Abstract
Context
CD34+ fibrocytes have been implicated in development of thyroid-associated ophthalmopathy (TAO), a consequential autoimmune manifestation of Graves disease (GD). In TAO, CD34+ fibrocytes appear to masquerade as CD34+ orbital fibroblasts mixed with CD34- OF (collectively, GD-OF). Slit2, an axon guidance glycoprotein, is expressed by CD34- OF and attenuates GD-OF gene expression. Cardinal features of TAO include hyaluronan (HA) accumulation and cytokine-driven inflammation.
Objective
Compare expression of HA synthase isoenzymes (HAS1-3), UDP-glucose dehydrogenase (UGDH), synthesis of HA, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in fibrocytes and GD-OF. Determine whether Slit2 alters gene expression patterns.
Design/Setting/Participants
Patients with TAO and healthy donors were recruited from an academic practice.
Main Outcome Measures
Real-time polymerase chain reaction, HA, IL-6, and TNF-α immunoassays.
Results
HA synthesis and release from fibrocytes is substantially lower than in GD-OF. HAS1 expression dominates in fibrocytes while HAS2 in GD-OF. In contrast, HAS2 and UGDH expression dominate GD-OF and localize to CD34- OF. Recombinant human Slit2 (rhSlit2) substantially upregulates HA synthesis and HAS2 expression in fibrocytes but attenuates IL-6 and TNF-α production in these cells. In contrast, knocking down Slit2 in GD-OF reduces HA synthesis and HAS2 and UGDH expression while upregulating IL-6 and TNF-α.
Conclusion
The dramatic differences in HA, IL-6, and TNF-α production, and HAS and UGDH expression found in fibrocytes and GD-OF appear, at least in part, to be attributable to Slit2. These findings provide novel insight into the differences in gene expression exhibited by CD34+ fibrocytes and CD34+ OF and therefore reveal important aspects of disease pathogenesis.
Keywords: Graves disease, orbit, fibroblasts, hyaluronan, cytokine, ophthalmopathy
Thyroid-associated ophthalmopathy (TAO) is a disfiguring and potentially blinding autoimmune periocular manifestation of Graves disease (GD) (1). The hallmark of GD centers around the dysregulated activation of the thyrotropin (TSH) receptor (TSHR). Until now, therapy for TAO has been limited to largely ineffective, nonspecific, and frequently hazardous agents with serious side effects (2). In TAO, the connective tissues of the orbit and upper face become inflamed and remodeled, consequences of fibroblast activation and their production of cytokines and hyaluronan (HA) (3, 4). The distinct phenotypic attributes of orbital fibroblasts from patients with GD (GD-OF) are currently thought to underlie the vulnerability of these tissues to involvement in TAO (5, 6). GD-OF comprise discrete cell subsets, namely those with the CD34+ CXCR4+Collagen 1+ phenotype (CD34+ OF) while others fail to display those cellular markers (CD34- OF) (7). CD34+ OF are currently thought to represent myeloid-derivative, monocyte progenitors infiltrating the orbit from circulating CD34+ fibrocytes (8). In contrast to GD-OF, orbital fibroblasts from healthy donors are uniformly CD34- OF (9). Fibrocytes from the peripheral circulation become more abundant in GD (9) and express several proteins thought historically to be expressed solely by thyroid epithelial cells, such as the TSHR, thyroglobulin, sodium iodide symporter, and thyroperoxidase (10, 11). Fibrocytes from patients with TAO exhibit phenotypic characteristics identical to those from healthy individuals (9, 12). Slit2 is an axon guidance/repellent glycoprotein (13) that has been shown to inhibit the differentiation of fibrocytes (14) and to modulate their distinctive gene expression repertoire (15). It acts through its cognate receptor, roundabout 1 (ROBO1) (16). We recently demonstrated that Slit2 is synthesized and released from the CD34- OF subset of GD-OF (15). Once expressed by CD34- OF, Slit2 attenuates in CD34+ OF the transcription of these thyroid-associated genes through a downregulation of the autoimmune regulator protein (11). Furthermore, Slit2 upregulates the relative expression of interleukin-12 (IL-12) while attenuating that of IL-23, members of a superfamily of cytokines implicated in GD and TAO (12). The concentrations of Slit2 in the tissues generating the molecule and at sites of its actions, such as the human orbit, are currently unknown.
An important biosynthetic activity of GD-OF is their substantial capacity for generating HA, particularly under the influence of proinflammatory cytokines (17-19). HA is an abundant glycosaminoglycan with distinct rheological properties (20-22). Among these characteristics is a remarkable hydrophilicity, electronegativity, and absence of a core protein (20). Further, unlike the other abundant glycosaminoglycans, HA is not sulfated. A cardinal feature of TAO is the disordered accumulation of HA within the orbit, resulting in increased connective tissue volume (20). HA synthesis within the TAO orbit results from the activities of GD-OF; however, the contributions of specific GD-OF subsets has not been examined previously. Furthermore, which subset expresses adequate levels of the necessary enzymes for HA synthesis is currently unknown. These include the hyaluronan synthase (HAS) isoenzymes and UDP-glucose dehydrogenase (UGDH) (23, 24). Such insights could aid in development of therapeutic strategies for normalizing pathological HA synthesis. Transcripts encoding all 3 HAS isoenzymes and UGDH have been detected in GD-OF (25, 26). HAS2 has been found to dominate the expression of these enzymes in dermal fibroblasts and GD-OF (26-28). In contrast to their capacity to synthesize HA, hyaluronidase activity was found to be undetectable in dermal fibroblasts (29) but the expression of mRNAs encoding the 3 hyaluronidase isoenzymes was detected more recently in GD-OF following treatment with prostaglandin D2 (30). Important aspects concerning the generation of HA in the context of TAO remain to be elucidated. Further, investigation of HA production has yet to be studied in fibrocytes.
Here we report that CD34+ fibrocytes cultivated from peripheral blood mononuclear cells (PBMCs) produce extremely low basal levels of HA, macromolecular synthesis that is minimally affected by bovine TSH (bTSH). In contrast, GD-OF synthesize and release considerably higher levels of the glycosaminoglycan under both control and bTSH-treated conditions. Furthermore, HA synthesis is enhanced by bTSH in GD-OF. rhSlit2 increases HA synthesis in fibrocytes, while knocking down Slit2 expression in GD-OF attenuates its production in those cells. The profiles of HAS isoenzyme and UGDH expression differ in the 2 cell types; low-level HAS1 predominates the HA synthesis-relevant gene expression repertoire in fibrocytes while HAS2 abundance is by far the most abundantly expressed in GD-OF. Slit2 alters this pattern of HAS and UGDH expression as well as cytokine synthesis in both fibrocytes and GD-OF. Thus, Slit2 appears to represent a molecular fulcrum for determining the pattern of HAS and UGDH expression and HA, IL-6, and TNF-α synthesis in these cells. It may represent an attractive therapeutic target for TAO.
Materials and Methods
Materials
Dulbecco’s modified Eagle medium (DMEM, cat.# 11965) containing 4.5 g/mL D-glucose and L-glutamine, penicillin-streptomycin mixture (cat.# 15140), and fetal bovine serum (FBS) (cat.# 16000–044) were purchased from Life Technologies (Grand Island, NY). bTSH (cat.# T1614) was from Scripps Laboratories, San Diego, CA. Human CD34+ nucleofection kit (cat.# VPA-1003) was supplied by Lonza, Allendale, NJ. Recombinant human Slit2 (rhSlit2) (cat.# SRP3155) was from Sigma-Aldrich (St. Louis, MO). The HA enzyme-linked immunosorbent assay (ELISA) kit (K-1200) came from Echelon Biosciences Inc. (Salt Lake City, UT). Human IL-6 (cat.# D6050) and TNF-α (DTA00D) ELISA kits were purchased from R&D Systems, Minneapolis, MN. Phycoerythrin-labeled (PE) mouse anti-human CD34 (cat.# 555822) and mouse IgG1 isotype control-PE (cat.# 555749) came from BD Biosciences, San Diego, CA. Accutase (cat.# SCR005) was from Millipore, Billerica, MA. Small interfering RNAs (siRNAs) targeting HAS1 (cat.# L-009664-01), HAS2 (cat.# L-012053-02), UGDH (cat.# L-019564-01), Slit2 (cat.# L-019853-00), and controls (cat.# D-001810–10) were from Dharmacon (Lafayette, CO).
Methods
Research participant recruitment
A total of 28 individuals with stable TAO and 21 healthy control subjects were recruited from a busy endocrine and oculoplastic academic practice in the University of Michigan Health System; these participants provided peripheral blood mononuclear cells (PBMCs). Another 12 individuals with stable, severe TAO provided strains of GD-OF at the time of their orbital decompression surgeries. Written informed consent was obtained from all subjects prior to their participation. These activities have been approved by the University of Michigan institutional review board. Additional blood samples were supplied by the Red Cross.
Fibrocyte and GD-OF cultivation
PBMCs were isolated by Ficoll Histopaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation and cultivated as originally described by Bucala et al (8) except that the culture substratum was not coated with fibronectin (9). Six-well plates were inoculated with 107 PBMCs and covered with DMEM containing 10% FBS. After 12 to 14 days in culture, adherent monolayers (<5% of the starting population) were rinsed and removed from substratum by mechanical scraping following Accutase treatment. Culture purity was confirmed routinely by fluorescence-activated cell sorting (FACS) analysis to be >90% fibrocytes on the basis of their consistent CD31-CD45+CD34+CXCR4+Col I+ TSHRhigh phenotype (31).
GD-OF were cultivated from fat removed during surgical orbital decompression as described (4). While the fraction of CD34+ OF inhabiting GD-OF strains from individuals with severe TAO varies somewhat, the ratios of CD34+/CD34- OF are nearly 50:50 in the early passages utilized in the studies reported (passages 2-11).
RNA preparation and real-time polymerase chain reaction
GD-OF strains were inoculated into 6 well plates in 10% FBS-supplemented DMEM. Confluent cultures were shifted to DMEM without or with bTSH (5 mIU/mL) for the final 6 hrs. Cellular RNA was extracted with an Aurum TM Total RNA Mini Kit (Bio-RAD, Hercules, CA, cat #732–6820). RNA (2 µg) was reverse-transcribed and real-time polymerase chain reaction (PCR) was performed using an Applied Biosystems instrument and QuantiTect SybrGreen PCR kits (Qiagen, cat #204143). Primer sequences synthesized by Life Technologies were as follows: GAPDH forward, 5′-TTGCCATCAATGACCCCTTCA-3′, reverse, 5′-GCCCCACTTGATTTTGGA, HAS1, CGATACTGGGTAGCCTTCAATG-3′, reverse, 5′-GGAGGTGTACTTGGTAGCATAA CC-3, HAS2, forward, 5′- GTGTTATACATGTCGAGTTTACTTCC -3′ reverse, 5′- GTC- ATATTGTTGTCCCTTCTTCCGC--3′; HAS3 forward, 5′- GGTACCATCAAGTTCCTAG-GCAGC-3′, reverse, 5′- GAGGAGAATGTTCCAGATGC-G-3′; IL-6; forward, 5′-TGAGAAAGGAGACATGTAACAAGAGT-3′, reverse, 5′-TTGTTCCTCACTACTCTCA-AATCTGT-3′; UGDH; forward, 5′- CATCCAGGTGTTTCAGAGGATGAC -3′, reverse, 5′- GAATGCGTTCATAATCCAATTCC -3′, Hyal1 forward, 5′- CAAACCACTTTCT-GCCCCTG-3′, reverse, 5′- GCTTGACTGCAGAGAAGGG -3, Hyal2, forward, 5′- TGGACCTCATCTCTACCATTGG-3′ reverse, 5′-CAGCCGTGTCAGGTAATCTT -3′; Hyal3 forward, 5′- TGATCACATAGGGGCCCAAG -3′, reverse, 5′- CCTCACACACC-GGAGATCTG-3. Quantitect primer assays were from Qiagen (Frederick, MD). Sample values were generated against a standard curve and normalized to GAPDH signals.
HA ELISA
Confluent cultures were treated with nothing or rhSlit2 (50 ng/mL) for 5 days, with or without bTSH (5 mU/mL) for the intervals indicated in the figure legends or for 72 hours. siRNA was used to knock down target mRNAs while control cultures received scramble siRNA without or with bTSH (5 mU/mL) for 72 hours. Media and rinsed cell layers were homogenized in 0.1M NaCl and assayed for HA content using an ELISA kit (K-1200) from Echelon Biosciences Inc. (Salt Lake City, UT) according to the manufacturer’s instructions.
Cell transfections
Confluent GD-OF and fibrocyte cultures were transfected with 3 µg control siRNA or siRNA targeting HAS1, HAS2, Slit2, UGDH, or SP1 using a human CD34+ nucleofection kit and Amaxa nucleofector II instrument from Amaxa Bio Systems (Gaithersburg, MD) (using the U-033 program.). After 3 to 4 days, cells were incubated without or with bTSH (5 mU/mL) for 72 hours. Cell layers were subjected to RNA extraction, followed by reverse transcription, and the cDNAs were subjected to quantitative RT-PCR analysis. Levels of transfection efficiency are shown in Fig. S1 (32). The siRNA knockdowns in fibrocytes were as follows: HAS1 (5.5 fold, P < 0.01), HAS2 (2.6 fold, P < 0.01), and ROBO1 (13 fold, P < 0.001), while those conducted in GD-OF were: Slit2 (26 fold, P < 0.001), UGDH (2.6 fold, P < 0.05), and HAS2 (3.7 fold, P < 0.01).
Flow cytometric analysis
GD-OF were subjected to cytometric cell sorting using a FACSAria III (BD Biosciences) instrument. Cell monolayers were stained for 30 minutes at 4 °C with fluorescein isothiocyanate-conjugated anti-mouse CD34 or its isotype control. Washed cells were sorted under sterile conditions into GD-OF (parental), CD34+ OF, and CD34- OF subsets which were then re-cultured for 48 hours and treated without or with bTSH (5 mU/mL) for 6 hours as indicated in the figure legends.
Statistics
Statistical significance was determined by a 2-tailed Student t test. Analyses were performed with SAS version 9.4 (Cary, NC).
Results
Divergent HA, IL-6, and TNF-α synthesis and release from fibrocytes and GD-OF
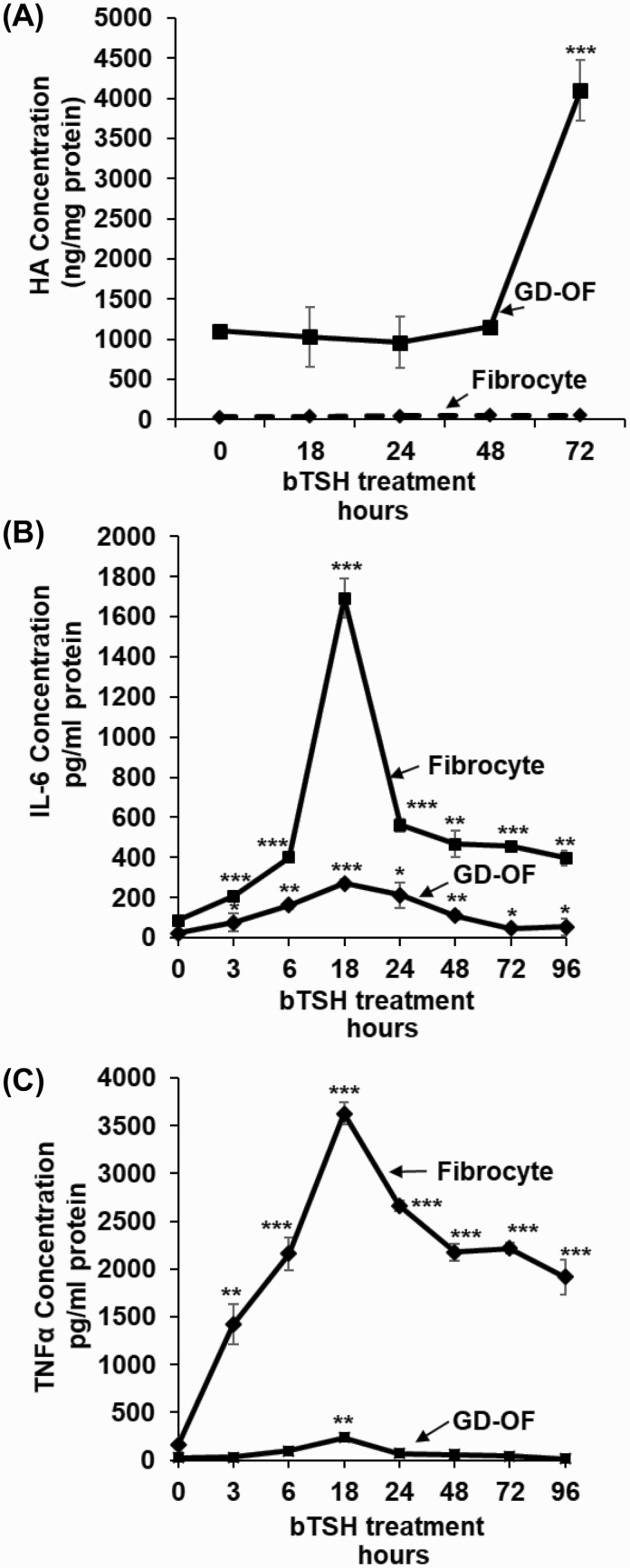
GD-OF generate HA under basal culture conditions and following treatment with insulin-like growth factor-I (IGF-I) and cytokines (18, 20, 27, 33). Compared with levels in fibrocytes, basal HA synthesis in GD-OF is substantially higher (Fig. 1A). HA release from GD-OF is increased substantially following treatment with bTSH (5 mIU/mL), a potent, nonphysiological activator of the human TSHR, in a time-dependent manner so that at 72 hours, the duration of the study, levels are 4-fold above baseline. This concentration of bTSH is associated with near-maximal cell responses mediated through the human TSHR (12). It can be regarded as representative of the concentrations occurring in extreme primary hypothyroidism. In contrast, fibrocytes continue to generate HA at extremely low levels, regardless of the bTSH treatment duration. HA synthesis in fibrocytes from patients with TAO and those from healthy donors are similarly low (data not shown), With regard to cytokine production, fibrocytes are substantially more active. bTSH treatment increases IL-6 production in fibrocytes by 20-fold at 18 hours versus baseline while the increase is substantially less robust in GD-OF (Fig. 1B). The cell-type divergence is even greater with regard to TNF-α, which is synthesized and released at a dramatically higher level in fibrocytes after 18 hours of bTSH treatment compared to GD-OF, where levels of the cytokine remain essentially undetectable (Fig. 1C). Thus, HA and cytokine production diverge in fibrocytes and GD-OF in a cell-type specific manner.
Figure 1.
Time-dependent effects of bTSH on hylauronan (HA), IL-6, and TNF-α synthesis in fibrocytes and GD-OF. Confluent cell cultures were shifted from growth medium containing 10% FBS to DMEM supplemented with 1% FBS for 18 hours. They remained untreated or received bTSH (5 mIU/mL) for the graded intervals indicated along the figure abscissas. At time “0,” all cultures were harvested. Media were collected and analyzed by specific ELISAs for (A), HA, (B) IL-6, and (C) TNF-α. These values were normalized to respective cell layer protein content. Data are expressed as mean ± SD of triplicate independent determinations. Experiments were performed 3 times; * P < 0.05; ** P < 0.01; *** P < 0.001.
HAS isoenzymes are expressed in distinct patterns in fibrocytes and GD-OF
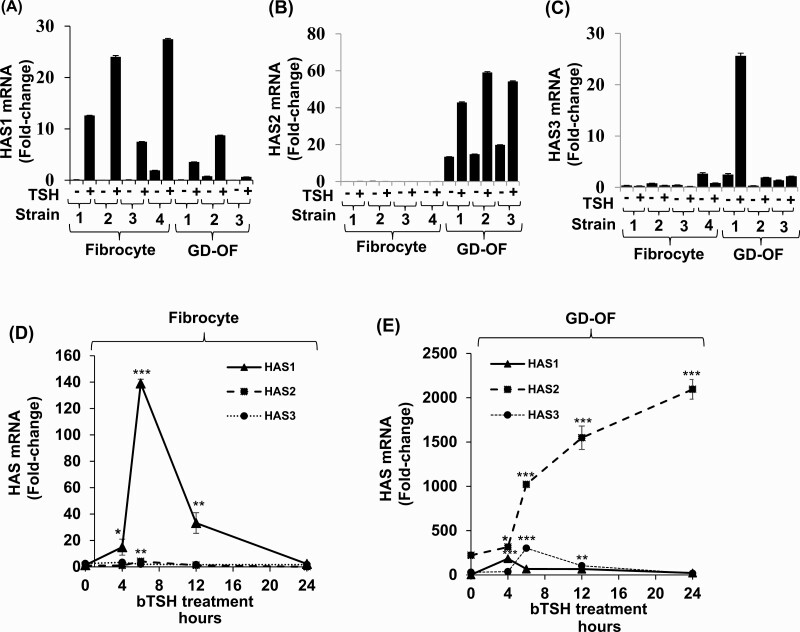
The expression patterns of the 3 HAS isoenzymes were next assessed in the 2 cell types to determine whether a basis for the different levels of HA production could be identified. As data in Fig. 2 demonstrate, very different expression patterns of HAS1 and HAS2 mRNAs were detected in fibrocytes and GD-OF. HAS1 mRNA levels following treatment with bTSH for 6 hours are higher in fibrocytes (Fig. 2A) while those of HAS2 predominate in GD-OF (Fig. 2B). HAS3 is expressed at extremely low levels in GD-OF and are undetectable in fibrocytes (Fig. 2C). These patterns can be seen in multiple strains of each cell type. The time course of HAS1 mRNA induction reveals a rapid increase peaking at 6 hours in fibrocytes and rapid decay with return to baseline at 24 hours (Fig. 2D). In contrast, HAS 2 induction, occurring predominantly in GD-OF, is initially detectable at 6 hours and continues in increase at 24 hours, the duration of the study, when it is 9-fold above basal levels (Fig. 2E).
Figure 2.
Divergent pattern of HAS isoenzyme expression and induction by bTSH in fibrocyte and GD-OF. (A-C) Confluent fibrocyte and GD-OF monolayers remained untreated or received bTSH (5 mIU/mL) for 6 hours. (D,E) Cultures were treated with bTSH for the graded intervals indicated along the abscissas. Rinsed cell layers were harvested and RNA was reverse-transcribed, cDNA was subjected to quantitative real-time PCR for HAS1, HAS2, and HAS3 mRNA levels. Values were normalized to their respective GAPDH signals and are expressed as the mean ± SD of 3 independent determinations. Experiments were repeated 3 times. * P < 0.05; ** P < 0.01; *** P < 0.001.
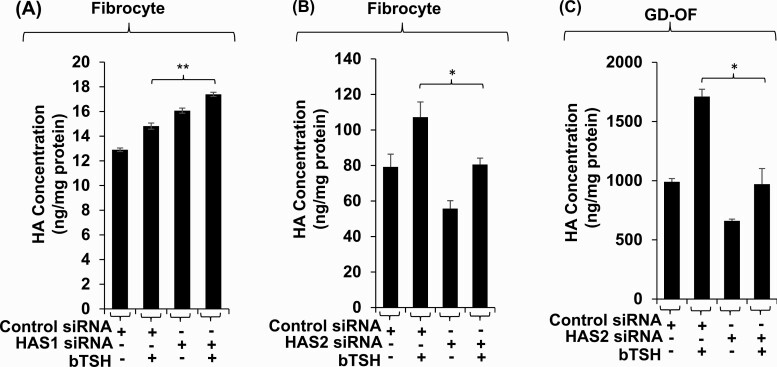
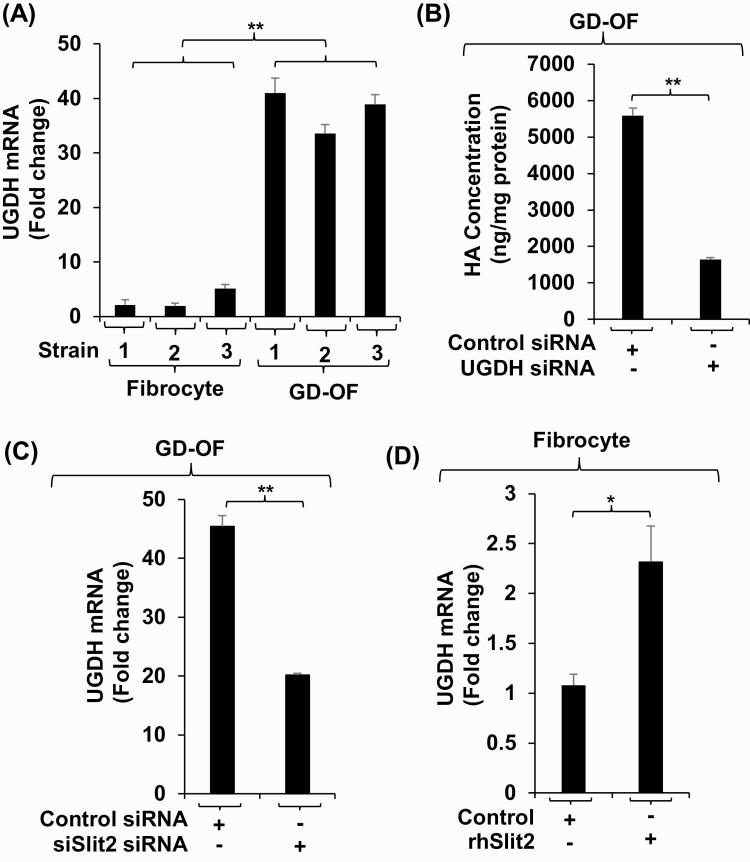
To determine whether HAS1 was a substantial contributor to the extremely low-level HA production in fibrocytes we knocked down its expression with a specific targeting siRNA and quantified the impact on glycosaminoglycan synthesis (Fig. 3A). As the data show, reducing HAS1 expression enhances both basal and bTSH-induced HA release from fibrocytes. We next knocked down HAS2 expression and found that this reduction resulted in significantly lower basal and bTSH-induced HA production (Fig. 3B). Finally, HAS2 was knocked down in GD-OF and found to reduce HA release from these cells (Fig. 3C). Furthermore, the pattern of HA release was similar when HAS2 expression was reduced in both fibrocytes and GD-OF. Thus despite the dramatically lower level of HA production in fibrocytes and the very low level of HAS2 expression in these cells, HAS2 represents the major determinant of HA synthesis in both cell types. Furthermore, HAS1 expression may attenuate HA synthesis in fibrocytes.
Figure 3.
Knocking down HAS isoenzymes reveals relative contributions of each to bTSH-induced HA synthesis n fibrocytes and GD-OF. Confluent cultures were transfected with either control siRNA (3µg) specific HAS1 (A) or HAS2 (B, C) targeting siRNA (3µg) using the CD34+ nucleofection kit as described in “Methods.” After 24 hours of re-culture, monolayers were treated with nothing or bTSH (5 mIU/mL) for 72 hours. Media were harvested and subjected to HA ELISA quantification. HA concentrations were normalized to respective monolayer protein content. Data are expressed as mean ± SD of triplicate independent determinations. Experiments were repeated 3 times. * P < 0.05; ** P < 0.01.
Slit2 reciprocally regulates HAS1, HAS2, and HA synthesis in fibrocytes and GD-OF
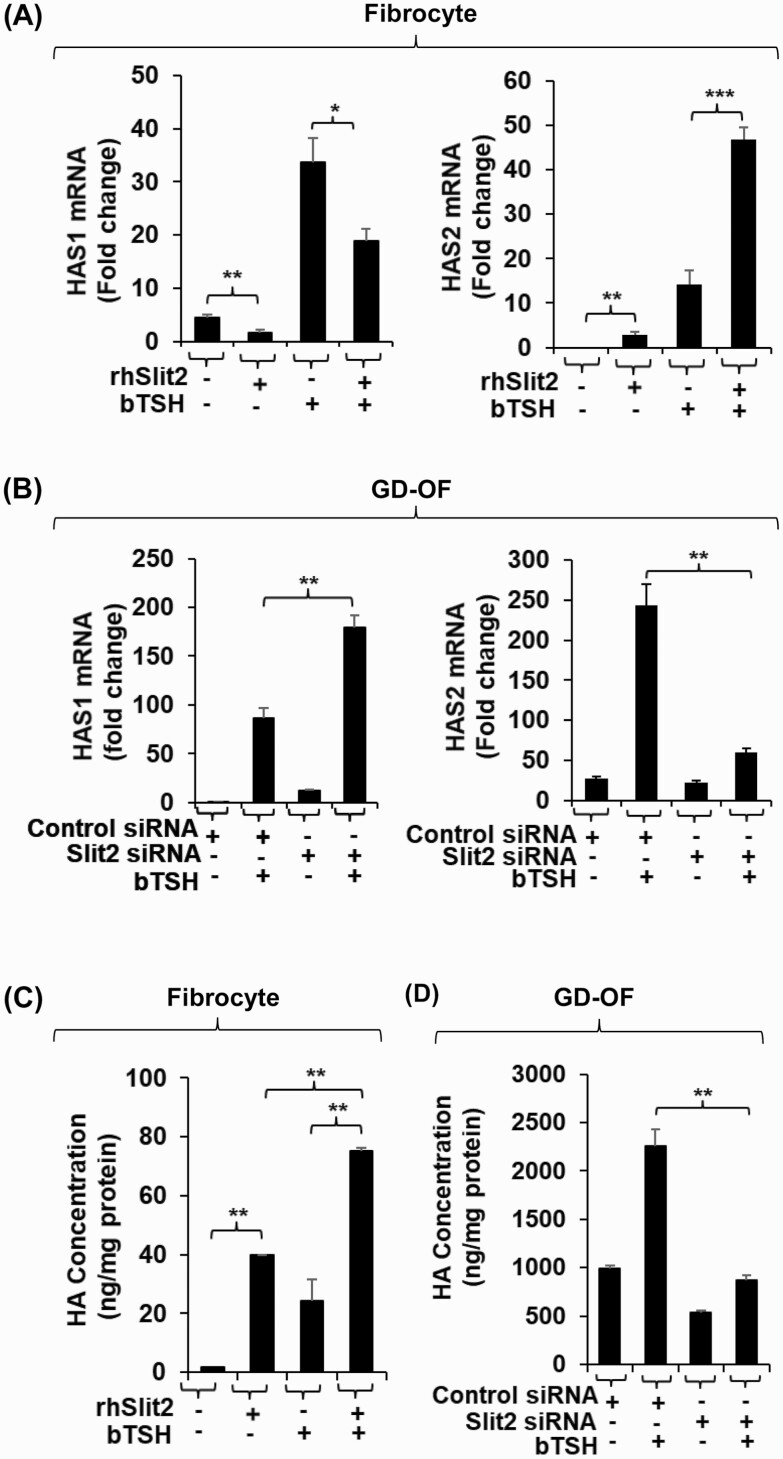
We have reported recently that Slit2 is a major determinant of gene expression in fibrocytes and GD-OF (12, 15). We thus tested the possibility that this glycoprotein might underlie the divergent expression of HAS isoenzymes and HA production in these cell types. To determine whether Slit2 alters HAS expression pattern in fibrocytes, cells that do not express the glycoprotein, confluent cultures remained untreated or were treated with rhSlit2 (50 ng/ml) for 7 to 9 days and received nothing or bTSH for the final 6 hours. Slit2 reduced HAS1 mRNA levels and the induction of that transcript by bTSH (Fig. 4A). In contrast, Slit2 upregulated basal and bTSH-dependent HAS2. To determine the impact of Slit2 generated in GD-OF, the CD34- subset, of which constitutively expresses the molecule, was transfected with either control or Slit2-targeting siRNA and then remained untreated or received bTSH for 6 hours. Knocking down Slit2 resulted in the enhanced induction of HAS1 by bTSH while reducing both basal and bTSH-dependent HAS2 expression (Fig. 4B). rhSlit2 treatment resulted in substantial upregulation of HA synthesis in fibrocytes under both basal and bTSH-treated conditions (Fig. 4C) while knocking down Slit2 expression in GD-OF reduced the induction by bTSH of HA synthesis (Fig. 4D). Thus, by utilizing very different approaches, we could document the impact of Slit2 on both fibrocytes and GD-OF.
Figure 4.
Slit2 alters HAS1 and HAS2 expression levels and HA synthesis in fibrocytes and GD-OF. (A) Fibrocytes were incubated in the absence or presence of rhSlit2 (50 ng/mL) for 7 to 9 days and then were untreated or received bTSH (5 mIU/mL) for 6 hours. (B) GD-OF were transfected with scrambled (control) siRNA (3 µg) or Slit2-targeting siRNA (3 µg) and then re-cultured for 3 days. They were then incubated in the absence or presence of bTSH for 6 hours. Cell layers were harvested, RNA was extracted, reverse-transcribed, and subjected to RT-PCR for the targets indicated. Values were normalized to their respective GAPDH levels. (C) Fibrocytes were untreated or received rhSlit2 as in panel A while in (D) GD-OF were transfected with control or Slit2-targeting siRNA and then in the absence or presence of bTSH (5 mIU/mL) for 72 hours. Media were harvested and subjected to HA ELISA. Values were normalized to cell layer protein content. Data are expressed as the mean ± SD; * P < 0.05; ** P < 0.01; *** P < 0.001 of 3 independent determinations. Experiments were performed 3 times.
Slit2 regulates UGDH expression and UGDH-dependent HA synthesis
UGDH is a critical enzyme in the biosynthesis of HA (34). The impact of Slit2 on UGDH expression was examined in fibrocytes and GD-OF. The enzyme is expressed in fibrocytes at extremely low levels compared to those in GD-OF (Fig. 5A). Furthermore, knocking expression down in GD-OF dramatically attenuates HA expression in these cells (Fig. 5B). Knocking down Slit2 in GD-OF attenuates UGDH expression (Fig. 5C) while rhSlit2 upregulates expression of the enzyme in fibrocytes (Fig. 5D).
Figure 5.
Assessment of UGDH expression, impact of knocking down UGDH levels on HA synthesis, and determining the consequences of Slit2 on UGDH expression. (A) Confluent fibrocyte and GD-OF monolayers were harvested, RNA extracted, reverse-transcribed, and subjected to RT-PCR for UGDH mRNA. Values were normalized to their respective GAPDH levels. (B) GD-OF were transfected with scrambled (control) (3 µg) or UGDH-targeting siRNA (3 µg), re-cultured for 3 days, and media collected and subjected to HA ELISA. Cell layers were collected and protein levels determined. (C) GD-OF were transfected with scrambled (3 µg) or Slit2-targeting siRNA (3 µg), re-cultured, and assessed for UGDH mRNA levels by RT-PCR. (D) Fibrocytes were incubated with nothing or rhSlit2 (50 ng/ml) for 7 to 9 days. Cell layers were processed as in panel (C). Data are expressed as the mean ± SD; * P < 0.05; **; P < 0.01 of 3 independent determinations. Experiments were performed 3 times.
Regulation of cytokine expression by Slit2 diverges from its effects on HAS expression and HA synthesis
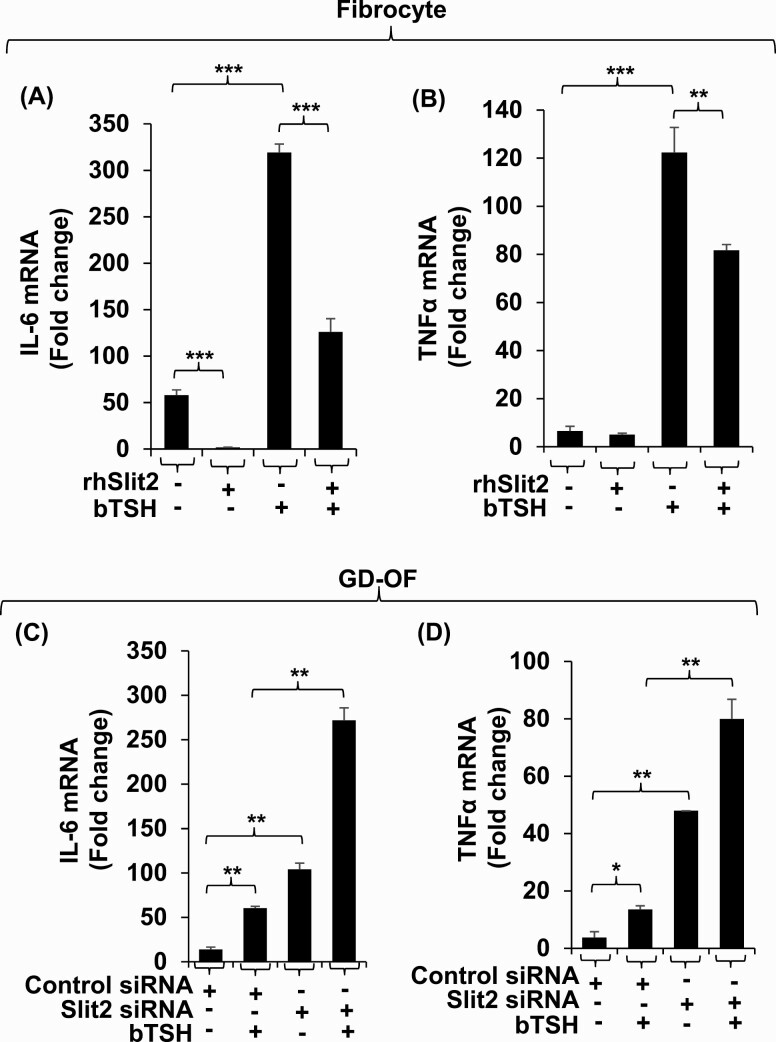
Presence of rhSlit2 attenuates the basal and bTSH-dependent expression of IL-6 in fibrocytes by 35-fold and 3-fold, respectively (Fig. 6A) and induction of TNF-α in these cells by 1.5-fold (Fig. 6B). Knocking down Slit2 in GD-OF upregulates basal and bTSH-induced IL-6 by 8-fold and 4.5-fold, respectively (Fig. 6C). Similar patterns were observed with regard to TNF-α in these cells (Fig. 6D).
Figure 6.
The impact of Slit2 on basal and bTSH-induced IL-6 and TNFα expression in fibrocytes and GD-OF. (A, B) Confluent fibrocytes were treated with nothing or rhSlit2 (50 ng/ml) for 7 to 9 days and incubated without or with bTSH (5 mIU/mL) for 6 hours. (C, D) Confluent GD-OF monolayers were transfected with scrambled (control) (3 µg) or Slit2-targeting siRNA (3 µg), re-cultured for 3 days, and treated with nothing or bTSH (5 mIU/mL) for 6 hours. Cell layers were harvested and RNA extracted, reverse-transcribed and subjected to quantitative RT-PCR for IL-6 (A and C) and TNF-α (B and D). Values were normalized to their respective GAPDH levels. Data are expressed as the mean ± SD; * P < 0.05; ** P < 0.01; *** P < 0.001 of 3 independent determinations. Experiments were repeated 3 times.
Localizing HAS and cytokine expression to specific GD-OF subsets
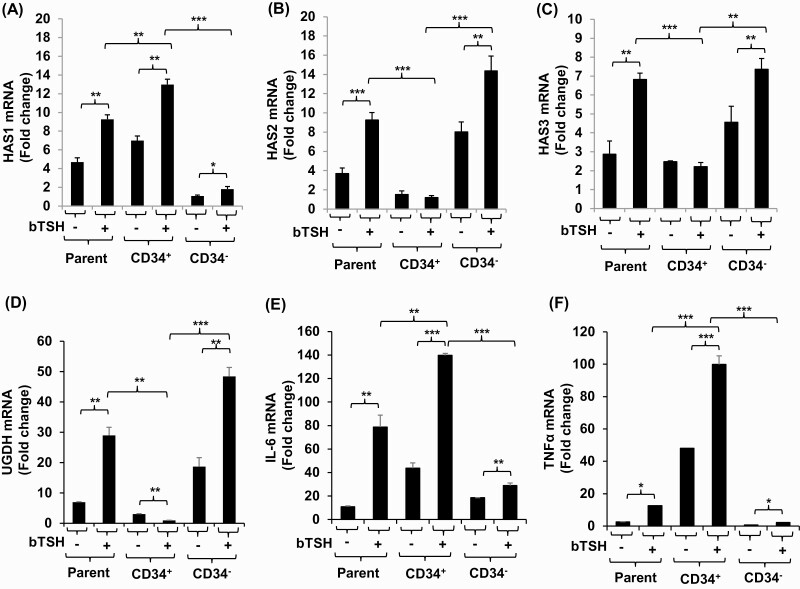
GD-OF strains comprise a mixture of CD34+ OF, putatively derived from circulating fibrocytes and CD34- OF, which are thought to be residential orbital fibroblasts (9). Parental strains, admixtures of these subsets, were subjected to cytometric cell sorting into pure cell populations. They were then cultured in the absence or presence of bTSH for 6 hours, harvested, and subjected to RT-PCR analyses. As the data in Fig. 7 demonstrate, the highest basal and bTSH-induced levels of HAS1 mRNA are found in CD34+ OF compared with CD34- OF (Fig. 7A), while induction of HAS2 dominates in CD34- OF (Fig. 7B). HAS3 (Fig. 7C) and UGDH (Fig. 7D) mRNA levels are also localized to CD34- OF. Both IL-6 and TNF-α mRNAs following induction with bTSH, mirroring HAS1, are higher in CD34+ OF (Fig. 7E, F).
Figure 7.
Localization of HAS1, HAS2, HAS3, UGDH, IL-6, and TNF-α expression and induction by bTSH to CD34+ OF and CD34- OF subsets of GD-OF. GD-OF were sorted into pure CD34+ and CD34- cell populations as described in “Methods.” These were re-cultured for 48 hours, the final 6 hours in the absence or presence of bTSH (5 mIU/mL). RNA was extracted, reverse-transcribed, and subjected for quantitative RT-PCR for the target genes indicated. Transcript levels were normalized to their respective GAPDH signals. Data are expressed as mean ± SD of triplicate independent determinations from a single experiment representative of 3 conducted. * P < 0.05; ** P < 0.01; *** P < 0.001.
ROBO1 mediates downregulation by Slit2 of bTSH-induced HAS1, IL-6, and TNF-α expression in fibrocytes
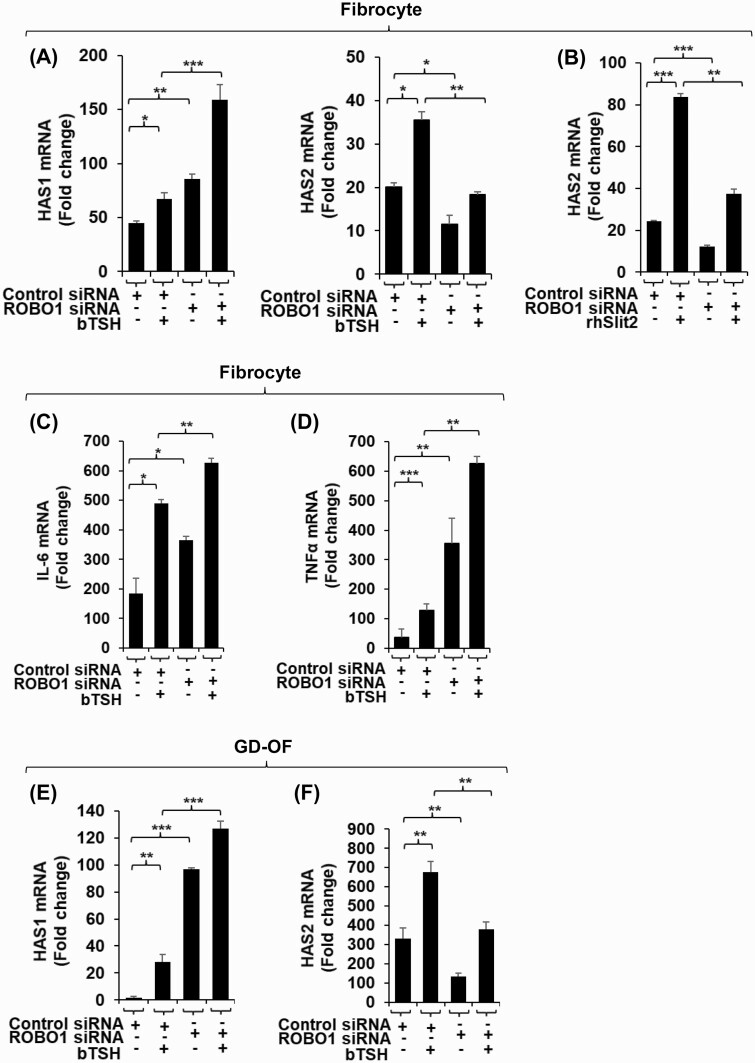
Like many receptor/ligand interactions, those between Slits and ROBOs can be promiscuous. We therefore determined whether ROBO1 mediates the actions of Slit2 in fibrocytes and GD-OF. We found that siRNA knockdown of ROBO1 in fibrocytes for 3 days resulted in enhancement of bTSH-dependent HAS1 expression while attenuating the HAS2 induction (Fig. 8A). Knockdown of ROBO1 could also attenuate the enhancement of HAS2 expression by rhSlit2(Fig. 8B). IL-6 expression was enhanced in fibrocytes with siROBO1 (Fig. 8C) as was that of TNF-α (Fig. 8D). Knocking down ROBO1 in GD-OF upregulated HAS1 induction (Fig. 8E) while attenuating that of HAS2 (Fig. 8F). Thus, the inhibitory effects of Slit2 appear to be mediated through their cognate receptor, ROBO1.
Figure 8.
Knockdown of ROBO1 enhances HAS1, IL-6 and TNF-α expression and induction by bTSH in fibrocytes while reducing that of HAS2. Confluent fibrocyte and GD-OF cultures were disrupted with accutase and cell pellets were transfected with control siRNA (3 µg) or ROBO1 siRNA according to the manufacturer’s protocol using the U-033 program. Cells were re-plated and incubated for 48 hours in the absence or presence of bTSH (5 mIU/mL) for the final 6 hours. RNA was extracted, reverse-transcribed and cDNAs were analyzed by RT-PCR for (panels A, B, E, F) HAS1, HAS2 or (panels C,D) IL-6, and TNF-α expression. Transcript levels were normalized to their respective GAPDH signals. Data are expressed as mean ± SD of triplicate independent determinations. Each experiment was performed 3 times. *, P < 0.05; **, P < 0.01, ***, P < 0.001.
Relative expression levels of hyaluronidases in fibrocytes compared with GD-OF
Basal hyaluronidase 2 (Hyal2) and (Hyal3) mRNAs appear to be more highly abundant in fibrocytes than in GD-OF (Fig. S2) (35). While none of the isoenzymes appears to be induced by bTSH, Hyal2 mRNA is consistently reduced by the hormone in fibrocytes after 24 hours. Hyal1 predominates in fibrocytes under basal and bTSH-induced conditions. Induction of that enzyme peaks at 4 hours (by 3-fold), and very rapidly returns to baseline. This finding is consistent with the general absence of hyaluronidase activity in human fibroblasts (29), including those from the orbit (17), despite the expression induced by prostaglandin D2 of the respective mRNAs (30).
Discussion
Results from the current studies indicate that glycosaminoglycan biosynthesis in GD-OF localizes to the CD34- OF subset while IL-6 and TNF-α expression primarily occurs in CD34+ OF. In aggregate, GD-OF, comprising both CD34+ OF and CD34- OF (9), exhibit unique phenotypes, including their remarkable capacity to generate inflammatory factors, including prostaglandins and eicosanoids (36, 37). Tissue remodeling in TAO is dominated by a disordered accumulation of HA within the orbit (20, 38), adipogenesis, and the local production of proinflammatory cytokines such as IL-6 and TNF-α (39). From earlier studies, we now understand many important aspects of the biosynthesis of these molecules. HA synthesis localizes to the plasma membrane, unlike the generation of other abundant glycosaminoglycans (40). HAS isoenzymes are integral proteins that have been molecularly cloned and their gene promoter regions characterized (41-43). Similarly, the UGDH gene and its promoter region have also been solved (28, 44). The latter enzyme catalyzes UDP-glucose oxidation forming UDP glucuronic acid, upstream from its utilization as a substrate for HA synthesis by all 3 HAS proteins. However, with specific regard to this process in GD-OF, HA synthesis has not been fully characterized previously, despite its importance in the development of TAO. Furthermore, a complete understanding of its regulation and the identification of which GD-OF subset(s) contributes primarily to glycosaminoglycan accumulation in the disease has yet to be accomplished. While earlier studies have disclosed that HA synthesis in these cells can be upregulated by several exogenous factors, including interferon γ, transforming growth factor β, IGF-I, leukoregulin, CD40 ligand (CD154), and IL-1β (3, 18, 33, 45), none identified endogenous factors regulating HA synthesis in GD-OF (20). The current studies disclose a divergent pattern of HAS isoenzymes, UGDH, and cytokine expression as well as quantitative differences in HA, IL-6, and TNF-α synthesis within GD-OF subsets. They allow partitioning of these activities between CD34+ OF and CD34- OF. These cell-specific differences appear to be related, at least in part, to the generation of Slit2 by CD34- OF and its actions on CD34+ OF (12, 15). Furthermore, the actions of exogenous rhSlit2 on fibrocytes provoke a transition from HAS1 dominating expression, albeit at a very low level, to that of HAS2, while attenuating the expression of both IL-6 and TNF-α. Regulation of HA synthesis in GD-OF differs fundamentally from that in dermal fibroblasts. In the latter, thyroid hormones and glucocorticoids attenuate this process whereas both hormones appear to have substantially less influence on HA synthesis and accumulation in GD-OF (17, 46, 47). HA production can be upregulated by several factors, including exogenous high-molecular-weight HA (48), multiple cytokines (3, 18, 19, 26, 49, 50), TSH (51), IGF-I, and GD-IgG (33, 52). Co-culturing GD-OF with T cells and HMC-1 mast cells can also enhance HA production (26, 53, 54) Differences in signaling upstream from the activation of HAS2 gene expression have been identified in subcutaneous fibroblasts and GD-OF (27, 28, 44). Forkhead box O is involved in determining levels of HAS2 and HA production in GD-OF (55). While high-molecular-weight HA enhances and low-molecular-weight HA inhibits fibrocyte differentiation (56), to our knowledge, HA synthesis by human fibrocytes has not been characterized previously.
The current findings underscore the complexities of cell-cell interactions present in the heterogeneous fibroblast population comprising GD-OF. These presumably mirror the interplay between those fibroblasts within the TAO orbit. Further studies will be necessary to directly assess these interactions in vivo. These studies identify several genes expressed by fibrocytes and CD34+ OF that are targeted by Slit2, dramatically altering the inflammatory phenotype of these cells. In our view, they suggest the imperative that studying GD-OF, not only as mixed populations, but also as pure CD34+ OF and CD34- OF, will allow identification of their intrinsic characteristics, masked by the other subset. Our current findings indicate that Slit2 may represent a factor previously unrecognized for its capacity to modulate HAS and UGDH expression and HA synthesis in human tissues. It is therefore possible that this glycoprotein can be targeted therapeutically to mitigate pathological HA accumulation in TAO and potentially in other diseases as well.
Acknowledgments
The authors are indebted to Ms. Dana Barnhart for her expert help in the preparation of this manuscript.
Financial Support: This work was supported in part by National Institutes of Health (NIH) grants R01 EY008976, NIH Autoimmune Center of Excellence grant AR088974, National Eye Institute (NEI) Core grant EY007003 and support from the Bell Charitable Family Foundation and Research to Prevent Blindness.
Glossary
Abbreviations
- bTSH
bovine thyrotropin (thyroid-stimulating hormone)
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GD
Graves disease
- GD-OF
orbital fibroblasts from patients with Graves disease
- HA
hyaluronan
- HAS1-3
HA synthase isoenzymes 1-3
- Hyal
hyaluronidase
- IGF-I
insulin-like growth factor-I
- IL-
interleukin
- OF
orbital fibroblasts
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- rhSlit2
recombinant human Slit2
- ROBO1
roundabout 1 (receptor)
- siRNA
small interfering RNA
- TAO
thyroid-associated ophthalmopathy
- TNF-α
tumor necrosis factor-α
- TSH
thyrotropin (thyroid-stimulating hormone)
- TSHR
thyrotropin (thyroid-stimulating hormone) receptor
- USDH
uridine diphosphate-glucose dehydrogenase
Additional Information
Disclosure Summary: T.J.S. was issued patents covering treatment of TAO and other autoimmune diseases with inhibitors of the insulin-like growth factor-I receptor. These are held by UCLA/Los Angeles Biomedical Foundation.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1565. [DOI] [PubMed] [Google Scholar]
- 2. Taylor PN, Zhang L, Lee RWJ, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104-116. [DOI] [PubMed] [Google Scholar]
- 3. Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268(2 Pt 1):C382-C388. [DOI] [PubMed] [Google Scholar]
- 4. Smith TJ. Role of orbital fat in thyroid-associated ophthalmopathy. Graves’ Associated Orbital Disease. In: Dutton JJ, Haik BG eds. Thyroid Eye Disease: Diagnosis and Treatment. New York, NY: Marcel Dekker, Inc. Publishers. 2002:215-221 [Google Scholar]
- 5. Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120(3):380-386. [DOI] [PubMed] [Google Scholar]
- 6. Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol. 2015. ;11(3):171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith TJ. Potential roles of CD34+ fibrocytes masquerading as orbital fibroblasts in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2019;104(2):581-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71-81. [PMC free article] [PubMed] [Google Scholar]
- 9. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012;109(19):7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99(7):E1236-E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernando R, Atkins SJ, Smith TJ. Slit2 may underlie divergent induction by thyrotropin of IL-23 and IL-12 in human fibrocytes. J Immunol. 2020;204(7):1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96(6):785-794. [DOI] [PubMed] [Google Scholar]
- 14. Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci U S A. 2014;111(51):18291-18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernando R, Grisolia ABD, Lu Y, Atkins S, Smith TJ. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol. 2018;200(12):3942-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ypsilanti AR, Chedotal A. Roundabout receptors. Adv Neurobiol. 2014;8:133-164. [DOI] [PubMed] [Google Scholar]
- 17. Smith TJ, Bahn RS, Gorman CA. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. J Clin Endocrinol Metab. 1989;69(5):1019-1023. [DOI] [PubMed] [Google Scholar]
- 18. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991;72(5):1169-1171. [DOI] [PubMed] [Google Scholar]
- 19. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615-29625. [DOI] [PubMed] [Google Scholar]
- 20. Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10(3):366-391. [DOI] [PubMed] [Google Scholar]
- 21. Smith TJ. n-Butyrate inhibition of hyaluronate synthesis in cultured human fibroblasts. J Clin Invest. 1987;79(5):1493-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kahaly G, Förster G, Hansen C. Glycosaminoglycans in thyroid eye disease. Thyroid. 1998;8(5):429-432. [DOI] [PubMed] [Google Scholar]
- 23. Garantziotis S, Savani RC. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019;78-79:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Passi A, Vigetti D, Buraschi S, Iozzo RV. Dissecting the role of hyaluronan synthases in the tumor microenvironment. Febs J. 2019;286(15):2937-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273(4):1923-1932. [DOI] [PubMed] [Google Scholar]
- 26. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1beta in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84(11):4079-4084. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Grennan-Jones F, Lane C, Rees DA, Dayan CM, Ludgate M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(2):653-662. [DOI] [PubMed] [Google Scholar]
- 28. Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273(39):25117-25124. [DOI] [PubMed] [Google Scholar]
- 29. Arbogast B, Hopwood JJ, Dorfman A. Absence of hyaluronidase in cultured human skin fibroblasts. Biochem Biophys Res Commun. 1975;67(1):376-382. [DOI] [PubMed] [Google Scholar]
- 30. Guo N, Baglole CJ, O’Loughlin CW, Feldon SE, Phipps RP. Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: implications for thyroid eye disease. J Biol Chem. 2010;285(21):15794-15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernando R, Smith TJ. Supplemental Figure 1 from: Slit2 regulates hyaluronan & cytokine synthesis in fibrocytes: potential relevance to thyroid associated ophthalmopathy. University of Michigan Deep Blue Repository. Deposited September 2, 2020. http://hdl.handle.net/2027.42/156503. [DOI] [PMC free article] [PubMed]
- 33. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076-5080. [DOI] [PubMed] [Google Scholar]
- 34. Arnold JM, Gu F, Ambati CR, et al. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene. 2020;39(15):3089-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernando R, Smith TJ. Supplemental Figure 2 from: Slit2 regulates hyaluronan & cytokine synthesis in fibrocytes: potential relevance to thyroid associated ophthalmopathy. University of Michigan Deep Blue Repository. Deposited September 2, 2020. http://hdl.handle.net/2027.42/156504. [DOI] [PMC free article] [PubMed]
- 36. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355-16364. [DOI] [PubMed] [Google Scholar]
- 37. Chen B, Tsui S, Boeglin WE, Douglas RS, Brash AR, Smith TJ. Interleukin-4 induces 15-lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves disease. Evidence for anatomic site-selective actions of Th2 cytokines. J Biol Chem. 2006;281(27):18296-18306. [DOI] [PubMed] [Google Scholar]
- 38. Mohyi M, Smith TJ. IGF1 receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol. 2018;61(1):T29-T43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85(3):1194-1199. [DOI] [PubMed] [Google Scholar]
- 40. Philipson LH, Schwartz NB. Subcellular localization of hyaluronate synthetase in oligodendroglioma cells. J Biol Chem. 1984;259(8):5017-5023. [PubMed] [Google Scholar]
- 41. Spicer AP, Augustine ML, McDonald JA. Molecular cloning and characterization of a putative mouse hyaluronan synthase. J Biol Chem. 1996;271(38):23400-23406. [DOI] [PubMed] [Google Scholar]
- 42. Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997;272(14):8957-8961. [DOI] [PubMed] [Google Scholar]
- 43. Monslow J, Williams JD, Guy CA, et al. Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J Biol Chem. 2004;279(20):20576-20581. [DOI] [PubMed] [Google Scholar]
- 44. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286(27):24487-24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao HJ, Hogg MG, Martino LJ, Smith TJ. Transforming growth factor-beta induces plasminogen activator inhibitor type-1 in cultured human orbital fibroblasts. Invest Ophthalmol Vis Sci. 1995;36(7):1411-1419. [PubMed] [Google Scholar]
- 46. Smith TJ, Murata Y, Horwitz AL, Philipson L, Refetoff S. Regulation of glycosaminoglycan synthesis by thyroid hormone in vitro. J Clin Invest. 1982;70(5):1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith TJ. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J Clin Invest. 1984;74(6):2157-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim HS, Back KO, Kim HJ, Choi YH, Park YM, Kook KH. Hyaluronic acid induces COX-2 expression via CD44 in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(11):7441-7450. [DOI] [PubMed] [Google Scholar]
- 49. van Steensel L, Paridaens D, van Meurs M, et al. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2012;97(3):E400-E408. [DOI] [PubMed] [Google Scholar]
- 50. Chu X, Pan CM, Zhao SX, et al. ; China Consortium for Genetics of Autoimmune Thyroid Disease . A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet. 2011;43(9):897-901. [DOI] [PubMed] [Google Scholar]
- 51. Zhang L, Grennan-Jones F, Draman MS, et al. Possible targets for nonimmunosuppressive therapy of Graves’ orbitopathy. J Clin Endocrinol Metab. 2014;99(7):E1183-E1190. [DOI] [PubMed] [Google Scholar]
- 52. Adams DD, Purves HD, Sirett NE. The response of hypophysectomized mice to injections of human serum containing long-acting thyroid stimulator. Endocrinology. 1961;68:154-155. [DOI] [PubMed] [Google Scholar]
- 53. Kahaly G, Otto E, Förster G, et al. T cells and orbital connective tissue in endocrine orbitopathy. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 4):79-83. [DOI] [PubMed] [Google Scholar]
- 54. Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in coculture: evidence for up-regulation of prostaglandin E2 and hyaluronan synthesis. Endocrinology. 1999;140(8):3518-3525. [DOI] [PubMed] [Google Scholar]
- 55. Zhang L, Ji QH, Ruge F, et al. Reversal of pathological features of Graves’ orbitopathy by activation of forkhead transcription factors, FOXOs. J Clin Endocrinol Metab. 2016;101(1):114-122. [DOI] [PubMed] [Google Scholar]
- 56. Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One. 2011;6(10):e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.