Abstract
Contemporary studies of long-term outcomes in children supported on extracorporeal membrane oxygenation (ECMO) in the U.S. are limited. We enrolled 99 ECMO patients between July 2010 and June 2015 in a two-center prospective observational study that included neurologic and neuropsychological evaluation at 6 and 12 months, using standardized outcome measures. Pre-ECMO, 20 (20%) had a pre-existing neurologic diagnosis, 40 (40%) had cardiac arrest, and 10 of 47 (21%) children with neuroimaging had acute abnormal findings. Of 50 children eligible for follow-up at 6 or 12 months, 40 (80%) returned for at least one visit. At the follow-up visit of longest interval from ECMO, the median Vineland Adaptive Behavior Scales-II (VABS-II) score was 91 (interquartile range [IQR], 81-98), the median Pediatric Stroke Outcome Measure (PSOM) score was 1 (IQR, 0-2), and the median Mullen Scales of Early Learning composite score was 85 (IQR, 72-96). Presence of new neuroimaging abnormalities during ECMO or within 6 weeks post-ECMO was associated with VABS-II score <85 or death within 12 months after ECMO. The Pediatric Cerebral Performance Category at hospital discharge showed a strong relationship with unfavorable VABS-II and PSOM scores at 6 or 12 months after ECMO. In this study, we report a higher prevalence of pre-ECMO neurologic conditions than previously described. In survivors to hospital discharge, median scores for adaptive behavior and cognitive, neurologic, and quality of life assessments were all below the general population means, but most deficits would be considered minor within each of the domains tested.
Keywords: extracorporeal life support, ECLS, extracorporeal membrane oxygenation, ECMO, child, outcome assessment (healthcare), quality of life
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a well-established life support technique that was first employed in intensive care units (ICUs) in 1972 to support patients with severe, refractory, cardiopulmonary failure and circulatory collapse.1,2 Though resource-intensive, costly, and high-risk, ECMO is life-saving in select populations. With advances in technology, ECMO utilization has doubled in children and increased 10-fold in adults in the last decade. More than 2,500 pediatric and 4,500 adult cases were reported to the Extracorporeal Life Support Organization (ELSO) registry yearly in 2015 and 2016.2 Patients who are cannulated onto ECMO are sicker than they were historically, yet, despite the increase in cases, mortality has remained stable during the same period at 35–45%.2
As many as 15–36% of ECMO patients suffer neurologic injury surrounding their ECMO course, including hypoxic-ischemic injury, thromboembolic stroke, and intracranial hemorrhage.3–6 Acute neurologic injury during ECMO is associated with an 89% increase in risk of mortality,4 and neurologic disability among survivors has been reported at a rate of 10–60%.7–10 Neurologic disability in former ECMO patients manifests as motor deficits,11 behavior problems, diminished processing speed,12 and verbal, visuospatial, and working memory deficits.13 Global cognitive ability in most survivors appears to be less affected than other neurofunctional domains.11,12,14 While the pathophysiology of neurodevelopmental impairment in ECMO patients is multifactorial, the added risk of ECMO to the baseline risk for neurologic injury in critically ill children has been documented in several patient populations, including children with congenital diaphragmatic hernia and congenital heart disease.15,16 Given our inability to stratify risk and classify outcomes early in the ECMO course, no attempts have been made to implement interventional trials for neuroprotective therapies during ECMO, with the exception of a negative randomized clinical trial of mild hypothermia in neonatal ECMO conducted in the U.K.17 Most published data on long-term outcomes after neonatal and pediatric ECMO in the last 15 years originate from outside the U.S., from cohorts in Canada, the U.K., and the Netherlands.12–14,16,18–20
The aims of this study were to determine the burden of acute neurologic injury surrounding the ECMO course in a contemporary cohort of infants and children in two U.S. centers, and to investigate the association between patient and ECMO course characteristics, survival, and 12-month neurologic, neuropsychological, and health-related quality of life (HRQOL) outcomes.
METHODS
Study Design and Patients
In this cohort study, we prospectively screened and enrolled consecutive children (age 0 to <18 years) who underwent ECMO for any indication at two academic, urban, pediatric ICUs between July 2010 and June 2015. We excluded patients cannulated at an outside hospital and transported to a study site >24 hours after cannulation, patients with ECMO duration <6 hours, patients whose guardians discussed withdrawal of support with the clinical team within 24 hours from cannulation, non-index ECMO cannulation during the same hospitalization, and patients who were in foster care or wards of state at the time of cannulation. We obtained written informed consent from guardians within 24 hours of ECMO cannulation. This study was approved by the Institutional Review Boards at both participating sites.
We collected demographic, clinical, and neuroimaging data prospectively during the ECMO course and up to 6 weeks after ECMO decannulation. We recorded the survival status at hospital discharge and performed in-person neurodevelopmental assessments of consenting survivors prospectively at 6 and 12 months after ECMO decannulation. Neuropsychological tests from both sites were scored at the Kennedy Krieger Institute by a pediatric neuropsychologist (CS). Clinical neuroimaging studies with head ultrasound (HUS), brain computed tomography (CT), and/or brain magnetic resonance imaging (MRI) were reviewed by a pediatric neuroradiologist (AT) blinded to patient outcomes.
The analysis included two primary outcomes in two stages. The first stage described factors related to in-hospital mortality among all children. The second stage investigated, among those who survived to discharge, the presence of unfavorable neuropsychological outcomes or death up to 12 months after ECMO initiation. Secondary outcomes were other neuropsychological and HRQOL scores at 6 and 12 months after ECMO decannulation, as described below.
Outcome Assessment Measures
Trained study personnel at both sites conducted in-person parent interviews regarding adaptive function with the Vineland Adaptive Behavior Scales-II (VABS-II)21,22 The VABS-II measures functional skills and provides age-corrected standard scores from birth to age 18 years (mean = 100, standard deviation [SD] = 15) for overall adaptive behavior composite (ABC) and four domains (communication, daily living, socialization, motor skills), with higher scores indicating higher adaptive behavior function.
Psychology associates at only one site administered the Mullen Scales of Early Learning (MSEL)23 and the Wechsler Intelligence Scale for Children-IV (WISC-IV)24 to test general cognitive ability in children <6 years and children ≥6 years, respectively. The MSEL is a measure of cognitive functioning for younger children with four primary scales that contribute to the total composite (visual reception, fine motor, receptive language, expressive language). Normative data and age-corrected standard scores are available from birth through age 5-years-8-months. Age-corrected standardized scores are available for each scale (t-scores [mean 50, SD 10]) and overall early learning composite (standard score [mean = 100, SD = 15]). The WISC-IV measures global intellectual functioning with a full scale intellectual quotient (IQ) score. Age-corrected standardized scores are available for children ages 6 to 16 (standard score [mean = 100, SD = 15]), with higher scores indicating higher cognitive function.
At both sites, HRQOL was measured using the Infant Toddler Quality of Life (ITQOL)25 questionnaire for children <2 years of age and the PedsQL26,27 questionnaire for children ≥2 years. The ITQOL has three global items and 10 multi-item subscales and measures the physical and emotional well-being of infants, toddlers, and their families.25 The PedsQL 4.0 Generic Core Scale is a 23-item questionnaire with established validity and reliability for health outcome measurements in pediatric populations ≥2 years.26,27 The PedsQL can be used as self-report in children 5–18 years and includes a parent proxy report for children between 2 and 18 years.26,27 The ITQOL and PedsQL use a 0–100 scale on which higher values reflect better HRQOL.
A standardized neurologic exam was conducted by a pediatric neurologist at both sites using the Pediatric Stroke Outcome Measure – Short Neurologic Exam (PSOM-NSE).28,29 The PSOM-NSE consists of five subdomains of neurologic function: sensorimotor – right, sensorimotor – left, language – expressive, language – receptive, and cognitive/behavioral. Each subdomain is scored as follows: 0=normal, 0.5=minimal impairment with no impact on function, 1=mild to moderate impairment with slowed function, 2=severe impairment with missing function. Each subdomain score is summed for a 10-point total, and higher scores indicate more severe neurologic deficit.28,29
Pediatric Cerebral Performance Category (PCPC)30,31 was used to assess global neurologic functioning at baseline, at hospital discharge, and at 6 and 12 months post-ECMO. The PCPC is a 6-point scale developed from the Glasgow Outcome Scales to assess changes in cognitive abilities in pediatric intensive care for children 0 to <18 years of age. The 6 categories are: 1 – normal, 2 – mild cerebral disability, 3 – moderate cerebral disability, 4 – severe cerebral disability, 5 – coma or vegetative state, and 6 – brain death.30,31
Statistical Analysis
Descriptive statistics summarizing patient and ECMO course characteristics are presented overall and stratified by in-hospital mortality. The primary outcome was in-hospital mortality, and the secondary outcome among survivors to hospital discharge was the VABS-II ABC Standardized Score dichotomized at 85 (i.e., −1 SD below the reference population mean). Study participants who survived to discharge were stratified by status at follow-up and those who did not complete a follow-up visit. Univariate associations between patient and ECMO course characteristics and outcomes were examined with the Wilcoxon’s rank sum and chi-square tests. To assess the relationships of characteristics before and during ECMO, we constructed logistic regression models with in-hospital mortality as the outcome for the full cohort. Next, we restricted analysis to those who survived to discharge and contributed follow-up data, and used the same logistic models with outcomes of VABS-II ABC < 85 or death (total n=43). Although this composite outcome does not discriminate between low VABS-II ABC and death, very few died after hospital discharge (n=3). Unadjusted models compared the univariate associations with each outcome; minimally adjusted models included age, gender, and primary ECMO indication (respiratory failure, cardiac failure, extracorporeal cardiopulmonary resuscitation [ECPR], and sepsis) as covariates separately. The other variables included pre-ECMO cardiac arrest and the presence of a new neuroimaging abnormality during ECMO or within 6 weeks post-ECMO. To assess the correlation of PCPC scores at discharge with scores at 6 and 12 months, we calculated the weighted kappa statistic. Overall differences between VABS-II scores and PSOM scores across PCPC scores at discharge were compared by one-way ANOVA. All analyses were conducted with SAS 9.4 software (SAS Institute, Cary, NC, USA).
RESULTS
Of 179 ECMO patients who were screened, 153 were eligible and 99 were enrolled in the study (65% enrollment rate; Figure 1). Median age was 35 days (interquartile range [IQR], 2 days-20 months), and 54 (55%) were male. ECMO course characteristics, neuroimaging and neurologic adverse events are presented below and in Tables 1 and 2.
Figure 1.

Study flowchart. ECMO, extracorporeal membrane oxygenation
Table 1.
Demographic and Clinical Characteristics of ECMO Cohort by Outcome Statusa
| Variable | All (n=99) |
Died in hospital (n=42) |
Survived to discharge (n=57) |
p-valueb | ||
|---|---|---|---|---|---|---|
| VABS-II <85 or death within 12 months after ECMO (n=17) |
VABS-II ≥85 (n=26) |
No follow-up data (n=14) |
||||
| Age group | 0.894 | |||||
| Neonate (<30 d) | 51 (52) | 23 (55) | 7 (41) | 16 (62) | 5 (36) | |
| Infant (30 d to <1 y) | 21 (21) | 9 (21) | 5 (29) | 3 (12) | 4 (29) | |
| Child (1 to <12 y) | 19 (19) | 7 (17) | 4 (24) | 3 (12) | 5 (36) | |
| Adolescent (≥12 y) | 8 (8) | 3 (7) | 1 (6) | 4 (15) | 0 (0) | |
| Male | 54 (55) | 24 (57) | 10 (59) | 14 (54) | 6 (43) | 0.687 |
| Race | 0.924 | |||||
| Caucasian | 51 (52) | 21 (50) | 8 (47) | 16 (62) | 6 (43) | |
| African American | 33 (33) | 15 (36) | 5 (29) | 8 (31) | 5 (36) | |
| Other race | 15 (15) | 6 (14) | 4 (24) | 2 (8) | 3 (21) | |
| Hispanic | 9 (9) | 4 (10) | 2 (12) | 1 (4) | 2 (14) | 1.000 |
| Weight (kg) | 4.0 [3.1, 11.8] | 3.9 [3, 8] | 4.5 [3.3, 10] | 3.8 [3.4, 12.0] | 7.8 [3.8, 16.4] | 0.237 |
| Primary indication for ECMO | 0.012 | |||||
| Cardiac failure | 39 (39) | 20 (48) | 7 (41) | 8 (31) | 4 (29) | |
| Respiratory failure | 33 (33) | 8 (19) | 6 (35) | 13 (50) | 6 (43) | |
| Sepsis | 8 (8) | 2 (5) | 1 (6) | 3 (12) | 2 (14) | |
| ECPR | 19 (19) | 12 (29) | 3 (18) | 2 (8) | 2 (14) | |
| Gestational age (weeks) (n=47) | 38 [37, 39] | 37.5 [37, 39] | 39 [37, 39] | 38 [37, 39] | 39 [39, 40] | 0.088 |
| Chromosomal defect | 18 (18) | 10 (24) | 5 (29) | 2 (8) | 1 (7) | 0.292 |
| Pre-ECMO neurologic diagnosisc | 20 (20) | 9 (21) | 6 (35) | 2 (8) | 3 (21) | 0.805 |
| Cardiac arrest within 7 days pre-ECMO | 40 (40) | 22 (52) | 4 (24) | 8 (31) | 6 (43) | 0.041 |
| Total duration of cardiopulmonary resuscitation (minutes) | 24 [5, 59] | 12 [5, 59] | 39 [24, 72] | 31 [10, 49] | 21 [1, 40] | 0.640 |
| Pre-ECMO inotrope use | 78 (80) | 32 (78) | 14 (82) | 20 (77) | 12 (86) | 0.802 |
| Inotropic score | 13.5 [0, 30] | 10 [0, 25] | 11 [5, 20] | 20 [0, 32] | 17.5 [3, 40] | 0.167 |
| Vasoactive inotropic score | 11.5 [5, 30] | 10 [4, 25] | 15 [6, 20] | 22.5 [0, 33] | 13.8 [5, 35] | 0.226 |
Median [interquartile range] or n (%).
P-value for difference between those who died in hospital and all survivors.
Pre-ECMO neurologic diagnosis: seizure disorder (6), neonatal encephalopathy (6), developmental delay (7), static encephalopathy (1), rabdoid tumor (1), Coffin Siris syndrome (1), tuberous sclerosis (1), agenesis of the corpus callosum (1).
ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Table 2.
ECMO and Hospital Course Characteristics by Outcome Statusa
| Variable | All (n=99) |
Died in hospital (n=42) |
Survived to discharge (n=57) |
p-valueb | ||
|---|---|---|---|---|---|---|
| VABS-II <85 or death within 12 months after discharge (n=17) |
VABS-II ≥85 (n=26) |
No follow-up data (n=14) |
||||
| Mode of ECMO | 0.233 | |||||
| Venoarterial | 92 (93) | 41 (98) | 16 (94) | 23 (88) | 12 (86) | |
| Venovenous | 7 (7) | 1 (2) | 1 (6) | 3 (12) | 2 (14) | |
| Duration of ECMO support (days) | 4.8 [3.2, 10.5] |
6.6 [3.2, 13.5] |
3.9 [3.1, 5.7] |
5.0 [2.6, 8.0] |
4.4 [3.5, 12.1] |
0.071 |
| Reason for ECMO discontinuation | <0.001 | |||||
| Improved organ function | 74 (75) | 19 (45) | 17 (100) | 25 (96) | 13 (93) | |
| Withdrawal of life support | 20 (20) | 20 (48) | 0 (0) | 0 (0) | 0 (0) | |
| Died on ECMO | 3 (3) | 3 (7) | 0 (0) | 0 (0) | 0 (0) | |
| Otherc | 2 (2) | 0 (0) | 0 (0) | 1 (4) | 1 (7) | |
| Clinical seizures during ECMO | 10 (10) | 9 (21) | 1 (6) | 0 (0) | 0 (0) | 0.002 |
| EEG obtained during ECMO | 43 (43) | 22 (52) | 8 (47) | 7 (27) | 6 (43) | 0.153 |
| Electrographic seizures, n=43 | 12 (29) | 10 (45) | 1 (14) | 1 (14) | 0 (0) | 0.019 |
| Neuroimaging during ECMOd | 75 (76) | 36 (86) | 11 (65) | 18 (69) | 10 (71) | 0.059 |
| Neuroimaging during or after ECMOe | 88 (89) | 38 (90) | 15 (88) | 22 (85) | 13 (93) | 0.755 |
| Neuroimaging abnormalities | 44 (50) | 20 (53) | 11 (73) | 8 (36) | 5 (38) | 0.830 |
| Embolic infarction | 20 (23) | 8 (21) | 5 (33) | 4 (18) | 3 (23) | |
| Intracranial hemorrhage | 32 (36) | 12 (32) | 10 (67) | 6 (27) | 4 (31) | |
| Postasphyxial brain injury | 17 (19) | 12 (32) | 2 (13) | 2 (9) | 1 (8) | |
| New tracheostomy | 6 (6) | 1 (2) | 1 (6) | 0 (0) | 4 (29) | 0.237 |
| New gastrostomy tube | 17 (17) | 3 (7) | 5 (29) | 4 (15) | 5 (36) | 0.030 |
| PICU length of stay (days) | 28 [12, 50] | 27.5 [8, 53] | 39 [26, 92] | 19 [12, 35] | 33.5 [12, 50] | 0.598 |
| Hospital length of stay (days) | 47 [23, 81] | 27.5 [11, 60] | 81 [47, 107] | 47 [26, 66] | 63.5 [28, 148] | <0.001 |
| Home discharge | 27 (47) | NA | 12 (71) | 10 (38) | 5 (36) | NA |
| Discharge to rehabilitation facility | 29 (51) | NA | 5 (29) | 15 (58) | 9 (64) | NA |
Median [interquartile range] or n (%).
P-value for difference between those who died in hospital and all survivors.
Transition to ventricular assist device (1), transition to cardiopulmonary bypass for heart transplantation (1).
Neuroimaging during ECMO: only daily head ultrasounds (HUS), 54 (55%); daily HUS and brain computed tomography (CT), 13 (13%); only brain CT, 8 (8%); no neuroimaging during ECMO, 24 (24%).
Neuroimaging during or within 6 weeks post-ECMO decannulation: daily HUS during and/or post-ECMO, 35 (35%); daily HUS during ECMO, brain CT during or post-ECMO, and/or brain MRI post-ECMO, 34 (34%); at least one brain CT during or post-ECMO and/or brain MRI post-ECMO, without HUS, 19 (19%); no neuroimaging, 11 (11%).
ECMO, extracorporeal membrane oxygenation; EEG, electroencephalography; NA, not applicable; PICU, pediatric intensive care unit; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Twenty children (20%) had developmental delay or carried a neurologic diagnosis before ECMO (Table 1). Eighteen children (18%) had confirmed chromosomal abnormalities. Forty children (40%) suffered cardiac arrest within 7 days pre-ECMO cannulation, including 19 ECPR cases. The median duration of cardiopulmonary resuscitation was 24 minutes (IQR, 5-59; range, 1-103 minutes).
Pre-ECMO, four children (4%) had known chronic neuroimaging abnormalities. In addition, of 47 children (47%) who had neuroimaging conducted within 7 days pre-ECMO exhibited acute abnormal findings, including basal ganglia and punctate white matter hemorrhage (n=4), postasphyxial injury (n=3), subdural hemorrhage (n=2), and subarachnoid hemorrhage (n=1).
Eighty-eight children (89%) had neuroimaging studies during ECMO or within 6 weeks after decannulation (Table 2). Of these, 44 (50%) were found to have new abnormalities, including intracranial hemorrhage (n=32, 36%), embolic infarction (n=20, 23%), and postasphyxial injury (n=17, 19%), alone or in combination.
Seventy-six children (76%) survived to ECMO decannulation and 57 (58%) survived to hospital discharge. Based on the ELSO definitions for ECMO discontinuation,32 74 children (75%) showed improved organ function, 20 (20%) had withdrawal of life support, 3 (3%) died on ECMO, and 2 (2%) transitioned to ventricular assist device or to cardiopulmonary bypass for heart transplantation. After the ECMO course, 6 survivors (6%) required new tracheostomy and 17 (17%) required a new gastrostomy tube prior to hospital discharge. Discharge disposition for the 57 survivors was home, 28 (49%) or rehabilitation facility, 29 (51%).
At 6 months post-ECMO, 55 of 99 children (56%) were alive, but 5 were still hospitalized. At 12 months post-ECMO, 54 of 99 children (55%) were alive, but 4 were still hospitalized (Figure 1). Forty of 50 children (80%) were available for follow-up. The median time to 6-month and 12-month follow-up visits was 6.8 months (IQR, 6.4-7.5) and 12.6 months (IQR, 12.2-13.2), respectively.
VABS-II ABC scores at the longest follow-up duration available were median 91 (IQR, 81-98; range, 53-130). Subdomain VABS-II scores and median changes from 6 months to 12 months are presented in Supplemental Table 1. For the subgroup of 35 survivors with neuroimaging during ECMO or within 6 weeks of ECMO decannulation, VABS-II scores at the longest follow-up duration available were lower among those with abnormal compared to normal neuroimaging for the overall VABS-II ABC (median, 82; IQR, 79-94 vs median 92; IQR, 86-102), and this difference was borderline significant (p=0.07). The distributions of VABS-II ABC and subdomain scores are displayed in Figure 2A–E.
Figure 2.
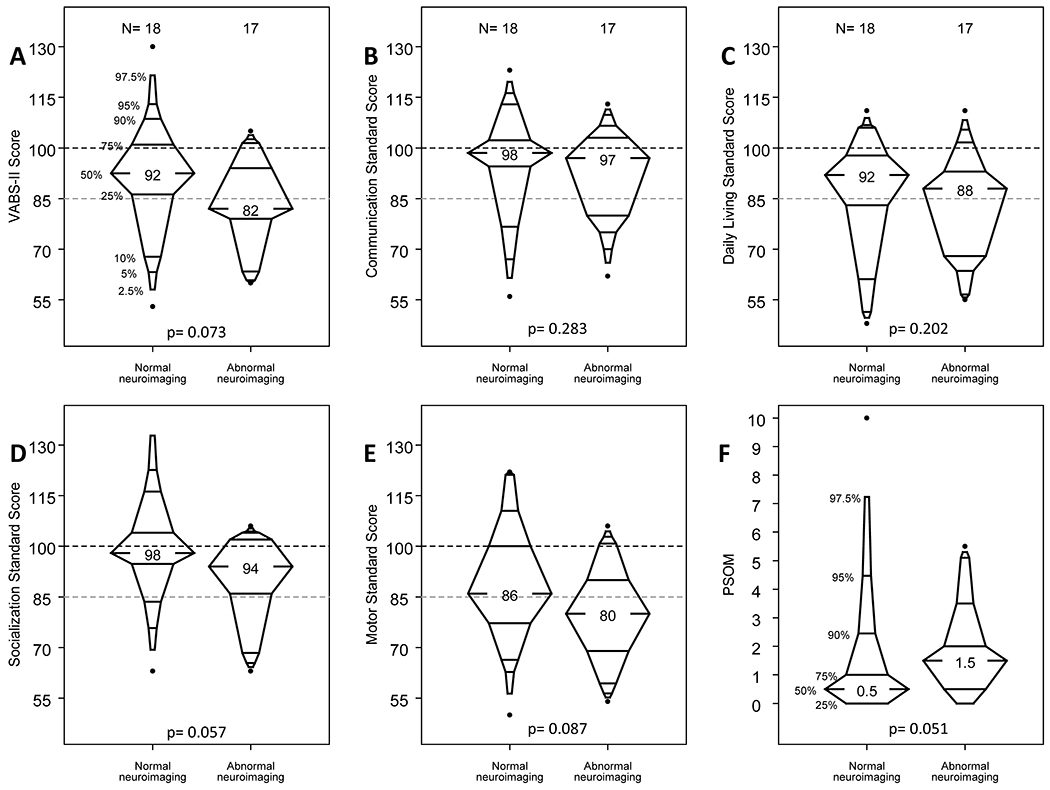
Relationship of Vineland Adaptive Behavior Scales, Second Edition (VABS-II) and Pediatric Stroke Outcome Measure (PSOM) at study follow-up to neuroimaging abnormalities during or within 6 weeks after ECMO. A-E, The VABS-II Composite Score and Communication, Daily Living, Socialization, and Motor Standard Scores at the follow-up visit of longest interval from ECMO, corresponding to neuroimaging category (p for difference not significant for all variables). F, The PSOM at the follow-up visit of longest interval from ECMO, corresponding to neuroimaging category (p for difference not significant).
Cognitive evaluation with the MSEL was carried out at only one center in 29 children < 6 years of age at the time of follow-up. The median Early Learning Composite Score at the longest follow-up duration available was 85 (IQR, 72-96) (Supplemental Table 1). Three children ≥ 6 years of age at the time of evaluation completed the WISC-IV test, with full scale intelligence quotients of 90, 89, and 107 (25th, 23rd, and 68th percentile, respectively).
HRQOL questionnaires in 32 children <2 years at the time of follow-up consisted of ITQOL, with overall health rated at median of 85 (IQR, 60-85). PedsQL was administered to caregivers of 8 children ≥ 2 years at the time of follow-up and the total score had a median of 69 (IQR, 46-83) (Supplemental Table 2).
Median PSOM score evaluated at the follow-up visit of longest interval from ECMO was 1 (IQR, 0-2). For those children seen at both follow-up visits, the median change from 6 to 12 months was 0 (IQR, −0.75 to 0) (Supplemental Table 1). Though PSOM scores at the follow-up visit of longest interval from ECMO were higher in children with abnormal neuroimaging than in those with normal neuroimaging, this difference was not statistically significant (median, 1.5; IQR, 0.5-2 vs median, 0.5; IQR, 0-1, p=0.051; Figure 2F).
PCPC scores for survivors at hospital discharge and 6-month and 12-month follow-up are presented in Supplemental Figure 1. Overall, the PCPC scores improved with time post-ECMO, such that 19% of survivors had a PCPC score of 1 at discharge and 47% of survivors who returned at 12 months had a PCPC score of 1. PCPC at discharge was more correlated with 6-month follow-up (weighted kappa=0.58 indicating good reproducibility) than with 12-month follow-up (weighted kappa=0.35 indicating marginal reproducibility) providing further evidence of improvement over time.
PCPC at hospital discharge was correlated with VABS-II ABC and VABS-II subdomain standardized scores at the follow-up visit of longest interval from ECMO (Figure 3A–E, p for difference <0.05 for all variables, with the exception of VABS communication standard score in which p=0.054). All children with PCPC of 1 at hospital discharge had VABS-II ABC ≥85 at the follow-up visit of longest interval from ECMO, and 74% of children with PCPC of 2 at hospital discharge had VABS-II ABC ≥85 at follow-up. Conversely, five of six children with PCPC of 3 at hospital discharge had VABS-II ABC <85 at the follow-up visit of longest interval from ECMO, as did both children with PCPC of 4 at hospital discharge.
Figure 3.
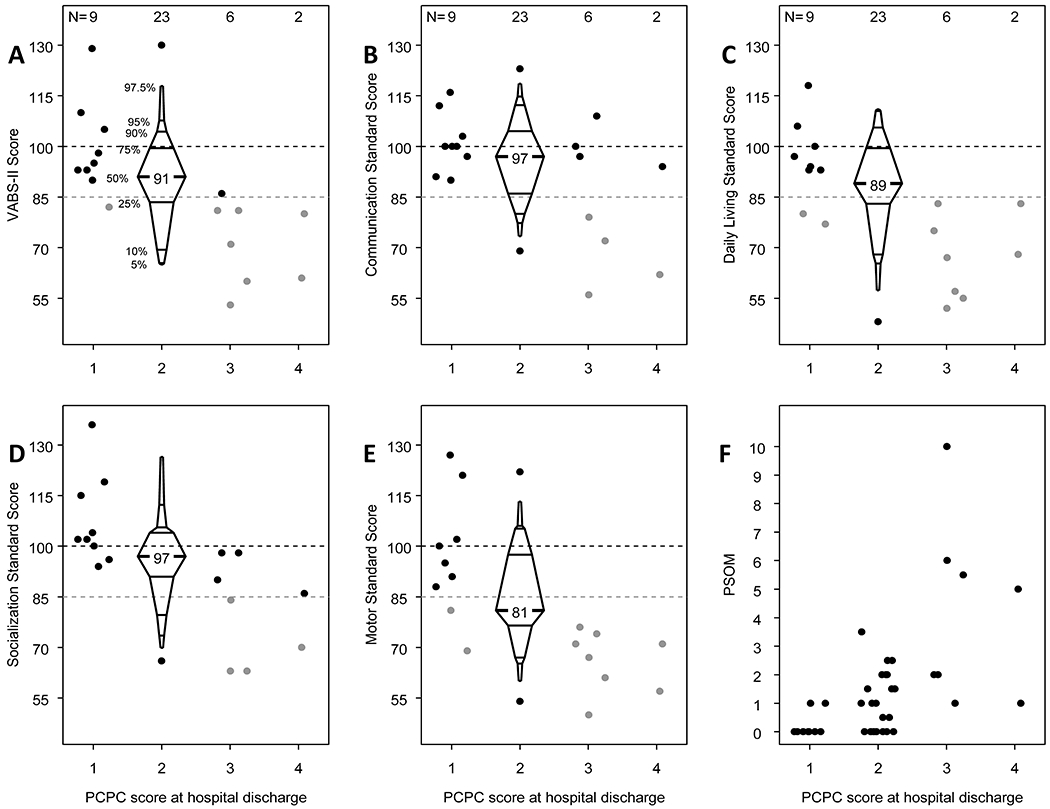
Relationship of Vineland Adaptive Behavior Scales, Second Edition (VABS-II) and Pediatric Stroke Outcome Measure (PSOM) at study follow-up to Pediatric Cerebral Performance Category (PCPC) scores at hospital discharge. A-E, The VABS-II Composite Score and Communication, Daily Living, Socialization, and Motor Standard Scores at the follow-up visit of longest interval from ECMO, corresponding to each PCPC score category at hospital discharge (p for difference <0.05 for all variables, with the exception of VABS communication standard score in which p=0.054). F, The PSOM at the follow-up visit of longest interval from ECMO, corresponding to each PCPC score category at hospital discharge (p for difference <0.001).
PCPC at hospital discharge was also correlated with the PSOM score at the follow-up visit of longest interval from ECMO (p for difference <0.001). Only 2 of 9 (22%) children with PCPC of 1 at hospital discharge had a PSOM score >0 (both had a PSOM score of 1). Among children with PCPC of 2 at hospital discharge, 15 of 23 (65%) had PSOM score >0 (range, 0.5 to 3.5), whereas all children with PCPC ≥3 at hospital discharge had PSOM score ≥1 at the follow-up visit of longest interval from ECMO (range, 1-10; Figure 3F).
In unadjusted models, primary indication for ECMO and cardiac arrest within 7 days pre-ECMO were associated with in-hospital mortality. Models adjusting for age and gender showed a strong association between primary indications of cardiac failure and ECPR and mortality. Associated risk was higher, though not significant, for neonates (less than 30 days) than for infants 30 days to 1 year, and for children with pre-ECMO cardiac arrest (Table 3).
Table 3.
Univariate and Multivariable Models for Death Prior to Hospital Discharge and for Vineland Adaptive Behavior Scales, Second Edition, Composite Score <85 or Death within 12 Months
| Death prior to hospital discharge (n=99) | VABS-II <85 or death within 12 months after ECMO among survivors to hospital discharge with follow-up data (n=43) | |||
|---|---|---|---|---|
| Variable/Outcome | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Age category | ||||
| Neonate (<30 d) | 1.10 (0.39, 3.05) | 3.25 (0.87, 12.20) | 0.26 (0.05, 1.42) | 0.18 (0.02, 1.84) |
| Infant (30 d to <1 y) | Ref | Ref | Ref | Ref |
| Child (1 to <12 y) | 0.78 (0.22, 2.77) | 1.61 (0.38, 6.77) | 0.80 (0.10, 6.35) | 0.57 (0.05, 6.23) |
| Adolescent (≥12 y) | 0.80 (0.15, 4.26) | 1.28 (0.21, 7.85) | 0.15 (0.01, 2.06) | 0.13 (0.01, 2.14) |
| Gender | ||||
| Female | Ref | Ref | Ref | Ref |
| Male | 1.20 (0.54, 2.68) | 0.89 (0.36, 2.22) | 1.22 (0.36, 4.21) | 3.04 (0.53, 17.33) |
| Primary ECMO indication | ||||
| Respiratory failure | Ref | Ref | Ref | Ref |
| Cardiac failure | 3.29 (1.19, 9.07) | 4.66 (1.53, 14.20) | 1.90 (0.47, 7.70) | 2.01 (0.37, 11.07) |
| ECPR | 5.36 (1.57, 18.25) | 10.65 (2.41, 47.04) | 3.25 (0.43, 24.84) | 1.82 (0.14, 23.65) |
| Sepsis | 1.04 (0.17, 6.22) | 1.41 (0.22, 8.99) | 0.72 (0.06, 8.46) | 0.80 (0.05, 11.89) |
| Pre-ECMO cardiac arrest | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 2.38 (1.05, 5.43) | 1.75 (0.56, 5.42) | 0.69 (0.17, 2.80) | 0.14 (0.01, 1.93) |
| Neuroimaging abnormality | ||||
| Absent | Ref | Ref | Ref | Ref |
| Present | 1.25 (0.56, 2.79) | 1.14 (0.46, 2.84) | 4.13 (1.13, 15.10) | 5.43 (1.13, 26.12) |
CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; OR, odds ratio; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Among the subgroup of survivors who were discharged and seen in follow-up at 6 or 12 months, the presence of a neuroimaging abnormality was associated with VABS-II ABC <85 in unadjusted and adjusted models (adjusted OR: 5.43, 95%CI: 1.13, 26.12). Age, gender, primary ECMO indication, and pre-ECMO cardiac arrest were not significantly associated with outcomes at 6-month and 12-month follow-up in survivors to hospital discharge (Table 3).
DISCUSSION
In this prospective cohort study, we comprehensively evaluated the neuropsychological, neurologic, and HRQOL outcomes of neonates and children who underwent ECMO between 2010 and 2015 in two centers. In the U.S., such prospective outcome data do not include the contemporary era,33 although ECMO epidemiology and practices have changed quite dramatically in recent years.34 We attained an enrollment rate of 65% with a consent window of 24 hours after ECMO cannulation, and an 80% rate of follow-up at 6 or 12 months post-ECMO in those survivors who were eligible for follow-up. These results demonstrate feasibility of conducting multicenter prospective studies in this critically ill population. However, prolonged hospitalizations (longer than 6 months in 5% of cases, and longer than 12 months in 4% of cases) and chronic medical conditions requiring frequent outpatient visits and re-hospitalizations were important barriers to follow-up and need to be taken into account when designing future studies.
For this study, we selected the VABS-II a priori as the primary outcome measure in hospital survivors. The median VABS-II ABC at the follow-up visit of longest interval from ECMO was 91, and the score ranges were wide. Almost two-thirds (65%) of study participants with follow-up had a VABS-II score ≥85 (i.e., above 1 SD below the population mean), 20% had VABS-II scores between 70 and 85 (i.e., 2 to 1 SD below the mean), and 15% had VABS-II scores <70 (i.e., more than 2 SD below the mean). Previous assessments of adaptive behavior in ECMO patients were conducted exclusively for neonatal ECMO, in single centers, and with follow-up duration ranging from a mean of 3.5 years to a mean of 7.1 years.35–39 Although the overall VABS-II scores in our cohort were similar to those reported in prior publications, comparability is limited by the fact that ECMO indications have changed in the intervening time (e.g., we enrolled fewer neonates with meconium aspiration syndrome or persistent pulmonary hypertension of the newborn). Almost half (48%) of the patients enrolled in this cohort were outside the neonatal age (≥30 days) at the time of ECMO cannulation, and the time to follow-up was shorter (6 and/or 12 months).
In the subgroup of patients who underwent cognitive ability evaluation using MSEL (age <6 years at follow-up), the median Early Learning Composite Standard Score was 85 (IQR, 72-96), falling at 1 SD below the general population mean. In five distinct studies that included mixed ECMO populations of all ages <18 years (neonatal as well as pediatric), among 672 cumulative individuals with IQ or Mental Development Index (MDI) scores reported using Wechsler Preschool and Primary Scale of Intelligence, Wechsler Intelligence Scale for Children, or Bayley Scales of Infant Development, as appropriate for age, the mean IQ or MDI score was 87 (SD 10.3) at a median duration to follow-up of 60 months (IQR, 9-84) after ECMO (all studies enrolled ECMO patients in the 1990s up to 2004).15,33,39–42
We also collected PCPC to allow for comparison with other ECMO outcome studies. Seventy-nine percent of survivors had PCPC ≤ 2, indicating no or mild disability at hospital discharge, and that proportion increased over time in study participants who were available for follow-up (i.e., 77% at 6 months, and 85% at 12 months). PCPC at hospital discharge was strongly correlated with VABS-II and PSOM at 6- or 12-month follow-up. In a systematic review of neurologic outcomes after neonatal and pediatric ECMO, PCPC was the most commonly used outcome measure identified in 18 of 60 (30%) studies included, although it was primarily evaluated at hospital discharge, and not long-term.33 Although findings from studies conducted in children with cardiac disease who required ECMO were similar to ours (proportion of survivors with PCPC ≤ 2 at hospital discharge or follow-up to 2.5 years of 81% to 91%),43–45 studies conducted in mixed populations of children requiring ECMO reported a cumulative proportion of survivors with PCPC ≤ 2 at follow-up of only 58%.33
Neuroimaging in this cohort was conducted for clinical purposes in both participating centers and included daily HUS in all infants with an open anterior fontanel (54% of study participants), brain CT in 34% of study participants, and post-ECMO brain MRIs in 36%. Overall, 89% of participants had brain imaging with at least one of the three modalities. The presence of new neuroimaging abnormalities during or post-ECMO was not significantly associated with in-hospital mortality but was significantly associated with poor outcome (lower VABS-II scores or death within 12 months after ECMO) among survivors to hospital discharge, after adjusting for potential confounders. Counseling and rehabilitation services are potential targets for future interventional studies to improve neurodevelopmental outcomes in critically ill children requiring ECMO, especially in those with abnormal neuroimaging.
It is notable that 20% of children enrolled had a pre-existing neurologic diagnosis and 40% had a cardiac arrest within 7 days prior to ECMO cannulation, and thus had possible pre-existing hypoxic-ischemic injury at the time of ECMO. In the subgroup of children who underwent neuroimaging before ECMO cannulation, 21% had an acute finding. Acute neurologic injury detected during or immediately after the ECMO course therefore needs to be interpreted with caution when pre-ECMO imaging was not obtained, as it could overestimate ECMO-related rates of injury. In adults with respiratory failure managed with and without venovenous ECMO, a recent study showed that after adjusting for potential confounders, use of ECMO was not independently associated with intracranial hemorrhage.46 Similarly, in the design of ECMO studies that include long-term neurodevelopmental evaluations, the continuum of neurologic insults, whether from pre-existing neurologic conditions or acute events such as cardiac arrest, needs to be accounted for at data collection and during data analysis.
This study has several limitations. We did not anticipate the high rate of long-term hospitalization, a scenario that needs to be taken into account in the design of future studies. We did not collect data on post-discharge burden of medical outpatient appointments, readmissions to the hospital, and amount of rehabilitation services. These are potential post-discharge confounders for the outcomes of interest and also constituted barriers to study follow-up. Among all survivors eligible for follow-up at 6 and 12 months, the follow-up rate was 80%. Neonatal and pediatric ECMO is regionalized, with only large academic children’s hospitals providing these services. Therefore in-person follow-up may be more difficult for these patients than for patients with other critical illnesses, even when plans for in-person follow-up are discussed prior to hospital discharge, as in our study. Perhaps outcomes that can be reliably ascertained by telephone interview of caregivers would decrease attrition. There are excellent data regarding agreement between in-person and by-phone administration of the VABS-II questionnaire,47,48 as well as measures such as the Recurrence and Recovery Questionnaire that could be administered by phone in lieu of the in-person PSOM evaluation.49 Lastly, results of multivariable logistic models for outcomes at 6 and 12 months among survivors to hospital discharge should be interpreted cautiously because of the small sample size (n=43).
CONCLUSIONS
In this two-center prospective cohort study of neonatal and pediatric ECMO, we report a higher prevalence of pre-ECMO neurologic conditions and acute neurologic events such as cardiac arrest or new findings on neuroimaging than previously described. Survival to hospital discharge was similar to that in previous reports. In survivors to hospital discharge, median scores for adaptive behavior and cognitive, neurologic, and quality of life assessments were all below the general population means, but most deficits would be considered minor within each of the domains tested. PCPC at hospital discharge showed a strong relationship with unfavorable VABS-II and PSOM scores at 6 months and/or 12 months after ECMO.
Supplementary Material
Supplemental Figure 1. Pediatric Cerebral Performance Category (PCPC) scores in survivors to hospital discharge. Each cell represents the proportion of survivors in each PCPC category in relationship to their hospital discharge PCPC. κ, kappa statistic.
Acknowledgments
The authors thank Claire F. Levine, MS, ELS (Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University), for editing the manuscript.
Funding: Support for this work included funding from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS076674 and R01NS106292 (MMB). This work was supported by U54 HD079123.
References
- 1.Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). use of the bramson membrane lung. N Engl J Med. 1972;286(12):629–634. [DOI] [PubMed] [Google Scholar]
- 2.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(1):60–67. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116(15):1693–1700. [DOI] [PubMed] [Google Scholar]
- 4.Barrett CS, Bratton SL, Salvin JW, Laussen PC, Rycus PT, Thiagarajan RR. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10(4):445–451. [DOI] [PubMed] [Google Scholar]
- 5.Weber TR, Kountzman B. Extracorporeal membrane oxygenation for nonneonatal pulmonary and multiple-organ failure. J Pediatr Surg. 1998;33(11):1605–1609. [DOI] [PubMed] [Google Scholar]
- 6.Cengiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS. Central nervous system complications during pediatric extracorporeal life support: Incidence and risk factors. Crit Care Med. 2005;33(12):2817–2824. [DOI] [PubMed] [Google Scholar]
- 7.Waitzer E, Riley SP, Perreault T, Shevell MI. Neurologic outcome at school entry for newborns treated with extracorporeal membrane oxygenation for noncardiac indications. J Child Neurol. 2009;24(7):801–806. [DOI] [PubMed] [Google Scholar]
- 8.Glass P, Miller M, Short B. Morbidity for survivors of extracorporeal membrane oxygenation: Neurodevelopmental outcome at 1 year of age. Pediatrics. 1989;83(1):72–78. [PubMed] [Google Scholar]
- 9.Adolph V, Ekelund C, Smith C, Starrett A, Falterman K, Arensman R. Developmental outcome of neonates treated with extracorporeal membrane oxygenation. J Pediatr Surg. 1990;25(1):43–46. [DOI] [PubMed] [Google Scholar]
- 10.Schiller RM, Madderom MJ, Reuser JJ, et al. Neuropsychological follow-up after neonatal ECMO. Pediatrics. 2016;138(5):e20161313 Epub 2016 Oct 6. [DOI] [PubMed] [Google Scholar]
- 11.Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: A population-based study. Crit Care. 2009;13(2):R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madderom MJ, Reuser JJ, Utens EM, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: A nationwide multicenter study. Intensive Care Med. 2013;39(9):1584–1593. [DOI] [PubMed] [Google Scholar]
- 13.Madderom MJ, Schiller RM, Gischler SJ, et al. Growing up after critical illness: Verbal, visual-spatial, and working memory problems in neonatal extracorporeal membrane oxygenation survivors. Crit Care Med. 2016;44(6):1182–1190. [DOI] [PubMed] [Google Scholar]
- 14.McNally H, Bennett CC, Elbourne D, Field DJ, UK Collaborative ECMO Trial Group. United kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: Follow-up to age 7 years. Pediatrics. 2006;117(5):845. [DOI] [PubMed] [Google Scholar]
- 15.Madderom MJ, Toussaint L, van der Cammen-van Zijp MH, et al. Congenital diaphragmatic hernia with(out) ECMO: Impaired development at 8 years. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):316. [DOI] [PubMed] [Google Scholar]
- 16.Ryerson LM, Guerra GG, Joffe AR, et al. Survival and neurocognitive outcomes after cardiac extracorporeal life support in children less than 5 years of age: A ten-year cohort. Circ Heart Fail. 2015;8(2):312–321. [DOI] [PubMed] [Google Scholar]
- 17.Field D, Juszczak E, Linsell L, et al. Neonatal ECMO study of temperature (NEST): A randomized controlled trial. Pediatrics. 2013;132(5):1247. [DOI] [PubMed] [Google Scholar]
- 18.Garcia Guerra G, Robertson CM, Alton GY, et al. Health-related quality of life in pediatric cardiac extracorporeal life support survivors. Pediatr Crit Care Med. 2014;15(8):720–727. [DOI] [PubMed] [Google Scholar]
- 19.Garcia Guerra G, Zorzela L, Robertson CM, et al. Survival and neurocognitive outcomes in pediatric extracorporeal-cardiopulmonary resuscitation. Resuscitation. 2015;96:208–213. [DOI] [PubMed] [Google Scholar]
- 20.Kakat S, O’Callaghan M, Smith L, et al. The 1-year follow-up clinic for neonates and children after respiratory extracorporeal membrane oxygenation support: A 10-year single institution experience. Pediatr Crit Care Med. 2017;18(11):1047–1054. [DOI] [PubMed] [Google Scholar]
- 21.Morris RD, Krawiecki NS, Wright JA, Walter LW. Neuropsychological, academic, and adaptive functioning in children who survive in-hospital cardiac arrest and resuscitation. J Learn Disabil. 1993;26(1):46–51. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. J Consult Clin Psychol. 1990;58(1):93–98. [DOI] [PubMed] [Google Scholar]
- 23.Mullen EM. Mullen scales of early learning. Circle Pine, MN: American Guidance Service; 1995. [Google Scholar]
- 24.Wechsler D Wechsler intelligence scale for children: Technical manual. 4th Edition ed. Antonio San, Texas: The Psychological Corporation; 2003. [Google Scholar]
- 25.Landgraf J The infant/toddler child health questionnaire: Conceptual framework, logic content, and preliminary psychometric results. ; 1994. [Google Scholar]
- 26.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–139. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 28.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15(5):316–324. [DOI] [PubMed] [Google Scholar]
- 29.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: A validation and reliability study. Stroke. 2012;43(6):1602–1608. [DOI] [PubMed] [Google Scholar]
- 30.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. [DOI] [PubMed] [Google Scholar]
- 31.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616–2620. [DOI] [PubMed] [Google Scholar]
- 32.ELSO registry form submission instructions, extracorporeal life support organization (ELSO). https://www.elso.org/Portals/0/Files/PDF/ELSO_Registry_Instructions_2017.pdf. Updated 2017. Accessed August 23, 2017.
- 33.Boyle K, Felling R, Yiu A, et al. Neurologic outcomes after extracorporeal membrane oxygenation - a systematic review. Pediatr Crit Care Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbaro RP, Xu Y, Borasino S, et al. Does extracorporeal membrane oxygenation improve survival in pediatric acute respiratory failure? Am J Respir Crit Care Med. 2018;197(9):1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nield TA, Langenbacher D, Poulsen MK, Platzker AC. Neurodevelopmental outcome at 3.5 years of age in children treated with extracorporeal life support: Relationship to primary diagnosis. J Pediatr. 2000;136(3):338–344. [DOI] [PubMed] [Google Scholar]
- 36.Rais-Bahrami K, Wagner AE, Coffman C, Glass P, Short BL. Neurodevelopmental outcome in ECMO vs near-miss ECMO patients at 5 years of age. Clin Pediatr (Phila). 2000;39(3):145–152. [DOI] [PubMed] [Google Scholar]
- 37.Langenbacher D, Nield T, Poulsen MK. Neurodevelopmental outcome of ECMO survivors at five years of age: The potential for academic and motor difficulties. The Journal of Special Education. 2001;35:156–160. [Google Scholar]
- 38.Parish AP, Bunyapen C, Cohen MJ, Garrison T, Bhatia J. Seizures as a predictor of long-term neurodevelopmental outcome in survivors of neonatal extracorporeal membrane oxygenation (ECMO). J Child Neurol. 2004;19(12):930–934. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg AA, Lee NR, Vaver KN, et al. School-age outcomes of newborns treated for persistent pulmonary hypertension. J Perinatol. 2010;30(2):127–134. [DOI] [PubMed] [Google Scholar]
- 40.Wagner K, Risnes I, Berntsen T, et al. Clinical and psychosocial follow-up study of children treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2007;84(4):1349–1355. [DOI] [PubMed] [Google Scholar]
- 41.Lequier L, Joffe AR, Robertson CM, et al. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008;136(4):976–983.e3. [DOI] [PubMed] [Google Scholar]
- 42.Rasheed A, Tindall S, Cueny DL, Klein MD, Delaney-Black V. Neurodevelopmental outcome after congenital diaphragmatic hernia: Extracorporeal membrane oxygenation before and after surgery. J Pediatr Surg. 2001;36(4):539–544. [DOI] [PubMed] [Google Scholar]
- 43.Chrysostomou C, Maul T, Callahan PM, et al. Neurodevelopmental outcomes after pediatric cardiac ECMO support. Front Pediatr. 2013;1:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrysostomou C, Morell VO, Kuch BA, O’Malley E, Munoz R, Wearden PD. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg. 2013;146(2):317–325. [DOI] [PubMed] [Google Scholar]
- 45.Nosaka N, Muguruma T, Fujiwara T, Enomoto Y, Toida C, Morishima T. Effects of the elective introduction of extracorporeal membrane oxygenation on outcomes in pediatric myocarditis cases. Acute Medicine & Surgery. 2015;2(2):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lockie CJA, Gillon SA, Barrett NA, et al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. 2017;45(10):1642–1649. [DOI] [PubMed] [Google Scholar]
- 47.Limperopoulos C, Majnemer A, Steinbach CL, Shevell MI. Equivalence reliability of the vineland adaptive behavior scale between in-person and telephone administration. Phys Occup Ther Pediatr. 2006;26(1-2):115–127. [PubMed] [Google Scholar]
- 48.Lux AL, Edwards SW, Hancock E, et al. The united kingdom infantile spasms study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: A multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717. [DOI] [PubMed] [Google Scholar]
- 49.Lo WD, Ichord RN, Dowling MM, et al. The pediatric stroke recurrence and recovery questionnaire: Validation in a prospective cohort. Neurology. 2012;79(9):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Pediatric Cerebral Performance Category (PCPC) scores in survivors to hospital discharge. Each cell represents the proportion of survivors in each PCPC category in relationship to their hospital discharge PCPC. κ, kappa statistic.


