Abstract
Recently, increasing public concern about hygiene has been driving many studies to investigate antimicrobial and antiviral agents. However, the use of any antimicrobial agents must be limited due to their possible toxic or harmful effects. In recent years, due to previous antibiotics’ lesser side effects, the use of herbal materials instead of synthetic or chemical drugs is increasing. Herbal materials are found in medicines. Herbs can be used in the form of plant extracts or as their active components. Furthermore, most of the world’s populations used herbal materials due to their strong antimicrobial properties and primary healthcare benefits. For example, herbs are an excellent material to replace nanosilver as an antibiotic and antiviral agent. The use of nanosilver involves an ROS-mediated mechanism that might lead to oxidative stress-related cancer, cytotoxicity, and heart diseases. Oxidative stress further leads to increased ROS production and also delays the cellular processes involved in wound healing. Therefore, existing antibiotic drugs can be replaced with biomaterials such as herbal medicine with high antimicrobial, antiviral, and antioxidant activity. This review paper highlights the antibacterial, antiviral, and radical scavenger (antioxidant) properties of herbal materials. Antimicrobial activity, radical scavenger ability, the potential for antimicrobial, antiviral, and anticancer agents, and efficacy in eliminating bacteria and viruses and scavenging free radicals in herbal materials are discussed in this review. The presented herbal antimicrobial agents in this review include clove, portulaca, tribulus, eryngium, cinnamon, turmeric, ginger, thyme, pennyroyal, mint, fennel, chamomile, burdock, eucalyptus, primrose, lemon balm, mallow, and garlic, which are all summarized.
Keywords: herbal material, reactive oxygen species (ROS), antimicrobial, antioxidant, medicine applications, antiviral, virucidal
1. Introduction
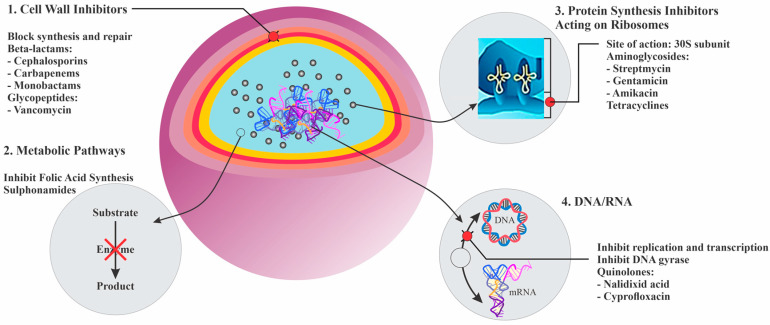
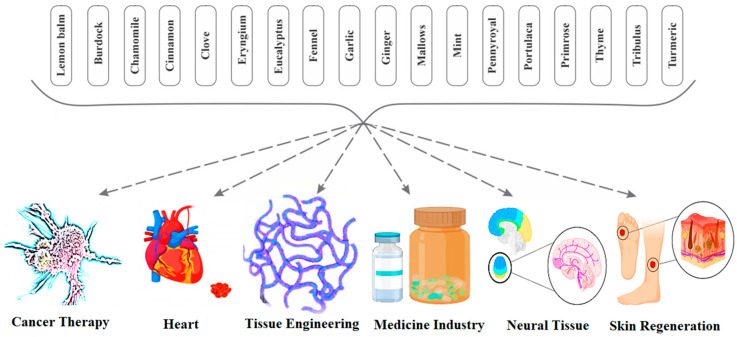
Due to increasing concerns about the sustainability of human living, the control of the damaging effects of microorganisms is becoming very important. A wide range of microorganisms exist in a biological balance with the human body and its living environments, but an uncontrolled and rapid growth of microbes can lead to some dangerous problems [1,2,3,4]. Antimicrobial agents are used as antibiotic drugs to control infections in the human body, but they can cause many side effects, especially increasing reactive oxygen species (ROS) in the human body [5,6]. ROS are very dangerous to human health and well-being and play a role in producing cancer [7,8,9]; further, they can increase potential health risks [10,11,12]. Figure 1 demonstrates the different antimicrobial mechanisms of antibiotics for disrupting bacterial cells [13,14]. The herbal materials used as medicinal plants include several types of plants. Many of these herbal materials show medicinal activities such as antioxidant, anticancer, anti-inflammatory, antimicrobial, and antiviral activities. Furthermore, these herbs can play the main role in drug synthesis and development. These materials show a significant role in different biological applications such as cancer therapy, cardiovascular disease treatment, neural disease treatment and skin regeneration [15,16,17]. The biomedical applications of these materials are illustrated in Figure 2 [18]. Herbal medicines performed the primary medicinal functions in ancient cultures in Africa, Europe, the Americas, and especially in Asia [19,20,21]. Herbal medicines are the primary medicine to treat infection in some developing countries. The extracts of herbal materials signify continuous attempts to investigate new compounds with potential antibacterial activity [22]. Several studies have shown that different herbal medicines are sources of diverse molecules, many of which exhibit radical scavenger and antimicrobial properties which can defend the human body against pathogens and also cellular oxidation reactions. Therefore, these materials are significant in synthesizing different types of herbal medicine for their antimicrobial, antiviral, and antioxidant potential [23,24,25,26,27]. These diverse molecules can control and inhibit pathogens with low toxicity to cells and are therefore considered as materials for new antimicrobial medicine research. Based on those above control methods for the terrible effect of the rapid growth of bacteria and viruses, many studies have focused on researching antibacterial and antiviral medicines with lower side effects [28,29,30,31].
Figure 1.
Illustration of the various antimicrobial mechanisms of antibiotics that interrupt bacterial cells [13,14].
Figure 2.
The biomedical applications of herbal materials [18].
2. Antimicrobial Agent
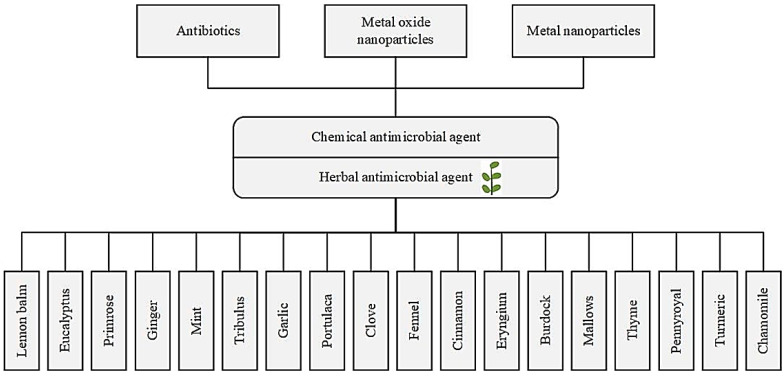
An agent which can kill microorganisms or stop their growth is known as an antimicrobial agent or antimicrobial medicine. Antimicrobial medicines are categorized based on the primary microorganisms they act against such as bacteria and viruses [32]. Antimicrobial agents are divided into two groups based on their different chemical substances. The first group is synthetic antimicrobial agents (chemical antimicrobial agents) including antibiotic drugs and metal and metal oxide nanoparticles including silver, silver oxide, and so on. The second group is herbal antimicrobial agents. Figure 3 shows the classification of the different antimicrobial agents based on their chemical substances.
Figure 3.
The antimicrobial agents’ classification based on their different substances.
Antibiotics and other chemical antimicrobial agents play a big role as antimicrobial agents, but they lead to various side effects. One of the main side effects is the generation of free oxygen radicals (ROS). ROS are very toxic and have been thought to play a main role in producing cancer [8,12,13].
The second group is related to herbal antimicrobial agents, such as clove, portulaca, tribulus, eryngium, cinnamon, turmeric, ginger, thyme, pennyroyal, mint, fennel, chamomile, burdock, eucalyptus, primrose, lemon balm, mallows, and garlic. These biomaterials can act as free radical scavengers and can therefore block the production of ROS. A brief list of essential biomaterials that can act as antimicrobial agents without any toxicity is shown in Table 1.
Table 1.
A brief list of herbal antimicrobial agents and its medicinal applications and main biological compounds.
| Herbal Materials | Medicinal Applications | Main Biological Compounds | Ref. |
|---|---|---|---|
| Clove | Antioxidant, antimicrobial, anti-inflammatory, anti-mutagenic, anti-allergic and anti-cancer. | Eugenol, eugenyl acetate, α-humulene, 2-heptanone, and β-caryophyllene |
[38,39] |
| Portulaca | Antioxidant, antimicrobial, anti-inflammatory, anticancer, neuroprotective and antidiabetic. | Ascorbic acid, a-tocopherols, omega-3 fatty acids, apigenin, gallotannins, quercetin, and kaempferol | [40,41] |
| Tribulus | Antioxidant, antimicrobial, analgesic, anti-inflammatory and cardiovascular protective. | flavonoid, tannin and phenolic acids | [42,43] |
| Eryngium | Antioxidant, antimicrobial, anticancer, antidiabetic, antimalarial, anti-Alzheimer and anti-inflammatory. | Flavonoids, phenolic acids, and coumarins | [44] |
| Cinnamon | Antioxidant, antimicrobial, anti-inflammatory, anticancer, cholesterol-lowering, immunomodulatory and cardiovascular. | Cinnamaldehyde and eugenol | [45,46,47] |
| Turmeric | Antioxidant, antimicrobial, anti-inflammatory, anticancer, hypoglycemia and anticoagulant. | Vitamin-C, cineole, tumerone, borneol, zingiberene, d-sabinene, and d-phellandrene |
[48,49] |
| Ginger | Antioxidant, antimicrobial, anti-diabetic, neuro- protective, analgesic, cardiovascular, gastrointestinal, anti-inflammatory, anticancer and antihypertensive. | Phenolic acids, gingerols, paradols and shogaols | [50,51] |
| Thyme | Antioxidant, antimicrobial, expectorant, spasmolytic, mucolytic and antitussive. | Carvacrol, thymol and phenols | [52,53] |
| Pennyroyal | Antioxidant, antimicrobial, anti-hepatic, Anti-genotoxic. | Neo-menthol, pulegone and menthone | [54,55] |
| Fennel | Antioxidant, antimicrobial and anti-inflammatory | Phenolic compounds | [56] |
| Chamomile | Antioxidant, antimicrobial, anti-inflammatory, anticancer, analgesic, anti-hypoglycemic, anti-stress and hepatoprotective. | Flavonoids, terpenoids, phenolic compounds, apigenin and matricin | [57,58] |
| Mint | Antioxidant, antimicrobial, anticancer and anti-inflammatory. | Phenolic compounds | [59] |
| Burdock | Antioxidant, antimicrobial, anti-proliferative and anti-inflammatory. | Caffeic acid, rutin, o-hydrobenzoic acid, chlorogenic acid, and p-coumaric acid |
[60,61] |
| Eucalyptus | Antioxidant, antimicrobial anti-inflammatory and antipyretic. | Flavonols, hydroxybenzoic acids and hydrolyzable tannins | [62,63] |
| Primrose | Antioxidant, antimicrobial, anti-neuropathic, anti-inflammatory, anticancer and anti-ulcerogenic. | Phenolic acids, flavonoids, sterols, hydrocarbons, and tocopherols | [64,65] |
| Lemon balm | Antioxidant, antimicrobial, anti-stress, anti-Alzheimer, anti-inflammatory, anticancer, anti-cardiovascular and antispasmodic. | Phenolic componds such as thymol and carvacrol | [66,67] |
| Mallows | Antioxidant, antimicrobial, antinociceptive, anti-inflammatory, hepatoprotective and anticancer. | β-carotene, flavonoids, vitamin E, polyphenols, and vitamin C | [68,69] |
| Garlic | Antioxidant, antimicrobial, antidiabetic, anticancer, cardioprotective, anti-neurologica and anti-inflammatory. | Organosulfur such as Allicin, phenolic and polysaccharides compounds | [70,71] |
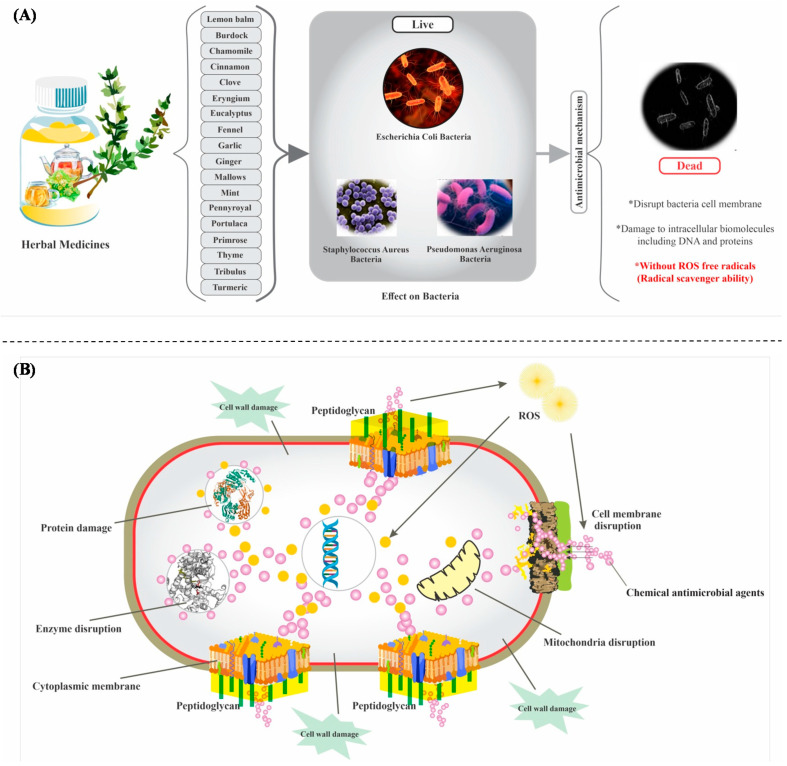
Recently, using the natural material has become ideal for treatment of microbial infections due to the possible toxic or harmful effects of many chemical antimicrobial agents [33]. Figure 4 shows the antimicrobial mechanisms of chemical antimicrobial agents against herbal antimicrobial agents [34,35]. The application of herbal materials would be an ideal alternative [36,37] and can also open up a new chance for producing new anticancer, antimicrobial, and antiviral drugs with lower side effects. There is much research that has demonstrated this material as a potential antimicrobial agent. This review paper is concerned with the pharmacological effects and properties of several herbal plants including clove, portulaca, tribulus, eryngium, cinnamon, turmeric, ginger, thyme, pennyroyal, mint, fennel, chamomile, burdock, eucalyptus, primrose, lemon balm, mallows, and garlic with good potential for antimicrobial, antiviral, and radical scavenger abilities.
Figure 4.
The antimicrobial mechanisms of herbal antimicrobial agents (A) against chemical antimicrobial agents (B) [34,35].
2.1. Clove
Clove (Syzygium aromaticum), from the Myrtaceae family, is one of the most effective antimicrobial and antioxidant herbs. This herb is one of the traditional herbs primarily local to Asia and Africa. Based on the bioactive components of clove such as eugenol, eugenyl acetate, α-humulene, 2-heptanone, and β-caryophyllene, it can display many pharmacological activities such as antimicrobial, antioxidant, anti-inflammatory, antimutagenic, anticancer, and anti-allergic properties. These bioactive components allow clove to demonstrate one of the highest potent antioxidant activities among other herbal medicines. Previous studies have reported the sufficient antibacterial property of clove extract and oil against different strains of bacteria (Gram-positive and Gram-negative) [39,72]. Other researchers have shown that clove has antimicrobial activity against many bacteria including Listeria monocytogenes, Klebsiella pneumoniae, S. aureus, E. coli and S. Typhimurium [73,74,75]. Clove based on eugenol plays an antimicrobial role. The antimicrobial mechanism of action of eugenol is that, at first, the eugenol molecule with high solubility can participate in the cytoplasmic membrane. Then, it creates disturbance as a consequence of its OH group. Finally, it can pass through the hydrophilic proportion of the cell. After that, the OH group of eugenol can bind to proteins of the membrane of bacteria and can permeate the fundamental cell components [76,77]. Clove extract with various amounts (1 and 3 mg mL−1) have been demonstrated to experience a great antimicrobial impact on S. typhi and E. coli [72]. Clove has strong antioxidant effects that can naturalize ROS and other free radicals in lipid chains. Therefore, they inhibit further oxidation of lipids [78]. Researchers have also observed that clove’s extract could inhibit the malondialdehyde formation from horse blood plasma oxidation [79]. Other researchers have reported the maximum antioxidant activity of clove against DPPH (2, 2-diphenyl-1-picryl hydracyl), than BHA (butylated hydroxyanisole), and BHT (butylated hydroxytoluene) radicals [80]. Similarly, the result of one research has shown the antioxidant activity of eugenol of clove extract against DPPH, ABTS and superoxide radicals [81]. Other studies have reported strong antioxidant activity of this herb against DPPH when compared to vitamin C [82]. The antiviral activity of this plant has been reported against different viruses such as herpes adenovirus, poliovirus, and coxsackievirus [83]. The high antioxidant activity of clove’s extract and essential oil is related to the chemical content of this herb, like a phenolic compound.
2.2. Portulaca
Portulaca is one of the traditional herbs from Asia. It has been reported to have potent antimicrobial and antioxidant activity. Several researchers have declared the biomedicine activities of portulaca over the past decades. The antioxidant effects of portulaca are the main factor of the biomedicine activity of this plant. Therefore, this plant can naturalize free radicals such as ROS in lipid chains. Hence, it can inhibit the further oxidation of lipids [40]. The antioxidant and antimicrobial property of portulaca is related to its components such as ascorbic acid, a-tocopherols, omega-3 fatty acids, apigenin, gallotannins, quercetin, and kaempferol. The antioxidant activity of portulaca is primarily related to omega-3 fatty acids [40,41]. Previous studies have shown the antimicrobial effects of this plant against different bacteria and fungi [84]. Furthermore, the pectic polysaccharide of this plant has been shown to have high antiviral properties against spatial viruses such as simplex virus type II [85]. Portulaca can be shown antibacterial activity against different bacteria (Gram-positive and Gram-negative) such as Pseudomonas aeruginosa, Neisseria gonorrhea, E. coli (Escherichia coli), Streptococcus faecalis, Bacillus and S. aureus (Staphylococcus aureus) [86,87]. Researchers in the last decade have found that portulaca’s extracts demonstrate inhibitory ability against different bacteria (Gram-negative and Gram-positive). Other studies have reported the antifungal activity of portulaca extracts against various fungi using an automatic single-cell bioassay system. The antifungal activity of the portulaca was also revealed against fungi such as Aspergillus yeast Candida and Trichophyton. The ethylacetate extract of portulaca shows positive activity against dermatophytes of the genera Trichophyton [88]. The ethanol extracted of this plant has also been tested against Bacillus [89]. The antifungal activity of organic and aqueous solvent extracts of this medical plant is also reported against Fusarium, Aspergillus niger, and Rhizopus artocarpi [90]. The antioxidant and antimicrobial activity of this medicinal plant is based on its chemical content including phenolic compounds [40,41].
2.3. Tribulus
Tribulus (Tribulus terrestris) is from the Zygophyllaceae family. This herb is native to Southern Asia, Europe, and Africa [91]. This plant has shown several pharmacological activities including antioxidant, cardiotonic, antimicrobial, antihypertensive, anticancer, and analgesic characteristics [92,93]. The Iranian and Turkish tribulus has been shown to have high antibacterial activity [94,95]. The antimicrobial activity of different extracts from tribulus was examined against eleven microorganisms including E. coli, S. aureus, Bacillus cereus, Corynebacterium diphtheria, Salmonella typhimurium, Candida albicans, Proteus vulgaris, K. pneumonia (Klebsiella pneumonia), S. marcescens (Serratia marcescens), and Pseudomonas aeruginosa. Previous studies have found that all types of tribulus extracts such as chloroform and ethanol extracts demonstrate high antimicrobial properties against various microorganisms [96,97,98]. Tribulus extract has been investigated for its antioxidant activity, radical scavenging, and its ability to strongly inhibit lipid peroxidation. Past studies have reported that this herb can play the primary role as an authoritative natural source of antioxidants. Therefore, it can be useful in inhibiting pathologies of free radicals. Previous studies have investigated the extract of tribulus as a high source of flavonoids [42,99]. Furthermore, it is well known that total polyphenols including flavonoid, tannin, and phenolic acids are strongly related to the antioxidant activity [43,100,101,102,103,104]. This plant also shows inhibitory activity against HIV virus [105].
2.4. Eryngium
Eryngium (Eryngium) is one of the plants native to Central and Southeast Europe, America, and Central Asia. This plant belongs to the Apiaceae family. The different components of eryngium include flavonoids, phenolic acids, and coumarins which are key factors of the pharmacological property of this plant [106,107,108,109,110]. Based on the presence of this component, eryngium shows high antimicrobial and antioxidant activity. Previous studies have proved that the high antioxidant activity of eryngium is due to the presence of flavonoids in the plant extract [111]. Therefore, eryngium is shown to have some pharmacological activity such as antimicrobial, antioxidant, antidiuretic, antitussive, aphrodisiac, appetizer, and stimulant characteristics [44,112,113]. Some researchers have reported that this plant shows antibacterial, anti-yeast, antiviral, and antifungal activity, while the results also indicate that polyacetylenes of this plant demonstrated antifungal and antibacterial abilities. The extracts and essential oil of this plant show high antibacterial properties against S. aureus, Listeria monocyatogenes, Bacillus, E. coli, Salmonella typhimurium, P. acnes., S. bovis, S. pyogenes, S. dysgalactiae, S. pneumonia, and Pseudomonas [114,115,116,117].
2.5. Cinnamon
Cinnamon (Cinnamomum verum and Cinnamomum zeylanicum) is one of the plants that belong to the Lauraceae family. This traditional herbal medicine is from Australia and Asia [118,119,120]. Based on the antioxidant, antimicrobial, and anticarcinogenic activities of this plant, it is widely used in medical industries [120,121,122,123]. Previous investigations have found cinnamon to have antimicrobial characteristics [124,125,126,127]. Cinnamon has been traditionally used for its antiseptic, antioxidant, and antimicrobial properties. Previous studies have investigated the antimicrobial activities of cinnamon against various bacteria, such as Bacillus and E. coli [128]. Cinnamon oil has shown antibacterial effects against E. coli, Listeria monocytogenes, Bacillus, Enterococcus faecalis, Salmonella typhimurium, Pseudomonas aeruginosa, Yersinia enterocolitica and Staphylococcus aureus [129,130,131,132]. This strong antimicrobial activity is based on the presence of cinnamaldehyde and eugenol in cinnamon essential oil. Bacteria such as Campylobacter jejuni have been shown to be more inhibited by the essential oil of cinnamon compared to other Gram-negative bacteria such as Escherichia coli [47]. Other researchers have demonstrated the mechanism of the antimicrobial action of the essential oil of cinnamon against cell walls of Listeria monocytogenes, E. coli, and S. aureus [133,134,135]. The appearance of phenolic substances in cinnamon results in potential antioxidant, antimutagenic, antidiabetic, anticancer, and anti-inflammatory activities. The essential oil of this herb has been shown to have antioxidant activity. Other studies have reported antioxidant activities of water, methanol, and ethanolic cinnamon extracts [45,46,136,137,138]. This plant shows high anti-influenza virus activity [139].
2.6. Turmeric
Turmeric (Curcuma longa) is one of the herbal medicines used traditionally. It belongs to the Zingiberaceae family. Due to the existence of curcumin (a polyphenolic compound), the extracts of turmeric have shown antimicrobial and antioxidant activity. Therefore, the phenolic compound of curcumin is responsible for its antioxidant activities [48]. The phytochemical structures in turmeric include vitamin C, cineole, tumerone, borneol, zingiberene, d-sabinene, and d-phellandrene. Many types of chemical compounds are found in turmeric including sesquiterpene Ketones, monoterpenes, and sesquiterpene alcohols (e.g., zingeberene). Fresh turmeric contains zingiberene, while the most significant curcuminoid presented in turmeric is curcumin. Previous literature has reported that turmeric has an antimicrobial (antibacterial and antifungal) effect [49,140,141,142]. Curcumin is known for its inhibitory action on microorganisms such as E. coli, S. aureus, Salmonella typhimurium, and Pseudomonas aeruginosa [141,143]. In numerous literature works, turmeric’s extracts have been shown to have strong antioxidant properties. The main active compound of turmeric (curcumin) shows strong radical scavenger activity. It can scavenge RNS (reactive nitrogen species) and ROS such as superoxide radicals, alkoxy radicals, peroxyl radicals, hydrogen peroxide, singlet oxygen, peroxynitrite, hydroxyl radicals, and nitricoxide by three active sites through the electron transfer and hydrogen abstraction [144,145]. Curcumin also shows indirect antioxidant properties through a reduction process of numerous cytoprotective proteins including catalase, γ-glutamylcysteine ligase, glutathione S transferase, glutathione reductase, heme oxygenase 1, superoxide dismutase, and glutathione peroxidase [146,147]. Treatment with turmeric can reduce plasma malondialdehyde with increased glutathione reductase, glutathione peroxidase, catalase activity, and plasma albumin levels [148]. The aqueous and ethanol extracts of turmeric show significant antioxidant characteristics through the increase in antioxidant enzymes, scavenging different free radicals, and inhibiting lipid peroxidation [149]. Some in vivo studies on rats have demonstrated that turmeric inhibits hydrogen peroxide in cells by preventing lipid peroxidation [150,151]. The different extracts of turmeric, such as chloroform, n-butanol, ethyl acetate, and n-hexane, show strong antioxidant characteristics. Analyses have revealed a high correlation between the scavenging ability and the phenolic contents of these extracts [152]. Furthermore, the ethanolic extract shows high antiradical activity in rats through increased activity of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase [153]. The research shows antiviral activity of this plant against HIV virus [154,155].
2.7. Ginger
Ginger (underground rhizome of Zingiber officinale roscoe and herbaceous perennial plants) is one of the essential herbal medicinal plants from the Zingiberaceae family [156]. Ginger (the rhizome of Zingiber officinale) is native to Asia and has been used as a medicine for more than two thousand years around the world [157,158,159,160]. Ginger contains polyphenol components, including phenolic acids, gingerols, paradols, and shogaols. These principal components are responsible for its biological properties such as antioxidant, antidiabetic, antimicrobial, renoprotective, antihypertensive, antiulcer, anti-inflammatory, cardiovascular, analgesic, and gastrointestinal activities [50,51,161,162,163]. The antioxidant activity of ginger is related to the chemical compounds present in ginger such as zingiberene, zingerone, shogaols, and gingerols [164]. Some studies have analyzed the antioxidant activities of ginger and its components in numerous in vivo and in vitro lab experiments. Some researchers have demonstrated the potential antioxidant properties of ginger extract [165,166,167]. One study on rats has shown that ginger extract have antioxidant effect [168].
This herbal medicine also affects insulin secretion, insulin action, or both [169,170,171]. It can affect type one and type two diabetes because it can inhibit the metabolism of carbohydrates and lipids [172,173,174]. Extract of ginger shows high antimicrobial activity against dissimilar strains of bacteria such as S. aureus, E. coli, and Salmonella typhi [49,175]. Ginger has been used in several countries as a one of the most significant anticancer medicines based on the exogenous antioxidant activity of this plant. Therefore, it can be used as a treatment of diseases caused by free radicals [176]. Furthermore, the safety of using ginger as an antibiotic medicine has been investigated. In general, ginger has been known as a safe herbal medicine with pharmacological activity [177]. The antiviral activity of this plant has been demonstrated against influenza virus [178].
2.8. Thyme
Thyme (Thymus vulgaris) is one of the active antimicrobial herbal medicine plants which belong to the Lamiaceae family. It is more active against different bacteria and can inhibit the growth of bacteria such as Lactobacillus plantarum, Brochothrix thermosphacta, and Brevibacterium linens. This potent antimicrobial ability is related to the presence of high concentrations of carvacrol, thymol, and phenols in the extracts and essential oils of thyme [53,179]. Thyme extracts and essential oil have demonstrated a long list of medicinal properties, such as antibacterial, antioxidant, antitussive, spasmolytic, anticancer, and anti-inflammatory characteristics [52]. The extract of this plant has been traditionally used as an antitumor medicine due to its antioxidant property [180]. Numerous studies in the literature have demonstrated that the higher phenolic content of thyme is responsible for the high radical scavenging and antioxidant properties of this medicinal plant [181,182,183,184]. The structural variability of extracts and oils of thyme has been the subject of numerous studies [185]. Some studies on the antimicrobial activity of thyme plants have evaluated the composition of thyme extracts and oil influenced by growing conditions, the genotype, and ontogenic development [186,187]. The antimicrobial ability of thyme has been reported against Pseudomonas aeruginosa, S. aureus, Klebsiella pneumonia, E. coli, and Bacillus [188]. The antiviral activity of thyme has been reported against herpes simplex virus type 1 [189].
2.9. Pennyroyal
Pennyroyal (Mentha pulegium) is one of the aromatic herbs belonging to the Lamiaceae family. This herb is native to Europe, Asia, and Africa [190]. Based on the high antioxidant and antimicrobial ability of this plant, its extracts and essential oils have been traditionally used in medicine, especially for skin diseases, aromatherapy, and cosmetics [191,192]. Numerous studies have demonstrated the bioactive properties of this plant in different countries around the world, including Portugal, Turkey, Iran, and Greece, by focusing on the chemical composition of pennyroyal [192,193,194,195,196]. In the last decade, some researchers have demonstrated the antioxidant properties of pennyroyal extracts and essential oil by focusing on the chemical composition of this plant [197]. Phenols are the most bioactive component of the extracts and essential oil of pennyroyal. This organic compound has been shown to contain an OH functional group that is bound directly to the aromatic ring, and the hydrogen atom of the OH functional group can snare peroxyl radicals. Therefore, it can prevent the oxidation of other compounds [192,198]. Finally, the presence of phenol compounds is the reason for the antioxidant characteristic of this herbal medicinal plant [199]. Some researchers have studied the relationship between concentrations of the extract and essential oil of pennyroyal and their antioxidant activity. These results have shown a direct relation between the antioxidant activity and phenol content. The essential oil of this plant has also been shown to possess high antibacterial activity [200]. The antimicrobial ability of this plant is related to the presence of neo-menthol, pulegone, and menthone. Most studies have shown a strong antimicrobial ability of pennyroyal against different bacteria including E. coli, S. typhimurim, Yersinia enterocolitica, Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Clostridium perfrigens, Helicobacter pylori, Brochothrix thermosphacta, Pseudomonas aeruginosa, and Klebsiella. The extract and essential oils of this plant pass through the cell wall of bacteria by damaging the membrane. This then results in disruption of the structure of polysaccharides, fatty acids, and phospholipids layers by an oxidation reaction [55,201]. This herbal medicine shows high inhibitory activity against herpes virus and influenza virus [202].
2.10. Fennel
Fennel (Foeniculum vulgare) is one of the herbal medicinal plants belonging to the Apiaceae family. Its native habitats include shores of Mediterranean Sea. There are some studies on the radical scavenging activity of fennel [203]. These studies have revealed that the antioxidant ability of this plant is due to the presence of high phenolic content in its extracts. Fennel has been shown to have high antioxidant ability. The antioxidant ability of the extract of this plant is due to numerous antioxidant processes such as free radical scavenging, superoxide anion radical scavenging, total antioxidant, and hydrogen peroxide scavenging [204,205]. The strong antioxidant characteristics of ethanol extracts and essential oil of this plant have been demonstrated by in vitro studies [206]. The hydro-ethanolic extracts of this plant have shown to possess free radical scavenging characteristics directly proportional to the content of phenolic compounds of fennel extract. The extracts and essential oil of this plant have been demonstrated to have significant antioxidant, antimicrobial, and anti-inflammatory properties [56]. The antimicrobial property of the essential oil (EO) and extract of fennel has been proven using the disk diffusion method [207]. Fennel extracts and essential oils have demonstrated high inhibitory activity against Bacillus megaterium, Eschericha coli, Bacillus pumilus, S. aureus, Pseudomonas putida, Pseudomonas syringae, Salmonella typhi, Bacillus cereus, Micrococcus luteus, Klebsiella pneumonia and Bacillus subtili [205,208]. The inhibitory ability of fennel also depends on its dosage. Consequently, fennel extract and oils could be a biosource of medicinal materials needed for the manufacturing of novel antimicrobial agents [209]. Fennel has been shown to be inhibitory against influenza A virus [210].
2.11. Chamomile
Chamomile (Matricaria chamomilla) is one of the traditional herbal medicines. This plant is part of the Asteraceae family and is still used in different medical applications, such as pharmaceutical and cosmetic industries [205,211]. Chamomile has a strong antimicrobial and antioxidant ability [212]. Various researchers have demonstrated the high antimicrobial ability of the extract and essential oil (EO) of this plant against various bacteria (Gram-positive and Gram-negative) including E. coli, Salmonella thyphimurium, S. aureus, and Bacillus. The high antimicrobial ability of this plant is also due to the high contents of phenolic compounds. Chamomile contains flavonoids, terpenoids, phenolic compounds, apigenin, and matricin [58,205,213,214,215]. The presence of flavonoids in the extract of this plant is the reason for its antioxidant characteristic. Chamomile shows various pharmacological activities including antioxidative, antibacterial, anti-inflammatory, antifungal, analgesic, anticancer, anti-hypoglycemic, anti-stress, antihypertensive, and hepatoprotective properties [57,58,216,217]. This plant shows antiviral activity against HSV-2 [218].
2.12. Mint
Mint (Mentha) is one of the aromatic perennial herbs belonging to the Lamiaceae family [219]. It has been used for various applications, such as pharmaceuticals and cosmetics applications [220]. The EO and aqueous extracts of mint potentially have antioxidant properties due to the existence of phenolic compounds [59,221,222,223]. Mint essential oil has been shown to be an effective alternative short-term treatment of irritable bowel syndrome in humans, due to its anti-inflammatory abilities [224]. The antioxidant activity of this plant exclusively relies on its chemical composition and can prevent oxidative stress at the cellular level or in a living organism. Other studies have reported the use of mint extract as an antioxidant and antimicrobial bioactive natural extract [225,226,227]. Numerous studies have revealed the inhibitory ability of this plant depending on the type of bacteria and its strong antimicrobial ability against Gram-positive bacteria, especially S. aureus. Other studies have reported that the antimicrobial effect of this plant with different oil concentrations. Mint oil shows strong antimicrobial ability against different bacteria including S. aureus, S. epidermidis, E. coli, Bacillus cereus, Enterococcus faecalis, and Cronobacter sakazakii [228,229]. This herbal medicine shows inhibitory activity against HSV-1 and HIV viruses [230].
2.13. Burdock
Burdock (Arctium lappa) is a popular traditional medical plant from Asia, America, and Europe belonging to the Asteraceae family. Based on the phenolic-rich compounds present in the extract, burdock has antimicrobial and antioxidant capabilities, as well as other biochemical activities including antipyresis and detoxifying [231,232,233,234,235]. Therefore, this plant could be used as an antioxidant, antimicrobial, and anti-inflammatory agent [60,236]. Studies have reported the antioxidant activity of burdock [237,238,239,240]. There is also information on the antimicrobial ability of burdock against different bacteria (Gram-negative and Gram-positive) including S. aureus, S. epidermidis, Proteus mirabilis, Enterococcus faecalis, B. cereus (Bacillus cereus), and E. coli because this plant is rich in phenolics [241,242,243]. In general, the extracts of burdock have demonstrated high antimicrobial activities and antioxidant capacity. The antioxidant and antimicrobial abilities of burdock extracts are due to the combined action of caffeic acid, rutin, o-hydrobenzoic acid, chlorogenic acid, and p-coumaric acid present in these extracts. Burdock leaves show high antimicrobial activities. The results of the previous studies have also conclusively revealed the antioxidant activities of the leaves of burdock [61,234]. The presence of phenolic-rich compounds in burdock is a potential source for its ability to inhibit oxidation activity [239]. This herbal medicine shows antiviral activity against herpesvirus (HSV-1, HSV-2), HIV, and adenovirus (ADV-3, ADV-11) [231].
2.14. Eucalyptus
Eucalyptus (Eucalyptus) is a member of the Myrtaceae family. It is called the fever tree based on its strong antimicrobial ability. This herbal medicine is native to the Mediterranean, Australia, and Tasmania area and it has been used as traditional medication for the treatment of numerous diseases including diabetes, pulmonary tuberculosis, bacteria and fungal infections, and influenza [244,245,246,247,248,249]. The medical applications of eucalyptus are based on the high antioxidant and antimicrobial abilities of its essential oil [62]. High concentrations of several polyphenolic compounds including flavonols, hydroxybenzoic acids, and hydrolyzable tannins have been found in the extract of eucalyptus [63]. These compounds are the reason for the high antimicrobial and antioxidant activity of eucalyptus. Recent studies have revealed the strong antibacterial ability of eucalyptus against S. aureus, Listeria monocytogenes, Bacillus, Klebsiella pneumoniae, Enterococcus faecalis, Pseudomonas aeruginosa, Salmonella Enteritidis, and Escherichia coli. These results suggest that eucalyptus has high antimicrobial and antioxidant abilities. The antioxidant ability of this herbal medicine is due to the presence of polyphenol, oenothein B, gallic acid, ellagic acid, flavonoids, and hydrolyzable tannin dimer in its extract. Eucalyptus extract also contains kaempferol-3-O-β-D-glucuronides and quercetin [250,251,252,253]. The presence of flavonoids also enhances the chances of having antioxidant ability in the extracts and essential oil of eucalyptus. Studies have reported that the radical scavenging ability of the eucalyptus essential oil increases by increasing the concentration of this herbal medicine plant [254]. This plant shows antiviral activity against herpes simplex virus (HSV) and influenza virus [255,256].
2.15. Primrose
Primrose (genus Oenothera) is a member of the Onagraceae family. Primrose shows numerous pharmacological effects including antimicrobial and antioxidant properties. This herbal medicine has been traditionally used for skin treatment [64,257]. The oils and extracts of primrose may also be used as natural antioxidants. The most important component of primrose includes various phenolic compounds which have been shown to be responsible for the antioxidant and free radical scavenging ability of primrose [258,259]. Numerous researchers have reported that the antioxidant activity of this plant is due to its phenolic components. Another group of chemical compounds present in primrose is triterpenoids. The antioxidant ability of primrose ethanolic extracts has been demonstrated in the literature [64,260,261]. It has been reported that the lipophilic triterpenoid esters present in essential primrose oil are the reason for the effective antioxidant activity of this plant [262,263]. Methanolic extracts of primrose were also demonstrated to possess potential antioxidant ability [264]. The radical scavenging ability of primrose was revealed against 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid). It was demonstrated that the alcoholic extract exhibits strong antioxidant ability [64]. The antioxidant ability of primrose was also demonstrated against DPPH, which was also revealed to possess strong antioxidant ability [265]. The oil of primrose has been used for the treatment of MS disease due to its immune-modulating and anti-inflammatory effects. Based on the concentration of primrose, it can show different inhibitory effects against various bacteria (Gram-positive and Gram-negative) [266,267,268]. The EO of primrose contains gamma linolenic acid (GLA). GLA has a strong ability in treating diabetes [269,270]. Primrose contains some alcohols, sterols, hydrocarbons, and tocopherols [65]. Sterols have been used for their antitumor properties and their cholesterol-lowering effects. Phytosterols present in primrose have also been shown to have antimicrobial and anti-inflammatory abilities [65]. Previous studies have demonstrated the high antimicrobial ability of the extracts and essential oil of primrose against different microorganisms such as S. aureus, and E. coli [267,271,272]. Recently, the methanolic and aqueous extracts of primrose have been shown to possess a high antibacterial effect against enterobacteria [273]. In vivo experiments have shown that primrose extract has high inhibitory effects on dental cavities caused by S. mutans in rats [274]. This plant shows antiviral activity against varicella virus, Epstein–Barr virus, and herpes simplex virus [64].
2.16. Lemon Balm
Lemon balm (Melissa officinalis) is one of the traditional herbal medicines belonging to the Laminaceae family. This herbal medicinal plant grows in North America, Europe, and Asia. Its use as a medicinal plant originated from Mediterranean countries [275]. The lemon balm has been used for several purposes such as medicine [276]. The extracts and EO of the lemon balm plant have some pharmacology effects including antimicrobial, anticancer, antibacterial, anti-cardiovascular diseases, antioxidant, anti-inflammatory, antispasmodic, and antiviral properties [66]. Some studies have demonstrated the effectiveness of lemon balm against different diseases such as HIV-1, cancer, and Alzheimer’s [277]. Lemon balm is a rich source of phenolic componds such as thymol and carvacrol which are the potential reason for the antibacterial and antioxidant activity of the lemon balm plant [67,278]. The antimicrobial characteristics of lemon balm have been used against Gram-negative bacteria including E. coli, Salmonella typhi, Pseudomonas aeruginosa, Proteus, and Klebsiella and Gram-positive bacteria including S. aureus, Sarcina lutea, beta-hemolytic Streptococcus, and Bacillus cereus [278,279,280]. The antimicrobial activities of lemon balm are mainly explained through the C15 and C10 terpenes with phenolic hydroxyl groups and aromatic rings, as well as other active terpenes, such as esters, aldehydes, and alcohols [278]. Moreover, these components have also demonstrated high antioxidant activity in lemon balm [281]. Some studies have reported the free radical scavenging ability of lemon balm together with positive results on lipid peroxidation. Other studies have revealed the antiviral activity of lemon balm’s essential oil [31,282,283].
2.17. Mallows
Mallow (Malva sylvestris) is another herbal medicinal plant which belongs to the Malvaceae family. This herb is native to Europe, America, and Asia [284]. The extract of this plant has high phenolic content and high antimicrobial properties. However, there is little clinical evidence regarding the use of mallow. The antibacterial effect of this herb has been reported against different bacteria (Gram-positive and Gram-negative) including S. aureus, Bacillus cereus, E. coli, Klebsiella pneumoniae, Salmonella typhimurium, Listeria monocytogenes, Proteus vulgaris, Streptococcus pyogenes, Micrococcus luteus, Pseudomonas aeruginosa, and Mycobacterium smegmatis [68,285,286,287]. In the last decade, mallow extract has been used for medicinal applications due to its excellent effect on the several systems of the body, including skin injuries and disorders, the muscular and skeletal systems, and the respiratory and digestive systems. Mallow has been shown to possess spasmolytic, lenitive, laxative, diuretic, demulcent, anti-diarrheal, bronchodilator, expectorant, and antitussive effects. It has high antimicrobial and antioxidant activity and is therefore highly recommended for skincare [288,289,290]. The antimicrobial and antioxidant activities of mallow are related to its chemical components including β-carotene, flavonoids, vitamin E, polyphenols, and vitamin C [69,291]. Polyphenols are the main reason for the excellent antioxidant and antimicrobial ability of mallow [292]. Due to the antioxidant effects of these phytochemicals, mallow is able to scavenge various free radicals, leading to the defence of biological molecules against oxidation. Therefore, the alcoholic and aqueous extracts of mallow have some pharmacological ability including antiviral, anti-inflammatory, antibacterial, and anti-cancerogenic effects [68,293].
2.18. Garlic
Garlic (Allium sativum) is an herbal medicine belonging to the Amaryllidaceae family [294]. Garlic is native to Central Asia, especially Iran [295]. Garlic has been demonstrated to possess biological activities including antioxidant, immunomodulatory activities, antidiabetic, anticancer, antibacterial, cardioprotective, and anti-inflammatory effects. The major components of garlic are phenolic, polysaccharides, and organosulfur contents. It also contains saponins, amino acid, flavonoids, vitamins A and C, B-complex vitamins, and minerals [70,296,297]. These chemical components are the reason for the biological activity of garlic. Garlic has been known as a natural antioxidant and can inhibit the harmful effects of free radicals in cells [298,299,300]. Antioxidant materials are naturally found in different plants and can neutralize free radicals through electron donation and by converting these harmful molecules to harmless products. Garlic is one of the traditional medicines with antimicrobial and antioxidant characteristics [301].
Numerous researchers have demonstrated the antioxidant property of garlic which is due to the presence of some chemical components including organosulfur, and phenolic compounds [70,302]. Garlic has a high content of phenolic compounds which are the reason for the high antioxidant activities of garlic [303]. Some researchers have indicated that the extract of garlic shows high scavenging of free radicals and therefore has powerful antioxidant ability [304,305]. Furthermore, other studies have reported that garlic shows antioxidant ability including one research which analyzed this ability using in vivo experiments [71,306]. Amino acids such as alliin represent one of the important components of garlic [307]. The alliinase enzyme can convert alliin to allicin which is one of the main components of garlic. This chemical compound is responsible for the antimicrobial activity of this herbal medicine [70,308]. Garlic was demonstrated to exhibit antibacterial activity against a varied range of different bacteria (Gram-positive and Gram-negative) such as Klebsiella, Enterococcus faecalis, Pseudomonas, Salmonella typhi, Proteus, Staphylococcus aureus, and Escherichia coli [309,310,311,312,313]. Some studies have also reported high antimicrobial and antioxidant activities of garlic based on the chemical reaction between allicin and thiol groups of various enzymes [314,315]. This plant also shows antiviral activity against influenza virus (H1N1) [316]. Table 2 demonstrates that some of the research has shown these herbal medicines including clove, portulaca, tribulus, eryngium, cinnamon, turmeric, ginger, thyme, pennyroyal, mint, fennel, chamomile, burdock, eucalyptus, primrose, lemon balm, mallows, and garlic to be potential antimicrobial agents. Table 3 and Table 4 show the antiviral and antioxidant activity of these materials.
Table 2.
The herbal antimicrobial agents developed and their properties.
| Herbal Biomaterials | Type | Minimum Inhibitory Concentration | Target of Bacteria | Result | Ref. |
|---|---|---|---|---|---|
| Clove | Essential oil | 0.304 mg mL−1 |
S. Typhimurium, S. aureus, L. monocytogenes, and E. coli. |
Good antibacterial efficiency against all tested bacteria | [73] |
| Portulaca | Extract | 10 mg mL−1 | S. aureus | High inhibitory activity against S. aureus | [86] |
| Tribulus | Metanolic extract | 2 mg mL−1 | E. faecali, S. aureus, P. aeruginosa, and E. coli | High effective antimicrobial activity against these bacteria | [317] |
| Eryngium | Essential oil | 12.5 mg mL−1 | S. aureus | Very interesting inhibitory activity against S. aureus | [117] |
| Cinnamon | Extract | 62.5 mg mL−1 | S. aureus | The good inhibitory effect against S. aureus | [318] |
| Turmeric | Essential oil | 0.05 mg mL−1 | B. coagulans, S. aureus, and B. subtilis | Maximum inhibitory activity was noted | [319] |
| Ginger | Essential oil | 1 mg mL−1 | S. aureus | Effective efficiency against S. aureus | [320] |
| Thyme | Essential oil | 2.857 mg mL−1 | Klebsiella pneumoniae | Good inhibitory activity | [321] |
| Pennyroyal | Alcoholic extract | 400 mg mL−1 | Klebsiella and S. aureus | Good inhibitory activity | [322] |
| Fennel | Essential oil | 0.125 mg mL−1 | S. dysenteriae | Effective inhibitory activity | [323] |
| Chamomile | Alcoholic extract | 8 mg mL−1 | Klebsiella pneumoniae | Effective antimicrobial activity | [324] |
| Mint | Extract | 64 µg mL−1 | E. coli | High inhibitory activity | [325] |
| Burdock | Extract | 256 μg mL−1 | S. aureus | The extract shows antibacterial activity against S. aureus | [241] |
| Eucalyptus | Essential oil | 1000 μg mL−1 | S. aureus and E. coli | Good bacteria inhibitory activty | [252] |
| Primrose | Methanol extract | 0.07 mg L−1 | Listeria monocytogenes | Highest inhibitory effect against Listeria monocytogenes | [326] |
| Lemon balm | Essential oils | 0.25 mg mL−1 | Listeria strain | Sensitive inhibitory effect against Listeria strain | [327] |
| Mallows | Ethanol extracts | 0.4–0.8 mg mL−1 |
S. pyogenes, P. vulgaris, S. aureus, and P. aeruginosa |
The extracts were active against S. pyogenes, P. vulgaris, S. aureus, and P. aeruginosa |
[286] |
| Garlic | Extract | 0.075 mg mL−1 | E. coli | Significant antimicrobial efficiency against E. coli |
[328] |
Table 3.
Antiviral activity of herbal materials and their virus target.
| Herbal Biomaterials | Target of Virus | Ref. |
|---|---|---|
| Clove | Herpes simplex and hepatitis C | [329] |
| Portulaca | Influenza A viruses | [330] |
| Tribulus | Newcastle disease virus | [331] |
| Eryngium | HIV | [332] |
| Cinnamon | Influenza A virus, herpes simplex virus type 1 and 2 | [333] |
| Turmeric | Epstein–Barr virus | [334] |
| Ginger | SARS-CoV-2 or COVID-19 | [335] |
| Thyme | Influenza virus | [336] |
| Pennyroyal | HSV-1 | [337] |
| Fennel | Para-influenza type 3 and Herpes simplex type 1 | [338] |
| Chamomile | Influenza A virus | [339] |
| Mint | COVID-19 | [340] |
| Burdock | COVID-19 | [341] |
| Eucalyptus | COVID-19 | [342] |
| Primrose | Hepatitis C virus (HCV) | [343] |
| Lemon balm | Influenza A virus (H9N2), SARS-CoV-2 | [31,344] |
| Mallows | Influenza virus | [293] |
| Garlic | Influenza B and herpes simplex | [345] |
Table 4.
Antioxidant activity of herbal materials.
| Herbal Biomaterials | Type of Free Radical | Type of Study | Medical Application | Ref. |
|---|---|---|---|---|
| Clove | DPPH | In vitro | Cancer therapy | [72] |
| Portulaca | DPPH | In vitro | Cancer therapy | [41] |
| Tribulus | DPPH, ABTS |
In vitro | Infertility therapy | [99] |
| Eryngium | DPPH | In vitro | Cancer therapy | [111] |
| Cinnamon | DPPH and ABTS | In vitro | Antidiabetic | [137] |
| Turmeric | ROS | In vitro | Cancer therapy and antidiabetic | [144] |
| Ginger | DPPH | In vitro | Cancer therapy | [163] |
| Thyme | DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals | In vitro | Cancer therapy | [181] |
| Pennyroyal | DPPH | In vitro | Cancer therapy | [196] |
| Fennel | DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals | In vitro | Cancer therapy | [205] |
| Chamomile | DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals | In vitro | Cancer therapy | [205] |
| Mint | ROS, 2,2-diphenyl-1-picrylhydrazyl, (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), (ABTS) |
In vitro and in vivo | Cancer therapy | [227] |
| Burdock | ROS | In vivo | Antidiabetic | [238] |
| Eucalyptus | 2,2-diphenyl-1-picrylhydrazyl, (DPPH) | In vitro | Antitumor | [250] |
| Primrose | Oxygen free radicals | In vivo | Anti-inflammatory | [261] |
| Lemon balm | ROS | In vitro | Infertility therapy | [283] |
| Mallows | DPPH | In vitro | Anti-inflammatory and anti-cancerogenic | [290] |
| Garlic | DPPH and ROS | In vitro andIn vivo | Cancer therapy and diabetes therapy | [301] |
3. Conclusions and the Future Outlook
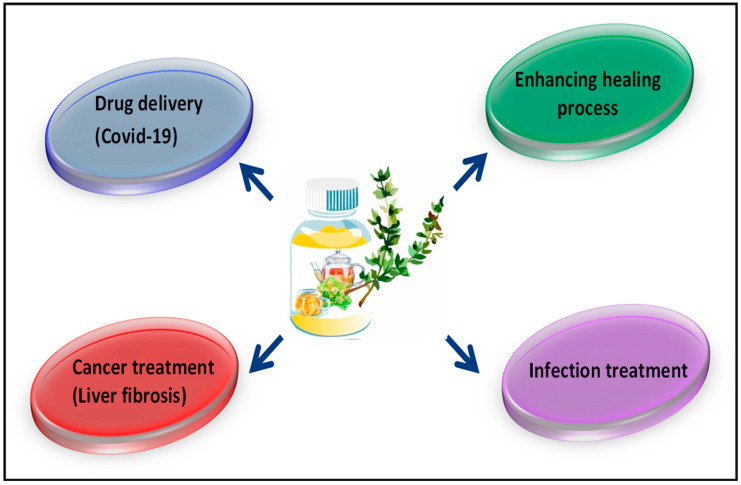
Many researchers have reported the biomedical applications of herbal medicines [346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365]. Antibiotics and other chemical antimicrobial agents including metal oxide and metal nanoparticles can inhibit bacteria growth but also lead to ROS generation and other side effects. On the other hand, herbal medicines such as clove, portulaca, tribulus, eryngium, cinnamon, turmeric, ginger, thyme, pennyroyal, mint, fennel, chamomile, burdock, eucalyptus, primrose, lemon balm, mallows, and garlic can eliminate bacteria by acting as a free radical scavenger. The antibacterial activity of these herbal medicines is different toward various kinds of bacteria and different kind of extract and essential oil of these herbal medicines [73,366]. In this light, it was exhibited [34,367] that the minimum inhibitory concentration (MIC) of these herbal medicines such as the essential oil of thyme towards various bacteria is in the range of 50–400 ppm for E. coli, S. aureus, P. mirabilis, S. typhimurium, P. vulgaris, Y. enterocolitica, S. marcescens, B. licheniformis, P. putida, S. flava, P. fluorescens, L. innocua, Micrococcus spp., and B. thuringiensis [34,367]. However, some other studies [368,369,370,371,372,373,374,375,376] evaluated positive aspects and restrictions of nanofibrous dressings with a great surface area and capacity to release antibiotics, herbal medicine, and antibacterial agents locally into the wound in order to the treatment of infection. It is worth noting that herbal medicines can act as direct antioxidants by blocking ROS generation and therefore can inhibit the planned cell death pathways. Thus, based on the lower concentration of ROS, the defense mechanism is started before the apoptosis pathway is prompted by glutamate. The presence of phenolic compounds in the extract or essential oil of these herbal medicines often demonstrates the potent antioxidant and antimicrobial properties. Therefore, herbal medicines are often preferred due to their less toxic and side effect-free nature compared to synthetic antimicrobial agents. Based on the high antimicrobial and antioxidant ability of these herbal medicines, they can be beneficial for healing all types of wounds. Herbal medicines also show a high antimicrobial potential at a lower price. Bioavailability is the primary essential measure for assessing the health benefits of herbal medicine for humans. In current years, the idea that natural remedies are more secure in comparison with prescription medications has acquired traction and contributed to a massive rise in phytopharmaceutical applications [377]. The bioavailability of these herbal medicinal products varies in blood, plasma, or tissue [378]. The bioavailability of these herbal medicinal products such as thyme [379], eucalyptus [380], turmeric [381], mint [378], garlic [382], ginger [377], cinnamon [377], and clove [377] was recorded in earlier research. These studies significantly lead to the precise scientific evaluation of the numerous remarks regarding the herbal products, which are progressively marketed with curative remarks throughout the world [378]. Therefore, the replacement of chemical antimicrobial agents with these herbal antimicrobial agents is a perfect solution due to their performance as an antimicrobial agent and also their radical scavenger ability. Herbal materials have created a novel exciting field in all sciences, especially in medicine, for future studies due to their unique properties. Their medicinal applications have already led to the development of new medical productions. Considering the decisive role of antimicrobial drugs in human life, these new fields in the drug industry have become increasingly more acceptable. However, designing new antimicrobial drugs free from side effects will not only create a new area of study but can also help meet the expanding human needs. Hence, this review suggests the use of herbal antimicrobial agents instead of synthetic ones due to their dual activity. In this paper, antioxidant, antimicrobial, and antiviral properties of individual herbs have been described comprehensively. Use of a combination of these herbs is also widespread in traditional medicine practice. Therefore, future research can focus on the characterization of the active component and the effect of herb–herb combinations for future therapeutic advancements and pharmaceutical product development. These materials shows high antiviral activity; therefore, these biomaterials are suggested for use against the COVID-19 virus. Therefore, these materials can be suggested to improve drug delivery (Covid-19), cancer therapy, infection, and acceleration of the healing rate, as shown in Figure 5.
Figure 5.
The future trend of herbal materials.
Acknowledgments
The authors would like to thank the Isfahan University of Medical Sciences, University Teknologi Malaysia (UTM) and Norwegian University of Science and Technology, Islamic Azad University, Najafabad for providing the facilities of this research.
Author Contributions
Conceptualization, supervision, formal analysis, writing—original draft preparation, methodology, S.P.; Conceptualization, supervision, writing—review and editing, H.R.B.-R., A.Z.K., S.S. and A.F.I.; writing—review and editing, H.N.; supervision, writing—review and editing, funding acquisition, S.R. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scheepmaker J.W., Busschers M., Sundh I., Eilenberg J., Butt T.M. Sense and nonsense of the secondary metabolites data requirements in the EU for beneficial microbial control agents. Biol. Control. 2019;136:104005. doi: 10.1016/j.biocontrol.2019.104005. [DOI] [Google Scholar]
- 2.Dastjerdi R., Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z.C., Wang B.C., Yang X.S., Wang Q., Ran L. The synergistic activity of antibiotics combined with eight traditional Chinese medicines against two different strains of Staphylococcus aureus. Colloids Surf. B. 2005;41:79–81. doi: 10.1016/j.colsurfb.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Parham S., Wicaksono D.H., Bagherbaigi S., Lee S.L., Nur H. Antimicrobial treatment of different metal oxide nanoparticles: A critical review. J. Chin. Chem. Soc. 2016;63:385–393. doi: 10.1002/jccs.201500446. [DOI] [Google Scholar]
- 5.Huh A.J., Kwon Y.J. Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh S., Nazam N., Rizvi SM D., Ahmad K., Baig M.H., Lee E.J., Choi I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raut P.K., Kim S.H., Choi D.Y., Jeong G.S., Park P.H. Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: Critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem. Pharmacol. 2019;161:73–88. doi: 10.1016/j.bcp.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y., Arakawa H. Discovery of Mieap-regulated mitochondrial quality control as a new function of tumor suppressor p53. Cancer Sci. 2017;108:809–817. doi: 10.1111/cas.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S.H., Kim K.Y., Yu S.N., Park S.G., Yu H.S., Seo Y.K., Ahn S.C. Monensin induces PC-3 prostate cancer cell apoptosis via ROS production and Ca2+ homeostasis disruption. Anticancer Res. 2016;36:5835–5843. doi: 10.21873/anticanres.11168. [DOI] [PubMed] [Google Scholar]
- 10.Panieri E., Santoro M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016;7:2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parham S., Nemati M., Sadir S., Bagherbaigi S., Wicaksono D.H., Nur H. In Situ Synthesis of Silver Nanoparticles for Ag-NP/Cotton Nanocomposite and Its Bactericidal Effect. J. Chin. Chem. Soc. 2017;64:1286–1293. doi: 10.1002/jccs.201700157. [DOI] [Google Scholar]
- 12.Parham S., Wicaksono D.H., Nur H. A proposed mechanism of action of textile/Al2O3–TiO2 bimetal oxide nanocomposite as an antimicrobial agent. J. Text. I. 2019;110:791–798. doi: 10.1080/00405000.2018.1526445. [DOI] [Google Scholar]
- 13.Simões D., Miguel S.P., Ribeiro M.P., Coutinho P., Mendonça A.G., Correia I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Homaeigohar S., Boccaccini A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020 doi: 10.1016/j.actbio.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Pohl P., Dzimitrowicz A., Jedryczko D., Szymczycha-Madeja A., Welna M., Jamroz P. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 2016;130:326–335. doi: 10.1016/j.jpba.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Xu X., Wang J., Yu H., Wang X., Yang H., Xu H., Tang S., Li Y., Yang L., et al. A system-level investigation into the mechanisms of Chinese Traditional Medicine: Compound Danshen Formula for cardiovascular disease treatment. PLoS ONE. 2012;7:e43918. doi: 10.1371/journal.pone.0043918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iid I.I., Kumar S., Shukla S., Kumar V., Sharma R. Putative antidiabetic herbal food ingredients: Nutra/functional properties, bioavailability and effect on metabolic pathways. Trends Food Sci. Technol. 2020;97:317–340. [Google Scholar]
- 18.Parham S., Kharazi A.Z., Bakhsheshi-Rad H.R., Ghayour H., Ismail A.F., Nur H., Berto F. Electrospun nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials. 2020;13:2153. doi: 10.3390/ma13092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim J.M. The relationship between the use of complementary and alternative medicine and the use of biomedical services: Evidence from East Asian medical systems. Asia Pac. J. Public Health. 2016;28:51–60. doi: 10.1177/1010539515613411. [DOI] [PubMed] [Google Scholar]
- 20.Abdullahi A.A. Trends and challenges of traditional medicine in Africa. Afr. J. Tradit. Complement. Altern. Med. 2011;8:115–123. doi: 10.4314/ajtcam.v8i5S.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton R., Graff A., Jolliffe G., Länger R., Williamson E. American Herbal Pharmacopoeia: Botanical Pharmacognosy-Microscopic Characterization of Botanical Medicines. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 22.Kennedy D.A., Lupattelli A., Koren G., Nordeng H. Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complem Altern M. 2016;16:102. doi: 10.1186/s12906-016-1079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saratale R.G., Benelli G., Kumar G., Kim D.S., Saratale G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. R. 2018;25:10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 24.Firenzuoli F., Gori L. Herbal medicine today: Clinical and research issues. Evid. Based Complement. Alternat. Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miraj S., Rafieian-Kopaei M., Kiani S. Melissa officinalis L.: A review study with an antioxidant prospective. Evid. Based Complement. Alternat Med. 2017;22:385–394. doi: 10.1177/2156587216663433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preethi R., Devanathan V.V., Loganathan M. Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Adv. Biol. Res. 2010;4:122–125. [Google Scholar]
- 27.Salazar-Aranda R., Pérez-Lopez L.A., Lopez-Arroyo J., Alanís-Garza B.A., Waksman de Torres N. Antimicrobial and antioxidant activities of plants from northeast of Mexico. Evid. Based Complement. Alternat. Med. 2011;2011:6. doi: 10.1093/ecam/nep127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Shanab B., Adwang M., Abu-Safiya D., Jarrar N., Adwan K. Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk. J. Biol. 2005;28:99–102. [Google Scholar]
- 29.Sharma A., del Carmen Flores-Vallejo R., Cardoso-Taketa A., Villarreal M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017;208:264–329. doi: 10.1016/j.jep.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Waris K., Saleem S., Arshad M.U., Iqbal J. A novel complementary alternative medicine: An In-Vitro evaluation of efficacy of Nigella sativa extract as an antibacterial agent against Porphyromonas gingivalis. Ann. Punjab. Med. Coll. 2017;11:247–251. doi: 10.29054/APMC/17.411. [DOI] [Google Scholar]
- 31.Pourghanbari G., Nili H., Moattari A., Mohammadi A., Iraji A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2) Virus Dis. 2016;27:170–178. doi: 10.1007/s13337-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett-Boothroyd S.C., McCarthy B.J. Textiles for Hygiene and Infection Control. Woodhead Publishing; Oxford, UK: 2011. Antimicrobial Treatments of Textiles for Hygiene and Infection Control Applications: An Industrial Perspective; pp. 196–209. [Google Scholar]
- 33.Bereksi M.S., Hassaïne H., Bekhechi C., Abdelouahid D.E. Evaluation of antibacterial activity of some medicinal plants extracts commonly used in Algerian traditional medicine against some pathogenic bacteria. Pharmacogn. J. 2018;10:3. doi: 10.5530/pj.2018.3.83. [DOI] [Google Scholar]
- 34.Najafloo R., Behyari M., Imani R., Nour S. A mini-review of Thymol incorporated materials: Applications in antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2020;60:101904. doi: 10.1016/j.jddst.2020.101904. [DOI] [Google Scholar]
- 35.Nithya P., Sundrarajan M. Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. JPPBEG. 2020;202:111706. doi: 10.1016/j.jphotobiol.2019.111706. [DOI] [PubMed] [Google Scholar]
- 36.Torkan S., Khamesipour F., Katsande S. Evaluating the effect of oral administration of Echinacea hydroethanolic extract on the immune system in dog. Auton Autacoid. Pharmacol. 2015;35:9–13. doi: 10.1111/aap.12024. [DOI] [PubMed] [Google Scholar]
- 37.Tribess B., Pintarelli G.M., Bini L.A., Camargo A., Funez L.A., de Gasper A.L., Zeni A.L.B. Ethnobotanical study of plants used for therapeutic purposes in the Atlantic Forest region, Southern Brazil. J. Ethnopharmacol. 2015;164:136–146. doi: 10.1016/j.jep.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Anwer M.K., Jamil S., Ibnouf E.O., Shakeel F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014;63:347–354. doi: 10.5650/jos.ess13213. [DOI] [PubMed] [Google Scholar]
- 39.Cortés-Rojas D.F., de Souza CR F., Oliveira W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop Bio. 2014;4:90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y.X., Xin H.L., Rahman K., Wang S.J., Peng C., Zhang H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Bio. Med. Res. Int. 2015;2015:925631. doi: 10.1155/2015/925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira I., Valentão P., Lopes R., Andrade P.B., Bento A., Pereira J.A. Phytochemical characterization and radical scavenging activity of Portulaca oleraceae L. leaves and stems. Microchem. J. 2009;92:129–134. doi: 10.1016/j.microc.2009.02.006. [DOI] [Google Scholar]
- 42.Tian C., Chang Y., Zhang Z., Wang H., Xiao S., Cui C., Liu M. Extraction technology, component analysis, antioxidant, antibacterial, analgesic and anti-inflammatory activities of flavonoids fraction from Tribulus terrestris L. leaves. Heliyon. 2019;5:e02234. doi: 10.1016/j.heliyon.2019.e02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durgawale P.P., Datkhile K.D. Study of Polyphenol content and anti-oxidative potential of tribulus terrestris dry fruit extract. Int. J. Pharmacogn. Phytochem. Res. 2017;9:716–721. [Google Scholar]
- 44.Soumia B. Polyphenols: Mechanisms of Action in Human Health and Disease. Academic Press; Cambridge, MA, USA: 2018. Eryngium campestre L.: Polyphenolic and Flavonoid Compounds; Applications to Health and Disease; pp. 69–79. [Google Scholar]
- 45.Willis S., Sunkara R., Hester F., Shackelford L., Walker L.T., Verghese M. Chemopreventive and anti-inflammatory potential of select herbal teas and cinnamon in an in-vitro cell model. Food Nutr. Sci. 2019;10:1142–1156. [Google Scholar]
- 46.Gruenwald J., Freder J., Armbruester N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 47.Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028X-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S., Ghataury S.K., Sarathe A., Dubey G., Parkhe G. Curcuma angustifolia Roxb, (Zingiberaceae): Ethnobotany, phytochemistry and pharmacology: A review. J. Pharmacogn. Phytochem. 2019;8:1535–1540. [Google Scholar]
- 49.Panpatil V.V., Tattari S., Kota N., Nimgulkar C., Polasa K. In vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. J. Pharmacogn Phytochem. 2013;2:143–148. [Google Scholar]
- 50.Singh A., Rani R., Sharma M. Medicinal Herbs of Punjab (India) Biol. Forum. 2018;10:10–27. [Google Scholar]
- 51.Idris N.A., Yasin H.M., Usman A. Voltammetric and spectroscopic determination of polyphenols and antioxidants in ginger (Zingiber officinale Roscoe) Heliyon. 2019;5:e01717. doi: 10.1016/j.heliyon.2019.e01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliviero M., Romilde I., Beatrice M.M., Matteo V., Giovanna N., Consuelo A., Claudio C., Giorgio S., Filippo M., Massimo N. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem. Biol. Interact. 2016;256:125–133. doi: 10.1016/j.cbi.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 53.Tzima K., Makris D., Nikiforidis C.V., Mourtzinos I. Potential use of rosemary, propolis and thyme as natural food preservatives. J. Nutri. Health. 2015;1:6. [Google Scholar]
- 54.Miraj S., Kiani S. Study of pharmacological effect of Mentha pulegium: A review. Der. Pharm. Lett. 2016;8:242–245. [Google Scholar]
- 55.Gaeini Z., Taghinezhad M., Sohrabvandi S., Mortazavian A.M., Mahdavi S.M. Healthful characteristics of pennyroyal essential oil. J. Paramed. Sci. 2013;4:2008–4978. [Google Scholar]
- 56.Rajić J.R., Đorđević S.M., Tešević V., Živković M., Đorđević N.O., Paunović D.M., Nedović V.A., Petrović T.S. The extract of fennel fruit as a potential natural additive in food industry. J. Agric. Sci. 2018;63:205–215. [Google Scholar]
- 57.Miraj S., Alesaeidi S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile) Electron. Physician. 2016;8:3024. doi: 10.19082/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh O., Khanam Z., Misra N., Srivastava M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011;5:82. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mimica-Dukic N., Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008;14:3141–3150. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- 60.Fierascu R.C., Georgiev M.I., Fierascu I., Ungureanu C., Avramescu S.M., Ortan A., Georgescu M.I., Sutan A.N., Zanfirescu A., Dinu-Pirvu C.E., et al. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L.(Asteraceae) and Veronica persica Poiret (Plantaginaceae) Food Chem. Toxicol. 2018;111:44–52. doi: 10.1016/j.fct.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Lou Z., Wang H., Li J., Chen S., Zhu S., Ma C., Wang Z. Antioxidant activity and chemical composition of the fractions from burdock leaves. J. Food Sci. 2010;75:C413–C419. doi: 10.1111/j.1750-3841.2010.01616.x. [DOI] [PubMed] [Google Scholar]
- 62.Mallard I., Bourgeois D., Fourmentin S. A friendly environmental approach for the controlled release of Eucalyptus essential oil. Colloid. Surf. A Physicochem. Eng. Asp. 2018;549:130–137. doi: 10.1016/j.colsurfa.2018.04.010. [DOI] [Google Scholar]
- 63.Luís Â., Neiva D.M., Pereira H., Gominho J., Domingues F., Duarte A.P. Bioassay-guided fractionation, GC–MS identification and in vitro evaluation of antioxidant and antimicrobial activities of bioactive compounds from Eucalyptus globulus stump wood methanolic extract. Ind. Crop. Prod. 2016;91:97–103. doi: 10.1016/j.indcrop.2016.06.022. [DOI] [Google Scholar]
- 64.Munir R., Semmar N., Farman M., Ahmad N.S. An updated review on pharmacological activities and phytochemical constituents of evening primrose (genus Oenothera) Asian Pac. J. Trop. Biomed. 2017;7:1046–1054. doi: 10.1016/j.apjtb.2017.10.004. [DOI] [Google Scholar]
- 65.Montserrat-de la Paz S., Fernández-Arche Á., Ángel-Martín M., García-Giménez M.D. The sterols isolated from Evening Primrose oil modulate the release of proinflammatory mediators. Phytomedicine. 2012;19:1072–1076. doi: 10.1016/j.phymed.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Miraj S., Azizi N., Kiani S. A review of chemical components and pharmacological effects of Melissa officinalis L. Der. Pharm. Lett. 2016;8:229–237. [Google Scholar]
- 67.Pirbalouti A.G., Nekoei M., Rahimmalek M., Malekpoor F. Chemical composition and yield of essential oil from lemon balm (Melissa officinalis L.) under foliar applications of jasmonic and salicylic acids. Biocatal. Agric. Biotechnol. 2019;19:101144. doi: 10.1016/j.bcab.2019.101144. [DOI] [Google Scholar]
- 68.Paul D. A review on biological activities of common Mallow (Malva sylvestris L.) Life Sci. 2016;4:1–5. [Google Scholar]
- 69.Nasiri E., Hosseinimehr S.J., Azadbakht M., Akbari J., Enayati-fard R., Azizi S. Effect of Malva sylvestris cream on burn injury and wounds in rats. Avicenna J. Phytomed. 2015;5:341. [PMC free article] [PubMed] [Google Scholar]
- 70.Martins N., Petropoulos S., Ferreira I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: A review. Food Chem. 2016;211:41–50. doi: 10.1016/j.foodchem.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 71.Toledano Medina M.Á., Merinas-Amo T., Fernández-Bedmar Z., Font R., del Río-Celestino M., Pérez-Aparicio J., Moreno-Ortega A., Alonso-Moraga A., Moreno-Rojas R. Physicochemical characterization and biological activities of Black and White Garlic: In Vivo and In Vitro assays. Foods. 2019;8:220. doi: 10.3390/foods8060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abd E.A.M.H., El-Mesallamy A.M.D., El-Gerby M., Awad A. Anti-Tumor, antioxidant and antimicrobial and the phenolic constituents of clove flower buds (Syzygium aromaticum) J. Microb. Biochem. Technol. 2014;10:s8-s007. [Google Scholar]
- 73.Radünz M., da Trindade M.L., Camargo T.M., Radünz A.L., Borges C.D., Gandra E.A., Helbig E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019;276:180–186. doi: 10.1016/j.foodchem.2018.09.173. [DOI] [PubMed] [Google Scholar]
- 74.Heredia-Guerrero J.A., Ceseracciu L., Guzman-Puyol S., Paul U.C., Alfaro-Pulido A., Grande C., Vezzulli L., Bandiera T., Bertorelli R., Russo D., et al. Antimicrobial, antioxidant, and waterproof rtv silicone-ethyl cellulose composites containing clove essential oil. Carbohyd. Polym. 2018;192:150–158. doi: 10.1016/j.carbpol.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 75.Wankhede T.B. Evaluation of antioxidant and antimicrobial activity of the Indian clove Syzygium aromaticum L. Merr. and Perr. Int. Res. J. Sci. Eng. 2015;3:166–172. [Google Scholar]
- 76.Cui H., Zhang C., Li C., Lin L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018;94:140–146. doi: 10.1016/j.foodcont.2018.07.007. [DOI] [Google Scholar]
- 77.Li W., Chen H., He Z., Han C., Liu S., Li Y. Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT-Food Sci. Technol. 2015;62:39–47. doi: 10.1016/j.lwt.2015.01.012. [DOI] [Google Scholar]
- 78.Nikousaleh A., Prakash J. Antioxidant components and properties of dry heat treated clove in different extraction solvents. J. Food Sci. Technol. 2016;53:1993–2000. doi: 10.1007/s13197-015-2113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jahanian E. Clove bud oil; a novel herbal medicine for future kidney researches. Ann. Res. Antioxid. 2016;1:27–29. [Google Scholar]
- 80.Jirovetz L., Buchbauer G., Stoilova I., Stoyanova A., Krastanov A., Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 81.Gülçin İ., Elmastaş M., Aboul-Enein H.Y. Antioxidant activity of clove oil–A powerful antioxidant source. Arab. J. Chem. 2012;5:489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
- 82.Adefegha S.A., Oboh G., Oyeleye S.I., Osunmo K. Alteration of starch hydrolyzing enzyme inhibitory properties, antioxidant activities, and phenolic profile of clove buds (Syzygium aromaticum L.) by cooking duration. Food Sci. Nutr. 2016;4:250–260. doi: 10.1002/fsn3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akthar M.S., Degaga B., Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Bio. Sci. Pharma. Res. 2014;2:001–007. [Google Scholar]
- 84.Jaiswal S., Rajwade D. A Review on Portulaca oleracea (Nonia bhaji): A wonderful weed of Chhattisgarh. Res. J. Pharm Technol. 2017;10:2415–2420. doi: 10.5958/0974-360X.2017.00426.7. [DOI] [Google Scholar]
- 85.Dong C.X., Hayashi K., Lee J.B., Hayashi T. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea L. Chem. Pharm. Bull. 2010;58:507–510. doi: 10.1248/cpb.58.507. [DOI] [PubMed] [Google Scholar]
- 86.Gismalla M.A.M. Ph.D. Thesis. University of Gezira; Wad Medani, Sudan: 2017. Antimicrobial Activity of Aqueous Methanol Extracted Rigla (Portulaca oleraceaLinn) [Google Scholar]
- 87.Supriya J.A., Kishor G.U., Aniket G.H. Phytochemical screening and antimicrobial activity of portulaca quadrifida linn. Asian J. Pharm Clin Res. 2019;12:78–81. doi: 10.22159/ajpcr.2019.v12i3.27587. [DOI] [Google Scholar]
- 88.Khanam B., Begum W., Tipo F.A. Pharmacological profile, phytoconstituents, and traditional uses of Khurfa (Portulaca oleracea L.): Unani perspective. J. Pharm. Innov. 2019;8:367–372. [Google Scholar]
- 89.Londonkar R., Nayaka H.B. Phytochemical and antimicrobial activities of Portulaca oleracea L. J. Pharm. Res. 2011;4:3553–3555. [Google Scholar]
- 90.Masoodi M.H., Ahmad B., Mir S.R., Zargar B.A., Tabasum N. Portulaca oleracea L. a review. J. Pharm. Res. 2011;4:3044–3048. [Google Scholar]
- 91.Yanala S.R., Sathyanarayana D., Kannan K. A Recent Phytochemical Review-Fruits of Tribulus terrestris Linn. J. Pharm. Sci. Res. 2016;8:132. [Google Scholar]
- 92.Chhatre S., Nesari T., Somani G., Kanchan D., Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014;8:45. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abubakar S., Akanbi B., Etim V., Segun O., Ogbu J. Comparative study of phytochemical and synergistic anti-bacterial activity of Tribulus terrestris (L.) and Pandiaka heudelotii (Moq.) Hien on some clinical bacterial isolates. Pharm. Biol. Eval. 2016;3:83–91. [Google Scholar]
- 94.Usman H., Abdulrahman F.I., Ladan A.H. Phytochemical and antimicrobial evaluation of Tribulus terrestris L. (Zygophylaceae). growing in Nigeria. Res. J. Bio. Sci. 2007;2:244–247. [Google Scholar]
- 95.Batoei S., Mahboubi M., Yari R. Antibacterial activity of Tribulus terrestris methanol extract against clinical isolates of Escherichia coli. Herba Pol. 2016;62:57–66. doi: 10.1515/hepo-2016-0011. [DOI] [Google Scholar]
- 96.Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbial. 2019;10:911. doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Bayati F.A., Al-Mola H.F. Antibacterial and antifungal activities of different parts of Tribulus terrestris L. growing in Iraq. J. Zhejiang Univ. Sci. 2008;9:154–159. doi: 10.1631/jzus.B0720251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satvati S.A.R., Shooriabi M., Amin M., Shiezadeh F. Evaluation of the Antimicrobial activity of Tribulus terrestris, Allium sativum, Salvia officinalis, and Allium hirtifolium Boiss against Enterococcus faecalis. Int. J. Enteric. Pathog. 2017;5:63–67. [Google Scholar]
- 99.Zheleva-Dimitrova D., Obreshkova D., Nedialkov P. Antioxidant activity of tribulus terrestris—A natural product in infertility therapy. Int. J. Pharm Pharm Sci. 2012;4:508–511. [Google Scholar]
- 100.Farooq S.A., Farook T.T., Al-Rawahy S.H. Natural Products and Their Active Compounds on Disease Prevention. Nova Science Publishers; Hauppauge, NY, USA: 2012. Bioactive compounds from Tribulus Terrestris L. (zygophyllaceae) pp. 245–268. [Google Scholar]
- 101.Hifnawy M.S., AbouZid S.F., Ali Z.Y., Fouda M.M. Phenolic contents and in vitro free radical scavenging activity of alcoholic extract of the fruits of Tribulus terrestris L. Pharm. Innov. 2015;4:92. [Google Scholar]
- 102.Ligor M., Ratiu I.A., Kiełbasa A., Al-Suod H., Buszewski B. Extraction approaches used for the determination of biologically active compounds (cyclitols, polyphenols and saponins) isolated from plant material. Electrophoresis. 2018;39:1860–1874. doi: 10.1002/elps.201700431. [DOI] [PubMed] [Google Scholar]
- 103.Ivanišová E., Farkaš A., Frančáková H., Kačániová M. Antioxidant activity and total polyphenol content of medicinal herbs with adaptogenic effect to human body. J. Anim. Sci. Biotechnol. 2018;51:119–123. Scientific papers. [Google Scholar]
- 104.Ali S.I., Gaafar A.A., Abdallah A.A., El-Daly S.M., El-Bana M., Hussein J. Mitigation of Alpha-Cypermethrin-Induced hepatotoxicity in rats by Tribulus terrestris rich in antioxidant compounds. Jordan J. Biol. Sci. 2018;11:5. [Google Scholar]
- 105.Song Y.H., Kim D.W., Curtis-Long M.J., Park C., Son M., Kim J.Y., Yuk H.J., Lee K.W., Park K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem. 2016;114:201–208. doi: 10.1016/j.ejmech.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 106.Aleksandrovna S.E., Mikhailovna E.L., Alexeevich K.D. Experience of introduction of two species of Eryngium in the North Caucasus. Pharmacogn. J. 2018;10:6. doi: 10.5530/pj.2018.6s.11. [DOI] [Google Scholar]
- 107.Meindl C., Brune V., Listl D., Poschlod P., Reisch C. Survival and postglacial immigration of the steppe plant Scorzonera purpurea to Central Europe. Plant. Syst. Evol. 2016;302:971–984. doi: 10.1007/s00606-016-1311-9. [DOI] [Google Scholar]
- 108.Kikowska M., Thiem B., Sliwinska E., Rewers M., Kowalczyk M., Stochmal A., Długaszewska J. Micropropagation of Eryngium campestre L. via shoot culture provides valuable uniform plant material with enhanced content of phenolic acids and antimicrobial activity. Acta Biol. Cracov. Bot. 2016;58:43–56. doi: 10.1515/abcsb-2016-0009. [DOI] [Google Scholar]
- 109.Kikowska M., Długaszewska J., Kubicka M.M., Kędziora I., Budzianowski J., Thiem B. In vitro antimicrobial activity of extracts and their fractions from three Eryngium L. species. Herba Pol. 2016;62:67–77. doi: 10.1515/hepo-2016-0012. [DOI] [Google Scholar]
- 110.Erdem S.A., Nabavi S.F., Orhan I.E., Daglia M., Izadi M., Nabavi S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015;23:53. doi: 10.1186/s40199-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daneshzadeh M.S., Abbaspour H., Amjad L., Nafchi A.M. An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J. Food Meas. Charact. 2020;14:708–715. doi: 10.1007/s11694-019-00317-y. [DOI] [Google Scholar]
- 112.Benmerache A., Magid A.A., Berrehal D., Kabouche A., Voutquenne-Nazabadioko L., Messaili S., Abedinib A., Harakatc D., Kabouche Z. Chemical composition, antibacterial, antioxidant and tyrosinase inhibitory activities of glycosides from aerial parts of Eryngium tricuspidatum L. Phytochem. Lett. 2016;18:23–28. doi: 10.1016/j.phytol.2016.08.018. [DOI] [Google Scholar]
- 113.Rjeibi I., Saad A.B., Ncib S., Souid S. Phenolic composition and antioxidant properties of Eryngium maritimum (sea holly) J. Coast. Life Med. 2017;5:212–215. doi: 10.12980/jclm.5.2017J7-18. [DOI] [Google Scholar]
- 114.Paşayeva L., Şafak E.K., Arıgün T., Fatullayev H., Tugay O. In vitro antioxidant capacity and phytochemical characterization of Eryngium kotschyi Boiss. J. Pharm. Pharmacogn. Res. 2020;8:18–31. [Google Scholar]
- 115.Traversier M., Gaslonde T., Lecso M., Michel S., Delannay E. Comparison of extraction methods for chemical composition, antibacterial, depigmenting and antioxidant activity of Eryngium maritimum. Int. J. Cosmet. Sci. 2020;42:127–135. doi: 10.1111/ics.12595. [DOI] [PubMed] [Google Scholar]
- 116.Dalukdeniya D.A.C.K., Rathnayaka R. Comparative study on antibacterial and selected antioxidant activities of different Eryngium Foetidum extracts. J. Appl Life Sci. Int. 2017;12:1–7. doi: 10.9734/JALSI/2017/34378. [DOI] [Google Scholar]
- 117.Kikowska M., Kalemba D., Dlugaszewska J., Thiem B. Chemical composition of essential oils from rare and endangered species—Eryngium maritimum L. and E. alpinum L. Plants. 2020;9:417. doi: 10.3390/plants9040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Azad R., Kumara K.W., Senanayake G., Ranawaka R., Pushpakumara D., Geekiyanage S. Flower morphological diversity of cinnamon (Cinnamomum verum Presl) in Matara District, Sri Lanka. Open Agric. 2018;3:236–244. doi: 10.1515/opag-2018-0025. [DOI] [Google Scholar]
- 119.Maridass M. Evaluation of brine shrimp lethality of Cinnamomum species. Ethnobotanical. Leaflets. 2008;2008:106. [Google Scholar]
- 120.Razali Z., Muhammad N.A.I., Salleh S.N.M. Cinnamaldehyde Constituent and Screening of Antibacterial Potential in Local Cinnamomum Zeylanicum Bark Zainab. J. Intelek. 2016;11:1. [Google Scholar]
- 121.Ribeiro-Santos R., Andrade M., Madella D., Martinazzo A.P., Moura L.D.A.G., de Melo N.R., Sanches-Silva A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Tech. 2017;62:154–169. doi: 10.1016/j.tifs.2017.02.011. [DOI] [Google Scholar]
- 122.Sharafeldin K., Rizvi M.R. Effect of traditional plant medicines (Cinnamomum zeylanicum and Syzygium cumini) on oxidative stress and insulin resistance in streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2015;72:126–134. doi: 10.1016/j.jobaz.2015.09.002. [DOI] [Google Scholar]
- 123.Hamidpour R., Hamidpour M., Hamidpour S., Shahlari M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer’s disease, and a series of functions such as antioxidant, anticholesterol, antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J. Tradit. Complement. Med. 2015;5:66–70. doi: 10.1016/j.jtcme.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng K., Wen P., Yang H., Li N., Lou W.Y., Zong M.H., Wu H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017;7:1572–1580. doi: 10.1039/C6RA25977D. [DOI] [Google Scholar]
- 125.Chuesiang P., Siripatrawan U., Sanguandeekul R., McClements D.J., McLandsborough L. Antimicrobial activity of PIT-fabricated cinnamon oil nanoemulsions: Effect of surfactant concentration on morphology of foodborne pathogens. Food Control. 2019;98:405–411. doi: 10.1016/j.foodcont.2018.11.024. [DOI] [Google Scholar]
- 126.Shabani N.R., Ismail Z., Ismail W.I., Zainuddin N., Rosdan N.H., Roslan M.N., Mohd Azahar N.M. Antimicrobial activity of cinnamon oil against bacteria that cause skin infections. J. Sci. Res. Dev. 2016;3:1–6. [Google Scholar]
- 127.Mazimba O., Wale K., Tebogo E., Tebogo E., Kwape Shetonde O. Cinnamomum verum: Ethylacetate and methanol extracts antioxidant and antimicrobial activity. J. Med. Plants Stud. 2015;3:28–32. [Google Scholar]
- 128.Mukhtar S., Ghori I. Antibacterial activity of aqueous and ethanolic extracts of garlic, cinnamon and turmeric against Escherichia coli ATCC 25922 and Bacillus subtilis DSM 3256. Int. J. Appl. Biol. Pharm. 2012;3:131–136. [Google Scholar]
- 129.Abbaszadegan A., Dadolahi S., Gholami A., Moein M.R., Hamedani S., Ghasemi Y., Abbott P.V. Antimicrobial and cytotoxic activity of Cinnamomum zeylanicum, Calcium Hydroxide, and triple antibiotic paste as root canal dressing materials. J. Contemp. Dent. Pract. 2016;17:105–113. doi: 10.5005/jp-journals-10024-1811. [DOI] [PubMed] [Google Scholar]
- 130.Brnawi W.I., Hettiarachchy N.S., Horax R., Kumar-Phillips G., Ricke S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella typhimurium in broth system and on celery. J. Food Process. Preserv. 2019;43:13888. doi: 10.1111/jfpp.13888. [DOI] [Google Scholar]
- 131.Reyes-Jurado F., Navarro-Cruz A.R., Ochoa-Velasco C.E., Palou E., López-Malo A., Ávila-Sosa R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2020;60:1641–1650. doi: 10.1080/10408398.2019.1586641. [DOI] [PubMed] [Google Scholar]
- 132.Bouhdid S., Abrini J., Amensour M., Zhiri A., Espuny M.J., Manresa A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010;109:1139–1149. doi: 10.1111/j.1365-2672.2010.04740.x. [DOI] [PubMed] [Google Scholar]
- 133.Vasconcelos N.G., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018;120:198–203. doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 134.Oussalah M., Caillet S., Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157, H7 and Listeria monocytogenes. J. Food Prot. 2006;69:1046–1055. doi: 10.4315/0362-028X-69.5.1046. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y., Liu X., Wang Y., Jiang P., Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 136.Tulini F.L., Souza V.B., Thomazini M., Silva M.P., Massarioli A.P., Alencar S.M., Palloneb EM J.A., Genovesed M.I., Favaro-Trindadeb C.S. Evaluation of the release profile, stability and antioxidant activity of a proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract co-encapsulated with α-tocopherol by spray chilling. Food Res. Int. 2017;95:117–124. doi: 10.1016/j.foodres.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 137.Gulcin I., Kaya R., Goren A.C., Akincioglu H., Topal M., Bingol Z., Cetin Ç.K., Ozturk S.S.B., Durmaz L., Alwasel S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019;22:1511–1526. doi: 10.1080/10942912.2019.1656232. [DOI] [Google Scholar]
- 138.Ervina M., Lie H.S., Diva J., Tewfik S., Tewfik I. Optimization of water extract of Cinnamomum burmannii bark to ascertain it’s in vitro antidiabetic and antioxidant activities. Biocatal. Agric. Biotechnol. 2019;19:101152. doi: 10.1016/j.bcab.2019.101152. [DOI] [Google Scholar]
- 139.Vimalanathan S., Hudson J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil Nat. Prod. 2014;2:47–53. [Google Scholar]
- 140.Gautam R.K., Arora D., Goyal S. Science of Spices and Culinary Herbs-Latest Laboratory, Pre-Clinical, and Clinical Studies. Volume 1. Bentham Science Publishers; Sharjah, UAE: 2019. Pre-clinical/animal studies conducted on Turmeric and Curcumin and their formulations; pp. 198–225. [Google Scholar]
- 141.Singh D.B., Maurya A.K., Rai D. Science of Spices and Culinary Herbs-Latest Laboratory, Pre-Clinical, and Clinical Studies. Volume 1. Bentham Science Publishers; Sharjah, UAE: 2019. Antibacterial and anticancer activities of Turmeric and its active ingredient Curcumin, and mechanism of action; pp. 74–103. [Google Scholar]
- 142.Gitika A., Mishra R., Panda S.K., Mishra C., Sahoo P.R. Evaluation of antifungal activity of Curcumin against Aspergillus flavus. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:2323–2329. doi: 10.20546/ijcmas.2019.807.284. [DOI] [Google Scholar]
- 143.Neyestani Z., Ebrahimi S.A., Ghazaghi A., Jalili A., Sahebkar A., Rahimi H.R. Review of anti-bacterial activities of Curcumin against Pseudomonas aeruginosa. Crit. Rev. Eukaryot. Gene Expr. 2019;29:5. doi: 10.1615/CritRevEukaryotGeneExpr.2019029088. [DOI] [PubMed] [Google Scholar]
- 144.Barzegar A., Moosavi-Movahedi A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE. 2011;6:e26012. doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Calabrese V., Bates T.E., Mancuso C., Cornelius C., Ventimiglia B., Cambria M.T., Renzo L.D., Lorenzo A.D., Dinkova-Kostova A.T. Curcumin and the cellular stress response in free radical-related diseases. Mol. Nutr. Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 146.Dinkova-Kostova A.T., Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008;52:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 147.Abu-Rizq H.A., Mansour M.H., Safer A.M., Afzal M. Cyto-protective and immunomodulating effect of Curcuma longa in Wistar rats subjected to carbon tetrachloride-induced oxidative stress. Inflammopharmacology. 2008;16:87–95. doi: 10.1007/s10787-007-1621-1. [DOI] [PubMed] [Google Scholar]
- 148.Pakfetrat M., Akmali M., Malekmakan L., Dabaghimanesh M., Khorsand M. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial. Int. 2015;19:124–131. doi: 10.1111/hdi.12204. [DOI] [PubMed] [Google Scholar]
- 149.Karimi N., Ghanbarzadeh B., Hamishehkar H., Mehramuz B., Kafil H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC) Colloid Interfac. Sci. 2018;22:18–24. doi: 10.1016/j.colcom.2017.11.006. [DOI] [Google Scholar]
- 150.Sahin K., Pala R., Tuzcu M., Ozdemir O., Orhan C., Sahin N., Juturu V. Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016;9:147. doi: 10.2147/JIR.S110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nasir A.S., Jaffat H.S. Protective role of turmeric extract (Curcuma longa) in the lipid profile and activity of antioxidant in the male rats treated by lithium carbonate. Int. J. Pharmtech. Res. 2016;9:98–105. [Google Scholar]
- 152.Riaz T., Abbasi M.A., Shahzadi T., Qureshi M.Z., Khan K.M. Antioxidant Activity and Radical Scavenging Effects of Various Fractions from Curcuma zedoaria. AJPBR. 2011;1:525–533. [Google Scholar]
- 153.Rezaei-Moghadam A., Mohajeri D., Rafiei B., Dizaji R., Azhdari A., Yeganehzad M., Shahidi M., Mazani M. Effect of turmeric and carrot seed extracts on serum liver biomarkers and hepatic lipid peroxidation, antioxidant enzymes and total antioxidant status in rats. BioImpacts. 2012;2:151. doi: 10.5681/bi.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hay E., Lucariello A., Contieri M., Esposito T., De Luca A., Guerra G., Perna A. Therapeutic effects of turmeric in several diseases: An overview. Chem. Biol. Interact. 2019;310:108729. doi: 10.1016/j.cbi.2019.108729. [DOI] [PubMed] [Google Scholar]
- 155.Chattopadhyay I., Biswas K., Bandyopadhyay U., Banerjee R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004;87:44–53. [Google Scholar]
- 156.Marwat S.K., Shoaib M., Khan E.A., Rehman F., Ullah H. Phytochemistry and Bioactivities of Quranic Plant, Zanjabil-Ginger (Zingiber officinale Roscoe): A Review. Am. Eurasian J. Agric. Environ. Sci. 2015;15:707–713. [Google Scholar]
- 157.Li H., Huang M., Tan D., Liao Q., Zou Y., Jiang Y. Effects of soil moisture content on the growth and physiological status of ginger (Zingiber officinale Roscoe) Acta Physiol. Plant. 2018;40:125. doi: 10.1007/s11738-018-2698-4. [DOI] [Google Scholar]
- 158.Chan E.W., Wong S.K., Chan H.T. Alpinia zerumbet, a ginger plant with a multitude of medicinal properties: An update on its research findings. J. Chin. Pharm. 2017;15:1. doi: 10.5246/jcps.2017.11.088. [DOI] [Google Scholar]
- 159.Babu K.N., Samsudeen K., Divakaran M., Pillai G.S., Sumathi V., Praveen K. Protocols for in vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants. 2nd ed. Humana Press; New York, NY, USA: 2016. Protocols for in vitro propagation, conservation, synthetic seed production, embryo rescue, microrhizome production, molecular profiling, and genetic transformation in ginger (Zingiber officinale Roscoe.) pp. 403–426. [DOI] [PubMed] [Google Scholar]
- 160.Mashhadi N.S., Ghiasvand R., Askari G., Hariri M., Darvishi L., Mofid M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013;4:36. [PMC free article] [PubMed] [Google Scholar]
- 161.Stoilova I., Krastanov A., Stoyanova A., Denev P., Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. doi: 10.1016/j.foodchem.2006.06.023. [DOI] [Google Scholar]
- 162.Takeuchi H., Trang V.T., Morimoto N., Nishida Y., Matsumura Y., Sugiura T. Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 2014;20:8971. doi: 10.3748/wjg.v20.i27.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shalaby M.T., Ghanem A.A., Maamon H.M. Protective effect of ginger and cactus saguaro extract against cancer formation cells. JFDS. 2016;7:487–491. doi: 10.21608/jfds.2016.46069. [DOI] [Google Scholar]
- 164.Höferl M., Stoilova I., Wanner J., Schmidt E., Jirovetz L., Trifonova D., Stanchev V., Krastanov A. Composition and comprehensive antioxidant activity of ginger (Zingiber officinale) essential oil from Ecuador. Nat. Prod. Commun. 2015;10:1085–1090. doi: 10.1177/1934578X1501000672. [DOI] [PubMed] [Google Scholar]
- 165.Tohma H., Gülçin İ., Bursal E., Gören A.C., Alwasel S.H., Köksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas Charact. 2017;11:556–566. doi: 10.1007/s11694-016-9423-z. [DOI] [Google Scholar]
- 166.Nile S.H., Park S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crops Prod. 2015;70:238–244. doi: 10.1016/j.indcrop.2015.03.033. [DOI] [Google Scholar]
- 167.Tung B.T., Thu D.K., Thu N.T.K., Hai N.T. Antioxidant and acetylcholinesterase inhibitory activities of ginger root (Zingiber officinale Roscoe) extract. J. Complement. Integr. Med. 2017;14:14. doi: 10.1515/jcim-2016-0116. [DOI] [PubMed] [Google Scholar]
- 168.Chan E.W., Lim Y.Y., Wong L.F., Lianto F.S., Wong S.K., Lim K.K., Joe C.E., Lim T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–483. doi: 10.1016/j.foodchem.2008.02.016. [DOI] [Google Scholar]
- 169.De las Heras N., Valero-Muñoz M., Martín-Fernández B., Ballesteros S., López-Farré A., Ruiz-Roso B., Lahera V. Molecular factors involved in the hypolipidemic-and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2016;42:209–215. doi: 10.1139/apnm-2016-0374. [DOI] [PubMed] [Google Scholar]
- 170.Adeniyi P.O., Sanusi R.A., Obatolu V.A. Effect of raw and cooked ginger (Zingiber officinale Roscoe) Extracts on insulin sensitivity in normal and high-fat diet-induced diabetic rats. J. Food Nutr. Res. 2017;5:838–843. [Google Scholar]
- 171.Abdulrazak A., Tanko Y., Mohammed A., Dikko A.A. Modulatory roles of clove and fermented ginger supplements on lipid profile and thyroid functions in high fat diet induced insulin resistance in rabbits. Asian J. Med. Sci. 2017;8:1–9. [Google Scholar]
- 172.Abdulrazaq N.B., Cho M.M., Win N.N., Zaman R., Rahman M.T. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. Br. J. Nutr. 2012;108:1194–1201. doi: 10.1017/S0007114511006635. [DOI] [PubMed] [Google Scholar]
- 173.Mozaffari-Khosravi H., Talaei B., Jalali B.A., Najarzadeh A., Mozayan M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014;22:9–16. doi: 10.1016/j.ctim.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 174.Mahluji S., Attari V.E., Mobasseri M., Payahoo L., Ostadrahimi A., Golzari S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013;64:682–686. doi: 10.3109/09637486.2013.775223. [DOI] [PubMed] [Google Scholar]
- 175.Akintobi O.A., Onoh C.C., Ogele J.O., Idowu A.A., Ojo O.V., Okonko I.O. Antimicrobial activity of Zingiber officinale (ginger) extract against some selected pathogenic bacteria. Nat. Sc. 2013;11:7–15. [Google Scholar]
- 176.Naghsh F. Nano drug delivery study of anticancer properties on ginger using QM/MM methods. Orient J. Chem. 2015;31:465–478. doi: 10.13005/ojc/310156. [DOI] [Google Scholar]
- 177.Shakya S.R. Medicinal uses of ginger (Zingiber officinale Roscoe) improves growth and enhances immunity in aquaculture. Int. J. Chem. Stud. 2015;3:83–87. [Google Scholar]
- 178.Dorra N., El-Berrawy M., Sallam S., Mahmoud R. Evaluation of Antiviral and Antioxidant Activity of Selected Herbal Extracts. JHIPH. 2019;49:36–40. doi: 10.21608/jhiph.2019.29464. [DOI] [Google Scholar]
- 179.Piccaglia R., Marotti M., Giovanelli E., Deans S.G., Eaglesham E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Ind. Crops Prod. 1993;2:47–50. doi: 10.1016/0926-6690(93)90010-7. [DOI] [Google Scholar]
- 180.Köksal E., Bursal E., Gülçin İ., Korkmaz M., Çağlayan C., Gören A.C., Alwasel S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017;20:514–525. doi: 10.1080/10942912.2016.1168438. [DOI] [Google Scholar]
- 181.Martins N., Barros L., Santos-Buelga C., Silva S., Henriques M., Ferreira I.C. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015;167:131–137. doi: 10.1016/j.foodchem.2014.06.094. [DOI] [PubMed] [Google Scholar]
- 182.Gavaric N., Mozina S.S., Kladar N., Bozin B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil-Bear Plants. 2015;18:1013–1021. doi: 10.1080/0972060X.2014.971069. [DOI] [Google Scholar]
- 183.Ghaderi-Ghahfarokhi M., Barzegar M., Sahari M.A., Azizi M.H. Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioproc. Tech. 2016;9:1187–1201. doi: 10.1007/s11947-016-1708-z. [DOI] [Google Scholar]
- 184.El-Guendouz S., Aazza S., Anahi Dandlen S., Majdoub N., Lyoussi B., Raposo S., Dulce Antunes M., Gomes V., Graça Miguel M. Antioxidant activity of Thyme waste extract in O/W emulsions. Antioxidants. 2019;8:243. doi: 10.3390/antiox8080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tohidi B., Rahimmalek M., Trindade H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crop. Prod. 2019;134:89–99. doi: 10.1016/j.indcrop.2019.02.038. [DOI] [Google Scholar]
- 186.Gömöri C., Vidács A., Kerekes E.B., Nacsa-Farkas E., Böszörményi A., Vágvölgyi C., Krisch J. Altered antimicrobial and anti-biofilm forming effect of thyme essential oil due to changes in composition. Nat. Pro. Commun. 2018;13:483–487. doi: 10.1177/1934578X1801300426. [DOI] [Google Scholar]
- 187.Ahmadi R., Alizadeh A., Ketabchi S. Antimicrobial activity of the essential oil of Thymus kotschyanus grown wild in Iran. Int. J. Biosci. 2015;6:239–248. [Google Scholar]
- 188.Anwar M.M., Nasr E.H., Ali S.E. Effect of gamma irradiation on chemical constituents, antimicrobials and antioxidants of Thyme and Cinnamon volatile oils. Appl Radiat. Isotopes. 2015;47:125–142. [Google Scholar]
- 189.Santoyo S., Jaime L., García-Risco M.R., Lopez-Hazas M., Reglero G. Supercritical fluid extraction as an alternative process to obtain antiviral agents from thyme species. Ind. Crop. Prod. 2014;52:475–480. doi: 10.1016/j.indcrop.2013.10.028. [DOI] [Google Scholar]
- 190.Mkaddem M., Boussaid M., Fadhel N.B. Variability of volatiles in Tunisian Mentha pulegium L.(Lamiaceae) J. Essent. Oil Res. 2007;19:211–214. doi: 10.1080/10412905.2007.9699263. [DOI] [Google Scholar]
- 191.Vaghardoost R., Ghavami Y., Sobouti B. The effect of Mentha Pulegium on healing of burn wound injuries in rat. World J. Plast Surg. 2019;8:43. doi: 10.29252/wjps.8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Teixeira B., Marques A., Ramos C., Batista I., Serrano C., Matos O., Neng N.R., Nogueira J.M., Saraiva J.A., Nunes M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crop. Prod. 2012;36:81–87. doi: 10.1016/j.indcrop.2011.08.011. [DOI] [Google Scholar]
- 193.Hassanpouraghdam M.B., Akhgari A.B., Aazami M.A., Emarat-Pardaz J. New menthone type of Mentha pulegium L. volatile oil from Northwest Iran. Zech. J. Food Sci. 2011;29:285–290. doi: 10.17221/165/2009-CJFS. [DOI] [Google Scholar]
- 194.Morteza-Semnani K., Saeedi M., Akbarzadeh M. Chemical composition and antimicrobial activity of the essential oil of Mentha pulegium L. J. Essent. Oil-Bear Plants. 2011;14:208–213. doi: 10.1080/0972060X.2011.10643923. [DOI] [Google Scholar]
- 195.Petrakis E.A., Kimbaris A.C., Pappas C.S., Tarantilis P.A., Polissiou M.G. Quantitative determination of pulegone in pennyroyal oil by FT-IR spectroscopy. J. Agr Food Chem. 2009;57:10044–10048. doi: 10.1021/jf9026052. [DOI] [PubMed] [Google Scholar]
- 196.Sarikurkcu C., Eryigit F., Cengiz M., Tepe B., Cakir A., Mete E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectrosc. Lett. 2012;45:352–358. doi: 10.1080/00387010.2012.666701. [DOI] [Google Scholar]
- 197.Hadi M.Y., Hameed I.H., Ibraheam I.A. Mentha pulegium: Medicinal uses, Anti-Hepatic, Antibacterial, Antioxidant effect and Analysis of Bioactive Natural Compounds: A Review. Res. J. Pharm Technol. 2017;10:3580–3584. doi: 10.5958/0974-360X.2017.00648.5. [DOI] [Google Scholar]
- 198.Salem N., Sriti J., Bachrouch O., Msaada K., Khammassi S., Hammami M. Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne. Asian Pac. J. Trop Biomed. 2018;8:207. doi: 10.4103/2221-1691.231283. [DOI] [Google Scholar]
- 199.Ferreres F., Bernardo J., Andrade P.B., Sousa C., Gil-Izquierdo A., Valentão P. Pennyroyal and gastrointestinal cells: Multi-target protection of phenolic compounds against t-BHP-induced toxicity. RSC Adv. 2015;5:41576–41584. doi: 10.1039/C5RA02710A. [DOI] [Google Scholar]
- 200.Abdelli M., Moghrani H., Aboun A., Maachi R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016;94:197–205. doi: 10.1016/j.indcrop.2016.08.042. [DOI] [Google Scholar]
- 201.Bonyadian M., Moshtaghi H. Bacteriocidal activity of some plants essential oils against bacillus cereus, salmonella typhimurium, listeria monocytogenes and yersinia enterocolitica. Res. J. Microbiol. 2008;3:648–653. doi: 10.3923/jm.2008.648.653. [DOI] [Google Scholar]
- 202.Yusuf U., Muhammad M. Antibacterial Properties of Mentha Pulegium. South. Asian Res. J. Med. Sci. 2019;1:43–45. doi: 10.36346/sarjams.2019.v01i02.004. [DOI] [Google Scholar]
- 203.Rather M.A., Dar B.A., Sofi S.N., Bhat B.A., Qurishi M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016;9:S1574–S1583. doi: 10.1016/j.arabjc.2012.04.011. [DOI] [Google Scholar]
- 204.Bettaieb ép Rebey I., Bourgou S., Marzouk B., Fauconnier M.L., Ksouri R. Salinity impact on seed yield, polyphenols composition and antioxidant activity of Fennel (Foeniculum vulgarae Mill) extracts. J. New Sci. 2017;2017:2610–2619. [Google Scholar]
- 205.Roby M.H., Sarhan M.A., Selim K.A., Khalel K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Ind. Crop. Prod. 2013;44:437–445. doi: 10.1016/j.indcrop.2012.10.012. [DOI] [Google Scholar]
- 206.Ahmed A.F., Shi M., Liu C., Kang W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness. 2019;8:67–72. doi: 10.1016/j.fshw.2019.03.004. [DOI] [Google Scholar]
- 207.AbduRahim S.A., Elamin B.E., Bashir A.A., Almagboul A.Z. In vitro test of antimicrobial activity of Foeniculum Vulgare Mill. (Fennel) essential oil. J. Multidiscip. Eng. Sci. Stud. 2017;3:1609–1614. [Google Scholar]
- 208.Al-Hadid K.J. Quantitative analysis of antimicrobial activity of ‘Foeniculum vulgare’: A review. Plant. Omics. 2017;10:28. doi: 10.21475/poj.10.01.17.322. [DOI] [Google Scholar]
- 209.Chang S., Mohammadi Nafchi A., Karim A.A. Chemical composition, antioxidant activity and antimicrobial properties of three selected varieties of Iranian fennel seeds. J. Essent. Oil Res. 2016;28:357–363. doi: 10.1080/10412905.2016.1146169. [DOI] [Google Scholar]
- 210.GabAllah M., Kandeil A., Mousa A.E., Ahmed Ali M. Antiviral activity of water extracts of some medicinal and nutritive plants from the Apiaceae family. NRMJ. 2020;4:725–735. doi: 10.21608/nrmj.2020.84021. [DOI] [Google Scholar]
- 211.Agatonovic-Kustrin S., Ortakand D.B., Morton D.W., Yusof A.P. Rapid evaluation and comparison of natural products and antioxidant activity in calendula, feverfew, and German chamomile extracts. J. Chromatogr. A. 2015;1385:103–110. doi: 10.1016/j.chroma.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 212.Stanojevic L.P., Marjanovic-Balaban Z.R., Kalaba V.D., Stanojevic J.S., Cvetkovic D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.) J. Essent. Oil Bear Plants. 2016;19:2017–2028. doi: 10.1080/0972060X.2016.1224689. [DOI] [Google Scholar]
- 213.Ismail M.C., Waleed S., Ibrahim K., Fakhri N.U. Synergistic interaction between Chamomile flower (Matricaria chamomilla L.) extracts and tetracycline against wound infection bacteria. Al-Nahrain J. Sci. 2013;16:191–195. doi: 10.22401/JNUS.16.3.27. [DOI] [Google Scholar]
- 214.Mekonnen A., Yitayew B., Tesema A., Taddese S. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016;2016:8. doi: 10.1155/2016/9545693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mekinić I.G., Skroza D., Ljubenkov I., Krstulović L., Možina S.S., Katalinić V. Phenolic acids profile, antioxidant and antibacterial activity of chamomile, common yarrow and immortelle (Asteraceae) Nat. Prod. Commun. 2014;9:1745–1748. doi: 10.1177/1934578X1400901222. [DOI] [PubMed] [Google Scholar]
- 216.Formisano C., Delfine S., Oliviero F., Tenore G.C., Rigano D., Senatore F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy) Ind. Crop. Prod. 2015;63:256–263. doi: 10.1016/j.indcrop.2014.09.042. [DOI] [Google Scholar]
- 217.Pereira S.V., Reis R.A., Garbuio D.C., de Freitas L.A. Dynamic maceration of Matricaria chamomilla inflorescences: Optimal conditions for flavonoids and antioxidant activity. Rev. Bras. Farmacogn. 2018;28:111–117. doi: 10.1016/j.bjp.2017.11.006. [DOI] [Google Scholar]
- 218.Allahverdiyev A.M., Bagirova M., Yaman S., Koc R.C., Abamor E.S., Ates S.C., Baydar S.Y., Elcicek S., Oztel O.N. Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components. Academic Press; Cambridge, MA, USA: 2013. Development of new antiherpetic drugs based on plant compounds; pp. 245–259. [Google Scholar]
- 219.Bajaj S., Urooj A., Prabhasankar P. Antioxidative properties of mint (Mentha spicata L.) and its application in biscuits. Curr. Res. Nutr. Food Sci. 2016;4:209–216. doi: 10.12944/CRNFSJ.4.3.07. [DOI] [Google Scholar]
- 220.Prakash O., Chandra M., Pant A.K., Rawat D.S. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; Cambridge, MA, USA: 2016. Mint (Mentha spicata L.) Oils; pp. 561–572. [Google Scholar]
- 221.Singh R., Shushni M.A., Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015;8:322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 222.Ben Haj Yahia I., Bouslimi W., Messaoud C., Jaouadi R., Boussaid M., Zaouali Y. Comparative evaluation of Tunisian Mentha L. species essential oils: Selection of potential antioxidant and antimicrobial agents. J. Essent. Oil Res. 2019;31:184–195. doi: 10.1080/10412905.2018.1550021. [DOI] [Google Scholar]
- 223.Benabdallah A., Rahmoune C., Boumendjel M., Aissi O., Messaoud C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. 2016;6:760–766. doi: 10.1016/j.apjtb.2016.06.016. [DOI] [Google Scholar]
- 224.Merat S., Khalili S., Mostajabi P., Ghorbani A., Ansari R., Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig. Dis. Sci. 2010;55:1385–1390. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 225.Džamić A.M., Soković M.D., Ristić M.S., Novaković M., Grujić-Jovanović S., Tešević V., Marin P.D. Antifungal and antioxidant activity of Mentha longifolia (L.) Hudson (Lamiaceae) essential oil. Bot Serb. 2010;34:57–61. [Google Scholar]
- 226.Elmastas M., Telci İ., Akşit H., Erenler R. Comparison of total phenolic contents and antioxidant capacities in mint genotypes used as spices. Turk. J. Bioch. 2015;40:456–462. doi: 10.1515/tjb-2015-0034. [DOI] [Google Scholar]
- 227.Stringaro A., Colone M., Angiolella L. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines. 2018;5:112. doi: 10.3390/medicines5040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Talei G.R., Mohammadi M., Bahmani M., Kopaei M.R. Synergistic effect of Carum copticum and Mentha piperita essential oils with ciprofloxacin, vancomycin, and gentamicin on Gram-negative and Gram-positive bacteria. Int. J. Pharm. Investig. 2017;7:82. doi: 10.4103/jphi.JPHI_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lim H.W., Kim H., Kim J., Bae D., Song K.Y., Chon J.W., Lee J.M., Kim S.H., Kim D.H., Seo K.H. Antimicrobial effect of Mentha piperita (Peppermint) oil against Bacillus cereus, Staphylococcus aureus, Cronobacter sakazakii, and Salmonella Enteritidis in various dairy foods: Preliminary study. J. Milk Sci. Biotechnol. 2018;36:146–154. doi: 10.22424/jmsb.2018.36.3.146. [DOI] [Google Scholar]
- 230.Anwar F., Abbas A., Mehmood T., Gilani A.H., Rehman N.U. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019;33:2548–2570. doi: 10.1002/ptr.6423. [DOI] [PubMed] [Google Scholar]
- 231.Chan Y.S., Cheng L.N., Wu J.H., Chan E., Kwan Y.W., Lee S.M., Leung G.P., Yu P.H., Chan S.W. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2011;19:245–254. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 232.Rodriguez J.M., de Souza A.R., Krüger R.L., Bombardelli M.C., Machado C.S., Corazza M.L. Kinetics, composition and antioxidant activity of burdock (Arctium lappa) root extracts obtained with supercritical CO2 and co-solvent. J. Supercrit. Fluids. 2018;135:25–33. doi: 10.1016/j.supflu.2017.12.034. [DOI] [Google Scholar]
- 233.Ku M.K., Liu H.C., Lin S.R. Efficacy analysis of preserved great burdock essence compounds. Biomark Genom. Med. 2013;5:67–70. doi: 10.1016/j.gmbhs.2013.04.002. [DOI] [Google Scholar]
- 234.Lou Z., Li C., Kou X., Yu F., Wang H., Smith G.M., Zhu S. Antibacterial, antibiofilm effect of Burdock (Arctium lappa L.) leaf fraction and its efficiency in meat preservation. J. Food Prot. 2016;79:1404–1409. doi: 10.4315/0362-028X.JFP-15-576. [DOI] [PubMed] [Google Scholar]
- 235.Kim Y.S., Kim S.H. Physicochemical and antioxidant characteristics of hot water extracts on pre-treatment conditions of Burdock (Arctium lappa L.) J. Korean Soc. Food Sci. Nutr. 2018;47:612–619. doi: 10.3746/jkfn.2018.47.6.612. [DOI] [Google Scholar]
- 236.Yen C.H., Chiu H.F., Huang S.Y., Lu Y.Y., Han Y.C., Shen Y.C., Venkatakrishnan K., Wang C.K. Beneficial effect of Burdock complex on asymptomatic Helicobacter pylori-infected subjects: A randomized, double-blind placebo-controlled clinical trial. Helicobacter. 2018;23:e12469. doi: 10.1111/hel.12469. [DOI] [PubMed] [Google Scholar]
- 237.Tian K., Wang J., Zhang Z., Cheng L., Jin P., Singh S., Prior B.A., Wang Z.X. Enzymatic preparation of fructooligosaccharides-rich burdock syrup with enhanced antioxidative properties. Electron. J. Biotechnol. 2019;40:71–77. doi: 10.1016/j.ejbt.2019.04.009. [DOI] [Google Scholar]
- 238.Ahangarpour A., Oroojan A.A., Heidari H., Ghaedi E., Taherkhani R. Effects of hydro-alcoholic extract from arctium Lappa L. (Burdock) root on gonadotropins, testosterone, and sperm count and viability in male mice with nicotinamide/streptozotocin-induced type 2 diabetes. Malays. J. Med. Sci. 2015;22:25. [PMC free article] [PubMed] [Google Scholar]
- 239.Park M.Y., Park Y.O., Park Y.H. Antioxidant activities of Burdock root (Arctium lappa L.) with various heat treatment conditions. J. Korean Soc. Food Cult. 2018;33:78–85. [Google Scholar]
- 240.Petkova N., Ivanova L., Filova G., Ivanov I., Denev P. Antioxidants and carbohydrate content in infusions and microwave extracts from eight medicinal plants. J. Appl Pharm Sci. 2017;7:055–061. [Google Scholar]
- 241.Pirvu L., Nicorescu I., Hlevca C., Albu B., Nicorescu V. Burdock (Arctium lappa) leaf extracts increase the in vitro antimicrobial efficacy of common antibiotics on gram-positive and gram-negative bacteria. Open Chem. J. 2017;15:92–102. doi: 10.1515/chem-2017-0012. [DOI] [Google Scholar]
- 242.Tonea A., Mandra Badea L.O., Sava S., Vodnar D. Antibacterial and antifungal activity of endodontic intracanal medications. Clujul. Med. 2017;90:344. doi: 10.15386/cjmed-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Keyhanfar M., Nazeri S., Bayat M. Evaluation of antibacterial activities of some medicinal plants, traditionally used in Iran. Iran. J. Pharm Sci. 2012;8:353–358. [Google Scholar]
- 244.Boulekbache-Makhlouf L., Slimani S., Madani K. Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Ind. Crops Prod. 2013;41:85–89. doi: 10.1016/j.indcrop.2012.04.019. [DOI] [Google Scholar]
- 245.Harkat-Madouri L., Asma B., Madani K., Said Z.B., Rigou P., Grenier D., Allalou H., Remini H., Adjaoud A., Boulekbache-Makhlouf L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crop. Prod. 2015;78:148–153. doi: 10.1016/j.indcrop.2015.10.015. [DOI] [Google Scholar]
- 246.De Mendonça Braga N.S., da Costa Silva M., Cunha A.L., Goulart A.E., Sant’Ana L.L., dos Santos A.F. Chemical characterization and biological potential of the essential oil of Eucalyptus globulus Labill. J. Pharm Pharmacol. 2018;6:979–988. doi: 10.17265/2328-2150/2018.12.001. [DOI] [Google Scholar]
- 247.Guleria S., Tiku A.K., Gupta S., Singh G., Koul A., Razdan V.K. Chemical composition, antioxidant activity and inhibitory effects of essential oil of Eucalyptus teretecornis grown in north-western Himalaya against Alternaria alternata. J. Plant. Biochem. Biot. 2012;21:44–50. doi: 10.1007/s13562-011-0073-2. [DOI] [Google Scholar]
- 248.Singh H.P., Kaur S., Negi K., Kumari S., Saini V., Batish D.R., Kohli R.K. Assessment of in vitro antioxidant activity of essential oil of Eucalyptus citriodora (lemon-scented Eucalypt; Myrtaceae) and its major constituents. LWT-Food Sci. Technol. 2012;48:237–241. doi: 10.1016/j.lwt.2012.03.019. [DOI] [Google Scholar]
- 249.Al-Ghzi S.H. Evaluation the hypoglycemic activity and anti-oxidative potential of polyphenol extract of eucalyptus. J. Thi-Qar Sci. 2012;3:107–116. [Google Scholar]
- 250.Amakura Y., Yoshimura M., Sugimoto N., Yamazaki T., Yoshida T. Marker constituents of the natural antioxidant Eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009;73:1060. doi: 10.1271/bbb.80832. [DOI] [PubMed] [Google Scholar]
- 251.Gilles M., Zhao J., An M., Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119:731–737. doi: 10.1016/j.foodchem.2009.07.021. [DOI] [Google Scholar]
- 252.Chaves T.P., Pinheiro R.E., Melo E.S., Soares M.J., Souza J.S., de Andrade T.B., de Lemos T.L., Coutinho H.D. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind. Crop. Prod. 2018;112:70–74. doi: 10.1016/j.indcrop.2017.10.048. [DOI] [Google Scholar]
- 253.Dhakad A.K., Pandey V.V., Beg S., Rawat J.M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018;98:833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 254.Mishra A.K., Sahu N., Mishra A., Ghosh A.K., Jha S., Chattopadhyay P. Phytochemical screening and antioxidant activity of essential oil of Eucalyptus leaf. Pharmacogn. J. 2010;2:25–28. doi: 10.1016/S0975-3575(10)80045-8. [DOI] [Google Scholar]
- 255.Brezáni V., Leláková V., Hassan S.T., Berchová-Bímová K., Nový P., Klouček P., Maršík P., Dall’Acqua S., Hošek J., Šmejkal K. Anti-infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Viruses. 2018;10:360. doi: 10.3390/v10070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sadatrasul M.S., Fiezi N., Ghasemian N., Shenagari M., Esmaeili S., Jazaeri E.O., Abdoli A., Jamali A. Oil-in-water emulsion formulated with eucalyptus leaves extract inhibit influenza virus binding and replication in vitro. AIMS Microbiol. 2017;3:899. doi: 10.3934/microbiol.2017.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Timoszuk M., Bielawska K., Skrzydlewska E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants. 2018;7:108. doi: 10.3390/antiox7080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pająk P., Socha R., Broniek J., Królikowska K., Fortuna T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019;275:69–76. doi: 10.1016/j.foodchem.2018.09.081. [DOI] [PubMed] [Google Scholar]
- 259.Sielicka M., Małecka M. Enhancement of oxidative stability of flaxseed oil through flaxseed, evening primrose and black cumin cake extracts. J. Food Process. Preserv. 2017;41:13070. doi: 10.1111/jfpp.13070. [DOI] [Google Scholar]
- 260.Kolivand M., Aghajani Z. Effects of drought stress on the components of the essential oil of evening primrose (Oenothera macrocarpa) and determination of the biological activities of its extracts. Bulg Chem. Commun. 2016;48:636–640. [Google Scholar]
- 261.Sałaga M., Lewandowska U., Sosnowska D., Zakrzewski P.K., Cygankiewicz A.I., Piechota-Polańczyk A., Sobczak M., Mosinska P., Chen C., Krajewska W.M., et al. Polyphenol extract from evening primrose pomace alleviates experimental colitis after intracolonic and oral administration in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014;387:1069–1078. doi: 10.1007/s00210-014-1025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zaugg J., Potterat O., Plescher A., Honermeier B., Hamburger M. Quantitative analysis of anti-inflammatory and radical scavenging triterpenoid esters in evening primrose seeds. J. Agric. Food Chem. 2006;54:6623–6628. doi: 10.1021/jf0611466. [DOI] [PubMed] [Google Scholar]
- 263.Peschel W., Dieckmann W., Sonnenschein M., Plescher A. High antioxidant potential of pressing residues from evening primrose in comparison to other oilseed cakes and plant antioxidants. Ind. Crop. Prod. 2007;25:44–54. doi: 10.1016/j.indcrop.2006.07.002. [DOI] [Google Scholar]
- 264.Mardani V., Alami M., Arabshahi S., Khodabakhshi R., Ghaderi M. Evaluating antioxidant and antimicrobial activities of phenolic essences extracted from Evening Primrose (Oenothera Biennis) flowers. Iran. Food Sci. Technol. Res. J. 2013;9:182–189. [Google Scholar]
- 265.Marzouk M.S., Moharram F.A., El Dib R.A., El-Shenawy S.M., Tawfike A.F. Polyphenolic profile and bioactivity study of Oenothera speciosa Nutt. aerial parts. Molecules. 2009;14:1456–1467. doi: 10.3390/molecules14041456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Majdinasab N., Namjoyan F., Taghizadeh M., Saki H. The effect of evening primrose oil on fatigue and quality of life in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2018;14:1505. doi: 10.2147/NDT.S149403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lodhia M.H., Bhatt K.R., Thaker V.S. Antibacterial activity of essential oils from palmarosa, evening primrose, lavender and tuberose. Indian J. Pharm. Sci. 2009;71:134. doi: 10.4103/0250-474X.54278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Burzynska-Pedziwiatr I., Bukowiecka-Matusiak M., Wojcik M., Machala W., Bienkiewicz M., Spolnik G., Danikiewicz W., Wozniak L.A. Dual stimulus-dependent effect of Oenothera paradoxa extract on the respiratory burst in human leukocytes: Suppressing for Escherichia coli and phorbol myristate acetate and stimulating for formyl-methionyl-leucyl-phenylalanine. Oxid. Med. Cell Longev. 2014;2014:1–13. doi: 10.1155/2014/764367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Senanayake S.N., Shahidi F. Incorporation of docosahexaenoic acid (DHA) into evening primrose (Oenothera biennis L.) oil via lipase-catalyzed transesterification. Food Chem. 2004;85:489–496. doi: 10.1016/S0308-8146(02)00412-0. [DOI] [Google Scholar]
- 270.Jamilian M., Karamali M., Taghizadeh M., Sharifi N., Jafari Z., Memarzadeh M.R., Mahlouji M., Asemi Z. Vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids. 2016;51:349–356. doi: 10.1007/s11745-016-4123-3. [DOI] [PubMed] [Google Scholar]
- 271.Sayegh F., Elazzazy A., Bellou S., Moustogianni A., Elkady A.I., Baeshen M.N., Aggelis G. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 2016;66:937–948. doi: 10.1007/s13213-015-1176-0. [DOI] [Google Scholar]
- 272.Ratz-Łyko A., Herman A., Arct J., Pytkowska K. Evaluation of antioxidant and antimicrobial activities of Oenothera biennis, Borago officinalis, and Nigella sativa seedcake extracts. Food Sci. Biotechnol. 2014;23:1029–1036. doi: 10.1007/s10068-014-0140-2. [DOI] [Google Scholar]
- 273.Gomez-Flores R., Reyna-Martínez R., Tamez-Guerra P., Quintanilla-Licea R. Antibacterial activity of Oenothera rosea (L’Hér) leaf extracts. J. Adv. Med. Med. Res. 2012;2:396–404. doi: 10.9734/BJMMR/2012/1480. [DOI] [Google Scholar]
- 274.Matsumoto-Nakano M., Nagayama K., Kitagori H., Fujita K., Inagaki S., Takashima Y., amesada M., Kawabata S., Ooshima T. Inhibitory effects of Oenothera biennis (evening primrose) seed extract on Streptococcus mutans and S. mutans-induced dental caries in rats. Caries Res. 2011;45:56–63. doi: 10.1159/000323376. [DOI] [PubMed] [Google Scholar]
- 275.Boneza M.M., Niemeyer E.D. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind. Crop. Prod. 2018;112:783–789. doi: 10.1016/j.indcrop.2018.01.003. [DOI] [Google Scholar]
- 276.Bogdanovic A., Tadic V., Arsic I., Milovanovic S., Petrovic S., Skala D. Supercritical and high pressure subcritical fluid extraction from Lemon balm (Melissa officinalis L., Lamiaceae) J. Supercrit. Fluid. 2016;107:234–242. doi: 10.1016/j.supflu.2015.09.008. [DOI] [Google Scholar]
- 277.Said-Al Ahl H.A., Sabra A.S., Gendy A.S., Astatkie T. Essential oil content and concentration of constituents of Lemon Balm (Melissa officinalis L.) at different harvest dates. J. Essent. Oil-Bear Plants. 2018;21:1410–1417. doi: 10.1080/0972060X.2018.1553636. [DOI] [Google Scholar]
- 278.Rostami H., Kazemi M., Shafiei S. Antibacterial activity of Lavandula officinalis and Melissa officinalis against some human pathogenic bacteria. Asian J. Biochem. 2012;7:133–142. doi: 10.3923/ajb.2012.133.142. [DOI] [Google Scholar]
- 279.Bağdat R.B., Coşge B. The essential oil of lemon balm (Melissa officinalis L.), its components and using fields. J. Fac. Agric. OMU. 2006;21:116–121. [Google Scholar]
- 280.Abdel-Naime W.A., Fahim J.R., Fouad M.A., Kamel M.S. Antibacterial, antifungal, and GC–MS studies of Melissa officinalis. S Afr. J. Bot. 2019;124:228–234. doi: 10.1016/j.sajb.2019.05.011. [DOI] [Google Scholar]
- 281.Moradkhani H., Sargsyan E., Bibak H., Naseri B., Sadat-Hosseini M., Fayazi-Barjin A., Meftahizade H. Melissa officinalis L., a valuable medicine plant: A review. J. Med. Plant. Res. 2010;4:2753–2759. [Google Scholar]
- 282.Duda S.C., Mărghitaş L.A., Dezmirean D., Duda M., Mărgăoan R., Bobiş O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crop. Prod. 2015;77:499–507. doi: 10.1016/j.indcrop.2015.09.045. [DOI] [Google Scholar]
- 283.Luño V., Gil L., Olaciregui M., Jerez R.A., de Blas I., Hozbor F. Antioxidant effect of lemon balm (Melissa officinalis) and mate tea (Ilex paraguensys) on quality, lipid peroxidation and DNA oxidation of cryopreserved boar epididymal spermatozoa. Andrologia. 2015;47:1004–1011. doi: 10.1111/and.12370. [DOI] [PubMed] [Google Scholar]
- 284.Mustafa A.K., Ali M. New steroidal lactones and homomonoterpenic glucoside from fruits of Malva sylvestris L. Acta Pol. Pharm. 2011;68:393–401. [PubMed] [Google Scholar]
- 285.Walter C.Y., Shinwari Z.K., Afzal I.M., Malik R.N. Antibacterial activity in herbal products used in Pakistan. Pak. J. Bot. 2011;43:155–162. [Google Scholar]
- 286.Zare P., Mahmoudi R., Shadfar S., Ehsani A., Afrazeh Y., Saeedan A., Niyazpour F., Pourmand B.S. Efficacy of chloroform, ethanol and water extracts of medicinal plants, Malva sylvestris and Malva neglecta on some bacterial and fungal contaminants of wound infections. J. Med. Plants Res. 2012;6:4550–4552. [Google Scholar]
- 287.Misak J.A., Mohammed H.J., Malik S.N. Screening of antibacterial properties for some Iraqi plants against Salmonella typhimurium. Iraqi J. Vet. Sci. 2011;35:28–35. [Google Scholar]
- 288.Delfine S., Marrelli M., Conforti F., Formisano C., Rigano D., Menichini F., Senatore F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crop. Prod. 2017;98:29–37. doi: 10.1016/j.indcrop.2017.01.016. [DOI] [Google Scholar]
- 289.Sharifi-Rad J., Melgar-Lalanne G., Hernández-Álvarez A.J., Taheri Y., Shaheen S., Kregiel D., Antolak H., Pawlikowska E., Brdar-Jokanović M., Rajkovic J., et al. Malva species: Insights on its chemical composition towards pharmacological applications. Phytother. Res. 2020;34:546–567. doi: 10.1002/ptr.6550. [DOI] [PubMed] [Google Scholar]
- 290.Petkova N., Popova A., Alexieva I. Antioxidant properties and some phytochemical components of the edible medicinal Malva sylvestris L. J. Med. Plant. Res. 2019;7:96–99. [Google Scholar]
- 291.Cecotti R., Bergomi P., Carpana E., Tava A. Chemical characterization of the volatiles of leaves and flowers from cultivated Malva sylvestris var. mauritiana and their antimicrobial activity against the aetiological agents of the European and American foulbrood of honeybees (Apis mellifera) Nat. Prod. Commun. 2016;11:1527–1530. doi: 10.1177/1934578X1601101026. [DOI] [PubMed] [Google Scholar]
- 292.DellaGreca M., Cutillo F., Abrosca B.D., Fiorentino A., Pacifico S., Zarrelli A. Antioxidant and radical scavenging properties of Malva sylvestris. Nat. Prod. Commun. 2009;4:893–896. doi: 10.1177/1934578X0900400702. [DOI] [PubMed] [Google Scholar]
- 293.Anuradha J., Muhtari K., Lone H., Tripathi S., Sanjeevi R. Potentials of herbs on the rescue of influenza prevention and control. J. Chem. Chem. Sci. 2018;8:898–903. doi: 10.29055/jccs/658. [DOI] [Google Scholar]
- 294.Divya B.J., Suman B., Venkataswamy M., Thyagaraju K. A study on phytochemicals, functional groups and mineral composition of Allium sativum (garlic) cloves. Int. J. Curr. Pharm. Res. 2017;9:42–45. doi: 10.22159/ijcpr.2017.v9i3.18888. [DOI] [Google Scholar]
- 295.Shaaf S., Sharma R., Kilian B., Walther A., Özkan H., Karami E., Mohammadi B. Genetic structure and eco-geographical adaptation of garlic landraces (Allium sativum L.) in Iran. Genet. Resour. Crop. Evol. 2014;61:1565–1580. doi: 10.1007/s10722-014-0131-4. [DOI] [Google Scholar]
- 296.Fratianni F., Ombra M.N., Cozzolino A., Riccardi R., Spigno P., Tremonte P., Coppola R., Nazzaro F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.) J. Funct. Foods. 2016;21:240–248. doi: 10.1016/j.jff.2015.12.019. [DOI] [Google Scholar]
- 297.Khan M.S., Quershi N.A., Jabeen F., Asghar M.S., Shakeel M. Analysis of minerals profile, phenolic compounds and potential of Garlic (Allium sativum) as antioxidant scavenging the free radicals. Int. J. Biosci. 2016;8:72–82. [Google Scholar]
- 298.Onyeoziri U.P., Romanus E.N., Onyekachukwu U.I. Assessment of antioxidant capacities and phenolic contents of Nigerian cultivars of onions (Allium cepa L.) and garlic (Allium sativum L.) Pak. J. Pharm. Sci. 2016;29:1183–1188. [PubMed] [Google Scholar]
- 299.Khanum F., Anilakumar K.R., Viswanathan K.R. Anticarcinogenic properties of garlic: A review. Crit. Rev. Food Sci. Nutr. 2004;44:479–488. doi: 10.1080/10408690490886700. [DOI] [PubMed] [Google Scholar]
- 300.Ghasemi K., Bolandnazar S., Tabatabaei S.J., Pirdashti H., Arzanlou M., Ebrahimzadeh M.A., Fathi H. Antioxidant properties of garlic as affected by selenium and humic acid treatments. New Zeal. J. Crop. Hort. 2015;43:173–181. doi: 10.1080/01140671.2014.991743. [DOI] [Google Scholar]
- 301.Batcioglu K., Yilmaz Z., Satilmis B., Uyumlu A.B., Erkal H.S., Yucel N., Gunal S., Serin M., Demirtas H. Investigation of in vivo radioprotective and in vitro antioxidant and antimicrobial activity of garlic (Allium sativum) Eur. Rev. Med. Pharmacol. Sci. 2012;16:47–57. [PubMed] [Google Scholar]
- 302.Chung L.Y. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food. 2006;9:205–213. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- 303.Chen S., Shen X., Cheng S., Li P., Du J., Chang Y., Meng H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE. 2013;8:79730. doi: 10.1371/journal.pone.0079730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Benkeblia N. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Braz. Arch. Biol. Technol. 2005;48:753–759. doi: 10.1590/S1516-89132005000600011. [DOI] [Google Scholar]
- 305.Daliri E.B., Kim S.H., Park B.J., Kim H.S., Kim J.M., Kim H.S., Oh D.H. Effects of different processing methods on the antioxidant and immune stimulating abilities of garlic. Food Sci. Nutr. 2019;7:1222–1229. doi: 10.1002/fsn3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sadrefozalayi S., Aslanipour B., Alan M., Calan M. Determination and comparison of in vitro radical scavenging activity of both garlic oil and aqueous garlic extracts and their in vivo antioxidant effect on schistosomiasis disease in mice. Turk. J. Agric. Food Sci. Technol. (TURJAF) 2018;6:820–827. doi: 10.24925/turjaf.v6i7.820-827.1647. [DOI] [Google Scholar]
- 307.Suleria H.A., Butt M.S., Khalid N., Sultan S., Raza A., Aleem M., Abbas M. Garlic (Allium sativum): Diet based therapy of 21st century–A review. Asian Pac. J. Trop Dis. 2015;5:271–278. doi: 10.1016/S2222-1808(14)60782-9. [DOI] [Google Scholar]
- 308.Mandal S.K., Das A., Dey S., Sahoo U., Bose S., Bose A., Dhiman N., Madan S., Ramadan M.A. Bioactivities of Allicin and related organosulfur compounds from garlic: Overview of the literature since 2010. Egypt J. Chem. 2019;62:2–3. doi: 10.21608/ejchem.2019.15787.1954. [DOI] [Google Scholar]
- 309.Rawat S. Evaluation of synergistic effect of Ginger, Garlic, Turmeric extracts on the antimicrobial activity of drugs against bacterial phatogens. Int. J. Biopharm. 2015;6:60–65. [Google Scholar]
- 310.Yadav S., Trivedi N.A., Bhatt J.D. Antimicrobial activity of fresh garlic juice: An in vitro study. Ayurveda. 2015;36:203. doi: 10.4103/0974-8520.175548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ismail R.M., Saleh A.H., Ali K.S. GC-MS analysis and antibacterial activity of garlic extract with antibiotic. J. Med. Plants Stud. 2020;8:26–30. [Google Scholar]
- 312.Bakri I.M., Douglas C.W. Inhibitory effect of garlic extract on oral bacteria. Arch. Oral. Biol. 2005;50:645–651. doi: 10.1016/j.archoralbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 313.Kuzelov A., Andronikov D., Taskov N., Sofijanova E., Saneva D. Oxidative stability effect of basil, garlic and muscat blossom extracts on lipids and microbiology of minced meat. Cr. Acad. Bulg. Sci. 2017;70:1227–1236. [Google Scholar]
- 314.Gruhlke M., Nicco C., Batteux F., Slusarenko A. The effects of allicin, a reactive sulfur species from garlic, on a selection of mammalian cell lines. Antioxidants. 2017;6:1. doi: 10.3390/antiox6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ge L., Xu Y., Jiang X., Xia W., Jiang Q. Broad-spectrum inhibition of proteolytic enzymes by allicin and application in mitigating textural deterioration of ice-stored grass carp (Ctenopharyngodon idella) fillets. Int. J. Food Sci. Tech. 2016;51:902–910. doi: 10.1111/ijfs.13047. [DOI] [Google Scholar]
- 316.Mehrbod P., Amini E., Tavassoti-Kheiri M. Antiviral activity of garlic extract on influenza virus. Iran. J. Virol. 2009;3:19–23. doi: 10.21859/isv.3.1.19. [DOI] [Google Scholar]
- 317.Kianbakht S., Jahaniani F. Evaluation of antibacterial activity of Tribulus terrestris L. growing in Iran. Iran. J. Pharmacol. Ther. 2003;2:22–24. [Google Scholar]
- 318.Gupta C., Garg A.P., Uniyal R.C., Kumari A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr. J. Microbiol. Res. 2008;2:247–251. [Google Scholar]
- 319.Negi P.S., Jayaprakasha G.K., Rao L.J.M., Sakariah K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 320.Wang X., Shen Y., Thakur K., Han J., Zhang J.G., Hu F., Wei Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules. 2020;25:3955. doi: 10.3390/molecules25173955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Benameur Q., Gervasi T., Pellizzeri V., Pľuchtová M., Tali-Maama H., Assaous F., Guettou B., Rahal K., Gruľová D., Dugo G., et al. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with cefotaxime against bla ESBL producing multidrug resistant Enterobacteriaceae isolates. Nat. Prod. Res. 2019;33:2647–2654. doi: 10.1080/14786419.2018.1466124. [DOI] [PubMed] [Google Scholar]
- 322.Gheisari Z., Kalani L., Khaledi M., Nouri A., Eshaghi Milasi Y., Hossainy N., Soleymani A. Antibacterial Effects of Hydro-alcoholic Extract of Pennyroyal, Cinnamon and Rhubarb on Klebsiella pneumoniae and Staphylococcus aureus: An In vitro Study. J. Pharm. Res. Inter. 2019;17:1–6. doi: 10.9734/jpri/2019/v28i630218. [DOI] [Google Scholar]
- 323.Diao W.R., Hu Q.P., Zhang H., Xu J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 324.Alkuraishy H.M., Al-Gareeb A.I., Albuhadilly A.K., Alwindy S. In vitro assessment of the antibacterial activity of Matricaria chamomile alcoholic extract against pathogenic bacterial strains. Microbiol. Res. J. Int. 2015;7:55–61. doi: 10.9734/BMRJ/2015/16263. [DOI] [Google Scholar]
- 325.Al-Sum B.A., Al-Arfaj A.A. Antimicrobial activity of the aqueous extract of mint plant. Sci. J. Clin. Med. 2013;2:110–113. doi: 10.11648/j.sjcm.20130203.19. [DOI] [Google Scholar]
- 326.Akarca G., Tomar O. The Antibacterial effects of the different extracts of Oenothera biennis and Origanum minutiflorum O. Schwarz et. PH davis on food-borne pathogenic bacteria. Selcuk. J. Agric. Food Sci. 2020;34:78–83. [Google Scholar]
- 327.Gutierrez J., Barry-Ryan C., Bourke P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26:142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 328.Petropoulos S., Fernandes Â., Barros L., Ciric A., Sokovic M., Ferreira I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018;245:7–12. doi: 10.1016/j.foodchem.2017.10.078. [DOI] [PubMed] [Google Scholar]
- 329.Chaieb K., Hajlaoui H., Zmantar T., Kahla-Nakbi A.B., Rouabhia M., Mahdouani K., Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 330.Li Y.H., Lai C.Y., Su M.C., Cheng J.C., Chang Y.S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. J. Ethnopharmacol. 2019;241:112013. doi: 10.1016/j.jep.2019.112013. [DOI] [PubMed] [Google Scholar]
- 331.Malik A., Mehmood M.D., Anwar H., Sultan U. In vivo antiviral potential of crude extracts derived from Tribulus terrestris against newcastle disease virus. J. Drug Deliv. Ther. 2018;8:149–154. doi: 10.22270/jddt.v8i6.2114. [DOI] [Google Scholar]
- 332.Wang P., Su Z., Yuan W., Deng G., Li S. Phytochemical constituents and pharmacological activities of Eryngium L. (Apiaceae) Pharm. Crop. 2012;3:99–120. doi: 10.2174/2210290601203010099. [DOI] [Google Scholar]
- 333.Lane T., Anantpadma M., Freundlich J.S., Davey R.A., Madrid P.B., Ekins S. The natural product eugenol is an inhibitor of the ebola virus in vitro. Pharm. Res. 2019;36:104. doi: 10.1007/s11095-019-2629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nisar T., Iqbal M., Raza A., Safdar M., Iftikhar F., Waheed M. Turmeric: A promising spice for phytochemical and antimicrobial activities. Am. Eur. J. Agric. Environ. Sci. 2015;15:1278–1288. [Google Scholar]
- 335.Goswami D., Kumar M., Ghosh S.K., Das A. Natural product compounds in alpinia officinarum and ginger are potent SARS-CoV-2 papain-like protease inhibitors. Chem. Rxiv. 2020 doi: 10.26434/chemrxiv.12071997.v1. [DOI] [Google Scholar]
- 336.Walther C., Schmidtke M. Anti-rhinovirus and anti-influenza virus activities of mucoactive secretolytic agents and plant extracts—A comparative in vitro study. Res. Sq. 2020 doi: 10.21203/rs.2.23461/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ben-Shabat S., Yarmolinsky L., Porat D., Dahan A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2019;10:354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Badgujar S.B., Patel V.V., Bandivdekar A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014;2014:1–32. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sokolova A.S., Yarovaya O.I., Semenova M.D., Shtro A.A., Orshanskaya I.R., Zarubaev V.V., Salakhutdinov N.F. Synthesis and in vitro study of novel borneol derivatives as potent inhibitors of the influenza A virus. MedChemComm. 2017;8:960–963. doi: 10.1039/C6MD00657D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang S.X., Wang Y., Lu Y.B., Li J.Y., Song Y.J., Nyamgerelt M., Wang X.X. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J. Integr. Med. 2020;18:275–283. doi: 10.1016/j.joim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu J., Zhang Y. Traditional Chinese Medicine treatment of COVID-19. Complement. Ther. Clin. Pract. 2020;39:101–165. doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sharma A.D. Eucalyptol (1, 8 cineole) from Eucalyptus essential oil a potential inhibitor of covid 19 corona virus infection by molecular docking studies. Preprints. 2020 doi: 10.20944/preprints202003.0455.v1. [DOI] [Google Scholar]
- 343.Tamura S., Yang G.M., Koitabashi T., Matsuura Y., Komoda Y., Kawano T., Murakami N. Oenothein B, dimeric hydrolysable tannin inhibiting HCV invasion from Oenothera erythrosepala. J. Nat. Med. 2019;73:67–75. doi: 10.1007/s11418-018-1239-1. [DOI] [PubMed] [Google Scholar]
- 344.Sampangi-Ramaiah M.H., Vishwakarma R., Shaanker R.U. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020;118:1087–1092. [Google Scholar]
- 345.Tsai Y., Cole L.L., Davis L.E., Lockwood S.J., Simmons V., Wild G.C. Antiviral properties of garlic: In vitro effects on influenza B, herpes simplex and coxsackie viruses. Planta Med. 1985;51:460–461. doi: 10.1055/s-2007-969553. [DOI] [PubMed] [Google Scholar]
- 346.Yamaguchi Y., Honma R., Yazaki T., Shibuya T., Sakaguchi T., Uto-Kondo H., Kumagai H. Sulfuric odor precursor S-allyl-L-cysteine sulfoxide in garlic induces detoxifying enzymes and prevents hepatic injury. Antioxidants. 2019;8:385. doi: 10.3390/antiox8090385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kicel A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology. Antioxidants. 2020;9:1002. doi: 10.3390/antiox9101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lee M.S., Chyau C.C., Wang C.P., Wang T.H., Chen J.H., Lin H.H. Flavonoids identification and pancreatic beta-cell protective effect of Lotus Seedpod. Antioxidants. 2020;9:658. doi: 10.3390/antiox9080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kim Y.S., Kim J., Kim C.S., Lee I.S., Jo K., Jung D.H., Lee Y.M., Kim J.S. The herbal combination cpa4-1 inhibits changes in retinal capillaries and reduction of retinal occludin in db/db mice. Antioxidants. 2020;9:627. doi: 10.3390/antiox9070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kim H.J., Kim D., Yoon H., Choi C.S., Oh Y.S., Jun H.S. Prevention of Oxidative Stress-Induced Pancreatic Beta Cell Damage by Broussonetia kazinoki Siebold Fruit Extract via the ERK-Nox4 Pathway. Antioxidants. 2020;9:406. doi: 10.3390/antiox9050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Mussard E., Jousselin S., Cesaro A., Legrain B., Lespessailles E., Esteve E., Berteina-Raboin S., Toumi H. Andrographis paniculata and its bioactive diterpenoids against inflammation and oxidative stress in keratinocytes. Antioxidants. 2020;9:530. doi: 10.3390/antiox9060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Tanaka Y., Ito T., Tsuji G., Furue M. Baicalein inhibits benzo [a] pyrene-induced toxic response by downregulating src phosphorylation and by upregulating NRF2-HMOX1 system. Antioxidants. 2020;9:507. doi: 10.3390/antiox9060507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Spiegel M., Andruniów T., Sroka Z. Flavones’ and flavonols’ antiradical structure–activity relationship—A quantum chemical study. Antioxidants. 2020;9:461. doi: 10.3390/antiox9060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Liou C.J., Chen Y.L., Yu M.C., Yeh K.W., Shen S.C., Huang W.C. Sesamol alleviates airway hyperresponsiveness and oxidative stress in asthmatic mice. Antioxidants. 2020;9:295. doi: 10.3390/antiox9040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chen C.C., Li H.Y., Leu Y.L., Chen Y.J., Wang C.J., Wang S.H. Corylin inhibits vascular cell inflammation, proliferation and migration and reduces atherosclerosis in apoe-deficient mice. Antioxidants. 2020;9:275. doi: 10.3390/antiox9040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Li X., Zeng J., Liu Y., Liang M., Liu Q., Li Z., Zhao X., Chen D. Inhibitory effect and mechanism of action of quercetin and quercetin diels-alder anti-dimer on erastin-induced ferroptosis in bone marrow-derived mesenchymal stem cells. Antioxidants. 2020;9:205. doi: 10.3390/antiox9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bacchetti T., Morresi C., Bellachioma L., Ferretti G. Antioxidant and pro-oxidant properties of carthamus tinctorius, hydroxy safflor yellow A, and safflor yellow A. Antioxidants. 2020;9:119. doi: 10.3390/antiox9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jung T.Y., Lee A.Y., Song J.H., Lee M.Y., Lim J.O., Lee S.J., Ko J.W., Shin N.R., Kim J.C., Shin I.S., et al. Scrophularia koraiensis nakai attenuates allergic airway inflammation via suppression of NF-κB and enhancement of Nrf2/HO−1 signaling. Antioxidants. 2020;9:99. doi: 10.3390/antiox9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Iciek M., Bilska-Wilkosz A., Górny M., Sokołowska-Jeżewicz M., Kowalczyk-Pachel D. The effects of different garlic-derived allyl sulfides on anaerobic sulfur metabolism in the mouse kidney. Antioxidants. 2016;5:46. doi: 10.3390/antiox5040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Junsi M., Takahashi Yupanqui C., Usawakesmanee W., Slusarenko A., Siripongvutikorn S. Thunbergia laurifolia leaf extract increased levels of antioxidant enzymes and protected human cell-lines in vitro against cadmium. Antioxidants. 2020;9:47. doi: 10.3390/antiox9010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Taghouti M., Martins-Gomes C., Schäfer J., Santos J.A., Bunzel M., Nunes F.M., Silva A.M. Chemical characterization and bioactivity of extracts from Thymus mastichina: A Thymus with a distinct salvianolic acid composition. Antioxidants. 2020;9:34. doi: 10.3390/antiox9010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sidor A., Drożdżyńska A., Brzozowska A., Szwengiel A., Gramza-Michałowska A. The effect of plant additives on the stability of polyphenols in cloudy and clarified juices from Black Chokeberry (Aronia melanocarpa) Antioxidants. 2020;9:801. doi: 10.3390/antiox9090801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Majdi C., Pereira C., Dias M.I., Calhelha R.C., Alves M.J., Rhourri-Frih B., Charrouf Z., Barros L., Amaral J.S., Ferreira I.C. Phytochemical characterization and bioactive properties of Cinnamon Basil (Ocimum basilicum cv.‘Cinnamon’) and Lemon Basil (Ocimum× citriodorum) Antioxidants. 2020;9:369. doi: 10.3390/antiox9050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Horn T., Bettray W., Noll U., Krauskopf F., Huang M.R., Bolm C., Slusarenko A.J., Gruhlke M.C. The sulfilimine analogue of allicin, s-allyl-s-(s-allyl)-n-cyanosulfilimine, is antimicrobial and reacts with glutathione. Antioxidants. 2020;9:1086. doi: 10.3390/antiox9111086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ansary J., Forbes-Hernández T.Y., Gil E., Cianciosi D., Zhang J., Elexpuru-Zabaleta M., Simal-Gandara J., Giampieri F., Battino M. Potential health benefit of garlic based on human intervention studies: A brief overview. Antioxidants. 2020;9:619. doi: 10.3390/antiox9070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bussmann R.W., Malca-García G., Glenn A., Sharon D., Chait G., Díaz D., Pourmand K., Jonat B., Somogy S., Guardado G., et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010;132:101–108. doi: 10.1016/j.jep.2010.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Marino M., Bersani C., Comi G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999;62:1017–1023. doi: 10.4315/0362-028X-62.9.1017. [DOI] [PubMed] [Google Scholar]
- 368.Bakhsheshi-Rad H.R., Chen X., Ismail A.F., Aziz M., Abdolahi E., Mahmoodiyan F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019;30:1333–1339. doi: 10.1002/pat.4563. [DOI] [Google Scholar]
- 369.Hadisi Z., Bakhsheshi-Rad H.R., Walsh T., Dehghan M.M., Farzad-Mohajeri S., Gholami H., Diyanoush A., Pagan E., Akbari M. In vitro and in vivo evaluation of silk fibroin-hardystonite-gentamicin nanofibrous scaffold for tissue engineering applications. Polym. Test. 2020;91:106698. doi: 10.1016/j.polymertesting.2020.106698. [DOI] [Google Scholar]
- 370.Bakhsheshi-Rad H.R., Ismail A.F., Aziz M., Akbari M., Hadisi Z., Omidi M., Chen X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020;149:513–521. doi: 10.1016/j.ijbiomac.2020.01.139. [DOI] [PubMed] [Google Scholar]
- 371.Hadisi Z., Farokhi M., Bakhsheshi-Rad H.R., Jahanshahi M., Hasanpour S., Pagan E., Dolatshahi-Pirouz A., Zhang Y.S., Kundu S.C., Akbari M. Hyaluronic Acid (HA)-based Silk Fibroin/Zinc oxide core–shell electrospun dressing for burn wound management. Macromol. Biosci. 2020;19:1900328. doi: 10.1002/mabi.201900328. [DOI] [PubMed] [Google Scholar]
- 372.Bakhsheshi-Rad H.R., Akbari M., Ismail A.F., Aziz M., Hadisi Z., Pagan E., Daroonparvar M., Chen X. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-akermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coat. Technol. 2019;377:124898. doi: 10.1016/j.surfcoat.2019.124898. [DOI] [Google Scholar]
- 373.Bakhsheshi-Rad H.R., Ismail A.F., Aziz M., Hadisi Z., Omidi M., Chen X. Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants. Ceram. Int. 2019;45:11883–11892. doi: 10.1016/j.ceramint.2019.03.071. [DOI] [Google Scholar]
- 374.Bakhsheshi-Rad H.R., Hadisi Z., Hamzah E., Ismail A.F., Aziz M., Kashefian M. Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy. Mater. Lett. 2017;207:179. doi: 10.1016/j.matlet.2017.07.072. [DOI] [Google Scholar]
- 375.Bakhsheshi-Rad H.R., Ismail A.F., Aziz M., Akbari M., Hadisi Z., Daroonparvar M., Chen X.B. Antibacterial activity and in vivo wound healing evaluation of polycaprolactone-gelatin methacryloyl-cephalexin electrospun nanofibrous. Mater. Lett. 2019;256:126618. doi: 10.1016/j.matlet.2019.126618. [DOI] [Google Scholar]
- 376.Bakhsheshi-Rad H.R., Hadisi Z., Ismail A.F., Aziz M., Akbari M., Berto F., Chen X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020;82:106298. doi: 10.1016/j.polymertesting.2019.106298. [DOI] [Google Scholar]
- 377.Percival S.S., Vanden Heuvel J.P., Nieves C.J., Montero C., Migliaccio A.J., Meadors J. Bioavailability of herbs and spices in humans as determined by ex vivo inflammatory suppression and DNA strand breaks. J. Am. Coll. Nutr. 2012;31:288–294. doi: 10.1080/07315724.2012.10720438. [DOI] [PubMed] [Google Scholar]
- 378.Bhattaram V.A., Graefe U., Kohlert C., Veit M., Derendorf H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine. 2002;9:1–33. doi: 10.1078/1433-187X-00210. [DOI] [PubMed] [Google Scholar]
- 379.Kohlert C., Schindler G., März R.W., Abel G., Brinkhaus B., Derendorf H., Gräfe E.U., Veit M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002;42:731–737. doi: 10.1177/009127002401102678. [DOI] [PubMed] [Google Scholar]
- 380.Brunner M., Davies D., Martin W., Leuratti C., Lackner E., Müller M. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects. Br. J. Clin. Pharmacol. 2011;71:852. doi: 10.1111/j.1365-2125.2011.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 382.Rahman M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007;10:245–268. doi: 10.1080/10942910601113327. [DOI] [Google Scholar]