Abstract
Mercury is among the most toxic nonradioactive elements which may cause toxicity even at low doses. Some studies showed release of mercury from dental amalgam fillings in individuals who used mobile phone. This study was conducted to assess the effect of high-field MRI on mercury release from dental amalgam filling. We studied two groups of students with identical tooth decays requiring a similar pattern of restorative dentistry. They were exposed to a magnetic flux density of 1.5 T produced by a MRI machine. 16 otherwise healthy students with identical dental decay participated in this study. They underwent similar restorative dentistry procedures and randomly divided into two groups of MRI-exposed and control arms. Urinary concentrations of mercury in the control subjects were measured before (hour 0) and 48 and 72 hrs after amalgam restoration, using cold vapor atomic absorption spectrometry. Urinary concentrations of mercury in exposed individuals were determined before (hour 0), and 24, 48, 72 and 96 hrs after amalgam restoration. Unlike control subjects, they underwent conventional brain MRI (15 min, 99 slices), 24 hrs after amalgam restoration. The mean±SD urinary mercury levels in MRI-exposed individuals increased linearly from a baseline value of 20.70±17.96 to 24.83±22.91 µg/L 72 hrs after MRI. In the control group, the concentration decreased linearly from 20.70±19.77 to 16.14±20.05 µg/L. The difference between urinary mercury in the exposed and control group, 72 hrs after MRI (96 h after restoration),was significant (p=0.046). These findings provide further support for the noxious effect of MRI (exposure to strong magnetic field)and release of mercury from dental amalgam fillings.
Keywords: Magnetic resonance imaging, Electromagnetic fields, Dental restoration, permanent, Mercury, Mercury poisoning
TAKE-HOME MESSAGE
We found a significant increase in the release of mercury from dental amalgam fillings if MRI is done within 24 hrs of amalgam restoration that lasts up to 72 hrs of MRI.
Mercury is one of the most toxic nonradioactive elements.
Dental amalgam that is one of the most commonly used materials in restorative dentistry, consists of around 50% elemental mercury and a mixture of silver, tin, copper, and zinc.
As children and some adults tend to be more sensitive to mercury, it is better clinicians use alternatives to amalgam in these groups, if possible.
Introduction
Mercury is one of the most toxic nonradioactive elements,1 and may cause toxicity even at low doses.2,3 Dental amalgam,which is one of the most commonly used materials in restorative dentistry, has been used for more than 150 years.4 It consists of around 50% elemental mercury and a mixture of silver, tin, copper and zinc. The level of mercury vapor, which is emitted from dental amalgam restorations, markedly increases by chewing, eating, brushing, and drinking hot liquids.5 Early experiments on undersea welders showed that electromagnetic fields (EMFs) might alter the evaporation of mercury from dental amalgam restorations.6
MRI is an ever increasing, efficient medical diagnostic imaging modality. During the procedure, patients are exposed to static and gradient magnetic fields as well as electromagnetic radiation in the radiofrequency range.7
Over the past years, our laboratory has focused on studying the health effects of exposure of laboratory animals and human to some common sources of EMFs, such as mobile phones and their base stations,8,9 laptop computers, MRI,10 and mobile phone jammers,11 as well as occupational exposure to EMFs generated by dental cavitron12 or radar13.
Previously, we showed that exposure to 0.23 T MRI significantly increased the mean±SD salivary mercury level from 8.6±3.0 mg/L before MRI to 11.3±5.3 mg/L 15 min after the imaging, in 30 people with dental amalgam restorations.14 However, our study had some basic limitations. The participants were referred to MRI department by their own physicians and we had no control over the number as well as the surface/volume of amalgam dental restorations. The temporal relationship between mercury concentrations in biological fluids (saliva), and exposure to magnetic fields could not be established as only two samples were available (before and after MRI exposure). And, the participants were exposed to low magnetic flux density MRI (0.23 T).
We therefore, conducted this study to assess the potential alterations in the release of mercury from human dental amalgam restorations among a group of participants who had identical tooth decays requiring a similar pattern of restorative dentistry and exposed them to a significantly higher magnetic flux density of 1.5 T.
Materials and Methods
Participants of this study were selected by a screening program on students who required dental restorations. They referred to a dentist for oral health examination. Based on the inclusion criteria, 16 healthy students were found eligible to participate in this study. The sample size was calculated based on the data of one of our previous studies.14
Initially, two matched groups, each consisting of eight individuals (three men and five women), were formed;then the groups were randomly divided into either control or MRI-exposed arms. Following approval by the Medical Ethics Committee of Shiraz University of Medical Sciences and obtaining informed written consents from the participants, identical dental amalgam restorations were performed for all participants. Mercury level was measured in the urine samples of the control participants before amalgam restoration (hour 0), and 48 and 72 hrs after the restoration.
The participants in the exposed group underwent conventional brain MRI (99 cuts in 15 min) using a 1.5 T GE scanner 24 hrs after amalgam restoration. Urinary mercury level in the exposed individuals was determined before amalgam restoration (hour 0), and 24, 48, 72 and 96 hrs after amalgam restoration, the time course during which the urinary mercury level in subjects who had undergone restorative dentistry procedures,normally returned to baseline levels.15,16 Mercury concentrations in samples were measured by cold vapor atomic absorption spectrophotometry.
Urinary creatinine concentration was also measured for both groups to ensure the level was within the acceptable range of 0.3 to 3 g/L (ACGIH, 2010) before urine samples were analyzed for mercury.17
Statistical Analysis
SPSS® for Windows® ver 17.0 was used for data analysis. Mann-Whitney U test was used to compare urinary mercury level in two studied groups. A p value <0.05 was considered statistically significant.
Results
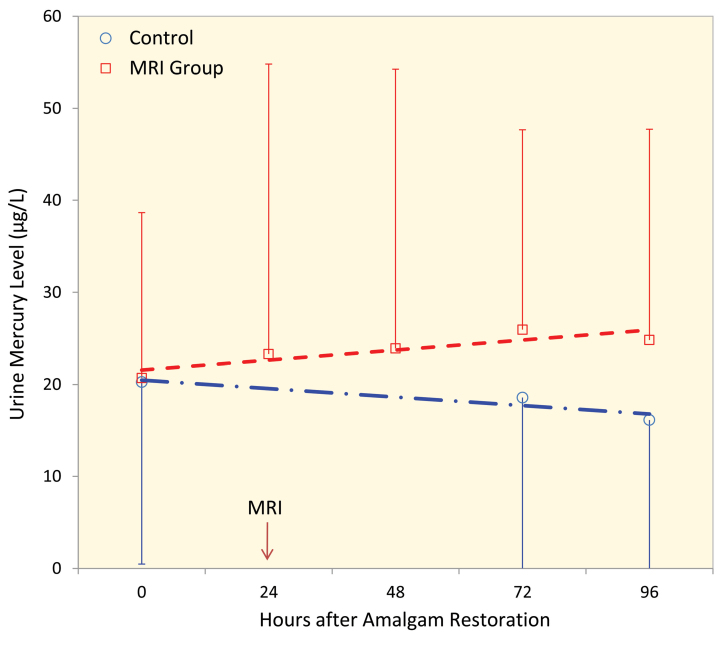
The mean±SD age of participants in the control and study groups was 24.4±4.2 and 25.6±3.7 years, respectively (p=0.67). Figure 1 shows the trend of urinary mercury level in the two studied groups. The mean±SD urinary mercury levels before amalgam restoration for the study and control groups were 20.70±17.96 and 20.25±19.77 µg/L, respectively (p=0.87); 72 hrs after amalgam restoration (48 hrs of MRI), the mercury level in the study and control groups were 25.95±21.72 and 18.57±19.04 µg/L, respectively (p=0.038). Later on, 96 hrs of amalgam restoration (72 hrs after MRI), the mercury level in the MRI group was still significantly (p=0.046) higher than that in the control group (p=0.046).
Figure 1.
Trend of urinary mercury concentration in the MRI exposed and unexposed group. Error bars represent SD.
Discussion
In our study, while there was no significant difference between the baseline urinary mercury level of the study and control groups, from 48 hrs after MRI on, the mercury level in those who underwent MRI increased to levels significantly higher than those in the control group. These findings confirmed the results obtained in our previous study,14 and indicated that MRI may significantly increase the release of mercury from amalgam dental fillings if administered within 24 hrs of the procedure. In another study conducted by Müller-Miny,et al , they examined the mercury release for typical MRI protocols, separated for both the static and the variable magnetic fields in a 1.5 T MRI unit.18 They could not demonstrate any significant increase in mercury release due to MRI. The main reason for the discrepancies observed between the results of our studies and those reported by Müller-Miny, et al , may be attributed to the in vitro nature of their experiment.
It should be noted that the saliva is a good electrolyte and may increase the release of mercury from dental amalgam due to induction of galvanic currents. Amalgam fillings may also produce electrical currents that accelerate the release of mercury.
In spite of the fact that mercury is a well-known health hazard,19 and that exposure to even low levels of this heavy metal has been associated with sub-clinical symptoms of intoxication,2,3 no unanimous agreements exists among investigators on the amount of released mercury from dental amalgam that under normal circumstances, would cause a toxic response in humans.20
Although some researchers believe that no conclusive evidence exists to indicate that dental amalgams cause health problems in the majority of the population,21 the effects of dental amalgam on specific groups including pregnant women, small children, elderly and people who are especially sensitive to mercury, might be different. This would be further complicated if these individuals are exposed to high-field MRI within the first 24 hrs of receiving dental amalgam fillings, as we showed.
About 15 years ago, the Federation of American Societies for Experimental Biology Journal published a paper calling mercury restorative material a major source of mercury exposure to the US population. The authors of this report suggested that both federal and state legislations be passed throughout their country to ensure that consent forms are given to patients receiving silver-mercury amalgam restorative material.5 As some people tend to be more sensitive to the effects of exposure to any chemical substances in their environment, in 1999, the National Health and Medical Research Council recommended clinicians use alternatives to amalgam in children where appropriate.22
Considering the importance of the issue, further studies are required to clarify whether other common sources of EMF exposure may also cause alterations in dental amalgam and accelerate the release of mercury, and to investigate whether these enhanced releases are of pathological importance, particularly among susceptible individuals.
Acknowledgements
This study was partially supported financially by the office of Vice Chancellor for Research, Shiraz University of Medical Sciences. The author would like to express their gratitude for the support received from the Center for Research in Health Sciences and the Center for Research on Protection against Ionizing and Non-ionizing Radiations, affiliated to Shiraz University of Medical Sciences. The materials presented in this article were a summary of the thesis of our MPH student, Dr. Anoosheh, under the supervision of Dr. Mortazavi and Dr. Neghab.
Conflicts of Interest:
None declared.
Cite this article as: Mortazavi SMJ, Neghab M, Anoosheh SMH, et al. High-field MRI and mercury release from dental amalgam fillings. Int J Occup Environ Med 2014;5:101-105.
References
- 1.Mutter J. Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J Occup Med Toxicol. 2011;6:2. doi: 10.1186/1745-6673-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neghab M, Choobineh A, Hassan Zadeh J, Ghaderi E. Symptoms of intoxication in dentists associated with exposure to low levels of mercury. Ind Health. 2011;49:249–54. doi: 10.2486/indhealth.ms1214. [DOI] [PubMed] [Google Scholar]
- 3.Neghab M, Norouzi MA, Choobineh A. et al. Health effects associated with long-term occupational exposure of employees of a chlor-alkali plant to mercury. Int J Occup Saf Ergon. 2012;18:97–106. doi: 10.1080/10803548.2012.11076920. [DOI] [PubMed] [Google Scholar]
- 4.Brigato Rde C, Costa LM, da Costa MR. et al. Mercury, copper, and zinc concentrations in extracted human teeth. Arch Environ Occup Health. 2009;64:266–9. doi: 10.1080/19338240903339955. [DOI] [PubMed] [Google Scholar]
- 5.Edlich RF, Greene JA, Cochran AA. et al. Need for informed consent for dentists who use mercury amalgam restorative material as well as technical considerations in removal of dental amalgam restorations. J Environ Pathol Toxicol Oncol. 2007;26:305–22. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i4.70. [DOI] [PubMed] [Google Scholar]
- 6.Ortendahl TW, Hogstedt P, Odelius H, Noren JG. Effects of magnetic fields from underwater electrical cutting on in vitro corrosion of dental amalgam. Undersea Biomed Res. 1988;15:443–55. [PubMed] [Google Scholar]
- 7.Formica D, Silvestri S. Biological effects of exposure to magnetic resonance imaging: an overview. Biomed Eng Online. 2004;3:11. doi: 10.1186/1475-925X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortazavi SM, Rouintan MS, Taeb S. et al. Human short-term exposure to electromagnetic fields emitted by mobile phones decreases computer-assisted visual reaction time. Acta Neurol Belg. 2012;112:171–5. doi: 10.1007/s13760-012-0044-y. [DOI] [PubMed] [Google Scholar]
- 9.Mortazavi SMJ, Mosleh-Shirazi MA, Tavassoli AR. et al. Increased radioresistance to lethal doses of gamma rays in mice and rats after exposure to microwave radiation emitted by a GSM mobile phone simulator. Dose Response. 2012;11:281–92. doi: 10.2203/dose-response.12-010.Mortazavi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortazavi SMJ, Daiee E, Yazdi A. et al. Mercury release from dental amalgam restorations after magnetic resonance imaging and following mobile phone use. Pakistan Journal of Biological Sciences. 2008;11:1142–6. doi: 10.3923/pjbs.2008.1142.1146. [DOI] [PubMed] [Google Scholar]
- 11.Mortazavi SMJ, Parsanezhad ME, Kazempour M. et al. Male reproductive health under threat: short term exposure to radiofrequency radiations emitted by common mobile jammers. J Hum Reprod Sci. 2013;6:124–8. doi: 10.4103/0974-1208.117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortazavi SM, Vazife-Doost S, Yaghooti M. et al. Occupational exposure of dentists to electromagnetic fields produced by magnetostrictive cavitrons alters the serum cortisol level. J Nat Sci Biol Med. 2012;3:60–4. doi: 10.4103/0976-9668.95958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortazavi SMJ, Taeb S, Dehghan N. Alterations of visual reaction time and short term memory in military radar personnel. Iran J Publ Health. 2013;42:428–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Mortazavi SM, Daiee E, Yazdi A. et al. Mercury release from dental amalgam restorations after magnetic resonance imaging and following mobile phone use. Pak J Biol Sci. 2008;11:1142–6. doi: 10.3923/pjbs.2008.1142.1146. [DOI] [PubMed] [Google Scholar]
- 15. WHO. Methylmercury. IPCS-Environmental health criteria. Geneva: World Health Organization, 1990.
- 16. IPCS. Inorganic mercury. International programme on chemical safety. Geneva: World Health Organization, 1991.
- 17. Chemical Analysis Branch: New South Wales government, 2011.
- 18.Muller-Miny H, Erber D, Moller H. et al. Is there a hazard to health by mercury exposure from amalgam due to MRI? J Magn Reson Imaging. 1996;6:258–60. doi: 10.1002/jmri.1880060146. [DOI] [PubMed] [Google Scholar]
- 19.Diez S. Human health effects of methylmercury exposure. Rev Environ Contam Toxicol. 2009;198:111–32. doi: 10.1007/978-0-387-09647-6_3. [DOI] [PubMed] [Google Scholar]
- 20.Richardson GM, Wilson R, Allard D. et al. Mercury exposure and risks from dental amalgam in the US population, post-2000. Sci Total Environ. 2011;409:4257–68. doi: 10.1016/j.scitotenv.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Ye X, Qian H, Xu P. et al. Nephrotoxicity, neurotoxicity, and mercury exposure among children with and without dental amalgam fillings. Int J Hyg Environ Health. 2009;212:378–86. doi: 10.1016/j.ijheh.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran LA, Messer LB. Clinicians’ choices of restorative materials for children. Aust Dent J. 2003;48:221–32. doi: 10.1111/j.1834-7819.2003.tb00035.x. [DOI] [PubMed] [Google Scholar]