Abstract
Context
Weight regain (WR) after bariatric surgery is emerging as a common clinical problem due to the increase in the number of procedures performed. Early interventions are necessary to curtail the potential recurrence of comorbid conditions. However, it is often difficult to recognize WR early enough to introduce mitigating measures because there are no current guidelines for timely diagnosis and assessment of the severity of this condition.
Objective
We present a practical approach for the early recognition of WR, based on 11-year follow-up data from our multiethnic bariatric surgery patient population.
Methods
We classify WR according to the rate of increase in weight relative to nadir weight, normalized per 30-day interval. We also review pertinent literature about the etiologic factors contributing to WR after bariatric surgery.
Results
According to our algorithm, mild, moderate, and rapid WR are defined as weight increases of 0.2% to <0.5%, 0.5% to 1.0%, and more than 1.0% of nadir weight per 30 days, respectively. Treatment options, including dietary counseling, use of antiobesity medication, and consideration of surgical revision, are described. A case is presented to illustrate the utility of timely identification of WR and the importance of collaboration between bariatric surgeons, obesity medicine specialists, and dietitians.
Conclusion
Our approach emphasizes the importance of regular long-term follow-up for all bariatric surgery patients.
Keywords: bariatric surgery, weight regain, weight loss surgery, gastric bypass, obesity, obesity treatment
The Patient
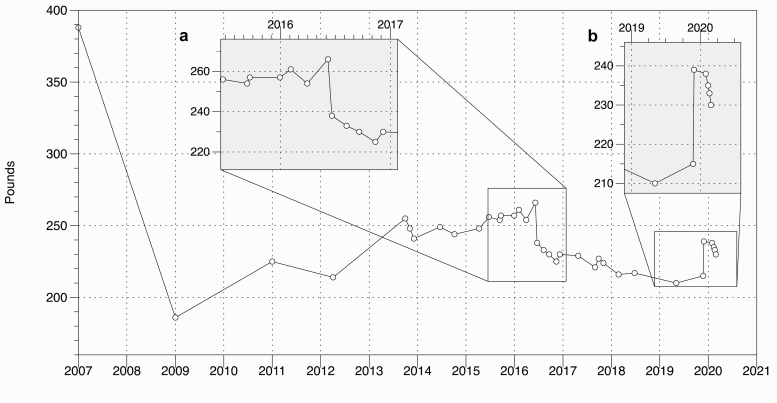
The patient is a 50-year-old White Caribbean female with a history of gastroesophageal reflux disease, asthma, and iron deficiency anemia who returned to the Nutrition and Weight Management clinic with the concern of weight regain 4 years after Roux-en-Y gastric bypass surgery (RYGB) in 2007 (Fig. 1). Her initial body weight was 389 lb (176.4 kg; BMI 65 kg/m2), and by the time she was at nadir, she weighed 183 lb (83.0 kg; BMI 30.5 kg/m2), which represents a weight loss of 53% of initial weight. During the next 2 years, she was treated with citalopram for mild depression, started to complain of hunger and regained 25 lb (11.3 kg; 13% over nadir). She continued to gain weight despite counseling for lifestyle modification; by 2014, she had regained another 20 lb (9.1 kg) for a total of 45 lb (20.4 kg) over nadir. Because of concern for a gastrogastric (GG) fistula or other anatomic abnormality, an upper gastrointestinal (GI) series was performed, which was normal. With fistula ruled out, she was started on phentermine and topiramate. During the next 3 years with the help of intermittent antiobesity medications (AOMs), she was able to maintain a stable weight of around 240 lb (108.9 kg). By 2016, however, she weighed 261 lb (118.4 kg) (Fig. 1A) and was still complaining of hunger and eating larger meal portions. The patient asked if any other procedure could be performed because she felt scared to regain more weight. An upper endoscopy was performed to look for a dilated anastomosis and/or a dilated pouch. Several abnormalities were found and a procedure was performed in June 2016. Over the next 3 years, she lost 40 lb (18.1 kg). However, her physical activities diminished as she complained of sciatica and fatigue from iron deficiency. Over the course of a 6-month interval between May and December of 2019, she gained 29 lb (13.2 kg) (Fig. 1B). AOMs were resumed in January 2020.
Figure 1.
Weight graph for the patient.
Background
Bariatric surgery is the most effective treatment for severe obesity (1) because it results in durable weight loss and improvement or remission of comorbidities such as type 2 diabetes mellitus (T2DM), hypertension, sleep apnea, and dyslipidemia. In the United States, the RYGB and the vertical sleeve gastrectomy (VSG) are the most commonly performed types of bariatric surgery (2). The mean weight loss after successful bariatric surgery is approximately 33% of the initial body weight (3). However, at least one-third of patients will regain more than 25% of total weight lost (4) and this weight regain typically occurs within 2 to 5 years of surgery (5).
Weight regain (WR) after bariatric surgery has the potential of adversely affecting health outcomes because of the reemergence of obesity-associated comorbidities (6), worsening quality of life (7), and increased health care costs (8). Therefore, it is important to recognize WR in patients with a history of bariatric surgery to mitigate its clinical and metabolic impact. Unfortunately, a clear definition of clinically significant WR has not been established. Furthermore, studies have used different methodologies to report WR, resulting in confusion about the prevalence of this condition and its occurrence. Thus, it is currently difficult to identify WR as a specific clinical entity. Here, we use data from our multiethnic patient population to define WR and present a working plan for assessment and management of the patient with clinically significant WR. The case we present is an example of a typical patient who regained a significant amount of weight after successful RYGB and required long-term follow-up and multidisciplinary weight-management interventions. The case also illustrates the utility of early recognition of WR and management with AOMs. In the following sections, we review the factors that contribute to WR and describe each management modality.
Defining Weight Regain Following Bariatric Surgery
Without a standard definition of WR, it is difficult to compare data from different studies. For example, in the Swedish Obese Subjects (SOS) study, long-term weight outcomes are reported as weight changes relative to the presurgical weight (9-12). This approach emphasizes the beneficial long-term outcomes of bariatric surgery since typically there is still significant weight loss from baseline to 10 years following the surgical procedure despite some WR. However, data presented as such do not provide clear and direct measures of the amount of WR. A discussion of the different ways of reporting WR and how they relate to clinical outcomes is presented by King et al (13). We have previously argued that because WR is the consequence of the specific physiological processes associated with nutrient excess and positive energy balance (termed metabolic overfeeding), it is better evaluated in terms of actual weight increase rather than by a decrease in previously achieved weight loss (3, 14). Since the energy needs are directly related to body mass, we use nadir weight as the reference for WR assessment. In this way, we regard WR/nadir as a clinical marker of the degree of positive energy balance in the post–bariatric surgery phase. Because total WR increases with time, we define WR/nadir as a rate taking into consideration the time interval during which the increase in weight occurs (3, 14).
Based on our previous studies (3), we propose that the rate of WR/nadir, arbitrarily expressed per 30-day interval, is a clinical tool to identify the occurrence of metabolic overfeeding and determine the severity of WR in “real time.” Until more definitive data are available, we define mild, moderate, and rapid WR as the regain of less than 0.5%, 0.5% to 1.0%, and greater than 1.0% of nadir weight, respectively, over a period of 30 days. This definition is based on the tertile distribution of WR data, adjusted to the nearest 0.5, in our bariatric surgery patient population during an observation period of 11 years (3). It should be emphasized that this classification is arbitrary and pertains to our specific patient population. Further studies are necessary to delineate the clinical consequences of WR and establish evidence-based classification criteria. However, we note that by our classification, “rapid WR” would result in weight increase in excess of 5% within 6 months, which is considered clinically significant (15). The utility of this classification for patient assessment and treatment planning is discussed in a subsequent section.
Factors Contributing to Weight Regain: Metabolic Overfeeding
WR is an active process in which energy consumed in excess of need is deposited as body fat. Its occurrence indicates channeling of intermediary metabolites toward fat synthesis and deposition. We refer to this process as metabolic overfeeding. Factors that lead to positive energy balance, metabolic overfeeding, and WR can be categorized as behavioral/environmental, metabolic/medical, and anatomic/surgical, and will be discussed in the following sections.
Behavioral/environmental
Dietary factors.
Food intake diminishes substantially after bariatric surgery, which is the main driver of weight loss in the first 6 months. At nadir weight, typically achieved by 12 to 24 months after surgery, patients are able to consume sufficient food to match their energy needs and maintain a stable weight without experiencing hunger or food-seeking behavior. Thus, it has been proposed that bariatric surgery changes the weight “set point.” This beneficial effect (16, 17) is frequently attributed to changes in regulatory gut hormones, such as glucagon-like peptide 1 (GLP-1), cholecystokinin, peptide YY, ghrelin, pancreatic polypeptide, and leptin. Patients who start experiencing WR often are aware of changes in their perception of satiety and report recurrence of overeating behavior. The physiological basis behind the regulation of satiety after bariatric surgery remains incompletely understood. Hypothetically, the beneficial effects of guthormone changes may become blunted or counterregulated by other pathways over time (18, 19).
Disordered eating habits such as grazing and binge eating disorder, night eating syndrome, postsurgical eating avoidance disorder, soft food syndrome, and eating disorders not otherwise specified have been identified as increasing the risk of WR after bariatric surgery (20). It is therefore critical that patients have frequent psychological assessments for eating disorders as well as screening for depression (21), which, along with other psychiatric illnesses, has been associated with increased risk of early weight plateau and WR (22, 23). The relationship of depression to emotional and disordered eating behavior is well recognized in the literature and needs to be considered whenever patients present with WR after bariatric surgery. In addition, some of the common psychotropic and antidepressant medications have side effects of weight gain (24) (Table 1). Thus, collaboration with the patient’s medical providers to choose the least weight-promoting or weight-neutral agent is important.
Table 1.
Drugs associated with weight gain (24)
| Diabetes medications |
| • Thiazolidinediones—pioglitazone, rosiglitazone |
| • Sulfonylureas—glimepiride, glipizide, glyburide |
| • Insulin |
| Steroids |
| • Glucocorticoids—dexamethasone, methylprednisolone, prednisolone, prednisone |
| • Contraceptives—medroxyprogesterone acetate |
| Antipsychotic medications |
| • First generation—thorazine, thioridazine, haloperidol, fluphenazine |
| • Second generation—clozapine, olanzapine, quetiapine, risperidone, paliperidone, asenapine, iloperidone, cariprazine |
| Antidepressants |
| • MAOIs—isocarboxazid, phenelzine, tranylcypromine |
| • SSRI—paroxetine |
| • TCAs—amitriptyline, doxepin, imipramine |
| • Mirtazapine |
| • Lithium |
| Antiepileptic medications |
| • carbamazepine, gabapentin, pregabalin, valproic acid |
| Allergy medications |
| • Antihistamines—diphenhydramine |
| Cardiovascular medications |
| • β-Adrenergic receptor blockers—atenolol, metoprolol, nadolol, propranolol |
| • α-Adrenergic receptor blockers—doxazosin, prazosin, terazosin |
Abbreviations: MAOI, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
It has been recognized that patients who have undergone bariatric surgery, especially RYGB, are at increased risk of developing alcohol use disorder (AUD) (25). AUD postoperatively can occur as an “addiction transfer” or “cross-addiction,” whereby binge eating is substituted by another addictive behavior, in this case alcohol abuse (26). Addiction transfer can be facilitated by decreased availability of alcohol dehydrogenase from gastric mucosa, causing “rapid intoxication” with lesser alcohol content after both RYGB and SG surgeries (27). The contribution of AUD to WR after bariatric surgery has been documented (26, 28).
Suboptimal diet composition and food choices have also been implicated in WR (29). A high-protein, low glycemic index, and low-fat content diet with at least 5 servings of fruits and vegetables per day is advised after bariatric surgery. Although exact needs after bariatric surgery have not been defined, 1.0 to 1.5 g/kg ideal body weight protein intake per day is recommended (30) to mitigate lean muscle loss during the weight loss period and to ensure continued satiety during weight maintenance from the protein load (31).
Post–bariatric surgery hypoglycemia, which is a late component of dumping syndrome, is a risk factor for WR. Although the exact incidence is unknown, one study suggests that about 13% of patients meet at least one criterion for a diagnosis of hypoglycemia (32, 33), although severe hypoglycemia with neuroglycopenic symptoms occurs in less than 1% of patients after bariatric surgery. Typically, a reactive drop in blood glucose occurs after consumption of sugars and refined carbohydrates and is caused by altered GI hormones and insulin secretion in response to the rapid transit of nutrients into the jejunum (34). Factors responsible for hypoglycemia include an increase in GLP-1 secretion, known to occur after gastric bypass, and enhanced insulin sensitivity resulting from the weight loss. The prevalence of dumping syndrome after bariatric surgery is well established and reported to vary between 15% and 70%, with lesser reported prevalence in VSG in comparison to RYGB (35). Unfamiliarity with the etiology of hypoglycemia associated with dumping syndrome and disregard of the principles of post–bariatric surgery dietary management trigger more carbohydrate and sugar consumption in response to the acute symptoms, thus further aggravating WR.
Finally, it has been established that a major factor in WR has been lack of adherence to recommended follow-up clinic visits, which is observed in more than 60% of patients 4 years after bariatric surgery (36). Long-term and frequent counseling with a bariatric surgery dietitian is associated with improved weight loss success (37) and should be routinely encouraged. Despite the initial extensive dietary education provided by bariatric surgery programs, we find that long-term dietary counseling remains beneficial for most patients postoperatively (38, 39).
Physical activity.
Physical activity guidelines for weight loss and weight maintenance after bariatric surgery have been difficult to establish. In one study, supervised physical activity training for 12 weeks has been observed to improve lean body mass and functional walking ability 12 to 24 months post-bariatric surgery (40). Based on clinical observations from many studies, 150 minutes per week of moderate to vigorous activity has been found to be effective in its ability to help patients achieve greater weight loss and weight loss maintenance in general. However, severe energy restriction, rapid weight loss, and limited protein intake add to the complexity of understanding the impact of exercise on fat-free muscle mass in the setting of bariatric surgery (41). It is estimated that loss of muscle mass accounts for nearly one-third (29.7%) of the total weight loss after bariatric surgery (40, 42, 43). Exercise training and specifically resistance exercise can potentially attenuate muscle loss (44); however, published data demonstrate that only a minority of patients reach the recommended 150 minutes of activity per week after bariatric surgery (45, 46). In addition, the decrease in total energy expenditure due to loss of muscle mass after bariatric surgery has not been thoroughly investigated as a mechanism for WR. Therefore, the value of a perioperative exercise program for bariatric surgery patients to attenuate WR has not yet been established. Prospective studies are needed to determine the long-term effects of physical activity including resistance exercise on weight maintenance and to establish physical activity guidelines that help reduce WR after bariatric surgery. We further note that adequate protein intake must be ensured for physical activity to be maximally beneficial (47).
Metabolic and racial factors
WR following weight loss has recently been recognized as a physiological process, and not the result of loss of “will power.” The universally accepted thermodynamic principles of energy conservation imply that consumption of calories in excess of energy expenditure drives WR. Consequently, the possibility that calorie restriction reduces energy expenditure to conserve body fat has been proposed as an important factor in WR. The gap between expected and actual energy expenditure after weight loss, or metabolic adaptation (MA) (48), imposes overfeeding conditions at energy intake levels below those predicted by body mass. However, the contribution of MA to WR is unclear and is not universally accepted. To begin with, this energy gap is thought to be relatively small (approximately 50-100 kcal/day) and diminishes with time. Indeed, there is conflicting evidence regarding whether MA even occurs and whether it persists (49-51) or is blunted (52) after bariatric surgery. Individual variation in this process may correlate with degree of WR (53).
In addition, there are racial disparities in weight loss with all treatments that have long been observed and seem to particularly affect African Americans (AAs) with obesity. Differences in energy metabolism between AAs and other racial groups have also been documented in relationship to obesity (54), weight loss (55), and body composition (54, 56). Racial disparities are also observed in the clinical outcomes after bariatric surgery. For example, a study of 84 adult patients suggested that Whites lose more weight than AAs after bariatric surgery (57). More recently, our group has demonstrated that AAs are more prone to WR (3) and recurrence of T2DM after RYGB than Whites (58). The etiology of these racial disparities is multifactorial and most likely includes behavioral as well as genetic and metabolic differences (57, 59).
Surgical and anatomical factors
A GG fistula can form between the gastric pouch and the excluded stomach after RYGB, allowing food to enter the excluded (bypassed) stomach and reducing the restrictive nature of RYGB surgery, resulting in WR and recurrence of T2DM (60, 61). The prevalence of GG fistula after RYGB is only about 1% and, in addition to WR, symptoms include abdominal pain, gastroesophageal reflux, marginal ulcers, and possible GI bleeding. Patients with these symptoms should be evaluated for GG fistula, for which the “gold-standard” test is the upper GI series (62, 63). Other potential anatomical factors contributing to WR after gastric bypass surgery are dilation of the gastrojejunal stoma and distention of the gastric pouch, which may develop over time (64, 65). These abnormalities can be evaluated and corrected endoscopically (66, 67), which can aid in modest short-term weight loss, although long-term effects are less than satisfactory (68). However, these procedures are typically not covered by third-party payers, which shifts the financial responsibility to the patient. An upper GI should be considered for WR after VSG as well, because dilation or the sleeve has been associated with weight gain (69).
Management of the Patient With Weight Regain
Rationale and stratification of weight regain
WR is a cumulative process that has the potential to progressively worsen with time. Thus, early detection is crucial for curtailing the negative impact of WR, especially in patients who have already benefited from successful bariatric surgery. We have developed an approach to detect and stratify the risk of WR based on the apparent rate of increase in body weight in patients who are post-bariatric surgery as they present to their follow-up clinic visits. Our approach is to define WR as a marker of overfeeding by relating the amount of weight gained to nadir weight and normalizing by time. The main advantage of expressing WR as a rate is to delineate the risk of overfeeding and make it a clear target for intervention. We find that this approach helps define treatment goals succinctly both to the patient and to the medical provider.
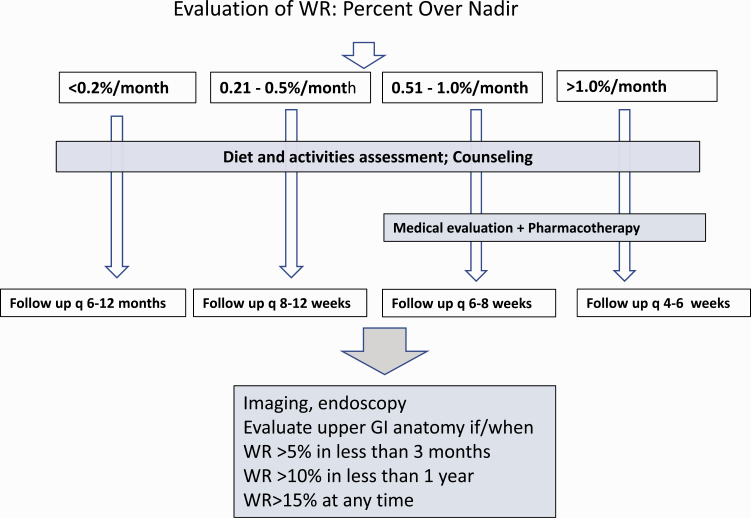
In the absence of established guidelines, we use data from our patient population to stratify WR. Expressed as a rate relative to nadir, the cutoff points for the quartile distribution are 0.2%, 0.7%, and 1.2% per 30-days (3). Fig. 2 summarizes our current multidisciplinary approach for the treatment of patients with post–bariatric surgery WR. We define mild, moderate, and rapid WR as WR/nadir 0.2% to <0.5%, 0.5% to 1.0%, and greater than 1.0% per 30-day interval, respectively. According to this approach, all patients coming for follow-up are offered diet and exercise counseling. Subsequent clinic visits are individualized. Patients with no or minimal WR (< 0.2% per month) may be followed every 6 to 12 months. This interval is shortened for patients with more rapid WR, as shown in Fig. 2. We suggest that those who gain weight at a rate of 0.5% per month or higher be considered for pharmacotherapy. This approach targets patients who are on a trajectory to gain 5% or more within a period of 6 months, which is likely to be clinically significant (15). Patients whose rate of WR exceeds 5% in 3 months, or 10% per year, and those who regain more than 15% over their nadir weight may be evaluated further to rule out correctable anatomic abnormalities.
Figure 2.
Multidisciplinary approach to the management of the patient with weight regain following bariatric surgery.
WR after bariatric surgery is multifactorial. It is important that patients have long-term follow-up with frequent visits (70) with a multidisciplinary team of surgeons, obesity medicine specialists, dietitians, and psychologists. Clinicians involved in the care of bariatric patients should be aware of WR and know how to treat it. Lifestyle modification, diet, and exercise guidance are integral to the management of all post–bariatric surgery patients. Pharmacotherapy and surgical revisions are considered for patients with moderate and rapid WR.
Diet and eating behaviors recommendations
Lifestyle behaviors influence WR following bariatric surgery. It is paramount that patients preparing for surgery understand the recommended postoperative dietary and physical activity behaviors that are essential to achieve optimal and durable weight loss. This requires appropriate nutrition education, dietary and physical activity counseling, and lifelong follow-up with the bariatric multidisciplinary team (30, 71). The ability of patients to tolerate solid food immediately after surgery is limited, thus necessitating a staged-approach progression of the diet as described elsewhere (72). Within the first 2 months after surgery, the main focus of dietary counseling is to optimize the intake of high-quality protein sources starting with liquids and slowly advancing to solid food. It should be noted that studies to determine protein requirements for bariatric surgery patients are not available; however, the suggested recommendations are between 60 and 120 g/day depending on the type of procedure or 1.2 to 1.5 g/kg ideal body weight per day (73). Additionally, the role of dietary counseling after bariatric surgery is to ensure that patients adhere to the prescribed vitamins and mineral supplementation (74).
Altered GI anatomy after bariatric surgery imposes specific limitations on food choices and eating behaviors. For example, the practice of eating slowly, chewing thoroughly, avoiding large volumes, and adhering to specific food restrictions is an essential component of dietary counseling after bariatric surgery. Post–bariatric surgery patients are instructed to avoid refined carbohydrates and simple sugars because of the potential for dumping syndrome and subsequent hypoglycemia. The general guidelines for the post–bariatric surgery diet include the following: limiting portion size of meals to one-half cup (125 g) per 30 minutes, and selecting protein-rich and high-fiber–containing foods such as poultry, fish, lean meats, eggs, low-fat dairy, legumes, fruits, vegetables, and whole grains (75). Other considerations are to advise patients to choose low glycemic index foods and proper spacing of meals (4-6 meals daily) to help optimize satiety and improve tolerance to foods (30, 76).
The patient presenting with WR poses specific dietary counseling challenges. Disordered eating, such as grazing and night eating, are common behaviors leading to suboptimal weight loss and WR (77). Careful dietary assessments are essential to identifying and addressing these behaviors early on in an effort to achieve optimal weight-loss results (37). Early identification of disordered eating habits and inappropriate food choices is important for reducing the risk of WR. A list of specific dietary and eating behaviors that could potentially promote WR, together with suggested counseling measures, is presented in Table 2. It is important to note that despite extensive preoperative nutrition education and counseling, patients continue to require reminders of the adverse effects of simple sugars and refined carbohydrates after bariatric surgery, and of the importance of adequate protein, fiber, and supplemental micronutrient intake.
Table 2.
Problematic eating behaviors associated with weight regain and intervention approaches following bariatric surgery
| Eating Behavior | Problem | Intervention |
|---|---|---|
| Erratic eating | Inconsistent/unplanned eating schedule | Preplan approximate timing and appropriate spacing of meals throughout the day based on daily schedule |
| Meal skipping | Not eating for an extended time period leading to subsequent hunger and overeating | Plan meals ahead of time; create a shopping list to ensure selected food and appropriate amounts are available to prepare these meals |
| Unhealthy food and beverage selections | Frequent intake of high-calorie/processed meals and snacks including fast food/take-out, fried food, concentrated sweets, and refined carbohydrates; calorie-rich beverages such as soda and juices | Education on balanced meal preparation containing protein and fiber-rich sources to help optimize satiety; encourage cooking classes and/or online cooking resources |
| Nibbling/Grazing | Continuous/repetitive and unplanned eating of modest portions of food throughout the day; often associated with previous binge-eating behaviors; leads to excessive cumulative energy intake | Avoidance of skipping meals; appropriate meal portion sizes (1/2-1 cup). Identify triggers including stress, boredom, and emotional factors or engaging in other activities such as watching television |
| Night eating | Consuming more calories before sleep favors positive energy balance and weight gain | Schedule time for meals during the day; self-monitor using food journal |
| Inappropriate portions | Portion sizes beyond the feeling of fullness resulting in discomfort | Weigh and measure foods, use smaller plates and utensils, and limit volume to 1 cup of food per meal |
| Alcohol use | Excess nonnutritive calories; promotes increased hunger, food cravings, and compromises judgment regarding proper food selection | Avoid or limit alcohol consumption; consider referral to treatment program if unable to control behavior |
| Insufficient protein and fiber intake | Protein and fiber promote optimal satiation; protein-rich foods optimize muscle integrity and energy metabolism | Education on quality sources of protein and fiber; assist with meal planning to achieve appropriate intake |
| Drinking fluids with meals | Potential enlargement of the gastric pouch and outlet with repeated behavior; leads to rapid emptying of the stomach | Delay fluid intake at least 30 minutes after consuming solid food |
Physical activity recommendations
As noted earlier, currently there are no established physical activity guidelines that specifically address the problem of WR after bariatric surgery. However, it is assumed some of the benefits of physical activity and exercise after bariatric surgery are higher daily energy expenditure, more optimal weight loss, and overall improvement in cardiometabolic health (78). Thus, it is intuitive to promote an increase in the level of physical activity for bariatric surgery patients, as it is for the general population. However, it is important to note that suboptimal protein consumption, which is common early after bariatric surgery, may pose limitations on the intensity of exercise because of enhancement of muscle loss as noted in some studies (79). Until more definitive data are available, we advocate moderate exercise activity, and particularly resistance training, to all post–bariatric surgery patients (80).
Pharmacotherapy
We consider initiating pharmacotherapy whenever postoperative bariatric surgery patients present with WR at a rate of 0.5% per month or higher (Fig. 2). Our group has recently published data showing the efficacy of this approach (14). Briefly, pharmacotherapy can significantly delay the progression of rapid WR as well as reduce its occurrence. However, it should be noted that the use of pharmacotherapy after bariatric surgery remains limited in scope. This is because of the lack of formal guidelines and recommendations on AOM use after bariatric surgery. In addition, some bariatric surgery centers are not staffed with obesity medicine specialists, which poses limitations on AOM use due to lack of familiarity of bariatric surgeons with these medications and the rapid evolution of this medical field (81).
The current US Food and Drug Administration (FDA)-approved AOMs are listed in Table 3 (82). One medication, lorcaserin, was recently withdrawn from the market because of safety concerns (83). Phentermine, which was approved in 1959, is a sympathomimetic amine that increases catecholamine secretion in the hypothalamus leading to appetite suppression. Alone or in combination with topiramate, it has shown to mitigate WR (14) and promote weight loss after inadequate results achieved after bariatric surgery (84, 85). The combination phentermine/topiramate extended release was approved by the FDA as an AOM in 2012.
Table 3.
Current Food and Drug Administration–approved antiobesity medications
| Drug (FDA-approved dosage) | Mechanism of action | Contraindications |
|---|---|---|
| Phentermine (8 mg [short-acting], 15 mg capsule, 37.5 mg tablet) | Sympathomimetic amine | Cardiovascular disease, uncontrolled hypertension, agitated states, history of drug use, hyperthyroidism, glaucoma, MAOI use within 14 d |
| Phentermine/topiramate ER (3.75/23 mg, 7.5/46 mg, 11.25/69 mg and 15/92 mg) | Combination of sympathomimetic amine, anorectic, and extended-release antiepileptic drug | Glaucoma, hyperthyroidism, MAOI use within 14 d |
| Naltrexone SR (8 mg)/bupropion SR (90 mg) | Combination opioid antagonist and aminoketone antidepressant | Uncontrolled hypertension, seizure disorders, anorexia nervosa or bulimia, chronic opioid use, MAOI use within 14 d, abrupt discontinuation of alcohol or seizure medications |
| Orlistat (60 mg OTC, 120 mg) | Pancreatic and gastric lipase inhibitor | Malabsorption syndrome, cholestasis |
| Liraglutide (3.0 mg) | GLP-1 agonist | Family or personal history of medullary thyroid cancer or multiple endocrine neoplasia type 2 |
Abbreviations: ER, extended release; FDA, Food and Drug Administration; GLP-1, glucagon-like peptide 1; MAOI, monoamine oxidase inhibitor; OTC, over the counter; SR, sustained release.
Topiramate, an antiepileptic and migraine medication, is not approved as a single-agent treatment for obesity although it has been used off label because of its anorexigenic effect both alone and in combination with phentermine and also for binge eating disorder (86). The exact mechanism of appetite suppression is not well understood. In one study, topiramate emerged as one of the most effective options for treating postsurgical WR in one cohort of postoperative patients (87).
Naltrexone SR/Bupropion SR is another FDA-approved combination AOM. Naltrexone alone is approved for the treatment of AUD and opioid use disorder. Bupropion has been used for smoking cessation as well as unipolar major depression. Both medications independently affect the central nervous system (CNS) reward system, and a synergistic effect has been hypothesized in humans based on animal studies for its effect on the pro-opiomelanocortin receptor to induce satiety and block feedback inhibition. However, there are no published data on the use of this combination in patients after bariatric surgery for WR.
Liraglutide is an injectable GLP-1 agonist and was approved by the FDA as an AOM in the 3.0-mg dosing. It seems to regulate appetite through peripheral and CNS pathways and has been used effectively in postoperative bariatric surgery patients with insufficient weight loss or WR (88, 89). In addition, limited data suggest that GLP-1 agonists could be useful in managing post–bariatric surgery hypoglycemia (90), thus providing dual benefit. Another GLP-1 agonist, semaglutide, which is approved for treatment of T2DM, has been shown to induce weight loss (91) and may even be superior to liraglutide (92). Although it has not been evaluated for WR, we anticipate that it will be particularly useful in patients who experience relapse of T2DM after bariatric surgery.
Orlistat is the only FDA-approved AOM that does not work through the CNS pathway—it is a lipase inhibitor that promotes the malabsorption of 25% to 30% of ingested fat from the GI tract (93). Currently there are no data on orlistat for WR. Given that patients after bariatric surgery already are at increased risk of fat-soluble vitamin deficiencies, orlistat might increase the risk and hence, should not be the first-line choice in bariatric patients with WR.
Finally, clinicians should use the same guidance in dose initiation and titration as in patients who have not had surgery. Treatment should be started with the lowest dose and adjusted as needed. Combination treatments are possible when single agents are ineffective; however, this option should be considered only by experienced practitioners.
Surgical interventions
The most commonly agreed on surgical intervention for weight gain is the repair of a GG fistula when identified on upper GI or endoscopy (60). Other anatomic abnormalities that can be addressed surgically are an enlarged gastric pouch and dilated stoma. These can be addressed both endoscopically as well as laparoscopically, with good short-term weight loss results but variable long-term results. When performed laparoscopically, the gastrojejunal anastomosis is typically taken down, and in doing so, the gastric pouch is resized, so it would address 2 potential mechanisms of WR from enlargement of both areas. The short-term weight loss can be adequate but the durability of this type of revisional procedure has yet to be demonstrated (94). With the advent of the laparoscopic approach to bariatric surgery, the complication rates have diminished for primary and revisional surgery (95), making revisional surgery a less risky option to improve on the weight loss achieved than it had been in the past.
The growing problem of WR after bariatric surgery has prompted evaluation of different revisional surgeries that could be employed even when there are no anatomic abnormalities identified in the primary procedure. Procedures such as distalization (96) of the jejunojejunostomy for a gastric bypass, conversion of a VSG to a gastric bypass, and single anastomosis duodenoilieal (SADI) bypass are performed to increase or add a malabsorptive component to the original procedure. For a distal gastric bypass, the jejunojejunostomy is moved to either increase the length of the biliopancreatic limb or alimentary limb with a short common channel usually between 75 and 150 cm. Although weight loss has been achieved after these procedures, protein calorie malnutrition and micronutrient deficiencies have been reported, necessitating total parenteral nutrition in up to 20% of the cases performed (68).
When considering a revision for WR after VSG, patients who also have acid reflux disease are good candidates for gastric bypass; but for those who would be converted for WR alone, the SADI procedure provides superior weight loss to the conversion to gastric bypass (97). In this procedure, the duodenum is divided 3 cm distal to the pylorus; the small bowel is measured backward from the ileocecal valve to a length of 150 to 300 cm, where it is connected to the duodenum. Because the surgical complications and malnutrition rates of this procedure are similar to a conversion to gastric bypass, the SADI may become the procedure of choice to treat inadequate weight loss after a VSG (97).
Back to the Patient
Our patient’s weight loss after RYGB, amounting to 51% of her initial body weight, exceeds the mean for this type of surgery. She seems to have started to regain weight only 2 years after the procedure, which is more common in patients with a history of depression. There is currently no evidence linking iron deficiency to WR. However, it is possible that easy fatigability could limit physical activity level and thus contribute to WR.
At her presentation to the Nutrition and Weight Management Clinic in 2013, our patient had already regained 13% over her nadir weight. Her weight change between April 4, 2012 and September 30, 2013, amounted to 41 lbs (18.6 kg; 21% over nadir), or approximately 1.2% per 30 days, which is considered rapid WR. The use of pharmacotherapy, in this case phentermine and topiramate, together with lifestyle and diet counseling, helped stabilize her weight. However, because of continued feelings of hunger, the bariatric surgery team was concerned about a pouch enlargement and/or dilated anastomosis, both of which were confirmed by endoscopy. With increasing acid reflux and presence of a hiatal hernia, the bypass was surgically revised. One year after the revision, she weighed 221 lbs (100.2 kg), which represents a 39 lb (17.7 kg) weight loss.
However, at this time the bariatric surgery dietitian noted that the patient was consuming large amounts of refined carbohydrates and not meeting the minimum requirement of 60 g protein. Recommendations to maintain weight loss included increasing protein and fiber with each meal and decreasing intake of simple carbohydrates. Her most recent bout of weight regain is attributed to lack of physical activity resulting from sciatica and iron deficiency anemia. A weight regain of 29 lb (13.2 kg), approximately 14% over the “second nadir” during a period of 6 months (> 2% per month) triggered more intensive diet counseling and the restart of pharmacotherapy.
Conclusions
With the increase in bariatric surgery procedures, WR is now recognized as a clinical problem. Defining “significant” and “rapid” weight regain is critical to help clinicians to recognize and implement the treatment for an improved outcome in a timely fashion. We have identified herein an algorithm to define and track significant WR after bariatric surgery as well as to offer treatment strategies to mitigate WR early in its course. This requires a multidisciplinary approach for diagnosis of the etiology of WR as well as its treatment with input from bariatric surgeons, obesity medicine specialists, dietitians, exercise specialists, and mental health providers. This method of approach to the patient with WR after bariatric surgery can significantly extend the benefits of the surgery with the use of AOMs combined with behavioral methods. Although we focused primarily on WR following RYGB, we believe this approach may also be applied to patients who regain weight following other surgical procedures. The case presented illustrates that even when bariatric surgery produces early and robust weight loss, a follow-up plan to monitor eating behaviors, diet, and exercise is necessary to ensure long-term weight maintenance.
Acknowledgments
The authors would like to thank Ashley McCarthy for her helpful feedback.
Financial Support: This work was supported in part by the National Institutes of Health (P30 DK046200). The funder had no role in the collection, management, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Glossary
Abbreviations
- AA
African American
- AOM
antiobesity medication
- AUD
alcohol use disorder
- BMI
body mass index
- CNS
central nervous system
- FDA
US Food and Drug Administration
- GG
gastrogastric
- GI
gastrointestinal
- GLP-1
glucagon-like peptide 1
- MA
metabolic adaptation
- RYGB
Roux-en-Y gastric bypass surgery
- SADI
single anastomosis duodenoilieal
- T2DM
type 2 diabetes mellitus
- VSG
vertical sleeve gastrectomy
- WR
weight regain
Additional Information
Disclosure Summary: Dr Apovian has participated on advisory boards for Orexigen, Gelesis, Allergan, Abbott Nutrition, EnteroMedics, Zafgen, Real Appeal, Nutrisystem, Novo Nordisk, Scientific Intake, Bariatrix Nutrition, SetPoint Health, Xeno Biosciences, Rhythm, Janssen, Tivity Health, Roman Health Ventures, and Jazz Pharmaceuticals. Dr Apovian has received research funding from Gelesis and Novo Nordisk. The other authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2018. 2018. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed June 29, 2020. [Google Scholar]
- 3. Thomas DD, Anderson WA, Apovian CM, et al. Weight recidivism after Roux-en-Y gastric bypass surgery: an 11-year experience in a multiethnic medical center. Obesity (Silver Spring). 2019;27(2):217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in weight regain following Roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes Surg. 2015;25(8):1474-1481. [DOI] [PubMed] [Google Scholar]
- 5. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sjöström L, Lindroos AK, Peltonen M, et al. ; Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683-2693. [DOI] [PubMed] [Google Scholar]
- 7. Voorwinde V, Steenhuis IHM, Janssen IMC, Monpellier VM, van Stralen MM. Definitions of long-term weight regain and their associations with clinical outcomes. Obes Surg. 2020;30(2):527-536. [DOI] [PubMed] [Google Scholar]
- 8. Sheppard CE, Lester EL, Chuck AW, Birch DW, Karmali S, de Gara CJ. The economic impact of weight regain. Gastroenterol Res Pract. 2013;2013:379564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zenténius E, Andersson-Assarsson JC, Carlsson LMS, Svensson PA, Larsson I. Self-reported weight-loss methods and weight change: ten-year analysis in the Swedish Obese Subjects Study Control Group. Obesity (Silver Spring). 2018;26(7):1137-1143. [DOI] [PubMed] [Google Scholar]
- 10. Kristensson FM, Andersson-Assarsson JC, Kanerva N, Peltonen M, Carlsson B, Carlsson LMS. Long-term effects of bariatric surgery in patients with obesity and chromosome 16 p11.2 microdeletion. Surg Obes Relat Dis. 2017;13(8):1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjöholm K, Pajunen P, Jacobson P, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58(7):1448-1453. [DOI] [PubMed] [Google Scholar]
- 12. Sarzynski MA, Jacobson P, Rankinen T, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond). 2011;35(5):676-683. [DOI] [PubMed] [Google Scholar]
- 13. King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320(15):1560-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Istfan NW, Anderson WA, Hess DT, Yu L, Carmine B, Apovian CM. The Mitigating effect of phentermine and topiramate on weight regain after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2020;28(6):1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keeney BJ, Fulton-Kehoe D, Wickizer TM, Turner JA, Chan KC, Franklin GM. Clinically significant weight gain 1 year after occupational back injury. J Occup Environ Med. 2013;55(3):318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lean ME, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond). 2016;40(4):622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bužga M, Zavadilová V, Holéczy P, et al. Dietary intake and ghrelin and leptin changes after sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. 2014;9(4):554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santo MA, Riccioppo D, Pajecki D, et al. Weight regain after gastric bypass: influence of gut hormones. Obes Surg. 2016;26(5):919-925. [DOI] [PubMed] [Google Scholar]
- 19. Xu HC, Pang YC, Chen JW, et al. Systematic review and meta-analysis of the change in ghrelin levels after Roux-en-Y gastric bypass. Obes Surg. 2019;29(4):1343-1351. [DOI] [PubMed] [Google Scholar]
- 20. Livhits M, Mercado C, Yermilov I, et al. Patient behaviors associated with weight regain after laparoscopic gastric bypass. Obes Res Clin Pract. 2011;5(3):e169-e266. [DOI] [PubMed] [Google Scholar]
- 21. Paul L, van der Heiden C, Hoek HW. Cognitive behavioral therapy and predictors of weight loss in bariatric surgery patients. Curr Opin Psychiatry. 2017;30(6):474-479. [DOI] [PubMed] [Google Scholar]
- 22. Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20(3):349-356. [DOI] [PubMed] [Google Scholar]
- 23. Rutledge T, Groesz LM, Savu M. Psychiatric factors and weight loss patterns following gastric bypass surgery in a veteran population. Obes Surg. 2011;21(1):29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apovian CM, Aronne LJ, Bessesen DH, et al. ; Endocrine Society Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. [DOI] [PubMed] [Google Scholar]
- 25. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C. Alcohol and other addictive disorders following bariatric surgery: prevalence, risk factors and possible etiologies. Eur Eat Disord Rev. 2015;23(6):442-450. [DOI] [PubMed] [Google Scholar]
- 27. Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK, Pepino MY. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis. 2018;14(3):277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanos BR, Saules KK, Schuh LM, Sogg S. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes Surg. 2015;25(8):1364-1370. [DOI] [PubMed] [Google Scholar]
- 29. Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4(5):640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allied Health Sciences Section Ad Hoc Nutrition Committee; Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73-S108. [DOI] [PubMed] [Google Scholar]
- 31. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23(5):373-385. [DOI] [PubMed] [Google Scholar]
- 32. Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity (Silver Spring). 2016;24(6):1210-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring). 2015;23(5):1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abrahamsson N, Börjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9): 2667-2675. [DOI] [PubMed] [Google Scholar]
- 35. Ahmad A, Kornrich DB, Krasner H, et al. Prevalence of dumping syndrome after laparoscopic sleeve gastrectomy and comparison with laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2019;29(5):1506-1513. [DOI] [PubMed] [Google Scholar]
- 36. Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18(6):648-651. [DOI] [PubMed] [Google Scholar]
- 37. Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition. 2012;28(1):53-58. [DOI] [PubMed] [Google Scholar]
- 38. da Silva FB, Gomes DL, de Carvalho KM. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition. 2016;32(11-12): 1250-1253. [DOI] [PubMed] [Google Scholar]
- 39. Rusch MD, Andris D. Maladaptive eating patterns after weight-loss surgery. Nutr Clin Pract. 2007;22(1):41-49. [DOI] [PubMed] [Google Scholar]
- 40. Herring LY, Stevinson C, Carter P, et al. The effects of supervised exercise training 12-24 months after bariatric surgery on physical function and body composition: a randomised controlled trial. Int J Obes (Lond). 2017;41(6):909-916. [DOI] [PubMed] [Google Scholar]
- 41. Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR. Weight-loss maintenance in successful weight losers: surgical vs non-surgical methods. Int J Obes (Lond). 2009;33(1):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obes Surg. 2011;21(1):61-70. [DOI] [PubMed] [Google Scholar]
- 43. Pouwels S, Wit M, Teijink JA, Nienhuijs SW. Aspects of exercise before or after bariatric surgery: a systematic review. Obes Facts. 2015;8(2):132-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansen D, Decroix L, Devos Y, et al. Towards optimized care after bariatric surgery by physical activity and exercise intervention: a review. Obes Surg. 2020;30(3):1118-1125. [DOI] [PubMed] [Google Scholar]
- 45. King WC, Hsu JY, Belle SH, et al. Pre- to postoperative changes in physical activity: report from the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2). Surg Obes Relat Dis. 2012;8(5):522-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. King WC, Chen JY, Bond DS, et al. Objective assessment of changes in physical activity and sedentary behavior: pre- through 3 years post-bariatric surgery. Obesity (Silver Spring). 2015;23(6):1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thivel D, Brakonieki K, Duche P, et al. Surgical weight loss: impact on energy expenditure. Obes Surg. 2013;23(2):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24(8):1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Butte NF, Brandt ML, Wong WW, et al. Energetic adaptations persist after bariatric surgery in severely obese adolescents. Obesity (Silver Spring). 2015;23(3):591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilms B, Ernst B, Thurnheer M, Schmid SM, Spengler CM, Schultes B. Resting energy expenditure after Roux-en Y gastric bypass surgery. Surg Obes Relat Dis. 2018;14(2): 191-199. [DOI] [PubMed] [Google Scholar]
- 51. Martins C, Gower BA, Hill JO, Hunter GR. Metabolic adaptation is not a major barrier to weight-loss maintenance. Am J Clin Nutr. 2020;112(3):558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Faria SL, Faria OP, Cardeal Mde A, Ito MK, Buffington C. Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass surgery: a prospective study. Surg Obes Relat Dis. 2014;10(1):138-143. [DOI] [PubMed] [Google Scholar]
- 53. Cardeal MA, Faria SL, Faria OP, Facundes M, Ito MK. Diet-induced thermogenesis in postoperatve Roux-en-Y gastric bypass patients with weight regain. Surg Obes Relat Dis. 2016;12(5):1098-1107. [DOI] [PubMed] [Google Scholar]
- 54. Cardel M, Higgins PB, Willig AL, et al. African genetic admixture is associated with body composition and fat distribution in a cross-sectional study of children. Int J Obes (Lond). 2011;35(1):60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Foster GD, Wadden TA, Swain RM, Anderson DA, Vogt RA. Changes in resting energy expenditure after weight loss in obese African American and white women. Am J Clin Nutr. 1999;69(1):13-17. [DOI] [PubMed] [Google Scholar]
- 56. Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI. A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. Am J Clin Nutr. 2001;73(2):308-315. [DOI] [PubMed] [Google Scholar]
- 57. Anderson WA, Greene GW, Forse RA, Apovian CM, Istfan NW. Weight loss and health outcomes in African Americans and whites after gastric bypass surgery. Obesity (Silver Spring). 2007;15(6):1455-1463. [DOI] [PubMed] [Google Scholar]
- 58. Istfan N, Anderson WA, Apovian C, Ruth M, Carmine B, Hess D. Racial differences in weight loss, hemoglobin A1C, and blood lipid profiles after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12(7):1329-1336. [DOI] [PubMed] [Google Scholar]
- 59. Weinsier RL, Hunter GR, Zuckerman PA, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71(5):1138-1146. [DOI] [PubMed] [Google Scholar]
- 60. Ribeiro-Parenti L, De Courville G, Daikha A, Arapis K, Chosidow D, Marmuse JP. Classification, surgical management and outcomes of patients with gastrogastric fistula after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(2):243-248. [DOI] [PubMed] [Google Scholar]
- 61. Jirapinyo P, Thompson AC, Kröner PT, Chan WW, Thompson CC. Metabolic effect of foregut exclusion demonstrated by the impact of gastrogastric fistula on recurrence of diabetes. J Am Coll Surg. 2018;226(3):259-266.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sugerman HJ, Wolper JL. Failed gastroplasty for morbid obesity. Revised gastroplasty versus Roux-Y gastric bypass. Am J Surg. 1984;148(3):331-336. [DOI] [PubMed] [Google Scholar]
- 63. Gao G, Nezami N, Mathur M, Balcacer P, Israel G, Spektor M. Diagnosis of gastrogastric fistula on computed tomography: a quantitative approach. Abdom Radiol (NY). 2018;43(6):1329-1333. [DOI] [PubMed] [Google Scholar]
- 64. Heneghan HM, Yimcharoen P, Brethauer SA, Kroh M, Chand B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis. 2012;8(4):408-415. [DOI] [PubMed] [Google Scholar]
- 65. Topart P, Becouarn G, Ritz P. Pouch size after gastric bypass does not correlate with weight loss outcome. Obes Surg. 2011;21(9):1350-1354. [DOI] [PubMed] [Google Scholar]
- 66. Yimcharoen P, Heneghan HM, Singh M, et al. Endoscopic findings and outcomes of revisional procedures for patients with weight recidivism after gastric bypass. Surg Endosc. 2011;25(10):3345-3352. [DOI] [PubMed] [Google Scholar]
- 67. Saliba C, El Rayes J, Diab S, Nicolas G, Wakim R. Weight regain after sleeve gastrectomy: a look at the benefits of re-sleeve. Cureus. 2018;10(10):e3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tran DD, Nwokeabia ID, Purnell S, et al. Revision of Roux-en-Y gastric bypass for weight regain: a systematic review of techniques and outcomes. Obes Surg. 2016;26(7):1627-1634. [DOI] [PubMed] [Google Scholar]
- 69. Lauti M, Stevenson S, Hill AG, MacCormick AD. Patient perspectives about follow-up care and weight regain following sleeve gastrectomy. Obes Surg. 2016;26(11):2724-2731. [DOI] [PubMed] [Google Scholar]
- 70. Lombardo M, Bellia A, Mattiuzzo F, et al. Frequent follow-up visits reduce weight regain in long-term management after bariatric surgery. Bariatr Surg Pract Patient Care. 2015;10(3):119-125. [Google Scholar]
- 71. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—executive summary. Endocr Pract. 2019;25(12):1346-1359. [DOI] [PubMed] [Google Scholar]
- 72. Bosnic G. Nutritional requirements after bariatric surgery. Crit Care Nurs Clin North Am. 2014;26(2):255-262. [DOI] [PubMed] [Google Scholar]
- 73. Mechanick JI, Youdim A, Jones DB, et al. ; American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013;21(Suppl 1):S1-S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Magallares A, Schomerus G. Mental and physical health-related quality of life in obese patients before and after bariatric surgery: a meta-analysis. Psychol Health Med. 2015;20(2):165-176. [DOI] [PubMed] [Google Scholar]
- 75. Parkes E. Nutritional management of patients after bariatric surgery. Am J Med Sci. 2006;331(4):207-213. [DOI] [PubMed] [Google Scholar]
- 76. Zalesin KC, Franklin BA, Miller WM, et al. Preventing weight regain after bariatric surgery: an overview of lifestyle and psychosocial modulators. Am J Lifestyle Med. 2010;4(2):113-120. [Google Scholar]
- 77. Leahy CR, Luning A. Review of nutritional guidelines for patients undergoing bariatric surgery. AORN J. 2015;102(2):153-160. [DOI] [PubMed] [Google Scholar]
- 78. Tettero OM, Aronson T, Wolf RJ, Nuijten MAH, Hopman MTE, Janssen IMC. Increase in physical activity after bariatric surgery demonstrates improvement in weight loss and cardiorespiratory fitness. Obes Surg. 2018;28(12):3950-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carnero EA, Dubis GS, Hames KC, et al. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity (Silver Spring). 2017;25(7):1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007;31(5):743-750. [DOI] [PubMed] [Google Scholar]
- 81. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175-247. [DOI] [PubMed] [Google Scholar]
- 82. Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12-24. [DOI] [PubMed] [Google Scholar]
- 83. US Food and Drug Administration. Drug Safety Communications. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market FDA. June 15, 2020. https://www.fda.gov/media/135189/download. Accessed June 29, 2020. [Google Scholar]
- 84. Toth AT, Gomez G, Shukla AP, et al. Weight loss medications in young adults after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Children (Basel). 2018;5(9):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schwartz J, Chaudhry UI, Suzo A, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: a retrospective review. Obes Surg. 2016;26(2):452-458. [DOI] [PubMed] [Google Scholar]
- 86. Grilo CM, Reas DL, Mitchell JE. Combining pharmacological and psychological treatments for binge eating disorder: current status, limitations, and future directions. Curr Psychiatry Rep. 2016;18(6):55. [DOI] [PubMed] [Google Scholar]
- 87. Stanford FC, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. 2017;13(3):491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miras AD, Pérez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549-559. [DOI] [PubMed] [Google Scholar]
- 89. Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9(4):e12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. 2013;169(6):885-889. [DOI] [PubMed] [Google Scholar]
- 91. Ahrén B, Atkin SL, Charpentier G, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018;20(9):2210-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-649. [DOI] [PubMed] [Google Scholar]
- 93. Avenell A, Robertson C, Skea Z, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. Health Technol Assess. 2018;22(68):1-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hamdi A, Julien C, Brown P, et al. Midterm outcomes of revisional surgery for gastric pouch and gastrojejunal anastomotic enlargement in patients with weight regain after gastric bypass for morbid obesity. Obes Surg. 2014;24(8): 1386-1390. [DOI] [PubMed] [Google Scholar]
- 95. Morales MP, Wheeler AA, Ramaswamy A, Scott JS, de la Torre RA. Laparoscopic revisional surgery after Roux-en-Y gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2010;6(5):485-490. [DOI] [PubMed] [Google Scholar]
- 96. Himpens J, Coromina L, Verbrugghe A, Cadière GB. Outcomes of revisional procedures for insufficient weight loss or weight regain after Roux-en-Y gastric bypass. Obes Surg. 2012;22(11):1746-1754. [DOI] [PubMed] [Google Scholar]
- 97. Dijkhorst PJ, Boerboom AB, Janssen IMC, et al. Failed sleeve gastrectomy: single anastomosis duodenoileal bypass or Roux-en-Y gastric bypass? A multicenter cohort study. Obes Surg. 2018;28(12):3834-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.