Abstract
Patients with gastric precancerous lesions (atrophic gastritis and intestinal metaplasia) have increased risk of developing gastric cancer, and adequate management and surveillance of these patients should allow to reduce gastric cancer-related mortality. The guidelines on the management of these patients have been recently published by the European Societies (MAPS II guidelines) and by the American Gastroenterological Association (AGA). The aim of this commentary is to compare these two guidelines by highlighting the common points and differences between them. Both guidelines recommend a systematic detection and eradication of Helicobacter pylori in all patients with gastric atrophy. However, there is a major difference in the recommendations for surveillance: while the MAPS II guidelines recommend systematic endoscopic surveillance in all patients with severe gastric atrophy (with or without intestinal metaplasia), the AGA guidelines focus only on intestinal metaplasia and plead against systematic surveillance, leaving the possibility of surveillance in individual patients based on shared decision between clinicians and patients. The difference between two guidelines comes essentially from the different arguments used by two authorities (randomized control studies by AGA and observational cohort studies by the European Societies), and may be, at least in part, related to the difference between the European and American health care systems and potential economic burden.
Keywords: Gastric precancerous lesions, Atrophic gastritis, Gastric intestinal metaplasia, Surveillance, Guidelines, Controversy
Introduction
Despite its decreasing incidence, gastric cancer (GC) remains one of the major global health problems, with over a million new cases registered every year worldwide and an overall mortality rate exceeding 70% [1]. GC is a heterogeneous disease; the vast majority of cases are adenocarcinomas, which can be classified based on anatomical location (cardia or non-cardia) and histological type (diffuse or intestinal). The most frequent type remains the non-cardia intestinal type, mainly related to chronic infection with Helicobacter pylori. The consecutive histological steps leading to the development of this type of GC are known as “Correa’s cascade” [2], [3]. According to this model, the process begins with an H. pylori infection leading to non-atrophic chronic gastritis, which may evolve into multifocal atrophic gastritis (without intestinal metaplasia, IM), atrophic gastritis with IM, dysplasia, and ultimately into adenocarcinoma. Surveillance of patients with these gastric precancerous lesions (GPL) should enable detection of early stage GC that can be effectively treated, with consequent excellent prognosis and reduced GC mortality.
Guidelines on the management of GPL related to noncardia adenocarcinomas have been recently published by two authoritative clinical groups: the European Society of Gastrointestinal Endoscopy in association with other European societies (The MAPS II guidelines [4], which correspond to an update of MAPS I guidelines [5]) and the American Gastroenterological Association (AGA) [6]. Although both guidelines used a high-quality methodology, their scopes are slightly different; whereas MAPS II guidelines are focused on the management (diagnosis, treatment, and surveillance) of individuals with atrophic gastritis, IM, and dysplasia, the AGA guidelines are exclusively focused on the management of patients with IM.
The authors endeavored to compare these two guidelines by highlighting their common points as well as their differences with respect to the management of gastric atrophy. We also refer occasionally to other guidelines, those of the British Society of Gastroenterology [7] and of the Italian Societies of Gastroenterology and Digestive Endoscopy [8]. This article complements a recent commentary on the same topic by Dinis-Ribeiro and Kuipers [9].
Before discussing the guidelines, it is crucial to define gastric atrophy and its variants. Gastric atrophy is a condition characterized by the loss of native epithelium, which may be replaced by fibrotic tissue (non-metaplastic atrophy) or by a different type of epithelium (IM or pseudopyloric metaplasia). The extension of gastric atrophy determines GC risk and may be evaluated using the operative link on gastritis assessment (OLGA) [10], and the operative link on gastric intestinal metaplasia assessment (OLGIM) [11] systems. Both systems rank GC risk in stages from 0 to IV based on five gastric biopsies [two from the antrum, two from the corpus (lesser and greater curvature each), and one from the incisura] according to the updated Sydney system [12]. OLGA and OLGIM systems are endorsed by MAPS, British, and Italian guidelines. Nevertheless, as highlighted by Rugge et al. [13], there is a profound misunderstanding of the OLGA system among gastroenterologists and pathologists. In the OLGA system, the entire spectrum of atrophic lesions (non-metaplastic, IM, and pseudopyloric metaplasia) is included in order to assess the extension of atrophy. IM is one of the variants of atrophy, not a different lesion. The OLGIM system solely evaluates IM extension. Therefore, the OLGA stage of an individual may be either equal or more advanced than the OLGIM stage, but never lower (Fig. 1). Another important issue to consider when applying the OLGA/OLGIM systems is that although high-risk stages III and IV certainly indicate extensive atrophic changes, individuals in low-risk stages I or II should not be excluded from surveillance based solely on their stage. As an example, an individual may have IM in both the antrum/incisura and corpus (even in all five biopsies) and still be classified in stage I or II with either system. Although the ideal approach for risk stratification would include both systems, limitations of the OLGA system are the higher inter-observer variability in the assessment of the extension of atrophy without IM as compared to IM [14], and the requirement of well-oriented biopsies that represent the entire thickness of the gastric mucosa.
Fig. 1.
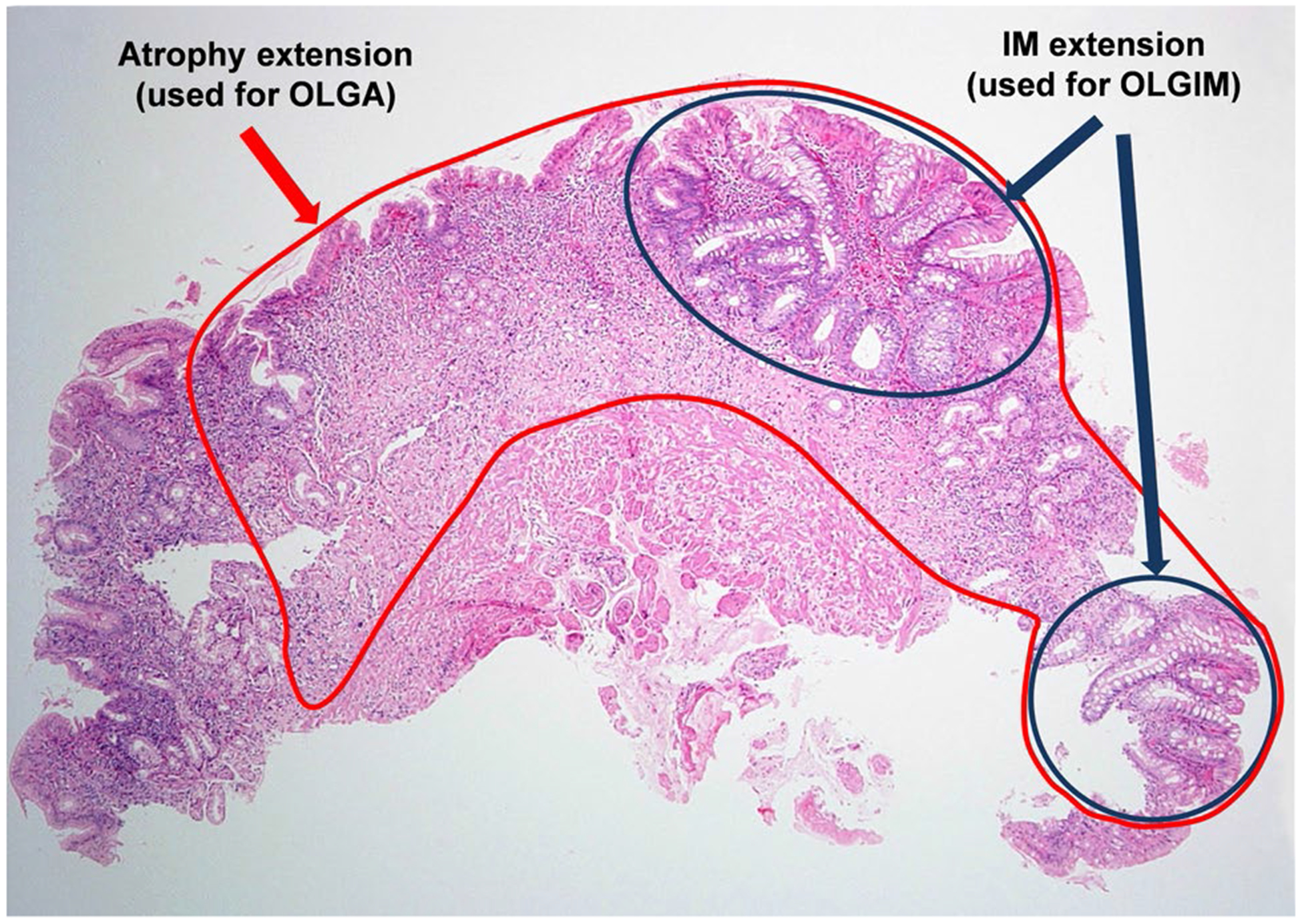
Assessment of atrophy at the single biopsy level. In this example, about 90% of the native epithelium is lost (circled in red) and about 30% has been replaced by IM (circled in dark blue). Therefore, there is 90% atrophy (for OLGA) and 30% IM (for OLGIM). Each biopsy is assessed individually. Then, the average of 3 antrum/incisura biopsies and the average of 2 corpus biopsies are used to assign the OLGA and OLGIM stages. For a detailed tutorial for applying OLGA system, see Rugge et al., Dig Liver Dis. 2008;40:650–8
A detailed comparison between MAPS II and AGA guidelines for endoscopic surveillance in individuals with histologically confirmed gastric atrophy is presented in Table 1.
Table 1.
MAPS II and AGA recommendations for endoscopic surveillance of histologically diagnosed gastric atrophy
| Condition | OLGA | OLGIM | Follow-up interval in years | ||
|---|---|---|---|---|---|
| MAPS II | AGA1 | ||||
| Atrophy without intestinal metaplasia (includes pseudopyloric metaplasia) | |||||
| Antrum/incisura only | Mild/moderate* | I/II | 0 | No F-U | NA |
| Severe | III | 0 | 3 | NA | |
| Corpus only | Mild/moderate | I/II | 0 | No F-U | NA |
| Severe | II | 0 | No F-U | NA | |
| Both | Mild | I | 0 | No F-U | NA |
| Moderate | III | 0 | 3 | NA | |
| Severe | IV | 0 | 3 | NA | |
| Other combinations | II-IV | 0 | No F-U versus 32 | NA | |
| IM | |||||
| Antrum/incisura only | Mild/moderate | I-IV | I | No F-U versus 32 | No F-U |
| Severe | III-IV | III | 3 | No F-U | |
| Complete type** | No F-U | No F-U | |||
| Incomplete/mixed type | 3 | 3–5 | |||
| Corpus only | Mild/moderate | I-IV | I/II | No F-U versus 32 | No F-U |
| Severe | II-IV | II | No F-U versus 32 | No F-U | |
| Complete type** | No F-U | No F-U | |||
| Incomplete/mixed type | 3 | 3–5 | |||
| Both | Mild/moderate/severe | I-IV | I-IV | 3 | 3–5 |
| Complete | 3 | 3–5 | |||
| Incomplete/mixed type | 3 | 3–5 | |||
| IM + family history of GC*** | 33 | 3–5 | |||
| IM + persistent H. pylori infection | 34 | NA | |||
| Autoimmune gastritis | 3–5 | NA | |||
F-U follow-up, IM intestinal metaplasia, NA non-applicable/not considered in the guidelines, OLGA operative link on gastritis assessment, OLGIM operative link on intestinal metaplasia assessment
Mild, moderate, or severe denote histological extension of atrophy (by OLGA and OLGIM systems). It is calculated separately in antrum/incisura (average of 3 biopsies) and corpus (average of 2 biopsies)
Follow-up for patients with complete-type IM in only one anatomical location should be based on IM extension and other risk factors
These recommendations do not apply to hereditary/familial diffuse GC
Using shared decision making, patients with IM and concerns about completeness of baseline endoscopy, extensive, or incomplete-type IM, and/or who are specifically at overall increased risk for GC (racial/ethnic minorities, immigrants from high GC risk regions, or family history of GC) may elect for repeat endoscopy within 1 year for risk stratification
Follow-up recommended if OLGA stage is III or IV
Individuals with advanced stages of gastric atrophy (not only IM) and family history of GC may benefit from follow-up every 1–2 years
In individuals with extensive IM, the follow-up interval may be 1–2 years
Common Statements and Recommendations by MAPS II and AGA Guidelines
Systematic H. pylori test-and-treat strategy in patients with GPL and after endoscopic resection of early stage GC.
Among patients with IM, those with extensive IM (defined as involving antrum and corpus), incomplete (colonic) type, or family history of GC, are considered at higher risk of GC. MAPS II guidelines add persistent H. pylori infection as a high-risk factor.
IM subtyping (not necessarily requiring special techniques) is not routinely recommended, but when available, it should be considered for surveillance decision.
Differences Between MAPS II and AGA Guidelines
MAPS II guidelines provide specific recommendations for surveillance of gastric atrophy, whereas AGA guidelines state that this is a decision that should be made by physicians in consultation with their patients.
Routine short interval (< 1 year) repeat endoscopy after accidental diagnosis (without Sydney protocol biopsies) of IM for risk stratification is not recommended by AGA. Although there is no specific statement in the MAPS II guidelines, it appears explicit as a correct evaluation of the severity of the lesions preferably by using OLGA/OLGIM systems is recommended. On this point, the British guidelines explicitly recommend that unless such biopsy protocol has been applied at the initial endoscopy, patients should undergo another endoscopy with an optimal biopsy protocol in order to guide proper management.
Since AGA correctly claims that there are no randomized trials demonstrating that endoscopic surveillance of GPL reduces the risk of GC, it therefore pleads against routine endoscopic surveillance in patients with IM, but suggests shared decision making for patients at higher risk of GC. In contrast, based on several observational studies indicating that screening and surveillance of GPL reduce GC risk, MAPS II guidelines give specific recommendations according to the severity of the lesions and associated risk factors.
MAPS II recognize the important role of a high-quality endoscopy [15] to better evaluate the gastric mucosa and guide targeted (rather than random) biopsies, which should include virtual chromo-endoscopy techniques whenever possible.
AGA emphasizes that information on race/ethnicity should be taken into account in the decision for surveillance, recognizing that some US minorities and immigrants from high GC risk regions are at higher risk of GC. This point is controversial, since once IM has developed, there are no conclusive data showing that individuals from particular racial/ethnic groups are at higher risk of progression to GC than others.
Conclusions and Future Directions
MAPS II and AGA guidelines agree on H. pylori detection and eradication in patients with gastric atrophy. The major difference between these guidelines is that MAPS II explicitly recommend surveillance of all patients with advanced gastric atrophy (with and without IM), whereas AGA focuses only on IM and suggests shared decision making between clinicians and patients. The difference between the two guidelines comes essentially from the difference in the arguments used by two authorities: While AGA underlines the absence of randomized controlled studies proving the efficacy of systematic surveillance, the European Societies consider that the data obtained from observational cohort studies are sufficient to propose this surveillance. Furthermore, this difference may be related to the differences between the American and European healthcare systems, and potential economic burden.
There is compelling evidence that the incidence of noncardia GC is increasing in low incidence rate countries including young adults in the USA [16] and Europe [17]. Considering this change in the epidemiology of GC, clear guidelines for screening and surveillance of GPL are continuously gaining importance.
Until highly discriminatory noninvasive biomarkers become available for risk stratification, a combined and organized effort among physicians from diverse specialties (mainly gastroenterologists and pathologists) is needed for an optimal management of patients with gastric atrophy and to continue improving guidelines. In terms of research, randomized trials of patients with IM addressing the effect of endoscopic surveillance on early detection and survival of GC would be the ultimate evidence. Nonetheless, well-designed and long-term observational studies of patients with IM in multiple populations would be also informative. Avoidable mortality from GC may be globally achieved with prompt identification and adequate management of GPL, particularly IM.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. [DOI] [PubMed] [Google Scholar]
- 3.Correa P Human gastric carcinogenesis: a multistep and multifactorial process—first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 4.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 5.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Li D, El Serag HB, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology. 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks M, Graham D, Jansen M, et al. British society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis. 2019;51:1621–1632. [DOI] [PubMed] [Google Scholar]
- 9.Dinis-Ribeiro M, Kuipers EJ. How to manage a patient with gastric intestinal metaplasia: an international perspective. Gastroenterology. 2020;158:1534–1537. 10.1053/j.gastro.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–1158. [DOI] [PubMed] [Google Scholar]
- 12.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol.. 1996;20:1161–1181. [DOI] [PubMed] [Google Scholar]
- 13.Rugge M, Fassan M, Farinati F, Genta RM. The war of the worlds: metaplastic versus nonmetaplastic atrophic gastritis Gastrointest Endosc. 2011;73:411–2; author reply 412–3. [DOI] [PubMed] [Google Scholar]
- 14.Isajevs S, Liepniece-Karele I, Janciauskas D, et al. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch. 2014;464:403–407. [DOI] [PubMed] [Google Scholar]
- 15.Committee ASoP, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr, Rosenberg PS, Camargo MC. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]


