Abstract
This study aimed to evaluate the radiologic response and adverse event rates of immune checkpoint inhibitor (ICI) therapy with or without radiotherapy for the treatment of non-small cell lung cancer (NSCLC) brain metastases. A systematic literature search was performed up to January 3, 2020. Studies evaluating the intracranial objective response rates (ORR) and/or disease control rates (DCR) of ICI with or without radiotherapy for treating NSCLC brain metastases were included. Consequently, twelve studies satisfied inclusion criteria. ICI combined with radiotherapy (pooled ORR, 95%; DCR, 97%) showed better local efficacy compared to ICI monotherapy (pooled ORR, 24%; DCR, 44%; p < 0.01 for both ORR and DCR). Grade 3 or 4 central nervous system (CNS)-related adverse event rates were not different (5% vs. 4%; p = 0.93). In conclusion, ICI combined with radiotherapy showed better intracranial efficacy than ICI monotherapy for treating NSCLC brain metastases. CNS-related grade 3 or 4 adverse event rate was not statistically different between the two groups. Several prospective trials are needed to compare the efficacy of ICI combined with radiotherapy and ICI monotherapy.
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, intracranial response, meta-analysis
1. Introduction
The brain is a common site of metastases in patients with advanced non-small cell lung cancer (NSCLC). Approximately 10–15% of NSCLC patients get detected with brain metastasis at the initial diagnosis [1], and 24–44% experience brain metastasis at some point during their medical treatment [2]. Multiple lesions are common in brain metastasis, and in case of NSCLC, it is observed in approximately half of the patients [3]. The prognosis is dismal, with median overall survival being approximately 7 months [4].
Immunotherapy is now a standard therapy for the patients with advanced NSCLC with PD-L1 expression based on multiple clinical trial results [5,6]. However, patients with brain metastases were mostly excluded from the pivotal trials using immunotherapy, and the efficacy of immunotherapy in those patients were not fully evaluated. Currently, the whole brain or stereotactic radiation therapy and surgery are the mainstay of treatment for the brain metastases. Recent studies showed promising results of immunotherapy with or without combined radiation therapy for the brain metastasis, with objective response rate (ORR) of 9–40% [7,8,9,10,11,12] using ICI therapy alone, and 95–100% with combination of ICI therapy and radiotherapy [13,14]. In one study, patients with NSCLC who were treated with pembrolizumab and who received previous radiation therapy showed improved therapeutic response [15]. These results suggest a synergistic effect between radiation and ICI. However, their data were not sufficient to compare differences in therapeutic response between the available treatment arms (i.e., ICI monotherapy, ICI combined with chemotherapy, ICI combined with radiotherapy). Therefore, the purpose of our meta-analysis was to evaluate the local efficacy and safety of different treatment options using ICI for the treatment of NSCLC brain metastases.
2. Materials and Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16].
2.1. Search Strategy and Eligibility Criteria
A computerized search of the MEDLINE/PubMed and EMBASE databases was performed using Medical Subject Headings (MeSH) or EMTREE terms to find relevant articles until 3 January 2020. The search keywords were as follows: ((lung cancer) or (non small cell lung cancer) or (NSCLC)) and (brain metasta*)) and ((CTLA4) or (CTLA-4) or (PD1) or (PD-1) or (PD-L1) or (ipilimumab) or (nivolumab) or (pembrolizumab) or (atezolizumab) or (avelumab) or (durvalumab)). The search was not limited by any filters.
After eliminating identical articles, we screened the articles by the titles and abstracts for relevance. Full-texts were evaluated depending on the following eligibility criteria: (a) patients: NSCLC patients with brain metastases; (b) intervention: ICI with or without radiotherapy; (c) comparator(s)/control: not applicable; (d) outcomes: intracranial ORR or disease control rate (DCR); and (e) study design: observational studies and clinical trials published as original articles. Studies were excluded if any of the following criteria were met: (a) other types of publications including conference abstracts, reviews, case reports, comments, editorials, and letters and (b) studies providing insufficient information for calculating the intracranial results of the intervention.
2.2. Data Extraction and Quality Assessment
Using a standardized extraction form we obtained the following data on study design and results: (a) study characteristics: country and institution of origin, recruitment period, study design (retrospective vs. prospective); (b) demographic and clinical characteristics: number of treated patients/lesions, presence vs. absence of symptoms associated with NSCLC brain metastasis; (c) intervention characteristics: treatment arms (ICI combined with chemotherapy vs. ICI combined with radiotherapy vs. ICI monotherapy), ICI used (e.g., atezolizumab, pembrolizumab, and nivolumab); and (d) characteristics account for outcomes: response assessment criteria, response assessment time after the first therapy. We evaluated the quality of the evidence in the included studies using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [17,18]. The GRADE system ranks the quality of evidence from very low to high based on study design, risk of bias, imprecision, inconsistency, indirectness, the magnitude of the effect, dose-response relationship, and consideration of all plausible residual confounders.
2.3. Data Synthesis and Analysis
The primary outcomes of this meta-analysis are (a) intracranial ORR (percentage of patients with NSCLC brain metastases who confirmed complete [CR] or partial response [PR]) and (b) intracranial DCR (percentage of patients with NSCLC brain metastases who confirmed CR, PR, or stable disease [SD]) evaluated with the response assessment criteria of each included study. Results were pooled according to the treatment arms (ICI monotherapy vs. ICI combined with radiotherapy vs. ICI combined with chemotherapy). In addition, the intracranial CR rate was also meta-analytically pooled.
We also assessed the safety-associated outcomes, including treatment-related grade 3 or 4 adverse events and CNS-related grade 3 or 4 adverse events, following the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 or 4.0, and according to each selected prespecified study.
Meta-analytic pooling was based on the inverse variance weighting method to calculate weights, and the clinical response and grade 3 or 4 adverse event rates with their 95% confidence intervals (CI) were meta-analytically pooled using DerSimonian–Laird (random-effects modeling) method and fixed-effects modeling, respectively. Heterogeneity was evaluated using the following tests: (a) Cochran’s Q test, with p < 0.05 indicating the presence of heterogeneity, and (b) Higgins inconsistency index (I2), with I2 > 50% indicating the presence of heterogeneity [19,20,21]. To test whether treatment arms as moderators have statistical effects on meta-regression, we used Wald-type chi-square tests with multiplicity adjustment and the regression coefficient obtained was used to estimate the intervention effect from a reference group [22,23]. Statistical analyses were conducted using R software (version 3.6.1.; R Foundation for Statistical Computing, Vienna, Austria) with the “meta” and the “metafor” packages.
3. Results
3.1. Literature Search
A flow chart describing the study selection process is presented in Figure 1. After excluding two duplicates, a total of 183 studies were identified. Of these, 155 articles were excluded according to their titles and abstracts for the following reasons: (a) conference abstract (n = 80); (b) reviews (n = 30); (c) case reports (n = 23); (d) not in the field of interest (n = 19); and (e) comments/editorial/letters (n = 3). On the basis of the eligibility criteria, a full-text review of 28 potentially eligible studies was performed. A further exclusion of 16 articles was made according to the following reasons: (a) not reporting intracranial response rates (n = 7); (b) reporting intracranial and extracranial outcomes in an inseparable way (n = 6); (c) reporting outcomes in NSCLC and non-NSCLC patients in an inseparable way (n = 2); and (d) reporting ICI outcomes and non-ICI outcomes in an inseparable way (n = 1). Finally, a total of 12 studies (separated according to the treatment arms; seven cohorts treated with ICI monotherapy; one cohort treated with ICI combined with chemotherapy; four cohorts treated with ICI combined with radiotherapy) were included in our study [7,8,9,10,11,12,13,14,24,25,26,27].
Figure 1.
Flowchart of the study selection process.
3.2. Characteristics of the Included Studies
Detailed study characteristics are described in Table 1. Four studies were multicenter studies [8,9,11,24], one study was a phase II clinical trial [10], and the remaining studies were retrospective. Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, Modified RECIST v1.1, Response Assessment in Neuro-Oncology-brain metastases (RANO-BM), and immunotherapy RANO (iRANO) were applied as tumor response criteria for four [7,9,12,25], three [8,10,11], two [13,14], and one [26] studies, respectively. Two studies did not report their response criteria [24,27]. Response assessment time after first therapy ranged from 1.5–3 months. Five studies did not mention their response assessment time [8,11,12,25,27]. Seven studies used ICI monotherapy [7,8,9,10,11,12,24], one study used both ICI and chemotherapy [25], and four studies used both ICI and stereotactic radiosurgery (SRS) [13,14,26,27]. Two studies recruited only asymptomatic NSCLC brain metastasis patients [8,10]. Nine studies used per-patient analysis [7,8,9,10,11,12,13,24,25], and the remaining three studies used per-lesion analysis [14,26,27].
Table 1.
Characteristics of 12 included studies.
| Study (Publication Year) | Nation | Multicenter | Study Design | Recruitment Period | Response Criteria | Response Assessment Time after Initiation of Therapy | ICI Used | Radio-Therapy | Symptoms | Analysis | Treated No. | ICI - Line of Therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 3rd> | ||||||||||||
| ICI monotherapy | |||||||||||||||
| Bjornhart B et al (2019) [7] | Denmark | No | Retrospective | 2015.09–2018.04 | RECIST 1.1 | 8~9 weeks | Pembrolizumab or nivolumab |
- | Mixed | Per-patient | 21 | 2 * | 7 * | 7 * | 5 * |
| Dudnik E et al (2016) [8] | Israel | Yes | Retrospective | 2015.02–2015.12 | mRECIST 1.1 | NR | Nivolumab | - | Asymptomatic | Per-patient | 5 | - | |||
| Gauvain C et al (2018) [9] | France | Yes | Retrospective | 2015.05–2016.08 | RECIST 1.1 | 2 months | Nivolumab | - | NR | Per-patient | 30 | - | |||
| Goldberg SB et al (2016) [10] | USA | No | Phase II trial | 2014.03–2015.05 | mRECIST 1.1 | 8 weeks | Pembrolizumab | - | Asymptomatic | Per-patient | 18 | 5 * | 6 * | 3 * | 4 * |
| Hendriks LEL et al (2019) [24] | France, Netherlands | Yes | Retrospective | 2012.11–2018.07 | NR | 6–9 weeks | Nivolumab | - | Mixed | Per-patient | 5 | 0 | 1 | 2 | 2 |
| Kim R et al (2019) [11] | Korea | Yes | Retrospective | 2014.02–2016.11 | mRECIST 1.1 | NR | Pembrolizumab or nivolumab | - | NR | Per-patient | 18 | 0 * | 6 * | 7 * | 5 * |
| Song P et al (2019) [12] | China | No | Retrospective | 2015.08–2018.02 | RECIST 1.1 | NR | Pembrolizumab or nivolumab or atezolizumab |
- | Mixed | Per-patient | 5 | - | |||
| ICI combined with chemotherapy | |||||||||||||||
| Afzal MZ et al (2018) [25] | USA | No | Retrospective | 2016.01–2017.12 | RECIST 1.1 | NR | Pembrolizumab | - | NR | Per-patient | 5 | 3 ** | 3 ** | ||
| ICI combined with radiotherapy | |||||||||||||||
| Ahmed KA et al (2017) [26] | USA | No | Retrospective | 2014.02–2016.10 | iRANO | 2–3 months | Nivolumab or durvalumab |
SRS or FSRT | NR | Per-lesion | 49 | - | |||
| Schapira E et al (2018) [27] | USA | No | Retrospective | 2012.01–2017.12 | NR | NR | Pembrolizumab or nivolumab or atezolizumab |
SRS | NR | Per-lesion | 85 | 0 | - | ||
| Shepard MJ et al (2019) [13] | USA | No | Retrospective | 2012.01–2018.12 | RANO-BM | 2–3 months | Pembrolizumab or nivolumab or atezolizumab |
SRS | NR | Per-patient | 16 | - | |||
| Singh C et al (2019) [14] | USA | No | Retrospective | 2013.01–2016.12 | RANO-BM | 1.5, 3, 6, 9, and 12 months | Pembrolizumab or nivolumab or nivolumab + ipilimumab or atezolizumab |
SRS | NR | Per-lesion | 291 | 0 | - | ||
ICI = immune checkpoint inhibitor; NR = not reported; RECIST = Response Evaluation Criteria in Solid Tumors; mRECIST = modified RECIST; RANO = Response Assessment in Neuro-Oncology; iRANO = immunotherapy RANO; RANO-BM = RANO brain metastases; SRS = stereotactic radiosurgery; FSRT = fractionated stereotactic radiotherapy. * Only previous systemic therapies were considered when determining the line of therapy. ** One patient was not radiographically evaluated.
3.3. Quality Assessment
Detailed quality assessments are described in Table 2. The phase II clinical trial by Goldberg et al. was initially rated with a high certainty rate [10], and the other eleven retrospective studies were initially rated with a low certainty rate [7,8,9,11,12,13,14,24,25,26,27]. In the risk of bias domain, three studies were down-rated as they performed per-lesion analysis [14,26,27]. In the imprecision domain, four studies were down-rated because of the wide 95% CI for the local efficacy, derived from a small sample size of 5 [8,12,24,25]. In the inconsistency domain, the study by Kim et al. was down-rated because of a discrepancy of local efficacy compared to the other studies using ICI monotherapy [11]. In the indirectness domain, the study by Hendriks et al. was down-rated because it only included patients who presented leptomeningeal seeding [24]. The study by Singh et al. was up-rated due to a large effect size (comprising 82% [291 out of 356 patients/lesions] among the studies including ICI combined with radiotherapy) [14]. Consequently, the quality of evidence was high in one study [10] low in four [7,9,13,14], and very low in seven studies [8,11,12,24,25,26,27].
Table 2.
Summary of quality assessment according to the GRADE system.
| Study (Publication Year) | Initial Rating | Risk of Bias | Imprecision | Inconsistency | Indirectness | Publication Bias | Large Magnitude of Effect | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|
| ICI monotherapy | ||||||||
| Bjornhart B et al (2019) [7] | Low | Not serious | Not serious | Not serious | Not serious | Not serious | Not large | Low |
| Dudnik E et al (2016) [8] | Low | Not serious | Serious | Not serious | Not serious | Not serious | Not large | Very low |
| Gauvain C et al (2018) [9] | Low | Not serious | Not serious | Not serious | Not serious | Not serious | Not large | Low |
| Goldberg SB et al (2016) [10] | High | Not serious | Not serious | Not serious | Not serious | Not serious | Not large | High |
| Hendriks LEL et al (2019) [24] | Low | Not serious | Serious | Not serious | Serious | Not serious | Not large | Very low |
| Kim R et al (2019) [11] | Low | Not serious | Not serious | Serious | Not serious | Not serious | Not large | Very low |
| Song P et al (2019) [12] | Low | Not serious | Serious | Not serious | Not serious | Not serious | Not large | Very low |
| ICI combined with chemotherapy | ||||||||
| Afzal MZ et al (2018) [25] | Low | Not serious | Serious | Not serious | Not serious | Not serious | Not large | Very low |
| ICI combined with radiotherapy | ||||||||
| Ahmed KA et al (2017) [26] | Low | Serious | Not serious | Not serious | Not serious | Not serious | Not large | Very low |
| Schapira E et al (2018) [27] | Low | Serious | Not serious | Not serious | Not serious | Not serious | Not large | Very low |
| Shepard MJ et al (2019) [13] | Low | Not serious | Not serious | Not serious | Not serious | Not serious | Not large | Low |
| Singh C et al (2019) [14] | Low | Serious | Not serious | Not serious | Not serious | Not serious | Large | Low |
3.4. Efficacy
The pooled intracranial ORR and DCR are described in Table 3. Six [7,8,9,10,11,12], one [25], and two [13,14] articles reported on intracranial ORR for ICI monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy, respectively.
Table 3.
Pooled analysis of the included studies evaluating efficacy (random-effects model).
| Treatment Arm | Intracranial ORR | Intracranial DCR | Intracranial CR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion | OR (95% CI) |
p-Value | Proportion | OR (95% CI) |
p-Value | Proportion | OR (95% CI) |
p-Value | |
| ICI monotherapy | 24 (14–39) | REF | 44 (35–55) | REF | 10 (4–22) | REF | |||
| ICI combined with chemotherapy | 80 (31–97) | 1.90 (0.76–4.79) | 0.14 | 80 (31–97) | 1.48 (0.73–3.00) | 0.23 | 20 (3–69) | 1.22 (0.75–2.00) | 0.36 |
| ICI combined with radiotherapy | 95 (92–97) | 2.32 (1.96–2.75) | <0.01 | 97 (94–98) | 1.81 (1.53–2.14) | <0.01 | 46 (40–51) | 1.58 (1.46–1.71) | <0.01 |
| Total | 51 (17–84) | - | - | 69 (42–88) | - | - | 19 (9–36) | - | - |
Values are expressed as proportion (95% confidence interval). ICI = immune checkpoint inhibitor; ORR = objective response rate (proportion of the patients who were confirmed as CR or PR); DCR = disease control rate (proportion of the patients who were confirmed as CR, PR, or SD); OR = odds ratio; REF = reference category.
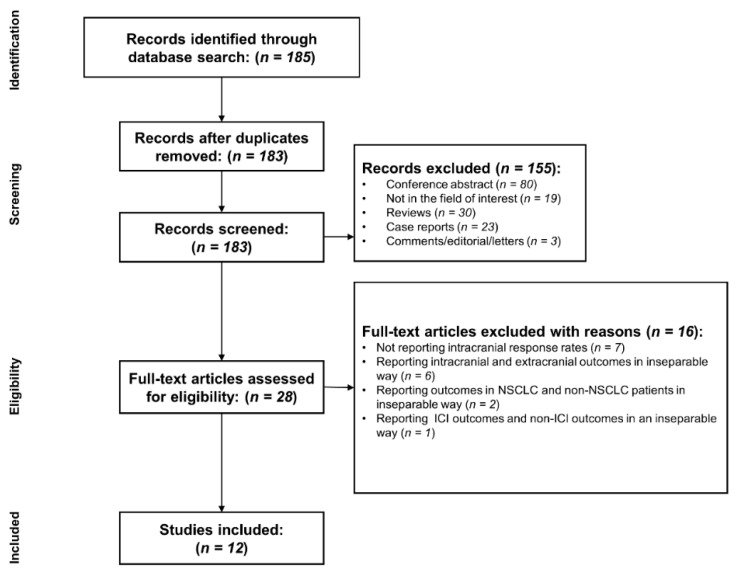
Overall intracranial ORR was 51% (95% CI, 17–84%), with a substantial heterogeneity (I2 = 94%; p < 0.01) (Figure 2). Pooled intracranial ORR with random-effects modeling was 24% (95% CI, 14–39%), 80% (95% CI, 31–97%), and 95% (95% CI, 92–97%) for ICI monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy, respectively. No substantial heterogeneity was found in any of the three subgroups. Compared to ICI monotherapy, intracranial ORR was significantly higher for ICI combined with radiotherapy (OR [95% CI], 2.32 [1.96–2.75]; p < 0.01), while no significant difference was found for ICI combined with chemotherapy (OR [95% CI], 1.90 [0.76–4.79]; p = 0.14).
Figure 2.
Forest plot of the intracranial (A) objective response rates and (B) disease control rates when using immune checkpoint inhibitor (ICI) monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy.
Seven [7,8,9,10,11,12,24], one [25], and three studies [13,14,26] reported intracranial DCR when using ICI monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy, respectively. Overall intracranial DCR was 69% (95% CI, 42–88%), with a substantial heterogeneity (I2 = 91%; p < 0.01) (Figure 2). When using ICI monotherapy, based on random-effects modeling, pooled intracranial DCR was 44% (95% CI, 35–55%), whereas for ICI combined with chemotherapy and ICI combined with radiotherapy, the value was 80% (95% CI, 31–97%) and 97% (95% CI, 94–98%), respectively. No substantial heterogeneity was found in any of the three subgroups. Intracranial DCR was significantly higher when using ICI combined with radiotherapy than when using ICI monotherapy (OR [95% CI], 1.81 [1.53–2.14); p < 0.01) but not different when using ICI combined with chemotherapy (OR [95% CI], 1.48 [0.73–3.00]; p = 0.23).
Six [7,8,9,10,11,12], one [25], and two [13,14] studies reported intracranial CR rates when using ICI monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy, respectively. Overall intracranial CR was 19% (95% CI, 9–36%), with substantial heterogeneity (I2 = 71%; p < 0.01) (see Supplementary Figure S1). Pooled intracranial CR, based on random-effects modeling, was 10% (95% CI, 4–22%), 20% (95% CI, 3–69%), and 46% (95% CI, 40–51%) when using ICI monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy, respectively. No substantial heterogeneity was found in any of the three subgroups. Intracranial CR was significantly higher when using ICI combined with radiotherapy than when using ICI monotherapy (OR [95% CI], 1.58 [1.46–1.71]; p < 0.01).
3.5. Safety
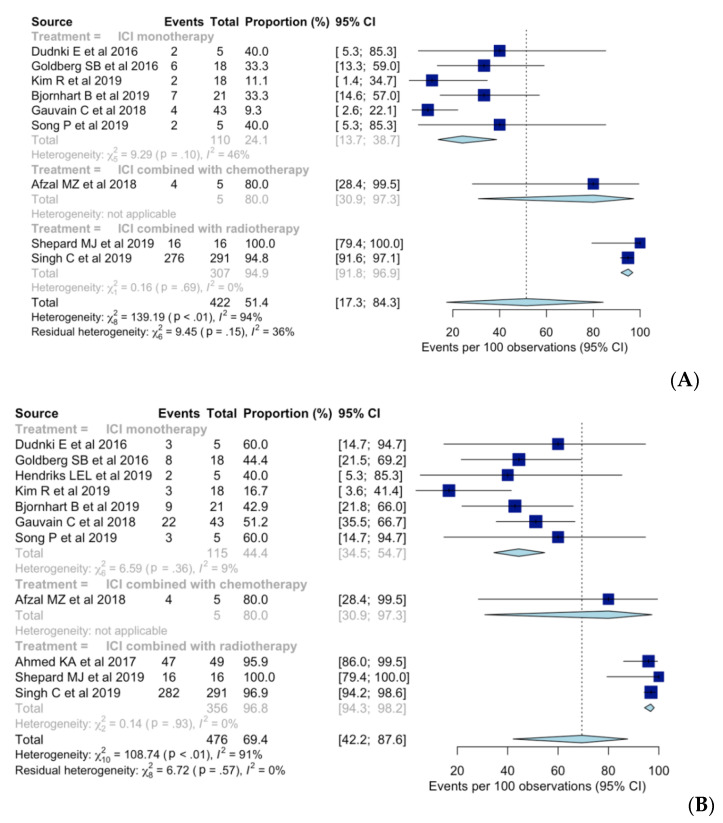
The pooled adverse event rates are summarized in Supplementary Table S1 and details of adverse events are described in Supplementary Table S2. Four [7,8,9,10] and three studies [13,26,27] reported grade 3 or 4 adverse event rates when using ICI monotherapy and ICI combined with radiotherapy, respectively. Overall grade 3 or 4 adverse event rates were 19% (95% CI, 13–27%) without substantial heterogeneity (I2 = 31%; p = 0.19) (Figure 3). The pooled grade 3 or 4 adverse event rates were 24% (95% CI, 16–34%) and 7% (95% CI, 3–17%) when using ICI monotherapy and ICI combined with radiotherapy, respectively.
Figure 3.
Forest plot of the (A) grade 3 or 4 adverse event rates and (B) grade 3 or 4 CNS-related adverse event rates when using immune checkpoint inhibitor (ICI) monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy.
Three [8,9,10] and three studies [13,26,27] reported grade 3 or 4 CNS-related adverse event rates when using ICI monotherapy and ICI combined with radiotherapy, respectively. Overall grade 3 or 4 CNS-related adverse event rates were 5% (95% CI, 2–10%), without a heterogeneity (I2 = 0%; p = 0.98) (Figure 3). The pooled grade 3 or 4 CNS-related adverse event rates were 5% (95% CI, 2–14%) and 4% (95% CI, 1–13%) when using ICI monotherapy and ICI combined with radiotherapy, respectively. Grade 3 or 4 CNS-related adverse event rates were not significantly different between the two arms (p = 0.93).
4. Discussion
In this meta-analysis, ICI treatment showed good intracranial responses across the treatment arms (overall ORR, 51%; DCR, 69%; CR, 19%). Although the number of included papers was small, ICI combined with radiotherapy (pooled ORR, 95%; DCR, 97%; CR, 46%) showed more promising results than ICI monotherapy (pooled ORR, 24%; DCR, 44%; CR, 10%). Regarding safety, the grade 3 or 4 adverse event rate was lower when using ICI combined with radiotherapy (7%) than when using ICI monotherapy (24%), and there was no significant difference in grade 3 or 4 CNS-related adverse event rate between the two groups (5% in ICI monotherapy, 4% in ICI combined with radiotherapy). Overall, these results indicate that ICI combined with radiotherapy seems to be a promising option for the treatment of NSCLC brain metastases. However, prospective trials are necessary to confirm this.
Radiation therapy acts on tumor via cytotoxicity and systemic pro-inflammatory effect which lead to activate host anti-tumor immune response which is enhanced by ICI treatment [28]. Therefore, it was hypothesized that and ICI treatment could have a synergistic effect, so-called abscopal effect, with an increase of drug efficacy. In a recent study for advanced NSCLC, pembrolizumab showed a better therapeutic response in patients who received previous radiation therapy than in those who did not [15] and in NCT02492568 trial, pembrolizumab after radiotherapy improved ORR at 12 weeks from 20% of pembrolizumab alone to 50% [29]. In the same context, our study showed that the patients who had combination therapy with ICI and radiation showed better intracranial response rate compared to ICI monotherapy, which can be explained by increased cytotoxicity and permeability of the blood-brain barrier induced by radiation therapy [30]. Several studies have been suggested that combining immunotherapy with radiotherapy can increase immune response [15,29], and many ongoing trials (NCT03168464, NCT03044626, NCT03391869) are investigating more detailed information about sequence of treatment arms, optimal dose and fractionations with different combinations of ICIs and radiotherapy. These further studies could provide an opportunity to establish new treatment strategies of NSCLC in specific situations (e.g., alternative option of surgery in early stage NSCLC, or neoadjuvant therapy before surgery).
Regarding safety, the pooled CNS-related grade 3 or 4 adverse event rate was similar between the two groups. Treatment-related necrosis, a representative CNS-related adverse event, has a significant effect on quality of life due to the accompanying focal neurologic deficits. A previous study showed that immunotherapy increases treatment-related necrosis in melanoma cases [31], especially when using ipilimumab, a drug targeting CTLA-4 [32]. However, in our meta-analysis study, subjects were patients with NSCLC, and the ICI combined with radiotherapy group used drugs targeting PD-1/PD-L1. Thus, pooled CNS-related grade 3 or 4 adverse events may have had no significant difference between the two treatment arms. This is in line with an existing study [33] that showed that the safety profile of PD-1/PD-L1 immunotherapy for NSCLC patients is acceptable.
With the introduction of immunotherapy, a growing number of patients experience pseudoprogression. Pseudoprogression occurs when an image initially looks like progression, but then a durable response is shown on following imaging. According to previous studies, the rate of pseudoprogression in NSCLC patients receiving immunotherapy varies from 0% to 6% [34,35,36,37,38,39,40]. RANO-BM and immuno-response evaluation criterion in solid tumors (iRECIST) are representative assessment criteria considering pseudoprogression. Unlike iRECIST, which is mainly used for solid tumors of the whole body, RANO-BM was developed for assessing the therapeutic response of brain metastasis only. The acceptance of RANO-BM for the evaluation of therapeutic responses to ICI and SRS has been recently increasing. As immunotherapy targeted approaches for brain metastasis increase, these new criteria will have more important meanings.
This meta-analysis has some limitations. First, the total number of included studies was rather small, and because each study did not report on both efficacy and safety indicators, in some groups, the number of included studies was even more insufficient. For example, only one study was included in the ICI combined with chemotherapy group [25]; so, there was no statistically significant result compared to that obtained from studies included in the ICI monotherapy group. In addition, the number of studies was insufficient to perform subgroup analysis. Second, with the exception of one study, all others [10] were conducted retrospectively. Due to this, there is a possibility of recall bias. Therefore, several prospective trials are needed to investigate the outcomes of ICI combined with radiotherapy, especially focusing on its comparison with ICI monotherapy and ICI combined with chemotherapy. Third, response assessment criteria were different across the included studies, and only two studies [13,14] used RANO-BM, which is most suitable for assessing the effect of ICI on brain metastasis. Furthermore, some studies [8,11,12,25,27] did not report their response assessment time. Lastly, comparison of grade 3 or 4 adverse event rate between the two groups was not possible due to incomplete data from the included studies. Nevertheless, this meta-analysis provides important information and suggests the need for future studies.
5. Conclusions
In conclusion, although the number of included studies was small, ICI combined with radiotherapy showed better intracranial efficacy than ICI monotherapy for the treatment of NSCLC brain metastases. CNS-related grade 3 or 4 adverse event rate was not statistically different between the two groups. Several prospective trials are needed to compare the efficacy of ICI combined with radiotherapy with that of ICI monotherapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/12/1098/s1, Figure S1. Forest plot of the intracranial complete response rates when using immune checkpoint inhibitor (ICI) monotherapy, ICI combined with chemotherapy, and ICI combined with radiotherapy; Table S1. Pooled analysis of the included studies evaluating safety (fixed-effect model); Table S2. Summary of grade 3 or 4 adverse events.
Author Contributions
Conceptualization, C.H.S.; Methodology, D.Y.K., P.H.K., C.H.S.; Software, D.Y.K., P.H.K.; Validation, P.H.K., C.H.S.; Formal analysis, D.Y.K., P.H.K.; Investigation, D.Y.K.; Resources, K.W.K.; Data curation, D.Y.K., P.H.K.; Writing, D.Y.K., P.H.K.; Visualization, P.H.K.; Supervision, C.H.S., H.S.K.; Project administration, C.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sorensen J.B., Hansen H.H., Hansen M., Dombernowsky P. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J. Clin. Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 2.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 3.Gavrilovic I.T., Posner J.B. Brain metastases: Epidemiology and pathophysiology. J. Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 4.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., Sneed P.K., Chao S.T., Weil R.J., Suh J., et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst R.S., Baas P., Kim D.W., Felip E., Pérez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Bjornhart B., Hansen K.H., Jorgensen T.L., Herrstedt J., Schytte T. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: A retrospective cohort study. Acta Oncol. 2019;58:953–961. doi: 10.1080/0284186X.2019.1615636. [DOI] [PubMed] [Google Scholar]
- 8.Dudnik E., Yust-Katz S., Nechushtan H., Goldstein D.A., Zer A., Flex D., Siegal T., Peled N. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98:114–117. doi: 10.1016/j.lungcan.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Gauvain C., Vauleon E., Chouaid C., Le Rhun E., Jabot L., Scherpereel A., Vinas F., Cortot A.B., Monnet I. Intracerebral efficacy and tolerance of nivolumab in non-small-cell lung cancer patients with brain metastases. Lung Cancer. 2018;116:62–66. doi: 10.1016/j.lungcan.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg S.B., Gettinger S.N., Mahajan A., Chiang A.C., Herbst R.S., Sznol M., Tsiouris A.J., Cohen J., Vortmeyer A., Jilaveanu L., et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R., Keam B., Kim S., Kim M., Kim S.H., Kim J.W., Kim Y.J., Kim T.M., Jeon Y.K., Kim D.W., et al. Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: Therapeutic implications for immune checkpoint inhibitors. BMC Cancer. 2019;19:19. doi: 10.1186/s12885-018-5214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song P., Zhang J., Shang C., Zhang L. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci. Rep. 2019;9:4278. doi: 10.1038/s41598-019-40748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepard M.J., Xu Z., Donahue J., Eluvathingal Muttikkal T.J., Cordeiro D., Hansen L., Mohammed N., Gentzler R.D., Larner J., Fadul C.E., et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: A matched cohort study. J. Neurosurg. 2019:1–8. doi: 10.3171/2019.4.Jns19822. [DOI] [PubMed] [Google Scholar]
- 14.Singh C., Qian J.M., Yu J.B., Chiang V.L. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J. Neurosurg. 2019;132:512–517. doi: 10.3171/2018.10.JNS181371. [DOI] [PubMed] [Google Scholar]
- 15.Shaverdian N., Lisberg A.E., Bornazyan K., Veruttipong D., Goldman J.W., Formenti S.C., Garon E.B., Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 17.Atkins D., Eccles M., Flottorp S., Guyatt G.H., Henry D., Hill S., Liberati A., O’Connell D., Oxman A.D., Phillips B., et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Brit. Med. J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.W., Lee J., Choi S.H., Huh J., Park S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean J. Radiol. 2015;16:1175–1187. doi: 10.3348/kjr.2015.16.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Kim K.W., Choi S.H., Huh J., Park S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015;16:1188–1196. doi: 10.3348/kjr.2015.16.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P., Thompson S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 23.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks L.E.L., Bootsma G., Mourlanette J., Henon C., Mezquita L., Ferrara R., Audigier-Valette C., Mazieres J., Lefebvre C., Duchemann B., et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur. J. Cancer. 2019;116:182–189. doi: 10.1016/j.ejca.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Afzal M.Z., Dragnev K., Shirai K. A tertiary care cancer center experience with carboplatin and pemetrexed in combination with pembrolizumab in comparison with carboplatin and pemetrexed alone in non-squamous non-small cell lung cancer. J. Thorac. Dis. 2018;10:3575–3584. doi: 10.21037/jtd.2018.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed K.A., Kim S., Arrington J., Naghavi A.O., Dilling T.J., Creelan B.C., Antonia S.J., Caudell J.J., Harrison L.B., Sahebjam S., et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J. Neurooncol. 2017;133:331–338. doi: 10.1007/s11060-017-2437-5. [DOI] [PubMed] [Google Scholar]
- 27.Schapira E., Hubbeling H., Yeap B.Y., Mehan W.A., Jr., Shaw A.T., Oh K., Gainor J.F., Shih H.A. Improved Overall Survival and Locoregional Disease Control With Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients With Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:624–629. doi: 10.1016/j.ijrobp.2018.02.175. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Ruiz M.E., Vanpouille-Box C., Melero I., Formenti S.C., Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theelen W., Peulen H.M.U., Lalezari F., van der Noort V., de Vries J.F., Aerts J., Dumoulin D.W., Bahce I., Niemeijer A.N., de Langen A.J., et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y., Tsien C.I., Shen Z., Tatro D.S., Ten Haken R., Kessler M.L., Chenevert T.L., Lawrence T.S. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J. Clin. Oncol. 2005;23:4127–4136. doi: 10.1200/JCO.2005.07.144. [DOI] [PubMed] [Google Scholar]
- 31.Colaco R.J., Martin P., Kluger H.M., Yu J.B., Chiang V.L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J. Neurosurg. 2016;125:17–23. doi: 10.3171/2015.6.JNS142763. [DOI] [PubMed] [Google Scholar]
- 32.Martin A.M., Cagney D.N., Catalano P.J., Alexander B.M., Redig A.J., Schoenfeld J.D., Aizer A.A. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4:1123–1124. doi: 10.1001/jamaoncol.2017.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubbeling H.G., Schapira E.F., Horick N.K., Goodwin K.E.H., Lin J.J., Oh K.S., Shaw A.T., Mehan W.A., Shih H.A., Gainor J.F. Safety of Combined PD-1 Pathway Inhibition and Intracranial Radiation Therapy in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018;13:550–558. doi: 10.1016/j.jtho.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Kurra V., Sullivan R.J., Gainor J.F., Hodi F.S., Gandhi L., Sadow C.A., Harris G.J., Flaherty K., Lee S. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J. Clin. Oncol. 2016;34:6580. doi: 10.1200/JCO.2016.34.15_suppl.6580. [DOI] [Google Scholar]
- 35.Hammer M., Bagley S., Aggarwal C., Bauml J., Nachiappan A.C., Simone C.B., 2nd, Langer C., Katz S.I. Thoracic imaging of non-small cell lung cancer treated with anti-programmed death receptor-1 therapy. Curr. Probl. Diagn. Radiol. 2019;48:142–147. doi: 10.1067/j.cpradiol.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gettinger S.N., Horn L., Gandhi L., Spigel D.R., Antonia S.J., Rizvi N.A., Powderly J.D., Heist R.S., Carvajal R.D., Jackman D.M., et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients with Previously Treated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiou V.L., Burotto M. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishino M., Ramaiya N.H., Chambers E.S., Adeni A.E., Hatabu H., Janne P.A., Hodi F.S., Awad M.M. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J. Immunother. Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H.K., Heo M.H., Lee H.S., Sun J.M., Lee S.H., Ahn J.S., Park K., Ahn M.J. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother. Pharmacol. 2017;80:591–598. doi: 10.1007/s00280-017-3396-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.