Abstract
Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. However, some studies in mast cell-deficient mice have suggested that these cells may not play a critical role in cutaneous scarring/fibrosis. Here, we will review the data for and against mast cells as key regulators of skin fibrosis and discuss scientific gaps in the field.
Keywords: mast cell, fibroblast, inflammation, hypertrophic scar, keloid, cutaneous fibrosis, scleroderma
1. Introduction
When the skin is injured, the body initiates a wound-healing response. Depending on the tissue type and the severity of the injury, one of three general outcomes can result: regeneration of normal tissue without scarring, repair of the tissue with a scar, or fibrosis with excessive scar tissue production. Although regeneration is the ideal response to skin injury, this type of healing rarely occurs except in developing fetal skin. The normal response to tissue damage in the skin is repair. This well-characterized process begins with an inflammatory response during which resident inflammatory cells (e.g., mast cells and macrophages) become activated and circulating inflammatory cells (e.g., neutrophils and monocytes) are recruited. This is followed by a period of marked cellular proliferation and eventually the production/remodeling of collagen by fibroblasts to form a scar [1,2,3].
Scars are a significant clinical concern, and they pose many problems. Scar tissue has altered biomechanical properties, causing it to be weaker and more rigid than normal skin [4,5]. Scar tissue also inhibits the normal function of the skin, which leads to problems with thermoregulation, impairment of normal tissue growth, and restriction of joint movement. Furthermore, the quality of life for individuals with visible scars is often negatively affected [6]. In some cases, abnormal scars such as keloids or hypertrophic scars can develop. Additionally, excessive formation of scar tissue is the fundamental concern in diseases that cause cutaneous fibrosis, where there is extensive replacement of normal skin with scar tissue. Cutaneous fibrosis often develops in disorders with underlying vascular damage/dysfunction and/or pathological immune responses, such as systemic sclerosis and graft-versus-host disease.
An imbalanced or persistent inflammatory response is believed to contribute to scar formation and fibrosis [7]. One inflammatory cell type that has been proposed to drive fibroblast activation and excessive collagen deposition is the mast cell. Mast cells are resident inflammatory cells present at high numbers in organs exposed to the external environment such as the skin. Although they are known for their role in allergic reactions, mast cells can become activated in response to many different stimuli and are believed to play a role in a number physiological and pathologic processes outside of allergic responses. For example, roles for mast cells have been described in maintaining normal homeostasis, defense against parasitic, viral, and bacterial infections, neutralization/resistance to venom and toxins, and the development of diseases such as cancer and diabetes [8,9,10,11]. Mast cells are also involved in the wound repair process. Studies in animal models and human wounds have shown that mast cells undergo degranulation in response to skin injury and that mast cell numbers increase during repair, which is likely from the recruitment of mast cell precursors from the circulation [12,13,14,15,16,17]. Mast cells have been suggested to play a role in each phase of the wound repair process, including the inflammatory, proliferative, and scar formation/remodeling phases. Early after injury, they contribute to inflammatory cell recruitment and help prevent infection, they can stimulate the proliferative phase by promoting keratinocyte/fibroblast activity as well as angiogenesis, and they can communicate with fibroblasts to influence scar formation [18,19,20].
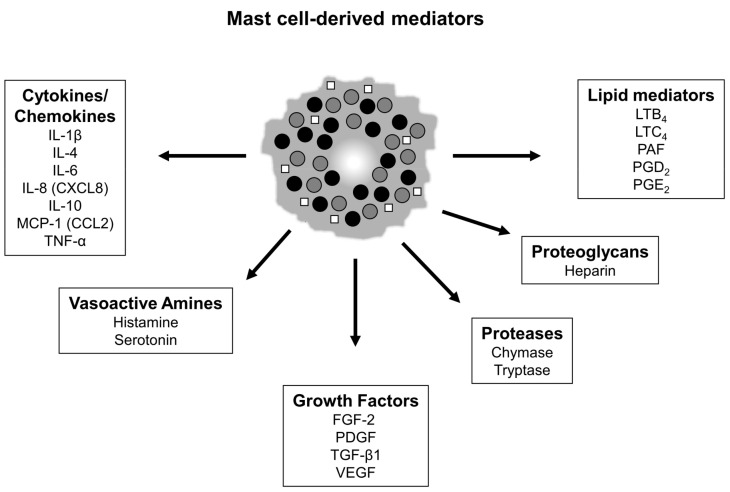
The diversity of activities described above that have been attributed to mast cells is likely due to the ability of these cells to produce a wide range of mediators [21] (Figure 1). Many mast cell mediators can stimulate neighboring fibroblasts, which has led to a large number of studies investigating the role of mast cells in scar formation and fibrosis. The majority of published data support a pro-fibrotic role for mast cells in the skin; however, some recent studies have questioned the functional importance of these cells in fibrosis. This review will summarize the data supporting and opposing a critical role for mast cells in skin scarring and fibrosis from both human and animal studies, and will discuss open questions and future opportunities for research in this area.
Figure 1.
Notable mast cell mediators. Mature mast cells can secrete a wide array of mediators that are either pre-made and stored in granules or are synthesized upon activation. Mediators can be secreted from mast cells through the release of granules (filled circles) or the release of secretory vesicles (open squares). (Note: this is not a complete list of mast cell mediators).
2. Mast Cells in Scarless and Fibrotic Fetal Skin Wounds
Although most types of injury in mature skin lead to the formation of a scar, there is a period during fetal development during which regenerative, scarless healing is known to occur. Fetal skin is capable of healing scarlessly prior to the third trimester of development, whereas more mature skin beyond this stage of development heals with the formation of a fibrotic scar [22,23,24]. The extent of inflammation induced after injury is one crucial difference between scarless and fibrotic fetal wounds, and this is believed to account, at least in part, for the divergent healing outcomes. A large number of studies have shown that there is little, if any, inflammation in scarless fetal wounds; by comparison, fibrotic fetal wounds display a vigorous inflammatory response early in the healing process [25,26]. As additional evidence for the importance of inflammation in dictating the healing phenotype, the artificial induction of an inflammatory response in fetal wounds that would otherwise heal scarlessly (e.g., by introducing an inflammatory stimulus) causes the wound to heal with a scar [27,28].
Interestingly, the density of dermal mast cells is known to increase as fetal skin becomes more developed and loses its ability to heal scarlessly. This has been shown by several groups in rat [29] and mouse [30,31] skin. This also appears to be true for human skin, as it has been reported that mid-gestation fetal skin (18–22 weeks) contains a lower number of mast cells compared to mature skin (adult) [32]. In addition to an increase in mast cell density during development, mast cells become more mature with regard to their size, granular density, and the biochemical makeup of their granules [30,31,33]. These differences in mast cell maturity may explain why mast cells from less developed fetal skin do not degranulate to the same degree in response to activation stimuli in ex vivo assays or in response to injury in early-gestation scarless fetal wounds in vivo [30,34]. More detailed experiments assessing the role of mast cells in scar formation in fetal wounds showed that mast cell lysates from late-gestation fetal skin disrupted scarless healing when injected into early-gestation fetal skin wounds and that late-gestation fetal wounds created in mast cell-deficient KitW/W-v mice healed with smaller scars compared to wild-type mice that contain normal dermal mast cell numbers [30]. Together, the association between elevated mast cell numbers, enhanced mast cell maturity, and increased mast cell degranulation with the generation of scar tissue in late-gestation fetal wounds, as well as the reduction in scar formation in late-gestation wounds from mast cell-deficient mice, suggest that mast cells are an important contributor to scar formation in fetal skin (summarized in Table 1).
Table 1.
Mast cell characteristics in developing skin.
| Early- to Mid-Gestation Skin (Scarless) |
Late-Gestation/ Postnatal Skin (Fibrotic) |
||
|---|---|---|---|
| Unwounded skin | Mast cell number | lower | higher |
| Mast cell maturity | less mature | more mature | |
| Mast cell size | smaller | larger | |
| Mast cell granularity | less dense | more dense | |
| Wounded skin | Mast cell degranulation | infrequent | pronounced |
3. Mast Cells in Normal Scarring
The normal cutaneous wound repair process leads to the formation of a scar. Several studies have quantified mast cells in normal human scar tissue and found that mast cells are more abundant in scars compared to normal skin. In excisional wounds, mast cells were shown to significantly increase after injury, and the number of mast cells in 4-week and 6-week scars was approximately double the number found in uninjured skin [17]. Several other studies have reported an increase in mast cells in normal scars compared to uninjured skin [35,36,37]. Differences in the types of mast cells present in scars have also been described, with a reduction in mast cells expressing high levels of chymase and mast cells with a mature phenotype in scars, despite the higher total numbers of mast cells in scars [36,37]. This suggests that the infiltration of immature mast cell precursors may account for the increase in overall mast cell numbers in scars. Interestingly, studies have also suggested that mast cell numbers differ depending on the age of the scars, with more mast cells present in scars of more advanced age [36,37,38]. Several studies have also suggested that mast cell numbers are reduced by therapies that may mitigate scarring. For example, topical treatment with epigallocatechin-3-gallate (EGCG) was shown to improve various scar parameters, and this coincided with a reduction in mast cells in human skin wounds [17]. Overall, there appears to be a positive correlation between mast cell numbers and the normal scar formation process in human skin.
In addition to the human studies discussed above, animal models have been used to carry out functional studies testing the importance of mast cells in the normal scar formation process. These types of studies have generally used mast cell stabilizers to block mast cell degranulation or mouse strains deficient in mast cells. Disodium cromoglycate, also known as cromolyn, is a mast cell stabilizer that has been used in several animal studies. Direct injection of this drug into rat wounds has been shown to reduce collagen content [39]. In another study, systemic administration with disodium cromoglycate was shown to reduce scar size and normalize collagen architecture and collagen fibril density in murine excisional wounds [40].
Aside from mast cell stabilizers, several different mast cell-deficient mouse strains have also been used to examine the role of mast cells in normal scar formation. KitW/W-v mice are naturally occurring mutant mice that lack mast cells as a result of mutations in the gene encoding the tyrosine kinase receptor Kit. This receptor mediates signaling in response to stem cell factor, which is required for mast cell development. In a scald wound model, KitW/W-v mice were reported to heal with less wound edge fibrosis [41]. In another burn wound model, KitW/W-v mice as well as mice lacking the mast cell enzymes murine mast cell protease (mMCP)-4 (chymase) or mMCP-5 (elastase) healed with hair regrowth and less scarring [42]. In large excisional wounds, collagen remodeling was suggested to differ between wild-type and KitW/W-v mice [43]. In another excisional wound study, KitW/W-v mice were shown to produce less granulation tissue and collagen in response to negative pressure, which causes microdeformation and enhances granulation tissue formation and collagen deposition in normal wild-type mice [44].
Other studies in various mast cell-deficient strains have suggested that mast cells do not play a strong role in scar formation. In splinted excisional wounds, no differences were found in scar size or collagen density in mast cell-deficient KitW/W-v, KitW-sh/W-sh, or Cpa3-Cre/Mcl-1fl/fl mice [45]. KitW/W-v and KitW-sh/W-sh strains have mutations in the Kit receptor, whereas Cpa3-Cre/Mcl-1fl/fl mice lack mast cells due to cre recombinase-mediated ablation of the anti-apoptotic factor Mcl-1 (myeloid leukemia cell differentiation protein-1) in carboxypeptidase A (Cpa3)-expressing mast cells [46]. Another study using Cpa3Cre/+ mice [47], which are deficient in mast cells due to cre-related genotoxicity in mast cells, showed no differences in granulation tissue/scar area or collagen organization between mice with and without mast cells in excisional wounds [48]. Finally, a study in Mcpt5-Cre/iDTR mice [49], which allow for inducible ablation of mast cells expressing the mast cell protease 5 (Mcpt5) using diphtheria toxin administration, showed no differences in granulation tissue/scar area or alpha-smooth muscle actin (α-SMA) staining after excisional wounding [50]. The reasons behind the mixed results from mast cell-deficient mouse studies are not completely clear, but differences in wound models and time points analyzed, as well as caveats with each mouse strain could play a role (see Section 7 for more discussion on this topic). It is also possible that mast cells play a role in the scar formation process but that their functions are redundant with those of other cells, in which case the depletion of mast cells alone may not lead to obvious effects [51].
4. Mast Cells in Abnormal Scarring
Although the production of scar tissue even at normal levels can be problematic, excessive or abnormal scarring poses even more of a concern from aesthetic, functional, and clinical points of view. Hypertrophic scars (HTS) and keloids are two types of abnormal scars that can develop after injury. HTS are raised red scars that fall within the borders of the original injury, whereas keloids are abnormal raised scars that spread beyond the margins of the original wound. A number of studies have assessed the potential role of mast cells in abnormal scars.
4.1. Hypertrophic Scars
Several published studies have examined mast cells in human HTS. Early studies showed an increase in histamine in HTS compared to normal skin, suggesting the presence of active mast cells in HTS [52]. An increase in the incidence of allergic symptoms has also been reported in HTS patients compared to control patients [53], and burn patients with pruritis have been shown to have more mast cells and more severe scarring compared to patients without pruritis [54], suggesting a possible role for mast cell activation in scarring. Several other studies directly examining mast cells have suggested higher numbers of mast cells in HTS tissue compared to either normal skin, granulation tissue, or mature scar tissue [55,56,57,58,59,60]. Another study suggested increased staining for tryptase-positive mast cells in HTS and surgical scars compared to normal skin, although staining for chymase-positive mast cells was similar between groups [61]. In addition, several studies have suggested that various therapeutic approaches to reduce hypertrophic scar thickness, such as electric current or pressure therapy, leads to reduced dermal mast cell numbers [60,62]. In contrast to the results described above, some studies have suggested that the density of tryptase-positive mast cells is similar in HTS compared to normal surgical scars or normal skin [38,63,64,65], and one study suggested that there are reduced numbers of chymase-positive mast cells in HTS compared to normal skin [64].
Mast cells have also been examined in animal models of HTS. The red Duroc pig is often used as a model of HTS and mimics several important features of human HTS. In this model, HTS tissue was shown to have more mast cells compared to normal skin [55]. Another study in red Duroc pigs showed that treatment with the mast cell stabilizer ketotifen resulted in reduced wound contraction and collagen deposition, with the formation of thinner and less dense collagen fibrils [66]. Small animal models have also been used to study mast cells in HTS formation. Studies have shown that mast cells are prominent in HTS-like lesions that develop in mechanically loaded wounds with increased tension [67]. Elevated mast cell numbers have also been reported in a mouse model of HTS contracture [68] and in a model that used human skin grafting in nude mice to induce HTS [69].
4.2. Keloids
Since there is no standardized, widely accepted animal model for keloids, studies on mast cells in keloid disease have primarily been limited to descriptive studies and ex vivo evaluation of human samples. Many of the studies published to date have suggested a potential role for mast cells in keloid scarring. Elevated histamine levels [52] and high numbers of histamine-containing mast cells [70] have been described in human keloid tissue, and allergic symptoms have been reported to be more frequent in keloid-forming patients compared to the normal population [53]. The number of mast cells and degranulated (i.e., activated) mast cells have been shown to be higher in keloids compared to normal skin and normal scar tissue [35]. Other studies have suggested an increase in the number of tryptase-positive mast cells [71,72] as well as higher total mast cells, chymase-positive mast cells, and chymase enzymatic activity in keloid tissue compared to normal skin [73]. There is also some experimental evidence that reduced mast cell numbers may correlate with therapeutic effects. In one study, treatment with silicone sheeting was shown to reduce pain, pruritis, and scar height in keloids, and a reduction in mast cells was observed after treatment compared to the numbers present before treatment [74]. In addition, the green tea polyphenol EGCG has been shown to inhibit the activity of keloid fibroblasts [75,76] and reduce keloid volume in an organ culture system [77]. These effects could be due in part to the action of EGCG on mast cells, as EGCG treatment significantly reduces mast cell numbers in keloid tissue ex vivo [77]. Despite the large number of studies suggesting a positive correlation between mast cell presence and keloid scarring, some studies have reported similar numbers of mast cells in keloids compared to normal tissue [61] or low mast cell numbers in keloids [65,78,79].
Overall, the majority of studies on HTS and keloids seem to indicate that the presence of mast cells may promote abnormal scarring, but the studies reporting results contrary to this idea raise questions about whether mast cell numbers are truly associated with scar formation. Although the exact reasons for the conflicting results are not known, there are a few items that should be considered when weighing the data. First, many of the studies use different histological or immunostaining methods to identify mast cells. These methods may stain mast cell granules (toluidine blue), mast cell proteases (tryptase or chymase), or membrane receptors on mast cells (CD117/Kit), which may yield different results. Of particular importance is the fact that mast cells can degranulate and may also release proteases upon activation, making it more difficult to accurately count the number of mast cells in settings with pronounced mast cell activation in toluidine blue- or protease-stained sections. In addition, the number of samples, type of samples, and the specific area(s) within the samples being analyzed could also impact the results. For example, mast cell numbers can vary depending on the age of the scar [38,63] and can fluctuate across different regions within a scar [35]. These caveats should all be accounted for when attempting to compare or reconcile results from different studies on mast cells.
5. Mast Cells in Cutaneous Fibrosis
5.1. Systemic Sclerosis
Systemic sclerosis (SSc) is a disease characterized by fibrosis in the skin and other internal organs, and it is associated with vascular damage and immune dysfunction [80]. Many studies have shown that the number of mast cells and the frequency of mast cell degranulation is higher in sclerotic skin lesions from SSc patients compared to either uninvolved skin or skin from normal control patients, and this can depend on the type and grade of SSc [81,82,83,84,85,86,87,88]. For instance, dermal mast cells appear to be more prevalent in patients with systemic sclerosis compared to those with localized scleroderma [83], and total mast cells or degranulating mast cells have been described to be more pronounced in lower grade (scleroedematous) lesions compared to higher grade (sclerotic) lesions [83,86]. Additionally, mast cells and mast cell degranulation have also been shown to be higher in progressive lesions compared to improving or regressing lesions [81,85]. Levels of the mast cell growth factor stem cell factor (SCF) have been reported to be elevated in the skin and serum of SSc patients, which may help explain the overrepresentation of mast cells in this disease [89]. Other studies have suggested differences in the protease or cytokine profiles of mast cells in SSc lesions. For example, an increase in the frequency of MCT (tryptase+/chymase−) cells compared to MCTC (tryptase+/chymase+) cells has been described in SSc skin lesions [90], and other studies have shown that SSc is associated with an increase in the number of dermal mast cells expressing transforming growth factor (TGF)-β1/2 [88] or interleukin (IL)-17A [91].
In skin from SSc patients, there appears to be a close association between mast cells and fibroblasts, and mast cell degranulation seems to be closely tied to fibrosis [81], with degranulating mast cells prevalent in areas of severe skin fibrosis [83] and less degranulation in skin from late, regressing skin lesions [85]. Furthermore, dermal mast cell hyperactivation has been reported in a case of rapidly progressing SSc [92]. Several other studies support these observations that mast cells are in a more activated state or degranulate more readily in SSc patients. For example, plasma histamine levels were shown to be higher in SSc patients compared to healthy controls [93] suggesting higher mast cell activity, and the size of wheals generated in response to the degranulation stimulating agent 48/80 were larger in SSc patients compared to normal controls [94].
5.2. Mouse Models of Scleroderma
Several animal models are used to study scleroderma [95], and most studies using these models have implicated mast cells in skin fibrosis. Repeated subcutaneous injection of bleomycin is commonly used to induce skin sclerosis/fibrosis in mice. Higher numbers of total mast cells and/or numbers of degranulating mast cells have been reported in the dermis with the bleomycin model [96,97,98,99]. In experiments where bleomycin-induced skin fibrosis was alleviated due to various treatments or genetic ablation of specific mediators that reduce fibrosis, reduced mast cell density or degranulation was evident [97,100,101,102,103,104,105]. Despite the clear correlation between mast cell density and activity in bleomycin-induced fibrosis, studies in mast-cell deficient mice have yielded mixed results. One study using KitW/W-v mice showed that in early phases (1 week), sclerosis did not develop in mast cell-deficient mice, but there were no significant differences compared to control mice at later stages (2–4 weeks), suggesting that mast cells may accelerate the formation of sclerotic lesions at early stages [106]. In a more recent study using a mouse model in which diphtheria toxin was used to induce the ablation of Mcpt5-expressing mast cells, mast cell-deficient and control mice developed skin fibrosis to a similar degree in response to bleomycin at 2 and 4 weeks [50]; however, early changes were not assessed in this study.
Tight skin (Tsk) mutant mice are also commonly used as a model of skin fibrosis [95]. Fibrosis in Tsk1+/− mice has been shown to be a result of a mutation in the fibrillin 1 gene [107]. An increase in mast cells and mast cell degranulation have been reported to be associated with fibrosis in Tsk1+/− mice [108,109,110]. It has been suggested that the increase in mast cells in Tsk1+/− mice is at least partially due to enhanced local production of cytokines such as TGF-β1, SCF, and IL-15 [109]. The mast cell stabilizers ketotifen and disodium cromoglycate, which block mast cell degranulation, were shown to inhibit fibrosis in Tsk1+/− mice [111,112]. Studies have shown that the expression and activity of the mast cell protease mMCP4 (chymase) is elevated in Tsk1+/− mice and treatment with a chymase inhibitor can reduce fibrosis [110,113]. In adoptive transfer experiments, the injection of bone marrow cells from Tsk1+/− mice into irradiated normal mice caused the development of skin fibrosis; however, this was not associated with an increase in mast cell numbers or degranulation [114]. These results raised questions about the importance of mast cells in skin fibrosis in Tsk1+/− mice. However, studies in which Tsk1+/− and KitW/W-v mice were crossed to generate mast cell-deficient Tsk1+/− mice suggested that while there was no change in the onset of fibrosis in the absence of mast cells, there was a reduction in the degree of fibrosis that developed in mice lacking mast cells [115].
More recent studies have suggested a role for mast cells in fibrosis using another model that has been reported to mimic several features of scleroderma. In this model, skin fibrosis develops in transgenic mice that overexpress the transcription factor Snail in epidermal keratinocytes [116]. Skin fibrosis in Snail transgenic mice is associated with an increase in dermal mast cells, which is dependent on the production of plasminogen activator inhibitor-1 (PAI-1) by keratinocytes. In vitro studies showed that PAI-1 stimulated mast cell chemotaxis and promoted mast cell-fibroblast interactions by increasing focal adhesion kinase signaling and enhancing the surface expression of intercellular adhesion molecule-1 [116].
5.3. Graft-Versus-Host Disease
Skin fibrosis is a recognized complication of graft-versus-host disease (GVHD), and the fibrotic process associated with GVHD shares several key features with progressive SSc [117]. An increase in mast cells has been reported in the skin of human GVHD samples compared to normal skin [118], and mast cells are commonly found in areas with irregular collagen fibers and active fibroblasts along with other immune cells [119]. Murine models of GVHD also develop cutaneous fibrosis, which is accompanied by prominent mast cell degranulation [118,120,121,122,123,124,125,126]. Studies have shown that GVHD mice treated with nedocromil sodium, a mast cell stabilizer, had less of a fibrotic response [126]. In contrast, mice treated with 48/80, which stimulates mast cell degranulation, developed more severe fibrosis [126]. In addition, less fibrosis and collagen deposition were noted in mast cell-deficient KitW-sh/W-sh mice with GVHD compared to control mice with GVHD [118].
6. Mechanistic Insights: Mast Cell–Fibroblast Interactions
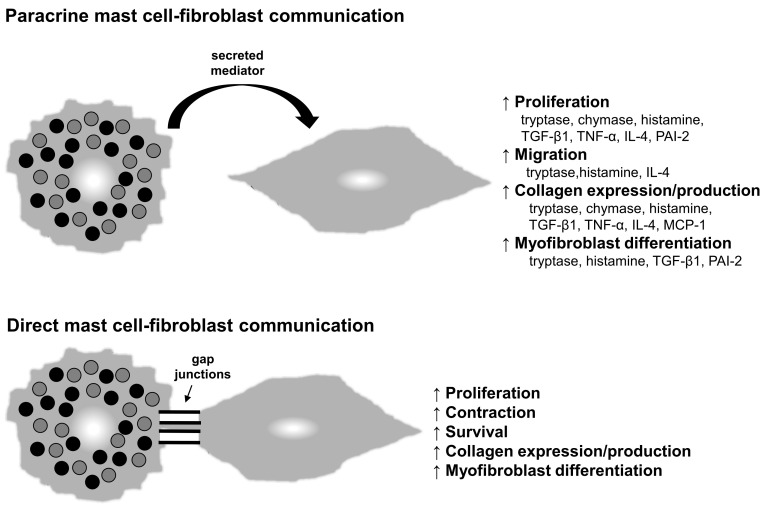
A number of mechanisms have been described by which mast cells can stimulate the activity of dermal fibroblasts and presumably promote scar formation and fibrosis. These include the secretion of paracrine mediators by mast cells and direct mast cell–fibroblast interactions (Figure 2).
Figure 2.
Mast cell–fibroblast interactions. Mast cells can influence the behavior of fibroblasts by secreting mediators that act in a paracrine manner (top) or by directly interacting with fibroblasts through heterocellular gap junctions (bottom).
6.1. Paracrine Communication
Activated mast cells are known to influence the activity of fibroblasts [127,128,129,130]. Mast cells store an array of mediators in cytoplasmic granules that can be quickly released after activation through the process of degranulation. In addition, mast cells can synthesize and subsequently secrete mediators in response to activation. Many of these mast cell-derived mediators have been shown to stimulate the activity of fibroblasts, including proteases, vasoactive amines, cytokines, and growth factors (Figure 2, top panel).
Tryptase and chymase are two mast cell proteases for which a wide range of effects on dermal fibroblasts have been reported. Tryptase has been shown to enhance fibroblast proliferation [64,131,132,133], chemotaxis [134], myofibroblast differentiation (α-SMA expression) [40,135], and collagen expression or production [40,132,133,134,136]. Chymase, at least at certain concentrations, has been reported to stimulate fibroblast proliferation [64,73,137,138] and collagen expression [58,73] as well as induce TGF-β1 production and signaling [73,137,138]. Interestingly, chymase may also play a direct role in collagen biosynthesis based on in vitro studies, as cleavage of a C-terminal region of type I pro-collagen by chymase leads to de novo collagen fibril formation [139].
Histamine is a vasoactive amine produced primarily by mast cells. Although the primary target of mast cell-derived histamine may be vascular endothelial cells, histamine also affects dermal fibroblasts. Studies have shown that histamine can increase the expression of collagen [133,140] and the production of various growth factors, including TGF-β1 [140]. Histamine has also been shown to increase α-SMA expression/myofibroblast differentiation [135] as well as fibroblast proliferation [133,141,142] and migration [142].
Several other mast cell-derived mediators can affect the activity of dermal fibroblasts. Studies have shown that the ability of activated mast cells to increase collagen expression/secretion [129] and proliferation [130] are partially dependent on TGF-β1 and TNF-α (tumor necrosis factor-α). IL-4 is produced by mast cells and increases fibroblast proliferation [143], chemotaxis [144], and collagen synthesis [145,146]. Mast cell-derived PAI-2 reportedly enhances fibroblast proliferation [131] and induces α-SMA expression in fibroblasts [147], suggesting that it may stimulate myofibroblast development. Mast cells are also known to produce monocyte chemoattractant protein-1 (MCP-1/CCL2) [148,149] and several mast cell mediators, including IL-4, TGF-β1, and TNF-α, also increase the release of MCP-1 from fibroblasts [150,151]. This chemokine has been shown to increase collagen production by fibroblasts [149] and has been linked to cutaneous fibrosis/scar formation by multiple groups [152,153].
6.2. Direct Cell–Cell Communication
Mast cells and fibroblasts are known to be closely associated in fibrotic tissues, and studies have shown that they directly adhere to and communicate with one another via heterocellular gap junctions in vitro (Figure 2, bottom panel). The co-culture of mast cells and fibroblasts, with direct contact between the two cell types, has been shown to stimulate myofibroblast conversion and increase collagen synthesis [154], increase the rate and degree of collagen contraction in various assays [135,155,156], and increase fibroblast proliferation [143]. Direct mast cell–fibroblast interactions also appear to enhance fibroblast survival in response to dexamethasone [157]. In one study, mast cells that were degranulated prior to co-culturing were just as effective as regular mast cells in stimulating fibroblasts, suggesting that direct mast cell–fibroblast contact was responsible for the observed effects [154]. Additional studies have supported direct communication between the cells via gap junction formation by showing that connexin 43 knockdown or treatment with amide hydrolase, which both inhibit gap junction formation, blocks the ability of mast cells to directly affect fibroblast activity [154,158]. While the ability of mast cells and fibroblasts to interact directly in vitro is fairly well established, whether these interactions take place in vivo is difficult to prove. However, ultrastructural analyses of tissue have shown that mast cells and fibroblasts are in very close contact with one another in fibrotic skin and abnormal scars [81,159,160]. One published study also presented limited in vivo data suggesting that mast cells can directly communicate with fibroblasts through gap junctions in a rat polyvinyl alcohol sponge implant model by showing the transfer of dye from labeled mast cells to fibroblasts [155]. Further study in this area will be needed to fully understand the scope of mast cell–fibroblast interactions and their biological significance in vivo.
7. Mast Cells in Fibrosis: Unanswered Questions and Future Directions
The review of the literature presented above highlights the broad scope of published studies that have examined mast cells in cutaneous scarring and fibrosis. There is a distinct period of mast cell degranulation in response to various types of skin injury that leads to the release of mediators, many of which have been shown to stimulate dermal fibroblasts. Furthermore, based on the bulk of results from both animal studies and human samples, it seems clear that most scars and fibrotic diseases in the skin contain elevated numbers of mast cells, and these conditions are also often associated with prominent mast cell activation. Despite these key pieces of information, questions still remain about whether mast cells are actually critical for the development of scar tissue and fibrosis, primarily due to conflicting data on the functional role of dermal mast cells.
7.1. Experimental Limitations—Mast Cell Stabilizers
Factors that may be contributing to lingering questions about the importance of mast cells in fibrosis include limitations in the currently existing experimental approaches used to study mast cells in vivo. Mast cell stabilizers, such as ketotifen and disodium cromoglycate, have been used to prevent the release of mast cell mediators via degranulation. However, no drugs have been described that can specifically target mast cells [11] and the mast cell stabilizers described to date are somewhat non-specific and may affect other cell types, complicating interpretation of the results. In addition, the utility of these drugs for topical treatment is limited due to their chemical properties, which prevent effective penetration into the skin. New mast cell stabilizers that are more appropriate for topical use are being developed [161,162]; however, thus far, they have only been tested with the intention of treating diabetic wounds, so their usefulness for preventing scarring/fibrosis is not known.
7.2. Experimental Limitations—Mouse Models
In addition to mast cell stabilizers, a variety of different mast cell-deficient mouse strains have been used to study mast cell function in scar formation and cutaneous fibrosis, but the results have been variable. Many of these studies have used mice with mutations in the Kit receptor, such as KitW/W-v and KitW-sh/W-sh mice. The Kit mutations lead to mast cell deficiency, but they also cause additional abnormalities such as macrocytic anemia and neutrophil defects that can confound the results when directly comparing Kit mutant and wild-type mice [163]. One method that has been used to overcome these concerns is to compare Kit mutant mice to mutant mice that have been reconstituted with mast cells from wild-type mice via intradermal injection (sometimes referred to mast cell knock-in mice) [164]. However, thus far, all published studies on scarring and fibrosis in Kit mutant mice have directly compared wild-type and Kit mutant mice without including reconstituted/knock-in control mice. As a result, questions remain about whether any observed changes between the mouse strains in these studies are truly due to the presence or absence of mast cells. Several newer mast cell-deficient mouse strains have been developed that do not rely on Kit mutations (discussed in Section 3) [165], but these models have drawbacks as well [166]. For example, both mast cells and basophils are depleted in several of these newer strains. In addition, these models do not account for the possible existence of mast cell subpopulations with opposing functions or potential changes in these subpopulations over time, since the entire mast cell population is depleted in these strains.
7.3. Mast Cell Heterogeneity
The concept of mast cell heterogeneity and the possible existence of different functional mast cell subpopulations in the skin is not new, but detailed studies in this area are lacking. Published reports [36,37] and our own unpublished staining from previously collected samples [17,167] (Figure 3 and Figure 4) suggest that the characteristics of mast cells change over time in a healing wound, and it is possible that there are different mast cell subpopulations that either promote or dampen fibrosis depending on the mediators they are producing. While this idea has not been examined in detail, published studies in other experimental systems have highlighted potential mast cell heterogeneity and plasticity, as well as the possible existence of mast cell subtypes with opposing roles (e.g., mast cells with either pro-inflammatory or anti-inflammatory/immunoregulatory activity) [168,169]. The existence of a population of anti-fibrotic mast cells in some organs has been proposed [170,171,172]; however, so far, there is no evidence of this in the skin. It would be interesting to know whether there is a population of mast cells that provides stop signals late in the repair process that functions to halt the production of scar tissue, but more work needs to be done to define different mast cell subpopulations and to characterize potential changes in these cells during the scar formation process.
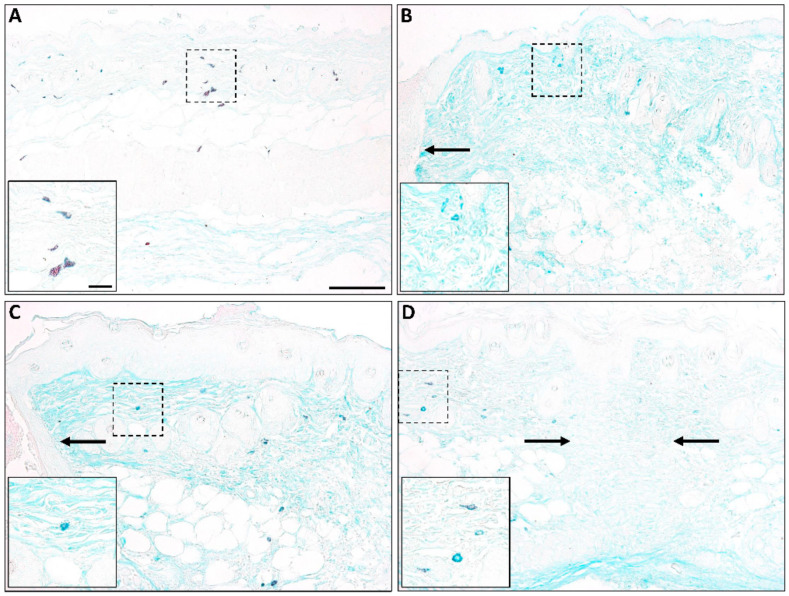
Figure 3.
Mast cells in murine wounds. Alcian blue-safranin staining of murine skin (A) and wounds at 1 day (B), 3 days (C), or 7 days (D) post-injury shows changes in mast cell maturity during healing (blue staining = immature; red staining = mature). High numbers of mature mast cells are found in unwounded skin, followed by mostly immature mast cells at early times post-injury. By 7 days, mast cells with some red granules begin to appear. Insets show a high magnification view of the boxed area; arrows show the wound edge (B,C) and healed wound margin (D). Scale bar = 100 µm; Inset scale bar = 20 µm.
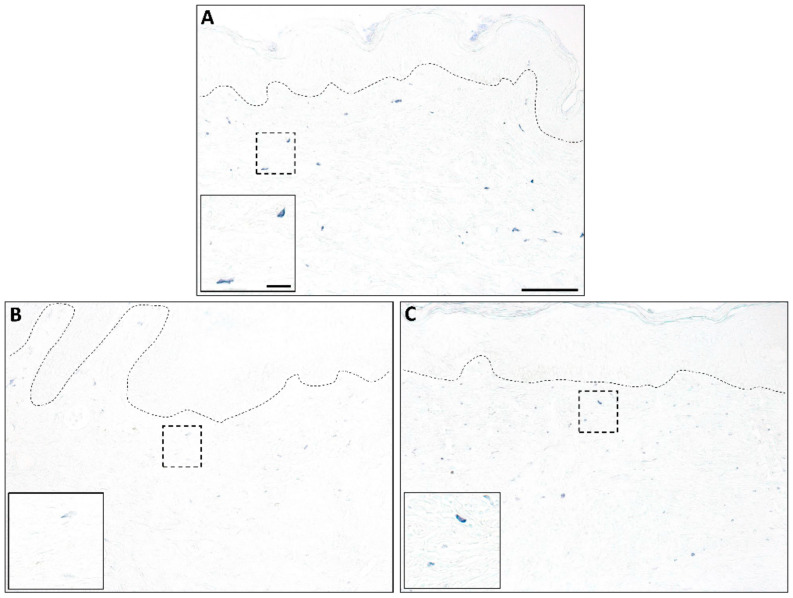
Figure 4.
Mast cells in human wounds. Toluidine blue staining of human skin (A) and wounds at 4 weeks (B) or 8 weeks (C) post-injury shows changes in mast cells during healing. Mast cells with dense staining are present in unwounded skin, and an increase in mast cell numbers can be seen in 4- and 8-week wounds. Mast cells in 4-week wounds stain less intensely than those in 8-week wounds, suggesting an increase in mast cell maturity as the wounds heal. Insets show a high magnification view of the boxed area; dotted lines denote the epidermal–dermal junction. Scale bar = 100 µm; Inset scale bar = 20 µm.
7.4. Mast Cells in Human Skin
Although there are data correlating high mast cell numbers with scarring and skin fibrosis in human skin, the relative importance of mast cells in these processes is not known. It is difficult to carry out cell-specific functional analyses in clinical studies, which is one reason animal models are commonly used to study in vivo mast cell function. Although murine models can be useful for understanding basic mechanisms of scar/fibrosis development in vivo and genetic alterations can be used to deplete mast cells or mast cell mediators, anatomical differences between mouse and human make it difficult to know how relevant results from murine studies are to human clinical disease. Further studies will have to be done to clarify the role of mast cells in cutaneous scarring and fibrosis in humans and to determine whether targeting mast cells could be a viable approach to prevent or limit scar tissue formation in human skin.
8. Conclusions
A comprehensive review of the published literature indicates a strong link between mast cells and cutaneous scarring/fibrosis, as well as strong evidence that mast cell-derived mediators influence fibroblast behavior. However, questions still remain about how critical mast cells are for the development of scar tissue. It is possible that mast cell activity is not absolutely required for scar formation but that mast cells promote this process. In order to better understand the role of mast cells in scar formation, more research needs to be done to characterize in detail the different mast cell subpopulations in the skin and how they change over time. More precise methods of depleting mast cells in animal models and more specific drugs to target mast cells are needed to advance the current knowledge about mast cell function in vivo. Overall, more work will be needed in order to gain a full understanding of dermal mast cells and how they contribute to the production of scar tissue during wound repair and the development of fibrosis in the skin.
Abbreviations
| CCL | CC chemokine ligand |
| Cpa3 | carboxypeptidase 3 |
| CXCL | CXC chemokine ligand |
| EGCG | epigallocatechin-3-gallate |
| FGF | fibroblast growth factor |
| GVHD | graft-versus-host disease |
| HTS | hypertrophic scar |
| iDTR | inducible diphtheria toxin receptor |
| IL | interleukin |
| LT | leukotriene |
| Mcl | myeloid leukemia cell differentiation protein |
| MCP-1 | monocyte chemoattractant protein-1 (also known as CCL2) |
| Mcpt | mast cell protease |
| mMCP | murine mast cell protease |
| PAF | platelet activating factor |
| PAI | plasminogen activator inhibitor |
| PDGF | platelet derived growth factor |
| PG | prostaglandin |
| SCF | stem cell factor |
| SMA | smooth muscle actin |
| SSc | systemic sclerosis |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| Tsk | tight skin |
| VEGF | vascular endothelial growth factor |
Author Contributions
Conceptualization, T.A.W., S.U.-D., A.B.; Writing—Original draft preparation, T.A.W.; Writing—Review and editing, T.A.W., S.U.-D., A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge support from the National Institutes of Health (NIH grant AR071115), the National Institute for Health Research Manchester Biomedical Research Centre (NIHR Q11 Manchester BRC) and the MRC (SA).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing—Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Corr D.T., Gallant-Behm C.L., Shrive N.G., Hart D.A. Biomechanical behavior of scar tissue and uninjured skin in a porcine model. Wound Repair Regen. 2009;17:250–259. doi: 10.1111/j.1524-475X.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunn M.G., Silver F.H., Swann D.A. Mechanical analysis of hypertrophic scar tissue: Structural basis for apparent increased rigidity. J. Investig. Dermatol. 1985;84:9–13. doi: 10.1111/1523-1747.ep12274528. [DOI] [PubMed] [Google Scholar]
- 6.Brown B.C., McKenna S.P., Siddhi K., McGrouther D.A., Bayat A. The hidden cost of skin scars: Quality of life after skin scarring. J. Plast. Reconstr. Aesthet. Surg. 2008;61:1049–1058. doi: 10.1016/j.bjps.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Wilgus T.A. Inflammation as an orchestrator of cutaneous scar formation: A review of the literature. Plast. Aesthet. Res. 2020;7:54. doi: 10.20517/2347-9264.2020.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J., Chen L., Zhang Y., Jayaswal N., Mezghani I., Zhang W., Veves A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020;37:4519–4537. doi: 10.1007/s12325-020-01499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli S.J., Metz M., Starkl P., Marichal T., Tsai M. Mast cells and IgE in defense against lethality of venoms: Possible “benefit” of allergy. Allergo J. Int. 2020;29:46–62. doi: 10.1007/s40629-020-00118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudeck A., Koberle M., Goldmann O., Meyer N., Dudeck J., Lemmens S., Rohde M., Roldan N.G., Dietze-Schwonberg K., Orinska Z., et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019;144:S4–S18. doi: 10.1016/j.jaci.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Varricchi G., de Paulis A., Marone G., Galli S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019;20:4397. doi: 10.3390/ijms20184397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak A.M., Kissell S. Granule changes of human skin mast cells characteristic of piecemeal degranulation and associated with recovery during wound healing in situ. J. Leukoc. Biol. 1991;49:197–210. doi: 10.1002/jlb.49.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Lateef Z., Stuart G., Jones N., Mercer A., Fleming S., Wise L. The Cutaneous Inflammatory Response to Thermal Burn Injury in a Murine Model. Int. J. Mol. Sci. 2019;20:538. doi: 10.3390/ijms20030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda H., Kitamura Y. Migration of stromal cells supporting mast-cell differentiation into open wound produced in the skin of mice. Exp. Hematol. 1981;9:38–43. [PubMed] [Google Scholar]
- 15.Trautmann A., Toksoy A., Engelhardt E., Brocker E.B., Gillitzer R. Mast cell involvement in normal human skin wound healing: Expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J. Pathol. 2000;190:100–106. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Nishikori Y., Kakizoe E., Kobayashi Y., Shimoura K., Okunishi H., Dekio S. Skin mast cell promotion of matrix remodeling in burn wound healing in mice: Relevance of chymase. Arch. Dermatol. Res. 1998;290:553–560. doi: 10.1007/s004030050351. [DOI] [PubMed] [Google Scholar]
- 17.Ud-Din S., Foden P., Mazhari M., Al-Habba S., Baguneid M., Bulfone-Paus S., McGeorge D., Bayat A. A Double-Blind, Randomized Trial Shows the Role of Zonal Priming and Direct Topical Application of Epigallocatechin-3-Gallate in the Modulation of Cutaneous Scarring in Human Skin. J. Investig. Dermatol. 2019;139:1680–1690. doi: 10.1016/j.jid.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Komi D.E.A., Khomtchouk K., Santa Maria P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020;58:298–312. doi: 10.1007/s12016-019-08729-w. [DOI] [PubMed] [Google Scholar]
- 19.Ud-Din S., Wilgus T.A., Bayat A. Mast Cells in Skin Scarring: A Review of Animal and Human Research. Front. Immunol. 2020;11:552205. doi: 10.3389/fimmu.2020.552205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann C., Troeltzsch D., Gimenez-Rivera V.A., Galli S.J., Metz M., Maurer M., Siebenhaar F. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc. Natl. Acad. Sci. USA. 2019;116:20500–20504. doi: 10.1073/pnas.1908816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore A.L., Marshall C.D., Barnes L.A., Murphy M.P., Ransom R.C., Longaker M.T. Scarless wound healing: Transitioning from fetal research to regenerative healing. Wiley Interdiscip. Rev. Dev. Biol. 2018;7:e309. doi: 10.1002/wdev.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilgus T.A. Regenerative healing in fetal skin: A review of the literature. Ostomy Wound Manag. 2007;53:16–31. [PubMed] [Google Scholar]
- 24.Wilgus T.A. Fetal wound healing. In: Bagchi D., Das A., Roy S., editors. Wound Healing, Tissue Repair, and Regeneration in Diabetes. Academic Press; Cambridge, MA, USA: 2020. [Google Scholar]
- 25.Armstrong J.R., Ferguson M.W. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev. Biol. 1995;169:242–260. doi: 10.1006/dbio.1995.1141. [DOI] [PubMed] [Google Scholar]
- 26.Cowin A.J., Brosnan M.P., Holmes T.M., Ferguson M.W. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 27.Kumta S., Ritz M., Hurley J.V., Crowe D., Romeo R., O’Brien B.M. Acute inflammation in foetal and adult sheep: The response to subcutaneous injection of turpentine and carrageenan. Br. J. Plast. Surg. 1994;47:360–368. doi: 10.1016/0007-1226(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 28.Wilgus T.A., Bergdall V.K., Tober K.L., Hill K.J., Mitra S., Flavahan N.A., Oberyszyn T.M. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am. J. Pathol. 2004;165:753–761. doi: 10.1016/S0002-9440(10)63338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savall R., Ferrer I. Mast cells in the skin of rats during development. Med. Cutanea Ibero Lat. Am. 1981;9:345–350. [PubMed] [Google Scholar]
- 30.Wulff B.C., Parent A.E., Meleski M.A., DiPietro L.A., Schrementi M.E., Wilgus T.A. Mast cells contribute to scar formation during fetal wound healing. J. Investig. Dermatol. 2012;132:458–465. doi: 10.1038/jid.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasugai T., Oguri K., Jippo-Kanemoto T., Morimoto M., Yamatodani A., Yoshida K., Ebi Y., Isozaki K., Tei H., Tsujimura T., et al. Deficient differentiation of mast cells in the skin of mi/mi mice. Usefulness of in situ hybridization for evaluation of mast cell phenotype. Am. J. Pathol. 1993;143:1337–1347. [PMC free article] [PubMed] [Google Scholar]
- 32.Walraven M., Talhout W., Beelen R.H., van Egmond M., Ulrich M.M. Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen. 2016;24:533–541. doi: 10.1111/wrr.12421. [DOI] [PubMed] [Google Scholar]
- 33.Combs J.W., Lagunoff D., Benditt E.P. Differentiation and proliferation of embryonic mast cells of the rat. J. Cell Biol. 1965;25:577–592. doi: 10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wulff B.C., Wilgus T.A. Examining the role of mast cells in fetal wound healing using cultured cells in vitro. Methods Mol. Biol. 2013;1037:495–506. doi: 10.1007/978-1-62703-505-7_29. [DOI] [PubMed] [Google Scholar]
- 35.Bagabir R., Byers R.J., Chaudhry I.H., Muller W., Paus R., Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br. J. Dermatol. 2012;167:1053–1066. doi: 10.1111/j.1365-2133.2012.11190.x. [DOI] [PubMed] [Google Scholar]
- 36.Hermes B., Feldmann-Boddeker I., Welker P., Algermissen B., Steckelings M.U., Grabbe J., Henz B.M. Altered expression of mast cell chymase and tryptase and of c-Kit in human cutaneous scar tissue. J. Investig. Dermatol. 2000;114:51–55. doi: 10.1046/j.1523-1747.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 37.Hermes B., Welker P., Feldmann-Boddeker I., Kruger-Krasagakis S., Hartmann K., Zuberbier T., Henz B.M. Expression of mast cell growth modulating and chemotactic factors and their receptors in human cutaneous scars. J. Investig. Dermatol. 2001;116:387–393. doi: 10.1046/j.1523-1747.2001.01284.x. [DOI] [PubMed] [Google Scholar]
- 38.Beer T.W., Baldwin H., West L., Gallagher P.J., Wright D.H. Mast cells in pathological and surgical scars. Br. J. Ophthalmol. 1998;82:691–694. doi: 10.1136/bjo.82.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabrowski R., Drobnik J. The effect of disodium cromoglycate on the skin wound healing and collagen content in the wounds of rats. Acta Physiol. Pol. 1990;41:195–198. [PubMed] [Google Scholar]
- 40.Chen L., Schrementi M.E., Ranzer M.J., Wilgus T.A., DiPietro L.A. Blockade of mast cell activation reduces cutaneous scar formation. PLoS ONE. 2014;9:e85226. doi: 10.1371/journal.pone.0085226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiota N., Nishikori Y., Kakizoe E., Shimoura K., Niibayashi T., Shimbori C., Tanaka T., Okunishi H. Pathophysiological role of skin mast cells in wound healing after scald injury: Study with mast cell-deficient W/W(V) mice. Int. Arch. Allergy Immunol. 2010;151:80–88. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- 42.Younan G., Suber F., Xing W., Shi T., Kunori Y., Abrink M., Pejler G., Schlenner S.M., Rodewald H.R., Moore F.D., Jr., et al. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J. Immunol. 2010;185:7681–7690. doi: 10.4049/jimmunol.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iba Y., Shibata A., Kato M., Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int. Immunopharmacol. 2004;4:1873–1880. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Younan G.J., Heit Y.I., Dastouri P., Kekhia H., Xing W., Gurish M.F., Orgill D.P. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast. Reconstr. Surg. 2011;128:649e–658e. doi: 10.1097/PRS.0b013e318230c55d. [DOI] [PubMed] [Google Scholar]
- 45.Nauta A.C., Grova M., Montoro D.T., Zimmermann A., Tsai M., Gurtner G.C., Galli S.J., Longaker M.T. Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS ONE. 2013;8:e59167. doi: 10.1371/journal.pone.0059167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lilla J.N., Chen C.C., Mukai K., BenBarak M.J., Franco C.B., Kalesnikoff J., Yu M., Tsai M., Piliponsky A.M., Galli S.J. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feyerabend T.B., Weiser A., Tietz A., Stassen M., Harris N., Kopf M., Radermacher P., Moller P., Benoist C., Mathis D., et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Antsiferova M., Martin C., Huber M., Feyerabend T.B., Forster A., Hartmann K., Rodewald H.R., Hohl D., Werner S. Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J. Immunol. 2013;191:6147–6155. doi: 10.4049/jimmunol.1301350. [DOI] [PubMed] [Google Scholar]
- 49.Dudeck A., Dudeck J., Scholten J., Petzold A., Surianarayanan S., Kohler A., Peschke K., Vohringer D., Waskow C., Krieg T., et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Willenborg S., Eckes B., Brinckmann J., Krieg T., Waisman A., Hartmann K., Roers A., Eming S.A. Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. J. Investig. Dermatol. 2014;134:2005–2015. doi: 10.1038/jid.2014.12. [DOI] [PubMed] [Google Scholar]
- 51.Galli S.J. Rethinking the potential roles of mast cells in skin wound healing and bleomycin-induced skin fibrosis. J. Investig. Dermatol. 2014;134:1802–1804. doi: 10.1038/jid.2014.142. [DOI] [PubMed] [Google Scholar]
- 52.Cohen I.K., Beaven M.A., Horakova Z., Keiser H.R. Histamine and collagen synthesis in keloid and hypertrophic scar. Surg. Forum. 1972;23:509–510. doi: 10.1097/00006534-197306000-00062. [DOI] [PubMed] [Google Scholar]
- 53.Smith C.J., Smith J.C., Finn M.C. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J. Burn Care Rehabil. 1987;8:126–131. doi: 10.1097/00004630-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Kwak I.S., Park S.Y., Choi Y.H., Cho S.I., Yang Y.S., Cho Y.S., Choi M.G., Seo C.H., Park C.W., Kim H.O. Clinical and Histopathological Features of Post Burn Pruritus. J. Burn Care Res. 2016;37:343–349. doi: 10.1097/BCR.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 55.Harunari N., Zhu K.Q., Armendariz R.T., Deubner H., Muangman P., Carrougher G.J., Isik F.F., Gibran N.S., Engrav L.H. Histology of the thick scar on the female, red Duroc pig: Final similarities to human hypertrophic scar. Burns. 2006;32:669–677. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tredget E.E., Shankowsky H.A., Pannu R., Nedelec B., Iwashina T., Ghahary A., Taerum T.V., Scott P.G. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: Effects of interferon alpha-2b. Plast. Reconstr. Surg. 1998;102:1317–1328. doi: 10.1097/00006534-199810000-00001. discussion 1329–1330. [DOI] [PubMed] [Google Scholar]
- 57.Choi Y.H., Kim K.M., Kim H.O., Jang Y.C., Kwak I.S. Clinical and histological correlation in post-burn hypertrophic scar for pain and itching sensation. Ann. Dermatol. 2013;25:428–433. doi: 10.5021/ad.2013.25.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H., Xu Y., Yang G., Zhang Q., Huang X., Yu L., Dong X. Mast cell chymase promotes hypertrophic scar fibroblast proliferation and collagen synthesis by activating TGF-beta1/Smads signaling pathway. Exp. Ther. Med. 2017;14:4438–4442. doi: 10.3892/etm.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kischer C.W., Bailey J.F. The mast cell in hypertrophic scars. Tex. Rep. Biol. Med. 1972;30:327–338. [PubMed] [Google Scholar]
- 60.Kischer C.W., Bunce H., 3rd, Shetlah M.R. Mast cell analyses in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J. Investig. Dermatol. 1978;70:355–357. doi: 10.1111/1523-1747.ep12543553. [DOI] [PubMed] [Google Scholar]
- 61.Gaber M.A., Seliet I.A., Ehsan N.A., Megahed M.A. Mast cells and angiogenesis in wound healing. Anal. Quant. Cytopathol. Histopathol. 2014;36:32–40. [PubMed] [Google Scholar]
- 62.Weiss D.S., Eaglstein W.H., Falanga V. Exogenous electric current can reduce the formation of hypertrophic scars. J. Dermatol. Surg. Oncol. 1989;15:1272–1275. doi: 10.1111/j.1524-4725.1989.tb03146.x. [DOI] [PubMed] [Google Scholar]
- 63.Niessen F.B., Schalkwijk J., Vos H., Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J. Pathol. 2004;202:121–129. doi: 10.1002/path.1502. [DOI] [PubMed] [Google Scholar]
- 64.Algermissen B., Hermes B., Feldmann-Boeddeker I., Bauer F., Henz B.M. Mast cell chymase and tryptase during tissue turnover: Analysis on in vitro mitogenesis of fibroblasts and keratinocytes and alterations in cutaneous scars. Exp. Dermatol. 1999;8:193–198. doi: 10.1111/j.1600-0625.1999.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 65.Hellstrom M., Hellstrom S., Engstrom-Laurent A., Bertheim U. The structure of the basement membrane zone differs between keloids, hypertrophic scars and normal skin: A possible background to an impaired function. J. Plast. Reconstr. Aesthetic Surg. 2014;67:1564–1572. doi: 10.1016/j.bjps.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Gallant-Behm C.L., Hildebrand K.A., Hart D.A. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen. 2008;16:226–233. doi: 10.1111/j.1524-475X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 67.Aarabi S., Bhatt K.A., Shi Y., Paterno J., Chang E.I., Loh S.A., Holmes J.W., Longaker M.T., Yee H., Gurtner G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim M.M., Bond J., Bergeron A., Miller K.J., Ehanire T., Quiles C., Lorden E.R., Medina M.A., Fisher M., Klitzman B., et al. A novel immune competent murine hypertrophic scar contracture model: A tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen. 2014;22:755–764. doi: 10.1111/wrr.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Ding J., Jiao H., Honardoust D., Momtazi M., Shankowsky H.A., Tredget E.E. Human hypertrophic scar-like nude mouse model: Characterization of the molecular and cellular biology of the scar process. Wound Repair Regen. 2011;19:274–285. doi: 10.1111/j.1524-475X.2011.00672.x. [DOI] [PubMed] [Google Scholar]
- 70.Hakanson R., Owman C., Sjoberg N.O., Sporrong B. Direct histochemical demonstration of histamine in cutaneous mast cells: Urticaria pigmentosa and keloids. Experientia. 1969;25:854–855. doi: 10.1007/BF01897918. [DOI] [PubMed] [Google Scholar]
- 71.Mantel A., Newsome A., Thekkudan T., Frazier R., Katdare M. The role of aldo-keto reductase 1C3 (AKR1C3)-mediated prostaglandin D2 (PGD2) metabolism in keloids. Exp. Dermatol. 2016;25:38–43. doi: 10.1111/exd.12854. [DOI] [PubMed] [Google Scholar]
- 72.Ammendola M., Zuccala V., Patruno R., Russo E., Luposella M., Amorosi A., Vescio G., Sammarco G., Montemurro S., De Sarro G., et al. Tryptase-positive mast cells and angiogenesis in keloids: A new possible post-surgical target for prevention. Updates Surg. 2013;65:53–57. doi: 10.1007/s13304-012-0183-y. [DOI] [PubMed] [Google Scholar]
- 73.Dong X., Zhang C., Ma S., Wen H. Mast cell chymase in keloid induces profibrotic response via transforming growth factor-beta1/Smad activation in keloid fibroblasts. Int. J. Clin. Exp. Pathol. 2014;7:3596–3607. [PMC free article] [PubMed] [Google Scholar]
- 74.Eishi K., Bae S.J., Ogawa F., Hamasaki Y., Shimizu K., Katayama I. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: Possible target of mast cells. J. Dermatol. Treat. 2003;14:248–252. doi: 10.1080/09546630310016808. [DOI] [PubMed] [Google Scholar]
- 75.Park G., Yoon B.S., Moon J.H., Kim B., Jun E.K., Oh S., Kim H., Song H.J., Noh J.Y., Oh C., et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J. Investig. Dermatol. 2008;128:2429–2441. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Q., Kelly A.P., Wang L., French S.W., Tang X., Duong H.S., Messadi D.V., Le A.D. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J. Investig. Dermatol. 2006;126:2607–2613. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
- 77.Syed F., Bagabir R.A., Paus R., Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab. Investig. 2013;93:946–960. doi: 10.1038/labinvest.2013.82. [DOI] [PubMed] [Google Scholar]
- 78.Theoret C.L., Olutoye O.O., Parnell L.K., Hicks J. Equine exuberant granulation tissue and human keloids: A comparative histopathologic study. Vet. Surg. 2013;42:783–789. doi: 10.1111/j.1532-950X.2013.12055.x. [DOI] [PubMed] [Google Scholar]
- 79.Craig S.S., DeBlois G., Schwartz L.B. Mast cells in human keloid, small intestine, and lung by an immunoperoxidase technique using a murine monoclonal antibody against tryptase. Am. J. Pathol. 1986;124:427–435. [PMC free article] [PubMed] [Google Scholar]
- 80.Allanore Y., Simms R., Distler O., Trojanowska M., Pope J., Denton C.P., Varga J. Systemic sclerosis. Nat. Rev. Dis. Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 81.Ozbilgin M.K., Inan S. The roles of transforming growth factor type beta3 (TGF-beta3) and mast cells in the pathogenesis of scleroderma. Clin. Rheumatol. 2003;22:189–195. doi: 10.1007/s10067-003-0706-5. [DOI] [PubMed] [Google Scholar]
- 82.Takeda K., Hatamochi A., Ueki H. Increased number of mast cells accompany enhanced collagen synthesis in linear localized scleroderma. Arch. Dermatol. Res. 1989;281:288–290. doi: 10.1007/BF00431065. [DOI] [PubMed] [Google Scholar]
- 83.Akimoto S., Ishikawa O., Igarashi Y., Kurosawa M., Miyachi Y. Dermal mast cells in scleroderma: Their skin density, tryptase/chymase phenotypes and degranulation. Br. J. Dermatol. 1998;138:399–406. doi: 10.1046/j.1365-2133.1998.02114.x. [DOI] [PubMed] [Google Scholar]
- 84.Prescott R.J., Freemont A.J., Jones C.J., Hoyland J., Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992;166:255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 85.Seibold J.R., Giorno R.C., Claman H.N. Dermal mast cell degranulation in systemic sclerosis. Arthritis Rheumatol. 1990;33:1702–1709. doi: 10.1002/art.1780331114. [DOI] [PubMed] [Google Scholar]
- 86.Nishioka K., Kobayashi Y., Katayama I., Takijiri C. Mast cell numbers in diffuse scleroderma. Arch. Dermatol. 1987;123:205–208. doi: 10.1001/archderm.1987.01660260075017. [DOI] [PubMed] [Google Scholar]
- 87.Hawkins R.A., Claman H.N., Clark R.A., Steigerwald J.C. Increased dermal mast cell populations in progressive systemic sclerosis: A link in chronic fibrosis? Ann. Intern. Med. 1985;102:182–186. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- 88.Hugle T., Hogan V., White K.E., van Laar J.M. Mast cells are a source of transforming growth factor beta in systemic sclerosis. Arthritis Rheumatol. 2011;63:795–799. doi: 10.1002/art.30190. [DOI] [PubMed] [Google Scholar]
- 89.Kihira C., Mizutani H., Asahi K., Hamanaka H., Shimizu M. Increased cutaneous immunoreactive stem cell factor expression and serum stem cell factor level in systemic scleroderma. J. Dermatol. Sci. 1998;20:72–78. doi: 10.1016/S0923-1811(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 90.Irani A.M., Gruber B.L., Kaufman L.D., Kahaleh M.B., Schwartz L.B. Mast cell changes in scleroderma. Presence of MCT cells in the skin and evidence of mast cell activation. Arthritis Rheumatol. 1992;35:933–939. doi: 10.1002/art.1780350813. [DOI] [PubMed] [Google Scholar]
- 91.Truchetet M.E., Brembilla N.C., Montanari E., Lonati P., Raschi E., Zeni S., Fontao L., Meroni P.L., Chizzolini C. Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheumatol. 2013;65:1347–1356. doi: 10.1002/art.37860. [DOI] [PubMed] [Google Scholar]
- 92.Claman H.N. Mast cell changes in a case of rapidly progressive scleroderma-ultrastructural analysis. J. Investig. Dermatol. 1989;92:290–295. doi: 10.1111/1523-1747.ep12276876. [DOI] [PubMed] [Google Scholar]
- 93.Falanga V., Soter N.A., Altman R.D., Kerdel F.A. Elevated plasma histamine levels in systemic sclerosis (scleroderma) Arch. Dermatol. 1990;126:336–338. doi: 10.1001/archderm.1990.01670270068011. [DOI] [PubMed] [Google Scholar]
- 94.Pearson M.E., Huff J.C., Giorno R.C., Panicheewa S., Claman H.N., Steigerwald J.C. Immunologic dysfunction in scleroderma: Evidence for increased mast cell releasability and HLA-DR positivity in the dermis. Arthritis Rheumatol. 1988;31:672–677. doi: 10.1002/art.1780310514. [DOI] [PubMed] [Google Scholar]
- 95.Marangoni R.G., Varga J., Tourtellotte W.G. Animal models of scleroderma: Recent progress. Curr. Opin. Rheumatol. 2016;28:561–570. doi: 10.1097/BOR.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto T., Takagawa S., Katayama I., Yamazaki K., Hamazaki Y., Shinkai H., Nishioka K. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Investig. Dermatol. 1999;112:456–462. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 97.Kitaba S., Murota H., Terao M., Azukizawa H., Terabe F., Shima Y., Fujimoto M., Tanaka T., Naka T., Kishimoto T., et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am. J. Pathol. 2012;180:165–176. doi: 10.1016/j.ajpath.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 98.Kohno S., Endo H., Hashimoto A., Hayashi I., Murakami Y., Kitasato H., Kojima F., Kawai S., Kondo H. Inhibition of skin sclerosis by 15deoxy delta12,14-prostaglandin J2 and retrovirally transfected prostaglandin D synthase in a mouse model of bleomycin-induced scleroderma. Biomed. Pharmacother. 2006;60:18–25. doi: 10.1016/j.biopha.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Kawai M., Masuda A., Kuwana M. A CD40-CD154 interaction in tissue fibrosis. Arthritis Rheumatol. 2008;58:3562–3573. doi: 10.1002/art.23994. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto T., Takagawa S., Katayama I., Mizushima Y., Nishioka K. Effect of superoxide dismutase on bleomycin-induced dermal sclerosis: Implications for the treatment of systemic sclerosis. J. Investig. Dermatol. 1999;113:843–847. doi: 10.1046/j.1523-1747.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 101.Yamamoto T., Takagawa S., Katayama I., Nishioka K. Anti-sclerotic effect of transforming growth factor-beta antibody in a mouse model of bleomycin-induced scleroderma. Clin. Immunol. 1999;92:6–13. doi: 10.1006/clim.1999.4720. [DOI] [PubMed] [Google Scholar]
- 102.Lakos G., Melichian D., Wu M., Varga J. Increased bleomycin-induced skin fibrosis in mice lacking the Th1-specific transcription factor T-bet. Pathobiology. 2006;73:224–237. doi: 10.1159/000098208. [DOI] [PubMed] [Google Scholar]
- 103.Ishikawa M., Yamamoto T. Antifibrogenic effects of C-C chemokine receptor type 2 antagonist in a bleomycin-induced scleroderma model. Exp. Dermatol. 2020 doi: 10.1111/exd.14088. [DOI] [PubMed] [Google Scholar]
- 104.Del Rio C., Cantarero I., Palomares B., Gomez-Canas M., Fernandez-Ruiz J., Pavicic C., Garcia-Martin A., Luz Bellido M., Ortega-Castro R., Perez-Sanchez C., et al. VCE-004.3, a cannabidiol aminoquinone derivative, prevents bleomycin-induced skin fibrosis and inflammation through PPARgamma- and CB2 receptor-dependent pathways. Br. J. Pharmacol. 2018;175:3813–3831. doi: 10.1111/bph.14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pamuk O.N., Can G., Ayvaz S., Karaca T., Pamuk G.E., Demirtas S., Tsokos G.C. Spleen tyrosine kinase (Syk) inhibitor fostamatinib limits tissue damage and fibrosis in a bleomycin-induced scleroderma mouse model. Clin. Exp. Rheumatol. 2015;33(Suppl. 91):S15–S22. [PubMed] [Google Scholar]
- 106.Yamamoto T., Takahashi Y., Takagawa S., Katayama I., Nishioka K. Animal model of sclerotic skin. II. Bleomycin induced scleroderma in genetically mast cell deficient WBB6F1-W/W(V) mice. J. Rheumatol. 1999;26:2628–2634. [PubMed] [Google Scholar]
- 107.Siracusa L.D., McGrath R., Ma Q., Moskow J.J., Manne J., Christner P.J., Buchberg A.M., Jimenez S.A. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 1996;6:300–313. doi: 10.1101/gr.6.4.300. [DOI] [PubMed] [Google Scholar]
- 108.Walker M., Harley R., Maize J., DeLustro F., LeRoy E.C. Mast cells and their degranulation in the Tsk mouse model of scleroderma. Proc. Soc. Exp. Biol. Med. 1985;180:323–328. doi: 10.3181/00379727-180-42183. [DOI] [PubMed] [Google Scholar]
- 109.Wang H.W., Tedla N., Hunt J.E., Wakefield D., McNeil H.P. Mast cell accumulation and cytokine expression in the tight skin mouse model of scleroderma. Exp. Dermatol. 2005;14:295–302. doi: 10.1111/j.0906-6705.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 110.Kakizoe E., Shiota N., Tanabe Y., Shimoura K., Kobayashi Y., Okunishi H. Isoform-selective upregulation of mast cell chymase in the development of skin fibrosis in scleroderma model mice. J. Investig. Dermatol. 2001;116:118–123. doi: 10.1046/j.1523-1747.2001.00165.x. [DOI] [PubMed] [Google Scholar]
- 111.Walker M., Harley R., LeRoy E.C. Ketotifen prevents skin fibrosis in the tight skin mouse. J. Rheumatol. 1990;17:57–59. [PubMed] [Google Scholar]
- 112.Walker M.A., Harley R.A., LeRoy E.C. Inhibition of fibrosis in TSK mice by blocking mast cell degranulation. J. Rheumatol. 1987;14:299–301. [PubMed] [Google Scholar]
- 113.Shiota N., Kakizoe E., Shimoura K., Tanaka T., Okunishi H. Effect of mast cell chymase inhibitor on the development of scleroderma in tight-skin mice. Br. J. Pharmacol. 2005;145:424–431. doi: 10.1038/sj.bjp.0706209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walker M.A., Harley R.A., DeLustro F.A., LeRoy E.C. Adoptive transfer of tsk skin fibrosis to +/+ recipients by tsk bone marrow and spleen cells. Proc. Soc. Exp. Biol. Med. 1989;192:196–200. doi: 10.3181/00379727-192-42979. [DOI] [PubMed] [Google Scholar]
- 115.Everett E.T., Pablos J.L., Harley R.A., LeRoy E.C., Norris J.S. The role of mast cells in the development of skin fibrosis in tight-skin mutant mice. Comp. Biochem. Physiol. A Physiol. 1995;110:159–165. doi: 10.1016/0300-9629(94)00127-F. [DOI] [PubMed] [Google Scholar]
- 116.Pincha N., Hajam E.Y., Badarinath K., Batta S.P.R., Masudi T., Dey R., Andreasen P., Kawakami T., Samuel R., George R., et al. PAI1 mediates fibroblast-mast cell interactions in skin fibrosis. J. Clin. Investig. 2018;128:1807–1819. doi: 10.1172/JCI99088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spielvogel R.L., Goltz R.W., Kersey J.H. Scleroderma-like changes in chronic graft vs host disease. Arch. Dermatol. 1977;113:1424–1428. doi: 10.1001/archderm.1977.01640100102020. [DOI] [PubMed] [Google Scholar]
- 118.Strattan E., Palaniyandi S., Kumari R., Du J., Hakim N., Huang T., Kesler M.V., Jennings C.D., Sturgill J.L., Hildebrandt G.C. Mast Cells Are Mediators of Fibrosis and Effector Cell Recruitment in Dermal Chronic Graft-vs.-Host Disease. Front. Immunol. 2019;10:2470. doi: 10.3389/fimmu.2019.02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janin-Mercier A., Devergie A., Van Cauwenberge D., Saurat J.H., Bourges M., Lapiere C.M., Gluckman E. Immunohistologic and ultrastructural study of the sclerotic skin in chronic graft-versus-host disease in man. Am. J. Pathol. 1984;115:296–306. [PMC free article] [PubMed] [Google Scholar]
- 120.Charley M.R., Bangert J.L., Hamilton B.L., Gilliam J.N., Sontheimer R.D. Murine graft-versus-host skin disease: A chronologic and quantitative analysis of two histologic patterns. J. Investig. Dermatol. 1983;81:412–417. doi: 10.1111/1523-1747.ep12522551. [DOI] [PubMed] [Google Scholar]
- 121.Claman H.N. Mast cell depletion in murine chronic graft-versus-host disease. J. Investig. Dermatol. 1985;84:246–248. doi: 10.1111/1523-1747.ep12265302. [DOI] [PubMed] [Google Scholar]
- 122.Claman H.N., Jaffee B.D., Huff J.C., Clark R.A. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell. Immunol. 1985;94:73–84. doi: 10.1016/0008-8749(85)90086-3. [DOI] [PubMed] [Google Scholar]
- 123.Claman H.N., Choi K.L., Sujansky W., Vatter A.E. Mast cell “disappearance” in chronic murine graft-vs-host disease (GVHD)-ultrastructural demonstration of “phantom mast cells”. J. Immunol. 1986;137:2009–2013. [PubMed] [Google Scholar]
- 124.Choi K.L., Giorno R., Claman H.N. Cutaneous mast cell depletion and recovery in murine graft-vs-host disease. J. Immunol. 1987;138:4093–4101. [PubMed] [Google Scholar]
- 125.Jaffee B.D., Claman H.N. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell. Immunol. 1983;77:1–12. doi: 10.1016/0008-8749(83)90001-1. [DOI] [PubMed] [Google Scholar]
- 126.Levi-Schaffer F., Goldenhersh M.A., Segal V., Nagler A. Nedocromil sodium ameliorates skin manifestations in a murine model of chronic graft-versus-host disease. Bone Marrow Transpl. 1997;19:823–828. doi: 10.1038/sj.bmt.1700734. [DOI] [PubMed] [Google Scholar]
- 127.Abe M., Yokoyama Y., Amano H., Matsushima Y., Kan C., Ishikawa O. Effect of activated human mast cells and mast cell-derived mediators on proliferation, type I collagen production and glycosaminoglycans synthesis by human dermal fibroblasts. Eur. J. Dermatol. 2002;12:340–346. [PubMed] [Google Scholar]
- 128.Levi-Schaffer F., Kupietzky A. Mast cells enhance migration and proliferation of fibroblasts into an in vitro wound. Exp. Cell Res. 1990;188:42–49. doi: 10.1016/0014-4827(90)90275-F. [DOI] [PubMed] [Google Scholar]
- 129.Gordon J.R., Galli S.J. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. J. Exp. Med. 1994;180:2027–2037. doi: 10.1084/jem.180.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kendall J.C., Li X.H., Galli S.J., Gordon J.R. Promotion of mouse fibroblast proliferation by IgE-dependent activation of mouse mast cells: Role for mast cell tumor necrosis factor-alpha and transforming growth factor-beta 1. Pt 1J. Allergy Clin. Immunol. 1997;99:113–123. doi: 10.1016/S0091-6749(97)70308-7. [DOI] [PubMed] [Google Scholar]
- 131.Albrecht M., Frungieri M.B., Kunz L., Ramsch R., Meineke V., Kohn F.M., Mayerhofer A. Divergent effects of the major mast cell products histamine, tryptase and TNF-alpha on human fibroblast behaviour. Cell. Mol. Life Sci. 2005;62:2867–2876. doi: 10.1007/s00018-005-5289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abe M., Kurosawa M., Ishikawa O., Miyachi Y., Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin. Exp. Allergy. 1998;28:1509–1517. doi: 10.1046/j.1365-2222.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 133.Garbuzenko E., Nagler A., Pickholtz D., Gillery P., Reich R., Maquart F.X., Levi-Schaffer F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: A direct role for mast cells in skin fibrosis. Clin. Exp. Allergy. 2002;32:237–246. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 134.Gruber B.L., Kew R.R., Jelaska A., Marchese M.J., Garlick J., Ren S., Schwartz L.B., Korn J.H. Human mast cells activate fibroblasts: Tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J. Immunol. 1997;158:2310–2317. [PubMed] [Google Scholar]
- 135.Gailit J., Marchese M.J., Kew R.R., Gruber B.L. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J. Investig. Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 136.Abe M., Kurosawa M., Ishikawa O., Miyachi Y. Effect of mast cell-derived mediators and mast cell-related neutral proteases on human dermal fibroblast proliferation and type I collagen production. Pt 2J. Allergy Clin. Immunol. 2000;106:S78–S84. doi: 10.1067/mai.2000.106058. [DOI] [PubMed] [Google Scholar]
- 137.Dong X., Chen J., Zhang Y., Cen Y. Mast cell chymase promotes cell proliferation and expression of certain cytokines in a dose-dependent manner. Mol. Med. Rep. 2012;5:1487–1490. doi: 10.3892/mmr.2012.851. [DOI] [PubMed] [Google Scholar]
- 138.Dong X., Zhang C., Ma S., Wen H. High concentrations of mast cell chymase facilitate the transduction of the transforming growth factor-beta1/Smads signaling pathway in skin fibroblasts. Exp. Ther. Med. 2015;9:955–960. doi: 10.3892/etm.2015.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kofford M.W., Schwartz L.B., Schechter N.M., Yager D.R., Diegelmann R.F., Graham M.F. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J. Biol. Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 140.Murota H., Bae S., Hamasaki Y., Maruyama R., Katayama I. Emedastine difumarate inhibits histamine-induced collagen synthesis in dermal fibroblasts. J. Investig. Allergol. Clin. Immunol. 2008;18:245–252. [PubMed] [Google Scholar]
- 141.Abe M., Kurosawa M., Igarashi Y., Ishikawa O., Miyachi Y. Influence of IgE-mediated activation of cultured human mast cells on proliferation and type I collagen production by human dermal fibroblasts. Pt 2J. Allergy Clin. Immunol. 2000;106:S72–S77. doi: 10.1067/mai.2000.106059. [DOI] [PubMed] [Google Scholar]
- 142.Kupietzky A., Levi-Schaffer F. The role of mast cell-derived histamine in the closure of an in vitro wound. Inflamm. Res. 1996;45:176–180. doi: 10.1007/BF02285158. [DOI] [PubMed] [Google Scholar]
- 143.Trautmann A., Krohne G., Brocker E.B., Klein C.E. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J. Immunol. 1998;160:5053–5057. [PubMed] [Google Scholar]
- 144.Postlethwaite A.E., Seyer J.M. Fibroblast chemotaxis induction by human recombinant interleukin-4. Identification by synthetic peptide analysis of two chemotactic domains residing in amino acid sequences 70–88 and 89–122. J. Clin. Investig. 1991;87:2147–2152. doi: 10.1172/JCI115247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Postlethwaite A.E., Holness M.A., Katai H., Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J. Clin. Investig. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fertin C., Nicolas J.F., Gillery P., Kalis B., Banchereau J., Maquart F.X. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell. Mol. Biol. 1991;37:823–829. [PubMed] [Google Scholar]
- 147.Kim C.D., Sohn K.C., Lee S.S., Lee J.H., Kim S., Lee Y.H., Ryu E.K., Park Y.S. Plasminogen activator inhibitor-2 (PAI-2) secreted from activated mast cells induces alpha-smooth muscle actin (alpha-SMA) expression in dermal fibroblasts. J. Dermatol. Sci. 2011;62:204–206. doi: 10.1016/j.jdermsci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 148.Gordon J.R. Monocyte chemoattractant peptide-1 expression during cutaneous allergic reactions in mice is mast cell dependent and largely mediates the monocyte recruitment response. Pt 1J. Allergy Clin. Immunol. 2000;106:110–116. doi: 10.1067/mai.2000.107036. [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto T., Hartmann K., Eckes B., Krieg T. Role of stem cell factor and monocyte chemoattractant protein-1 in the interaction between fibroblasts and mast cells in fibrosis. J. Dermatol. Sci. 2001;26:106–111. doi: 10.1016/S0923-1811(00)00164-X. [DOI] [PubMed] [Google Scholar]
- 150.Nabeshima Y., Hiragun T., Morita E., Mihara S., Kameyoshi Y., Hide M. IL-4 modulates the histamine content of mast cells in a mast cell/fibroblast co-culture through a Stat6 signaling pathway in fibroblasts. FEBS Lett. 2005;579:6653–6658. doi: 10.1016/j.febslet.2005.09.104. [DOI] [PubMed] [Google Scholar]
- 151.Gordon J.R. TGFbeta1 and TNFalpha secreted by mast cells stimulated via the FcepsilonRI activate fibroblasts for high-level production of monocyte chemoattractant protein-1 (MCP-1) Cell. Immunol. 2000;201:42–49. doi: 10.1006/cimm.2000.1631. [DOI] [PubMed] [Google Scholar]
- 152.Ferreira A.M., Takagawa S., Fresco R., Zhu X., Varga J., DiPietro L.A. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Investig. Dermatol. 2006;126:1900–1908. doi: 10.1038/sj.jid.5700302. [DOI] [PubMed] [Google Scholar]
- 153.Wong V.W., Rustad K.C., Akaishi S., Sorkin M., Glotzbach J.P., Januszyk M., Nelson E.R., Levi K., Paterno J., Vial I.N., et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2011;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Foley T.T., Ehrlich H.P. Through gap junction communications, co-cultured mast cells and fibroblasts generate fibroblast activities allied with hypertrophic scarring. Plast. Reconstr. Surg. 2013;131:1036–1044. doi: 10.1097/PRS.0b013e3182865c3f. [DOI] [PubMed] [Google Scholar]
- 155.Au S.R., Au K., Saggers G.C., Karne N., Ehrlich H.P. Rat mast cells communicate with fibroblasts via gap junction intercellular communications. J. Cell. Biochem. 2007;100:1170–1177. doi: 10.1002/jcb.21107. [DOI] [PubMed] [Google Scholar]
- 156.Yamamoto T., Hartmann K., Eckes B., Krieg T. Mast cells enhance contraction of three-dimensional collagen lattices by fibroblasts by cell-cell interaction: Role of stem cell factor/c-kit. Immunology. 2000;99:435–439. doi: 10.1046/j.1365-2567.2000.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Foley T.T., Ehrlich H.P. Mast cells prevent dexamethasone-induced cell death of cultured fibroblasts: Relationship to gap junctional intercellular communications. Plast. Reconstr. Surg. 2014;133:638e–644e. doi: 10.1097/PRS.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 158.Pistorio A.L., Ehrlich H.P. Modulatory effects of connexin-43 expression on gap junction intercellular communications with mast cells and fibroblasts. J. Cell. Biochem. 2011;112:1441–1449. doi: 10.1002/jcb.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Arbi S., Eksteen E.C., Oberholzer H.M., Taute H., Bester M.J. Premature collagen fibril formation, fibroblast-mast cell interactions and mast cell-mediated phagocytosis of collagen in keloids. Ultrastruct. Pathol. 2015;39:95–103. doi: 10.3109/01913123.2014.981326. [DOI] [PubMed] [Google Scholar]
- 160.Shaker S.A., Ayuob N.N., Hajrah N.H. Cell talk: A phenomenon observed in the keloid scar by immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2011;19:153–159. doi: 10.1097/PAI.0b013e3181efa2ef. [DOI] [PubMed] [Google Scholar]
- 161.Tellechea A., Bai S., Dangwal S., Theocharidis G., Nagai M., Koerner S., Cheong J.E., Bhasin S., Shih T.Y., Zheng Y., et al. Topical Application of a Mast Cell Stabilizer Improves Impaired Diabetic Wound Healing. J. Investig. Dermatol. 2020;140:901–911. doi: 10.1016/j.jid.2019.08.449. [DOI] [PubMed] [Google Scholar]
- 162.Bai S., Nagai M., Koerner S.K., Veves A., Sun L. Structure-activity relationship study and discovery of indazole 3-carboxamides as calcium-release activated calcium channel blockers. Bioorg. Med. Chem. Lett. 2017;27:393–397. doi: 10.1016/j.bmcl.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reber L.L., Marichal T., Galli S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grimbaldeston M.A., Chen C.C., Piliponsky A.M., Tsai M., Tam S.Y., Galli S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katz H.R., Austen K.F. Mast cell deficiency, a game of kit and mouse. Immunity. 2011;35:668–670. doi: 10.1016/j.immuni.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 166.Galli S.J., Gaudenzio N., Tsai M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020;38:49–77. doi: 10.1146/annurev-immunol-071719-094903. [DOI] [PubMed] [Google Scholar]
- 167.Wulff B.C., Pappa N.K., Wilgus T.A. Interleukin-33 encourages scar formation in murine fetal skin wounds. Wound Repair Regen. 2019;27:19–28. doi: 10.1111/wrr.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gaudenzio N., Marichal T., Galli S.J., Reber L.L. Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity. Front. Immunol. 2018;9:1275. doi: 10.3389/fimmu.2018.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsai M., Grimbaldeston M., Galli S.J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 170.Amiot L., Vu N., Drenou B., Scrofani M., Chalin A., Devisme C., Samson M. The anti-fibrotic role of mast cells in the liver is mediated by HLA-G and interaction with hepatic stellate cells. Cytokine. 2019;117:50–58. doi: 10.1016/j.cyto.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 171.Beghdadi W., Madjene L.C., Claver J., Pejler G., Beaudoin L., Lehuen A., Daugas E., Blank U. Mast cell chymase protects against renal fibrosis in murine unilateral ureteral obstruction. Kidney Int. 2013;84:317–326. doi: 10.1038/ki.2013.98. [DOI] [PubMed] [Google Scholar]
- 172.Legere S.A., Haidl I.D., Legare J.F., Marshall J.S. Mast Cells in Cardiac Fibrosis: New Insights Suggest Opportunities for Intervention. Front. Immunol. 2019;10:580. doi: 10.3389/fimmu.2019.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]