Abstract
Objective:
Current information on opioid use in nursing home residents, particularly those with dementia, is unknown. We examined the temporal trends in opioid use by dementia severity and the association of dementia severity with opioid use in long-term care nursing home residents.
Design:
Repeated measures cross-sectional study
Setting:
Long-term care nursing homes
Participants:
Using 20% Minimum Data Set (MDS) and Medicare claims from 2011–2017, we included long-term care residents (n=734,739) from each year who had 120 days of consecutive stay. In a secondary analysis, we included residents who had an emergency room visit for a fracture (n=12,927).
Measurements:
Dementia was classified as no, mild, moderate and severe based on the first MDS assessment each year. In the 120 days of nursing home stay, opioid use was measured as any, prolonged (>90 days) and high-dose (≥90 morphine milligram equivalent dose/day). For residents with a fracture, opioid use was measured within 7 days after emergency room discharge. Association of dementia severity with opioid use was evaluated using logistic regression.
Results:
Overall, any opioid use declined by 8.4% (35.2% to 32.2%, p<0.001), prolonged use by 4.6% (14.1% to 13.4%, p<0.001) and high-dose by 19.7% (1.4% to 1.1%, p<0.001) from 2011 to 2017. Opioid use declined across four dementia severity groups. Among residents with fracture, opioid use declined by 9% in mild, 9.5% in moderate and 12.3% in severe dementia. The odds of receiving any, prolonged and high-dose opioids decreased with increasing severity of dementia. For example, severe dementia reduced the odds of any (23.5% vs. 47.6%; OR=0.56, 95%CI=0.55–0.57), prolonged (9.8% vs. 20.7%; OR=0.69, 95%CI=0.67–0.71) and high-dose (1.0% vs. 2.3%; OR=0.69, 95%CI=0.63–0.74) opioids.
Conclusions and Implications:
Use of opioids declined in nursing home residents from 2011 to 2017, and the use was lower in residents with dementia, possibly reflecting suboptimal pain management in this population.
Keywords: Opioid use, pain, dementia, nursing home
Brief Summary
This nationally representative study found that opioid use declined in nursing home residents from 2011 to 2017, and the use was lower in residents with dementia.
Introduction
Nearly 1.4 million people live in long-term care nursing homes and over two-thirds have Alzheimer’s disease or related dementia.1 Over half of those with dementia regularly experience pain, and the prevalence of pain is even greater in nursing home residents with dementia.2 Appropriate management of pain can improve quality of life and the behavioral and psychological symptoms of dementia.3, 4
There is no evidence that dementia reduces sensitivity to pain.2, 5 However, pain management is not optimal in dementia patients. Over the years, use of analgesic medications to manage pain has improved in nursing homes. For example, the proportion of nursing home residents with pain receiving analgesic medications increased from 75% before 20006–9 to 83% in 2007–200810 and 94.6% in 2011–2012.11 However, residents with moderate to severe dementia are less likely to receive analgesic medications.10–12 Opioids are useful to reduce pain in nursing home residents. However, clinicians often face challenges in managing the benefits of opioids against potential side effects such as falls and delirium.13 The 2019 American Geriatric Society’s Beers criteria caution on using opioids in residents with a history of falls or fracture – most nursing home residents.14
In the United States, opioid use declined after 2012 due to emerging evidence on the safety and dangers of opioids, the 2014 Drug Enforcement Agency (DEA) law reclassifying and restricting hydrocodone prescribing, new Guidelines from the Centers for Disease Control and Prevention (CDC) issued in 2016 and changes in several state laws and federal regulations.15–18 In Canada, opioid use increased by 39% among nursing home residents from 2009–10 to 2016–17.19 It is unclear how policy changes in the US affected opioid use in the current years among nursing home residents, particularly those with dementia.
Lower use of opioids in nursing home residents with dementia may reflect under-treatment of pain or greater use of alternative treatment strategies. An analysis of 2012 nursing home data found that 25% of residents with severe cognitive impairement received opioids, compared to 44% with no/mild impirement. Long-term opioid use was lower in residents with moderate to severe cognitive impairment.12 We were interested in determining how US policies restricting opioid use affected its use in long-term care residents with dementia.
The study objectives were to: (1) determine time trends in opioid use among long-term care nursing home residents with no, mild, moderate and severe dementia; and (2) determine the association of dementia severity with opioid use. We hypothesized that the use of any, prolonged and high-dose opioids would decline from 2011 to 2017, and opioid use would be lower in patients with moderate to severe dementia. We studied opioid use in long-term care nursing home residents and residents who had a new fracture while in the nursing home.
Methods
Data Source
We used the Minimum Data Set (MDS) 3.0 linked to Medicare claims data for the 20% national Medicare samples from 2011 to 2017. The MDS captures information on nursing home residents’ clinical and functional characteristics. The following Medicare claims files were used: beneficiary summary (patient demographic and enrollment data), Medicare Provider Analysis and Review (MedPAR) (claims for hospitalization), outpatient file (claims for outpatient visits), carrier file (claims for physician services) and Part D file (claims for prescription drugs). The Institutional Review Board approved the study.
Study Design and Cohort
We employed a repeated measures cross-sectional study design and developed two cohorts. In the primary cohort, we included long-term care nursing home residents with at least 120 days of consecutive stay in a nursing home in each year from January 1, 2011 to December 31, 2017. We selected the first available nursing home assessment per resident each year. We identified long-term care nursing home stay using the MDS data. Any stay that overlapped during a resident’s long-term care nursing home stay was identified from part A claims data and excluded.20, 21 The first MDS assessment date was considered as the index date. Residents were included if they were 65 years or older, had continuous enrollment in Medicare part D during their 120 days of stay and for 6 months prior to the index date, and had continuous Medicare parts A and B enrollment in the year prior to the index date.
In the secondary cohort, we included patients who experienced an acute fracture event. For this, we included long-term care residents with an outpatient emergency room visit for a fracture (head, neck, thorax, abdomen, lower back, lumbar spine, pelvis, external genitals, shoulder and upper arm, elbow and forearm, wrist, hand and fingers, knee and lower leg, hip and thigh or ankle and foot) from January 1, 2012 to December 24, 2017. We used only the first emergency room visit for each resident in each year. To avoid the possibility of carryover supply of opioids, we excluded residents who had a fracture within the 6 months prior to the emergency room admission date. Residents hospitalized within 7 days following the emergency room discharge date were excluded to eliminate severe cases of fractures. The emergency room visit discharge date was considered as the index date. Residents were included if they were 65 years or older, had continuous enrollment in Medicare part D for 7 days after and 6 months prior to the index date, and had continuous Medicare parts A and B enrollment in the year prior to the index date. We additionally excluded residents who had an opioid prescription within the 90 days prior to the emergency room admission date.
Opioid Use
In the primary cohort, we followed residents for 120 days from the index MDS assessment date to determine opioid use. Opioid prescriptions were identified from the Medicare Part D files using national drug codes.22, 23 Both short- and long-acting opioids were included. A new opioid prescription as well as a carryover supply from an opioid prescription prior to the index date were used to calculate opioid use during 120 days. We used the following three measures: (1) any opioid use: at least one prescription of an opioid during the follow-up period; (2) prolonged opioid use: opioid use of 90 days or more during the follow-up period; (3) high-dose opioid use: an average daily dose of opioid ≥90 milligram morphine equivalent (MME). We calculated the daily MME, which summarizes opioids with different ingredients and strengths, to estimate the daily opioid level. Average daily MME was calculated as [(strength per unit)x(quantity prescribed)x (MME conversion factor)]/daily supply.
For the secondary cohort, we followed residents for 7 days after the emergency room discharge date to determine if they received at least one prescription of an opioid.
Dementia Severity
Dementia severity was measured using the Brief Interview for Mental Status (BIMS) and the Cognitive Performance Scale (CPS). The BIMS is administered to residents who can be interviewed and the CPS is administered to those who cannot be interviewed. Lower scores on the BIMS (range 0-15) indicate greater impairment, whereas higher scores on the CPS (range 0–6) indicate greater impairment. We combined results from both the BIMS and the CPS and classified all nursing home residents as having no (BIMS: 13–15), mild (BIMS: 8–12 or CPS: 0–2), moderate (BIMS: 0–7 or CPS 3–4) or severe (CPS: 5-6) dementia.24, 25
Patient Characteristics
We determined patients’ age, sex, race, region, marital status and Medicare and Medicaid dual eligibility. Education was defined at a zip code–level. The patient’s comorbidity burden was assessed using the Elixhauser comorbidity score.26, 27 We used a chronic disease score to determine each patient’s prescription medication use burden.28 Chronic disease score includes prescription drugs as a surrogate marker for 28 chronic conditions. All patient characteristics from the MDS data were identified based on the first valid MDS assessment, i.e., index date. We defined mood disorders (no depression, minimal to mild depression, moderate to severe depression), activities of daily living (ADL) (6 items with a score 0–24; higher score indicating greater dependence), hallucination/delusions and aggressive behavior (none, mild/moderate, severe). For residents who could self-report pain, frequency and severity of pain was recorded. We used the CMS quality indicator definition of pain to classify this as no pain, mild/infrequent pain (mild to severe pain occurring rarely or occasionally) and moderate to severe pain (moderate/severe pain occurring frequently or almost constantly or very severe/horrible occurring at any frequency).22 For residents who could not self-report pain, staff review the medical records, consult other staff and observe the resident to determine indicators of pain in the previous five days. This was classified as no pain, infrequent pain (1–2 days) or frequent pain (≥ 3 days). We combined self-reported and staff-assessed pain in the following categories: no pain, mild/infrequent pain, moderate/severe/frequent pain.11, 25
Statistical Analysis
In the primary cohort of all long-term care nursing home residents, we compared patient characteristics across the level of dementia severity, i.e., no, mild, moderate and severe dementia, using chi-square for categorical variables and analysis of variance for continuous variables.. The Cochran-Armitage trends test was used to evaluate trends in opioid use from 2011 to 2017. Three separate generalized estimating equations with a binomial distribution and logit link were constructed to determine the association of dementia severity with the level of opioid use. Nursing home resident was included once in each year of data. Only the first 120 days of nursing home stay for each resident was included per year, but a resident could contribute to multiple years of data. To account for this, we used a repeated patient identifier and compound symmetry covariance structure in the generalized estimating equation. All regression models controlled for patient characteristics. Odds ratios (OR) and 95% confidence intervals (CI) were reported to show the association of dementia severity with opioid use.
In the secondary cohort of nursing home residents with a fracture, we reported trends in opioid use from 2012 to 2017. We constructed a generalized estimating equation with a binomial distribution and logit link, while accounting for the repeated patient identifier, to determine the association of dementia severity with opioid use. Because all patients had acute pain due to a fracture, we controlled for only age, gender, race and year.
All analyses were performed using SAS Enterprise version 7.12 (SAS Institute, Inc., Cary, NC).
Results
Patient Characteristics
The primary study cohort included 734,739 long-term care nursing home residents from 2011 to 2017 (Supplementary Figure 1) and the secondary cohort included 12,927 residents who had an emergency room visit for a fracture (Supplementary Figure 2). The mean age of nursing home residents (n=734,739) was 83.60±8.6 years, 74.0% were female and 80.8% were White; 27.1% had no, 24.3% had mild, 38.1% had moderate and 10.5% had severe dementia. Table 1 reports the patient characteristics by dementia severity. Residents with mild, moderate and severe dementia were more likely to be older, Black, reside in the South region, had lower comorbidity and chronic disease scores and had greater dependence in ADLs. The proportion of patients reporting mild/infrequent or moderate/severe/frequent pain decreased significantly with increasing level of dementia severity.
Table 1.
Patient Characteristics by Dementia Severity in Long-term Care Nursing Home Residents
| Patient Characteristics | No Dementia | Mild Dementia | Moderate Dementia | Severe Dementia | P-value |
|---|---|---|---|---|---|
| N=198,738 (27.1%) | N=178,842 (24.3%) | N=280,009 (38.1%) | N=77,150 (10.5%) | ||
| Age, mean (SD) | 81.02 (8.9) | 83.31 (8.7) | 85.40 (8.1) | 84.37 (8.3) | <0.01 |
| Gender | <0.01 | ||||
| Male | 143,599 (72.3%) | 127,289 (71.2%) | 212,259 (75.8%) | 60,777 (78.8%) | |
| Female | 55,139 (27.7%) | 51,553 (28.8%) | 67,750 (24.2%) | 16,373 (21.2%) | |
| Race | <0.01 | ||||
| White | 168,260 (84.7%) | 145,069 (81.1%) | 220,255 (78.7%) | 59,965 (77.7%) | |
| Black | 19,599 (9.9%) | 20,439 (11.4%) | 34,873 (12.5%) | 10,139 (13.1%) | |
| Hispanic | 6,386 (3.2%) | 8,376 (4.7%) | 16,639 (5.9%) | 4,549 (5.9%) | |
| Other | 4,493 (2.3%) | 4,958 (2.8%) | 8,242 (2.9%) | 2,497 (3.2%) | |
| Region | <0.01 | ||||
| Northeast | 44,315 (22.3%) | 39,552 (22.1%) | 63,280 (22.6%) | 18,838 (24.4%) | |
| Midwest | 63,053 (31.7%) | 52,412 (29.1%) | 76,760 (27.4%) | 19,083 (24.7%) | |
| West | 21,474 (10.8%) | 19,016 (10.6%) | 30,774 (11.0%) | 8,202 (10.6%) | |
| South | 69,896 (35.2%) | 67,862 (38.0%) | 109,195 (39.0%) | 31,027 (40.2%) | |
| Marital status | <0.01 | ||||
| Married | 33,290 (16.8%) | 31,642 (17.7%) | 52,579 (18.8%) | 18,335 (23.8%) | |
| Unmarried | 165,448 (83.2%) | 147,200 (82.3%) | 227,430 (81.2%) | 58,815 (76.2%) | |
| Education by zip code | <0.01 | ||||
| Q1 (<=81.8) | 48,386 (24.4%) | 45,703 (25.6%) | 73,003 (26.1%) | 19,479 (25.3%) | |
| Q2 (>81.8;<= 88.0) | 50,304 (25.3%) | 45,056 (25.2%) | 69,053 (24.7%) | 18,602 (24.1%) | |
| Q3 (>88.0; <=92.4) | 51,541 (25.9%) | 44,683 (24.4%) | 68,379 (24.4%) | 18,886 (24.9%) | |
| Q4 (>92.4) | 48,507 (24.4%) | 43,400 (24.3%) | 69,574 (24.9%) | 20,183 (26.2%) | |
| Medicaid eligibility | <0.01 | ||||
| No | 68,265 (34.4%) | 63,714 (35.6%) | 104,034 (37.2%) | 29,248 (37.9%) | |
| Yes | 130,473 (65.6%) | 115,128 (64.4%) | 175,975 (62.9%) | 47,902 (62.1%) | |
| Elixhauser comorbidity score*, mean (SD) | 6.19 (3.1) | 5.76 (3.0) | 5.04 (2.8) | 4.52 (2.7) | <0.01 |
| <=3 | 39,431 (19.8%) | 42,477 (23.8%) | 89,428 (31.9%) | 31,020 (40.2%) | |
| 4,5 | 53,866 (27.1%) | 51,349 (28.7%) | 85,528 (30.5%) | 22,982 (29.8%) | |
| 6,7 | 45,903 (23.1%) | 40,429 (22.6%) | 56,331 (20.1%) | 13,089 (17.0%) | |
| 8 and above | 59,538 (30.0%) | 44,587 (24.9%) | 48,722 (17.4%) | 10,059 (13.0%) | |
| Chronic disease score, mean (SD) | 2.91 (1.5) | 2.65 (1.5) | 2.31 (1.3) | 2.00 (1.3) | <0.01 |
| Pain | <0.01 | ||||
| None | 100,523 (50.6%) | 108,917 (60.9%) | 202,371 (72.3%) | 55,970 (72.6%) | |
| Mild/Infrequent | 90,619 (45.6%) | 65,009 (36.3%) | 73,561 (26.3%) | 20,914 (27.1%) | |
| Moderate/Severe/Frequent | 7,596 (3.8%) | 4,916 (2.8%) | 4,077 (1.4%) | 266 (0.3%) | |
| Mood disorder | <0.01 | ||||
| No depression | 91,865 (46.2%) | 78,732 (44.0%) | 132,558 (47.3%) | 32,579 (42.2%) | |
| Mild or moderate depression | 97,463 (19.0%) | 89,809 (50.2%) | 130,424 (46.6%) | 36,268 (47.0%) | |
| Severe depression | 9,410 (4.7%) | 10,301 (5.8%) | 17,027 (6.1%) | 8,303 (10.8%) | |
| Activities of daily living score (higher score indicates greater dependence) | <0.01 | ||||
| 0–6 | 45,935 (23.1%) | 27,756 (15.5%) | 23,136 (8.3%) | 1,356 (1.8%) | |
| 7–12 | 41,875 (21.1%) | 33,618 (18.8%) | 42,796 (15.3%) | 3,637 (4.7%) | |
| 13–18 | 92,619 (46.6%) | 89,753 (50.2%) | 132,822 (47.4%) | 23,445 (30.4%) | |
| 19–24 | 18,309 (9.2%) | 27,715 (15.5%) | 81,255 (29.0%) | 48,712 (63.1%) | |
| Hallucinations or delusions | 6,874 (3.5%) | 10,956 (6.1%) | 27,138 (9.7%) | 6,987 (9.1%) | <0.01 |
| Aggressive behavior | <0.01 | ||||
| None | 179,920 (90.5%) | 151,989 (85.0%) | 213,624 (76.3%) | 54,432 (70.6%) | |
| Mild/moderate | 14,734 (7.4%) | 19,771 (11.1%) | 43,600 (15.6%) | 12,960 (16.8%) | |
| Severe | 4,084 (2.1%) | 7,082 (3.9%) | 22,785 (8.1%) | 9,758 (12.6%) | |
| Year | <0.01 | ||||
| 2011 | 27,086 (13.6%) | 28,133 (15.7%) | 45,755 (16.3%) | 12,599 (16.3%) | |
| 2012 | 29,036 (14.6%) | 27,015 (15.1%) | 42,905 (15.3%) | 12,252 (15.9%) | |
| 2013 | 30,156 (15.2%) | 27,366 (15.3%) | 42,759 (15.3%) | 11,632 (15.1%) | |
| 2014 | 30,794 (15.5%) | 27,017 (15.1%) | 42,270 (15.1%) | 11,560 (15.0%) | |
| 2015 | 28,558 (14.4%) | 25,011 (14.0%) | 38,654 (13.8%) | 10,583 (13.7%) | |
| 2016 | 28,494 (14.3%) | 24,034 (13.4%) | 36,534 (13.1%) | 10,061 (13.0%) | |
| 2017 | 24,614 (12.4%) | 20,266 (11.3%) | 31,132 (11.1%) | 8,463 (11.07%) |
Trends in Opioid Use
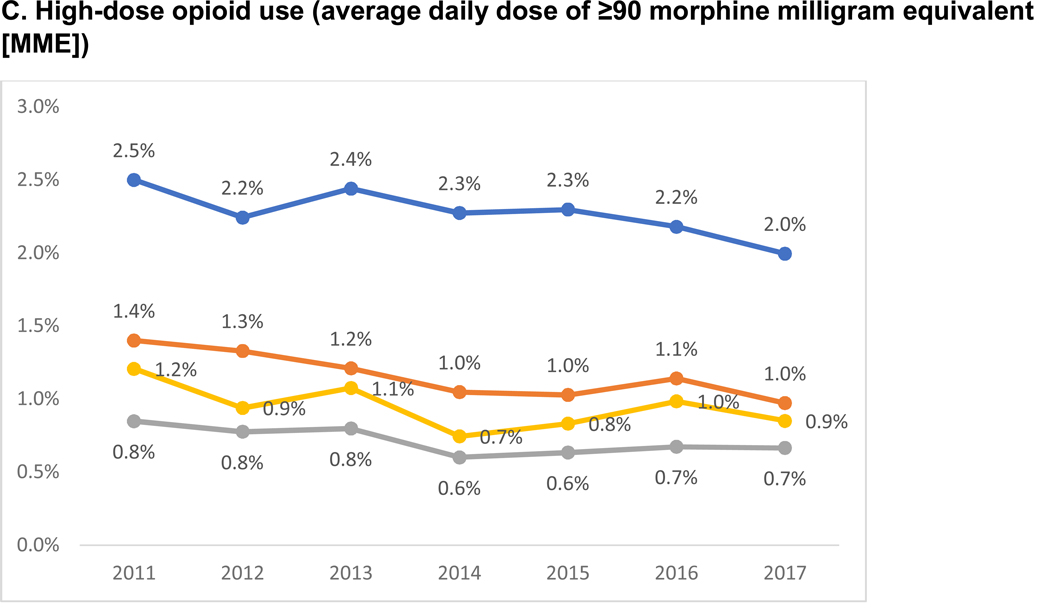
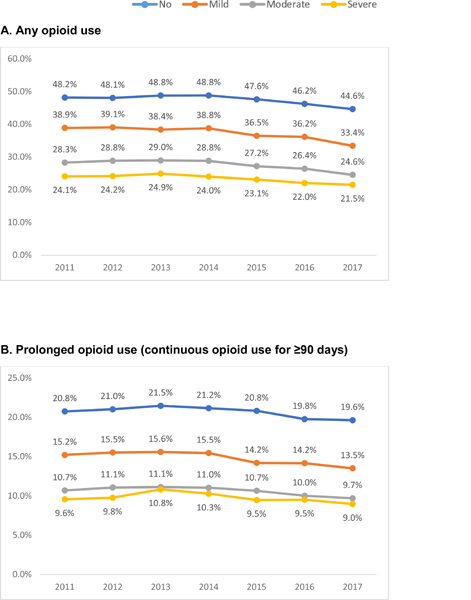
Figure 1 reports the trends in any, prolonged and high-dose opioid use among long-term care nursing home residents. From 2011 to 2017, the use of any opioids decreased by 7.4% in residents with no (48.2% to 44.6%, p<0.001), 14.1% in mild (38.9% to 33.4, p<0.001), 13.2% in moderate (28.3% to 24.6%, p<0.001) and 10.7% in severe (24.1% to 21.5%, p<0.001) dementia. The relative reduction in prolonged opioid use from 2011 to 2017 was 5.8% for no, 11.1% for mild, 9.3% for moderate and 6.3% for severe dementia, and for high-dose opioid use was 20.2% for no, 30.6% for mild, 21.6% for moderate and 29.5% for severe dementia.
Figure 1. Trends in Opioid Use by Dementia Severity in Long-term Care Nursing Home Residents.
P value for trends from Cochrane-Armitage trends test: no <0.001; mild <0.001; moderate <0.001; severe <0.001
P value for trends from Cochrane-Armitage trends test: no <0.001; mild <0.001; moderate <0.001; severe 0.0437
P value for trends from Cochrane-Armitage trends test: no <0.001; mild <0.001; moderate <0.001; severe 0.009
Among nursing home residents with a fracture, opioid use declined from 2012 to 2017 by 9.0% in those with mild (50.4% to 45.8%, p=0.045), 9.5% with moderate (45.7% to 41.4%, p=0.032) and 12.3% with severe (38.6% to 33.8%, p=0.015) dementia; no decline was observed among those with no dementia (relative reduction 2.4%; 52.2% to 50.9%, p=0.78) (Supplementary Figure 3).
Association of Dementia Severity with Opioid Use in Long-term Care Nursing Home Residents
Table 2 reports the rate of any, prolonged and high-dose opioid use and the adjusted OR from the generalized estimating equation regression models. Among long-term care residents (n=734,739), 35.0% used at least one opioid, 14.3% used for over 90 days and 1.3% used high-dose opioids.
Table 2.
Association of Dementia Severity with Any Opioid Use, Prolonged Opioid Use and High-dose Opioid Use in Long-term Care Nursing Home Residents
| Patient Characteristics | Sample size, N | Any opioid use, % | Odds ratio (95% CI) | Prolonged opioid use, % | Odds ratio (95% CI)a | High-dose opioid use, % | Odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Overall use | 734,739 | 35.0% | 14.3% | 1.4% | |||
| Dementia severity | |||||||
| No | 198,739 | 47.6% | Reference | 20.7% | Reference | 2.3% | Reference |
| Mild | 178,842 | 37.5% | 0.80 (0.79, 0.81) | 14.9% | 0.83 (0.81, 0.84) | 1.2% | 0.70 (0.66, 0.74) |
| Moderate | 280,009 | 27.8% | 0.64 (0.63, 0.65) | 10.7% | 0.70 (0.69, 0.72) | 0.7% | 0.58 (0.54, 0.61) |
| Severe | 77,150 | 23.5% | 0.56 (0.55, 0.57) | 9.8% | 0.69 (0.67, 0.71) | 1.0% | 0.69 (0.63, 0.74) |
| Age, mean (SD) | 734,739 | 0.99 (0.98, 0.99) | 0.99 (0.98, 1.01) | 0.96 (0.95, 0.96) | |||
| Gender | |||||||
| Male | 190,815 | 29.9% | Reference | 11.0% | Reference | 1.3% | Reference |
| Female | 543,924 | 36.9% | 1.36 (1.34, 1.38) | 15.5% | 1.40 (1.37, 1.43) | 1.3% | 1.06 (0.99, 1.13) |
| Race | |||||||
| White | 593,549 | 36.5% | Reference | 15.3% | Reference | 1.4% | Reference |
| Black | 85,050 | 30.1% | 0.82 (0.80, 0.84) | 10.9% | 0.76 (0.73, 0.78) | 1.0% | 0.77 (0.69, 0.84) |
| Hispanic | 35,950 | 28.7% | 0.72 (0.69, 0.74) | 9.7% | 0.65 (0.62, 0.69) | 0.9% | 0.58 (0.49, 0.84) |
| Other | 20,190 | 25.6% | 0.61 (0.59. 0.64) | 9.3% | 0.62 (0.58. 0.67) | 0.9% | 0.58 (0.49. 0.67) |
| Region | |||||||
| Northeast | 165,985 | 24.8% | Reference | 10.5% | Reference | 1.1% | Reference |
| Midwest | 211,308 | 37.5% | 1.63 (1.60, 1.66) | 16.9% | 1.55 (1.50, 1.59) | 1.2% | 0.97 (0.89, 1.05) |
| South | 277,980 | 38.3% | 1.77 (1.74, 1.81) | 14.5% | 1.32 (1.29, 1.36) | 1.1% | 1.00 (0.92, 1.08) |
| West | 79,466 | 38.5% | 2.06 (2.01, 2.12) | 14.9% | 1.55 (1.49, 1.61) | 2.4% | 2.21 (2.02, 2.42) |
| Marital status | |||||||
| Married | 135,846 | 33.4% | Reference | 12.7% | Reference | 1.3% | Reference |
| Unmarried | 598,893 | 35.4% | 1.04 (1.04, 1.08) | 14.7% | 1.13 (1.09, 1.15) | 1.3% | 1.06 (0.99, 1.13) |
| Education by zip code | |||||||
| Q1 (<=81.8) | 186,571 | 35.1% | Reference | 13.6% | Reference | 1.1% | Reference |
| Q2 (>81.8;<= 88.0) | 183,015 | 36.5% | 1.05 (1.03, 1.07) | 14.9% | 1.03 (1.01, 1.06) | 1.3% | 1.21 (1.12, 1.31) |
| Q3 (>88.0; <=92.4) | 183,489 | 35.6% | 1.05 (1.03, 1.07) | 15.2% | 1.05 (1.02, 1.08) | 1.4% | 1.33 (1.23, 1.44) |
| Q4 (>92.4) | 181,664 | 33.0% | 0.99 (0.97, 1.01) | 13.7% | 0.99 (0.96, 1.01) | 1.4% | 1.37 (1.27, 1.48) |
| Medicaid eligibility | |||||||
| No | 265,261 | 32.7% | Reference | 13.4% | Reference | 1.1% | Reference |
| Yes | 469,478 | 36.4% | 1.05 (1.04, 1.07) | 14.9% | 1.09 (1.07, 1.11) | 1.4% | 1.06 (0.99, 1.12) |
| Elixhauser comorbidity score* | |||||||
| <=3 | 202,356 | 28.5% | Reference | 12.5% | Reference | 0.9% | Reference |
| 4,5 | 213,725 | 32.8% | 1.04 (1.02, 1.05) | 14.2% | 0.97 (0.96, 0.99) | 1.0% | 1.00 (0.94, 1.07) |
| 6,7 | 155,752 | 37.2% | 1.06 (1.04, 1.07) | 15.3% | 0.91 (0.89, 0.93) | 1.4% | 1.11 (1.04, 1.18) |
| 8 and above | 162,906 | 44.0% | 1.07 (1.05, 1.09) | 15.8% | 0.79 (0.77, 0.80) | 1.9% | 1.17 (1.09, 1.25) |
| Chronic disease score | 734,739 | 1.32 (1.31, 1.32) | 1.26 (1.25, 1.26) | 1.14 (1.13, 1.16) | |||
| Pain | |||||||
| None | 467,781 | 24.1% | Reference | 10.3% | Reference | 0.6% | Reference |
| Mild/Infrequent | 250,103 | 53.0% | 1.96 (1.94, 1.98) | 20.4% | 1.20 (1.18, 1.21) | 2.4% | 2.31 (2.21, 2.41) |
| Moderate/Severe/Frequent | 16,855 | 72.7% | 2.77 (2.69, 2.85) | 37.3% | 1.64 (1.58, 1.71) | 4.1% | 3.16 (2.87, 3.47) |
| Mood disorder | |||||||
| No depression | 335,734 | 31.1% | Reference | 12.5% | Reference | 1.0% | Reference |
| Mild or moderate depression | 353,964 | 38.2% | 1.09 (1.08, 1.10) | 15.7% | 1.05 (1.03, 1.06) | 1.4% | 1.18 (1.13, 1.23) |
| Severe depression | 45,041 | 40.1% | 1.16 (1.14, 1.19) | 17.0% | 1.09 (1.06, 1.12) | 1.9% | 1.43 (1.32, 1.55) |
| Activities of daily living score (higher score indicates greater dependence) | |||||||
| 0–6 | 98,183 | 35.8% | Reference | 15.6% | Reference | 1.4% | Reference |
| 7–12 | 121,926 | 34.0% | 1.02 (1.01, 1.04) | 13.6% | 0.98 (0.95, 0.99) | 1.2% | 0.96 (0.89, 1.04) |
| 13–18 | 338,639 | 36.8% | 1.16 (1.14, 1.18) | 14.8% | 1.04 (1.01, 1.06) | 1.3% | 1.06 (0.99, 1.14) |
| 19–24 | 175,991 | 31.9% | 1.19 (1.17, 1.22) | 13.2% | 1.09 (1.06, 1.12) | 1.3% | 1.36 (1.26, 1.48) |
| Hallucinations or delusions | |||||||
| No | 682,784 | 35.2% | Reference | 14.4% | Reference | 1.3% | Reference |
| Yes | 51,955 | 32.4% | 0.93 (0.92, 0.95) | 13.2% | 0.96 (0.93, 0.98) | 0.8% | 0.76 (0.69, 0.83) |
| Aggressive behavior | |||||||
| None | 599,965 | 35.5% | Reference | 14.5% | Reference | 1.3% | Reference |
| Mild/moderate | 91,065 | 33.9% | 1.03 (1.02, 1.04) | 13.9% | 1.04 (1.02, 1.06) | 1.2% | 0.98 (0.92, 1.04) |
| Severe | 43,709 | 31.5% | 1.01 (0.99, 1.04) | 12.5% | 0.98 (0.95, 1.01) | 1.0% | 0.85 (0.77, 0.94) |
| Year | |||||||
| 2011 | 113,517 | 35.2% | Reference | 14.1% | Reference | 1.4% | Reference |
| 2012 | 111,208 | 35.8% | 0.88 (0.86, 0.89) | 14.6% | 0.93 (0.92, 0.95) | 1.3% | 0.87 (0.82, 0.92) |
| 2013 | 111,913 | 36.2% | 0.88 (0.86, 0.89) | 15.0% | 0.98 (0.96, 0.99) | 1.4% | 0.90 (0.85, 0.96) |
| 2014 | 111,641 | 36.3% | 0.88 (0.87, 0.90) | 14.8% | 1.01 (0.99, 1.03) | 1.2% | 0.80 (0.75, 0.86) |
| 2015 | 102,806 | 34.7% | 0.83 (0.81, 0.84) | 14.2% | 1.00 | (0.98, 1.02) 1.2% | 0.83 (0.77, 0.89) |
| 2016 | 99,123 | 34.0% | 0.84 (0.82, 0.85) | 13.8% | 1.05 (1.03, 1.08) | 1.3% | 0.88 (0.82, 0.94) |
| 2017 | 84,475 | 32.2% | 0.84 (0.83, 0.85) | 13.4% | 1.11 (1.08, 1.14) | 1.1% | 0.88 (0.82, 0.95) |
Any opioid use:
Nursing home residents with mild (37.5% vs. 47.6%; OR 0.80, 95% CI 0.79–0.81), moderate (27.8% vs. 47.6%; OR 0.64, 95% CI 0.63–0.65) or severe (23.5% vs. 47.6%; OR 0.56, 95% CI 0.55–0.57) dementia were less likely to receive opioids compared to residents with no dementia.
Prolonged opioid use:
Nursing home residents with mild (14.9% vs. 20.7%; OR 0.83, 95% CI 0.81–0.84), moderate (10.7% vs. 20.7%; OR 0.70, 95% CI 0.69–0.72) or severe (9.8% vs. 20.7%; OR 0.69, 95% CI 0.67–0.71) dementia were less likely to receive prolonged opioids compared to residents with no dementia.
High-dose opioid use:
Mild (1.2% vs. 2.3%; OR 0.70, 95% CI 0.66–0.74), Moderate (0.7% vs. 2.3%; OR 0.58, 95% CI 0.54–0.61) and severe (1.0% vs. 2.3%; OR 0.69, 95% CI 0.63–0.74) dementia reduced the odds of receiving high-dose opioids compared to no dementia.
Association of Dementia Severity and Opioid Use in Nursing Home Residents with a Fracture
Opioid use was lower in nursing home residents with a fracture who had mild (47.5% vs. 53.3%; OR 0.77, 95% CI 0.69–0.86), moderate (43.6% vs. 53.3%; OR 0.62, 95% CI 0.57–0.69) and severe (35.7% vs. 53.3%; OR 0.46, 95% CI 0.41–0.52) dementia compared to those with no dementia (Table 3).
Table 3.
Association of Dementia Severity with Opioid Use during the Year that a Fracture was Diagnosed, among Long-term Care Nursing Home Residents
| Patient Characteristics | Sample size, N | Any opioid use within 7 days of emergency room discharge, % | Odds ratio (95% CI)a |
|---|---|---|---|
| Overall use | |||
| Dementia severity | |||
| No | 2,632 | 53.3% | Reference |
| Mild | 2,663 | 47.5% | 0.77 (0.69, 0.86) |
| Moderate | 5,377 | 43.6% | 0.62 (0.57, 0.69) |
| Severe | 2,255 | 35.7% | 0.46 (0.41, 0.52) |
Adjusted for age, gender, race and year.
CI, confidence interval.
Discussion
Our study found that any, prolonged and high-dose opioid use declined from 2011 to 2017 in nursing home residents. Among residents with a fracture, opioid use declined in those with dementia but not in residents with no dementia. Mild, moderate and severe dementia reduced the odds of any, prolonged and high-dose opioid use in all nursing home residents, and opioid use upon discharge from an emergency room after a fracture.
The decline in opioid use in nursing homes from 2012 to 2017 can be attributed to several state and federal intiatives aimed to reduce opioid use.15, 18 Our findings of declining opioid use among residents with emergency room visit for a fracture were consistent with prior study.29 No decline of opioid prescribing among reidents with no dementia who had a fracture can be explained by better communication in expressing pain and seeking medication for pain relief.
Lower use of opioids in patients with dementia is consistent with prior studies. In early 2000, lower use of opioid was observed in dementia patients.30–32 A nationally representative study of US community-dwelling elderly with chronic pain conditions from 2006 to 2013 found that dementia patients were 19% less likely to receive opioids.33 Similarly, lower use of opioids were reported in nursing home residents with dementia in Denmark.34 Both studies did not consider the severity of dementia or the degree of pain.33, 34 In contrast to prior studies, our study controlled for pain level and showed lower use of opioids. Several factors may explain these findings. It is possible that pain cues from patients with dementia may not be recognized by staff because of lack of training in assessing pain, no use of dementia-specific pain assessment tools or the patient’s difficulty in expressing pain. Nursing home residents are frail, have multiple chronic conditions and are at increased risk of adverse events.35 Opioid use increases the risk of falls and fractures13 and further deteriorates cognitive function in patients with dementia.36 Therefore, providers may be reluctant to use opioids in patients with dementia. Moreover, policy makers, regulators, and surveyors have been encouraging the use of alternative non-opioid pain treatments.
Prolonged opioid use in nursing home residents (14.3%) was comparable to opioid use in community-dwelling elderly (13.9%).37 In US nursing homes, high-dose opioid use was 1.1% in 2017, which was higher than the national rate of 0.36%.15 The CDC Guideline recommends limiting long-term opioid use and dosage.38, 39 However, the CDC has advised not to apply recommendations to populations outside the scope of the Guideline.39 Residents with acute fracture events or those who are at the end of life may be adversely affected if the CDC guideline is applied without clinical judgement. It is possible that nursing home residents may require prolonged or long-term opioids to manage their pain.38, 40 From Medicare part D claims data, it is difficult to assess the clinical appropriateness of prolonged or high-dose opioid use in nursing home residents. Future studies can address this question.
The lower use of opioids in residents with dementia may suggest under-treatment of pain or the use of other treatment modalities. Under-treatment of pain increases the risk of delirium, behavioral disturbances, accelerated functional decline, malnutrition, poor response to rehabilitation and decreased recovery. Appropriate pain treatment can reduce both pain and behavioral disturbances in dementia patients.3, 41 It is important that as-needed analgesics (opioids and non-opioids) be avoided in the setting of severe dementia and severe pain, given the patients’ inability to communicate pain and ask for medication. A scheduled pain medication regiment would be more appropriate in this population.
Our study has limitations. First, we may have overestimated prolonged and high-dose opioid use if a prescription for opioids was filled but the drug was never administered or administered at less than the prescribed dose. Second, we did not capture opioids used during hospitalization. Third, patients can have multiple measurements for dementia severity and pain. However, we only used the initial measurements. Fourth, we included only residents with fractures who were treated in the emergency room and did not require hospital admission and, therefore, more severe fractures were excluded. Fifth, we did not study the use of alternative non-opioid therapies such as acetaminophen or non-pharmacological management of pain. It is possible that decreasing use of opioids over time in dementia patients may be offset by increasing use of non-opioid therapies for pain.
Conclusions and Implications
In conclusion, opioid use declined from 2011 to 2017 in nursing home residents and the use was lower in patients with mild, moderate and severe dementia compared to those with no dementia, indicating shifts in pain management, which could reflect increasing use of other modalities or suboptimal pain management. To improve quality of care, systemic efforts from healthcare professionals, nursing home administrators and policymakers are needed to evaluate opioid use in nursing home residents with dementia in the context of overall pain detection and management and promotion of quality of life. Future prospective and quality improvement studies should investigate the clinical appropriateness of opioid use in nursing home residents with dementia.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Health (5R01DA039192 and 3R01DA039192–03S1), National Cancer Institute (K05-CA134923) and the Claude D. Pepper Older Americans Independence Center Award (P30-AG024832–12).
Footnotes
Author Disclosures: None
Conflicts of Interest: All authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaugler JE, Yu F, Davila HW, et al. Alzheimer’s disease and nursing homes. Health Aff (Millwood) 2014;33(4):650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbett A, Husebo B, Malcangio M, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol 2012;8(5):264–274. [DOI] [PubMed] [Google Scholar]
- 3.Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343:d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson EL, White N, Lord K, et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain 2015;156(4):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell MJ, Katz B, Helme RD. The impact of dementia on the pain experience. Pain 1996;67(1):7–15. [DOI] [PubMed] [Google Scholar]
- 6.Horgas AL, Tsai PF. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs Res 1998;47(4):235–242. [DOI] [PubMed] [Google Scholar]
- 7.Loeb JL. Pain management in long-term care. Am J Nurs 1999;99(2):48–52. [PubMed] [Google Scholar]
- 8.Ferrell BA, Ferrell BR, Rivera L Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995;10(8):591–598. [DOI] [PubMed] [Google Scholar]
- 9.Won A, Lapane K, Gambassi G, et al. Correlates and management of nonmalignant pain in the nursing home. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. J Am Geriatr Soc 1999;47(8):936–942. [DOI] [PubMed] [Google Scholar]
- 10.Fain KM, Alexander GC, Dore DD, et al. Frequency and Predictors of Analgesic Prescribing in U.S. Nursing Home Residents with Persistent Pain. J Am Geriatr Soc 2017;65(2):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunnicutt JN, Ulbricht CM, Tjia J, et al. Pain and pharmacologic pain management in long-stay nursing home residents. Pain 2017;158(6):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunnicutt JN, Chrysanthopoulou SA, Ulbricht CM, et al. Prevalence of Long-Term Opioid Use in Long-Stay Nursing Home Residents. J Am Geriatr Soc 2018;66(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshikawa A, Ramirez G, Smith ML, et al. Opioid Use and the Risk of Falls, Fall Injuries and Fractures among Older Adults: A Systematic Review and Meta-Analysis. J Gerontol A Biol Sci Med Sci 2020. [DOI] [PubMed] [Google Scholar]
- 14.By the American Geriatrics Society Beers Criteria Update Expert, P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 15.Bohnert ASB, Guy GP Jr., Losby JL. Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention’s 2016 Opioid Guideline. Ann Intern Med 2018;169(6):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieber LZ, Guy GP Jr., Seth P, et al. Trends and Patterns of Geographic Variation in Opioid Prescribing Practices by State, United States, 2006–2017. JAMA Netw Open 2019;2(3):e190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo YF, Raji MA, Liaw V, et al. Opioid Prescriptions in Older Medicare Beneficiaries After the 2014 Federal Rescheduling of Hydrocodone Products. J Am Geriatr Soc 2018;66(5):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raji MA, Kuo YF, Adhikari D, et al. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol Drug Saf 2018;27(5):513519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iaboni A, Campitelli MA, Bronskill SE, et al. Time trends in opioid prescribing among Ontario long-term care residents: a repeated cross-sectional study. CMAJ Open 2019;7(3):E582–E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin JS, Li S, Zhou J, et al. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res 2017;17(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah R, Chou LN, Kuo YF, et al. Long-Term Opioid Therapy in Older Cancer Survivors: A Retrospective Cohort Study. J Am Geriatr Soc 2019;67(5):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jena AB, Goldman D, Karaca-Mandic P Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med 2016;176(7):990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downer B, Thomas KS, Mor V, et al. Cognitive Status of Older Adults on Admission to a Skilled Nursing Facility According to a Hospital Discharge Diagnosis of Dementia. J Am Med Dir Assoc 2017;18(8):726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nursing Home Data Compendium 2015. Edition; https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf. Accessed April 12, 2020.
- 26.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 27.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47(6):626–633. [DOI] [PubMed] [Google Scholar]
- 28.Von Korff M, Wagner EH, Saunders K A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992;45(2):197–203. [DOI] [PubMed] [Google Scholar]
- 29.Rui P, Santo L, JJ A. Trends in opioids prescribed at discharge from emergency departments among adults: United States, 2006–2017. National Health Statistics Reports; no 135. Hyattsville, MD: National Center for Health Statistics; 2020. . [PubMed] [Google Scholar]
- 30.Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. J Pain Symptom Manage 2000;19(4):240–248. [DOI] [PubMed] [Google Scholar]
- 31.Nygaard HA, Jarland M Are nursing home patients with dementia diagnosis at increased risk for inadequate pain treatment? Int J Geriatr Psychiatry 2005;20(8):730737. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta M, Bercovitz A, Harris-Kojetin LD. Prevalence and management of pain, by race and dementia among nursing home residents: United States, 2004. NCHS Data Brief 2010;(30):1–8. [PubMed] [Google Scholar]
- 33.Shen C, Zhao X, Dwibedi N, et al. Opioid use and the presence of Alzheimer’s disease and related dementias among elderly Medicare beneficiaries diagnosed with chronic pain conditions. Alzheimers Dement (N Y) 2018;4:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen-Dahm C, Gasse C, Astrup A, et al. Frequent use of opioids in patients with dementia and nursing home residents: A study of the entire elderly population of Denmark. Alzheimers Dement 2015;11(6):691–699. [DOI] [PubMed] [Google Scholar]
- 35.Guerriero F, Sgarlata C, Maurizi N, et al. Pain management in dementia: so far, not so good. Journal of Gerontology and Geriatrics 2016;50:75. [Google Scholar]
- 36.Dublin S, Walker RL, Gray SL, et al. Prescription Opioids and Risk of Dementia or Cognitive Decline: A Prospective Cohort Study. J Am Geriatr Soc 2015;63(8):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin JS, Kuo YF, Brown D, et al. Association of Chronic Opioid Use With Presidential Voting Patterns in US Counties in 2016. JAMA Netw Open 2018;1(2):e180450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin R Limits on Opioid Prescribing Leave Patients With Chronic Pain Vulnerable. JAMA 2019;321(21):2059–2062. [DOI] [PubMed] [Google Scholar]
- 39.CDC Advises Against Misapplication of the Guideline for Prescribing Opioids for Chronic Pain; https://www.cdc.gov/media/releases/2019/s0424-advises-misapplication-guidelineprescribing-opioids.html. Accessed April 12, 2020.
- 40.Dowell D, Haegerich T, Chou R No Shortcuts to Safer Opioid Prescribing. N Engl J Med 2019;380(24):2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandvik RK, Selbaek G, Seifert R, et al. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: a cluster randomized trial. Eur J Pain 2014;18(10):1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.