Abstract
Background:
Rotator cuff (RC) muscle atrophy and fatty infiltration (FI) are independent factors correlated with failure of attempted tendon repair in larger RC tears. However, there is no effective treatment for RC muscle atrophy and FI at this time. The recent discovery of beige adipose tissue (BAT) in adults shed light on a new avenue in treating obesity and excessive fat deposition by promoting BAT activity. The goal of this study was to define the role of intramuscular BAT in RC muscle FI and the effect of β3-adrenergic receptor agonists in treating RC muscle FI by promoting BAT activity.
Materials and methods:
Three-month-old wild-type C57BL/6J, platelet derived growth factor receptor-alpha (PDGFRα) green fluorescent protein (GFP) reporter and uncoupling protein 1 (UCP-1) knockout mice underwent a unilateral RC injury procedure, which included supraspinatus (SS) and infraspinatus tendon resection and suprascapular nerve transection. To stimulate BATactivity, amibegron, a selective β3-adrenergic receptor agonist, was administered to C57BL/6J mice either on the same day as surgery or 6 weeks after surgery through daily intraperitoneal injections. Gait analysis was conducted to measure forelimb function at 6 weeks or 12 weeks (in groups receiving delayed amibegron treatment) after surgery. Animals were killed humanely at 6 weeks (or 12 weeks for delayed amibegron groups) after surgery. SS muscles were harvested and analyzed histologically and biochemically.
Results:
Histologic analysis of SS muscles from PDGFRα-GFP reporter mice showed that PDGFRα-positive fibroadipogenic progenitors in RC muscle expressed UCP-1, a hallmark of BAT during the development of FI after RC tears. Impairing BAT activity by knocking out UCP-1 resulted in more severe muscle atrophy and FI with inferior forelimb function in UCP-1 knockout mice compared with wild-type mice. Promoting BAT activity with amibegron significantly reduced muscle atrophy and FI after RC tears and improved forelimb function. Delayed treatment with amibegron reversed muscle atrophy and FI in muscle.
Conclusions:
Fat accumulated in muscle after RC tears possesses BAT characteristics. Impairing BAT activity results in worse RC muscle atrophy and FI. Amibegron reduces and reverses RC atrophy and FI by promoting BAT activity.
Level of evidence:
Basic Science Study; Histology; In Vivo Animal Model
Keywords: Rotator cuff, fatty infiltration, atrophy, beige fat, amibegron
Rotator cuff (RC) tear is one of the most common upper-extremity injuries that can result in chronic pain and reduction of arm function. Symptomatic RC tears not responsive to conservative interventions usually lead to surgical repair. Secondary muscle pathologies after RC injury, including muscle atrophy and fatty infiltration (FI), are identified to be critical factors in determining functional outcomes after cuff repair.7,17 Muscle atrophy is loss of muscle mass, whereas FI is proliferation of fatty tissue within muscle.19 Some researchers have reported that atrophy caused by small- and medium-sized RC tears could partially recover after successful repair; however, RC muscle atrophy and FI have not been shown to recover after massive RC tears even after successful tendon repair.12,14,17,20,28
The pathogenesis and molecular mechanisms of FI in muscles are still not fully defined. Joshi et al24 reported that the mTOR pathway regulates FI through SREBP-1 and PPARγ pathways in a rodent cuff tear model. Lee et al27 found that fatty acid–binding protein 4 regulates FI by hypoxia-inducible factor 1 after RC tears in mice. Recent studies, including our work, have demonstrated that a group of muscle residential interstitial progenitor cells, named “fibroadipogenic progenitors” (FAPs), are the primary cellular source of pathologic muscle fat deposition, including that in RC muscles.30,42
Different from classic adipocytes, adipocytes developed from FAPs have unique characteristics.36 Adipocytes derived from FAP express uncoupling protein 1 (UCP-1), a hallmark for beige adipose tissue (BAT).18,40 Different from white fat, brown fat is characterized by dense packing of mitochondria and multilocular intracellular lipid droplets, as well as its expression of UCP-1. A distinct intermediate adipose type, namely “beige” or “brite” fat, was discovered recently in both rodents and humans.5,16,21,47 Although mature beige fat morphologically resembles white fat, it can become brown-like in response to specific stimuli.15,32 The mechanism of BAT in muscle regeneration is not fully understood. Crichton et al9 concluded that after activation of β3-adrenergic receptor on the cell membrane surface, the intracellular lipid droplets are separated into free fatty acids through the cAMP-PKA pathway, and the proton conduction of UCP-1 is activated to generate energy in the mitochondria. Meyer et al33 have demonstrated that healthy RC muscles are encapsulated by epimuscular adipose tissue that appears to adopt a beige phenotype when the RC tendon is torn, and transplantation of these cells promotes muscle regeneration.4 In our previous work, we also demonstrated that transplantation of BAT FAPs promotes muscle regeneration and forelimb function after massive RC tear and repair.25,26
Brown fat used to be thought to exist only in infants. However, recent radiologic observations, especially 18F-fluorodeoxyglucose positron emission tomography–computed tomography scanning, have discovered metabolically active beige fat tissue in adults.39 The existence of BAT in adult humans has led to substantial interest in its therapeutic potential as a means to increase whole-body energy expenditure in obesity. Thermogenic action of BAT is activated through sympathetic stimulation of β3-adrenergic receptors, which are highly abundant on mature adipocytes.23,41 In humans, β3-adrenergic receptor expression has been reported in several tissues, such as white and brown adipose, heart, blood vessels, gall bladder, gastrointestinal tract, prostate and urinary bladder detrusor, brain, and near-term myometrium, suggesting that β3-adrenergic receptor is an important regulator of various physiological functions.44 Recently, Cypess et al11 have shown that stimulation of selective β3-adrenergic receptors can activate BAT in humans. Fat oxidation in BAT and lipolysis in white adipose tissue were also observed after β3-adrenergic receptor activation in humans, suggesting that fat oxidation was dependent on mobilization of fat from white adipose tissue depots to increase circulating free fatty acids.52
We hypothesized that fat accumulated in muscle after RC tears would possess BAT characteristics. Moreover, impairing BAT activity would result in increased RC muscle atrophy and FI. Finally, we hypothesized that amibegron, a selective β3-adrenergic receptor agonist, can prevent and reverse RC atrophy and FI by promoting BAT activity.
Materials and methods
All reagents used in this study were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless otherwise indicated.
Surgical procedures
Seventy 3-month-old C57BL/6J mice (half male and half female mice purchased from Jackson Laboratory (stock no. 000664; Sacramento, CA, USA), twenty PDGFRα-GFP reporter mice, twenty UCP-1 knockout (KO) mice, and twenty of their colony control C57BL/6J wild-type (WT) mice (all 3 months old) bred from our colonies were used in this study. The C57BL/6J strain is the most common WT mouse. PDGFRα-GFP reporter mice express the H2B-eGFP fusion gene from the endogenous PDGFRα locus. PDGFRα is a specific marker of FAPs in muscle used to trace the presence of FAPs in muscle. UCP-1 KO mice are homozygous for UCP-1 KO. All mice used in the study had the C57BL/6J genetic background. The mice weighed between 23.2 and 25.3 g. No significant difference was found between groups.
The sample size was determined based on a previous power analysis demonstrating that a sample size (n) of 4 would be sufficient to detect a significant difference in the fat area fraction of muscle in our RC injury model.49 An additional power analysis with α = .05 and a power of 0.80 was run for the fat area fraction of muscle based on means and standard deviations from a pilot study using amibegron to treat a tendon transection + denervation (TT + DN) mouse model. We found that a minimum of 4 mice per group was required to detect a difference of 15% in the fat area fraction of muscle between the treatment and control groups. To increase the power and address possible sex differences, we included 5 male and 5 female animals in each group in this study. Animals were randomly divided into sham and massive RC tear groups (which included supraspinatus [SS] and infraspinatus [IS] tendon and suprascapular nerve transection [TT + DN]); detailed grouping information is summarized in Table I.
Table I.
Experimental groups*
| Group | Mice | Surgical procedure | Intervention |
|---|---|---|---|
| I | PDGFRα-GFP | Sham | No injection |
| II | PDGFRα-GFP | TT + DN | No injection |
| III | C57BL/6J | Sham | No injection |
| V | C57BL/6J | TT + DN | No injection |
| V | UCP-1 KO | Sham | No injection |
| VI | UCP-1 KO | TT + DN | No injection |
| VII | C57BL/6J | Sham | 5% DMSO at time of surgery |
| Vili | C57BL/6J | TT + DN | 5% DMSO at time of surgery |
| IX | C57BL/6J | TT + DN | Amibegron, 1 mg/kg, at time of surgery |
| X | C57BL/6J | TT + DN | Amibegron, 10 mg/kg, at time of surgery |
| XI | C57BL/6J | Sham | 5% DMSO at 6 weeks after surgery |
| XII | C57BL/6J | TT + DN | 5% DMSO at 6 weeks after surgery |
| XIII | C57BL/6J | TT + DN | Amibegron, 10 mg/kg, at 6 weeks after surgery |
UCP-1, uncoupling protein 1; KO, knockout; DMSO, dimethyl sulfoxide; TT+DN, tendon transection + denervation.
Five male and five female mice were randomly assigned to each group. Groups I-X were killed humanely at 6 weeks after surgery, and groups XI-XIII were killed humanely at 12 weeks after surgery.
After general anesthesia was achieved by 1%–5% isoflurane inhalation, the right-side SS and IS tendons and suprascapular nerve were transected as described previously.29 In the sham group, we performed skin and muscle incisions, exposed the SS and IS tendons and suprascapular nerve, and then closed the muscle and skin. To stimulate BAT activity, amibegron (SR-58611A, SML1070–25MG; Sigma-Aldrich, Burlington, MA, USA) was administered to C57BL/6J mice either at the same time as surgery (immediate treatment) or 6 weeks after surgery (delayed treatment) through daily intraperitoneal injections for 6 weeks (5 days per week). Amibegron was dissolved in 5% dimethyl sulfoxide (DMSO) in saline solution. To evaluate the effect of amibegron on preventing and reversing FI in muscle, we set 2 starting time points. According to previous literature, we used 2 doses of amibegron for immediate treatment: 1 mg/kg and 10 mg/kg.41,50 In the delayed-treatment groups, we used 10 mg/kg based on our pilot experiments. Control-group mice were injected with 5% DMSO in saline solution.All experiments were approved by our local Institutional Animal Care and Use Committee.
Gait data analysis
We conducted DigiGait (Mouse Specifics, Quincy, MA, USA) analysis as described previously to measure the forelimb function at 6 weeks or 12 weeks (for the 6-week delayed-treatment groups) after surgery.3 All mice walked at 10 cm/s for 10 seconds on the DigiGait system. To assess forelimb function, we normalized stride length, stance width, and paw area at the peak stance to the animal’s weight and reported these values as the percentage of body weight.8,35,48
Muscular harvesting and histologic analysis
Mice were killed humanely at 6 weeks or 12 weeks (for the 6-week delayed-treatment groups) after surgery. The wet weight of the bilateral SS muscle was measured as follows: ([SS on right side – SS on left side]/SS on left side) × 100%.13,48 Muscles were subsequently flash frozen and underwent cryosection at a thickness of 10 μm. Oil Red O staining (Sigma-Aldrich) was conducted to evaluate FI as described previously.29,48 For immunostaining, after fixation in 4% paraformaldehyde and rinsing in phosphate-buffered saline solution (PBS), sections were incubated in blocking solution (0.3% Triton X-100 and 5% bovine serum albumin in PBS) for 1 hour and then incubated with the primary antibodies (anti-laminin [L9393; Sigma-Aldrich], diluted 1:500, and anti–UCP-1 [sc-6529; Santa Cruz Biotechnology, Santa Cruz, CA, USA], diluted 1:50) at 4○C for >15 hours. After PBS rinses, the sections were treated with secondary antibodies (diluted 1:250) for 2 hours. For PDGFRα-GFP reporter mice, the secondary antibodies were Alexa Fluor 594–conjugated anti-rabbit IgG (ab150076) and Alexa Fluor 647–conjugated anti-goat IgG (ab150131) (Alexa Fluor, Thermo Fischer Scientific). For C57BL/6J mice, the secondary antibodies were Alexa Fluor 488–conjugated anti-rabbit IgG (ab150077) and Alexa Fluor 594–conjugated anti-goat IgG (ab150140). After a PBS rinse, we mounted the slides using VectaShield (VectaShield, Vector Laboratories, Burlingame, CA, USA) with DAPI (4’,6-diamidino-2-phenylindole) and coverslips.
Image capture and data quantification
Histologic pictures were obtained with the Axio Imager 2 microscope (Zeiss, Oberkochen, Germany). Pictures were captured with a Zeiss digital camera and analyzed using ImageJ (National Institutes of Health, Bethesda, MD) as described previously.29 All images were evaluated by 2 researchers blinded to treatment group. The cross-sectional area (CSA) was measured for muscle fibers from the mid bellies of muscles. Five slides from different places in the muscle belly were measured for 1 sample (600–2000 fibers). CSA loss was calculated as follows: ([CSA on right side – CSA on left side]/CSA on left side) × 100%. The fat area fraction was evaluated by dividing the Oil Red O–stained area by the entire sample area. The UCP-1 area fraction was assessed by dividing the UCP-1–stained area by the entire sample area.48 The ratio of PDGFRα-GFP and UCP-1 double-positive cells was calculated by dividing the number of double-positive cells by the number of PDGFRα-GFP–positive cells’ and multiplying by 100%. The ratio of PDGFRα-GFP–negative UCP-1–positive cells and PDGFRα-GFP–positive UCP-1–positive cells in the TT + DN group was calculated by dividing the number of PDGFRα-GFP–negative UCP-1–positive cells or the number of PDGFRα-GFP–positive UCP-1–positive cells by the total number of UCP-1–positive cells and multiplying by 100%.
Statistical analysis
Analyses of variance with Tukey post hoc comparisons were applied to determine the statistical difference in wet muscle weight loss, CSA loss, fat area fraction, UCP-1 area fraction, ratio of PDGFRα and UCP-1 double-positive cell number, and gait analysis findings among all groups in each experiment. Analysis of variance is an appropriate method for comparison of >2 group means. Tukey post hoc comparisons can help compare the differences between 2 group. All data were presented as mean ± standard deviation. A statistically significant difference was indicated at P < .05.
Results
PDGFRα-positive FAPs express UCP-1 during development of FI
SS muscle from PDGFRα-GFP reporter mice showed increased PDGFRα-positive FAPs at 6 weeks after TT + DN when compared with the sham group. For male mice, the ratio of PDGFRα-GFP and UCP-1 double-positive cells was 23.28% ± 3.12% in the TT + DN group vs. 0.15% in the sham group (P <.001) (Fig. 1, A and B). For female mice, the ratio of PDGFRα-GFP and UCP-1 double-positive cells was 25.22% ± 3.66% in the TT + DN group vs. 0.23% ± 0.09% in the sham group (P < .001) (Fig. 1, C and D). Although there were almost no UCP-1–positive FAPs in the SS after sham surgery, >20% of FAPs stained positive for UCP-1 after massive RC tears. The majority of UCP-1–positive cells overlapped with PDGFRα-GFP signal. No sex difference was found (P >.05) (Fig. 1).
Figure 1.
Immunofluorescent staining (original magnification ×200) of supraspinatus in PDGFRα-GFP reporter mice. (A-D) Compared with sham surgery, PDGFRα-GFP–positive cells and uncoupling protein 1 (UCP-1) expression were significantly increased in the supraspinatus at 6 weeks after TT + DN surgery. UCP-1 is pink; PDGFRα-GFP, green; laminin, red; and DAPI (4’,6-diamidino-2-phenylindole), blue. (E) Ratio of PDGFRα-GFP and UCP-1 double-positive cells, calculated as the number of double-positive cells divided by the total number of PDGFRα-GFP–positive cells. (F) In the TT + DN group, the ratio of PDGFRα-GFP–negative UCP-1–positive cells was calculated as the number of PDGFRα-GFP–negative UCP-1–positive cells divided by the total number UCP-1–positive cells. Likewise, the ratio of PDGFRα-GFP–positive UCP-1–positive cells was calculated as the number of PDGFRα-GFP–positive UCP-1–positive cells divided by the total number of UCP-1–positive cells. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Knocking out UCP-1 results in increased RC muscle atrophy and FI and decreased limb function
To test the functional role of BAT in RC muscle FI, we used age- and sex-matched UCP-1 KO mice, which had impaired BAT function, and their colony control C57BL/6J WT mice in this study. Although both WT and UCP-1 KO mice had remarkable muscle weight loss, FI, and forelimb function reduction after TT + DN, UCP-1 KO mice had significantly increased muscle weight loss and muscle fiber CSA reduction compared with their control WT mice. Muscle weight loss measured −46.16% ± 4.17% in WT male mice in the TT + DN group vs. −54.02% ± 2.45% in UCP-1 KO male mice (P = .007); it measured −42.52% ± 5.61% in WT female mice vs. −52.97% ± 3.25% in UCP-1 KO female mice (P =.007). CSA loss measured −51.77% ± 4.44% in WT male mice in the TT + DN group vs. −59.70% ± 4.93% in UCP-1 KO male mice (P = .028); it measured −54.65% ± 2.81% in WT female mice vs. −60.71% ± 2.51% in UCP-1 KO female mice (P = .007) (Fig. 2). UCP-1 KO mice had significantly increased FI after TT + DN when compared with WT mice. The fat area fraction measured 16.92% ± 2.13% in WT male mice in the TT + DN group vs. 20.95% ± 1.04% in UCP-1 KO male mice (P = .005); it measured 15.55% ± 2.58% in WT female mice vs. 20.55% ± 3.10% in UCP-1 KO female mice (P = .024) (Fig. 3). UCP-1 KO mice exhibited worse forelimb function after TT + DN when compared with WT mice (Fig. 4). No sex difference was detected (P >.05).
Figure 2.
Muscle weight loss and muscle fiber cross-sectional area loss in supraspinatus in wild-type (WT) and uncoupling protein 1 (UCP-1) knockout (KO) mice. Muscle weight loss and cross-sectional area loss were more severe in the TT + DN group of UCP-1 KO mice when compared with the TT + DN group of wild-type mice. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Figure 3.

(A-H) Oil Red O staining (original magnification ×200) of supraspinatus in wild-type (WT) and uncoupling protein 1 (UCP-1) knockout (KO) mice. Significant fatty infiltration was found in the supraspinatus muscles in the TT + DN groups of both WT and UCP-1 KO mice. (I) A higher fat area fraction was found in the supraspinatus in the TT + DN group of UCP-1 KO mice when compared with the TT + DN group of wild-type mice. Fat area fraction (as a percentage) was calculated as the area of Oil Red O staining divided by the entire sample area. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Figure 4.
Gait analysis. On the basis of the normalized data of stride length, stance width, and paw area at peak stance, the TT + DN group of uncoupling protein 1 (UCP-1) knockout (KO) mice had worse forelimb function than the TT + DN group of wild-type (WT) mice. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Amibegron reduces SS muscle atrophy and FI by promoting BAT activity
To test the role of β3-adrenergic receptor agonists in treating RC muscle atrophy and FI after massive tendon tears, we administered amibegron (dissolved in 5% DMSO) to C57BL/6J WT mice immediately after TT + DN surgery at a dose of either 1 mg/kg or 10 mg/kg. Six weeks after daily intraperitoneal injections, mice receiving 10-mg/kg amibegron showed significantly reduced muscle weight loss and muscle fiber CSA reduction compared with the DMSO-treated control group. Muscle weight loss measured −31.56% ± 4.99% in male mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. −43.26% ± 2.88% in male mice in the TT + DN group immediately receiving vehicle (P = .002); it measured −31.05% ± 4.21% in female mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. −44.49% ± 3.33% in female mice in the TT + DN group immediately receiving vehicle (P < .001). CSA loss measured −42.72% ± 7.31% in male mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. −55.73% ± 8.98% in male mice in the TT + DN group immediately receiving vehicle (P = .036); it measured −40.91% ± 6.42% in female mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. −57.24% ± 5.85% in female mice in the TT + DN group immediately receiving vehicle (P =.003) (Fig. 5).
Figure 5.
Muscle weight loss and muscle fiber cross-sectional area (CSA) loss in supraspinatus in wild-type mice undergoing sham surgery vs. TT + DN surgery combined immediately with treatment. All groups that underwent TT + DN surgery combined immediately with 5% dimethyl sulfoxide (DMSO), 1-mg/kg amibegron, and 10-mg/kg amibegron had significant muscle weight loss and CSA loss when compared with the group that underwent sham surgery combined with 5% DMSO. However, the group that underwent TT + DN surgery combined immediately with 10-mg/kg amibegron showed improved muscle weight loss and CSA loss when compared with the groups that underwent TT + DN surgery combined immediately with 5% DMSO and 1-mg/kg amibegron. Solid lines indicate P < .05, and dashed lines indicate P <.01. Error bars indicate standard deviations.
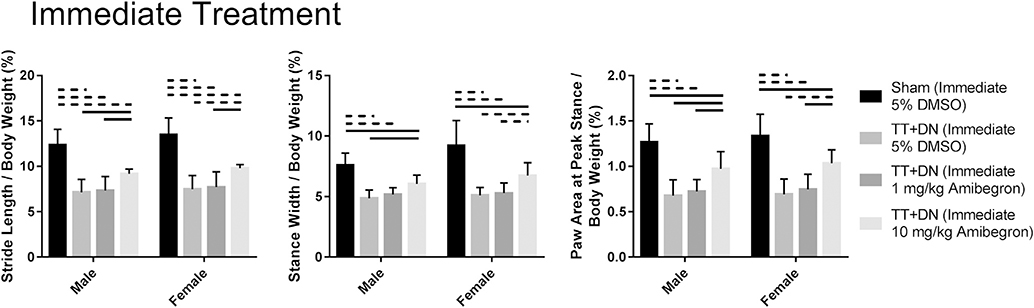
Animals receiving 10-mg/kg amibegron also had significantly reduced FI and increased UCP-1 expression compared with vehicle-treated controls. The fat area fraction was 5.03% ± 1.81% in male mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. 16.49% ± 3.45% in male mice in the TT + DN group immediately receiving vehicle (P < .001); it was 5.26% ± 0.98% in female mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. 15.84% ± 2.80% in female mice in the TT + DN group immediately receiving vehicle (P < .001). The UCP-1 area fraction measured 5.12% ± 0.85% in male mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. 2.83% ± 0.47% in male mice in the TT + DN group immediately receiving vehicle (P < .001); it measured 5.32% ± 0.86% in female mice in the TT + DN group immediately receiving 10-mg/kg amibegron vs. 2.93% ± 0.50% in female mice in the TT + DN group immediately receiving vehicle (P < .001) (Fig. 6). Daily injection of 10-mg/kg amibegron also significantly improved forelimb function compared with the vehicle control group when assessed with the DigiGait system (Fig. 7). No sex difference was detected (P > .05).
Figure 6.
Histologic staining in immediate treatment groups. (A-H) Oil Red O staining (original magnification ×200) showed significant fatty infiltration in the supraspinatus muscles in the groups that underwent TT + DN surgery combined immediately with 5% dimethyl sulfoxide (DMSO) and 1-mg/kg amibegron. However, fatty infiltration was significantly recovered in the group that underwent TT + DN surgery combined immediately with 10-mg/kg amibegron. (I-P) Immunofluorescent staining (original magnification ×200) indicated that, compared with the other 3 groups, the group that underwent TT + DN surgery combined immediately with 10-mg/kg amibegron showed significantly increased uncoupling protein 1 (UCP-1) expression. UCP-1 is red; laminin, green; and DAPI (4’,6-diamidino-2-phenylindole), blue. (Q) The fat area fraction (as a percentage) was calculated as the area of Oil Red O staining divided by the entire sample area. (R) The UCP-1 area fraction (as a percentage) was calculated as the area of UCP-1 staining divided by the entire sample area. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Figure 7.
Gait analysis. All groups that underwent TT + DN surgery combined immediately with treatment had inferior forelimb function when compared with the group that underwent sham surgery combined with 5% dimethyl sulfoxide (DMSO). However, the group that underwent TT + DN surgery combined immediately with 10-mg/kg amibegron had improved forelimb function when compared with the groups that underwent TT + DN surgery combined immediately with 5% DMSO and 1-mg/kg amibegron. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Amibegron reverses SS muscle atrophy and FI by promoting BAT activity
To test if β3-adrenergic receptor agonist can reverse FI in RC muscle, we administered amibegron at a dose of 10 mg/kg to C57BL/6J WT mice at 6 weeks after TT + DN surgery, when muscle atrophy and FI had already developed. We treated the animals with daily intraperitoneal injections for another 6 weeks. After 6 weeks’ treatment, animals receiving 10-mg/kg amibegron showed significantly reduced muscle weight loss and fiber CSA reduction compared with the DMSO-treated control group. Muscle weight loss measured −38.53% ± 5.87% in male mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. −51.01% ± 2.91% in male mice in the TT + DN group receiving vehicle after a 6-week delay (P = .003); it measured −39.48% ± 6.30% in female mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. −50.44% ± 5.48% in female mice in the TT + DN group receiving vehicle after a 6-week delay (P = .019). CSA loss measured −48.91% ± 8.62% in male mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. −59.31% ± 3.31% in male mice in the TT + DN group receiving vehicle after a 6-week delay (P = .036); it measured −46.84% ± 5.67% in female mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. −56.84% ± 7.62% in female mice in the TT + DN group receiving vehicle after a 6-week delay (P = .046) (Fig. 8).
Figure 8.
Muscle weight loss and muscle fiber cross-sectional area (CSA) loss in supraspinatus in wild-type mice that underwent sham and TT + DN surgery combined with treatment after a 6-week (w) delay. All groups that underwent TT + DN surgery combined with 5% dimethyl sulfoxide (DMSO) and 10-mg/kg amibegron after a 6-week delay had significant muscle weight loss and CSA loss when compared with the group that underwent sham surgery combined with 5% DMSO after a 6-week delay. However, the group that underwent TT + DN surgery combined with 10-mg/kg amibegron after a 6-week delay had improved muscle weight loss and CSA loss when compared with the group that underwent TT + DN surgery combined with 5% DMSO after a 6-week delay. Solid lines indicate P < .05, and dashed linesf indicate P < .01. Error bars indicate standard deviations.
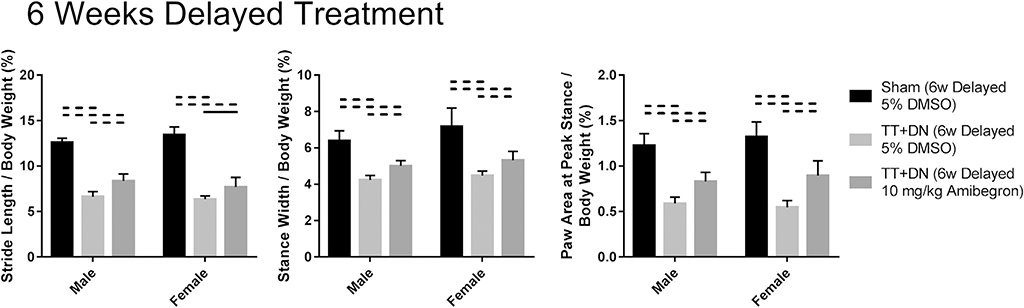
Delayed amibegron treatment also significantly reduced FI and increased UCP-1 expression in SS muscle compared with the DMSO-treated control group. The fat area fraction was 9.77% ± 3.30% in male mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. 18.71% ± 3.47% in male mice in the TT + DN group receiving vehicle after a 6-week delay (P = .003); it was 10.01% ± 3.93% in female mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. 18.55% ± 1.74% in female mice in the TT + DN group receiving vehicle after a 6-week delay (P = .002). The UCP-1 area fraction measured 4.18% ± 0.91% in male mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. 2.44% ± 0.54% in male mice in the TT + DN group receiving vehicle after a 6-week delay (P = .006); it measured 4.13% ± 0.56% in female mice in the TT + DN group receiving 10-mg/kg amibegron after a 6-week delay vs. 2.46% ± 0.20% in female mice in the TT + DN group receiving vehicle after a 6-week delay (P <.001) (Fig. 9). Amibegron also significantly improved forelimb function compared with the DMSO-treated control group when assessed with the DigiGait system (Fig. 10). No sex difference was detected (P >.05).
Figure 9.
Histologic staining in delayed-treatment groups. (A-F) Oil Red O staining (original magnification ×200) showed significant fatty infiltration in the supraspinatus muscles from the group that underwent TT + DN surgery combined with 5% dimethyl sulfoxide (DMSO) after a 6-week delay (w). However, fatty infiltration was significantly reduced in the group that underwent TT + DN surgery combined with 10-mg/kg amibegron after a 6-week delay. (G-L) Immunofluorescent staining (original magnification ×200) indicated that, compared with the other 2 groups, the group with TT + DN surgery combined with 10-mg/kg amibegron showed significantly increased uncoupling protein 1 (UCP-1) expression. UCP-1 is red; laminin, green; and DAPI (4’,6-diamidino-2-phenylindole), blue. (M) The fat area fraction (as a percentage) was calculated as the area of Oil Red O staining divided by the entire sample area. (N) The UCP-1 area fraction (as a percentage) was calculated as the area of UCP-1 staining divided by the entire sample area. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Figure 10.
Gait analysis. All groups that underwent TT + DN surgery combined with treatment after a 6-week delay had inferior forelimb function when compared with the group that underwent sham surgery combined with 5% dimethyl sulfoxide (DMSO). However, the group that underwent TT + DN surgery combined with 10-mg/kg amibegron after a 6-week delay also had better forelimb function when compared with the group that underwent TT + DN surgery combined with 5% DMSO after a 6-week delay. Solid lines indicate P < .05, and dashed lines indicate P < .01. Error bars indicate standard deviations.
Discussion
The results from this study in a mouse model demonstrate that fat accumulated in muscle after RC tears possesses BAT characteristics. Promoting BAT activity with amibegron, a selective β3-adrenergic receptor agonist, can reduce and reverse RC atrophy and FI. This result challenges the current concept that RC muscle FI is irreversible. If the results are proved in humans, β3-adrenergic receptor agonists could serve as a new treatment for muscle atrophy and FI in patients with RC injuries.
Previous studies from our group demonstrated that FAPs are the primary cellular source of fat in RC muscles after RC tears.30 In this study, we further demonstrated that FAPs can adopt a BAT phenotype during the development of FI, as evidenced by expression of UCP-1, the hallmark of BAT. Tracing the cellular origin of human RC muscle FI is difficult. However, similarities between human and mouse FAPs reported in previous studies1,43 allow us to reasonably hypothesize that FAPs are the primary cellular source in RC muscle FI in humans as well. Similar to mouse FAPs, FAPs from human muscle have been found to be able to express UCP-1.18,22 Future work is needed to determine if fat accumulated in human RC muscles possesses BAT characteristics by use of positron emission tomography–computed tomography.
β3-Adrenergic receptors are selectively expressed in adipose tissue, and selective β3-adrenergic receptor agonists can effectively stimulate BAT activity in humans without significant cardiovascular system side effects.10,11 Mirabegron, a β3-adrenergic receptor agonist with a similar structure to amibegron, has been approved by the US Food and Drug Administration for treating overactive bladder.6,38 Though mildly increasing heart rate and blood pressure in patients, mirabegron could be considered a potential new treatment for treating FI in the future. However, amibegron, which has the same effect as mirabegron with a lower cost, is more commonly used in animal experiments.
Because UCP-1 plays a critical role in beige adipogenesis, we investigated the phenotype of RC muscle atrophy and FI in mice deficient in UCP-1. We saw significantly more muscle atrophy and FI in RC muscles compared with WT mice. Impaired BAT differentiation of FAPs in UCP-1 KO mice is likely the main reason for this phenotype. However, because the strain of UCP-1 KO mice used in this study is a global KO strain, it is possible that the worsening of RC muscle FI was at least partially due to the loss of some cytokines secreted by brown fat, which was recently discovered as an endocrine organ.45,46 FAP-specific UCP-1 KO mice, when developed in the future, could provide a definitive answer to this question.
Previous clinical studies have reported a higher prevalence of RC tears in male patients than in female patients,34,51 whereas female patients have been shown to have more disability than male patients both prior to and after decompression or repair surgical procedures.31,37 In a previous study, we found that FI of the SS was related to age, sex, tear severity, and muscle atrophy; older women with severe RC tears and atrophic muscles were more likely to show substantial FI.2 To address possible sex differences in RC muscle atrophy and FI, as well as the response to β3-adrenergic receptor agonist treatment, we included both male and female animals in all groups in this study. However, we failed to see sex differences in all parameters, including muscle atrophy, FI, and UCP-1 expression level, as well as gait analysis findings. Male and female animals responded similarly to either immediate or delayed amibegron treatment. We note that the mice used in this study were relatively young and the time points assessed were relatively short. Thus, we think that that differences in species, age, and course of illness may be responsible for this discrepancy.
Some limitations of this study should be noted. First, the difference in BAT between species, that is, mice and humans, is significant. Adult mice contain a significant amount of BAT, whereas the amount of BAT in adult humans is relatively small. Most BAT in adult humans remains inactive without appropriate physical or chemical stimulation. Second, we only investigated the function of BAT in RC muscle FI after tendon tears; no tendon repair was conducted. Although there is some spontaneous reconnection of torn tendon to the humeral head by scar tissue, neither real healing of torn RCs nor complete restoration of forelimb function could be achieved without surgical repair. Thus, the effect of amibegron in treating muscle FI and improving forelimb function could be limited by the model. We have recently developed a chronic RC repair model in mice. We will test the role of amibegron in treating RC muscle FI and improving forelimb function after tendon repair in the near future. Third, because of limited supply of KO mice, we did not include UCP-1 KO mice in the amibegron treatment experiment. We will consider using FAP-specific UCP-1 KO mice when they become available in the future. We will further study the mechanism of β3-adrenergic receptor agonists in preventing and reversing FI in the near future. Clinical study of mirabegron in both acute and chronic RC tear patients using both systemic and local treatment could be considered in the future.
Conclusion
We discovered that fat accumulated in muscle after RC tears possessed BAT characteristics in our mouse model. Impairing BAT activity results in worse RC muscle atrophy and FI. Amibegron, a selective β3-adrenergic receptor agonist, can prevent and reverse RC atrophy and FI by promoting BAT activity. No sex difference was detected in all parameters tested in this study, suggesting that amibegron may work equally efficiently in both male and female animals.
Acknowledgments
Disclaimers
This work was supported by a research grant from the National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01AR072669-01A1; principal investigator [PI]: Brian T. Feeley); a merit review grant from the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (1I01BX002680; PI: Hubert Kim); and a grant from the National Natural Science Foundation of China (81902245; PI: Zili Wang). In addition, Zili Wang received support from the China Scholarship Council for this study.
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
All experiments including animal studies have been approved by the San Francisco Veterans Affairs Animal Studies Subcommittee (Institutional Animal Care and Use Committee, protocol no. 15-015-01).
References
- 1.Arrighi N, Moratal C, Clément N, Giorgetti-Peraldi S, Peraldi P, Loubat A, et al. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 2015;6:e1733 10.1038/cddis.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry JJ, Lansdown DA, Cheung S, Feeley BT, Ma CB. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg 2013;22:18–25. 10.1016/j.jse.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 3.Bell R, Taub P, Cagle P, Flatow EL, Andarawis-Puri N. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res 2015;33:25–32. 10.1002/jor.22727 [DOI] [PubMed] [Google Scholar]
- 4.Bryniarski AR, Meyer GA. Brown fat promotes muscle growth during regeneration. J Orthop Res 2019;37:1817–26. 10.1002/jor.24324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey AL, Kingwell BA. Brown adipose tissue in humans: therapeutic potential to combat obesity. Pharmacol Ther 2013;140:26–33. 10.1016/j.pharmthera.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 6.Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B, et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J 2013; 24:1447–58. 10.1007/s00192-013-2042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhury S, Dines JS, Delos D, Warren RF, Voigt C, Rodeo SA. Role of fatty infiltration in the pathophysiology and outcomes of rotator cuff tears. Arthritis Care Res (Hoboken) 2012;64:76–82. 10.1002/acr.20552 [DOI] [PubMed] [Google Scholar]
- 8.Coulthard P, Pleuvry BJ, Brewster M, Wilson KL, Macfarlane TV. Gait analysis as an objective measure in a chronic pain model. J Neurosci Methods 2002;116:197–213. 10.1016/s0165-0270(02)00042-0 [DOI] [PubMed] [Google Scholar]
- 9.Crichton PG, Lee Y, Kunji ERS. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie 2017;134:35–50. 10.1016/j.biochi.2016.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 2012;109:10001–5. 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 2015;21:33–8. 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014;134:985–90. 10.1007/s00402-014-2009-5 [DOI] [PubMed] [Google Scholar]
- 13.Eliasberg CD, Dar A, Jensen AR, Murray IR, Hardy WR, Kowalski TJ, et al. Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J Bone Joint Surg Am 2017;99:331–41. 10.2106/JBJS.16.00645 [DOI] [PubMed] [Google Scholar]
- 14.Fuchs B, Gilbart MK, Hodler J, Gerber C. Clinical and structural results of open repair of an isolated one-tendon tear of the rotator cuff. J Bone Joint Surg Am 2006;88:309–16. 10.2106/jbjs.e.00117 [DOI] [PubMed] [Google Scholar]
- 15.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2000;82:505–15. [DOI] [PubMed] [Google Scholar]
- 16.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 2013;154:2992–3000. 10.1210/en.2013-1403 [DOI] [PubMed] [Google Scholar]
- 17.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35: 719–28. 10.1177/0363546506297539 [DOI] [PubMed] [Google Scholar]
- 18.Gorski T, Mathes S, Kr€utzfeldt J. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle 2018;9:384–99. 10.1002/jcsm.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78–83. [PubMed] [Google Scholar]
- 20.Hamano N, Yamamoto A, Shitara H, Ichinose T, Shimoyama D, Sasaki T, et al. Does successful rotator cuff repair improve muscle atrophy and fatty infiltration of the rotator cuff? A retrospective magnetic resonance imaging study performed shortly after surgery as a reference. J Shoulder Elbow Surg 2017;26:967–74. 10.1016/j.jse.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 21.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19:1252–63. 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- 22.Herz CT, Kiefer FW. Adipose tissue browning in mice and humans. J Endocrinol 2019;241:R97–109. 10.1530/JOE-18-0598 [DOI] [PubMed] [Google Scholar]
- 23.Himms-Hagen J, Cui J, Danforth E Jr, Taatjes DJ, Lang SS, Waters BL, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol 1994;266:R1371–82. [DOI] [PubMed] [Google Scholar]
- 24.Joshi SK, Liu X, Samagh SP, Lovett DH, Bodine SC, Kim HT, et al. mTOR regulates fatty infiltration through SREBP-1 and PPARγ after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res 2013;31:724–30. 10.1002/jor.22254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Liu M, Agha O, Kim HT, Feeley BT, Liu X. Beige FAPs transplantation improves muscle quality and shoulder function after massive rotator cuff tears. J Orthop Res 2020;38:1159–66. 10.1002/jor.24558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, Liu M, Agha O, Kim HT, Liu X, Feeley BT. Beige fibroadipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg 2020;29:719–27. 10.1016/j.jse.2019.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y, Kim J, Oh K, Chung S. Fatty acid-binding protein 4 regulates fatty infiltration after rotator cuff tear by hypoxia-inducible factor 1 in mice. J Cachexia Sarcopenia Muscle 2017;8:839–50. 10.1002/jcsm.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am 2007;89:1770–6. 10.2106/jbjs.f.00749 [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am 2012;94:e41 10.2106/JBJS.K.00620 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 2016;6:6–15. 10.11138/mltj/2016.6.1.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maher A, Leigh W, Brick M, Young S, Millar J, Walker C, et al. Gender, ethnicity and smoking affect pain and function in patients with rotator cuff tears. ANZ J Surg 2017;87:704–8. 10.1111/ans.13921 [DOI] [PubMed] [Google Scholar]
- 32.Maman E, Harris C, White L, Tomlinson G, Shashank M, Boynton E. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am 2009;91:1898–906. 10.2106/JBJS.G.01335 [DOI] [PubMed] [Google Scholar]
- 33.Meyer GA, Gibbons MC, Sato E, Lane JG, Ward SR, Engler AJ. Epimuscular fat in the human rotator cuff is a novel beige depot. Stem Cells Transl Med 2015;4:764–74. 10.5966/sctm.2014-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop 2013;10:8–12. 10.1016/j.jor.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardes AM, Freedman BR, Soslowsky LJ. Ground reaction forces are more sensitive gait measures than temporal parameters in rodents following rotator cuff injury. J Biomech 2016;49:376–81. 10.1016/j.jbiomech.2015.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potter R, Havlioglu N, Thomopoulos S. The developing shoulder has a limited capacity to recover after a short duration of neonatal paralysis. J Biomech 2014;47:2314–20. 10.1016/j.jbiomech.2014.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razmjou H, Davis AM, Jaglal SB, Holtby R, Richards RR. Disability and satisfaction after rotator cuff decompression or repair: a sex and gender analysis. BMC Musculoskelet Disord 2011;12:66 10.1186/1471-2474-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossanese M, Novara G, Challacombe B, Iannetti A, Dasgupta P, Ficarra V. Critical analysis of phase II and III randomised control trials (RCTs) evaluating efficacy and tolerability of a β3-adrenoceptor agonist (mirabegron) for overactive bladder (OAB). BJU Int 2015;115: 32–40. 10.1111/bju.12730 [DOI] [PubMed] [Google Scholar]
- 39.Sampath SC, Sampath SC, Bredella MA, Cypess AM, Torriani M. Imaging of brown adipose tissue: state of the art. Radiology 2016;280: 4–19. 10.1148/radiol.2016150390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 2011;108: 143–8. 10.1073/pnas.1010929108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, et al. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem 1995;270:29483–92. [DOI] [PubMed] [Google Scholar]
- 42.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143–52. 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- 43.Uezumi A, Kasai T, Tsuchida K. Identification, isolation, and characterization of mesenchymal progenitors in mouse and human skeletal muscle. Methods Mol Biol 2016;1460:241–53. 10.1007/978-1-4939-3810-0_17 [DOI] [PubMed] [Google Scholar]
- 44.Ursino MG, Vasina V, Raschi E, Crema F, Ponti FD. The beta3-adrenoceptor as a therapeutic target: current perspectives. Pharmacol Res 2009;59:221–34. 10.1016/j.phrs.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 45.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 2017;13:26–35. 10.1038/nrendo.2016.136 [DOI] [PubMed] [Google Scholar]
- 46.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 2013;305:E567–72. 10.1152/ajpendo.00250.2013 [DOI] [PubMed] [Google Scholar]
- 47.Vosselman MJ, van Marken Lichtenbelt WD, Schrauwen P. Energy dissipation in brown adipose tissue: from mice to men. Mol Cell Endocrinol 2013;379:43–50. 10.1016/j.mce.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Feeley BT, Kim HT, Liu X. Reversal of fatty infiltration after suprascapular nerve compression release is dependent on UCP1 expression in mice. Clin Orthop Relat Res 2018;476:1665–79. 10.1097/CORR.0000000000000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Liu X, Davies MR, Horne D, Kim H, Feeley BT. A mouse model of delayed rotator cuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med 2018;46:2981–9. 10.1177/0363546518793403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Liu X, Jiang K, Kim H, Kajimura S, Feeley BT. Intramuscular brown fat activation decreases muscle atrophy and fatty infiltration and improves gait after delayed rotator cuff repair in mice. Am J Sports Med 2020;48:363546520910421 10.1177/0363546520910421 [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 2010;19:116–20. 10.1016/j.jse.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Tao Y. Physiology and pathophysiology of the β3-adrenergic receptor. Prog Mol Biol Transl Sci 2019;161:91–112. 10.1016/bs.pmbts.2018.09.003 [DOI] [PubMed] [Google Scholar]