Abstract
Background:
Multiple myeloma (MM), is a plasma cell neoplasm characterized by destructive bony lesions, anemia, and renal impairment. Access to effective therapy is limited globally. We report the rates and utilization of hematopoietic cell transplantation (HCT) globally from 2006–2015 to better characterize access to HCT for patients with MM.
Methods:
This was an analysis of a retrospective survey of Worldwide Network of Blood and Marrow Transplant sites, conducted annually between 2006–2015. Incidence estimates were from the Global Burden of Disease study. Outcome measures included total number of autologous and allogeneic HCTs by world regions, and percentage of newly diagnosed MM patients who underwent HCT, calculated by the number of transplants per region in calendar year / gross annual incidence of MM per region.
Results:
From 2006–2015, the number of autologous HCT performed worldwide for MM increased by 107%. Utilization of autologous HCT was highest in Northern America and European regions, increasing from 13% to 24% in Northern America, and an increase from 15% to 22% in Europe. In contrast, the utilization of autologous HCT was lower in the Africa/Mediterranean region, with utilization only changing from 1.8% in 2006 to 4% in 2015. The number of first allogeneic HCT performed globally for MM declined after a peak in 2012 by −3% since 2006.
Discussion:
Autologous HCT utilization for MM has increased worldwide in high-income regions but remains poorly utilized in Africa and the East Mediterranean. More work is needed to improve access to HCT for MM patients, especially in low to middle income countries.
Introduction
Multiple myeloma (MM) is a hematologic malignancy with substantial morbidity and mortality, characterized by the presence of abnormal clonal plasma cells, and end-organ destruction with renal failure, osteolytic bone lesions, and anemia. Although less common on a global level compared to lung cancer or breast cancer, MM is a global disease; in 2016, there were an estimated 138 509 incident cases, with an age-standardized incidence rate of 2.1 cases per 100 000 persons, and MM incident cases have increased globally from 1990 – 2016 by 126%, largely attributable to an aging world population (1). There are heterogeneities with respect to the burden of MM, which may in part be due to under ascertainment in certain world regions.
Although MM is considered an incurable disease, modern treatments have dramatically improved the long-term outcomes for patients. Starting with the introduction of autologous hematopoietic cell transplantation (HCT) in the 1990’s, and more recently, with the introduction of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs) – particularly lenalidomide and bortezomib – survival for MM patients has improved dramatically (2–5). Recent FDA and EMA approvals of daratumumab, a CD38 antibody, elotuzumab, an SLAMF7 antibody have expanded options for patients as well, and are being studied in the newly diagnosed setting (6–12).
Autologous HCT, with melphalan conditioning, was one of the first therapeutic options to improve outcomes for MM patients. Pioneered in the 1980’s, and dramatically expanded use in the 1990’s and 2000’s, autologous HCT results in durable remissions in many patients (2–4). Transplant eligibility is largely determined by several factors, including burden of comorbidities, physiologic health, and, in some cases, chronologic age. The most recently reported randomized clinical trial to examine the effect of autologous HCT in the novel agent era was the IFM-2009 trial, a randomized study of lenalidomide, bortezomib, and dexamethasone (RVd) induction and consolidation followed by lenalidomide maintenance, compared with RVd induction, autologous HCT, RVd consolidation, and lenalidomide maintenance (4). Although there was no difference in overall survival between the two groups, the autologous HCT arm had improved PFS, 50 vs 36 months. This trial, and others, continue to support autologous HCT as a standard of care amongst eligible patients.
Although autologous HCT is effective in MM, the availability and utilization of HCT in general, is limited outside of high-income regions of the world. A landmark publication by the Worldwide Network of Blood and Marrow Transplantation (WBMT) published in 2010 (13), assessed the global use of HCT and explored associations with macroeconomic factors associated with HCT utilization (14). There was significant variability between regions with respect to use of HCT; this was associated with government health expenditures, number of transplant teams per 1 million people, and gross national income. A follow-up publication with more follow-up described similar findings, that transplant rates were higher in countries with more resources, more transplant teams, and an unrelated donor infrastructure (15).
Given the central importance of autologous HCT for MM, and data showing global disparities in utilization of HCT in general, we sought to determine the numbers, and utilization of autologous and allogeneic HCT for MM globally and regionally.
Methods
Study Design
This was a retrospective survey of all the HCT teams, organized by the Worldwide Network for Blood and Marrow Transplantation (WBMT) through well-established international and regional organizations and, where no organizations were in place, directly from the transplant centers. Informed consent of the individual patients was waived because no individualized data was transferred to the investigators.
The main outcome measures were ascertainment of numbers of first autologous and allogeneic stem cell transplantation for plasma cell disorders (PCD) by region type on a global level. Secondary outcomes included determination of first autologous and allogeneic stem cell transplant utilization, for all ages, and a subset of patients less than 70 years of age. We determine transplant utilization by dividing the number of transplants per region, divided by the gross incidence of MM per region, per year (first HCT for MM/MM incidence in a given year).
Participating Groups, Continents, Countries, and Teams
The organizations providing information to the WBMT were the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR), Asia-Pacific Blood and Marrow Transplantation Group (APBMT), Bone Marrow Donors Worldwide (BMDW), Canadian Blood and Marrow Transplantation Group (CBMGT), the Center for International Blood and Marrow Transplant Research (CIBMTR), European Society for Blood and Marrow Transplantation (EBMT), Eastern Mediterranean Blood and Marrow Transplantation Group (EMBMT), Latin American Blood and Marrow Transplantation Group (LABMT), and the World Marrow Donor Association (WMDA).
Data Collection
Global transplant numbers for PCD by country of origin, year of transplant, and donor type (autologous vs allogeneic) were searched for using the reporting member organization. The WBMT registry includes all HCT for PCD, the majority of which were for MM. We worked under the assumption that the majority of HCT were for MM in our calculations and reporting. In the European region only, first allogeneic HCT included both tandem auto-allo HCT and first allogeneic HCT; all other regions reported first allogeneic HCT only.
Global raw incidence data, unadjusted for MM by year and World Bank Region were obtained from the Global Burden of Disease (GBD) Study 2017, using the GBD source tool(16). Separate data were obtained for all ages, and for those less than 70 years of age.
Definitions
We reported the number of HCT per year by region and by donor type from the years 2006 – 2015. We did not adjust for patients who crossed a border to receive an HCT in a different country. Given that some regions and teams do not routinely use a strict age cut off for HCT in MM, 2 analyses were performed, for all ages, and for under 70. We calculated utilization of HCT by donor type, determined by number of HCT per calendar year, divided by the gross annual incidence of MM for a given region.
Results
Transplant Activity
From 2006 through 2015, there was a 100% increase in activity of HCT for MM worldwide, increasing from 11446 transplants in 2006, to 22896 transplants in 2015 (Figure 1). Autologous HCT activity outnumbered allogeneic donor HCT through all years reported, and autologous HCT activity increased from 10673 to 22144 transplant globally from 2006 to 2015 (107% increase). Global first allogeneic HCT for MM numbers, in contrast, remained largely stable (Table 1).
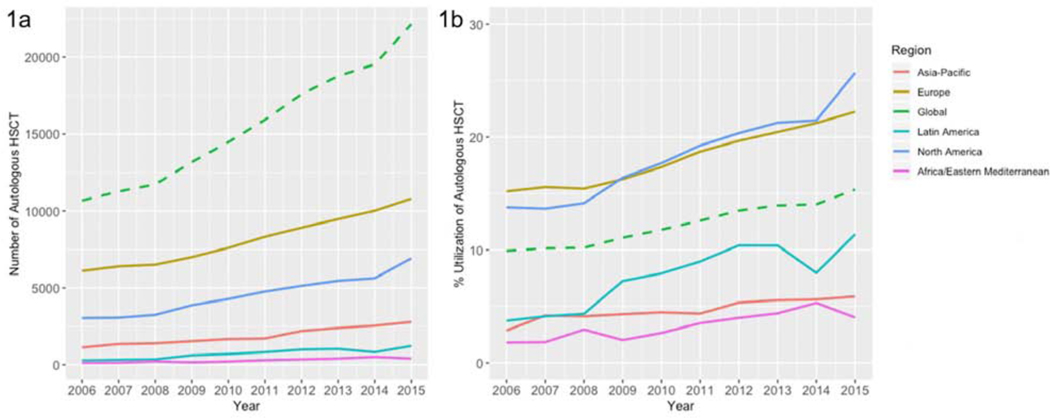
Figure 1:
Number (1a) and Utilization of Autologous HCT (1b), All Ages, Worldwide, by Region, 2006 – 2015
Table 1.
Transplant Utilization by Region
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | % Change, 2006–2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| North America | |||||||||||
| Autologous | 3038 | 3062 | 3245 | 3872 | 4282 | 4763 | 5132 | 5458 | 5627 | 6922 | 128% |
| Allogeneic | 204 | 238 | 213 | 228 | 172 | 211 | 184 | 186 | 221 | 105 | −49% |
| Incidence, Gross, All Ages | 22050.76 | 22418.30 | 22952.77 | 23610.29 | 24209.06 | 24742.40 | 25224.68 | 25663.29 | 26212.15 | 26937.22 | 22% |
| Incidence, Gross, Age < 70 | 10843.45 | 11093.57 | 11431.86 | 11807.79 | 12107.55 | 12337.44 | 12568.47 | 12696.61 | 12902.74 | 13268.20 | 22% |
| Utilization auto / allo HSCT, all ages | 13.78/0.93 | 13.66/1.06 | 14.14/0.93 | 16.40/0.97 | 17.69/0.71 | 19.25/0.85 | 20.35/0.73 | 21.27/0.72 | 21.47/0.84 | 25.70/0.39 | +87% / −58% |
| Utilization auto / allo HSCT, age < 70 | 28.02/1.88 | 27.60/2.15 | 28.39/1.86 | 32.79/1.93 | 35.37/1.42 | 38.61/1.71 | 40.83/1.46 | 42.99/1.46 | 43.61/1.71 | 52.17/0.79 | +86% / −58% |
| Asia Pacific | |||||||||||
| Autologous | 1127 | 1352 | 1398 | 1535 | 1676 | 1712 | 2195 | 2394 | 2548 | 2790 | 148% |
| Allogeneic | 53 | 67 | 63 | 77 | 65 | 77 | 93 | 90 | 95 | 70 | 32% |
| Incidence, Gross | 30709.17 | 32202.62 | 33897.37 | 35604.72 | 37443.57 | 39231.98 | 41120.69 | 43094.63 | 45312.55 | 47447.92 | 55% |
| Incidence, Gross, Age < 70 | 18631.45 | 19508.90 | 20535.75 | 21631.75 | 22783.67 | 23927.04 | 25190.52 | 26556.55 | 28067.64 | 29537.14 | 59% |
| Utilization auto / allo HSCT, all ages | 3.67/0.17 | 4.20/0.21 | 4.12/0.19 | 4.31/0.22 | 4.48/0.17 | 4.36/0.20 | 5.34/0.23 | 5.56/0.21 | 5.62/0.21 | 5.88/0.15 | +60% / −15% |
| Utilization auto / allo HSCT, age < 70 | 6.05/0.28 | 6.93/0.34 | 6.81/0.31 | 7.10/0.36 | 7.36/0.29 | 7.16/0.32 | 8.71/0.37 | 9.01/0.34 | 9.08/0.34 | 9.45/0.24 | +56% / −17% |
| Africa and East Mediterranean | |||||||||||
| Autologous | 125 | 132 | 220 | 157 | 215 | 298 | 352 | 404 | 512 | 406 | 225% |
| Allogeneic | 2 | 12 | 17 | 6 | 15 | 28 | 22 | 17 | 24 | 26 | 1200% |
| Incidence, Gross | 6931.16 | 7192.70 | 7470.27 | 7785.06 | 8125.95 | 8456.45 | 8824.53 | 9214.89 | 9645.25 | 10111.78 | 46% |
| Incidence, Gross, Age < 70 | 4729.58 | 4907.72 | 5101.99 | 5322.56 | 5558.37 | 5799.91 | 6073.47 | 6363.84 | 6686.38 | 7037.35 | 49% |
| Utilization auto / allo HSCT, all ages | 1.80/0.03 | 1.84/0.17 | 2.95/0.23 | 2.02/0.08 | 2.65/0.18 | 3.52/0.33 | 3.99/0.25 | 4.38/0.18 | 5.31/0.25 | 4.02/0.26 | +123% / +791% |
| Utilization auto / allo HSCT, age < 70 | 2.64/0.04 | 2.69/0.24 | 4.31/0.33 | 2.95/0.11 | 3.87/0.27 | 5.14/0.48 | 5.80/0.36 | 6.35/0.27 | 7.66/0.36 | 5.77/0.37 | +118% / +774% |
| Latin America | |||||||||||
| Autologous | 282 | 323 | 354 | 617 | 701 | 825 | 996 | 1037 | 825 | 1228 | 335% |
| Allogeneic | 11 | 8 | 11 | 2 | 4 | 8 | 6 | 14 | 10 | 9 | −18% |
| Incidence, Gross | 7551.52 | 7826.32 | 8152.86 | 8509.68 | 8847.85 | 9189.38 | 9567.46 | 9968.37 | 10353.19 | 10784.06 | 43% |
| Incidence, Gross, Age < 70 | 4908.45 | 5078.10 | 5297.63 | 5526.58 | 5729.29 | 5942.42 | 6191.24 | 6452.62 | 6697.50 | 6973.91 | 42% |
| Utilization auto / allo HSCT, all ages | 3.73/0.15 | 4.13/0.10 | 4.34/0.13 | 7.25/0.02 | 7.92/0.05 | 8.98/0.09 | 104.1/0.06 | 10.40/0.14 | 7.97/0.10 | 11.39/0.08 | +205% / −43% |
| Utilization auto / allo HSCT, age < 70 | 5.75/0.22 | 6.36/0.16 | 6.68/0.21 | 11.16/0.04 | 12.24/0.07 | 13.88/0.13 | 16.09/0.1 | 16.07/0.22 | 12.32/0.15 | 17.61/0.13 | +206% / −42% |
| Europe | |||||||||||
| Autologous | 6101 | 6398 | 6506 | 6998 | 7604 | 8328 | 8915 | 9473 | 10017 | 10798 | 77% |
| Allogeneic | 503 | 503 | 515 | 552 | 552 | 583 | 658 | 588 | 562 | 542 | 8% |
| Incidence, Gross | 40177.92 | 41091.70 | 42166.11 | 43024.01 | 43844.04 | 44503.89 | 45316.02 | 46330.43 | 47165.95 | 48549.73 | 21% |
| Incidence, Gross, Age < 70 | 19773.47 | 20067.16 | 20430.98 | 20658.60 | 20917.67 | 21058.37 | 21389.82 | 21916.59 | 22362.32 | 23130.78 | 17% |
| Utilization auto / allo HSCT, all ages | 15.18/1.25 | 15.57/1.22 | 15.43/1.22 | 16.27/1.28 | 17.34/1.26 | 18.71/1.31 | 19.67/1.45 | 20.45/1.27 | 21.24/1.19 | 22.24/1.12 | +46% / −11% |
| Utilization auto / allo HSCT, age < 70 | 30.85/2.54 | 31.88/2.51 | 31.84/2.52 | 33.87/2.67 | 36.35/2.64 | 39.55/2.77 | 41.68/3.08 | 43.22/2.68 | 44.79/2.51 | 46.68/2.34 | +51% / −8% |
| Global Total | |||||||||||
| Autologous | 10673 | 11267 | 11723 | 13179 | 14478 | 15926 | 17590 | 18766 | 19529 | 22144 | 107% |
| Allogeneic | 773 | 828 | 819 | 865 | 808 | 907 | 963 | 895 | 912 | 752 | −3% |
| Incidence, Gross | 107420.54 | 110731.64 | 114639.38 | 118533.76 | 122470.47 | 126124.10 | 130053.38 | 134271.60 | 138689.09 | 143830.71 | 34% |
| Incidence, Gross, Age < 70 | 58886.39 | 60655.46 | 62798.21 | 64947.28 | 67096.54 | 69065.18 | 71413.51 | 73986.21 | 76716.59 | 79947.38 | 36% |
| Utilization auto / allo HSCT, all ages | 9.94/0.72 | 10.18/0.75 | 10.23/0.71 | 11.12/0.73 | 11.82/0.66 | 12.63/0.72 | 13.53/0.74 | 13.98/0.67 | 14.08/0.66 | 15.40/0.52 | +55% / −27% |
| Utilization auto / allo HSCT, age < 70 | 18.12/1.31 | 18.58/1.37 | 18.67/1.30 | 20.29/1.33 | 21.58/1.20 | 23.06/1.31 | 24.63/1.35 | 25.36/1.21 | 25.46/1.19 | 27.70/0.94 | +53% / −28% |
| Total HSCT | 11446 | 12095 | 12542 | 14044 | 15286 | 16833 | 18553 | 19661 | 20441 | 22896 | 100% |
All regions studied reported increasing frequency of HCT for MM. The regions with the largest increase in autologous HCT were Latin America and Africa/Eastern Mediterranean Regions, increasing by 335%, and 225%, respectively, from 2006–2015 (for African and Eastern Mediterranean region, this corresponded to an absolute increase of 281 transplants). Asia-Pacific also had large increase in activity, by 148%. In contrast, most regions had relatively stable numbers of first allogeneic HCT from 2006 – 2015, with some regions, notably, North America, reporting declines in allogeneic HCT frequency.
Transplant Utilization for All Ages
We first analyzed the utilization of HCT by donor type, amongst MM patients of all ages, using data from the Global Burden of Disease study from years 2006 – 2015 (Table 1). Globally, there was a 55% increase in utilization of autologous HCT for MM, increasing from 9.9% to 15.4% from 2006–2015. The North American and European regions saw among the highest utilization at baseline and modest increases over 2006–2015. In 2015, the utilization of autologous HCT for all patients with MM in North America was 25.7%, a 87% increase from 2006, and in Europe, the utilization was 22.2%, a 46% increase from 2006. In contrast, all other reporting regions had much lower baseline utilization of autologous HCT, but also saw amongst the largest increases from 2006–2015. The utilization in Africa and East Mediterranean increased from 1.8% to 4.02% by 2015, a 123% increase; and in Latin American, autologous HCT for MM increased from 3.7% up to 11.4% in 2015, a 205% increase.
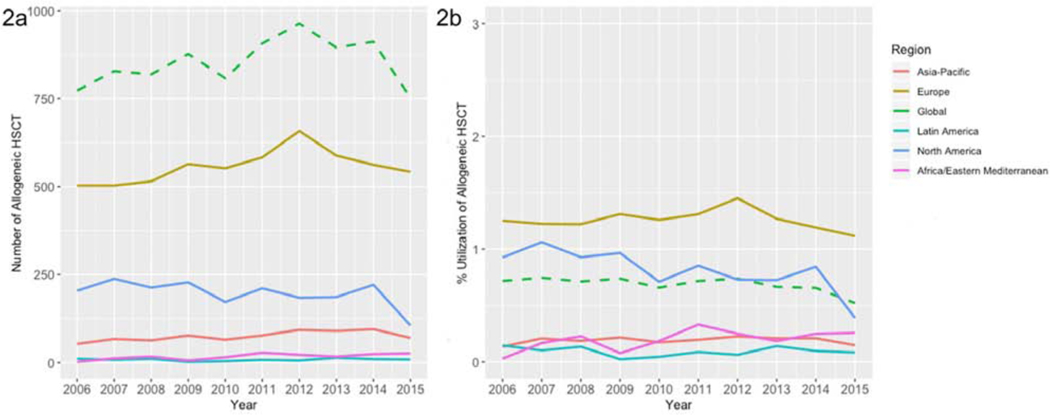
Although autologous HCT saw increasing utilization globally, in contrast, there was a 27% decreased utilization of first allogeneic HCT for MM, declining from 0.7% to 0.5% from 2006–2015 (Figure 2). The North American region saw the largest decline, from 0.93% in 2006 to 0.4% in 2015, a 58% decrease. The European region had the smallest decline over time, with first allogeneic HCT performed for MM in 1.3% of patients in 2006, and 1.1% of patients in 2015, a 11% decrease.
Figure 2:
Number (2a) and Utilization of Allogeneic HCT (2b), All Ages, Worldwide, by Region, 2006–2015
Transplant Utilization, Age < 70
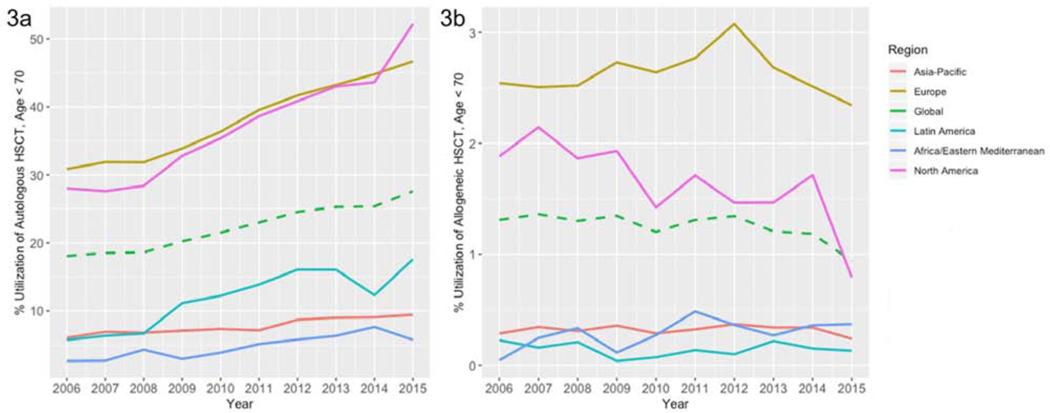
Given that many healthcare systems restrict receipt of autologous and allogeneic HCT for MM to patients under the age of 65–70, we then examined the utilization of HCT in MM in the age < 70 population using the GBD source tool for data. Globally, utilization of autologous HCT was 27.7% in 2015, representing a 53% increase since 2006, and utilization of allogeneic HCT was only 0.94%, a decline of 28% since 2006 (Figure 3). Both North American and European regions reported the highest use of autologous HCT amongst MM patients < 70 years; as of 2015, at 52.2% in North America, and 46.7% in Europe.
Figure 3:
Utilization of Autologous (3a) and Allogeneic (3b) HCT Worldwide, Age < 70, by Region, 2006–2015
Allogeneic HCT in European Region
As discussed previously, the reporting of allogeneic HCT in the European region includes both tandem auto-allo HCT and first allogeneic HCT in the total numbers of allogeneic HCT reported to the WBMT. Including only first allogeneic HCT and excluding auto-allo HCT, the absolute numbers of allogeneic HCT and amounted 270 and 250 in 2006 and 2015, respectively.
Discussion
Autologous HCT for eligible patients with newly diagnosed MM remains a standard of care globally, despite introduction of effective drugs such as PIs, IMiDs, and monoclonal antibodies. In our analysis of the global state of HCT for MM, autologous HCT gross numbers have increased – globally by 107% from 2006–2015, in all studied world regions. Utilization of autologous HCT has also increased globally, by 66% - from 9.9% to 15.4% in 2015. Particularly encouraging are the findings that autologous HCT activity has increased amongst world regions with predominantly lower middle income countries – such as Latin America, where autologous HCT utilization has increased from 3% to greater than 10% over this 9-year period (an absolute increase from 282 to 1228 HCT per year). Notably, autologous HCT utilization in North America and Europe in the under 70 age group has increased dramatically, exceeding 50% in North America and 45% in Europe. It is encouraging that an effective therapy such as autologous HCT is being highly utilized amongst eligible patients in these high-income regions.
Nevertheless, there are disparities in HCT activity in general, when comparing high income regions such as North America and Europe with all other world regions, particularly Asia-Pacific, and Africa/Eastern Mediterranean regions. This is likely multifactorial, and due to a combination of factors – including lower numbers of active transplant teams/10 million inhabitants, differences in healthcare infrastructure, and potentially, lack of awareness of the effectiveness of autologous HCT for MM. One potential reason for the lower frequency of HCT usage in Asia-Pacific may be prioritization of HCT for curable disease such as aplastic anemia, acute leukemia, and hemoglobinopathy tends to take priority over autologous HCT (17). Prior work by the WBMT has established a clear relationship between transplant activity and economic factors such as gross national income and health expenditures, and it is likely that the same factors are involved with autologous HCT for MM in these data(14, 15). Potential remedies for this imbalance include efforts by non-profits (e.g. WBMT) and governments alike to increase the number of functioning transplant teams in low-utilization countries and improve education and awareness of HCT through teaching and training programs that partner with high-volume transplant centers. Such activities are currently operative in a few pilot centers using telemedicine supervision.
In contrast to the marked gains seen globally with autologous HCT for MM, the pattern of use for allogeneic HCT is dramatically different. Throughout the 9-year period of study, allogeneic HCT has been much less utilized by all regions across the world except Africa and Asia-Pacific. The region that saw the greatest utilization, Europe, has also seen the most stable rates of use. In contrast, North America has had a decline in utilization of allogeneic HCT – at a peak of greater than 2% in 2007 for those under 70, currently, in 2015, at much less than 1%. There are likely several reasons for the global decline of allogeneic HCT. Although some studies have demonstrated improvement in outcomes with and over autologous HCT alone, other large randomized trials have failed to reproduce these findings (18–20). It is very likely that the results of these conflicting trials have led to decreased use, globally. Moreover, with the addition of several new agents for treatment of relapsed and newly diagnosed MM, the indication for allogeneic HCT may have changed to second line or after autologous(6–8, 11). Despite these factors, allogeneic HCT has remained utilized at a steady, but low constant rate in Europe. We note that these numbers represent only the first allogeneic HCT – and that trends over the last decade have increasingly leaned towards allogeneic HCT in the relapsed setting in Europe (21).
With respect to allogeneic HCT in Europe, there may be differences in reporting here compared with other world regions. The European data includes both first allogeneic HCT and tandem auto-allo HCT as part the allogeneic HCT numbers for MM. However, the differences between the absolute numbers and the utilization do not change the overall picture in Europe – that allogeneic HCT is not commonly utilized. Although there may be some discrepancies in reporting methods amongst different world regions, it is clear that allogeneic HCT remains utilized at a consistent, but low level.
With respect to pre-autologous HCT utilization of novel agents – Pis, IMiDs, and monoclonal antibodies – we did not have the ability to assess for this given the data collected by the WBMT survey. For a full picture of global therapeutic utilization for MM, future research should investigate utilization of, and access to novel agent therapy prior to autologous HCT. The limited data that is published, showed that as of 2017, global regulatory approval of lenalidomide, a commonly used IMiD, and bortezomib, a PI, were widely approved, but some regions still lacked regulatory approval such as Central Asia and Sub-Saharan Africa (1).
Our report has some important limitations. We have used data from the GBD study to determine gross incidence of MM globally – and since these statistics themselves may have inherent flaws (such as under ascertainment – acknowledged specifically in the recent publication on the global burden of MM) these should be taken as rough estimates (1). Additionally, the population burden of MM may vary significant between countries, especially those with a different age structure – adding to some uncertainty about the true utilization of HCT across different regions. However, we note that it is difficult to adjust for these factors, and conclude that although our report may have limitations, it is also useful as a benchmark for understanding trends and disparities in how HCT is used on global level. Due to the nature of the data source from the WBMT and the GBD, we lack long term outcomes data, which are critical in the full evaluation of HCT on a global level. Finally, our WBMT registry data for HCT for PCD include other diagnoses, at a low frequency, such as immunoglobulin light-chain AL amyloidosis and does not represent a totally clean dataset – however, given that the vast majority of HCT worldwide for PCD are for MM, we feel confident that the data represent a good assessment of the numbers and utilization of HCT for MM worldwide.
In summary, we have reported here data on the global use of HCT for MM. Though Europe and North America remain heavy utilizers of autologous HCT for MM, the rest of the world is dramatically different. While economic factors may play into this finding, this cannot be the only reason. As an example, in Asia-Pacific, one of the regions with comparatively lower use of autologous HCT for MM, human development and gross national income have increased rapidly over the past 15 years, yet HCT rates for MM have not kept pace. Thus, other factors may also be more important, including improved training, education, and awareness of the optimal management of MM pathways that includes autologous HCT. Further efforts need to be focused on building better healthcare infrastructure globally, and expanding education and training in transplant, to improve MM patients access to transplant on a global level.
Highlights:
We report the rates and utilization of HCT for MM globally from 2006–2015
Autologous HCT for MM has increased worldwide by 107% from 2006 to 2015
Allogeneic HCT for MM has continued to decline worldwide from 2006–2015, with the exception of the European region
Utilization of autologous HCT for MM in Latin America has increased dramatically from 3% to over 10% in this 9-year period
More work is needed to improve access to effective therapy such as HCT for MM on a global level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4(9):1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–502. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attal M, Richardson PG, Moreau P. Drug Combinations with Transplantation for Myeloma. N Engl J Med. 2017;377(1):93–4. [DOI] [PubMed] [Google Scholar]
- 6.Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med. 2018;379(19):1811–22. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Lonial S, Betts KA, Chen C, Zichlin ML, Brun A, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer. 2018;124(20):4032–43. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60. [DOI] [PubMed] [Google Scholar]
- 11.San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–206. [DOI] [PubMed] [Google Scholar]
- 12.Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016;51(6):778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. Jama. 2010;303(16):1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2(3):e91–100. [DOI] [PubMed] [Google Scholar]
- 16.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida M, Kodera Y, Dodds A, Ho AYL, Nivison-Smith I, Akter MR, et al. Advances in hematopoietic stem cell transplantation in the Asia-Pacific region: the second report from APBMT 2005–2015. Bone Marrow Transplant. 2019;54(12):1973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gahrton G, Iacobelli S, Apperley J, Bandini G, Bjorkstrand B, Blade J, et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: reduced relapse risk in female to male transplants. Bone Marrow Transplant. 2005;35(6):609–17. [DOI] [PubMed] [Google Scholar]
- 19.Gahrton G, Iacobelli S, Bjorkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121(25):5055–63. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobh M, Michallet M, Gahrton G, Iacobelli S, van Biezen A, Schonland S, et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia. 2016;30(10):2047–54. [DOI] [PubMed] [Google Scholar]