Abstract
Consumption of red raspberries has been reported to exert acute beneficial effects on postprandial glycemia, insulinemia, triglyceridemia, and cytokine levels in metabolically disturbed subjects. In a two-arm parallel-group, randomized, controlled trial, 59 subjects with overweight or abdominal obesity and with slight hyperinsulinemia or hypertriglyceridemia were randomized to consume 280 g/day of frozen raspberries or to maintain their usual diet for 8 weeks. Primary analyses measured metabolic differences between the groups. Secondary analyses performed with omics tools in the intervention group assessed blood gene expression and plasma metabolomic changes following the raspberry supplementation. The intervention did not significantly affect plasma insulin, glucose, inflammatory marker concentrations, nor blood pressure. Following the supplementation, 43 genes were differentially expressed, and several functional pathways were enriched, a major portion of which were involved in the regulation of cytotoxicity, immune cell trafficking, protein signal transduction, and interleukin production. In addition, 10 serum metabolites were found significantly altered, among which β-alanine, trimethylamine N-oxide, and bioactive lipids. Although the supplementation had no meaningful metabolic effects, these results highlight the impact of a diet rich in raspberry on the immune function and phospholipid metabolism, thus providing novel insights into potential immune-metabolic pathways influenced by regular raspberry consumption.
Keywords: berry fruits, metabolic syndrome, multi-omics, immunity, gene expression, sphingolipids, phenolic compounds
1. Introduction
Both clinical and epidemiological studies highlight the contribution of a plant-food-predominant diet in the maintenance, or even the improvement, of metabolic homeostasis owing to its fiber and phytochemical contents [1]. Long-term consumption of a diet poor in these components constitutes the main predisposing factor to metabolic homeostasis dysregulation [2,3]. Metabolic dysregulations can be seen as a broad range of intermediate phenotypes, all converging with time toward metabolic syndrome, a cluster of interrelated immune-metabolic abnormalities increasing the risk of developing type 2 diabetes or atherosclerotic diseases [4]. Although public health authorities promote plant-food-predominant diets [5], efforts to increase vegetable and fruit consumption have been hardly efficient. Accordingly, it has been demonstrated that dietary habits are difficult to change over the long term [6], highlighting the need to identify simple nutritional interventions that increase the fiber and phytochemical contents of the diet. In this regard, growing evidence has supported the role of berry fruits in the prevention and management of metabolic disorders [7]. Among the most commonly consumed berries, red raspberry (Rb; Rubus idaeus L.) is low in sugar and particularly rich in both fiber and phenolic compounds, mostly anthocyanins, and ellagitannins [8].
Collectively, in vitro and ex vivo research conducted with Rb extracts or purified components have revealed various antioxidative, anti-inflammatory, and metabolic properties through which Rb components may help treat or improve immune-metabolic abnormalities [9,10]. Several animal studies have confirmed these beneficial effects with both Rb components and the entire fruit [11,12,13]. Moreover, some of these studies brought to light the immunomodulatory effects of Rb phenolic compounds [14,15]. Altogether, these results are consistent with those observed with black raspberry (Rubus occidentalis), although of reduced amplitude for an equal amount, presumably attributable to its lower phenolic content [16,17]. Unlike the black raspberry, for which the health-promoting effects have been confirmed in numerous human studies [18,19], very few human randomized controlled intervention studies have been undertaken to assess the health impact of whole Rb or its components, and most of them looked only at the short-term metabolic impact [20,21,22,23,24,25].
In that respect, the aims of this randomized controlled clinical trial were to investigate the health effects of Rb consumption on immune-metabolic features in subjects at risk of developing metabolic syndrome and to delineate the mechanisms underlying these effects through transcriptomics and metabolomics. In the context of personalized nutrition, this holistic approach is part of the efforts undertaken to further understand the impact of foods or nutrients on metabolic syndrome.
2. Materials and Methods
2.1. Study Participants and Eligibility Criteria
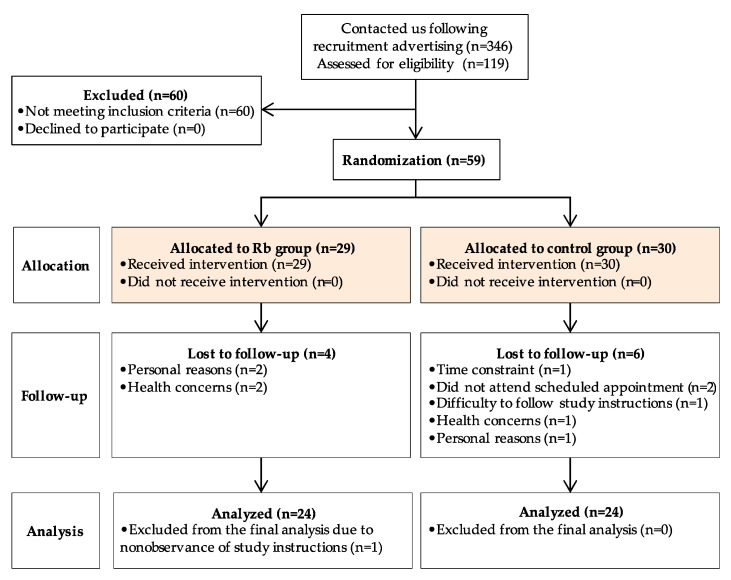
A two-arm parallel-group, randomized, controlled trial of the effects of Rb consumption on immune-metabolic parameters was conducted at the Institute of Nutrition and Functional Foods (INAF) at Université Laval between January 2018 and August 2019. Study participants from the greater Quebec City metropolitan area were recruited through emails, newspapers, and media advertisements. A total of 468 individuals contacted our research team for information related to the study, among which 119 were scheduled for a screening visit at the clinical investigation unit, and 59 were found to be eligible to participate in the study (Figure 1).
Figure 1.
Patient flow diagram for recruitment, randomization, and data collection.
The age and medical history of participants were assessed at week 0, before the beginning of the intervention. To be included in the study, subjects had to be men or pre-menopausal women, Caucasian, aged between 18 to 60 years, have a body-mass index (BMI) between 25 and 40 kg/m2 or a waist circumference ≥ 94 cm for men and ≥80 cm for women, as well as meet at least one of the following criteria: Plasma triglycerides (TG) > 1.35 mmol/L, or insulin concentration > 42 pmol/L, using our new analytic method and corresponding to a threshold value of 60 pmol/L with the former method that was predictive of a higher risk of cardiovascular disease in the Quebec population [26]. Subjects were excluded if they had a diagnosis for diabetes, hypercholesterolemia, or hypertension, had a taste aversion for or were allergic or intolerant to Rb, were taking medication known to affect study parameters, had taken antibiotics, supplements, or natural health products on a regular basis over the past 3 months, had undergone surgery in the last 3 months, or had planned surgery during the duration of the study. Nicotine usage, non-conventional dietary patterns (e.g., vegan, gluten-free or ketogenic diets), bodyweight loss or gain greater than 5% in the last 3 months, or daily alcohol consumption greater than 2 drinks were also considered exclusion criteria. The study was approved by the Université Laval Ethics Committee in January 2018 (CER-Université 2017–218). Upon recruitment, written informed consent was obtained from all subjects prior to the beginning of the study procedures. This trial was registered at clinicaltrials.gov as NCT03620617. Changes in anthropometric and metabolic variables in response to the Rb supplementation were defined as the primary outcomes, whereas pre- and post-supplementation changes in metabolomics and transcriptomics were considered as the secondary outcomes.
2.2. Nutritional Intervention
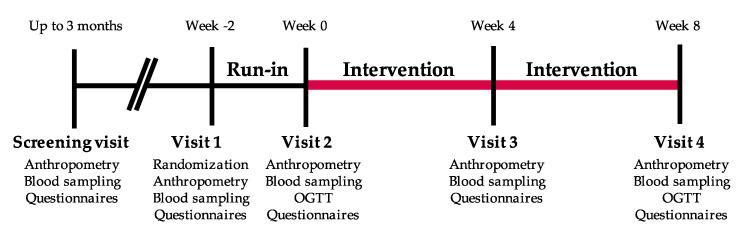
The randomization occurred after eligibility was confirmed. Participants were randomized to study arms using an in-house electronic management platform. A sex-stratified block randomization ensuring a 2:2 treatment allocation for each sex to either the intervention group consuming frozen Rb, or the control group was used (Figure 2). The randomization process was under the responsibility of the lead study coordinator. Three clinical coordinators and one graduate student were responsible for the recruitment and follow-up of participants in this study. None of the participants dropped out when they found out which group they had been assigned to. After a 2-week run-in period, participants assigned to the intervention group were invited to consume 280 g of frozen Rb daily (roughly 2 cups) for 8 weeks. Control group participants were invited to maintain their health and food habits stable for an 8-week period. Participants of both groups were scheduled for clinical visits after the run-in period (week 0), during the intervention (week 4), and at the end of the protocol (week 8). During the protocol, including the run-in period, participants were asked to avoid the use of supplements, natural health products, wine, or products with a polyphenolic profile similar to that of Rb and to limit the consumption of berries other than those provided, and any other products containing berries, to 2 portions per week. They were also instructed to limit coffee and tea consumption to 1 serving/day, and alcohol to 2 drinks/week. Participants had to complete a journal reporting any deviation from the dietary instructions listed above, as well as the Rb quantity consumed for the intervention group. The count of empty Rb packages returned by the participants was used to assess their compliance with the study protocol. Participants received regular emails to follow-up on any issues that may arise and to enhance adherence to the protocol.
Figure 2.
Graphical representation of the study protocol.
2.3. Anthropometric Measurements
At each clinical visit, waist and hip circumferences were measured to the nearest millimeter according to procedures recommended at the Airlie conference [27]. Height was also measured to the nearest millimeter. Bodyweight was measured using a BWB-800 electronic scale (Tanita, Arlington Heights, IL, USA) to an accuracy of 0.1 kg. For weight measurements, participants were asked to wear light indoor clothes and to remove their shoes. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Systolic (SBP) and diastolic (DBP) blood pressure measurements were assessed while sitting on a chair after a 10 min rest. The mean of 3 measurements performed at 3 min intervals was used for the analyses.
2.4. Nutritional and Physical Activity Assessments
Nutritional habits were assessed at each clinical visit to the clinical investigation unit through the use of a web-based and self-administered Food Frequency Questionnaire (FFQ), validated for French-speaking Canadian adults [28]. The FFQ was based on 136 items grouped into the 8 following categories: Dairy products, fruits, vegetables, meat and alternatives, cereals and grain products, beverages, other foods, and supplements. Participants reported how often they consumed each item per day, per week, per month, or none at all in the last month. Finally, pictures with examples of portion sizes were used to reflect a better estimation of the portion consumed by the participant. Physical activity habits were evaluated using the Leisure Time Activity questionnaire, which was completed by participants at the beginning and at the end of the intervention [29].
2.5. Biochemical Analyses
Fasting plasma samples were collected on weeks 0, 4, and 8, and used to measure total-cholesterol (Total-C), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), TG, glucose, and insulin levels. Total-C and TG concentrations were measured using enzymatic assays [30]. Apolipoprotein B (ApoB) was measured by immunonephelometry assay using a Siemens Dimension Vista™ 1500 Analyzer. The HDL-C fraction was obtained after precipitation of VLDL and LDL particles in the infranatant with heparin manganese chloride. LDL-C was calculated with the Friedewald formula [31]. High-sensitivity C-reactive protein (CRP) levels were measured by immunoassay [32]. Fasting glycated hemoglobin (HbA1c) was measured in plasma by immunoturbidimetry (Biorad HPLC D-100). Oral glucose tolerance tests (OGTTs) using a 75 g glucose solution were conducted twice during the intervention period (weeks 0 and 8). Blood samples were drawn at −15, 0, 15, 30, 60, 90, and 120 min during the OGTT to assess plasma insulin and glucose levels. Glucose concentrations were enzymatically measured [33], while insulin concentrations were measured by chemiluminescence (Siemens Advia Centaur XPT). The homeostatic model assessment of insulin resistance (HOMA-IR) index and the Matsuda index were calculated from OGTT values [34,35].
2.6. Transcriptomic Analyses
Gene expression profiling before and after the supplementation was conducted in whole blood, commonly used to assess the effects of dietary interventions in human trials [36]. The effects of Rb supplementation on the gene expression profile was examined in blood samples collected into PAXgene preparation tubes (Qiagen, Valencia, CA, USA) at week 0 and 8, and stored at −80 °C until the analyses. Total RNA was extracted, and samples were sent to the Génome Québec Innovation Centre (McGill University, Montreal, Canada) for sequencing. The purity and integrity of RNA samples were assessed using a 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). RNA samples were converted to cDNA with the Illumina NEB stranded mRNA library preparation kit (Illumina, San Diego, CA, USA) based on the manufacturer’s protocol. The prepared libraries were then sequenced on an Illumina NovaSeq6000 S4 sequencer using paired-end, 100 bp reads. Quality trimming of the raw reads was carried out to remove bases with a Phred33 score < 30 and reads with less than 50 bases using Trim Galore (v0.6.5), a wrapper tool around Cutadapt (v1.15) and FastQC (v0.11.9). Kallisto (0.46.2) with 100 bootstraps and default parameters was run for alignment and normalization. Reads were aligned to the GRCh38 human reference transcriptome [37]. Transcriptomic data reported as counts per million (cpm), were filtered based on a worthwhile number of counts in a minimum number of samples in the edgeR v3.28.1 package [38]. The differential expression analysis was conducted using a generalization of a paired t-test implemented in the quasi-likelihood functionality of edgeR. Differentially expressed transcripts between pre- vs. post-intervention at a nominal p-value < 0.05 were considered for further analysis. The functional significance of genes showing at least a 25% difference (1.25-fold change) between pre- and post-supplementation states was explored by pathway enrichment analysis using the clusterProfiler v3.16.0 R package [39]. The clusterProfiler package implements statistical methods to analyze and visualize functional profiles of genes and gene clusters and produces adjusted p-values using the Benjamini–Hochberg procedure (BH-p) to indicate pathways that were significantly enriched. The following pathway databases were used for functional enrichment analysis: Gene Ontology Biological Processes (GO-BP) and the Kyoto Encyclopedia of Genes and Genomes (KEGG). Transcriptomic data analysis was implemented in R v3.6.3.
2.7. Metabolomic Analyses
The quantitative analysis of 630 metabolites from 26 biochemical classes was performed in paired plasma samples from 24 participants before and after the Rb supplementation with the MxP® Quant 500 kit (Biocrates Life Sciences AG, Innsbruck, Austria). The targeted metabolomic profiling was performed by the Analytical Facility for Bioactive Molecules at the Hospital for Sick Children (Toronto, Canada). Metabolite data from 24 matched participants were processed using the MetaboAnalystR package (v3.0) [39]. First, 117 features with a constant or single value across samples were found and deleted. Non-informative signals were further filtered out based on the interquartile range estimate, and samples were normalized by quantile normalization. Metabolite data were log-transformed and scaled by Pareto scaling. After quantile normalization, one more feature with a constant value was found and deleted. From the original 630 metabolites analyzed, a total of 382 were finally retained for statistical analysis. Paired t-tests were used to analyze within-subject changes in plasma metabolite levels between pre- and post-supplementation states. A volcano plot associated with paired t-tests was further used to identify the most differentially abundant metabolites between pre- and post-supplementation groups. A p-value < 0.05, along with a count of significant pairs higher than 50% showing at least 25% difference (1.25-fold change), were the criteria used to consider metabolite plasma levels differing significantly between pre- and post-supplementation states. The dimensional reduction was conducted by using the partial least squares discriminant analysis (PLS-DA), a supervised algorithm able to reduce the number of metabolites in high-dimensional metabolomics data to produce robust and easy-to-interpret models. This method was able to differentiate the class membership through multivariate regression of a given set of metabolites. In the present study, we used a variation of PLS-DA, the multilevel PLS-DA (mPLS-DA), given its ability to exploit the paired structure of the multivariate data obtained before and after the Rb supplementation in the same group of participants [39,40]. Concretely, a sparse mPLS-DA (smPLS-DA) was used to identify the most important metabolites helping to discriminate matched study groups [41]. The smPLS-DA algorithm was implemented using the mixOmics R package (v6.12.1) [42]. Variable importance in projection (VIP) coefficients were computed as a weighted sum of squares of the smPLS-DA loadings to depict the relative importance of each metabolite in the classification model. Predictors with large VIP were the most relevant for discriminating class membership [43].
2.8. Statistical Analyses
The normality of distribution of all variables was assessed using skewness and kurtosis, and none of the variables needed transformation before analysis. Descriptive characteristics between the Rb and the control group were presented as means (±SD) or frequencies. Chi-square tests for categorical variables and analyses of variance (general linear model, type III sum of squares) for continuous variables were used to seek for inter-group differences in baseline characteristics and in changes between follow-up and baseline. The mixed procedure in SAS with special provisions for repeated measures that incorporated data from all visits was used to examine the effects of time, treatment, and their interaction on clinical variables. Data were adjusted for age, sex, BMI, and baseline values. For all analyses, a p-value < 0.05 was considered for statistical significance. Statistical analyses were performed using SAS version 3.8 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Trial Flow, Baseline Characteristics, and Compliance
Of the 119 subjects screened for eligibility, 59 were enrolled in the study, 29 were randomized to the Rb group, and 30 to the control group (Figure 2). Eleven participants (6 and 5 from the Rb and control group, respectively) withdrew during follow-up by the inability to comply with study procedures or schedule conflict. Therefore, 48 subjects were included in the per-protocol analysis.
According to BMI values, 22 subjects had obesity (BMI > 30 kg/m2), 19 were overweight (BMI between 25 and 30 kg/m2), and 7 were in the normal weight range (BMI < 25 kg/m2). The mean BMI of participants was 29.9 kg/m2 (ranging from 22.3–43.7), and the mean age was 32.2 years (ranging from 22 to 57). Upon randomization, baseline characteristics were well-distributed at baseline and, therefore, no significant differences between the Rb group and the control group were observed for age, sex, anthropometric, physiologic, or metabolic variables (Table 1). However, although not statistically significant, the HOMA-IR difference between groups may be considered as clinically meaningful. No adverse events related to Rb supplementation were reported during the study. Adherence to the study protocol was good, with an overall compliance of 92.8%.
Table 1.
Clinical and laboratory baseline characteristics of study participants.
| Variable | N | Control | N | Rb | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sex (men/women) | 24 | 9/15 | 24 | 7/17 | 0.54 | ||||
| Age (years) | 24 | 31.92 | ± | 8.05 | 24 | 32.46 | ± | 10.12 | 0.83 |
| BMI (kg/m2) | 24 | 29.38 | ± | 3.94 | 24 | 30.42 | ± | 5.00 | 0.43 |
| Waist circumference (cm) | 24 | 98.10 | ± | 11.81 | 24 | 98.53 | ± | 13.32 | 0.90 |
| Fasting glucose (mmol/L) | 24 | 4.84 | ± | 0.47 | 23 | 4.98 | ± | 0.59 | 0.38 |
| Fasting insulin (pmol/L) | 22 | 73.91 | ± | 33.57 | 19 | 83.11 | ± | 43.54 | 0.21 |
| HbA1C (%) | 24 | 5.05 | ± | 0.29 | 24 | 5.03 | ± | 0.31 | 0.85 |
| Plasma TG (mmol/L) | 24 | 1.56 | ± | 0.78 | 24 | 1.46 | ± | 0.80 | 0.66 |
| ApoB-100 (g/L) | 24 | 0.88 | ± | 0.17 | 24 | 0.92 | ± | 0.24 | 0.48 |
| HDL-C (mmol/L) | 24 | 1.33 | ± | 0.23 | 24 | 1.32 | ± | 0.40 | 0.94 |
| LDL-C (mmol/L) | 24 | 2.55 | ± | 0.74 | 24 | 2.65 | ± | 0.86 | 0.69 |
| CRP (mg/L) | 24 | 2.72 | ± | 2.82 | 23 | 2.68 | ± | 2.34 | 0.95 |
| HOMA-IR | 13 | 1.78 | ± | 0.76 | 11 | 2.84 | ± | 1.73 | 0.06 |
| MATSUDA | 13 | 5.79 | ± | 2.43 | 11 | 4.54 | ± | 2.24 | 0.21 |
Data are means ± standard deviations. Sex is presented as counts. p-values were obtained by a general linear model, type III sum of squares, and chi-square tests (sex). BMI, body mass index. HbA1C, glycated hemoglobin. TG, triglycerides. ApoB-100, Apolipoprotein B. HDL-C, high-density lipoprotein cholesterol. LDL-C, low-density lipoprotein cholesterol. CRP, C-reactive protein. HOMA-IR, homeostatic model assessment of insulin resistance.
3.2. Food Intake and Physical Activity
There was no significant difference in energy intake between groups at any time point during the protocol. Accordingly, no significant treatment by time interactions was found in macronutrients or other nutritional compounds, with the exception of those deriving directly from Rb composition, namely glucose, fructose, soluble and insoluble fiber (Table 2). According to the FFQ data, adding 2 cups/day of Rb accounted, respectively, for 17.6%, 17.1%, 13.3%, and 25.5% of total dietary glucose, fructose, soluble, and insoluble fibers intakes. Moreover, when analyzing dietary intakes based on food groups (bread and cereals, fruits, vegetables, dairy products, animal proteins), there were no significant differences between groups except for fruit consumption (p < 0.0001, a mean of 2, 3 portions for the control group vs. 6 portions for the Rb group during the intervention). The intervention resulted in a nutritionally significant shift (around 3 servings) in fruit consumption in the Rb group. Physical activity remained stable throughout the protocol (Table 2).
Table 2.
Daily dietary intakes by nutrients and by food group over time within and between groups.
| Time | Week 0 | Week 4 | Week 8 | p-Values | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Rb | Control | Rb | Control | Rb | Time | tx | tx*Time | ||||||||||||
| Energy (kcal) | 2132.8 | ± | 823.2 | 2059.7 | ± | 662.1 | 1992.7 | ± | 692.4 | 1983.3 | ± | 682.1 | 2060.7 | ± | 638.7 | 2075.8 | ± | 623.2 | 0.32 | 0.70 | 0.83 |
| Lipids (g) | 93.5 | ± | 44.9 | 82.7 | ± | 28.6 | 87.7 | ± | 40.9 | 76.4 | ± | 31.8 | 91.4 | ± | 37.7 | 80.9 | ± | 28.7 | 0.25 | 0.60 | 0.99 |
| Proteins (g) | 90.1 | ± | 37.3 | 87.2 | ± | 30.9 | 87.4 | ± | 32.1 | 84.0 | ± | 30.1 | 91.9 | ± | 28.6 | 88.3 | ± | 30.8 | 0.53 | 0.68 | 1.00 |
| Soluble Fiber (g) | 8.3 | ± | 4.1 | 7.7 a | ± | 3.3 | 7.3 x | ± | 3.4 | 8.5 y | ± | 3.1 | 7.5 x | ± | 3.1 | 8.9 by | ± | 3.1 | 0.69 | 0.01 | 0.02 |
| Insoluble Fiber (g) | 15.6 | ± | 7.8 | 15.0 x | ± | 6.8 | 13.8 a | ± | 6.9 | 23.1 by | ± | 6.6 | 14.4 x | ± | 6.2 | 23.8 by | ± | 6.3 | <0.0001 | <0.0001 | <0.0001 |
| Fructose (g) | 24.1 a | ± | 14.4 | 24.9 a | ± | 9.9 | 19.3 bx | ± | 8.9 | 29.7 by | ± | 9.4 | 22.2 x | ± | 9.0 | 29.9 by | ± | 8.9 | 0.41 | <0.0001 | 0.002 |
| Glucose (g) | 23.7 a | ± | 13.3 | 25.1 a | ± | 8.9 | 19.8 bx | ± | 8.8 | 30.2 by | ± | 7.9 | 22.2 x | ± | 8.8 | 30.9 by | ± | 7.8 | 0.18 | <0.0001 | 0.0005 |
| Alcohol (g) | 3.2 | ± | 3.6 | 2.8 | ± | 2.8 | 2.5 | ± | 2.1 | 1.9 | ± | 1.7 | 2.8 | ± | 2.5 | 2.3 | ± | 1.4 | 0.07 | 0.37 | 0.95 |
| Caffeine (mg) | 147.7 | ± | 148.9 | 103.6 | ± | 114.9 | 147.9 | ± | 142.7 | 95.2 | ± | 126.9 | 150.0 | ± | 149.7 | 106.6 | ± | 142.7 | 0.59 | 0.65 | 0.73 |
| Bread and cereals (serving) | 3.9 | ± | 2.2 | 4.6 | ± | 2.3 | 4.0 | ± | 2.0 | 4.1 | ± | 2.6 | 4.1 | ± | 2.3 | 4.1 | ± | 1.9 | 0.45 | 0.36 | 0.26 |
| Fruits (serving) |
2.9 a | ± | 2.6 | 3.1 a | ± | 1.6 | 2.2 bx | ± | 1.7 | 6.0 by | ± | 1.3 | 2.9 x | ± | 1.9 | 5.9 by | ± | 1.3 | <0.0001 | <0.0001 | <0.0001 |
| Vegetables (serving) | 3.6 | ± | 1.9 | 3.6 | ± | 2.3 | 3.3 | ± | 1.6 | 2.6 | ± | 1.6 | 3.4 | ± | 1.6 | 3.2 | ± | 2.1 | 0.02 | 0.10 | 0.41 |
| Dairy products (serving) | 2.0 | ± | 1.7 | 2.2 | ± | 1.1 | 2.0 | ± | 1.3 | 2.3 | ± | 1.3 | 1.8 | ± | 0.9 | 2.2 | ± | 1.4 | 0.69 | 0.51 | 0.74 |
| Animal proteins (serving) | 2.3 | ± | 1.2 | 1.8 | ± | 0.9 | 2.2 | ± | 1.2 | 1.8 | ± | 0.8 | 2.5 | ± | 1.2 | 2.0 | ± | 1.1 | 0.13 | 0.39 | 0.93 |
| Physical activity (AMI) | 298.1 | ± | 147.0 | 259.0 | ± | 131.9 | 280.8 | ± | 190.5 | 218.3 | ± | 159.9 | 0.13 | 0.34 | 0.54 | ||||||
Data are means ± standard deviations. A mixed model adjusted for age, sex, BMI, and baseline values was used to obtain p-values. Time effect, treatment effect, and the interaction of treatment by time were significant at ⍺ = 0,05. Significant p-values are in bold. Letters stand for significant differences (a,b for time effect and x,y for treatment effect). N = 24 for both Rb and control. tx, treatment. AMI, Activity Metabolic Index. tx*Time stands for the interaction between treatment and time effects.
3.3. Primary Outcomes
We found no significant differences between follow-up and baseline values between control and intervention groups for any of the parameters analyzed (Table 3). The statistical adjustment for age, sex, and BMI had no effect on the lack of differences between the Rb and control groups (data not shown).
Table 3.
Differences between follow-up and baseline.
| Variable | N | Control | N | Rb | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 24 | −0.01 | ± | 0.60 | 24 | +0.10 | ± | 0.57 | 0.43 |
| Waist circumference (cm) | 24 | −0.18 | ± | 2.18 | 24 | +0.39 | ± | 3.12 | 0.46 |
| Fasting glucose (mmol/L) | 23 | +0.01 | ± | 0.35 | 22 | −0.09 | ± | 0.29 | 0.27 |
| Fasting insulin (pmol/L) | 19 | +3.10 | ± | 22.98 | 16 | +3.12 | ± | 26.49 | 0.99 |
| HbA1C (%) | 23 | −0.03 | ± | 0.13 | 23 | +0.03 | ± | 0.14 | 0.13 |
| Plasma TG (mmol/L) | 22 | −0.04 | ± | 0.53 | 23 | −0.17 | ± | 0.68 | 0.48 |
| ApoB-100 (g/L) | 23 | +0.01 | ± | 0.09 | 23 | −0.03 | ± | 0.12 | 0.30 |
| HDL-C (mmol/L) | 23 | −0.04 | ± | 0.10 | 23 | −0.01 | ± | 0.18 | 0.43 |
| LDL-C (mmol/L) | 22 | +0.01 | ± | 0.42 | 23 | +0.03 | ± | 0.42 | 0.89 |
| CRP (mg/L) | 20 | +0.90 | ± | 2.33 | 22 | −0.04 | ± | 1.59 | 0.14 |
| HOMA-IR | 13 | +0.11 | ± | 0.74 | 11 | −0.16 | ± | 0.53 | 0.33 |
| Matsuda index | 13 | −0.60 | ± | 2.08 | 11 | +0.14 | ± | 1.43 | 0.33 |
Data are means ± standard deviations. p-values were obtained by a general linear model, type III sum of squares. BMI, body mass index. HbA1C, glycated hemoglobin. TG, triglycerides. ApoB-100, Apolipoprotein B. HDL-C, high-density lipoprotein cholesterol. LDL-C, low-density lipoprotein cholesterol. CRP, C-reactive protein. HOMA-IR, homeostatic model assessment of insulin resistance.
Repeated measures analysis revealed a significant treatment by time interaction effect on SBP (p = 0.03) and plasma ApoB levels (p = 0.03) (Table 4). Indeed, in the control group, we found a significant increase in plasma ApoB concentrations from the beginning of the intervention (0.86–0.16 g/L) to the mid-point visit (0.95–0.17 g/L), before regaining its initial level at the end of the study (0.87–0.19 g/L). Again, a significant effect of time was observed for both HDL-C (p = 0.03) and LDL-C (p = 0.01) in the control group. There was no other singular or interaction effect of time and treatment for any other metabolic or anthropometric variable. In accordance to the lack of change in fasting and postprandial plasma insulin and glucose concentrations in response to the intervention, both HOMA-IR and MATSUDA indexes derived from OGGT were not found to differ significantly.
Table 4.
Variations of anthropometrics, body composition, and blood pressure parameters over time within and between groups.
| Time | Week 0 | Week 4 | Week 8 | p-Values | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | N Control | N Rb | Control | Rb | Control | Rb | Time | tx | tx*Time | ||||||||||||||
| SBP (mmHg) | 24 | 110.8 | ± | 11.2 | 24 | 112.8 | ± | 11.0 | 110.7 | ± | 12.9 | 113.1 | ± | 10.4 | 112.8 | ± | 11.9 | 110.9 | ± | 11.0 | 0.99 | 0.37 | 0.03 |
| DBP (mmHg) | 24 | 68.9 | ± | 8.7 | 24 | 71.9 | ± | 8.9 | 68.8 | ± | 10.4 | 71.8 | ± | 9.0 | 69.6 | ± | 11.1 | 70.3 | ± | 9.3 | 0.86 | 0.67 | 0.34 |
| BMI (kg/m2) | 24 | 29.4 | ± | 3.9 | 24 | 30.4 | ± | 4.9 | 29.5 | ± | 3.9 | 30.5 | ± | 5.0 | 29.4 | ± | 3.9 | 30.5 | ± | 5.1 | 0.44 | 0.69 | 0.69 |
| Waist circumference (cm) | 24 | 98.1 | ± | 11.8 | 24 | 98.5 | ± | 13.3 | 98.5 | ± | 12.1 | 98.4 | ± | 13.3 | 97.9 | ± | 12.9 | 98.9 | ± | 14.1 | 0.93 | 0.73 | 0.23 |
| Hips circumference (cm) | 24 | 108.9 | ± | 7.5 | 24 | 112.1 | ± | 10.2 | 109.5 | ± | 8.4 | 112.3 | ± | 10.5 | 109.3 | ± | 8.4 | 112.1 | ± | 10.5 | 0.63 | 0.59 | 0.77 |
| CRP | 20 | 2.50 | ± | 2.50 | 21 | 2.1 | ± | 1.64 | 2.65 | ± | 2.60 | 2.86 | ± | 2.59 | 2.46 | ± | 2.42 | 3.28 | ± | 2.67 | 0.30 | 0.16 | 0.26 |
| ApoB-100 | 23 | 0.86 a | ± | 0.16 | 23 | 0.92 | ± | 0.24 | 0.95 b | ± | 0.17 | 0.92 y | ± | 0.21 | 0.87 a | ± | 0.19 | 0.89 | ± | 0.25 | 0.002 | 0.02 | 0.03 |
| Total-C (mmol/L) | 23 | 4.53 a | ± | 0.79 | 23 | 4.60 | ± | 0.91 | 4.85 b | ± | 0.81 | 4.70 | ± | 0.84 | 4.51 a | ± | 0.88 | 4.54 | ± | 0.86 | 0.001 | 0.14 | 0.22 |
| HDL-C (mmol/L) | 23 | 1.33 | ± | 0.23 | 23 | 1.30 | ± | 0.40 | 1.39 a | ± | 0.29 | 1.32 | ± | 0.35 | 1.29 b | ± | 0.27 | 1.30 | ± | 0.33 | 0.03 | 0.49 | 0.17 |
| LDL-C (mmol/L) | 22 | 2.44 a | ± | 0.67 | 23 | 2.62 | ± | 0.87 | 2.78 b | ± | 0.68 | 2.74 | ± | 0.78 | 2.45 a | ± | 0.55 | 2.65 | ± | 0.82 | 0.001 | 0.42 | 0.14 |
| TG (mmol/L) | 22 | 1.42 | ± | 0.61 | 23 | 1.47 | ± | 0.82 | 1.32 | ± | 0.60 | 1.39 | ± | 0.64 | 1.38 | ± | 0.61 | 1.29 | ± | 0.61 | 0.37 | 0.59 | 0.72 |
| Fasting glucose (mmol/L) | 23 | 4.82 | ± | 0.47 | 22 | 5.00 | ± | 0.59 | 4.85 | ± | 0.49 | 5.11 | ± | 0.57 | 4.83 | ± | 0.52 | 4.91 | ± | 0.57 | 0.12 | 0.99 | 0.25 |
| Fasting insulin (pmol/L) | 17 | 65.3 | ± | 29.0 | 16 | 80.1 | ± | 37.4 | 65.9 | ± | 29.1 | 77.5 | ± | 33.2 | 64.0 | ± | 23.2 | 83.3 | ± | 44.2 | 0.77 | 0.59 | 0.10 |
| HbA1C (%) | 23 | 5.02 | ± | 0.27 | 23 | 5.04 | ± | 0.32 | 4.98 | ± | 0.29 | 5.03 | ± | 0.30 | 5.00 | ± | 0.27 | 5.07 | ± | 0.29 | 0.11 | 0.22 | 0.23 |
Data are means ± standard deviations. A mixed model adjusted for age, sex, BMI, and baseline values was used to obtain p-values (except for BMI and waist circumference, which were adjusted for age, sex, and baseline values only). Time effect, treatment effect, and the interaction of treatment by time were significant at ⍺ = 0.5. Significant p-values are in bold. Letters stand for significant differences (a,b for time effect and x,y for treatment effect). tx, treatment. SPB, systolic blood pressure. DBP, diastolic blood pressure. BMI, body mass index. CRP, C-reactive protein. ApoB-100, Apolipoprotein B. Total-C, total cholesterol. HDL-C, high-density lipoprotein cholesterol. LDL-C, low-density lipoprotein cholesterol. TG, triglycerides. HbA1C, glycated hemoglobin. HOMA-IR, homeostatic model assessment insulin resistance. tx*Time stands for the interaction between treatment and time effects.
3.4. Secondary Outcomes
3.4.1. Effects of Rb Supplementation on Gene Expression
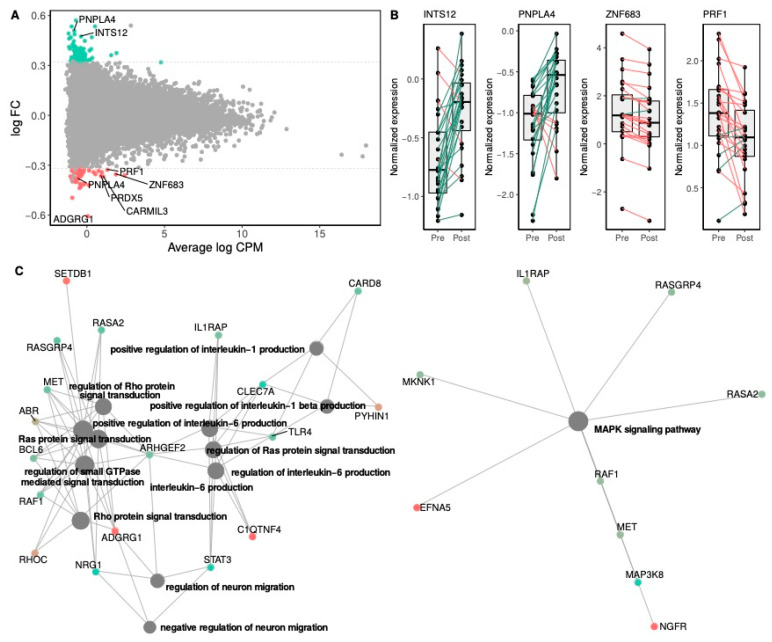
The study of differential gene expression in blood cells in the Rb group revealed a total of 1384 genes differentially expressed following Rb supplementation (p < 0.05), from which 119 showed a fold change greater than 1.25. Following multiple testing correction, none of these genes reached the significance threshold of FDR-p < 0.05. We then relaxed the significance criteria in order to identify those genes differentially expressed at p < 0.001 (Table 5). A total of 43 genes were identified, among which 9 were found to be upregulated and 34 downregulated, with fold changes ranging from −1.5 (ADGRG1) to 1.4 (PNPLA4), with 8 of the 43 genes demonstrating at least a 1.25-fold change (Figure 3A). Individual changes in gene expression levels for the top differentially up- and down-regulated genes presented in Figure 3B illustrate the inter-individual variability of gene expression changes among participants.
Table 5.
List of the most significant differentially expressed genes in Rb group after 8 weeks of Rb supplementation.
| RefSeq | Gene Symbol |
Gene Name | Nominal p-Value |
FDR | FC |
|---|---|---|---|---|---|
| NM_001114759 | ZNF683 | Zinc finger protein 683 | 4.5 × 10−6 | 0.07 | −1.28 |
| NM_001470 | GABBR1 | Gamma-aminobutyric acid type B receptor subunit 1 | 7.4 × 10−6 | 0.07 | 1.22 |
| NM_033423 | GZMH | Granzyme H | 2.5 × 10−5 | 0.12 | −1.19 |
| NM_031950 | FGFBP2 | Fibroblast growth factor binding protein 2 | 2.6 × 10−5 | 0.12 | −1.20 |
| NM_030760 | S1PR5 | Sphingosine-1-phosphate receptor 5 | 4.8 × 10−5 | 0.18 | −1.21 |
| NM_139355 | MATK | Megakaryocyte-associated tyrosine kinase | 8.4 × 10−5 | 0.26 | −1.16 |
| NM_020395 | INTS12 | Integrator complex subunit 12 | 9.5 × 10−5 | 0.26 | 1.39 |
| NM_001144884 | SLC30A7 | Solute carrier family 30 member 7 | 1.7 × 10−4 | 0.28 | 1.23 |
| NM_005170 | ASCL2 | Achaete-scute family bHLH transcription factor 2 | 1.8 × 10−4 | 0.28 | −1.16 |
| NM_025069 | ZNF703 | Zinc finger protein 703 | 1.9 × 10−4 | 0.28 | −1.14 |
| NM_001083116 | PRF1 | Perforin 1 | 2.1 × 10−4 | 0.28 | −1.25 |
| NM_138360 | CARMIL3 | Capping protein regulator and myosin 1 linker 3 | 2.1 × 10−4 | 0.28 | −1.29 |
| NM_005601 | NKG7 | Natural killer cell granule protein 7 (1) | 2.2 × 10−4 | 0.28 | −1.20 |
| NM_001024401 | SBK1 | SH3 domain binding kinase 1 | 2.2 × 10−4 | 0.28 | −1.12 |
| NM_006653 | FRS3 | Fibroblast growth factor receptor substrate 3 | 2.2 × 10−4 | 0.28 | 1.17 |
| NR_110601 | PGS1 | Phosphatidylglycerophosphate synthase 1 | 2.9 × 10−4 | 0.33 | 1.20 |
| NR_110030 | LINC01215 | Long intergenic non-protein coding RNA 1215 | 3.0 × 10−4 | 0.33 | 1.24 |
| NM_001363693 | NKG7 | Natural killer cell granule protein 7 (2) | 3.2 × 10−4 | 0.33 | −1.24 |
| NM_001145770 | ADGRG1 | Adhesion G protein-coupled receptor G1 | 3.5 × 10−4 | 0.34 | −1.52 |
| NR_024618 | LINC02035 | Long intergenic non-protein coding RNA 2035 | 3.6 × 10−4 | 0.34 | 1.14 |
| NM_000234 | LIG1 | DNA ligase 1 | 3.9 × 10−4 | 0.34 | −1.23 |
| NM_001122630 | CDKN1C | Cyclin dependent kinase inhibitor 1C | 3.9 × 10−4 | 0.34 | -1.18 |
| NM_170783 | ZNRD1 | Zinc ribbon domain containing 1 | 4.1 × 10−4 | 0.34 | −1.22 |
| NM_013432 | TONSL | Tonsoku like, DNA repair protein | 4.8 × 10−4 | 0.36 | −1.15 |
| NM_001145777 | FKBP5 | FKBP prolyl isomerase 5 | 4.9 × 10−4 | 0.36 | 1.19 |
| NM_198053 | CD247 | CD247 molecule | 5.1 × 10−4 | 0.36 | −1.10 |
| NM_032737 | LMNB2 | Lamin B2 | 5.2 × 10−4 | 0.36 | −1.09 |
| NM_004650 | PNPLA4 | Patatin like phospholipase domain containing 4 (1) | 6.2 × 10−4 | 0.37 | −1.30 |
| NM_001271822 | SERPINB6 | Serpin family B member 6 | 6.2 × 10−4 | 0.37 | −1.17 |
| NM_001358511 | PRDX5 | Peroxiredoxin 5 | 6.4 × 10−4 | 0.37 | −1.29 |
| NM_005686 | SOX13 | SRY-box transcription factor 13 | 6.4 × 10−4 | 0.37 | −1.14 |
| NM_017931 | TTC38 | Tetratricopeptide repeat domain 38 | 6.4 × 10−4 | 0.37 | −1.12 |
| NM_001142389 | PNPLA4 | Patatin like phospholipase domain containing 4 (2) | 6.8 × 10−4 | 0.37 | 1.43 |
| NM_012483 | GNLY | Granulysin | 6.9 × 10−4 | 0.37 | −1.18 |
| NM_001004310 | FCRL6 | Fc receptor like 6 | 6.9 × 10−4 | 0.37 | −1.20 |
| NM_024310 | PLEKHF1 | Pleckstrin homology and FYVE domain containing 1 | 7.2 × 10−4 | 0.37 | −1.16 |
| NM_013351 | TBX21 | T-box transcription factor 21 | 7.3 × 10−4 | 0.37 | −1.18 |
| NM_007182 | RASSF1 | Ras association domain family member 1 | 7.6 × 10−4 | 0.37 | −1.14 |
| NM_006056 | NMUR1 | Neuromedin U receptor 1 | 7.7 × 10−4 | 0.37 | −1.13 |
| NM_001110556 | FLNA | Filamin A | 8.9 × 10−4 | 0.42 | −1.12 |
| NM_004669 | CLIC3 | Chloride intracellular channel 3 | 9.3 × 10−4 | 0.42 | −1.22 |
| NM_001136044 | TMUB1 | Transmembrane and ubiquitin-like domain containing 1 | 9.3 × 10−4 | 0.42 | −1.11 |
| NM_001335 | CTSW | Cathepsin W | 9.5 × 10−4 | 0.42 | −1.14 |
RefSeq stands for the reference sequence accession number. A negative fold change stands for a decrease in gene expression levels following Rb supplementation. FDR stands for False Discovery Rate-adjusted p-values obtained by paired t-test. FC stands for fold change. Numbers in parentheses in the Gene name column stand for transcript variants.
Figure 3.
Gene expression change between pre- and post-supplementation states in the Rb group. Panel (A) shows the log2 average abundance of transcripts in counts per million mapped reads (log CPM) on the x-axis and the log2 fold-change (log FC) on the y-axis. Non-significant genes are represented by grey dots. Over- and under-expressed genes (FC > 1.25) with unadjusted significant differences (paired t-test p-value < 0.05) are colored in green and red, respectively. Significant differentially expressed genes from paired t-tests (p-value < 0.001) and showing at least a 1.25 FC are labeled with gene names. The dashed lines represent 1.25 FC. Panel (B) shows the top differentially expressed genes between pre- and post-supplementation states in the Rb group. Box and whisker plots show median, first, and third quartiles, and maximum and minimum values for the 24 sample pairs before (Pre) and after (Post) the Rb supplementation. The three transcripts, which exhibited the most significant (p-value < 0.001) over- and under-expression derived from paired t-tests (post vs. pre), are shown on the top and bottom rows, respectively. Green and red lines stand for increasing or decreasing gene expression levels between pre- and post-supplementation states within individual paired samples. Panel (C) shows network plots of enriched terms following the Rb supplementation. Network plots depict the linkages among differentially regulated gene clusters and functional enriched terms in the Gene Ontology Biological Processes (GO-BP) (left) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (right) pathway databases. The size of the grey dots is proportional to the number of genes in the enriched pathway (from 7 to 13 genes), and the red-to-green color gradient of gene dots represents the direction of the gene expression fold-change following the Rb supplementation.
The pathway enrichment analysis was conducted with all of the 119 genes showing at least a p-value < 0.05 and a fold change > 1.25 in response to the Rb supplementation (Figure 3C). Most differentially expressed genes were clustered into intracellular signal transduction pathways, as shown by the top-12 significantly enriched GO-BP categories, including those involved in the regulation of Ras and Rho signal transduction and several pathways related to the production of interleukins IL-6 and IL-1β. A relevant signal transduction pathway related to MAPK signaling was also found to be significantly enriched in the KEGG database.
3.4.2. Effects of Rb Supplementation on Metabolite Levels
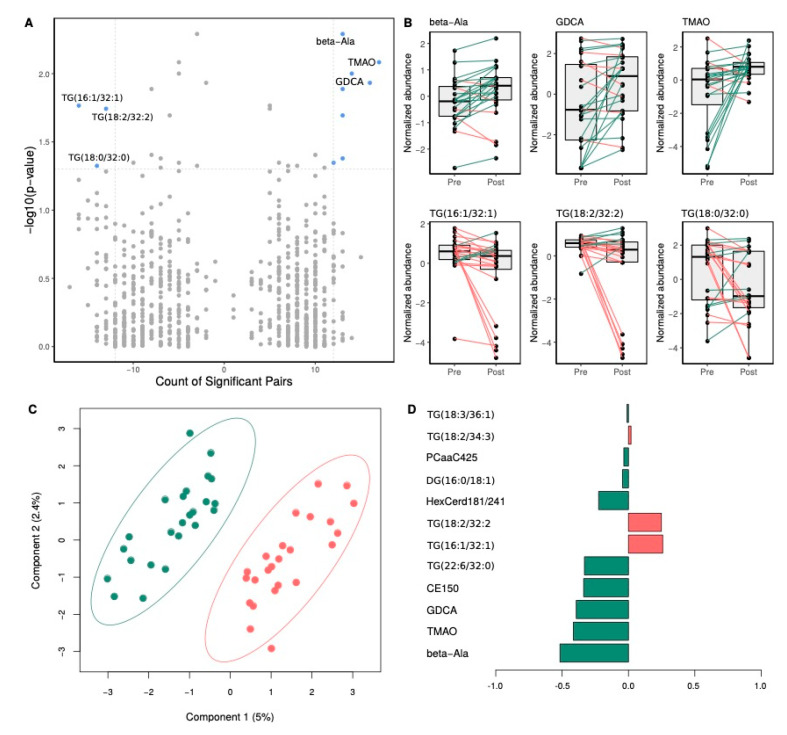
Paired t-test analysis brought out that, following Rb supplementation, a total of 14 metabolites had significantly different plasma levels (paired t-test p-value < 0.05), as compared to pre-supplementation levels. This number was reduced to 10 metabolites after applying more restricted statistical significance criteria (fold-change >1.25 and significant metabolite counts > 50%) (Figure 4A and Table 6). Plasma metabolites showing a significant reduction following Rb supplementation are shown on the top-left corner of the paired volcano plot, and those showing a significant increase are shown on the top-right corner (Figure 4A). Top-three under- and over-abundant metabolites are shown in paired boxplots in Figure 4B. More specifically, we identified β-alanine, deoxycholic acid glycine conjugate (GDCA), and trimethylamine N-oxide (TMAO) among the metabolites showing the most significant increase following Rb supplementation. On the other hand, three TG species, TG (16:1/32:1), TG (18:2/32:2) and TG (18:0/32:0), were among the metabolites undergoing a significant decrease (respectively –0.96 ± 1.83, –1.04 ± 1.99 and –0.93 ± 2.17 μmol/L, p = 0.02, 0.02 and 0.04). The score plot derived from smPLS-DA shows the complete separation of pre- and post-supplementation groups, without overlap (Figure 4C). Component 1 was the main one responsible for group discrimination, accounting for 5% of the variance, with component 2 accounting for 2.4%, respectively. The 10 metabolites associated with the first component and underlying the discrimination between pre- and post-supplementation groups are shown in the loadings panel of the first component (Figure 4D). Most of these metabolites were also identified as differentially abundant between pre- and post-supplementation groups (Figure 4B).
Figure 4.
Impact of Rb supplementation on plasma metabolite levels. Panel (A) shows a volcano plot of paired comparisons between metabolite plasma levels in pre- and post-supplementation groups. On the x-axis, a count of significant sample pairs is shown. On the y-axis, the minus logarithm of paired t-test p-values is shown. Metabolites showing statistically significant changes following the Rb supplementation (p-value < 0.05 and fold change > 1.25) are depicted as blue dots on the right (increase) and left (decrease) top corners. Top-three significantly different metabolites of both increasing and decreasing values are labeled. Panel (B) shows top metabolites showing significant changes following Rb supplementation. Box and whisker plots show median, first, and third quartiles, and maximum and minimum values for the 24 sample pairs before (Pre) and after (Post) the Rb supplementation. The three metabolites, which exhibited the most significant decrease and increase following the supplementation, are shown on the top and bottom rows, respectively. Green and red lines stand for increasing or decreasing plasma metabolite levels between pre- and post-supplementation states within individual paired samples. Panel (C) shows a bi-dimensional score plot depicting the distinct plasma metabolomic profile between pre- (red dots) and post-supplementation (green dots) paired participants. The two principal components of the smPLS-DA model along with their corresponding variance in group discrimination are shown on y- and x-axis, respectively. Panel (D) shows a loading plot representing the top 10 metabolites selected on the first component of the smPLS-DA model. Most important metabolites in group discrimination are ordered according to their loading weights (horizontal bars), from bottom to top. Bar color indicates the group for which the mean value is the highest for each feature (orange and green stand for pre- and post-supplementation groups, respectively).
Table 6.
List of the plasma metabolites showing significant changes in plasma levels in Rb group after 8 weeks of Rb supplementation.
| Metabolite Name | Common Name | Super Pathway | p-Value | HMDB |
|---|---|---|---|---|
| beta-Ala | β-Alanine | Biogenic Amines | 0.005 | HMDB0000056 |
| TMAO | Trimethylamine N-oxide | Amine Oxides | 0.008 | HMDB0000925 |
| GDCA | Deoxycholic acid glycine conjugate | Bile Acids | 0.01 | HMDB00631 |
| CE15:0 | Cholesterol 1-pentadecanoate | Cholesterol Esters | 0.01 | HMDB0060057 |
| TG (22:6/32:0) | 1-Palmitoyl-2-palmitoyl-3-docosahexaenoyl-glycerol | Triacylglycerols | 0.01 | HMDB10418 |
| TG (16:1/32:1) | 1-Octadecanoyl-2-(9Z-hexadecenoyl)-3-(9Z-tetradecenoyl)-glycerol | Triacylglycerols | 0.02 | HMDB0044888 |
| TG (18:2/32:2) | 1-Palmitoleoyl-2-palmitoleoyl-3-linoleoyl-glycerol | Triacylglycerols | 0.02 | HMDB05435 |
| HexCer(d18:1/24:1) | Hexosylceramide | Glucosylceramides | 0.02 | - |
| PC aa C42:5 | 1-Arachidonyl-2-docosapentaenoyl-sn-glycero-3-phosphocholine | Glycerophospholipids | 0.04 | HMDB0008287 |
| TG (18:0/32:0) | 1-Octadecanoyl-2-octadecanoyl-3-(9Z-tetradecenoyl)-glycerol | Triacylglycerols | 0.04 | HMDB0044753 |
p-values stand for paired t-tests. HMDB, Human Metabolome Database.
4. Discussion
To our knowledge, this is the first randomized controlled trial to investigate immune-metabolic changes in response to Rb supplementation over such a long timescale. Briefly, the present intervention resulted in the modulation of the expression of numerous genes and circulating levels of some plasma metabolites being potential biomarkers of metabolic syndrome. Despite the effect of Rb supplementation at transcriptomic and metabolomic levels, its impact on traditional metabolic syndrome features was relatively modest, with no significant effects observed on relevant metabolic outcomes. However, very few clinical trials have looked at the health effects of Rb, and most of them were based on acute postprandial interventions with small numbers of subjects. That way, findings from two recent studies indicated that acute Rb supplementation, with daily consumptions of 125 and 250 g, may lower postprandial hyperglycemia, hypertriglyceridemia, and inflammatory response (IL-6 and TNF-α), as well as SBP when extended for four weeks, in diabetic or prediabetic individuals [20,21]. By contrast, two other acute postprandial studies conducted on healthy subjects offered less conclusive results, with no lowering effects on glycemic and insulinemic responses [23,24]. Overall, results from clinical trials suggest that Rb consumption can acutely attenuate metabolic syndrome features in subjects with a pre-existing metabolic condition, lowering postprandial glucose, plasma TG and inflammatory biomarkers levels. The mild clinical impact of the present Rb supplementation is, therefore, not surprising and may be attributable to the fact that participants were at risk of developing metabolic syndrome but displayed limited metabolic alterations. Thus, it appears that Rb effects may be clinically noticeable on individuals whose homeostatic set points have shifted to altered states. However, the significant increase in glucose and fructose intake observed in the Rb group, and attributable to the reported increase in fruit servings, might have masked the effects of Rb on cardiometabolic health.
We further used transcriptomics and metabolomics to reveal the effects of Rb at a molecular level. Two of the most significantly down-regulated genes, ZNF683 and ADGRG1, are both linked to immune system functioning. ZNF683 encodes for a key regulatory transcription factor of natural killer (NK) cell differentiation [44] that also drives the expression of ADGRG1 [45]. Furthermore, ADGRG1 is down-regulated upon NK cell activation, controls NK cell effector functions, and is thus closely associated with the production of cytolytic proteins, such as perforin and granzyme, which are major players of the cytolytic process of NK cells and T lymphocytes [45]. Accordingly, PRF1, GZMH, GNLY, FGFBP2, and two transcript variants of NKG7, which code, respectively, for the perforin-1 protein, granzyme H, granulysin, fibroblast growth factor-binding protein 2, and NK cell granule protein 7 were down-regulated. In addition, the gene encoding for the sphingosine-1-phosphate receptor 5 (S1PR5), which is implicated in monocyte trafficking and the exit of mature NK cells from the bone marrow and lymph nodes into blood and lymph circulation, was also significantly downregulated [46,47]. S1PR5 belongs to the G protein-coupled receptor family S1PR1-5, known to mediate the effects of sphingosine-1-phosphate (S1P), a bioactive phospholipid, which exerts pleiotropic effects. S1P stems from the phosphorylation by sphingosine kinases of sphingosine that is derived from the hydrolysis of ceramide species [48]. With the class of hexosylceramides (HexCer) being involved in the extensive network of sphingolipids metabolism [49], it is worth highlighting the potential connection between S1P and HexCer(d18:1/24:1), a ceramide whose plasma levels significantly increased in response to the intervention. Similarly, GDCA, another metabolite undergoing a significant increase following the intervention, has been shown, amongst other conjugated bile acids, to activate S1PR2, which belongs to the receptor family mentioned above [50].
Several signaling pathways related to IL-1β and IL-6 production, as well as to Ras, Rho, and MAPK protein signal transduction, were found to be significantly enriched in response to Rb supplementation. Interestingly, Ras, Rho, and MAPK are part of the signal transduction pathways on which S1PR1-5 action relies [51], suggesting a link with the downregulation of S1PR5, as well as with the increase of plasma HexCer(d18:1/24:1) and GDCA. Taken together, this points toward a slight modulation of the “inside-out signaling” process of immune cells. Of interest, animal studies have demonstrated beneficial effects of black raspberry on colorectal and pancreatic cancer, in conjunction with enhanced infiltration of NK cells into the colon and modulations of cytotoxic NK and T cell subsets [16,52]. Part of Rb effects has been linked to its richness in ellagitannins and anthocyanins. Previous research has shown that ellagitannins can modulate mice and human leucocytes activity and cytokine production [14,53]. Based on these findings, part of the transcriptional variations observed herein could be attributed to the high ellagitannins content of Rb.
Amongst metabolites tested, β-alanine exhibited the largest increase following Rb supplementation. It is the intermediate between glycine and gamma-aminobutyric acid (GABA), and acts as a partial GABA receptor antagonist. GABAergic signaling is known to play a role in gastrointestinal motility through vagus nerve stimulation [54]. Components of the GABA signaling machinery are also expressed in immune cells and are involved in the regulation of cytokine production, immune cell proliferation, and chemotaxis [55]. Moreover, we found that GABBR1, which is coding for the subunit 1 of GABA type B receptor, was the most upregulated gene in response to Rb. Interestingly, an agonist of the GABAB receptor has been shown to reduce the secretion of cytokines, including TNF-α and IL-6, in blood mononuclear cells and to interfere with their chemotaxis [56]. Based on these findings, one may speculate that the significant increase in plasma β-alanine concentration is causally related to GABBR1 upregulation, as well as to IL-6 pathways enrichment. Surprisingly, TMAO, a gut microbiota-derived metabolite from dietary choline, choline-containing phospholipids, betaine, and carnitine, also underwent a large increase. It has been previously found that TMAO increases monocyte recruitment and activation, leading to the upregulation of inflammatory cytokine genes, monocyte adhesion, and to the formation of foam cells [57]. TMAO is then considered as a pro-atherogenic metabolite associated with an increased risk of cardiovascular disease. This increase in TMAO may be owed to an increase in animal protein consumption of the participants, which was not the case in the present study. More startling is that the reverse trend is generally observed with small fruit extract supplementation, which suggests the effect of an unknown factor that remains to be identified [58,59]. Finally, in addition to GDCA and HexCer (d18:1/24:1), other lipids belonging to cholesterol esters and glycerophospholipids pathways significantly increased, whilst triacylglycerols increased for some and decreased for others. Similarly, in a randomized controlled trial conducted on individuals with metabolic syndrome who consumed 300 g/day of berries comprising 100 g of Rb for 8 weeks, serum lipidomic profiling revealed several lipids discriminating berry consumers from controls, notably cholesterol esters, phosphatidylethanolamines, phosphatidylcholines, and TGs [24]. Of note here, are the shared biochemical pathways undergoing modulations following both interventions, first the cholesterol ester and triacylglycerols pathways, but also the functional connections between the phosphatidylcholines, TMAO, sphingosine and hexosylceramides. Our findings, together with those of Puupponen-Pimiä et al. [24], point toward sphingolipids and choline metabolism. Accordingly, studies linked sphingolipid metabolism dysregulation to insulin resistance and ceramide accumulation to insulin signaling inhibition [60]. In addition, an interconnection between polyphenols intake and sphingolipid metabolism has been established [61]. Specifically, anthocyanins were reported to attenuate insulin resistance by modulating sphingomyelin conversion and ceramide de novo synthesis [62], while ellagic acid has been identified as a potential inhibitor of sphingosine kinase [63].
Taken together, clinical, transcriptional, and metabolomical prisms have shed some light on the potential mechanisms underlying the health effects of Rb. A non-negligible part of these transcriptional results points toward slight modulations in the tight interplay between the cytotoxicity of lymphocytes and immune cell trafficking. This phenomenon may be viewed in relation to changes in phospholipid metabolism, S1PR5 downregulation being potentially linked to the modulation of sphingolipid and choline metabolism, reflected in the upregulation of HexCer (d18:1/24:1) and TMAO, respectively. In addition, according to the heterogeneity observed in the response of genes and metabolites, these results point to the need for further research on the determinants of the inter-individual variability in response to foods and nutrients.
Strengths and Limitations
This study has several limitations. First, participants were Caucasians at risk of metabolic syndrome, which limits the generalization of the results. Second, the use of a crossover instead of a parallel design could have reduced the inter-individual variability in baseline characteristics. In addition, freezing small fruits could alter their content in phenolic compounds and, therefore, reduce their effects. For instance, it has been reported that the anthocyanin content of Haskap berries was reduced by nearly 60% after being frozen at −18 °C for 6 months [64]. Due to high hemolysis in some plasma samples, which prevents adequate measurement of plasma insulin levels, HOMA-IR and Matsuda indexes have been calculated for only a limited number of subjects in each group. Finally, the free-living design of the present study led to a significant increase in fruits consumption in the Rb group. Accordingly, glucose and fructose intakes were significantly higher in the Rb group, as compared to the control group, thus limiting the possibilities of clearly delineating the effects of raspberries on cardiometabolic health. Nonetheless, this study has strengths such as its randomization, which prevents selection bias in allocating intervention to participants. Compliance to the treatment was high, with no differences observed between groups in withdrawals from the study. Thus, although a per protocol analysis was used, exclusion of data are unlikely to have introduced bias.
5. Conclusions
In conclusion, clinical results are in line with those previously reported in human studies [24,25], as it appears that Rb health effects are clinically noticeable only in subjects with advanced metabolic disorders [20,21]. Omics profiling provided an overview of Rb health effects at the molecular level. Future investigations should be deepened to increase our understanding of the impact of Rb consumption on phospholipid metabolism and immune system and the links in-between.
Acknowledgments
The authors thank the study participants for their excellent collaboration and acknowledge the work of the clinical investigation unit staff. M.-C.V. is a Tier 1 Canada Research Chair in Genomics Applied to Nutrition and Metabolic Health. M.F. received a studentship from the Centre Nutrition, Santé et Société (NUTRISS).
Author Contributions
Conceptualization, V.G. (Véronique Garneau), D.R., C.C., A.M., and M.-C.V.; data curation, J.d.T.-M. and V.G. (Véronique Garneau); formal analysis, M.F. and J.d.T.-M.; funding acquisition, D.R., C.C., A.M., and M.C.-V.; investigation, M.F., V.G. (Véronique Garneau), V.G. (Valérie Guay), M.K. and P.C.; methodology, J.d.T.-M. and M.C.-V.; project administration, V.G. (Véronique Garneau) and M.C.-V.; resources, M.C.-V.; software, J.d.T.-M.; visualization, M.F. and J.d.T.-M.; writing—original draft, M.F.; writing—review and editing, J.d.T.-M., V.G. (Véronique Garneau), V.G. (Valérie Guay), M.K., G.P., D.R., P.C., C.C., A.M., and M.C.-V., M.F., J.d.T.-M. and M.C.-V. have primary responsibility for final content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by WA Red Raspberry Commission (WRRC).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, data collection and analysis, interpretation of results, decision to publish, or redaction of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eichelmann F., Schwingshackl L., Fedirko V., Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016;17:1067–1079. doi: 10.1111/obr.12439. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Morenga L.T. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019;4:984–996. doi: 10.1016/S2468-1253(19)30257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti K., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.-C., James W.P.T., Loria C.M., Smith S.C. Harmonizing the Metabolic Syndrome. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Health Canada Canada’s Dietary Guidelines. [(accessed on 31 October 2020)];2019 Available online: https://food-guide.canada.ca/en/guidelines.
- 6.Shepherd R. Resistance to Changes in Diet. Proceedings of the Nutrition Society. Volume 61. CABI Publishing; Wallingford, UK: 2002. pp. 267–272. [DOI] [PubMed] [Google Scholar]
- 7.Luís Â., Domingues F., Pereira L. Association between berries intake and cardiovascular diseases risk factors: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018;9:740–757. doi: 10.1039/c7fo01551h. [DOI] [PubMed] [Google Scholar]
- 8.Rao A.V., Snyder D.M. Raspberries and Human Health: A Review†. J. Agric. Food Chem. 2010;58:3871–3883. doi: 10.1021/jf903484g. [DOI] [PubMed] [Google Scholar]
- 9.Seeram N.P., Adams L.S., Zhang Y., Lee R., Sand D., Scheuller H.S., Heber D. Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry Extracts Inhibit Growth and Stimulate Apoptosis of Human Cancer Cells In Vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 10.Wang P.-W., Cheng Y.-C., Hung Y.-C., Lee C.-H., Fang J.-Y., Li W.-T., Wu Y.-R., Pan T.-L. Red Raspberry Extract Protects the Skin against UVB-Induced Damage with Antioxidative and Anti-inflammatory Properties. Oxidative Med. Cell. Longev. 2019;2019:1–14. doi: 10.1155/2019/9529676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu L., Sun H., Tang M., Zhao J., Zhang Z., Sun X., He S. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019;133:110796. doi: 10.1016/j.fct.2019.110796. [DOI] [PubMed] [Google Scholar]
- 12.Noratto G., Chew B.P., Atienza L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017;227:305–314. doi: 10.1016/j.foodchem.2017.01.097. [DOI] [PubMed] [Google Scholar]
- 13.Kirakosyan A., Seymour E.M., Kondoleon N., Gutierrez-Albanchez E., Wolforth J., Bolling S. The intake of red raspberry fruit is inversely related to cardiac risk factors associated with metabolic syndrome. J. Funct. Foods. 2018;41:83–89. doi: 10.1016/j.jff.2017.12.033. [DOI] [Google Scholar]
- 14.Allen C.T., Peden-Adams M.M., Eudaly J., Keil D.E. Subchronic Exposure to Ellagic Acid Impairs Cytotoxic T-Cell Function and Suppresses Humoral Immunity in Mice. Immunopharmacol. Immunotoxicol. 2003;25:409–422. doi: 10.1081/IPH-120024508. [DOI] [PubMed] [Google Scholar]
- 15.Nowak A., Sójka M., Klewicka E., Lipińska L., Klewicki R., Kołodziejczyk K. Ellagitannins from Rubus idaeus L. Exert Geno- and Cytotoxic Effects against Human Colon Adenocarcinoma Cell Line Caco-2. J. Agric. Food Chem. 2017;65:2947–2955. doi: 10.1021/acs.jafc.6b05387. [DOI] [PubMed] [Google Scholar]
- 16.Pan P., Kang S., Wang Y., Liu K., Oshima K., Huang Y.-W., Zhang J., Yearsley M., Yu J., Wang L.-S. Black Raspberries Enhance Natural Killer Cell Infiltration into the Colon and Suppress the Progression of Colorectal Cancer. Front. Immunol. 2017;8:997. doi: 10.3389/fimmu.2017.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knobloch T., Ryan N.M., Bruschweiler-Li L., Wang C., Bernier M.C., Somogyi Á., Yan P., Cooperstone J.L., Mo X., Brüschweiler R., et al. Metabolic Regulation of Glycolysis and AMP Activated Protein Kinase Pathways during Black Raspberry-Mediated Oral Cancer Chemoprevention. Metabolites. 2019;9:140. doi: 10.3390/metabo9070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong H.S., Kim S., Hong S.J., Choi S.C., Choi J.-H., Kim J.-H., Park C.-Y., Cho J.Y., Lee T.-B., Kwon J.-W., et al. Black Raspberry Extract Increased Circulating Endothelial Progenitor Cells and Improved Arterial Stiffness in Patients with Metabolic Syndrome: A Randomized Controlled Trial. J. Med. Food. 2016;19:346–352. doi: 10.1089/jmf.2015.3563. [DOI] [PubMed] [Google Scholar]
- 19.Sardo C., Kitzmiller J.P., Apseloff G., Harris R.B., Roe D.J., Stoner G.D., Jacobs E.T. An Open-Label Randomized Crossover Trial of Lyophilized Black Raspberries on Postprandial Inflammation in Older Overweight Males. Am. J. Ther. 2016;23:e86–e91. doi: 10.1097/MJT.0b013e3182a40bf8. [DOI] [PubMed] [Google Scholar]
- 20.Xiao D., Zhu L., Edirisinghe I., Fareed J., Brailovsky Y., Burton-Freeman B.M. Attenuation of Postmeal Metabolic Indices with Red Raspberries in Individuals at Risk for Diabetes: A Randomized Controlled Trial. Obesity. 2019;27:542–550. doi: 10.1002/oby.22406. [DOI] [PubMed] [Google Scholar]
- 21.Schell J., Betts N.M., Lyons T.J., Basu A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019;74:165–174. doi: 10.1159/000497226. [DOI] [PubMed] [Google Scholar]
- 22.Istas G., Feliciano R.P., Weber T., Garcia-Villalba R., Tomás-Barberán F.A., Heiss C., Rodriguez-Mateos A. Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial. Arch. Biochem. Biophys. 2018;651:43–51. doi: 10.1016/j.abb.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Clegg M.E., Pratt M., Meade C.M., Henry C.J.K. The addition of raspberries and blueberries to a starch-based food does not alter the glycaemic response. Br. J. Nutr. 2011;106:335–338. doi: 10.1017/S0007114511001450. [DOI] [PubMed] [Google Scholar]
- 24.Puupponen-Pimiä R., Seppänen-Laakso T., Kankainen M., Maukonen J., Törrönen R., Kolehmainen M., Leppänen T., Moilanen E., Nohynek L., Aura A.-M., et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013;57:2258–2263. doi: 10.1002/mnfr.201300280. [DOI] [PubMed] [Google Scholar]
- 25.Törrönen R., Kolehmainen M., Sarkkinen E., Poutanen K., Mykkänen H., Niskanen L. Berries Reduce Postprandial Insulin Responses to Wheat and Rye Breads in Healthy Women. J. Nutr. 2013;143:430–436. doi: 10.3945/jn.112.169771. [DOI] [PubMed] [Google Scholar]
- 26.Scarsella C., Alméras N., Mauriège P., Blanchet C., Sauvé L., Dewailly E., Bergeron J., Després J.-P. Prevalence of metabolic alterations predictive of cardiovascular disease risk in the Québec population. Can. J. Cardiol. 2003;19:51–57. [PubMed] [Google Scholar]
- 27.Norgan N.G. A Review of: Anthropometric Standardization Reference Manual Edited by T. G. LOHMAN, A.F. ROCHE and R. MARTORELL. (Champaign, IL.: Human Kinetics Books, 1988.) ISBN 087322 121 4. Ergonomics. 1988;31:1493–1494. doi: 10.1080/00140138808966796. [DOI] [Google Scholar]
- 28.Labonté M.-È., Cyr A., Baril-Gravel L., Royer M.-M., Lamarche B. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur. J. Clin. Nutr. 2011;66:166–173. doi: 10.1038/ejcn.2011.163. [DOI] [PubMed] [Google Scholar]
- 29.Taylor H.L., Jacobs D.R., Schucker B., Knudsen J., Leon A.S., Debacker G. A questionnaire for the assessment of leisure time physical activities. J. Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 30.Burstein M., Samaille J. Sur un dosage rapide du cholesterol lié aux α-et aux β-lipoprotéines du sérum. Clin. Chim. Acta. 1960;5:609. doi: 10.1016/0009-8981(60)90075-9. [DOI] [PubMed] [Google Scholar]
- 31.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 32.LeDue T.B., Weiner D.L., Sipe J.D., Poulin S.E., Collins M.F., Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann. Clin. Biochem. Int. J. Lab. Med. 1998;35:745–753. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- 33.Richterich R., Dauwalder H. [Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method] Schweiz. Med. Wochenschr. 1971;101:615–618. [PubMed] [Google Scholar]
- 34.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 36.Reynés B., Priego T., Cifre M., Oliver P., Palou A. Peripheral Blood Cells, a Transcriptomic Tool in Nutrigenomic and Obesity Studies: Current State of the Art. Compr. Rev. Food Sci. Food Saf. 2018;17:1006–1020. doi: 10.1111/1541-4337.12363. [DOI] [PubMed] [Google Scholar]
- 37.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong J., Xia J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics. 2018;34:4313–4314. doi: 10.1093/bioinformatics/bty528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao M.D., Giskeødegård G.F., Bathen T.F., Sitter B., Bofin A.M., Lønning P.E., Lundgren S., Gribbestad I.S. Prognostic value of metabolic response in breast cancer patients receiving neoadjuvant chemotherapy. BMC Cancer. 2012;12:39. doi: 10.1186/1471-2407-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westerhuis J.A., Van Velzen E.J.J., Hoefsloot H.C.J., Smilde A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics. 2010;6:119–128. doi: 10.1007/s11306-009-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Cao K.-A., Boitard S., Besse P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohart F., Gautier B., Singh A., Le Cao K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017;13:e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Post M., Cuapio A., Osl M., Lehmann D., Resch U., Davies D.M., Bilban M., Schlechta B., Eppel W., Nathwani A., et al. The Transcription Factor ZNF683/HOBIT Regulates Human NK-Cell Development. Front. Immunol. 2017;8:535. doi: 10.3389/fimmu.2017.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang G.-W., Hsiao C.-C., Peng Y.-M., Braga F.A.V., Kragten N.A., Remmerswaal E.B., Van De Garde M.D., Straussberg R., König G.M., Kostenis E., et al. The Adhesion G Protein-Coupled Receptor GPR56/ADGRG1 Is an Inhibitory Receptor on Human NK Cells. Cell Rep. 2016;15:1757–1770. doi: 10.1016/j.celrep.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 46.Debien E., Mayol K., Biajoux V., Daussy C., De Agüero M.G., Taillardet M., Dagany N., Brinza L., Henry T., Dubois B., et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur. J. Immunol. 2013;43:1667–1675. doi: 10.1002/eji.201343312. [DOI] [PubMed] [Google Scholar]
- 47.Drouillard A., Mathieu A.-L., Marçais A., Belot A., Viel S., Mingueneau M., Guckian K., Walzer T. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J. Allergy Clin. Immunol. 2018;141:2265–2268.e1. doi: 10.1016/j.jaci.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Maceyka M., Milstien S., Spiegel S. Sphingosine kinases, sphingosine-1-phosphate and sphingolipidomics. Prostaglandins Other Lipid Mediat. 2005;77:15–22. doi: 10.1016/j.prostaglandins.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J., Walsh M.T., Hammad S.M., Cuchel M., Tarugi P., Hegele R.A., Davidson N.O., Rader D.J., Klein R.L., Hussain M.M. Microsomal Triglyceride Transfer Protein Transfers and Determines Plasma Concentrations of Ceramide and Sphingomyelin but Not Glycosylceramide. J. Biol. Chem. 2015;290:25863–25875. doi: 10.1074/jbc.M115.659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M., Pandak W.M., Dent P., Spiegel S., Shi R., et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2011;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryan A.M., Del Poeta M. Sphingosine-1-phosphate receptors and innate immunity. Cell. Microbiol. 2018;20:e12836. doi: 10.1111/cmi.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan P., Zhu Z., Oshima K., Aldakkak M., Tsai S., Huang Y.-W., Dong W., Zhang J., Lin C.-W., Wang Y., et al. Black raspberries suppress pancreatic cancer through modulation of NKp46 + CD8 +, and CD11b + immune cells. Food Front. 2020;1:70–82. doi: 10.1002/fft2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bessler H. On the Link between Ellagic Acid and the Immune Balance between Human Mononuclear and Colon Carcinoma Cells. Immunol Curr Res. 2017;1:1. [Google Scholar]
- 54.Wu F.-S., Gibbs T.T., Farb D.H. Dual activation of GABAA and glycine receptors by β-alanine: Inverse modulation by progesterone and 5α-pregnan-3α-ol-20-one. Eur. J. Pharmacol. Mol. Pharmacol. 1993;246:239–246. doi: 10.1016/0922-4106(93)90037-A. [DOI] [PubMed] [Google Scholar]
- 55.Jin Z., Mendu S.K., Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids. 2013;45:87–94. doi: 10.1007/s00726-011-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duthey B., Hübner A., Diehl S., Boehncke S., Pfeffer J., Boehncke W.-H. Anti-inflammatory effects of the GABAB receptor agonist baclofen in allergic contact dermatitis. Exp. Dermatol. 2010;19:661–666. doi: 10.1111/j.1600-0625.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 57.Geng J., Yang C., Wang B., Zhang X., Hu T., Gu Y., Li J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018;97:941–947. doi: 10.1016/j.biopha.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Annunziata G., Maisto M., Schisano C., Ciampaglia R., Narciso V., Tenore G.C., Novellino E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo®) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients. 2019;11:139. doi: 10.3390/nu11010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bresciani L., Dall’Asta M., Favari C., Calani L., Del Rio D., Brighenti F. An in vitro exploratory study of dietary strategies based on polyphenol-rich beverages, fruit juices and oils to control trimethylamine production in the colon. Food Funct. 2018;9:6470–6483. doi: 10.1039/C8FO01778F. [DOI] [PubMed] [Google Scholar]
- 60.Mandal N., Grambergs R., Mondal K., Basu S.K., Tahia F., Dagogo-Jack S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J. Diabetes Complicat. 2020;10:107734. doi: 10.1016/j.jdiacomp.2020.107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cas M.D., Ghidoni R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients. 2018;10:940. doi: 10.3390/nu10070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si X., Tian J., Shu C., Wang Y., Gong E.S., Zhang Y., Zhang W., Cui H., Li B. Serum Ceramide Reduction by Blueberry Anthocyanin-Rich Extract Alleviates Insulin Resistance in Hyperlipidemia Mice. J. Agric. Food Chem. 2020;68:8185–8194. doi: 10.1021/acs.jafc.0c01931. [DOI] [PubMed] [Google Scholar]
- 63.Gupta P., Mohammad T., Khan P., Alajmi M.F., Hussain A., Rehman T., Hassan I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019;118:109245. doi: 10.1016/j.biopha.2019.109245. [DOI] [PubMed] [Google Scholar]
- 64.Khattab R., Celli G.B., Ghanem A., Brooks M.S.-L. Effect of frozen storage on polyphenol content and antioxidant activity of haskap berries (Lonicera caerulea L.) J. Berry Res. 2015;5:231–242. doi: 10.3233/JBR-150105. [DOI] [Google Scholar]