Abstract
Flos Lamii albi has a high biological activity and is widely used in herbal medicine. The aim of the study was to characterize the secretory structures present in Lamium album subsp. album corolla and the location of phenolic compounds. Additionally, we carried out qualitative phytochemical analyses of flavonoids and phenolic acids. Light, fluorescence, and scanning electron microscopy were used to analyze the structure of the floral organs. The main classes of phenolic compounds and their localization were determined histochemically. Phytochemical analyses were performed with high-performance thin-layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC). Six types of glandular trichomes were found which contained flavonoids, phenolic acids, and tannins. The phytochemical studies demonstrated the presence of caffeic, chlorogenic, ferulic, gallic, p-coumaric, protocatechuic, syringic, gentisic, and vanillic phenolic acids as well as rutoside, isoquercetin, and quercetin flavonoids. The corolla in L. album subsp. album has antioxidant properties due to the presence of various polyphenols, as shown by the histo- and phytochemical analyses. The distribution and morphology of trichomes and the content of phenolic compounds in the corolla have taxonomic, pharmacognostic, and practical importance, facilitating the identification of the raw material.
Keywords: phenolic acids, flavonoids, tannins, secretory trichomes, white nettle corolla
1. Introduction
The genus Lamium L. (f. Lamiaceae) comprises approximately 40 species native to Europe, Asia, and North Africa [1,2]. They are mostly annual or perennial herbaceous plants.
Lamium album L. is a 50–100 cm-high perennial herb [2]. It often grows in clusters and covers roadsides, debris, scrubs, and ditches. The species is characteristic of Lamio-albi Urticenea dioicae Dengler x Wollert subcl. nov. hoc. loco [3]. Its stem is rectangular in cross section. It has opposite, petiolate, cordate, or ovate leaves with serrated margins [4]. Its bilabiate flowers with a 19–21 mm-long corolla are white or creamy white. Whorls of 5–22 flowers grow from leaf axils in the upper part of the stem [5]. The aboveground plant parts are densely covered with glandular and non-glandular trichomes [4,5].
To date, the morphology of glandular trichomes located on various aboveground parts of some Lamium species has been described [6,7,8]; however, corolla trichomes have only been characterized in L. lycium [9] and L. pisidicum [10].
Lamium album has been used as a famine food and as alternative nourishment in different countries in Europe, China, and Japan [11]. Its young shoots, leaves, and flowers are edible. They are used for the preparation of teas and raw or cooked food supplements [12]. Supplements with L. album extracts are claimed to detoxify the organism [13] (p. 100).
L. album flowers contain flavonoids, tannins, phenolic acids, choline, glycosides, saponins, mucilage, iridoids, essential oils, triterpenes, isoscutellarein derivatives, fatty acids, polysaccharides, and amines [2,14,15]. With its considerable diversity of secondary metabolites, the raw material exhibits a high biological activity. Given its anti-inflammatory, antiseptic, bacteriostatic, astringent, mucolytic, and antispasmodic activities, it is widely used in herbal medicine [2,12,15,16].
The bioactivity of L. album extracts has been evidenced in many scientific investigations. Various pharmacological properties have been determined. The constituents of L. album extracts can act as inhibitory factors of virus entry [16], have antioxidant properties [17], and exhibit a protective effect on liver tissue [18] and human fibroblast cell lines [14]. L. album can be used as a source of antimicrobial substances. Chloroform, ethanolic, and water extracts have been tested to assess their activity against E. coli, S. aureus, and C. albicans [19]. Extracts of the plant have been reported to reduce the growth of cancer cell lines [20]. Water plant extracts have been found to decrease the inflammation of ocular tissues and the level of human neutrophils [21]. They also exhibit antianemic effects [2,22].
The ecological functions of plant secondary metabolites include plant protection against fungal infection and herbivore attack as well as being attractants to pollinators and seed-dispersing animals and allelopathic agents [23] (pp. 1250–1318), [24] (pp. 229–265).
The whole Lamiaceae family is rich in various active substances [25,26]. The genus Lamium is primarily an abundant source of phenolic acids and flavonoids, which are considered as the main groups of substances with multiple biological activities [15,27]. Therefore, these phenolic compounds were chosen for the phytochemical and histochemical tests in our work.
Since the L. album corolla is a valuable medicinal raw material and the morphotypes of bioactive compound-producing trichomes located on this organ have not been described, the aim of this study was to examine the content of phenolic compounds and their distribution in glandular and non-glandular trichomes. We also carried out qualitative analysis of flavonoids and phenolic acids in the L. album subsp. album corolla raw material.
2. Results
The compact Lamium album subsp. album inflorescences (Figure 1a,b) growing from the axils are composed of flowers with a white two-lipped corolla, which is substantially longer than the calyx. Strongly tapered sepals fuse at an almost half the length (Figure 1c).
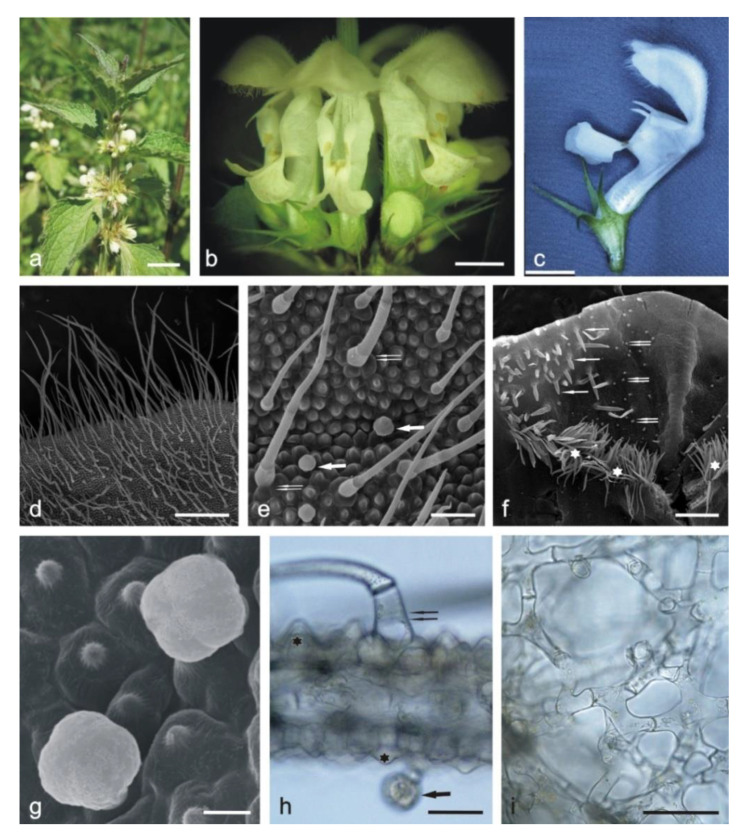
Figure 1.
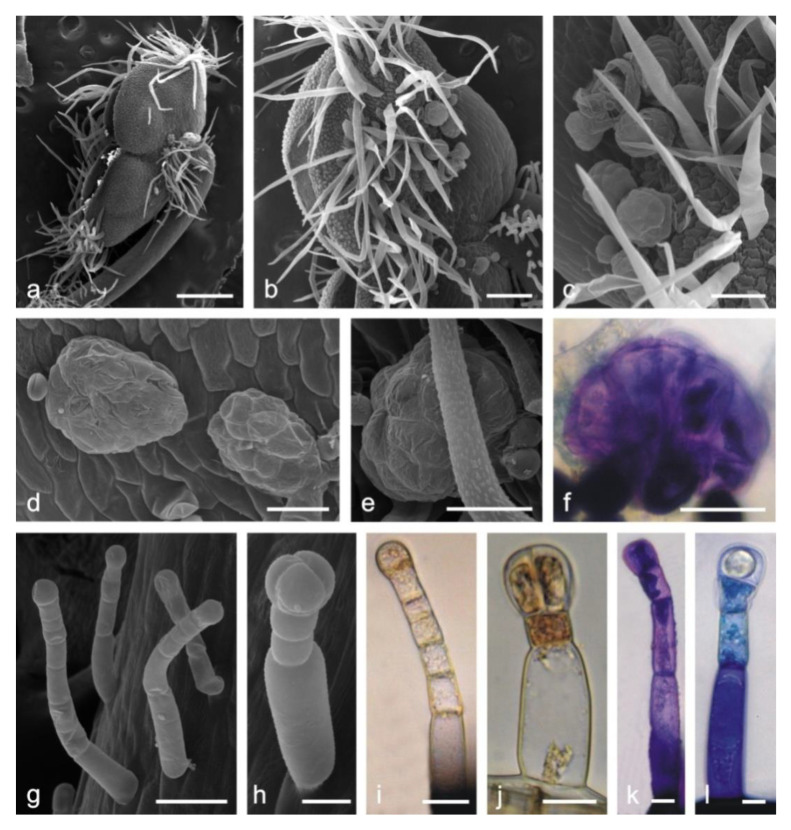
Lamium album subsp. album flowers and fragments of the corolla. c—stereoscopic microscopy (SM), (d–g)— scanning electron microscopy (SEM), (h,i)—light microscopy (LM). (a) Blooming plants. (b) Fragment of an inflorescence. (c) Lateral view of the flower. (d) Margin of the upper lip with long non-glandular trichomes. (e) Various types of trichomes and papillae on the abaxial surface of the upper lip: glandular trichomes (arrows), non-glandular trichomes (double arrows). (f) Fragment of the adaxial surface of the corolla tube with unevenly distributed non-glandular conical trichomes (double arrows), glandular trichomes (arrows) and a ring of flattened non-glandular trichomes (stars) located above the ovary. (g) Papillae and glandular trichomes with a 4-celled head on the adaxial surface of the upper lip. (h) Cross section of the upper lip with visible papillae (asterisks), glandular trichomes (arrow), and a non-glandular trichome (double arrow). (i) Loose arrangement of mesophyll cells in the cross section of the upper lip. Scale bars: 1 cm (a), 5 mm (b), 4 mm (c), 500 µm (d,f,i), 100 µm (e), 30 µm (h), 20 µm (g).
2.1. Anatomical Analyses
2.1.1. Corolla
The adaxial and abaxial surfaces of both corolla lips are covered by different types of trichomes. Long single-layered 1–4 celled non-glandular trichomes cover densely the upper margin on the abaxial surface of the upper lip (Figure 1d) but are less dense on other fragments of the surface. There are substantially smaller glandular trichomes and papillae between non-glandular trichomes (Figure 1e). The lower lip is covered by similar types of trichomes. Inside the corolla tube, there are non-glandular and glandular trichomes. The non-glandular trichomes are long, unicellular, flattened, and pointed. They form a specific ring located above the ovary and the nectary at the bottom of the tube (Figure 1f). Conical trichomes with varying lengths are unique for the corolla tube (Figure 1f and Figure 2e,w). All the glandular trichomes present on the petals are short-stalked (Figure 1g,h). They are composed of a basal cell; a 1–2-celled stalk; and a 1-celled (Figure 2a), 2-celled (Figure 2b,f), 3-celled (Figure 2g), and 4-celled head (Figure 2c,n). The peltate trichomes on the corolla petals have a basal cell, a 1-celled stalk, and a 4–8-celled head (Figure 2d,j,o).
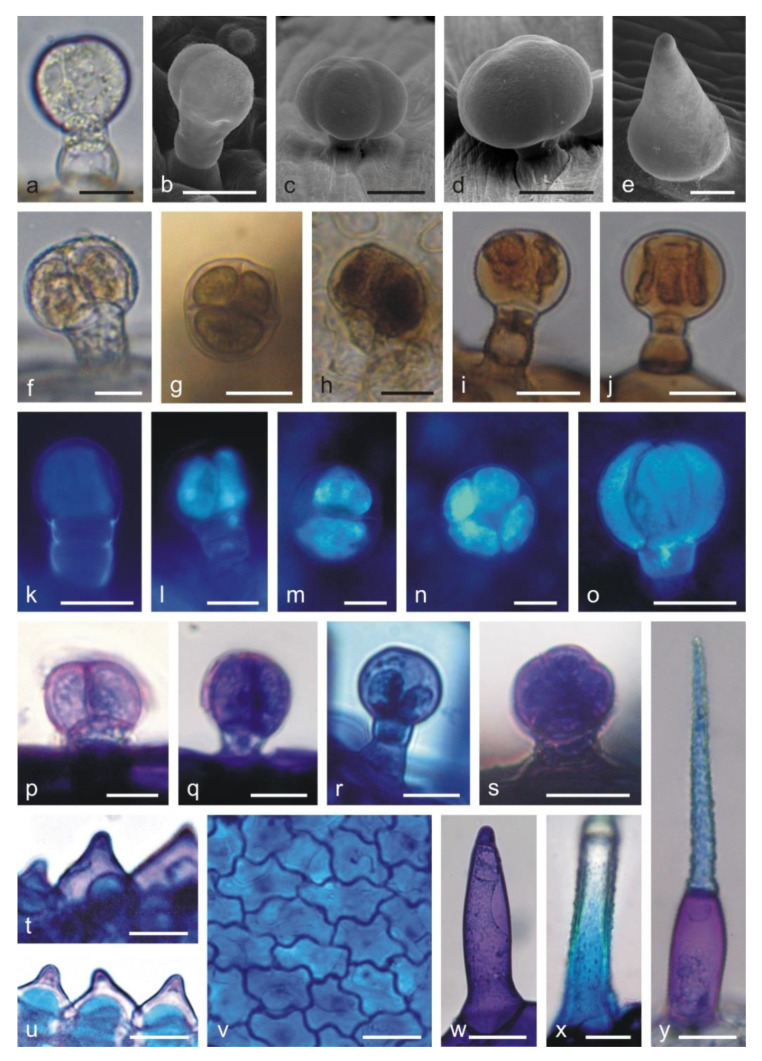
Figure 2.
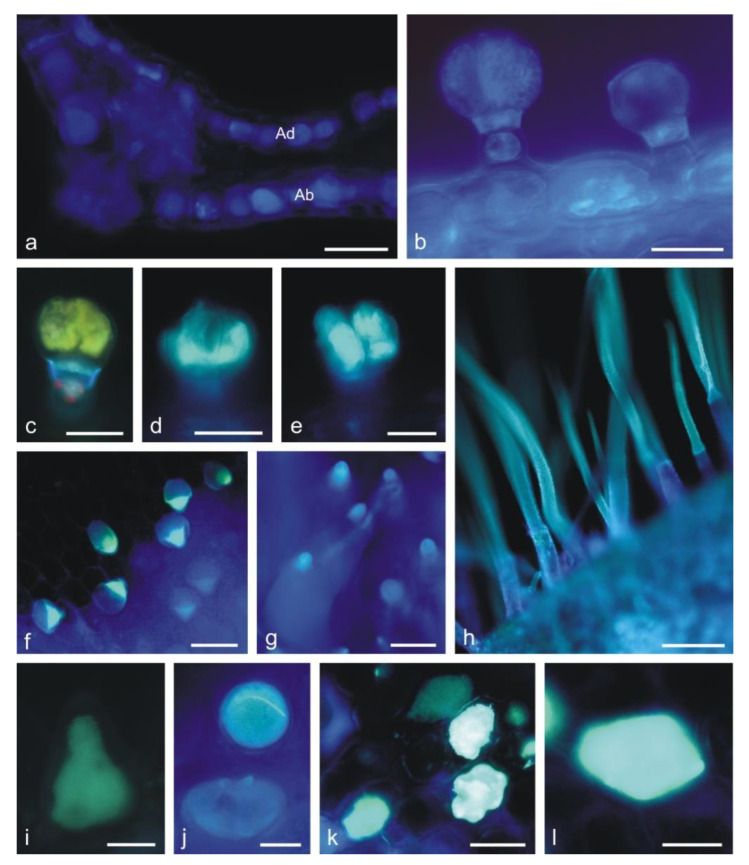
Types of trichomes and papillae on the L. album subsp. album corolla and the results of histochemical assays. (a,f) Control trichomes without staining (LM). (b–e) SEM images of trichomes. (g–j,p–y) Histochemical reactions (LM). (k–o) fluorescence microscopy (FM) images of trichomes. (a) Capitate trichome with a 1-celled head. (b) Capitate trichome with a 2-celled head. (c) Capitate trichome with a 4-celled head. (d) Peltate trichome. (e) Conical trichome. (f) Capitate trichome with a bicellular head and yellow content in the head cells. (g,h) Phenolic compounds stained black in the heads of trichomes after the application of ferric trichloride. (g) Capitate trichome with a 3-celled head. (h) Peltate trichome. (i,j) Brown color of tannins in trichomes stained with potassium dichromate. (k–o) Blue autofluorescence in different capitate and peltate (o) trichomes indicating the presence of phenolic acids. (p–r) Tannins in capitate trichomes stained blue after Toluidine blue O treatment. (s) Peltate trichome stained blue after Toluidine blue O treatment. (t–u) Phenolic compounds in papillae stained blue after Toluidine blue O treatment. (v) Phenolic compounds visible in abaxial epidermis cells of the upper lip after Toluidine blue O treatment. (w) Non-glandular conical trichome from the corolla tube stained purple (pectins) after Toluidine blue O treatment. (x,y) Non-glandular trichomes containing phenolic compounds (blue) visible after Toluidine blue O treatment. Scale bars: 30 µm (c,d,h,i,q,r,w,y), 20 µm (a,b,f,g,j,k,o,p,s,t,u,v), 10 µm (e,l,m,n,x).
The cross section of the upper lip shows papillae in both the adaxial and abaxial epidermis (Figure 1h). The mesophyll of the petals consists of interconnected branched parenchyma cells arranged loosely with large intercellular spaces (Figure 1i).
2.1.2. Calyx
The calyx consists of five pointed sepals (Figure 3a). In contrast to the adaxial epidermis, the abaxial surface is covered with numerous glandular and non-glandular trichomes (Figure 3b–d).
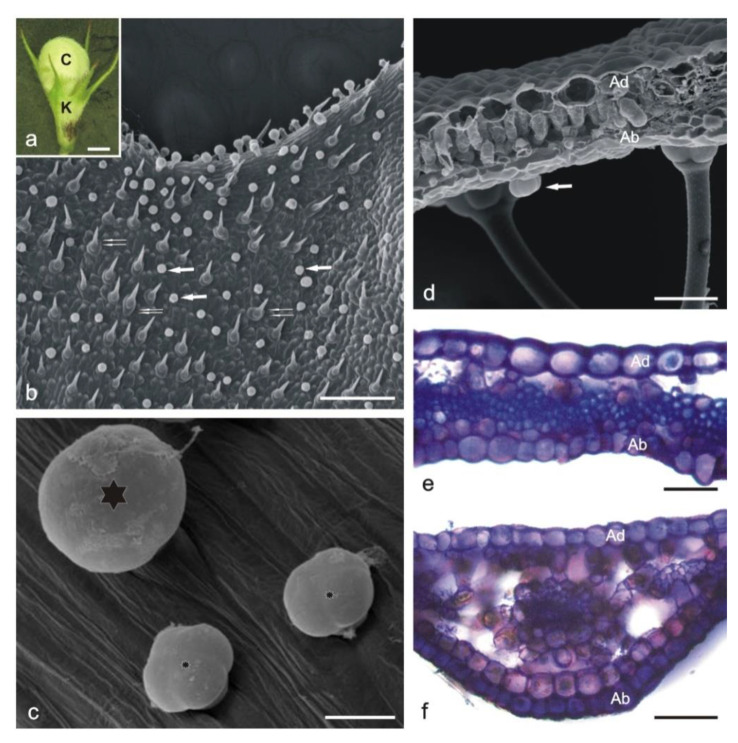
Figure 3.
General habit and fragments of the L. album subsp. album calyx. (b–d)—SEM; (e,f)—LM. (a) Flower bud with an open calyx. (b) Glandular (arrows) and non-glandular (double arrows) trichomes on the abaxial surface of the calyx. (c) Peltate trichome (large asterisk) and capitate trichomes (small asterisks) on the abaxial surface of a sepal nerve. (d) Cross section of a sepal with fragments of two non-glandular trichomes and a glandular trichome (arrow) on the abaxial surface. (e,f) Cross sections of sepals (Toluidine blue O staining). Abbreviations: K calyx, C corolla, Ad adaxial epidermis, Ab abaxial epidermis. Scale bars: 2 mm (a), 200 µm (b), 50 µm (d–f), 20 µm (c).
The capitate glandular trichomes are similar to this type of trichomes on the corolla (Figure 4a,b,f). The peltate trichomes on the calyx are longer than on the corolla and consist of a basal cell, a 3–4-celled stalk, and a 4–8-celled head (Figure 4c–e,j–n). The adaxial epidermis cells are larger than those in the abaxial layer and have thicker outer cell walls (Figure 3d,e). The mesophyll consists of palisade parenchyma (1 layer) and sponge parenchyma (2–5 layers), with large intercellular spaces (Figure 3d,f). Only the subepidermal parenchyma cells located below vascular bundles form a compact system (Figure 3f).
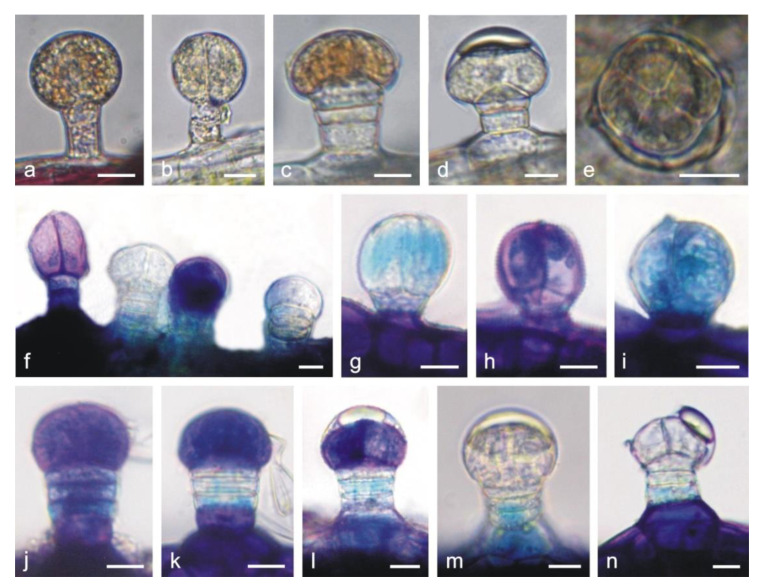
Figure 4.
Glandular trichomes present on the L. album subsp. album calyx in LM. (a–e) Trichomes from non-stained control preparations. (f–n) Trichomes stained with Toluidine blue O. (a) Capitate trichome with 1-celled head. (b) Capitate trichome with 2-celled head. (c–e) Peltate trichomes at various stages of development. (f–i) Differently stained (blue and green) capitate trichomes after Toluidine blue O treatment indicating various contents of phenolic compounds. (j–n) Peltate trichomes with differently stained head cells at different stages of development after Toluidine blue O treatment. In the older trichomes, the secretion is visible in the subcuticular space of the head (l,m) or on its surface with a ruptured cuticle (n). Scale bars: 10 µm (a–n).
The cross sections of the upper zone of the sepals show a layer of mechanical tissue cells with lignified walls and a small diameter in the central part (Figure 3e). Non-glandular trichomes are uniseriate, 2–3-celled, with swollen basal cells (Figure 3d).
2.1.3. Stamens
The trichomes on the stamens have a different structure than those on the calyx and corolla. Some part of the surface of anther in L. album subsp. album flowers is covered by relatively long one-celled non-glandular trichomes (Figure 5a–c). Additionally, there are large glandular trichomes with a multicellular head and a very short stalk in close proximity to the attachment of the anther and filament (Figure 5c–f). The upper part of filaments bears numerous long capitate trichomes with a 2–5-celled stalk and a mostly rounded 1–4-celled head (Figure 5g–l).
Figure 5.
Fragments of L. album subsp. album stamens with various types of trichomes. (a–e,g–h) SEM; (f,l)—LM. (a–c) Long non-glandular trichomes and multicellular glandular trichomes anthers. (d–f) Multicellular glandular trichomes. (g–l) Long-stalked capitate trichomes on filaments. (f,k,l) Tannins stained blue after Toluidine blue O treatment. (i,j) Tannins stained brown after potassium dichromate treatment. Scale bars: 500 µm (a), 200 µm (b), 100 µm (c), 50 µm (d–g), 20 µm (h–j), 10 µm (k,l).
2.2. Histochemical Analyses
The application of fluorescence microscopy and histochemical assays facilitated the identification of phenolic compounds—i.e., phenolic acids, tannins, and flavonoids—in the L. album subsp. album corolla trichomes (Table 1).
Table 1.
Histochemical reactions of Lamium album subsp. album corolla trichomes.
| Target Compounds | Stain Reagent | Reaction Color | Trichomes | Papillae | Epidermis Cells | ||
|---|---|---|---|---|---|---|---|
| Peltate | Capitate | Non-Glandular | |||||
| General phenolic compounds | FeCl3 | black, brown | + | + | nd | + | + |
| Toluidine blue O | green or blue | + | + | + | + | + | |
| Tannins | Potassium dichromate | brown | + | + | nd | + | - |
| Toluidine blue O | deep blue | + | + | + | + | + | |
| Phenolic acids | Autofluorescence UV | light blue | + | + | - | + | + |
| Flavonoids | AlCl3 under UV | yellow | + | + | + | + | + |
| Pectins | Toluidine blue O | purple or pink | + | + | + | + | - |
+ present, —absent, nd not determined.
In the control preparations, the secretion contained in the head cells of capitate and peltate trichomes was yellowish (Figure 2f). The presence of phenolic compounds was confirmed by the appearance of dark brown staining in the heads of all glandular trichomes (Figure 2g,h) and in papilla-forming epidermis cells of the corolla after the treatment with iron trichloride (not shown). Phenolic acids were detected in the stalk cells of capitate trichomes (Figure 2k and Figure 4b) and in the head cells of all types of capitate and peltate trichomes on the corolla (Figure 5l–o) and in epidermis cells (Figure 4a,b). This was evidenced by blue ultra-violet (UV) autofluorescence, which is characteristic of phenolic acids.
Tannins staining brown in the reaction with potassium dichromate (Figure 2i,j) and deep blue after treatment with Toluidine blue O (Figure 2p–s) were detected in all corolla glandular trichomes. A similar color was recorded in the content and cell walls of papilla epidermis (Figure 2t,u), epidermis (Figure 2v), and non-glandular trichomes (Figure 2x,y), which indicated the presence of phenolic compounds in these cells as well. Additionally, treatment with Toluidine blue O revealed the presence of pectin compounds, which was evidenced by the pink color of the heads of glandular trichomes (Figure 2p,q,s), papillae (Figure 2t,u), and non-glandular trichomes (Figure 2w,y).
The treatment with Toluidine blue O showed the presence of phenolic compounds staining blue and green in the stalk and head cells in the capitate (Figure 4f–i) and peltate trichomes (Figure 4j–n) on the calyx. The difference in the staining of the trichome cells was probably associated with their different ages. The heads of some trichomes, which probably underwent the final stages of their activity, were not stained by Toluidine blue O, indicating no presence of phenolic compounds (Figure 4f,m,n). The stalk and head cells of the long glandular trichomes covering the stamens contained tannins, which were visualized with the use of potassium dichromate (Figure 5i,j) and Toluidine blue O (Figure 5f,k,l).
The presence of the flavonoids in all types of glandular trichomes on the corolla (Figure 6c–e) and in the non-glandular trichomes (Figure 6f–h), papillae (Figure 6i,j), and epidermis cells (Figure 6k,l) was demonstrated with the use of aluminum trichloride as a fluorochrome, inducing a yellowish secondary fluorescence.
Figure 6.
Fragments of corolla petals L. album subsp. album imaged by a fluorescence microscope. (a,b) Light blue autofluorescence of phenolic acids (a) in epidermis cells in the cross section of the corolla tube, (b) in epidermis cells and capitate trichomes. (c–l) Yellowish secondary fluorescence of flavonoids observed after the application of aluminum trichloride under the Cy 5 filter. (c–e) Capitate trichomes containing flavonoids. (f,g) Different-length conical trichomes visible on the adaxial surface of the corolla tube with flavonoids in the apical part. (h) Non-glandular trichomes from the upper lip: flavonoid fluorescence in the cell walls. (i,j) Papillae on the upper lip containing flavonoids. (k,l) Epidermis cells from the upper lip with flavonoid content. Abbreviation: Ad adaxial epidermis, Ab abaxial epidermis. Scale bars: 100 µm (g,h), 50 µm (a), 30 µm (b,c,f,k), 20 µm (d,e,l), 10 µm (i,j).
2.3. Phytochemical Analyses
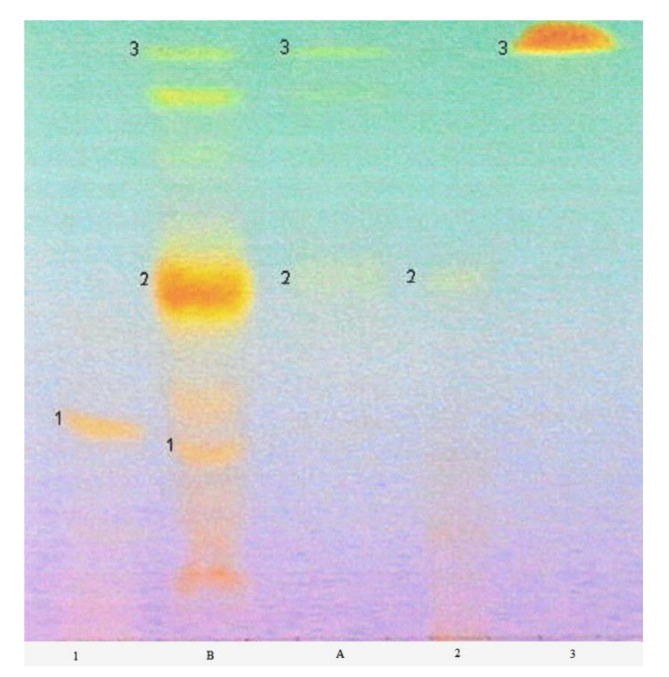
The flavonoid analysis carried out with the HPTLC method confirmed the presence of rutoside, quercetin, and isoquercetin in the methanol extract of Lamium album subsp. album flowers (Figure 7). The separation of flavonoids was achieved with the use of the MGD (multiple gradient development) technique. In the visualization of flavonoid compounds (Table S1), aluminum chloride stains the standards blue (Figure S1). The staining of standards of flavonoids with FeCl3 is visible as a brown and yellow color in visible light (not shown).
Figure 7.
Chromatogram of the separation of flavonoids in methanol extract from L. album subsp. album flowers with the multiple gradient development technique (MGD). The bands of the standards correspond with the bands of the compounds in the extract: 1—rutoside; 2—isoquercetin; 3—quercetin; A—ethyl acetate extract-excluded from the investigations; B—methanol extract. Visible light, derivatization with the use of a 1% methanolic solution of aluminum chloride. The mobile phase elution program is given in Table 2. Chromatographic plate HPTLC Si 60 F 254.
Three groups of phenolic acids were analyzed in the methanol extract with the HPTLC method: free phenolic acids present in L. album subsp. album flower cells and phenolic acids bound with glycosides and esters. Phenolic acids bound with glycosides were submitted to acid hydrolysis and analyzed afterwards. Phenolic acids bound with esters were released in the basic hydrolysis process (Figure 8).
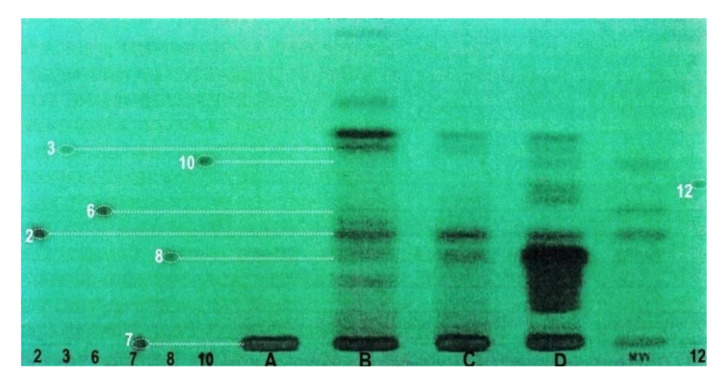
Figure 8.
Separation of phenolic acids in methanol extracts of L. album subsp. album flowers. A—ethyl acetate extract-excluded from the investigations; B—methanol extract; C—acid hydrolysis; D—basic hydrolysis; MW—mixture of standards; 2—protocatechuic acid (3,4-dihydroxybenzoic); 3—vanillic acid (4-hydroxy-3-metoxybenzoic); 6—syringic acid (4-hydroxy-3,5-dimetoxybenzoic); 7—chlorogenic acid (depside of caffeic and quinic acids); 8—caffeic acid (3,4-dihydroxycinnamic); 10—p-coumaric acid (4-hydroxycinnamic); 12—ferulic acid (4-hydroxy-3-metoxycinnamic). Videoscaner Desaga (Germany). Stationary phase—chromatographic plate: Si 60 HPTLC F 254 (Merck). Elution program in Table 3. Wavelength 254 nm.
The analysis of methanolic extracts in the chromatography experiments was carried out with the use of two methods. First, HPTLC was applied. Protocatechuic, vanillic, and caffeic acids were identified among the free phenolic acids. After acid hydrolysis, the same acids were identified, which implies that these three acids are present in L. album subsp. album flowers in two forms: as free acids and as glycosides. Furthermore, the highest amount of caffeic acid was found in the extract after basic hydrolysis, which suggests that this phenolic acid exists also in an esterified form in the analyzed material. Protocatechuic and ferulic acids were detected as esters.
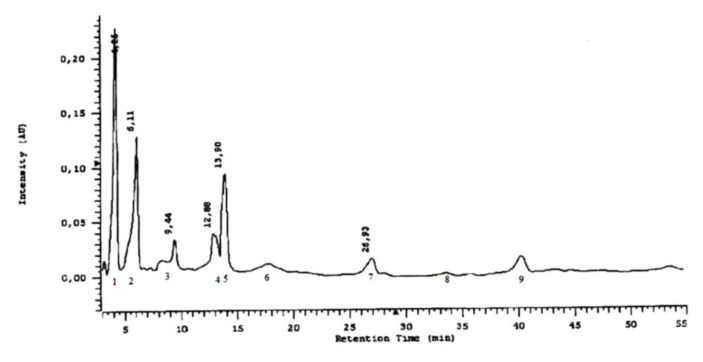
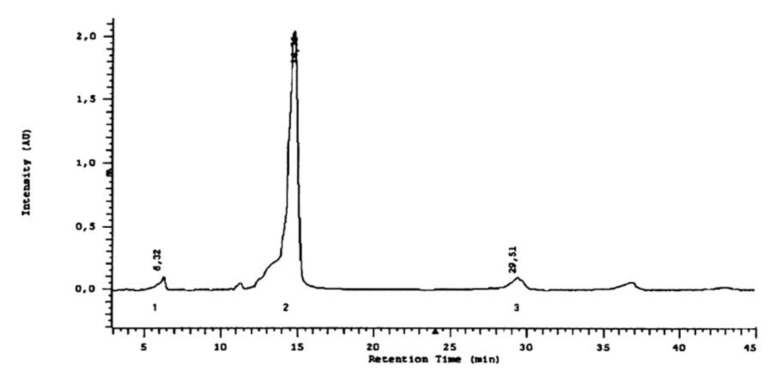
The use of the HPLC method provided a more detailed characterization of the forms of phenolic acids in the L. album subsp. album flowers (Figure 9). Nine acids were detected in esterified forms: protocatechuic, vanillic, caffeic, syringic, gallic, gentisic, p-coumaric, and chlorogenic acids, and trace amounts of ferulic acid (Figure 10). Besides the acids detected with the HPTLC method in the extracts after basic hydrolysis, gallic acid was detected by HPLC. This acid exists only in an esterified form in the analyzed flowers. Caffeic and small amounts of protocatechuic and p-coumaric acids were identified with the HPLC method in the extracts after acid hydrolysis (Figure 11). Caffeic acid is the main phenolic acid in a glycosylated form.
Figure 9.
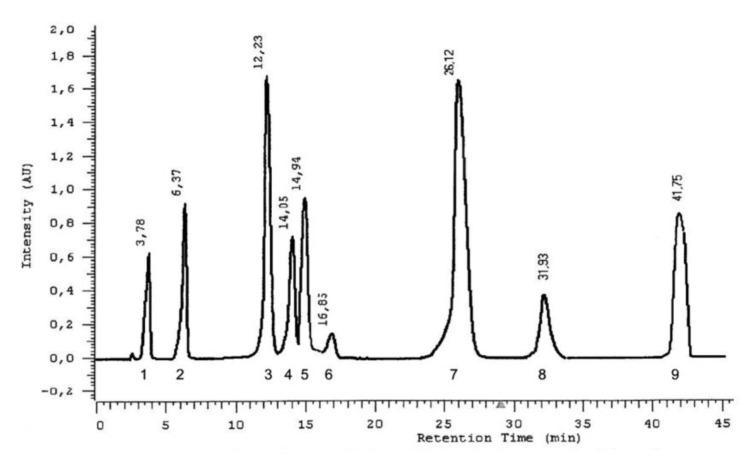
Separation of a mixture of phenolic acid standards in HPLC. Stationary phase—RP-18, mobile phase: water: methanol: formic acid, (75:25:0.5 v/v). Each compound is characterized by retention time in minutes (given above peaks): (1)—gallic acid (3,4,5-trihydroxybenzoic); (2)—protocatechuic acid (3,4-dihydroxybenzoic); (3)—gentisic acid (2,5-dihydroxybenzoic); (4)—vanillic acid (4-hydroxy-3-metoxybenzoic); (5)—caffeic acid (3,4-dihydroxycinnamic); (6)—syringic acid (4-hydroxy-3,5-dimetoxybenzoic); (7)—p-coumaric acid (4-hydroxycinamic); (8)—ferulic acid (4-hydroxy-3-metoxycinnamic); (9)—chlorogenic acid (depside of caffeic and quinic acids). Wavelength 254 nm.
Figure 10.
Separation of phenolic acids in methanol extract after basic hydrolysis in HPLC. Stationary phase—RP-18; mobile phase: water: methanol: formic acid (75:25:0.5 v/v). (1)—gallic acid (3,4,5-trihydroxybenzoic); (2)—protocatechuic acid (3,4-dihydroxybenzoic); peak (3) is not identified; (4)—gentisic acid (2,5-dihydroxybenzoic); (5)—caffeic acid (3,4-dihydroxycinnamic) and vanillic acid (4-hydroxy-3-metoxybenzoic); (6)—syringic acid (4-hydroxy-3,5-dimetoxybenzoic); (7)—p-coumaric acid (4-hydroxycinamic); (8)—ferulic acid (4-hydroxy-3-metoxycinnamic); (9)—chlorogenic acid (depside of caffeic and quinic acids). Wavelength 254 nm.
Figure 11.
Separation of phenolic acids after the acid hydrolysis of methanolic extract in HPLC. Stationary phase—RP-18; mobile phase: water: methanol: formic acid (75:25:0.5 v/v). (1)—protocatechuic acid (3,4-dihydroxybenzoic); (2)—caffeic acid (3,4-dihydroxycinnamic); (3)—p-coumaric acid (4-hydroxycinamic). Wavelength 254 nm.
3. Discussion
3.1. Anatomy and Histochemistry
In aromatic plants, phenolic compounds are one of the three classes of natural products, besides fatty acid derivates and terpenes, from which volatile organic compounds originate [28].
Using histochemical assays and fluorescence microscopy, we determined the location of phenolic compounds, including phenolic acids, flavonoids, and tannins, in tissues of the L. album subsp. album corolla, stamens, and calyx. As shown by the analysis, phenolic compounds were present in all types of glandular trichomes and in the non-glandular trichomes of the L. album subsp. album corolla and calyx, corolla epidermis cells, papillae, and stamen trichomes.
As shown by the available literature, the types of trichomes on L. album subsp. album stems, leaves, and calyces have been described to date, but those located on corolla petals have not been studied [8]. We demonstrated differences in the types of trichomes developing on the corolla and the calyx, as well as their diverse morphology. The corolla petals had a greater number of morphotypes of trichomes than the sepals.
Three morphotypes of corolla non-glandular trichomes were distinguished: (i) uniseriate multicellular trichomes formed by 2–4 cells; (ii) one-celled conical trichomes of variable length; and (iii) long, flattened, pointed 1-celled trichomes. In turn, the calyx was covered by only one type of non-glandular trichomes—i.e., unieseriate multicellular hairs (i). This type of trichomes contained tannin-like phenolic compounds, as shown by the reaction with Toluidine blue O. A similar distribution of phenolic compounds in non-glandular trichomes on leaves of another Lamiaceae representative—i.e., Dracocephalum moldavica—was reported earlier by Dmitruk et al. [29].
The conical one-celled (ii) trichomes located in the corolla tube as well as some epidermis cells and papillae were found to contain flavonoids emitting secondary yellow fluorescence. The flavonoid content in some leaf epidermis cells and glandular trichomes and in the basal cells of non-glandular trichomes was determined previously in D. moldavica [29].
In L. album subsp. album flowers, the long non-glandular trichomes forming a ring at the bottom of the corolla tube located above the ovary and nectary were an exception, as we did not detect phenolic compounds in these structures.
Peltate and capitate trichomes were distinguished among the glandular hairs present on the calyx and corolla. The peltate trichomes on the calyx epidermis differed from those on the corolla by the greater number of stalk cells (2–3). A similar structure of peltate trichomes containing additional stalk cells was described by other authors on the leaves and calyx of L. lycium and L. pisidicum [9,10]. In turn, Atalay et al. [8] found short-stalked peltate trichomes with a head formed of 4–8 cells on L. album subsp. album leaves, which corresponds to the structure of the peltate trichomes on the corolla epidermis observed in this species in the present study.
The morphology of trichomes covering the L. album subsp. album corolla corresponds to the data provided by Evert [30] and Bosabalidis and Sawidis [31], who found that peltate trichomes in Lamiaceae consist of a basal cell, a short stalk cell, and a broad head formed of 4–18 secretory cells. However, the peltate trichomes present in the epidermis of the L. album subsp. album calyx had a greater number of stalk cells, and therefore they do not fit the description.
The most abundant peltate trichomes on the L. album subsp. album corolla of flowers analyzed in this study had a four-celled head. Peltate trichomes with a four-celled head were also found in other Lamiaceae species—i.e., Lamium truncatum [6], Ocimum basilicum [32], O. obovatum [33], and Isodon rubescens [34]. The epidermis of the L. album subsp. album calyx and corolla in our study exhibited four types of short-stalked capitate trichomes with a one-, two-, three- and four-celled head. Our research results differ significantly from the observations reported by Atalay et al. [8], as these authors found only short-stalked and sessile capitate trichomes with a one-celled head on the stems, leaves, and calyx in L. album subsp. album. The differences may have been related to the younger age of the plants analyzed by these authors, which had not fully developed trichomes with a simpler structure. It is also probable that the ecotypes of L. album subsp. album growing in Turkey differ in the morphology their trichomes from the ecotypes growing in Poland.
In the present study, long-stalked capitate trichomes with a 1–4-celled head were observed only on stamen filaments in L. album subsp. album. These trichomes contained phenolic compounds. In turn, Baran and Özdemir [10] reported the presence of similar long-stalked trichomes with a one-celled head on the corolla and filaments in L. pisidicum.
The histochemical microscopic examinations carried out in the present study demonstrated that phenolic compounds were present not only in the glandular trichomes of the L. album subsp. album corolla but also in the non-glandular trichomes and epidermis cells. All the glandular trichomes contained phenolic compounds—i.e., phenolic acids, tannins, and flavonoids.
As reported by other authors, phenolic compounds in many Lamiaceae species are contained in glandular trichomes, although these compounds have not been detected in some taxa. In Stachys, Prasium, Sideritis, and Scutelaria, most types of glandular trichomes contained phenolic compounds [35,36]. Similar results were obtained in the case of Dracocephalum [29], Isodon [34], Satureja [37], Melissa [38], Marrubium [39], and Hyssopus [40]. In contrast, no phenolic compounds in glandular trichomes were detected in Ocimum obovatum [33], Plectranthus ornatus [41], and Salvia aegyptiaca [42].
Flavonoids have also been identified in glandular trichomes in other Lamiaceae species—e.g., in Salvia officinalis [43]; 11 species from the genera Stachys, Prasium, Sideritis, and Scutellaria [35]; Dracocephalum moldavica [29]; Marrubium vulgare [39]; and Thymus quinquecostatus [44].
The presence of secondary metabolites representing phenolic compounds only in some types of trichomes in many species may confirm the hypothesis postulated by various authors on the different functions of individual types of trichomes [41,43].
The results of the present research and the studies cited above are consistent with the findings reported by Richardson [45] on the wide distribution of flavonoids in the Lamiaceae family.
Our investigations have revealed that phenolic compounds are present in different parts of epidermis cells and trichomes. Flavonoids were detected in the cells of the epidermis and papillae and in glandular trichomes located on the L. album subsp. album corolla. Furthermore, we observed the secondary fluorescence of these compounds in the walls of the non-glandular trichomes. In their previous studies, other authors have reported the presence of flavonoids in the epicuticular waxes, cuticle, and fruit epidermis cells in various plant species [46,47]. Flavonoids were also detected in the protoplasts of epidermis cells and in the basal cells of non-glandular trichomes in Dracocephalum moldavica [29].
Tannins were detected in epidermis cell vacuoles, papilla vacuoles, and protoplasts of the glandular trichomes in the studied species. It has been found in other plants that vacuoles in epidermis cells serve as accumulation sites of tannins in the soluble form or in stable complexes with proteins, fats, mucilage, and pectins [48]. Tannin deposits can also accumulate in cell walls, thereby increasing their rigidity [48,49].
We identified phenolic acids in the protoplasts of epidermis cells and in the protoplasts and the cell walls of glandular trichomes. Other authors have detected phenolic acids bound to cell walls in which they serve a structural function—i.e., they enhance wall rigidity [50].
Our anatomical studies and histochemical assays facilitated the localization of phenolic compounds in L. album subsp. album corollas—i.e., flavonoids and phenolic acids—which were subjected to the qualitative analysis presented in the phytochemical part of the study.
3.2. Phytochemistry
Natural plant products give many possibilities of treatment of various illnesses, and their active substances were investigated in many phytochemical studies [51]. Phenolics are one of the principal constituents responsible for the bioactivities of Lamium species [27]. It has also been found that phenylpropanoids, flavonoids, iridoids, and phenolic acids are the main compounds of the aerial parts of L. album [22,52].
The present anatomical studies have shown that flavonoids and phenolic acids are accumulated in the epidermis cells in L. album subsp. album corolla petals and in the superficial structures of this tissue—i.e., in the outer cell walls, including the cuticle, and in the glandular and non-glandular trichomes. The function of these compounds in the epidermis layer and its structures in the plant consists of protection against UV-B radiation and their role as the first line of defense against pathogens and insects [49]. The flavonoids contained in deeper plant tissues protect them against oxidative damage, and the phenolic acids present in the cell walls are substrates for the synthesis of lignin and suberin [49]. As demonstrated in previous studies, ferulic and p-coumaric acids in plants are located in the cuticle or in glandular and non-glandular trichomes [53].
Phenolic compounds exert a wide spectrum of therapeutic effects on the human organism [49]. Flavonoids and phenolic acids are responsible for the high antioxidant activity of the plant material [27]. The impact of dietary flavonoids on the human organism is associated with a reduced risk of cancer [54], and with the protection of human skin fibroblasts against oxidative stress [55]. Previous studies have shown that quercetin has an inhibitory of effect on cell growth [15].
The present phytochemical studies performed with the HPTLC method revealed the presence of three flavonoids, quercetin, isoquercetin, and rutoside, in the methanol extract of L. album subsp. album flowers. In purified ethanol extracts of underground parts of L. album, Pereira et al. [12] detected for the first time the presence of derivatives of flavone isoscutellarein, which constituted one third of the total amount of phenolics. Kaempferol and quercetin derivatives have been identified in 80% ethanol extracts from L. album by other authors [56].
Using the HPTLC and HPLC methods, we detected nine phenolic acids—i.e., caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, protocatechuic acid, syringic acid, gentisic acid, and vanillic acid—in the methanol extracts after basic hydrolysis of L. album subsp. album flowers. Caffeic, protocatechuic, and vanillic acids are present in these flowers also as glycosides, and caffeic acid is the main acid released from glycosidic forms. In the methanol and ethyl acetate extracts from L. album flowers, Paduch et al. [14] detected six phenolic acids, which were found in the herb as free phenolic acids. Noteworthily, we additionally detected gallic, gentisic, and syringic acid after basic hydrolysis, which was not reported by the authors of the cited study. Caffeic and vanillic acids have essential pharmacological effects in the treatment of depression [57] and periodontal diseases [58].
Comparative studies of phenolic compounds and flavonoids in 15 plant species, including six representatives of the Lamiaceae family, demonstrated the lowest content of these substances in plant shoots with flowers in L. album [59]. The authors also found a correlation between the content of phenolic constituents and the antioxidant activity of plants. Other authors compared the phenolic content and antioxidant capacity of L. album and L. maculatum [60]. Their study showed that L. album had a lower content of phenolic compounds and a lower antioxidant capacity than L. maculatum.
The information about the location of flavonoids and phenolic acids in L. album subsp. album corolla tissues and the qualitative analysis of these constituents confirms the achievement of the goal of the present study. The complementary anatomical and phytochemical studies of the plant material can greatly contribute to the elucidation of the pharmacological effect of the plant raw material.
4. Materials and Methods
Flowering Lamium album L. subsp. album plants intended for the anatomical and phytochemical analysis were collected in the UMCS Botanical Garden in Lublin, SE Poland (51°15′44″ N, 22°30′48″ E). The study was conducted in 2016–2018. The botanical identification of plants was performed using Illustrierte Flora von Mittel Europa [61] and a taxonomic revision of Lamium (Lamiaceae) [62]. Voucher specimens of Lamium album subsp. album supporting this study were deposited in the Herbarium of Department of Botany and Plant Physiology (University of Life Sciences in Lublin, Akademicka 15, 20-950 Lublin, Poland).
Since the corolla is the main medicinal raw material [63], particular attention was focused on this part of the flower. The micromorphology and anatomy as well as the content of phenolic compounds of petals and the structure of trichomes were examined. Corollas of L. album subsp. album flowers were the material for the phytochemical and histochemical analyses.
The corolla parts (lower lip, upper lip, tube) were observed using stereoscopic (SM), light (LM), fluorescence (FM), and scanning electron microscopy (SEM). Stamens were examined as well, as they constitute the medicinal raw material together with corolla petals. Additionally, sepals were analyzed as a comparative material to check whether the corolla and calyx bear the same types of trichomes. The pistil was not analyzed in the study, as no trichomes were found in the epidermis of this organ. Stamens and sepals were analyzed only with the use of light (LM) and electron scanning (SEM) microscopy.
4.1. Histochemical Tests and FM
The content of phenolic compounds in corolla epidermis, its structures, and the stamens was determined using fresh free-hand sections and the following histochemical assays: ferric trichloride for total phenolic compounds [64]; potassium dichromate for tannins [65]; and Toluidine blue O (pH 4) for phenolic compounds, tannins [66,67,68,69], and pectins, the staining of which is limited at their lower content in the cell walls [68,70] (pp. 25–40).
Fluorescence microscopy was employed to detect phenolic acids that exhibit light blue autofluorescence in the presence of UV [71,72]. A FITC (fluorescein isothiocyanate) filter set was used at excitation light of 465–495 nm and a barrier filter was used at a wavelength of 515–555 nm [73]. Additionally, aluminum trichloride was used as a fluorochrome under ultraviolet light with a Cy5 (cyanine dye) filter set (excitation light of 590–650 nm and a barrier filter wavelength 663–738 nm) to determine the presence of flavonoids emitting yellow secondary fluorescence [74].
Since some histochemistry and fluorescence techniques do not allow the specific separation of a complex mixture of components [75], we used a relevant number of repetitions (n = 10) for each object and each technique and independent parallel reaction to avoid incorrect interpretation. The experiments produced similar positive results 8–9 times. We compared the results with the control. Standard control procedures were carried out simultaneously. Water with glycerine (1:1) slides were used for standard control procedures. Photographs were taken with a Coolpix 4500 (Nikon, Tokyo, Japan) camera coupled to an Eclipse 400 Nikons light microscope (Nikon).
4.2. SEM
Fragments of the corolla petals and calyx as well as stamens were fixed in a 4% glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.0). After 12 h incubation at a temperature of 4 °C, the samples were washed in the same buffer four times at 20 min intervals. An ethanol series (30, 50, 70, 90, and 95%) was used to dehydrate the plant material. Next, the samples were immersed three times in absolute alcohol and transferred to acetone. The samples were critical point dried in liquid CO2 using Bal-Tec CPD 030 (Balzers, Liechtenstein).
Dried fragments of the examined floral parts were glued onto stubs with the use of a double-sided carbon tape. The samples were coated with a 10 μm gold layer using a Polaron SC 7640 sputter coater (Emitech). A scanning electron microscope TESCAN/VEGA LMU (Tescan) at an accelerating voltage of 30 kV was used for the examination of the material.
4.3. Phytochemical Analyses
4.3.1. Extract Preparation and Purification
Flowers of Lamium album subsp. album without calyces were collected in summer 2017 (June to September), dried at room temperature without the direct sunlight, and stored at a temperature of 17 °C. Two groups of bioactive compounds were analyzed: flavonoids and phenolic acids. The liquid-liquid extraction process was used in the extract preparation.
To prepare the extract for the analysis of flavonoids, 10 g of L. album subsp. album flowers were taken and exhaustively extracted with methanol. Three-step extraction, with three portions of methanol (50 mL), was conducted in an ultrasonic bath (Sonorex Typ RK 102 HB, Bandelin, Berlin, Germany) according to the method described before [76]. The extract was concentrated to 20 mL and used in HPTLC experiments.
Purification and fractionation of phenolic acids was conducted to use the samples in HPTLC and HPLC experiments. After exhaustive extraction of plant material (10 g) with three portions of methanol in ultrasonic bath, the extracts were pooled, evaporated, dissolved in hot distilled water (100 mL), and purified with petroleum ether [77]. The extract of free phenolic acids was obtained after extraction with diethyl ether. To analyze the phenolic acids bound with glycosides and in combination with esters, the hydrolysis process was conducted according to the method described before [78]. The water fraction remaining after the extraction of free phenolic acids was halved (two portion of 50 mL volume) and subjected to acid and basic hydrolysis. The extract of phenolic acids from esterified conjugations was obtained after basic hydrolysis at pH 12. The extract of phenolic acids unbounded from glycosides was obtained in acid hydrolysis (pH 2). In both cases, unbounded phenolic acids were extracted with the use of diethyl ether. Ether fractions were evaporated to dryness and washed with methanol (5 mL) to obtain extracts for use in the HPTLC and HPLC experiments.
4.3.2. Planar Chromatography Experiments
Chromatography experiments were performed on 100 × 100 mm glass plates percolated with a 0.25 mm layer of silica-HPTLC Kieselgel Si 60 with a fluorescent indicator (Merck, Darmstad, Germany). Before use, the plates were washed with methanol and acetone and dried for five minutes at 105 °C for activation. During the experiments, the plates were developed in horizontal Teflon DS chambers (Chromdes, Lublin, Poland). The distance was 90 mm. Before the development, the plates were conditioned for 15 min above the mobile phase. All the solvents used in the planar chromatography experiments were of pro-analytical grade and were purchased from Polish Reagents (POCh, Gliwice, Poland). Techniques of isocratic elution and MGD (multiple gradient development) techniques were used. In the MGD technique, the plate was developed several times (2–9 times). The migration of the mobile phase was dependent on the composition of eluents—the elution strength of the mobile phase was adjusted to the type of the sample [79]. After each development step, the eluent was evaporated from the chromatographic plates.
4.3.3. Analysis of Flavonoids
Flavonoid standards were purchased from Sigma (St. Louis. MO, USA) and prepared as 0.1% solutions in methanol. The best separation of flavonoid standards was obtained with the use of a mobile phase consisting of toluene, hexane, formic acid, ethyl acetate, and methanol (Table 2)
Table 2.
Multiple gradient development program in the analysis of flavonoids.
| Elution Step | Development Distance [cm] | Mobile Phase Composition |
|---|---|---|
| 1 | 3 | 6 mL solution A (toluene hexane: formic acid, 7:3: 0.1), 3 mL ethyl acetate, 1 mL methanol |
| 2 | 9 | 5 mL solution A (toluene hexane: formic acid, 7:3: 0.1), 2.5 mL ethyl acetate, 2.5 mL methanol |
4.3.4. Visualization of Flavonoids
Flavonoids have an ability to form complex compounds with metal ions. This was used in the derivatization of chromatographic plates with ethanol solutions of aluminum chloride and FeCl3 (Figure S1). A total of 2 µL of flavonoid standards were applied with the use of a Hammilton syringe.
4.3.5. Analysis of Phenolic Acids
Phenolic acid standards were purchased from Sigma (St. Louis. MO, USA) and prepared as 0.1% solutions in methanol. They are specified in the description of in Figure 8 and Figure 9.
Many mobile phases were tested in the HPTLC analyses of phenolic acids. The best separation of phenolic acid standards was achieved with the use of two mobile phases in the gradient multiple program of chromatogram development (Table 3), distance 90 mm. Plates were dried at room temperature for approximately 20 min in a stream of air after each step of development.
Table 3.
Multiple gradient development program in the analysis of phenolic acids.
| Development Step | Mobile Phase Composition |
|---|---|
| 1, 2 | 3 mL solution A (heptane: dichlorometane, 7:3), 2 mL diisopropyl ether, 0.1 mL 85% formic acid, 1 mL distilled water |
| 3–7 | 4 mL solution A (heptane: dichlorometane, 7:3), 1 mL diisopropyl ether, 0.1 mL 85% formic acid |
The best separation of standards and application of chosen mobile phases for analysis of three extracts of L. album subsp. album flowers are shown in Figure 8. The plates were conditioned with the use of the mobile phase from the first step of development. The plate was photographed with the use of a Videoscanner (Desaga, Germany) at λ = 254 nm.
4.3.6. Chromatographic HPLC Experiments
All the solvents used in the HPLC method were pro-HPLC grade and were purchased from Polish Reagents (POCh, Gliwice, Poland). Standard solutions of phenolic acids were prepared as 0.01% methanol solutions, as presented in Figure 9. The separation of phenolic acid in methanol extract was carried out after acid and basic hydrolysis. The investigated compounds were identified by retention times and by a comparison of the absorption maxima of the standards. Each phenolic acid in the HPLC analysis is characterized by retention time in minutes and by absorption maxima in the range 200–400 nm (Table 4).
Table 4.
Retention time in minutes and absorption maxima in the range 200–400 nm for phenolic acids in the HPLC method.
| Phenolic Acid Standard | Retention Time [min] | λmax [nm] |
|---|---|---|
| gallic acid | 3.78 | 227.5; 271.0 |
| protocatechuic acid | 6.37 | 227.5; 258.6; 294.0 |
| gentisic acid | 12.23 | 234.9; 326.9 |
| vanillic acid | 14.05 | 226.4; 260.5; 291.0 |
| caffeic acid | 14.95 | 239.0; 322.6 |
| syringic acid | 16.85 | 224.7; 273.3 |
| p-coumaric acid | 26.12 | 228.7; 308.9 |
| ferulic acid | 31.93 | 234.9; 322.6 |
| chlorogenic acid | 41.75 | 234.9; 300.3 |
The HPLC analyses were carried out with the use of a Liquid Chromatograph La-Chrom—Merck with a diode array detector DAD (L-7455), pump (L-7100), degasser (L-7612), thermostat (L-7250), rheodyne injector (20 µL), and Zorbax steel column SB-C 18, dimensions 250 mm × 4.6 mm with a 5 µm grain stationary phase. The investigations were conducted in reverse mode phases. The stationary phase was silica gel with octadecyl groups (RP-18). The mobile phase consisted of methanol and water (25:75, v/v), with a 0.25% addition of 40% formic acid. The flow rate was 1 mL/min. The measurements were conducted at 25 °C, and the injection volume was 20 µL. The wavelength range was 200–400 nm.
Supplementary Materials
Figure S1: Flavonoid standards after visualization with aluminum chloride in VIS light; Table S1: Names of standards with CAS number, producer, and product number.
Author Contributions
Conceptualization, E.W.-C. and A.S.-K.; Formal analysis, A.S., A.K., A.M.-W., M.D., E.W-C., A.S-K.; Investigation, A.S., A.K., A.M.-W., M.D., E.W.-C. and A.S.-K.; Methodology, A.S., A.K., A.M.-W., M.D., E.W.-C., A.S.-K. and R.R.; Visualization, A.S., A.K., A.M.-W. and M.D.; Writing—original draft, A.S., A.K., E.W.-C. and A.S.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by THE POLAND’S MINISTRY OF SCIENCE AND HIGHER EDUCATION as part of the statutory activities of the Department of Botany and Plant Physiology, University of Life Sciences in Lublin (project LKR/S/49/2020RiO) and the Department of General Ophthalmology, Medical University of Lublin (project DS 180).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mabberley D.J. The Plant Book. 2nd ed. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- 2.Yordanova Z.P., Zhiponova M.K., Iakimova E.T., Dimitrova M.A., Kapchina-Toteva V.M. Revealing the reviving secret of the white dead nettle (Lamium album L.) Phytochem. Rev. 2014;13:375–389. doi: 10.1007/s11101-014-9356-2. [DOI] [Google Scholar]
- 3.Dengler J., Berg C., Eisenberg M., Isermann M., Jansen F., Koska I., Löbel S., Manthey M., Pazolt J., Spangenberg A., et al. New descriptions and typifications of syntaxa within the project ‘Plant communities of Mecklenburg-Vorpommern and their vulnerability’—Part I. Feddes Repertorium Zeitschrift für Botanische Taxonomie und Geobotanik. 2003;114:587–631. doi: 10.1002/fedr.200311017. [DOI] [Google Scholar]
- 4.Metcalfe C.R., Chalk L. Anatomy of the Dicotyledons. Volume 2 Oxford Press; London, UK: 1972. [Google Scholar]
- 5.Sulborska A., Dmitruk M., Konarska A., Weryszko-Chmielewska E. Adaptations of Lamium album L. flowers to pollination by Apoidea. Acta Sci. Pol. Hortorum Cultus. 2014;13:31–43. [Google Scholar]
- 6.Celep F., Kahraman A., Atalay Z., Doğan M. Morphology, anatomy and trichome properties of Lamium truncatum Boiss. (Lamiaceae) and their systematic implications. Aust. J. Crop Sci. 2011;5:147–153. [Google Scholar]
- 7.Özdemir C., Baran P. Morphological, anatomical and cytological investigation on alpine Lamium cymbalariifolium endemic to Turkey. Aust. J. Crop Sci. 2012;6:532–540. [Google Scholar]
- 8.Atalay Z., Celep F., Bara F., Doğan M. Systematic significance of anatomy and trichome morphology in Lamium (Lamioideae; Lamiaceae) Flora. 2016;225:60–75. doi: 10.1016/j.flora.2016.10.006. [DOI] [Google Scholar]
- 9.Baran P., Özdemir C. The morphological and anatomical properties of Lamium lyceum (Lamiaceae), endemic to Turkey. Nord. J. Bot. 2009;27:388–396. doi: 10.1111/j.1756-1051.2009.00417.x. [DOI] [Google Scholar]
- 10.Baran P., Özdemir C. Morphological, anatomical and cytological studies on endemic Lamium pisidicum. Pak. J. Bot. 2013;45:73–85. [Google Scholar]
- 11.Turner N.J., Łukasz Ł.J., Migliorini P., Pieroni A., Dreon A.L., Sacchetti L.E., Paoletti M.G. Edible and Tended Wild Plants, Traditional Ecological Knowledge and Agroecology. Crit. Rev. Plant Sci. 2011;30:198–225. doi: 10.1080/07352689.2011.554492. [DOI] [Google Scholar]
- 12.Pereira O.R., Domingues M.D.R., Silva A.M., Cardoso S.M. Phenolic constituents of Lamium album: Focus on isoscutellarein derivatives. Food Res. Int. 2012;48:330–335. doi: 10.1016/j.foodres.2012.04.009. [DOI] [Google Scholar]
- 13.Xu F. Chinese Medicine e.g. for Treating Arthropathy, Comprises Broad Cocklebur, Vervain, Condyle Grass, Motherwort, Saxifrage, Cactus, Mullberry Branch, White Dead Nettle, Boston Ivy, Folium Photiniae, Water Pepper and Chinese Fever Vine. 2008. p. 10. XUFF-Individual. [Google Scholar]
- 14.Paduch R., Wójciak-Kosior M., Matysik G. Investigation of biological activity of Lamii albi flos extracts. J. Ethnopharmacol. 2007;110:69–75. doi: 10.1016/j.jep.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Kelayeh T.P.S., Abedinzade M., Ghorbani A. A review on biological effects of Lamium album (white dead nettle) and its components. J. Herbmed Pharm. 2019;8:185–193. doi: 10.15171/jhp.2019.28. [DOI] [Google Scholar]
- 16.Zhang H.-J., Rothwangl K., Mesecar A.D., Sabahi A., Rong L., Fong H.H.S. Lamiridosins, Hepatitis C Virus Entry Inhibitors from Lamium album. J. Nat. Prod. 2009;72:2158–2162. doi: 10.1021/np900549e. [DOI] [PubMed] [Google Scholar]
- 17.Valyova M.S., Dimitrova M.A., Ganeva Y.A., Kapchina-Toteva V.M., Yordanova Z.P. Evaluation of antioxidant and free radical scavenging potential of Lamium album L. growing in Bulgaria. J. Pharm. Res. 2011;4:945–947. [Google Scholar]
- 18.Pereira O.R., Macias R.I.R., Perez M.J., Marin J.J.G., Cardoso S.M. Protective effects of phenolic constituents from Cytisus mulitflorus, Lamium album L. and Thymus citriodorus on liver cells. J. Funct. Foods. 2013;5:1170–1179. doi: 10.1016/j.jff.2013.03.014. [DOI] [Google Scholar]
- 19.Kokoska L., Polesny Z., Rada V., Nepovim A., Vanek T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002;82:51–53. doi: 10.1016/s0378-8741(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 20.Topouzova-Hristova T., Moskova-Doumanova V., Keremidarska M., Doumanov J., Miteva G., Petkova B., Kapchina-Toteva V. Anticancer effect of plant extracts from Lamium album L. by induction od cell death in vitro. Medicine. 2012;2:55–59. [Google Scholar]
- 21.Gao J., Morgan G., Tieu D., A Schwalb T., Luo J.Y., A Wheeler L., E Stern M. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp. Eye Res. 2004;78:823–835. doi: 10.1016/j.exer.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Czerwińska M.E., Świerczewska A., Woźniak M., Kiss A.K. Bioassay-Guided Isolation of Iridoids and Phenylpropanoids from Aerial Parts of Lamium album and Their Anti-inflammatory Activity in Human Neutrophils. Planta Med. 2017;83:1011–1019. doi: 10.1055/s-0043-107031. [DOI] [PubMed] [Google Scholar]
- 23.Croteau R., Kutchan T.M., Lewis N.G. Natural products (secondary metabolites) In: Buchanan B., Gruissem W., Jones R., Dekker M., editors. Biochemistry & Molecular Biology of Plants. American Society of Plant Physiologists; New York, NY, USA: 2000. pp. 1250–1318. [Google Scholar]
- 24.Lotina-Hennsen B., King-Díaz B., Aguilar M., Terrones M.G.H. Plant secondary metabolites. Targets and mechanisms of allelopathy. In: Reigosa M.J., Pedrol N., Gonzáles L., editors. Allelopathy: A Physiological Proccess with Ecological Implications. Springer; Dordrecht, The Netherlands: 2006. pp. 229–265. [Google Scholar]
- 25.Tošić S., Stojičić D., Slavkovska V., Mihailov-Krstev T., Zlatković B., Budimir S., Uzelac B. Phytochemical composition and biological activities of native and in vitro-propagated Micromeria croatica (Pers.) Schott (Lamiaceae) Planta. 2019;249:1365–1377. doi: 10.1007/s00425-018-03071-5. [DOI] [PubMed] [Google Scholar]
- 26.Frezza C., Venditti A., Serafini M., Bianco A. Studies in Natural Products Chemistry. Volume 62. Elsevier; Amsterdam, The Netherlands: 2019. Phytochemistry, chemotaxonomy, ethnopharmacology, and nutraceutics of Lamiaceae; pp. 125–178. [Google Scholar]
- 27.Salehi B., Armstrong L., Rescigno A., Yeskaliyeva B., Seitimova G., Beyatli A., Jugreet S., Mahomoodally M.F., Sharopov F., Durazzo A., et al. Lamium Plants—A Comprehensive Review on Health Benefits and Biological Activities. Molecules. 2019;24:1913. doi: 10.3390/molecules24101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caissard J.-C., Joly C., Bergougnox V., Hugueney P., Mauriat M., Baudino S. Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Rec. Res. Develop. Cell Biol. 2004;2:1–15. [Google Scholar]
- 29.Dmitruk M., Sulborska A., Żuraw B., Stawiarz E., Weryszko-Chmielewska E. Sites of secretion of bioactive compounds in leaves of Dracocephalum moldavica L.: Anatomical, histochemical, and essential oil study. Braz. J. Bot. 2019;42:701–715. doi: 10.1007/s40415-019-00559-6. [DOI] [Google Scholar]
- 30.Evert R.F. Esau’s Plant Anatomy. 3rd ed. Wiley-Interscience; Hoboken, NJ, USA: 2006. [Google Scholar]
- 31.Bosabalidis A.M., Sawidis T. Glandular and non-glandular hairs in the seasonally dimorphic Origanum dictamnus L. (Lamiaceae) as a means of adaptation to cold stress. Acta Agrobot. 2014;67:15–20. doi: 10.5586/aa.2014.010. [DOI] [Google Scholar]
- 32.Werker E., Putievsky E., Ravid U., Dudai N., Katzir I. Glandular Hairs and Essential Oil in Developing Leaves of Ocimum basilicum L. (Lamiaceae) Ann. Bot. 1993;71:43–50. doi: 10.1006/anbo.1993.1005. [DOI] [Google Scholar]
- 33.Naidoo Y., Kasim N., Heneidak S., Nicholas A., Naidoo G. Foliar secretory trichomes of Ocimum obovatum (Lamiaceae): Micromorphological structure and histochemistry. Plant Syst. Evol. 2013;299:873–885. doi: 10.1007/s00606-013-0770-5. [DOI] [Google Scholar]
- 34.Liu M.-Q. Structure and histochemistry of the glandular trichomes on the leaves of Isodon rubescens (Lamiaceae) Afr. J. Biotechnol. 2012;11:4069–4078. doi: 10.5897/ajb11.4024. [DOI] [Google Scholar]
- 35.Giuliani C., Bini L.M. Insight into the structure and chemistry of glandular trichomes of Labiatae, with emphasis on subfamily Lamioideae. Plant Syst. Evol. 2008;276:199–208. doi: 10.1007/s00606-008-0085-0. [DOI] [Google Scholar]
- 36.Venditti A., Bianco A., Quassinti L., Bramucci M., Lupidi G., Damiano S., Papa F., Vittori S., Bini L.M., Giuliani C., et al. Phytochemical Analysis, Biological Activity, and Secretory Structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from Central Italy. Chem. Biodivers. 2015;12:1172–1183. doi: 10.1002/cbdv.201400275. [DOI] [PubMed] [Google Scholar]
- 37.Marin M., Ascensão L., Lakušić B. Trichomes of Satureja horvatii Šilić (Lamiaceae): Micromorphology and histochemistry. Arch. Biol. Sci. 2012;64:995–1000. doi: 10.2298/ABS1203995M. [DOI] [Google Scholar]
- 38.Chwil M., Nurzyńska-Wierdak R., Chwil S., Matraszek R., Neugebauerová J. Histochemistry and micromorphological diversity of glandular trichomes in Melissa officinalis L. leaf epidermis. Acta Sci. Pol. Hortorum Cultus. 2016;15:153–172. [Google Scholar]
- 39.Haratym W., Weryszko-Chmielewska E. Ultrastructural and histochemical analysis of glandular trichomes of Marrubium vulgare L. (Lamiaceae) Flora Morphol. Distrib. Funct. Ecol. Plants. 2017;231:11–20. doi: 10.1016/j.flora.2017.04.001. [DOI] [Google Scholar]
- 40.Venditti A., Bianco A., Frezza C., Conti F., Bini L.M., Giuliani C., Bramucci M., Quassinti L., Damiano S., Lupidi G., et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Ind. Crop. Prod. 2015;77:253–363. doi: 10.1016/j.indcrop.2015.09.002. [DOI] [Google Scholar]
- 41.Ascensão L., Mota L., Castro M.D.M. Glandular Trichomes on the Leaves and Flowers of Plectranthus ornatus: Morphology, Distribution and Histochemistry. Ann. Bot. 1999;84:437–447. doi: 10.1006/anbo.1999.0937. [DOI] [Google Scholar]
- 42.Janošević D., Budimar S., Alimpić A., Marin P., Al Sheef N., Giweli A., Dulitić-Laušević S. Micromorphology and histochemistry of leaf trichomes of Salvia aegyptica (Lamiaceae) Arch. Biol. Sci. 2016;68:291–301. doi: 10.2298/ABS150602018J. [DOI] [Google Scholar]
- 43.Corsi G. Glandular Hairs of Salvia officinalis: New Data on Morphology, Localization and Histochemistry in Relation to Function. Ann. Bot. 1999;84:657–664. doi: 10.1006/anbo.1999.0961. [DOI] [Google Scholar]
- 44.Jia P., Gao T., Xin H. Changes in Structure and Histochemistry of Glandular Trichomes of Thymus quinquecostatus Celak. Sci. World J. 2012;2012:1–7. doi: 10.1100/2012/187261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson P.M. The chemistry of the Labiatea: An introduction and overview. In: Harley R.M., Reynolds T., editors. Advances in Labiate Science. Kew, Royal Botanical Gardens; Richmond, UK: 1992. [Google Scholar]
- 46.Usenik V., Stampar F., Kastelec D. Phytochemicals in fruits of two Prunus domestica L. plum cultivars during ripening. J. Sci. Food Agric. 2013;93:681–692. doi: 10.1002/jsfa.5783. [DOI] [PubMed] [Google Scholar]
- 47.Konarska A. Morphological, anatomical, and ultrastructural changes in Vaccinium corymbosum fruits during ontogeny. Botany. 2015;93:589–602. doi: 10.1139/cjb-2015-0050. [DOI] [Google Scholar]
- 48.Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Karabourniotis G., Liakopoulos G., Nikolopoulos D., Bresta P., Stavroulaki V., Sumbele S. “Carbon gain vs. water saving, growth vs. defence”: Two dilemmas with soluble phenolics as a joker. Plant Sci. 2014;227:21–27. doi: 10.1016/j.plantsci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Padayachee A., Netzel G., Netzel M., Day L., Zabaras D., Mikkelsen D., Gidley M.J. Binding of polyphenols to plant cell wall analogues—Part 2: Phenolic acids. Food Chem. 2012;135:2287–2292. doi: 10.1016/j.foodchem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Bazzo Y.M., Catini C.D., Vicentini F.T., Cardoso J.C., Junior R.L.C.D.A., Fonseca M.J.V. Efficacy of Marigold Extract-Loaded Formulations Against UV-induced Oxidative Stress. J. Pharm. Sci. 2011;100:2182–2193. doi: 10.1002/jps.22438. [DOI] [PubMed] [Google Scholar]
- 52.Alipieva K., Kokubun T., Taskova R., Evstatieva L., Handjieva N. LC–ESI-MS analysis of iridoid glucosides in Lamium species. Biochem. Syst. Ecol. 2007;35:17–22. doi: 10.1016/j.bse.2006.07.004. [DOI] [Google Scholar]
- 53.Karabourniotis G., Liakopoulos G. Phenolic compounds in plant cuticles: Physiological and ecophysiological aspects. In: Hemantaranjan A., editor. Advances in Plant Physiology. Volume 8. Scientific Publishers; Jodhpur, India: 2005. pp. 33–47. [Google Scholar]
- 54.O’Prey J., Brown J., Fleming J., Harrison P.R. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem. Pharm. 2003;66:2075–2088. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Spencer J.P.E., El Mohsen M.M.A., Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: Implications for their bioactivity. Arch. Biochem. Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Botirov E.K. Flavonoids and Phenolcarboxylic Acids from Lamium album. Chem. Nat. Compd. 2019;55:1159–1160. doi: 10.1007/s10600-019-02921-2. [DOI] [Google Scholar]
- 57.Monteiro Á.B., Rodrigues C.K.D.S., Nascimento E.P.D., Sales V.D.S., Delmondes G.D.A., Da Costa M.H.N., De Oliveira V.A.P., Pereira-De-Morais L., Boligon A.A., Barbosa R., et al. Anxiolytic and antidepressant-like effects of Annona coriacea (Mart.) and caffeic acid in mice. Food Chem. Toxicol. 2020;136:111049. doi: 10.1016/j.fct.2019.111049. [DOI] [PubMed] [Google Scholar]
- 58.Karatas O., Yuce H.B., Taskan M.M., Gevrek F., Yarkac F.U., Keskin A., Karatas S.F.O., Toker H. The effect of vanillic acid on ligature-induced periodontal disease in Wistar rats. Arch. Oral Biol. 2019;103:1–7. doi: 10.1016/j.archoralbio.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Tupec M., Hýsková V., Bělonožníková K., Hraníček J., Červený V., Ryšlavá H. Characterization of some potential medicinal plants from Central Europe by their antioxidant capacity and the presence of metal elements. Food Biosci. 2017;20:43–50. doi: 10.1016/j.fbio.2017.08.001. [DOI] [Google Scholar]
- 60.Danila D., Adriana T., Camelia S., Valentin G., Anca M. Antioxidant activity of methanolic extracts of Lamium album and Lamium maculatum species from wild populations in the Romanian Eastern Carpathians. Planta Med. 2015:81. doi: 10.1055/s-0035-1565816. [DOI] [Google Scholar]
- 61.Hegi G. Illustrierte Flora von Mittel-Europa. Volume 4 Carl Hanser Verlag; München, Germany: 1965. [Google Scholar]
- 62.Mennema J. A Taxonomic Revision of Lamium (Lamiaceae) Volume 11 E.J. Brill; Leiden, The Netherlands: 1989. (Leiden Botanical Series). [Google Scholar]
- 63.Kohlmünzer S. Farmakognozja: Podręcznik dla Studentów Farmacji. PZWL Wydawnictwo Lekarskie; Warszawa, Poland: 2017. [Google Scholar]
- 64.Gahan P.B. Plant Histochemistry and Cytochemistry. Academic Press; London, UK: 1984. [Google Scholar]
- 65.Gabe M. Techniques Histologiques. Masson; Paris, France: 1968. [Google Scholar]
- 66.Baker J.R. Cytological Technique. 5th ed. Methven; London, UK: 1966. [Google Scholar]
- 67.Gutmann M. Improved staining procedures for photographic documentation of phenolic deposits in semithin sections of plant tissue. J. Microsc. 1995;179:277–281. doi: 10.1111/j.1365-2818.1995.tb03642.x. [DOI] [Google Scholar]
- 68.O’Brien T.P., McCully M.E. The Study of Plant Structure: Principles and Selected Methods. Termarcarphi Pty Ltd.; Melbourne, Australia: 1981. [Google Scholar]
- 69.Ribeiro V.C., Leitão C.A.E. Utilisation of Toluidine blue O pH 4.0 and histochemical inferences in plant sections obtained by free-hand. Protoplasma. 2019;257:993–1008. doi: 10.1007/s00709-019-01473-0. [DOI] [PubMed] [Google Scholar]
- 70.Soukup A. Plant Cell Morphogenesis. Humana Press; Totowa, NJ, USA: 2014. Selected Simple Methods of Plant Cell Wall Histochemistry and Staining for Light Microscopy; pp. 25–40. [DOI] [PubMed] [Google Scholar]
- 71.Buschmann C., Langsdorf G., Lichtenthaler H. Imaging of the Blue, Green, and Red Fluorescence Emission of Plants: An Overview. Photosynthetica. 2000;38:483–491. doi: 10.1023/A:1012440903014. [DOI] [Google Scholar]
- 72.Chaerle L., Lenk S., Hagenbeek D., Buschmann C., Van Der Straeten D. Multicolor fluorescence imaging for early detection of the hypersensitive reaction to tobacco mosaic virus. J. Plant Physiol. 2007;164:253–262. doi: 10.1016/j.jplph.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Huang S.S., Kirchoff B.K., Liao J.P. The capitate and peltate glandular trichomes in Lavandula pinnata L. (Lamiaceae): Histochemistry, ultrastructure and secretion. J. Torrey Bot. Soc. 2008;135:155–167. doi: 10.3159/07-RA-045.1. [DOI] [Google Scholar]
- 74.Charrière-Ladreix Y. Répartition intracellulaire du secrétat flavonique de Populus nigra L. Planta. 1976;129:167–174. doi: 10.1007/BF00390024. [DOI] [PubMed] [Google Scholar]
- 75.Badria F.A., Aboelmaaty W.S. Plant Histochemistry: A Versatile and Indispensible Tool in Localization of Gene Expression, Enzymes, Cytokines, Secondary Metabolites and Detection of Plants Infection and Pollution. Acta Sci. Pharm. Sci. 2019;3:88–100. doi: 10.31080/ASPS.2019.03.0318. [DOI] [Google Scholar]
- 76.Wójciak-Kosior M., Matysik G., Skalska A. Densitometric determination of kinetics of hydrolysis of flavonoid glycosides. J. Planar Chromatogr. Mod. TLC. 2004;17:286–289. doi: 10.1556/JPC.17.2004.4.8. [DOI] [Google Scholar]
- 77.Chernetskyy M., Woźniak A., Skalska-Kamińska A., Żuraw B., Blicharska E., Rejdak R., Donica H., Weryszko-Chmielewska E. Structure of Leaves and Phenolic Acids in Kalanchoë daigremontiana Raym.-Hamet & H. Perrier. Acta Sci. Pol. Hortorum Cultus. 2018;17:137–155. doi: 10.24326/asphc.2018.4.13. [DOI] [Google Scholar]
- 78.Tyszczuk-Rotko K., Skalska-Kamińska A., Woźniak A. Voltammetric method using a lead film electrode for the determination of caffeic acid in a plant material. Food Chem. 2011;125:1498–1503. doi: 10.1016/j.foodchem.2010.10.075. [DOI] [Google Scholar]
- 79.Matysik G. Modified programmed multiple gradient development (MGD) in the analysis of complex plant extracts. Chromatographia. 1996;43:39–43. doi: 10.1007/BF02272819. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.