]
- 82.Cottreau C.M., Dashevsky I., Andrade S.E., Li D.K., Nekhlyudov L., Raebel M.A., Ritzwoller D.P., Partridge A.H., Pawlowski P.A., Toh S. Pregnancy-associated cancer: A U.S. population-based study. J. Womens Health. 2019;28:250–257. doi: 10.1089/jwh.2018.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andersson T.L., Johansson A.V., Fredriksson I., Lambe M. Cancer during pregnancy and the postpartum period: A population-based study. Cancer. 2015;121:2072–2077. doi: 10.1002/cncr.29325. [DOI] [PubMed] [Google Scholar]
- 84.Nazer A., Czuzoj-Shulman N., Oddy L., Abenhaim H.A. Incidence of ma-ternal and neonatal outcomes in pregnancies complicated by ovarian masses. Arch. Gynecol. Obstet. 2015;292:1069–1074. doi: 10.1007/s00404-015-3700-7. [DOI] [PubMed] [Google Scholar]
- 85.Eibye S., Kjær S.K., Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013;122:608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 86.Kwon Y.S., Mok J.E., Lim K.T., Lee I.H., Kim T.J., Lee K.H., Shim J.U. Ovarian cancer Turing pregnancy: Clinical and pregnancy outcome. J. Korean Med. Sci. 2010;25:230–234. doi: 10.3346/jkms.2010.25.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stensheim H., Møller B., van Dijk T., Fossa S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J. Clin. Oncol. 2009;27:45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 88.Hallum S., Jakobsen M.A., Gerds T.A., Pinborg A., Tjønneland A., Kamper-Jørgensen M. Mare origin microchimerism and ovarian cancer. Int. J. Epidemiol. 2020 doi: 10.1093/ije/dyaa019. [DOI] [PubMed] [Google Scholar]
- 89.Killock D. Viral gene therapy active in ovarian cancer. Nat. Rev. Clin. Oncol. 2020;17:391. doi: 10.1038/s41571-020-0371-5. [DOI] [PubMed] [Google Scholar]
- 90.Xi Y., Nie X., Wang J., Gao L., Lin B. Overexpression of BMPER in ovaria cancer and the mechanism by which it promotes malignant biological bahaviour in tumor cells. BioMed Res. Int. 2020 doi: 10.1155/2020/3607436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrreira R., Roela R.A., Lopez R.V.M., Del Estevez-Diz M.P. The prognostic role of microRNA in epithelial ovarian cancer: A systematic review of literature with an overall survival meta-analysis. Oncotarget. 2020;11:1085–1095. doi: 10.18632/oncotarget.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin X., Tang X., Zheng T., Qiu J., Hua K. Long non-coding RNA AOC4P suppresses epithelial ovarian cancer metastasis by regulating epithelial-mesenchymal transition. J. Ovarian. Res. 2020;13:45. doi: 10.1186/s13048-020-00644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salamini-Montemurri M., Lamas-Maceiras M., Barreiro-Alonso A., Vizoso-Vázquez A., Rodriguez-Belmonte E., Qiundós-Varela M., Cerdán M.E. The challenges and opportunities of lncRNAs in ovarian cancer research and clinical use. Cancers. 2020;12:1020. doi: 10.3390/cancers12041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blake E.A., De Zoysa M.Y., Marocco E.B., Kaiser S.B., Kodama M., Grubbs B.H., Matsuo K. Teenage pregnancy complicated by primary invasive ovarian cancer: Association for oncologic outcome. J. Gynecol. Oncol. 2018;29:e79. doi: 10.3802/jgo.2018.29.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]