Abstract
Simple Summary
Doublecortin-like kinase 1 (DCLK1) is a tumor stem cell marker in colon, pancreatic, and potentially other cancers that has received wide attention recently. Aside from its role as a tuft cell marker in normal tissue and as a tumor stem cell marker in cancer, previous studies have demonstrated that silencing DCLK1 functionally reduces stemness, epithelial mesenchymal transition (EMT), and tumorigenesis in cancers. More recently, DCLK1′s role in regulating the inflammatory, pre-cancer, and tumor microenvironment including its ability to modulate immune cell mechanisms has started to come into focus. Importantly, clinically viable therapeutic means of targeting DCLK1 have finally become available in the form of kinase inhibitors, monoclonal antibodies, and chimeric antigen receptor T cells (CAR-T). Herein, we comprehensively review the mechanistic role of DCLK1 in the tumor microenvironment, assess the potential for targeting DCLK1 in colon, pancreatic and renal cancer.
Abstract
Microtubule-associated doublecortin-like kinase 1 (DCLK1) is an accepted marker of tuft cells (TCs) and several kinds of cancer stem cells (CSCs), and emerging evidence suggests that DCLK1-positive TCs participate in the initiation and formation of inflammation-associated cancer. DCLK1-expressing CSCs regulate multiple biological processes in cancer, promote resistance to therapy, and are associated with metastasis. In solid tumor cancers, tumor epithelia, immune cells, cancer-associated fibroblasts, endothelial cells and blood vessels, extracellular matrix, and hypoxia all support a CSC phenotype characterized by drug resistance, recurrence, and metastasis. Recently, studies have shown that DCLK1-positive CSCs are associated with epithelial-mesenchymal transition, angiogenesis, and immune checkpoint. Emerging data concerning targeting DCLK1 with small molecular inhibitors, monoclonal antibodies, and chimeric antigen receptor T-cells shows promising effects on inhibiting tumor growth and regulating the tumor immune microenvironment. Overall, DCLK1 is reaching maturity as an anti-cancer target and therapies directed against it may have potential against CSCs directly, in remodeling the tumor microenvironment, and as immunotherapies.
Keywords: DCLK1, tuft cells, cancer stem cells, microenvironment, immunotherapies
1. Introduction
Microtubule-associated doublecortin-like kinase 1 (DCLK1) was originally thought to be a brain-specific protein before 2006 [1] when Giannakis et al. first reported DCLK1 as a potential marker of stem-like cells of the small intestine [2]. However, further research has identified these cells as differentiated tuft cells (TCs) possessing a variety of unique molecular and functional characteristics [3]. DCLK1+ tuft cells of the gastrointestinal tract are characterized by microvilli and may be long-lived and display self-renewal or progenitor functionality under some conditions [4,5,6]. Importantly, they regulate the immune microenvironment through IL-25/IL-17RB signaling in order to affect epithelial repair after injury, and may initiate inflammation-associated tumorigenesis after mutation [7,8,9,10,11,12]. In 2008, the Houchen group proposed that DCLK1 is a specific marker protein for intestinal adenoma stem cells [13], which brought attention to DCLK1 in cancer research and was the first of a series of research reports providing evidence that it might be an effective target for oncology drug development. To date, DCLK1 has been demonstrated to be a relatively selective marker of several kinds of cancer stem-like cells or cancer stem cells (CSCs) including in colon, breast, pancreas, kidney, and esophageal cancers [14,15,16,17]. After twenty years of research, DCLK1 is accepted as a specific marker of TCs and several kinds of CSCs, and is well known for its ability to regulate tumor growth, invasion, metastasis, epithelial-mesenchymal transition (EMT), pluripotency, angiogenesis, and pro-survival signaling [18,19,20,21].
CSCs are an important subpopulation of cells in the immunosuppressive tumor microenvironment (TME), which in turn provides a niche to support stem cell characteristics including self-renewal, differentiation, and immunosuppressive cell recruitment. Tumors create an immunosuppressive microenvironment by secreting a variety of chemokines and cytokines which may recruit tumor associated macrophages (TAM), tumor associated neutrophils (TAN), myeloid derived suppressor cells (MDSC), and other regulatory immune cells. TAM and TAN differentiate from polarized macrophages and neutrophils respectively, and remodel the TME to support tumor growth and angiogenesis [22]. TAM have been shown to promote the degradation of extracellular matrix and secrete exosomes containing mRNA and miRNA which ultimately promote tumor invasion and metastasis. Both TAM and CD4+ T-cells secrete tumor necrosis factor alpha (TNF-α) and up-regulate NF-κB signal pathway to induce the expression of EMT transcription factors Snail and Twist [23]. Moreover, they enhance transforming growth factor-β (TGF-β) signaling to promote the self-renewal of CSCs [24]. Presently, CSCs are considered a key driver of chemotherapy resistance, recurrence, and metastasis. Recent work shows that DCLK1 promotes CSC self-renewal and drug-resistance and can be targeted to inhibit tumorigenesis in kidney cancer [25]. Furthermore, several recent studies show that DCLK1 affects tumor growth and metastasis via regulating TAM and immune checkpoint. Finally, monoclonal antibodies and chimeric antigen receptor T-Cells (CAR-T) based on DCLK1 have demonstrated potential as novel cancer immunotherapies [26,27,28]. Herein we review key advances in the understanding of DCLK1 and DCLK1+ TCs function in the context of the tumor and immune microenvironment and discuss future directions for DCLK1-based research and development.
2. Function of DCLK1-Expressing Gastrointestinal Tuft Cells
TCs are present above the +4 position of the intestinal crypt and in the villus where they function as a chemosensory and secretory cell type. Additionally, TCs are found in the respiratory tract, salivary gland, gallbladder, pancreatic duct, auditory tube, urethra, and thymus [29,30,31,32,33]. The majority within the intestinal epithelium express DCLK1, and accumulating evidence suggests that DCLK1+ tuft cells take part in a diffuse chemosensory system where they serve a sentinel function to detect chemical signals in the microenvironment and orchestrate the repair of local epithelial tissue [12]. For instance, TCs located in the lung, colon and stomach epithelium can sense alterations to pH, nutrients, or the microbiota using taste receptors including GTP-binding protein α-gustducin and transient receptor potential cation channel subfamily M member 5 (TRPM5), or regulate capillary resistance to hypoxia by inducing an epithelial response via secretion of IL-25, leading to the activation of innate lymphoid type 2 cells (ILC2) and IL-13 secretion [34,35,36,37]. Using a transgenic intestinal epithelium specific DCLK1 knockout mouse model (VillinCre;Dclk1fl/fl), May et al. reported that DCLK1 deletion in tuft cells resulted in altered gene expression in pathways for epithelial growth, stemness, barrier function, and taste reception signaling further suggesting its importance in TCs [9]. Furthermore, several studies have reported that DCLK1-expressing TCs secrete various kinds of regulatory molecules such as leukotrienes, prostaglandins, nitric oxide and IL-25, which lead to ILC2 and LGR5+ stem cell-mediated tuft and goblet cell differentiation in chronic inflammation and injury [35,38]. This is a key emerging area of interest that will provide new knowledge about the inflammatory, pre-cancer, and tumor microenvironments as well as immune–tumor interactions as they relate to tumorigenesis and progression.
There is strong evidence that DCLK1 expression in TCs play an important functional role in epithelial repair processes of the gut. Intestinal epithelium-specific knockout of DCLK1 (VilCre;Dclk1flox/flox) leads to increased severity of injury and death in mouse whole body irradiation and dextran sulfate sodium (DSS)-induced colitis models [6,9,39]. A recent study expounded on this idea more directly. Yi et al. reported the deletion of DCLK1 in the mucin-type O-glycan deficient model of ulcerative colitis (UC) resulted in greater severity of disease characterized by enhanced mucosal thickening and increased inflammatory cell infiltration. They found that in the absence of DCLK1, epithelial proliferative responses to chronic inflammation were impaired. However, the deletion of DCLK1 did not affect the numbers of intact TCs. These results indicate that DCLK1 expression is a regulator of TC activation status, despite not being involved in TC expansion [10]. Moreover, this function has consequences to the entire intestinal epithelial response to injury as supported by previous findings [6,9,39]. Although these findings are highly suggestive, further studies will be necessary to fully determine the exact mechanisms by which DCLK1 in TCs regulate this response.
DCLK1-expressing TC expansion has been observed in human Barrett’s esophagus, chronic gastritis in transgenic mice, rat gastric mucosa and intestinal neoplasia [14,40,41]. While TCs are not usually proliferative, it appears that mutations acquired by stem cells or progenitors can be passed on to TCs, which might then interconvert into tumor initiating cells under inflammatory or injurious conditions. Alternatively, putative “long-lived” TCs might acquire and maintain mutations, finally initiating tumorigenesis after a secondary insult such as colitis [6]. During the early stages of tumorigenesis, DCLK1+ TC expansion is observed in the gastrointestinal niche where they interact with neurons and promote tumorigenesis by secreting acetylcholine to stimulate enteric nerves. Notably, intestinal epithelial cells can express acetylcholine receptors to activate Wnt signaling and regulate the differentiation of intestinal epithelial cells which may be required for tumorigenesis [42]. Using lineage tracing mouse models, Nakanishi et al. and Westphalen et al. concurrently demonstrated the DCLK1+ TC’s cell-of-origin status in Wnt-driven tumorigenesis. In the Nakanishi study, the ApcMin/+ model of intestinal polyposis was crossed with a Dclk1Cre-ERT mouse to generate lineage tracing (ApcMin/+;Dclk1Cre-ERT;R26LacZ) and diptheria-toxin receptor TC-specific deletion (ApcMin/+;Dclk1Cre-ERT;iDTR) mice. Dclk1+ TC-based lineage tracing specifically traced the entirety of the adenoma in these mice. In comparison, an intestinal stem cell marker Lgr5-based model traced the entirety of the normal epithelium and the polyp. Moreover, deletion of DCLK1+ TCs using the diptheria-toxin receptor model resulted in a complete collapse of polyps within days [43]. The Westphalen study made use of an alternative Dclk1Cre model which was crossed to an Apcflox/flox mouse. In this model, spontaneous tumorigenesis did not occur. However, lineage tracing experiments demonstrated a small, but abnormally long-lived, population of DCLK1+ TCs in the intestinal epithelium. In conditions of colitis induced chemically via DSS, these long-lived TCs gave rise to tumors with a severe adenocarcinoma-like phenotype [6]. Importantly, this study was the first to ascertain the existence of multiple functionally unique populations of TCs. This finding has now been confirmed by single-cell RNA-Sequencing studies which identified a separate immunomodulatory population of TCs [44].
In summary, DCLK1-expressing TCs play an important role in stimulating gastrointestinal epithelial stem cells in the microenvironment and contributing to cancer progression [45]. Moreover, studying the two distinct subpopulations of TCs separately may clarify their dual-role in epithelial restitution and tumorigenesis. Promisingly, specific markers for each TC subtype have already been identified [44]. Finally, limited evidence suggests that DCLK1-expressing intestinal TCs in the gut can promote tumor progression in hepatocellular carcinoma (HCC) through activating alternative macrophages in tumor microenvironment via secreting IL-25 [46]. This distant signaling functionality across the gut-liver axis adds an interesting new dimension to understanding the role of TCs.
3. Function of DCLK1+ Acinar and Tuft Cells in Pancreatitis and Pancreatic Cancer
In the pancreas, DCLK1 is a marker of a population of pancreatic cancer-initiating cells, some of which have morphological and molecular features of gastrointestinal TCs [47]. However, DCLK1 also notably marks pancreatic acinar cells, which are a likely source of tumorigenesis through the acinar-ductal metaplasia process. Genetic lineage tracing experiments show that Dclk1+ pancreatic epithelial cells are necessary for pancreatic regeneration following injury and chronic inflammation. Moreover, KRAS mutation in Dclk1+ pancreatic epithelial cells leads to pancreatic cancer in the presence of induced pancreatitis [43]. In pancreatic tumors, it has recently been shown that immune cell-derived IL-17 regulates the development of TCs via increased expression of DCLK1, POU domain class 2 transcription factor 3 (POU2F3), aldehyde dehydrogenase 1 family member A1 (ALDH1A1), and IL17RC [48]. Intriguingly, DCLK1 kinase inhibitor can inhibit DCLK1+ organoids derived from pancreatic ductal adenocarcinoma patient tumors, indicating that DCLK1 activity and perhaps DCLK1+ TCs or acinar cells are a potential target for pancreatic ductal adenocarcinoma [49]. Although the underlying signaling mechanisms of DCLK1+ epithelial cell-mediated tumorigenesis require further elaboration, DCLK1 and DCLK1+ epithelial cells such as TCs are likely to be a target for new classes of immunotherapies and TME-remodeling drugs in gastrointestinal tract cancers.
4. Interactions between DCLK1 and the Tumor Microenvironment
CSCs depend on the surrounding microenvironment to maintain immune evasion, EMT, drug efflux, DNA repair, signaling pathway regulation, metabolic reprogramming, and epigenetic reprogramming to enhance tumor metastasis, multi-drug resistance and antitumor immunity [50]. Hypoxia is a key regulator of the TME and evidence indicates that DCLK1-positive colorectal cancer cells have increased stemness in hypoxic conditions [51]. Hypoxia famously induces CSCs to express hypoxia inducible factor (HIF) which is a key factor in inducing vascular endothelial growth factor (VEGF) and angiogenesis, which in turn further fuel CSCs. Knockdown of DCLK1 with siRNA or downregulation of DCLK1 with a kinase inhibitor (XMD8-92) results in decreased expression of angiogenic markers/VEGF receptors (VEGFR1 and VEGFR2) and EMT-related transcription factors ZEB1, ZEB2, Snail and Slug [19,52] in pancreatic tumor xenografts. Hypoxia can also be increased epigenetically by histone lysine demethylase 3A (KDM3A) overexpression in pancreatic cancer cells, which leads to increased expression of DCLK1. Knockdown of KDM3A in this context results in reduced invasion, spheroid formation, and orthotopic tumor formation [53]. Finally, in renal cell carcinoma, siRNA-mediated knockdown of DCLK1 significantly sensitized co-cultured endothelial cells to the vascular endothelial growth factor receptor (VEGFR) inhibitor sunitinib in an in vitro angiogenesis assay, demonstrating that expression of DCLK1 on neoplastic cells directly modulates this component of the tumor microenvironment. However, further studies will be needed to determine if this effect is direct [17]. Together these results link the activity of DCLK1, hypoxia, and angiogenesis and further research should be promising.
EMT and CSCs are both linked by key biological characteristics, such as resistance to cytotoxic T lymphocytes (CTLs) and reliance on TGF-β signaling pathway [54]. Microenvironmental changes, such as hypoxia, induce CSCs and in turn CSCs maintain plasticity in their niche via inflammation, EMT, and hypoxia through various signaling pathways [55]. Strong evidence demonstrates that DCLK1 is a regulator of EMT in gastric, colorectal, pancreatic, breast, renal, and other cancers [56,57]. EMT is defined as cellular phenotypic changes from epithelial to mesenchymal type with high expression of N-cadherin and Vimentin [58], and is further associated with TGF-β signaling pathway and functional migration, invasion, metastasis, extracellular matrix (ECM) alteration, apoptosis and drug resistance [59]. Enhanced EMT features after exposure to inflammatory cytokines (i.e., TGF-β, interferon gamma (IFN-γ) and TNF-α) can impact proliferation, differentiation and apoptosis of natural killer cells (NKs) and T and B cells [60], suggesting the importance of EMT in the tumor immune microenvironment. Indeed, evidence suggests that blocking TGF-β signaling may sensitize tumors to immune checkpoint inhibitors [61], and checkpoint ligand programmed cell death 1 (PD-1) ligand (PD-L1) is frequently upregulated in EMT-high tumors.
Additionally, miRNAs are known to play an important role in regulating EMT [62]. Members of miR-200 family directly inhibit ZEB1/ZEB2 activity and overexpression of the miR-200 family can suppress EMT and sensitize cancer cells to chemotherapeutic agents [63]. Knockdown of DCLK1 expression leads to down-regulation of miR-200a, miR-144, and miR-let7a along with downregulation of EMT-associated transcription factors ZEB1, ZEB2, Snail, Slug, and Twist in human pancreatic and colon cancer cells [20,64]. Therefore, DCLK1-mediated EMT could be a target in decreasing HIF levels to regulate angiogenesis and suppress migration by inhibiting cell-to-cell adhesion. Moreover, targeting DCLK1-mediated EMT to regulate TGF-β pathway may alter resistance to CTLs and other anti-tumor immune cells. Blocking DCLK1-mediated EMT also may damage CSC homeostatic processes through several correlated signaling pathways. A study using lung cancer models showed that downregulation of miR-200 family members and upregulation of ZEB1 not only drive EMT, but also lead to upregulation of the PD-L1 in association with exhaustion of intratumoral CD8+ T lymphocytes, which ultimately promotes metastasis [65]. These results suggest that targeting DCLK1-mediated EMT may increase PD-L1 regulated CD8+ T lymphocyte infiltration via regulation of the miR-200 family. Indeed, some evidence shows that DCLK1 marked CSCs support growth, metastasis, and escape from eradication in the tumor microenvironment [25,66,67]. These results are not the only ones demonstrating a relationship between DCLK1 and miRNA activity. Razi et al. showed that DCLK1 is expressed at higher levels in colorectal cancer (CRC) tissue compared to pre-cancerous polyps and that it is inversely correlated with the expression of functional tumor suppressor miRNAs miR-137 and miR-15a. The combined effect of miR-137/miR-15a loss could be significant in CRC as loss of the first is associated with more severe pathological characteristics, and loss of the second has anti-apoptotic, pro-proliferative, and pro-invasive effects [68].
Another key area of focus for DCLK1′s role in the tumor microenvironment involves its basic activity in cell signal transduction. Unlike other prominent target kinases, little is known about DCLK1′s ligands, interacting proteins, and substrates. This perhaps results from the difficulty in studying DCLK1′s complex isoforms, two of which are initiated from an upstream CpG-island regulated promoter (alpha-promoter) and another two of which are initiated from a downstream TATA-box promoter (beta-promoter). However, strides in understanding DCLK1′s basic molecular function have been made in recent years. Notably, DCLK1 has been identified as a potential RAS effector and activator in multiple studies, and DCLK1 expression in pancreatic cancer patients is correlated with RAS downstream signaling pathways ERK, PI3K, and MTOR [49,69,70,71]. DCLK1-AL (transcribed from the α-promoter and characterized by a lengthened C-terminus) can complex with RAS and increase GTP-bound active RAS [71].
Kato et al. showed that loss of the G9a (EHMT2) histone methyl transferase results in a decrease in the number of Dclk1-positive cells and correlated reduction in Erk phosphorylation in mouse pancreatic intraepithelial neoplasia (mPanIN) lesions of a pancreatic cancer mouse model [70], which concurs with findings in the Dclk1Cre;KrasLSL-G12D model of pancreatic tumorigenesis [69]. Ferguson et al. also provided evidence for the importance of the interaction between DCLK1 and ERK in a subset of KRAS-mutant pancreatic cancers [49]. In regards to substrates of DCLK1, Liu et al. used the novel and specific inhibitor DCLK1-IN-1 as a tool to identify several candidates including ERK2, GSK3B, CDK1, CDK2, CHK1, and PKACA. Additional potential substrates in nucleic acid processing such as CDK11, MATR3, and DNA topoisomerase 2-beta (TOP2B) were also identified and phosphopeptides including TOP2B, CDK11B, and MATR3 were significantly decreased after treatment with DCLK1-IN-1. Pathway analysis suggested substrate involvement in RNA processing, insulin signaling, ErbB signaling, proteoglycan synthesis, and maintenance of focal adhesion and tight junction pathways [72]. Finally, Koizumi et al. experimentally identified MAP7D1 (microtubule-associated protein 7 domain containing 1) as a substrate of DCLK1 in cortical neurons and the phosphomimetic MAP7D1 fully rescued the impaired callosal axon elongation in neurons after DCLK1 knockdown [73]. All together, these findings are some of the first to unravel DCLK1′s complex molecular mechanisms and may have implications for future translational research and biomarker development.
5. Regulation of Immune Checkpoint and Macrophage Polarization by DCLK1
The tumor immune microenvironment (TIME) refers to the microenvironment as it relates to immune cells including: infiltrated-excluded TIME in which there is a relative lack of cytotoxic T lymphocytes in the core location of the tumor; infiltrated–inflamed TIME in which infiltration occurs to a large degree with expression of immune negative regulatory receptor PD-1 of CTLs and inhibitory PD-L1 of leukocytes; and tertiary lymphoid structure TIME, which contains a large number of lymphocytes, including initial and activated T-cells, regulatory T-cells, B cells, and dendritic cells [74]. Overexpression of PD-L1 on tumor cells inhibits the activation of immune cells by binding PD-1 on the surface of T cells after a T-cell receptor binds to cancer cells to promote PD-1 expression. PD-1 and PD-L1 antibodies can block PD-1/PD-L1 co-inhibition signaling and relieve the inhibition of T cells and induce their cytotoxicity. A recent study reported that DCLK1 regulates the level of PD-L1 expression by affecting the corresponding expression level of yes-associated protein (YAP) in the Hippo pathway in pancreatic tumors [26]. These findings concur with others in renal cancer which also show a direct relationship between DCLK1 and PD-L1 expression [26]. CSCs maintain TME stemness as well as increase angiogenesis, which is associated with reduced recognition of T cells and evasion of the immune system via lack of T-cell recognition [75]. PD-L1 perhaps has a key role in helping DCLK1-positive CSCs to evade the immune system, leading to an immune suppressive microenvironment. Moreover, this process may be linked to DCLK1 regulatory activity on EMT, as the EMT process is also strongly associated with immune checkpoint [76].
In the TME, TAMs can serve an anti-tumorigenic or pro-tumorigenic role depending on their status. Typically, the M1 pro-inflammatory macrophage status is correlated to tumor suppression, while the M2 anti-inflammatory/tissue-repair status promotes tumor progression and metastasis. CSCs can secrete various cytokines and chemokines to recruit TAMs to infiltrate the tumor and maintain the M2 phenotype. In turn, M2 macrophages activate STAT3 signaling via secreting IL-6 and epidermal growth factor to increase the expression of Sox2 and enhance the tumorigenic potential of CSCs. In addition, M2 macrophages can also secrete TGF-β to induce EMT and maintain stemness. Overexpression of DCLK1 has been related to worse clinical prognosis via increasing immune and stromal components in colon and gastric cancer patients, and DCLK1 affects multiple immune cell types such as TAMs and Treg and notably inhibits CD8+ T-cells by increasing inhibitor proteins TGF-β1 and chemokine (C-X-C motif) ligand 12 (CXCL12) and their receptors [77]. A recent study demonstrated that overexpression of DCLK1-AL in pancreatic tumor cells can lead to polarization of M1-macrophages towards an M2-phenotype characterized by secretion of chemokines and cytokines such as IL-6, IL-10, and CXCL12, which enhance tumor cell migration, invasion, and self-renewal [28]. In addition, DCLK1-AL induced M2-macrophages inhibited CD8+ T-cell proliferation and Granzyme-B activation, resulting in immunosuppression. Interestingly, silencing DCLK1 caused macrophages to retain the M1 phenotype and abrogated the M2-macrophage ability to enhance aggressiveness and self-renewal in pancreatic cancer cells. Together, these findings suggest that DCLK1 is a promising target to enhance antitumor effect through regulating TIME in some types of cancer (Figure 1).
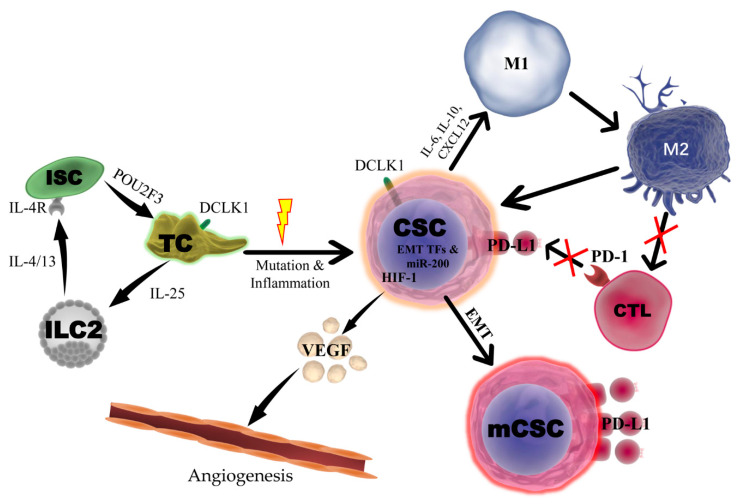
Figure 1.
Potential Role of DCLK1 and DCLK1+ Cells in the Intestinal Tumor Microenvironment. DCLK1+ tuft cells (TC) will recruit innate lymphoid type-2 cells (ILC2) by secreting IL-25 which in turn reprograms intestinal stem cells (ISC) through IL4/13-IL4R signaling to express transcription factor POU2F3 leading to TC hyperplasia. In the presence of mutation and inflammation, DCLK1+ TCs can be converted to cancer stem cells (CSC) and initiate a tumor. Under hypoxia, DCLK1+ CSCs may induce angiogenesis by upregulation of HIF-1 and secretion of VEGF. Furthermore, DCLK1+ CSCs promote EMT via the miR-200 family leading to metastatic CSCs (mCSC) with high levels of PD-L1. DCLK1+ CSCs further regulate the immune tumor microenvironment by polarization of M1 macrophages towards an M2 status by secreting IL-6, IL-10 and CXCL12, which leads to inhibition of T-cell proliferation and activation. DCLK1-positive CSCs also express programmed death ligand 1 (PD-L1) expression to inhibit CD8/PD1++ CTL function.
6. Development of DCLK1-Targeted Therapeutic Agents and Biologics
The recent discovery of new CSC surface markers and functional membrane proteins has led to suitable candidate targets such as CD13 and α3β1 for hepatocellular carcinoma (HCC) and bladder cancer, respectively [78,79]. DCLK1 is an optimal target as it represents a more specific CSC marker for colorectal, pancreatic, and possibly other cancers such as gastric cancer, esophageal cancer, breast cancer and renal carcinoma. A small molecule kinase inhibitor, LRRK2-IN-1, was first reported to regulate DCLK1-mediated stemness and EMT by suppressing DCLK1 kinase activity in colorectal and pancreatic cancer [80]. LRRK2-IN-1 impaired cell proliferation, induced apoptosis, and decreased colony formation capacity in cholangiocarcinoma primary cells. Interestingly, it was also shown that DCLK1 marks a subpopulation of LGR5+ and CD133+ CSC-like cells in cholangiocarcinoma, suggesting its potential as a target in this disease [81]. However, LRRK2-IN-1 has notable activity against ERK5 and sub-optimal properties for in vivo delivery. Recently a more specific and in vivo-compatible inhibitor, DCLK1-IN-1, was developed to target the DCLK1 kinase domain based on chemo-proteomic profiling and structure-activity based design. Importantly, this inhibitor showed significant activity against clinically relevant DCLK1+ patient-derived pancreatic ductal adenocarcinoma organoids [49]. Additionally, DCLK1-IN-1 was shown to be effective in CRC using kinase-modified engineered DCLK1 in the DLD-1 cell line [72]. Further studies are needed using DCLK1-IN-1 and other specific DCLK1 kinase inhibitors, and an assessment of its ability to influence anti-tumor immunity is especially desirable as the clinical use of kinase inhibitors in conjunction with immune checkpoint therapies is emerging [82].
Overexpression of DCLK1-AL induces the expression of aldehyde dehydrogenase, stimulates CSC self-renewal, and enhances resistance to FDA-approved receptor tyrosine kinase inhibitors (sunitinib/sorafenib) and mammalian target of rapamyoin inhibitors (everolimus/temsirolimus) in renal cell carcinoma (RCC), suggesting its value as a target in this cancer. A novel monoclonal antibody (CBT-15) was developed to target DCLK1′s extracellular C-terminus and effectively blocked RCC tumorigenesis in an RCC xenograft model [25]. Notably, DCLK1 variants containing the extracellular domain show restricted expression in normal tissue but overexpression in tumor tissue. CBT-15 also showed a significant effect in inhibiting tumor growth in vivo in mouse models of pancreatic cancer [71]. Another DCLK1-targeted mAb, DCLK1-87, stains tissue regions with CSC-marker ALDH expression in CRC, and CRC patients bearing tumors with low tissue staining intensity from this mAb showed improved survival [83].
Utilizing well-characterized CSC markers makes it possible to develop CAR-T cells with the potential to eliminate CSCs. As a CAR-T target, DCLK1 single-chain antibody variable fragment (CBT-511), showed a prominent cytotoxic effect against tumor cells and reduced tumor growth in CRC [27]. CBT-511 also increased IFN-γ release in CRC cells (2D and 3D). It has been reported previously that CSCs decrease the number of activated dendritic cells which is accompanied by decreased secretion of IL-10, IL-12 and IFN-γ cytokines, resulting in the inhibition of proliferation and differentiation of immature T lymphocytes [84]. Further investigation of DCLK1-targeted CAR-T in this context is warranted (Table 1).
Table 1.
Investigational targeted therapies against doublecortin-like kinase 1.
| Name of Drug | Class of Drug | DCLK1 Affinity | Other Significant Targets | Cancer Types Tested | Level of Evidence | Functional Target of Drug | Author (Year) | PMID |
|---|---|---|---|---|---|---|---|---|
| LRRK2-IN-1 | Kinase inhibitor | <60 nM | LRRK2, ERK5 | CRC, PDAC, CCA | In vitro, in vivo, and ex vivo | Stemness, proliferation, migration, invasion, apoptosis, cell cycle, DNA damage, EMT and tumor growth | Weygant et al. (2014) [80] | 24885928 |
| Kawamura et al. (2017) [85] | 29048622 | |||||||
| Nevi et al. (2020) [81] | 32978808 | |||||||
| Suehiro et al. (2018) [86] | 30396941 | |||||||
| XMD8-92 | Kinase inhibitor | < 100 nM | ERK5, DCLK2 | Mesothelioma, PDAC | In vitro and in vivo | Stemness, EMT, angiogenesis, proliferation and tumor growth | Sureban et al. (2014) [52] | 24880079 |
| Wang et al. (2017) [87] | 28560410 | |||||||
| DCLK1-IN-1 | Kinase inhibitor | < 60 nM | DCLK2 | PDAC, CRC | In vitro and ex vivo | Proliferation, invasion and stemness | Ferguson et al. (2020) [49] | 32251410 |
| Ferguson et al. (2020) [88] | 32530623 | |||||||
| NP-siDCAMKL-1 | Nanoparticle-encapsulated siRNA | N/A | None | CRC, HCC, PDAC | In vitro and in vivo | Tumor growth | Sureban et al. (2011) [64] | 21929751 |
| Sureban et al. (2015) [89] | 26468984 | |||||||
| Sureban et al. (2013) [19] | 24040120 | |||||||
| CBT-15 | Monoclonal antibody | <1 nM | None | PDAC, RCC | In vitro and in vivo | ADCC and tumor growth | Ge et al. (2018) [25] Qu et al. (2019) [71] |
29577277 31467540 |
| DCLK1–HA–PEG–PLGA | Bifunctional-antibody/nanoparticle conjugate | N/A | CD44 | Breast cancer | In vitro and in vivo | Qiao et al. (2016) [90] | 27994463 | |
| CBT-511 | Chimeric antigen receptor T-cells | <1 nM | None | CRC | In vitro and in vitro | Proliferation and tumor growth | Sureban et al. (2019) [27] | 31878090 |
7. Future Directions for DCLK1 Research and Drug Development
Although traditional radiotherapy and chemotherapy have therapeutic effects on tumors, clinical data show that CSCs are resistant to chemotherapy and radiotherapy, which is a key reason for tumor metastasis and recurrence. Therefore, it is desirable to develop specific and effective targeted therapies against CSCs. DCLK1 is a promising therapeutic target as shown by studies using kinase inhibitor, mAb or CAR-T. However, further studies of DCLK1-expressing TCs and their biological effect in normal conditions and in initiating cancer will be needed to safely target DCLK1. Furthermore, exploration of DCLK1′s relationship to other biological aspects are sorely needed.
Under hypoxia, DCLK1 overexpression induces stemness, but the intermediary mechanisms remain unknown. Hypoxia is known to protect CSCs from chemotherapy and radiation therapy-mediated damage in the TME, and to induce angiogenesis by secreting VEGF and recruiting monocytes, macrophages, macrophages and endothelial cells. Moreover, it limits the proliferation and activation of cytotoxic CD8+ T-cells, activates WNT and Notch signaling pathways to maintain self-renewal, and induces TGF-β signaling to promote EMT. Although DCLK1 is upregulated in angiogenesis and regulates chemotherapy resistance and cancer stemness by WNT signaling, knowledge of the effect of DCLK1 on hypoxia-driven immune cells, endothelial cells, blood vessels, and ECM remains limited [91]. Prior studies show that hypoxia induce CSCs to different metabolic phenotypes including glycolysis for the quiescent M state and oxidative phosphorylation for the proliferative E-state to enhance chemoresistance and acquire other stem-cell characteristics [92,93,94]. Strong evidence shows that the metabolism of CSCs is context-dependent and reliant on glycolysis or mitochondrial oxidative metabolism [95,96,97,98,99,100]. Currently, conventional therapies such as chemotherapy and radiotherapy have a low effect on CSCs because of increased expression of drug transporters, maintenance of a slow dividing state (quiescence), and efficient DNA repair mechanisms. Metabolic phenotypes are directly related with CSC dividing state and it is thought that targeting CSC metabolism may be an effective way to eliminate chemo-resistance and tumor relapse. One interesting study showed that Doublecortin-like (a splice-variant produced from DCLK1′s alpha promoter) knockdown is associated with reduced mitochondrial activity which significantly decreases tumor growth by regulating cytochrome c oxidase activity and ATP synthesis in neuroblastoma tumor xenografts. Another study showed that glycolysis promotes the expression of DCLK1 and maintains the CSC and EMT phenotypes via low reactive oxygen species levels in chemo-resistant pancreatic cancer cells [101]. Tumor microenvironmental factors including hypoxia, glucose deprivation, low pH, oxygen stress, and others are key in promoting CSC selection of metabolic pathways leading to metastasis or drug-resistance [102]. Metabolic alterations may cause cells to acquire stem-cell-like characteristics, and DCLK1+ TCs are long-lived and quiescent before they are activated by injury. Recent studies demonstrate that targeting oxidative phosphorylation may inhibit CSC metabolic processes and proliferation in some cancers [103]. Switching metabolic phenotypes of CSCs and TCs by enhancing oxidative phosphorylation to inhibit their tumorigenesis or tumor growth may be a feasible direction for further study. Future studies should focus on whether targeting DCLK1 to regulate metabolic processes or targeting metabolic activity of DCLK1+ TCs or CSCs may be a viable focus for therapy.
Targeting the TIME has already resulted in remarkable achievements including CAR-T and CAR-NK technologies that can potentially kill CSCs. The composition of immune cells in the tumor microenvironment will affect their response to specific immunotherapies and alter antigen presentation and macrophage polarization. Inhibition of IL-6 secretion from TAMs can inhibit the activation of CSCs to improve therapy, and overexpression of immune checkpoint ligand PD-L1 on CSCs blocks the cytotoxic CD8+ T-cell response [104]. Although existing data is relatively limited, DCLK1 has shown promising prospects in the above areas and a full assessment of DCLK1′s impact on immune checkpoint and pro-tumor macrophages is warranted.
8. Conclusions
DCLK1+ TCs are closely related with tumor initiation and chronic inflammatory diseases. Currently, the TC biological effect on cancer initiation and progression is not fully understood. Although CSCs drive chemotherapy and radiotherapy failures, there is still no effective therapeutic strategy against them. Due to these limitations, targeting the tumor microenvironment provides a prospective option for cancer treatment. However, in different tumor types or different developmental stages of the tumor, the interaction between CSCs and the microenvironment varies and will complicate developing these therapeutic strategies. Finding a reliable molecular target is crucial, and DCLK1 is one such potential marker that should be pursued in this context. DCLK1 is a multifaceted target due to several isoforms with variable functions and cellular localization. However, new evidence of their various roles is emerging. Importantly, efforts are underway to determine how DCLK1 functions within the TC to promote the response to injury, including how it modulates the immune microenvironment and how it balances this role with potential pro-tumorigenic signaling. When sufficient knowledge is gained, a variety of DCLK1-specific targeting modalities are already available for translation including specific kinase inhibitors and targeted monoclonal antibodies and CAR-T therapies.
Author Contributions
Conceptualization: Z.C., N.W., J.P. and D.Q.; writing—original draft preparation: Z.C., N.W. and D.Q.; writing—review and editing, P.C., C.W.H. and J.P.; visualization: Z.C. and N.W.; supervision: D.Q.; project administration: J.P. and D.Q.; funding acquisition: D.Q. and C.W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by an NIH R50 grant to D.Q. (R50CA233186) and two NIH R01 grants to C.W.H. (5R01CA214017 and 1R01DK119495).
Conflicts of Interest
C.W.H., D.Q., and N.W. are inventors on a patent for CBT-15 DCLK1-targeted monoclonal antibodies. C.W.H. is an inventor on a patent for CBT-511 DCLK1-targeted CAR-T. C.W.H. is an inventor on a patent for nanoparticle-encapsulated DCLK1-targeted siRNAs.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Des Portes V., Pinard J.M., Billuart P., Vinet M.C., Koulakoff A., Carrié A., Gelot A., Dupuis E., Motte J., Berwald-Netter Y., et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/S0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 2.Giannakis M., Stappenbeck T.S., Mills J.C., Leip D.G., Lovett M., Clifton S.W., Ippolito J.E., Glasscock J.I., Arumugam M., Brent M.R., et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J. Biol. Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 3.Gerbe F., Brulin B., Makrini L., Legraverend C., Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137:2179–2180. doi: 10.1053/j.gastro.2009.06.072. author reply 2180–2181. [DOI] [PubMed] [Google Scholar]
- 4.Chandrakesan P., May R., Qu D., Weygant N., Taylor V.E., Li J.D., Ali N., Sureban S.M., Qante M., Wang T.C., et al. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget. 2015;6:30876–30886. doi: 10.18632/oncotarget.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leppanen J., Helminen O., Huhta H., Kauppila J.H., Miinalainen I., Ronkainen V.P., Saarnio J., Lehenkari P.P., Karttunen T.J. Doublecortin-like kinase 1-positive enterocyte—A new cell type in human intestine. APMIS. 2016;124:958–965. doi: 10.1111/apm.12599. [DOI] [PubMed] [Google Scholar]
- 6.Westphalen C.B., Asfaha S., Hayakawa Y., Takemoto Y., Lukin D.J., Nuber A.H., Brandtner A., Setlik W., Remotti H., Muley A., et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middelhoff M., Westphalen C.B., Hayakawa Y., Yan K.S., Gershon M.D., Wang T.C., Quante M. Dclk1-expressing tuft cells: Critical modulators of the intestinal niche? Am. J. Physiol. Liver Physiol. 2017;313:G285–G299. doi: 10.1152/ajpgi.00073.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto N., Fukuda A., Yamaga Y., Yoshikawa T., Maruno T., Maekawa H., Inamoto S., Kawada K., Sakai Y., Miyoshi H., et al. Lineage tracing and targeting of IL17RB(+) tuft cell-like human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA. 2019;116:12996–13005. doi: 10.1073/pnas.1900251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May R., Qu D., Weygant N., Chandrakesan P., Ali N., Lightfoot S.A., Li L., Sureban S.M., Houchen C.W. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 2014;32:822–827. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi J., Bergstrom K., Fu J., Shan X., McDaniel J.M., McGee S., Qu D., Houchen C.W., Liu X., Xia L. Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis. Cell Death Differ. 2019;26:1656–1669. doi: 10.1038/s41418-018-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aladegbami B., Barron L., Bao J., Colasanti J., Erwin C.R., Warner B.W., Guo J. Epithelial cell specific Raptor is required for initiation of type 2 mucosal immunity in small intestine. Sci. Rep. 2017;7:5580. doi: 10.1038/s41598-017-06070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrakesan P., May R., Weygant N., Qu D., Berry W.L., Sureban S.M., Ali N., Rao C., Huycke M., Bronze M.S., et al. Intestinal tuft cells regulate the ATM mediated DNA Damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury. Sci. Rep. 2016;6:37667. doi: 10.1038/srep37667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May R., Riehl T.E., Hunt C., Sureban S.M., Anant S., Houchen C.W. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 14.Vega K.J., May R., Sureban S.M., Lightfoot S.A., Qu D., Reed A., Weygant N., Ramanujam R., Souza R., Madhoun M., et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J. Gastroenterol. Hepatol. 2012;27:773–780. doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May R., Sureban S.M., Hoang N., Riehl T.E., Lightfoot S.A., Ramanujam R., Wyche J.H., Anant S., Houchen C.W. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May R., Sureban S.M., Lightfoot S.A., Hoskins A.B., Brackett D.J., Postier R.G., Ramanujam R., Rao C.V., Wyche J.H., Anant S., et al. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am. J. Physiol. Liver Physiol. 2010;299:G303–G310. doi: 10.1152/ajpgi.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weygant N., Qu D., May R., Tierney R.M., Berry W.L., Zhao L., Agarwal S., Chandrakesan P., Chinthalapally H.R., Murphy N.T., et al. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget. 2015;6:2193–2205. doi: 10.18632/oncotarget.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphalen C.B., Quante M., Wang T.C. Functional implication of Dclk1 and Dclk1-expressing cells in cancer. Small GTPases. 2017;8:164–171. doi: 10.1080/21541248.2016.1208792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sureban S.M., May R., Qu D., Weygant N., Chandrakesan P., Ali N., Lightfoot S.A., Pantazis P., Rao C.V., Postier R.G., et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS ONE. 2013;8:e73940. doi: 10.1371/journal.pone.0073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sureban S.M., May R., Lightfoot S.A., Hoskins A.B., Lerner M., Brackett D.J., Postier R.G., Ramanujam R., Mohammed A., Rao C.V., et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito H., Tanaka S., Akiyama Y., Shimada S., Adikrisna R., Matsumura S., Aihara A., Mitsunori Y., Ban D., Ochiai T., et al. Dominant Expression of DCLK1 in Human Pancreatic Cancer Stem Cells Accelerates Tumor Invasion and Metastasis. PLoS ONE. 2016;11:e0146564. doi: 10.1371/journal.pone.0146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura T., Qian B.Z., Pollard J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith A.L., Robin T.P., Ford H.L. Molecular pathways: Targeting the TGF-β pathway for cancer therapy. Clin. Cancer Res. 2012;18:4514–4521. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 25.Ge Y., Weygant N., Qu D., May R., Berry W.L., Yao J., Chandrakesan P., Zheng W., Zhao L., Zhao K.L., et al. Alternative splice variants of DCLK1 mark cancer stem cells, promote self-renewal and drug-resistance, and can be targeted to inhibit tumorigenesis in kidney cancer. Int. J. Cancer. 2018;143:1162–1175. doi: 10.1002/ijc.31400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan R., Li J., Zhou Y., Yao L., Sun R., Xu Y., Ge Y., An G. Inhibition of DCLK1 down-regulates PD-L1 expression through Hippo pathway in human pancreatic cancer. Life Sci. 2020;241:117150. doi: 10.1016/j.lfs.2019.117150. [DOI] [PubMed] [Google Scholar]
- 27.Sureban S.M., Berahovich R., Zhou H., Xu S., Wu L., Ding K., May R., Qu D., Bannerman-Menson E., Golubovskaya V., et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers. 2019;12:54. doi: 10.3390/cancers12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrakesan P., Panneerselvam J., May R., Weygant N., Qu D., Berry W.R., Pitts K., Stanger B.Z., Rao C.V., Bronze M.S., et al. DCLK1-Isoform2 Alternative Splice Variant Promotes Pancreatic Tumor Immunosuppressive M2-Macrophage Polarization. Mol. Cancer Ther. 2020;19:1539–1549. doi: 10.1158/1535-7163.MCT-19-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luciano L., Reale E., Ruska H. On a “chemoreceptive” sensory cell in the tachea of the rat. Z. fur Zellforsch. Mikrosk. Anat. 1968;85:350–375. doi: 10.1007/BF00328847. [DOI] [PubMed] [Google Scholar]
- 30.Chang L.Y., Mercer R.R., Crapo J.D. Differential distribution of brush cells in the rat lung. Anat. Rec. 1986;216:49–54. doi: 10.1002/ar.1092160109. [DOI] [PubMed] [Google Scholar]
- 31.Luciano L., Reale E. Brush cells of the mouse gallbladder. A correlative light- and electron-microscopical study. Cell Tissue Res. 1990;262:339–349. doi: 10.1007/BF00309889. [DOI] [PubMed] [Google Scholar]
- 32.Krasteva G., Hartmann P., Papadakis T., Bodenbenner M., Wessels L., Weihe E., Schütz B., Langheinrich A.C., Chubanov V., Gudermann T., et al. Cholinergic chemosensory cells in the auditory tube. Histochem. Cell Biol. 2012;137:483–497. doi: 10.1007/s00418-012-0911-x. [DOI] [PubMed] [Google Scholar]
- 33.Sato A., Miyoshi S. Fine structure of tuft cells of the main excretory duct epithelium in the rat submandibular gland. Anat. Rec. 1997;248:325–331. doi: 10.1002/(SICI)1097-0185(199707)248:3<325::AID-AR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Hass N., Schwarzenbacher K., Breer H. A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem. Cell Biol. 2007;128:457–471. doi: 10.1007/s00418-007-0325-3. [DOI] [PubMed] [Google Scholar]
- 35.Sbarbati A., Bramanti P., Benati D., Merigo F. The diffuse chemosensory system: Exploring the iceberg toward the definition of functional roles. Prog. Neurobiol. 2010;91:77–89. doi: 10.1016/j.pneurobio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Schütz B., Jurastow I., Bader S., Ringer C., von Engelhardt J., Chubanov V., Gudermann T., Diener M., Kummer W., Krasteva-Christ G., et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerbe F., Sidot E., Smyth D.J., Ohmoto M., Matsumoto I., Dardalhon V., Cesses P., Garnier L., Pouzolles M., Brulin B., et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadjsombati M.S., McGinty J.W., Lyons-Cohen M.R., Jaffe J.B., DiPeso L., Schneider C., Miller C.N., Pollack J.L., Nagana Gowda G.A., Fontana M.F., et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity. 2018;49:33–41.e37. doi: 10.1016/j.immuni.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu D., Weygant N., May R., Chandrakesan P., Madhoun M., Ali N., Sureban S.M., An G., Schlosser M.J., Houchen C.W. Ablation of Doublecortin-Like Kinase 1 in the Colonic Epithelium Exacerbates Dextran Sulfate Sodium-Induced Colitis. PLoS ONE. 2015;10:e0134212. doi: 10.1371/journal.pone.0134212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuchi M., Nagata H., Watanabe N., Watanabe H., Tatemichi M., Hibi T. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol. 2010;10:65. doi: 10.1186/1471-230X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okumura T., Ericksen R.E., Takaishi S., Wang S.S., Dubeykovskiy Z., Shibata W., Betz K.S., Muthupalani S., Rogers A.B., Fox J.G., et al. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435–8445. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labed S.A., Wani K.A., Jagadeesan S., Hakkim A., Najibi M., Irazoqui J.E. Intestinal Epithelial Wnt Signaling Mediates Acetylcholine-Triggered Host Defense against Infection. Immunity. 2018;48:963–978. doi: 10.1016/j.immuni.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi Y., Seno H., Fukuoka A., Ueo T., Yamaga Y., Maruno T., Nakanishi N., Kanda K., Komekado H., Kawada M., et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet. 2013;45:98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 44.Haber A.L., Biton M., Rogel N., Herbst R.H., Shekhar K., Smillie C., Burgin G., Delorey T.M., Howitt M.R., Katz Y., et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konishi M., Hayakawa Y., Koike K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines. 2019;7:58. doi: 10.3390/biomedicines7030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedrich M., Jasinski-Bergner S., Lazaridou M.-F., Subbarayan K., Massa C., Tretbar S., Mueller A., Handke D., Biehl K., Bukur J., et al. Tumor-induced escape mechanisms and their association with resistance to checkpoint inhibitor therapy. Cancer Immunol. Immunother. 2019 doi: 10.1007/s00262-019-02373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey J.M., Alsina J., Rasheed Z.A., McAllister F.M., Fu Y.Y., Plentz R., Zhang H., Pasricha P.J., Bardeesy N., Matsui W., et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Zoltan M., Riquelme E., Xu H., Sahin I., Castro-Pando S., Montiel M.F., Chang K., Jiang Z., Ling J., et al. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology. 2018;155:210–223. doi: 10.1053/j.gastro.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson F.M., Nabet B., Raghavan S., Liu Y., Leggett A.L., Kuljanin M., Kalekar R.L., Yang A., He S., Wang J., et al. Discovery of a selective inhibitor of doublecortin like kinase 1. Nat. Chem. Biol. 2020;16:635–643. doi: 10.1038/s41589-020-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weygant N., Ge Y., Westphalen C.B., Ma W.W., Vega K.J. Role of the Microenvironment in Gastrointestinal Tumors. J. Oncol. 2019;2019:2153413. doi: 10.1155/2019/2153413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Bellows C.F. Doublecortin-like kinase 1 exhibits cancer stem cell-like characteristics in a human colon cancer cell line. Chin. J. Cancer Res. 2013;25:134–142. doi: 10.3978/j.issn.1000-9604.2013.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sureban S.M., May R., Weygant N., Qu D., Chandrakesan P., Bannerman-Menson E., Ali N., Pantazis P., Westphalen C.B., Wang T.C., et al. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351:151–161. doi: 10.1016/j.canlet.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Dandawate P., Ghosh C., Palaniyandi K., Paul S., Rawal S., Pradhan R., Sayed A.A.A., Choudhury S., Standing D., Subramaniam D., et al. The Histone Demethylase KDM3A, Increased in Human Pancreatic Tumors, Regulates Expression of DCLK1 and Promotes Tumorigenesis in Mice. Gastroenterology. 2019;157:1646–1659. doi: 10.1053/j.gastro.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terry S., Chouaib S. EMT in immuno-resistance. Oncoscience. 2015;2:841–842. doi: 10.18632/oncoscience.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plaks V., Kong N., Werb Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z.Q., He W.F., Wu Y.J., Zhao S.L., Wang L., Ouyang Y.Y., Tang S.Y. LncRNA SNHG1 promotes EMT process in gastric cancer cells through regulation of the miR-15b/DCLK1/Notch1 axis. BMC Gastroenterol. 2020;20:156. doi: 10.1186/s12876-020-01272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W., Wang S., Sun Q., Yang Z., Liu M., Tang H. DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int. J. Cancer. 2018;142:2068–2079. doi: 10.1002/ijc.31232. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe S., Quader S., Cabral H., Ono R. Interplay of EMT and CSC in Cancer and the Potential Therapeutic Strategies. Front. Pharmacol. 2020;11:904. doi: 10.3389/fphar.2020.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peixoto P., Etcheverry A., Aubry M., Missey A., Lachat C., Perrard J., Hendrick E., Delage-Mourroux R., Mosser J., Borg C., et al. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019;10:205. doi: 10.1038/s41419-019-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ricciardi M., Zanotto M., Malpeli G., Bassi G., Perbellini O., Chilosi M., Bifari F., Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br. J. Cancer. 2015;112:1067–1075. doi: 10.1038/bjc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., III, Koeppen H., Astarita J.L., Cubas R., et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lou Y., Diao L., Cuentas E.R., Denning W.L., Chen L., Fan Y.H., Byers L.A., Wang J., Papadimitrakopoulou V.A., Behrens C., et al. Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin. Cancer Res. 2016;22:3630–3642. doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T., Choi H., El Rayes T., Ryu S., Troeger J., et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sureban S.M., May R., Mondalek F.G., Qu D., Ponnurangam S., Pantazis P., Anant S., Ramanujam R.P., Houchen C.W. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnol. 2011;9:40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Gibbons D.L., Goswami S., Cortez M.A., Ahn Y.H., Byers L.A., Zhang X., Yi X., Dwyer D., Lin W., et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ong B.A., Vega K.J., Houchen C.W. Intestinal stem cells and the colorectal cancer microenvironment. World J. Gastroenterol. 2014;20:1898–1909. doi: 10.3748/wjg.v20.i8.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliveras-Ferraros C., Vazquez-Martin A., Cuyas E., Corominas-Faja B., Rodriguez-Gallego E., Fernandez-Arroyo S., Martin-Castillo B., Joven J., Menendez J.A. Acquired resistance to metformin in breast cancer cells triggers transcriptome reprogramming toward a degradome-related metastatic stem-like profile. Cell Cycle. 2014;13:1132–1144. doi: 10.4161/cc.27982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Razi S., Sadeghi A., Asadi-Lari Z., Tam K.J., Kalantari E., Madjd Z. DCLK1, a promising colorectal cancer stem cell marker, regulates tumor progression and invasion through miR-137 and miR-15a dependent manner. Clin. Exp. Med. 2020 doi: 10.1007/s10238-020-00665-w. [DOI] [PubMed] [Google Scholar]
- 69.Westphalen C.B., Takemoto Y., Tanaka T., Macchini M., Jiang Z., Renz B.W., Chen X., Ormanns S., Nagar K., Tailor Y., et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell. 2016;18:441–455. doi: 10.1016/j.stem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kato H., Tateishi K., Fujiwara H., Ijichi H., Yamamoto K., Nakatsuka T., Kakiuchi M., Sano M., Kudo Y., Hayakawa Y., et al. Deletion of Histone Methyltransferase G9a Suppresses Mutant Kras-driven Pancreatic Carcinogenesis. Cancer Genom. Proteom. 2020;17:695–705. doi: 10.21873/cgp.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu D., Weygant N., Yao J., Chandrakesan P., Berry W.L., May R., Pitts K., Husain S., Lightfoot S., Li M., et al. Overexpression of DCLK1-AL Increases Tumor Cell Invasion, Drug Resistance, and KRAS Activation and Can Be Targeted to Inhibit Tumorigenesis in Pancreatic Cancer. J. Oncol. 2019;2019:6402925. doi: 10.1155/2019/6402925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Ferguson F.M., Li L., Kuljanin M., Mills C.E., Subramanian K., Harshbarger W., Gondi S., Wang J., Sorger P.K., et al. Chemical Biology Toolkit for DCLK1 Reveals Connection to RNA Processing. Cell Chem. Biol. 2020;27:1229–1240. doi: 10.1016/j.chembiol.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koizumi H., Fujioka H., Togashi K., Thompson J., Yates J.R., 3rd, Gleeson J.G., Emoto K. DCLK1 phosphorylates the microtubule-associated protein MAP7D1 to promote axon elongation in cortical neurons. Dev. Neurobiol. 2017;77:493–510. doi: 10.1002/dneu.22428. [DOI] [PubMed] [Google Scholar]
- 74.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruttel V.S., Wischhusen J. Cancer stem cell immunology: Key to understanding tumorigenesis and tumor immune escape? Front. Immunol. 2014;5:360. doi: 10.3389/fimmu.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koh Y.W., Han J.H., Haam S. Expression of PD-L1, cancer stem cell and epithelial-mesenchymal transition phenotype in non-small cell lung cancer. Pathology. 2020 doi: 10.1016/j.pathol.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Wu X., Qu D., Weygant N., Peng J., Houchen C.W. Cancer Stem Cell Marker DCLK1 Correlates with Tumorigenic Immune Infiltrates in the Colon and Gastric Adenocarcinoma Microenvironments. Cancers. 2020;12:274. doi: 10.3390/cancers12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haraguchi N., Ishii H., Mimori K., Tanaka F., Ohkuma M., Kim H.M., Akita H., Takiuchi D., Hatano H., Nagano H., et al. CD13 is a therapeutic target in human liver cancer stem cells. J. Clin. Investig. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li C., Du Y., Yang Z., He L., Wang Y., Hao L., Ding M., Yan R., Wang J., Fan Z. GALNT1-Mediated Glycosylation and Activation of Sonic Hedgehog Signaling Maintains the Self-Renewal and Tumor-Initiating Capacity of Bladder Cancer Stem Cells. Cancer Res. 2016;76:1273–1283. doi: 10.1158/0008-5472.CAN-15-2309. [DOI] [PubMed] [Google Scholar]
- 80.Weygant N., Qu D., Berry W.L., May R., Chandrakesan P., Owen D.B., Sureban S.M., Ali N., Janknecht R., Houchen C.W. Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol. Cancer. 2014;13:103. doi: 10.1186/1476-4598-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nevi L., Di Matteo S., Carpino G., Zizzari I., Safarikia S., Ambrosino V., Costantini D., Overi D., Giancotti A., Monti M., et al. DCLK1, a putative novel stem cell marker in human cholangiocarcinoma. Hepatology. 2020 doi: 10.1002/hep.31571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ragavan M., Das M. Systemic Therapy of Extensive Stage Small Cell Lung Cancer in the Era of Immunotherapy. Curr. Treat. Options Oncol. 2020;21:64. doi: 10.1007/s11864-020-00762-8. [DOI] [PubMed] [Google Scholar]
- 83.Dai T., Hu Y., Lv F., Ozawa T., Sun X., Huang J., Han X., Kishi H., Muraguchi A., Jin A. Analysis of the clinical significance of DCLK1(+) colorectal cancer using novel monoclonal antibodies against DCLK1. OncoTargets Ther. 2018;11:5047–5057. doi: 10.2147/OTT.S169928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szaryńska M., Olejniczak A., Kobiela J., Łaski D., Śledziński Z., Kmieć Z. Cancer stem cells as targets for DC-based immunotherapy of colorectal cancer. Sci. Rep. 2018;8:12042. doi: 10.1038/s41598-018-30525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawamura D., Takemoto Y., Nishimoto A., Ueno K., Hosoyama T., Shirasawa B., Tanaka T., Kugimiya N., Harada E., Hamano K. Enhancement of cytotoxic effects of gemcitabine by Dclk1 inhibition through suppression of Chk1 phosphorylation in human pancreatic cancer cells. Oncol Rep. 2017;38:3238–3244. doi: 10.3892/or.2017.5974. [DOI] [PubMed] [Google Scholar]
- 86.Suehiro Y., Takemoto Y., Nishimoto A., Ueno K., Shirasawa B., Tanaka T., Kugimiya N., Suga A., Harada E., Hamano K. Dclk1 inhibition cancels 5-FU-induced cell-cycle arrest and decreases cell survival in colorectal cancer. Anticancer Res. 2018;38:6225–6230. doi: 10.21873/anticanres.12977. [DOI] [PubMed] [Google Scholar]
- 87.Wang H., Dai Y.Y., Zhang W.Q., Hsu P.C., Yang Y.L., Wang Y.C., Chan G., Au A., Xu Z.D., Jiang S.J., et al. DCLK1 is correlated with MET and ERK5 expression, and associated with prognosis in malignant pleural mesothelioma. Int. J. Oncol. 2017;51:91–103. doi: 10.3892/ijo.2017.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferguson F.M., Liu Y., Harshbarger W., Huang L., Wang J., Deng X., Cappuzzi S.J., Muratov E.N., Tropsha A., Muthuswamy S., et al. Synthesis and structure-activity relationships of DCLK1 kinase inhibitors based on a 5,11-dihydro-6H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6-one scaffold. J. Med. Chem. 2020;63:7817–7826. doi: 10.1021/acs.jmedchem.0c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sureban S.M., Madhoun M.F., May R., Qu D., Ali N., Fazili J., Weygant N., Chandrakesan P., Ding K., Lightfoot S.A., et al. Plasma DCLK1 is a marker of hepatocellular carcinoma (HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a microRNA-dependent mechanism. Oncotarget. 2015;6:37200–37215. doi: 10.18632/oncotarget.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiao S., Zhao Y., Geng S., Li Y., Hou X., Liu Y., Lin F.H., Yao L., Tian W. A novel double-targeted nondrug delivery system for targeting cancer stem cells. Int. J. Nanomed. 2016;11:6667–6678. doi: 10.2147/IJN.S116230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia W., Deshmukh A., Mani S.A., Jolly M.K., Levine H. A possible role for epigenetic feedback regulation in the dynamics of the epithelial-mesenchymal transition (EMT) Phys. Biol. 2019;16:066004. doi: 10.1088/1478-3975/ab34df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo M., Shang L., Brooks M.D., Jiagge E., Zhu Y., Buschhaus J.M., Conley S., Fath M.A., Davis A., Gheordunescu E., et al. Targeting Breast Cancer Stem Cell State Equilibrium through Modulation of Redox Signaling. Cell Metab. 2018;28:69–86.e66. doi: 10.1016/j.cmet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vazquez F., Lim J.H., Chim H., Bhalla K., Girnun G., Pierce K., Clish C.B., Granter S.R., Widlund H.R., Spiegelman B.M., et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang G., Frederick D.T., Wu L., Wei Z., Krepler C., Srinivasan S., Chae Y.C., Xu X., Choi H., Dimwamwa E., et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J. Clin. Investig. 2016;126:1834–1856. doi: 10.1172/JCI82661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng W., Gentles A., Nair R.V., Huang M., Lin Y., Lee C.Y., Cai S., Scheeren F.A., Kuo A.H., Diehn M. Targeting unique metabolic properties of breast tumor initiating cells. Stem Cells. 2014;32:1734–1745. doi: 10.1002/stem.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song K., Kwon H., Han C., Zhang J., Dash S., Lim K., Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: Regulation by MIR-122. Oncotarget. 2015;6:40822–40835. doi: 10.18632/oncotarget.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emmink B.L., Verheem A., Van Houdt W.J., Steller E.J., Govaert K.M., Pham T.V., Piersma S.R., Borel Rinkes I.H., Jimenez C.R., Kranenburg O. The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J. Proteom. 2013;91:84–96. doi: 10.1016/j.jprot.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 98.Liao J., Qian F., Tchabo N., Mhawech-Fauceglia P., Beck A., Qian Z., Wang X., Huss W.J., Lele S.B., Morrison C.D., et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Y., Zhou Y., Shingu T., Feng L., Chen Z., Ogasawara M., Keating M.J., Kondo S., Huang P. Metabolic alterations in highly tumorigenic glioblastoma cells: Preference for hypoxia and high dependency on glycolysis. J. Biol. Chem. 2011;286:32843–32853. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Viale A., Pettazzoni P., Lyssiotis C.A., Ying H., Sánchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V., et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao H., Duan Q., Zhang Z., Li H., Wu H., Shen Q., Wang C., Yin T. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J. Cell. Mol. Med. 2017;21:2055–2067. doi: 10.1111/jcmm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiche J., Brahimi-Horn M.C., Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell. Mol. Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dzobo K., Senthebane D.A., Ganz C., Thomford N.E., Wonkam A., Dandara C. Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review. Cells. 2020;9:1896. doi: 10.3390/cells9081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee Y., Shin J.H., Longmire M., Wang H., Kohrt H.E., Chang H.Y., Sunwoo J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clinical Cancer Res. 2016;22:3571–3581. doi: 10.1158/1078-0432.CCR-15-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]