Abstract
The significant increase in the production and variety of nanoparticles (NPs) has led to their release into the environment, especially into the marine environment. Titanium dioxide nanoparticles (TiO2-NPs) are used in different industrial sectors, from the food industry to several consumer and household products. Since the aquatic environment is highly sensitive to contamination by TiO2-NPs, this work aimed to give a preliminary assessment of the contamination of packaged seafood, where the food additive TiO2 (E171) is not to be intentionally added. This allowed providing a chemical characterization and quantification of TiO2-NPs in processed canned fish products belonging to different trophic positions of the pelagic compartment and in canned clam. The new emerging technique called single-particle inductively coupled plasma mass spectrometry (spICP-MS) was applied, which allows the determination of nanoparticle number-based concentration, as well as the dissolved titanium. This study highlights how processed food, where the pigment E171 was not intentionally added, contains TiO2 in its nanoparticle form, as well as dissolved titanium. Processed clam represented the seafood with the highest content of TiO2-NPs. In pelagic fish species, we found progressively higher levels and smaller sizes of TiO2-NPs from smaller to larger fish. Our results highlight the importance of planning the characterization and quantification of TiO2-NPs in food both processed and not, as well as where the pigment E171 is intentionally added and not, as it is not the only source of TiO2-NPs. This result represents a solid step toward being able to estimate the real level of dietary exposure to TiO2-NPs for the general population and the related health risks.
Keywords: titanium dioxide, nanoparticle, spICP-MS, processed food, dietary intake, E171
1. Introduction
In Earth science, nanoparticles (1–100 nm) are important components of the biogeochemical system, but human impact on the environment has altered their natural cycle [1] by introducing engineered nanoparticles with several physicochemical characteristics and applications [2]. Nanoparticles represents a blooming industrial sector destined to increase because of the numerous investments that it is able to attract. Due to their peculiar characteristics (e.g., different optical properties, greater flexibility, resistance, reactivity, electrical conductivity, or absorption), nanoparticles (NPs) are finding more and more applications in many consumer products, such as conductors, cosmetics, plastics, and agents used in environmental recovery, as well as in various sectors such as the pharmaceutical, food, biomedical, military, and automotive industries [3,4].
Our study focuses on titanium dioxide (TiO2), a metal oxide naturally occurring in three polymorphic forms known as rutile, anatase, and brookite [2]. It is synthetized as titanium dioxide nanoparticles (TiO2-NPs) for several industrial sectors. In the food industry, TiO2 is authorized as a food additive (E 171) in accordance with the European Regulation (EC) No. 1333/2008, in both anatase and rutile forms (Commission Regulation (EU) No. 231/2012) [5]. It is added for whitening and brightening purposes in many foods including milk and dairy products, chewing gum, ice cream, and all sweets where it is included in the coating of sugar confectionary [5,6]. Nevertheless, it is not considered a nanomaterial by the current “European Commission recommendation for the definition of nanomaterials”. Accordingly, a nanomaterial should consist for 50% or more of particles having a size between 1 and 100 nm (Regulation 211/696/EU), but E171 may contain up to 3.2% of its weight in nanoparticles. TiO2 in its nanoform is also used in plastic packaging, as titanium dioxide surface-treated with fluoride-modified alumina, to improve the quality and the shelf-life of products. According to the European Food Safety Authority (EFSA) safety assessment, the substance does not raise safety concerns for the consumer if used as an additive up to 25% w/w in polymers in contact with all food types in any time and temperature conditions [7]. TiO2-NPs are also used in several consumer and household products to which humans can be potentially exposed such as toothpaste, sun cream, creams, and lip balm [8,9,10]. Other uses of the manufacturer TiO2 nanomaterial known as P25 include antimicrobial applications, paints, catalysts for air and water purification, medical applications, and energy storage [11]. Despite their favorable properties, NPs are known to cause local adverse effects and/or systemic toxicity [12].
The significant increase in the production and variety of nanomaterials has led to their release into the environment [13], especially into the marine environment, as the NPs are not completely removed from domestic and industrial wastes after water treatment [14,15]. This increases both the ecological risks for the ecosystems and the likelihood of inhalation or dermal and oral exposure for humans [16]. In toxicological studies concerning TiO2-NPs, most researchers utilized the industrial-grade nanoform of TiO2, although food grade one represents the majority of TiO2-containing materials that enter the ecosystem today [17]. The choice depends on the fact that it is commonly used, due to the primary crystals being relatively uniform and less than 50 nm in size. Studies also demonstrated a difference in the removal efficiency of food-grade TiO2, which is lower than industrial-grade TiO2. For this reason, food-grade TiO2 accumulates preferably in the environment, involving an environmental and human health risk [17].
The oral exposure route of TiO2-NPs has been the least investigated. To date, despite the existing numerous applications of TiO2-NPs, little or nothing is known about the bioaccumulation potential of TiO2-NPs along the food chain and the dietary exposure dose to the general population. Nevertheless, as this compound has a limited elimination rate and as it can be absorbed by the gastrointestinal tract in a size-dependent manner and pass through the mucus pores to enter other organs [18], some studies suggested a potential health risk such as disorders of the gut microbiota and gut-associated metabolism in vivo [19], apoptosis induction [20], hepatic toxicity [21], a potential risk for liver, ovaries, and testes [22], an impact on lipid metabolism [23], and an increased abundance of proinflammatory immune cells and cytokines in the colonic mucosa [24].
The risk to humans is through food chain transport because TiO2 has a high tendency to bioaccumulate in aquatic organisms, and this implies that TiO2 particles could be transferred from one species to another [17].
Since the aquatic environment is highly sensitive to contamination by TiO2-NPs [13], this work aimed to give a preliminary assessment of the contamination of packaged food derived from marine environment. According to the literature, very few studies reported the levels of nanoparticles in marine organisms [25,26,27,28,29].
With this study, we wanted to provide a chemical characterization and quantification of TiO2-NPs (<100 nm), TiO2 total particles (TiO2-Ps-Tot, all particle sizes), dissolved Ti, and total Ti in processed canned seafood products belonging to different trophic positions. Our choice to use canned seafood for this evaluation study was because it is a fundamental component in human nutrition, representing an important source of unsaturated fatty acids, protein, and different micronutrients [30].
Furthermore, we also provided data related to the estimated daily intake for both adults and children, which may be useful to risk assessors for developing a provisional tolerable daily intake for TiO2-NPs.
2. Methods
2.1. Alkaline Digestion and Single-Particle Inductively Coupled Plasma Mass Spectrometry (spICP-MS) Analysis
Different brands of canned tuna (five), mackerel (four), anchovy (three), and clam (three), among the best-selling and low-cost brands, were purchased from the main Italian supermarket chains in the city of Catania, (Italy), in the period between June and July 2019, and stored at −80 °C until analysis. For each brand of seafood product, we also chose to purchase three different batches, which were extracted and processed in triplicate. Thus, we performed a total of 45 extractions for canned tuna, 36 for canned mackerel, 27 for canned anchovy, and 37 for canned clam.
Assessment of TiO2 was performed using a NexION® 350D (Perkin Elmer, Waltham, MA, USA) with the Syngistix Nano Application software (Perkin Elmer, Waltham, MA, USA). The instrumental operating conditions for the determination of TiO2 are listed in Table 1. The dwell time used was chosen on the basis of other studies [27,31].
Table 1.
NexION® 350D inductively coupled plasma mass spectrometry (ICP-MS) instrumental condition for single particles analysis. RF, radiofrequency.
| Parameter | Value |
|---|---|
| Nebulizer, flow | Meinhard, 1 mL/min |
| Spray chamber | Glass cyclonic |
| Sample uptake rate | 0.26–0.28 mL/min |
| RF power | 1600 W |
| Analysis mode | Standard |
| Quadrupole settling time | 0 µs |
| Analyte | Ti 48.9 |
| Dwell time | 50 µs |
| Data acquisition time | 60 s |
| Density | 4.23 g/cm3 |
| Ti mass fraction | 60% |
The new emerging technique of single-particle inductively coupled plasma mass spectrometry (spICP-MS) allows the determination of particle number-based concentration, with rapid simultaneous characterization of the elemental composition, number of particles, size and size distribution, and dissolved concentration. Furthermore, by modifying the integration window, it is possible to collect data related to a specific size distribution. Accordingly, for each sample, we captured data on the total TiO2 particles (Ps-Tot) and TiO2 nanoparticles (NPs < 100 nm) only.
A titanium nanoparticle stock solution was prepared from a TiO2-NP standard (60 nm TiO2 nanopowder, rutile, 99.9%, AEM) purchased from Nanovision (Brugherio, MB, Italy), while a Ti ion standard (1000 mg/L, CPAchem) was used for the spICP-MS calibration of titanium.
To support the quality of spICP-MS measurements, the particle size distribution of the powder TiO2-NPs standard was assessed as follows: a TiO2-NP stock suspension (215 ng/L or 4.5 × 105 particles/mL) was prepared in ultrapure water and dispersed for 30 min at 37 °C using an ultrasonic bath, to maximize a homogeneous dispersion. The transport efficiency (TE%) was calculated with the certified reference material PELCO (Ag-NPs, 39 ± 5 nm, 110 ng/L, monitoring m/z 107) under the same instrumental conditions as the samples, obtaining a value of TE% 2.54. This solution gave a TiO2-NP concentration of 4.6 × 105 ± 0.16 × 105 particles/mL (n = 10), in agreement with the particle concentration prepared. TiO2-NPs showed a size range of 44–85 nm with a mean size of 66.2 ± 3.0 (n = 10) and a modal size of 56.6 ± 4.4 (n = 10). The results obtained were in compliance with the size of the TiO2-NP standard supplied by the manufacturer (60 nm).
Before performing the spICP-MS analysis, an alkaline digestion of the samples was performed using the method described by Gray et al. (2013) [32]. Approximately 0.5 g of wet sample tissue was weighed in DigiTUBEs (SCP Science, Baie D’Urfé, Québec, Canada) and mixed with 5 mL of tetramethylammonium hydroxide (TMAH, 20% v/v), which is a strong organic base capable of digesting tissues and releasing nanoparticles without altering them. A vortex was used, at first, to prevent the tissues from sticking to the walls of the container used for digestion. The TiO2 extraction was obtained through sonication for 30 min at 37 °C using an ultrasonic bath to accelerate tissue breakdown and prevent particle aggregation. Subsequently, the samples were left to digest for another 24 h at room temperature. At the end of digestion, samples were diluted with MilliQ water (Millipore, Bedford, MA, USA) to 1% TMAH concentration before analysis and 0.1% Triton X-100 to allow the detection of single particles.
All samples and calibration solutions were sonicated for 30 min before being analyzed. To avoid contamination between samples, the system was rinsed with nitric acid (2%, v/v) prior to the measurement. The TiO2-NP standard was also used for the determination of the transport efficiency within the 3–8% range, in agreement with the TE obtained with an Ag certified reference material.
The effect of the extracting solution on the size distribution and particle concentration was studied in triplicate, simultaneously evaluating the TiO2 standard in ultrapure water (n1 = 3) and TMAH 1% (n2 = 3), at the same concentration (215 ng/L or 4.5 × 105 particles/mL) we used for determining the transport efficiency. We obtained a particle concentration of 4.3 × 105 ± 5.0 × 104 in water and 4.9 × 105 ± 3.6 × 104 in TMAH 1%, with recoveries of 97.6% ± 10.5% and 108.8% ± 7.2%, respectively. In addition, the results revealed that the alkaline digestion in TMAH 1% did not affect the TiO2 particle size distribution (mean size and modal size). The mean size of TiO2 in ultrapure water (63.8 nm ± 3.0) and mean size of TiO2 in TMAH 1% (61.1 nm ± 4.3) were statistically homogeneous according to a two tailed t-test (p = 95%; n1 + n2 − 2) (tcalculated = 1.5 < ttabulated = 2.8). Moreover, the modal size was statistically homogeneous in ultrapure water (57.6 ± 3.2) and in TMAH 1% (56.0 ± 4.0) (tcalculated = 0.88; ttabulated = 2.8).
The limit of detection (LOD) and the limit of quantification (LOQ) were calculated by analyzing 10 alkaline extract blanks, in the same analytical condition of the samples, on the basis of the mean ± 3 SD and the mean ± 10 SD criteria of the number of particles/mL obtained, respectively. The LOD was 1.3 × 103 particles/mL, while the LOQ was 2.5 × 103 particles/mL. Referring to the sample weight and digestion volume used, these values were equivalent to 2.6 × 105/g and 5.0 × 105/g, respectively.
In addition, the LOD in size (LODnm) was estimated at 35 nm using Equation (1) [26,33].
| (1) |
where is three times the standard deviation of the counts/dwell time of alkaline blanks (1% TMAH), R is the slope of the calibration curve of ionic Ti solutions, fa is the mass fraction of the analyzed metallic element in the TiO2 NPs, and ρ is the density of the TiO2 NPs.
For each batch of analysis, a quality control was performed with analytical recovery before and after spiking with 60 nm TiO2-NPs at a concentration of 5 µg/L, corresponding to a concentration of 1.1 × 106 parts/mL. The values (range 90–95%) were calculated for the whole size distribution by dividing the TiO2-NP concentration by the TiO2-NP concentration found in the solution of TiO2-NPs used for spiking and multiplying by 100.
Accuracy was tested using a laboratory-fortified matrix (LFM) with a seafood sample spiked with 5 µg/L of TiO2-NPs (60 nm). An LFM was processed at each batch of digestion, obtaining a concentration of 9.8 × 105 ± 4.6 × 105 particles/mL (1.9 × 108 ± 9.3 × 107 particles/g) and a recovery range of 87–121% (n = 5). The measured mean size of TiO2-NPs in alkaline digested samples was 64.2 ± 5.1 nm.
2.2. Acid Digestion and ICP-MS Analysis of Total Ti
For the determination of total titanium, the same samples were processed by acid digestion. Aliquots of 0.5 g of wet samples were weighed in Teflon reactors using an analytical balance (Mettler Toledo) and then digested in a microwave oven (Ethos, TC, Milestone, Sorisole (BG), Italy), by adding 6 mL of 67% super-pure nitric acid (HNO3; Carlo Erba, Italy) and 2 mL of 30% hydrogen peroxide (H2O2; Carlo Erba, Italy) for 1 h at 80 °C. After acid digestion, all samples were diluted to 50 mL with ultrapure water and were filtrated through a 0.45 µm membrane filter before analysis. Total Ti was quantified with the same inductively coupled plasma mass spectrometer (ICP-MS NexION® 350D, Perkin Elmer, Waltham, MA, USA) in standard mode, using the standard addition technique covering the concentration from 0 to 50 µg/L. Instrumental condition for Total Ti determination are showed in Table 2.
Table 2.
NexION® 350D ICP-MS instrumental condition for Total Ti in standard mode.
| Parameter | Value |
|---|---|
| Nebulizer, flow | Meinhard, 0.89 mL/min |
| Spray chamber | Glass cyclonic |
| RF power | 1600 W |
| Analogic phase voltage | −1950 V |
| Pulses voltage | 1300 V |
| Discriminator threshold | 12 |
| Deflector voltage | −12 V |
| Analysis mode | Standard |
| Analyte | Ti 48.9 |
| Internal standard | Y |
A single-element standard solution of Ti (1000 mg/L in 5% di HNO3, 0.5% HF) was purchased from CPAchem. Standards for instrument calibration were prepared in the same acid matrix, and yttrium (Y) was used as an internal standard to verify the accuracy.
To verify if acid digestion of the samples allowed the total dissolution of TiO2 particles and then the quantification of total Ti, we conducted a preliminary acid digestion of 4 µg/L ionic Ti (n = 6), canned clam (n = 6), and canned tuna (n = 6). The quantification of TiO2 particles was detected using Syngistix Nano Application software, and the resulting background signals, expressed as particles/mL, are shown in Table 3.
Table 3.
Descriptive statistics concerning the quantification of TiO2 particles (Ps-Tot) expressed as particles/mL detected with Syngistix Nano Application software after acid digestion of the samples.
| Statistics | 4 µg/L Ionic Ti (n = 6) | Canned Clam (n = 6) | Canned Tuna (n = 6) |
|---|---|---|---|
| Mean of particles/mL | 564 | 588 | 653 |
| SD | 155 | 267 | 237 |
| Min | 324 | 267 | 252 |
| Max | 758 | 901 | 918 |
| Mean + 3 SD | 1029 | - | - |
The threshold value of TiO2 particles was estimated as the mean + 3 SD given by ionic Ti, which resulted in 1029 particles/mL. Digested samples of canned clam and canned tuna did not show background values of TiO2 particles higher than the calculated threshold, demonstrating the effectiveness of the acid digestion in dissolving all TiO2 particles.
The LOD and LOQ were calculated by analyzing 10 acid extract blanks according to the mean ± 3 SD and mean ± 10 SD criteria, respectively. They resulted 0.06 and 0.16 mg/kg, respectively.
For each batch of analysis, a quality control was performed with analytical recovery after spiking with 20 µg/L of ionic Ti.
Accuracy was tested using a laboratory-fortified matrix with a seafood sample (use of ionic Ti at 20 µg/L) processed at each batch of digestion, obtaining a recovery range of ionic Ti from 92–115%.
2.3. Estimated Meal Intake
An exposure assessment derived from the consumption of selected seafood products was conducted for TiO2-NPs (TiO2 particles <100 nm), TiO2-Ps-Tot (TiO2 total particles) and dissolved Ti according to the method described in a previous paper [34]. Briefly, the estimated meal intake (EDI) (mg/kg body weight (BW) per day) was determined according to Equation (2).
| EMI = (C × M)/BW, | (2) |
where C is the TiO2-NPs, TiO2-Ps-Tot, or dissolved Ti (mg/kg wet weight), M is the meal size (0.227 kg for adults and 0.114 kg for children) [34], and BW is the body weight, considered as 16 kg for children (6 years) and 70 kg for adults (70 years) [35].
2.4. Statistical Analysis
The statistical software package IBM SPSS 20.0 (IBM, Armonk, NY, USA) was used for statistical analysis. One-way ANOVA and a post hoc Tukey test were performed to evaluate differences in TiO2 and dissolved Ti levels among canned products. We processed a total of 45 samples of canned tuna, 36 of canned mackerel, 27 of canned anchovy, and 37 of canned clam.
3. Results
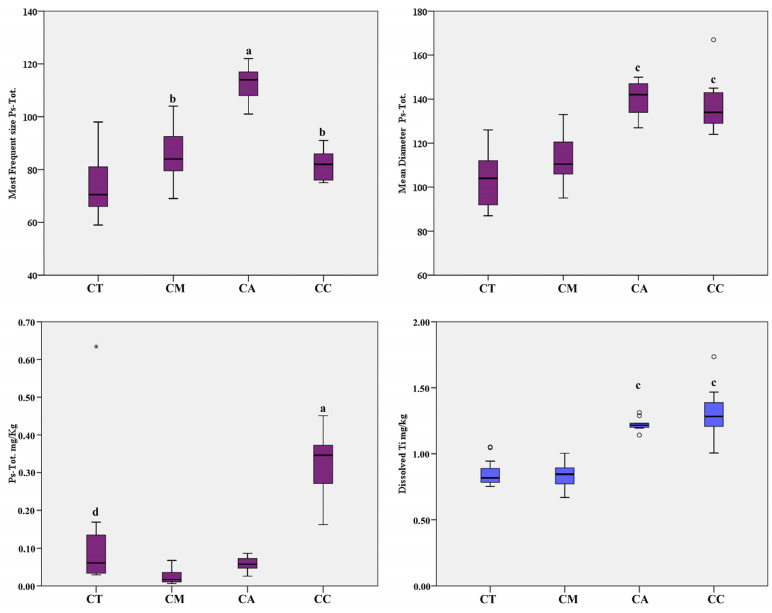
The total TiO2 particles present (Ps-Tot size), as well as the level of Ti in its dissolved form, were analyzed using the NexION® 350D (Perkin Elmer, Waltham, MA, USA) with the Syngistix Nano Application software, by processing the samples with ultrasound-assisted alkaline digestion. In a separate working session, we also determined the level of total Ti measured with ICP-MS in standard mode, by processing the samples digested with acid. As shown in Table 4, the quantification of dissolved Ti did not differ significantly from the total Ti. The slightly higher result of total Ti can be related to the acid dissolution of the Ti bonded in the particles, as supported in the experimental data shown in Table 3. As shown in Table 4 and Figure 1, canned anchovy and canned clam revealed the highest mean diameters of Ps-Tot size, close to 140 nm, significantly higher than the mean diameters found in canned tuna and canned mackerel, close to 100 nm. The modal size was found to be significantly higher in canned anchovy versus the other seafood products, followed by canned mackerel, canned clam, and canned tuna. With regard to the Ps-Tot level, the samples of canned clam had the highest levels of TiO2-NPs compared to the fish species, with a mean value of 0.326 mg/kg. Among fish species, canned tuna had levels of TiO2-NPs significantly higher than canned mackerel and anchovy, with a mean value of 0.117 mg/kg. Considering the ionic Ti determined using the single-particle technique, canned clam and canned anchovy had higher levels than the other seafood products, with mean values of 1.31 and 1.22 mg/kg, respectively.
Table 4.
Descriptive statistics concerning the chemical characterization and quantification of total TiO2 particles (Ps-Tot), dissolved Ti (mg/kg), and total Ti in packaged seafood products.
| Canned Tuna | Most Frequent Size Ps-Tot. (nm) | Mean Diameter Ps-Tot. (nm) | Number of Ps-Tot/g. | Ps-Tot. mg/kg | Dissolved Ti a mg/kg | Total Ti b mg/kg |
| Mean | 73 | 104 | 9.77 × 107 | 0.117 | 0.851 | 1.015 |
| SD | 11 | 12 | 6.27 × 107 | 0.156 | 0.099 | 0.121 |
| Min. | 59 | 87 | 1.93 × 107 | 0.029 | 0.752 | 0.857 |
| Max. | 98 | 126 | 2.86 × 108 | 0.634 | 1.052 | 1.152 |
| Canned Mackerel | Most Frequent size Ps-Tot. (nm) | Mean Diameter Ps-Tot. (nm) | Number of Ps-Tot/g. | Ps-Tot. mg/kg | Dissolved Ti a mg/kg | Total Ti b mg/kg |
| Mean | 85 | 112 | 1.47 × 107 | 0.025 | 0.835 | 1.154 |
| SD | 11 | 11 | 7.17 × 106 | 0.021 | 0.095 | 0.108 |
| Min. | 69 | 95 | 8.15 × 106 | 0.007 | 0.669 | 0.951 |
| Max. | 104 | 133 | 2.72 × 107 | 0.068 | 1.003 | 1.185 |
| Canned Anchovy | Most Frequent size Ps-Tot. (nm) | Mean Diameter Ps-Tot. (nm) | Number of Ps-Tot/g. | Ps-Tot. mg/kg | Dissolved Ti a mg/kg | Total Ti b mg/kg |
| Mean | 113 | 141 | 1.67 × 107 | 0.059 | 1.223 | 1.385 |
| SD | 7 | 8 | 3.12 × 106 | 0.019 | 0.051 | 0.148 |
| Min. | 101 | 127 | 1.06 × 107 | 0.026 | 1.141 | 0.985 |
| Max. | 122 | 150 | 2.02 × 107 | 0.087 | 1.312 | 1.485 |
| Canned Clam | Most Frequent size Ps-Tot. (nm) | Mean Diameter Ps-Tot. (nm) | Number of Ps-Tot/g. | Ps-Tot. mg/kg | Dissolved Ti a mg/kg | Total Ti b mg/kg |
| Mean | 82 | 138 | 1.05 × 108 | 0.326 | 1.313 | 1.458 |
| SD | 6 | 13 | 1.52 × 107 | 0.084 | 0.210 | 0.341 |
| Min. | 75 | 124 | 7.00 × 107 | 0.162 | 1.005 | 1.145 |
| Max. | 91 | 167 | 1.00 × 108 | 0.451 | 1.735 | 1.915 |
a Ultrasound-assisted alkaline digestion and spICP-MS determination. b Microwave-assisted acid digestion and ICP-MS determination in standard mode.
Figure 1.
Box-plot distribution of TiO2-Ps-Tot modal size (nm), mean diameter (nm), level of Ps-Tot, and dissolved Ti (mg/kg) in packaged seafood products. Legend: CT, canned tuna; CM, canned mackerel; CA, canned anchovy; CC, canned clam; a, p < 0.001 vs. all; b, p < 0.05 vs. CT; c, p < 0.001 vs. CT and CM; d p < 0.05 vs. CM and CA. ° Outliers values of the distribution. * Extreme values of the distribution.
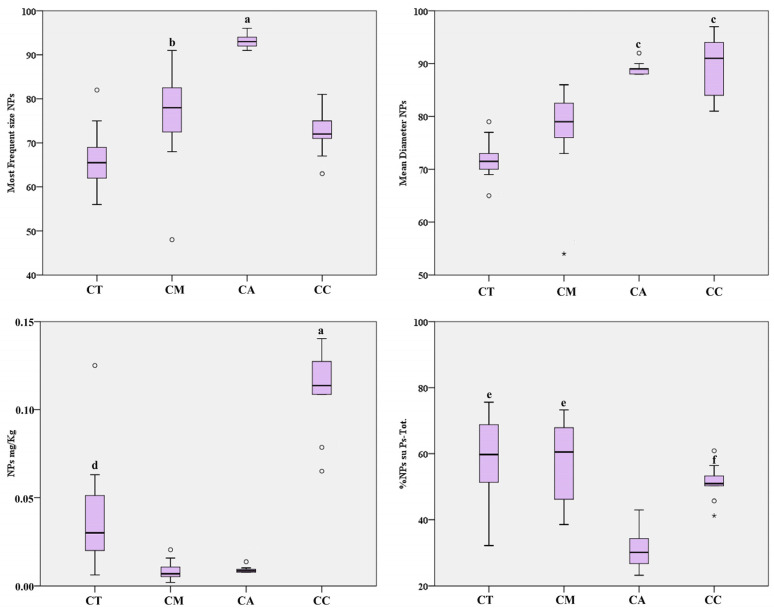
For each sample, by reducing the integration window of particle size, we selected the particle size distribution <100 nm to quantify the nanoparticles only. The analyses carried out showed the presence of TiO2-NPs, with a diameter lower than 100 nm, in all the seafood samples. As shown in Table 5 and Figure 2, with regard to NPs size, canned anchovy and canned clam revealed the highest mean diameters, close to 90 nm, higher than the mean diameters found in canned tuna and canned mackerel (both below 80 nm). The modal size was found to be significantly higher in canned anchovy versus the other seafood products with a mean value of 93 nm, followed by canned mackerel, canned clam, and canned tuna. With regard to the NP level, the samples of canned clam had the highest levels of TiO2-NPs compared to the fish species, with a mean value of 0.112 mg/kg. Among fish species, canned tuna had levels of TiO2-NPs fourfold higher than canned mackerel and anchovy, with a mean value of 0.038 mg/kg. Furthermore, the percentage of TiO2-NPs in the TiO2 total particles was close to 60% in all seafood products except for canned anchovy, which showed a percentage close to 30% (Table 5 and Figure 2).
Table 5.
Descriptive statistics concerning the chemical characterization and quantification of TiO2 nanoparticles (TiO2-NPs) (<100 nm) in packaged seafood products.
| Canned Tuna | Most Frequent Size NPs (nm) | Mean Diameter NPs (nm) | Number of NPs/g | NPs mg/kg | %NPs on Ps-Tot. |
| Mean | 66 | 72 | 5.26 × 107 | 0.0380 | 58.5 |
| SD | 7 | 4 | 2.19 × 107 | 0.0298 | 13.3 |
| Min. | 56 | 65 | 9.34 × 106 | 0.0062 | 32 |
| Max. | 82 | 79 | 1.02 × 108 | 0.1251 | 76 |
| Canned Mackerel | Most Frequent size NPs (nm) | Mean Diameter NPs (nm) | Number of NPs/g | NPs mg/Kg | %NPs on Ps-Tot. |
| Mean | 77 | 78 | 7.84 × 106 | 0.0085 | 57.4 |
| SD | 11 | 8 | 2.42 × 106 | 0.0053 | 12.2 |
| Min. | 48 | 54 | 5.24 × 106 | 0.0020 | 39 |
| Max. | 91 | 86 | 1.23 × 107 | 0.0206 | 73 |
| Canned Anchovy | Most Frequent size NPs (nm) | Mean Diameter NPs (nm) | Number of NPs/g | NPs mg/Kg | %NPs on Ps-Tot. |
| Mean | 93 | 89 | 5.16 × 106 | 0.0092 | 31.6 |
| SD | 2 | 1 | 9.30 × 105 | 0.0019 | 6.78 |
| Min. | 91 | 88 | 4.36 × 106 | 0.0078 | 23 |
| Max. | 96 | 92 | 7.24 × 106 | 0.0137 | 43 |
| Canned Clam | Most Frequent size NPs (nm) | Mean Diameter NPs (nm) | Number of NPs/g | NPs mg/Kg | %NPs on Ps-Tot. |
| Mean | 72 | 89 | 5.37 × 107 | 0.1116 | 51.4 |
| SD | 5 | 6 | 8.34 × 106 | 0.0254 | 5.70 |
| Min. | 63 | 81 | 4.00 × 107 | 0.0650 | 41 |
| Max. | 81 | 97 | 7.00 × 107 | 0.1403 | 61 |
Figure 2.
Box-plot distribution of TiO2-NP modal size (nm), mean diameter (nm), %NPs in Ps-Tot, and level (mg/kg) in packaged seafood products. Legend: CT, canned tuna; CM, canned mackerel; CA, canned anchovy; CC, canned clam; a, p < 0.001 vs. all; b, p < 0.01 vs. CT; c, p < 0.001 vs. CT and CM; d, p < 0.01 vs. CM and CA; e, p < 0.001 vs. CA; f, p < 0.01 vs. CA. ° Outliers values of the distribution. * Extreme values of the distribution.
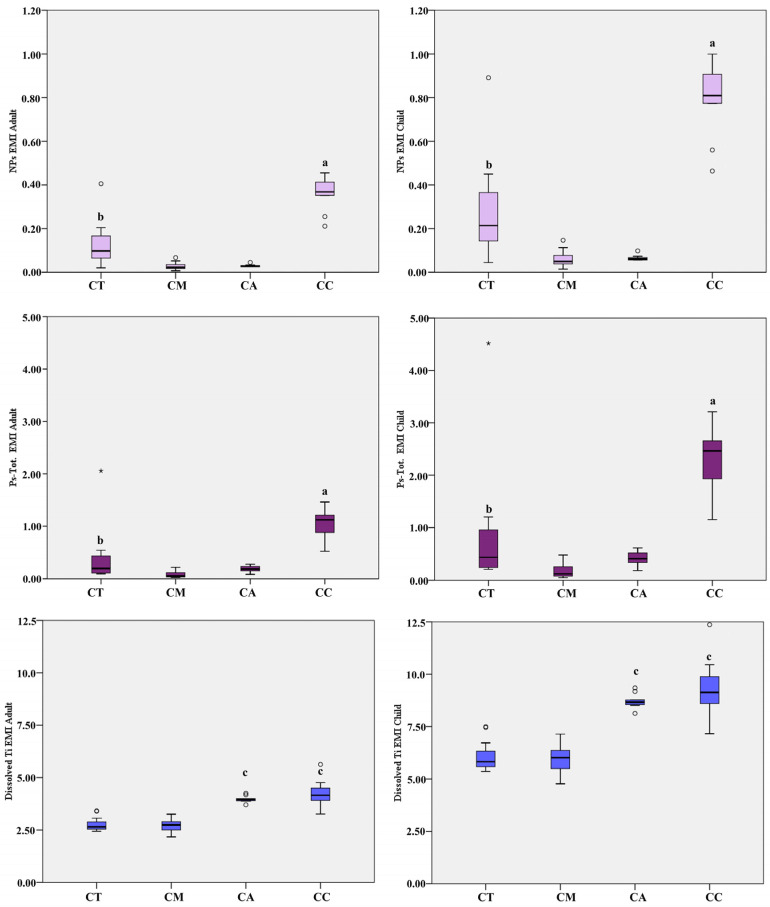
Regarding the estimated meal intake, shown in Table 6 and Figure 3, bas significantly higher levels were found in children than in adults, due to the low body weight of the considered age class. According to the different seafood products, the EMI of both NPs and Ps-Tot was highest with a meal of canned clam, followed by the EMI calculated for canned tuna, significantly higher than the EMI derived from a meal of canned mackerel or canned anchovy. With regard to the dissolved Ti, the EMIs calculated for canned clam and canned anchovy were both significantly higher than the EMI calculated for the other fish species (p < 0.001).
Table 6.
Descriptive statistics of estimated meal intake (EMI mg/kg BW) calculated for adults (70 years) and children (6 years) concerning the TiO2-NPs, TiO2-Ps-Tot, and dissolved Ti.
| Canned Tuna | EMI Adult NPs | EMI Child NPs | EMI Adult Ps-Tot. | EMI Child Ps-Tot. | EMI Adult Dissolved Ti | EMI Child Dissolved Ti |
| Mean | 0.124 | 0.272 | 0.379 | 0.832 | 2.759 | 6.063 |
| SD | 0.097 | 0.212 | 0.507 | 1.113 | 0.321 | 0.706 |
| Min. | 0.020 | 0.044 | 0.095 | 0.209 | 2.438 | 5.357 |
| Max. | 0.406 | 0.891 | 2.056 | 4.517 | 3.412 | 7.497 |
| Canned Mackerel | EMI Adult NPs | EMI Child NPs | EMI Adult Ps-Tot. | EMI Child Ps-Tot. | EMI Adult Dissolved Ti | EMI Child Dissolved Ti |
| Mean | 0.028 | 0.060 | 0.082 | 0.180 | 2.709 | 5.952 |
| SD | 0.017 | 0.038 | 0.066 | 0.146 | 0.307 | 0.674 |
| Min. | 0.007 | 0.014 | 0.021 | 0.046 | 2.171 | 4.769 |
| Max. | 0.067 | 0.147 | 0.219 | 0.482 | 3.253 | 7.147 |
| Canned Anchovy | EMI Adult NPs | EMI Child NPs | EMI Adult Ps-Tot. | EMI Child Ps-Tot. | EMI Adult Dissolved Ti | EMI Child Dissolved Ti |
| Mean | 0.030 | 0.066 | 0.191 | 0.419 | 3.967 | 8.716 |
| SD | 0.006 | 0.013 | 0.062 | 0.135 | 0.164 | 0.361 |
| Min. | 0.025 | 0.055 | 0.084 | 0.184 | 3.699 | 8.129 |
| Max. | 0.044 | 0.098 | 0.280 | 0.616 | 4.254 | 9.346 |
| Canned Clam | EMI Adult NPs | EMI Child NPs | EMI Adult Ps-Tot. | EMI Child Ps-Tot. | EMI Adult Dissolved Ti | EMI Child Dissolved Ti |
| Mean | 0.362 | 0.795 | 1.056 | 2.319 | 4.259 | 9.358 |
| SD | 0.082 | 0.181 | 0.273 | 0.600 | 0.681 | 1.496 |
| Min. | 0.211 | 0.464 | 0.524 | 1.152 | 3.259 | 7.162 |
| Max. | 0.455 | 0.999 | 1.462 | 3.211 | 5.628 | 12.36 |
Figure 3.
Box-plot distribution of estimated meal intake (EMI mg/kg BW) of TiO2-NPs, TiO2-Ps-Tot, and dissolved Ti concentration calculated for adults (70 years) and children (6 years). Legend: CT, canned tuna; CM, canned mackerel; CA, canned anchovy; CC, canned clam; a, p < 0.001 vs. all; b, p < 0.01 vs. CM and CA; c, p < 0.001 vs. CT and CM. ° Outliers values of the distribution. * Extreme values of the distribution.
4. Discussion
Our results are the first to provide a quantitative analysis of TiO2-Ps-Tot in packaged pelagic fish and to deepen findings related to bivalve mollusks. It is important to specify that, in all the products we chose, the use of the additive E171 was not indicated on the label, indicating that it was not intentionally added during food processing. Thus, the presence of titanium dioxide in particle or nanoparticle form may be attributed to contamination within the food industry, in addition to the bioaccumulation of organisms in their natural environment. As shown in Table 5 and Figure 2, our findings highlighted how the longest-lived and larger-sized organisms, such as tuna and mackerel, had a lower TiO2 nanoparticle diameter than anchovy, a small pelagic fish, and clams, as well as the highest percentage of NPs in the total particles. Accordingly, it seems that nanoparticles of smaller diameter have a higher potential of bioaccumulation and biomagnification along the food chain in aquatic systems. This environmental behavior of TiO2-NPs seems to be promoted by their poor water solubility and long-term persistence in aquatic systems [36].
Nevertheless, both TiO2-Ps-Tot and TiO2-NP concentrations were found to be significantly higher in clam, surely due to their filter-feeding behavior, making it a great biological model to test for the bioavailability of contamination at the sediment–water interface. The sedimentation capacity of TiO2-NPs is strictly related to the availability and characteristic of natural organic matter (NOM). NOM can act to maintain smaller particle sizes and more negative surface charges on the NPs [37], reducing the rate of sedimentation of metal oxide NPs [38,39]. As experimentally proven, the low NOM content and high ionic strength of seawater promote a high rate of sedimentation of metal oxide nanoparticles [38]. In this way, NPs are less mobile and their interaction with filter-feeders and sediment-dwelling organisms is favored [40], supporting our findings in stating that benthic mollusks are more exposed to TiO2-NP bioaccumulation.
The first occurrence of data related to the single-particle characterization and quantification of TiO2-Ps-Tot in shellfish was reported in [26,27], although the authors did not reduce the particle acquisition spectrum to the nano-fraction of 100 nm in diameter. Taboada-López et al. (2018) processed different shellfish species collected both fresh and frozen at the supermarket, by applying an ultrasound-assisted enzyme digestion and spICP-MS analysis. The levels of dissolved Ti they found in fresh clam species (0.802–1.31 mg/kg wet weight) are comparable to ours (1.31 ± 0.21 mg/kg wet weight), while they found a slightly lower particle concentration (4.16–7.56 × 107 parts/g vs. 10.5 ± 1.52 × 107 parts/g). Furthermore, Taboada-López et al. (2018), by analyzing the variegated scallop, found a significantly higher concentration of TiO2 particles and dissolved Ti in frozen samples than fresh ones, suggesting contamination by the food industry. Overall, the mean size of TiO2-NP diameter given for these shellfish was in the range of 60–84 nm versus a mean size of 89.8 ± 6 nm found in our packaged clams. Xu et al. (2018) also performed an enzyme digestion and spICP-MS analysis in shellfish collected from an offshore aquaculture farm. In clams, they found comparable concentrations of dissolved Ti (1.11 ± 0.35 mg/kg wet weight) and mean size of TiO2-NPs (70.9 ± 12.4 nm), but a lower concentration of TiO2-NPs (2.10 ± 0.31 × 107 parts/g). Furthermore, they did not find any significant differences with the other shellfish species analyzed.
We also provided a first estimate of TiO2-NPs, TiO2-Ps-Tot, and dissolved Ti intake derived from a meal consisting of the different packaged seafood, both for adults of 70 years and children of 6 years. Results were significantly higher in children than in adults, due to the low body weight of the considered age class.
According to a carcinogenicity study with TiO2 (as E171) in mice and in rats, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) chose the lowest no observed adverse effects levels (NOAEL), which was 2250 mg TiO2/kg BW/day [5], but it may contain up to 3.2% of its weight in nanoparticles. For these reasons, E171 as a food additive is not considered as a nanomaterial according to the EU recommendation on the definition of a nanomaterial [5]. In our samples, we found a high occurrence of TiO2 in its nanoparticle structure, with the lowest in canned anchovy (32%) and the highest in canned tuna (59%); thus, we cannot refer to the established NOAEL for toxicological evaluation.
Most studies available evaluated the oral intake of total TiO2 and TiO2-NPs for TiO2 added during the production process in food, toothpaste, and supplements [8,11,41]. Bachler et al. (2015), by developing a physiologically based pharmacokinetic (PBPK) model, calculated the dietary intake of total titanium for the German population as between 0.5 and 1.0 mg/kg BW for all age groups except the age group “other children”, who had the highest titanium intake of all age classes (approximately 2.0 mg/kg BW). Furthermore, by assuming that 10% of the total titanium intake is in the nano-range, which is a conservative estimation according to the ratio of ionic to particulate titanium intake (1:19) and the amount of TiO2-NPs in E171 (approximately 11% by weight) [11], they calculated the 95th percentile of TiO2-NPs dietary intake as below 170 ng/g for all age groups. Moreover, Rompelberg et al. (2016) estimated the long-term intake of TiO2-NPs for the Dutch population as ranging from 0.19 µg/kg BW/day for the elderly to 0.55 µg/kg BW/day for 7–69-year-olds and 2.16 µg/kg BW/day for young children. The results found in the literature are significantly lower than the potential exposure calculated in this study. Nevertheless, considering that the presence of E171 as a food additive in canned fish is not expected, our results give an estimate of the dietary intake probably related to TiO2-NP bioaccumulation in fish and seafood species through environmental contamination. However, we cannot be sure regarding the potential total amount of TiO2-NPs which can be accumulated in human tissue after oral ingestion, considering that part of the compound can be altered in the gastrointestinal environment by extreme pH and co-ingested food [42], although intestinal epithelial cells are affected at a functional level [43]. Lastly, a study limitation was the inability to discern the origin of TiO2-NPs, i.e., natural or anthropogenic. Taking into account the numerous applications of TiO2-NPs, a proper improvement of the wastewater depuration process could be a step to prevent the contamination of the water bodies.
5. Conclusions
This study provided, for the first time, an evaluation of the estimated daily intake of TiO2-NPs starting from quantitatively measured data. Our findings highlighted how processed seafood, where the pigment E171 is not intentionally added, may contain amounts of TiO2 in its particle and nanoparticle form, as well as dissolved titanium. In particular, our results revealed that the consumption of selected types of foods could be an important route for the uptake of TiO2, especially for a vulnerable group such as children. Processed clam was the seafood with the highest content of TiO2-NPs, while, among pelagic fish species, we found progressively higher levels and smaller sizes from small to large fish. Nevertheless, seafood consumption represents only a small part of the human total diet; thus, to provide a realistic exposure assessment, it is important to carry out TiO2-NP characterization and quantification in a large number of food items, both processed and not, as well as where the pigment E171 is intentionally added and not, as it is not the only source of TiO2-NPs. This information would be a solid step toward being able to actually estimate the TiO2-NP dietary exposure of populations and the related health risks. The new emerging technique of spICP-MS, which is able to distinguish between the particulate and the dissolved fraction, is crucial for a better understanding of the fate and transport characteristics of the particles and ionic forms, as well as their possible (independent or synergistic) environmental and (eco) toxicological impacts.
Author Contributions
Conceptualization, A.G., C.C., and M.F. (Margherita Ferrante); methodology, A.G., C.C., and G.A.; formal analysis, A.G., A.C., and P.Z.; data curation, A.G., C.C., G.A., and M.F. (Margherita Ferrante); writing—original draft preparation, A.G., C.C., and P.Z.; writing—review and editing, T.F., M.F. (Maria Fiore), G.O.C., and M.F. (Margherita Ferrante); supervision, C.C. and M.F. (Margherita Ferrante). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lespes G., Faucher S., Slaveykova V.I. Natural Nanoparticles, Anthropogenic Nanoparticles, Where Is the Frontier? Front. Environ. Sci. 2020;8:71. doi: 10.3389/fenvs.2020.00071. [DOI] [Google Scholar]
- 2.Ziental D., Czarczynska-Goslinska B., Mlynarczyk D.T., Glowacka-Sobotta A., Stanisz B., Goslinski T., Sobotta L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials. 2020;10:387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boverhof D.R., David R.M. Nanomaterial characterization: Considerations and needs for hazard assessment and safety evaluation. Anal. Bioanal. Chem. 2010;396:953–961. doi: 10.1007/s00216-009-3103-3. [DOI] [PubMed] [Google Scholar]
- 4.Piccinno F., Gottschalk F., Seeger S., Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012;14:1109. doi: 10.1007/s11051-012-1109-9. [DOI] [Google Scholar]
- 5.EFSA Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016;14:e04545. doi: 10.2903/j.efsa.2016.4545. [DOI] [Google Scholar]
- 6.Filippini T., Tancredi S., Malagoli C., Malavolti M., Bargellini A., Vescovi L., Nicolini F., Vinceti M. Dietary Estimated Intake of Trace Elements: Risk Assessment in an Italian Population. Expo. Health. 2019 doi: 10.1007/s12403-019-00324-w. [DOI] [Google Scholar]
- 7.Silano V., Baviera J.M.B., Bolognesi C., Tlustos C., Loveren H.V., Vernis L., Zorn H., Castle L., Cravedi J.P., Kolf-Clauw M., et al. Safety assessment of the substance, titanium dioxide surface treated with fluoride-modified alumina, for use in food contact materials. EFSA J. 2019;17:e05737. doi: 10.2903/j.efsa.2019.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rompelberg C., Heringa M.B., van Donkersgoed G., Drijvers J., Roos A., Westenbrink S., Peters R., van Bemmel G., Brand W., Oomen A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10:1404–1414. doi: 10.1080/17435390.2016.1222457. [DOI] [PubMed] [Google Scholar]
- 9.Schneider S.L., Lim H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019;35:442–446. doi: 10.1111/phpp.12439. [DOI] [PubMed] [Google Scholar]
- 10.Ruszkiewicz J.A., Pinkas A., Ferrer B., Peres T.V., Tsatsakis A., Aschner M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017;4:245–259. doi: 10.1016/j.toxrep.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weir A., Westerhoff P., Fabricius L., von Goetz N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostan H.B., Rezaee R., Valokala M.G., Tsarouhas K., Golokhvast K., Tsatsakis A.M., Karimi G. Cardiotoxicity of nano-particles. Life Sci. 2016;165:91–99. doi: 10.1016/j.lfs.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Klaine S.J., Alvarez P.J.J., Batley G.E., Fernandes T.F., Handy R.D., Lyon D.Y., Mahendra S., McLaughlin M.J., Lead J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 14.Carmo T.L.L., Azevedo V.C., Siqueira P.R., Galvão T.D., Santos F.A., Martinez C.B.R., Appoloni C.R., Fernandes M.N. Mitochondria-rich cells adjustments and ionic balance in the Neotropical fish Prochilodus lineatus exposed to titanium dioxide nanoparticles. Aquat. Toxicol. 2018;200:168–177. doi: 10.1016/j.aquatox.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Westerhoff P., Song G., Hristovski K., Kiser M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011;13:1195–1203. doi: 10.1039/c1em10017c. [DOI] [PubMed] [Google Scholar]
- 16.Borm P.J.A., Robbins D., Haubold S., Kuhlbusch T., Fissan H., Donaldson K., Schins R., Stone V., Kreyling W., Lademann J., et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C., Marcus I.M., Waller T., Walker S.L. Comparison of filtration mechanisms of food and industrial grade TiO2 nanoparticles. Anal. Bioanal. Chem. 2018;410:6133–6140. doi: 10.1007/s00216-018-1132-5. [DOI] [PubMed] [Google Scholar]
- 18.Jovanović B. Critical review of public health regulations of titanium dioxide, a human food additive: Titanium Dioxide in Human Food. Integr. Environ. Assess. Manag. 2015;11:10–20. doi: 10.1002/ieam.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z., Han S., Zhou D., Zhou S., Jia G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale. 2019;11:22398–22412. doi: 10.1039/C9NR07580A. [DOI] [PubMed] [Google Scholar]
- 20.Schneider T., Westermann M., Glei M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol. 2017;91:3517–3527. doi: 10.1007/s00204-017-1976-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., Zhou D., Zhou S., Jia G. Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats. J. Appl. Toxicol. 2019;39:807–819. doi: 10.1002/jat.3769. [DOI] [PubMed] [Google Scholar]
- 22.Heringa M.B., Geraets L., van Eijkeren J.C.H., Vandebriel R.J., de Jong W.H., Oomen A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology. 2016;10:1515–1525. doi: 10.1080/17435390.2016.1238113. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Han S., Zheng P., Zhou D., Zhou S., Jia G. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague-Dawley rats. Nanoscale. 2020;12:5973–5986. doi: 10.1039/C9NR10947A. [DOI] [PubMed] [Google Scholar]
- 24.Cao X., Han Y., Gu M., Du H., Song M., Zhu X., Ma G., Pan C., Wang W., Zhao E., et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small. 2020:e2001858. doi: 10.1002/smll.202001858. [DOI] [PubMed] [Google Scholar]
- 25.Taboada-López M.V., Herbello-Hermelo P., Domínguez-González R., Bermejo-Barrera P., Moreda-Piñeiro A. Enzymatic hydrolysis as a sample pre-treatment for titanium dioxide nanoparticles assessment in surimi (crab sticks) by single particle ICP-MS. Talanta. 2019;195:23–32. doi: 10.1016/j.talanta.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Taboada-López M.V., Iglesias-López S., Herbello-Hermelo P., Bermejo-Barrera P., Moreda-Piñeiro A. Ultrasound assisted enzymatic hydrolysis for isolating titanium dioxide nanoparticles from bivalve mollusk before sp-ICP-MS. Anal. Chim. Acta. 2018;1018:16–25. doi: 10.1016/j.aca.2018.02.075. [DOI] [PubMed] [Google Scholar]
- 27.Xu L., Wang Z., Zhao J., Lin M., Xing B. Accumulation of metal-based nanoparticles in marine bivalve mollusks from offshore aquaculture as detected by single particle ICP-MS. Environ. Pollut. 2020;260:114043. doi: 10.1016/j.envpol.2020.114043. [DOI] [PubMed] [Google Scholar]
- 28.Yin C., Zhao W., Liu R., Liu R., Wang Z., Zhu L., Chen W., Liu S. TiO2 particles in seafood and surimi products: Attention should be paid to their exposure and uptake through foods. Chemosphere. 2017;188:541–547. doi: 10.1016/j.chemosphere.2017.08.168. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q., Liu L., Liu N., He B., Hu L., Wang L. Determination and characterization of metal nanoparticles in clams and oysters. Ecotoxicol. Environ. Saf. 2020;198:110670. doi: 10.1016/j.ecoenv.2020.110670. [DOI] [PubMed] [Google Scholar]
- 30.Domingo J.L., Bocio A., Falcó G., Llobet J.M. Benefits and risks of fish consumption: Part I. A quantitative analysis of the intake of omega-3 fatty acids and chemical contaminants. Toxicology. 2007;230:219–226. doi: 10.1016/j.tox.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Wu S., Zhang S., Gong Y., Shi L., Zhou B. Identification and quantification of titanium nanoparticles in surface water: A case study in Lake Taihu, China. J. Hazard. Mater. 2020;382:121045. doi: 10.1016/j.jhazmat.2019.121045. [DOI] [PubMed] [Google Scholar]
- 32.Gray E.P., Coleman J.G., Bednar A.J., Kennedy A.J., Ranville J.F., Higgins C.P. Extraction and Analysis of Silver and Gold Nanoparticles from Biological Tissues Using Single Particle Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Technol. 2013;47:14315–14323. doi: 10.1021/es403558c. [DOI] [PubMed] [Google Scholar]
- 33.Lee S., Bi X., Reed R.B., Ranville J.F., Herckes P., Westerhoff P. Nanoparticle Size Detection Limits by Single Particle ICP-MS for 40 Elements. Environ. Sci. Technol. 2014;48:10291–10300. doi: 10.1021/es502422v. [DOI] [PubMed] [Google Scholar]
- 34.Copat C., Arena G., Fiore M., Ledda C., Fallico R., Sciacca S., Ferrante M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food Chem. Toxicol. 2013;53:33–37. doi: 10.1016/j.fct.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 35.US-EPA . Guidance for Assessing Chemical Contamination Data for Use in Fish Advisories—Volume II. Risk Assessment and Fish Consumption Limits EPA/823-B94-004. Environmental Protection Agency; Washington DC, USA: 2000. [Google Scholar]
- 36.Gupta G.S., Kumar A., Shanker R., Dhawan A. Assessment of agglomeration, co-sedimentation and trophic transfer of titanium dioxide nanoparticles in a laboratory-scale predator-prey model system. Sci. Rep. 2016;6 doi: 10.1038/srep31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Chen Y., Westerhoff P., Crittenden J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009;43:4249–4257. doi: 10.1016/j.watres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Keller A.A., Wang H., Zhou D., Lenihan H.S., Cherr G., Cardinale B.J., Miller R., Ji Z. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010;44:1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 39.Pettibone J.M., Cwiertny D.M., Scherer M., Grassian V.H. Adsorption of Organic Acids on TiO2 Nanoparticles: Effects of pH, Nanoparticle Size, and Nanoparticle Aggregation. Langmuir. 2008;24:6659–6667. doi: 10.1021/la7039916. [DOI] [PubMed] [Google Scholar]
- 40.Farré M., Gajda-Schrantz K., Kantiani L., Barceló D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem. 2009;393:81–95. doi: 10.1007/s00216-008-2458-1. [DOI] [PubMed] [Google Scholar]
- 41.Bachler G., Goetz N von Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2015;9:373–380. doi: 10.3109/17435390.2014.940404. [DOI] [PubMed] [Google Scholar]
- 42.Walczak A.P., Kramer E., Hendriksen P.J., Helsdingen R., van der Zande M., Rietjens I.M., Bouwmeester H. In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology. 2015;9:886–894. doi: 10.3109/17435390.2014.988664. [DOI] [PubMed] [Google Scholar]
- 43.Guo Z., Martucci N.J., Moreno-Olivas F., Tako E., Mahler G.J. Titanium Dioxide Nanoparticle Ingestion Alters Nutrient Absorption in an In Vitro Model of the Small Intestine. NanoImpact. 2017;5:70–82. doi: 10.1016/j.impact.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]