Abstract
Aims
To compare the safety and efficacy of edoxaban combined with P2Y12 inhibition following percutaneous coronary intervention (PCI) in patients with atrial fibrillation (AF) presenting with an acute coronary syndrome (ACS) or chronic coronary syndrome (CCS).
Methods and results
In this pre-specified sub-analysis of the ENTRUST-AF PCI trial, participants were randomly assigned 1:1 to edoxaban- or vitamin K antagonist (VKA)-based strategy and randomization was stratified by ACS (edoxaban n = 388, VKA n = 389) vs. CCS (edoxaban n = 363, VKA = 366). Participants received edoxaban 60 mg once-daily plus a P2Y12 inhibitor for 12 months, or VKA combined with a P2Y12 inhibitor and aspirin 100 mg (for 1–12 months). The primary bleeding endpoint at 12 months occurred in 59 (15.2%) vs. 79 (20.3%) ACS patients [hazard ratio (HR): 0.73, 95% confidence interval (CI): 0.59–1.02, P = 0.063], and in 69 (19.0%) vs. 73 (19.9%) CCS patients (HR: 0.94, 95%CI: 0.68–1.31, P = 0.708) with edoxaban- and VKA-based therapy, respectively [P for interaction (P-int) = 0.2741]. The main secondary endpoint (composite of CV death, myocardial infarction, stroke, systemic embolic events, or definite stent thrombosis) in ACS patients was 33 (8.5%) vs. 28 (7.2%) (HR: 1.16, 95%CI: 0.70–1.92), compared with 16 (4.4%) vs. 18 (4.9%) (HR: 0.91, 95%CI: 0.47–1.78) CCS patients with edoxaban and VKA-based therapy, respectively (P-int = 0.5573).
Conclusions
In patients with AF who underwent PCI, the edoxaban-based regimen, as compared with VKA-based regimen, provides consistent safety and similar efficacy for ischaemic events in patients with AF regardless of their clinical presentation.
Keywords: Atrial fibrillation, Anticoagulation, Edoxaban, Percutaneous coronary intervention, Acute coronary syndromes, Stable coronary artery disease
See page 4505 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa683)
Introduction
Anticoagulation with currently marketed non-vitamin K antagonist oral anticoagulants (NOACs), in patients with atrial fibrillation (AF) who develop an acute coronary syndrome (ACS) and/or undergo percutaneous coronary intervention (PCI) has been studied in four large controlled randomized trials.1–4
The ENTRUST-AF PCI trial, the most recent among the four NOAC trials, included patients with AF and either ACS or chronic coronary syndrome (CCS), and demonstrated similar rates of bleeding and ischaemic events with an antithrombotic regimen consisting of edoxaban and a P2Y12 inhibitor without aspirin compared with a regimen that included a vitamin K antagonist (VKA) in combination with a P2Y12 inhibitor and aspirin (for 1–12 months).4 Randomization was stratified according to clinical presentation (ACS vs. CCS).4 , 5
The comparative safety and efficacy of combination regimens of antiplatelets and anticoagulants may differ among patients with AF according to their clinical presentation. Therefore, we pre-specified to assess the consistency of the treatment effects among patients with ACS or CCS who underwent PCI and were included in the ENTRUST-AF PCI trial.
Methods
Patients
The details of the ENTRUST-AF PCI trial (ClinicalTrials.gov, NCT02866175) design and results have been reported.4 , 5 In brief, the ENTRUST-AF PCI trial was an international, randomized, open-label phase 3b trial with masked outcome assessment in AF patients undergoing PCI and taking a P2Y12 inhibitor who were assigned to edoxaban or VKA and aspirin 100 mg once-daily.
The trial was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice, as defined by the International Conference on Harmonization. The study protocol was approved by ethics committees or institutional review boards at participating sites; patients provided written informed consent prior to inclusion in the study.
Randomization and treatment
Once written informed consent was obtained, randomization took place between 4 h to 5 days after successful PCI and before hospital discharge. If a staged PCI was planned, consent and randomization took place after completion of the last stage.
Randomization was performed with the use of a central, 24-h, interactive web-response system and stratified according to geographic location (country), clinical presentation (ACS or CCS), and fulfilment of edoxaban dose-reduction criteria. Stratification by geographic region was opted to ensure balance across potential local differences in treatment practices.
The edoxaban-based regimen consisted of treatment with edoxaban 60/30 mg once-daily and clopidogrel bisulphate 75 mg once-daily [or in the presence of a documented clinical need, prasugrel (5 or 10 mg once-daily) or ticagrelor (90 mg twice-daily)] for 12 months. A reduced dose of edoxaban 30 mg once-daily was used in patients if any of the following characteristics were present at the time of randomization or during the study period: moderate or severe renal impairment (calculated creatinine clearance 15–50 mL/min), low body weight of ≤60 kg (132 lbs), or concurrent use of certain potent P-glycoprotein inhibitors.
The VKA-based regimen was dose-adjusted to achieve an international normalized ratio (INR) between 2.0 and 3.0 inclusive, in combination with clopidogrel bisulphate 75 mg once-daily [or in the presence of a documented clinical need, prasugrel (5 or 10 mg once-daily) or ticagrelor (90 mg twice-daily)] for 12 months and aspirin (100 mg once-daily) for a minimum of 1 month and up to 12 months’ duration at the investigator’s discretion. In the control group, INR management was according to the standard of care and performed by the Investigator, the patient’s primary care physician, or other private physician, at a specialized anticoagulation clinic or by patient self-monitoring. The duration of the aspirin treatment in the VKA-based regimen was pre-declared by the investigator prior to randomization guided by the clinical presentation (ACS or CCS) and based upon the CHA2DS2-VASc and HAS-BLED scores. The investigator followed the applicable regional clinical guidelines.5
The assigned regimen was implemented after randomization without any undue delay and the randomized regimens (edoxaban-based or VKA-based) and clopidogrel (or other P2Y12 inhibitor) were continued for 12 months, when the scheduled end-of-treatment was reached. The use of gastric protection drugs (such as pantoprazole) was recommended.
Study visits were performed at 1, 3, 6, 9, and 12 months after randomization, and telephone assessments at 2, 4, 5, 7, 8, 10, and 11 months after randomization. INR monitoring was in accordance with routine care at the respective centre, but the level of INR control was collected and centrally monitored. To assure study drug compliance and maintain INR in target range, at least monthly contact was scheduled in both the edoxaban and VKA treatment groups.
Outcomes
The primary outcome was the first occurrence of International Society on Thrombosis and Haemostasis (ISTH) major or clinically relevant non-major (CRNM) bleeding. ISTH major bleeding is defined as clinically overt bleeding with a haemoglobin drop of ≥2 g/dL or transfusion of ≥2 units of packed red cells, or bleeding occurring at a critical site or resulting in death. CRNM bleeding is defined as any overt bleeding that did not meet the criteria for major bleeding and met ≥1 of the following criteria: requires hospitalization; requires a physician-guided medical or surgical intervention to treat the bleeding, or results in unscheduled contact with a physician (visit or telephone call); results in pain or impairment of daily activities; or results in a physician-guided change in antithrombotic therapy.6
Secondary efficacy outcomes included the composite of cardiovascular death (CVD), myocardial infarction (MI), stroke (modified Valve Academic Research Consortium-2 definition),7 systemic embolic event (SEE), or definite stent thrombosis [according to the academic research consortium (ARC) criteria]; and net clinical benefit, defined as the composite of CVD, stroke, SEE, spontaneous MI, definite stent thrombosis (according to the ARC criteria),8 and ISTH-defined major bleeding. Other outcomes included ISTH major bleeding, CRNM and individual components of the composite outcomes. All outcomes were adjudicated according to standard definitions by an independent committee blinded to treatment assignment.
Statistical analysis
Baseline characteristics were summarized for each of the two subgroups (ACS and CCS) by treatment allocation (edoxaban-based treatment or VKA-based treatment). Categorical variables were compared with the use of the chi-square test or Fisher’s exact test. Continuous variables were compared with the use of Student’s t-test or the Wilcoxon’s rank-sum test for non-normally distributed data.
For each of the outcomes, the proportion of patients with at least one event was calculated and summarized.
All analyses were performed according to the intention-to-treat (ITT) principle, including all patients in the analysis according to the clinical presentation, taking into account all outcome events irrespective of whether the randomized treatment was taken or not; and repeated, for sensitivity reasons, for the on-treatment population. All events were counted from randomization through the 12-month visit or the last day of known outcome status, whichever came first.
We analysed primary and secondary endpoints separately for ACS and CCS patients, based on time to occurrence of first event using a Cox proportional hazard model including treatment group, and the two stratification factors: geographic region and requirement for dose adjustment to derive hazard ratios (HR) with 95% confidence intervals (CIs). In addition, we used an extended Cox model adding clinical presentation and the interaction term between treatment and clinical presentation to perform treatment-by-subgroup interaction tests. There was no pre-specified hierarchical testing of endpoints.
The differences between anticoagulant treatments within each of the subgroups were tested and characterized by HRs and corresponding 95% CIs from a Cox proportional hazards model.
To evaluate whether the low INR rates shortly after randomization led to the apparent violation of the proportional hazards assumption, we performed a post hoc landmark analysis with a landmark in the first 14 days. The 14 days’ time frame was selected based on the INR distribution over time and visual inspection of the Kaplan–Meier (KM) curve.
The primary statistical analysis was performed by statisticians contracted by a contract research organization (Chiltern) and checked for consistency by a second statistician employed by the sponsor. All statistical analyses were performed in SAS (version 9·4).
Results
A total of 1506 patients were randomized from 186 sites in 18 countries (Supplementary material online, Figure S1). Among them, 51.6% (n = 777) presented with an ACS and 48.4% (n = 729) with CCS (Supplementary material online, Table S1). A total of 320 (21.1%) patients were documented with ST-elevation myocardial infarction (STEMI) and 265 (17.6%) with non-STEMI (NSTEMI). Acute coronary syndrome patients (59.3% and 58.1% in the edoxaban and VKA treatment groups, respectively) had more frequently presented with paroxysmal AF than CCS patients (47.4% and 36.2%, respectively) who had in turn a higher incidence of previous cardiovascular events or congestive heart failure (Supplementary material online, Table S1). The median (Q1; Q3) CHA2DS2-VASc scores for the ACS and CCS groups were identical at 4.0 (3.0; 5.0), whereas the mean (SD) scores were 3.85 (1.60) and 3.86 (1.57), respectively. The median HAS-BLED (Q1; Q3) scores were identical at 3.0 (2.0; 3.0), and the mean (SD) scores were 2.82 (0.83) among ACS and 2.91 (0.80) among CCS patients.
The baseline characteristics for the comparison of edoxaban with VKA treatment groups were well balanced across subgroups (Supplementary material online, Table S1). Among patients assigned to the VKA regimen, triple-antithrombotic therapy was taken for a median of 90.0 days (IQR 30–266) in ACS patients and 34.0 days (IQR 29–161) in CCS patients. The median time in therapeutic range (INR 2.0–3.0) was 62.4% (IQR 45.5–74.5) and 64.4% (IQR 46.9–76.4) respectively. Based on the on-treatment analysis set, 71 (18.4%) of 385 ACS patients vs. 59 (16.3%) of 361 CCS patients prematurely discontinued the edoxaban-based regimen and 83 (21.8%) of 380 ACS patients vs. 77 (21.4%) of 360 CCS patients prematurely discontinued the VKA regimen. Adherence to the intended aspirin duration is reported in Supplementary material online, Table S2.
Safety and efficacy outcomes comparing edoxaban with VKA across subgroups at 12 months
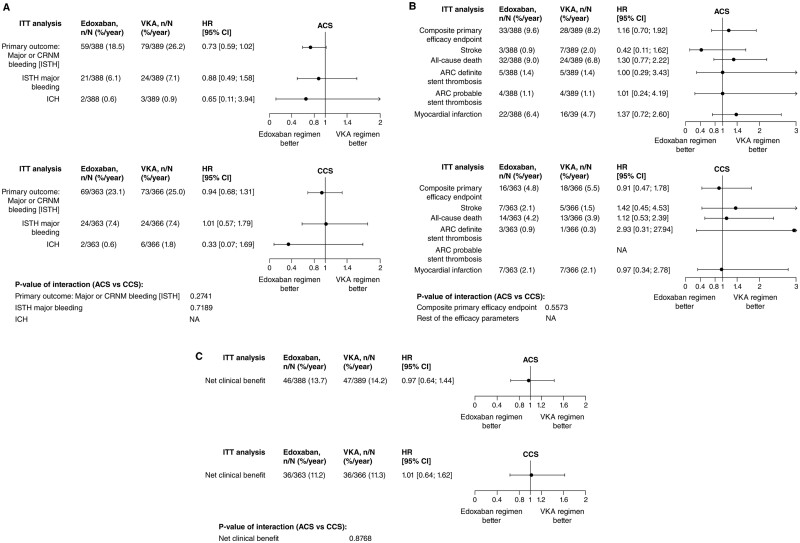
Among patients treated with edoxaban or VKA, the primary endpoint of ISTH major or CRNM bleeding at 12 months occurred in 59 (15.2%) vs. 79 (20.3%) patients, respectively, in the ACS group (HR 0.73, 95% CI 0.59–1.02, P = 0.063), and in 69 (19.0%) vs. 73 (19.9%) patients, respectively, in the CCS group (HR 0.94, 95% CI 0.68–1.31, P = 0.708) in CCS patients, P for interaction (P-int) = 0.2741 (Figure 1A and Supplementary material online, Table S3). Figure 2A–C summarizes the primary, the main secondary, and the net clinical benefit outcomes comparing edoxaban with VKA across both subgroups.
Figure 1.
Study outcomes, Intent-to-Treat analysis (N = 1506) separately by clinical presentation (ACS, chronic coronary syndrome). (A) Safety outcomes; (B) Efficacy outcomes; and (C) Net clinical benefit. ACS, acute coronary syndrome; ARC, academic research consortium; CCS, chronic coronary syndrome; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; ICH, intracranial haemorrhage; ISTH, International Society on Thrombosis and Haemostasis; ITT, intent-to-treat; MI, myocardial infarction; NA, not available; SEE, systemic embolic event; VKA, vitamin K antagonist. P-value of interaction results are based on a Cox model with treatment group, the three stratification factors: geographic region, requirement for dose adjustment and clinical presentation, and the interaction term between clinical presentation and treatment.
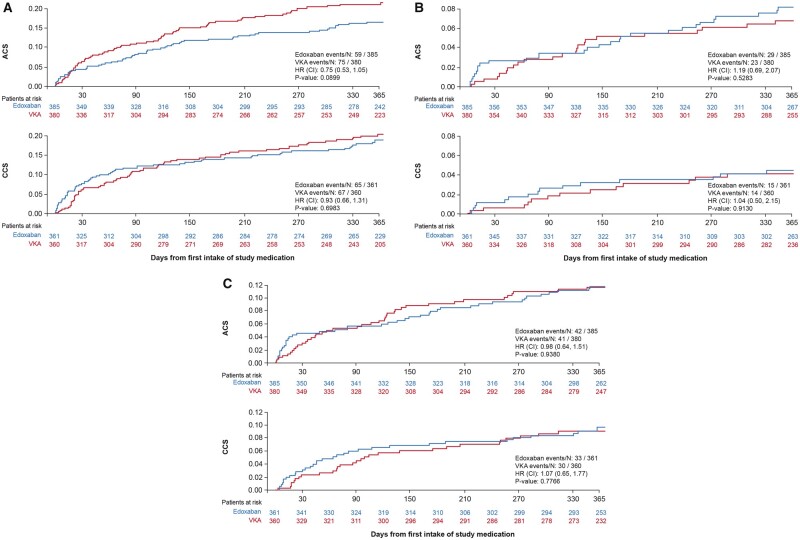
Figure 2.
The primary, the main secondary, and the net clinical benefit outcomes comparing edoxaban with vitamin K antagonist across acute coronary syndrome and chronic coronary syndrome groups over 12 months, mITT analysis set, on-treatment analysis. (A) The primary outcome of major or CRNM bleeding [ISTH] by clinical presentation (acute coronary syndrome or chronic coronary syndrome) over 12 months. (B) The secondary efficacy outcome [composite of cardiovascular death, stroke (VARC-2), systemic embolic event, myocardial infarction or definite stent thrombosis] by clinical presentation (acute coronary syndrome or chronic coronary syndrome) over 12 months. (C) The net clinical benefit defined as the composite of CV death, stroke (VARC-2), systemic embolic event, myocardial infarction, definite stent thrombosis and major bleeding (ISTH) by clinical presentation (acute coronary syndrome or chronic coronary syndrome) over 12 months. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; MITT, modified intention-to-treat; SEE, systemic embolic event; VARC, valve academic research consortium; VKA, vitamin K antagonist.
Rates of bleeding according to ISTH, Thrombolysis in Myocardial Infarction (TIMI), and bleeding Academic Research Consortium (BARC) definitions were consistent (Supplementary material online, Table S4).
The main secondary efficacy endpoint was similar when comparing edoxaban vs. VKA in both subgroups and occurred in 33 (8.5%) vs. 28 (7.2%) ACS patients [HR 1.16 (0.70–1.92), P: 0.575], and in 16 (4.4%) vs. 18 (4.9%) CCS patients [HR 0.91 (0.47–1.78), P: 0.783] with no statistical evidence for treatment-by-subgroup interaction (P-int: 0.5573) (Figure 1B and Supplementary material online, Table S3).
The net clinical benefit outcomes occurred in 46 (11.9%) vs. 47 (12.1%) ACS patients [HR 0.97 (0.64–1.44), P: 0.867], and 36 (9.9%) vs. 36 (9.8%) CCS patients [HR 1.01 (0.64–1.62), P: 0.957], with no statistical evidence for interaction (P-int: 0.8768) (Figure 1C and Supplementary material online, Table S3).
Risk estimates for the comparison of both groups regarding Academic Research Consortium (ARC)-defined definite stent thrombosis were imprecise due to the low number of events. However, tests for treatment-by-subgroup interaction were not significant with respect to MI (P-int = 0.409), stroke (P-int = 0.195), and definite stent thrombosis (P-int = 0.518). Most stent thrombosis in the edoxaban-treated patients occurred early after randomization (Table 1). The type of MI and timing, by clinical presentation are shown in Table 2.
Table 1.
Occurrence of probable and definite stent thrombosis after randomization
| N (n) | Edoxaban | VKA | Total | |
|---|---|---|---|---|
| Definite ST | ACS | 5 (4) | 5 (0) | 10 (4) |
| CCS | 3 (2) | 1 (0) | 4 (2) | |
| total | 8 (6) | 6 (0) | 14 (6) | |
| Probable ST | ACS | 4 (4) | 4 (2) | 8 (6) |
| CCS | 1 (1) | 0 (0) | 1 (1) | |
| Total | 5 (5) | 4 (2) | 9 (7) | |
| Definite or probable ST | ACS | 9 (8) | 9 (2) | 18(10) |
| CCS | 4 (3) | 1 (0) | 5 (3) | |
| Total | 13 (11) | 10 (2) | 23 (13) |
N is total number of patients (n = within 30 days after randomization).
ACS, acute coronary syndromes; CCS, chronic coronary syndromes; ST, stent thrombosis; VKA, vitamin K antagonist.
Table 2.
Type of myocardial infarction by clinical presentation
| ACS | CCS | ||||||
|---|---|---|---|---|---|---|---|
| Type of MI | Groups (N) |
Type of MI | Groups (N) |
||||
| Edoxaban | VKA | Total | Edoxaban | VKA | Total | ||
| Type 1 | 11 | 6 | 17 | Type 1 | 0 | 6 | 6 |
| Type 2 | 3 | 3 | 6 | Type 2 | 2 | 0 | 2 |
| Type 3 | 1 | 0 | 1 | Type 3 | 0 | 0 | 0 |
| Type 4aa | 0 | 0 | 0 | Type 4aa | 0 | 0 | 0 |
| Type 4b | 4 (4) | 4 (0) | 8 (4) | Type 4b | 3 (2) | 1 (0) | 4 (2) |
| Type 4c | 3 | 3 | 6 | Type 4c | 2 | 0 | 2 |
| Total | 22 | 16 | 38 | Total | 7 | 7 | 14 |
Type 4b denotes MI associated with stent thrombosis as detected by coronary angiography or autopsy in the setting of myocardial ischaemia in combination with a rise and/or fall of cardiac biomarkers with at least one value above the 99th percentile URL. Depicted as N = total number (n = number from randomisation to 30 days).
Analysed according to the SCAI consensus definition.9
All-cause death occurred in 32 (8.20%) vs. 24 (6.20%) ACS patients [HR 1.30 (0.77–2.21), P: 0.328], and 14 (3.90%) vs. 13 (3.60%) CCS patients [HR 1.12 (0.53–2.39), P: 0.770], with no statistical evidence for interaction (P-int: 0.7074) (Figure 1B and Supplementary material online, Table S5).
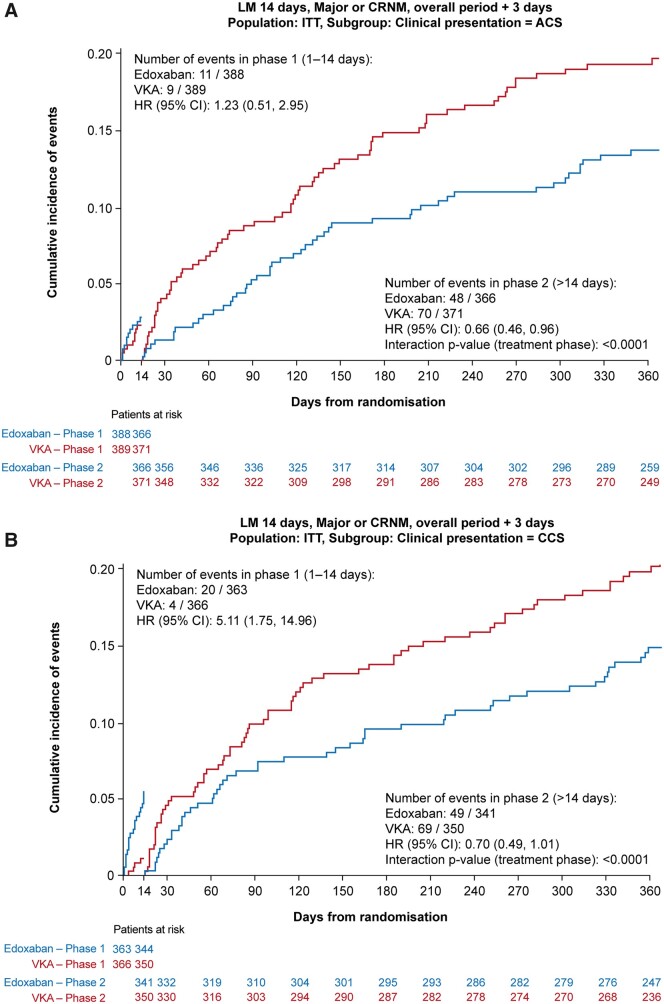
Post hoc landmark analysis
The landmark analyses are presented in Figure 3A and B. Analyses up to 14 days, and from 15 days to 1 year did not show any significant interactions according to clinical presentation with respect to the primary bleeding endpoint.
Figure 3.
The landmark primary bleeding endpoint analyses up to 14 days, and from 15 days to 1 year according to clinical presentation (A) acute coronary syndrome or (B) chronic coronary syndrome, ITT analysis. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; CI, confidence interval; HR, hazard ratio; CRNM, clinically relevant non-major; ITT, intention-to-treat; KM, Kaplan–Meier; VKA, vitamin K antagonist.
Discussion
In this pre-specified subgroup analysis of the ENTRUST-AF PCI trial, we analysed safety and efficacy endpoints according to clinical presentation over 12 months. For the primary composite endpoint of ISTH major and CRNM bleeding, we did not find a difference in treatment effects between ACS and CCS patients treated with edoxaban and a P2Y12 inhibitor and VKA in combination with a P2Y12 inhibitor plus aspirin for 1–12 months. Furthermore, there was no evidence for differences in treatment effects between subgroups in terms of the main ischaemic endpoint, the net clinical benefit, or each of the individual components of the composite endpoints in terms of CVD, MI, stroke, SEE, or definite stent thrombosis. The results of this analysis among two key patient subgroups are consistent with the main ENTRUST-AF PCI study outcomes for the primary and main secondary outcomes. The numerically higher risk of ISTH major or CRNM bleeding in the VKA-treated patients compared with edoxaban at 12 months was consistent across both subgroups. There was a favourable HR for VKA relative to edoxaban in the first 2 weeks that converted to an almost similar HR for both subgroups that continuously favoured edoxaban throughout the remainder of the study period. The early hazard of bleeding events with edoxaban over VKA was more pronounced in the CCS patients as compared to the ACS patients. The lower than expected bleeding rate on VKA in the first 2 weeks may be explained by the high proportion of patients with an INR <2, no bridging with low molecular weight heparins was used in VKA patients.4 The time from index PCI to randomization was shorter in the CCS patients as compared with the ACS patients.
In the ACS group, the numerically lower risk of bleeding with edoxaban-treated patients compared with VKA-treated patients was offset by the higher risk of MI without net difference in terms of all ischaemic events. However, there was a numerically higher incidence in all-cause death in ACS patients. The latter may be due to chance considering that there was no difference in definite or probable stent thrombosis (Table 1 and Supplementary material online, Table S5) between the two treatment groups.
In the AUGUSTUS trial, an antithrombotic regimen consisting of apixaban 5 mg twice-daily and a P2Y12 inhibitor without aspirin demonstrated superior safety and similar efficacy in patients with AF who had ACS, whether managed medically or with PCI, and those undergoing elective PCI compared with regimens that include VKA, aspirin, or both.10 Oldgren et al.11 reported consistent findings from RE-DUAL PCI in favour of both dabigatran doses (110 and 150 mg twice-daily) as part of dual-antithrombotic therapy over VKA-based triple therapy in the pre-specified subgroup analysis of ACS patients undergoing PCI.
In a meta-analysis of four randomized trials that included 7168 AF patients after PCI or with ACS12; compared with triple-antithrombotic therapy with warfarin, dual-antithrombotic therapy with NOACs was associated with less major bleeding and less major or CRNM bleeding, and a non-significantly higher composite of death and ischaemic events but with no difference in mortality. Likewise, similar findings were observed in a pooled analysis of 10 938 patients as well as a meta-analysis of four randomized trials encompassing 10 234 AF patients after PCI or with ACS.13 , 14 The meta-analysis showed that compared with triple-antithrombotic therapy with warfarin, dual-antithrombotic therapy with NOACs was associated with less major bleeding and less major or CRNM bleeding, but increased risk of stent thrombosis and MI, however, with no difference in all-cause and cardiovascular mortalities, stroke and major adverse cardiovascular events.14 Furthermore, pooled data from NOAC trials (PIONEER AF-PCI, RE-DUAL PCI and AUGUSTUS) suggest that despite the safety of dual therapy, an initial course of NOAC-based triple therapy may be desirable in most AF patients.15
Of note, this subgroup analysis of ENTRUST-AF PCI trial points to a potential benefit of aspirin in the prevention of stent thrombosis and MI in the specific group of patients with ACS undergoing PCI who are at high risk for recurrent ischaemic events. We concur with the AUGUSTUS trial investigators that clinical decision-making is needed with regards to a 14- to 30-day course of aspirin in patients with AF receiving an NOAC in combination with a P2Y12-inhibitor immediately after PCI.10 , 16 , 17
In both the ENTRUST-AF PCI and the AUGUSTUS trials, the approved doses of edoxaban and apixaban, respectively, for the prevention of stroke and SEE in patients with AF, were tested. Dose adjustments were as per the drug label.
Limitations
The results of this pre-specified subgroup analysis of the ENTRUST-AF PCI trial need to be considered in view of several limitations. Although the main study was powered for the primary safety endpoint of ISTH major or CRNM bleeding, the results reported here separately for both patient subgroups are underpowered. The main study was not powered for the main efficacy outcome, nor was this subgroup analysis. All patients received aspirin during hospitalization for the index procedure. Therefore, the term dual-antithrombotic therapy refers only to the period after randomization.
Conclusion
An antithrombotic regimen consisting of edoxaban and a P2Y12 inhibitor without aspirin provides consistent safety and efficacy in patients with AF regardless of their clinical presentation throughout 1 year. A trend towards a safety benefit with the edoxaban-based strategy compared with the VKA-based strategy was noticed in ACS patients but not in CCS patients. This finding merits further investigation in a patient-based pooled analysis of the four major trials in the field, or in a dedicated trial.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported and funded by Daiichi Sankyo.
Conflict of interest: P.V. discloses personal fees from Daiichi Sankyo during the conduct of the study; and personal fees from AstraZeneca, Bayer Health Care, CLS Behring, and Terumo outside the submitted work. A.G. discloses honoraria and speaker fees from Astra Zeneca, Bayer Health Care, Berlin-Chemie, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Medtronic, Novartis, and Omeicos. Research has been supported by Josef-Freitag Stiftung and Deutsche Herzstiftung e. V. outside the submitted work. M.V. reports grants and personal fees from Abbott, Alvimedica, Amgen, Bayer, Bristol-Myers Squibb SA, Coreflow, Daiichi Sankyo, Vifor, Idorsia, Terumo, and iVascular; grants and personal fees from grants from AstraZeneca and Medicure, outside the submitted work. L.E. discloses consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boehringer, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and sanofi-aventis. Research has been supported by German Research Foundation (DFG) and German Heart Foundation outside the submitted work. T.L. reports personal fees from Abbott, Boston Scientific, Bayer, Boehringer, Daiichi Sankyo, and Pfizer outside the submitted work. R.U. reports personal fees from Daiichi Sankyo, Abbott, Bayer Healthcare, Boston Scientific, Medtronic, Astra Zeneca, Berlin-Chemie Menarini, Servier and Terumo, outside the submitted work. F.M. reports personal fees from AstraZeneca, Bayer, Pfizer-BMS, Boehringer-Ingelheim and Daiichi Sankyo outside the submitted work. F.S. reports personal fees from Astra Zeneca, Bayer, Amgen, Novartis, Sanofi, MSD, Daiichi Sankyo, Servier, outside of the submitted work. P.L., P.-E.R., R.S., and W.Z. are employees of Daiichi Sankyo Europe. J.T. reports personal fees from AstraZeneca, Bayer, and Boehringer Ingelheim outside the submitted work.
Supplementary Material
Contributor Information
Pascal Vranckx, Department of Cardiology & Critical Care Jessaziekenhuis Hasselt, Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium.
Marco Valgimigli, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Lars Eckardt, Atrial Fibrillation Network (AFNET), Münster, Germany; Department of Cardiology and Angiology, Division of Electrophysiology, University of Muenster, Muenster, Germany.
Thorsten Lewalter, Atrial Fibrillation Network (AFNET), Münster, Germany; Department of Cardiology, Hospital Munich South, Munich, Germany; University of Bonn, Bonn, Germany.
Ramunas Unikas, University of Health Sciences hospital, Kaunas, Lithuanian, Lithuania.
Francisco Marin, Hospital Universitario Virgen de la Arrixaca, IMIB-Arrixaca, CIBERCV, Murcia, Spain.
François Schiele, Chru Jean Minjoz, Besancon, France.
Petra Laeis, Daiichi Sankyo Europe GmbH, München, Germany.
Paul-Egbert Reimitz, Daiichi Sankyo Europe GmbH, München, Germany.
Rüdiger Smolnik, Daiichi Sankyo Europe GmbH, München, Germany.
Wolfgang Zierhut, Daiichi Sankyo Europe GmbH, München, Germany.
Jan Tijssen, Department of Cardiology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands; Cardialysis, Rotterdam, The Netherlands.
Andreas Goette, Atrial Fibrillation Network (AFNET), Münster, Germany; Department of Cardiology, St. Vincenz-Hospital, Paderborn, Germany and Working Group of Molecular Electrophysiology, University Hospital Magdeburg Magdeburg, Germany.
References
- 1. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH; RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 2. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 3. Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, Van de Werf F, Armstrong PW, Mahaffey KW, Harrington RA, Ohman EM, White HD, Wallentin L, Granger CB. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart 2008;94:867–873. [DOI] [PubMed] [Google Scholar]
- 4. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335–1343. [DOI] [PubMed] [Google Scholar]
- 5. Vranckx P, Lewalter T, Valgimigli M, Tijssen JG, Reimitz PE, Eckardt L, Lanz HJ, Zierhut W, Smolnik R, Goette A. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: rationale and design of the ENTRUST-AF PCI trial. Am Heart J 2018;196:105–112. [DOI] [PubMed] [Google Scholar]
- 6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 7. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 8. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 9. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Windecker S, Lopes RD, Massaro T, Jones-Burton C, Granger CB, Aronson R, Heizer G, Goodman SG, Darius H, Jones WS, Aschermann M, Brieger D, Cura F, Engstrom T, Fridrich V, Halvorsen S, Huber K, Kang HJ, Leiva-Pons JL, Lewis BS, Malaga G, Meneveau N, Merkely B, Milicic D, Morais J, Potpara TS, Raev D, Sabate M, de Waha-Thiele S, Welsh RC, Xavier D, Mehran R, Alexander JH; AUGUSTUS Investigators. Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome treated medically or with percutaneous coronary intervention or undergoing elective percutaneous coronary intervention: insights from the AUGUSTUS trial. Circulation 2019;140:1921–1932. [DOI] [PubMed] [Google Scholar]
- 11. Oldgren J, Steg PG, Hohnloser SH, Lip GYH, Kimura T, Nordaby M, Brueckmann M, Kleine E, Ten Berg JM, Bhatt DL, Cannon CP. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur Heart J 2019;40:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shurrab M, Danon A, Alnasser S, Glover B, Kaoutskaia A, Henderson M, Newman D, Crystal E, Ko D. Dual-antithrombotic therapy with DOACs after acute coronary syndrome or percutaneous coronary intervention in atrial fibrillation: a meta-analysis of randomized controlled trials. Can J Cardiol 2020;36:135–142. [DOI] [PubMed] [Google Scholar]
- 13. Ando G, Costa F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol 2020;302:95–102. [DOI] [PubMed] [Google Scholar]
- 14. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–3767. [DOI] [PubMed] [Google Scholar]
- 15. Potpara TS, Mujovic N, Proietti M, Dagres N, Hindricks G, Collet JP, Valgimigli M, Heidbuchel H, Lip G. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace 2020;22:33–46. [DOI] [PubMed] [Google Scholar]
- 16. Alexander JH, Wojdyla D, Vora AN, Thomas L, Granger CB, Goodman SG, Aronson R, Windecker S, Mehran R, Lopes RD. Risk/benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation 2020;141:1618–1627. [DOI] [PubMed] [Google Scholar]
- 17. Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RF, Vinereanu D, Granger CB, Goodman SG, Aronson R, Windecker S, Thiele H, Valgimigli M, Mehran R, Alexander JH. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation 2020;141:781–783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.