Abstract
Respiratory viral infections represent the leading cause of hospitalization in infants and young children worldwide and the second leading cause of infant mortality. Among these, Respiratory Syncytial Virus (RSV) represents the main cause of lower respiratory tract infections (LRTIs) in young children worldwide. RSV manifestation can range widely from mild upper respiratory infections to severe respiratory infections, mainly bronchiolitis and pneumonia, leading to hospitalization, serious complications (such as respiratory failure), and relevant sequalae in childhood and adulthood (wheezing, asthma, and hyperreactive airways). There are no specific clinical signs or symptoms that can distinguish RSV infection from other respiratory pathogens. New multiplex platforms offer the possibility to simultaneously identify different pathogens, including RSV, with an accuracy similar to that of single polymerase chain reaction (PCR) in the majority of cases. At present, the treatment of RSV infection relies on supportive therapy, mainly consisting of oxygen and hydration. Palivizumab is the only prophylactic method available for RSV infection. Advances in technology and scientific knowledge have led to the creation of different kinds of vaccines and drugs to treat RSV infection. Despite the good level of these studies, there are currently few registered strategies to prevent or treat RSV due to difficulties related to the unpredictable nature of the disease and to the specific target population.
Keywords: antiviral therapy, pneumonia, respiratory virus, RSV, vaccine
1. Introduction
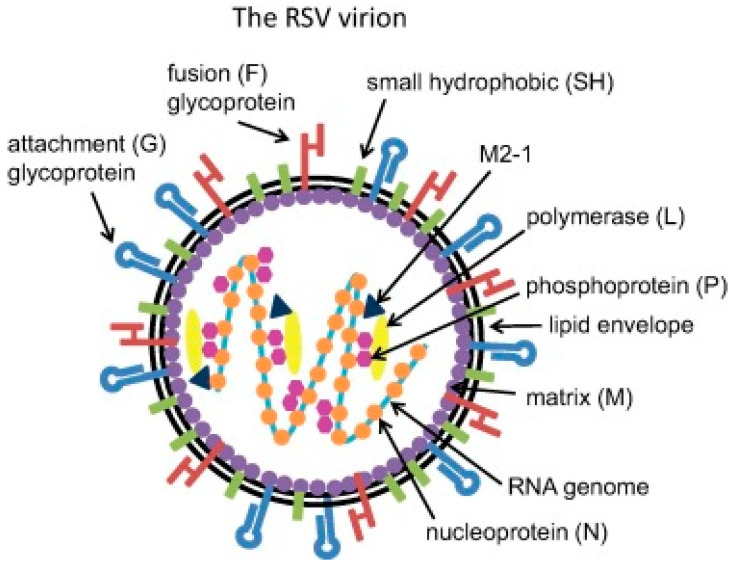
Respiratory viral infections represent the leading cause of hospitalization in infants and young children worldwide and are the second leading cause of infant mortality [1,2]. Among these infections, Respiratory Syncytial Virus (RSV) represents the main cause of lower respiratory tract infections (LRTIs) in young children worldwide [3,4,5,6,7,8,9]. RSV is an enveloped, non-segmented, negative-strand RNA virus belonging to the Paramyxoviridae family [10]. RSV is divided into two antigenic subtypes, A and B, based on the reactivity of the F and G surface proteins to monoclonal antibodies [11]. The subtypes tend to circulate simultaneously within local epidemics, although subtype A tends to be more prevalent. Figure 1 shows the structure of RSV.
Figure 1.
Structure of Respiratory Syncytial Virus (RSV).
The global burden of RSV-associated acute LRTI is estimated at 33 million annually, resulting in more than 3 million hospitalizations and 59,600 in-hospital deaths in children aged under 5 years and 6.7% of all deaths in infants younger than one year old [12,13]. Furthermore, RSV-associated acute LRTIs account for 1.4 million hospitalizations and 27,300 in-hospital deaths in infants aged under 6 months [13,14,15,16]. Globally, RSV represents the major contributor to infant death in children worldwide [17]. An RSV wave starts in most countries in the Southern Hemisphere between March and June and in countries in the Northern Hemisphere between September and December [18,19,20,21,22]. A decrease in RSV activity was observed from August to October in the Southern Hemisphere and from February to May in the Northern Hemisphere [18,19,20,21,22].
RSV clinical manifestation ranges widely from mild upper respiratory infections to severe LRTIs, mainly bronchiolitis and pneumonia, leading to hospitalization, serious complications (such as respiratory failure) and relevant sequalae in childhood and adulthood (i.e., wheezing, asthma, and hyperreactive airways) [23,24,25,26,27].
Children in their first 2 years of life comprise the major risk group for RSV severe disease, with a peak in infants approximately 3 months old, after which the incidence gradually declines with age [28,29]. It has been speculated that almost all children aged < 2 years old experience at least one episode of RSV infection, and half of them are re-infected during their second or third year of life [30,31,32,33,34]. Risk factors for severe RSV infections are prematurity, low birth weight, male sex, bronchopulmonary dysplasia, congenital heart disease, immunodeficiency, cerebral palsy, and Down’s syndrome [35,36,37]. Moreover, children with so-called medical complexity (CMC), not only including subjects with previously cited specific chronic medical problems but also those with other potential lifelong conditions associated with medical fragility or relevant functional limitations necessitating care and/or require specific technological assistance, are at major risk of developing serious problems in cases of RSV infections [38,39,40,41]. Nevertheless, approximately 50–80% of emergency admissions related to RSV bronchiolitis occur in otherwise healthy term infants [41].
As RSV infections and their related problems represent a global burden worldwide, the World Health Organization (WHO) created a surveillance programme similar to that of influenza infection in 2017, with the aim of better understanding the incidence, seasonality, and regional patterns of this infection and the clinical aspects that lead to hospitalization. This programme has already entered phase II, which is planned to last until the end of 2021 [14,42]. Before this surveillance programme, Lam and colleagues gathered information about different respiratory viruses, including RSV, from 2010 to 2015 in 14 different countries, analysing the seasonal peaks in different parts of the world; the data revealed a notable pattern of synchrony for RSV (and influenza and parainfluenza viruses) incidence peaking globally, despite significant distances among the sites considered [43]. Moreover, collecting data from 27 countries between 2016 and 2017, the Obando-Pachebo group provided information that may allow the prediction of the beginning of RSV outbreaks worldwide [44].
This review aims to gather state-of-the-art information about RSV infection in children, specifically RSV pneumonia.
2. Incidence of Respiratory Syncytial Virus (RSV)
Globally, pneumonia is a major cause of paediatric infectious disease mortality and the greatest cause of death in children under 5 years old (with a 12.8% percentage of annual death beyond the neonatal period) [44,45,46,47]. Analysing an important sample of 36,500 paediatric pneumonia cases, the Tian group examined the meteorological conditions that influence RSV incidence: meteorological factors play an important role in the incidence of RSV, with the strongest correlation at the lowest temperature [48,49]. During the Pneumonia Aetiology Research for Child Health (PERCH) study, the authors reported a high prevalence of RSV infections in children living in high-burden, low-resource regions (of Africa and Asia), with 61% of children requiring hospital admission [50,51,52,53,54]. Moreover, the Global Approach to Biological Research, Infectious Disease and Epidemics in Low-income countries (GABRIEL) study reported an RSV incidence among pneumonia cases of 11.7%, placing it as the second most relevant pathogen, after influenza A, due to its high adjusted population attributable fraction (aPAF) of 18.2%; an inverse relationship between incidence of RSV pneumonia and age was also observed [55].
In 230 Australian children with pneumonia evaluated by the Bhuiyan group, RSV was detected in 20.2% (among the 44.4% amount of respiratory virus detectable globally), with a prevalence between May and November [56]. Nascimento and colleagues reported a lower incidence (approximately 15% for RSV-A and 12% for RSV-B) of RSV cases in patients with community-acquired pneumoniae (CAP), which was not as severe as hospitalization [57]. Additionally, the Khuri-Bulos group detected >95% viral infections during the winter period in their studied population, with a predominance of RSV, mainly in younger patients (approximately 20% in subjects younger than 6 months) and in those with pneumonia (approximately 35%) [58].
Romero-Espinoza and colleagues reported a high percentage of RSV, particularly serotype B, as the predominant species, along with Rhinovirus C, in subjects under 15 years old affected by pneumonia and/or asthma [59,60]. Moreover, Esposito et al. reported Italian circulating RSV types and serotypes (79.4% carried RSV-A and 20.6% RSV-B) among children presenting with influenza-like syndromes in five consecutive winter seasons [61].
RSV epidemics are seasonal, with peak infection occurring during late autumn/winter to early spring in the Northern Hemisphere [62,63,64].
3. Pathophysiology of Respiratory Syncytial Virus (RSV) Infection
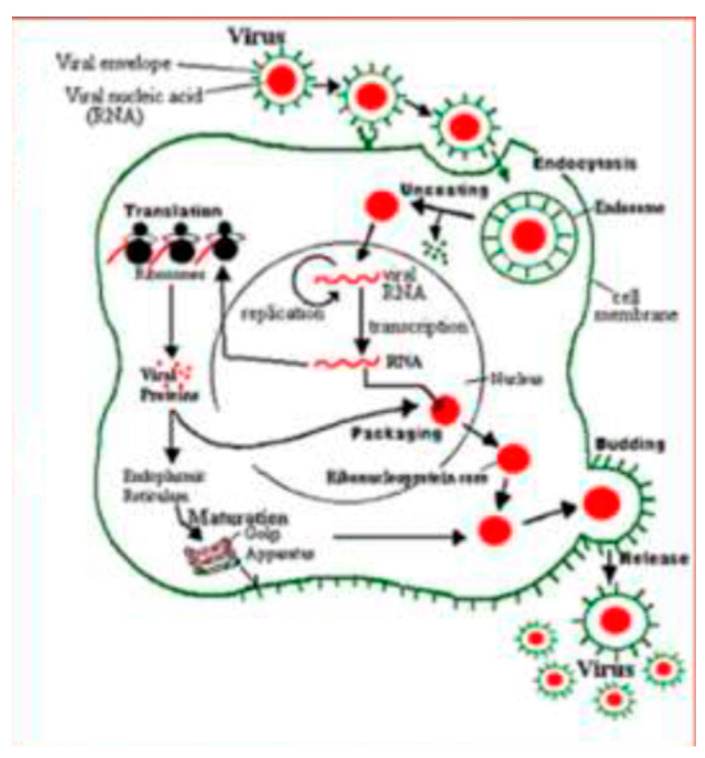
Many studies have attempted to explain the mechanism of entrance and the interaction of RSV with the human host (Figure 2) [65,66,67]. Lay and colleagues proposed a new model of pathogenesis. Firstly, RSV enters airway epithelial cells through interaction with the G protein; secondly, this attachment determines the binding of the RSV F protein to nucleolin (rich in cholesterol domain), which leads to activation of cytoskeleton and actin filament reorganization of the RSV envelope to introduce its content in the cytoplasm of the host cell [68].
Figure 2.
Binding and entry of Respiratory Syncytial Virus (RSV) into the host cell.
RSV presents direct cytopathic activity, but signs and symptoms, which are virus dependent, are also due to the local host inflammatory response (innate and adaptative immune responses) created by its presence in airway epithelial cells [69,70,71,72,73]. Many in vitro and animal models have been employed to infer the pathological pathway of RSV, though its interaction with human hosts, and particularly with children, differs greatly from other models [74].
4. Clinical Manifestation of Respiratory Syncytial Virus (RSV)
RSV infections can range from mild, eventually even asymptomatic, to severe clinical infections, such as bronchiolitis and pneumonia. RSV can also cause severe pneumonia and acute respiratory distress syndrome (ARDS), which can lead to hospitalization and access to the intensive care unit (ICU) [75].
Focusing on pneumonia, which is the target of this review, and omitting better-known RSV bronchiolitis, subjects affected by RSV pneumonia usually present with the following features: fever, signs and symptoms of acute respiratory infections (cough, wheezing, tachypnoea), hypoxaemia and oxygen necessity, alteration of reactive C protein (CRP), and radiographic evidence (increased pulmonary texture or mottled shadows) [48,50,55,76,77].
In general, signs and symptoms appear after a short incubation period, as for the majority of viruses, of approximately 4 days [78]. The already cited prospective viral surveillance study of Khuri-Bulos and colleagues reported a significantly lower presence of fever but a high probability of cough, shortness of breath, and flaring in patients with RSV infection [58]. It has been observed that RSV-A causes more severe illness than RSV-B; in fact, the two serotypes elicit a different response in terms of cytokine and neutrophil production [74,79].
As mentioned before, there are some risk factors for RSV infection. Different studies have suggested the importance of any of these and of their association: prematurity, low birth weight, multiple birth, siblings, male sex, atopy, passive smoking and pollution, day care attendance, crowding, and malnutrition [80]. In a retrospective study, the Ravindranath group reported RSV as an important cause of paediatric acute respiratory distress syndrome (P-ARDS): analysing 161 RSV-positive patients admitted to the Paediatric Intensive Care Unit (PICU), the authors observed 20% P-ARDS among these subjects, who were younger than those with a milder presentation and had comorbidities [81].
RSV can cause symptomatic reinfection throughout life, even in subjects with healthy and mature immune systems. These infections can appear every two or three years, with different clinical manifestations according to age: the older the subject is, the milder is the infection; in fact, data highlight that in healthy adults, RSV only causes the common cold or limited upper respiratory tract infection [82].
Although there is partial protection against a specific RSV strain, consistent and durable protection is never achieved [83]. Some authors report evidence that the medium- and long-term alteration in mucosal innate responses may lead to protective innate memory and inflammatory signals that are essential for adaptative memory; indeed, the modulation of innate and adaptative responses may be responsible for the ability of RSV to re-infect [83,84].
RSV has been reported to modify airways and lead to problems later in childhood and even in adulthood. Acute wheezing illnesses, which are extremely common in young children, have a viral root in more than 60% of cases [85]. RSV has been associated with recurrent wheezing episodes and/or asthma [86,87], and the risk of wheezing is higher in patients who had more severe RSV infections [88]. The link between RSV infection and asthma has been well studied, though not completely understood. Rossi and colleagues proposed a role of inducer, rather than a trigger, for RSV in predisposing an individual towards asthma [69]. Other authors have suggested that asthma-related genetic traits themselves might predispose a person with RSV infection towards severe disease [68,89,90,91].
5. Diagnosis of Respiratory Syncytial Virus (RSV)
There are no specific clinical signs or symptoms that can distinguish RSV infection from other respiratory pathogens. However, identifying the aetiology of infection can enhance antimicrobial stewardship and reduce individual antibiotic-related complications [92,93]. The possibility of using technological point-of-care tests appears extremely important in terms of cost savings and even time used to organize where to place patients, isolated or not isolated [94].
Before the advent of novel rapid molecular diagnostic assays, the following were strategies available for detecting RSV: (1) cell culture (which has historically been considered the gold standard before the development of other techniques); (2) reverse transcriptase polymerase chain reaction (RT-PCR), which has recently been considered the new gold standard due to its excellent sensitivity and specificity and rapid turnaround time; (3) direct immunofluorescence assays (DFAs); and (4) rapid antigen detection tests (RADTs). RADTs are further divided into: (a) traditional antigen detection tests, such as immunochromatographic tests, enzyme immunoassays, and optical immunoassays, which required an operator to interpret the presence/absence of a positive signal, leading to variability; and (b) newer automated immunoassays [93].
Some authors have reported a relevant difference in RSV rapid antigen test sensitivity according to the age of the patient: the younger the patient is, the more accurate the test is. Starting from more than 80% in children younger than 6 months, sensitivity decreased to 60% in the 24–35-month-old group [95,96]. New multiplex platforms offer the possibility to simultaneously identify different pathogens, with an accuracy similar to that of single PCR in the majority of cases [61,78] (Table 1). Once the virus in the patient sample is identified, clinicians must decide whether the virus is truly responsible for the disease. As the state of RSV asymptomatic carriage is relatively uncommon, when RSV is detected, it is likely to be the cause of the clinical manifestation [78]. Nonetheless, the reported indisputable positive effects of molecular tests have some limitations. Firstly, viral RNA can persist beyond the period of clinical significance, and thus tests can detect both viable and nonviable pathogens, creating difficulties in interpreting results. Secondly, the majority of the available tests produce positive or negative results, with no information about the cycle threshold. Moreover, low-complexity assays do not differentiate between RSV subtypes A and B and do not provide a quantitative result. The number of samples analysed together can also vary widely. Finally, for some less recent tests, it might take longer to obtain results [78,93,97,98].
Table 1.
Diagnostic strategies for RSV detection.
| Test | Turnaround Time | Sensitivity | Specificity | Note |
|---|---|---|---|---|
| Cell culture | Days | Low | Excellent | Traditional gold standard; long time for results |
| RT-PCR | Hours | Excellent | Excellent | New gold standard; expensive; needs expertise; positive results may not indicate active infection; possibility of multiplex tests |
| DFAs | <1 h | Low | Low | Needs expertise; rapid results |
| RADTs | Minutes | Low | Variable | Traditional tests depend on operator skills; newer platforms have better sensitivity/specificity |
| Novel rapid molecular diagnostic assays | Minutes–few hours | Excellent | Good | Variability; not detection of RSV types; qualitative but not quantitative results |
RT-PCR = reverse transcriptase polymerase chain reaction; DFAs = direct immunofluorescence assays; RADTs = rapid antigen detection tests.
One of the best biological samples on which to perform RSV analysis with molecular testing is a nasopharyngeal swab obtained by trained personnel [99]. Therefore, the majority of studies rely on this technique, though the Turi group reported the successful identification of urinary metabolites in patients with acute respiratory infections due to RSV [100].
6. Treatment of Respiratory Syncytial Virus (RSV) Infection
Currently, the treatment of RSV infection relies on supportive therapy, mainly consisting of oxygen and hydration [101,102]. RSV pneumonia and severe LRTI represent the condition for which a treatment may be necessary. However, there are two main important obstacles to the creation of specific therapeutic strategies for RSV. Firstly, the virus undergoes changes during replication, which allows it to escape antiviral therapies. Secondly, treatments should be targeted to a specific population of young children, as RSV infection in older children or adults can be milder or even asymptomatic [103]. Despite these difficulties, antiviral drugs against a specific target of the virus are currently under study, as follows: (1) nucleoside analogues; (2) RNA interference; (3) fusion inhibitors; and (4) immunoglobulins other than palivizumab [103,104,105]. Among the antivirals studied to date, ribavirin represents the only licensed drug for RSV treatment. Nonetheless, its use is extremely limited to selected cases due to its potential toxicity [106].
7. Respiratory Syncytial Virus (RSV) Prevention
RSV prevention strategies include passive and active immunization. The two major RSV antigens, the F and G proteins, are the only two proteins targeted by neutralizing antibodies [107,108]. At present, the only prophylactic available for RSV infection is palivizumab, a humanized monoclonal antibody directed against a conserved epitope on the RSV F fusion protein that is administered once monthly during the winter season in selected patients at high risk of RSV-related complications [103,109,110,111,112].
The Domachowske group reported their positive experience with another prophylactic strategy against RSV: MEDI8897, a recombinant monoclonal antibody against the RSV F protein that has shown greater neutralizing activity and a longer serum half-life than palivizumab when administered in a single fixed dose to preterm infants (born between 32 and 34 weeks), for whom palivizumab was not recommended [113].
With regard to the development of vaccines, there are problems due to the following reasons: the target population, which is not easy to test; difficulties of transport evidence derived from adult studies, in whom RSV disease is different; and the capacity of RSV to evade the immune response. [64,73,114,115]. The history of vaccines dates backs to the 1960s, when a formalin-inactivated alum-adjuvated RSV vaccine (FI-RSV) administered in young (<2 years old) RSV-seronegative children resulted in enhanced respiratory disease during a subsequent natural RSV infection [83,107,116,117]. Recent advances in understanding RSV F protein structure and instability as well as improvements in technology have provided new targets for vaccines. Currently, there are approximately 60 vaccine candidates, some of which are at a good clinical phase. They can be divided into the following classes: (1) live attenuated vaccines, (2) particle-based vaccines, (3) subunit-based vaccines, and (4) vector-based vaccines [73,118,119]. Among these candidate vaccines are also ones for maternal immunization, which have been driven by the increasing number of countries that currently adopt vaccination in pregnant women against influenza and pertussis [73,120,121,122]. Vaccination against RSV during pregnancy may present two main benefits: (1) to protect mothers against this infection and its potential adverse birth outcomes and (2) to increase transplacental RSV-specific antibodies to protect the infant in the first months of life [37]. The Shi group estimated that achieving protection of 80% due to maternal immunization and prophylaxis in the first 6 months of life may prevent up to 1.1 million hospitalizations and 22,000 in-hospital deaths globally due to RSV [15].
Another possibility in terms of both RSV infection prevention and treatment that is currently being explored is the focus on potential host factor targets, such as RSV receptors, co-receptors, intracellular adhesion molecule, Toll-like receptor 4, and nucleolin [106,123,124,125].
At present, we found 23 active clinical trials on RSV prophylaxis and treatment, 10 of which are now in the recruitment phase. Of these, 2 are observational, multicentre studies, 4 are evaluating new vaccines (three in phase 1 and one in phase 2), 1 involves an RSV F protein inhibitor administered orally (RV521), and 3 are related to monoclonal antibodies (two, in phase 1 and 2, of the already mentioned MDI8897, and one involving MK-1654 in phase 2) [126].
8. Conclusions
RSV represents an insidious burden, especially for younger children, with a high mortality rate and a high percentage of serious infection and sequelae. Much progress has been made in understanding its mode of entry and interaction with the human host and its ability to cause disease. Advances in technology and scientific knowledge have led to the development of different kinds of vaccines and drugs to treat RSV infection. Despite the good level of some studies (that are now at phase 3 of clinical trials), there are still few registered strategies to prevent or treat RSV due to difficulties related to the unpredictable nature of the disease and the specific target population. However, it is highly likely that in the near future new preventive and therapeutic strategies against RSV will appear in the market, reducing its clinical and socio-economic impact.
Author Contributions
S.B. wrote the first draft of the manuscript; E.S. and A.A. performed the literature review; V.F. and G.P. gave a substantial scientific contribution; S.E. supervised the project, gave a scientific contribution and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript was supported by a grant from the University of Perugia and Parma University Hospital, Italy.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heinonen S., Rodriguez-Fernandez R., Diaz A., Rodriguez-Pastor S.O., Ramilo O., Mejias A. Infant immune response to respiratory viral infections. Immunol. Allergy Clin. N. Am. 2019;39:361–376. doi: 10.1016/j.iac.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J., Liu C., Xiao Y., Xiang Z., Zhou H., Chen L., Shen K., Xie Z., Ren L., Wang J. Respiratory syncytial virus seasonality, Beijing, China, 2007–2015. Emerg. Infect. Dis. 2019;25:1127–1135. doi: 10.3201/eid2506.180532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C., Stockmann C., Anderson E.J., Grijalva C.G., Self W.H., et al. Community-Acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockman L.J., Curns A.T., Anderson L.J., Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatrics Infect. Dis. J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 6.Esposito S., Bianchini S., Argentiero A., Neglia C., Principi N. How does one choose the appropriate pharmacotherapy for children with lower respiratory tract infections? Expert Opin. Pharmacother. 2020;21:1–9. doi: 10.1080/14656566.2020.1781091. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Pneumonia. [(accessed on 7 September 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia.
- 8.Taylor S.M., Lopez P., Weckx L., Borja-Tabora C., Ulloa-Gutierrez R., Lazcano-Ponce E., Kerdpanich A., Weber M.A.R., Santos A.M.D.L., Tinoco J.-C., et al. Respiratory viruses and influenza-like illness: Epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J. Infect. 2017;74:29–41. doi: 10.1016/j.jinf.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavia A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect. Dis. 2011;52:S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rey-Jurado E., Kalergis A.M. Immunological features of respiratory syncytial virus-caused pneumonia—Implications for vaccine design. Int. J. Mol. Sci. 2017;18:556. doi: 10.3390/ijms18030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosis S., Esposito S., Niesters H.G.M., Zuccotti G.V., Marseglia G., Lanari M., Zuin G., Pelucchi C., Osterhaus A.D.M.E., Principi N. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin. Microbiol. Infect. 2008;14:677–684. doi: 10.1111/j.1469-0691.2008.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taleb S.A., Al Thani A.A., Al Ansari K., Yassine H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1817–1827. doi: 10.1007/s10096-018-3289-4. [DOI] [PubMed] [Google Scholar]
- 13.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization WHO Strategy to Pilot Global Respiratory Syncytial Virus Surveillance Based on the Global Influenza Surveillance and Response System (GISRS) [(accessed on 7 September 2020)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/259853/9789241513203-eng.pdf;jsessionid=876497B8465C841C0AA1F4A9F6F8F95D?sequence=1.
- 15.Histoshi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/s0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rath B., Conrad T., Myles P., Alchikh M., Ma X., Hoppe C., Tief F., Chen X., Obermeier P., Kisler B., et al. Influenza and other respiratory viruses: Standardizing disease severity in surveillance and clinical trials. Expert Rev. Anti-Infect. Ther. 2017;15:545–568. doi: 10.1080/14787210.2017.1295847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgson D., Pebody R., Panovska-Griffiths J., Baguelin M., Atkins K.E. Evaluating the next generation of RSV intervention strategies: A mathematical modelling study and cost-effectiveness analysis. BMC Med. 2020;18:348. doi: 10.1186/s12916-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korsun N., Angelova S., Tzotcheva I., Georgieva I., Lazova S., Parina S., Alexiev I., Perenovska P. Prevalence and genetic characterisation of respiratory syncytial viruses circulating in Bulgaria during the 2014/15 and 2015/16 winter seasons. Pathog. Glob. Health. 2017;111:351–361. doi: 10.1080/20477724.2017.1375708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baymakova M. Fever of unknown origin in a bulgarian hospital: Evaluation of 54 cases for a four year-period. J. Clin. Anal. Med. 2016;7:70–75. doi: 10.4328/JCAM.3897. [DOI] [Google Scholar]
- 20.Korsun N., Angelova S., Trifonova I., Voleva S., Grigorova I., Tzotcheva I., Mileva S., Alexiev I., Perenovska P. Predominance of ON1 and BA9 genotypes of Respiratory Syncytial Virus (RSV) in Bulgaria, 2016–2018. J. Med. Virol. 2020;11 doi: 10.1002/jmv.26415. [DOI] [PubMed] [Google Scholar]
- 21.Pavlova S., Hadzhiolova T., Abadjieva P., Kotseva R. Application of RT-PCR for diagnosis of respiratory syncytial virus and human metapneumovirus infections in Bulgaria, 2006–7 and 2007–8. Eurosurveillance. 2009;14:19233. doi: 10.2807/ese.14.23.19233-en. [DOI] [PubMed] [Google Scholar]
- 22.Dimova-Yaneva D.N., Helms P.J. The role of leukotrienes and eosinophil cationic protein in acute respiratory syncytial virus bronchiolitis. Folia Med. 2003;45:5–11. [PubMed] [Google Scholar]
- 23.Stein R.T., Bont L.J., Zar H., Polack F.P., Park C., Claxton A., Borok G., Butylkova Y., Wegzyn C.M. Respiratory syncytial virus hospitalization and mortality: Systematic review and meta-analysis. Pediatrics Pulmonol. 2016;52:556–569. doi: 10.1002/ppul.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanken M.O., Rovers M.M., Molenaar J.M., Winkler-Seinstra P.L., Meijer A., Kimpen J.L., Bont L., Dutch RSV Neonatal Network Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 25.Feddema J.J., Claassen E. Prevalence of viral respiratory infections amongst asthmatics: Results of a meta-regression analysis. Respir. Med. 2020;173:106020. doi: 10.1016/j.rmed.2020.106020. [DOI] [PubMed] [Google Scholar]
- 26.Kitcharoensakkul M., Bacharier L.B., Schweiger T.L., Wilson B., Goss C.W., Lew D., Schechtman K.B., Castro M. Lung function trajectories and bronchial hyperresponsiveness during childhood following severe RSV bronchiolitis in infancy. Pediatrics Allergy Immunol. 2020 doi: 10.1111/pai.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigelman A., Bacharier L.B. The role of early life viral bronchiolitis in the inception of asthma. Curr. Opin. Allergy Clin. Immunol. 2013;13:211–216. doi: 10.1097/ACI.0b013e32835eb6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim A., Butt M.L., Dix J., Elliott L., Paes B. Respiratory syncytial virus (RSV) infection in children with medical complexity. Eur. J. Clin. Microbiol. Infect. Dis. 2018;38:171–176. doi: 10.1007/s10096-018-3409-1. [DOI] [PubMed] [Google Scholar]
- 29.Bont L., Checchia P.A., Fauroux B., Figueras-Aloy J., Manzoni P., Paes B., Simões E.A.F., Carbonell-Estrany X. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect. Dis. Ther. 2016;5:271–298. doi: 10.1007/s40121-016-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y., Hua J., Wang D., Chen L., Zhang J., Zhu H., Tian J., Zhang T., Zhao G. Risk factors of respiratory syncytial virus infection among pediatric influenza-like illness and severe acute respiratory infections in Suzhou, China. J. Med. Virol. 2018;90:397–404. doi: 10.1002/jmv.24961. [DOI] [PubMed] [Google Scholar]
- 31.Hall C.B. Respiratory syncytial virus in young children. Lancet. 2010;375:1500–1502. doi: 10.1016/S0140-6736(10)60401-1. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths C., Drews S.J., Marchant D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017;30:277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazur N.I., Martinón-Torres F., Baraldi E., Fauroux B., Greenough A., Heikkinen T., Manzoni P., Mejias A., Nair H., Papadopoulos N.G., et al. Lower respiratory tract infection caused by respiratory syncytial virus: Current management and new therapeutics. Lancet Respir. Med. 2015;3:888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 34.Green C.A., Sande C., De Lara C., Thompson A., Silva-Reyes L., Napolitano F., Pierantoni A., Capone S., Vitelli A., Klenerman P., et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine. 2018;36:6183–6190. doi: 10.1016/j.vaccine.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 35.Beckhaus A.A., Castro-Rodriguez J.A. Down Syndrome and the Risk of Severe RSV Infection: A Meta-analysis. Pediatrics. 2018;142:e20180225. doi: 10.1542/peds.2018-0225. [DOI] [PubMed] [Google Scholar]
- 36.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J. Glob. Health. 2015;5:010408. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray A., Chu H.Y. RSV, antibodies and the developing world. Pediatrics Infect. Dis. J. 2019;38:S24–S27. doi: 10.1097/INF.0000000000002333. [DOI] [PubMed] [Google Scholar]
- 38.Cohen E., Kuo D.Z., Agrawal R., Berry J.G., Bhagat S.K.M., Simon T.D., Srivastava R. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leyenaar J.K., Lagu T., Shieh M.-S., Pekow P.S., Lindenauer P.K. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr. Infect. Dis. J. 2014;33:907–911. doi: 10.1097/INF.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manzoni P., Figueras-Aloy J., Simões E.A.F., Checchia P.A., Fauroux B., Bont L., Paes B., Carbonell-Estrany X. Defining the incidence and associated morbidity and mortality of severe respiratory syncytial virus infection among children with chronic diseases. Infect. Dis. Ther. 2017;6:383–411. doi: 10.1007/s40121-017-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray J., Bottle A., Sharland M., Modi N., Aylin P., Majeed A., Saxena S., On Behalf of the Medicines for Neonates Investigator Group Risk factors for hospital admission with RSV bronchiolitis in England: A population-based birth cohort study. PLoS ONE. 2014;9:e89186. doi: 10.1371/journal.pone.0089186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization WHO RSV Surveillance Phase-2 (2019–2021) [(accessed on 7 September 2020)]; Available online: https://www.who.int/influenza/rsv/RSV_surveillance_phase2/en/
- 43.Lam T.T., Tang J.W., Lai F.Y., Zaraket H., Dbaibo G., Bialasiewicz S., Tozer S., Heraud J.-M., Drews S.J., Hachette T., et al. Comparative global epidemiology of influenza, respiratory syncytial and parainfluenza viruses, 2010–2015. J. Infect. 2019;79:373–382. doi: 10.1016/j.jinf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A., O’Brien K.L., Campbell H., Black R.E. Global burden of childhood pneumonia and diarrhea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of under-5 mortality in 2000–2015: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prayle A.P., Atkinson M., Smyth A.R. Pneumonia in the developed world. Paediatr. Respir. Rev. 2011;12:60–69. doi: 10.1016/j.prrv.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Berg A.S., Inchley C.S., Aase A., Fjaerli H.O., Bull R., Aaberge I., Leegaard T.M., Nakstad B. Etiology of pneumonia in a pediatric population with high pneumococcal vaccine coverage: A prospective study. Pediatrics Infect. Dis. J. 2016;35:e69–e75. doi: 10.1097/INF.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 48.Tian D.-D., Jiang R., Chen X.-J., Ye Q. Meteorological factors on the incidence of MP and RSV pneumonia in children. PLoS ONE. 2017;12:e0173409. doi: 10.1371/journal.pone.0173409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du Prel J., Puppe W., Gröndahl B., Knuf M., Weigl J.A.I., Schaaff F., Schmitt H. Are meteorological parameters associated with acute respiratory tract infections? Clin. Infect. Dis. 2009;49:861–868. doi: 10.1086/605435. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien K.L., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Higdon M.M., Howie S.R., Knoll M.D., Kotloff K.L., Levine O.S., et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine O.S., O’Brien K.L., Deloria-Knoll M., Murdoch D.R., Feikin D.R., DeLuca A.N., Driscoll A.J., Baggett H.C., Brooks W.A., Howie S.R.C., et al. The pneumonia etiology research for child health project: A 21st century childhood pneumonia etiology study. Clin. Infect. Dis. 2012;54:S93–S101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott J.A.G., Wonodi C., Moïsi J.C., Deloria-Knoll M., DeLuca A.N., Karron R.A., Bhat N., Murdoch D.R., Crawley J., Levine O.S., et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clin. Infect. Dis. 2012;54:S109–S116. doi: 10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knoll M.D., Feikin D.R., Scott J.A.G., O’Brien K.L., DeLuca A., Driscoll A.J., Levine O.S., The Pneumonia Methods Working Group Identification and selection of cases and controls in the pneumonia etiology research for child health project. Clin. Infect. Dis. 2012;54:S117–S123. doi: 10.1093/cid/cir1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higdon M.M., Le T., O’Brien K.L., Murdoch D.R., Prosperi C., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Howie S.R.C., et al. Association of C-reactive protein with bacterial and respiratory syncytial virus-associated pneumonia among children aged <5 years in the PERCH Study. Clin. Infect. Dis. 2017;64:S378–S386. doi: 10.1093/cid/cix150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bénet T., Picot V.S., Messaoudi M., Chou M., Eap T., Wang J., Shen K., Pape J.-W., Rouzier V., Awasthi S., et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: The GABRIEL Pneumonia Multicenter, prospective, case-control study. Clin. Infect. Dis. 2017;65:604–612. doi: 10.1093/cid/cix378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhuiyan M.U., Snelling T.L., West R., Lang J., Rahman T., Granland C., De Gier C., Borland M.L., Thornton R.B., Kirkham L.-A.S., et al. The contribution of viruses and bacteria to community-acquired pneumonia in vaccinated children: A case-control study. Thorax. 2019;74:261–269. doi: 10.1136/thoraxjnl-2018-212096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nascimento-Carvalho A.C., Vilas-Boas A.-L., Fontoura M.-S.H., Vuorinen T., Nascimento-Carvalho C.M., PNEUMOPAC-Efficacy Study Group Respiratory viruses among children with non-severe community-acquired pneumonia: A prospective cohort study. J. Clin. Virol. 2018;105:77–83. doi: 10.1016/j.jcv.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khuri-Bulos N., Lawrence L., Piya B., Wang L., Fonnesbeck C., Faouri S., Shehabi A., Vermund S.H., Williams J.V., Halasa N.B. Severe outcomes associated with respiratory viruses in newborns and infants: A prospective viral surveillance study in Jordan. BMJ Open. 2018;8:e021898. doi: 10.1136/bmjopen-2018-021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Espinoza J.A., Moreno-Valencia Y., Coronel-Tellez R.H., Torres-Espíndola L.M., Hernández A., Dominguez A., Miliar-García A., Barbachano-Guerrero A., Perez-Padilla R., Alejandre-Garcia A., et al. Virome and bacteriome characterization of children with pneumonia and asthma in Mexico City during winter seasons 2014 and 2015. PLoS ONE. 2018;13:e0192878. doi: 10.1371/journal.pone.0192878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ching N.S., Kotsanas D., Easton M.L., Francis M.J., Korman T.M., Buttery J.P. Respiratory virus detection and co-infection in children and adults in a large Australian hospital in 2009–2015. J. Paediatr. Child. Health. 2018;54:1321–1328. doi: 10.1111/jpc.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esposito S., Piralla A., Zampiero A., Bianchini S., Di Pietro G., Scala A., Pinzani R., Fossali E., Baldanti F., Principi N. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in northern Italy in five consecutive winter seasons. PLoS ONE. 2015;10:e0129369. doi: 10.1371/journal.pone.0129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kazakova A., Kakkola L., Päkkilä H., Teros-Jaakkola T., Soukka T., Peltola V., Waris M., Julkunen I. Serological array-in-well multiplex assay reveals a high rate of respiratory virus infections and reinfections in young children. mSphere. 2019;4:e00447-19. doi: 10.1128/mSphere.00447-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A., Auinger P., Griffin M.R., Poehling K.A., Erdman D., et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017;23:107–112. doi: 10.1016/j.coviro.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welliver T.P., Garofalo R.P., Hosakote Y., Hintz K.H., Avendano L., Sanchez K., Velozo L., Jafri H., Chavez-Bueno S., Ogra P.L., et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villenave R., Thavagnanam S., Sarlang S., Parker J., Douglas I., Skibinski G., Heaney L.G., McKaigue J.P., Coyle P.V., Shields M.D., et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. USA. 2012;109:5040–5045. doi: 10.1073/pnas.1110203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teng M.N., Whitehead S.S., Collins P.L. Contribution of the respiratory syncytial virus g glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology. 2001;289:283–296. doi: 10.1006/viro.2001.1138. [DOI] [PubMed] [Google Scholar]
- 68.Lay M.K., González P.A., León M.A., Céspedes P.F., Bueno S.M., Riedel C.A., Kalergis A.M. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 2013;15:230–242. doi: 10.1016/j.micinf.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Rossi G.A., Colin A.A. Infantile respiratory syncytial virus and human rhinovirus infections: Respective role in inception and persistence of wheezing. Eur. Respir. J. 2014;45:774–789. doi: 10.1183/09031936.00062714. [DOI] [PubMed] [Google Scholar]
- 70.Guo-Parke H., Canning P., Douglas I., Villenave R., Heaney L.G., Coyle P.V., Lyons J.D., Shields M.D., Power U.F. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 2013;188:842–851. doi: 10.1164/rccm.201304-0750OC. [DOI] [PubMed] [Google Scholar]
- 71.Legg J.P., Hussain I.R., Warner J.A., Johnston S.L., Warner J.O. Type 1 and Type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2003;168:633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 72.Johnson J.E., A Gonzales R., Olson S.J., Wright P.F., Graham B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 73.Noor A., Krilov L.R. Respiratory syncytial virus vaccine: Where are we now and what comes next? Expert Opin. Biol. Ther. 2018;18:1247–1256. doi: 10.1080/14712598.2018.1544239. [DOI] [PubMed] [Google Scholar]
- 74.González-Parra G., Dobrovolny H.M. A quantitative assessment of dynamical differences of RSV infections in vitro and in vivo. Virology. 2018;523:129–139. doi: 10.1016/j.virol.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Roe M., O’Donnell D., Callif C.G. Respiratory viruses in the intensive care unit. Paediatr. Respir. Rev. 2003;4:166–171. doi: 10.1016/S1526-0542(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 76.Cherian T., Mulholland E.K., Carlin J.B., Ostensen H., Amin R., De Campo M., Greenberg D., Lagos R., Lucero M., Madhi S.A., et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 77.Fancourt N., Knoll M.D., Barger-Kamate B., De Campo J., De Campo M., Diallo M., Ebruke B.E., Feikin D.R., Gleeson F., Gong W., et al. standardized interpretation of chest radiographs in cases of pediatric pneumonia from the PERCH study. Clin. Infect. Dis. 2017;64:S253–S261. doi: 10.1093/cid/cix082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esposito S., Mencacci A., Cenci E., Camilloni B., Silvestri E., Principi N. Multiplex platforms for the identification of respiratory pathogens: Are they useful in pediatric clinical practice? Front. Cell. Infect. Microbiol. 2019;9:196. doi: 10.3389/fcimb.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez-Fernandez R., Tapia L.I., Yang C.-F., Torres J.P., Chavez-Bueno S., Garcia C., Jaramillo L.M., Moore-Clingenpeel M., Jafri H.S., Peeples M.E., et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J. Infect. Dis. 2018;217:24–34. doi: 10.1093/infdis/jix543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi T., Balsells E., Wastnedge E., Singleton R., Rasmussen Z.A., Zar H.J., Rath B.A., Madhi S.A., Campbell S., Vaccari L.C., et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta–analysis. J. Glob. Health. 2015;5:020416. doi: 10.7189/jogh.05.020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ravindranath T.M., Gomez A., Harwayne-Gidansky I., Connors T.J., Neill N., Levin B., Howell J.D., Saiman L., Baird J.S. Pediatric acute respiratory distress syndrome associated with human metapneumovirus and respiratory syncytial virus. Pediatrics Pulmonol. 2018;53:929–935. doi: 10.1002/ppul.24044. [DOI] [PubMed] [Google Scholar]
- 82.Agoti C.N., Mwihuri A.G., Sande C.J., Onyango C.O., Medley G.F., Cane P.A., Nokes D.J. Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J. Infect. Dis. 2012;206:1532–1541. doi: 10.1093/infdis/jis570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Openshaw P.J., Chiu C., Culley F.J., Johansson C. Protective and harmful immunity to RSV infection. Annu. Rev. Immunol. 2017;35:501–532. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 84.Netea M.G., Joosten L.A.B., Latz E., Mills K.H.G., Natoli G., Stunnenberg H.G., O’Neill L.A.J., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kusel M.M., Kebadze T., Johnston S.L., Holt P.G., Sly P.D. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur. Respir. J. 2011;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 86.Coverstone A.M., Wang L., Sumino K. Beyond respiratory syncytial virus and rhinovirus in the pathogenesis and exacerbation of asthma: The role of metapneumovirus, bocavirus and influenza virus. Immunol. Allergy Clin. N. Am. 2019;39:391–401. doi: 10.1016/j.iac.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacharier L.B., Cohen R., Schweiger T., Yin-DeClue H., Christie C., Zheng J., Schechtman K.B., Strunk R.C., Castro M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012;130:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sigurs N., AlJassim F., Kjellman B., Robinson P.D., Sigurbergsson F., Bjarnason R., Gustafsson P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 89.Mohapatra S.S., Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin. Microbiol. Rev. 2008;21:495–504. doi: 10.1128/CMR.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daley D., Park J.E., He J.-Q., Yan J., Akhabir L., Stefanowicz D., Becker A., Chan-Yeung M., Bossé Y., Kozyrskyj A.L., et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J. Allergy Clin. Immunol. 2012;130:1284–1293. doi: 10.1016/j.jaci.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 91.James K.M., Peebles R.S., Jr., Hartert T.V. Response to infections in patients with asthma and atopic disease: An epiphenomenon or reflection of host susceptibility? J. Allergy Clin. Immunol. 2012;130:343–351. doi: 10.1016/j.jaci.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferronato Â.E., Gilio A.E., Ferraro A.A., De Paulis M., Vieira S.E. Etiological diagnosis reduces the use of antibiotics in infants with bronchiolitis. Clinics. 2012;67:1007–1011. doi: 10.6061/clinics/2012(09)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hogan C.A., Caya C., Papenburg J. Rapid and simple molecular tests for the detection of respiratory syncytial virus: A review. Expert Rev. Mol. Diagn. 2018;18:617–629. doi: 10.1080/14737159.2018.1487293. [DOI] [PubMed] [Google Scholar]
- 94.Mahony J.B., Blackhouse G., Babwah J., Smieja M., Buracond S., Chong S., Ciccotelli W., O’Shea T., Alnakhli D., Griffiths-Turner M., et al. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J. Clin. Microbiol. 2009;47:2812–2817. doi: 10.1128/JCM.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chartrand C., Tremblay N., Renaud C., Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: Systematic review and meta-analysis. J. Clin. Microbiol. 2015;53:3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papenburg J., Buckeridge D.L., De Serres G., Boivin G. Host and viral factors affecting clinical performance of a rapid diagnostic test for respiratory syncytial virus in hospitalized children. J. Pediatrics. 2013;163:911–913. doi: 10.1016/j.jpeds.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 97.Chan M., Koo S.H., Jiang B., Lim P.Q., Tan T.-Y. Comparison of the biofire filmarray respiratory panel, seegene AnyplexII RV16, and Argene for the detection of respiratory viruses. J. Clin. Virol. 2018;106:13–17. doi: 10.1016/j.jcv.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beckmann C., Hirsch H.H. Comparing luminex NxTAG-respiratory pathogen panel and respifinder-22 for multiplex detection of respiratory pathogens. J. Med. Virol. 2016;88:1319–1324. doi: 10.1002/jmv.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moe N., Pedersen B., Nordbø S.A., Skanke L.H., Krokstad S., Smyrnaios A., Døllner H. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS ONE. 2016;11:e0159196. doi: 10.1371/journal.pone.0159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turi K.N., Romick-Rosendale L., Gebretsadik T., Watanabe M., Brunwasser S., Anderson L.J., Moore M.L., Larkin E.K., Peebles R.S., Hartert T.V. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics. 2018;14:135. doi: 10.1007/s11306-018-1431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barr R., Green C.A., Sande C.J., Drysdale S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect. Dis. 2019;6 doi: 10.1177/2049936119865798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.National Institute for Health and Care Excellence Bronchiolitis in Children: Diagnosis and Management. NICE Guideline [NG9] [(accessed on 7 September 2020)];2015 Available online: https://www.nice.org.uk/guidance/NG9. [PubMed]
- 103.Xing Y., Proesmans M. New therapies for acute RSV infections: Where are we? Eur. J. Pediatrics. 2019;178:131–138. doi: 10.1007/s00431-018-03310-7. [DOI] [PubMed] [Google Scholar]
- 104.Simões E.A.F., DeVincenzo J.P., Boeckh M., Bont L., Crowe J.E., Griffiths P., Hayden F.G., Hodinka R.L., Smyth R.L., Spencer K., et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J. Infect. Dis. 2015;211:S1–S20. doi: 10.1093/infdis/jiu828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jorquera P.A., Tripp R.A. Respiratory syncytial virus: Prospects for new and emerging therapeutics. Expert Rev. Respir. Med. 2017;11:609–615. doi: 10.1080/17476348.2017.1338567. [DOI] [PubMed] [Google Scholar]
- 106.Heylen E., Neyts J., Jochmans D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem. Pharmacol. 2017;127:1–12. doi: 10.1016/j.bcp.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 107.Ke Z., Dillard R.S., Chirkova T., Leon F., Stobart C.C., Hampton C.M., Strauss J.D., Rajan D., Rostad C.A., Taylor J.V., et al. The morphology and assembly of respiratory syncytial virus revealed by cryo-electron tomography. Viruses. 2018;10:446. doi: 10.3390/v10080446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Graham B.S., Modjarrad K., McLellan J.S. Novel antigens for RSV vaccines. Curr. Opin. Immunol. 2015;35:30–38. doi: 10.1016/j.coi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ralston S.L., Lieberthal A.S., Meissner H.C., Alverson B.K., Baley J.E., Gadomski A.M., Johnson D.W., Light M.J., Maraqa N.F., Mendonca E.A., et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 110.Walpert A.S., Thomas I.D., Lowe M.C., Jr., Seckeler M.D. RSV prophylaxis guideline changes and outcomes in children with congenital heart disease. Congenit. Heart Dis. 2018;13:428–431. doi: 10.1111/chd.12590. [DOI] [PubMed] [Google Scholar]
- 111.Blake S.M., Tanaka D., Bendz L.M., Staebler S., Brandon D. Evaluation of the financial and health burden of infants at risk for respiratory syncytial virus. Adv. Neonatal Care. 2017;17:292–298. doi: 10.1097/ANC.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 112.Ambrose C.S., Chen X., Kumar V.R. A population-weighted, condition-adjusted estimate of palivizumab efficacy in preventing RSV-related hospitalizations among US high-risk children. Hum. Vaccines Immunother. 2014;10:2785–2788. doi: 10.4161/hv.32082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Domachowske J.B., Khan A.A., Esser M.T., Jensen K., Takas T., Villafana T., Dubovsky F., Griffin M.P. Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatrics Infect. Dis. J. 2018;37:886–892. doi: 10.1097/INF.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bianchini S., Argentiero A., Camilloni B., Silvestri E., Alunno A., Esposito S. Vaccination against paediatric respiratory pathogens. Vaccines. 2019;7:168. doi: 10.3390/vaccines7040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Esposito S., Scarselli E., Lelii M., Scala A., Vitelli A., Capone S., Fornili M., Biganzoli E., Orenti A., Nicosia A., et al. Antibody response to respiratory syncytial virus infection in children <18 months old. Hum. Vaccines Immunother. 2016;12:1700–1706. doi: 10.1080/21645515.2016.1145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rostad C.A., Stobart C.C., Gilbert B.E., Pickles R.J., Hotard A.L., Meng J., Blanco J.C.G., Moin S.M., Graham B.S., Piedra P.A., et al. A recombinant respiratory syncytial virus vaccine candidate attenuated by a low-fusion F protein is immunogenic and protective against challenge in cotton rats. J. Virol. 2016;90:7508–7518. doi: 10.1128/JVI.00012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Melero J.A., Mas V., McLellan J.S. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine. 2017;35:461–468. doi: 10.1016/j.vaccine.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazur N.I., Higgins D., Nunes M.C., Melero J.A., Langedijk A.C., Horsley N., Buchholz U.J., Openshaw P.J., McLellan J.S., A Englund J., et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018;18:e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 119.Crank M.C., Ruckwardt T.J., Chen M., Morabito K.M., Phung E., Costner P.J., Holman L.A., Hickman S.P., Berkowitz N.M., Gordon I.J., et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365:505–509. doi: 10.1126/science.aav9033. [DOI] [PubMed] [Google Scholar]
- 120.Giersing B.K., Modjarrad K., Kaslow D.C., Moorthy V.S., WHO Product Development for Vaccines Advisory Committee. WHO Product Development for Vaccines Product Development Advisory Committee Report from the World Health Organization’s Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7–9th September 2015. Vaccine. 2016;34:2865–2869. doi: 10.1016/j.vaccine.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abu-Raya B., Maertens K., Edwards K.M., Omer S.B., Englund J.A., Flanagan K.L., Snape M.D., Amirthalingam G., Leuridan E., Van Damme P., et al. Global perspectives on immunization during pregnancy and priorities for future research and development: An international consensus statement. Front. Immunol. 2020;11:1282. doi: 10.3389/fimmu.2020.01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Esposito S., Principi N. Strategies to develop vaccines of pediatric interest. Expert Rev. Vaccines. 2017:175–186. doi: 10.1080/14760584.2017.1237875. [DOI] [PubMed] [Google Scholar]
- 123.Chirkova T., Lin S., Oomens A.G.P., Gaston K.A., Boyoglu-Barnum S., Meng J., Stobart C.C., Cotton C.U., Hartert T.V., Moore M.L., et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015;96:2543–2556. doi: 10.1099/vir.0.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marr N., Turvey S.E. Role of human TLR4 in respiratory syncytial virus-induced NF-κB activation, viral entry and replication. Innate Immun. 2012;18:856–865. doi: 10.1177/1753425912444479. [DOI] [PubMed] [Google Scholar]
- 125.Tayyari F., Marchant D.R., Moraes T.J., Duan W., Mastrangelo P., Hegele R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 126.Clinical Trials Gov RSV Infection. [(accessed on 7 September 2020)]; Available online: https://clinicaltrials.gov/ct2/results?cond=RSV+Infection&Search=Apply&recrs=a&age_v=&age=0&gndr=&type=&rslt=