Abstract
Personalized medicine strives to optimize drug treatment for the individual patient by taking into account both genetic and non-genetic factors for drug response. Inflammation is one of the non-genetic factors that has been shown to greatly affect the metabolism of drugs—primarily through inhibition of cytochrome P450 (CYP450) drug-metabolizing enzymes—and hence contribute to the mismatch between the genotype predicted drug response and the actual phenotype, a phenomenon called phenoconversion. This review focuses on inflammation-induced drug metabolism alterations. In particular, we discuss the evidence assembled through human in-vitro models on the effect of inflammatory mediators on clinically relevant CYP450 isoform levels and their metabolizing capacity. We also present an overview of the current understanding of the mechanistic pathways via which inflammation in hepatocytes may modulate hepatic functions that are critical for drug metabolism. Furthermore, since large inter-individual variability in response to inflammation is observed in human in-vitro models and clinical studies, we evaluate the potential role of pharmacogenetic variability in the inflammatory signaling cascade and how this can modulate the outcome of inflammation on drug metabolism and response.
Keywords: cytochrome P-450 enzyme system, drug metabolism, hepatocytes, inflammation, inter-individual variability, pharmacogenomics, phenoconversion, phenotype, pregnane X receptor
1. Introduction
The clinical outcome of drug treatments can vary greatly between individuals and even within the same individual. Consequently, certain patients may (suddenly) experience reduced efficacy or exhibit an increased risk for developing adverse events [1]. While part of this variability can be explained by genetic variations in drug-metabolizing enzymes (DMEs)—mainly stemming from the cytochrome P450 (CYP) enzyme family—other non-genetic factors may also greatly contribute to the observed variability in drug response [2].
Inflammation or disease state is shown to have major effects on the metabolism of drugs through downregulation of CYP enzymes and hence contribute to the mismatch between the genotype predicted drug response and the actual phenotype—a phenomenon better known as phenoconversion [3]. However, the impact of inflammation-induced phenoconversion may differ greatly between individual patients and can be dependent on multiple factors. First, the degree of inflammation can significantly influence the extent of CYP suppression [4]. Indeed, signature markers of inflammation are often inversely correlated with drug metabolism [5,6]. Secondly, the type of inflammation or cytokine profile is an important determinant in the effect of inflammation on drug metabolism. Evidently, interleukin-targeting biologics have shown cytokine-specific successes in reversing the repressing effects of inflammatory cytokines towards CYP proteins [7,8]. Thirdly, the extent of inflammation-induced phenoconversion might be dependent on the metabolic pathway of a drug since inflammation is shown to downregulate CYP activities in an isoform-specific manner [9]. Lastly, since drug metabolism is also greatly dependent on genetic variability this might be a fourth factor that alters the extent of inflammation-induced phenoconversion in patients [2,10].
To personalize and optimize drug treatments, a better understanding is needed of how inflammation affects pharmacokinetic behavior and clinical effectiveness of drugs. One major hurdle is that the specific effects of inflammation on pharmacokinetics cannot be easily assessed with in-vivo studies, due to the presence of many interfering covariables (e.g., age, genetic backgrounds, kidney function, co-medication). Therefore, in-vitro liver models may be valuable tools to elucidate the specific effects of inflammation on drug metabolism. Earlier studies with in-vitro models have already demonstrated that various inflammatory mediators associated with inflammation and infection can modulate drug metabolism by reducing the expression of CYPs [2,3,11,12]. However, since the effect of inflammation-induced phenoconversion depends on the degree and type of inflammation as well as the metabolic pathway of the drug, it is necessary to better understand the different effects of various pro-inflammatory mediators and focus on the differential sensitivity between CYP isoforms in response to them.
The aim of this literature review is therefore to (1) summarize and update the available evidence on the effects of inflammatory stimuli on CYP expression levels and activity in human in-vitro liver models, with a specific focus on type of inflammation and metabolic pathway of the drug. (2) Provide an overview of our current understanding of the mechanistic pathways via which inflammation in hepatocytes modulates hepatic functions (e.g., transcription factors, enzymes, nuclear receptors) that are critical for drug metabolism. (3) Define how genetic variation in these defined mechanistic pathways may modulate the effect of inflammation on drug metabolism and drug response.
2. Effects of Inflammatory Stimuli on CYP Expression Levels and Activity in Human In-Vitro Liver Models
Experimental laboratory studies have been instrumental for our understanding of how inflammation may modulate drug metabolism in the clinic. Through the use of in-vitro hepatocyte models, researchers have investigated which inflammatory mediators can be held responsible for the observed changes in drug metabolism. These studies primarily emphasize the effects of inflammatory stimuli on either the mRNA expression of the major CYPs responsible for drug metabolism or the actual metabolism of probe substrates for these CYPs. Since DMEs show substantial interspecies differences in terms of metabolizing activity and isoform composition, rodent data may not be useful in extrapolation to the clinic [13]. Therefore, we describe the effects of inflammatory stimuli on CYPs in relevant human in-vitro models, summarized in Table 1.
Table 1.
Regulation of Clinically Important Drug-Metabolizing Cytochrome P450 Enzymes by Pro-Inflammatory Stimuli in Relevant Human in-Vitro Models.
| Stimulus | Model | Effect on CYP mRNA Expression | Effect on Drug Metabolism | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | Studied Concentration | Maximal Effect (%) | Potency (EC50 ng/mL) | Duration | Drug | Metabolite | Potency (EC50 ng/mL) | Maximal Effect (%) | |||
| IL-6 | PHH | 24 h | 10 ng/mL | CYP3A4 (↓95%) | [14] | ||||||
| CYP2C9 (↓35%) | |||||||||||
| CYP2C19 (↓40%) | |||||||||||
| PHH | 24 h | 10 ng/mL | CYP3A4 (↓85%) | 72 h | atorvastatin | o-OH-atorvastatin | NS | [15] | |||
| CYP1A2 (↓76%) | phenacetin | acetaminophen | NS | ||||||||
| CYP2C9 (↓65%) | tolbutamide | OH-tolbutamide | NS | ||||||||
| CYP2C19 (↓41%) | S-mephenytoin | 4′-OH-mephentoin | NS | ||||||||
| CYP2D6 (↓41%) | |||||||||||
| CYP2E1 (↑402%) | |||||||||||
| PHH | 24 h | 10 ng/mL | CYP3A4 (↓97%) | [16] | |||||||
| CYP1A2 (↓96%) | |||||||||||
| PHH | 48 h | 0.0006–50 ng/mL | CYP3A4 (↓90%) | 0.454 | [17] | ||||||
| CYP1A2 (↓80%) | 5.49 | ||||||||||
| PHH * | 48 h | 10 ng/mL | CYP3A4 (↓98%) | 72 h | testosterone | 6β-hydroxytestosterone | ↓76% | [18] | |||
| CYP1A2 (↓27%) | phenacetin | acetaminophen | ↓22% | ||||||||
| CYP2C19 (↓72%) | S-mephenytoin | 4′-OH-mephentoin | ↓65% | ||||||||
| CYP2C9 (↓63%) | tolbutamide | OH-tolbutamide | ↓35% | ||||||||
| CYP2D6 (↑240%) | dextromethorphan | dextrorphan | ↓39% | ||||||||
| PHH | 72 h | 0.005–50 ng/mL | CYP3A4 (↓95%) | 0.0032 | 72 h | testosterone | 6β-hydroxytestosterone | 0.073 | ↓70% | [19] | |
| CYP3A5 (↓95%) | 0.051 | ||||||||||
| CYP1A2 (↓85%) | 0.271 | phenacetin | acetaminophen | 1.25 | ↓90% | ||||||
| CYP2C19 (↓80%) | 0.071 | ||||||||||
| CYP2C9 (↓90%) | 0.121 | ||||||||||
| CYP2D6 (↓70%) | 0.151 | ||||||||||
| PHH/PHH:KC (10:4) | 2–200 ng/mL | CYP3A4 (?) | 72 h | testosterone | 6β-hydroxytestosterone | ↓90% | [20] | ||||
| PHH:KC * | 0.001–10 ng/mL | CYP3A4 (?) | 96 h | luminogenic P450-Glo™ substrate | proluciferin substrate | 0.463 | ↓80% | [21] | |||
| PHH:KC (10:4) | 96 h | 0.00625–5 ng/mL | CYP3A4 (↓95%) | 96 h | luminogenic P450-Glo™ substrate | proluciferin substrate | 0.252 | ↓ > 95% | [22] | ||
| HepaRG | 24 h | 10 ng/mL | CYP3A4 (↓95%) | 24 h | midazolam | 1′-hydroxymidazolam | decreased | [23] | |||
| CYP3A5 (↓90%) | |||||||||||
| CYP1A2 (↓80%) | phenacetin | acetaminophen | decreased | ||||||||
| CYP2C9 (↓85%) | tolbutamide | OH-tolbutamide | NS | ||||||||
| CYP2C19 (↓85%) | S-mephenytoin | 4′-OH-mephentoin | decreased | ||||||||
| CYP2D6 (NS) | propafenone | 5-hydroxypropafenone | NS | ||||||||
| CYP2E1 (NS) | |||||||||||
| HepaRG | 24 h | 10 ng/mL | CYP3A4 (↓93%) | 72 h | atorvastatin | o-OH-atorvastatin | ↓ > 80% | [15] | |||
| CYP3A5 (↓89%) | |||||||||||
| CYP1A2 (↓90%) | phenacetin | acetaminophen | ↓ > 60% | ||||||||
| CYP2C9 (↓83%) | tolbutamide | OH-tolbutamide | ↓ > 60% | ||||||||
| CYP2C19 (↓83%) | S-mephenytoin | 4′-OH-mephentoin | ↓ > 60% | ||||||||
| CYP2E1 (NS) | |||||||||||
| HepaRG | 48 h | 0.123–30 ng/mL | CYP3A4 (↓99%) | <0.123 | 72 h | midazolam | 1′-hydroxymidazolam | 2.89 | ↓60% | [17] | |
| CYP1A2 (↓90%) | 0.452 | phenacetin | acetaminophen | 8.96 | ↓65% | ||||||
| HepaRG | 48 h | 10 ng/mL | CYP3A4 (↓ > 95%) | 4 h | midazolam | 1′-hydroxymidazolam | ↓61% | [16] | |||
| CYP1A2 (↓ > 95%) | phenacetin | acetaminophen | ↓68% | ||||||||
| HepaRG | 336 h (14 days) | 10 ng/mL | CYP3A4 (NS) | 336 h (14 days) | midazolam | 1′-hydroxymidazolam | decreased | [23] | |||
| CYP3A5 (↓80%) | |||||||||||
| CYP1A2 (↓95%) | phenacetin | acetaminophen | decreased | ||||||||
| CYP2C9 (NS) | tolbutamide | OH-tolbutamide | NS | ||||||||
| CYP2C19 (↓90%) | S-mephenytoin | 4′-OH-mephentoin | NS | ||||||||
| CYP2D6 (NS) | |||||||||||
| CYP2E1 (NS) | |||||||||||
| IL-1β | PHH | 24 h | 5 ng/mL | CYP3A4 (↓95%) | [14] | ||||||
| CYP2C9 (NS) | |||||||||||
| CYP2C19 (NS) | |||||||||||
| PHH | 72 h | 0.0001–10 ng/mL | CYP3A4 (↓95%) | 0.294 | 72 h | testosterone | 6β-hydroxytestosterone | 0.416 | ↓90% | [24] | |
| CYP3A5 (↓62%) | 0.347 | ||||||||||
| CYP1A2 (↓73%) | 0.531 # | phenacetin | acetaminophen | 0.45 | ↓65% | ||||||
| CYP2C9 (↓79%) | 0.229 # | ||||||||||
| CYP2C19 (↓58%) | 0.153 # | ||||||||||
| CYP2D6 (↓75%) | 0.945 # | ||||||||||
| PHH/ PHH:KC (10:4) | 0.2–200 ng/mL | CYP3A4 (?) | 72 h | Testosterone | 6β-hydroxytestosterone | ↓85% | [20] | ||||
| PHH:KC (10:4) | 96 h | 0.00625–5 ng/mL | CYP3A4 (↓ > 95%) | 96 h | luminogenic P450-Glo™ substrate | proluciferin substrate | 0.098 | ↓ > 95% | [22] | ||
| HepaRG | 24 h | 5 ng/mL | CYP3A4 (↓97%) | 72 h | atorvastatin | o-OH-atorvastatin | ↓ > 80% | [15] | |||
| CYP3A5 (↓91%) | |||||||||||
| CYP1A2 (↓93%) | phenacetin | acetaminophen | ↓ > 80% | ||||||||
| CYP2C9 (↓90%) | tolbutamide | OH-tolbutamide | ↓ > 80% | ||||||||
| CYP2C19 (↓93%) | S-mephenytoin | 4′-OH-mephentoin | ↓ > 80% | ||||||||
| CYP2E1 (↓75%) | |||||||||||
| HepaRG | 24 h | 1 ng/mL | CYP3A4 (↓98%) | [16] | |||||||
| CYP1A2 (↓99%) | |||||||||||
| IL-18 | PHH | 48 h | 1.95–500 ng/mL | CYP3A4 (NS) | 72 h | midazolam | 1′-hydroxymidazolam | NS | [17] | ||
| CYP1A2 (NS) | phenacetin | acetaminophen | NS | ||||||||
| HepaRG | 48 h | 2.06–600 ng/mL | CYP3A4 (NS) | 72 h | midazolam | 1′-hydroxymidazolam | NS | [17] | |||
| CYP1A2 (NS) | phenacetin | acetaminophen | NS | ||||||||
| TNF-α | PHH | 24 h | 10 ng/mL | CYP3A4 (↓80%) | [14] | ||||||
| CYP2C9 (NS) | |||||||||||
| CYP2C19 (NS) | |||||||||||
| PHH * | 48 h | 10 ng/mL | CYP3A4 (↓87%) | 72 h | testosterone | 6β-hydroxytestosterone | ↓70% | [18] | |||
| CYP1A2 (↓45%) | phenacetin | acetaminophen | ↓72% | ||||||||
| CYP2C19 (NS) | S-mephenytoin | 4′-OH-mephentoin | ↓82% | ||||||||
| CYP2C9 (NS) | tolbutamide | OH-tolbutamide | ↓17% | ||||||||
| CYP2D6 (↓40%) | dextromethorphan | dextrorphan | ↓42% | ||||||||
| HepaRG | 24 h | 10 ng/mL | CYP3A4 (↓90%) | 72 h | atorvastatin | o-OH-atorvastatin | ↓ > 80% | [15] | |||
| CYP3A5 (↓79%) | |||||||||||
| CYP1A2 (↓87%) | phenacetin | acetaminophen | ↓ > 80% | ||||||||
| CYP2C19 (↓64%) | S-mephenytoin | 4′-OH-mephentoin | ↓ > 80% | ||||||||
| CYP2C9 (↓62%) | tolbutamide | OH-tolbutamide | ↓ > 80% | ||||||||
| CYP2E1 (↓54%) | |||||||||||
| TGF-β | PHH | 24 h | 10 ng/mL | CYP3A4 (↓75%) | [14] | ||||||
| CYP2C9 (↓50%) | |||||||||||
| CYP2C19 (↓50%) | |||||||||||
| IFN-y | PHH | 24 h | 10 ng/mL | CYP3A4 (↓75%) | [14] | ||||||
| CYP2C9 (NS) | |||||||||||
| CYP2C19 (NS) | |||||||||||
| IL-22 | PHH | 48 h | 10 ng/mL | CYP3A4 (↓70%) | [25] | ||||||
| CYP1A2 (↓45%) | |||||||||||
| CYP2C9 (↓50%) | |||||||||||
| HepaRG | 24 h | 10 ng/mL | CYP3A4 (↓75%) | 1.7 | 48 h | midazolam | 1′-hydroxymidazolam | ↓50% | [25] | ||
| CYP1A2 (↓60%) | phenacetin | acetaminophen | ↓50% | ||||||||
| CYP2C9 (↓50%) | |||||||||||
| IL-2 | PHH * | 2–200 ng/mL | CYP3A4 (?) | 72 h | testosterone | 6β-hydroxytestosterone | NS | [20] | |||
| PHH * | 48 h | 10 ng/mL | CYP3A4 (NS) | 72 h | testosterone | 6β-hydroxytestosterone | NS | [18] | |||
| CYP1A2 (NS) | phenacetin | acetaminophen | NS | ||||||||
| CYP2C19 (NS) | S-mephenytoin | 4′-OH-mephentoin | ↓21% | ||||||||
| CYP2C9 (NS) | tolbutamide | OH-tolbutamide | NS | ||||||||
| CYP2D6 (↑150%) | dextromethorphan | dextrorphan | ↓22% | ||||||||
| PHH:KC (10:4) * | 200 ng/mL | CYP3A4 (?) | 72 h | testosterone | 6β-hydroxytestosterone | ↓70% | [20] | ||||
| PHH:KC (10:4) | 200 ng/mL | CYP3A4 (?) | 96 h | luminogenic P450-Glo™ substrate | proluciferin substrate | NS | [22] | ||||
| IL-12 | PHH | 48 h | 10 ng/mL | CYP3A4 (NS) | 48 h | testosterone | 6β-hydroxytestosterone | NS | [26] | ||
| CYP2C19 (NS) | S-mephenytoin | 4′-OH-mephentoin | |||||||||
| CYP2C9 (NS) | tolbutamide | OH-tolbutamide | |||||||||
| IL-23 | PHH | 48 h | 10 ng/mL | CYP3A4 (NS) | 48 h | testosterone | 6β-hydroxytestosterone | NS | [26] | ||
| CYP2C19 (NS) | S-mephenytoin | 4′-OH-mephentoin | |||||||||
| CYP2C9 (NS) | tolbutamide | OH-tolbutamide | |||||||||
| PHH:KC (10:4) | 200 ng/mL | CYP3A4 (?) | 96 h | luminogenic P450-Glo™ substrate | proluciferin substrate | NS | [22] | ||||
| LPS | PHH | 24 h | 10 µg/ml | CYP3A4 (↓95%) | [14] | ||||||
| CYP2C9 (NS) | |||||||||||
| CYP2C19 (NS) | |||||||||||
| HepaRG | 48 h | 1.37–333 ng/mL | CYP3A4 (↓95%) | <1.37 | 72 h | midazolam | 1′-hydroxymidazolam | 7.85 | ↓60% | [17] | |
NS = not significant. * = effect of inflammatory mediator on CYP expression and activity was investigated after treatment with a standard CYP-inducer. # = excluding non-responders. ↑ = increased CYP expression. ↓ = decreased CYP expression. ? = effect of inflammatory mediator on CYP mRNA expression was not determined.
2.1. Interleukin-6 (IL-6)
IL-6 is the chief stimulator cytokine in activation of innate immunity in the liver to contribute to host defense [27]. Owing to its role as the main cytokine in the acute phase response (APR), multiple studies have focused on investigating the effect of IL-6 on CYP levels in-vitro.
2.1.1. Maximal Effect (Emax) (mRNA)
Numerous investigators have confirmed that IL-6 is a potent downregulator of CYP enzymes in both primary human hepatocytes (PHHs) and in the HepaRG cell line, an immortalized human hepatic cell system that retains PHH characteristics but lacks donor variability. Aitken et al. investigated the effect of inflammatory mediators including IL-6 on mRNA expression of CYP2C9, CYP2C19, and CYP3A4 in PHHs [14]. Treatment with IL-6 downregulated mRNA levels for all isoforms studied, but simultaneously revealed profound differences in the magnitude of downregulation, as the expression of CYP3A4 was markedly more reduced than that of CYP2C9 or CYP2C19. A similar observation was made by Dickmann et al. [19] and Klein et al. [15] in PHHs and by Tanner et al. [23] in the HepaRG cell line, who all reported that IL-6 exerted the strongest downregulation on CYP3A4, whereas the effects of IL-6 on other CYPs, most notably CYP2D6, seemed to be more limited. It should be noted from the work of Klein et al. that IL-6 may also induce expression of CYP2E1 in PHHs, which could be relevant for the metabolism of certain anesthetics [15]. Beyond this exception, IL-6 predominantly reduces CYP expression and thus impairs the biotransformation of a wide range of (pro) drugs that are metabolized through CYP enzymes.
2.1.2. Sensitivity between CYPs (mRNA)
The strength of the Dickmann study is that it examined the effects of IL-6 at different concentrations, allowing determination of the potency (EC50) and thus rank ordering the responsiveness of the major CYP enzymes following IL-6 exposure [19]. Through this approach this study was able to demonstrate that the exerted effects of IL-6 in PHH occur at physiological relevant concentrations, as similar concentrations of IL-6 have been detected in the circulation of patients with either chronic or acute inflammation [28,29]. Importantly, these investigations revealed that CYP3A4 was also by potency most sensitive to downregulation by IL-6, as IL-6 downregulated CYP3A4 mRNA with an EC50 of 0.0032 ng/mL, whereas a 20- to 500-fold higher concentration of IL-6 was needed for downregulation of other CYPs. A similar difference in CYP sensitivity to IL-6 was observed by Rubin et al. in both PHHs and HepaRG cells [17]. Such differences in sensitivity are potentially important as these data suggest that drugs metabolized by CYP3A4 may be affected already at an earlier state during inflammation than drugs that rely on other CYP enzymes.
2.1.3. Sensitivity between PHH Donors
Another point of attention is the observed interindividual variability in response to IL-6 between donors in a single experimental setup, excluding inconsistencies observed between studies due to model variations or treatment differences [30]. For example, Dickmann et al. reported EC50 values for CYP1A2 activity suppression between 0.142 and 4.07 ng/mL (ranging 29-fold) over five donors and a range between 0.0042 and 0.176 ng/mL (ranging 42-fold) for CYP3A4 activity suppression [19]. Evers et al. also reported that CYP3A4 downregulation upon IL-6 stimulation varied largely between donors in one experimental setup, reporting EC50 suppression values over approximately a 20-fold range between donors. [30]. The observed different susceptibility to inflammation between donors may be a consequence of both genetic variability and differences in disease state or medical history of the studied donors.
2.1.4. Drug-Metabolizing Activity
Determination of the cytochrome P450 enzymatic activity is important because beyond the described transcriptional effects, posttranscriptional mechanisms may also contribute to the effects of inflammation on drug metabolism [14,31]. As can be observed from Table 1, effects of inflammation have commonly been assessed by measuring metabolite formation of probe substrates of CYP3A4 (midazolam/testosterone), CYP1A2 (phenacetin), CYP2C9 (tolbutamide), CYP2C19 (S-mephenytoin), and CYP2D6 (propafenone/dextromethorfan). Klein et al. showed in PHHs trends for reduced metabolite formation upon IL-6 treatment but statistical power was lacking, presumably because of the heterogeneity of the donors and potential pharmacogenetic variation in CYP450 enzymes as confounding factors [15]. The HepaRG cell line lacks interindividual variability and showed stable CYP expression in the control group over at least 72 h, increasing the reproducibility of the results. In this model, the highest suppression of activity was noted for CYP3A4 as compared to other CYPs, in line with the observed transcriptional downregulation. Tanner et al. showed decreased downregulation of activity for CYP3A4, CYP1A2, and CYP2C19 but not CYP2C9 and CYP2D6 after 24 h [23].
2.1.5. Pathways
IL-6 may exert its effects in hepatocytes via distinct pathways, as the binding of IL-6 to its receptor initiates cellular signaling pathways via three arms, the Janus kinase (JAK)/STAT protein-3 (STAT3) pathway, the mitogen activated protein kinase (MAPK)/extracellular regulated kinase 1 and 2 (ERK1/2) pathway, and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway [32]. Keller et al. found that, using chemical inhibitors in IL-6 treated PHHs, especially the MAPK/ERK and PI3K/AKT signaling pathways—and not the canonical JAK/STAT pathway—were critical for downregulation of CYP enzymes during inflammation [33]. However, it should be noted that the effect of individual kinase inhibitors was tested in only one individual donor. In contrast, Febvre-James et al. found that treatment with the JAK inhibitor ruxolitinib completely reversed the IL-6-mediated suppression of CYP1A2 and CYP3A4 mRNA levels in both HepaRG cells and PHHs, suggesting a prominent role of the JAK/STAT pathway in CYP downregulation [16]. As such, multiple signaling arms of the IL-6 pathways can be held responsible for the observed downregulation of CYP enzymes.
2.1.6. Long-Term Studies
Implementing long-term investigation on inflammation-induced CYP suppression in-vitro could aid in a better understanding of the chronic inflammation frequently observed in a clinical setting. Long-term investigations on the effect of inflammatory mediators on CYP expression are scarce, especially in PHHs since CYP expression rapidly declines over time in this model [34]. Long et al. investigated the effect of IL-6 on CYP3A4 activity in a 3D microreactor platform with PHH and Kupffer cells [21]. They tested the effect of tocilizumab, an anti-IL-6 receptor antibody, on inflamed hepatocytes and found that coadministration of tocilizumab with IL-6 after initial 4-day IL-6 treatment prevented the CYP3A4 activity decrease across donors. This highlights that the model is capable of capturing physiological adaptation to inflammation, since CYP3A4 desuppression occurred. Tanner et al. collected data on the long-term effects of IL-6 treatment (14 days) in HepaRG cells, which resulted in more pronounced downregulation of P450 expression as compared to short-term treatment [23]. Still, current studies do not address the impact of long-term low concentrations of cytokines as compared to single high-dose treatment, which leaves an open question.
2.1.7. Clinic
Interestingly, the effects of inflammation on drug metabolism in the clinic, most commonly assessed through the IL-6 regulated marker C-reactive protein (CRP), seems to be most reported for CYP3A4 substrates including midazolam, tacrolimus, and/or voriconazole, and less for drugs metabolized through other metabolic pathways. This is in line with data from in-vitro hepatocyte models where IL-6 exerts most profound effects on CYP3A4 [4]. Altogether, these data confirm isoform specific effects of IL-6 and suggest that drugs metabolized via CYP3A4 may be more prone to the effects of inflammation.
2.2. Interleukin 1 (IL-1)-Family: Interleukin-1β and Interleukin-18
The IL-1 family compromises a group of 11 proteins that play a role in the initiation and regulation of inflammatory responses, of which IL-1β is the most studied member [35].
2.2.1. Maximal Effect (Emax) (mRNA)
In PHHs, IL-1β treatment reduced CYP3A4 mRNA expression with 95%, but it had no effect on CYP2C9 or CYP2C19 mRNA levels [14]. Protein levels of CYP3A4, but also of CYP2C9, were significantly downregulated after 24 h of treatment with IL-1β. Dickmann et al. showed donor-wide suppression (n = 5) for CYP3A4/A5, however, IL-1β-mediated suppression of other CYP isoforms (CYP2C9, C19, and 1A2) was not consistently observed among all donors [24]. The observed nonresponse towards IL-1β of certain CYP isoforms cannot simply be explained by a lack of effect, since IL-1β consistently reduced CYP3A4 expression by >80% in all donors. Alternatively, because the nonresponsive CYP isoforms (CYP2C9 or CYP2C19) differed between donors, nonresponse to IL-1β can perhaps be explained by pharmacogenetic variation within these CYP isoforms. IL-18 treatment in HepaRG cells and PHHs did not result in significant downregulation of mRNA levels nor CYP activity [17].
2.2.2. Sensitivity between Models
Interestingly, although Klein et al. showed that the maximal impact of Il-1β and IL-6 on the mRNA expression of CYP isoforms was comparable in HepaRG cells, IL-1β showed an approximate 100-fold higher potency than IL-6 in inducing the same downregulation [15]. This described difference in potency might be underlined by the fact that the HepaRG cell line displays morphological heterogeneity, including clusters with nonparenchymal cells which could aggravate or sensitize the response to an inflammatory mediator [36]. For example, IL-1β release is associated with activation of the inflammasome in Kupffer cells [37], providing a feed-forward stimulus for production of more inflammatory cytokines which could potentially aggravate cytokine-induced downregulation of CYPs. Indeed, coculturing of Kupffer cells increased responsiveness to IL-1β as compared to monocultures of hepatocytes, as evident from an EC50 shift from >5 to 0.098 ng/mL for CYP3A4 suppression upon coculturing, an effect not seen with IL-6 treatment [20,22]. Since IL-18 is also reported to mediate its effect through Kupffer cells [38], this can explain the lack of effect on CYPs in HepaRG or PHHs cell models described by Rubin et al. Thus, inclusion of nonparenchymal cells in model systems might increase the responsiveness to IL-1β and IL-18 and hence better reflect the potential effect these inflammatory cytokines may have in an intact human liver.
2.2.3. Sensitivity between PHH Donors
Looking at the suppression of activity of CYP3A4 and CYP1A2 in PHHs upon IL-1β treatment, again large interdonor variation is evident [24]. Dickmann et al. found an average EC50 value for two donors of 0.450 ng/mL (three donors showed no response) regarding CYP1A2 activity. For CYP3A4, EC50 values for activity ranged from 0.005 to 1.06 ng/mL over five donors.
2.2.4. Pathways
The effects of IL-1β are presumed to be mediated via activation of the nuclear factor kappa B (NF-κB) pathway [39]. Importantly, IL-1β may also rapidly (within 2–4 h) induce IL-6 expression, which raises the possibility that part of the observed actions of IL-1β are actually mediated by IL-6 [40]. Interestingly, a recent study by Febvre-James et al. found that the IL-1β repression of CYP enzymes could not be reversed by the JAK inhibitor ruxolitinib, confirming that IL-1β and IL-6 induce distinct pathways upon inflammation and may complement one another in altering drug metabolism [16].
2.3. Tumor Necrosis Factor α (TNF-α)
TNF-α is another main cytokine involved in inducing the acute phase response in the liver during inflammation. Hepatocytes express the tumor necrosis factor receptor 1 (TNFR1) that upon binding by TNF-α results in the activation of the major NF-κB pathway and the MAPK/ERK pathway [41]. Aitken et al. found that TNF-α treatment induced CYP3A4 mRNA downregulation but not protein downregulation after 24 h [14]. They saw no effect on CYP2C9 and CYP2C19 mRNA levels upon TNF-α treatment, but interestingly the CYP2C9 protein levels were reduced by >95% after 24 h treatment, pointing to a mismatch between the effects on mRNA and protein expression levels. This suggests that post-transcriptional mechanisms, i.e., protein degradation or regulation by miRNAs, are involved in downregulation of CYP protein levels by TNF-α. In line, Dallas et al. reported no effects of TNF-α on CYP2C19 and CYP2C9 mRNA levels, but found significantly downregulated CYP2C19 and CYP2C9 activity [18]. Klein et al. found that TNF-α treatment resulted in similar downregulation of CYP gene expression in HepaRG cells as observed with IL-6 treatment, presuming that part of the effect of TNF-α is mediated via nonparenchymal cells [15]. After 72 h of exposure to TNF-α, all P450 activities were reduced by more than 80%.
2.4. Pathogen Associated Molecular Patterns (PAMPs)
PAMPs, such as lipopolysaccharide (LPS), are microbial molecules that can signal immune cells to destroy intruding pathogens associated with infection [42]. Upon LPS recognition, the toll like receptor 4 (TLR4) signaling pathway ultimately activates NF-kB. The study by Aitken et al. found LPS to be the most efficacious inflammatory stimulus in downregulating mRNA levels of CYP3A4, and CYP3A4 protein levels were decreased by about 75% of control 24 h after treatment [14]. Whereas LPS treatment did not influence mRNA levels of CYP2C9 or CYP2C19, CYP2C9 protein levels were reduced by 80% after 24 h of treatment, again indicating a mismatch between mRNA and protein levels. This is in accordance with data presented by Rubin et al. in HepaRG cells and PHHs, where LPS downregulated CYP3A4 and CYP1A2 mRNA levels in both models [17]. LPS showed comparable potency in downregulating CYP3A4 compared to IL-6, but was much less potent in downregulating CYP1A2 levels.
2.5. Other Cytokines: Transforming Growth Factor β (TGF-β), Interferon γ (IFN-γ), Interleukin-22 (IL-22), Interleukin-23 (IL-23), and Interleukin-2 (IL-2)
The effect of other pro-inflammatory mediators beyond IL-6, IL-1β, TNF-α, and PAMPs has also been studied in in-vitro hepatocyte models. TGF-β, an inflammatory mediator linked to fibrosis, caused significant downregulation of CYP3A4, CYP2C9, and CYP2C19 mRNA levels and subsequent protein levels (only shown for CYP3A4 and CYP2C9) [14]. Interestingly, only TGF-β and IL-6 downregulated CYP2C9 mRNA, but protein expression levels of CYP2C9 were strongly downregulated by all inflammatory stimuli tested. IFN-γ, a mediator that is associated with the immune response to viral infections, only reduced mRNA levels of CYP3A4 in PHHs. Conversely, IL-22, a pro-inflammatory mediator found in different auto-immune disorders, was found to repress mRNA levels of CYP1A2, CYP3A4, and CYP2C9 in PHHs and HepaRG cells [25]. Studies investigating the effect of IL-2 on CYP3A4, 1A2, 2C9, 2C19, and 2D6 expression found no suppression of mRNA levels upon treatment in PHHs [18,20]. Interestingly, when culturing the hepatocytes together with Kupffer cells, a concentration-dependent decrease (50–70%) of CYP3A4 activity was observed with IL-2 at 72 h, suggesting that Kupffer cells are essential for the suppressive effect of IL-2 [20]. IL-12 and IL-23, pro-inflammatory mediators associated with inflammatory autoimmune responses, did not impact CYP3A4 levels [26] and a coculture model did not change this [22]. The effect of other cytokines on CYP expression and activity is yet to be determined.
2.6. Summary
The in-vitro data summarized here suggests that direct treatment with inflammatory stimuli can suppress DMEs stemming from the CYP1, CYP2, and CYP3 family. This suppressive effect is most convincingly demonstrated for IL-6, IL-1β, TNF-α, and LPS. CYP3A4 seems to be most susceptible to cytokine-induced downregulation in human in-vitro hepatocyte models, whereas CYP2D6 seems to be the least sensitive. The enzyme expression of CYP1A2, CYP2C9, and CYP2C19 was also sensitive to the effects of inflammatory mediators, though higher concentrations of cytokines were in general required to downregulate these enzymes and the response was not always conserved among all studied donors. Interestingly, model-dependent responses were observed which could be reliant on the presence of nonparenchymal cells. The effect of inflammatory mediators should therefore be divided into direct effects on hepatocytes and indirect effects through inflammatory signaling in nonparenchymal cells.
Importantly, interdonor variation in response to inflammation within the same experimental setup was observed. Translating these findings to the clinic, the consequences of inflammation-induced phenoconversion for drug treatments may differ therefore greatly between individuals and between the metabolic CYP pathways via which drugs are metabolized.
3. Mechanistic Pathways via Which Inflammation Modulates Hepatic Functions That Are Critical for Drug Metabolism
The above described findings from in-vitro models show that the sensitivity to inflammation may differ between CYP isoforms and inflammatory stimuli. This implies that distinct mechanisms are involved in the downregulation of CYP enzyme expression and activity.
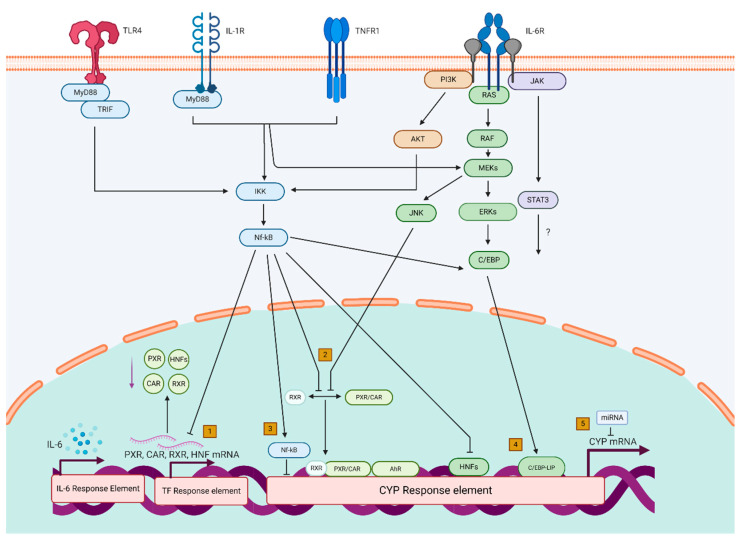
Mechanistically, regulation of hepatic CYP levels and interactions with CYP gene regulators is complicated and includes a wide variety of ligand-activated transcription factors and mediators. Cytokine-mediated alteration of gene transcription is thought to be the main regulatory mechanism accountable for changing CYP450 activity upon inflammation. It is essential to note that no single common pathway is recognized for all the CYP enzymes and underlying mechanisms are cytokine-specific. Here we describe, summarized in Figure 1, how repression of important CYP enzymes during inflammation may proceed through (1) transcriptional downregulation of transcription factors, (2) interference with dimerization/translocation of (nuclear) transcription factors, (3) altered liver-enriched C/EBP signaling, (4) direct regulation by NF-κB, or (5) post-transcriptional mechanisms.
Figure 1.
Mechanistic insights into the effects of inflammation on CYP expression and activity. Transcriptional repression of important CYP enzymes during inflammation may proceed through (1) transcriptional downregulation of nuclear receptors and other transcription factors, (2) interference with dimerization/translocation of nuclear transcription factors, (3) altered liver-enriched C/EBP signaling, (4) direct regulation by NF-κB, or (5) posttranscriptional mechanisms.
3.1. Transcriptional Downregulation of Transcription Factors
Transcription factors involved in the regulation of CYP mRNA levels, including the nuclear receptors pregnane X receptor (PXR), the constitutive androstane receptor (CAR), their dimerization partner retinoid X receptor (RXR), the aryl hydrocarbon receptor (AhR), as well as human nuclear factors (HNFs) are held responsible for the observed downregulation of DMEs upon inflammation. It is important to distinguish between the role of nuclear transcription factors in the constitutive expression of CYP enzymes versus drug- or inflammation-mediated expression. Here we will focus on the nuclear hormone receptor mechanisms likely to be involved in inflammation-altered CYP expression.
3.1.1. Downregulation of Nuclear Receptors
The PXR (gene: NR1I2) and the CAR (gene: NR1I3) are members of the nuclear receptor superfamily highly expressed in the enterohepatic system of mammals [43]. These ligand activated transcription factors have been identified as key transcriptional regulators of the cytochrome P450 xenobiotic-metabolizing enzymes, mostly for the CYP2C9, CYP2C19, CYP3A4, and CYP3A5 enzyme expression [44]. Upon binding with the RXR, the heterodimer nuclear receptor-RXR complex binds to responsive elements present in the 5′-flanking regions of target genes, usually resulting in an upregulation of gene expression aimed at increased metabolism of drugs. Studies have indeed shown that PXR and CAR increase transcription of the human CYP3A4/5, CYP2C9, CYP2C19, and CYP1A2 genes upon drug treatment [45,46,47].
One mechanism by which inflammation changes gene transcription of major DMEs is through repression of the nuclear receptor PXR and CAR. A vast body of evidence shows that inflammation represses PXR levels, leading to downregulation of important CYP enzymes. Pascussi et al. pioneered in showing that IL-6 downregulates PXR mRNA in PHH and inhibits the rifampicine-induced induction of CYP3A4 [48]. Upon LPS treatment in HepG2 cells, the mRNA and protein levels of PXR are reduced [49]. Mechanistically, a decrease in PXR expression within the nucleus was observed, leading to reduced transactivation of the CYP3A4 promotor and subsequent inhibited transcriptional activity of CYP3A4. Additionally, LPS treatment in mice led to functional repression of PXR’s dimerization partner RXR [50]. Yang et al. showed that inhibition of a CYP3A4 promotor reporter after IL-6 treatment in human hepatocytes was greater in the presence of PXR than after its knockdown, suggesting a role for PXR in IL-6-facilitated suppression of CYP3A4 [51]. Knockdown of PXR in human hepatocytes reversed the IL-6-induced CYP3A downregulation. Furthermore, the authors suggest that downregulation of PXR by inflammatory stimuli is causative for decreased transcription of CYP3A4: a continuous decrease in PXR levels was observed already after 1.5 h of treatment, whereas a significant decrease in CYP3A4 mRNA levels occurred only after 3 h. A likely scenario is that the suppressive effect of inflammation on PXR expression is mediated through NF-κB activation, since Zhou X et al. showed that NF-kB directly interacts with a functional binding site in the PXR promotor to suppress its transcriptional expression [52]. Transcriptional downregulation of CAR upon inflammatory stimuli has also been reported. A study by Assenat et al. investigated the negative regulation of CAR via pro-inflammatory cytokines IL-1β and LPS in human hepatocytes [53]. IL-1β treatment reduced mRNA levels of CYP2B6, CYP2C9, and CYP3A4 through NF-κB p65 activation. This p65 subunit of the NF-κB complex interfered with the distal glucocorticoid response element present in the CAR promotor, leading to repressed transcription of CAR. In contrast, the AhR is not substantially affected by IL-6 treatment [15,23]. As such, it appears that the response to inflammation is substantial for PXR and CAR and their dimerization partner RXR, but not for AhR.
Still, some debate remains about the role of nuclear receptors in the downregulation of CYP enzymes during inflammation, mostly stemming from conflicting rodent vs. human studies. In the rodent field, a study by Beigneux et al. suggested that downregulation of PXR and CAR was causative for CYP450 downregulation [50], whereas other experiments suggest that downregulation of important P450 enzymes does not necessitate the nuclear receptor PXR. As an example, Richardson et al. found that downregulation of multiple CYP mRNAs was similar in LPS-treated control and PXR-null mice, suggesting a PXR independent mechanism [54]. For the human situation, transcription factors responsible for the homeostasis of CYPs are evidently downregulated through inflammation. However, up to what extent downregulation of these transcription factors can actually be held responsible for the inflammation driven changes in expression of DMEs and drug metabolism itself remains to be further investigated.
3.1.2. Downregulation of Hepatocyte Nuclear Factors
Hepatocyte nuclear factors (HNFs), including HNF-1α and HNF-4α, form another important family of transcription factors. They can modulate CYP expression in the liver through DNA-binding interactions in CYP promotors or via modulation of PXR and CAR expression [55,56,57,58,59]. Despite their well-documented role in CYP homeostasis, the contribution of HNFs for the inflammation-induced changes in CYP expression remain, however, scarcely investigated.
The binding activities of HNF-1α and HNF-4α to DNA were quickly reduced in rat livers treated with LPS in parallel with downregulated hepatic CYP mRNA levels [60]. In HepG2 cells, treatment with IL-6 and IL-1β resulted in a 10% decrease of HNF-4α activity as a result of an altered phosphorylation status [61]. Acute and prolonged treatment with IL-6 reduced mRNA levels of HNF-4a in HepaRG cells, but this effect was not seen for HNF-1a and the changes shrink into insignificance compared to the observed downregulation of, e.g., PXR [23]. In contrast, Klein et al. found that mRNA levels of the HNF-4α were downregulated (≈40%) by IL-6 only at the early time point of 8 h and seemed to have normalized after 24 h [15]. However, a direct link between the fast transcriptional suppression of P450 genes and the reduced mRNA levels/activity of HNFs is still lacking, questioning a prominent role of transcriptional HNF downregulation as a factor in IL-6-induced DME suppression.
3.2. Interference with Dimerization/Nuclear Translocation of (Nuclear) Transcription Factors
Impairment of the activity of important transcription factors could, in addition to the above described transcriptional repression of transcription factors, also contribute to repression of CYPs during inflammation. Tanner et al. questioned whether transcriptional downregulation of PXR and CAR mRNA levels itself can fully explain the observed downregulation of CYP enzymes [23]. They suggested that the transactivation potential of PXR and CAR might be simultaneously influenced by inflammation. They found a clear correlation between downregulated PXR and CYP mRNA levels after short-term treatment with IL-6. However, the reduction in PXR expression following prolonged treatment (14-days) with IL-6 was very modest compared to the downregulation observed for the CYP enzymes. As such, downregulation of nuclear receptor target genes (e.g., CYPs) during inflammation could be a consequence of decreased availability of PXR itself, or an impairment of the translocation/activity of the receptor.
The existence for such interactions between inflammation and hepatic transcription factors (PXR, CAR, and AhR) have been suggested for both the NF-κB pathway and pathways related to IL-6 signaling. A hypothesized mechanism for this is interference of NF-κB with the dimerization of PXR to RXR and subsequent binding to DNA, thereby inhibiting the activity of PXR. The inhibited transcriptional activity of PXR leads to downregulation of DMEs in HepG2 cells [62]. As NF-κB interferes with the binding of RXR to PXR, this mechanism of repression by NF-κB may also hold true for more nuclear receptor-controlled systems where RXR is the dimerization partner (e.g., CAR), but no experimental evidence exists that can yet support this. AhR-regulated CYP1A2 is likely not regulated by this mechanism. Studies in mouse hepatoma cells have shown that interactions between the P65 subunit of NF-κB and AhR may result in the formation of an inactive complex, with possible consequences for the translocation to the nucleus [63]. In addition, NF-κB has been shown to inhibit transcriptional activity of AhR by reducing histone acetylation of promotors of CYP enzymes (e.g., CYP1A2), thereby altering the accessibility of the DNA for nuclear transcription factors [64]. Thus, activation of the NF-κB pathway may modulate the activity of nuclear transcription factors through changes in dimerization, translocation, or chromatin remodeling.
Kinases involved in the IL-6 signaling pathway can also alter the protein status and translocation of nuclear receptors. Cell signaling protein kinases such as Jun-N-terminal kinase (JNK) and protein kinase C (PKC) can repress the activity of the nuclear receptors PXR and CAR, thereby altering their function and impact on downstream transcriptional CYP activity [65,66,67]. One hypothesized mechanism is that kinases can alter the phosphorylation status of these nuclear receptor proteins. IL-1β treatment induces JNK expression which can phosphorylate RXR, leading to reduced nuclear binding activity and subsequently inhibited RXR-dependent hepatic gene expression [68]. Additionally, LPS-induced downregulation of P450 genes was attenuated upon treatment with a specific JNK inhibitor in a primary mouse hepatocyte model [69]. Thus, JNK can play a role in inflammation-mediated downregulation of nuclear receptors with RXR as partner. This was backed up by findings from Ghose et al., who showed that an increase in JNK signaling is associated with higher export of RXR out of the nucleus upon low-dose LPS treatment, leading to less RXR-mediated hepatic gene expression [70]. Additionally, ERK signaling has been proven to impair nuclear translocation of CAR in a mouse primary hepatocyte model [71]. Altogether, these findings indicate that kinases play an important role in the regulation of nuclear receptors and their dimerization with RXR, thereby offering a general mechanism for the suppression of genes regulated by nuclear receptors during inflammation. How other important inflammatory cell-signaling components in the IL-6 pathway, such as STAT3, mechanistically regulate CYP repression remains to be investigated.
3.3. Direct Regulation by NF-κB
NF-κB can precisely control the expression of CYP1A1, CYP2B1, CYP2C11, CYP2D5, CYP2E1, and CYP3A7 via interaction with the promotors of these genes, leading to downregulation in most cases [72]. For example, Iber et al. reported that the CYP2C11 promotor region contains a low-affinity binding place for NF-κB and mutations in the 3′-end or 5′-end in this NF-κB response element reduced the binding affinity for NF-κB and subsequently suppressed CYP2C11 transcription by IL-1 or LPS in rat hepatocytes [73]. However, experimental evidence for this hypothesis has only been obtained in animal models. Although there is high conservation of CYP enzymes amongst species, the extent and catalytic activity between species differs, highlighting that caution should be taken in extrapolation of results to a human situation [13].
3.4. Altered Liver-Enriched C/EBP Signaling
The expression and DNA binding activity of the transcription factor C/EBPβ is severely enhanced during the acute phase liver response through activation of the NF-κB pathway [74]. One mechanism that is hypothesized to contribute to CYP repression upon IL-6 stimulation is altered balance between two isoforms of the transcription factor C/EBP-β: the liver-enriched transcriptional activating protein (LAP) and the liver-enriched transcriptional inhibitory protein (LIP). The LIP isoform is a shortened variant of C/EBPβ deficient of transactivation activity. Jover et al. found upregulation of the C/EBPβ-LIP protein isoform in HepG2 cells treated with IL-6 [75]. They demonstrated that LIP antagonized transactivation of CYP3A4 by the functional LAP isoform. This altered LAP:LIP ratio correlated with a downregulation of CYP3A4 enzyme levels. Martinez et al. showed a novel enhancer site located in the CYP3A4 gene where the LAP isoform can bind and initiate transcription, whereas the antagonizing action of the truncated LIP isoform on LAP resulted in CYP3A4 gene repression, confirming that the LAP:LIP ratio is of importance in regulation of constitutive expression of CYP3A4 [76]. A C/EBPβ-based mechanism was also found to be involved in transcriptional repression of CYP2A6 [77]. It is yet to be determined whether this mechanism can also explain repression of other CYPs upon IL-6 stimulation in a human model.
3.5. Posttranscriptional Mechanisms (miRNA)
The mechanisms behind downregulation of DMEs upon inflammation, as described above, remain an area of intense study. Increasing attention is being given to the potential post-transcriptional mechanisms that could regulate P450 enzymes in inflammation as well. MicroRNAs (miRNAs) can influence the translation and stability of cellular mRNAs at their 3′-UTR side, offering a broad mechanism for gene expression regulation [78]. Previous research has already shown that miRNA activity regulates phase I and II metabolizing enzymes and transcriptional factors through posttranscriptional modification [31,79,80].
A recent study by Kugler et al. questions whether the previously observed mechanisms are sufficient to explain the huge downregulation of DMEs observed upon inflammation and investigated the possible role of miRNA in this process [81]. They performed transfections with five inflammation-associated miRNAs in HepaRG cells and looked at the CYP mRNA levels and activity. They found miRNA-dependent downregulation of several CYP mRNA and expression levels after 96 h, where CYP2C19 and CYP3A4 were amongst the top downregulated genes. Thus, miRNAs might be an extra factor in downregulating drug metabolizing capacity during inflammation. Potentially, this could also explain the sometimes observed mismatch between CYP mRNA levels and CYP protein levels after inflammatory stimuli, as was the case for CYP2C9 in the study from Aitken et al. [14]. Since the 3′-UTR region of CYP2C9 can directly be regulated by miR-130b, this could explain the downregulation of CYP2C9 enzyme expression. As such, miRNA regulation could (in part) be responsible for the effects of inflammatory mediators on protein levels in the absence of preceding downregulation of mRNA.
Other post-transcriptional mechanisms, such as the role of nitric oxide in the cytokine-mediated regulation of CYPs were excellently reviewed by Morgan et al. [82].
3.6. Concluding Remark
Concluding from previous sections, we hypothesize that the variation in sensitivity of different CYP enzymes for inflammation stems from the distinct mechanisms that regulate them. It seems like PXR- and CAR-regulated CYP enzymes (3A4/5, 2C9, 2C19) are more sensitive to inflammation, whereas the AhR regulated isoform CYP1A2 is less sensitive. CYP2D6 shows to be least sensitive to inflammation, which might be due to the fact that it is not inducible by nuclear receptors and therefore not sensitive to inflammation-induced alterations of the levels of PXR, CAR, and AhR that regulate the expression of other CYPs [83,84,85]. Most interestingly, deduction of CYP specific inflammatory mechanisms of downregulation can shed light on the distinct sensitivities towards inflammation.
4. Pharmacogenetic Variation in Inflammatory Pathways and the Effect on Drug Pharmacokinetics
The available data from in-vitro experiments with PHHs on drug metabolism have indicated that the response to inflammation or its inflammatory mediators may differ substantially between donors under controlled experimental conditions [19,24,30]. This raises the question whether the observed distinct response to inflammation between persons is also observed in the clinic. Clinical studies by van Wanrooy et al. and Vet et al. have shown that the metabolism of voriconazole and midazolam at similar concentrations of CRP and corrected for other known confounding factors may still vary considerably between patients [5,6]. These findings from both in-vitro models and clinical studies suggests the existence of interindividual variability with regards to the effects of inflammation on drug metabolism. This distinct response towards inflammation between subjects may in part be caused by genetic variability in the described pathways via which inflammation modulates the activity of DMEs.
By presenting examples from the available literature we illustrate how genetic variability within the different elements presented in Figure 1 can modulate the outcomes of the effect of inflammation on drug metabolism and consequently may contribute to the observed interindividual variability in the effect of inflammation.
4.1. Genetic Variation: Inflammatory Mediators
It is well established that genetic variability within inflammatory mediators (e.g., cytokines) can predispose individuals to an altered susceptibility to immune-related disease [86]. For this reason, it is plausible that polymorphisms in cytokine genes could shape the immune response that affects drug metabolism. One prominent example relates to the rs1946518 (-607C/A) variant within the promoter of IL-18 and its effects on the metabolism of the immunosuppressive tacrolimus. Xing et al. and Zhang et al. demonstrated that Han-Chinese patients carrying the AA genotype (19–29% of the patients) exhibited lower concentration/dose (C/D) ratios of tacrolimus within the first month after lung or kidney transplantation than patients with an AC or CC genotype [87,88]. Interestingly, this relationship for the rs1946518 variant was exclusively shown for patients expressing CYP3A5*1 and functionally linked to lower expression of IL-18 mRNA in the liver. These results imply that the rs1946518 variant reduced the IL-18 driven inflammation in the liver, which prevents the inflammation-induced downregulation of CYP3A5 and consequently reduces the impact of inflammation on drug metabolism in these patients. Importantly, rs1946518 did not modulate C/D ratios in liver transplant patients who were already treated for 1 year with tacrolimus [89]. These results suggest that the variant only affects drug metabolism shortly after transplantation when the immune/inflammatory responses are highest. Altogether, this example illustrates that genetic variability within inflammatory mediators has the potential to modulate the effects of inflammation on drug metabolism.
4.2. Genetic Variation: Inflammatory Receptors
As described above, toll-like receptor (TLR) activation by pathogen-associated molecules may downregulate CYP3A4 expression and modulate drug metabolism. However, TLR activation may also be triggered by endogenous molecules (e.g., DNA) that are released during ischemia-reperfusion injury that develops during organ transplantations [90]. Therefore, it has been postulated that genetic variability in TLRs may alter the effect of inflammation and its consequences for drug metabolism. Ou et al. showed that liver transplant patients with the TLR9-rs352139 AA genotype exhibited lower C/D tacrolimus levels than carriers of the AG/GG genotype [91]. Subsequent cellular experiments provided functional support for these observations and demonstrated that the TLR9-rs352139 variant impaired TLR9 expression and consequently reduced NF-κB activation. TLR9-rs352139 AA genotype carriers were thus protected from the effects of ischemia-reperfusion-induced inflammation, which resulted in conservation of their metabolic capacity. The opposite effect was observed for carriers of the TLR4-rs1927907-GG phenotype who exhibited higher tacrolimus C/D ratios than AA/AG carriers, indicating that these patients were more susceptible to the effects of inflammation on their drug-metabolizing capacity [91,92]. These studies illustrate that genetic variants in receptors can be important modulators of inflammation, which may be particularly relevant for receptors (e.g., IL6R or IL-1R) that are directly involved in the downregulation of CYP enzymes, but this remains to be investigated.
4.3. Genetic Variation: Inflammatory Transcription Factors (NF-κB)
Genetic variability within NF-κB is of great interest given its essential role in inflammatory signaling [93]. One common polymorphism in the NFKB1 gene is the promotor -94 ATTG insertion/deletion mutation (rs28362491), with a minor allele frequency of 0.43. Deletion of the ATTG alleles is shown to reduce synthesis of the NF-κB p50 subunit [94]. Zhang et al. showed that patients with the NFKB1 -94 ATTG ins/ins genotype had higher CYP3A4-metabolized dose-adjusted cyclosporine trough concentrations than patients with the -94 ATTG del/del genotype [95]. The impact of the same polymorphism in NFKB1 on the pharmacokinetics of lovastatin, a cholesterol-lowering drug mainly metabolized by CYP3A4, was also investigated [96]. In accordance, the area under the plasma concentration–time curve (AUC) of the metabolite lovastatin lactone was twofold higher in subjects with two copies of the NFKB1-94 ATTG ins/ins mutation and the plasma clearance was lower as compared to the NFKB1-94del/del genotype. The NFKB1-94del/del mutation may thus impair inflammatory signaling and hence attenuate the inflammation-induced downregulation of CYP3A4. Consequently, patients with the NFKB1-94del/del genotype may perceive milder consequences of inflammation on drug metabolism than people lacking this variant. Since NF-κB is a downstream effector molecule of several inflammatory cytokines, genetic variability has the potential to simultaneously alter the actions of multiple inflammatory mediators on CYP gene expression. The potential impact of genetic variability within NF-κB or within the genes of NF-κB adaptor proteins on the effects of inflammation on drug metabolism is therefore predicted to be greater than genetic variability in the receptors or the mediators themselves.
4.4. Genetic Variation: Nuclear Receptors (PXR, CAR)
The nuclear receptors PXR and CAR are, as highlighted earlier, important for the transcriptional regulation of CYP450 enzymes. Pharmacogenetic variations within the genes encoding PXR (NR1I2) or CAR (NR1I3) has therefore been thoroughly investigated in relation to their effects on pharmacokinetics and efficacy of drug treatments, as reviewed comprehensively by Mbatchi et al. [97]. However, the influence of genetic variants within NR1I2 or NR1I3 has primarily been linked to homeostatic regulation of CYP expression in the absence of inflammation. Until now, it remains therefore largely unclear which genetic polymorphisms in NR1I2 or NR1I3 might be candidates for modulating the effects of inflammation on drug metabolism.
Since PXR is regulated by NF-κB, either through direct transcriptional repression or via interference with RXR-PXR binding, we hypothesize that polymorphisms within NR1I2 that present themselves in or near NF-κB binding sites might influence the impact of inflammation on drug metabolism [98]. For this reason we used the computational databases “gene transcription regulation database” (GTRD) and “Alggen PROMO database” for identification of polymorphisms in NR1I2 that would be susceptible to the effects of inflammation [99,100,101]. Using information on confirmed NF-κB binding sites by chromatin immunoprecipitation-sequencing (CHIP-seq) or predicted NF-κB binding spots, we were able to identify four common variants (minor-allele frequency > 0.01) in NR1I2 that are located in or near NF-κB binding spots, as summarized in Table 2. Importantly, the variant NR1I2-rs3814055 that has initially been linked to a NF-κB binding site was not confirmed by this approach, which is in accordance with observations from Dring et al. who also did not find evidence for a NF-κB binding site positioned at the rs2814055 location [102].
Table 2.
SNPs in NR1I2 Located in A Predicted or Confirmed NF-κB Binding Site 1.
| SNP | Variation | Location | Allele Frequency | In Binding Site (Proximity) of: | Distance to Binding Site (bp) | Binding Spot Predicted in: |
|---|---|---|---|---|---|---|
| rs10934498 | G > A, C, T | intron | G = 0.5024 | NFκB1-p105 subunit | 0 | GTRD |
| A = 0.4976 | ||||||
| rs1403526 | A > C, G | Intron | A = 0.64900 | RelA-p65 subunit | 0 | Alggen PROMO |
| G = 0.35100 | ||||||
| rs12721602 | G > A | 5 -UTR | G = 0.98303 | RelA-p65 subunit | 13 | Alggen PROMO |
| A = 0.01697 | ||||||
| rs1054191 | G > A, C | 3′-UTR | G = 0.87745 | NF-κB, NF-κB1 p105 | 17 | Alggen PROMO |
| A = 0.12255 |
1 To cover relevant NF-kB binding sites, we took into consideration the human NF-κB p105 subunit, the NF-κB p100 subunit, and the RelA-p65 subunit binding sites. An arbitrary threshold of 25 base pairs from confirmed or predicted NF-κB binding spot was set to identify relevant NR1I2 SNPs [98]. Matching score to consensus sequence was set at 85% for the Alggen PROMO database. For the GTRD, CHIP-seq derived data was collected from the meta clusters data set. Allele frequencies were obtained from the GnomeAD or 1000Genomes database.
The effects on drug metabolism of these four genetic variants in the NF-kB binding spots in NR1I2 are sparsely reported in the literature. This may suggest that these variants contribute less than other common SNPs within NR1I2 (e.g., rs3814055, rs2472677) to the variability of drug metabolism in the absence of inflammation. However, some data is available from studies conducted in cancer patients. Inflammatory reactions are frequently observed in cancer patients and a common cause of phenoconversion [3,103].
Interestingly, in a cohort of 109 patients with colon cancer, the “inflammatory” variant NR1I2 rs10934498 (G > A) was identified, from a panel of NR1I2 variants, as one of the main determinants of Irinotecan pharmacokinetics [104]. Irinotecan is a prodrug that is converted into its active metabolite SN-38 and subsequently detoxified into SN-38G. Patient with the rs10934498 AA genotype exhibited reduced SN-38 AUC levels and increased metabolic ratios of SN-38G compared to AG or GG carriers, which indicates that the metabolism of Irinotecan is more conserved in patients with the rs10934498 AA genotype. Based on our observation that rs10934498 is located in an NF-κB binding site, we hypothesize that PXR may no longer be downregulated by inflammation in patients carrying the rs10934498 AA genotype, resulting in a conserved drug-metabolizing activity compared to patients lacking this variant.
Altogether, the computational identification of common “inflammatory” variants within NR1I2 suggest that genetic variability may modulate PXR-dependent outcomes of inflammatory signaling. However, further (functional) studies are needed to elucidate the impact of these NR1I2 polymorphisms on drug metabolism in the context of inflammation.
4.5. Genetic Variation: Cytochrome P450 Enzymes
Ultimately, the output of the inflammatory signaling cascade regulates CYP expression and subsequent drug metabolic capacity. Even though it is well established that genetic polymorphisms in CYP enzymes contribute to the interindividual variability in pharmacokinetics [2], it remains uncertain how and to what extent CYP polymorphisms may modulate the impact of inflammation on drug metabolism. Some studies hint towards a genotype-dependent effect of inflammation-induced phenoconversion, as summarized by Klomp et al. [105]. CYP2C19 is highly polymorphic and shown to be affected by inflammation. For example, in a study of 34 patients with an invasive fungal infection receiving voriconazole, it was shown that the effect of inflammation was modulated by the CYP2C19 genotype: the metabolic ratio of voriconazole and its metabolite was more decreased by inflammation in CYP2C19 ultrarapid metabolizers compared to CYP2C19 intermediate metabolizers [106]. Similarly, Ohnishi et al. aimed to investigate the consequences of inflammation for different CYP2C19 genotypes by examining the metabolic ratios of omeprazole and its metabolite in hepatitis C virus (HCV)-positive patients and healthy volunteers [107]. The shift in metabolic ratio between healthy patients and HCV-positive patients was largest for genotype-predicted normal metabolizers (21.1-fold change), followed by intermediate metabolizers (12.4-fold change) and least evident for poor metabolizers. Although these examples only illustrate the effects of inflammation on CYP2C19 mediated drug metabolism, and other CYP isoforms remain to be investigated, they clearly indicate that inflammation-induced changes in CYP450-mediated drug metabolism are affected by an individual’s CYP metabolizer genotype.
5. Conclusions
Concluding, data from in-vitro models have been instrumental to elucidate that CYP isoforms show distinct susceptibility to downregulation by inflammatory mediators wherein CYP3A4 seems to be most affected by inflammation, supporting clinical observations on CYP3A4 drug substrates. Additionally, the pattern of downregulation of CYP isoforms was dependent on the inflammatory stimulus. Interestingly, interindividual variability in response to inflammation is observed in both in-vitro models and clinical studies. Genetic variability in the described pathways via which inflammation modulates the expression and activity of DMEs might in part explain the distinct response towards inflammation between subjects, but this remains to be further investigated. Ultimately, a better understanding of inflammation-induced phenoconversion may aid in optimizing treatment for the individual patient.
Acknowledgments
We are very thankful for the assistance of Jan Schoones of the Walaeus library (LUMC) with the literature search. For creation of the figure, BioRender.com. was used.
Author Contributions
Conceptualization, L.M.d.J., M.L.M. and J.J.S.; writing—original draft preparation, L.M.d.J. and M.L.M.; writing—review and editing, L.M.d.J., M.L.M., W.J. and J.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilkinson G.R. Drug therapy: Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005;352:2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 2.Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Shah R.R., Smith R.L. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: Hypothesis with implications for personalized medicine. Drug Metab. Dispos. 2015;43:400–410. doi: 10.1124/dmd.114.061093. [DOI] [PubMed] [Google Scholar]
- 4.Stanke-Labesque F., Gautier-Veyret E., Chhun S., Guilhaumou R. Inflammation is a major regulator of drug metabolizing enzymes and transporters: Consequences for the personalization of drug treatment. Pharmacol. Ther. 2020 doi: 10.1016/j.pharmthera.2020.107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vet N.J., Brussee J.M., De Hoog M., Mooij M.G., Verlaat C.W.M., Jerchel I.S., Schaik R.H.N.V., Koch B.C.P., Tibboel D., Knibbe C.A.J., et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am. J. Respir. Crit. Care Med. 2016;194:58–66. doi: 10.1164/rccm.201510-2114OC. [DOI] [PubMed] [Google Scholar]
- 6.Van Wanrooy M.J.P., Span L.F.R., Rodgers M.G.G., Van Heuvel E.R.D., Uges D.R.A., Van Werf T.S.D., Kosterink J.G.W., Alffenaara J.W.C. Inflammation is associated with voriconazole trough concentrations. Antimicrob. Agents Chemother. 2014;58:7098–7101. doi: 10.1128/AAC.03820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Y., de Vries D.E., Xu Z., Marciniak S.J., Chen D., Leon F., Davis H.M., Zhou H. Evaluation of disease-mediated therapeutic protein-drug interactions between an anti-interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach. J. Clin. Pharmacol. 2015;55:1386–1394. doi: 10.1002/jcph.561. [DOI] [PubMed] [Google Scholar]
- 8.Davis J.D., Bansal A., Hassman D., Akinlade B., Li M., Li Z., Swanson B., Hamilton J.D., DiCioccio A.T. Evaluation of Potential Disease-Mediated Drug–Drug Interaction in Patients with Moderate-to-Severe Atopic Dermatitis Receiving Dupilumab. Clin. Pharmacol. Ther. 2018;104:1146–1154. doi: 10.1002/cpt.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravel S., Chiasson J.L., Turgeon J., Grangeon A., Michaud V. Modulation of CYP450 Activities in Patients With Type 2 Diabetes. Clin. Pharmacol. Ther. 2019;106:1280–1289. doi: 10.1002/cpt.1496. [DOI] [PubMed] [Google Scholar]
- 10.Sim S.C., Kacevska M., Ingelman-Sundberg M. Pharmacogenomics of drug-metabolizing enzymes: A recent update on clinical implications and endogenous effects. Pharm. J. 2013;13:1–11. doi: 10.1038/tpj.2012.45. [DOI] [PubMed] [Google Scholar]
- 11.Aitken A.E., Richardson T.A., Morgan E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J., Li F. Potential pharmacokinetic interactions of therapeutic cytokines or cytokine modulators on small-molecule drugs: Mechanistic understanding via studies using in vitro systems. Drug Metabol. Drug Interact. 2014;29:17–28. doi: 10.1515/dmdi-2013-0028. [DOI] [PubMed] [Google Scholar]
- 13.Martignoni M., Groothuis G.M.M., de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 14.Aitken A.E., Morgan E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab. Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein M., Thomas M., Hofmann U., Seehofer D., Damm G., Zanger U.M. A systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG Cells. Drug Metab. Dispos. 2015;43:273–283. doi: 10.1124/dmd.114.060962. [DOI] [PubMed] [Google Scholar]
- 16.Febvre-James M., Bruyère A., Le Vée M., Fardel O. The jak1/2 inhibitor ruxolitinib reverses interleukin-6-mediated suppression of drug-detoxifying proteins in cultured human hepatocytes. Drug Metab. Dispos. 2018;46:131–140. doi: 10.1124/dmd.117.078048. [DOI] [PubMed] [Google Scholar]
- 17.Rubin K., Janefeldt A., Andersson L., Berke Z., Grime K., Andersson T.B. HepaRG cells as human-relevant in vitro model to study the effects of inflammatory stimuli on cytochrome P450 isoenzymes. Drug Metab. Dispos. 2015;43:119–125. doi: 10.1124/dmd.114.059246. [DOI] [PubMed] [Google Scholar]
- 18.Dallas S., Sensenhauser C., Batheja A., Singer M., Markowska M., Zakszewski C., Mamidi R.N.V.S., McMillia M., Han C., Zhou H., et al. De-Risking Bio-therapeutics for Possible Drug Interactions Using Cryopreserved Human Hepatocytes. Curr. Drug Metab. 2012;13:923–929. doi: 10.2174/138920012802138589. [DOI] [PubMed] [Google Scholar]
- 19.Dickmann L.J., Patel S.K., Rock D.A., Wienkers L.C., Slatter J.G. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab. Dispos. 2011;39:1415–1422. doi: 10.1124/dmd.111.038679. [DOI] [PubMed] [Google Scholar]
- 20.Sunman J.A., Hawke R.L., LeCluyse E.L., Kashuba A.D.M. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab. Dispos. 2004;32:359–363. doi: 10.1124/dmd.32.3.359. [DOI] [PubMed] [Google Scholar]
- 21.Long T.J., Cosgrove P.A., Dunn R.T., Stolz D.B., Hamadeh H., Afshari C., McBride H., Griffith L.G. Modeling therapeutic antibody-small molecule drug-drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab. Dispos. 2016;44:1940–1948. doi: 10.1124/dmd.116.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.V., Ukairo O., Khetani S.R., McVay M., Kanchagar C., Seghezzi W., Ayanoglu G., Irrechukwu O., Evers R. Establishment of a hepatocyte-kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab. Dispos. 2015;43:774–785. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 23.Tanner N., Kubik L., Luckert C., Thomas M., Hofmann U., Zanger U.M., Böhmert L., Lampen A., Braeuning A. Regulation of drug metabolism by the interplay of inflammatory signaling, steatosis, and xeno-sensing receptors in HepaRG cells. Drug Metab. Dispos. 2018;46:326–335. doi: 10.1124/dmd.117.078675. [DOI] [PubMed] [Google Scholar]
- 24.Dickmann L.J., Patel S.K., Wienkers L.C., Greg Slatter J. Effects of Interleukin 1β (IL-1β) and IL-1β/Interleukin 6 (IL-6) Combinations on Drug Metabolizing Enzymes in Human Hepatocyte Culture. Curr. Drug Metab. 2012;13:930–937. doi: 10.2174/138920012802138642. [DOI] [PubMed] [Google Scholar]
- 25.Le Vée M., Bruyère A., Jouan E., Fardel O. Janus kinase-dependent regulation of drug detoxifying protein expression by interleukin-22 in human hepatic cells. Int. Immunopharmacol. 2020;83:106439. doi: 10.1016/j.intimp.2020.106439. [DOI] [PubMed] [Google Scholar]
- 26.Dallas S., Chattopadhyay S., Sensenhauser C., Batheja A., Singer M., Silva J. Interleukins-12 and -23 do not alter expression or activity of multiple cytochrome p450 enzymes in cryopreserved human hepatocytes. Drug Metab. Dispos. 2013;41:689–693. doi: 10.1124/dmd.112.048884. [DOI] [PubMed] [Google Scholar]
- 27.Taub R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 28.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robak T., Gladalska A., Stepień H., Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediat. Inflamm. 1998;7:347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evers R., Dallas S., Dickmann L.J., Fahmi O.A., Kenny J.R., Kraynov E., Nguyen T., Patel A.H., Slatter J.G., Zhang L. Critical review of preclinical approaches to investigate cytochrome P450-mediated therapeutic protein drug-drug interactions and recommendations for best practices: A white paper. Drug Metab. Dispos. 2013;41:1598–1609. doi: 10.1124/dmd.113.052225. [DOI] [PubMed] [Google Scholar]
- 31.Dluzen D.F., Lazarus P. MicroRNA regulation of the major drug-metabolizing enzymes and related transcription factors. Drug Metab. Rev. 2015;47:320–334. doi: 10.3109/03602532.2015.1076438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeh H., Rudolph N., Billing U., Christen H., Streif S., Bullinger E., Schliemann-Bullinger M., Findeisen R., Schaper F., Huber H.J., et al. Response to IL-6 trans- A nd IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019;17:46. doi: 10.1186/s12964-019-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller R., Klein M., Thomas M., Dräger A., Metzger U., Templin M.F., Joos T.O., Thasler W.E., Zell A., Zanger U.M. Coordinating Role of RXRα in Downregulating Hepatic Detoxification during Inflammation Revealed by Fuzzy-Logic Modeling. PLoS Comput. Biol. 2016;12:e1004431. doi: 10.1371/journal.pcbi.1004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Antona C., Donato M.T., Boobis A., Edwards R.J., Watts P.S., Castell J.V., Gómez-Lechón M.J. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: Molecular mechanisms that determine lower expression in cultured cells. Xenobiotica. 2002;32:505–520. doi: 10.1080/00498250210128675. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parent R., Marion M.J., Furio L., Trépo C., Petit M.A. Origin and Characterization of a Human Bipotent Liver Progenitor Cell Line. Gastroenterology. 2004;126:1147–1156. doi: 10.1053/j.gastro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Szabo G., Petrasek J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann M., Pfeilschifter J., Mühl H. A prominent role of interleukin-18 in acetaminophen-induced liver injury advocates its blockage for therapy of hepatic necroinflammation. Front. Immunol. 2018;9:161. doi: 10.3389/fimmu.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Z., Xu M.-J., Gao B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016 doi: 10.1038/cmi.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahill C.M., Rogers J.T. Interleukin (IL) 1β induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IκB kinase α pathway targeting activator protein-1. J. Biol. Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi J.H., Sun S.C. Tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front. Immunol. 2018;9:1. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchi M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 43.Xie W., Uppal H., Saini S.P.S., Mu Y., Little J.M., Radominska-Pandya A., Zemaitis M.A. Orphan nuclear receptor-mediated xenobiotic regulation in drug metabolism. Drug Discov. Today. 2004;9:442–449. doi: 10.1016/S1359-6446(04)03061-2. [DOI] [PubMed] [Google Scholar]
- 44.Kojima K., Nagata K., Matsubara T., Yamazoe Y. Broad but distinct role of pregnane x receptor on the expression of individual cytochrome p450s in human hepatocytes. Drug Metab. Pharmacokinet. 2007;22:276–286. doi: 10.2133/dmpk.22.276. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y., Ferguson S.S., Negishi M., Goldstein J.A. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol. Pharmacol. 2003;64:316–324. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson S.S., Lecluyse E.L., Negishi M., Goldstein J.A. Regulation of human CYP2C9 by the constitutive androstane receptor: Discovery of a new distal binding site. Mol. Pharmacol. 2002;62:737–746. doi: 10.1124/mol.62.3.737. [DOI] [PubMed] [Google Scholar]
- 47.Xie W., Barwick J.L., Simon C.M., Pierce A.M., Safe S., Blumberg B., Guzelian P.S., Evans R.M. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascussi J.M., Gerbal-Chaloin S., Pichard-Garcia L., Daujat M., Fabre J.M., Maurel P., Vilarem M.J. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem. Biophys. Res. Commun. 2000;274:707–713. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- 49.Sun H.Y., Yan Y.J., Li Y.H., Lv L. Reversing effects of ginsenosides on LPS-induced hepatic CYP3A11/3A4 dysfunction through the pregnane X receptor. J. Ethnopharmacol. 2019;229:246–255. doi: 10.1016/j.jep.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Beigneux A.P., Moser A.H., Shigenaga J.K., Grunfeld C., Feingold K.R. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem. Biophys. Res. Commun. 2002;293:145–149. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 51.Yang J., Hao C., Yang D., Shi D., Song X., Luan X., Hu G., Yan B. Pregnane X receptor is required for interleukin-6-mediated down-regulation of cytochrome P450 3A4 in human hepatocytes. Toxicol. Lett. 2010;197:219–226. doi: 10.1016/j.toxlet.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X., Li X., Wang X., Jin X., Shi D., Wang J., Bi D. Cecropin B represses CYP3A29 expression through activation of the TLR2/4-NF-κ B/PXR signaling pathway. Sci. Rep. 2016;6 doi: 10.1038/srep27876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assenat E., Gerbal-Chaloin S., Larrey D., Saric J., Fabre J.M., Maurel P., Vilarem M.J., Pascussi J.M. Interleukin 1β inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40:951–960. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- 54.Richardson T.A., Morgan E.T. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J. Pharmacol. Exp. Ther. 2005;314:703–709. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- 55.Chung I., Bresnick E. Regulation of the constitutive expression of the human CYP1A2 gene: Cis elements and their interactions with proteins. Mol. Pharmacol. 1995;47:677–685. [PubMed] [Google Scholar]
- 56.Kamiyama Y., Matsubara T., Yoshinari K., Nagata K., Kamimura H., Yamazoe Y. Role of Human Hepatocyte Nuclear Factor 4α in the Expression of Drug-Metabolizing Enzymes and Transporters in Human Hepatocytes Assessed by Use of Small Interfering RNA. Drug Metab. Pharmacokinet. 2007;22:287–298. doi: 10.2133/dmpk.22.287. [DOI] [PubMed] [Google Scholar]
- 57.Tirona R.G., Lee W., Leake B.F., Lan L.B., Cline C.B., Lamba V., Parviz F., Duncan S.A., Inoue Y., Gonzalez F.J., et al. The orphan nuclear receptor HNF4α determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat. Med. 2003;9:220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Jiménez C.P., Castell J.V., Gómez-Lechón M.J., Jover Atienza R. Transcriptional activation of CYP2C9, CYP1A1, and CYP1A2 by hepatocyte nuclear factor 4α requires coactivators peroxisomal proliferator activated receptor-γ coactivator 1α and steroid receptor coactivator 1. Mol. Pharmacol. 2006;70:1681–1692. doi: 10.1124/mol.106.025403. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson S.S., Chen Y., LeCluyse E.L., Negishi M., Goldstein J.A. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4α. Mol. Pharmacol. 2005;68:747–757. doi: 10.1124/mol.105.013169. [DOI] [PubMed] [Google Scholar]
- 60.Cheng P.Y., Wang M., Morgan E.T. Rapid Transcriptional Suppression of Rat Cytochrome P450 Genes by Endotoxin Treatment and Its Inhibition by Curcumin. J. Pharmacol. Exp. Ther. 2003;307:1205–1212. doi: 10.1124/jpet.103.057174. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Salisbury-Rowswell J., Murdock A.D., Forse R.A., Burke P.A. Hepatocyte nuclear factor 4 response to injury involves a rapid decrease in DNA binding and transactivation via a JAK2 signal transduction pathway. Biochem. J. 2002;368:203–211. doi: 10.1042/bj20020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu X., Ke S., Liu D., Sheng T., Thomas P.E., Rabson A.B., Gallo M.A., Xie W., Tian Y. Role of NF-κB in regulation of PXR-mediated gene expression: A mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J. Biol. Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 63.Tian Y., Ke S., Denison M.S., Rabson A.B., Gallo M.A. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 64.Ke S., Rabson A.B., Germino J.F., Gallo M.A., Tian Y. Mechanism of Suppression of Cytochrome P-450 1A1 Expression by Tumor Necrosis Factor-α and Lipopolysaccharide. J. Biol. Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 65.Ding X., Staudinger J.L. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem. Pharmacol. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 66.Lichti-Kaiser K., Xu C., Staudinger J.L. Cyclic AMP-dependent protein kinase signaling modulates Pregnane x receptor activity in a species-specific manner. J. Biol. Chem. 2009;284:6639–6649. doi: 10.1074/jbc.M807426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taneja G., Chu C., Maturu P., Moorthy B., Ghose R. Role of c-Jun-N-terminal kinase in pregnane X receptor-mediated induction of human cytochrome P4503A4 in vitro. Drug Metab. Dispos. 2018;46:397–404. doi: 10.1124/dmd.117.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D., Zimmerman T.L., Thevananther S., Lee H.Y., Kurie J.M., Karpen S.J. Interleukin-1β-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J. Biol. Chem. 2002;277:31416–31422. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- 69.Shah P., Omoluabi O., Moorthy B., Ghose R. Role of adaptor protein toll-like interleukin domain containing adaptor inducing interferon b in toll-like receptor 3- and 4-mediated regulation of hepatic drug metabolizing enzyme and transporter genes. Drug Metab. Dispos. 2016;44:61–67. doi: 10.1124/dmd.115.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghose R., Zimmerman T.L., Thevananther S., Karpen S.J. Endotoxin leads to rapid subcellular re-localization of hepatic RXRα: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl. Recept. 2004;2 doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koike C., Moore R., Negishi M. Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear Constitutive Active/androstane Receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol. Pharmacol. 2007;71:1217–1221. doi: 10.1124/mol.107.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zordoky B., El-Kadi A. Role of NF-kB in the Regulation of Cytochrome P450 Enzymes. Curr. Drug Metab. 2009;10:164–178. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]
- 73.Iber H., Chen Q., Cheng P.Y., Morgan E.T. Suppression of CYP2C11 gene transcription by interleukin-1 mediated by NF-κB binding at the transcription start site. Arch. Biochem. Biophys. 2000;377:187–194. doi: 10.1006/abbi.2000.1772. [DOI] [PubMed] [Google Scholar]
- 74.Ruminy P., Gangneux C., Claeyssens S., Scotte M., Daveau M., Salier J.P. Gene transcription in hepatocytes during the acute phase of a systemic inflammation: From transcription factors to target genes. Inflamm. Res. 2001;50:383–390. doi: 10.1007/PL00000260. [DOI] [PubMed] [Google Scholar]
- 75.Jover R., Bort R., Gómez-Lechón M.J., Castell J.V. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: Molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–1801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- 76.Martínez-Jiménez C.P., Gómez-Lechón M.J., Castell J.V., Jover R. Transcriptional regulation of the human hepatic CYP3A4: Identification of a new distal enhancer region responsive to CCAAT/enhancer-binding protein β isoforms (liver activating protein and liver inhibitory protein) Mol. Pharmacol. 2005;67:2088–2101. doi: 10.1124/mol.104.008169. [DOI] [PubMed] [Google Scholar]
- 77.Pitarque M., Rodríguez-Antona C., Oscarson M., Ingelman-Sundberg M. Transcriptional regulation of the human CYP2A6 gene. J. Pharmacol. Exp. Ther. 2005;313:814–822. doi: 10.1124/jpet.104.081570. [DOI] [PubMed] [Google Scholar]
- 78.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 79.Li D., Tolleson W.H., Yu D., Chen S., Guo L., Xiao W., Tong W., Ning B. Pharmacoepigenetics. Elsevier; Amsterdam, The Netherlands: 2019. MicroRNA-Dependent Gene Regulation of the Human Cytochrome P450; pp. 129–138. [Google Scholar]
- 80.Papageorgiou I., Court M.H. Identification and validation of microRNAs directly regulating the UDP-glucuronosyltransferase 1A subfamily enzymes by a functional genomics approach. Biochem. Pharmacol. 2017;137:93–106. doi: 10.1016/j.bcp.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kugler N., Klein K., Zanger U.M. MiR-155 and other microRNAs downregulate drug metabolizing cytochromes P450 in inflammation. Biochem. Pharmacol. 2020;171 doi: 10.1016/j.bcp.2019.113725. [DOI] [PubMed] [Google Scholar]
- 82.Morgan E.T., Skubic C., Lee C.M., Cokan K.B., Rozman D. Regulation of cytochrome P450 enzyme activity and expression by nitric oxide in the context of inflammatory disease. Drug Metab. Rev. 2020;52:455–471. doi: 10.1080/03602532.2020.1817061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rae J.M., Johnson M.D., Lippman M.E., Flockhart D.A. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: Studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 2001;299:849–857. [PubMed] [Google Scholar]
- 84.Eichelbaum M., Mineshita S., Ohnhaus E., Zekorn C. The influence of enzyme induction on polymorphic sparteine oxidation. Br. J. Clin. Pharmacol. 1986;22:49–53. doi: 10.1111/j.1365-2125.1986.tb02879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madan A., Graham R.A., Carroll K.M., Mudra D.R., Alayne Burton L., Krueger L.A., Downey A.D., Czerwinski M., Forster J., Ribadeneira M.D., et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab. Dispos. 2003;31:421–431. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 86.Hill A.V.S. Evolution, revolution and heresy in the genetics of infectious disease susceptibility. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:840–849. doi: 10.1098/rstb.2011.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X., Xu J., Fan J., Zhang T., Li Y., Xie B., Zhang W., Lin S., Ye L., Liu Y., et al. Influence of IL-18 and IL-10 Polymorphisms on Tacrolimus Elimination in Chinese Lung Transplant Patients. Dis. Markers. 2017;2017 doi: 10.1155/2017/7834035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xing J., Zhang X., Fan J., Shen B., Men T., Wang J. Association between interleukin-18 promoter variants and tacrolimus pharmacokinetics in Chinese renal transplant patients. Eur. J. Clin. Pharmacol. 2015;71:191–198. doi: 10.1007/s00228-014-1785-8. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Zou Y., Cai B., Yang B., Ying B., Shi Y., Wang L. The associations of IL-18 serum levels and promoter polymorphism with tacrolimus pharmacokinetics and hepatic allograft dysfunction in Chinese liver transplantation recipients. Gene. 2012;491:251–255. doi: 10.1016/j.gene.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 90.Dar W.A., Sullivan E., Bynon J.S., Eltzschig H., Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ou B., Liu Y., Zhang T., Sun Y., Chen J., Peng Z. TLR9 rs352139 Genetic Variant Promotes Tacrolimus Elimination in Chinese Liver Transplant Patients During the Early Posttransplantation Period. Pharmacotherapy. 2019;39:67–76. doi: 10.1002/phar.2204. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z., Wu S., Chen D., Guo F., Zhong L., Fan J., Peng Z. Influence of TLR4 rs1927907 locus polymorphisms on tacrolimus pharmacokinetics in the early stage after liver transplantation. Eur. J. Clin. Pharmacol. 2014;70:925–931. doi: 10.1007/s00228-014-1673-2. [DOI] [PubMed] [Google Scholar]
- 93.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karban A.S., Okazaki T., Panhuysen C.I.M., Gallegos T., Potter J.J., Bailey-Wilson J.E., Silverberg M.S., Duerr R.H., Cho J.H., Gregersen P.K., et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum. Mol. Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y., Li J.L., Fu Q., Wang X.D., Liu L.S., Wang C.X., Xie W., Chen Z.J., Shu W.Y., Huang M. Associations of ABCB1, NFKB1, CYP3A, and NR1I2 polymorphisms with cyclosporine trough concentrations in Chinese renal transplant recipients. Acta Pharmacol. Sin. 2013;34:555–560. doi: 10.1038/aps.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao G., Liu M., Wu X., Li G., Qiu F., Gu J., Zhao L. Effect of polymorphisms in CYP3A4, PPARA, NR1I2, NFKB1, ABCG2 and SLCO1B1 on the pharmacokinetics of lovastatin in healthy Chinese volunteers. Pharmacogenomics. 2017;18:65–75. doi: 10.2217/pgs.16.31. [DOI] [PubMed] [Google Scholar]
- 97.Mbatchi L.C., Brouillet J.P., Evrard A. Genetic variations of the xenoreceptors NR1I2 and NR1I3 and their effect on drug disposition and response variability. Pharmacogenomics. 2018;19:61–77. doi: 10.2217/pgs-2017-0121. [DOI] [PubMed] [Google Scholar]
- 98.Mulero M.C., Wang V.Y.F., Huxford T., Ghosh G. Genome reading by the NF-κB transcription factors. Nucleic Acids Res. 2019;47:9967–9989. doi: 10.1093/nar/gkz739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farré D., Roset R., Huerta M., Adsuara J.E., Roselló L., Albà M.M., Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Messeguer X., Escudero R., Farré D., Núñez O., Martínez J., Albà M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 101.Yevshin I., Sharipov R., Kolmykov S., Kondrakhin Y., Kolpakov F. GTRD: A database on gene transcription regulation—2019 update. Nucleic Acids Res. 2019;47:D100–D105. doi: 10.1093/nar/gky1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dring M.M., Goulding C.A., Trimble V.I., Keegan D., Ryan A.W., Brophy K.M., Smyth C.M., Keeling P.W.N., O’Donoghue D., O’Sullivan M., et al. The Pregnane X Receptor Locus Is Associated with Susceptibility to Inflammatory Bowel Disease. Gastroenterology. 2006;130:341–348. doi: 10.1053/j.gastro.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Slaviero K.A., Clarke S.J., Rivory L.P. Inflammatory response: An unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003;4:224–232. doi: 10.1016/S1470-2045(03)01034-9. [DOI] [PubMed] [Google Scholar]
- 104.Mbatchi L.C., Robert J., Ychou M., Boyer J.C., Del Rio M., Gassiot M., Thomas F., Tubiana N., Evrard A. Effect of Single Nucleotide Polymorphisms in the Xenobiotic-sensing Receptors NR1I2 and NR1I3 on the Pharmacokinetics and Toxicity of Irinotecan in Colorectal Cancer Patients. Clin. Pharmacokinet. 2016;55:1145–1157. doi: 10.1007/s40262-016-0392-5. [DOI] [PubMed] [Google Scholar]
- 105.Klomp S.D., Manson M.L., Guchelaar H.-J., Swen J.J. Phenoconversion of Cytochrome P450 Metabolism: A Systematic Review. J. Clin. Med. 2020;9:2890. doi: 10.3390/jcm9092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veringa A., ter Avest M., Span L.F.R., van den Heuvel E.R., Touw D.J., Zijlstra J.G., Kosterink J.G.W., van der Werf T.S., Alffenaar J.W.C. Voriconazole metabolism is influenced by severe inflammation: A prospective study. J. Antimicrob. Chemother. 2017;72:261–267. doi: 10.1093/jac/dkw349. [DOI] [PubMed] [Google Scholar]
- 107.Ohnishi A., Murakami S., Akizuki S., Mochizuki J., Echizen H., Takagi I. In vivo metabolic activity of CYP2C19 and CYP3A in relation to CYP2C19 genetic polymorphism in chronic liver disease. J. Clin. Pharmacol. 2005;45:1221–1229. doi: 10.1177/0091270005280787. [DOI] [PubMed] [Google Scholar]