Abstract
Integrin ligands containing the tripeptide sequences Arg-Gly-Asp (RGD) and iso-Asp-Gly- Arg (isoDGR) were actively investigated as inhibitors of tumor angiogenesis and directing unit in tumor-targeting drug conjugates. Reported herein is the synthesis, of two RGD and one isoDGR cyclic peptidomimetics containing (1S,2R) and (1R,2S) cis-2-amino-1-cyclopentanecarboxylic acid (cis-β-ACPC), using a mixed solid phase/solution phase synthetic protocol. The three ligands were examined in vitro in competitive binding assays to the purified αvβ3 and α5β1 receptors using biotinylated vitronectin (αvβ3) and fibronectin (α5β1) as natural displaced ligands. The IC50 values of the ligands ranged from nanomolar (the two RGD ligands) to micromolar (the isoDGR ligand) with a pronounced selectivity for αvβ3 over α5β1. In vitro cell adhesion assays were also performed using the human skin melanoma cell line WM115 (rich in integrin αvβ3). The two RGD ligands showed IC50 values in the same micromolar range as the reference compound (cyclo[RGDfV]), while for the isoDGR derivative an IC50 value could not be measured for the cell adhesion assay. A conformational analysis of the free RGD and isoDGR ligands by NMR (VT-NMR and NOESY experiments) and computational studies (MC/EM and MD), followed by docking simulations performed in the αVβ3 integrin active site, provided a rationale for the behavior of these ligands toward the receptor.
Keywords: peptidomimetics, integrin ligands, beta-amino acids, NMR conformational analysis
1. Introduction
Integrins are a large family of heterodimeric cell adhesion protein receptors involved in physiological and pathological processes concerning cell adhesion, cell motility, and cell survival [1,2]. In particular, αvβ3, αvβ5, and α5β1 integrins are involved in tumor angiogenesis and are overexpressed in tumor vascular tissues [3,4,5]. These integrins recognize and bind the Arg-Gly-Asp (RGD) sequence in their natural ligands [6], but also the isoDGR sequence was shown to fit into the RGD-binding pocket of αvβ3 integrin, establishing the same interactions [7,8,9,10]. For both sequences, flanking residues combined to the 3D presentation determine the recognition specificity. Since the pioneering work of Kessler and coworkers [11], many different RGD peptides and peptidomimetics have been developed as integrin ligands and investigated as potential antitumor drugs with antiangiogenic properties [12,13,14] or directing ligands in molecular imaging and targeted anticancer therapy, which emerged as powerful weapon for reducing the toxicity of the antitumor treatments and the insurgence of drug resistance [15,16,17,18,19,20,21].
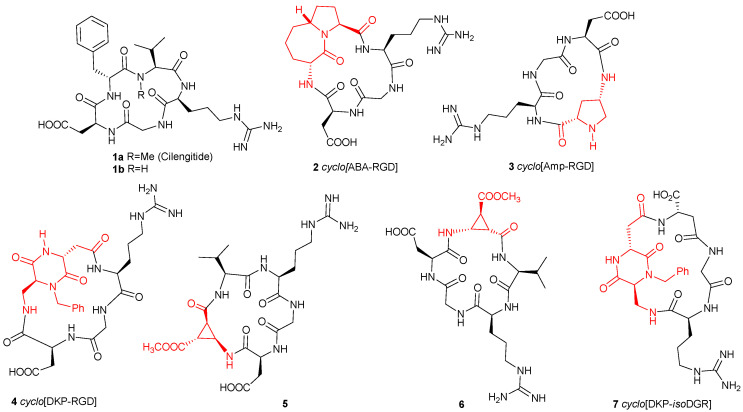
In many cases, the RGD sequence is constrained in cyclic peptides such as the cyclopentapeptide cyclo[Arg-Gly-Asp-NMe Val-D-Phe], Cilengitide 1a [22], the most studied ligand and forefather of a whole series of cyclic ligands, in which the complementary dipeptide sequence was varied to optimize the interaction of the RGD sequence with the integrin receptor. Based on this notion, several peptidomimetic scaffolds were inserted to mimic this complementary dipeptide moiety such as the bicyclic lactam in 2 [23,24,25], the 4-aminoproline in 3 [26], or the bifunctional diketopiperazine in 4 [27,28] (Figure 1).
Figure 1.
Cyclic RGD and isoDGR integrin ligands (azabicyclic alkane (ABA), aminoproline (Amp), and diketopiperazine scaffolds (DKP)).
By contrast, the number of isoDGR containing cyclic peptides and peptidomimetics which were synthesized and tested is much more limited and, among them, the cyclic isoDGR compound 7 (Figure 1) containing the bifunctional diketopiperazine mentioned above is one of the few low nM αvβ3 binder reported so far [29,30,31,32].
β-Amino acids have received considerable attention as possible substituents of α-amino acids in peptides to probe the structural specificity of α-amino acid-binding sites or as inhibitors of enzymes [33]. In addition, their incorporation into peptides of pharmacological interest was sometimes advantageous in terms of biological activity, metabolic stability, and conformational characteristics. In particular, β-amino acids stabilize distinct overall conformations of cyclopeptides and they act as γ-turn mimetics: [34,35], if a single β-amino acid is incorporated into a cyclic pentapeptide, it preferably occupies the central position of a γ-turn. These conformational preferences were thoroughly investigated in the field of RGD-cyclopeptides by the introduction of β-aminocyclopropane carboxylic acids (β-ACCs) [36], which are among the most restricted β-alanine derivatives, and whose rigidity is conferred by the small-sized ring closure. Reiser and co-workers synthesized both cis- and trans-β-aminocyclopropane carboxylic acids [37] and incorporated two enantiomeric cis-β-aminocyclopropanecarboxylic acids (cis-β-ACC) into two 16-membered cyclic RGD peptidomimetics 5–6 (Figure 1). These peptides showed nanomolar affinity toward αvβ3 and α5β1 integrins in in vitro cell adhesion assays.
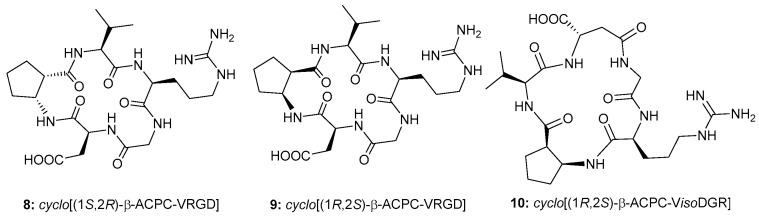
These considerations prompted us to investigate the properties of other β-aminocycloalkane carboxylic acids and herein we describe the synthesis, the conformational analysis, and some biological investigation of two RGDs, 8–9 (Figure 2), and one isoDGR, 10 (Figure 2) cyclic peptidomimetics, containing two cis-2-amino-1-cyclopentanecarboxylic (cis-β-ACPC) scaffolds. The RGD ligands feature a 16-membered ring containing the RGD sequence as well as a Val residue and either (1S,2R)-cis-β-ACPC (8) or (1R,2S)-cis-β-ACPC (9). In the case of the isoDGR ligand only the (1R,2S)-cis-β-ACPC scaffold was used and a 17-membered ring is obtained, since the Asp residue participates to the sequence through its β-carboxylic group (isoAsp residue). Integrin receptor competitive binding assays (Table 1) and cell adhesion assays with an αVβ3 positive cell line (WM115, a human skin melanoma cell line) (Table 2) were performed for all the compounds. The conformational preferences of the free ligands were investigated by NMR and computational methods. The conformational study of cyclic peptides and peptidomimetics is generally a complex matter, yet an essential step prior to docking calculations that are in most cases unable to perform a rigorous sampling of the macrocycle conformations [38,39]. In this paper, minimized structures satisfying the characteristic NOE contacts and H-bonds were employed as starting geometries for docking studies in the αvβ3 integrin.
Figure 2.
Cyclic peptidomimetics containing cis-β-ACPC scaffolds.
Table 1.
IC50 values measured from competitive binding assays to αvβ3 and α5β1 integrins.
| Compound | αvβ3 IC50 [nM] [a] | α5β1 IC50 [nM] [a] |
|---|---|---|
| Cilengitide (1a) | 0.71 ± 0.06 | 14.4 ± 3.1 |
| cyclo-[-Arg-Gly-Asp-d-Phe-Val-] (1b) | 3.2 ± 1.3 | 166 ± 28 |
| cyclo-[-DKP-Arg-Gly-Asp-] (4) | 4.5 ± 1.1 | 532 ± 35 |
| cyclo-[-Arg-Gly-Asp-(1S,2R)-β-ACPC-Val-] (8) | 44.3 ± 4.0 | 3227 ± 1468 |
| cyclo-[-Arg-Gly-Asp-(1R,2S)-β-ACPC-Val-] (9) | 39.0 ± 1.1 | 468 ± 114 |
| cyclo-(-DKP-isoAsp-Gly-Arg-) (7) | 9.2 ± 1.1 | 1066 ± 228 |
| cyclo-[-isoAsp-Gly-Arg-(1R,2S)-β-ACPC-Val-] (10) | 5362 ± 281 | 2331 ± 134 |
[a] IC50 values were calculated as the concentration of compound required for 50% inhibition of biotinylated vitronectin or fibronectin binding. Screening assays were performed by incubating the immobilized integrin αvβ3 or α5β1 with increasing concentrations (10−12–10−5 M/10−11–10−4 M) of the RGD and isoDGR ligands in the presence of the corresponding biotinylated ECM (extracellular matrix) protein (1 μg mL−1), and measuring bound protein in the presence of the competitive ligand. Each data point is the result of the average of triplicate wells and was analyzed by nonlinear regression analysis with the GraphPad Prism software. Each experiment was repeated in duplicate. All values shown are the arithmetic mean ± the standard deviation (SD) of these duplicate determinations.
Table 2.
IC50 values of the cyclic peptides as determined by cell adhesion assays with WM115 cells.
| Compound | IC50 [μM] [a] |
|---|---|
| cyclo-(-Arg-Gly-Asp-d-Phe-Val-) (1b) | 5.1 ± 1.8 |
| cyclo-[-Arg-Gly-Asp-(1S,2R)-β-ACPC-Val-] (8) | 75 ± 32.5 |
| cyclo-[-Arg-Gly-Asp-(1R,2S)-β-ACPC-Val-] (9) | 124.5 ± 10.6 |
| cyclo-[-isoAsp-Gly-Arg-(1R,2S)-β-ACPC-Val-] (10) | >300 |
[a] IC50 values were calculated as the concentration of compound required for 50% inhibition of cell adhesion to vitronectin. Cell adhesion assays were performed by incubating the immobilized vitronectin with fluorescein labeled cells and increasing concentrations (10−7–10−4 M) of the RGD and isoDGR ligands, and measuring the fluorescence of bound cells. All values are the arithmetic mean ± the standard deviation (SD) of two independent assays each with four replicate determinations.
2. Results
2-Aminocyclopentanecarboxylic acids (β-ACPCs) and their heterocyclic analogs represent a widely studied class of cyclic unnatural β-amino acids, showing interesting biological and conformational properties. For example, cis-(1R,2S)-2-aminocyclopentanecarboxylic acid (Cispentacin) is a potent antifungal agent [40], racemic cis-4-aminopyrrolidine-3-carboxylic acid has been used to probe the structure of the GABA (gamma-aminobutyric acid) receptor [41], while cis-N-Boc-4-aminopyrrolidine-3-carboxylic acid is a modestly active influenza neuraminidase inhibitor [42]. 2-Aminocyclopentanecarboxylic acid and 4-aminopyrrolidine-3-carboxylic acid have also found applications in the area of foldamers as promoters of helical structures, both as oligomeric structures or in combination with α-amino acids [43,44], and were recently used by Reiser and co-workers for the synthesis of helical peptidic foldamers in the synthesis of Neuropeptide Y analogs [45,46].
2.1. Synthesis
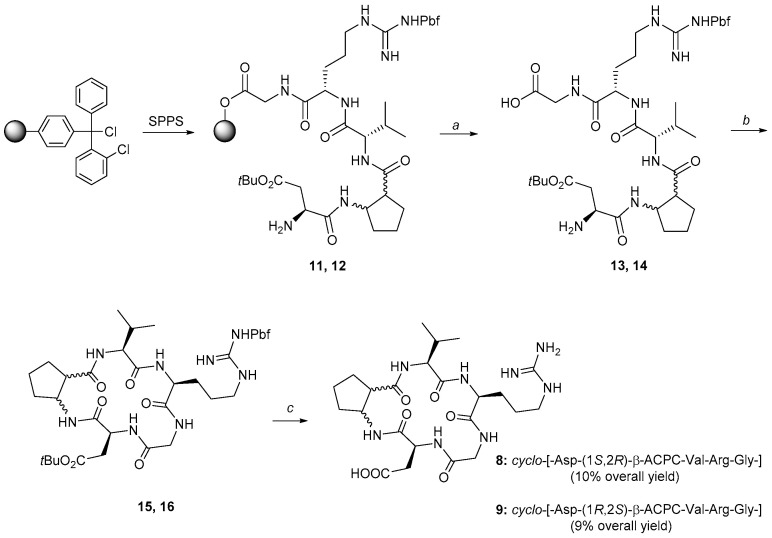
For the synthesis of the cyclic peptidomimetics 8–9 either (1S,2R)-cis-β-ACPC or (1R,2S)-cis-β-ACPC were used. The synthesis of the cyclic peptidomimetics was obtained by a mixed solid phase/solution phase approach (Scheme 1). The linear precursors 11 and 12 were assembled by SPPS on O-chlorotritylchloride resin as solid support using a Fmoc protection on the α-amino groups. Fmoc-ortogonal protecting groups were selected for the side chains of the amino acids (Pbf for Arg and tBu for Asp). The cleavage of the linear precursor from the resin was accomplished in weakly acidic conditions.
Scheme 1.
Synthesis of cyclo-[-Arg-Gly-Asp-(1S,2R)-β-ACPC-Val-] (8) and cyclo-[-Arg-Gly-Asp-(1R,2S)-β-ACPC-Val-] (9) via solid phase peptide synthesis of the linear counterparts 13 and 14 after cleavage from the resin, via in solution phase macrocyclization (15 and 16) and their full deprotection. a: 1% TFA in DCM, rt, 5 min for each cycle; b: HATU, DIPEA, DMF, rt, 30 min. c: TFA/Et3SiH/H2O, 95%/2.5%/2.5%, rt, 8 h. DIPEA = diisopropyl ethylamine, TFA = trifluoroacetic acid.
The macrocyclization on compounds 13 and 14 was performed under pseudo-high dilution conditions [47], and the final deprotection was carried out by treatment with TFA/Et3SiH/water, 95%/2.5%/2.5% and was followed by purification of the cyclic peptidomimetics by preparative HPLC. An overall yield of 10% and 9% and a purity of 96% and 99% was obtained for compounds 8 and 9, respectively.
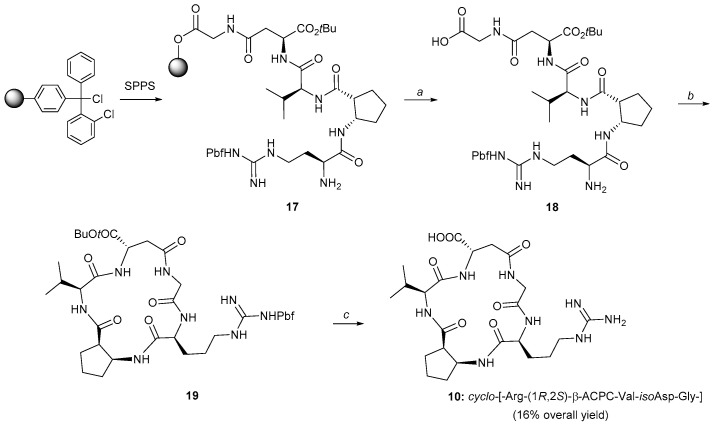
The same strategy (Scheme 2) was followed for the isoDGR cyclic peptidomimetic 10, using only the (1R,2S)-cis-β-ACPC scaffold and changing the loading sequence of the amino acids on the resin. The cyclic isoDGR compound 10 was obtained in an overall 16% yield, with a 99% purity. The overall yields for compounds 8–10 were calculated based on the weight and loading of the resin used for the SPPS and the millimoles of the final purified cyclic peptides (i.e., loading of the first amino acid on the resin, all the coupling steps, cleavage, cyclization, final deprotection and purifications). We believe that the cyclization is the crucial step lowering the yields in our case. Conformational preorganization is in general essential for cyclization, and the presence of elements able to impart a turn to the linear peptide chains (especially if located in the central region of the polypeptide), greatly improves the yields [34,35]. In our case this preorganization is missing and the final yields are only satisfying.
Scheme 2.
Synthesis of cyclo-[-isoAsp-Gly-Arg-(1R,2S)-β-ACPC-Val-] (10) via solid phase peptide synthesis of the linear counterpart 18 after cleavage from the resin, via in solution phase macrocyclization (19) and its full deprotection. a: 1% TFA in DCM, rt, 5 min for each cycle; b: HATU, DIPEA, DMF, rt, 30 min. c: TFA/Et3SiH/H2O, 95%/2.5%/2.5%, rt, 8 h. DIPEA = diisopropyl ethylamine, TFA = trifluoroacetic acid.
2.2. Biological Assays
The cyclic peptidomimetics 8–10 were analyzed in a first instance for their competitive binding to purified αvβ3 and α5β1 receptors with respect to the biotinylated vitronectin or fibronectin, respectively. The IC50 values are collected in Table 1, where the values of Cilengitide (1a), cyclo-[RGDfV] (1b), cyclo-[DKP-RGD] (4), and cyclo-[DKP-isoDGR] (7), measured using the same procedure [29,48,49,50], are included as reference compounds for the RGD and isoDGR series. The new RGD compounds cyclo-[-Arg-Gly-Asp-(1S,2R)-β-ACPC-Val-] (8) and cyclo-[-Arg-Gly-Asp-(1R,2S)-β-ACPC-Val-] (9) were found to be nanomolar αvβ3 integrin ligands, with a binding IC50 value one order of magnitude higher than 4. On the other hand, the IC50 values measured for α5β1 were higher than those for αvβ3, displaying a selectivity ratio (IC50α5β1/IC50αvβ3) ranging from about 12 (compound 9) to 73 (compound 8), and confirming the trend already observed in the case of ligand 4 [49], On the other hand, the new isoDGR ligand cyclo-[-isoAsp-Gly-Arg-(1R,2S)-β-ACPC-Val-] (10) is a micromolar binder for both integrins, much weaker than cyclo-[DKP-isoDGR] (7) in the case of αvβ3.
In vitro cell adhesion assays were also performed using WM115 cells (a human skin melanoma cell line in which adhesion to ECM proteins is mediated predominantly by integrin αvβ3). The inhibition of the adhesion of the cells was evaluated against plate-coated vitronectin and the IC50 values are collected in Table 2. The IC50 value of cyclo-[RGDfV] (1b), measured using the same procedure, is also listed for comparison.
The trend observed in the competitive binding test on the isolated integrin (Table 1) was substantially confirmed in the cell adhesion assay with WM115 cells. Also in this case, despite the different stereochemistry of the two contained cis-β-ACPC scaffolds, the two cyclic RGD peptidomimetics (8 and 9) showed IC50 values in the same micromolar range, one order of magnitude higher than the value of the reference compound (Table 2). Similar to the binding data, the isoDGR peptidomimetic 10 is the weakest inhibitor of cell adhesion among the tested compounds (very high micromolar IC50 values).
2.3. NMR Studies
The structure and connectivity of ligands 8–10 were unambiguously assigned by means of mono- and two-dimensional 1H and 13C NMR spectroscopy in H2O solution. The preferred conformations of the cyclic peptidomimetics 8–10 were then investigated, with the aim of rationalizing the affinity of these compounds for the αvβ3 receptor at a molecular level. The cyclic peptides are characterized by a high density of carbonyl groups and amide protons close together. In this situation, the formation of hydrogen bonds is favored as well as the presence of β-turn type folds. H-bonds were detected by variable temperature-NMR (VT-NMR) experiments observing the change of NH chemical shift with temperature, while NHi-NHi+1 NOE contacts, which are indicative of β-turn motifs, were identified by NOESY experiments.
The chemical shifts and temperature coefficients (Δδ/ΔT) of the amide protons relative to compounds 8–10 are reported in Table 3. The chemical shift of amide protons not involved in H-bonds generally shows significant temperature dependence (e.g., temperature coefficients in the range −7/−9.5 ppb K−1), whereas temperature coefficients between −2 and −5 ppb K−1 are indicative of H-bond formation.
Table 3.
Chemical shifts (δ, ppm) and temperature coefficients (Δδ/ΔT, ppb K−1) of amide protons of peptidomimetics 8–10 in H2O solution.
| Compound | NH-Gly δ (Δδ/ΔT) [a] | NH-Arg δ (Δδ/ΔT) [a] | NH-Asp δ (Δδ/ΔT) [a] | NH-ACPC δ (Δδ/ΔT) [a] | NH-Val δ (Δδ/ΔT) [a] |
|---|---|---|---|---|---|
| 8 | 8.37 (−7) | 9.10 (−9.5) | 7.73 (−2) | 7.33 (−4) | 7.92 (−6) |
| 9 | 8.62 (−7) | 8.30 (−5) | 8.38 (−8) | 7.59 (−5) | 7.52 (−6) |
| 10 | 8.04 (−3) | 8.44 (−7) | 8.42 (−7) | 7.91 (−4) | 7.74 (−9) |
[a] The temperature variable experiments were performed in the range of 298–328 K.
The relevant long range NOE contacts, classified as strong or medium on the basis of a qualitative assessment of their intensities, are shown in Table 4. Since these small cyclic molecules can fluctuate between different conformations, NMR data were used to evaluate the most representative 3D structures.
Table 4.
Relevant long range NOE contacts for compounds 8–10.
| Compound | NOE Contact [a] | NOE Contact [a] |
|---|---|---|
| 8 | NH-Asp—NH-ACPC (s) | NH-Gly—NH-Asp (m) |
| 9 | NH-Asp—NH-ACPC (s) | |
| 10 | NH-Arg—NH-ACPC (s) | NH-Gly—NH-isoAsp (m) |
[a] s = strong, m = medium.
2.3.1. Conformational Analysis of Compound 8
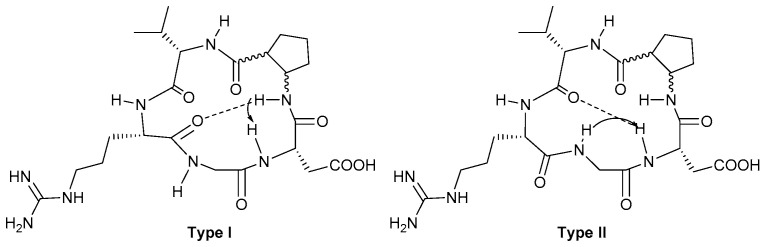
The NMR data of compound 8 suggest that the NH-Asp amide proton (Δδ/ΔT −2 ppb K−1, chemical shift 7.73 ppm) is involved in an intramolecular hydrogen bond. In addition, also the Δδ/ΔT of NH-ACPC amide proton (−4 ppb K−1) suggests that it is experiencing an intramolecular hydrogen bonding. The other Δδ/ΔT values are in the −6/−9.5 ppb K−1 range which is typical of solvent exposed amide protons. In the NOESY spectrum (mixing time 700 ms) two cross peaks involving NH-Asp can be detected: a strong NOE contact between NH-Asp and NH-ACPC, and a medium intensity NOE with NH-Gly. These long range NOEs are exclusive and provide evidence of two preferred conformations characterized by β-turn motifs. The NH-Asp—NH-ACPC NOE is indicative of a β-turn at Gly-Asp (Type I conformation in Figure 3) which is possibly stabilized by a hydrogen bond between NH-ACPC and C=O-Arg. The medium intensity NH-Asp—NH-Gly contact suggests a β-turn at Arg-Gly (Type II conformation in Figure 3) which might be stabilized by a hydrogen bond between NH-Asp and C=O-Val.
Figure 3.
Preferred intramolecular H-bond/β-turn patterns proposed for peptidomimetics 8 and 9 based on spectroscopic data. The arrows indicate significant NOE contacts. Type I H-bonding pattern, Gly-Asp β-turn motif. Type II H-bonding pattern, Arg-Gly β-turn motif (the pattern definition is consistent with that previously reported for other cyclic RGD pentapeptide mimics) [28].
2.3.2. Conformational Analysis of Compound 9
The NMR data of compound 9 point out a large conformational equilibrium, possibly also due to a pseudo-chair ring inversion of the β-ACPC scaffold. A cross-peak of strong intensity between NH-Asp and NH-ACPC is observed in the NOESY spectrum, suggesting that the Type I conformation (Figure 3) might contribute also to the conformational equilibrium of the pseudopeptide 9 (in agreement with the Δδ/ΔT value of NH-ACPC of −5 ppb K−1) [51].
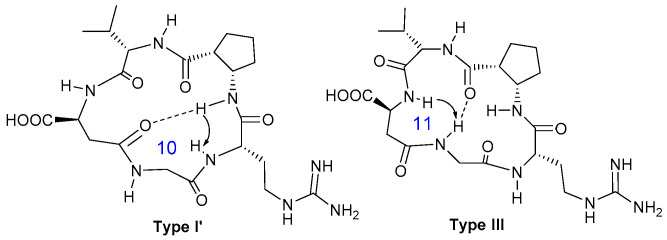
2.3.3. Conformational Analysis of Compound 10
The chemical shift and the Δδ/ΔT values of the amide protons of the isoDGR derivative 10 (Table 3) indicate that NH-Gly and NH-ACPC are involved in hydrogen bonds. Their values suggest the presence of an equilibrium between conformations in which these protons either form intramolecular hydrogen bonds or they are exposed to the solvent, forming hydrogen bonds with it. The other amide protons experience only H-bonds with the solvent. Two mutually exclusive long range NOE contacts are indicative of two different conformations, hereafter referred to as Type I’ and Type III conformations (Figure 4). The strong cross-peak between NH-Arg and NH-ACPC is consistent with a β-turn motif at Gly-Arg, which might be stabilized by a hydrogen bond between NH-ACPC and C=O-isoAsp (Figure 4). This Type I’ H-bond/β-turn pattern suggested for the isoDGR cyclopeptide 10 is very similar to the Type I pattern defined for the RGD derivatives 8 and 9 (Figure 3). The medium dipolar interaction between NH-Gly and NH-isoAsp detected in the NOESY spectrum of compound 10 suggests the presence of a pseudo β-turn at Val-isoAsp, which could be stabilized by a H-bond between NH-Gly and C=O-ACPC through the formation of a 11-membered ring (Type III, Figure 4).
Figure 4.
Preferred intramolecular H-bond/β-turn patterns proposed for the isoDGR cyclopeptide 10 spectroscopic data. The arrows indicate significant NOE contacts. Type I’ H-bonding pattern, Gly-Arg β-turn motif. Type III H-bonding pattern, Val-isoAsp pseudo β-turn motif.
2.4. Computational Studies
The conformation and the interaction with the αvβ3 integrin of the cyclic peptidomimetics 8–10 were investigated by means of computational studies to generate models fitting spectroscopic and biological data.
Monte Carlo/Energy Minimization (MC/EM) conformational searches [52] of simplified cyclic pentapeptide analogs (containing β-ACPC and methyl groups in place of Val, Arg and Asp/isoAsp side chains), followed by molecular dynamics (MD) simulations of the RGD or isoDGR peptidomimetic ligands, were run in water, as implicitly represented by the generalized Born/surface area (GB/SA) solvation model [53]. The compounds displayed high flexibility, by adopting different backbone geometries characterized by specific H-bond and β-turn patterns. Notably, the macrocycle conformations found for the new ligands are very similar to the geometries previously detected for other cyclic RGD pentapeptide mimics [28]. The analysis of the dihedral angle between the amino and the carboxylic group in the β-ACPC scaffold revealed absolute values of about 60°, with a preference for negative or positive values that depend on the stereochemistry of the cis-β-ACPC unit (i.e., preferred negative values for compound 8, positive values for compound 9). The inversion of the pseudo-chair ring conformation of the β-ACPC scaffold was indeed observed in the calculated structures of each compound, even if rarely and independently from the macrocycle conformation.
Among the conformers within 3 kcal/mol of the global minimum calculated for the (β-ACPC-Ala-Ala-Gly-Ala) analog of 8, cyclopeptide geometries were identified that nicely fit the Type I and II patterns suggested on the basis of the NMR data (Figure 3). Three-dimensional structures satisfying the characteristic NOE contact and H-bond of each pattern were selected from the MD trajectory of ligand 8 and then employed as starting geometries for docking studies in the αVβ3 integrin active site (see the Experimental section for computational details) [54]. The best pose of each pattern was re-docked to generate optimized poses that were compared to the crystal structure of the cyclic pentapeptide Cilengitide in complex with the extracellular segment of integrin αvβ3 (PDB code 1L5G).
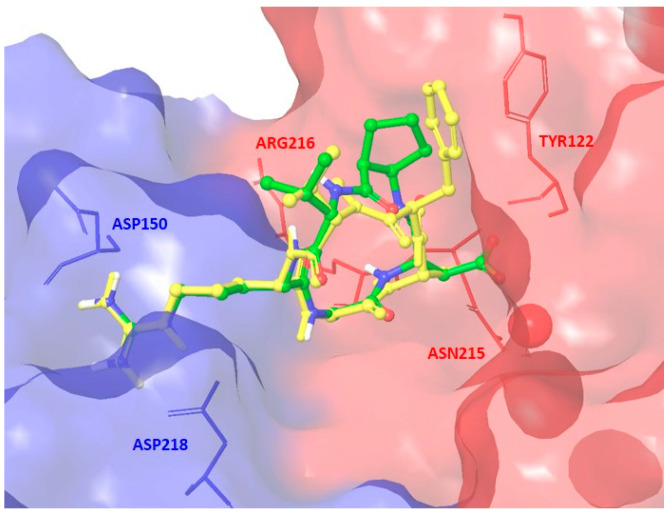
Docking runs starting from geometries of 8 adopting the Type I pattern produced top-ranked poses conserving most of the key interactions observed in the X-ray complex. In the best pose shown in Figure 5, the positively charged Arg guanidinium group of ligand 8 interacts with the negatively charged side chains of Asp218 and Asp150 in the α unit, one carboxylate oxygen of the ligand Asp side chain is coordinated to the metal cation in the metal-ion-dependent adhesion site (MIDAS) of the β unit, and the second carboxylate oxygen forms hydrogen bonds with the backbone amides of Asn215 and Tyr122 in the β unit. However, several other docking poses, including those obtained from Type II starting geometries (see the Supporting Information for the docking best pose of Type II conformation), lose some H-bond interactions or poorly reproduce the crystallographic binding mode mainly interacting with the β-subunit.
Figure 5.
Docking best pose of compound 8 (Type I, green C atoms, Gscore = −9.26 kcal/mol) in the crystal structure of the extracellular domain of αvβ3 integrin (α unit blue, β unit red) overlaid on the bound conformation of Cilengitide (yellow). Only selected integrin residues involved in interactions with the ligand are shown. The metal ion at MIDAS is shown as a red CPK sphere (space-filling model by Corey, Pauling, Koltun). Nonpolar hydrogens are hidden for clarity.
A possible explanation of this behavior may be found in the lack of the aromatic moiety and of the corresponding stabilizing interaction with the side chain of Tyr122 in the β subunit, as well as in the less extended arrangement of the RGD sequence in Type II conformations that is known to prevent the optimal binding with the integrin pocket. Indeed, in agreement with previous studies on different cyclic RGD pentapeptide mimics, Type I conformations are characterized by extended arrangements of the recognition motif (showing Cβ(Arg)–Cβ(Asp) distance values of about 9 Å), while the β-turn motif at Arg-Gly of the Type II pattern induces a reduced extension of the RGD sequence (showing Cβ(Arg)–Cβ(Asp) distance values of about 8 Å) [28].
In summary, although peptidomimetic 8 can adopt extended conformations of the recognition motif that should be able to properly fit into the receptor active site and establish the key polar interactions, the absence of an aromatic group and the conformational flexibility of the cyclopeptide induce a notable mobility of the ligand in the integrin binding pocket. This fact is possibly responsible for the non-optimal interaction pattern and of the IC50 value in competitive binding assay to αVβ3 receptor about 10 times higher than that of other cyclic RGD ligands (e.g., compound 4 in Table 1).
Similarly, different cyclopeptide geometries can be identified for compound 9 by means of MC/EM conformational searches and MD simulations, including geometries that nicely fit the Type I pattern suggested on the basis of the NMR data (Figure 3). Docking calculations (see the Experimental section for computational details and the Supporting Information for the docking best pose) showed that the conformations adopting this pattern produced top-ranked poses conserving most of the key interactions observed in the X-ray complex. However, the non-extended arrangement adopted by the RGD sequence in some other geometries prevents an optimal interaction with the integrin pocket. Similar considerations to those made for the compound 8 might explain the binding affinity of ligand 9: the lack of an aromatic moiety and the conformational flexibility of the cyclopeptide make the interaction and the geometrical preorganization less favorable than in other cyclic peptidomimetic RGD ligands.
Finally, docking studies of the isoDGR peptidomimetic 10 revealed that both Type I’ and Type III geometries are not able to fit unhindered the αVβ3 binding site, producing non-optimal binding modes (as shown in the Supplementary Materials).
3. Material and Methods
3.1. General Procedures
The synthesis of (1S,2R)-cis-2-amino-1-cyclopentanecarboxylic acid and (1R,2S)-cis-2-amino-1-cyclopentanecarboxylic acid were performed following published procedures [55,56]. The synthetic procedures for the preparation of compounds 8–10 and their characterization are reported in the Supporting Information, along with the 1H NMR and 13C NMR spectra, HPLC traces.
3.2. Competitive Binding Assays to the Purified αvβ3 and α5β1 Receptors
The inhibition assays of biotinylated vitronectin and fibronectin binding to the αvβ3 and α5β1 receptors, for compounds 8–10 were carried out as previously reported [29,48,49,50]. IC50 values were calculated as the concentration of compound required for 50% inhibition of biotinylated vitronectin or fibronectin binding. Screening assays were performed by incubating the immobilized integrin αvβ3 or α5β1 with increasing concentrations (10−12–10−5 M/10−11–10−4 M) of the RGD and isoDGR ligands in the presence of the corresponding biotinylated ECM protein (1 μg mL−1), and measuring bound protein in the presence of the competitive ligand. Each data point is the result of the average of triplicate wells and was analyzed by nonlinear regression analysis with the GraphPad Prism software. Each experiment was repeated in duplicate. IC50 values are the arithmetic mean ± the standard deviation (SD) of these duplicate determinations.
3.3. Cell Adhesion Assays
The cell adhesion studies on WM115 cell line with the compounds 8–10 are described in the Supporting Information.
3.4. NMR Studies
NMR experiments were performed on a Bruker Avance spectrometer (Bruker, Málaga, Spain) operating at 500 MHz at 298 K. The concentration of each compounds was 5 mM and they are analyzed dissolved in H2O-D2O 9:1 in a 5 mm NMR tube.
1H and 13C resonance assignment for all the ligands was performed on the base of 1D 1H, 2D COSY, TOCSY and 1H, 13C-HSQC experiments. For conformational analysis, NOESY experiments were acquired varying the mixing time from 400 to 700 ms. Water suppression was achieved by excitation sculpting sequence from standard Bruker library.
For the variable temperature analysis (VT-NMR), monodimensional 1H spectra were acquired from 298 K to 333 K.
3.5. Computational Studies
All calculations were performed using the Schrödinger suite of programs through the Maestro graphical interface [57]. Conformational preferences of compounds were investigated by molecular mechanics calculations using the MacroModel v11.1 implementation of the Amber all-atom force field (denoted AMBER *) and the implicit water GB/SA solvation model [53,58]. Monte Carlo/energy minimization (MC/EM) conformational search of the cyclopeptide analog containing methyl groups instead of the Val, Arg and Asp/isoAsp side chains was performed as the first step to generate starting cyclopeptide conformations for MD simulations that are not biased by electrostatic interactions. For the search, 1000 starting structures for each variable torsion angle were generated and minimized until the gradient was < 0.05 kJ Å−1 mol−1 using the truncated Newton-Raphson method implemented in MacroModel [59]. Duplicate conformations and those with energy > 5 kcal mol−1 above the global minimum were discarded. Free MD simulations of the RGD cyclic peptides (Asp and Arg side chains were considered ionized) were then performed at 300 K using MacroModel v11.1, the force field and the solvent defined above (1 fs integration step, 20 ns simulation time for each run, 5000 structures saved for the analysis), starting from the cyclopeptide backbone geometries located by the previous MC/EM step. Each significant long range interaction between amide protons described by a NOE was employed as a filter (distance between protons involved in the NOE contact < 3.5 Å) to select the conformations fitting a specific experimental contact. Representative 3D structures filtered out from MD trajectories and reproducing the H-bond patterns pointed out by NMR data, were employed as starting geometries for docking studies.
The crystal structure of the extracellular domain of the integrin αvβ3 in complex with the cyclic pentapeptide Cilengitide (PDB code 1L5G) was used for docking studies [60]. Flexible-ligand docking calculations were performed using Glide version 7.0 in the Standard Precision (SP) mode [61]. The settings of the protein preparation, grid generation and docking step were defined as previously reported [27,28,29]. The Glide program was initially tested for its ability to reproduce the X-ray binding geometry of Cilengitide. The program was successful in reproducing the experimentally found binding mode of this compound, as it corresponds to the best-scored pose (the superimposition of the docked ligand to the crystal structure is shown in the Supporting Information, docked vs. X-ray peptide heavy atoms RMSD = 0.3287 Å).
4. Conclusions
The synthesis, conformational analysis, and some biological investigation of two RGD, 8–9 (Figure 2), and one isoDGR, 10 (Figure 2) cyclic peptidomimetics, containing two cis-2-amino-1-cyclopentanecarboxylic (either (1S,2R)-cis-β-ACPC or (1R,2S)-cis-β-ACPC) scaffolds were described. The synthesis of the linear precursors was performed on the solid phase and, after cleavage from the resin and cyclization, the integrin ligands were obtained in satisfying yields and excellent purity. The RGD compounds 8 and 9 are good αvβ3 integrin ligands, confirming this behavior both in an isolated receptor competitive binding assay (IC50 values 44 and 39 nM, respectively, one order of magnitude higher than the reference compound cyclo-[RGDfV]), and in the cell adhesion assay performed on the αVβ3 positive human skin melanoma cell line WM115 (IC50 values 75 and 124 μM, respectively), again one order of magnitude higher than the reference compound cyclo-[RGDfV]). The ligands were also tested for competitive binding to purified α5β1 giving IC50 values above 450 nM, thus confirming the trend, albeit with a selectivity ratio (IC50α5β1/IC50αvβ3) ranging from about 12 (compound 9) to 73 (compound 8). On the contrary, the isoDGR ligand 10 is a micromolar for both αvβ3 and α5β1 binder (5362 and 2331 nM respectively) in the competitive binding assay and an IC50 value could not be measured for the cell adhesion assay. The behavior of these ligands could be explained by the conformational NMR and computational studies of the ligands and the docking simulations performed in the αVβ3 integrin active site. The RGD ligands display intramolecular hydrogen bonds imposing well defined conformations and extended presentation of the RGD recognition motif that should be able to properly fit into the receptor active site and establish the key polar interactions. However, the non-extended arrangement adopted by the RGD sequence in other geometries as well as the absence of an aromatic moiety and of the corresponding stabilizing interaction with the side chain of Tyr122 in the β subunit, prevent an optimal interaction with the integrin pocket and reduce the activity of these ligands as integrin binders. On the other hand, the geometries adopted by the isoDGR ligand 10 are not able to fit the αvβ3 binding site, producing non-optimal binding modes.
Further studies aimed at improving the activity and selectivity of these ligands (based on a rational design and plan to modification of the structure of the peptidomimetic scaffold) are currently in progress in our laboratories.
Supplementary Materials
Detailed synthetic procedures and characterization for compounds 8–10. Figures S1–S3: HPLC traces of compounds 8–10. Biological Tests (cell culture, determination of IC50 values, cell adhesion experiments). Figures S4 and S5: Cell adhesion curves for compounds 8–10. Computational studies, docking calculations. Figures S6–S9: Docking best poses for Cilengitide and compounds 8–10.
Author Contributions
Conceptualization, U.P., O.R. and N.S.; Synthesis, S.P. and T.E.; Biological assays: D.A. and I.K. NMR studies, F.V. and D.P.; Computational studies, M.C. and L.B.; data curation, S.G.; writing—original draft preparation, S.G., F.V., M.C.; writing—review and editing, S.G., U.P., O.R.; supervision, U.P., O.R.; funding acquisition, U.P., O.R., L.B. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by FONDAZIONE CARIPLO (Project RE-D DRUG TRAIN 2010–1373 FOR a PhD fellowship to S.P.), EUROPEAN COMMISSION (Marie Skłodowska-Curie ITN MAGICBULLET RELOADED 861316) MINISTERO DELL’UNIVERSITA’ E DELLA RICERCA (PRIN 2015 project 20157WW5EH) and DEUTSCHE FORSCHUNGSGEMEINSCHAFT (Project RE 948-9/1).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hynes R.O. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Takada Y., Ye X., Simon S. The Integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avraamides C.J., Garmy-Susini B., Varner J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danhier F., Le Breton A., Préat V. RGD-based strategies to target αVβ3 integrin in cancer therapy and diagnosis. Mol. Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 5.Nieberler M., Reuning U., Reichart F., Notni J., Wester H.-J., Schwaiger M., Weinmüller M., Räder A., Steiger K., Kessler H. Exploring the role of RGD-recognizing integrins in cancer. Cancers. 2017;9:116. doi: 10.3390/cancers9090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierschbacher M.D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 7.Spitaleri A., Mari S., Curnis F., Traversari C., Longhi R., Bordignon C., Corti A., Rizzardi G.-P., Musco G. Structural basis for the interaction of isoDGR with the RGD-binding site of αVβ3 integrin. J. Biol. Chem. 2008;283:19757–19768. doi: 10.1074/jbc.M710273200. [DOI] [PubMed] [Google Scholar]
- 8.Curnis F., Cattaneo A., Longhi R., Sacchi A., Gasparri A.M., Pastorino F., Di Matteo P., Traversari C., Bachi A., Ponzoni M., et al. Critical role of flanking residues in NGR-to-isoDGR transition and CD13/integrin receptor switching. J. Biol. Chem. 2010;285:9114–9123. doi: 10.1074/jbc.M109.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghitti M., Spitaleri A., Valentinis B., Mari S., Asperti C., Traversari C., Rizzardi G.-P., Musco G. Molecular dynamics reveal that isoDGR-containing cyclopeptides are true αVβ3 antagonists unable to promote integrin allostery and activation. Angew. Chem. Int. Ed. 2012;51:7702–7705. doi: 10.1002/anie.201202032. [DOI] [PubMed] [Google Scholar]
- 10.Paladino A., Civera M., Curnis F., Paolillo M., Gennari C., Piarulli U., Corti A., Belvisi L., Colombo G. The Importance of Detail: How Differences in Ligand Structures Determine Distinct Functional Responses in Integrin αVβ3. Chem. Eur. J. 2019;25:5959–5970. doi: 10.1002/chem.201900169. [DOI] [PubMed] [Google Scholar]
- 11.Aumailley M., Gurrath M., Müller G., Calvete J., Timpl R., Kessler H. Arg-Gly-Asp constrained within cyclic pentapoptides Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50–54. doi: 10.1016/0014-5793(91)81101-D. [DOI] [PubMed] [Google Scholar]
- 12.Auzzas L., Zanardi F., Battistini L., Burreddu P., Carta P., Rassu G., Curti C., Casiraghi G. Targeting αVβ3 integrin: Design and applications of mono- and multifunctional RGD-based peptides and semipeptides. Curr. Med. Chem. 2010;17:1255–1299. doi: 10.2174/092986710790936301. [DOI] [PubMed] [Google Scholar]
- 13.Hatley R.J.D., Macdonald S.J.F., Slack R.J., Le J., Ludbrook S.B., Lukey P.T. An αv-RGD Integrin Inhibitor Toolbox: Drug Discovery Insight, Challenges and Opportunities. Angew. Chem. Int. Ed. 2018;57:3298–3321. doi: 10.1002/anie.201707948. [DOI] [PubMed] [Google Scholar]
- 14.Fanelli R., Schembri L., Piarulli U., Pinoli M., Rasini E., Paolillo M., Galiazzo M.C., Cosentino M., Marino F. Effects of a novel cyclic RGD peptidomimetic on cell proliferation, migration and angiogenic activity in human endothelial cells. Vasc. Cell. 2014;6:11. doi: 10.1186/2045-824X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo R., Mingozzi M., Belvisi L., Arosio D., Piarulli U., Carenini N., Perego P., Zaffaroni N., De Cesare M., Castiglioni V., et al. Synthesis and biological evaluation (in Vitro and in Vivo) of cyclic arginine-glycine-aspartate (RGD) peptidomimetic-paclitaxel conjugates targeting integrin αVβ3. J. Med. Chem. 2012;55:10460–10474. doi: 10.1021/jm301058f. [DOI] [PubMed] [Google Scholar]
- 16.Dal Corso A., Caruso M., Belvisi L., Arosio D., Piarulli U., Albanese C., Gasparri F., Marsiglio A., Sola F., Troiani S., et al. Synthesis and biological evaluation of RGD peptidomimetic-paclitaxel conjugates bearing lysosomally cleavable linkers. Chem. Eur. J. 2015;21:6921–6929. doi: 10.1002/chem.201500158. [DOI] [PubMed] [Google Scholar]
- 17.Zanella S., Angerani S., Pina A., López Rivas P., Giannini C., Panzeri S., Arosio D., Caruso M., Gasparri F., Fraietta I., et al. Tumor Targeting with an isoDGR–Drug Conjugate. Chem. Eur. J. 2017;23:7910–7914. doi: 10.1002/chem.201701844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López Rivas P., Bodero L., Korsak B., Hechler T., Pahl A., Müller C., Arosio D., Pignataro L., Gennari C., Piarulli U. Synthesis and biological evaluation of RGD and isoDGR peptidomimetic-α-amanitin conjugates for tumor-targeting. Beilstein J. Org. Chem. 2018;14:407–415. doi: 10.3762/bjoc.14.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raposo Moreira Dias A., Bodero L., Martins A., Arosio D., Gazzola S., Belvisi L., Pignataro L., Steinkühler C., Dal Corso A., Gennari C., et al. Synthesis and Biological Evaluation of RGD and isoDGR–Monomethyl Auristatin Conjugates Targeting Integrin αVβ3. ChemMedChem. 2019;14:938–942. doi: 10.1002/cmdc.201900049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feni L., Parente S., Robert C., Gazzola S., Arosio D., Piarulli U., Neundorf I. Kiss and run: Promoting effective and targeted cellular uptake of a drug delivery vehicle composed of an integrin-targeting diketopiperazine peptidomimetic and a cell-penetrating peptide. Bioconjugate Chem. 2019;307:2011–2022. doi: 10.1021/acs.bioconjchem.9b00292. [DOI] [PubMed] [Google Scholar]
- 21.Agnello S., Brand M., Chellat M.F., Gazzola S., Riedl R. A Structural View on Medicinal Chemistry Strategies against Drug Resistance. Angew. Chem. Int. Ed. 2019;58:3300–3345. doi: 10.1002/anie.201802416. [DOI] [PubMed] [Google Scholar]
- 22.Dechantsreiter M.A., Planker E., Mathä B., Lohof E., Hölzemann G., Jonczyk A., Goodman S.L., Kessler H. N-methylated cyclic RGD peptides as highly active and selective αVβ3 integrin antagonists. J. Med. Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 23.Belvisi L., Riccioni T., Marcellini M., Vesci L., Chiarucci I., Efrati D., Potenza D., Scolastico C., Manzoni L., Lombardo K., et al. Biological and molecular properties of a new αVβ3/αVβ5 integrin antagonist. Mol. Cancer Ther. 2005;4:1670–1680. doi: 10.1158/1535-7163.MCT-05-0120. [DOI] [PubMed] [Google Scholar]
- 24.Belvisi L., Bernardi A., Colombo M., Manzoni L., Potenza D., Scolastico G., Giannini G., Marcellini M., Riccioni T., Castorina M., et al. Targeting integrins: Insights into structure and activity of cyclic RGD pentapeptide mimics containing azabicycloalkane amino acids. Bioorg. Med. Chem. 2006;14:169–180. doi: 10.1016/j.bmc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Manzoni L., Belvisi L., Arosio D., Civera M., Pilkington-Miksa M., Potenza D., Caprini A., Araldi E.M.V., Monferrini E., Mancino M., et al. Cyclic RGD-Containing functionalized azabicycloalkane peptides as potent integrin antagonists for tumor targeting. ChemMedChem. 2009;4:615–632. doi: 10.1002/cmdc.200800422. [DOI] [PubMed] [Google Scholar]
- 26.Zanardi F., Burreddu P., Rassu G., Auzzas L., Battistini L., Curti C., Sartori A., Nicastro G., Menchi G., Cini N., et al. Discovery of subnanomolar arginine-glycine-aspartate-based αVβ3/αVβ5 integrin binders embedding 4-aminoproline residues. J. Med. Chem. 2008;51:1771–1782. doi: 10.1021/jm701214z. [DOI] [PubMed] [Google Scholar]
- 27.Ressurreiçao A.S.M., Vidu A., Civera M., Belvisi L., Potenza D., Manzoni L., Ongeri S., Gennari C., Piarulli U. Cyclic RGD-peptidomimetics containing bifunctional diketopiperazine scaffolds as new potent integrin ligands. Chem. Eur. J. 2009;15:12184–12188. doi: 10.1002/chem.200902398. [DOI] [PubMed] [Google Scholar]
- 28.Marchini M., Mingozzi M., Colombo R., Guzzetti I., Belvisi L., Vasile F., Potenza D., Piarulli U., Arosio D., Gennari C. Cyclic RGD peptidomimetics containing bifunctional diketopiperazine scaffolds as new potent integrin ligands. Chem. Eur. J. 2012;18:6195–6207. doi: 10.1002/chem.201200457. [DOI] [PubMed] [Google Scholar]
- 29.Mingozzi M., Dal Corso A., Marchini M., Guzzetti I., Civera M., Piarulli U., Arosio D., Belvisi L., Potenza D., Pignataro L., et al. Cyclic isoDGR peptidomimetics as low-nanomolar αVβ3 integrin ligands. Chem. Eur. J. 2013;19:3563–3567. doi: 10.1002/chem.201204639. [DOI] [PubMed] [Google Scholar]
- 30.Panzeri S., Zanella S., Arosio D., Vahdati L., Dal Corso A., Pignataro L., Paolillo M., Schinelli S., Belvisi L., Gennari C., et al. Cyclic isoDGR and RGD Peptidomimetics Containing Bifunctional Diketopiperazine Scaffolds are Integrin Antagonists. Chem. Eur. J. 2015;21:6265–6271. doi: 10.1002/chem.201406567. [DOI] [PubMed] [Google Scholar]
- 31.Nardelli F., Paissoni C., Quilici G., Gori A., Traversari C., Valentinis B., Sacchi A., Corti A., Curnis F., Ghitti M., et al. Succinimide-Based Conjugates Improve IsoDGR Cyclopeptide Affinity to αVβ3 without Promoting Integrin Allosteric Activation. J. Med. Chem. 2018;61:7474–7485. doi: 10.1021/acs.jmedchem.8b00745. [DOI] [PubMed] [Google Scholar]
- 32.Frank A.O., Otto E., Mas-Moruno C., Schiller H.B., Marinelli L., Cosconati S., Bochen A., Vossmeyer D., Zahn G., Stragies R., et al. Conformational control of integrin-subtype selectivity in isoDGR peptide motifs: A biological switch. Angew. Chem. Int. Ed. 2010;49:9278–9281. doi: 10.1002/anie.201004363. [DOI] [PubMed] [Google Scholar]
- 33.Cabrele C., Martinek T.A., Reiser O., Berlicki L. Peptides containing β-amino acid patterns: Challenges and successes in medicinal chemistry. J. Med. Chem. 2014;57:9718–9739. doi: 10.1021/jm5010896. [DOI] [PubMed] [Google Scholar]
- 34.Gentilucci L., Gallo F., Meloni F., Mastandrea M., Del Secco B., De Marco R. Controlling Cyclopeptide Backbone Conformation with β/α-Hybrid Peptide–Heterocycle Scaffolds. Eur. J. Org. Chem. 2016;2016:3243–3251. doi: 10.1002/ejoc.201600448. [DOI] [Google Scholar]
- 35.Schumann F., Müller A., Koksch M., Müller G., Sewald N. Are β-amino acids γ-turn mimetics? Exploring a new design principle for bioactive cyclopeptides. J. Am. Chem. Soc. 2000;122:12009–12010. doi: 10.1021/ja0016001. [DOI] [Google Scholar]
- 36.Urman S., Gaus K., Yang Y., Strijowski U., Sewald N., De Pol S., Reiser O. The constrained amino acid β-Acc confers potency and selectivity to integrin ligands. Angew. Chem. Int. Ed. 2007;46:3976–3978. doi: 10.1002/anie.200605248. [DOI] [PubMed] [Google Scholar]
- 37.Beumer R., Bubert C., Cabrele C., Vielhauer O., Pietzsch M., Reiser O. The synthesis of diastereo- and enantiomerically pure β-aminocyclopropanecarboxylic acids. J. Org. Chem. 2000;65:8960–8969. doi: 10.1021/jo005541l. [DOI] [PubMed] [Google Scholar]
- 38.Allen S.E., Dokholyan N.V., Bowers A.A. Dynamic Docking of Conformationally Constrained Macrocycles: Methods and Applications. ACS Chem. Biol. 2016;11:10–24. doi: 10.1021/acschembio.5b00663. [DOI] [PubMed] [Google Scholar]
- 39.Vasile F., Civera M., Belvisi L., Potenza D., Tiana G. Thermodynamically-Weighted Conformational Ensemble of Cyclic RGD Peptidomimetics from NOE Data. J. Phys. Chem. B. 2016;120:7098–7107. doi: 10.1021/acs.jpcb.6b04941. [DOI] [PubMed] [Google Scholar]
- 40.Ohki H., Inamoto Y., Kawabata K., Kamimura T., Sakane K. Synthesis and antifungal activity of FR109615 analogs. J. Antibiot. 1991;44:546–549. doi: 10.7164/antibiotics.44.546. [DOI] [PubMed] [Google Scholar]
- 41.Thorbeck P., Hjeds H., Schaumburg K. Syntheses and 1H NMR spectroscopic investigations of some pyrrolidine carboxylic acids designed as potential glial GABA uptake inhibitors. Acta Chem. Scand. 1981;B35:473–479. doi: 10.3891/acta.chem.scand.35b-0473. [DOI] [Google Scholar]
- 42.Bunnage M.E., Davies S.G., Roberts P.M., Smith A.D., Withey J.M. Asymmetric synthesis of the cis- and trans-stereoisomers of 4-aminopyrrolidine-3-carboxylic acid and 4-aminotetrahydrofuran-3-carboxylic acid. Org. Biomol. Chem. 2004;2:2763–2776. doi: 10.1039/b407558g. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Espinosa J.F., Gellman S.H. 12-Helix formation in aqueous solution with short β-peptides containing pyrrolidine-based residues. J. Am. Chem. Soc. 2000;122:4821–4822. doi: 10.1021/ja000093k. [DOI] [Google Scholar]
- 44.Schmitt M.A., Choi S.H., Guzei I.A., Gellmann S.H. New helical foldamers: Heterogeneous backbones with 1:2 and 2:1 α:β-amino acid residue patterns. J. Am. Chem. Soc. 2006;128:4538–4539. doi: 10.1021/ja060281w. [DOI] [PubMed] [Google Scholar]
- 45.Berlicki Ł., Pilsl L., Wéber E., Mándity I., Cabrele C., Martinek T.A., Fülöp F., Reiser O. Unique α,β- and α,α,β,β-peptide foldamers based on cis-β-aminocyclopentanecarboxylic acid. Angew. Chem. Int. Ed. 2012;51:2208–2212. doi: 10.1002/anie.201107702. [DOI] [PubMed] [Google Scholar]
- 46.Berlicki Ł., Kaske M., Gutiérrez-Abad R., Bernhardt G., Illa O., Ortuňo R.M., Cabrele C., Buschauer A., Reiser O. Replacement of Thr32 and Gln34 in the C -terminal neuropeptide y fragment 25-36 by cis -cyclobutane and cis -cyclopentane β-amino acids shifts selectivity toward the Y4 receptor. J. Med. Chem. 2013;56:8422–8431. doi: 10.1021/jm4008505. [DOI] [PubMed] [Google Scholar]
- 47.Malešević M., Strijowski U., Bächle D., Sewald N. An improved method for the solution cyclization of peptides under pseudo-high dilution conditions. J. Biotechnol. 2004;112:73–77. doi: 10.1016/j.jbiotec.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Civera M., Arosio D., Bonato F., Manzoni L., Pignataro L., Zanella S., Gennari C., Piarulli U., Belvisi L. Investigating the interaction of cyclic RGD peptidomimetics with αVβ6 integrin by biochemical and molecular docking studies. Cancers. 2017;9:128.1. doi: 10.3390/cancers9100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzzetti I., Civera M., Vasile F., Arosio D., Tringali C., Piarulli U., Gennari C., Pignataro L., Belvisi L., Potenza D. Insights into the Binding of Cyclic RGD Peptidomimetics to α5β1 Integrin by using Live-Cell NMR And Computational Studies. ChemistryOpen. 2017;6:128–136. doi: 10.1002/open.201600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borbély A., Figueras E., Martins A., Bodero L., Raposo Moreira Dias A., López Rivas P., Pina A., Arosio D., Gallinari P., Frese M., et al. Conjugates of Cryptophycin and RGD or isoDGR Peptidomimetics for Targeted Drug Delivery. ChemistryOpen. 2019;8:737–742. doi: 10.1002/open.201900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.IMarelli U.K., Frank A.O., Wahl B., La Pietra V., Novellino E., Marinelli L., Herdtweck E., Groll M., Kessler H. Receptor-Bound Conformation of Cilengitide Better Represented by Its Solution-State Structure than the Solid-State Structure. Chem. Eur. J. 2014;20:14201–14206. doi: 10.1002/chem.201403839. [DOI] [PubMed] [Google Scholar]
- 52.Chang G., Guida W.C., Still W.C. An Internal Coordinate Monte Carlo Method for Searching Conformational Space. J. Am. Chem. Soc. 1989;111:4379–4386. doi: 10.1021/ja00194a035. [DOI] [Google Scholar]
- 53.Still W.C., Tempczyk A., Hawley R.C., Hendrickson T. Semianalytical Treatment of Solvation for Molecular Mechanics and Dynamics. J. Am. Chem. Soc. 1990;112:6127–6129. doi: 10.1021/ja00172a038. [DOI] [Google Scholar]
- 54.Weinstock D.S., Narayanan C., Felts A.K., Andrec M., Levy R.M., Wu K.P., Baum J. Distinguishing Among Structural Ensembles of the Gb1 Peptide: Remd Simulations and NMR experiments. J Am Chem Soc. 2007;129:4858–4859. doi: 10.1021/ja0677517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies S.G., Ichihara O., Walters A.S. An expeditious asymmetric synthesis of (-)-(1R,2S)-cispentacin. Synlett. 1993;1993:461–462. doi: 10.1055/s-1993-22490. [DOI] [Google Scholar]
- 56.Davies S.G., Ichihara O., Lenoir I., Walters A.S. Asymmetric synthesis of (-)-(1R,2S)-cispentacin and related cis- and trans-2-amino cyclopentane- and cyclohexane-1-carboxylic acids. J. Chem. Soc. Perkin Trans. 1994;1:1411–1415. doi: 10.1039/P19940001411. [DOI] [Google Scholar]
- 57.Maestro Version 10.5. Schrödinger; New York, NY, USA: 2016. [Google Scholar]
- 58.Macromodel, Version 11.1. Schrödinger; New York, NY, USA: 2016. [Google Scholar]
- 59.Ponder J.W., Richards F.M. An efficient newton-like method for molecular mechanics energy minimization of large molecules. J. Comput. Chem. 1987;8:1016–1024. doi: 10.1002/jcc.540080710. [DOI] [Google Scholar]
- 60.Xiong J.P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S.L., Arnaout M.A. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 61.Glide, Version 7.0. Schrödinger; New York, NY, USA: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.