Abstract
Exogenous antioxidant applications enable salt-stressed plants to successfully cope with different environmental stresses. The objectives of this investigation were to study the effects of sequential treatments of proline (Pro), ascorbic acid (AsA), and/or glutathione (GSH) on 100 mM NaCl-stressed cucumber transplant’s physio-biochemical and growth traits as well as systems of antioxidant defense. Under salinity stress, different treatment of AsA, Pro, or/and GSH improved growth characteristics, stomatal conductance (gs), enhanced the activities of glutathione reductase (GR), superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) as well as increased contents of AsA, Pro, and GSH. However, sequential application of antioxidants (GSH-Pro- AsA) significantly exceeded all individual applications, reducing leaf and root Cd2+ and Na+ contents in comparison to the control. In plants grown under NaCl-salt stress, growth characteristics, photosynthetic efficiency, membrane stability index (MSI), relative water content (RWC), contents of root and leaf K+ and Ca2+, and ratios of K+/Na+ and Ca2+/Na+ were notably reduced, while leaf contents of non-enzymatic and enzymatic antioxidants, as well as root and leaf Cd2+ and Na+ concentrations were remarkably increased. However, AsA, Pro, or/and GSH treatments significantly improved all investigated growth characteristics, photosynthetic efficiency, RWC and MSI, as well as AsA, Pro, and GSH, and enzymatic activity, leaf and root K+ and Ca2+ contents and their ratios to Na+, while significantly reduced leaf and root Cd2+ and Na+ contents.
Keywords: sequential application of antioxidants, salinity, Cucumis sativus, photosynthetic efficiency, antioxidant defense systems
1. Introduction
Cucumber (Cucumis sativus L.) is one of the most economically important and widely distributed vegetable crops, as well as being useful for human health and nutrition because it has several important mineral nutrients and vitamins and has reasonable amounts of proteins and carbohydrates [1]. In addition, it has many natural antioxidants and vitamins used as a nutritive source and for medical purposes such as headaches and anti-acne lotions due to its analgesic activities [2]. Also, cucumber can have antioxidants phenolic, especially flavonoids can be involved in reactive oxygen species (ROS) detoxification.
Cucumber has been found to be moderately sensitive to salinity [3]. In particular, salt stress causes inhibition of cucumber plant growth and productivity through affecting the key physiological and biochemical processes [4,5,6,7]. Due to the climatic changes occurring presently, salinization of plant-growing media is gradually aggravated, which will lead to further inhibition and loss in plant growth and yield, respectively. Therefore, considerable attention has been paid to boost salinity tolerance in cucumber plants by using many exogenous supports [6,8,9].
Generally, salinity negatively affects growth and restricts the productivity and quality of crops, especially in arid and semi-arid agricultural regions [10,11,12,13,14,15]. Plant growth and productivity are declined at varying degrees subjecting to the salt stress levels [1,16,17]. Salt stress causes plant performance reduction through the dysfunction occurred in the biochemical and physiological and processes, and the antioxidant defenses as a result of ROS excessive production such as 1O2, O2•−, H2O2, and OH‒, as well as the instabilities of cell membranes and lipid peroxidation occurred due to stress of increased Na+ ions along with increased ROS [18,19,20,21,22].
Plants have different antioxidative mechanisms for ROS scavenging through inspiring the antioxidative enzymes for instance CAT, GR, SOD, and APX, which are functioned along with the non-enzymatic antioxidants to mitigate the adverse impacts of different stresses in plant species [23,24,25]. The main non-enzymatic plant antioxidants are ascorbate, glutathione, carotenoids, tocopherols, and phenolic compounds. Non-enzymatic antioxidants such as AsA, GSH, and Pro can represent a fundamental portion of the endogenous defense mechanisms and adaptive strategies to fight the stress. They are often insufficient to cope with stress; therefore, plants are exogenously provided by these antioxidants singly or in combinations to promote and enhance their antioxidant defense systems in contrast to different environmental stresses, including salinity [26,27,28]. As reported in these works, the exogenous treatments (i.e., AsA, Pro, and GSH) applied singly or in sequences had boosted plant performance (i.e., growth and yield) through a positive stimulation of the key physio-biochemical and molecular attributes and increasing the capacity of antioxidant defenses. In addition, the above works have reported ROS detoxification, cell membrane stability, ion balance, and Na+ ions decline.
To our knowledge, only little previous works [24,28,29] showed that the sequential application of Pro, AsA, and GSH is proved to be more effective strategy for plants to efficiently cope with various abiotic stresses (i.e., heavy metals, drought, and salinity) than their individual or combined applications. Therefore, the aims of the current investigation were to assess the effects of exogenously applied AsA, Pro and GSH in a sequence method in comparison with individual treatments on the growth, efficiency of photosynthesis, tissue water content, and membrane health in terms of MSI and the contents of Cd2+ and Na+ as well as non-enzymatic and enzymatic defenses of cucumber transplants.
2. Materials and Methods
2.1. Experimental Setup
Seeds of cucumber (hybrid Bahi®) were separated to five groups, each consisted of 80 seeds. Then, seeds were soaked in distilled water (group 1), 0.5 mM AsA solution (group 2), 0.5 mM Pro solution (group 3), or 0.5 mM GSH solution (group 4). The duration of soaking was 4 h. Furthermore, seeds of group number 5 were soaked in sequential application of different antioxidants as follows: 0.5 mM AsA for 90 min, 0.5 mM Pro for 80 min, and then 0.5 mM GSH for 70 min in a sequence, respectively. Concentrations of antioxidants applied singly or in AsA-Pro-GSH and soaking duration were selected according to our data (not shown) of a preliminary study. In addition, AsA-Pro-GSH was generated greatest response among different tested sequences of our preliminary study (data not shown).
In each group, the seeds were divided into two sub-groups (i.e., n = 40 seeds) to represent 10 treatments. After germinating the seeds of all groups, irrigation was applied using a distilled water for all first sub-groups in the same time, while all second sub-groups were irrigated with 100 mM NaCl solution. Foam trays (209 cells) were used for the current work conducted on 28 May 2017 for 28 d. Trays were arranged in an open greenhouse under which the conditions were 62–68% humidity, 29 ± 3/19 ± 1 °C day/night temperatures, and average 13/11 h day/night length. The light intensity was the intensity of natural sunlight throughout the season (28th of May–24th of June). Peat moss and vermiculite mixture (1:2 v/v) was a germinating and growing medium of transplants. The completely randomized designs was used for the experiment with three foam trays/replicates for each treatment. Twenty-eight days after sowing, transplants of cucumber were collected for different measurements of morphology and physiological traits and biochemical attributes.
2.2. Measurements
After 28 days, seedlings (n = 20) were randomly selected from each treatment to record growth attributes i.e., leaf area, shoot length, stem diameter, and shoot FW (fresh weight) and DW (dry weight). Leaf area was determined as detailed by [30].
Stomatal conductance (gs) mmol−2 S−1 was measured by leaf porometer (Decagon Devices Inc., Pullman, WA, USA). The measurements were conducted 10, 14, 18, and 22 days after onset of NaCl applications from 7:00 am: 5:00 pm with 2 h intervals. SPAD chlorophyll meter (SPAD-502; Minolta, Osaka, Japan) was used to measure relative contents of chlorophyll. Chlorophyll fluorescence (performance index, PI; = maximum quantum yield of PSII = efficiency of the photosystem 2, Fv/Fm; Fv/F0), as a convenient tool to assess photosynthetic efficiency, was determined according to [31] using Handy PEA (Hansatech Instruments Ltd., Kings Lynn, UK). Leaves relative water content (RWC) and cell membrane stability index (MSI) were measured according to [32].
The method detailed by [33] was followed to determine leaf content of free proline after extraction of 500 mg fresh leaf using 3%, v/v, sulphosalicylic acid. Following the [34] method, leaf AsA was extracted and its content was determined. Leaf GSH content was determined as detailed in the [35] method. Protein content and activity of SOD (EC 1.15.1.1) was analyzed as described by Bradford [36] and Kono [37] respectively. CAT (EC 1.11.1.6) activity was analyzed as described by Aebi [38]. Potassium phosphate (KH2PO4; a buffer) and hydrogen peroxide (H2O2; a substrate) were used, and the absorbance was read using spectrophotometer at 240 nm. The activity of APX (EC 1.11.1.11) was analyzed using the method of [39] and the absorbance was read using spectrophotometer at 290 nm. GR (EC 1.6.4.1) activity was analyzed and the NADPH oxidation was monitored for three absorbance readings recorded at 340 nm [39].
A weight of 100 mg dried powdered leaf or root samples was digested for 12 h by using 80% HClO4 (2 mL) + concentrated H2SO4 (10 mL). The digested samples were then diluted each to 100 mL. The digested leaf samples were used to analyze the concentrations of K+ and Ca2+ via flame photometry [40]. In addition, the digested leaf and root samples were used to analyze Cd2+ contents via Atomic Absorption Spectrophotometer (a Perkin-Elmer, Model 3300) as detailed in [41] method.
2.3. Statistical Analysis
The data obtained from the effects of exogenously applied AsA, Pro and GSH in a sequence method in comparison with individual treatments on growth, physiological and biochemical traits as well as Cd2+ and Na+ contents of cucumber transplants gown under 100 mM NaCl-salt stress were statistically analyzed using the GLM procedure of Gen STAT (version 11; VSN International Ltd., Oxford, UK). Least significant differences (LSD) was calculated to compare the differences among means at 5% probability (p ≤ 0.05).
3. Results
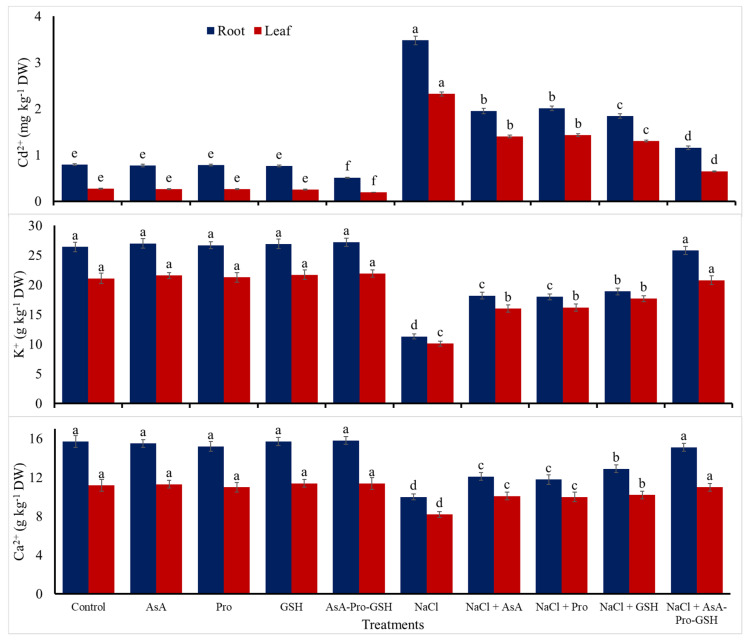
Concerning the antioxidant applications under normal conditions, the three antioxidants; AsA, Pro, and GSH that applied individually for cucumber seed improved all of the investigated growth characteristics (Table 1), gs (Table 2) and endogenous contents of AsA, Pro, and GSH (Table 3), as well as the enzymatic activities of SOD, CAT, GR, and APX (Table 4). On the other side, all of these individual antioxidant applications did not affect Fv/Fm, and PI and chlorophyll content (Table 2), RWC and MSI (Table 3), leaf and root contents of the poisonous elements Cd2+ and Na+, and the beneficial elements K+ and Ca2+ (Figure 1 and Figure 2). However, AsA-Pro-GSH treatment leads to better results for most of investigated parameters than those obtained with the individual applications, along with significant reductions of Cd2+ contents of leaves and roots upon comparing to the controls (Figure 1).
Table 1.
Effects of seed single or sequential application of antioxidants on growth traits of 100 mM NaCl-salt-stressed cucumber transplants.
| Treatments | Shoot Length (cm) | Leaf Area (cm2) | Stem Diameter (mm) | Shoot FW (g) | Shoot DW (g) |
|---|---|---|---|---|---|
| Control | 15.6 ± 0.4 cd | 64.0 ± 2.2 cd | 3.6 ± 0.1 b | 1.71 ± 0.07 b | 0.13 ± 0.02 d |
| AsA | 17.0 ± 0.6 abc | 70.6 ± 5.8 ab | 3.8 ± 0.1 ab | 2.00 ± 0.16 a | 0.15 ± 0.03 bc |
| Pro | 16.6 ± 0.7 bcd | 69.3 ± 3.6 bc | 3.7 ± 0.1 b | 2.03 ± 0.08 a | 0.15 ± 0.04 bc |
| GSH | 17.9 ± 0.7 ab | 70.8 ± 3.3 ab | 3.8 ± 0.1 ab | 2.02 ± 0.08 a | 0.15 ± 0.02 bc |
| AsA-Pro-GSH | 18.3 ± 0.6 a | 75.5 ± 5.0 a | 4.0 ± 0.1 a | 2.03 ± 0.14 a | 0.17 ± 0.04 a |
| NaCl | 11.8 ± 0.6 e | 47.2 ± 2.0 e | 3.0 ± 0.1 c | 1.41 ± 0.05 c | 0.10 ± 0.02 e |
| NaCl + AsA | 16.8 ± 0.3 abc | 63.4 ± 4.2 d | 3.6 ± 0.1 b | 1.95 ± 0.12 a | 0.14 ± 0.02 cd |
| NaCl + Pro | 15.4 ± 0.6 cd | 61.1 ± 2.3 d | 3.6 ± 0.1 b | 1.98 ± 0.06 a | 0.13 ± 0.02 d |
| NaCl + GSH | 15.1 ± 0.5 d | 64.1 ± 3.6 cd | 3.6 ± 0.1 b | 1.98 ± 0.09 a | 0.14 ± 0.03 cd |
| NaCl + AsA-Pro-GSH | 17.9 ± 0.4 ab | 70.7 ± 2.5 ab | 3.7 ± 0.1 b | 2.00 ± 0.07 a | 0.16 ± 0.04 ab |
Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants.
Table 2.
Effects of seed single or sequential application of antioxidants on photosynthetic efficiency of 100 mM NaCl-salt-stressed cucumber transplants.
| Treatments | Stomatal Conductance (mmol−2 S−1) | SPAD Chlorophyll | Fv/Fm | Performance Index (%) |
|---|---|---|---|---|
| Control | 193 ± 5 d | 42.1 ± 2.2 ab | 0.83 ± 0.06 a | 3.64 ± 0.24 ab |
| AsA | 241 ± 7 abc | 42.4 ± 1.0 ab | 0.83 ± 0.05 a | 3.75 ± 0.10 ab |
| Pro | 247 ± 5 abc | 42.8 ± 0.6 ab | 0.83 ± 0.05 a | 3.73 ± 0.12 ab |
| GSH | 254 ± 6 a | 42.9 ± 0.9 ab | 0.83 ± 0.04 a | 3.78 ± 0.14 a |
| AsA-Pro-GSH | 257 ± 8 a | 44.7 ± 1.4 a | 0.84 ± 0.05 a | 3.78 ± 0.12 a |
| NaCl | 142 ± 4 e | 33.8 ± 2.0 e | 0.76 ± 0.04 b | 3.03 ± 0.35 c |
| NaCl + AsA | 232 ± 8 c | 38.9 ± 1.8 cd | 0.82 ± 0.05 a | 3.46 ± 0.30 b |
| NaCl + Pro | 234 ± 5 bc | 38.6 ± 1.9 d | 0.82 ± 0.04 a | 3.46 ± 0.29 b |
| NaCl + GSH | 234 ± 7 bc | 39.0 ± 0.8 cd | 0.82 ± 0.04 a | 3.48 ± 0.31 b |
| NaCl + AsA-Pro-GSH | 252 ± 6 ab | 41.4 ± 1.5 bc | 0.84 ± 0.05 a | 3.72 ± 0.19 ab |
Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants. Fv/Fm = maximum quantum yield of PSII = efficiency of the photosystem 2.
Table 3.
Effects of seed single or sequential application of antioxidants on leaf relative water content (RWC), membrane stability index (MSI), and non-enzymatic antioxidant system of cucumber transplants grown under 100 mM NaCl-salt stress.
| Treatments | RWC (%) | MSI (%) | Free Proline (mg g−1 DW) |
AsA (μmol Ascorbate g−1 DW) | GSH (nmol GSH g-1 DW) |
|---|---|---|---|---|---|
| Control | 87.6 ± 0.7 abc | 76.3 ± 1.1 a | 0.27 ± 0.00 f | 1.48 ± 0.04 g | 0.14 ± 0.01 g |
| AsA | 88.3 ± 1.0 abc | 78.5 ± 0.4 a | 0.31 ± 0.01 ef | 3.54 ± 0.08 d | 0.19 ± 0.01 f |
| Pro | 89.2 ± 0.8 a | 78.3 ± 0.5 a | 0.65 ± 0.04 c | 1.85 ± 0.02 f | 0.20 ± 0.00 f |
| GSH | 88.7 ± 2.1 ab | 79.0 ± 1.1 a | 0.32 ± 0.02 e | 1.97 ± 0.04 f | 0.35 ± 0.01 d |
| AsA-Pro-GSH | 91.3 ± 2.6 a | 79.7 ± 0.8 a | 0.76 ± 0.00 b | 4.27 ± 0.08 c | 0.40 ± 0.00 c |
| NaCl | 74.5 ± 0.4 d | 63.6 ± 2.1 c | 0.33 ± 0.00 e | 2.89 ± 0.08 e | 0.18 ± 0.01 fg |
| NaCl + AsA | 82.7 ± 0.9 c | 69.8 ± 0.7 b | 0.40 ± 0.02 d | 6.32 ± 0.05 b | 0.25 ± 0.00 e |
| NaCl + Pro | 83.2 ± 2.7 bc | 69.9 ± 1.5 b | 0.80 ± 0.01 b | 3.34 ± 0.03 d | 0.28 ± 0.01 e |
| NaCl + GSH | 82.8 ± 1.2 bc | 70.4 ± 0.8 b | 0.41 ± 0.01 d | 3.40 ± 0.04 d | 0.49 ± 0.01 b |
| NaCl + AsA-Pro-GSH | 87.8 ± 1.4 abc | 76.8 ± 1.0 a | 0.87 ± 0.01 a | 7.58 ± 0.13 a | 0.59 ± 0.01 a |
Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants.
Table 4.
Effects of seed single or sequential application of antioxidants on enzymatic antioxidant system of cucumber transplants grown under 100 mM NaCl-salt stress.
| Treatments | Superoxide Dismutase | Catalase | Glutathione Reductase | Ascorbate Peroxidase |
|---|---|---|---|---|
| μmol mg‒1 Protein min‒1 | ||||
| Control | 0.23 ± 0.01 e | 0.23 ± 0.00 d | 0.27 ± 0.01 d | 0.30 ± 0.01 e |
| AsA | 0.29 ± 0.01 cd | 0.28 ± 0.01 c | 0.32 ± 0.01 c | 0.43 ± 0.02 c |
| Pro | 0.28 ± 0.01 d | 0.30 ± 0.02 c | 0.34 ± 0.02 c | 0.42 ± 0.01 c |
| GSH | 0.28 ± 0.01 d | 0.28 ± 0.01 c | 0.34 ± 0.02 c | 0.42 ± 0.02 c |
| AsA-Pro-GSH | 0.31 ± 0.02 bcd | 0.35 ± 0.00 b | 0.41 ± 0.02 b | 0.52 ± 0.02 b |
| NaCl | 0.25 ± 0.01 e | 0.29 ± 0.00 c | 0.32 ± 0.01 c | 0.36 ± 0.01 d |
| NaCl + AsA | 0.31 ± 0.01 bcd | 0.35 ± 0.01 b | 0.39 ± 0.02 b | 0.49 ± 0.02 b |
| NaCl + Pro | 0.31 ± 0.02 bcd | 0.37 ± 0.00 b | 0.39 ± 0.01 b | 0.50 ± 0.02 b |
| NaCl + GSH | 0.33 ± 0.02 b | 0.37 ± 0.01 b | 0.40 ± 0.02 b | 0.50 ± 0.02 b |
| NaCl + AsA-Pro-GSH | 0.38 ± 0.02 a | 0.41 ± 0.02 a | 0.47 ± 0.02 a | 0.55 ± 0.02 a |
Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants.
Figure 1.
Effects of seed single or sequential application of antioxidants on Cd2+, K+ and Ca2+ contents in roots and leaves of 100 mM NaCl-salt-stressed cucumber transplants. Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants.
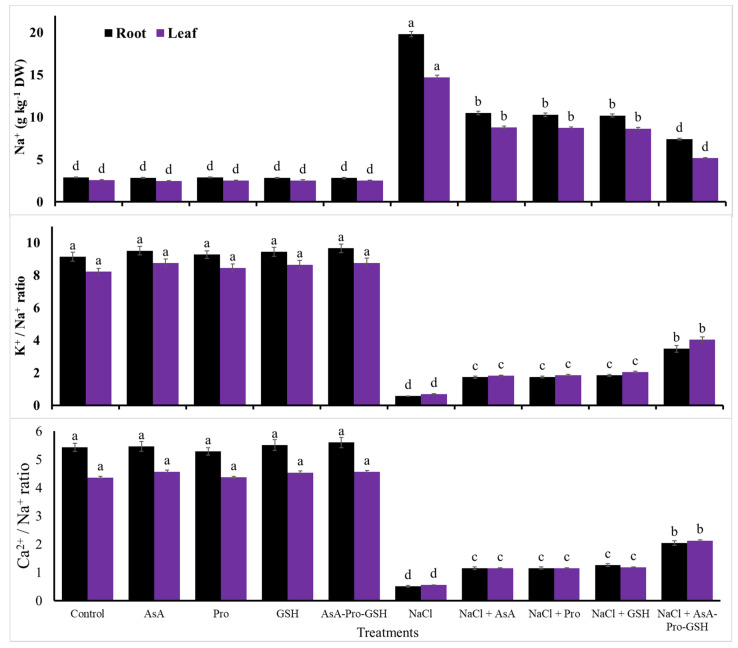
Figure 2.
Effects of seed single or sequential application of antioxidants on the content of Na+ and its relation to K+ and Ca2+ in roots and leaves of 100 mM NaCl-salt-stressed cucumber transplants. Means with the same letter within each trait are not significantly differed at p ≤ 0.05. AsA = ascorbic acid, Pro = proline, GSH = glutathione, and AsA-Pro-GSH = sequential application of different antioxidants.
Regarding the stress impacts of salinity, treatment with 100 mM NaCl considerably suppressed cucumber transplant growth parameters compared to those grown under control. For instance, it reduced the length of plant shoot by 24.4%, leaf area by 26.3%, stem diameter by 16.7%, shoot FW by 17.5%, and shoot DW by 23.1% (Table 1), photosynthetic efficiency (i.e., gs by 26.4%, SPAD chlorophyll by 19.7%, Fv/Fm by 8.4%, and PI by 16.8%; Table 2), leaf RWC and MSI (by 15.0 and 16.6%, respectively; Table 3), respectively compared to the controls. However, treatment with 100 mM NaCl significantly increased leaf contents of antioxidants, which are non-enzymatic and have low-molecular-weights such as Pro by 22.2%, AsA by 95.3%, and GSH by 28.6% (Table 3), leaf enzymatic activities such as SOD by 8.7%, CAT by 26.1%, GR by 18.5%, and APX by 20.0%, respectively in comparison to the plants grown under control treatment (Table 4).
Treatment with 100 mM NaCl resulted in a reduction for leaf and root contents of Ca2+ and K+ by 26.8 and 36.3%, and 52.0 and 57.2% in comparison to controls, respectively (Figure 1). Moreover, it reduced the ratios of Ca2+/Na+ and K+/Na+ of leaves and roots by 87.2 and 90.6%, and 91.6 and 93.8% compared to the controls, respectively (Figure 2). Nevertheless, salt stress treatment with 100 mM NaCl resulted in an increments in the contents of root and leaf Cd2+ and Na+ by 340.1 and 759.3%, and 585.1 and 472.0%, respectively compared to the controls (Figure 1 and Figure 2).
However, the application of non-enzymatic antioxidants for cucumber seed attenuated the stress impacts of 100 mM NaCl salinity by modifying, positively, the above salt stress effects. Where compared to NaCl treatment, the individual use of each of AsA, Pro, and GSH for cucumber seed markedly elevated all of the investigated growth characteristics, photosynthetic efficiency, RWC, MSI, endogenous AsA, Pro, and GSH levels (further increase), and the activity of antioxidant enzymes; CAT, APX, GR, and SOD (further increase), the leaf and root Ca2+ and K+ contents and their ratios to Na+, while considerably reduced Cd2+ and Na+ contents in leaves and roots. However, AsA-Pro-GSH treatment significantly exceeded all of the individual applications for all of the investigated parameters. This AsA-Pro-GSH applied sequentially elevated the length of the shoot by 51.7%, total area of leaves by 49.8%, stem diameter by 23.3%, shoot FW by 41.8%, shoot DW by 60.0%, gs by 77.5%, SPAD chlorophyll by 22.5%, Fv/Fm by 10.5%, PI by 22.8%, RWC by 17.9%, and MSI by 20.8%. This best treatment also increased the activity of SOD by 52.0%, CAT by 41.4%, GR by 46.9%, and APX by 52.8%, the content of Pro by 163.6%, AsA by 162.3%, GSH by 227.8%, root K+ by 128.3%, leaf K+ by 105.9%, root Ca2+ by 51.0%, and leaf Ca2+ by 34.1%, and the ratio of root K+/Na+ by 510.5%, leaf K+/Na+ by 484.1%, root Ca2+/Na+ by 300.0%, and leaf Ca2+/Na+ by 280.4% compared to NaCl treatment without antioxidants. AsA, Pro, and GSH applied individually or sequentially were more noticeable under salt stress than their applications under the control condition, especially for the AsA-Pro-GSH applied sequentially.
4. Discussion
Salinity significantly limited cucumber transplant growth characteristics (Table 1). This growth limitation might be due to alterations in osmotic potential emerging from the limited availability of water [42]. An increased concentration of salts restricts the ability of the plant to absorb enough water and forces it to absorb Na+ and Cl‒ in excessive amounts, early emerging osmotic stress and accumulating ionic Na+ and Cl‒ stresses. This mainly impairs the metabolic processes, negatively affecting photosynthetic efficiency and limiting the growth [43]. However, sequence use of AsA-Pro-GSH conferred significant results, mitigating the devastating impacts of salinity stress. AsA, Pro, or GSH applied exogenously as individual treatments have been reported to overcome the disastrous impacts of salinity on metabolic processes related to plant growth [21,44,45,46]. However, the exogenous use of AsA, Pro, and GSH as AsA-Pro-GSH treatment has rarely been shown to attenuate the salt stress influences [28]. AsA, proline, and GSH as antioxidants have great power to counter and rectify the damages constructed by the salt-induced ROS. This enables stressed plants to qualify a complex antioxidative system to maximize defensive strategies in plant cells to tolerate the NaCl-induced oxidative stress [18,28,47]. Soaked seeds using AsA-Pro-GSH generated significantly better growth results than seeds soaked in individual AsA, Pro, or GSH solution. This may be attributed to that application of the three antioxidants in an integrative sequential method confers a balance for the so-called “AsA-GSH cycle” to effectively control the ROS radicals along with the osmoprotectant Pro to save water for cellular processes under salt stress in favor of plant growth. As proved for the AsA-Pro-GSH treatment in our earlier study [28], AsA was the most helpful when applied first because it has been involved in several biological activity types associated with oxidative stress resistance in plant cells, where it functions as a donor/acceptor in electron transport in the chloroplasts or at the cellular plasma membranes, in addition to acting as an antioxidant and enzymatic cofactor, etc. [48]. In chloroplasts, AsA is oxidized to monodehydroascorbate (MDA) in the presence of APX in the “Halliwell-Asada” pathway. MDA might trigger dehydroascorbate (DHA), which is reduced with MDA to cause regeneration of the ascorbate pool. This scavenging type can occur close to the PSII, leading to minimizing the ROS escape risk and/or their reacting with each other [49]. Also, the AsA acts as the “terminal antioxidant” due to the redox potential of the AsA/MDA pair (280 mv), which is lower than the redox potential of most bio-radicals [50]. These into the elucidation of [51] that the biosynthesis of AsA from hexose phosphate and its inclusion in protecting against the stress effects of photo-oxidation suggests that there could be links between the size of AsA pool and the photosynthesis process.
Under salt (100 mM NaCl) stress, photosynthesis efficiency attributes (i.e., gs, SPAD chlorophyll, Fv/Fm, and PI; Table 2) of cucumber transplants were significantly decreased. This may be due to the inhibited or insufficient nutrient uptake [52], which accompanied by the increased uptake of undesired elements (i.e., Na+ and Cd2+) as shown in our results (Table 4 and Table 5). It has also been explained that the diminished contents of chlorophyll could be attributed to the inhibited biosynthesis of chlorophyll and/or the increased chlorophyll-degrading enzyme chlorophyllase [53]. Since chlorophyll is reported as an indicator of oxidative damage, ROSs induced by salt also contribute to chlorophyll degradation and loss of pigment. Fv/Fm ratio reflects the master photochemistry of PSII capacity that is very susceptible to different stress impacts induced by environmental stressors [54]. The ratio of Fv/Fm shows, firmly, a noteworthy susceptibility to stress influences of salinity and it is reported as an indicator of photo-inhibition and/or other PS2 complexes injuries [55]. In the current work, our findings displayed that in the same time of which various antioxidant applications did not affect the photosynthetic efficiency attributes under normal conditions, they significantly improved these attributes in salt-stressed cucumber transplants, with a preference for the AsA-Pro-GSH treatment. The improvements in the values of SPAD chlorophyll, gs, PI, and Fv/Fm were positively reflected in transplants growth improvement. This may be elucidated according to the integrative roles giving integrative mode of actions of AsA, Pro, and GSH as a major mechanism in mitigating the impacts of salt stress in plants [28].
Table 5.
Changes (%) in seedling physiological, biochemical and growth traits, and antioxidant system components relative to the control in cucumber under normal (N) and saline (100 mM NaCl) conditions. Three color scale heatmap as follow: yellow = midpoint of control and different traits with insignificant values compared to control, green = changes over control, and red = changes below control.
| Parameters | Control (N) |
Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N+ AsA |
N+ Pro |
N+ GSH |
N+ A.P.G |
NaCl (S) |
S+ AsA |
S+ Pro |
S+ GSH |
S+ A.P.G |
||
| Shoot length | 15.6 | 9.0 | 6.4 | 15 | 17 | −24 | 7.7 | −1.3 | −3.2 | 15 |
| Leaf area | 64.0 | 10 | 8.3 | 11 | 18 | −26 | −0.9 | −4.5 | 0.2 | 10 |
| Stem diameter | 3.6 | 5.6 | 2.8 | 5.6 | 11 | −17 | 0.0 | 0.0 | 0.0 | 2.8 |
| Shoot FW | 1.71 | 17 | 19 | 18 | 19 | −18 | 14 | 16 | 16 | 19 |
| Shoot DW | 0.13 | 15 | 15 | 15 | 31 | −23 | 7.7 | 0.0 | 7.7 | 23 |
| gs | 193 | 25 | 28 | 32 | 33 | −26 | 20 | 21 | 21 | 31 |
| SPAD value | 42.1 | 0.7 | 1.7 | 1.9 | 6.2 | −20 | −7.6 | −8.3 | -7.4 | -1.7 |
| Fv/Fm | 0.83 | 0.0 | 0.0 | 0.0 | 1.2 | −8.4 | −1.2 | −1.2 | −1.2 | 1.2 |
| PI | 3.64 | 3.0 | 2.5 | 3.8 | 3.8 | −17 | −4.9 | -4.9 | −4.4 | 2.2 |
| RWC | 87.6 | 0.8 | 1.8 | 1.3 | 4.2 | −15 | −5.6 | −5.0 | −5.5 | 0.2 |
| MSI | 76.3 | 2.9 | 2.6 | 3.5 | 4.5 | −17 | −8.5 | −8.4 | −7.7 | 0.7 |
| Free proline | 0.27 | 15 | 141 | 19 | 181 | 22 | 48 | 196 | 52 | 222 |
| Ascorbate | 1.48 | 139 | 25 | 33 | 189 | 95 | 327 | 126 | 130 | 412 |
| Glutathione | 0.14 | 36 | 43 | 150 | 186 | 29 | 79 | 100 | 250 | 321 |
| SOD activity | 0.23 | 26 | 22 | 22 | 35 | 8.7 | 35 | 35 | 43 | 65 |
| CAT activity | 0.23 | 22 | 30 | 22 | 52 | 26 | 52 | 61 | 61 | 78 |
| GR activity | 0.27 | 19 | 26 | 26 | 52 | 19 | 44 | 44 | 48 | 74 |
| APX activity | 0.30 | 43 | 40 | 40 | 73 | 20 | 63 | 67 | 67 | 83 |
| Cd2+ content | 0.53 | −31 | −2.5 | −5.6 | -33 | 550 | 283 | 292 | 257 | 94 |
| K+ content | 23.8 | 2.3 | 1.0 | 2.4 | 3.4 | −55 | −28 | −28 | −22 | −1.9 |
| Ca2+ content | 13.5 | −0.4 | -2.5 | 0.9 | 1.2 | −31 | -16 | −18 | −13 | −2.8 |
| Na+ content | 2.73 | −2.8 | −1.1 | −1.9 | -2.5 | 529 | 253 | 248 | 245 | 129 |
| K+/Na+ ratio | 8.67 | 5.4 | 2.2 | 4.4 | 6.2 | −93 | −80 | −79 | −77 | −57 |
| Ca2+/Na+ ratio | 4.90 | 2.7 | −2.6 | 2.8 | 3.9 | −89 | −77 | −76 | −75 | −56 |
AsA = ascorbate, Pro = proline, GSH = glutathione, A.P.G. = ascrobate-proline-glutathione, gs = stomatal conductance, PI = performance index, RWC = relative water content, MSI = membrane stability index, CAT = catalase, GR = glutathione reductase, SOD = superoxide dismutase, APX = ascorbate peroxidase, Cd2+ = cadmium, Ca2+ = calcium, K+ = potassium, and Na+ = sodium.
Our findings reported that RWC and MSI were markedly decreased under NaCl-salt stress (Table 3), indicating the destruction of the membrane stability and increasing the membrane lipid peroxidation [22]. The undesirable alterations in the membranes that underwent stress were identified as inorganic leakage, in which salt stress was demonstrated repeatedly to induce peroxidative damage to cellular plasma membranes [56]. However, pretreatment with antioxidants, especially sequenced AsA-Pro-GSH showed significant RWC and MSI improvements under NaCl-salt stress, preventing partially or the peroxidative damage to plasma membranes especially due to the fact that MSI value of cucumber tissue was reached equally to the MSI unstressed control value that was also true for the RWC value (Table 3). [57] showed that AsA treatment prevented the peroxidation of membrane lipids and reduced malondialdehyde production as a final product of membrane lipid peroxidation, positively modifying the membrane properties and functions to minimize inorganic leakage and consequently improve the stability of cell membranes [28]. The same results were explained both with Pro [58] and GSH [21]. RWC is a proper measurement of tissue status of water in the term physiological result of cellular water scarcity, while water potential is a measurement of plant water transport in the soil-plant-atmosphere continuum [59]. It is a key marker for various studies of salinity stress. It is also a general measurement used to assess the balance of water in plant tissues over periods of water deficit and measures the amount of leafy water in a plant as a portion of the total volumetric water, which can be held in the leaf at its full aqueous capacity. In plant cells, RWC maintaining allows the metabolic activities to continue by osmotic adjustments and other attributes of salinity and/or drought adaptation [60]. The use of AsA-Pro-GSH resulted in a significant increase of RWC, helping positive modifying of the plasma membrane that is reported as a target of unacceptable environmental stressors. As found with AsA-Pro-GSH treatment, it is often proved that maintaining cell membrane stability and integrity is a key component to achieving satisfactory plant performance [28,61]. The favorable effects of the sequential AsA-Pro-GSH treatment on MSI and RWC can be explained based on the integrative positive modification of osmotic adjustment by Pro addition and improving the efficiency of AsA-GSH cycle by AsA and GSH addition for scavenging the ROS effectively. Additionally, the advantageous effects, in this regard, of AsA, Pro, and GSH, which form a pivotal component of the abiotic stress response in plant cells [21,60].
Conferring several mechanisms to overcome salt stress effects, Pro, AsA, and GSH, as low-molecular-weight antioxidants with others, comprise a major part of the plant defense system, rendering safeguarding roles to withstand oxidative stress effects [62]. Due to the fact that Pro acts as osmo- and/or enzyme protectant and might make itself as a reserve of N, as well as it considers as a free radical scavenger, there is a potent connection between its level in plant cells and the ability to withstand the effects of stress. [63]. Our findings showed a noticeable proline level in cucumber transplants (Table 3), positively reflecting in transplant growth, tissue water content, and photosynthetic efficiency (Table 1 and Table 2) by AsA, Pro, and GSH pretreatment, especially when applied in a sequencing method. This result can be obtained on account of the expression of key genes-encoding enzymes for Pro biosynthesis (the biosynthetic P5CS) and oxidizing enzyme low activities [28,64]. In addition, Pro acts to modify toxic ions (Cd2+; Figure 1 and Na+; Figure 2) and organic solute contents [64]. It was also effective in districting the oxidative damages of NaCl by the plant’s antioxidant system, which includes various enzymes and low-molecular-weight antioxidants (Table 3 and Table 4). Another mechanism that may be acted in transplants under NaCl stress, the Pro/P5C cycle transfers the NAD(P)H equivalents, which are reduced to the mitochondria to support the maintenance of the pool of NAD(P)+ [65]. In the salt-stressed leaves of the plant, there is marked ProDH activity and decreased levels of Pro and P5C. These compounds (Pro and P5C) can act as sources of N due to the increase in soluble protein and N compound contents that contribute to the osmotic adjustment [64].
As shown in Table 3, AsA and GSH levels in AsA-, Pro-, and/or GSH-pretreated salt-stressed cucumber transplants were found to be markedly greater in comparison with the control levels. The AsA-Pro-GSH as an integrative treatment was more effective than individuals. These results are in a parallel line with those of [28]. Minutely, the pool of AsA and GSH must be balanced along with appropriate APX activity to improve the plant’s antioxidant ability to avoid damage of oxidative stress [66]. AsA is a highly potent ROS scavenger due to its electron donation ability in various reactions that occurred enzymatically and non-enzymatically. It keeps safe the cell membrane integrity by direct ROS (O2•− and OH−) scavenging [67]. Since the AsA-GSH cycle contains both AsA and GSH as major components, they can control the level of H2O2 in plant cells. By forming AsA and GSH, GR together with MDHAR and DHAR the all are mostly responsible for providing substrates for APX [68]. Under the stress of salinity, Desoky et al. [69] have reported increases in the state of redox activity of AsA and GSH in conjunction with an increase in their contents to reduce the level of H2O2. The reactions occurring in the transformation of oxidized glutathione; catalyzes the reduction of oxidized glutathione to reduced glutathione; GSH are catalyzed by GR.
In addition to non-enzyme-based antioxidants that play important roles in counteracting the effects of salinity, plants make use of antioxidant enzymes. This has become evident that comparatively raised activities of enzymes that scavenge different ROS have been reached in wheat seedlings [69]. Results of the current study (Table 4) have supported this finding. Also, the antioxidative enzymes assayed in the present work such as CAT, APX, GR, and SOD have a special role in mitigating the effects of oxidative stress stimulated by NaCl salinity. The activities of these enzymes were measured in cucumber transplants under normal or 100 mM NaCl stress in response to AsA, Pro, or GSH (singly) or AsA-Pro-GSH (sequential method as antioxidative integration). Our results showed that the activities of all enzymes raised under the stress of NaCl salinity and further increased with antioxidants pretreatment, especially with AsA-Pro-GSH pretreatment. Because SOD is an effective O2•– scavenger, it is the plant’s first defense employee against ROS [28], demonstrating the SOD defensive role for biosystems. Besides, CAT is considered to be the main scavenger of H2O2, producing H2O and O2. It may also be a protective agent against the formation of OH– radicals, which peroxidize cell membrane lipids and severely affect plant growth [70]. As CAT does, APX eliminates H2O2, and its elevated activity has also been noticed under salinity in different plant species [25,69]. Salt stress stimulates excess ROS accumulation in plant cells such as H2O2, which its metabolism depends on several antioxidant enzymes that functionally interconnected to eliminate H2O2 from cells of stressed plants [71,72].
AsA, Pro, and GSH contribute to reduce the salt-induced K+ efflux and increase Ca2+/Na+ and K+/Na+ ratios in the transplants stressed with NaCl salinity (Figure 1 and Figure 2), conferring proper ionic homeostasis in salt-stressed transplants. This suggests an effective mechanism in the roots of transplants stressed with NaCl salinity to avert Na+ xylem tonnage. Another efficient mechanism that may occur by applied antioxidants is the compartmentalization of excess Na+ authorizing a maximal K+ influx to transplant leaves [24,73,74], leading to an increased cytosolic ratio of K+/Na+ (along with Ca2+/Na+ ratio) that represents a crucial indicator for tolerance to salinity stress in plants [69]. An adaptive mechanism such as elevated K+ re-uptake lets plant cell to avert starvation of K+ under increased salts. Also, the three applied antioxidants may have integrated crucial roles as the main mechanism in reducing Cd2+ content in transplants (Figure 1). This may be due to the partitioning of Cd2+ to different organs to overcome the toxicity impacts of Cd2+ in the transplants of cucumber. Exogenous AsA-Pro-GSH improved ROS removal and metal ions chelation activities, establishing an important portion of the plant cell response to abiotic stress [24].
In general, sequential pretreatment with AsA-Pro-GSH resulted in the best findings as compared to their individuals, attenuating the stress harmful impacts of NaCl salinity. Exogenously applied AsA-Pro-GSH as a sequential pretreatment has been proved to attenuate the stress adverse impacts of 100 mM NaCl salinity on metabolic processes related to plant growth [28]. These antioxidants also reduced the Na+ and Cd2+ ion contents within plants (Figure 1 and Figure 2) due to the decreased uptake of these injurious ions and/or compartmentalization of them into transplant organs.
If a look is taken at the potential plant salt tolerance mechanisms proved in several works and the supporting role of exogenous AsA-Pro-GSH pretreatment performed in the current study, it has been found that: (1) Accumulation of osmotic adjustment substances is an important salt tolerance mechanism. This mechanism is supported by the exogenous addition of Pro in the AsA-Pro-GSH application, wherein plant cells Pro is considered to be a primary substance for osmotic adjustment and is also acted as a scavenger of ROS, a buffer of redox reactions, and/or molecular chaperone. These potential functions of Pro contribute to stabilizing the structures of plasma membranes and proteins under the conditions of stress, reflecting positive results in our study. (2) Selective absorption of ions and their compartmentalization is another pivotal mechanism of tolerance to salinity stress in plants. A high cytosolic K+/Na+ ratio contributes to the cells emptying of Na+ ions or conveying them to the region of inactive metabolism, the translocation of Na+ ions to the extracellular zone by the Na+/H+ antiporter at plasma membranes, and/or the partitioning of Na+ by the Na+/H+ antiporter in the vacuoles [75]. Besides, the gene A. thaliana AtNHX1 encodes the Na1yH1 antiporter at the tonoplast and is functioned by compartmentalization of Na+ into the cell vacuoles [76]. These results are in a parallel line with our results concerning the reduction of Na+ ion and the increase of K+ ion and K+/Na+ ratio by AsA-Pro-GSH application. (3) Enzymatically or non-enzymatically scavenging of the ROS by various antioxidants is a very crucial salt tolerance mechanism. The exogenous application of AsA, Pro, and GSH (non-enzymatic, low-molecular-weight antioxidants) in the sequential AsA-Pro-GSH treatment significantly supported their endogenous concentrations to effectively scavenge the ROS, reflecting in positive results in our study. These important mechanisms may be supported by another crucial salt tolerance mechanism; (4) Salt tolerance genes. Tolerance to the effects of salinity stress is a polygenic genetic trait. The plasma membrane Na+/H+ antiporter gene SOS1 and vacuolar Na+/H+ antiporter gene AtNHX1 in A. thaliana, in addition to rice OsbZIP71 gene and wheat genes Ta-UPnP, TaZNF, TaSST, TaDUF1, and TaSP are proved to promote the tolerance to the impacts of salinity stress in transgenic plants [75,77,78].
Improvements occurred by exogenous antioxidants in the seedling photosynthetic efficiency (Table 2 and Table 5), the relative content of water and stability index of cell membranes (Table 3 and Table 5), endogenous levels of AsA, free proline, and glutathione (Table 3 and Table 5), and the activities of antioxidative enzymes (Table 4 and Table 5) positively affected the growth traits of salt-stressed cucumber seedlings. Besides, the increased K+ and Ca2+ contents contributed to higher ratios of Ca2+/Na+ and K+/Na+, which were associated with a markedly lower Na+ content with the exogenous use of AsA-Pro-GSH (Table 5; Figure 1 and Figure 2) and all finally contributed to the increases in growth traits of salt-stressed cucumber seedlings (Table 1 and Table 5).
5. Conclusions
When applied exogenously as individuals or sequentially to soaking the seeds of cucumber, AsA, Pro, and GSH significantly enhanced the transplant water content and the cell membranes stabilities. Besides, photosynthetic activity, nutrients contents, and antioxidant defense systems were also improved along with the decrease of Na+ and Cd2+ contents under 100 mM NaCl-salt stress. These positive findings have led to the healthy growth of cucumber transplants. Pretreatment with AsA-Pro-GSH applied sequentially was more effective than any of the three individuals; Pretreatment with AsA, Pro, or GSH. Therefore, the elevation of the tolerance to salinity stress impacts in the transplants of cucumber, and the improvement of transplant growth and health would occur with pretreatment with AsA-Pro-GSH applied sequentially upon growth under 100 mM NaCl-salt stress.
Acknowledgments
The authors extend their appreciation to Taif University for funding current work by Taif University Researchers Supporting Project number (TURSP-2020/59), Taif University, Taif, Saudi Arabia. This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Author Contributions
Conceptualization, W.M.S., M.F.S., M.M.R., G.F.M., K.A.H., B.A.A., A.S. and M.M.H.; Data curation, W.M.S., M.M.R., G.F.M., K.A.H., and M.M.H.; Formal analysis, W.M.S., M.F.S., M.M.R., G.F.M., K.A.H. and B.A.A., A.S.; Investigation, W.M.S., M.F.S., M.M.R., G.F.M., and K.A.H., B.A.A., A.S.; Methodology, W.M.S., M.M.R., G.F.M., K.A.H.; Resources, W.M.S., M.F.S., M.M.R., G.F.M., and K.A.H.; Software, M.F.S., M.M.R., B.A.A. and M.M.H., A.S.; Writing—original draft, W.M.S., M.F.S., M.M.R., G.F.M., K.A.H., B.A.A., A.S. and M.M.H.; Writing—review and editing, W.M.S., M.F.S., M.M.R., G.F.M., K.A.H., B.A.A., A.S. and M.M.H. All authors have read and agree to the published version of the manuscript.
Funding
The current work was funded by Taif University Researchers Supporting Project number (TURSP-2020/59), Taif university, Taif, Saudi Arabia. This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rady M.O.A., Semida W.M., El-Mageed T.A.A., Hemida K.A., Rady M.M. Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Hortic. 2018;240:614–622. doi: 10.1016/j.scienta.2018.06.069. [DOI] [Google Scholar]
- 2.Kumar N., Pal M., Singh A., SaiRam R.K., Srivastava G.C. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L. ‘Grand Gala’) Sci. Hortic. 2010;127:79–85. doi: 10.1016/j.scienta.2010.09.009. [DOI] [Google Scholar]
- 3.Jones R.W., Jr., Pike L.M., Yourman L.F. Salinty influences cucumber growth and yield. J. Am. Soc. Hortic. Sci. 1989;114:547–551. [Google Scholar]
- 4.Abu-zinada I.A. Effect of salinity levels and application stage on cucumber and soil under greenhouse condition. Int. J. Agric. Crop Sci. 2015;8:73–80. [Google Scholar]
- 5.Furtana G.B., Tipirdamaz R. Physiological and antioxidant response of three cultivars of cucumber (Cucumis sativus L.) to salinity. Turk. J. Biol. 2010;34:287–296. doi: 10.3906/biy-0812-10. [DOI] [Google Scholar]
- 6.Peykanpour E., AM G., Fallahzade J., Najarian M. Interactive effects of salinity and ozonated water on yield components of cucumber. Plant Soil Environ. 2016;62:361–366. doi: 10.17221/170/2016-PSE. [DOI] [Google Scholar]
- 7.Wan S., Kang Y., Wang D., Liu S. Effect of saline water on cucumber (Cucumis sativus L.) yield and water use under drip irrigation in North China. Agric. Water Manag. 2010;98:105–113. doi: 10.1016/j.agwat.2010.08.003. [DOI] [Google Scholar]
- 8.Wu Y., Jin X., Liao W., Hu L., Dawuda M.M., Zhao X. 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018;9:1–16. doi: 10.3389/fpls.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildirim B., Yasar F., Özpay T., Türközü D., Terziodlu Ö., Tamkoç A. Variations in response to salt stress among field pea genotypes (Pisum sativum sp.arvense L.) J. Anim. Vet. Adv. 2008;7:907–910. [Google Scholar]
- 10.Seleiman M.F., Kheir A.S. Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere. 2018;193:538–546. doi: 10.1016/j.chemosphere.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 11.Seleiman M.F. Use of plant nutrients in improving abiotic stress tolerance in wheat. In: Hasanuzzaman M., Nahar K., Hossain A., editors. Wheat Production in Changing Environments: Management, Adaptation and Tolerance. Springer Nature; Singapore: 2019. pp. 481–495. [Google Scholar]
- 12.Seleiman M.F., Kheir A.M.S., Al-Dhumri S., Alghamdi A.G., Omar E.H., Aboelsoud H.M., Abdella K.A., AbouElHassan W.H. Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy. 2019;9:233. doi: 10.3390/agronomy9050233. [DOI] [Google Scholar]
- 13.Al-Ashkar I., Alderfasi A., Ben Romdhane W., Seleiman M.F., El-Said R.A., Al-Doss A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants. 2020;9:287. doi: 10.3390/plants9030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha R.S., Seleiman M.F., Alotaibi M., Alhammad B.A., Rady M.M., Mahdi A.H.A. Exogenous potassium treatments elevate salt tolerance and performances of glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy. 2020;10:1741. doi: 10.3390/agronomy10111741. [DOI] [Google Scholar]
- 15.Ding Z., Kheir A.S., Ali O.A., Hafez E., Elshamey E.A., Zhou Z., Wang B., Lin X., Ge Y., Fahmy A.F., et al. Vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2020;277:111388. doi: 10.1016/j.jenvman.2020.111388. [DOI] [PubMed] [Google Scholar]
- 16.Bargaz A., Nassar R.M.A., Rady M.M., Gaballah M.S., Thompson S.M., Brestic M., Schmidhalter U., Abdelhamid M.T. Improved salinity tolerance by phosphorus fertilizer in two phaseolus vulgaris recombinant inbred lines contrasting in their P-efficiency. J. Agron. Crop Sci. 2016 doi: 10.1111/jac.12181. [DOI] [Google Scholar]
- 17.Semida W.M., Abd El-Mageed T.A., Hemida K., Rady M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019;94:632–642. doi: 10.1080/14620316.2019.1592711. [DOI] [Google Scholar]
- 18.Abd El-Mageed T.A., Semida W.M., Mohamed G.F., Rady M.M. Combined effect of foliar-applied salicylic acid and deficit irrigation on physiological—Anatomical responses, and yield of squash plants under saline soil. S. Afr. J. Bot. 2016;106:8–16. doi: 10.1016/j.sajb.2016.05.005. [DOI] [Google Scholar]
- 19.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 20.Baker N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008;113:59–89. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 21.Rady M.M., Taha S.S., Kusvuran S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Sci. Hortic. 2018;62:361–366. doi: 10.1016/j.scienta.2018.01.047. [DOI] [Google Scholar]
- 22.Zhu J., Bie Z., Li Y. Physiological and growth responses of two different salt-sensitive cucumber cultivars to NaCl stress. Soil Sci. Plant Nutr. 2008;54:400–407. doi: 10.1111/j.1747-0765.2008.00245.x. [DOI] [Google Scholar]
- 23.Parida A.K., Das A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Semida W.M., Hemida K.A., Rady M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018;154:171–179. doi: 10.1016/j.ecoenv.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Desoky E.M., El-maghraby L.M.M., Awad A.E., Abdo A.I., Rady M.M., Semida W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata) Sci. Hortic. 2020;272:109576. doi: 10.1016/j.scienta.2020.109576. [DOI] [Google Scholar]
- 26.Ejaz B., Sajid Z.A., Aftab F. Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp. hybrid cv. HSF-240 under salt stress. Turk. J. Biol. 2012;36:630–640. doi: 10.3906/biy-1201-37. [DOI] [Google Scholar]
- 27.Rady M.M., Taha R.S., Semida W.M., Alharby H.F. Modulation of salt stress effects on vicia faba l. plants grown on a reclaimed-saline soil by salicylic acid application. Rom. Agric. Res. 2017;34:175–185. [Google Scholar]
- 28.Rady M.M., Hemida K.A. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016;133:252–259. doi: 10.1016/j.ecoenv.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Rady M.M., Hemida K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015;119:178–185. doi: 10.1016/j.ecoenv.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Semida W.M., El-Mageed T.A.A., Mohamed S.E., El-Sawah N.A. Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch. Agron. Soil Sci. 2017;63:1227–1239. doi: 10.1080/03650340.2016.1264579. [DOI] [Google Scholar]
- 31.Maxwell K., Johnson G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 32.Rady M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011;129:232–237. doi: 10.1016/j.scienta.2011.03.035. [DOI] [Google Scholar]
- 33.Bates L.S., Waldeen R.P., Teare I.D. Rapid determination of free proline for water—Stress studies. Plant Soil. 1973;207:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 34.Kampfenkel K., Vanmontagu M., Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 35.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 36.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 37.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 38.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 39.Rao M.V., Paliyath G., Ormrod P. Ultraviolet-9- and ozone-lnduced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams V., Twine S. Modern Methods of Plant Analysis. Springer; Berlin/Heidelberg, Germany: 1960. Flame photometric method for sodium, potassium and calcium; pp. 3–5. [Google Scholar]
- 41.Chapman H.D., Pratt P.F. Methods of Analysis for Soil, Plants and Water. University of California, Division of Agricultural Science; Berkeley, CA, USA: 1961. [Google Scholar]
- 42.Mousavi S.A.R., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- 43.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 44.Aliniaeifard S., Hajilou J., Tabatabaei S.J., Sifi-Kalhor M. Effects of ascorbic acid and reduced glutathione on the alleviation of salinity stress in olive plants. Int. J. Fruit Sci. 2016;16:395–409. doi: 10.1080/15538362.2015.1137533. [DOI] [Google Scholar]
- 45.Rady M.M., Semida W.M., Hemida K.A., Abdelhamid M.T. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int. J. Recycl. Org. Waste Agric. 2016;5:311–321. doi: 10.1007/s40093-016-0141-7. [DOI] [Google Scholar]
- 46.Shalata A., Neumann P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J. Exp. Bot. 2001;52:2207–2211. doi: 10.1093/jexbot/52.364.2207. [DOI] [PubMed] [Google Scholar]
- 47.Wutipraditkul N., Wongwean P., Buaboocha T. Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biol. Plant. 2015;59:547–553. doi: 10.1007/s10535-015-0523-0. [DOI] [Google Scholar]
- 48.Conklin P.L. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001;24:383–394. doi: 10.1046/j.1365-3040.2001.00686.x. [DOI] [Google Scholar]
- 49.Foyer C.H., Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between Stress perception and physiological responses. Plant Cell Online. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scandalios J.G., Guan L., Polidoros A.N. Catalases in plants: Gene structure, properties, regulation, and expression. Cold Spring Harb. Monogr. Ser. 1997;34:343–406. [Google Scholar]
- 51.Reddy K.R., Kakani V.G., Zhao D., Koti S., Gao W. Interactive effects of ultraviolet-B radiation and temperature on cotton physiology, growth, development and hyperspectral reflectance. Photochem. Photobiol. 2004;79:416–427. doi: 10.1562/2003-11-19-RA.1. [DOI] [PubMed] [Google Scholar]
- 52.Kanokwan S., Tanatorn S., Aphichart K. Effect of salinity stress on antioxidative enzyme activities in tomato cultured in vitro. Pak. J. Bot. 2015;47:1–10. [Google Scholar]
- 53.Rezende R.A.L.S., Rodrigues F.A., Soares J.D.R., Silveira H.R.O., Pasqual M., Dias G.M.G. Salt stress and exogenous silicon influence physiological and anatomical features of in vitro-grown cape gooseberry. Cienc. Rural. 2018;48 doi: 10.1590/0103-8478cr20170176. [DOI] [Google Scholar]
- 54.Sheng M., Tang M., Chen H., Yang B., Zhang F., Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18:287–296. doi: 10.1007/s00572-008-0180-7. [DOI] [PubMed] [Google Scholar]
- 55.Ranjbarfordoei A., Samson R., Van Damme P. Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica. 2006;44:513–522. doi: 10.1007/s11099-006-0064-z. [DOI] [Google Scholar]
- 56.Younis M.E., Mohamed S., Tourky N. Influence of salinity and adaptive compounds on growth parameters, carbohydrates, amino EISSN: Acids and nucleic acids in two cultivars of Vicia faba contrasting in salt tolerance. Plant Knowl. J. 2014;3:47–56. [Google Scholar]
- 57.Dolatabadian A., Jouneghani R.S. Impact of exogenous ascorbic acid on antioxidant activity and some physiological traits of common bean subjected to salinity stress. Not. Bot. Horti Agrobot. Cluj-Napoca. 2009;37:165–172. doi: 10.15835/nbha3723406. [DOI] [Google Scholar]
- 58.Valizadeh M., Asgari A., Shiri M. Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. J. BioSci. Biotechnol. 2015;4:313–319. [Google Scholar]
- 59.Darvishan M., Tohidi-moghadam H.R., Zahedi H. The effects of foliar application of ascorbic acid (vitamin C) on physiological and biochemical changes of corn (Zea mays L) under irrigation withholding in different growth stages. Maydica. 2013;58:195–200. [Google Scholar]
- 60.Slabbert M.M., Krüger G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014;95:123–128. doi: 10.1016/j.sajb.2014.08.008. [DOI] [Google Scholar]
- 61.Rady M.M., Abd El-Mageed T.A., Abdelhamid M.T., Abd El-Azeem M.M.M. Integrative potassium humate and biochar application reduces salinity effects and contaminants and improves growth and yield of eggplant grown under saline conditions. Int. J. Empir. Educ. Res. 2018;1:37–56. doi: 10.35935/edr/22.5637. [DOI] [Google Scholar]
- 62.Valero E., Macià H., De la Fuente I.M., Hernández J.A., González-Sánchez M.I., García-Carmona F. Modeling the ascorbate-glutathione cycle in chloroplasts under light/dark conditions. BMC Syst. Biol. 2016;10:11. doi: 10.1186/s12918-015-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sairam R.K., Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004;86:407–421. [Google Scholar]
- 64.Rady M.M., Kuşvuran A., Alharby H.F., Alzahrani Y., Kuşvuran S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019;38:449–462. doi: 10.1007/s00344-018-9860-5. [DOI] [Google Scholar]
- 65.Miller G., Honig A., Stein H., Suzuki N., Mittler R., Zilberstein A. Unraveling Δ1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 2009;84:19–36. doi: 10.1074/jbc.M109.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foyer C.H., Noctor G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semida W.M., Rady M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014;89:338–344. doi: 10.1080/14620316.2014.11513088. [DOI] [Google Scholar]
- 68.Zhou Y., Wen Z., Zhang J., Chen X., Cui J., Xu W., Liu H.-y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017;220:90–101. doi: 10.1016/j.scienta.2017.02.021. [DOI] [Google Scholar]
- 69.Desoky E.S.M., Elrys A.S., Rady M.M. Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxicol. Environ. Saf. 2019;169:50–60. doi: 10.1016/j.ecoenv.2018.10.117. [DOI] [PubMed] [Google Scholar]
- 70.Abogadallah G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010;5:369–374. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S.-Y., Lim J.-H., Park M.-R., Kim Y.-J., Park T.-I., Seo Y.-W., Choi K.-G., Yun S.-J. Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. BMB Rep. 2005;38:218–224. doi: 10.5483/BMBRep.2005.38.2.218. [DOI] [PubMed] [Google Scholar]
- 72.Desoky E.M., Merwad A.M., Semida W.M., Ibrahim S.A., El-saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198:110685. doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- 73.Assaha D.V.M., Ueda A., Saneoka H., Al-Yahyai R., Yaish M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017;8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abd El-mageed T.A., Abd El-mageed S.A., Semida W.M., Rady M.O.A. Silicon Defensive Role in Maize (Zea mays L.) against Drought Stress and Metals-Contaminated Irrigation Water. Silicon. 2020 doi: 10.1007/s12633-020-00690-0. [DOI] [Google Scholar]
- 75.Liang W., Ma X., Wan P., Liu L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018;495:286–291. doi: 10.1016/j.bbrc.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Gaxiola R.A., Rao R., Sherman A., Grisafi P., Alper S.L., Fink G.R. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA. 1999;96:1480–1485. doi: 10.1073/pnas.96.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He C., Yan J., Shen G., Fu L., Holaday A.S., Auld D., Blumwald E., Zhang H. Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiol. 2005;46:1848–1854. doi: 10.1093/pcp/pci201. [DOI] [PubMed] [Google Scholar]
- 78.Liu C., Mao B., Ou S., Wang W., Liu L., Wu Y., Chu C., Wang X. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2014;84:19–36. doi: 10.1007/s11103-013-0115-3. [DOI] [PubMed] [Google Scholar]