Abstract
Simple Summary
Understanding fat sensing in chickens has the potential to improve least cost feed formulation relevant to poultry feeds. Acid oils (soybean acid oil and palm fatty acid distillate) are economical and sustainable feedstuffs with similar fatty acid composition to crude oils (soybean oil and palm oil) but richer in free fatty acids. However, potential issues relevant to the palatability of these oils have been raised. Four experimental diets were offered in a series of double-choice tests to study the effect of free fatty acid content and the unsaturated:saturated ratio on dietary preferences in hens. Hens showed a feed preference for palm oil added diets over soybean oil diets, with palm oil and palm fatty acid distillate being equally preferred. However, the hens demonstrated a preference for soybean oil when offered in choice with soybean acid oil. In conclusion, free fatty acid content and saturation degree affected feed preferences in hens. The use of oils with greater preference values may give rise to greater feed palatability, enhancing feed intake at critical stages.
Abstract
Behavioural and genetic evidence shows that the taste system is intimately related to the sensing of nutrients with consequences for poultry nutrition practices. A better understanding of how chickens may sense fat could provide the background for selecting feedstuffs used in poultry feeds. Acid oils have the potential to be economical and sustainable feedstuffs. These fat by-products from the edible oil refining industry possess a similar fatty acid composition to the crude oils but are richer in free fatty acids (FFA). An experiment was conducted to study the effect of FFA content and the unsaturated:saturated ratio (U:S) on dietary preferences in hens. Four fat sources were added to a basal diet at an inclusion rate of 6%, determining the experimental diets: soybean oil (SO; high U:S, 5% FFA); soybean acid oil (SA; high U:S, 50% FFA); palm oil (PO; low U:S, 5% FFA); and palm fatty acid distillate (PFAD; low U:S, 50% FFA). The experimental diets were offered in a series of double-choice tests to forty-eight Lohmann Brown laying hens housed individually in cages. Each hen was offered the ten potential binary combinations of the four diets including each diet compared to itself (referred to as four control double-choices). Feed intake was measured for two hours twice a day after one hour of fasting. Consumption was analysed as a standard preference index (% of test diet intake in comparison with the total intake). Preference values were compared to the random choice value of 50% using the Student’s t-test. None of the four control comparisons differ significantly from 50% (p > 0.05), indicating that the changes in preference values observed in the other binary comparisons were related to the dietary changes associated to fat ingredients. Hens showed a feed preference for palm oil added diets over soybean oil diets (p < 0.05), with PO and PFAD being equally preferred (p < 0.05). However, in this trial the hens demonstrated a preference for SO (low %FFA) when offered in choice with SA (high %FFA) (p < 0.05). These results suggest that the degree of saturation plays an important role in dietary fat preferences: hens prefer predominantly saturated oils even when these are rich in FFA. Furthermore, when presented with a choice between predominantly unsaturated oils, hens prefer feed with a low %FFA. In conclusion, %FFA and the U:S ratio affected feed preferences in hens. The use of oils with greater preference values may give rise to greater feed palatability, enhancing feed intake at critical stages.
Keywords: fat source, soybean oil, palm oil, laying hen, palatability, double-choice, preference
1. Introduction
Taste perception influences feed intake guiding nutrient choices [1,2]. It has been widely assumed that the sense of taste in avian species is not as accurate as in mammals, as they have fewer taste buds [3]. However, the ratio of taste buds to oral cavity volume in birds appears to be higher than in most mammals [4]. Recent behavioural and genomic studies have found that birds have a highly developed sense of taste [1,5,6].
Poultry possess a well-developed capacity to differentiate feed, ingredients and toxic components [7,8]. When choice is limited, animals modulate feed consumption mainly based on energy and amino acid requirements and feed presentation [9,10]. When offered different diets simultaneously, dietary choices in chickens are mainly influenced by potential nutrient deficiencies [11,12]. However, feed preferences may also be influenced by other factors involved in palatability [13,14].
It is generally accepted that there are five basic taste qualities: sweet, sour, umami, bitter and salty. Compared to mammals, chickens have fewer bitter taste receptors and lack the mammalian sweet taste receptor T1R2 [6,15]. In contrast, the ability of chickens to sense fatty acids has been recently uncovered by identifying the involvement of taste receptors FFAR2 and FFAR4 [16,17,18,19,20].
Supplemental dietary fats are widely used to satisfy feed energy requirements, due to their high-energy value. Fat by-products such as acid oils from the edible oil refining industry, represent an economical alternative compared to conventional fats and oils [21]. These by-products are characterized by possessing a high proportion of free fatty acids (FFA) [22] which may affect palatability when added to poultry feeds. Due to the importance of the sense of taste in avian nutrition [4,23], it seems necessary to assess palatability and, if possible, optimize the use of cheaper and environmentally sustainable feedstuffs, improving the profitability of egg production by replacing high-cost ingredients.
Most published studies on chicken dietary preferences assessed long-term feed intake associated to physiological needs [9,11,12,24,25,26]. In contrast, much less is known on short-term feed intake drivers in chickens. Short-term trials assessing palatability are usually performed by double-choice tests, where animals choose between two different feeds offered simultaneously, enabling palatability to be determined [27,28,29,30,31,32].
The present study aimed at documenting laying hen preferences for a range of fat sources differing in FFA content and the degree of saturation (unsaturated:saturated; UFA:SFA) using double-choice tests.
2. Materials and Methods
2.1. Animals and Housing
All experimental procedures followed European Union guidelines for animal care and handling [33] and were approved by Animal Research Ethics Committee of the Universidad Cardenal Herrera-CEU (CEEA 17/018). The animal study was conducted at the Universidad Cardenal Herrera-CEU Teaching and Research Farm (Náquera, Valencia, Spain).
Forty-eight Lohmann Brown laying hens of 54 weeks of age were selected, possessing a body weight of between 2073 and 2363 g (2221 ± 77 g, mean ± standard deviation). The hens were individually housed in cages (76.2 × 63.0 cm2 with a minimum height of 45.0 cm) and animal interaction was avoided by using dividers. The cages were equipped with a nest, perch and three freshwater nipple drinkers. Prior to the experimental trial, a training period (7 days) was implemented to facilitate the hens’ adaptation to the experimental facilities and management.
2.2. Experimental Design
Four test diets were evaluated in pairs using a double-choice protocol adapted from Solà-Oriol et al. [29]. Each hen was fed the six possible binary combinations of the experimental feeds offered in two adjacent identical aluminium containers (31.0 × 10.5 × 5.5 cm3).
Potential position effects (right vs. left and hen location) were accounted by including as treatment four controls (one for each diet) where the same diet was offered in both feeders. In total, 10 double-choice tests were assessed. The duration of the tests was 10 days. With the objective of reducing the experimental error, a Latin square design was carried out. All potential binary combinations were tested once in each hen, row, column and floor and all were tested every day. The order of the double-choice tests was randomized for each hen.
2.3. Diets and Feeding
All experimental diets consisted of a basal diet (94%) and the remaining 6% from one of four dietary oil sources: soybean oil (SO), soybean acid oil (SA), palm oil (PO) or palm fatty acid distillate (PFAD) (Table 1). Oils were kept refrigerated (2 °C for 2 months) prior to mix with the basal diet (spiral automatic feed mixer; Gruber Hermanos, S.A., Spain). Two of the diets (SO and SA) had a high ratio of unsaturated (UFA) to saturated fatty acids (SFA), while the palm-oil-based diets (PO and PFAD) had a low UFA to SFA ratio. Two of the diets (SO and PO) had a low FFA content (5%) while SA and PFAD had a high FFA content (50%). The SO, SA and PO diets had a single oil source while PFAD had an oil blend, consisted of 33% crude palm oil combined with 66% palm FA distillate (and thus possessing the same FFA content as SA). The fatty acid composition of the experimental diets is shown in Table 2. Butylated hydroxytoluene (BHT), a synthetic antioxidant, was incorporated into soybean diets (SO and SA) at 100 ppm. The diets were formulated to cover the nutrient requirements of laying hens in accordance with Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA) recommendations [34] and remained stored in a cool, dry place until the beginning of the test (2 months).
Table 1.
Composition of the experimental diets.
| Ingredients, % | SO | SA | PO | PFAD |
|---|---|---|---|---|
| Barley 2 rows | 49.9 | 49.9 | 49.9 | 49.9 |
| Soybean meal 47.5% Crude Protein | 24.3 | 24.3 | 24.3 | 24.3 |
| Corn | 6.9 | 6.9 | 6.9 | 6.9 |
| Calcium carbonate (fine-grained) | 6.8 | 6.8 | 6.8 | 6.8 |
| Crude soybean oil (SO) | 6.0 | - | - | - |
| Acid soybean oil (SA) | - | 6.0 | - | - |
| Crude palm oil (PO) | - | - | 6.0 | 1.98 |
| Palm fatty acid distillate (PFAD) | - | - | - | 4.02 |
| Calcium carbonate (coarse-grained) | 2.1 | 2.1 | 2.1 | 2.1 |
| Sunflower 36% CP | 1.9 | 1.9 | 1.9 | 1.9 |
| Monocalcium phosphate | 1.1 | 1.1 | 1.1 | 1.1 |
| Sodium chloride | 0.3 | 0.3 | 0.3 | 0.3 |
| Methionine (MHA) | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin and mineral premix 1 | 0.2 | 0.2 | 0.2 | 0.2 |
| Feed sanitizer 2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 | 0.1 |
| Choline chloride 75% | 0.1 | 0.1 | 0.1 | 0.1 |
| White cereal enzymes | 0.05 | 0.05 | 0.05 | 0.05 |
| Red synthetic pigment | 0.03 | 0.03 | 0.03 | 0.03 |
| 6-phytase 100 | 0.03 | 0.03 | 0.03 | 0.03 |
| BHT 3 | 0.01 | 0.01 | - | - |
| Estimated nutrient composition | ||||
| Gross energy, kcal/kg | 3836 | 3772 | 3699 | 3625 |
| Dry matter, % | 90.79 | 90.79 | 90.79 | 90.79 |
| Crude protein, % | 17.55 | 17.55 | 17.55 | 17.55 |
| Lysine, % | 0.914 | 0.914 | 0.914 | 0.914 |
| Methionine, % | 0.433 | 0.433 | 0.433 | 0.433 |
| Methionine + Cysteine, % | 0.751 | 0.751 | 0.751 | 0.751 |
| Threonine, % | 0.657 | 0.657 | 0.657 | 0.657 |
| Tryptophan, % | 0.219 | 0.219 | 0.219 | 0.219 |
| Ash, % | 12.52 | 12.52 | 12.52 | 12.52 |
| Crude fat, % | 7.48 | 7.48 | 7.48 | 7.48 |
| Crude fibre, % | 4.16 | 4.16 | 4.16 | 4.16 |
| Calcium, % | 3.79 | 3.79 | 3.79 | 3.79 |
| Total Phosphorus, % | 0.720 | 0.720 | 0.720 | 0.720 |
| Digestible Phosphorus, % | 0.455 | 0.455 | 0.455 | 0.455 |
| Chloride, % | 0.225 | 0.225 | 0.225 | 0.225 |
| Sodium, % | 0.161 | 0.161 | 0.161 | 0.161 |
| Choline, % | 0.031 | 0.031 | 0.031 | 0.031 |
| Xanthophylls, mg/kg | 8.344 | 8.344 | 8.344 | 8.344 |
1 Provides per kg of feed: vitamin A, 9000 IU; vitamin D3, 3000 IU; vitamin E, 13 IU; vitamin B1, 1 mg; vitamin B2, 4 mg; vitamin B6, 1.8 mg; vitamin B12, 10 μg; vitamin K3, 1.7 mg; folic acid, 0.3 mg; niacin, 20 mg; pantothenic acid, 8mg; biotin, 52 mg; choline, 200 mg; Fe (from FeSO4·7H2O), 32 mg; Co (from 2CoCO3·3Co(OH)2·H2O), 7 mg; Zn (from ZnO), 65 mg; Mn (from MnO), 85 mg; Se (from Na2SeO3), 0.35 mg; I (from Ca(I2O3)2), 0.7 mg. 2 Form-Ad Plus (Adiveter). 3 Butylated hydroxytoluene.
Table 2.
Fatty acid and lipid fraction composition of the diets 1. Results expressed as % total fatty acids.
| Fatty Acid Composition | SO | SA | PO | PFAD |
|---|---|---|---|---|
| C12:0 | - | - | - | 0.41 |
| C14:0 | - | - | 0.81 | 0.95 |
| C16:0 | 11.82 | 12.70 | 34.59 | 36.42 |
| C16:1n7 | - | 0.21 | - | - |
| C18:0 | 4.04 | 3.84 | 4.00 | 4.11 |
| C18:1n9 | 23.21 | 28.88 | 34.79 | 33.70 |
| C18:1n7 | 1.26 | 1.08 | 0.80 | 0.77 |
| C18:2n6 | 51.59 | 48.38 | 22.76 | 21.53 |
| C18:3n3 | 6.92 | 2.64 | 1.50 | 1.48 |
| C20:0 | 0.41 | 0.61 | 0.43 | 0.41 |
| C20:1n9 | 0.26 | 0.28 | 0.21 | 0.21 |
| C22:0 | 0.49 | 0.93 | 0.10 | - |
| C24:0 | - | 0.46 | - | - |
| SFA | 16.75 | 18.55 | 39.93 | 42.31 |
| MUFA | 23.48 | 29.36 | 35.01 | 33.91 |
| PUFA | 58.51 | 51.01 | 24.26 | 23.01 |
| UFA: SFA | 4.90 | 4.33 | 1.48 | 1.35 |
| FFA | 5 | 50 | 5 | 50 |
1 Fatty acid determination by direct transesterification [35]; fatty acid quantification by gas chromatography (HP 6890 Series II GC System with Flame Ion Detection (FID), Agilent Technologies). SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; UFA: unsaturated fatty acid; FFA: free fatty acid. The symbol “-” indicates a non-quantifiable value.
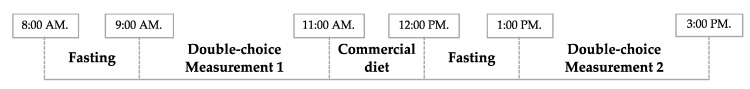
Figure 1 shows the feeding protocol [36]. In brief, after an hour of fasting, two test-containers, each one filled with 100 g of one of the experimental feeds, were fixed side-by-side to the front of each cage. Feed intake was measured for the following 120 min. This procedure was repeated twice daily. In order to avoid bias, the position of the test-containers was switched from one measurement to the next. Between the two measurements, birds were offered a commercial diet for the first 60 min and fasted during the next 60 min before the start of the second test of the day.
Figure 1.
Feeding protocol scheme.
The overall feed consumption was calculated as measurement 1 plus measurement 2. During the non-test period the hens were fed with a commercial diet and water was provided ad libitum during all tests. Cages were checked to ensure access to feed and to minimize any possible feed wastage.
2.4. Measurements and Calculations
Feed intake was determined by weighing the test-containers before and after each test. The consumption was analysed as a standard preference index (% of test diet intake compared to total intake) [29]. The following mathematical equation was used (1):
| Preference (%) = Test diet intake/Total intake × 100 | (1) |
Preference values ranged from 0 to 100% with 50% indicating no preference. Values significantly higher or lower than 50% indicated a preference or aversion, respectively.
2.5. Statistical Analysis
Prior to evaluate the preference, the Shapiro–Wilk normality test was performed for each set of preference values. Then, preference values were compared with the 50% no-preference value using the One-sample Student’s t-test, for each compared feed. Cohen’s d was calculated after every t-test to calculate size effect, with the following code: d = 0.2: small effect; d = 0.5: medium effect; d = 0.8: large effect [37]. Significant differences were considered for p < 0.05. The statistical analysis was carried out entirely with IBM SPSS Statistics 24.0 for Windows [38].
3. Results
The preference values registered for the 10 double-choice tests are presented in Table 3. All of them resulted in a normal distribution.
Table 3.
Feed preference values for different fat sources offered in a double-choice setup (left diet in every pair is used as the test one and compared with the 50% preference).
| Double Choice | N | Preference, % | S.E.M *, % | p ** | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|---|
| SO | vs. | SA | 48 | 62.10 | vs. | 37.90 | 3.26 | 0.001 | 0.530 |
| SO | vs. | PO | 48 | 39.12 | vs. | 60.88 | 3.40 | 0.002 | 0.462 |
| SO | vs. | PFAD | 48 | 42.96 | vs. | 57.04 | 3.31 | 0.039 | 0.310 |
| SA | vs. | PO | 48 | 31.02 | vs. | 68.98 | 3.48 | <0.001 | 0.787 |
| SA | vs. | PFAD | 48 | 27.10 | vs. | 72.90 | 2.93 | <0.001 | 1.127 |
| PO | vs. | PFAD | 48 | 50.88 | vs. | 49.12 | 2.53 | 0.729 | 0.050 |
| SO | vs. | SO *** | 48 | 51.86 | vs. | 48.14 | 2.44 | 0.450 | 0.110 |
| SA | vs. | SA *** | 48 | 48.68 | vs. | 51.32 | 2.52 | 0.603 | 0.075 |
| PO | vs. | PO *** | 48 | 52.09 | vs. | 47.91 | 1.91 | 0.278 | 0.158 |
| PFAD | vs. | PFAD *** | 48 | 47.30 | vs. | 52.70 | 2.13 | 0.212 | 0.182 |
* S.E.M.: Standard error of the mean. ** p: Preference values were compared with the 50% no-effect level. Significance was taken as p < 0.05. *** Indicate the four control treatments consisting of double choices between two identical feeds.
As expected, when hens were offered the same diet in both test-containers, the preference measured for control pairs did not differ (p > 0.05) from 50% for any of the four experimental diets (SO, SA, PO and PFAD).
The results obtained showed a clear preference for palm diets (PO and PFAD) over soybean diets (SO and SA), irrespective of the %FFA. In particular, PO was significantly (p < 0.01) preferred to SO and SA with preference values of 60.88% and 68.98%, respectively. Similarly, PFAD was preferred to SO and SA with values 57.04% (p < 0.05) and 72.9% (p < 0.01), respectively. PO and PFAD treatments resulted in the greatest preference values and there were no significant differences in palatability between the two (p > 0.05), regardless of the degree of acidity of the fat source. However, an effect of the %FFA on preference was observed when comparing SO to SA indicating that hens significantly preferred the low (SO) to the high (SA) FFA content (p < 0.01; preference value = 62.1%).
Hens demonstrated a consistent preference for palm-oil formulations over soybean-oil formulations, particularly over SA. Highly significant effect on preference values for PFAD compared to SA (Cohen’s d > 0.8) was observed.
4. Discussion
The double-choice test is probably the most widely used method compare palatability values between two diets. Any pair of feeds can be offered to determine which one is preferred by measuring the amount consumed after a given period of time. To study the palatability of more than two diets, it is common to use a reference diet and deduce the relative palatability of feeds that have not been compared directly [29,30]. In this study, four different experimental diets were evaluated in a double-choice protocol without a reference diet: each hen was fed once with each possible two-way combination in a complete block design. Likewise, to rank the four fat sources, it was necessary to make six comparisons.
The specific role of fats, and particularly of acid oils, in palatability has not yet been investigated. It has been reported that chickens have higher intakes when long chain triglycerides are abundant in feed compared to medium chain triglycerides [13,39,40]. Furthermore, Sawamura et al. [19] observed that chickens prefer corn oil (that contains oleic acid and is linoleic acid-rich) rather than mineral oil. The current study showed robust evidence that hens prefer palm oils to soybean oils when given a free choice (p < 0.05). Similar results have been observed in pigs [41]. In contrast, a significant preference for SO over PO was reported in a long-term preference trial in laying hens [42]. Understanding the potential effects of the duration of experimental tests (short vs. long term) remains an elusive aspect in feeding choices that deserves additional research.
The aim of our study focused on the short-term reaction of the hens to the feed offered to assess palatability. Previous studies suggest that, if two isoenergetic feeds are compared (one with low and the other with high-fat content), birds will present higher intakes of the high-fat feed, presumably due to better palatability [4,43]. The different fat sources studied were added to a basal diet on a weight basis (6%) and it was decided not to adjust for energy value as this might affect feed palatability.
However, palatability depends not only on the sensory properties of the feed. There are many factors that may affect feed preference in double-choice trials: previous experiences, genetic background, physiological state and environmental and social context [30,44]. In the current study, all the hens were of the same breed and age and had a similar body weight. The birds were placed in individual cages to avoid animal interaction and eliminate errors associated with feeding competition. Furthermore, a training period was implemented prior to the start of the trial, as the absence of a prior learning process might reduce the ability of the hens to make sensible choices [45,46].
Potential preferences for feeder location were also taken into account in the experimental design. Switching container positions between measurements served to minimize the effect of hens favouring a particular position when feeds are clearly differentiated by flavour [8,19,27,47], although some authors maintain that this can confuse the animals and confound the results of the tests [30,48,49].
To validate the trial, control comparisons were included and no differences between the fat sources were observed for any of the controls tested (p > 0.05). The double reference control comparisons did not deviate significantly from 50% in any of the possible options (SO vs. SO, SA vs. SA, PO vs. PO and PFAD vs. PFAD). Therefore, any deviation from equal amounts of feed consumed when two types were presented together can be attributed to some desire on the part of the bird [23,28,29].
On another note, the energy density of the diets is a strong driver of intake [10]. However, small differences in energy content do not explain the differences in feed preference. For example, the SO is only 64 kcal/kg more energetic than SA diets (this is <2%) while difference in preference values was very large (62.10% and 37.90%, respectively). Thus, the results presented are consistent with the reports from Solà-Oriol et al. [41] in pigs in that the degree of saturation of fat sources contributes to palatability. Soybean diets have higher polyunsaturated fatty acid (PUFA) (SO = 58.51%; SA = 51.01%) than palm diets (PO = 24.26%; PFAD = 23.01%), which are more susceptible to oxidation than monounsaturated fatty acid (MUFA) and SFA [50]. The rate of FA oxidation increases in relation to their degree of unsaturation. Therefore, predominantly unsaturated oils (i.e., soybean oil) are more unstable than predominantly saturated oils (i.e., palm oil) [51,52,53]. Lipid oxidation produces peroxides which may further break down to form unpleasant flavour and aroma compounds characteristic of what is known as “oxidative rancidity” or “flavour reversion” [53]. 2-Pentyl furan has been identified as a major contributor to flavour reversion of SO [50,54]. On that note, these results are in partial contradiction with previous studies suggesting that unsaturated fatty acids maybe less preferred due to oxidation. FFA are more susceptible to autoxidation than esterified fatty acids and also act as strong pro-oxidants [55,56,57,58]. Although acid oils (having a high %FFA) also have a rancid odour, in this trial no aversion to PFAD was found in hens. Furthermore, we observed a highly significant effect (Cohen’s d > 0.8) between PFAD and SA diets. The lack of significant differences between preference levels for PO and PFAD suggest that high %FFA is acceptable to hens when it comes from a predominantly saturated oil. On the other hand, hens are sensitive to levels of FFA when provided with a choice between SO and SA. This is consistent with the findings of Mistry and Min [56], who reported that FFA decreased the surface tension of SO and increased the diffusion rate of oxygen into the oil, accelerating the oxidation process.
5. Conclusions
During diet formulation, the nutritional value of feedstuffs is usually the main criterion for selecting feed composition and palatability is often not considered. Few studies have addressed the effects of the UFA:SFA ratio, rancidity and FFA concentrations on avian taste preference. This study showed that the amount and type of dietary fat sources (%FFA and U:S ratio) affect feed preferences. Hens prefer predominantly saturated oils over unsaturated oils even when they are rich in FFA. However, a clear effect of FFA can be observed in unsaturated oils, with hens preferring feed with a low %FFA. These findings may allow nutritionists to choose oils to better suit laying hen preferences, especially at critical stages when feed intake may be otherwise depressed.
Acknowledgments
The authors wish to thank the support received from María Calero, Araceli Orozco, Patricia Rodríguez, Paula Úbeda and Zucami Poultry Equipment, SLU.
Author Contributions
Conceptualization, M.P., M.D.S., E.R. and C.G.-N.; data curation, M.P. and C.G.-N.; formal analysis, M.P., M.D.S. and C.G.-N.; funding acquisition, M.D.S.; investigation, M.P., R.S., O.P. and C.G.-N.; methodology, M.P., M.D.S., E.R. and C.G.-N.; validation, M.D.S. and C.G.-N.; visualization, M.P.; writing—original draft, M.P.; writing—review and editing, M.D.S., E.R., R.S., O.P. and C.G.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Generalitat Valenciana and the European Social Fund (GV/188/2018 and ACIF/2019/201).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu H.X., Rajapaksha P., Wang Z., Kramer N.E., Marshall B.J. An Update on the Sense of Taste in Chickens: A Better Developed System than Previously Appreciated. J. Nutr. Food Sci. 2018;8:686. doi: 10.4172/2155-9600.1000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roura E., Foster S.R. Nutrient-Sensing Biology in Mammals and Birds. Annu. Rev. Anim. Biosci. 2018;6:197–225. doi: 10.1146/annurev-animal-030117-014740. [DOI] [PubMed] [Google Scholar]
- 3.Kassarov L. Do cyanogenic glycosides and pyrrolizidine alkaloids provide some butterflies with a chemical defense against their bird predators? A different point of view. Behaviour. 2001;138:45–67. doi: 10.1163/156853901750077781. [DOI] [Google Scholar]
- 4.Roura E., Baldwin M.W., Klasing K.C. The avian taste system: Potential implications in poultry nutrition. Anim. Feed Sci. Tech. 2013;180:1–9. doi: 10.1016/j.anifeedsci.2012.11.001. [DOI] [Google Scholar]
- 5.Clark L., Hagelin J., Werner S. The chemical senses in birds. In: Scanes C., editor. Sturkie’s Avian Physiology. 6th ed. Academic Press; Boston, MA, USA: Elsevier Inc.; Boston, MA, USA: 2014. [(accessed on 30 November 2020)]. pp. 89–111. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=2612&context=icwdm_usdanwrc. [Google Scholar]
- 6.Niknafs S., Roura E. Nutrient sensing, taste and feed intake in avian species. Nutr. Res. Rev. 2018;31:256–266. doi: 10.1017/S0954422418000100. [DOI] [PubMed] [Google Scholar]
- 7.Goff S.A., Klee H.J. Plant Volatile Compounds: Sensory Cues for Health and Nutritional Value? Science. 2006;311:815–819. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- 8.Kalmendal R., Bessei W. The preference for high-fiber feed in laying hens divergently selected on feather pecking. Poult. Sci. 2012;91:1785–1789. doi: 10.3382/ps.2011-02033. [DOI] [PubMed] [Google Scholar]
- 9.Forbes J.M., Shariatmadari F.S. Diet selection for protein by poultry. World’s Poult. Sci. J. 1994;50:7–24. doi: 10.1079/WPS19940002. [DOI] [Google Scholar]
- 10.Bouvarel I., Nys Y., Lescoat P. Hen nutrition for sustained egg quality. In: Nys Y., Bain M., Van Immerseel V., editors. Improving the Safety and Quality of Eggs and Egg Products. 1st ed. Volume 1. Woodhead Publishing Limited; Cambridge, UK: 2011. pp. 261–299. [DOI] [Google Scholar]
- 11.Forbes J.M. Why did the chicken choose the food?; Proceedings of the 17th Australian Poultry Science Symposium; Sydney, Australia. 7–9 February 2005; Sydney, Australia: Poultry Research Foundation; 2005. pp. 145–152. [Google Scholar]
- 12.Molnár A., Hamelin C., Delezie E., Nys Y. Sequential and choice feeding in laying hens: Adapting nutrient supply to requirements during the egg formation cycle. World’s Poult. Sci. J. 2018;74:1–12. doi: 10.1017/S0043933918000247. [DOI] [Google Scholar]
- 13.Mabayo R.T., Okumura J., Hirao A., Sugita S., Sugahara K., Furuse M. The role of olfaction in oil preference in the chicken. Physiol. Behav. 1996;59:1185–1188. doi: 10.1016/0031-9384(95)02152-3. [DOI] [PubMed] [Google Scholar]
- 14.Bruce E.H., Prescott N.B., Wathes C.M. Preferred food rewards for laying hens in behavioural experiments. Br. Poult. Sci. 2003;44:345–349. doi: 10.1080/0007166031000085490. [DOI] [PubMed] [Google Scholar]
- 15.Cheled-Shoval S.L., Reicher N., Niv M.Y., Uni Z. Detecting thresholds for bitter, umami, and sweet tastants in broiler chicken using a 2-choice test method. Poult. Sci. 2017;96:2206–2218. doi: 10.3382/ps/pex003. [DOI] [PubMed] [Google Scholar]
- 16.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., Coutre J.I., Ninomiya Y., Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Running C.A., Craig B.A., Mattes R.D. Oleogustus: The Unique Taste of Fat. Chem. Senses. 2015;40:507–516. doi: 10.1093/chemse/bjv036. [DOI] [PubMed] [Google Scholar]
- 18.Meslin C., Desert C., Callebaut I., Djari A., Klopp C., Pitel F., Leroux S., Martin P., Froment P., Guilbert E., et al. Expanding Duplication of Free Fatty Acid Receptor-2 (GPR43) Genes in the Chicken Genome. Genome Biol. Evol. 2015;7:1332–1348. doi: 10.1093/gbe/evv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawamura R., Kawabata Y., Kawabata F., Nishimura S., Tabata S. The role of G-protein-coupled receptor 120 in fatty acids sensing in chicken oral tissues. Biochem. Biophys. Res. Commun. 2015;458:387–391. doi: 10.1016/j.bbrc.2015.01.125. [DOI] [PubMed] [Google Scholar]
- 20.Besnard P., Passilly-Degrace P., Khan N.A. Taste of fat: A sixth taste modality? Physiol. Rev. 2016;96:151–176. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Sanchez R., Tres A., Sala R., Garcés-Narro C., Guardiola F., Gasa J., Barroeta A.C. Effects of dietary free fatty-acid content and saturation degree on lipid-class composition and fatty-acid digestibility along the gastrointestinal tract in broiler starter chickens. Poult. Sci. 2019;98:4929–4941. doi: 10.3382/ps/pez253. [DOI] [PubMed] [Google Scholar]
- 22.Nuchi C.D., Guardiola F., Bou R., Bondioli P., Della Bella L., Codony R. Assessment of the levels of degradation in fat by-products for feed uses and their relationship with some lipid composition parameters. J. Agric. Food Chem. 2009;57:1952–1959. doi: 10.1021/jf803369h. [DOI] [PubMed] [Google Scholar]
- 23.Seabolt B.S., Heugten E., Kim S.W., Heugten A., Roura E. Feed preferences and performance of nursery pigs fed diets containing various inclusion amounts and qualities of distillers coproducts and flavor. J. Anim. Sci. 2010;88:3725–3738. doi: 10.2527/jas.2009-2640. [DOI] [PubMed] [Google Scholar]
- 24.Uzu G., Picard M., Dunnington E.A., Siegel P.B. Feed intake adjustments by hens to feeding regimens in which dietary methionine is varied. Poult. Sci. 1993;72:1656–1662. doi: 10.3382/ps.0721656. [DOI] [PubMed] [Google Scholar]
- 25.Zuberbuehler C.A., Messikommer R.E., Wenk C. Choice Feeding of Selenium-Deficient Laying Hens Affects Diet Selection, Selenium Intake and Body Weight. J. Nutr. 2002;132:3411–3417. doi: 10.1093/jn/132.11.3411. [DOI] [PubMed] [Google Scholar]
- 26.Barkley G.R., Miller H.M., Forbes J.M. The ability of laying hens to regulate phosphorus intake when offered two feeds containing different levels of phosphorus. Br. J. Nutr. 2004;92:233–240. doi: 10.1079/BJN20041182. [DOI] [PubMed] [Google Scholar]
- 27.Chagneau A.M., Bessonneau D., Bouchot C., Lescoat P., Picard M., Lessire M. Broiler Short-Term Feed Preferences Measured with SRAbox, a New Feed Choice Procedure. Poult. Sci. 2006;85:808–815. doi: 10.1093/ps/85.4.808. [DOI] [PubMed] [Google Scholar]
- 28.Cerrate S., Coto C., Wang Z., Yan F., Costa F.G., Waldroup P.W. Choice Feeding of Two Different Broiler Strains Using Diets with Constant Energy Level. Int. J. Poult. Sci. 2008;7:726–737. doi: 10.3923/ijps.2008.726.737. [DOI] [Google Scholar]
- 29.Solà-Oriol D., Roura E., Torrallardona D. Use of double-choice feeding to quantify feed ingredient preferences in pigs. Livest. Sci. 2009;123:129–137. doi: 10.1016/j.livsci.2008.10.015. [DOI] [Google Scholar]
- 30.Forbes J.M. Palatability: Principles, methodology and practice for farm animals. CAB Rev. 2010;5:1–15. doi: 10.1079/PAVSNNR20105052. [DOI] [Google Scholar]
- 31.Roura E., Navarro M. Physiological and metabolic control of diet selection. Anim. Prod. Sci. 2018;58:613–626. doi: 10.1071/AN16775. [DOI] [Google Scholar]
- 32.Yoshida Y., Kawabata F., Kawabata Y., Nishimura S., Tabata S. Short-term perception of and conditioned taste aversion to umami taste, and oral expression patterns of umami taste receptors in chickens. Physiol Behav. 2018;191:29–36. doi: 10.1016/j.physbeh.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. [(accessed on 30 November 2020)];Off. J. Eur. Union L. 2010 276:33–79. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF. [Google Scholar]
- 34.FEDNA . Necesidades Nutricionales para Avicultura: Normas FEDNA. 2nd ed. Fundación Española para el Desarrollo de la Nutrición Animal; Madrid, Spain: 2018. [(accessed on 30 November 2020)]. pp. 108–109. Available online: http://www.fundacionfedna.org/sites/default/files/NORMAS_FEDNA_AVES_2018v.pdf. [Google Scholar]
- 35.Sukhija P.S., Palmquist D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988;36:1202–1206. doi: 10.1021/jf00084a019. [DOI] [Google Scholar]
- 36.Kim J.-H. Diet Selection for Protein Quality by Growing Broiler Chickens. Int. J. Poult. Sci. 2014;13:461–466. doi: 10.3923/ijps.2014.461.466. [DOI] [Google Scholar]
- 37.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 38.IBM Corp. IBM SPSS Statistics for Windows. IBM Corp.; Armonk, NY, USA: 2016. Version 24.0; Released. [Google Scholar]
- 39.Furuse M., Mabayo R.T., Choi Y.H., Denbow D.M., Okumura J. Feeding behaviour in chickens given diets containing medium chain triglyceride. Br. Poult. Sci. 1993:211–217. doi: 10.1080/00071669308417577. [DOI] [PubMed] [Google Scholar]
- 40.Furuse M., Mabayo R.T., Okumura J. The role of gustation in oil preference in the chicken. [(accessed on 30 November 2020)];Jpn. Poult. Sci. 1996 33:256–260. Available online: https://www.jstage.jst.go.jp/article/jpsa1964/33/4/33_4_256/_pdf. [Google Scholar]
- 41.Solà-Oriol D., Roura E., Torrallardona D. Feed preference in pigs: Effect of selected protein, fat, and fiber sources at different inclusion rates. J. Anim. Sci. 2011;89:3219–3227. doi: 10.2527/jas.2011-3885. [DOI] [PubMed] [Google Scholar]
- 42.Dänicke S., Halle I. Effects of iso-energetic low-fat feed, soybean oil or palm oil containing feed offered to laying hens, either separately or for self-selection, on feed intake, laying and reproductive performance and on egg quality. [(accessed on 30 November 2020)];Landbauforschung Völkenrode. 2002 52:239–247. Available online: https://literatur.thuenen.de/digbib_extern/zi028458.pdf. [Google Scholar]
- 43.Klasing K.C. Lipids. In: Klasing K.C., editor. Comparative Avian Nutrition. CAB International; Wallingford, UK: 1998. pp. 171–200. [Google Scholar]
- 44.Nielsen B.L. On the interpretation of feeding behaviour measures and the use of feeding rate as an indicator of social constraint. Appl. Anim. Behav. Sci. 1999;63:79–91. doi: 10.1016/S0168-1591(99)00003-9. [DOI] [Google Scholar]
- 45.Kyriazakis I., Tolkamp B.J., Emmans G. Diet selection and animal state: An integrative framework. Proc. Nutr. Soc. 1999;58:765–772. doi: 10.1017/S0029665199001044. [DOI] [PubMed] [Google Scholar]
- 46.Waldburg-Zeil C.G., Staaveren N., Harlander-Matauschek A. Do laying hens eat and forage in excreta from other hens? Animal. 2018;13:367–373. doi: 10.1017/S1751731118001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blavi L., Solà-Oriol D., Crespo F.J., Serra M.M., Pérez J.F. The effects of including increasing doses of stevia and neohesperidine dihydrochalcone on feed preference in young piglets. J. Anim. Sci. 2016;94:138–141. doi: 10.2527/jas.2015-9810. [DOI] [Google Scholar]
- 48.Steinruck U., Kirchgessner M., Roth F.X. Selective methionine intake of broilers by changing the position of the diets. Arch. Geflugelkd. 1990;54:245–250. [Google Scholar]
- 49.Ettle T., Roth F.X. Dietary selection for lysine by piglets at differing feeding regimen. Livest. Sci. 2009;122:259–263. doi: 10.1016/j.livsci.2008.09.007. [DOI] [Google Scholar]
- 50.Kim H.J., Min D.B. Chemistry of Lipid Oxidation. In: Akoh C.C., Min D.B., editors. Food Lipids: Chemistry, Nutrition, and Biotechnology. 3rd ed. CRC Press; Boca Raton, FL, USA: Taylor & Francis Group; Boca Raton, FL, USA: 2008. pp. 299–320. [DOI] [Google Scholar]
- 51.Frankel E.N. Chemistry of autoxidation: Mechanism, products and flavor significance. In: Min D.B., Smouse T.H., editors. Flavor Chemistry of Fats and Oils. 1st ed. American Oil Chemists’ Society; Champaign, IL, USA: 1985. pp. 1–37. [Google Scholar]
- 52.Erickson M. Lipid Oxidation of Muscle Foods. In: Akoh C.C., Min D.B., editors. Food Lipids: Chemistry, Nutrition, and Biotechnology. 3rd ed. CRC Press; Boca Raton, FL, USA: Taylor & Francis Group; Boca Raton, FL, USA: 2008. pp. 321–364. [DOI] [Google Scholar]
- 53.ISEO . Food, Fats and Oils. 10th ed. Institute of Shortening and Edible Oils; Washington, DC, USA: 2016. [(accessed on 30 November 2020)]. pp. 19–21. Available online: http://www.iseo.org/httpdocs/FoodFatsOils2016.pdf. [Google Scholar]
- 54.Smouse T.H., Chang S.S. A systematic characterization of the reversion flavor of soybean oil. J. Am. Oil. Chem. Soc. 1967;44:509–514. doi: 10.1007/BF02908549. [DOI] [PubMed] [Google Scholar]
- 55.Miyashita K., Takagi T. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 1986;63:1380–1384. doi: 10.1007/BF02679607. [DOI] [Google Scholar]
- 56.Mistry B.S., Min D.B. Effects of Fatty Acids on the Oxidative Stability of Soybean Oil. J. Food Sci. 1987;52:831–832. doi: 10.1111/j.1365-2621.1987.tb06741.x. [DOI] [Google Scholar]
- 57.Choe E. Effects and Mechanisms of Minor Compounds in Oil on Lipid Oxidation. In: Akoh C.C., Min D.B., editors. Food Lipids: Chemistry, Nutrition, and Biotechnology. 3rd ed. CRC Press; Boca Raton, FL, USA: Taylor & Francis Group; Boca Raton, FL, USA: 2008. pp. 449–474. [DOI] [Google Scholar]
- 58.Waraho T., McClements D.J., Decker E.A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011;129:854–859. doi: 10.1016/j.foodchem.2011.05.034. [DOI] [PubMed] [Google Scholar]