Abstract
Purpose:
To determine the relative impact of contact lens-assisted corneal cross-linking (CACXL) on regional corneal stiffness and Standard protocol CXL (CXL) using Brillouin microscopy.
Methods:
CXL and CACXL were performed on 30 intact fresh porcine eyes. Depth profile of stiffness variation and averaged elastic modulus of anterior, middle and posterior stroma were determined by Brillouin maps. Corneas were cut into strips to conduct mechanical stress-strain tests after Brillouin microscopy to verify stiffness difference between CXL and CACXL. Each eye served as its own control.
Results:
CXL had a greater impact on corneal stiffness, with a maximum increase of 5.74 % compared to 3.99 % for CACXL (P<0.001). CXL increased longitudinal modulus by 7.8 % in the anterior, 1.7 % in the middle, and −0.7 %. in the posterior regions as compared to CACXL, which increased longitudinal modulus by 5.5 % in the anterior (P<0.001), 1.2 % in the middle (P=0.15), and −0.4 % in the posterior regions (P=0.6). Mechanical stress-strain tests showed that at 10% strain averaged Young’s modulus for CXL was 5 MPa and for CACXL was 2.97 MPa (P<0.001).
Conclusions:
Both CACXL and Standard protocol CXL induced significant corneal stiffening primarily concentrated in the anterior cornea for both techniques. CACXL leads to less stiffening compared with CXL. An Attenuated but continuous stiffening effect can be observed through the whole cornea for both CACXL and CXL, though CACXL has a smaller stiffness gradient.
Keywords: Brillouin, microscopy, corneal cross-linking, keratoconus, contact lens, contact lens associated corneal cross linking, CXL
Corneal cross-linking (CXL) is an effective treatment to halt progression of progressive keratoconus.1 With extra fibrillar covalent bonds induced by photosensitizer riboflavin and ultraviolet A light of 370 nm, corneal Young’s modulus can be increased by a factor of 4.5 in human eyes.2 However, there is increased risk of endothelial damage in thin corneas.3–6
Several modified CXL protocols have been developed to address thin corneas, which are frequently observed in advanced keratoconus. These modified protocols can be categorized into two groups: decreasing UV exposure or increasing stromal thickness. To decrease UV exposure, thicker riboflavin film or higher riboflavin concentration can lead to greater absorption of the UV light, thereby reducing efficacy but also potential endothelial toxicity.7,8 Accelerated CXL protocols, with higher UV intensity for shorter irradiation time with the same total UV irradiation dose can also halt the progression of keratoconus with potentially less depth of corneal penetration and therefore less significant endothelial cell loss.9 To increase stromal thickness, stroma can be swelled by hypoosmolar riboflavin,10 covered by partial epithelium with customized epithelial debridement,11 or shielded by a riboflavin-soaked contact lens.12–14 Among these protocols, hypo-osmolar swelling has been found to be less efficacious,15,16 while contact lens-assisted corneal cross-linking (CACXL) provides a straightforward option for CXL in thin corneas without requiring complicated equipment or precise manipulation. The safety of this protocol has been demonstrated recently.12,13 Ex vivo overall biomechanical properties evaluated with stress-strain tests have shown reduced efficacy,14 most probably due to a relative lack of oxygen in the deeper layers of the cornea,17 depth-dependent stiffness distribution remains unknown.
In this study, a non-contact optical method, confocal Brillouin microscopy, was used to determine axial stiffness distribution before and after Standard CXL and CACXL in porcine eyes, and these results were compared with traditional mechanical stress-strain evaluation to determine the comparative difference in both total and regional stiffening effects induced by each protocol.
METHODS
Specimen preparation
The study was performed on 30 fresh porcine eyes which were kept in BSS during shipment and were used within 24 hours after collection. These 30 eyes were equally divided into two groups: standard CXL and CACXL. Pachymetry (Pachette 4, DGH Technology) was performed on all eyes before experiments to guarantee similar hydration status for the two groups. The average corneal thickness in standard CXL group was 1092 μm ± 37 μm, while in CACXL group the thickness was 1082 ±28 μm (p=0.38). For all eyes, adherent muscle and adipose tissue was detached without damaging the whole globe to allow for stable fixation, and epithelium was carefully removed with a crescent knife. Riboflavin solution was prepared by diluting riboflavin and dextran to 0.1% and 10% separately with 1X PBS. Unlike 20% dextran, which is generally used in standard CXL, hypo-osmolar riboflavin solution was used because of long-term disputes over the influence of the water content in Brillouin microscopy.18,19 10% dextran solution was used in previous Brillouin measurements to avoid corneas dehydration during CXL,20 which was also confirmed by Brillouin microscopy, so that stiffening effect mainly derived from CXL and dehydration had little influence on it. Contact lenses without UV filters were selected for CACXL (Bausch+Lomb, made of hilafilcon B, hydration 59%, diameter 14.2 mm, PWR −0.25). Brillouin scan was performed on all eyes before and after standard CXL or CACXL to make each eye serve as its own control.
Cross-linking protocol
Cross-linking protocols are shown in Figure 1. For Standard CXL, intact porcine eyes were soaked by dropping the riboflavin solution on corneas directly every 2 minutes for 30 minutes. After soaking, eyes were placed under a UV-A light (CCL-365 Vario, MLase AG) and exposed to a power density of 3 mW/cm2 for 30 minutes. During exposure, riboflavin was added on corneas every 2 minutes.
Figure 1.
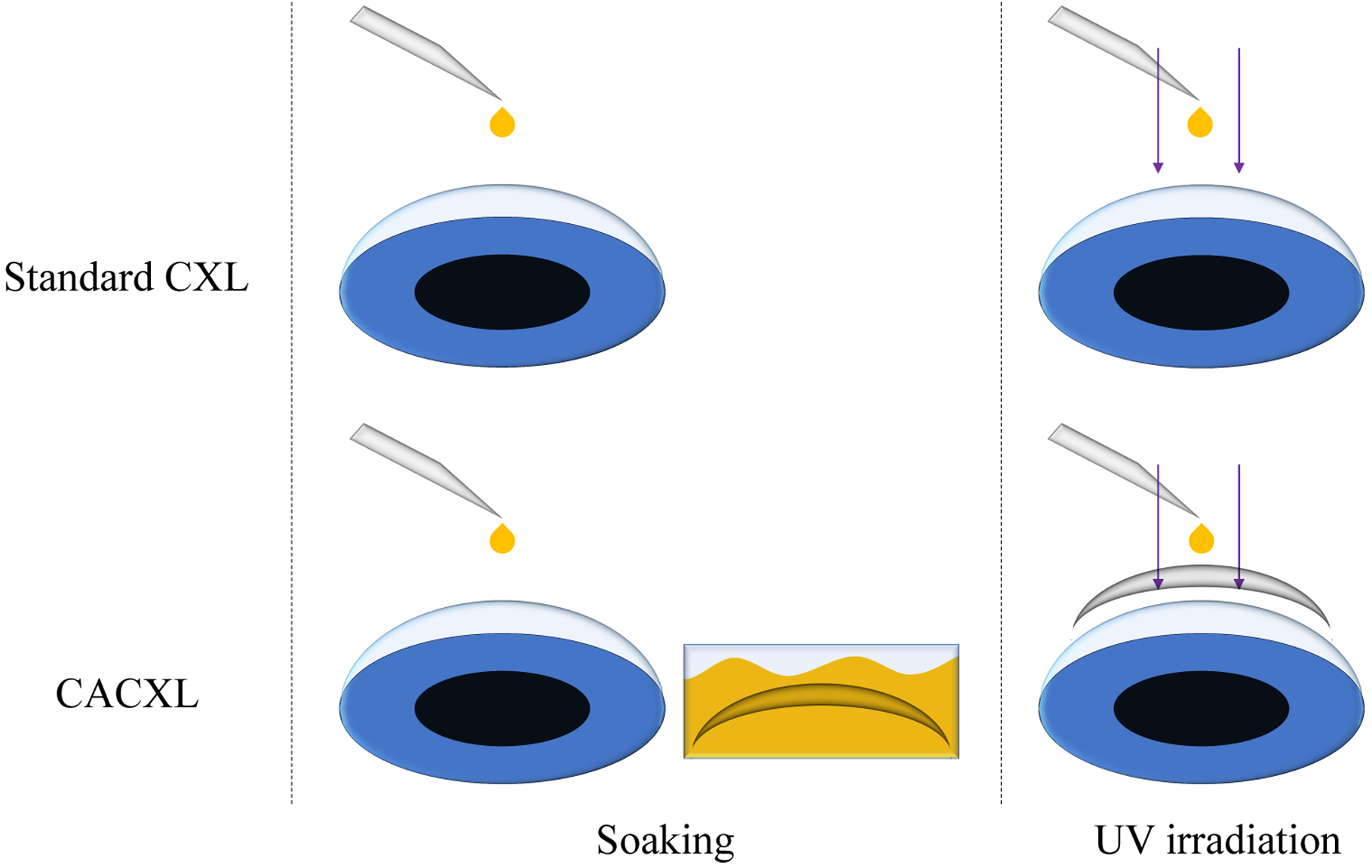
CXL protocols for standard CXL and CACXL. Hydrophilic contact lenses were used to make riboflavin solution penetrate through contact lenses and diffuse into the stroma. Thus, there was no need to lift contact lenses during UV irradiation.
Similar soaking procedure was conducted on eyes for CACXL. The difference was that the contact lens was immersed in the riboflavin solution for 30 minutes prior to UV-A irradiation. Then, the fully soaked contact lens was placed on the cornea to serve as a barrier between the UV-A light and the cornea. During irradiation, riboflavin solution was dropped on the contact lens directly every 2 minutes for 30 minutes.
Biomechanical properties measured by Brillouin microscopy
Spontaneous Brillouin scattering derives from the interaction between light and acoustic phonons generated by inherent density fluctuation, providing direct information on the speed of sound and viscoelastic properties.21,22 The relation between the Brillouin shift Δf and the speed of sound vA can be expressed as
| (1) |
where ρ is the density, n is refractive index, λ is probing laser wavelength and M’ is the longitudinal modulus which can represent the stiffness of the specimen.
To measure the optical frequency shift, a Brillouin spectrometer was built, using two virtually imaged phased arrays (VIPAs) as the core dispersion components.23,24 A continuous-wave 532 nm laser (torus 532, Laser Quantum) served as the probing light. 12 mW was delivered onto the specimen through a 40x objective lens with a numerical aperture of 0.6 (LUCPLFLN 40x, Olympus). The scattered light, collected by the same objective lens, was coupled into the Brillouin spectrometer and imaged by an EMCCD (iXon-L-897, Andor Technology) with an exposure time of 0.2 s. When calculating M’, ρ/n2 was treated as a constant of 0.57 g/cm3 based on previous researches.25–27 This estimation introduced a 0.3% uncertainty throughout the cornea.28–30
Brillouin image analysis
A cross-section of 200 μm (lateral) × 1200 μm (axial) in the center of a cornea was selected for each scan to image Brillouin shifts as a function of depth. The step sizes in both directions are 10 μm. In Brillouin maps, red represented softness and blue rigidity. To compare depth-dependent stiffness variation after different CXL protocols, maximum increase ratios of Brillouin shifts were used. Moreover, the cross-sections were equally divided into three segments (anterior, middle and posterior) and averaged percentage changes of longitudinal modulus in these 3 segments were calculated to indicate depth information.28,30
Stress-strain tests
To verify CXL effect and support longitudinal modulus measured by Brillouin microscopy, mechanical stretching tests were operated on all corneas after cross-linking once Brillouin scan was completed, using a stress-strain extensometer (BT2-FZ0.5TS, Zwick/Roell) equipped with a 5 N capacity load cell. The stretching tests were not performed on untreated corneas because intact eyeballs were needed for CXL and the goal was to investigate CXL efficacy on the same cornea. A corneoscleral strip (5 mm width) was prepared centrally in the horizontal axis from each eye. Its thickness was determined by performing pachymetry on the intact eyeball immediately after standard CXL or CACXL. Two ends of each strip were clamped between two grips with an initial gap of 5 mm.
A preload of 0.2 N was applied on the strip first. After 30 s hanging, three conditioning cycles were applied to realign fibril orientation and stabilize mechanical properties. In each cycle, distance between the two grips increased at a speed of 2 mm/min until a force of 2 N was reached. Then, a new stretching test was operated to measure Young’s modulus. Forces and corresponding strains were recorded by the extensometer automatically. The thickness and width of the strip were used to convert force to stress. After the transformation, stress-strain dots were plotted and fitted by an exponential function σ=Aexp(B×ε). Young’s moduli, E, were calculated from the gradient of the exponential function at strains of 4%, 6%, 8% and 10% separately.
RESULTS
Brillouin images of standard CXL and CACXL
Representative Brillouin shifts for standard CXL and CACXL are shown in Figures 2 and 3. To demonstrate the stiffening effect of the CXL treatments, untreated corneas were scanned from the anterior to aqueous humor before CXL (Figure 2(a) and Figure 3(a)) and again after CXL (Figure 2(b) and Figure 3(b)). For better visualization of depth-dependent stiffness change, depth profiles were calculated from the cross-sectional images, Figure 2(a) and (b), by averaging over the transverse axis. Figure 2(c) shows the depth profiles of this representative sample before and after standard CXL. The abscissa represented geometric thickness, rescaled by thickness measured by the pachymeter, taking the refractive index into account. As corneas became stiffer after CXL, theoretically the speed of sound set in the pachymeter should be updated to a larger value to accurately measure corneas after CXL. However, in reality there was minimal effect on measurements based on our experimental results. The influence can be estimated in the following way. In light of Eq. 1, the speed of sound is proportional to the Brillouin shift. Assuming the maximum frequency shift after CXL is fmax, the minimum shift after CXL is fmin, and the averaged shift before CXL is still f0, the variation, [(fmax+fmin)/2-f0]/f0, for the standard CXL group was (1.53±0.44) %, while that for the CACXL group was (1.18±0.48) %. Thus, the thickness error caused by the speed of sound (vA) was limited to ~1.5 %. If taking the increase of the refractive index after CXL into account, the influence was likely even less. A horizontal shift was added to the depth profile after CXL to make the two depth profiles overlap in the posterior for clearer comparison.
Figure 2.

Representative Brillouin results for the standard CXL group. (a) Distribution of Brillouin shifts in an untreated eye. Aqueous humor is the bottom part of the color map. (b) Distribution of Brillouin shifts in the same eye after standard CXL. According to Eq. (1), a higher Brillouin shift correlates to a larger longitudinal modulus. Increase of longitudinal modulus is elucidated with different colors. (c) Profiles of Brillouin shifts in (a) and (b) along the depth, averaged in lateral direction. The way to divide the cornea into 3 segments is also shown.
Figure 3.
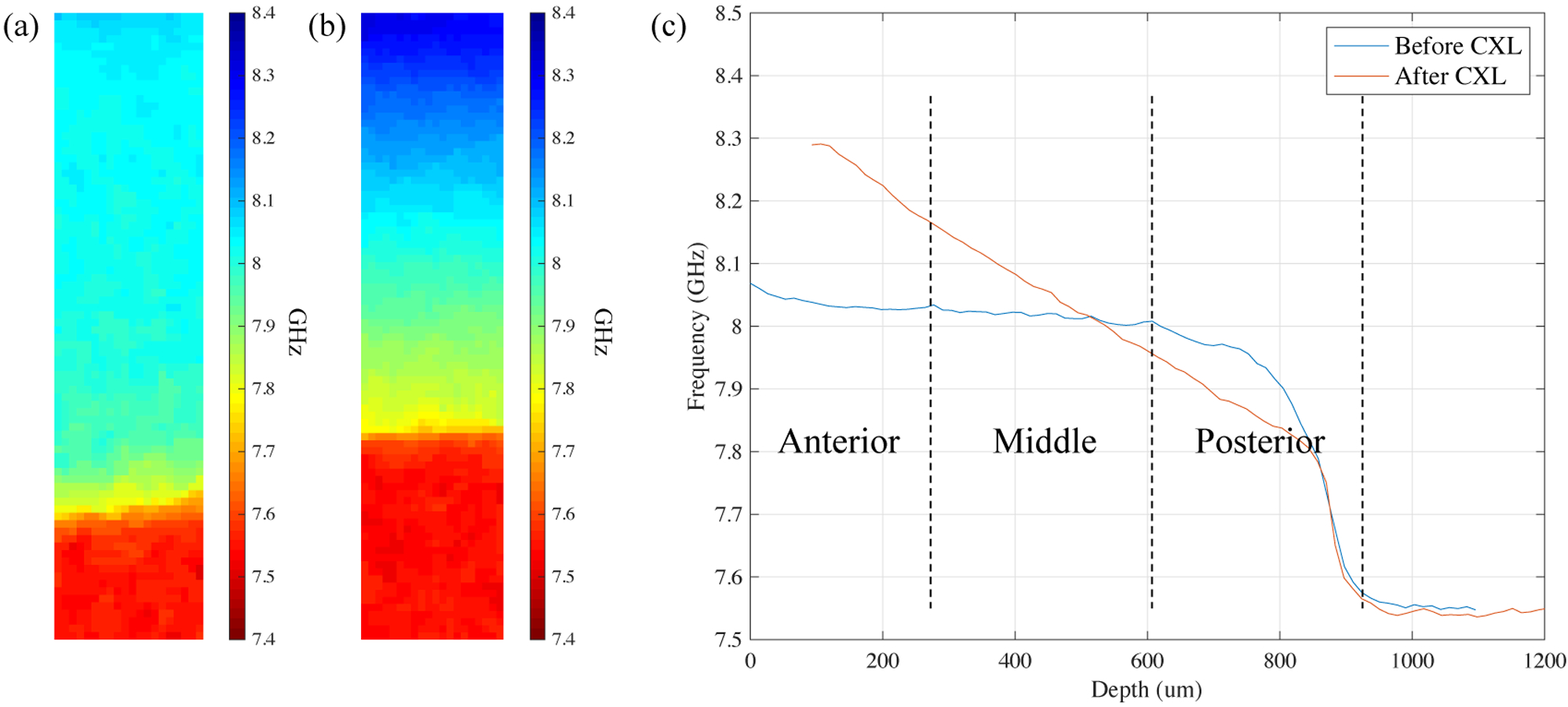
Representative Brillouin results for the CACXL group. (a) Distribution of Brillouin shifts for an untreated eye. Though thickness of this cornea is different from the one in Figure 2(a), similar stiffness distribution can be seen. (b) Distribution of Brillouin shifts for the same eye after CACXL. (c) Lateral averaged depth profiles of Brillouin shifts in (a) and (b).
Besides information of depth-dependent elasticity, Figure 2(c) also illustrates the way of dividing the cornea into 3 segments for depth analysis. In Figure 2(c), softer posterior part after CXL was confirmed by Brillouin microscopy, which was anticipated due to the use of the hypo-osmolar riboflavin solution designed to suppress the influence of dehydration on artifactual stiffening effects anteriorly. Compared with standard CXL, representative Brillouin shifts of CACXL are shown in Figure 3(b).
After Standard CXL, compared to pre-treatment there was a significant increase in the longitudinal modulus in the Anterior (2.56 GPa vs 2.76 GPa, P<0.001) and Middle (2.55 GPa vs 2.60 GPa, P<0.001) regions, while there was no significant stiffening induced in the posterior region (2.48 GPa vs 2.46 GPa, P=0.096). After CACXL, compared to pre-treatment there was a significant increase in the Anterior (2.56 GPa vs 2.70 GPa, P<0.001) and Middle (2.55 GPa vs 2.59 GPa, P<0.001) regions, while there was no significant stiffening induced in the posterior region (2.47 GPa vs 2.46 GPa, P=0.318).
When comparing techniques, Similar stiffening profiles can be seen in standard CXL and CACXL, as shown in Figures 2 and 3. The difference existed in the maximum Brillouin shift. For the maximum Brillouin shift, increased ratios, instead of absolute values, were used, considering diversity and relative flat Brillouin distribution of untreated corneas. The averaged shift of the anterior region before CXL, f0, served as the denominator and difference between the maximum shift and the averaged shift, (fmax-f0), was used as the numerator when calculating the ratios. An increase of 5.74±1.29% was discovered in the standard CXL group, while the increase for CACXL was 3.99±0.83% (P<0.001).
To demonstrate the axial stiffening difference (from anterior to posterior stroma) between standard CXL and CACXL with statistical analysis, depth-averaged modulus in each of the anterior, middle, and posterior regions were calculated. The increase of mean modulus after standard CXL was 7.8% in the anterior, 1.7% in the middle and −0.7% in the posterior regions. In comparison, the increase of mean modulus after CACXL was 5.5% in the anterior, 1.2% in the middle and −0.4% in the posterior regions. Significant difference existed between techniques in the anterior third of the cornea (P<0.001). No statistical differences were present between techniques in the middle or posterior regions, as shown in Figure 4. The negative variation in the posterior region demonstrated that hydration was induced in the posterior regions.
Figure 4.

Percentage change of mean longitudinal modulus of the anterior, middle and posterior for the standard CXL (N=15) versus CACXL (N=15). ***=P<0.001.
Stress-strain measurement
Stress values at selected strain points were first averaged, then exponential functions were used to fit the stress-strain curves (Figure 5). Gradient of these curves indicates Young’s modulus; corresponding results are listed in the Table. Standard CXL provided greater stiffening effect than CACXL at all selected strains (P<0.001).
Figure 5.
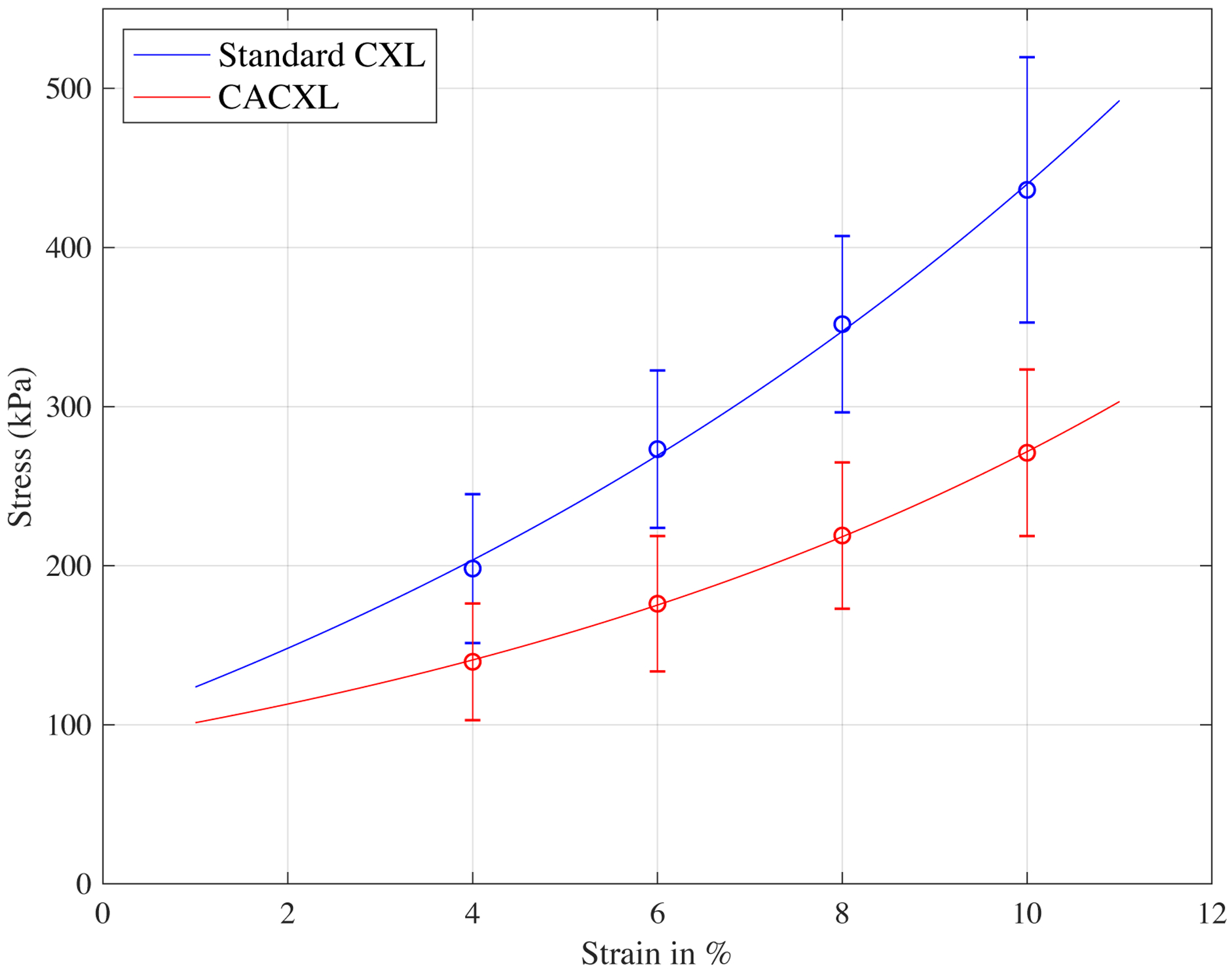
Stress-strain curves fitted by mean values at strains of 4%, 6%, 8% and 10%. Standard CXL had greater stiffness than CACXL at all selected Strains.
| 4% strain | 6% strain | 8% strain | 10% strain | |
|---|---|---|---|---|
| Standard CXL | 2.97±0.40 | 3.61±0.42 | 4.28±0.47 | 5.00±0.71 |
| CACXL | 1.53±0.40 | 1.93±0.47 | 2.40±0.50 | 2.97±0.57 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
DISCUSSION
Based on Brillouin results in enucleated porcine eyes, a larger maximum increase of the Brillouin shift could be found in the standard CXL group, with CACXL achieving 70% of the total stiffening effect of standard CXL. Both techniques induced significant corneal stiffening in the anterior and middle corneal regions, while no stiffening as induced in the posterior region of the cornea for either technique. Stiffening differences between techniques only existed in the anterior third of the cornea, with CACXL achieving 71% of the stiffening effect of standard CXL in this region, while in the other two regions no statistical difference could be observed between techniques (Figure 4). Stretching tests confirmed the verified that CACXL induced less stiffening effect than standard CXL (Figure 5).
The application of a contact lens did not appear to shift the CXL effect anteriorly, but rather blunted the cross-linking effect in the anterior and middle regions as compared to the standard technique without a contact lens in place (Figure 2 and Figure 3). This has potential clinical implications, since the cross-linking effect seems merely to be less than standard CXL rather than having the same stiffening effect but having that effect be anteriorly shifted. If the goal is to provide similar overall stiffening for thin corneas, future alternative strategies for treating thin cornea still need to be evaluated. As the primary concern for thin corneas, however, is endothelial protection, safety, a blunted response may be sufficient to protect the endothelium in thin corneas.
Due to the design of the current study, we were not able to evaluate the safety of CACXL. Endothelial safety of CACXL has been reported previously in human studies, with no significant effect on endothelial mosaic or endothelial cell count (ECC) reported during 6 months follow-up in two studies.12,13 Endothelial safety of CACXL has also been shown with using hydroxypropyl methylcellulose (HPMC)-based riboflavin solutions which tend to provide significantly deeper demarcation line than dextran-based riboflavin solutions. In the study conducted by Malhotra et al., ECC within 6 months follow-up was not significantly different from baseline in both types of the solutions.31
Biomechanical efficacy of CACXL has been evaluated in mouse eyes using stress-strain extensiometry. Kling et al. reported that the biomechanical effect of CACXL was overall reduced by approximately 30% when compared to standard CXL.17 This difference is most likely due to the essential role of oxygen in the cross-linking process.32 When applying a contact lens onto the cornea in CACXL, oxygen availability can be limited, considering the presence of riboflavin film over and under the contact lens and reduced oxygen solubility in liquid.14 Similarly, Wollensak et al. reported that in porcine corneas, the biomechanical effect of the CACXL in the anterior 400 microns was approximately two-thirds that of standard CXL.14 Applying the non-destructive method of Brillouin microscopy, we obtained a similar outcome. Figure 4 showed that the stiffening result after CACXL is about 70% of the CXL, which was validated by mechanical extensometer shown in Figure 5. Wollensak et al. also performed anterior segment OCT to measure the thickness of riboflavin film over and under the contact lens during the CACXL treatment.14 Their result showed that a thick riboflavin film on the contact lens eliminated the CXL effect. They recommended that the efficacy of CACXL might be improved by reducing or eliminating the riboflavin film over the contact lens. In our study, we instilled riboflavin solution on the contact lens every two minutes during the UV irradiation time. In contrast to previous results,14 we measured significant biomechanical changes with both Brillouin and extensometer in eyes that received frequent riboflavin drops during UV irradiation. Taking into account that the same contact lens, energy level and timing was used in both studies, the 10% dextran concentration in our study might lead to lower riboflavin film viscosity and thickness in comparison to 20% dextran in their study. However, CACXL in the in vivo clinical setting has showed clinical effectiveness with applying riboflavin with dextran 20% over and under contact lens every 3 minutes during 30 minutes of UV irradiation.12,13 Difference between the shape and size of the human and pig cornea may have altered the contact lens fit, which might affect the riboflavin film over and under the contact lens.
Similar to previous work, we found no stiffening effect in the posterior portion of the cornea with either CXL technique. Decreasing the frequency of riboflavin application and/or increasing dextran concentration might reduce the stiffen the posterior region. However, it might dehydrate the anterior region and exaggerate CXL efficacy.
This is not surprising, as it is unlikely the posterior corneal region can undergo significant cross-linking regardless of technique due to its ultrastructural differences as compared the anterior and middle corneal regions.33
Brillouin microscopy has two major advantages to traditional mechanical testing. As a non-invasive method for evaluation of the biomechanical properties, it allows for repeat, longitudinal measurements that are not possible with destructive testing methods. In the current study all samples were measured at baseline and then again after CXL, which allowed each to serve as its own control. Brillouin imaging also provides a 3-dimensional depth map of longitudinal modulus.21,22,28
There are limitations to our study. Hydration status impacts Brillouin measurements, and this must be taken into account for in-vitro experiments, especially when the corneal stroma is exposed for a long period of time such as this experiment. With the protection of the contact lens, evaporation ought to be slower in CACXL group theoretically, which could amplify difference between CACXL and standard CXL. To reduce the influence of hydration status, frequency of riboflavin application was shortened to every 2 minutes and hypoosmolar riboflavin solution was used. Based on results shown in Figure 4, CACXL and standard CXL had similar hydration status in the posterior corneal region and standard CXL had a more hydrated posterior region than the CACXL group, which confirmed that evaporation with subsequent dehydration was not a major factor to this study. The relatively stiffer posterior region of the CACXL group could be caused by hydrophilia of the contact lens. As the hydrophilia of the contact lens is not 100%, hypo-osmolar riboflavin solution dropped during UV exposure could not be absorbed totally, which led to a less hydrated posterior region.
Our data show that ex vivo analysis using Brillouin microscopy led to biomechanical results similar to the ones obtained in ex vivo stress-strain measurements.14 Moreover, Brillouin microscopy allowed us to analyze the biomechanical stiffening effect in a depth-dependent manner, which is not possible using stress-strain extensiometry. Brillouin microscopy thus is an ideal investigation method to support and even replace some of the testing done under ex vivo conditions.
In conclusion, the stiffening effect of CACXL was approximately 70% of the standard CXL technique in porcine eyes as measured by Brillouin microscopy and confirmed through extensiometry testing. There did not appear to be any major shift in depth of treatment but rather a blunted treatment response in the anterior corneal stromal region. It remains to be determined what aspects of the contact lens protocol, particularly related to contact lens used, oxygen diffusion capacity and method of riboflavin application, could be altered to maximize the stiffening effect and minimize risk to the corneal endothelium.
Financial Disclosures:
FH - Shareholder/investor for EMAGine AG (Zug, Switzerland), consultant for GroupAdvance Consulting GmbH (Zug, Switzerland), exclusive patent owner for PCT patent/application (corneal apparatus used for CXL and chromophore for CXL application), recipient of travel funds from Light for Sight Foundation (Zurich, Switzerland), directed research funds from Light for Sight Foundation (Zurich, Switzerland), Schwind Eye Tech Solutions (Kleinostheim, Germany), VELUX Foundation (Søborg, Denmark), Gelbert Foundation (Geneva, Switzerland), and in-kind financial contribution for research materials from SOOFT Italia (Montegiorgio, Italy).
The remaining authors have no financial disclosures
Funding: Supported in part by National Institutes of Health Grant R01 EY028666 (J.B.R., G.S.) and an unrestricted departmental grant to the Cole Eye Institute, Cleveland Clinic, from Research to Prevent Blindness (New York, NY, USA).
References
- 1.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology 2003;135(5):620–7. [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin–ultraviolet-A-induced cross-linking. Journal of Cataract & Refractive Surgery 2003;29(9):1780–5. [DOI] [PubMed] [Google Scholar]
- 3.Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. Journal of Refractive Surgery 2009;25(9):S824–S8. [DOI] [PubMed] [Google Scholar]
- 4.Kymionis GD, Portaliou DM, Diakonis VF, et al. Corneal collagen cross-linking with riboflavin and ultraviolet-A irradiation in patients with thin corneas. American Journal of Ophthalmology 2012;153(1):24–8. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed-Noriega K, Butrón-Valdez K, Vazquez-Galvan J, et al. Corneal melting after collagen cross-linking for keratoconus in a thin cornea of a diabetic patient treated with topical nepafenac: A Case Report with a Literature Review. Case Reports in Ophthalmology 2016;7(1):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007;26(4):385–9. [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G, Aurich H, Wirbelauer C, Sel S. Significance of the riboflavin film in corneal collagen crosslinking. Journal of Cataract & Refractive Surgery 2010;36(1):114–20. [DOI] [PubMed] [Google Scholar]
- 8.Raiskup F, Spoerl E. Corneal Crosslinking with Riboflavin and Ultraviolet A. I. Principles. The Ocular Surface 2013;11(2):65–74. [DOI] [PubMed] [Google Scholar]
- 9.Ozgurhan EB, Akcay BIS, Kurt T, et al. Accelerated corneal collagen cross-linking in thin keratoconic corneas. Journal of Refractive Surgery 2015;31(6):386–90. [DOI] [PubMed] [Google Scholar]
- 10.Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. Journal of Cataract & Refractive Surgery 2009;35(4):621–4. [DOI] [PubMed] [Google Scholar]
- 11.Kymionis GD, Diakonis VF, Coskunseven E, et al. Customized pachymetric guided epithelial debridement for corneal collagen cross linking. BMC Ophthalmology 2009;9(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob S, Kumar DA, Agarwal A, et al. Contact lens-assisted collagen cross-linking (CACXL): a new technique for cross-linking thin corneas. Journal of Refractive Surgery 2014;30(6):366–72. [DOI] [PubMed] [Google Scholar]
- 13.Mazzotta C, Jacob S, Agarwal A, Kumar DA. In vivo confocal microscopy after contact lens-assisted corneal collagen cross-linking for thin keratoconic corneas. Journal of Refractive Surgery 2016;32(5):326–31. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Spörl E, Herbst H. Biomechanical efficacy of contact lens-assisted collagen cross-linking in porcine eyes. Acta ophthalmologica 2019;97(1):e84–e90. [DOI] [PubMed] [Google Scholar]
- 15.Hafezi F Limitation of collagen cross-linking with hypoosmolar riboflavin solution: failure in an extremely thin cornea. Cornea 2011;30(8):917–9. [DOI] [PubMed] [Google Scholar]
- 16.Hatami-Marbini H, Rahimi A. Interrelation of hydration, collagen cross-linking treatment, and biomechanical properties of the cornea. Current eye research 2016;41(5):616–22. [DOI] [PubMed] [Google Scholar]
- 17.Kling S, Richoz O, Hammer A, et al. Increased biomechanical efficacy of corneal crosslinking in thin corneas due to higher oxygen availability. Journal of Refractive Surgery 2015;31(12):840–6. [DOI] [PubMed] [Google Scholar]
- 18.Wu P-J, Kabakova IV, Ruberti JW, et al. Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials. Nature Methods 2018;15(8):561–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarcelli G, Yun SH. Reply to ‘Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials.’ Nature Methods 2018;15(8):562–3. [DOI] [PubMed] [Google Scholar]
- 20.Shao P, Seiler TG, Eltony AM, et al. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Investigative Ophthalmology & Visual Science 2018;59(7):3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarcelli G, Yun SH. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat Photon 2008;2(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarcelli G, Polacheck WJ, Nia HT, et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Meth 2015;12(12):1132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarcelli G, Yun SH. Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Optics Express 2011;19(11):10913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berghaus KV, Yun SH, Scarcelli G. High speed sub-GHz spectrometer for Brillouin scattering analysis. JoVE (Journal of Visualized Experiments) 2015(106):e53468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He X, Liu J. A quantitative ultrasonic spectroscopy method for noninvasive determination of corneal biomechanical properties. Investigative ophthalmology & visual science 2009;50(11):5148–54. [DOI] [PubMed] [Google Scholar]
- 26.Wilson G, O’Leary D, Vaughan W. Differential swelling in compartments of the corneal stroma. Investigative ophthalmology & visual science 1984;25(9):1105–8. [PubMed] [Google Scholar]
- 27.Ortiz S, Siedlecki D, Grulkowski I, et al. Optical distortion correction in optical coherence tomography for quantitative ocular anterior segment by three-dimensional imaging. Optics express 2010;18(3):2782–96. [DOI] [PubMed] [Google Scholar]
- 28.Scarcelli G, Kling S, Quijano E, et al. Brillouin Microscopy of Collagen Crosslinking: Noncontact Depth-Dependent Analysis of Corneal Elastic Modulus. Investigative Ophthalmology & Visual Science 2013;54(2):1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarcelli G, Pineda R, Yun SH. Brillouin Optical Microscopy for Corneal Biomechanics. Investigative Ophthalmology & Visual Science 2012;53(1):185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb JN, Su JP, Scarcelli G. Mechanical outcome of accelerated corneal crosslinking evaluated by Brillouin microscopy. Journal of Cataract & Refractive Surgery 2017;43(11):1458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra C, Jain AK, Gupta A, et al. Demarcation line depth after contact lens–assisted corneal crosslinking for progressive keratoconus: Comparison of dextran-based and hydroxypropyl methylcellulose–based riboflavin solutions. Journal of Cataract & Refractive Surgery 2017;43(10):1263–70. [DOI] [PubMed] [Google Scholar]
- 32.Richoz O, Hammer A, Tabibian D, et al. The Biomechanical Effect of Corneal Collagen Cross-Linking (CXL) With Riboflavin and UV-A is Oxygen Dependent. Translational Vision Science & Technology 2013;2(7):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatami-Marbini H Influence of Microstructure on Stiffening Effects of Corneal Crosslinking Treatment. J Refract Surg. 2018;34(9):622–627 [DOI] [PubMed] [Google Scholar]


