Abstract
Background:
Detection of early-stage lung cancer improves during subsequent rounds of screening with low-dose CT and potentially leads to saving lives with curative treatment. Therefore, adherence to annual lung screening is important. We hypothesized that adherence to annual screening would increase after hiring of a dedicated program coordinator.
Methods:
We performed a mixed-methods study in a retrospective cohort of patients who underwent lung screening at our academic institution between January 1, 2014, and March 31, 2018. Patients with baseline lung screening examinations performed between January 1, 2014, and September 30, 2016, with Lung CT Screening Reporting & Data System 1 or 2 scores and a 12-month follow-up recommendation were included. We tracked patient adherence to annual follow-up lung screening over time (before and after hiring of a program coordinator) and conducted a cross-sectional survey of patients nonadherent to annual follow-up to elicit quantitative and qualitative feedback.
Results:
Of the 319 patients who completed baseline lung screening with normal results, 189 (59%) were adherent to annual follow-up recommendations and 130 (41%) were nonadherent. Patient adherence varied over time: 21.7% adherence (10 of 46) before hiring a program coordinator and 65.6% adherence (179 of 273) after the program coordinator’s hire date. Patients reported the following reasons for nonadherence to annual lung screening: lack of transportation, financial cost, lack of communication by physicians, and lack of current symptoms.
Conclusions:
Adherence to annual lung screening after normal baseline studies increased significantly over time. Hiring a full-time program coordinator was likely associated with this increased in adherence.
Keywords: Adherence, low-dose CT, lung cancer, screening
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in the United States [1]. Most patients are diagnosed with lung cancer at advanced stages when there is little chance for cure. Early detection with low-dose CT (LDCT) offers a strategy to diagnose lung cancer at earlier stages when there are more curative treatment options. In two large randomized clinical trials, the National Lung Screening Trial (NLST) and the Dutch-Belgian Randomized Controlled Trial for Lung Cancer Screening in High-Risk Subjects, annual LDCT was found to reduce lung cancer related mortality compared with chest x-ray (NLST) or no screening (Dutch-Belgian Randomized Controlled Trial for Lung Cancer Screening in High-Risk Subjects) in high-risk patients [2,3].
Since publication of the landmark NLST, US policies have been supportive of screening high-risk patients with LDCT [4–6]. These policies include a US Preventive Services Taskforce grade B recommendation for annual screening in the high-risk population, CMS coverage of screening for high-risk individuals, and an American Cancer Society guideline recommending annual screening in high-risk individuals. Although screening with baseline LDCT can identify a small number of early-stage lung cancers, detection improves during subsequent follow-up annual screening [7]. Therefore, annual adherence to annual lung screening is important to decrease lung cancer deaths [8].
In this study, we sought to evaluate adherence to 12-month follow-up LDCT examinations after baseline lung screening. We hypothesized that adherence to annual screening would increase over time after hiring of a dedicated program coordinator. A secondary aim was to understand why patients were nonadherent to the recommended follow-up examinations.
MATERIALS AND METHODS
Participants
We retrospectively collected data on patients who underwent LDCT lung screening at our academic institution between January 1, 2014, and March 31, 2018, and performed a mixed-methods study. Eligible patients had a baseline LDCT lung screening examination performed between January 1, 2014, and September 30, 2016, and had LDCT results described as “negative” (Lung CT Screening Reporting & Data System [Lung-RADS] 1) or “benign appearing or behaving” (Lung-RADS 2) [9]. Lung-RADS is a quality assurance reporting tool that was designed to standardize reporting of lung screening CT examinations [9]. Higher Lung-RADS scores indicate a higher level of suspicion for malignancy of the study findings. For both Lung-RADS 1 and 2 scores, a 12-month follow-up recommendation is standard of care. Exclusion criteria included patients who died, were diagnosed with cancer, had Lung-RADS 3 or 4 scores, had LDCT follow-up recommendation other than 12 months, or had LDCT performed for follow-up rather than baseline purposes.
This single-institution, HIPAA-compliant study was approved by our institutional review board. Survey participants provided informed consent via telephone. A waiver of written consent was obtained from the institutional review board.
Study Setting
Our Lung Screening Program is led by a multidisciplinary team at an academic medical center in the mid-South. All LDCT lung screening studies were performed on one of three CT scanners located in hospital-based outpatient imaging facilities (one GE Revolution EVO 128-slice scanner [GE Healthcare Waukesha, Wisconsin], one Philips CT Ingenuity 128-slice scanner, one Philips Brilliance 64-slice scanner [Philips Healthcare - Andover, Massachusetts]). These CT scanners are accredited by the ACR with acquisition protocols designed specifically for lung screening to maximize dose reduction while maintaining image quality. All patients are scanned in the supine position during a single breath-hold with the field of view extending from the thoracic inlet to the L1 vertebral body. A maximum slice thickness of 1.25 mm was used with volume computed tomography dose index (CTDIvol) < 3.0 millisieverts as required by the ACR. All lung screening LDCT studies were interpreted by radiologists with subspecialty training in chest imaging and reported using Lung-RADS. The official radiology reports of lung screening studies were sent to the patients’ referring providers.
Our Lung Screening Program provides both shared decision making for lung screening that includes face-to-face counseling with use of a decision aid and tobacco treatment counseling that includes behavioral recommendations, pharmaceutical recommendations, and referral to the state Quitline. Before hiring of the program coordinator (a full-time nurse practitioner) in October 2015, shared decision making and tobacco treatment counseling were performed by three thoracic radiologists and two nurse practitioners. After hiring, the program coordinator performed shared decision making, tobacco treatment counseling, and tracking of program participants.
We utilized data from the Lung Screening Program database and supplemented any missing data (such as race or ethnicity and full radiology reports) through the electronic health record. The Program database contains information collected from January 1, 2014, through the present on enrolled patients. Variables include demographic information such as age, race and ethnicity, dates of baseline and follow-up LDCT studies, and Lung-RADS scores.
Primary Outcome: Adherence
The primary outcome was patient adherence to annual follow-up LDCT examination. We defined the recommended follow-up study date as 12 months after the baseline study. The actual follow-up study date was defined as the date of next lung screening study. The interval between the recommended follow-up study date and actual follow-up study was calculated. Patients were considered adherent if this interval was no more than 90 days. Any patient with follow-up LDCT performed more than 90 days after the recommended date of follow-up was considered nonadherent. This adherence window was based on previous literature for adherence in the lung screening setting [10]. This conservative 90-day (3-month) adherence window allowed for scheduling difficulties and other logistical challenges, such as other health issues or transportation [11,12]. We also performed a sensitivity analysis with a longer (180-day) adherence window.
Secondary Outcome: Reasons for Nonadherence
We conducted a cross-sectional survey of patients who completed their baseline LDCT in the first year of the program (January 1, 2014, to December 31, 2014) and either did not return for follow-up or returned for follow-up more than 12 months after the recommended date (more than 2 years after the baseline study). A single study team member (S.L.) contacted patients via telephone and conducted the surveys using a standardized script to elicit quantitative and qualitative feedback. The survey included questions about the lung screening experience and took approximately 10 min to complete. Patients were asked the following questions with responses recorded on a 5-point Likert-like scale (“not at all important,”, “slightly important,” “neutral,” “moderately important,” “extremely important”):
“How important to you is it that tests are free of charge?”
“How important to you is it that you get a personal explanation of the test?”
“How important to you is it that the testing takes place in an environment that is convenient and comfortable?”
Patients were also asked an open-ended question (“Are there any other factors that kept you from returning for your recommended follow-up imaging?”) and were asked to rate potential barriers to returning for lung screening, including transportation, out-of-pocket cost, insurance, time spent at appointments, or support from family or friends.
At the conclusion of the telephone survey, patients were asked if they would like to contact the program coordinator with any additional questions.
Statistical Methods
Participant characteristics were summarized using descriptive statistics, including means and SDs for continuous variables as well as percentages and frequencies for categorical variables. We compared the differences between patient characteristics among those adherent to follow-up LDCT screening and those nonadherent to follow-up LDCT screening using Pearson’s χ2 and Fisher’s exact test. The Fisher’s exact test was used when the expected cell frequency was less than or equal to 5.
The primary analysis utilized a statistical process control chart, the p-control chart, to plot adherence rates over time. The p-control chart offers a graphical display of a proportion in a time series analysis [13]. To produce the p-chart, we collapsed individual participant adherence data to quarterly (3-month blocks) adherence rates and plotted the quarterly adherence rates over time. The rate denominator included all patients eligible for annual follow-up lung screening during the quarter. Patients were considered eligible for annual screening if the recommended follow-up study date fell within the quarter. The rate numerator represented the number of patients eligible for follow-up in the quarter who were categorized as adherent to annual follow-up lung screening. Adherence status for an individual participant was included only in the quarter in which the patient was eligible to return for annual screening (even if the participant returned for screening in a later quarter).
The control limits of a p-chart are standardly positioned 3 SDs from the central line. The central line of this p-chart reflects the mean adherence. The width of the control limits varies by quarter because of the varying sample size within each quarter, with the control limits closer to the central line in quarters with larger sample sizes. As the central line is a proportion, the lower control limit must be greater than or equal to zero.
Pearson’s χ2 test was used to assess differences in overall adherence rates before and after hiring of the program coordinator.
Study sample size was selected by the number of eligible patients enrolled in our institution’s Lung Screening Program.
All analyses were performed using Stata (release 14, 2015; StataCorp, College Station, Texas). A two-sided P < .05 was considered statistically significant.
RESULTS
Participants
The study cohort can be seen in Figure 1. There were 392 patients with LDCT studies having Lung-RADS 1 or Lung-RADS 2 scores between January 1, 2014, and September 30, 2016. We excluded a total of 73 patients (68 studies performed for follow-up rather than baseline, 1 deceased patient, and 4 patients with follow-up recommendation shorter than 12 months). Of the four patients with follow-up recommendation less than 12 months, three were given recommendation for 3-month follow-up because of probable infectious etiology of findings, and one was followed at shorter diagnostic intervals for other findings related to history of breast cancer. Thus, 319 patients with baseline LDCT screening reported as Lung-RADS 1 or 2 and 12-month follow-up recommendation were identified. Of these patients, a total of 189 (59%) were categorized as adherent to annual follow-up recommendations and 130 (41%) patients were categorized as nonadherent to the recommended 12-month LDCT.
Fig 1.
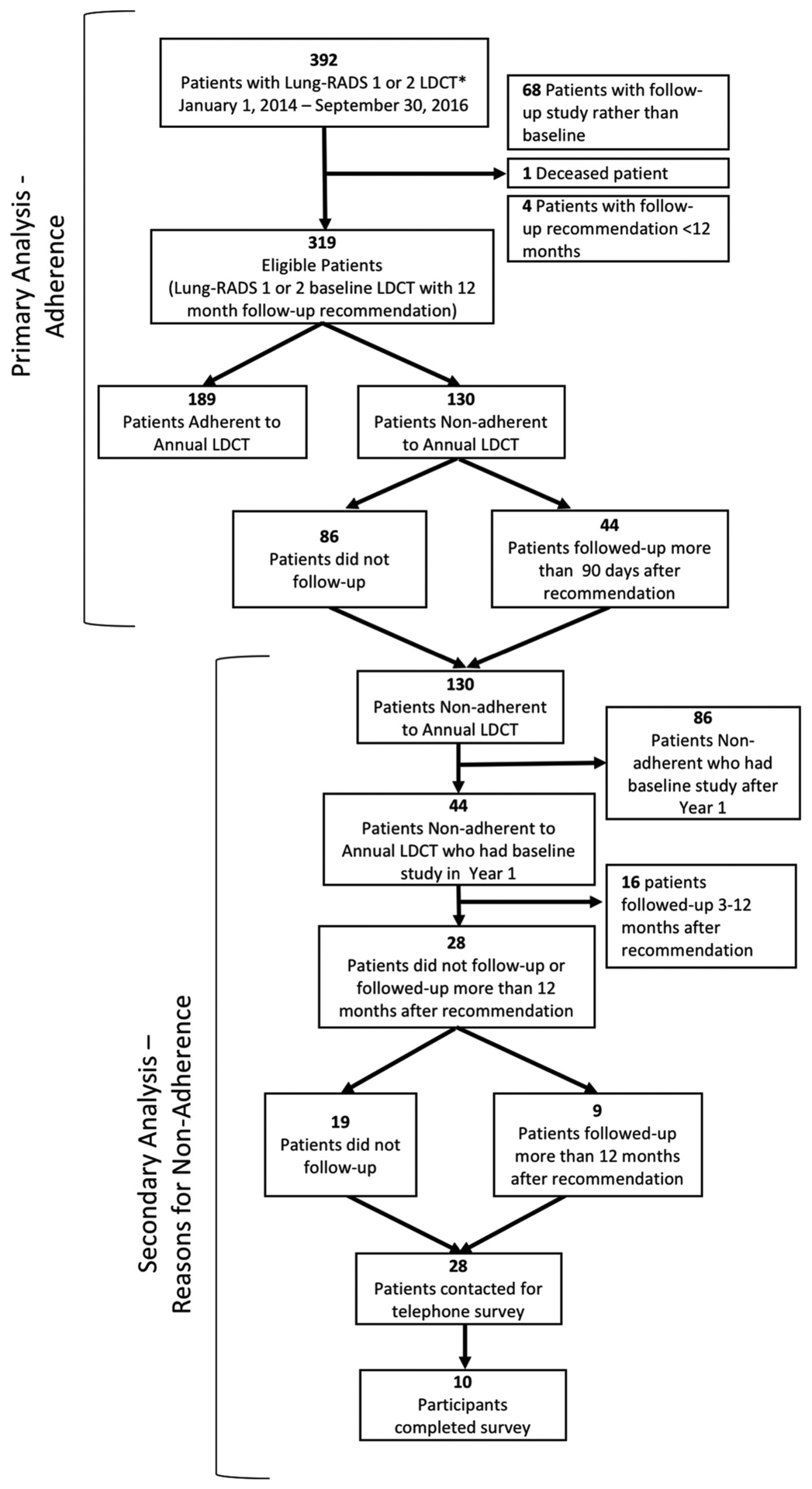
Study cohort. Flowchart demonstrating number of patients meeting inclusion and exclusion criteria for study participation. Primary analysis inclusion criteria: Lung-RADS 1 or 2 LDCT lung screening study between January 1, 2014, and September 30, 2016. Primary analysis exclusion criteria: nonbaseline study, death, follow-up recommendation less than 12 months, cancer diagnosis. *LDCT = low-dose CT; Lung-RADS = Lung CT Screening Reporting & Data System.
Demographics
Study cohort demographics are presented and compared with the NLST LDCT group in Table 1. In the study population, average age was 64 (SD 5.8), 49.2% were female, 86.8% were white, and 1.3% were Hispanic or Latino ethnicity. Patients adherent to annual follow-up LDCT were slightly older (mean age 65.2 [SD 5.4] versus 62.6 [SD 6.1], P < .0005) than patients who were non adherent to annual follow-up LDCT. There was no statistically significant difference in sex, race, or ethnicity between groups.
Table 1.
Study cohort demographics
| Characteristic | Adherent to Annual LDCT (n = 189) | Nonadherent to Annual LDCT (n = 130) | P value* | Total Study Population (n = 319) | NLST LDCT Group† (n = 26,722) |
|---|---|---|---|---|---|
| Age, mean (SD) | 65.2 (5.4) | 62.6 (SD 6.1) | <.0005 | 64.1 (5.8) | |
| Age group, n (%) | .001 | ||||
| <55 | 1 (0.5) | 5 (3.9) | 6 (1.9) | 2 (<0.1) | |
| 55–59 | 36 (19.1) | 35 (26.9) | 71 (22.3) | 11,440 (42.8) | |
| 60–64 | 39 (20.6) | 42 (32.3) | 81 (25.4) | 8,170 (30.6) | |
| 65–69 | 71 (37.6) | 31 (23.9) | 102 (32.0) | 4,756 (17.8) | |
| 70–74 | 35 (18.5) | 12 (9.2) | 47 (14.7) | 2,353 (8.8) | |
| ≥75 | 7 (3.7) | 5 (3.9) | 12 (3.8) | 1 (<0.1) | |
| Gender, female n (%) | 92 (48.7) | 65 (50.0) | .82 | 157 (49.2) | 10,952 (41.0) |
| Race, n (%) | .12 | ||||
| White | 168 (88.9) | 109 (83.9) | 277 (86.8) | 24,289 (90.9) | |
| African American | 14 (7.4) | 9 (6.9) | 23 (7.2) | 1,198 (4.5) | |
| Other or missing | 7 (3.7) | 12 (9.2) | 19 (6.0) | 905 (3.4) | |
| Ethnicity, Hispanic or Latino, n (%) | 2 (1.1) | 2 (1.5) | .41 | 4 (1.3) | 479 (1.8) |
Continuous variable P values obtained using independent two-sample t tests. Categorical variable P values obtained using Pearson’s χ2 test. The Fisher’s exact test was used when the expected cell frequency was less than or equal to 5.
National Lung Screening Trial (NLST) low-dose CT (LDCT) group data from: The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395–409 [2].
Primary Outcome: Adherence
The overall adherence rate was 59.3% (189 of 319). There were 130 patients who were nonadherent to annual follow-up lung screening. Of the 130 patients nonadherent to follow-up, 86 did not follow up with any additional screening and 44 returned for LDCT screening more than 90 days after the recommended follow-up date. Patient adherence (using the 90-day window) varied over time: 21.7% adherence (10 of 46) before hiring a program coordinator and 65.6% adherence (179 of 273) after the program coordinator’s hire date (P < .005). The sensitivity analysis using a 180-day adherence window showed similar results: 30.4% adherence (14 of 46) before hiring the program coordinator and 69.6% adherence (190 of 273) after the program coordinator’s hire (P < .005).
Figure 2 demonstrates increasing trends in quarterly adherence and number of patients eligible for follow-up by quarter over time.
Fig 2.
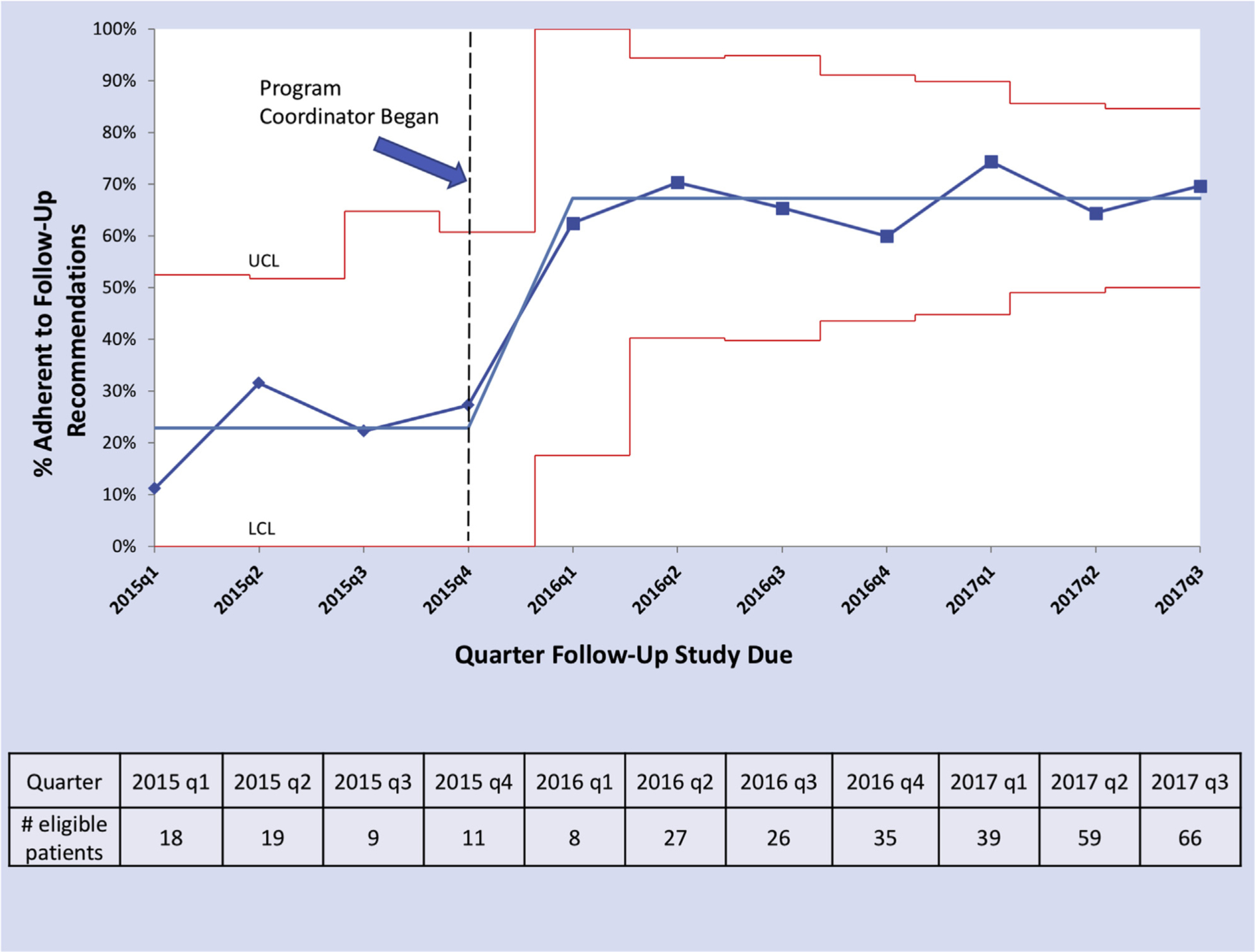
Adherence to annual lung screening follow-up over time. P-chart demonstrating quarterly adherence among those eligible for follow-up screening over time. Dotted black vertical line represents when the program coordinator was hired. Grey horizontal lines represent mean adherence values (before and after hiring of the program coordinator). Red lines represent upper control limit (UCL) and lower control limit (LCL). Control limits vary by quarter and represent 3 SDs from the central line (based on standard statistical process control methodology). As the central line (mean adherence) is a proportion, the LCL must be greater than or equal to zero. Number of eligible patients in each quarter is presented below the p-chart.
Secondary Outcome: Reasons for Nonadherence
Of the 130 patients who were nonadherent to annual follow-up recommendations, 44 were nonadherent in the first year of the program. Of these 44, 28 did not follow up at all or followed up more than 1 year after the recommended follow-up date. We attempted to contact these 28 patients for the telephone survey, of which 10 agreed to participate (36%). Of the 10 participating in the survey, 4 did not follow up with additional screening and 6 returned for LDCT screening more than 12 months after the recommended follow-up date. Of those participating in the survey, 70% indicated tests free of charge are extremely important, 100% indicated a personal explanation of the test is extremely important, and 80% indicated a convenient or comfortable testing environment is extremely important.
Patients indicated reasons for not returning for follow-up included lack of transportation, financial cost, lack of communication by physicians, and lack of current symptoms. Sample patient quotations explaining why he or she did not return for annual lung screening can be seen in Table 2.
Table 2.
Sample patient quotations explaining why he or she did not return for annual lung screening
| Sample Quotations |
|---|
| “If I had symptoms that I was concerned about then I would definitely go for a follow-up. As of now, I feel fine, so I don’t feel there is a need to go back for another follow-up.” |
| “It all depends on the cost.” |
| “I return for follow-up on the recommendation of my doctor. If he says I should return, then I will. If he doesn’t, then I won’t return.” |
| “I skipped my last follow-up because I live too far away. The interstate is poorly designed and I almost got into a serious accident … I wish [the institution] had a satellite facility.” |
| “When I had my lung cancer screening I was a smoker … I had a heart attack and quit smoking cold turkey. I went to determine if 40-plus years of smoking gave me spots on my lungs. Once I quit smoking, lung cancer screening fell off my priority list, so I did not come back for a follow-up.” |
| “Insurance considerations kept us away because I was not sure if my insurance would cover screening.” |
| “As soon as I got the call from my doctor, I was willing to go for my screening.” |
DISCUSSION
We found an increase in adherence to annual lung screening after baseline LDCT examinations after the hiring of a dedicated program coordinator (21.7% before hiring the program coordinator and 65.6% after hiring of the program coordinator, P < .005). The adherence rate in the NLST, a randomized controlled lung screening trial, was more than 90% for follow-up scans [2,7]. Adherence rates to lung screening in the real-world setting have been reported to be range from 77.6% to 85.7% in a centralized program with dedicated program navigators, use of a standardized patient discharge protocol, and use of a dedicated program management system and database [10,14]. The overall lower adherence rate than that reported in previous literature may be because of analysis of adherence at the very start of a lung screening program, the real-world setting rather than clinical trial setting, the small study population, and inclusion of only baseline normal or benign studies; other reported studies also included studies with abnormalities.
Patients identified several barriers to returning for annual lung screening, including lack of transportation, financial cost, lack of communication, and lack of current symptoms. These support barriers previously identified in the literature [15]. Our Lung Screening Program has made attempts to address reported barriers by improving communication with patients (including discussion of cost, insurance coverage, and importance of returning if asymptomatic) and instituting annual follow-up orders created by the program coordinator. These orders are independent of the referring provider and do not require an additional physician signatory. Our outpatient imaging centers send patients reminder letters in advance of the annual screening return visit due date and call patients up to three times to remind them of return annual screening appointments. If patients are unreached after attempted contact from the imaging center, the program coordinator sends an additional letter.
Both the adherence rate in our program and the number of new patients participating in the program increased over time. Although our study was not designed to assess why adherence increased after the hiring of a program coordinator, we speculate that this may be due to programmatic changes to address barriers to lung screening and an increase in program resources. Future work will better inform individual strategies to increase adherence.
Our study provides important results on the impact of resources for lung screening programs on program outcomes and the impact of a lung screening program coordinator on retaining patients with normal studies for annual lung screening. The strengths of this study include its pragmatic design to evaluate adherence to annual lung screening recommendations in a real-world setting and to evaluate adherence to lung screening before and after hiring of a dedicated program coordinator. However, the small population size and single institution limit generalizability. The small sample size before the hiring of the program coordinator limits our ability to establish baseline trends. Additionally, secular trends may account at least partially for the increased trend in adherence noted in our study. The lack of data on national adherence during the time period of this study limits our ability to assess for other factors that may have impacted the observed trend in adherence (ie, CMS coverage policy and US Preventive Services Taskforce guidelines). The secondary analysis is limited by the small number of participants in the survey, the low response rate, and potential for nonresponse and social desirability biases.
Assessment of trends in adherence to annual lung screening follow-up and identification of barriers to annual lung screening are important to understand the resources necessary for lung screening programs. Potential necessary resources include but are not limited to multidisciplinary interprofessional clinical teams; dedicated, trained program personnel; appropriate provider and patient education materials; shared decision-making tools; integrated smoking cessation; evidence-based nodule management algorithms; outreach to marginalized populations; and adequate data collection tools [16]. Appropriation of resources is likely necessary to achieve high-quality, evidence-based lung screening.
TAKE-HOME POINTS.
Adherence to annual lung screening after normal baseline studies increased significantly over time after hiring a full-time program coordinator.
Identification of barriers to and facilitators of annual lung screening is important to understand the resources necessary for lung screening programs.
Appropriation of resources is likely necessary to achieve high-quality, evidence-based lung screening.
Acknowledgments
This study was supported in part by the Department of Veterans Affairs-Office of Academic Affiliations, VA National Quality Scholars Program (L.B.S. and J.A.L.) with resources and the use of facilities at Veterans Affairs-Tennessee Valley Healthcare System, Nashville; Veterans Affairs Geriatric Research, Education, and Clinical Center (GRECC) (L.B.S. and C.L.R.), and the Vanderbilt-Ingram Cancer Center Support Grant CA68485. The authors state that they have no conflict of interest related to the material discussed in this article.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 2.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Koning H, Van Der Aalst C, Ten Haaf K, Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial Abstract PL02.05. Presented at: 19th IASLC World Conference on Lung Cancer, Toronto, Ontario, Canada, September 25, 2018. [Google Scholar]
- 4.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–8. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed May 10, 2019.
- 6.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2019;69:184–210. [DOI] [PubMed] [Google Scholar]
- 7.Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willén L, Berglund A, Bergström S, et al. Educational level and management and outcomes in non-small cell lung cancer. A nationwide population-based study. Lung Cancer 2019;131:40–6. [DOI] [PubMed] [Google Scholar]
- 9.ACR. Lung CT Screening Reporting & Data System. Available at: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed May 10, 2019.
- 10.Alshora S, McKee BJ, Regis SM, et al. Adherence to radiology recommendations in a clinical CT lung screening program. J Am Coll Radiol 2018;15:282–6. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard RA, O’Meara ES, Henderson LM, et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the U.S. Prev Med 2016;89:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gierisch JM, Earp JA, Brewer NT, Rimer BK. Longitudinal predictors of nonadherence to maintenance of mammography. Cancer Epidemiol Biomarkers Prev 2010;19:1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care 2010;22:402–7. [DOI] [PubMed] [Google Scholar]
- 14.Brasher P, Tanner N, Yeager D, Silvestri G. Adherence to annual lung cancer screening within the Veterans Health Administration Lung Cancer Demonstration Project. Chest 2018;154;636A–7A.29705221 [Google Scholar]
- 15.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to lung cancer screening engagement from the patient and provider perspective. Radiology 2019;290:278–87. [DOI] [PubMed] [Google Scholar]
- 16.Mckee BJ, Mckee AB, Borondy-Kitts A, Regis SM, Wald C. Low-dose computed tomography screening for lung cancer in a clinical setting: essential elements of a screening program. J Thorac Imaging 2015;30: 115–29. [DOI] [PubMed] [Google Scholar]


