Abstract
Simple Summary
Metal-based nanoparticles of different microbial pest control agents have been effective against several pests. This study reports the synthesis of Beauveria brongniartii based Fe0 nanoparticles (Fe0NPs) and their bio-efficacy against Spodoptera litura that was observed during this study. The median lethal concentration (LC50) of Fe0NPs against S. litura after 7 days was 59 ppm, whereas the median survival time (LT50) for 500 ppm concentrations of Fe0NPs was 2.93 days. B. brongniartii Fe0NPs caused a significant reduction in feeding and growth parameters as well as detoxifying enzyme production by S. litura at the end of the experimental period. These findings suggest that B. brongniartii Fe0NPs can potentially be used in environmentally friendly S. litura management programs.
Abstract
Nanotechnology has clear potential in the development of innovative insecticidal products for the biorational management of major insect pests. Metal-based nanoparticles of different microbial pest control agents have been effective against several pests. Synthesis of Beauveria brongniartii based Fe0 nanoparticles (Fe0NPs) and their bio-efficacy against Spodoptera litura was observed during this study. Beauveria brongniartii conidia were coated with Fe0NPs and characterized by applying a selection of different analytical techniques. Ultraviolet (UV) spectroscopy showed the characteristic band of surface plasmon at 430 nm; Scanning electron microscopy (SEM) images showed spherical shaped nanoparticles with a size ranging between 0.41 to 0.80 µm; Energy-dispersive X-ray (EDX) spectral analysis revealed characteristic Fe peaks at 6.5 and 7.1 Kev; the X-ray diffractogram showed three strong peaks at 2θ values of 45.72°, 64.47°, and 84.05°. The bioassay studies demonstrated that mortality of 2nd instar S. litura larvae following Fe0NPs treatment increased with increasing concentrations of Fe0NPs at different time intervals. The median lethal concentration (LC50) values of Fe0NPs against S. litura after seven days of fungal treatment was 59 ppm, whereas median survival time (LT50) values for 200 and 500 ppm concentrations of Fe0NPs against S. litura seven days post-treatment were 5.1 and 2.29 days, respectively. Beauveria brongniartii-Fe0NPs caused significant reductions in feeding and growth parameters (relative growth rate, relative consumption rate, and efficiency of conversion of ingested food) of S. litura. Beauveria brongniartii Fe0NPs induced reduction in glutathione-S-transferase activities throughout the infection period whereas activities of antioxidant enzymes decreased during later periods of infection. These findings suggest that B. brongniartii Fe0NPs can potentially be used in biorational S. litura management programs.
Keywords: biological control, entomopathogenic fungus, feeding, growth, toxicity
1. Introduction
Spodoptera litura (Lepidoptera; Noctuidae) is an important cosmopolitan pest with a wide range of hosts, including different vegetable and field crops such as cotton, groundnut, soybean, tomato, sweet potato, and tobacco [1,2]. There are numerous reports of the development of resistance in S. litura against a wide range of insecticides, resulting in many sporadic outbreaks of the pests which have led to the failure of crops [3]. Since reports of the presence of this pest in the Hunan province of China, the damage has increased continually. Currently, local farmers mainly depend on synthetic pesticides to control the pest. However, the mismanagement and over-application of synthetic chemicals have caused a high level of resistance to appear in several invertebrate pest populations including S. litura [4,5].
Entomopathogenic fungi have been suggested as potential agents for the biological control of different insects for over a century. Under favorable conditions, fungi occur frequently and often cause a significant reduction in insect pest populations [6]. There are several advantages of using entomopathogenic fungi: they have broad-spectrum insecticidal activity, a diversified species range, have complex metabolic types, and offer appropriate safety levels for humans and other non-target organisms [7]. They are also easy to mass-produce and the development of host resistance against them is unlikely to occur. However, one of the most widely used fungi as a biological pesticide, Beauveria brongniartii, can be affected by the environment. New agents with better efficacy, durability, and stability therefore need to be developed.
Nanotechnology is an emerging science that will potentially significantly revolutionize the agricultural and food industry by using nanoparticles and nanomaterials for disease and pest control [8]. Nanoparticles can be used in different ways and could be useful for pest control as the mixed formulation of metal and various other materials have already been reported effective against pests [9]. Nanoparticles of silver using different entomopathogenic fungi (Metarhizium anisopliae, Beauveria bassiana, and Isaria fumosorosea) have been reported as effective biopesticides against mosquitoes [10,11,12]. Wang et al. [13] studied the synthesis, characterization, and demonstrated the toxicity of I. fumosorosea based zero-valent iron nanoparticles against the sweet potato whitefly, Bemisia tabaci.
In this context, the current investigations were carried out to synthesize and characterize Beauveria brongniartii Fe0 nanoparticles in conjunction with studies on their bio-efficacy against S. litura. The main objectives of the current work were: (a) to synthesize and characterize Beauveria brongniartii Fe0 nanoparticles through different standardized analytical techniques (scanning electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffractometry, Fourier transmission infrared spectroscopy); (b) to study the concentration mortality responses of S. litura to Fe0NPs; (c) to measure the influence of Fe0NPs on feeding and growth parameters of S. litura, and; (d) to measure the effects of Fe0NPs on activities of detoxifying enzymes produced by S. litura.
2. Materials and Methods
2.1. Insect Cultures
Spodoptera litura individuals, used during these experiments, were collected from experimental fields of South China Agricultural University from cotton plants and reared for multiple generations on a semi-synthetic diet under insecticide-free conditions as outlined by David et al. [14] at the Engineering Research Centre of Biological Control, Ministry of Education, South China Agricultural University.
2.2. Fungal Inoculum
Beauveria brongniartii strain SB010 (obtained from the Key Laboratory of Biopesticides Innovation and Application of Guangdong Province, South China Agricultural University, Guangzhou, P.R. China) was cultured on Potato Dextrose agar (PDA) plates following the method of Ali et al. [15]. The basal fungal suspension (1 × 108 conidia/mL) to be used during the current experiments was prepared using the method of Ali et al. [16].
2.3. Preparation of Fe0NPs
Beauveria brongniartii Fe0 nanoparticles were synthesized extracellularly following the methods of Amerasan et al. [12] and Wang et al. [13]. Five milliliters of fungal suspension (1 × 108 conidia/mL) were inoculated in Erlenmeyer flasks (150 mL) containing freshly sterilized PDA broth. The flasks were placed in a rotary shaker at 150 revolutions per minute (rpm), 26 ± 2 °C for 72 h. Following 72 h, fungal biomass was separated from the broth via filtration across Whatman filter paper No. 01 and washed three times with ddH2O to remove any debris. Ten grams of fungal mycelia were then inoculated into the sterilized ddH2O (100 mL) and then incubated again on the rotary shaker at 150 rpm, 26 ± 2 °C for 72 h. The fungal broth was again filtered after 72 h and 100 mg zero-valent iron (particle size ≤ 100 nm) was added to the culture filtrates and again incubated at 120 rpm, 26 ± 2 °C for 72 h. The extra-cellularly prepared culture was stored in a refrigerator until required.
2.4. Characterization of Fe0NPs
The extracellular synthesis of Fe0NPs was characterized by applying different analytical techniques used for nanoparticle characterization in previous studies [12,13]. To perform scanning electron microscopy, Fe0NPs were processed and fixed by following the method of Wang et al. [13]. The images were captured under SU8010 (Hitachi Ltd, Hitachi, Japan) scanning electron microscope operating at an accelerated voltage of 5.0 kV. The UV-spectroscopy of Fe0NPs was performed at different wavelengths (300, 400, 500, 600 nm) in a Nanodrop one spectrophotometer (Thermo Scientific, USA) after 48 and 72 h. Energy-dispersive X-ray spectroscopy (EDX) was performed for further characterization of structure, as well as the composition of Fe0NPs. X-ray diffractometry (XRD) analysis was undertaken (using Co-Kα radiation in a Bruker D8 diffractometer, Karlshure, Germany) for the calculation of the crystalline structure of Fe0NPs. The Fe0NPs were characterized qualitatively through Fourier transformation infrared spectroscopy (FTIR) using a MIR8035 FTIR spectrometer (Thermo Fisher, Bemen, Germany). All the analytical studies were performed on three occasions with fresh samples.
2.5. Concentration Mortality Responses of Spodoptera litura to B. brongniartii Fe0NPs
The concentration mortality responses of S. litura to Fe0NPs were studied by following the method of Wu et al. [17]. Briefly, seven different concentrations of Fe0NPs, named as T1–T7 in Table 1 were added to an artificial diet before the diet has solidified. The artificial diet was treated with 1 × 108 conidia/mL B. brongniartii (T8) to compare the efficacy of Fe0NPs with fungal conidia alone while the artificial diet acted as a negative control (T9). The prepared diets were allowed to cool and then maintained at 4 °C until further use. Newly molted S. litura larvae (2nd instar) were starved for 3 h. Each larva was transferred to a small plastic cup (4 cm diameter) where they were individually fed on a treated or control diet (1 g). There were five replicates of each treatment and every replicate contained thirty larvae within each treatment. The plastic cups having insects belonging to different treatments were placed in an incubator (25 ± 2 °C; 80 ± 5% relative humidity and 16 h:8 h (light:dark) photoperiod). A freshly treated diet was placed in each plastic cup daily and the mortality of larvae was recorded until 7 days post-treatment.
Table 1.
Details of different Beauveria brongniartii Fe0 nanoparticles and B. brongniartii treatments used in the bio-efficacy studies.
| Treatment | Treatment Description | Concentration |
|---|---|---|
| T1 | B. brongniartii Fe0NPs | 6.25 ppm of diet |
| T2 | B. brongniartii Fe0NPs | 12.5 ppm of diet |
| T3 | B. brongniartii Fe0NPs | 25 ppm of diet |
| T4 | B. brongniartii Fe0NPs | 50 ppm of diet |
| T5 | B. brongniartii Fe0NPs | 100 ppm of diett |
| T6 | B. brongniartii Fe0NPs | 200 ppm of diet |
| T7 | B. brongniartii Fe0NPs | 500 ppm of diet |
| T8 | B. brongniartii conidial suspension | 108 conidia/g of diet |
| T9 | Control (ddH2O) | 0 |
2.6. Influence of B. brongniartii Fe0NPs on Feeding and Growth of Spodoptera litura
The changes in the feeding and growth parameters of S. litura were measured to determine the behavioral changes in response to Fe0NPs treatment. The 3rd instar S. litura (10 individuals) were fed on an artificial diet (1 g) treated with different treatments as shown in Table 1. The larvae (10 individuals) were individually placed in plastic cups (diameter: 3 cm, height: 4 cm) to feed on 1 g of pre-treated as well as the control diet. The experimental setup was incubated at 25 ± 2 °C, 80 ± 5% relative humidity, and 16 h:8 h (light:dark) photoperiod. The whole experimental setup was performed on three occasions. The larvae and artificial diet were weighed every day by using an electronic balance (precision: 0.0001 g) to record the larval weight gain and food weight loss until 15 days post-application on daily basis. The weight of the 3rd instar larvae at 12 h post-molting was used as the starting weight.
The feeding and larval growth data were used to calculate relative growth rate per unit weight of insect (RGR), relative consumption rate (RCR), and efficiency of conversion of ingested food (ECI); all of which were calculated through the following equations [18,19].
| RGR = Change in larval weight per day/initial larval weight |
| RCR = Change in weight of larval diet per day/initial weight of larval diet |
| ECI = (Weight gained by insects per day)/(weight of food consumed per day) × 100 |
2.7. Effects of B. brongniartii Fe0NPs on Activities of Detoxifying Enzymes
Newly molted 4th instar S. litura larvae were fed on an artificial diet treated with one of four different treatments (Fe0NPs: 250 ppm; Fe0NPs: 500 ppm; B. brongniartii conidial suspension: 1 × 108 conidia/mL and ddH2O as control) for enzymatic studies. The treated larvae were placed in plastic cups followed by incubation at 26 ± 2 °C; 65 ± 5% relative humidity. Following 3, 5, and 7 days, the samples (two larvae) were collected and dissected to get access to their fat bodies [19,20]. Each treatment was performed three times and each replicate consisted of twenty larvae.
Fat bodies were homogenized in phosphate buffer pH 7.3 (Nanjing Jiancheng Bioengineering, nanjing, China) (SOD, CAT and POD assay) and pH 7.5 (Nanjing Jiancheng Bioengineering, nanjing, China) (GST assay) at 4 °C followed by centrifugation at 10,000 g for 10 min at 4 °C The supernatant was retained for enzyme assays.
The total protein content of the supernatant was determined by Bradford assay, using bovine serum albumin (BSA) as standard [21].
The total superoxide dismutase (SOD) activity was analyzed following Beauchamp and Fridovich [22] through nitroblue tetrazolium reduction. The unit of SOD activity was described as the quantity of SOD needed to inhibit nitroblue tetrazolium reduction by 50%.
The method of Beers and Sizer [23] was adopted to analyze total catalase (CAT) activity. The decomposition of hydrogen peroxide was observed at 240 nm and one unit of enzyme activity was defined as the quantity of enzyme which can decompose 1 mM H2O2/min at an initial H2O2 concentration of 30 mM at 25 °C and pH 7.0.
The peroxidase (POD) activity was quantified through the method of Simon et al. [24] by observing changes in absorbance at 420 nm. Enzyme activity was expressed as units per mg protein (U/mg protein).
The glutathione S- transferase (GSTs) was conducted using the procedures developed by Habig and Jakoby [25]. Incubation was carried out at 25 °C for 5 min in 0.1 M Na-phosphate buffer (pH 6.5) containing 1 mM glutathione, 1 mM dinitrochilorobenzene, and 20 μL of the sample. The reaction was initiated by adding dinitrochilorobenzene solution in acetone. The concentration of 5-(2,4-dinitrophenyl) glutathione produced during the reaction was measured spectrophotometrically at a wavelength of 340 nm. One unit of enzyme will conjugate 10.0 nM of 1-Chloro-2,4-dinitrobenzene with reduced glutathione per minute.
2.8. Data Analysis
A percentage of insect mortality was subjected to probit analysis to calculate median lethal concentration and median lethal time values. The remaining data were analyzed by analysis of variance and significant differences between means were calculated by Tukey’s HSD test (p ≤ 0.05). All data analyses were performed by using SAS 8.1 software [26].
3. Results
3.1. Characterization of B. brongniartii Fe0 Nanoparticles
The supplementation of Fe0 into conidial filtrate of B. brongniartii induced a reduction process resulting in a change of the suspension’s color (black to dark grey) at 3 days post-incubation which confirmed the formation of Fe0NPs (Supplementary Figure S1).
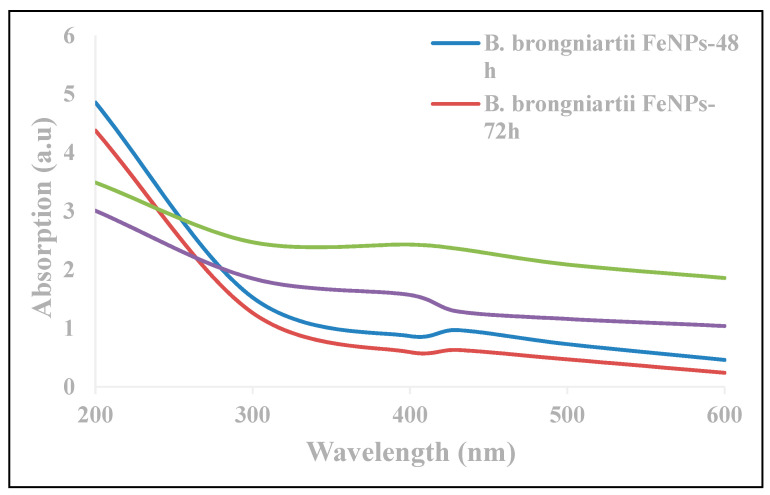
The UV analysis of aqueous suspension further confirmed the synthesis of Fe0NPs. The UV-absorption spectra of samples obtained at different time intervals showed a consistent decrease in UV spectra at different wavelengths with a specific surface plasmon absorption band between 400–450 nm (Figure 1).
Figure 1.
Absorption spectra of Beauveria brongniartii Fe0 nanoparticles and Beauveria brongniartii conidia at different wavelengths after 48 and 72 h.
The SEM images revealed adherence of Fe0 particles onto the B. brongniartii conidial surface indicating the proper formation of Fe0NPs (Figure 2).
Figure 2.
Scanning electron micrographs of Beauveria brongniartii Fe0NPs.
The EDX spectral analysis revealed characteristic Fe peaks at 6.5 and 7.1 Kev along with a signal of Mn (Supplementary Figure S2).
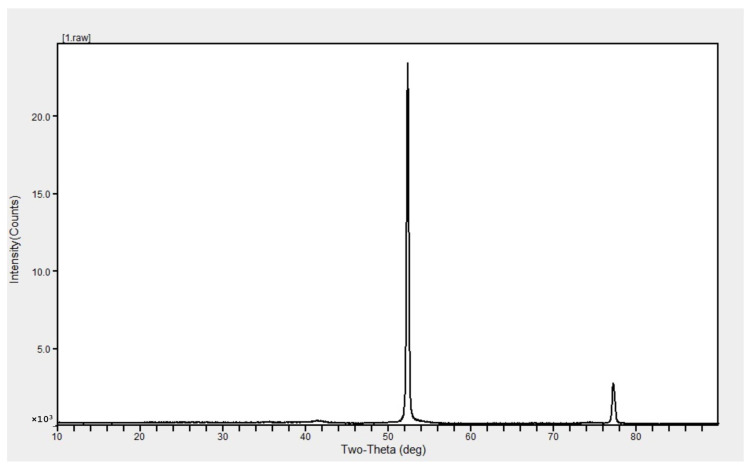
X-ray diffractometry (XRD) analysis of Fe0NPs showed diffracted intensities at 2θ angles ranging from 10° to 90°. The target of XRD analysis was CoKα with a wavelength of 1.743 Å. The X-ray diffractogram showed two strong peaks at 2θ values of 52.42° and 77.25° (Figure 3).
Figure 3.
X-ray diffraction pattern of Beauveria brongniartii Fe0NPs.
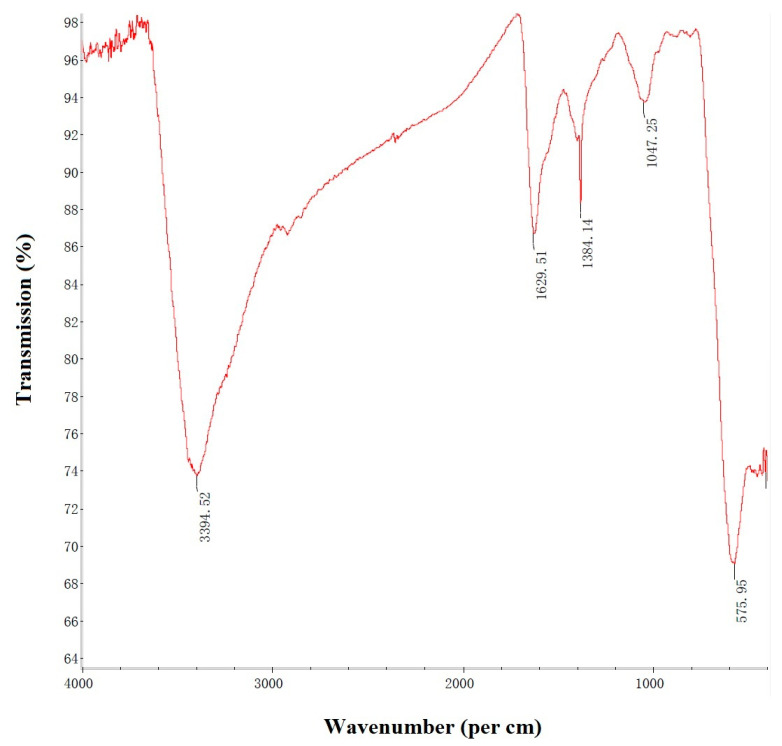
The FTIR spectroscopic analysis was carried out for the identification of biomolecules responsible for the reduction of Fe0 ions as well as the capping of bio-reduced Fe0NPs. The FTIR spectra of Fe0NPs showed absorption beaks at 3394.52, 1629.51, 1384.41, 1047.25, and 575.95 in the region of 4000–400 cm−1 (Figure 4). The analysis of spectral beaks showed the existence of O-H giving a very strong and broad beak, C≡C showed a medium-strong beak, C-H showed a sharp beak, O-H showed a weak beak, and C-O showed a very strong beak having a very strong structure between amino acid residues and synthesized proteins.
Figure 4.
Fourier-transform infrared spectroscopy pattern of Beauveria brongniartii Fe0NPs.
3.2. Dose Mortality Responses of S. litura to B. brongniartii Fe0NPs
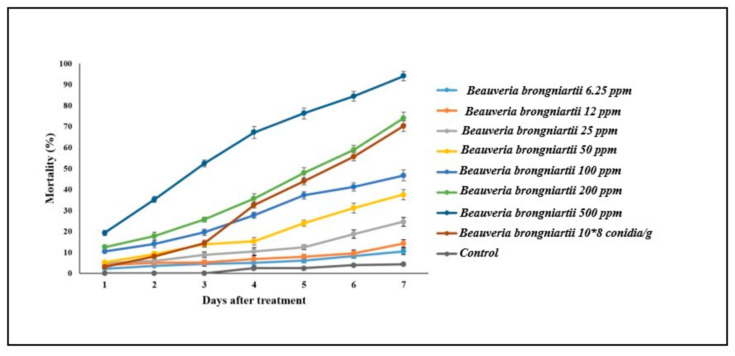
Our results revealed that 100, 200, and 500 ppm concentrations of Fe0NPs induced higher S. litura mortality compared with B. brongniartii conidial suspension (1 × 108 conidia/mL) alone until 3 days post-treatment. After this period 200 and 500 ppm concentrations of Fe0NPs caused higher S. litura mortality than B. brongniartii conidial suspension following 4 to 7 days of fungal treatment (Figure 5).
Figure 5.
Percentage mortality of 2nd instar Spodoptera litura larvae in response to different treatments of Beauveria brongniartii Fe0NPs, B. brongniartii, and control at different time intervals. * Treatments (T1–T9) are outlined in Table 1. Error bars indicate the standard error of means.
The LC50 values of Fe0NPs against S. litura after 7 days of fungal treatment was 58 ppm (Table 2). The LT50 values for 200 and 500 ppm concentrations of Fe0NPs against S. litura 7 days post-treatment were 5.10 and 2.29 days, respectively (Table 3).
Table 2.
Median lethal concentration (LC50) values of Beauveria brongniartii Fe0NPs against Spodoptera litura estimated by probit regression.
| Treatment Time (Days) |
LC50 (ppm) |
95% Fiducial Limit | Slope ± S.E | χ2 (df = 4) | p |
|---|---|---|---|---|---|
| 7 | 58 | 36–95 | 1.63 ± 0.13 | 3.92 | 0.870 |
Table 3.
Median lethal time (LT50) values of Beauveria brongniartii Fe0NPs nanoparticles against Spodoptera litura estimated by probit regression.
| Concentration (ppm) |
LT50 (Days) |
95% Fiducial Limit | Slope | χ2 (df = 4) | p |
|---|---|---|---|---|---|
| 200 | 5.10 | 3.72–6.99 | 2.32 ± 0.24 | 4.77 | 0.869 |
| 500 | 2.29 | 1.72–3.06 | 2.63 ± 0.19 | 3.92 | 0.964 |
3.3. Influence of B. brongniartii Fe0NPs on Feeding and Growth of Spodoptera litura
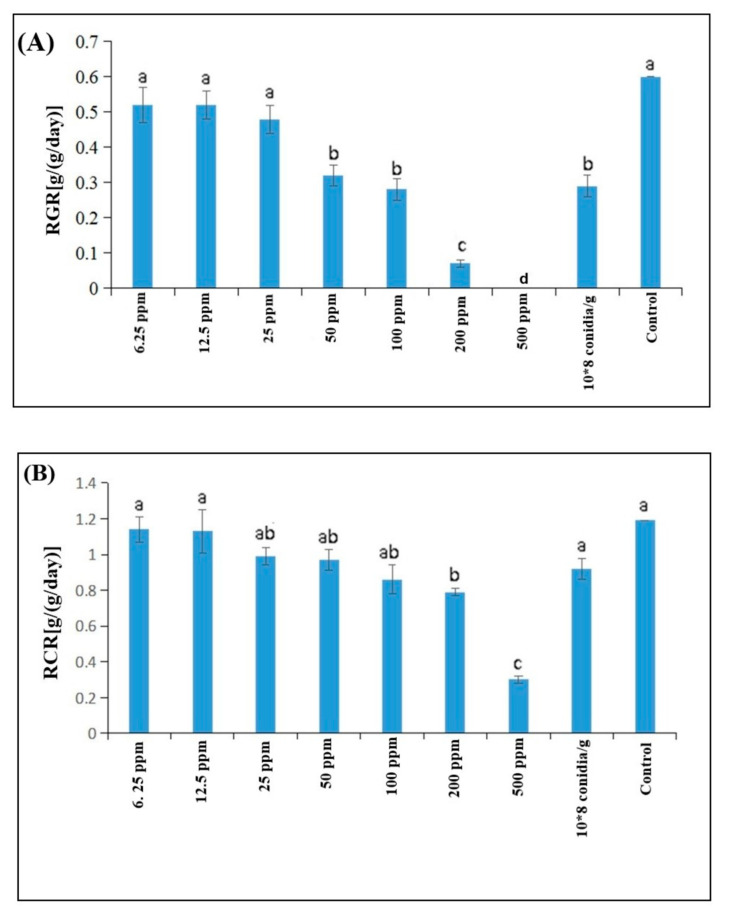
The relative growth rate (RGR) of S. litura in response to B. brongniartii Fe0NPs application showed significant differences among different treatments at the end of the experimental period (F8,18 = 57.92; p < 0.001). The highest RGR of S. litura was observed for control whereas the lowest RGR was observed for Fe0NPs 500 ppm) (Figure 6A). The RGR observed for Fe0NPs 6.25 ppm, Fe0NPs 12.5 ppm, and Fe0NPs 25 ppm were similar to that of control whereas RGR values observed for Fe0NPs 50 ppm and Fe0NPs 100 ppm were similar to that of B. brongniartii conidial suspension 1 × 108 conidia/g (Figure 6A).
Figure 6.
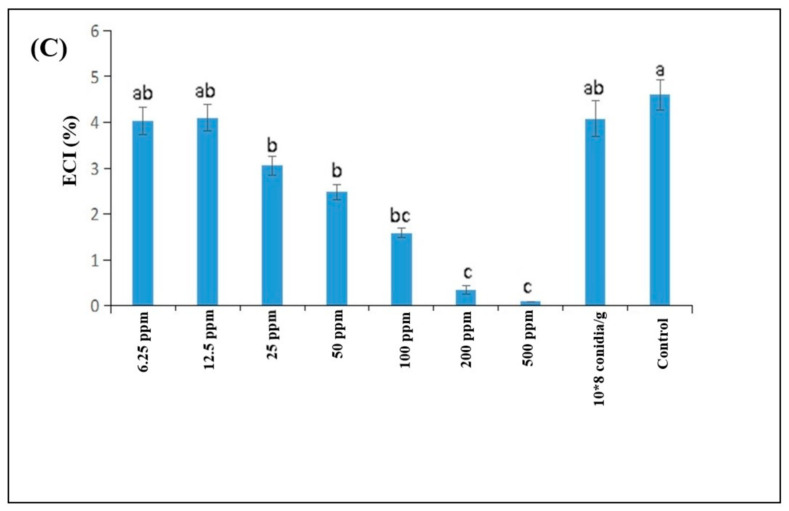
Feeding and growth parameters of 2nd instar Spodoptera litura larvae in response to different treatments of Beauveria brongniartii Fe0NPs, B. brongniartii conidia alone and control. (A) relative growth rate; (B) relative consumption rates and (C) percentage food conservation efficiency. Error bars indicate standard error of means. Different letters above columns indicate significant differences between means at a 5% level of significance.
Different treatments of B. brongniartii Fe0NPs significantly affected the relative consumption rates (RCR) of S. litura at the end of the experimental period (F8,18 = 49.54; p < 0.001). The highest RCR of S. litura was observed for control whereas the lowest RCR was observed for Fe0NPs 500 ppm (Figure 6B). The RGR observed for Fe0NPs 6.25 ppm, Fe0NPs 12.5 ppm, and B. brongniartii conidial suspension 1 × 108 conidia/g were significantly similar to control whereas RGR values observed for Fe0NPs 25 ppm, Fe0NPs 50 ppm, and Fe0NPs 100 ppm were statistically similar (Figure 6B).
The index of food conservation efficiency (ECI) of S. litura in response to B. brongniartii Fe0NPs application differed significantly among different treatments at the end of the experimental period (F8,18 = 41.97; p < 0.001). The highest ECI values of S. litura were observed for control whereas the lowest ECI was observed for Fe0NPs 500 ppm. The ECI observed for Fe0NPs 200 ppm and Fe0NPs 500 ppm were similar to each other whereas ECI values observed for Fe0NPs 6.25 ppm and Fe0NPs 12.5 ppm were statistically similar to B. brongniartii conidial suspension 1 × 108 conidia/g (Figure 6C).
3.4. Effects of B. brongniartii Fe0NPs on Activities of Detoxifying Enzymes
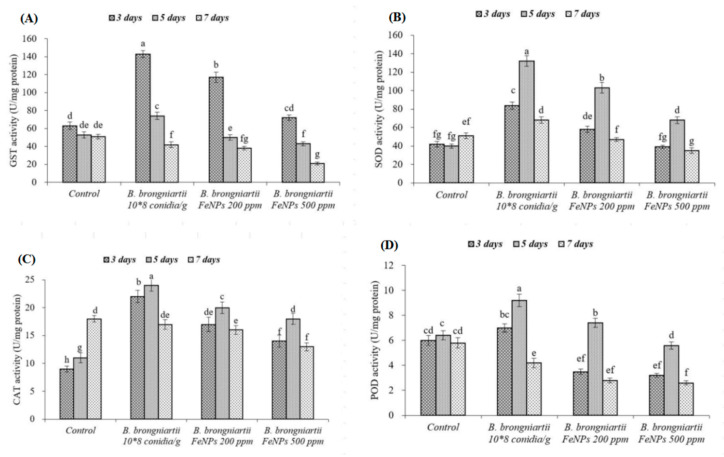
The glutathione-S-transferase (GST) activities in S. litura fat bodies when treated with different concentrations of B. brongniartii Fe0NPs or B. brongniartii conidial suspension differed significantly from the control at different time intervals (F11,19 = 36.78; p < 0.001). (Figure 7A). The highest GST activity was observed 3 days post-treatment followed by a consistent decrease in enzyme activity in response to different treatments at 5 and 7 days of application (except for the control). The GST activities observed following Fe0NPs treatments were significantly lower than GST activities observed for B. brongniartii conidial suspension at 3, 5, and 7 days of treatment (Figure 7A).
Figure 7.
Detoxifying enzyme produced by 2nd instar Spodoptera litura larvae in response to different treatments of Beauveria brongniartii Fe0NPs, B. brongniartii and control after 3, 5, and 7 days of treatment. (A): glutathione-S-transferase (GST); (B) superoxide dismutase (SOD); (C) catalase (CAT); and (D) peroxidase (POD). Error bars indicate standard error of means. Different letters above columns indicate significant differences between means at a 5% level of significance.
The activities of different antioxidant enzymes (SOD, CAT, and POD) in S. litura fat bodies when treated with different concentrations of B. brongniartii Fe0NPs or B. brongniartii conidial suspension also differed significantly from the control at 3, 5, and 7 days of treatment (SOD: F11,19 = 49.23, p < 0.001; CAT: F11,19 = 39.08, p < 0.001; POD: F11,19 = 28.21, p < 0.001). The enzyme activity values of antioxidant enzymes (SOD, CAT, and POD) showed an increasing-trends up to 5 days post-treatment followed then by a decrease in enzyme activity after 7 days of treatment. The antioxidant enzyme (SOD, CAT, and POD) activities observed for Fe0NPs treatments were significantly different from enzyme activities observed following the treatment of B. brongniartii conidial suspension at 3, 5, and 7 days of treatment (Figure 7B–D).
4. Discussion
The application of nanotechnology in combination with insect pathogens can be a promising biological alternative to synthetic chemicals for insect pest management [27]. Nanoparticles/nanomaterials-based formulations of insect pathogens have been recently tested as fungicides or insecticides for effective management of different insect pests and diseases [28,29]. During our previous studies, I. fumosorosea-Fe0NPs (100 ppm) application resulted in a 68% reduction in egg hatchability as well as 80–98% mortality of first, second, and third instar B. tabaci nymphs, respectively [13].
This study reports the use of B. brongniartii with Fe0NPs synthesis and their application for S. litura management. The treatment of B. brongniartii with Fe0 under dark conditions resulted in a change of filtrate color from light gray to dark gray showing the synthesis of Fe0NPs. The visualization of a characteristic peak between 400–450 nm in UV profile further confirmed the Fe0NPs synthesis. Our results are consistent with the findings of Wang et al. [13] who reported a similar change in the color of culture filtrate and a characteristic peak at 470 nm during bio-synthesis of I. fumosorosea-Fe0NPs. These findings are also in line with the results of Mukherjee et al. [30], Banu and Balasubramanian [10,11], and Amersan et al. [12] who also observed similar changes in culture filtrates during the synthesis of metal-based nanoparticles from different species of entomopathogenic fungi. The color change during the extracellular synthesis of Fe0NPs can be related/explained by two phenomena: (i) the excitation of surface plasmon vibration of nanoparticles [31]; (ii) metabolic utilization of nitrate through reduction of nitrate and ammonia during bio-reduction of metals [32]. The existence of Fe0 on B. brongniartii conidial surface was confirmed through scanning electron micrographs. The EDX spectroscopy showed two sharp peaks of iron at 6.5 and 7.1 KeV further confirming the absorption of metallic iron [33]. The XRD analysis showed characteristic peaks at 2θ values of 52.42° and 77.25°. The Fourier transformation infra-red spectrum also confirmed the presence of different biomolecules e.g., proteins which can be responsible for B. brongniartii Fe0NPs [11]. All these results are consistent with the findings of Wang et al. [13] who observed similar EDX, XRD, and FTIR patterns (with slight differences in peak values) during the extracellular synthesis of I. fumosorosea Fe0NPs.
During this study, 2nd instar S. litura larvae were treated with different concentrations of B. brongniartii Fe0NPs, resulting in larval mortality between 10% and 94% in response to different concentrations. The findings of this investigation are in line with previous studies on the toxicity of I. fumosorosea Fe0NPs against B. tabaci by Wang et al. [13] who reported 23–98% mortality of 2nd instar B. tabaci nymphs in response to different concentrations of I. fumosorosea Fe0NPs. The indices probit analysis such as median lethal concentration (LC50) and median lethal time (LT50) are commonly used parameters to gauge the efficacy of any pest control agent. The median lethal concentration (LC50) values of B. brongniartii Fe0NPs against 2nd instar S. litura larvae were 58 ppm after 7 days of treatment. Wang et al. [15] reported the median lethal concentration (LC50) value of 19.17 for I. fumosorosea Fe0NPs against 2nd instar B. tabaci nymphs. Banu and Balasubramanian [10] found the LC50 value of 0.79 ppm for B. bassiana AgNPs against 2nd instar larvae of Aedes aegypti. The median lethal time (LT50) values for 200 and 500 ppm concentrations of B. brongniartii Fe0NPs against 2nd instar S. litura larvae were 5.10 and 2.29 days, respectively. The LT50 values of this study were a little higher than the findings of Wang et al. [13] who reported an LT50 value of 3.15 days for 100 ppm concentration of I. fumosorosea Fe0NPs against 2nd instar B. tabaci nymphs following 7 days of fungal treatment.
The nutrition analysis to study the effects of B. brongniartii Fe0NPs on feeding and growth parameters of S. litura showed a significant reduction in RCR, RGR, and ECI values compared with the control following the application of different B. brongniartii Fe0NPs concentrations. Although B. brongniartii conidial suspension also caused a reduction in RCR, RGR, and ECI values compared with the control, the changes were more prominent with higher concentrations of B. brongniartii Fe0NPs. These changes in RCR, RGR, and ECI values can be explained by different phenomenon or hypothesis such as: (1) enhanced degradation of the insect cuticle or delayed ecdysis in response to B. brongniartii Fe0NPs due to a combined action of extracellular hydrolytic enzymes produced by fungi known for their effect on an insect cuticle [34,35] and Fe0 particles known to degrade hydrocarbons in nature or alkanes present in an insect cuticle [13,36]; (2) possible degradation of the insect gut peritrophic membranes by chitinase produced by the fungi [20,37,38] and also the degradation of the peritrophic membrane being further enhanced by the addition of Fe0 which is known for its ability to degrade hydrocarbons and aromatic structures in nature [39]; (3) decreased efficiency to convert the consumed food into a growth and energy source through the possible diversion of energy from growth to the detoxification process [19,20].
In insects, the detoxification of foreign pathogens or chemicals is accomplished by different detoxifying and antioxidant enzymes [16,40,41]. Glutathione-S-transferase (GST) is the main enzyme involved in the detoxification of insecticides or pathogens in insect bodies [40]. Our findings demonstrated an increase in GSTs activity during the initial 72 h followed then by a reduction in enzyme activity throughout the experimental period in response to the application of B. brongniartii or different concentrations of B. brongniartii Fe0NPs. This increase in enzyme activity during the initial infection period is a clear indication of the possible involvement of GSTs in the insect detoxification mechanism. The reduction in enzyme activity during 72 h post-infection can make the target host more susceptible to infection leading to a metabolic imbalance and insect death [41]. The antioxidant enzymes (SOD, CAT, and POD) are well known for their role in detoxifying destructive antioxidant species [42]. In our study, the activities of antioxidant enzymes (SOD, CAT, and POD) increased (until 5 days post-treatment) followed then by a decrease compared with the control when treated with B. brongniartii or different concentrations of B. brongniartii Fe0NPs. The antioxidant enzyme (SOD, CAT, and POD) activities in response to B. brongniartii Fe0NPs were significantly lower than B. brongniartii after 3, 5, and 7 days of treatment. The reduced activities of different antioxidant enzymes (SOD, CAT, and POD) during the later experimental period can lead to a lower elimination of reactive oxygen species which in turn can denature different bio-molecules within an insect body. The denaturation of bio-molecules stops all cellular processes which ultimately leads to the death of the insect [43].
5. Conclusions
In summary, this study characterized the bio-insecticidal activity of B. brongniartii Fe0NPs against S. litura but also provides information on the effects of B. brongniartii Fe0NPs on insect growth parameters as well as the detoxifying mechanism within S. litura. All the above findings suggest that B. brongniartii Fe0NPs can potentially be used within S. litura management programs. However, further work is required to determine the efficacy and persistence of B. brongniartii Fe0NPs under field conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/12/895/s1, Figure S1: Changes in colour of culture filtrate during extracellular synthesis of Beauveria brongniartii Fe0NPs (A) B. brongniartii conidial filtrate; (B) 1 mM Fe0; and (C) B. brongniartii Fe0NPs, Figure S2: Energy dispersive X-ray spectroscopy profile of Beauveria brongniartii Fe0NPs.
Author Contributions
S.A. designed and conceived the experiment. J.X., K.Z., and C.D. performed the experiment and data curation. K.Z. and C.D. performed the data analysis. J.X. and C.D. wrote the original draft. A.G.S.C. and S.A. revised the original draft and prepared the final manuscript. S.A. arranged the funding and supervised the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from The Science and Technology Program of Guangzhou, China (201807010019) and Key Area Research and Development Program of Guangdong Province (2018B020205003).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsuura H., Naito A. Studies on the cold-hardiness and overwintering of Spodoptera litura F. (Lepidoptera: Noctuidae) VI. Possible overwintering areas predicted from meteorological data in Japan. Appl. Entomol. Zool. 1997;32:167–177. doi: 10.1303/aez.32.167. [DOI] [Google Scholar]
- 2.Sahayara K., Gabriel P.M. Screening the relative toxicity of some plant extracts to Spodoptera litura fab. (Insecta: Lepidoptera: Noctuidae) of groundnut. Fresenius Environ. Bull. 1998;7:557–560. [Google Scholar]
- 3.Ahmad M., Arif M.I., Ahmad M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007;26:809–817. doi: 10.1016/j.cropro.2006.07.006. [DOI] [Google Scholar]
- 4.Ahmad M., Mehmood R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera, Noctuidae) in Pakistan. J. Econ. Entomol. 2015;108:1279–1288. doi: 10.1093/jee/tov085. [DOI] [PubMed] [Google Scholar]
- 5.Ishtiaq M., Saleem M.A., Razaq M. Monitoring of resistance in Spodoptera exigua (Lepidoptera: Noctuidae) from four districts of the Southern Punjab, Pakistan to four conventional and six new chemistry insecticides. Crop Prot. 2012;33:13–20. doi: 10.1016/j.cropro.2011.11.014. [DOI] [Google Scholar]
- 6.Driver F., Milner R.J., Trueman J.W.H. A taxonomic revision of Metarhizium based on a phylogenetic analysis of rDNA sequence data. Mycol. Res. 2000;104:134–150. doi: 10.1017/S0953756299001756. [DOI] [Google Scholar]
- 7.Sundh I., Goettel M.S. Regulating biocontrol agents: A historical perspective and a critical examination comparing microbial and macrobial agents. Biocontrol. 2013;58:575–593. doi: 10.1007/s10526-012-9498-3. [DOI] [Google Scholar]
- 8.Tarafdar J.C., Sharma S., Raliya R. Nanotechnology: Interdisciplinary science of applications. Afr. J. Biotechnol. 2013;12:219–226. [Google Scholar]
- 9.Goswami A., Roy I., Sengupta S., Debnath N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films. 2010;519:1252–1257. doi: 10.1016/j.tsf.2010.08.079. [DOI] [Google Scholar]
- 10.Banu A.N., Balasubramanian C. Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae) Parasitol. Res. 2014;113:2869–2877. doi: 10.1007/s00436-014-3948-z. [DOI] [PubMed] [Google Scholar]
- 11.Banu A.N., Balasubramanian C. Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol. Res. 2014;113:3843–3851. doi: 10.1007/s00436-014-4052-0. [DOI] [PubMed] [Google Scholar]
- 12.Amerasan D., Nataraj T., Murugan K., Panneerselvam C., Madhiyazhagan P., Nicoletti M., Benelli G. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae) J. Pest Sci. 2016;89:249–256. doi: 10.1007/s10340-015-0675-x. [DOI] [Google Scholar]
- 13.Wang X.S., Xu J., Wang X.M., Qiu B.L., Cuthbertson A.G.S., Du C.L., Wu J.H., Ali S. Isaria fumosorosea-based-zero-valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisia tabaci (Gennadius) Pest Manag. Sci. 2019;75:2174–2181. doi: 10.1002/ps.5340. [DOI] [PubMed] [Google Scholar]
- 14.David W., Ellaby S., Taylor G. Rearing Spodoptera exempta on semi synthetic diets and on growing maize. Entomol. Exp. Appl. 1975;19:226–236. doi: 10.1111/j.1570-7458.1975.tb02374.x. [DOI] [Google Scholar]
- 15.Ali S., Huang Z., Ren S.X. Media composition influences on growth, enzyme activity and virulence of the entomopathogen hyphomycete Isaria fumosorosea. Entomol. Exp. Appl. 2009;131:30–38. doi: 10.1111/j.1570-7458.2009.00833.x. [DOI] [Google Scholar]
- 16.Ali S., Zhang C., Wang Z., Wang X.M., Wu J.H., Cuthbertson A.G., Shao Z., Qiu B.L. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius) Sci. Rep. 2017;7:46558. doi: 10.1038/srep46558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J., Yu X., Wang X., Tang L., Ali S. Matrine enhances the pathogenicity of Beauveria brongniartii against Spodoptera litura (Lepidoptera: Noctuidae) Front. Microbiol. 2019;10:1812. doi: 10.3389/fmicb.2019.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koul O., Shankar J.S., Mehta N., Taneja S.C., Tripathi A.K., Dhar K.L. Bio-efficacy of crude extracts of Aglaia species (Meliaceae) and some active fractions against lepidopteran larvae. J. Appl. Entomol. 1997;121:245–248. doi: 10.1111/j.1439-0418.1997.tb01400.x. [DOI] [Google Scholar]
- 19.Rizwan M.H., Hu Q.B., Hu M.Y., Zhong G.H., Weng Q.F. Study of destruxin B and tea saponin, their interaction and synergism activities with Bacillus thuringiensis kurstaki against Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Appl. Entomol. Zool. 2009;44:419–428. doi: 10.1303/aez.2009.419. [DOI] [Google Scholar]
- 20.Ali S., Huang Z., Ren S.X. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010;83:361–370. doi: 10.1007/s10340-010-0305-6. [DOI] [Google Scholar]
- 21.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—Dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Beauchamp C., Fridovich I. Superoxide dismutase-improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 23.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 24.Simon L.M., Fatrai Z., Jonas D.E., Matkovics B. Study of metabolism enzymes during the development of Phaseolus vulgaris L. Biochem. Physiol. Pflanz. 1974;166:387–392. doi: 10.1016/S0015-3796(17)30073-2. [DOI] [Google Scholar]
- 25.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione-S-transferase. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 26.SAS Institute . SAS User’s Guide. Statistics SAS Institute; Cary, NC, USA: 2000. [Google Scholar]
- 27.Sabbour M.M., El-Aziz S.E.A. Efficacy of some nano-imidacloprid against red flour beetle Tribolium castaneum and confused flour beetle, Tribolium confusum (Coleoptera:Tenebrionidae) under laboratory and store conditions. Bull. Environ. Pharmacol. Life Sci. 2015;4:54–59. [Google Scholar]
- 28.Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- 29.Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus mediated synthesis of silver nanoparticles and its activity against pathogenic fungi in combination of fluconazole. Nanomedicine. 2009;5:282–286. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Ramani R., Parischa R., Ajayakumar P.V., Alam M., et al. Bioreduction of AuCl4-ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001;40:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::AID-ANIE3585>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Vigneshwaran N., Ashtaputrea N.M., Varadarajana P.V., Nachanea R.P., Paralikara K.M., Balasubramanyaa R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus Flavus. Mater. Lett. 2007;61:1413–1418. doi: 10.1016/j.matlet.2006.07.042. [DOI] [Google Scholar]
- 32.Lengke F.M., Fleet E.M., Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir. 2007;23:2694–2699. doi: 10.1021/la0613124. [DOI] [PubMed] [Google Scholar]
- 33.Chandra J.H., Raj L.F.A.A., Namasivayam S.K.R., Bharani R.S.A. Book of Abstracts International Conference on Advanced Nanomaterials and Emerging Engineering Technologies (ICANMEET) Chennai, India. IEEE; New York, NY, USA: 2013. Improved pesticidal activity of fungal metabolite from Nomuraea rileyi with chitosan nanoparticles; pp. 387–390. [DOI] [Google Scholar]
- 34.Ali S., Huang Z., Ren S.X. Production and regulation of extracellular proteases from the entomopathogenic fungus Isaria fumosoroseus (Cordycipitaceae; Hypocreales) in the presence of diamondback moth, Plutella xylostella cuticle. Biocontrol Sci. Technol. 2009;19:523–535. doi: 10.1080/09583150902887800. [DOI] [Google Scholar]
- 35.Ali S., Wu J.H., Huang Z., Ren S.X. Production and regulation of extracellular chitinase from the entomopathogenic fungus Isaria fumosorosea. Biocontrol Sci. Technol. 2010;20:723–738. doi: 10.1080/09583151003714091. [DOI] [Google Scholar]
- 36.Jamei M.R., Khosravi M.R., Anvaripour B. Soil remediation using nano zero-valent iron synthesized by an ultrasonic method. Iran. J. Oil Gas Sci. Technol. 2012;1:1–12. [Google Scholar]
- 37.Brandt C.R., Adang M.J., Spence K.D. The peritrophic membrane: Ultrastructural analysis and function as a mechanical barrier to microbial infection in Orgyia pseudotsugata. J. Invertebr. Pathol. 1978;32:12–24. doi: 10.1016/0022-2011(78)90169-6. [DOI] [Google Scholar]
- 38.Shahabuddin M., Toyoshima T., Aikawa M., Kaslow D.C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl. Acad. Sci. USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang M.C., Shu H.Y., Hsieh W.P., Wang M.C. Using nanoscale zero-valent iron for the remediation of polycyclic aromatic hydrocarbons contaminated soil. J. Air Waste Manag. Assoc. 2012;55:1200–1207. doi: 10.1080/10473289.2005.10464703. [DOI] [PubMed] [Google Scholar]
- 40.Claudianos C., Ranson H., Johnson R.M., Biswas S., Schuler M.A., Berenbaum M.R., Feyereisen R., Oakeshott J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006;15:615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian J., Diao H.L., Liang L., Hao C., Arthurs S., Ma R.Y. Pathogenicity of Isaria fumosorosea to Bemisia tabaci, with some observations on the fungal infection process and host immune response. J. Invertebr. Pathol. 2015;130:147–153. doi: 10.1016/j.jip.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Jia M., Cao G.C., Li Y.B., Tu X.B., Wang G.J., Nong X.Q., Whitman D.W., Zhang Z.H. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen) Sci. Rep. 2016;6:28424. doi: 10.1038/srep28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J.J., Li J.Y., Zhang C., Yu X.T., Cuthbertson A.G.S., Ali S. Biological impact and enzyme activities of Spodoptera litura (Lepidoptera: Noctuidae) in response to synergistic action of Matrine and Beauveria brongniartii. Front. Physiol. 2020;1:584405. doi: 10.3389/fphys.2020.584405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.