Abstract
Exposure to air pollution has been linked to elevated blood pressure (BP) and hypertension, but most research has focused on short-term (hours, days, or months) exposures at relatively low concentrations. We examined the associations between long-term (a 3-year average) concentrations of outdoor PM2.5 and household air pollution (HAP) from cooking with solid fuels with BP and hypertension in the Prospective Urban and Rural Epidemiology (PURE) study. Outdoor PM2.5 exposures were estimated at year of enrollment for 137,809 adults aged 35-70 years from 640 urban and rural communities in 21 countries using satellite and ground-based methods. Primary use of solid fuel for cooking was used as an indicator of HAP exposure, with analyses restricted to rural participants (n= 43,313) in 27 study centers in 10 countries. BP was measured following a standardized procedure and associations with air pollution examined with mixed-effect regression models, after adjustment for a comprehensive set of potential confounding factors. Baseline outdoor PM2.5 exposure ranged from 3 to 97 μg/m3 across study communities and was associated with an increased odds ratio (OR) of 1.04 (95% CI: 1.01, 1.07) for hypertension, per 10 μg/m3 increase in PM2.5. This association demonstrated non-linearity and was strongest for the fourth (PM2.5 > 62 μg/m3) compared to the first (PM2.5 < 14 μg/m3) quartiles (OR=1.36, 95% CI: 1.10, 1.69). Similar non-linear patterns were observed for SBP (β=2.15, 95% CI: −0.59, 4.89), DBP (β=1.35, 95% CI: −0.20, 2.89) while there was no overall increase in ORs across the full exposure distribution. Individuals who used solid fuels for cooking had lower BP measures compared to clean fuel users (e.g. 34% of solid fuels users compared to 42% of clean fuel users had hypertension), and even in fully adjusted models had slightly decreased odds of hypertension (OR = 0.93; 95% CI: 0.88, 0.99) and reductions in systolic (−0.51 mmHg; 95% CI: −0.99, −0.03) and diastolic (−0.46 mmHg; 95% CI: −0.75, −0.18) BP. In this large international multi-center study, chronic exposures to outdoor PM2.5 was associated with increased BP and hypertension while there were small inverse associations with HAP.
Keywords: Air pollution, global health, household, hypertension, blood pressure, cardiovascular
Introduction
High blood pressure (BP) is considered to be the leading global risk factor for overall disease burden (1–3). Exposure to both outdoor (4–10) and household air pollution (HAP) (11–14) has been linked to elevated BP. However, studies of outdoor PM2.5 (particles below 2.5 microns in aerodynamic diameter) and BP have focused on short-term exposures and been conducted mainly in high income countries (4; 9; 15; 16), while most studies of HAP and BP have included relatively small study populations and been restricted to specific communities or populations (13; 14).
The overall evidence regarding outdoor PM2.5 and BP is less established for chronic exposures (4; 6–10; 17), with very limited data from the developing regions of the world which bears some of the highest PM2.5-cardiovascular disease burden (18; 19). Only a few studies, mostly focused on specific communities or sub-populations, have demonstrated associations between HAP and increased BP (13; 14), and only one of which included populations from multiple regions of the world (14). In this and other HAP studies, the estimated effect size of HAP on BP and hypertension has been small or weak (13; 14).
To further understand the potential role of long-term exposure to outdoor PM2.5 and HAP on BP, we leveraged the Prospective Urban and Rural Epidemiological (PURE) study and examined outdoor PM2.5 for 137,809 adults aged 35 to 70 years from 640 urban and rural communities in 21 countries. We also examined HAP from solid fuel use for cooking in a subset of 43,313 individuals living in rural communities where more than 10% of participants used solid fuels. These cross-sectional analyses capture an extremely large range of outdoor PM2.5 exposures, various household cooking settings, and diverse individual and community characteristics, thus contributing important new information on the relationship between air pollution with BP and hypertension.
Methods
Study design and population:
We included the original 141,471 PURE study participants recruited between 2001 (India) and 2014 (Saudi Arabia) from 640 urban and rural communities in 21 low-, middle-, and high-income countries. After excluding participants with incomplete systolic and diastolic BP measurements (~3%), the final population used in this analysis involved 137,809 adults aged 35-70 years (Table 1). PURE study countries were purposively selected to reflect varied income regions of the world, with more emphasis placed on low- and middle-income regions. Study “communities” in each country represented neighborhoods in urban areas and small villages in rural areas, but were not meant to be representative of the country. However, PURE was designed to achieve a representative sample of adults and households in each study community (20).
Table 1:
Selected demographic, BP, exposure, and other health characteristics of the study participants by outdoor PM2.5 quartiles and solid fuel use for cooking (HAP analysis restricted to rural communities).
| PM2.5 quartile (μg/m3) | PM2.5 Analysis | HAP Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Entire sample (n=137,809) | Q1 (2.6-14.0) | Q2 (14.0-25.4) | Q3 (25.4-62.0) | Q4 (62.0-97.0) | Entire sample (n=43313) | Solid fuel (n=30,008) | Electricity/Gas (n=13,305) | |
| Systolic BP (mmHg) [(sd)] | 131.4 (21.7) | 132.6 (21.4) | 128.9 (21.7) | 130.8 (21.6) | 133 (21.9) | 131.1 (22.9) | 130.1 (23.1) | 133.3 (22.1) |
| Diastolic BP (mmHg) [(sd)] | 81.7 (12.4) | 82.3 (12.5) | 80 (12.2) | 81.8 (12.4) | 82.8 (12.2) | 81.8 (13) | 81.1 (13) | 83.2 (12.7) |
| Age (years) [(sd)] | 50.6 (9.8) | 51.9 (9.5) | 50 (9.8) | 49.9 (9.7) | 50.7 (9.9) | 49.5 (9.8) | 49.4 (9.8) | 49.6 (9.6) |
| BMI (Kg/m2) [(sd)] | 25.9 (5.2) | 27.2 (5.5) | 25.8 (5.8) | 25.6 (4.9) | 24.8 (4.2) | 23.8 (4.5) | 23.2 (4.3) | 25.2 (4.6) |
| % Rural | 46.2 | 58.4 | 36.7 | 49.0 | 40.6 | na | na | na |
| % Women | 58.0 | 58.2 | 58.2 | 58.1 | 57.0 | 42.4 | 57.6 | 57.8 |
| % Dirty fuel a | 28.7 | 14.0 | 28.5 | 38.2 | 33.1 | na | na | na |
| % Current smokers | 20.9 | 18.0 | 22.7 | 22.9 | 21.1 | 25.1 | 26.7 | 22.6 |
| % Hypertension | 40.2 | 44.5 | 36.2 | 38.1 | 41.8 | 36.7 | 34.2 | 42.2 |
| % Diabetes | 8.0 | 6.9 | 11.1 | 7.0 | 7.2 | 4.7 | 3.8 | 6.7 |
| % Anti-hypertensive medication | 13.8 | 17.8 | 12.9 | 11.7 | 13.0 | 8.7 | 6.7 | 13.2 |
| PM2.5 (μg/m3) [(sd)] | 36.2 (25.9) | 9.5 (2.5) | 19.6 (3.3) | 43.3 (12.8) | 73.2 (9.9) | 40.2 (24.7) | 41.1 (23.7) | 38.0 (26.6) |
Solid fuel + kerosene
At study entry, a comprehensive set of individual, household, and community-level information was collected (see Teo et al (20) and Yusuf et al (3)). This included individual socio-demographic characteristics, lifestyle behaviors (e.g. smoking, physical activity and dietary profiles), medical history, anthropometric measures and BP. In addition, household data were collected on cooking methods as well as fuel types, which were used as a surrogate for HAP. Hamilton Health Sciences Research Ethics Board, Oregon State University Research Ethics Board, the University of British Columbia Behavioral Research Ethics Board (H14-02982), and the local ethics committees in the participating countries approved this study.
BP measurement:
Sitting BP during the morning was measured at baseline by trained research assistants following a standardized procedure using Omron digital BP measuring devices (Omron HEM-757) (2; 21). The measurements occurred for different amounts of time (days to months) in each study community depending on the number of participants and the size of the local field staff. Considering that BP readings could be influenced by subject’s immediate activities and “white coat effect” (22), subjects were asked not to smoke, ingest food or caffeine beverages, or exercise (including stair climbing) in the previous 30 minutes prior to the time of measurement. Also, just before the measurement, subjects were made to rest quietly for at least 5 minutes. Two systolic and diastolic BP measurements in the right arm were taken about one minute apart using the brachial artery. The mean of the two measurements was used for all analyses (20). We assumed that these home BP measurements were representative of the participants’ average BP.
We assessed hypertension as an average systolic BP ≥ 140 mm Hg and average diastolic BP ≥ 90 mm Hg, or reported use of anti-hypertensive medication, which was defined as regular use of any or combination of angiotensin-converting enzyme inhibitors, diuretics, beta-blockers, angiotensin-receptor blockers, calcium-channel blockers, α-blockers, and other BP lowering medications. We also assessed pulse pressure (PP=systolic-diastolic BP) and mean arterial pressure (MAP=PP/3+diastolic BP) (6), to explore possible mechanisms through which exposures may affect BP. Individual BP records (continuous) and the hypertension status (yes vs. no) were analyzed separately.
Community outdoor PM2.5 exposure assessment:
PM2.5 concentrations were from a 1 x 1 km global model created by van Donkelaar et al (23). Briefly, the estimates were from a geographically weighted regression model using data from various satellite-, simulation-, and ground monitor-based sources. The raw satellite and ground monitor input data covered years 2001-2013 and valid model predictions were made for years 1999-2015, covering the study enrollment period (2001 – 2014). The model prediction of out-of-sample cross-validated PM2.5 concentrations from available ground monitors was R2 = 0.81. We assigned annual PM2.5 concentrations (from 1 × 1 km resolution) to the 640 PURE communities with each estimate representing a 3-year average centered on the year the community was enrolled in the study. For example, a community which was enrolled in 2011, would be assigned annual PM2.5 concentrations of the average of the 2010, 2011, and 2012 means. As all study subjects were established residents in the community prior to the study baseline (and had indicated their intention to remain into the foreseeable future), we assumed that the 3-year mean exposure centered on the enrollment year was reasonable estimates of their long-term PM2.5 exposures.
Household air pollution assessment:
Questionnaires completed at study enrollment were used to collect information on household characteristics. We used records on households’ primary cooking fuel type as an indicator of HAP exposure in terms of solid (coal, charcoal, wood, agriculture/crop products, shrub/grass and animal dung) vs. clean fuel (electricity and gas). This is a common proxy indicator for HAP exposure (24), and was our a-priori exposure measure for HAP.
Statistical analyses:
Mixed-effect linear and logistic regression models were used to examine the association of community chronic average PM2.5 levels and HAP exposure with individual BP measurements and hypertension status (yes/no). We adjusted for numerous covariates selected a priori from existing literature on BP and air pollution. To accommodate the clustered nature of the PURE study design (i.e. individuals nested within communities nested within countries), and to capture unmeasured variables at such large geographic scales, we included a nested random effect for country and community or center as specified in the following models:
| (A) |
| (B) |
where BP is the mean systolic BP, diastolic BP, PP, or MAP (mm Hg); X is a vector of individual-, household-, and community-level covariates; b and λ are country and community (equation A) or center (equation B) random intercepts; β0, β and γ are regression coefficients. We did not include random effects for household to account for multiple subjects from the same household as our results remained identical with or without this factor. For the same reason, we did not include the year of community enrollment as a way to control for potential time trends in the PM data.
We assessed model sensitivity in both the outdoor (Equation A) and HAP models (Equation B). First, we specified a base model (Model 1) which included age (years), gender (male, female), and race/ethnicity; and then added progressively more groups of socioeconomic and clinical covariates in subsequent models. Model 2 included factors related to socioeconomic status, involving education (none, primary, secondary/high school/higher secondary, trade school, college/university, unknown), marital status (married, not married), and a household wealth index (based on household assets, and calculated separately for each country). The fully adjusted model (Model 3) further included cardiovascular disease (CVD) risk factors of smoking status (never smoked, former smoker, current smoker), alcohol use (never used, former user, current user), body mass index (BMI; Kg/m2), alternative healthy eating index (a composite indicator for overall diet quality based on the dietary guidelines for Americans, computed for each household)(25), as well as community-level factors comprising of temperature (°C), geographical latitude (26), country income-level, and location of residence (urban, rural), in addition to the use of BP lowering medication (medication use, no medication use). All models included random intercepts for country and community or study center. Together, these models contained a vast amount of individual-, household-, and community-level covariates on participants from both rural and urban communities, and thus reduced the possibility of omitted variable bias or residual confounding.
Through stratified analyses for both the outdoor (Equation A) and HAP models (Equation B), we used the full model (Model 3) to explore potential differences in effect estimates by gender, urban/rural residence (equation A/outdoor model only), hypertension status (equation A/outdoor model only), use of anti-hypertensive medication, body mass index (BMI, categorized as underweight, normal weight, overweight, and obese), smoking, alcohol use, age, and CVD and diabetes mellitus disease status. Additional analysis was conducted for participants who were free of CVD, diabetes mellitus, and did not take BP medication.
The HAP analysis was restricted to rural communities in study centers where >10% of study participants used solid fuel for cooking. This was done to ensure a balanced sample, as most high- and upper-middle income countries contained in the outdoor analysis do not use solid fuel, and even in low-income countries the vast majority of solid fuel use by PURE participants was in the rural communities (93%). Those who used kerosene (1.2%) or other unclassified fuel types (0.7%) for cooking were also excluded, leaving 43 313 rural participants who cooked primarily with either solid or clean fuels for this analysis.
We also conducted additional sensitivity analysis for HAP by pooling center-specific models using random-effects meta-analysis. This was done to explore the potential heterogeneity of HAP and BP results by region, since HAP exposure could vary by unmeasured household or community characteristics, fuel conditions/types, cooking practices, customs, etc. Thus, the adjusted pooled effect estimates represent the weighted mean of the center-specific effect estimates, after assigning weights based on the inverse variance which included both within- and between-center variances.
We further explored the impact of joint exposures from both outdoor PM2.5 and HAP by examining the interaction between PM2.5 and HAP as well as by comparing the association for PM2.5 separately among clean versus dirty fuel users, using the fully-adjusted outdoor model (Model 3 in Equation A). We also compared HAP association among the lowest versus highest PM2.5 quartiles (Model 3 in Equation B).
We report the odds of having hypertension and changes in BP parameters for a 10 μg/m3 increase in PM2.5 to facilitate comparison with other studies. In contrast, HAP analyses compared estimates in individuals who cooked with solid fuel to clean fuel users. All analyses were implemented with the open-source statistical package R version 3.4.1 (R Project for Statistical Computing).
Results
The final population used in this analysis included 137,809 individuals from 97,708 households in 640 communities (342 urban and 298 rural) in 21 countries (4 high-income, 12 lower and upper middle-income, and 5 low-income) on five continents (Figure 1A). About 2/3 of the participants were from lower middle- and low-income countries. The mean age (standard deviation [SD]) of all participants was 50.6 (9.7) years. Mean systolic and diastolic BP across all participants were 131 (22) and 82 (12) mmHg, respectively. Men had higher BP than women (133 vs. 130 mmHg, differences in the means 95% CI: 3.0-3.5 for systolic BP; and 83 vs. 81, differences in the means 95% CI: 1.4-1.6 for diastolic BP). BP was higher in participants with diabetes, and among those with CVD. 46% of the study population resided in rural communities, however, BP did not differ substantially by rural-urban residency (~ 1 mmHg). Two in every five participants were hypertensive, but the prevalence of hypertension (and treatment) varied across income regions, ranging from 29.9% (4.4%) in low-income to 49.6% (21.0%) in upper middle-income countries. Details on the prevalence, awareness, treatment, and control of hypertension among PURE participants are provided elsewhere (2).
Figure 1:
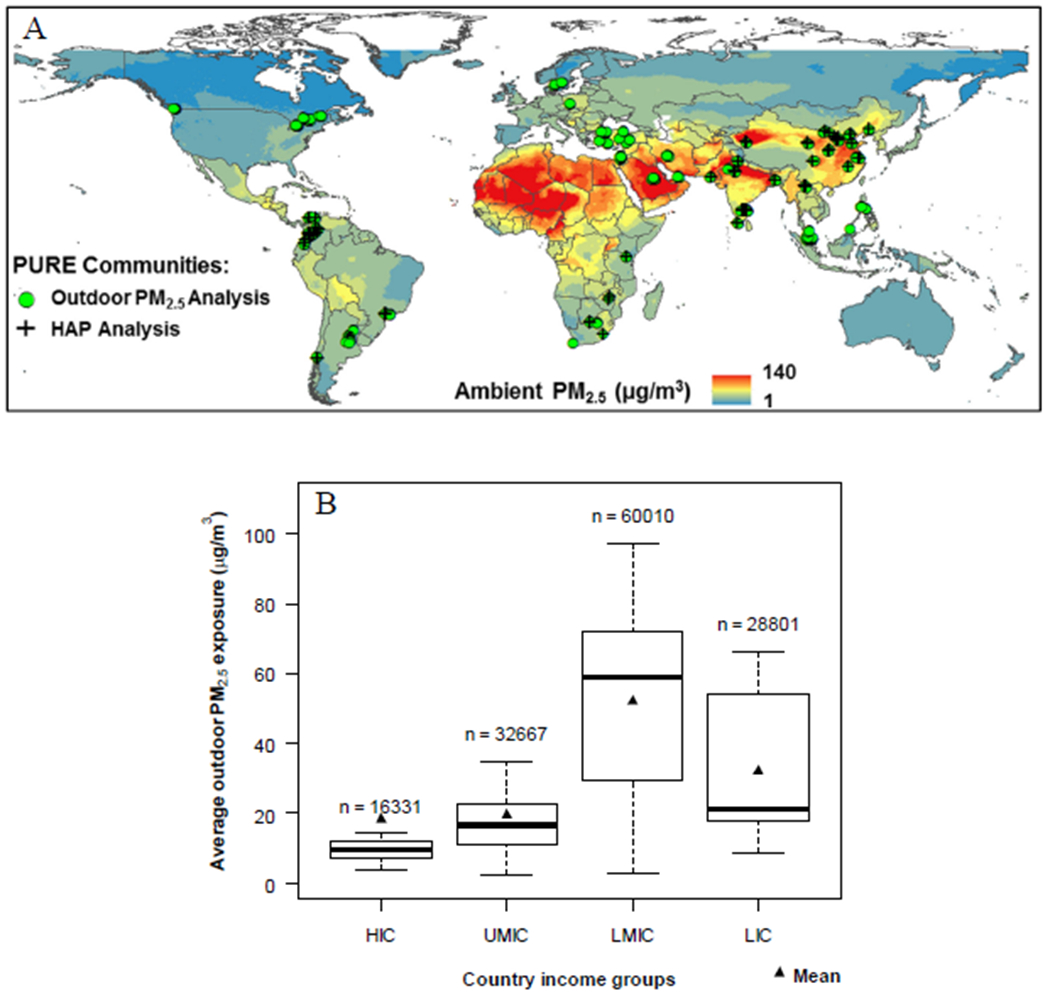
Global outdoor PM2.5 concentrations (μg/m3) (A) with locations of study countries in both outdoor and HAP analyses; and (B) by boxplots showing distribution by study country income classification a. Sample sizes show the total number of participants from countries in that income category. In each plot, the middle line represents the median, and the bottom and top of the box show the 25th and 75th percentiles of data.
High-income countries (HICs): Canada, Saudi Arabia, Sweden, and United Arab Emirates; Upper Middle-income countries (UMICs): Argentina, Brazil, Chile, Malaysia, Poland, South Africa, Turkey; Lower Middle-income countries (LMICs): China, Colombia, Iran, Palestine, Philippines; Low-income countries (LICs): Bangladesh, India, Pakistan, Tanzania, Zimbabwe.
a Categorization of economic level of a country was based on information from the World Bank in 2006
Outdoor PM2.5:
Figure 1 illustrates the location of the PURE study communities and PM2.5 concentration estimates (Figure 1A) and its distributions by country income status (Figure 1B). Community long-term average outdoor PM2.5 levels (defined as a 3-year average PM centered on the year the community was enrolled) ranged from < 10 μg/m3 in Canada, Colombia, and Sweden to > 60 μg/m3 in Bangladesh, China, and UAE. Residents of communities in lower middle-income countries had highest PM2.5 exposures, largely driven by high exposures in communities in China. Levels were significantly higher on average in low-income and lower middle-income country communities than in upper middle- and high-income countries (46 vs. 18 μg/m3). PM2.5 concentrations were higher in urban (40 μg/m3) compared to rural (32 μg/m3) communities.
Selected demographic, health, and exposure characteristics of the participants by outdoor PM2.5 quartiles are summarized in Table 1. There were no clear-cut variations in BP by PM2.5 quartiles, although both systolic and diastolic BP were relatively lowest among participants whose community average PM2.5 levels fell within the second quartile (Q2), and highest among those in the top 25th percentile (Q4) (Table 1). However, hypertension and the use of anti-hypertensive medications were highest among those in the lower 25th percentile (Q1). Solid fuel use was relatively higher among those in the top 50th (Q3 & Q4) percentile.
Association of outdoor PM2.5 with hypertension and BP:
Chronic PM2.5 exposure was associated with slightly increased odds of having hypertension in the entire cohort, with an adjusted odds ratio (OR) of 1.04 (95% CI: 1.01, 1.07) per 10 μg/m3 increase in PM2.5 (Table 2). Higher ORs were observed at higher PM2.5 concentrations; the OR for Q4 was 1.36 (95% CI: 1.10, 1.69), Q3 was 1.31 (95% CI: 1.08, 1.58), and Q2 was 0.90 (95% CI: 0.77, 1.05) compared to Q1 as reference. For BP parameters, we observed no associations in linear models (Table 1). However, we observed increased estimates for systolic and diastolic BP and MAP at the highest concentrations. For example, compared to Q1, systolic BP was 2.15 mmHg higher (95% CI: −0.59, 4.89) in Q4, 3.72 mmHg higher (95% CI: 1.37, 6.07) in Q3, and −0.78 mmHg (95% CI: −2.73, 1.17) in Q2 (Table 2).
Table 2:
Associations between outdoor PM2.5 (per 10 μg/m3 increase), use of solid fuels for cooking, versus clean fuels, and BP parameters and the odds of having hypertension.
| No. samples | Systolic BP β (95% CI) | Diastolic BP β (95% CI) | Pulse Pressure β (95% CI) | Mean Arterial Pressure β (95% CI) | Hypertension Odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Outdoor PM2.5 | ||||||
| Unadjusted | ||||||
| Linear | 137809 | 0.00 (−0.04, 0.04) | 0.01 (−0.01, 0.03) | 0.00 (−0.03, 0.02) | 0.01 (−0.02, 0.03) | 0.00 (0.00 0.01) |
| Q1 | 34267 | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| Q2 | 32009 | −3.72 (−5.89, −1.62) | −1.42 (−2.56, −0.27) | −2.28 (−3.75, −0.81) | −2.19 (−3.56, −0.82) | 0.80 (0.68, 0.94) |
| Q3 | 35511 | 0.72 (−1.71, 3.16) | 1.06 (−0.27, 2.40) | −0.25 (−1.96, 1.45) | 0.94 (−0.65, 2.53) | 1.18 (0.97, 1.43) |
| Q4 | 36022 | −0.35 (−3.19, 2.50) | 0.85 (−0.71, 2.41) | −1.12 (−3.11, 0.87) | 0.45 (−1.40, 2.31) | 1.22 (0.97, 1.54) |
| Adjusted | ||||||
| Linear | 137809 | 0.01 (−0.02, 0.05) | 0.01 (−0.01, 0.03) | 0.00 (−0.03, 0.03) | 0.01 (−0.01, 0.04) | 1.04 (1.01, 1.07) |
| Q1 | 34267 | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| Q2 | 32009 | −0.78 (−2.73, 1.17) | −0.49 (−1.58, 0.61) | −0.3 (−1.73, 1.14) | −0.58 (−1.85, 0.69) | 0.90 (0.77, 1.05) |
| Q3 | 35511 | 3.72 (1.37, 6.07) | 1.61 (0.29, 2.93) | 2.15 (0.42, 3.87) | 2.31 (0.77, 3.84) | 1.31 (1.08, 1.58) |
| Q4 | 36022 | 2.15 (−0.59, 4.89) | 1.35 (−0.2, 2.89) | 0.84 (−1.17, 2.85) | 1.62 (−0.17, 3.4) | 1.36 (1.10, 1.69) |
| Solid Fuel Use | ||||||
| Unadjusted | 43313 | −0.81 (−0.81, 0.26) | −1.21 (−1.50, −0.92) | 0.39 (0.06, 0.72) | −1.08 (−1.42, −0.73) | 0.90 (0.86, 0.95) |
| Adjusted | 43313 | −0.48 (−0.95, −0.01) | −0.44 (−0.72, −0.15) | −0.05 (−0.37, 0.27) | −0.45 (−0.78, −0.13) | 0.93 (0.88, 0.99) |
In stratified analyses (Table 3) using the full model (Model 3), we found 1.08 (95% CI: 1.02, 1.12) higher OR for hypertension in rural residents compared to weak 1.04 (95% CI: 1.00, 1.08) in urban residents. Further, we found higher ORs for men (1.05; 95% CI: 1.01, 1.09), individuals younger than age 50 (1.06; 95% CI: 1.02, 1.10), those with diabetes (1.06; 95% CI: 1.01, 1.11), and in those who were underweight (1.17; 95% CI: (1.11, 1.25) and obese (1.05; 95% CI: 1.01, 1.09) (Table 3). For other BP parameters we observed weak positive associations between outdoor PM2.5 (per 10 μg/m3 increase) and diastolic BP among subjects who were diabetic (0.35 mmHg; 95% CI: 0.04, 0.65); were younger than 50 years of age (0.27 mmHg; 95% CI: 0.06, 0.48); and were underweight (0.76 mmHg; 95% CI: 0.40, 1.12) (Table 3). Among those underweight, we also observed similar weak positive association for systolic BP (0.87 mmHg; 95% CI: 0.27, 1.46).
Table 3:
Model results for multivariable analysis of the association of BP parameters and odds of having hypertension with outdoor PM2.5 (per 10 μg/m3 increase), stratified by selected variables a
| No. samples | Systolic BP β (95% CI) | Diastolic BP β (95% CI) | Pulse Pressure β (95% CI) | Mean Arterial Pressure β (95% CI) | Hypertension Odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Entire sample | 137809 | 0.13 (−0.24, 0.49) | 0.12 (−0.09, 0.33) | 0.0 (−0.26, 0.26) | 0.12 (−0.12, 0.36) | 1.04 (1.01, 1.07) |
| Gender | ||||||
| Female | 79950 | 0.11 (−0.29, 0.50) | 0.17 (−0.04, 0.39) | −0.07 (−0.35, 0.20) | 0.15 (−0.11, 0.41) | 1.03 (0.99, 1.07) |
| Male | 57859 | 0.26 (−0.09, 0.62) | 0.16 (−0.05, 0.38) | 0.1 (−0.16, 0.35) | 0.20 (−0.05, 0.44) | 1.05 (1.01, 1.09) |
| Residence | ||||||
| Urban | 74601 | 0.32 (−0.10, 0.73) | 0.17 (−0.08, 0.42) | 0.14 (−0.22, 0.49) | 0.22 (−0.05, 0.50) | 1.04 (1.00, 1.08) |
| Rural | 63208 | 0.19 (−0.37, 0.75) | 0.29 (−0.03, 0.61) | −0.11 (−0.48, 0.26) | 0.25 (−0.13, 0.63) | 1.07 (1.02, 1.12) |
| Hypertension | ||||||
| No | 82346 | 0.14 (−0.08, 0.36) | 0.15 (0.01, 0.29) | −0.01 (−0.19, 0.16) | 0.15 (0.00, 0.30) | na |
| Yes | 55463 | −0.05 (−0.35, 0.25) | 0.05 (−0.13, 0.22) | −0.12 (−0.45, 0.2) | 0.02 (−0.15, 0.19) | na |
| Anti-hypertensive medication use | ||||||
| No | 118721 | 0.16 (−0.20, 0.51) | 0.17 (−0.04, 0.37) | −0.01 (−0.27, 0.25) | 0.16 (−0.07, 0.40) | 1.03 (1.00, 1.07) |
| Yes | 19088 | −0.29 (−0.82, 0.25) | −0.22 (−0.51, 0.07) | −0.09 (−0.47, 0.28) | −0.26 (−0.60, 0.09) | na |
| Heart disease | ||||||
| No | 132058 | 0.12 (−0.24, 0.49) | 0.13 (−0.08, 0.33) | −0.01 (−0.27, 0.25 | 0.13 (−0.12, 0.37) | 1.03 (1.00, 1.07) |
| Yes | 5568 | 0.07 (−0.57, 0.72) | 0.15 (−0.19, 0.50) | −0.05 (−0.50, 0.40) | 0.11 (−0.30, 0.53) | 1.06 (1.00, 1.13) |
| Diabetes mellitus | ||||||
| No | 126536 | 0.13 (−0.23, 0.49) | 0.13 (−0.08, 0.33) | −0.01 (−0.27, 0.25) | 0.13 (−0.11, 0.37) | 1.03 (1.00, 1.07) |
| Yes | 11120 | 0.37 (−0.16, 0.91) | 0.35 (0.04, 0.65) | 0.03 (−0.37, 0.42) | 0.35 (0.00, 0.70) | 1.06 (1.01, 1.11) |
| BMI | ||||||
| Underweight | 7075 | 0.87 (0.27, 1.46) | 0.76 (0.40, 1.12) | 0.01 (−0.34, 0.36) | 0.80 (0.39, 1.21) | 1.17 (1.11, 1.25) |
| Normal weight | 58189 | 0.05 (−0.33, 0.42) | 0.1 (−0.11, 0.32) | −0.05 (−0.31, 0.22) | 0.08 (−0.17, 0.33) | 1.03 (0.99, 1.07) |
| Overweight | 47896 | 0.09 (−0.28, 0.46) | 0.12 (−0.1, 0.35) | −0.03 (−0.30, 0.23) | 0.11 (−0.14, 0.36) | 1.03 (1.00, 1.07) |
| Obese | 24649 | 0.10 (−0.33, 0.54) | 0.07 (−0.18, 0.33) | 0.02 (−0.31, 0.34) | 0.09 (−0.20, 0.38) | 1.05 (1.01, 1.09) |
| Smoking status | ||||||
| Never | 16094 | −0.08 (−0.55, 0.38) | −0.03 (−0.31, 0.25) | −0.08 (−0.4, 0.25) | −0.05 (−0.37, 0.27) | 1.01 (0.96, 1.06) |
| Ever | 121715 | 0.13 (−0.25, 0.50) | 0.14 (−0.07, 0.35) | −0.02 (−0.29, 0.25) | 0.13 (−0.11, 0.38) | 1.03 (1.00, 1.07) |
| Alcohol use | ||||||
| Never | 6292 | 0.00 (−0.54, 0.53) | −0.03 (−0.37, 0.31) | 0.05 (−0.32, 0.42) | −0.02 (−0.40, 0.36) | 0.98 (0.93, 1.03) |
| Ever | 131517 | 0.07 (−0.30, 0.44) | 0.1 (−0.11, 0.31) | −0.03 (−0.30, 0.23) | 0.09 (−0.15, 0.33) | 1.03 (0.99, 1.06) |
| Age (years) | ||||||
| ≤ 50 | 70376 | 0.30 (−0.05, 0.64) | 0.27 (0.06, 0.48) | 0.03 (−0.22, 0.28) | 0.28 (0.05, 0.52) | 1.06 (1.02, 1.10) |
| > 50 | 67433 | −0.03 (−0.46, 0.41) | 0.09 (−0.13, 0.32) | −0.12 (−0.43, 0.19) | 0.05 (−0.23, 0.32) | 1.01 (0.98, 1.05) |
| Healthy participants b | 108656 | 0.14 (−0.22, 0.49) | 0.16 (−0.04, 0.37) | −0.03 (−0.29, 0.23) | 0.15 (−0.08, 0.39) | 1.03 (1.00, 1.07) |
Model 3: age, sex and race/ethnicity, education (none, primary, secondary/high school/higher secondary, trade school, college/university, unknown), marital status (married, not married), and a household wealth index (based on household assets and the index calculated separately for each country), smoking status (never smoked, former smoker, current smoker), alcohol use (never used, former user, current user), body mass index (BMI; Kg/m2), alternative healthy eating index (an indicator for overall diet quality based on the dietary guidelines for Americans), temperature (oC), geographical latitude, country income-level, and location of residence (urban, rural), and use of BP lowering medication (medication use, no medication use).
All models included random intercepts for country and community.
Participants who were free of CVD, diabetes mellitus, and did not take BP medication
Household air pollution:
A subset of 43,313 participants in 192 rural communities in 10 countries constituted the population used in the HAP analysis. The ten countries included here had >10% solid fuel use prevalence in at least one study center at study baseline. Overall, close to 70% of the rural population used solid fuel as their primary cooking fuel. Across countries, the predominant cooking fuel types in this rural sample were wood (32%), gas (27%) and coal (20%). Use of coal was highest (> 60%) in parts of China while wood was dominant in Pakistan, India, Zimbabwe and Tanzania. Selected demographic, health, and exposure characteristics of the participants by solid versus clean fuels for cooking are summarized in Table 1. Mean age and proportion of women were similar between solid and clean fuel users. The share of individuals living with hypertension (and its treatment) and diabetes were higher in clean fuel users (42%) compared to solid fuel users (34%). Smoking was more prevalent in those cooking with solid fuels than clean fuels. Outdoor PM2.5 was also higher in communities where solid fuel was predominant (Table 1). By individual fuel category, mean systolic BP did not follow any patterns; it was highest among those who cooked with electricity (137 mmHg), approximately 12 mmHg higher than in wood users (who had lowest mean systolic BP), followed by coal and shrub/grass. Diastolic BP was also higher by 9 mmHg among those who cooked primarily with electricity compared to wood.
Association of household solid fuel use with hypertension and BP:
The inverse relation between clean fuels and BP in our HAP dataset persisted in the multivariable analyses. We observed lower odds of having hypertension among those cooking primarily with solid fuels compared with clean fuels (OR= 0.93; 95% CI: 0.88, 0.99) (Table 2). The overall initial mean difference in BP levels among individuals who cooked with solid fuels was significantly lower than among those who used clean fuels (130 vs. 133 mmHg for systolic BP [95% CI: 2.7, 3.6] and 81 vs. 83 mmHg for diastolic BP [95% CI: 1.9, 2.4]). This lower BP among solid fuel users also persisted in the adjusted models. In the full model (Model 3), use of solid fuel was associated with 0.51 mmHg lower systolic BP (95% CI: −0.99, −0.03), 0.46 mmHg lower diastolic BP (95% CI: −0.75, −0.18), and 0.48 lower MAP (95% CI: −0.81, −0.15) (Figure S2).
Stratified analyses of BP and HAP did not show clear patterns by individual, household or community characteristics (Table 4). Use of solid fuels was associated with lower odds of hypertension in men, those younger than age 50, and among smokers, and alcohol users by approximately 6-11% (Table 4). It was about 0.47-0.87 mmHg lower for other BP parameters.
Table 4:
Model results for multivariable analysis of the association of BP parameters and odds of hypertension with solid fuel use, stratified by selected variables a
| No. samples | Systolic BP β (95% CI) | Diastolic BP β (95% CI) | Pulse Pressure β (95% CI) | Mean Arterial Pressure β (95% CI) | Hypertension Odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Entire sample | 43313 | −0.48 (−0.95, −0.01) | −0.44 (−0.72, −0.15) | −0.05 (−0.37, 0.27) | −0.45 (−0.78, −0.13) | 0.93 (0.88, 0.99) |
| Gender | ||||||
| Female | 24966 | −0.26 (−0.90, 0.38) | −0.19 (−0.56, 0.18) | −0.08 (−0.51, 0.35) | −0.21 (−0.64, 0.22) | 0.96 (0.88, 1.04) |
| Male | 18347 | −0.87 (−1.57, −0.17) | −0.82 (−1.26, −0.38) | −0.05 (−0.51, 0.41) | −0.84 (−1.33, −0.34) | 0.90 (0.83, 0.99) |
| Hypertension | ||||||
| No | 27430 | −0.54 (−0.87, −0.20) | −0.38 (−0.63, −0.14) | −0.17 (−0.42, 0.09) | −0.43 (−0.68, −0.19) | na |
| Yes | 15883 | 0.35 (−0.39, 1.09) | −0.02 (−0.45, 0.41) | 0.40 (−0.19, 1.00) | 0.10 (−0.37, 0.58) | na |
| Anti-hypertensive medication use | ||||||
| No | 39547 | −0.71 (−1.19, −0.22) | −0.46 (−0.76, −0.17) | −0.25 (−0.58, 0.07) | −0.54 (−0.88, −0.20) | 0.94 (0.88, 0.99) |
| Yes | 3766 | 1.25 (−0.48, 2.99) | −0.3 (−1.28, 0.68) | 1.59 (0.36, 2.82) | 0.21 (−0.94, 1.36) | na |
| Heart disease | ||||||
| No | 41653 | −0.45 (−0.93, 0.02) | −0.43 (−0.72, −0.14) | −0.03 (−0.35, 0.29) | −0.44 (−0.77, −0.11) | 0.89 (0.61, 1.30) |
| Yes | 1578 | −1.57 (−4.42, 1.29) | −0.86 (−2.55, 0.83) | −0.67 (−2.63, 1.29) | −1.08 (−3.02, 0.85) | 0.94 (0.88, 1.00) |
| Diabetes mellitus | ||||||
| No | 41209 | −0.47 (−0.96, 0.01) | −0.48 (−0.77, −0.18) | −0.01 (−0.33, 0.32) | −0.48 (−0.81, −0.14) | 0.80 (0.61, 1.05) |
| Yes | 2034 | 0.15 (−2.16, 2.46) | 0.11 (−1.18, 1.4) | −0.09 (−1.71, 1.52) | 0.14 (−1.38, 1.66) | 0.95 (0.89, 1.01) |
| BMI | ||||||
| Underweight | 4612 | −0.82 (−2.56, 0.93) | −0.19 (−1.27, 0.88) | −0.64 (−1.79, 0.52) | −0.4 (−1.62, 0.82) | 0.92 (0.70, 1.20) |
| Normal weight | 23300 | −0.62 (−1.26, 0.02) | −0.43 (−0.81, −0.05) | −0.20 (−0.62, 0.22) | −0.49 (−0.93, −0.06) | 0.94 (0.87, 1.03) |
| Overweight | 11854 | −0.14 (−1.01, 0.73) | −0.4 (−0.92, 0.12) | 0.24 (−0.34, 0.83) | −0.31 (−0.91, 0.28) | 0.93 (0.84, 1.03) |
| Obese | 3547 | −0.59 (−2.32, 1.14) | −0.4 (−1.42, 0.63) | −0.18 (−1.36, 0.99) | −0.47 (−1.65, 0.71) | 0.92 (0.77, 1.11) |
| Smoking status | ||||||
| Never | 2524 | 0.13 (−1.89, 2.15) | 0.00 (−1.25, 1.25) | 0.06 (−1.28, 1.4) | 0.05 (−1.37, 1.46) | 0.97 (0.76, 1.25) |
| Ever | 40789 | −0.54 (−1.03, −0.06) | −0.49 (−0.78, −0.20) | −0.06 (−0.39, 0.26) | −0.51 (−0.84, −0.17) | 0.94 (0.88, 1.00) |
| Alcohol use | ||||||
| Never | 1780 | 0.48 (−1.97, 2.92) | 0.14 (−1.37, 1.64) | 0.30 (−1.30, 1.90) | 0.25 (−1.46, 1.96) | 0.80 (0.59, 1.08) |
| Ever | 41533 | −0.54 (−1.02, −0.05) | −0.47 (−0.76, −0.18) | −0.07 (−0.40, 0.25) | −0.50 (−0.83, −0.16) | 0.94 (0.88, 0.99) |
| Age (years) | ||||||
| ≤ 50 | 24230 | −0.72 (−1.28, −0.16) | −0.55 (−0.92, −0.19) | −0.19 (−0.55, 0.17) | −0.61 (−1.02, −0.20) | 0.89 (0.82, 0.97) |
| > 50 | 19083 | −0.31 (−1.12, 0.51) | −0.35 (−0.79, 0.10) | 0.04 (−0.53, 0.60) | −0.34 (−0.87, 0.20) | 0.98 (0.90, 1.06) |
| Healthy participants b | 36947 | −0.65 (−1.15, −0.14) | −0.46 (−0.76, −0.15) | −0.2 (−0.53, 0.14) | −0.52 (−0.87, −0.17) | 0.95 (0.89, 1.01) |
Model 3: age, sex and race/ethnicity, education (none, primary, secondary/high school/higher secondary, trade school, college/university, unknown), marital status (married, not married), and a household wealth index (based on household assets and the index calculated separately for each country), smoking status (never smoked, former smoker, current smoker), alcohol use (never used, former user, current user), body mass index (BMI; Kg/m2), alternative healthy eating index (an indicator for overall diet quality based on the dietary guidelines for Americans), temperature (oC), geographical latitude, country income-level, and use of BP lowering medication (medication use, no medication use).
All models included random intercepts for country and center.
Participants who were free of CVD, diabetes mellitus, and did not take BP medication
In sensitivity analysis we used the full model (Model 3) to pool center-specific estimates through random-effects meta-analysis, and observed similar lower pooled odds of hypertension among solid fuel users when compared to clean fuels (Pooled OR = 0.93; 95% CI: 0.86, 1.00) (Figure 2). Similar findings were observed for other BP parameters in the center pooled estimates (Figure S3). There was statistically significant heterogeneity across study centers, and within and between countries, especially for systolic BP. For instance, in China, results generally demonstrate positive associations for systolic BP while centers in India and other countries showed negative associations (Figure S3).
Figure 2:
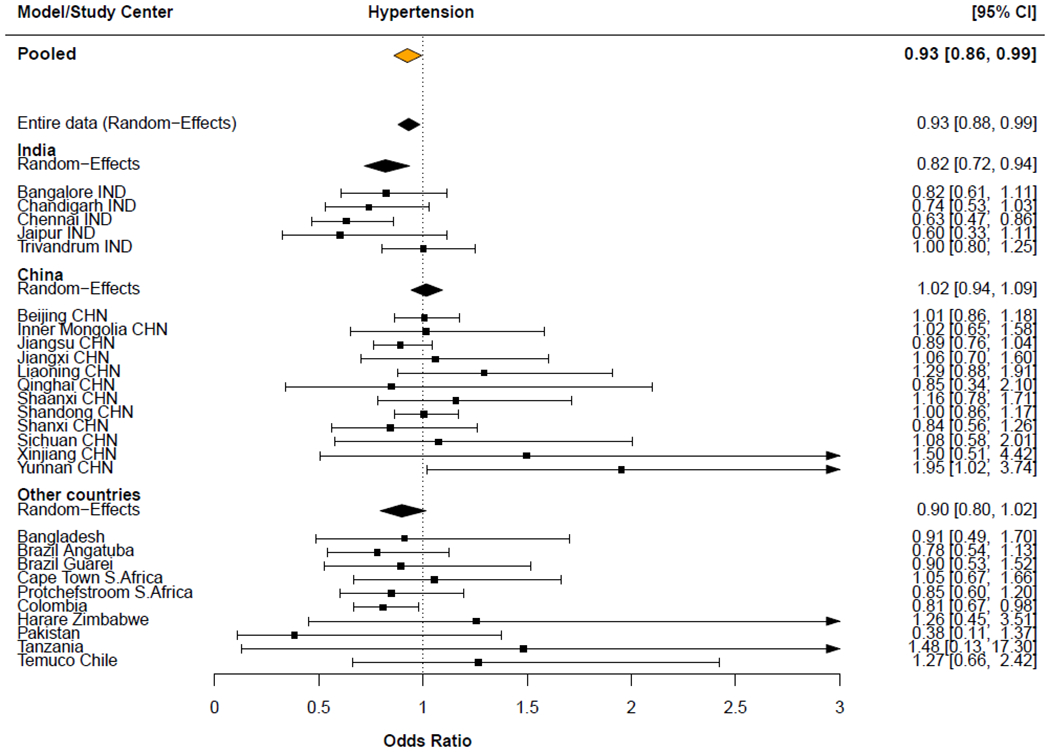
Model estimates of multivariable, country-, and center-specific, and meta-analysis of the odds (odds ratio; OR) for having hypertension among solid fuel users compared with clean fuel users.
Country-, and center-specific models adjusted for age, sex and race/ethnicity, education (none, primary, secondary/high school/higher secondary, trade school, college/university, unknown), marital status (married, not married), and a household wealth index (based on household assets and the index calculated separately for each country), smoking status (never smoked, former smoker, current smoker), alcohol use (never used, former user, current user), body mass index (BMI; Kg/m2), alternative healthy eating index (an indicator for overall diet quality based on the dietary guidelines for Americans), temperature (°C), geographical latitude, country income-level (where appropriate), and use of BP lowering medication (medication use, no medication use).
Models included random intercepts for either country and/or study center where appropriate.
Joint outdoor PM2.5 and HAP exposures versus BP parameters
The interaction term between outdoor PM2.5 and solid fuel use for cooking was not statistically significant. For outdoor PM2.5 among only clean fuel (electricity/gas) users, we observed small positive associations for systolic (0.43 mmHg; 95%CI: 0.06, 0.81), diastolic BP (0.30 mmHg; 95%CI: 0.08, 0.52), and MAP (0.35 mmHg; 95%CI: 0.10, 0.59). For every 10 μg/m3 increase in outdoor PM2.5, there was 1.34 (95%CI: 1.12, 1.59) higher odds of having hypertension among those who cooked primarily with clean fuels compared to 1.17 (95%CI: 0.95, 1.46) among solid fuel users (Table S1). We found no differences in the associations for solid fuel use among either the lowest or the highest PM2.5 quartiles (Table S1) for BP.
Discussion
This is the first multi-country and multi-center study of the relationship between chronic air pollution exposure, including both outdoor PM2.5 and household solid fuel use for cooking, with BP. We examined 137,809 adults aged 35 to 70 years from 640 urban and rural communities in 21 countries, capturing a wide range of outdoor PM2.5 exposures, household cooking settings, and diverse individual and community characteristics. For outdoor PM2.5, we observed increased odds of hypertension 1.04 (95% CI: 1.01, 1.07) per 10 μg/m3 increase in PM2.5. BP measures demonstrated non-linear relationship with an indication of positive associations at higher PM2.5 concentrations. In contrast, for HAP, we observed indications of decreases in odds of hypertension, and decreases in systolic and diastolic BP among solid fuel users, but these associations varied notably by study country and study center. The associations between HAP and BP likely reflect residual confounding in this multi-country analysis, especially in relation to affluence as we observed significantly higher baseline systolic and diastolic BP and hypertension prevalence among individuals using clean fuels (gas/electricity) compared to solid fuels.
Chronic PM2.5–BP association:
While evidence exists for short-term exposures (4; 9; 15; 16), data on chronic outdoor PM2.5 exposures and hypertension and BP are limited and inconsistent (6; 7; 10; 27–33). The few previous studies of chronic exposures were conducted mainly in a single country/city/center setting. While such homogenous population helps in reducing ecological confounding, the PM exposure levels are considerably lower or cover narrower ranges of exposure. In our multi-country and center analyses, which relied on long-term averaged exposures, and with one of the widest range of exposures (~3-97 μg/m3), the results demonstrate positive associations with hypertension, as reported in some studies (10; 31; 32), but which is also contrary to findings elsewhere (6; 28). The associations with BP itself were evident only at higher PM concentrations. Evidence non-linear relationship between PM and BP likely made it difficult for us to detect associations in our multi-country, multi-center study as countries and centers within country potentially occupy different parts of the exposure-response curve. We accounted for country income level, along with other factors like urban-rural location, but countries/centers likely have BP levels that are lower/higher than would be predicted based on PM alone.
A few population-based cohort studies from the US (6), Germany (7), and Taiwan (10; 34) found that long-term average PM2.5 was modestly associated with BP. Unlike these single setting studies, PM2.5 across our 21 study countries (on 5 continents) likely represented more heterogeneous mixtures that originated from more diverse sources (e.g. traffic, biomass, coal, and crustal dust) than in previous study settings (mostly traffic). It is possible that combustion PM, as proxied by black carbon (35), has a stronger association with BP parameters than the total mass, hence our weak findings using data from diverse locations globally.
In general, the body of evidence so far suggest that the impact of chronic PM2.5 exposure may be stronger for hypertension than for BP parameters. Even in the extreme case of chronic smoking, which exposes smokers to high concentrations of PM, and is a proven risk factor for heart attack and stroke, PM has not been conclusively linked to elevated BP. Epidemiological studies mostly found that BP levels among cigarette smokers were the same as or lower than those of non-smokers; and if any, the independent chronic effect of smoking on BP is small or clinically insignificant (37), which may be the case for the impact of long-term PM exposure on BP.
Solid fuel use–BP association:
There are few large epidemiological studies of HAP and BP (13; 38–40), but fewer multi-country analysis (13; 14). Contrary to our study, a recent multi-country analyses of nationally representative and internationally comparable data for younger women (aged 15-49) found links between history of solid fuel use and small increases in BP and odds of hypertension (14). Cook-stove intervention studies in Latin America have reported larger associations between decreased BP in women with reduced exposure from household smoke (11; 13; 41), and personal exposure studies of rural women also indicated evidence of associations between acute exposure to solid fuel combustion-related air pollutants and BP in China (12) and Ghana (42). In our study of 43,313 rural residents from 10 countries, we found an indication of lower BP among solid fuel users when compared to electricity/gas users, but with significant heterogeneity by centers and country; with generally positive associations for systolic BP in China and negative associations in India and in other countries. The positive association with systolic BP in China seems to be in accordance with findings of other studies conducted in China (12; 35; 39; 43).
Overall, unadjusted mean BP across our study countries is low, and hypertension prevalence was very low in solid fuel users. For instance, 34% of solid fuels users, compared to 42% of clean fuel users, had hypertension; and the mean systolic/diastolic BP in solid fuels users was 130/81 which was significantly lower when compared to 133/83 in the clean fuel group. It appears that solid fuel use, and thus HAP exposure in this cohort may be inversely correlated with other BP and hypertension risk factors, even after our comprehensive use of individual, household and community covariates, as was suggestive in smokers compared with nonsmokers (37).
Strengths and limitation:
The main strength of our study was its size and global scope, which enabled us to examine long-term average PM2.5 concentrations over the full global exposure distribution (range: 3-97), and with history of solid fuel use across multiple sites in different countries. Our data also included a wide range of individual-, household-, and community-level covariates on participants from both rural and urban communities. Our BP measures were also collected using a standardized protocol across study centers.
However, given the global nature of our analysis, there are also important limitations that must be considered. First, the diversity of sites in our study is a weakness in itself when making inference as adjustments such as country random effects will not remove all the ecological residual confounding. Residual confounding is a concern, especially for the HAP analysis given the larger baseline differences between solid and clean fuel users. While we included a comprehensive set of confounding factors, we could not account for personal or occupational PM2.5 exposures. It would be too costly and logistically prohibitive to conduct detail exposure measurement in a large multi-country study as ours, particularly in centers where both household and outdoor sources are related. Studies of actual personal exposure measurement have demonstrated wide variations in PM2.5 among individuals living in the same community due to local emission sources and personal time activity (12; 44). By relying on 3-year average outdoor PM2.5 and on proxy indicators for HAP, our study likely captured only regional differences in exposures, with no information on between-persons variations among participants in the same community. Consequently, exposure errors stemming from variability in individual’s long-term average exposures remain; but this also applies equally to all study communities and across the exposed versus unexposed groups. However, our PM2.5 predictions assigned at a 1 x 1 km resolution potentially minimized the magnitude of the misclassified exposures.
Similarly, we used surrogate measures to assess HAP, which likely led to large exposure misclassification stemming from factors like stove stacking, ventilation, cooking practices, and emissions from neighbors’ cookstoves, just to name a few (24; 45; 46). Nevertheless, history of solid fuel use for cooking is a commonly used metric in large HAP epidemiologic studies (24) and our center meta-analysis approach yielded similar results to our overall analyses. Further, although the sampling frame in each study country was not nationally representative, it was shown that the overall prevalence of hypertension was similar to global estimates (2). We could not account for acute temperature and PM2.5 exposures, which might be partially responsible for changes in BP on the day of measurement (47; 48). Importantly, we observed large initial differences in mean BP between individuals using solid fuel for cooking (130/81 mmHg) compared to individuals using electricity or gas (133/83 mmHg). BP is known to vary with diet, sedentary lifestyle, and other individual-level characteristics (e.g. BMI) that are related to low socio-economic status or poverty, which are also likely highly related to solid fuel use. Including covariates in the model may not totally control for such confounding effects. Residual confounding of our HAP results is therefore a possibility, but this would similarly be a limitation in any large existing studies of HAP and BP.
Conclusion:
In this large international multi-center study, chronic exposure to chronic outdoor PM2.5 was associated with increased odds of hypertension and small increases in BP at higher exposure levels, while HAP from cooking with solid fuels showed small decreases in odds of hypertension and BP, but the associations were inconsistent across countries and sub-populations. Future longitudinal analyses within the PURE cohort with center specific quantitative exposure data will clarify these findings.
Supplementary Material
Acknowledgments:
This work was supported by the Canadian Institutes of Health Research [grant #136893]; by the Office of The Director, National Institutes of Health of the National Institutes of Health [Award Number DP5OD019850]; and by the Michael Smith Foundation for Health Research Trainee Award [Award Number 28160]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Canadian Institutes of Health Research. Dr S Yusuf is supported by the Mary W Burke endowed chair of the Heart and Stroke Foundation of Ontario.
Additional acknowledgements are included at the end of the article.
Footnotes
Conflict of interests: We have no actual or potential competing financial interests
References
- 1.Collaborators RF. GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–68. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818–27. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. [DOI] [PubMed] [Google Scholar]
- 5.Pope CA, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circulation research. 2015;116(1):108–15. [DOI] [PubMed] [Google Scholar]
- 6.Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, et al. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environmental health perspectives. 2015;123(10):951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, et al. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environmental health perspectives. 2011;119(12):1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2010;52(3):258–62. [DOI] [PubMed] [Google Scholar]
- 9.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Guo C, Lau AK, Chan T-C, Chuang YC, Lin C, et al. Long-Term Exposure to Fine Particulate Matter, Blood Pressure, and Incident Hypertension in Taiwanese Adults. Environmental health perspectives. 2018;126(1):017008-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environmental health perspectives. 2007;115(7):996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environmental health perspectives. 2011;119(10):1390–5.21724522 [Google Scholar]
- 13.McCracken JP, Wellenius GA, Bloomfield GS, Brook RD, Tolunay HE, Dockery DW, et al. Household Air Pollution from Solid Fuel Use: Evidence for Links to CVD. Glob Heart. 2012;7(3):223–34. [DOI] [PubMed] [Google Scholar]
- 14.Arku RE, Ezzati M, Baumgartner J, Fink G, Zhou B, Hystad P, et al. Elevated blood pressure and household solid fuel use in premenopausal women: Analysis of 12 Demographic and Health Surveys (DHS) from 10 countries. Environ Res. 2017;160:499–505. [DOI] [PubMed] [Google Scholar]
- 15.Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8(1):E8–E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett RT, Pope CA 3rd, Ezzati M, Olives C, Lim SS, Mehta S, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environmental health perspectives. 2014;122(4):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzoulaki I, Elliott P, Kontis V, Ezzati M. Worldwide Exposures to Cardiovascular Risk Factors and Associated Health Effects: Current Knowledge and Data Gaps. Circulation. 2016;133(23):2314–33. [DOI] [PubMed] [Google Scholar]
- 19.Kwan GF, Mayosi BM, Mocumbi AO, Miranda JJ, Ezzati M, Jain Y, et al. Endemic Cardiovascular Diseases of the Poorest Billion. Circulation. 2016;133(24):2561–75. [DOI] [PubMed] [Google Scholar]
- 20.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S, Group PI-W. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. American heart journal. 2009;158(1):1–7 e1. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):937–52. [DOI] [PubMed] [Google Scholar]
- 22.Ramli A, Halmey N, Teng C. White coat effect and white coat hypertension: one and the same? Malays Fam Physician. 2008;3(3):158–61. [PMC free article] [PubMed] [Google Scholar]
- 23.van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. Global Estimates of Fine Particulate Matter using a Combined Geophysical-Statistical Method with Information from Satellites, Models, and Monitors. Environmental science & technology. 2016;50(7):3762–72. [DOI] [PubMed] [Google Scholar]
- 24.Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. [DOI] [PubMed] [Google Scholar]
- 25.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108(11):1896–901. [DOI] [PubMed] [Google Scholar]
- 26.Cabrera SE, Mindell JS, Toledo M, Alvo M, Ferro CJ. Associations of Blood Pressure With Geographical Latitude, Solar Radiation, and Ambient Temperature: Results From the Chilean Health Survey, 2009-2010. Am J Epidemiol. 2016;183(11):1071–3. [DOI] [PubMed] [Google Scholar]
- 27.Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA). Environmental health perspectives. 2008;116(4):486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuks KB, Weinmayr G, Hennig F, Tzivian L, Moebus S, Jakobs H, et al. Association of long-term exposure to local industry- and traffic-specific particulate matter with arterial blood pressure and incident hypertension. Int J Hyg Environ Health. 2016;219(6):527–35. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension: A Systematic Review and Meta-Analysis. Hypertension. 2016;68(1):62–70. [DOI] [PubMed] [Google Scholar]
- 30.Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014;32(11):2130–40; discussion 41. [DOI] [PubMed] [Google Scholar]
- 31.Honda T, Pun VC, Manjourides J, Suh H. Associations of long-term fine particulate matter exposure with prevalent hypertension and increased blood pressure in older Americans. Environ Res. 2018;164:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, Wang Y, Yang Y, Xu J, Zhang Y, Tang W, et al. Long-Term Effects of Ambient Particulate Matter (With an Aerodynamic Diameter </=2.5 mum) on Hypertension and Blood Pressure and Attributable Risk Among Reproductive-Age Adults in China. J Am Heart Assoc. 2018;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bo Y, Guo C, Lin C, Chang LY, Chan TC, Huang B, et al. Dynamic Changes in Long-Term Exposure to Ambient Particulate Matter and Incidence of Hypertension in Adults. Hypertension. 2019;74(3):669–77. [DOI] [PubMed] [Google Scholar]
- 34.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occupational and environmental medicine. 2011;68(1):64–8. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci U S A. 2014;111(36):13229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuks KB, Weinmayr G, Foraster M, Dratva J, Hampel R, Houthuijs D, et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Environmental health perspectives. 2014;122(9):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. American heart journal. 1986;111(5):932–40. [DOI] [PubMed] [Google Scholar]
- 38.Young BN, Clark ML, Rajkumar S, Benka-Coker ML, Bachand A, Brook RD, et al. Exposure to household air pollution from biomass cookstoves and blood pressure among women in rural Honduras: A cross-sectional study. Indoor Air. 2019;29(1):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Z, Liu Y, Yin Q, Qiu M. Impact of household solid fuel use on blood pressure and hypertension among adults in China. Air Quality, Atmosphere & Health. 2016:1–10. [Google Scholar]
- 40.Abtahi M, Koolivand A, Dobaradaran S, Yaghmaeian K, Mohseni-Bandpei A, Khaloo SS, et al. National and sub-national age-sex specific and cause-specific mortality and disability-adjusted life years (DALYs) attributable to household air pollution from solid cookfuel use (HAP) in Iran, 1990-2013. Environ Res. 2017;156:87–96. [DOI] [PubMed] [Google Scholar]
- 41.Alexander D, Larson T, Bolton S, Vedal S. Systolic blood pressure changes in indigenous Bolivian women associated with an improved cookstove intervention. Air Quality, Atmosphere & Health. 2015;8(1):47–53. [Google Scholar]
- 42.Quinn AK, Ae-Ngibise KA, Jack DW, Boamah EA, Enuameh Y, Mujtaba MN, et al. Association of Carbon Monoxide exposure with blood pressure among pregnant women in rural Ghana: Evidence from GRAPHS. Int J Hyg Environ Health. 2016;219(2):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arku RE, Dionisio KL, Hughes AF, Vallarino J, Spengler JD, Castro MC, et al. Personal particulate matter exposures and locations of students in four neighborhoods in Accra, Ghana. J Expos Sci Environ Epidemiol. 2015;25(6):557–66. [DOI] [PubMed] [Google Scholar]
- 45.Bruce N, Pope D, Rehfuess E, Balakrishnan K, Adair-Rohani H, Dora C. WHO indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure–risk functions. Atmospheric Environment. 2015;106:451–7. [Google Scholar]
- 46.Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environmental health perspectives. 2013;121(10):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modesti PA. Season, temperature and blood pressure: a complex interaction. Eur J Intern Med. 2013;24(7):604–7. [DOI] [PubMed] [Google Scholar]
- 48.Lewington S, Li L, Sherliker P, Guo Y, Millwood I, Bian Z, et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J Hypertens. 2012;30(7):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


