Abstract
Simple Summary
Invasive ants are often highly dominant competitors, having strong impacts on native species. Such invaders often exploit resources better than native species, finding them first or collecting them faster. They are also often more efficient when interfering with other species, suffering fewer losses or preventing access to resources. We assessed the competitive behavior of the invasive Argentine ant when facing another invasive species or a native dominant species. The exploratory behavior of the Argentine ant was strongly inhibited by the native dominant species. The Argentine ant brought very few prey resources to its nest and killed few opponents. Conversely, the other invasive species had low impact on the Argentine ant. Contrary to expectations, the invasive species lacked the ability to hinder resource exploitation by the Argentine ant, whereas the native dominant species did. These results suggest that a native species could impact invasive populations of the Argentine ant by interference competition, perhaps better so than some invasive species. In the northern half of Europe, it could prevent further expansion of this highly invasive species.
Abstract
Within ant communities, the biotic resistance of native species against invasive ones is expected to be rare, because invasive species are often highly dominant competitors. The invasive Argentine ant (Linepithema humile (Mayr)) often demonstrated numerical dominance against its opponents, increased aggressiveness, and ability to quickly recruit to food. The present study aimed to assess the behavioral mechanisms involved in the interspecific competition between L. humile, facing either an invasive species (Lasius neglectus Van Loon, Boomsma and Andrásfalvy) or a native dominant species (Lasius niger (Linnaeus)). The resource exploitation by the Argentine ant was investigated during one-hour competitive interactions using 10 dead Drosophila flies as prey. When facing La. niger, L. humile exploratory behavior was strongly inhibited, it brought very few prey resources, and killed few opponents. Conversely, La. neglectus had a low impact on L. humile. Contrarily to expectations, the invasive La. neglectus lacked the ability to hinder L. humile resource exploitation, whereas the native La. niger did. These results suggest that La. niger could impact invasive populations of L. humile by interference competition, perhaps better so than some invasive species. While L. humile has become invasive in Southern Europe, the invasion process could be slowed down in the northern latitudes by such native dominant species.
Keywords: aggression, Argentine ant, dominant species, interference competition, invasive species, resource exploitation
1. Introduction
Competition between species occurs in two ways. Exploitation competition involves the ability of species to find and exploit rapidly a resource before others, thereby making it unavailable to competitors. Interference competition involves the ability of species to prevent resource use by others (or to expulse them from the resource), either directly by aggression or indirectly by maintaining a territory [1,2]. Within ant communities, species co-occurrence could be partly explained by the fact that each species is assumed to excel in either competition by interference or competition by exploitation. Species depending on similar resources can coexist by means of a trade-off between the species’ ability to dominate resources and to discover them. This discovery–dominance trade-off occurs when species’ ability to excel at interference competition results in specialized morphological, behavioral, and physiological characteristics that reduce its ability to discover resources in the first place [3]. For instance, through competition by interference, most ant species affect abundance, spatial distribution, and behavior of other species through aggressive techniques that range from the use of chemical repellents to the establishment of territories [4,5]. Ant species can therefore be classified as dominant, subordinate, and submissive species [6]. Invasive ants especially are often highly aggressive, dominant competitors that displace many native species, through both interference and exploitative competition [7,8,9]. The dominance–discovery trade-off is indeed broken by these invasive species [5,10,11]. Finally, because the species previously established is more familiarized with the nesting and foraging site (hereinafter called “resident effect”), such species could have an advantage during the competitive interactions [12], for instance due to local numerical dominance.
During the invasion process, the resistance of local communities mainly depends on the presence of dominant ants [13,14] rather than species diversity [15]. The competition between invasive and native ant species has been substantially studied for a long time [7,16]. The interactions between the invasive Argentine ant (Linepithema humile (Mayr)) and native species have demonstrated that the competitive ability of L. humile stems from numerical dominance, aggressiveness, superior interference and exploitation competition, and the ability to quickly recruit to food [7,16]. For instance, Carpintero and Reyes-López [9] conducted a bait experiment where L. humile aggressively displaced large numbers of native ant species from the bait, whereas native species did not. In the study of Buczkowski and Bennett [12], Argentine ants aggressively outcompeted the native ant Tapinoma sessile (Say) from the baits through efficient interference competition and monopolized bait resources (exploitation competition). Studies considering the competition of invasive species facing dominant native species, and especially facing other invasive species, are less frequent. However, although invasions by the Argentine ant lead to an almost systematic exclusion of native ants and decreased ant community richness, some dominant native ants can resist (e.g., Iridomyrmex rufoniger (Lowne) [17]; Tapinoma group nigerrimum [18]). Similarly, some invasive species can also resist, as shown for Solenopsis invicta (Buren), where the competitive outcomes depended on the number of workers in each colony [19].
The present study therefore aimed to assess the behavioral mechanisms involved in the interspecific interference competition between invasive species or between an invasive and a native dominant species through laboratory experiments. More precisely, the main objective was to study how a competitor modulates resource exploitation using one the most successful invaders, the Argentine ant (L. humile) accounting for the resident effect of the established species. We studied interactions between L. humile workers facing workers, either of the invasive Lasius neglectus Van Loon, Boomsma and Andrásfalvy or of the native dominant Lasius niger (Linnaeus) species. Although most studies of the competitive ability of L. humile measured competition between individual ants or very small groups of workers, we conducted competition tests with groups of one hundred individuals to capture the group effect [18]. We also studied the effects of either a resident or colonizer status (i.e., familiarized or not with the foraging arena). Lasius neglectus is, as L. humile, one of the 19 species listed as highly invasive by the IUCN invasive species specialist group [IUCN SSC Invasive Species Specialist Group, 2019]. It is a more recent invader, described as a new species in 1990 [20], and shown to have a behavioral superiority over L. humile [21]. Both species are widely distributed across Europe and interact with invaded native communities, which often include the native black garden ant La. niger, one of the most common and dominant species in Europe. Lasius niger is characterized by its opportunism, its aggressiveness, and its ability to use mass recruitment [22,23], giving it a strong competitor’s potential. We measured 33 behavioral descriptors of the interactions covering the space occupation, the ability to exploit resources and the aggressiveness of L. humile, as well as the strength of the competition by the other species. We used controlled laboratory experiments where the behavioral characteristics that discriminate against the competitive ability of the three species can be assessed in the absence or presence of the competitor. As an invasive species, we expected to find evidence that La. neglectus has greater competitive abilities than La. niger, and therefore may hinder L. humile resource exploitation. The species established before the other, and therefore more familiarized with the nesting and foraging site, was also expected to have an advantage in the competitive interactions.
2. Materials and Methods
2.1. Studied Species
Three distinct species have been studied: the Argentine ant L. humile, the invasive garden ant La. neglectus and the black garden ant La. niger. Linepithema humile is currently a widespread and abundant invasive species, forming supercolonies (arising from the low levels of intraspecific aggression among colonies), with polydomic, highly polygynous nests and totally sterile workers [7]. Lasius neglectus also forms polygynous colonies with the presence of several functional queens within the nest [24]. This invasive species has ecological impacts on the biodiversity of Formicidae and other invertebrates (e.g., reducing the spatial and temporal foraging of native ants; [25,26]). The range of La. neglectus has increased rapidly and steadily in non-native locations over the last 30 years [27]. These two invasive species are present across almost all Europe and their distribution partly overlaps, especially in the easternmost part of Europe (Supplementary Material, Figure S1). Lasius niger is a widespread monogynous species, inhabiting all of Europe and parts of Asia and North America and colonizing a broad diversity of environments, but particularly abundant in arable land, as well as in cities, parks and gardens [23,28]. This species is aggressive towards other ants, including conspecifics [29].
2.2. Biological Material
The biological material used for the experiments came from colonies sampled between 23 April 2019 and 5 July 2019, from different sites: two super-colonies of La. neglectus (LnA—45.8248, 5.1004, Balan, France, collected on 19 June and 4 July 2019 and LnB—45.785258, 4.872009, Villeurbanne, France, collected on 21 June and 4 July 2019), two super-colonies of L. humile (LhA—36.992291, −6.451077, Huelva, Spain, collected on 23 April 2019, LhA’—43.209564, 5.629815, La Ciotat, France, collected on 15 May 2019 and LhB—43.402946, 6.730125, Fréjus, France, collected on 16 May 2019) and two colonies of La. niger from Villeurbanne, France (LniA—45.779328, 4.866604, collected 20 June and 5 July 2019 and LniB—45.781247, 4.867727, collected 21 June and 5 July 2019). In L. humile, the letter A indicates that it corresponds to the main supercolony and the letter B indicates that it corresponds to the Corsican supercolony [30,31]; in La. neglectus, the workers from colonies A and B demonstrate strong aggressiveness between each other, suggesting different supercolonies [32]. Each collected colony corresponds to three or more pooled nests on the same site. The colonies collected comprised several thousand workers, brood in substantial quantities, and at least five queens for nests belonging to polygynous species (only workers have been collected in La. niger monogynous colonies, preventing the colonies’ destruction).
Colony fragments were kept in the laboratory at 25 ± 3 °C with a mean hygrometry at 47%, and maintained in their original nesting substrate in boxes of 370 × 255 × H160 mm3. Colonies were supplied with a water-honey dilution (50%), proteinate food (insects such as mealworms, fruit flies or crickets) and water ad libitum. The interaction experiment was conducted in the two months after the installation and acclimation of the colonies.
2.3. Interaction Experiments
Interaction experiments were conducted between 20 June and 15 August 2019. The experimental set-up was as follows (Supplementary Material, Figure S2): two small peripheral plastic boxes (79 × 79 × H34 mm3) were connected by small plastic tubes (diameter 0.8 cm, length 2 cm) to a central arena (165 × 100 × H85 mm3). Each box was closed with stainless steel mesh to prevent leakage. These boxes were characterized by a resting site opposite the arena, with a source of humidity (small tube with wet cotton), with a 15 × 15 mm2 area covered with a red filter (favorable brightness). For each interaction, one hundred (±2) “naive” workers (i.e., never tested before) were taken from the donor colony, as closely as possible from the food resources to select preferentially foraging workers, and placed in a peripheral box with five brood elements. Water was supplied ad libitum. The taken ants were starved for 72 h prior to each trial [18]. During this period, one side of the device was open, allowing exploration of the central arena by one of the groups (resident), whereas the other did not have access to the arena (colonizer). The entrance of the resident was re-closed one hour prior to the prey being added.
Prior to the experiment, 10 dead Drosophila melanogaster (Meigen) individuals (adequate prey in field experiments with L. humile and native species; [33]) were placed in the center of the arena, equidistant from the two groups. At the start of the trial, the two entrances to the arena were simultaneously opened, and recruitment and interactions were filmed for one hour with two consumer-electronics RGB cameras BRIO 4K Ultra HD (Logitech, Lausanne, Switzerland). On each movie recorded, the species, the source colony, the date, and the conditions (temperature, hygrometry, status) were indicated on the video. All combinations of interactions with L. humile facing (i) no opponent, (ii) La. neglectus opponent and (iii) La. niger opponent were tested for both colonies of each species and successively for resident and colonizer statuses. Each individual test was replicated three times, resulting in 60 one-hour interactions recorded, covering a wide panel of situations encountered by a new colonizer, e.g., from no competitor (n = 12 videos), to an invasive or native competitor (n = 48 videos).
2.4. Video Analyses
Each video was analyzed by one of three observers familiar with the species and the ant’s behaviors. To ensure the consistency of results, ten percent of the videos were monitored by two observers simultaneously and ten percent of the videos were examined by two observers separately. All the videos have been analyzed twice (one time per interacting species). For each analysis, several global metrics were measured separately for each species: the time before the first worker leaves the peripheral box, the time between the entrance in the arena and the discovery of the resource and the time before the first interspecific interaction. We systematically recorded the temperature and hygrometry of the testing environment. Kinetic metrics were measured every five minutes for each species: the number of ongoing fights in the arena, the number of dead workers, the number of preys brought into the nest, moved in the arena or still available on the bait, the number of workers in the whole arena and the number of workers on the bait.
2.5. Statistical Analyses
All statistics were carried out using R v. 3.3 (RC Team, Vienna, Austria) software. Based on the kinetic and global variables resulting from the video analyses, 33 ethological descriptors of the interactions have been summarized in four categories, reflecting various aspects of the interactions: the space occupation by L. humile (5 descriptors), L. humile’s ability to exploit resources (8 descriptors), the aggressiveness of L. humile (8 descriptors) and the strength of the competition by the other species (12 descriptors; for interactions with opponents only; Appendix A, Table A1). We reduced these variables following three consecutive procedures: (i) remove correlated variables, (ii) run two principal component analyses (PCAs) for the different sets of descriptors, and (iii) run linear discriminant analyses (LDAs), based on the opponent species (coupled in previous PCAs), as explained below.
First, for each category of descriptors, we ran a collinearity analysis, eliminating the descriptors having a Spearman correlation value >0.7 with others, to establish a set of uncorrelated variables [34]. In each pair of correlated variables, the variable with the highest absolute correlation (i.e., the maximum average correlation with other variables) was identified using the find correlation function of the package caret [35] and removed (in italics in Appendix A, Table A1).
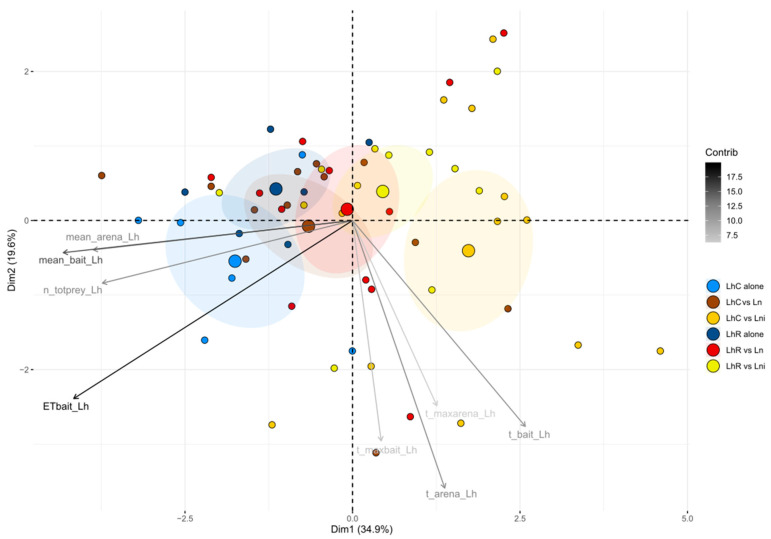
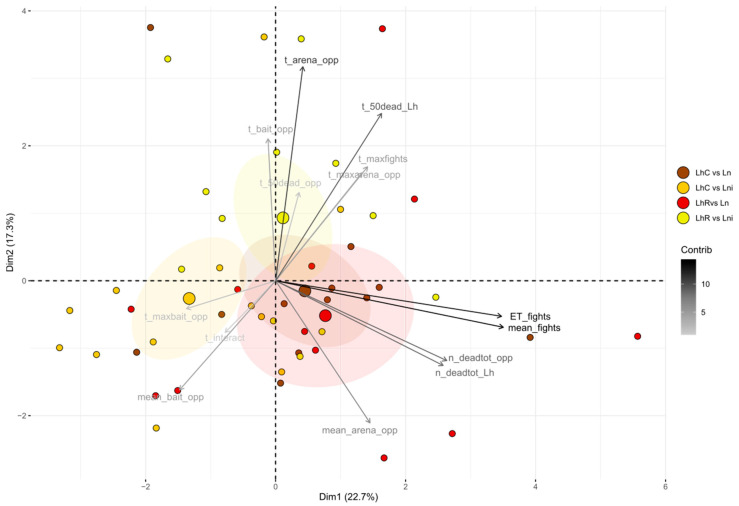
Second, the 22 remaining descriptors from the four categories (Appendix A, Table A1) have been used to perform two PCAs. The first PCA (Appendix A, Figure A1) was performed on 60 trials, including all variables regarding space occupation by L. humile and its ability to exploit resources, to reveal contrasts between tests with L. humile (i) alone, (ii) facing La. neglectus and (iii) facing La. niger. The second PCA (Appendix A, Figure A2) was performed on 48 trials, including all variables regarding the aggressiveness of L. humile and strength of the competition by the other species, to reveal contrasts between tests with L. humile facing (i) La. neglectus and (ii) La. niger.
Third, to identify variables varying according to the Argentine ant opponent (La. neglectus, La. niger or no opponent), we performed two discriminant analyses (LDAs) based on the two previous PCAs (package ade4; [36]). The significance of the eigenvalues was evaluated using a non-parametric version of Pillai’s test. Variables having a high proxy of the contribution to the discriminant function, i.e., whose cosines between the variables and the first axe of the linear discriminant analysis were >0.5 (as an absolute value) were considered the best drivers of the differences between species. The discriminant analyses suggested that eight variables were mainly responsible for the differences between the situations with different status and opponent species: the mean number of L. humile workers simultaneously present in the whole arena (mean_arena_Lh, M1), the number of preys brought by L. humile (n_totprey_Lh, M2), the standard deviation of the numbers of L. humile workers on the bait over time (ETbait_Lh, M3), the mean number of L. humile workers simultaneously present on the bait (mean_bait_Lh, M4), the number of dead L. humile individuals (n_deadtot_Lh, M5), the number of dead opponent individuals (n_deadtot_opp, M6), the mean number of simultaneous fights during the contest (mean_fights, M7) and the time when the maximal number of simultaneous fights occurs (t_maxfights, M8) (Appendix A, Table A2).
After reducing the 33 variables to eight, using the described three-step procedures, these eight variables were used to investigate the importance of (i) the opponent (La. neglectus, La. niger or no opponent), (ii) the status (resident vs. colonizer) and their interaction. In order to do that, we performed eight mixed models using the package glmmTMB [37]. The identity of the 20 combinations of source colonies and status was introduced in the model as a random effect. M1, M3, M7 and M8 were fitted with linear models, M2 and M4 were fitted with linear models performed following a square root transformation of the dependent variables, M5 and M6 were fitted with generalized linear models using Poisson family. The significance of each explanatory term was tested using Chi-squared tests. For every significant variable, post hoc, pairwise contrasts among treatments were performed by calculating the least square means (package lsmeans; [38]), and t-tests were adjusted to the number of tests using Tukey corrections. The raw data and scripts are available on the Zenodo repository (https://doi.org/10.5281/zenodo.4327129).
3. Results
Results of the PCA and LDA indicated likely differences in L. humile space occupation and ability to exploit resources according to the opponent species, but not regarding the colonizer/resident status, with more individuals in the arena and on the bait when alone in the arena than when in the presence of La. niger. The response seemed to be intermediate in the presence of La. neglectus (Appendix A, Figure A1, Table A2). The aggressive and competitive behaviors of L. humile seemed to differ against La. neglectus or La. Niger, and also differed according to status when facing La. niger (Appendix A, Figure A2, Table A2).
The models confirmed that six of these eight discriminant variables significantly varied among the opponent species (see below), whereas only one has been detected as significantly impacted by the status of L. humile (resident/colonizer) and its interaction with the opponent species (mean_arena_Lh, the mean number of L. humile workers simultaneously present in the whole arena) (Table 1).
Table 1.
Effects of the opponent species (Ln—Lasius neglectus, Lni—Lasius niger or No—no opponent) and the status of Linepithema humile (C—colonizer or R—resident) in the behavioral descriptors. A. Main effects. B. Post hoc tests of the significant variables. Interaction means the interaction between opponent and status. Significant statistics are marked in bold.
| A | Opponent | Status | Interaction | |||
|---|---|---|---|---|---|---|
| χ2 | p | χ2 | p | χ2 | p | |
| M1: Mean_arena_Lh | 6.95 | 0.03 | 1.16 | 0.281 | 24.89 | <0.001 |
| M2: n_totprey_Lh | 32.41 | <0.001 | 1.69 | 0.194 | 0.52 | 0.771 |
| M3: ETbait_Lh | 11.44 | 0.003 | 0.31 | 0.578 | 1.06 | 0.589 |
| M4: mean_bait_Lh | 37.91 | <0.001 | 0.02 | 0.903 | 1.13 | 0.568 |
| M5: n_deadtot_Lh | 0.38 | 0.538 | 1.73 | 0.188 | 0.11 | 0.738 |
| M6: n_deadtot_opp | 30.38 | <0.001 | 2.57 | 0.109 | 3.36 | 0.067 |
| M7: mean_fights | 9.21 | 0.002 | 0.02 | 0.887 | 0.25 | 0.615 |
| M8: t_maxfights | 0.82 | 0.367 | 2.46 | 0.117 | 2.17 | 0.141 |
| B | Estimate | SE | t.ratio | p value | ||
| M1: Mean_arena_Lh | A: No vs. Ln opponent | 5.34 | 5.73 | 0.93 | 0.954 | |
| B: No vs. Lni opponent | 13.48 | 5.69 | 2.37 | 0.142 | ||
| C: Ln vs. Lni opponent | 8.14 | 4.63 | 1.76 | 0.462 | ||
| D: C vs. R Status. | −10.72 | 6.58 | −1.63 | 0.556 | ||
| E: Interaction A:D | 3.84 | 5.73 | 0.67 | 0.992 | ||
| F: Interaction B:D | 23.42 | 5.69 | 4.12 | <0.001 | ||
| G: Interaction C:D | 19.58 | 4.63 | 4.23 | <0.001 | ||
| M2: n_totprey_Lh | A: No vs. Ln opponent | 0.53 | 0.74 | 0.71 | 0.861 | |
| B: No vs. Lni opponent | 3.47 | 0.74 | 4.68 | <0.001 | ||
| C: Ln vs. Lni opponent | 2.95 | 0.61 | 4.87 | <0.001 | ||
| M3: ETbait_Lh | A: No vs. Ln opponent | 0.72 | 0.37 | 1.96 | 0.158 | |
| B: No vs. Lni opponent | 1.23 | 0.37 | 3.36 | 0.004 | ||
| C: Ln vs. Lni opponent | 0.51 | 0.3 | 1.72 | 0.251 | ||
| M4: mean_bait_Lh | A: No vs. Ln opponent | 0.78 | 0.26 | 3.05 | 0.011 | |
| B: No vs. Lni opponent | 1.55 | 0.26 | 6.02 | <0.001 | ||
| C: Ln vs. Lni opponent | 0.76 | 0.21 | 3.64 | 0.002 | ||
| M6: n_deadtot_opp | A: Ln vs. Lni opponent | 2.41 | 0.42 | 5.72 | <0.001 | |
| B: C vs. R Status. | −0.86 | 0.42 | −2.04 | 0.135 | ||
| C: Interaction A:B | 0.77 | 0.42 | 1.83 | 0.206 | ||
| M7: mean_fights | A: Ln vs. Lni opponent | 3.51 | 1.16 | 3.02 | 0.004 | |
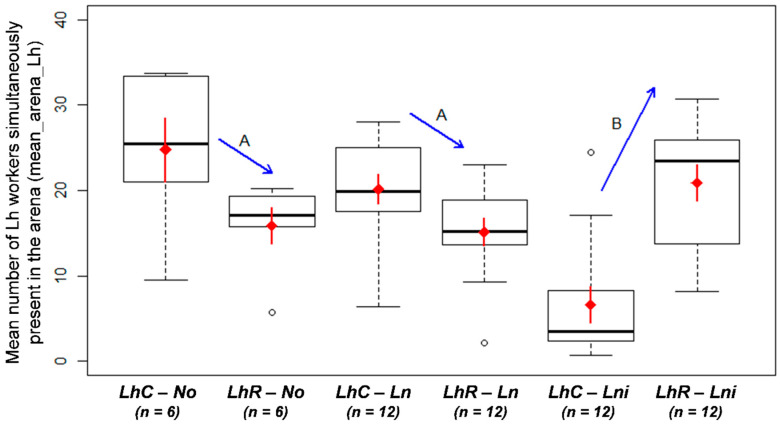
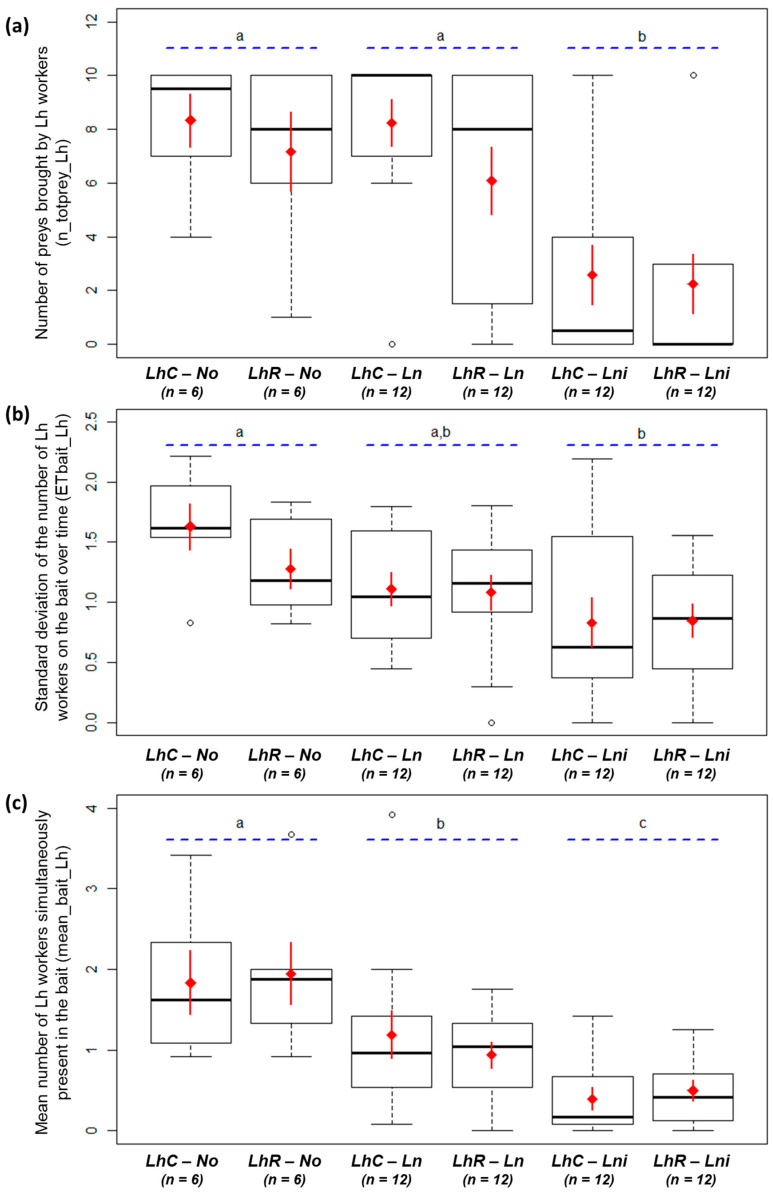
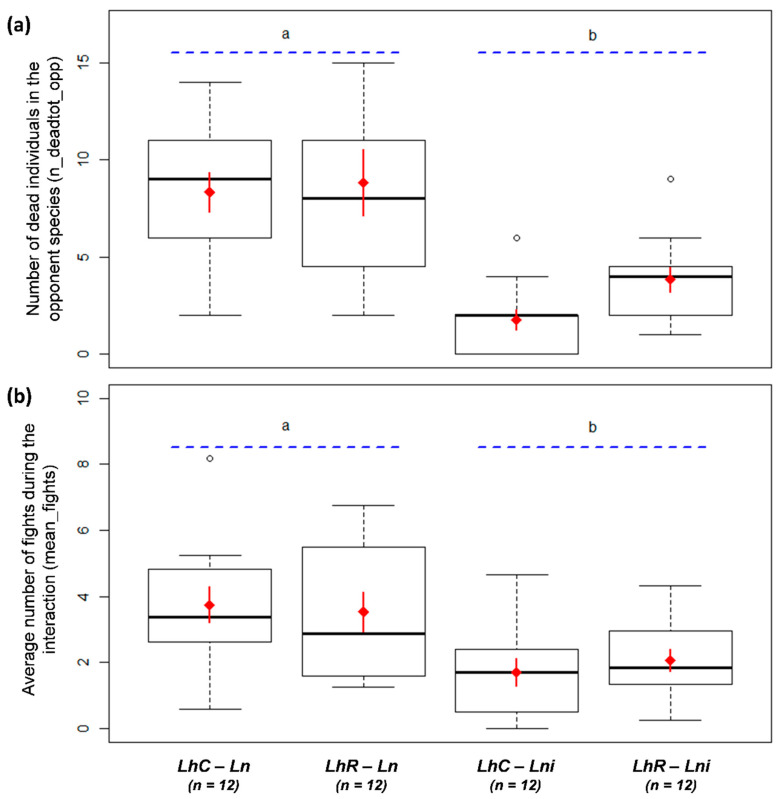
When facing La. niger, L. humile were fewer in the arena and the presence of resident La. niger seemed to strongly inhibit the foraging activity of colonizer L. humile (M1; Table 1, Figure 1). Facing La. niger, L. humile brought fewer preys than when facing La. neglectus or alone (M2; Table 1, Figure 2a), and was present on the bait with fewer workers (M3-4; Table 1, Figure 2b-c). Linepithema humile fought less against La. niger than against La. neglectus (M7; Table 1, Figure 3a) and thus killed fewer opponents of La. niger than of La. neglectus (M6; Table 1, Figure 3b). The number of L. humile in the arena was the same with and without La. neglectus (M1; Table 1, Figure 1). Despite a lower number of L. humile on the bait when facing La. neglectus than when alone (M4; Table 1, Figure 2c), the occupation of the bait by L. humile did not differ over time (M3; Table 1, Figure 2b), as well as the total number of preys brought by L. humile (M2; Table 1, Figure 2a).
Figure 1.
Average number of Linepithema humile in the arena during the interaction (mean_arena_Lh; n = 60). LhC: L. humile colonizer; LhR: L. humile resident; No: no opponent; Ln: Lasius neglectus opponent; Lni: Lasius niger opponent. Red diamond: mean value; red solid line: standard error of the mean. Letters A, B (blue arrows) indicate significant differences between opponent species × status interaction.
Figure 2.
(a) Number of preys brought by Linepithema humile during the interaction (n_totprey_Lh; n = 60). (b) Standard deviation of the numbers of L. humile on the bait over time (ETbait _Lh; n = 60). (c) Average number of L. humile on the bait during the interaction (mean_bait_Lh; n = 60). LhC: L. humile colonizer. LhR: L. humile resident. No: no opponent; Ln: Lasius neglectus opponent, Lni: Lasius niger opponent. White dots are outlier individuals; thick black horizontal line: median value; box ends: upper and lower quartiles; whiskers: max and min values. Red diamond: mean value; red solid line: standard error of the mean (SEM). Letters a, b, and c (blue dotted lines) indicate significant differences between opponent species.
Figure 3.
(a) Average number of fights during the interaction (mean_fights; n = 48). (b) Number of dead individuals in the opponent species (n_deadtot_opp; n = 48). Mean number of dead Linepithema humile: 14.08 ± 7.28 when facing Lasius. neglectus; 15.08 ± 6.70 when facing Lasius. niger. LhC: L. humile colonizer. LhR: L. humile resident. No: no opponent; Ln: Lasius neglectus opponent, Lni: L niger opponent. White dots are outlier individuals; thick black horizontal line: median value; box ends: upper and lower quartiles; whiskers: max and min values. Red diamond: mean value; red solid line: standard error of the mean (SEM). Letters a and b (blue dotted lines) indicate significant differences between opponent species.
4. Discussion
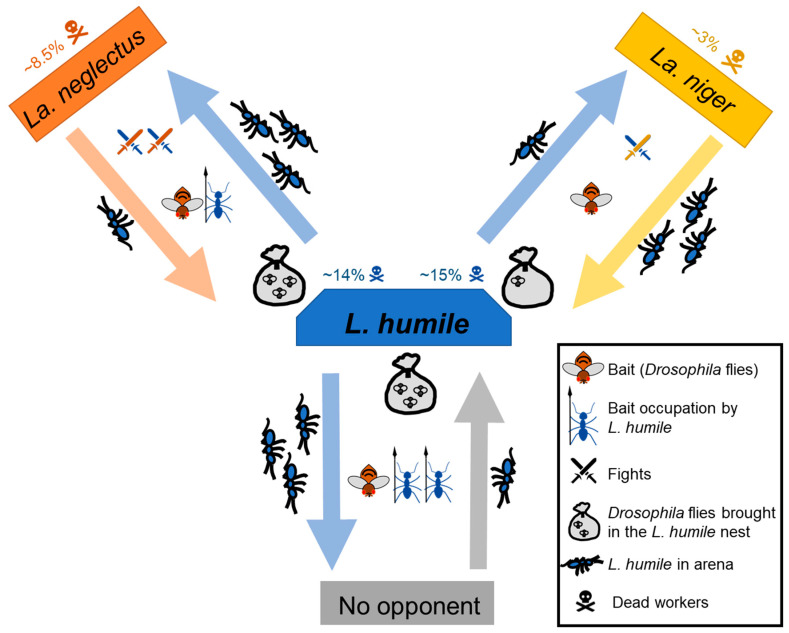
Among the behavioral descriptors of the interactions between the competing ant species, eight significantly discriminated against the opponent species (invasive or native opponents) or status of the opponent (resident or colonizer). When facing La. niger, the exploratory behavior of L. humile workers was inhibited, especially when L. humile was colonizer. Workers of L. humile brought very few resources, were almost absent from the resources, engaged in fewer fights and killed fewer opponents when facing La. niger. In contrast, La. neglectus was not so impactful, not modifying the number of L. humile in the arena or the quantity of resources brought by L. humile, although L. humile engaged in more fights with La. neglectus and killed more opponents. Contrary to what we expected, La. niger showed greater competitive ability to hinder L. humile resource exploitation than La. neglectus (Figure 4).
Figure 4.
Summary of the patterns observed in the interactions between Linepithema humile (blue) and Lasius neglectus (orange) or Lasius niger (yellow) or without opponent (grey). The arrows point towards the resident species, indicating the direction of the colonization.
Our main outcomes suggested that the native, and not the invasive Lasius species, may reduce the propensity of the Argentine ant to collect resources through interference competition. This finding is in contrast with the general established knowledge that aggressiveness and fight strategies of L. humile may explain its superiority over native ant species [8,12]. For instance, Carpintero and Reyes-López [9] showed that the Argentine ant is a competitively dominant species, because of its aggressive behavior and relative abundance, compared to native species such as Cataglyphis floricola Tinaut, Camponotus pilicornis (Roger) or Pheidole pallidula (Nylander) or Aphaenogaster senilis Mayr. When confronted with the Argentine ant, eight native species tended to retreat more frequently than Argentine ants (which also had initiated most of the encounters), which could help them to displace native species [16]. However, some cases in the literature have also found that specific local ants can offer strong resistance and delay or prevent the spread of Argentine ants, especially when encountering ecologically dominant or functionally similar native species (e.g., T. group nigerrimum [18], I. rufoniger [17], La. grandis Forel [14] or Prenolepis imparis (Say) [39]).
When facing La. niger, the risk perceived by L. humile appeared to decrease strongly its propensity to explore the arena, both for fighting and foraging, especially when exploring novel areas already colonized by La. niger. It has been suggested that chemicals laid in the environment could be used by L. humile as cues for the presence of local species, as they play an important role in the interactions [39]. A behavioral response to allocolonial or allospecific footprint cues could prevent encounters of potential competitors and thus be beneficial by reducing costs from competition [40]. Subordinate ant species avoided cuticular hydrocarbons of dominant species [41]. Lasius niger strongly differentiated between different cue types and avoided cues of allospecifics and allocolonial conspecifics [40]. In the present study, L. humile could thus have used chemical signals as well as direct allospecific interactions to evaluate the risk, and limited more its exploration activity when resident La. niger were present than when La. neglectus was present. By decreasing the ability of L. humile to collect resources, La. niger could also limit its expansion. This result is even more important as this dominant species is widely established in many environments, and the Argentine ant could therefore be challenged by habitats already occupied by the native ant La. niger when spreading north along Europe.
Conversely, some invasive species could be ineffective when facing L. humile, such as La. neglectus in the present study. For instance, T. magnum Mayr has shown considerable potential as an invasive species in Northern Europe, where it thrives in many urban areas and is considered a pest [42]. A recent study showed that T. magnum was systematically excluded by L. humile from resources and underwent a visible reduction in activity [43]. The foraging activity of L. humile was nevertheless reduced in the presence of T. magnum due to an increased worker commitment, both in the arena fights and directly in the colony of the competitor. In the present study, the native species La. niger was not excluded from the arena by L. humile, and its presence also resulted in a reduced foraging activity of L. humile due to an increased worker commitment in the arena fights. Moreover, Leonetti et al. [43] suggested that the nest of the opponent could have been perceived as a new potential threat or resource and, therefore, a more valid target for recruitment than the trophic resource. However, this result could differ for other food contents, such as carbohydrate food that could be more attractive than protein sources [44]. For instance, La. niger showed clear avoiding behavior when encountering the dominant species Formica fuscocinerea (Forel) at a carbohydrate-rich food source [45].
Our results showed that La. niger suffered very few losses during interactions with colonizer Argentine ants (mortality <5%), especially compared to La. neglectus (mortality ~10%). This finding contrasts with Bertelsmeier et al. [21], where dyadic interactions between La. neglectus and L. humile led to 90% mortality for L. humile and 35% for La. neglectus. The latter ranked second in the dominance hierarchy established between seven highly invasive ant species, and ranked before L. humile. The results observed here when L. humile interacted with La. niger or La. neglectus were therefore unexpected. The fact that the study of Bertelsmeier et al. [21] was based only on dyadic or ten versus ten interactions could explain the differences observed with our results. Although interference competition involves aggressive encounters between workers, including physical and chemical aggressions, it does not imply an important effect at the colony level [46,47]. In our experiments, we used colony fragments constituted by a hundred of workers with brood and queens for both L. humile and La. neglectus. In these situations, the possibility of many recruits implicated in the fights could thus be the cause of reduced differences in the outcomes of interactions with respect to the results of Bertelsmeier et al. [21]. This is especially relevant for L. humile, which often relies on group-level processes to successfully displace other species based on cooperative fighting [7,12,16]. Moreover, irrespective of their position in the dominance hierarchy, L. humile can adopt ‘‘the bourgeois strategy’’ during agonistic encounters with other species, changing its behavior based on numerical dominance: lone workers tend to be submissive in encounters, whereas, when numerically dominant, workers are aggressive. Linepithema humile has been shown to adopt this strategy against native species such as P. pallidula, T. group nigerrimum, or Monomorium antarcticum (Smith) [9,18,48]. Moreover, the bourgeois strategy could be a mechanism used by L. humile to co-occur with La. neglectus [49]. In this sense, because of the recent expansion of La. neglectus in Europe, where L. humile already invades, it was unexpected that the “resident effect” was not a primary determinant of the behavioral syndrome involved in competitive interactions for these species. Most of the parameters studied suggested that the species’ identity was more important than the status as resident or colonizer. However, we evaluated the resident effect as the access to the foraging arena, without accounting for the advantage of an earlier establishment and the consequent increase in density and numerical dominance [50], which could partly limit our results.
5. Conclusions
Our results suggest that native La. niger species could impact invasive ant populations of L. humile by means of interference competition. Although rare, this report is not the first instance of biotic resistance of native species against Argentine ants through physical aggression or chemicals to successfully defend themselves and their territory (e.g., [39]). Whereas L. humile has become invasive in Southern Europe, the invasion process could be slowed down in Northern Europe by such native dominant species rather than by other invasive ones, even under the scenario of co-occurrence between several highly invasive species [21]. However, the antagonistic behavior observed in this study does not necessarily imply that interspecific competition would take place in real conditions, because competition is a process of populations, not of individuals [5]. Further studies on the natural invasion progress and species competitive interactions in the field, especially including the study of long-term interactions, would be needed to eventually extend our conclusions.
Acknowledgments
We thank Rumsaïs Blatrix (CNRS, UMR 5175—CEFE) and Bernard Kaufmann (CNRS, UMR 5023—LEHNA) for their invaluable help in the field, and Bianca Dupont, Zoé Simon and Eliabelle Mauduit for their major help to monitor the competitive interactions in the lab. We also thank Frederic Mery for the equipment loan, Muriel Deparis and Palmyre Pasteau for their help in colonies maintenance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/12/2451/s1, Figure S1: Occurrences of the introduced species Lasius neglectus (left) and Linepithema humile (right) in Europe; Figure S2: Schema of the experimental design used for the interactions study.
Appendix A
Table A1.
Description of the sets of 33 variables used to describe the competitive interactions. Descriptors in italics were removed from the study following the collinearity analysis (Spearman correlation value >0.7 with others).
| Aggressiveness of L. humile | |
| n_deadtot_opp | total number of dead opponent workers |
| ETdead_opp | standard deviation of the number of dead opponent workers over time |
| t_50dead_opp | time when 50% of the opponent mortality load have been diagnosed |
| t_interact | time of the first interaction between L. humile and opponent workers |
| t_maxfights | time when the maximal number of simultaneous fights occurs |
| ET_fights | standard deviation of the numbers of fights over time |
| mean_fights | mean number of simultaneous fights during the contest |
| max_fights | max number of simultaneous fights during the contest |
| Strength of the competition by the other species | |
| n_deadtot_Lh | total number of dead workers of L. humile |
| t_50dead_Lh | time when 50% of the L. humile mortality load have been diagnosed |
| ETdead_Lh | standard deviation of the number of dead L. humile workers over time |
| t_arena_opp | time of the opponent entrance in the arena |
| t_bait_opp | time of opponent resources’ discovery |
| t_maxarena_opp | time when the max. number of opponent workers occurs in the arena |
| mean_arena_opp | mean number of opponent workers simultaneously present in the whole arena |
| max_arena_opp | max number of opponent workers simultaneously present in the whole arena |
| ETarena_opp | standard deviation of the numbers of opponent workers on the arena over time |
| t_maxbait_opp | time when the maximal number of opponent workers on the bait occurs |
| ETbait_opp | standard deviation of the numbers of opponent workers on the bait over time |
| mean_bait_opp | mean number of opponent workers on the bait |
| Space occupation by L. humile | |
| t_arena_Lh | time of the entrance in the arena of L. humile |
| t_maxarena_Lh | time when the max. number of workers of L. humile occurs in the arena |
| max_arena_Lh | max number of L. humile workers simultaneously present in the whole arena |
| mean_arena_Lh | mean number of L. humile workers simultaneously present in the whole arena |
| ETarena_Lh | standard deviation of the numbers of workers on the arena over time |
| L. humile ability to exploit resources | |
| n_totprey_Lh | total number of preys brought by L. humile |
| t_bait_Lh | time of resources’ discovery by L. humile |
| t_maxbait_Lh | time when the maximal number of L. humile individuals on the bait occurs |
| ETbait_Lh | standard deviation of the numbers of L. humile workers on the bait over time |
| mean_bait_Lh | mean number of L. humile workers on the bait |
| ETprey_Lh | standard deviation of the preys brought over time |
| t_50prey_Lh | time when 50% of the final prey load |
| t_maxprey_Lh | time when the maximal number of preys have been brought in the nest |
Figure A1.
PCA1 biplot along the two principal components; associating opponent species (LhC: Linepithema humile colonizer, LhR: L. humile resident; Ln: facing Lasius neglectus, Lni: facing Lasius niger, alone: without opponent) with eight variables reflecting space occupation by L. humile and its ability to exploit resources (n = 60; ellipses confidence intervals of 0.9; large points = mean points of groups (barycenters)).
Figure A2.
PCA2 biplot along the two principal components; associating opponent species (LhC: Linepithema humile colonizer, LhR: L. humile resident; Ln: facing Lasius neglectus, Lni: facing Lasius niger) with fourteen variables reflecting aggressiveness of L. humile and strength of the competition with the opponent species (n = 48; ellipses confidence intervals of 0.9; large points = mean points of groups (barycenters)).
Table A2.
Cosines between the variables and the first (DS1) and second (DS2) axes of the linear discriminant analyses 1 and 2 (LDA1 and LDA2). Cosines >0.5 (as an absolute value) were indicated in bold.
| L. humile Space Occupation and Ability to Exploit Resources | ||
| LDA1—p-value: 0.011 | DS1 | DS2 |
| t_arena_Lh | −0.0655 | −0.1174 |
| t_maxarena_Lh | 0.1374 | 0.2499 |
| mean_arena_Lh | 0.8011 | 0.4890 |
| n_totprey_Lh | 0.1467 | −0.7476 |
| t_bait_Lh | −0.3118 | −0.1410 |
| t_maxbait_Lh | 0.2970 | −0.1489 |
| ETbait_Lh | −0.6629 | 0.2491 |
| mean_bait_Lh | 0.4920 | −0.6903 |
| Aggressiveness of L. humile and Strength of the Competition | ||
| LDA2—p-value: 0.008 | DS1 | DS2 |
| n_deadtot_opp | 0.6639 | 0.4283 |
| t_interact | 0.1372 | 0.0774 |
| t_maxfights | −0.3212 | 0.5014 |
| ET_fights | −0.1470 | −0.3897 |
| mean_fights | 0.5459 | −0.3383 |
| t_50dead_opp | −0.1996 | −0.1825 |
| n_deadtot_Lh | −0.5596 | 0.2339 |
| t_arena_opp | −0.2872 | −0.0800 |
| t_bait_opp | 0.2391 | 0.2269 |
| t_maxarena_opp | 0.0462 | 0.4280 |
| mean_arena_opp | 0.1062 | 0.2755 |
| t_maxbait_opp | 0.1972 | −0.4622 |
| mean_bait_opp | −0.0549 | −0.2018 |
| t_50dead_Lh | 0.3425 | 0.2054 |
Author Contributions
Conceptualization, M.C. and F.C.; methodology, M.C., O.B. and E.A.; formal analysis, M.C. and O.B.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, M.C., O.B., E.A. and F.C.; supervision, F.C.; funding acquisition, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CNRS and the InvaCost grant from the French National Research Agency, grant number ANR-14-CE02-0021. Funds for the E.A. contract came from the AXA Research Fund Chair of Invasion Biology of University Paris Saclay.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vance R.R. Interference competition and the coexistence of two competitors on a single limiting resource. Ecology. 1984;65:1349–1357. doi: 10.2307/1939115. [DOI] [Google Scholar]
- 2.Fellers J.H. Interference and exploitation in a guild of woodland ants. Ecology. 1987;68:1466–1478. doi: 10.2307/1939230. [DOI] [Google Scholar]
- 3.Parr C.L., Gibb H. Competition and the role of dominant ants. In: Lach L., Parr C., Abbott K., editors. Ant Ecology. Oxford University Press; Oxford, UK: 2010. pp. 77–96. [Google Scholar]
- 4.Hölldobler B., Wilson E.O. The Ants. Harvard University Press; Cambridge, MA, USA: 1990. [Google Scholar]
- 5.Cerdá X., Arnan X., Retana J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol. News. 2013;18:131–147. [Google Scholar]
- 6.Savolainen R., Vepsäläinen K. A competition hierarchy among boreal ants: Impact on resource partitioning and community structure. Oikos. 1988;51:135–155. doi: 10.2307/3565636. [DOI] [Google Scholar]
- 7.Holway D.A. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology. 1999;80:238–251. doi: 10.1890/0012-9658(1999)080[0238:CMUTDO]2.0.CO;2. [DOI] [Google Scholar]
- 8.Rowles A.D., O’Dowd D.J. Interference competition by Argentine ants displaces native ants: Implications for biotic resistance to invasion. Biol. Invasions. 2007;9:73–85. doi: 10.1007/s10530-006-9009-5. [DOI] [Google Scholar]
- 9.Carpintero S., Reyes-López J. The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile) Biol. Invasions. 2008;10:25–35. doi: 10.1007/s10530-007-9103-3. [DOI] [Google Scholar]
- 10.Lebrun E.G., Feener D.H., Jr. When trade-offs interact: Balance of terror enforces dominance discovery trade-off in a local ant assemblage. J. Anim. Ecol. 2007;76:58–64. doi: 10.1111/j.1365-2656.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Parr C.L., Gibb H. The discovery–dominance trade-off is the exception, rather than the rule. J. Anim. Ecol. 2012;81:233–241. doi: 10.1111/j.1365-2656.2011.01899.x. [DOI] [PubMed] [Google Scholar]
- 12.Buczkowski G., Bennett G.W. Aggressive interactions between the introduced Argentine ant, Linepithema humile and the native odorous house ant, Tapinoma sessile. Biol. Invasions. 2008;10:1001–1011. doi: 10.1007/s10530-007-9179-9. [DOI] [Google Scholar]
- 13.Krushelnycky P.D., Gillespie R.G. Correlates of vulnerability among arthropod species threatened by invasive ants. Biodivers. Conserv. 2010;19:1971–1988. doi: 10.1007/s10531-010-9819-8. [DOI] [Google Scholar]
- 14.Wetterer J.K., Espadaler X., Wetterer A.L., Aguin-Pombo D., Franquinho-Aguiar A.M. Long-term impact of exotic ants on the native ants of Madeira. Ecol. Entomol. 2006;31:358–368. doi: 10.1111/j.1365-2311.2006.00790.x. [DOI] [Google Scholar]
- 15.Menke S.B., Fisher R.N., Jetz W., Holway D.A. Biotic and abiotic controls of Argentine ant invasion success at local and landscape scales. Ecology. 2007;88:3164–3173. doi: 10.1890/07-0122.1. [DOI] [PubMed] [Google Scholar]
- 16.Human K.G., Gordon D.M. Behavioral interactions of the invasive Argentine ant with native ant species. Insectes Soc. 1999;46:159–163. doi: 10.1007/s000400050127. [DOI] [Google Scholar]
- 17.Walters A.C., Mackay D.A. Importance of large colony size for successful invasion by Argentine ants (Hymenoptera: Formicidae): Evidence for biotic resistance by native ants. Austral Ecol. 2005;30:395–406. doi: 10.1111/j.1442-9993.2005.01481.x. [DOI] [Google Scholar]
- 18.Blight O., Provost E., Renucci M., Tirard A., Orgeas J. A native ant armed to limit the spread of the Argentine ant. Biol. Invasions. 2010;12:3785–3793. doi: 10.1007/s10530-010-9770-3. [DOI] [Google Scholar]
- 19.Kabashima J.N., Greenberg L., Rust M.K., Paine T.D. Aggressive interactions between Solenopsis invicta and Linepithema humile (Hymenoptera: Formicidae) under laboratory conditions. J. Econ. Entomol. 2007;100:148–154. doi: 10.1603/0022-0493(2007)100[148:AIBSIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Cremer S., Ugelvig L.V., Drijfhout F.P., Schlick-Steiner B.C., Steiner F.M., Seifert B., Hughes D.P., Schulz A., Petersen K.S., Konrad H., et al. The evolution of invasiveness in garden ants. PLoS ONE. 2008;3:e3838. doi: 10.1371/journal.pone.0003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertelsmeier C., Avril A., Blight O., Confais A., Diez L., Jourdan H., Orivel J., Saint Germès N., Courchamp F. Different behavioural strategies among seven highly invasive ant species. Biol. Invasions. 2015;17:2491–2503. doi: 10.1007/s10530-015-0892-5. [DOI] [Google Scholar]
- 22.Lenoir A. Ph.D. Thesis. Université de Tours; Tours, France: 1979. Feeding Behavior and Division of Labor in the Ant, Lasius niger. [Google Scholar]
- 23.Arnan X., Cerdá X., Retana J. Relationships among taxonomic, functional, and phylogenetic ant diversity across the biogeographic regions of Europe. Ecography. 2017;40:448–457. doi: 10.1111/ecog.01938. [DOI] [Google Scholar]
- 24.Espadaler X., Rey S., Bernal V. Queen number in a supercolony of the invasive garden ant, Lasius neglectus. Insectes Soc. 2004;51:232–238. doi: 10.1007/s00040-003-0732-y. [DOI] [Google Scholar]
- 25.Paris C., Espadaler X. Foraging activity of native ants on trees in forest fragments colonized by the invasive ant Lasius neglectus. Psyche. 2012;2012:261316. [Google Scholar]
- 26.Nagy C., Tartally A., Vilisics F., Merkl O., Szita E., Szél G., Podlussány A., Rédei D., Csősz S., Pozsgai G., et al. Effects of the invasive garden ant, Lasius neglectus Van Loon, Boomsma & András-Falvy, 1990 (Hymenoptera: Formicidae), on arthropod assemblages: Pattern analyses in the type supercolony. Myrmecol. News. 2009;12:171–181. [Google Scholar]
- 27.Espadaler X., Tartally A., Schultz R., Seifert B., Nagy C. Regional trends and preliminary results on the local expansion rate in the invasive garden ant, Lasius neglectus (Hymenoptera, Formicidae) Insectes Soc. 2007;54:293–301. doi: 10.1007/s00040-007-0944-7. [DOI] [Google Scholar]
- 28.Seifert B. A taxonomic revision of the Palaearctic members of the ant subgenus Lasius s. str. (Hymenoptera, Formicidae) Abh. Ber. Nat. Görlitz. 1992;66:1–67. [Google Scholar]
- 29.Devigne C., Detrain C. Collective exploration and area marking in the ant Lasius niger. Insectes Soc. 2002;49:357–362. doi: 10.1007/PL00012659. [DOI] [Google Scholar]
- 30.Giraud T., Pedersen J.S., Keller L. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA. 2002;99:6075–6079. doi: 10.1073/pnas.092694199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berville L., Blight O., Renucci M., Hefetz A., Provost E. A peaceful zone bordering two Argentine ant (Linepithema humile) supercolonies. Chemoecology. 2013;23:213–218. doi: 10.1007/s00049-013-0135-0. [DOI] [Google Scholar]
- 32.Kaufmann B. (University Claude Bernard Lyon 1, Villeurbanne, France). Personal Communication. 2019.
- 33.Angulo E., Caut S., Cerdá X. Scavenging in Mediterranean ecosystems: Effect of the invasive Argentine ant. Biol. Invasions. 2011;13:1183–1194. doi: 10.1007/s10530-011-9953-6. [DOI] [Google Scholar]
- 34.Dormann C.F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., García Marquéz J.R., Gruber B., Lafourcade B., Leitão P.J., et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- 35.Kuhn M. Caret: Classification and regression training. Ascl. 2015:ascl-1505. [Google Scholar]
- 36.Dray S., Dufour A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 37.Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Mächler M., Bolker B.M. Glmmtmb balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- 38.Lenth R., Lenth M.R. Package lsmeans. Am. Stat. 2018;34:216–221. [Google Scholar]
- 39.Sorrells T.R., Kuritzky L.Y., Kauhanen P.G., Fitzgerald K., Sturgis S.J., Chen J., Dijamco C.A., Basurto K.N., Gordon D.M. Chemical defense by the native winter ant (Prenolepis imparis) against the invasive Argentine ant (Linepithema humile) PLoS ONE. 2011;6:e18717. doi: 10.1371/journal.pone.0018717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wüst M., Menzel F. I smell where you walked–how chemical cues influence movement decisions in ants. Oikos. 2017;126:149–160. doi: 10.1111/oik.03332. [DOI] [Google Scholar]
- 41.Binz H., Foitzik S., Staab F., Menzel F. The chemistry of competition: Exploitation of heterospecific cues depends on the dominance rank in the community. Anim. Behav. 2014;94:45–53. doi: 10.1016/j.anbehav.2014.05.024. [DOI] [Google Scholar]
- 42.Seifert B., d’Eustacchio D., Kaufmann B., Centorame M., Lorite P., Modica M. Four species within the supercolonial ants of the Tapinoma nigerrimum complex revealed by integrative taxonomy (Hymenoptera: Formicidae) Myrmecol. News. 2017;24:123–144. [Google Scholar]
- 43.Leonetti D., Centorame M., Fanfani A. Differences in exploitation and interference ability between two dominant ants: The invasive Argentine ant (Linepithema humile) and Tapinoma magnum. Ethol. Ecol. Evol. 2019;31:369–385. doi: 10.1080/03949370.2019.1620341. [DOI] [Google Scholar]
- 44.Rust M.K., Reierson D.A., Paine E., Blum L.J. Seasonal activity and bait preferences of the Argentine ant (Hymenoptera: Formicidae) J. Agric. Urban Entomol. 2000;17:201–212. [Google Scholar]
- 45.Pohl A., Ziemen V., Witte V. Mass Occurrence and Dominant Behavior of the European Ant Species Formica fuscocinerea (Forel) J. Insect Behav. 2018;31:12–28. doi: 10.1007/s10905-017-9654-9. [DOI] [Google Scholar]
- 46.Pontin A.J. Population stabilization and competition between the ants Lasius flavus (F.) and L. niger (L.) J. Anim. Ecol. 1961;30:47–54. doi: 10.2307/2112. [DOI] [Google Scholar]
- 47.Ribas C.R., Schoereder J.H. Are all ant mosaics caused by competition? Oecologia. 2002;131:606–611. doi: 10.1007/s00442-002-0912-x. [DOI] [PubMed] [Google Scholar]
- 48.Sagata K., Lester P.J. Behavioural plasticity associated with propagule size, resources and the invasion success of the Argentine ant Linepithema humile. J. Appl. Ecol. 2009;46:19–27. doi: 10.1111/j.1365-2664.2008.01523.x. [DOI] [Google Scholar]
- 49.Trigos-Peral G., Abril S., Angulo E. Behavioral responses to numerical differences when two invasive ants meet: The case of Lasius neglectus and Linepithema humile. Biol. Invasions. 2020 doi: 10.1007/s10530-020-02412-4. [DOI] [Google Scholar]
- 50.Diaz M., Abril S., Enríquez M.L., Gómez C. Assessment of the Argentine ant invasion management by means of manual removal of winter nests in mixed cork oak and pine forests. Biol. Invasions. 2014;16:315–327. doi: 10.1007/s10530-013-0520-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.