Abstract
Maturity-onset diabetes of the young, or MODY-monogenic diabetes, is a not-so-rare collection of inherited disorders of non-autoimmune diabetes mellitus that remains insufficiently diagnosed despite increasing awareness. These cases are important to efficiently and accurately diagnose, given the clinical implications of syndromic features, cost-effective treatment regimen, and the potential impact on multiple family members. Proper recognition of the clinical manifestations, family history, and cost-effective lab and genetic testing provide the diagnosis. All patients must undergo a thorough history, physical examination, multigenerational family history, lab evaluation (glycated hemoglobin A1c [HbA1c], glutamic acid decarboxylase antibodies [GADA], islet antigen 2 antibodies [IA-2A], and zinc transporter 8 [ZnT8] antibodies). The presence of clinical features with 3 (or more) negative antibodies may be indicative of MODY-monogenic diabetes, and is followed by genetic testing. Molecular genetic testing should be performed before attempting specific treatments in most cases. Additional testing that is helpful in determining the risk of MODY-monogenic diabetes is the MODY clinical risk calculator (>25% post-test probability in patients not treated with insulin within 6 months of diagnosis should trigger genetic testing) and 2-hour postprandial (after largest meal of day) urinary C-peptide to creatinine ratio (with a ≥0.2 nmol/mmol to distinguish HNF1A- or 4A-MODY from type 1 diabetes). Treatment, as well as monitoring for microvascular and macrovascular complications, is determined by the specific variant that is identified. In addition to the diagnostic approach, this article will highlight recent therapeutic advancements when patients no longer respond to first-line therapy (historically sulfonylurea treatment in many variants).
Learning Objectives
Upon completion of this educational activity, participants should be able to:
Identify risk factors and learn when to suspect MODY-monogenic diabetes
Obtain appropriate diagnostic testing and evaluation prior to treatment
Determine appropriate monitoring and treatment for microvascular and macrovascular comorbidities
Recognize the recommended approach to treatment and monitoring that was performed in a patient with MODY-monogenic diabetes.
Target Audience
This continuing medical education activity should be of substantial interest to endocrinologists and all health care professionals who care for people with diabetes mellitus.
Keywords: MODY, diagnosis, treatment, pregnancy, monitoring
Maturity-onset diabetes of the young (MODY), the historic term for some forms of monogenic diabetes, is a collection of inherited disorders of non-autoimmune diabetes mellitus (antibody-negative) that present at a young age of onset. The prevalence is estimated to be 1/10 000 in adults and 1/23 000 in children, although the prevalence of MODY in different ethnic and racial groups may be underrepresented (3-5) as studies thus far have predominantly involved White European populations (6). GCK mutations have been estimated to be as high ~1/1000 individuals (7).
Relevant mutations in multiple genes (more than 15) have been associated with a MODY-monogenic phenotype with different therapeutic strategies (Table 1), in addition to different microvascular and macrovascular complication risks. Experts in the field are moving away from listing these as MODY-X (where “X” denotes the corresponding numerical MODY) and now prefer simply using the gene name for clarity, such as HNF1A diabetes (or HNF1A-MODY). Some of these MODY-X reports are now in doubt, as additional data on these variants has accumulated. Monogenic diabetes reported by genetic testing companies associated with variants in BLK, KLF11, NEUROD1, PAX4, and PDX1 should be carefully reviewed with experts in the field before coming to the conclusion that these are pathogenic. This is due to variants in BLK, KLF11, NEUROD1, PAX4, and PDX1 being reported in the Human Gene Mutation Database (HGMD) as pathogenic, but are present in the Exome Aggregation Consortium (ExAC) Database in the Genome Aggregation Database (gnomAD) at population frequencies that are not consistent with their potential clinical significance (6). Therefore, we recommend reviewing these variants with experts before determining their clinical significance. Although MODY-monogenic diabetes is rare, the diagnosis, treatment, and screening for complications have important implications and will be emphasized throughout this article.
Table 1.
MODY-Monogenic Diabetes: Gene, Protein, Function, Treatment, and Inheritance
| Gene (OMIM) | Protein | Function | Treatment | Inheritance | References |
|---|---|---|---|---|---|
| HNF4A (600281) – Formerly MODY1 | Hepatocyte nuclear factor 4α | Beta-cell transcription factor | First-line treatment: Sulfonylurea | Autosomal dominant | (3, 6, 8, 9) |
| Second-line treatments: GLP-1 RA (10), insulin | |||||
| GCK (138079) – Formerly MODY2 | Glucokinase | Glucose-sensor, first rate-limiting enzyme in glycolysis | No medication or diet, unless pregnancy (see Tables 2A and 2B) | Autosomal dominant | (6, 7, 11) |
| OR | |||||
| Neonatal diabetes: Autosomal recessive (neonatal cases) | |||||
| HNF1A (142410) – Formerly MODY3 | Hepatocyte nuclear factor 1α | Beta-cell transcription factor | First-line: Low-dose sulfonylurea or meglitinides | Autosomal dominant | (6, 12–17) |
| Second-line treatments: GLP-1 RA, DPP-4 inhibitors, insulin | |||||
| PDX1 a (606392) – Formerly MODY4 | Pancreas/duodenum homeobox protein 1 | Pancreatic and beta-cell development and function | First-line: OHAs/sulfonylureas | Most are Autosomal recessive | (6, 18, 19) |
| Second-line: Insulin | |||||
| HNF1B (189907) – Formerly MODY5 | Hepatocyte nuclear factor 1β | Beta-cell transcription factor | Minority respond to sulfonylureas, insulin | Autosomal dominant | (6, 20–23) |
| NEUROD1 a (601724) – Formerly MODY6 | Neurogenic differentiation factor 1 | Beta-cell transcription factor | First-line: Diet, OHA/sulfonylureas, | Autosomal dominant | (6, 24, 25) |
| Second-line: Insulin | |||||
| KLF11 a (603301) – Formerly MODY7 | Krueppel-like factor 11 | Zinc finger transcription factor that binds to SP-1-like sequences in epsilon and gamma-globin gene promoters. This binding (when functioning normally) inhibits cell growth and apoptosis | Insulin | Autosomal Dominant | (6, 18, 19) |
| CEL (114840) – Formerly MODY8 | Carboxyl ester lipase | Exocrine pancreas function (if mutated, leads to pancreatic atrophy and exocrine pancreatic insufficiency) Fibrosis and lipomatosis leading to diabetes | First-line: Oral hypoglycemic agents (OHAs)/sulfonylureas | Deletion of variable number of tandem repeat | (6, 26) |
| Second-line: Insulin (the pancreas needs to be damaged/destroyed to necessitate treatment) | |||||
| PAX4 a (167413) – Formerly MODY9 | Paired box 4 | Differentiation of endoderm-derived endocrine pancreas | First-line: Diet, OHAs/sulfonylureas | Autosomal dominant | (6, 18, 19) |
| Second-line: Insulin | |||||
| INS (176730) – Formerly MODY10 | Insulin | Production of insulin or insulin action | Diet, OHAs/sulonfylureas or insulin (may be small doses of insulin) | Autosomal dominant | (6, 27, 28) |
| OR | |||||
| Neonatal diabetes: Dominant, often de novo or recessive | |||||
| BLK a (191305) – Formerly MODY11 | Tyrosine-protein kinase BLK (Nonreceptor tyrosine kinase of the src family of proto-oncogenes) | Expressed in β-cells where it enhances insulin synthesis and secretion in response to glucose by up-regulating transcription factors Pdx1 and Nkx6.1 | First-line: Diet, OHAs/sulfonylureas | Autosomal dominant | (6, 18, 19, 29) |
| Second-line: Insulin | |||||
| ABCC8 (600509) – Formerly MODY12 | Sulfonylurea receptor subunit of β-cell KATP channel | Closure of ATP-sensitive potassium channel leads to beta-cell membrane depolarization, calcium influx and fusion of insulin secretory granules with β-cell membrane | First-line: Sulfonylurea | Autosomal Dominant | (6, 30–32) |
| Second-line: SGLT-2 inhibitors, insulin | |||||
| OR | |||||
| Neonatal diabetes: Dominant, often de novo or recessive | |||||
| KCNJ11 (600937) – Formerly MODY13 | Potassium channel subunit of β-cell KATP channel | Closure of the ATP-sensitive potassium channel leads to beta-cell membrane depolarization, calcium influx and fusion of insulin secretory granules with β-cell membrane | OHAs/sulfonylurea, insulin | Autosomal dominant | (6, 33, 34) |
| OR | |||||
| Neonatal diabetes: Dominant, often de novo | |||||
| APPL1 (604299) - Formerly MODY14 | Adaptor protein, phosphotyrosine interaction, PH domain, and leucine zipper containing 1 | Protein that bind to AKT in the insulin-signaling pathway | First-line: Diet, OHAs/sulfonylureas | Autosomal dominant | (6, 35) |
| Second-line: insulin | |||||
| WSF1 (606201) | Wolframin | Function of the endoplasmic reticulum | Multidisciplinary approach (DI, DM, hypogonadism, psychiatric manifestations, neurologic manifestations) | Autosomal recessive | (18, 36) |
aVariants in APPL1, CEL, BLK, KLF11, NEUROD1, PAX4, and PDX1 have previously been published as variants at population frequencies that are not consistent with clinical significance (6). This is due to variants in BLK, KLF11, NEUROD1, PAX4, and PDX1 being reported in the Human Gene Mutation (HGMD) as pathogenic, but are present in the Exome Aggregation Consortium (ExAC) Database in the Genome Aggregation Database (gnomAD) at population frequencies that are not consistent with their potential clinical significance (6). Additional studies are needed and consultation with an expert is necessary to understand their association with clinical pathogenesis.
Abbreviations: DPP-4, dipeptidyl peptidase-4; GLP-1 RA, glucagon-like peptide-1 receptor agonist; KATP, potassium-sensitive ATP channel; MODY, maturity-onset diabetes of the young; OHA, oral hypoglycemia agent; SGLT-2, sodium–glucose co-transporter-2.
Given the significant implications of MODY-monogenic diabetes, this article aims to provide the reader with a comprehensive approach to diagnostic testing, treatment, and screening for complications with relevance to the currently known MODY-monogenic diabetes variants. In this article, a patient case of MODY-monogenic diabetes is presented and the relevant literature for diagnostic testing, treatment, and screening for complications is discussed.
Patient Case
A 20-year-old man of Indian descent (South Asian) is referred for evaluation of new-onset hyperglycemia. He had a medical history significant for anxiety, tachycardia, and neonatal hypoglycemia. His father had a history of diabetes diagnosed at age 30, and a paternal grandfather with diabetes diagnosed at age 28. His mother had gestational diabetes mellitus during pregnancy (which resolved after delivery), and he was born at 37 weeks with a birth weight of 6 pounds 0 ounces (2721 grams) via cesarean section for fetal distress. He was treated in the neonatal intensive care unit (NICU) in the neonatal period for hypoglycemia. He had been maintained on diazoxide from infancy until age 18, at which point his hypoglycemia and neuroglycopenic symptoms resolved and his diazoxide was tapered off. On examination, blood pressure was 116/67 mmHg; heart rate 70 beats per minute; weight 71.6 kg; height 174 cm; and body mass index 23.6 kg/m2. He was thin, muscular, and without acanthosis nigricans. He had normal distribution of body fat and his feet were sensitive to 10-gram monofilament exam bilaterally. His glycated hemoglobin A1c (HbA1c) measurement was 6.7%, increased from 6.4% at the last office visit with his pediatric endocrinologist 3 months prior.
A fasting glucose measurement with concomitant C-peptide, HbA1c, and 3 antibody tests (glutamic acid decarboxylase antibodies [GADA], islet antigen 2 antibodies [IA-2A], and zinc transporter 8 [ZnT8] antibodies) were obtained.
Diagnostic Strategy and Evaluation
Monogenic defects that cause beta-cell dysfunction, including MODY-monogenic diabetes, represent a small but important fraction of patients with diabetes (<5%). The American Diabetes Association recommends that all children diagnosed with diabetes in the first 6 months of life (or patients who report at a later date they were diagnosed at age ≤6 months) should have immediate genetic testing for neonatal diabetes (a form of monogenic diabetes) (37-39). Additionally, children and those diagnosed in early adulthood who have diabetes not characteristic of type 1 or type 2 diabetes that occurs in successive generations (suggestive of an autosomal dominant pattern of inheritance) should have genetic testing for MODY-monogenic diabetes (39).
MODY-monogenic diabetes is characterized by the onset of hyperglycemia at an early age (classically before age 25 years, although diagnosis may occur at older ages). Beta-cell–specific gene pathways cause impaired insulin secretion with minimal or no defects in insulin action (in the absence of coexistent obesity), but genes related to specific defects in insulin action are not considered here (InsR [insulin receptor gene] for example) (39). Inheritance in most cases is autosomal dominant. For individuals with MODY-monogenic diabetes, genetic testing should be performed due to the significant treatment implications, identification of other family members, and its cost-effectiveness and increasing availability (Table 1) (3, 40).
Clinical characteristics
A diagnosis of MODY-monogenic diabetes should be considered in individuals who have atypical features of diabetes based on age <35 (with age <25 being more suggestive), negative antibodies, the presence of neonatal hypoglycemia and/or multiple family members with diabetes not characteristic of type 1 or type 2 diabetes (see Clinical Characteristics below) (1, 3-5, 40, 41). In most cases, the presence of antibodies for type 1 diabetes precludes further testing, although the presence of coexistent type 1 diabetes with MODY-monogenic diabetes has been reported (42, 43).
Clinical characteristics of MODY-monogenic diabetes
• Presence of transient neonatal hyperinsulinemic hypoglycemia (in HNF4A-MODY)
• Family history of diabetes with parent affected by MODY-monogenic diabetes
• Early-onset diabetes in adolescence or young adulthood (typically age <35 years; <25 years with higher likelihood);
-
• Features atypical for type 1 diabetes mellitus including:
o Absence of pancreatic antibodies, especially when measured at diagnosis (44)
o Low insulin requirement for treatment (eg, <0.5 U/kg/d).
o Evidence of endogenous insulin production outside the “honeymoon” phase (after more than 3-5 years after diagnosis of diabetes) with detectable C-peptide (>0.6 ng/mL [>0.2 nmol/L] [>200 pmol/L]) when glucose >72 mg/dL (>4 mmol/L) that will persist (more than 3-5 years) (44-47)
o Lack of ketoacidosis when insulin omitted from treatment
-
• Features atypical for type 2 diabetes mellitus including:
o Onset of diabetes before age 45 years with a normal or low body mass index
o Lack of acanthosis nigricans
o Normal triglyceride levels and/or normal or elevated high-density lipoprotein cholesterol (HDL-C) seen in HNF1A-MODY.
• Mild, stable fasting hyperglycemia that does not progress or respond appreciably to pharmacologic therapy
• Extreme sensitivity to sulfonylureas
• Extrapancreatic features (eg, renal, hepatic, gastrointestinal)
• A personal history of family history of neonatal diabetes or neonatal hypoglycemia
-
• A family history of diabetes consistent with autosomal dominant inheritance that contrasts with type 1 diabetes and type 2 diabetes in the following ways:
o Type 1 diabetes can run in families but is often sporadic: only 2–6% of individuals with type 1 diabetes have an affected parent (48)
o Type 2 diabetes often runs in families with shared environment and risk alleles. Family history that helps distinguish MODY are onset of diabetes before age 35 years with lack of obesity (in MODY) compared to onset of diabetes after age 45 years associated with obesity (type 2 diabetes) (6).
Family history
A 3-generation (or more) family history should be obtained either through discussion with the patient and family members and through the medical records when possible and permission is given. Particular attention should be given to the relevant details in the pedigree: age at onset of diabetes mellitus, body habitus at onset, insulin independence, medications used, hypoglycemia, and presence of molecular genetic testing. Since type 2 diabetes has a strong genetic component, shared risk alleles and shared environment can lead to occurrence of type 2 diabetes in multiple family members. In some families this can be hard to distinguish from autosomal dominant inheritance. Family history features can help distinguish between type 2 diabetes and MODY-monogenic diabetes.
Physical examination
In addition to the clinical characteristics listed above, MODY-monogenic diabetes is characterized by under-to-normal weight or mildly overweight status, but it has been noted that at least 4.5% of obese and overweight adolescents enrolled in a clinical trial to treat type 2 diabetes had MODY-monogenic diabetes (mostly HNF4A-MODY, GCK-MODY, or HNF1A-MODY) (6, 49). Since MODY-monogenic diabetes is not protective against becoming overweight or obese, it may occur together with insulin resistance. Inappropriate treatment of monogenic diabetes with insulin has been suggested to lead to or promote obesity due to treatment or consideration of therapy-induced hypoglycemia.
Diagnostic testing
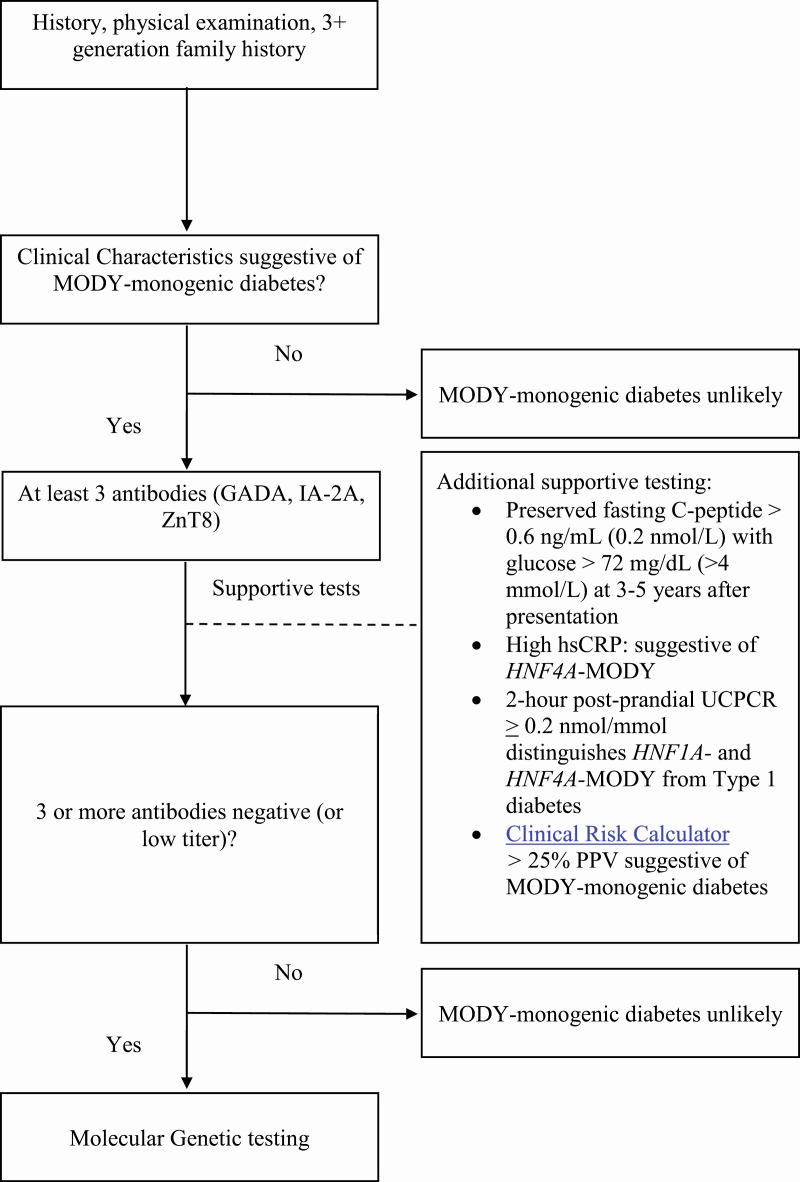
A fasting serum glucose should be obtained, (50) along with C-peptide, HbA1c (51), and 3 islet-cell antibodies (GADA, IA-2A, and ZnT8), preferably near the time of diagnosis. The most cost-effective way of ruling out type 1 diabetes prior to genetic testing for MODY-monogenic diabetes is to obtain islet antibodies if these can be obtained near the time of diabetes diagnosis. Adding a fourth antibody (insulin autoantibodies [IAA]), if the patient is not yet treated with insulin, is not cost-effective, as it only reduced the number of patients who were antibody-negative from 13% to 12% in the pediatric population (52). When patients have received insulin treatment in the past, the IAA test may be a false positive (53). Twenty-six percent of patients with presumed antibody-negative type 1 diabetes (insulin antibody, GADA, IA-2A, and islet cell antibody negative) are positive for ZnT8 antibodies (54-57). The initial approach is to obtain HbA1c, fasting glucose, and C-peptide values (at presentation and after 3-5 years), and test for at least 3 autoimmune antibodies (GADA, IA-2A, and ZnT8) (Fig. 1).
Figure 1.
MODY-monogenic diabetes diagnostic algorithm.
In addition to antibody testing, other diagnostic tools are helpful in determining the likelihood of and diagnosing MODY-monogenic diabetes. The appropriate timing for obtaining a C-peptide in order to distinguish MODY-monogenic diabetes from type 1 diabetes is an area of controversy. Endogenous insulin production outside of the “honeymoon” phase (more than 3-5 years) with a detectable C-peptide (>0.6 ng/mL or >0.2 nmol/L [>200 pmol/L) and a corresponding glucose > 72 mg/dL (> 4 mmol/L) indicate that MODY-monogenic diabetes is the likely diagnosis as opposed to type 1 diabetes (44, 45). The MODY calculator developed by the University of Exeter group is a clinical prediction tool that can calculate an estimate of an individual’s probability of having MODY (58, 59). The clinical risk calculator tool was validated in individuals younger than age 35 years. A genetic risk score has been developed to estimate a patient’s propensity for monogenic diabetes (60). Both prediction tools (clinical prediction tool and genetic risk calculator) have been largely validated in homogeneous populations of White Europeans. Candidates with a MODY calculator post-test probability > 25% should trigger genetic testing for MODY in patients treated with insulin within 6 months of diagnosis. The detection rate for MODY mutations is lower in the South Asian population than in the White European population. Even if a MODY mutation was not detected, the South Asian population is more likely to meet the clinical criteria for referral than White Europeans (61). More research is needed to validate this clinical prediction tool in diverse populations to ensure its accuracy. An additional noninvasive test that is useful in distinguishing long-standing type 1 diabetes (more than 3-5 years) from HNF1A-MODY and HNF4A-MODY, is a 2-hour postprandial (after largest meal of the day) UCPCR evaluation. A 2-hour postprandial UCPCR of ≥0.2 nmol/mmol is 97% sensitive and 96% specific for distinguishing HNF1A- and HNF4A-MODY from type 1 diabetes (2). Lastly, high-sensitivity C-reactive protein (hsCRP) is known to be lower in HNF1A-MODY, and can distinguish HNF1A-MODY from type 2 diabetes and HNF4A-MODY (62, 63). The clinical prediction calculator, 2-hour postprandial UCPCR, and hsCRP are useful, but more research is warranted prior to widespread implementation as the standard of care (Fig. 1).
Molecular genetic testing
Genetic testing is cost-effective in all patients suspected of having monogenic diabetes or MODY (64), and can include a combination of gene-targeted testing (serial single-gene or multigene panel) and comprehensive genomic testing (chromosomal microarray analysis or exome sequencing).
If patients have demonstrated distinct clinical features consistent with MODY (Table 1), serial single-gene testing, use of a multigene panel and/or chromosomal microarray analysis should be performed (6). The cost-effectiveness is dependent on availability and the cost of the genetic testing itself (64) (Fig. 1).
If the phenotype is determined to not be consistent with GCK-MODY and difficult to distinguish from other genetic causes of MODY, then a MODY multigene panel is most appropriate (consisting of the most common and validated MODY-related genes and other genes of interest) with specific scenarios including chromosomal microarray analysis. Large deletions in HNF1B are common and are associated with distinct phenotypic features that have had a contiguous gene deletion. One example is the 17q12 recurrent deletion syndrome that is associated with a 1.2-megabase or larger deletion that includes HNF1B, in which multigene testing has been able to estimate the breakpoint and size of a whole gene deletion (6).
Lastly, if the genetic cause is not identified with clinically available tests, or if the patient has additional clinical features, referral to a specialty center and exome sequencing should be considered (6).
Diagnostic Tests and Treatment in Our Patient
The patient had 3 negative antibody tests (GADA, IA-2A, and ZnT8). According to the clinical risk calculator he had a 75.5% posttest probability (PPV) of testing positive for MODY (1). He subsequently underwent molecular genetic testing using a multigene panel. The multigene panel revealed a pathogenic mutation in HNF4A (c.790: del “G” resulting in p.Val264Cys frameshift mutation), previously reported (65, 66), and he was diagnosed with HNF4A-MODY. He was started on glimepiride (sulfonylurea) at 0.5 mg once daily and titrated to a maximum dose of 4 mg daily. He maintained on this dose and initially achieved adequate glycemic control (HbA1c ≤7.0%).
Two years later, and between 3-month visits, the patient’s HbA1c rose to 8.7% without significant changes in his activity, dietary intake, or lifestyle (ie, stressors). He was given options for other secretagogues including a trial of glucagon-like peptide 1 receptor agonist (GLP-1 RA) treatment given strong family history of coronary artery disease as well as the risk of hypoglycemia related to insulin use. The patient had evidence of residual β-cell function with C-peptide of 1.2 ng/mL (reference 0.8-3.2 ng/mL), and therefore, he was switched from glimepiride (sulfonylurea) to once-weekly subcutaneous semaglutide (a GLP-1 RA) at a dose of 0.25 mg once-weekly, which was titrated to a maximum dose of 1 mg weekly over 8 weeks. The patient’s HbA1c improved to 6.2% after 6 months of GLP-1 RA treatment and he reported fewer and less severe hypoglycemic events (10).
His father also had HNF4A-MODY (same pathogenic mutation diagnosed in his 20s) and had a myocardial infarction at age 50 years. Seven years prior to initiation of GLP-1 RA treatment, he stopped responding to glimepiride (sulfonylurea) treatment and at that time, insulin (on multiple daily injections) and metformin (titrated to 1gram twice daily) were added. His average total daily dose of insulin was 128 Units (1.5 Units/kg/day, on multiple daily injections), and his HbA1c was 9.6%. Liraglutide was initiated and titrated to a maximum of 1.8mg over 3 weeks, and his insulin requirements decreased to an average total daily dose of 70 Units (0.8 Units/kg/day, basal insulin only) with a HbA1c of 7.0%. One year later, his HbA1c improved further to 5.9% and his insulin requirements remained stable with basal insulin, metformin, and GLP-1 RA treatment.
Treatment Approach
The treatment of MODY-monogenic diabetes is tailored to the specific genetic variant that is identified (Table 1). Despite sulfonylurea treatment, patients with MODY-monogenic diabetes have glucose-induced insulin secretion that decreases over time (at a rate of 1% to 4% per year) (67, 68). The adjuvant or alternative treatments lack prospective randomized controlled data supporting their use. In this article, we review the 3 most common forms of MODY-monogenic diabetes and suggest an approach to the treatment and monitoring of microvascular and macrovascular complications. Other mutations, with their respective protein expression, inheritance pattern(s), and treatment options are included in Table 1.
GCK-MODY
Patients with GCK-MODY (formerly referred to as MODY-2) have mild, stable fasting hyperglycemia (HbA1c range of 5.6%-7.6% (6, 51)) and do not require anti-hyperglycemic therapy except in specific circumstances during pregnancy (6, 39). Without treatment, there is no significant effect on their HbA1c or the incidence of micro and macrovascular complications in GCK-MODY compared to those who do receive treatment. Therefore, patients do not require therapy, unless they have co-occurrence of type 1 or type 2 diabetes, obesity (extremely rare), or are pregnant (6, 7, 11). For sodium–glucose co-transporter-2 (SGLT-2) inhibitors, caution is advised due to the risk of euglycemic diabetic ketoacidosis (DKA) in a patient with GCK-MODY (69). Withdrawal of treatment or not treating is difficult advice to follow given that HbA1c results may rise above 7%, but so far, the data on the lack of complications has been very strong (16).
In pregnant women with a known GCK-MODY mutation, recommendations for treatment are based on the fetal genotype and growth as outlined below in Tables 2A and 2B (6, 7, 70). When the mother has the GCK mutation and the fetus does not have the GCK mutation (or it is not inferred), adequate glycemic control is a challenge, as mothers have higher fasting and postprandial glycemic excursions in the first trimester (in comparison to HNF1A-MODY pregnancies) despite insulin treatment. This is due to exogenous insulin suppressing endogenous insulin secretion and counter-regulation occurring, which lower blood glucose values. These patients require higher doses of insulin, and have higher insulin requirements than their predicted replacement doses (71).
Table 2.
Management of GCK-MODY in Pregnancy (A) When GCK Variant Present in Fetus or Inferred(6, 7, 70, 72, 73) and (B) When GCK Variant Is Not Present in the Fetus or Inferred(6, 7, 70, 72, 73)
| Table 2A | ||
|---|---|---|
| GCK mutation source | GCK variant present in fetus (or inferreda when fetal abdominal circumference >75th percentile on second-trimester ultrasound) | |
| Fetal growth | Treatment | |
| Mother | Normal growth with normal insulin amount | None |
| Father (or de novo) | Restricted birth weight <400 grams compared to normalb | None |
| Table 2B | ||
| GCK mutation source | GCK variant NOT present in fetus (and NOT inferreda by abdominal circumference >75th percentile on second-trimester ultrasound) | |
| Fetal growth | Treatment | |
| Mother | Birth weight >700 grams compared to normal | Insulin is recommended (at dose required to lower mother’s fasting glucose. Dose is generally higher than replacement doses): |
| Fetus responds to maternal hyperglycemia with excess insulin production/excess growth | First-trimester average TDD insulin reported: 0.3 Units/kg (range, 0.1-0.5) | |
| Second-trimester average TDD insulin reported: 0.4 Units/kg (range 0.2-0.7) | ||
| Third-trimester average TDD insulin reported: 0.8 Units/kg (range 0.8-1.5) (68) | ||
| Consider delivery at 38 weeks gestation when fetus abdominal circumference >75th percentile | ||
| Father (or de novo) | Normal | None |
aInferred GCK variant in the fetus: abdominal circumference obtained during the second-trimester ultrasound examination, it is assumed that a fetal abdominal circumference > 75th percentile indicates that the fetus has NOT inherited the maternal GCK pathogenic variant.
bRestricted birth weight < 400grams: if the fetus inherits the GCK Mutation from the father, the fetus may have a difference in birth weight < 400grams of the expected birth weight (compared to normal).
Abbreviations: GCK, glucokinase; TDD, total daily dose.
Long-term complications are extremely rare in GCK-MODY, given that patients have mild stable fasting hyperglycemia, are nonobese, have normal cholesterol panels, and are not hypertensive. Currently, nonproliferative (also known as background) retinopathy is the only microvascular complication in GCK-MODY (mean age of 48.6 years) and is only slightly increased when compared to healthy controls (74). It is not recommended to perform annual screening, as it is low yield, unless there is clinical suspicion of underlying retinopathy (6, 39). The management of GCK-MODY differs significantly from other types that necessitate treatment for adequate glycemic control and to reduce micro- and macrovascular complications.
HNF1A-MODY
HNF1A-MODY (formerly referred to as MODY-3) is the most commonly reported genetic variant, representing 30% to 65% of all MODY cases (6). First-line therapy for HNF1A-MODY is low-dose sulfonylurea, which acts on potassium-sensitive ATP (KATP) channels to increase insulin secretion via a partially glucose-dependent mechanism (12). The starting dose of sulfonylureas should be low and should be titrated to target. On initiation of sulfonylurea, the insulin dose is lowered or discontinued to avoid hypoglycemia (6). Additionally, the dose of sulfonylurea should be lowered (if insulin already lowered/discontinued) if hypoglycemia occurs. In the US, glyburide is the most commonly prescribed sulfonylurea for HNF1A-MODY (6). In a single study evaluating the use of nateglinide, the shorter acting agent causes lower postprandial glucose levels and reduces incidence of hypoglycemia compared to the sulfonylurea glyburide (also known as glibenclamide) (16). Therefore, in most circumstances, sulfonylurea medication is used as first-line treatment. With any sulfonylurea treatment, glycemic control will likely deteriorate over a course of 3 to 25 years (68). This depends on the age at which sulfonylurea treatment is instituted.
An alternative treatment to sulfonylurea for beta-cell failure is GLP-1 RA (75, 76). Øsfoft et al demonstrated in a small (n = 16), randomized, double-blind, crossover trial that both liraglutide and glimepiride lower fasting plasma glucose and postprandial glucose excursion with no significant difference between treatments (13). Glimepiride is associated with a higher risk of exclusively mild hypoglycemia when compared with GLP-1 RA treatment. GLP-1 receptor activation on beta-cells results in stimulation of adenylate cyclase and subsequent elevation of cyclic adenosine monophosphate (cAMP). Both cAMP and activated protein kinase A influence secretory events distal to the genetic defect, which bypasses the decreased concentration of ATP associated with the genetic defect, and thereby stimulates the secretion of insulin and reduces postprandial glucose (13, 77-79). Additionally GLP-1 RA has direct effect on the KATP channel (80, 81).
SGLT-2 inhibitor use in HNF1A-MODY is cautioned against. One report demonstrated severe dehydration and DKA due to high inhibition of the remaining SGLT-2 activity in patients with HNF1A-MODY (82). Glycosuria in HNF1A-MODY is due to reduced expression of SGLT2, which is known to be under transcriptional control of HNF1A (83). Dapagliflozin (a SGLT-2 inhibitor) induced higher glycosuria in 14 HNF1A-MODY patients and 19 GCK-MODY patients when compared with 12 patients with type 2 diabetes (83). The risks of SGLT-2 inhibitor use in HNF1A-MODY have been reported to outweigh the potential benefits of this medication (eg, higher risk of dehydration, potential vascular collapse, genital infections, and DKA) (11, 82-84).
Patients with HNF1A-MODY have similar risk of all-cause mortality and cardiovascular disease when compared to patients with type 2 diabetes (85). Insulin or GLP-1 RA treatment are both options to augment sulfonylurea treatment in patients who are no longer achieving adequate glycemic control with sulfonylurea monotherapy. GLP-1 RA treatment has received recent attention, given its ability to reduce the risk of cardiovascular disease and since its mechanism of action leads to insulin release downstream from the genetic defect (86, 87). It is recommended as an add-on treatment to sulfonylurea (88), but in the cases presented in this article it was used as a replacement to sulfonylurea treatment (10). Although its role as first-line therapy is not established as superior to sulfonylurea treatment, it is an area of ongoing investigation. Lastly, dipeptidyl peptidase-4 (DPP-4) inhibitors demonstrate modest efficacy (14), and provide an additional option to consider as adjuvant treatment. In a randomized controlled trial, linagliptin as an add-on treatment to glimepiride demonstrated improved glycemic variability (by continuous glucose monitoring) and control without an increased risk of hypoglycemia in patients with HNF1A-MODY (15).
HNF4A-MODY
HNF4A-MODY (formerly referred to as MODY-1). Some patients with HNF4A-MODY present with transient hyperinsulinemic hypoglycemia in the neonatal period followed by diabetes in late adolescence or adulthood (6, 89). Similar to HNF1A-MODY, patients respond to sulfonylureas and meglitinide medications, and sulfonylurea is the first-line treatment for HNF4A-MODY (8, 16, 67, 90–92). However, HNF4A-MODY patients do not respond as well to sulfonylurea or meglitinide treatment as in HNF1A-MODY (for reasons that remain unclear). Despite the efficacy of sulfonylurea and meglitinides, most will require insulin as β-cell dysfunction progresses (68).
In this report, a father and son with HNF4A-MODY demonstrated a significant response to GLP-1 RA as a replacement to sulfonylurea treatment (after not achieving adequate glycemic control with sulfonylurea), and the mechanism of action is similar to that reported for GLP-1 RA use in HNF1A-MODY (10).
Patients with HNF4A-MODY are vulnerable to microvascular and macrovascular complications associated with diabetes mellitus (8, 90), and routine screening for these complications is required. We recommend that patients undergo yearly screening for all diabetes complications including hyperlipidemia, albuminuria, neuropathy, renal disease, and retinopathy.
Patient’s Treatment Course and Monitoring of Complications
This patient underwent transition from glimepiride to once-weekly 1 mg semaglutide (GLP-1 RA), and reported improvement in his glycemic control, reduced hypoglycemic frequency, and reduced hypoglycemia severity. This agent was chosen as his father (with the same genotype and similar phenotype) had coronary artery disease and history of myocardial infarction by age 50. Insulin could have been added, but the GLP1-RA offers a low risk of hypoglycemia, and the added benefit of reduction in cardiovascular morbidity and mortality (in patients with type 2 diabetes) (86, 87). Although GLP-1 RA treatment has not been formerly studied in this population, nor has it been studied as a replacement treatment for sulfonylurea; it is an area of future research interest. This patient was able to continue treatment without insulin, and his father was able to reduce his insulin need from basal-bolus insulin to once-daily long-acting basal insulin and GLP-1 RA (liraglutide).
In follow-up evaluation, the son was monitored with annual screening of his lipid profile, dilated retinal examination, and urine albumin:creatinine ratio. His lipid panel showed a total cholesterol of 137 mg/dL, HDL of 25 mg/dL, LDL of 76 mg/dL, and a triglyceride level of 182 mg/dL. His dilated retinal examination did not demonstrate retinopathy, and his urine albumin:creatinine ratio was 17 mg/g. He has remained on monotherapy with once-weekly semaglutide (GLP-1 RA) treatment for more than 1 year with HbA1c ≤6.5% and without notable side effects or complications. The father continued on his medication regimen of metformin 1 gram twice daily, GLP-1 RA (liraglutide) 1.8 mg once daily, and basal insulin once daily (at similar doses reported above) and was well controlled with a HbA1c ≤6.5% without notable side effects or complications.
Conclusion
In this article, we have emphasized the importance of identifying clinical manifestations of MODY-monogenic diabetes, ordering an appropriate diagnostic evaluation, mutation-tailored treatment of the most common forms of MODY-monogenic diabetes, and monitoring of micro- and macrovascular complications.
Acknowledgments
We would like to thank Graeme Bell, Siri Atma Greeley, Rochelle Naylor, Lisa Letourneau-Freiberg, Andrew Hattersley, and the entire monogenic research group at the University of Exeter for their contributions to this field and ongoing thoughtful discussions.
Financial Support: L.H.P. acknowledges the following grant support from the National Institutes of Health (NIH): DK118612-01, R01DK104942, P30DK020595.
Glossary
Abbreviations
- ATP
adenosine triphosphate
- DKA
diabetic ketoacidosis
- GADA
glutamic acid decarboxylase antibodies
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- IA-2A
islet antigen 2 antibodies
- IAA
insulin autoantibodies
- MODY
maturity-onset diabetes of the young
- PPV
Positive predictive value
- SGLT-2
sodium–glucose co-transporter-2
- UCPCR
urinary C-peptide to creatinine ratio
- ZnT8
zinc transporter 8
Additional Information
Disclosure Summary: D.T.B. has no relevant conflicts of interest to declare. In the past 12 months, K.M.P. reports receiving consulting honoraria from AstraZeneca, Bayer Inc., Corcept Therapeutics, Eli Lilly, Novo Nordisk, Merck, speaker honoraria from AstraZeneca, Novo Nordisk, and Merck. K.M.P. reports serving as a research investigator for Novo Nordisk and receiving research support from Bayer Inc., Merck, and Novo Nordisk. S.R.K. has no relevant conflicts of interest to declare. L.H.P. has no relevant conflicts of interest to declare.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. MODY Probability Calculator // Diabetes Genes. https://www.diabetesgenes.org/mody-probability-calculator/. Accessed May 4, 2020.
- 2. Besser RE, Shepherd MH, McDonald TJ, et al. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care. 2011;34(2):286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504-2508. [DOI] [PubMed] [Google Scholar]
- 4. Pihoker C, Gilliam LK, Ellard S, et al. ; SEARCH for Diabetes in Youth Study Group . Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98(10):4055-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shepherd M, Shields B, Hammersley S, et al. ; UNITED Team . Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care. 2016;39(11):1879-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naylor R, Knight Johnson A, del Gaudio D. Maturity-onset diabetes of the young overview. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews®. Seattle: University of Washington; 1993. http://www.ncbi.nlm.nih.gov/books/NBK500456/. Accessed April 27, 2020. [PubMed] [Google Scholar]
- 7. Chakera AJ, Steele AM, Gloyn AL, et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38(7):1383-1392. [DOI] [PubMed] [Google Scholar]
- 8. Pearson ER, Pruhova S, Tack CJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48(5):878-885. [DOI] [PubMed] [Google Scholar]
- 9. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971-980. [DOI] [PubMed] [Google Scholar]
- 10. Broome DT, Tekin Z, Pantalone KM, Mehta AE. Novel use of GLP-1 receptor agonist therapy in HNF4A-MODY. Diabet Care. 2020. doi: 10.2337/dc20-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stride A, Shields B, Gill-Carey O, et al. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014;57(1):54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bacon S, Kyithar MP, Rizvi SR, et al. Successful maintenance on sulphonylurea therapy and low diabetes complication rates in a HNF1A–MODY cohort. Diabet Med. 2016;33(7): 976-984. [DOI] [PubMed] [Google Scholar]
- 13. Østoft SH, Bagger JI, Hansen T, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care. 2014;37(7):1797-1805. [DOI] [PubMed] [Google Scholar]
- 14. Lumb AN, Gallen IW. Treatment of HNF1-alpha MODY with the DPP-4 inhibitor Sitagliptin1. Diabet Med. 2009;26(2):189-190. [DOI] [PubMed] [Google Scholar]
- 15. Christensen AS, Hædersdal S, Støy J, et al. Efficacy and safety of glimepiride with or without linagliptin treatment in patients with HNF1A diabetes (maturity-onset diabetes of the young type 3): a randomized, double-blinded, placebo-controlled, crossover trial (GLIMLINA). Diabetes Care. 2020. doi: 10.2337/dc20-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3. Diabetes Care. 2006;29(2):189-194. [DOI] [PubMed] [Google Scholar]
- 17. Shepherd M, Pearson ER, Houghton J, Salt G, Ellard S, Hattersley AT. No deterioration in glycemic control in HNF-1alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care. 2003;26(11):3191-3192. [DOI] [PubMed] [Google Scholar]
- 18. Misra S, Owen KR. Genetics of Monogenic Diabetes: Present Clinical Challenges. Curr Diab Rep. 2018;18(12):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delvecchio M, Pastore C, Giordano P. Treatment Options for MODY Patients: A Systematic Review of Literature. Diabetes Ther. 2020;11(8):1667-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montoli A, Colussi G, Massa O, et al. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1 beta gene: description of a new family with associated liver involvement. Am J Kidney Dis. 2002;40(2):397-402. [DOI] [PubMed] [Google Scholar]
- 21. Bellanné-Chantelot C, Chauveau D, Gautier JF, et al. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140(7):510-517. [DOI] [PubMed] [Google Scholar]
- 22. Ulinski T, Lescure S, Beaufils S, et al. Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006;17(2):497-503. [DOI] [PubMed] [Google Scholar]
- 23. Faguer S, Decramer S, Chassaing N, et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80(7):768-776. [DOI] [PubMed] [Google Scholar]
- 24. Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23(3):323-328. [DOI] [PubMed] [Google Scholar]
- 25. Kristinsson SY, Thorolfsdottir ET, Talseth B, et al. MODY in Iceland is associated with mutations in HNF-1alpha and a novel mutation in NeuroD1. Diabetologia. 2001;44(11):2098-2103. [DOI] [PubMed] [Google Scholar]
- 26. Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38(1):54-62. [DOI] [PubMed] [Google Scholar]
- 27. Edghill EL, Flanagan SE, Patch AM, et al. ; Neonatal Diabetes International Collaborative Group . Insulin mutation screening in 1044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57(4):1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meur G, Simon A, Harun N, et al. Insulin gene mutations resulting in early-onset diabetes: marked differences in clinical presentation, metabolic status, and pathogenic effect through endoplasmic reticulum retention. Diabetes. 2010;59(3):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borowiec M, Liew CW, Thompson R, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106(34):14460-14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowman P, Flanagan SE, Edghill EL, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012;55(1):123-127. [DOI] [PubMed] [Google Scholar]
- 31. Ovsyannikova AK, Rymar OD, Shakhtshneider EV, et al. ABCC8-Related Maturity-Onset Diabetes of the Young (MODY12): Clinical Features and Treatment Perspective. Diabetes Ther. 2016;7(3):591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riveline J-P, Rousseau E, Reznik Y, et al. Clinical and metabolic features of adult-onset diabetes caused by ABCC8 mutations. Diabetes Care. 2012;35(2):248-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonnefond A, Philippe J, Durand E, et al. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. Plos One. 2012;7(6):e37423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu L, Nagashima K, Yasuda T, et al. Mutations in KCNJ11 are associated with the development of autosomal dominant, early-onset type 2 diabetes. Diabetologia. 2013;56(12):2609-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prudente S, Jungtrakoon P, Marucci A, et al. Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am J Hum Genet. 2015;97(1):177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urano F. Wolfram syndrome: diagnosis, management, and treatment. Curr Diab Rep. 2016;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Franco E, Flanagan SE, Houghton JA, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greeley SA, Naylor RN, Philipson LH, Bell GI. Neonatal diabetes: an expanding list of genes allows for improved diagnosis and treatment. Curr Diab Rep. 2011;11(6):519-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 40. Awa WL, Schober E, Wiegand S, et al. Reclassification of diabetes type in pediatric patients initially classified as type 2 diabetes mellitus: 15 years follow-up using routine data from the German/Austrian DPV database. Diabetes Res Clin Pract. 2011;94(3):463-467. [DOI] [PubMed] [Google Scholar]
- 41. SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25(5):458-471. [DOI] [PubMed] [Google Scholar]
- 42. Urbanová J, Rypáčková B, Procházková Z, et al. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med. 2014;31(4):466-471. [DOI] [PubMed] [Google Scholar]
- 43. Draznin B. Diabetes Case Studies: Real Problems, Practical Solutions. American Diabetes Association; 2015. [Google Scholar]
- 44. Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009;10 Suppl 12: 33-42. [DOI] [PubMed] [Google Scholar]
- 45. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hope SV, Knight BA, Shields BM, et al. Random non-fasting C-peptide testing can identify patients with insulin-treated type 2 diabetes at high risk of hypoglycaemia. Diabetologia. 2018;61(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hope SV, Knight BA, Shields BM, Hattersley AT, McDonald TJ, Jones AG. Random non-fasting C-peptide: bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med. 2016;33(11):1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harjutsalo V, Lammi N, Karvonen M, Groop PH. Age at onset of type 1 diabetes in parents and recurrence risk in offspring. Diabetes. 2010;59(1):210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kleinberger JW, Copeland KC, Gandica RG, et al. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet Med. 2018;20(6):583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ellard S, Bellanné-Chantelot C, Hattersley AT; European Molecular Genetics Quality Network (EMQN) MODY group . Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51(4):546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Steele AM, Wensley KJ, Ellard S, et al. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. Plos One. 2013;8(6):e65326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carlsson A, Shepherd M, Ellard S, et al. Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year pediatric Swedish National Cohort Study. Diabetes Care. 2020;43(1):82-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61(1):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114(4):589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151-156. [DOI] [PubMed] [Google Scholar]
- 56. Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11-18. [DOI] [PubMed] [Google Scholar]
- 57. Wenzlau JM, Walter M, Gardner TJ, et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab. 2010;95(10):4712-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012;55(5):1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomas ER, Brackenridge A, Kidd J, et al. Diagnosis of monogenic diabetes: 10-year experience in a large multi-ethnic diabetes center. J Diabetes Investig. 2016;7(3):332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel KA, Oram RA, Flanagan SE, et al. Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes. 2016;65(7):2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Misra S, Shields B, Colclough K, et al. South Asian individuals with diabetes who are referred for MODY testing in the UK have a lower mutation pick-up rate than white European people. Diabetologia. 2016;59(10):2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McDonald TJ, Shields BM, Lawry J, et al. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011;34(8):1860-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011;54(11):2801-2810. [DOI] [PubMed] [Google Scholar]
- 64. Naylor RN, John PM, Winn AN, et al. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37(1): 202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34(5):669-685. [DOI] [PubMed] [Google Scholar]
- 66. VCV000447521.1—ClinVar—NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/447521/. Accessed May 24, 2020.
- 67. Fajans SS, Brown MB. Administration of sulfonylureas can increase glucose-induced insulin secretion for decades in patients with maturity-onset diabetes of the young. Diabetes Care. 1993;16(9):1254-1261. [DOI] [PubMed] [Google Scholar]
- 68. Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34(8):1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Magdaleno A, Venkataraman S, Perilli G. Euglycemic DKA in MODY patient: empagliflozin to blame. Endocr Pract. 2017;23(No. Supplement 3):41-42. [Google Scholar]
- 70. Spyer G, Hattersley AT, Sykes JE, Sturley RH, MacLeod KM. Influence of maternal and fetal glucokinase mutations in gestational diabetes. Am J Obstet Gynecol. 2001;185(1):240-241. [DOI] [PubMed] [Google Scholar]
- 71. Bacon S, Schmid J, McCarthy A, et al. The clinical management of hyperglycemia in pregnancy complicated by maturity-onset diabetes of the young. Am J Obstet Gynecol. 2015;213(2):236.e1-236.e7. [DOI] [PubMed] [Google Scholar]
- 72. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60(5):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Colom C, Corcoy R. Maturity onset diabetes of the young and pregnancy. Best Pract Res Clin Endocrinol Metab. 2010;24(4):605-615. [DOI] [PubMed] [Google Scholar]
- 74. Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. Jama. 2014;311(3):279-286. [DOI] [PubMed] [Google Scholar]
- 75. Docena MK, Faiman C, Stanley CM, Pantalone KM. Mody-3: novel HNF1A mutation and the utility of glucagon-like peptide (GLP)-1 receptor agonist therapy. Endocr Pract. 2014;20(2):107-111. [DOI] [PubMed] [Google Scholar]
- 76. Fantasia KL, Steenkamp DW. Optimal Glycemic Control in a Patient With HNF1A MODY With GLP-1 RA Monotherapy: Implications for Future Therapy. J Endocr Soc. 2019;3(12):2286-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89(18):8641-8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gromada J, Ding WG, Barg S, Renström E, Rorsman P. Multisite regulation of insulin secretion by cAMP-increasing agonists: evidence that glucagon-like peptide 1 and glucagon act via distinct receptors. Pflugers Arch. 1997;434(5):515-524. [DOI] [PubMed] [Google Scholar]
- 79. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes. 2010;59(5):1117-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aaboe K, Knop FK, Vilsboll T, et al. KATP channel closure ameliorates the impaired insulinotropic effect of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):603-608. [DOI] [PubMed] [Google Scholar]
- 81. Gutniak MK, Juntti-Berggren L, Hellström PM, Guenifi A, Holst JJ, Efendic S. Glucagon-like peptide I enhances the insulinotropic effect of glibenclamide in NIDDM patients and in the perfused rat pancreas. Diabetes Care. 1996;19(8):857-863. [DOI] [PubMed] [Google Scholar]
- 82. Pruhova S, Dusatkova P, Neumann D, et al. Two cases of diabetic ketoacidosis in HNF1A-MODY linked to severe dehydration: is it time to change the diagnostic criteria for MODY? Diabetes Care. 2013;36(9):2573-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hohendorff J, Szopa M, Skupien J, et al. A single dose of dapagliflozin, an SGLT-2 inhibitor, induces higher glycosuria in GCK- and HNF1A-MODY than in type 2 diabetes mellitus. Endocrine. 2017;57(2):272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Menzel R, Kaisaki PJ, Rjasanowski I, Heinke P, Kerner W, Menzel S. A low renal threshold for glucose in diabetic patients with a mutation in the hepatocyte nuclear factor-1α (HNF-1α) gene. Diabet Med. 1998;15(10):816-820. [DOI] [PubMed] [Google Scholar]
- 85. Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med. 2010;27(2):157-161. [DOI] [PubMed] [Google Scholar]
- 86. Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. [DOI] [PubMed] [Google Scholar]
- 88. Christensen AS, Hædersdal S, Storgaard H, et al. GIP and GLP-1 potentiate sulfonylurea-induced insulin secretion in hepatocyte nuclear factor 1-alpha mutation carriers. Diabetes. 2020. doi: 10.2337/db20-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bacon S, Kyithar MP, Condron EM, Vizzard N, Burke M, Byrne MM. Prolonged episodes of hypoglycaemia in HNF4A-MODY mutation carriers with IGT. Evidence of persistent hyperinsulinism into early adulthood. Acta Diabetol. 2016;53(6):965-972. [DOI] [PubMed] [Google Scholar]
- 90. Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003;362(9392):1275-1281. [DOI] [PubMed] [Google Scholar]
- 91. Becker M, Galler A, Raile K. Meglitinide analogues in adolescent patients with HNF1A-MODY (MODY 3). Pediatrics. 2014;133(3):e775-e779. [DOI] [PubMed] [Google Scholar]
- 92. Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009;26(4):437-441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.