Cervical cancer is a preventable disease. Lei et al. [1] recently demonstrated using the Swedish nationwide registry that human papillomavirus (HPV) vaccines protected not only precancerous cervical lesions but also invasive cervical cancers. In this study, the risk of cervical cancer among women who had been vaccinated <17 years of age was 88% lower than the unvaccinated population. This effective vaccination results in a survival benefit when ‘herd immunity’ is achieved through high vaccination coverage in a population. One systematic review and meta-analysis by Drolet et al. [2] found that unvaccinated persons would indirectly benefit from HPV vaccination if vaccination coverage of girls and women in a population exceeds 50%. Vaccine coverage of the study of Lei et al. [1] in Sweden was 83.9% in the 2002 birth cohort and as high as 83.3%–97.7% in Australia where cervical cancer incidence continually declined over the years of vaccination [3,4]. This critical role of high vaccine coverage rates in reduction of cervical cancer incidence suggest that the HPV vaccination program must be scaled to national levels.
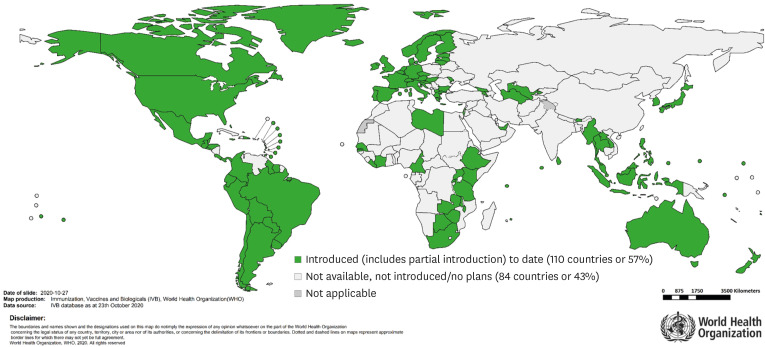
The World Health Organization (WHO) reported countries with HPV vaccines in the National Immunization Program (NIP) (Fig. 1), which showed that HPV vaccine coverages of most Asian countries was very low [5]. Whereas 83.4% and 62.5% of 15-year-old girls in Malaysia and Korea completed HPV vaccinations in 2018, respectively, vaccine coverage in Indonesia, the Philippines and Singapore ranged from just 0.1% to 0.7%. In Thailand, 91% of 12,500 girls aged 11 years in Ayutthaya province received at least 2 doses of HPV vaccine within a pilot vaccination program from 2014 to 2016, with excellent safety and high acceptability. Thereafter, HPV vaccination gradually expanded to the national level in 2020, pending the announcement of national HPV vaccination coverage. In India, several local governments started HPV vaccination programs after vaccines were made available in 2008 [6]. HPV vaccination was suspended after several deaths occurred during these initial programs. However, local governments, including Punjab and Delhi, resumed sponsoring their HPV vaccine programs. A recent study for 11-year-old girls in Punjab state found that HPV vaccination appeared highly cost-effective, suggesting a rationale of implementing HPV vaccination as a NIP in India [7]. Nevertheless, their national coverage is still low. In China, recent rates of both HPV vaccination and cervical cancer screening remain low [8]. According to a recent study conducted on Chinese college students [9], only 3.1% of the study group had been vaccinated and 36.9% of women were willing to be vaccinated. To overcome this situation, the Chinese government included a HPV vaccination program within national basic public health services in 2019. However, there was no national HPV vaccination program in China as of August 2020.
Fig. 1. Countries with human papillomavirus vaccine in the national immunization program as of October 23, 2020 (with permission from the World Health Organization). Notably, the HPV vaccination program was implemented in Taiwan in 2018, and it is a partial introduction in Indonesia and the Philippines.
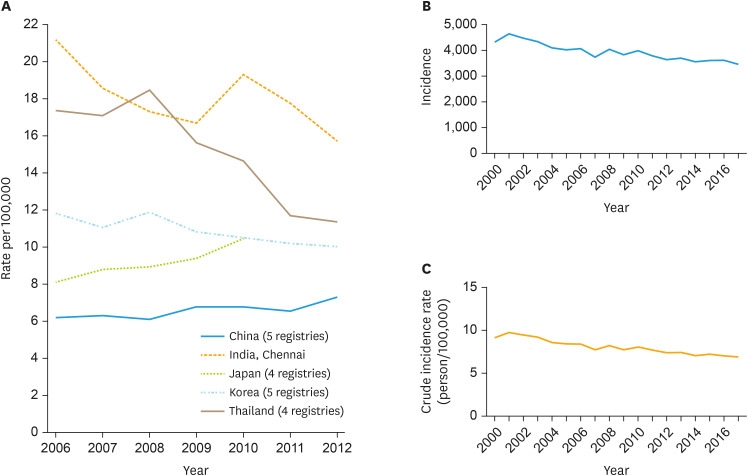
Very low coverage of HPV vaccination below 1% in Japan seems mainly due to the anti-vaccine movement, which excessively claims rare adverse events such as complex regional pain syndrome, despite the absolute benefit [10,11]. Japan had a NIP which was then suspended in 2013. The Japanese government has estimated that 67,305 deaths would be attributed to cervical cancer in Japan over the next 20 years without resuming the NIP [11]. In Korea, the NIP began in 2016, which provides HPV vaccination for girls at the age of 12 years. HPV vaccine coverage is high only for the specific birth cohorts which are covered by NIP: 86.3% for 2007 births and 47.2% for 2008 births [12]. Since HPV vaccines were introduced in Korea in 2006, the incidence of cervical cancer has been slowly decreasing for the last 15 years. However, vaccine coverage in all age groups was estimated around 26% [13]. The absence of a steeper decline of cervical cancer incidence (Fig. 2) [14], which was expected through herd immunity, is probably because of this low vaccine coverage in Korea.
Fig. 2. The age-standardized incidence rate of cervical cancer in 5 Asian countries (A), International Agency for Research on Cancer. [Access December 10, 2020]. Incidence (B) and crude incidence rate (C) of cervical cancer in Korea [14].
WHO recently launched the strategy to eliminate cervical cancer with the goals of achieving 90% HPV vaccination coverage, 70% screening coverage, and 90% access to treatment for cervical pre-cancer and cancer [2]. Many relevant societies worldwide including the Korean Society of Gynecologic Oncology have been scrambling to release their own recommendations for HPV vaccines [15,16,17,18]. Despite these consistent efforts of WHO and various academic societies, the HPV vaccination strategies of governments in Japan and Korea do not seem satisfactory. There was no policy change of HPV vaccination program in Japan, and the NIP has remained suspended since 2013 [11]. There was no further advancement of NIP after its commencement in 2016 in Korea.
Both countries have demonstrated the capacity for efficient resource allocation in the midst of the COVID-19 pandemic. However, with respect to allocating resources for HPV-related disease prevention, the health-related departments of both countries have failed. Based on the solid scientific evidence of the benefits of the HPV vaccine [1], the governing bodies should respond actively to step up, restarting the HPV vaccination program, improving current coverage by extending the age groups and incorporating nonavalent vaccines into NIP, and promoting the benefits of HPV vaccination which include reduction in morbidity and mortality as well as costs incurred from treating cervical cancer. Establishing an appropriate outcome-measuring system including a national registry for HPV vaccination is also an essential element to effectively save lives threatened by this preventable disease [19].
ACKNOWLEDGMENTS
We would like to thank Dr. Sudeep Gupta (Director ACTREC, Mumbai, India) for providing information of India.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 2.Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbaro B, Brotherton JM. Measuring HPV vaccination coverage in Australia: comparing two alternative population-based denominators. Aust N Z J Public Health. 2015;39:326–330. doi: 10.1111/1753-6405.12372. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Australia. Cervical cancer in Australia statistics [Internet] Strawberry Hills: Cancer Australia; 2020. [cited 2020 Dec 3]. Available from: https://www.canceraustralia.gov.au/affected-cancer/cancer-types/cervical-cancer/cervical-cancer-australia-statistics. [Google Scholar]
- 5.World Health Organization, The Global Health Observatory. Girls aged 15 years old that received the recommended doses of HPV vaccine [Internet] Geneva: World Health Organization; 2020. [cited 2020 Nov 26]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/girls-aged-15-years-old-that-received-the-recommended-doses-of-hpv-vaccine. [Google Scholar]
- 6.Sankaranarayanan R, Basu P, Kaur P, Bhaskar R, Singh GB, Denzongpa P, et al. Current status of human papillomavirus vaccination in India's cervical cancer prevention efforts. Lancet Oncol. 2019;20:e637–e644. doi: 10.1016/S1470-2045(19)30531-5. [DOI] [PubMed] [Google Scholar]
- 7.Prinja S, Bahuguna P, Faujdar DS, Jyani G, Srinivasan R, Ghoshal S, et al. Cost-effectiveness of human papillomavirus vaccination for adolescent girls in Punjab state: Implications for India's universal immunization program. Cancer. 2017;123:3253–3260. doi: 10.1002/cncr.30734. [DOI] [PubMed] [Google Scholar]
- 8.Zou Z, Fairley CK, Ong JJ, Hocking J, Canfell K, Ma X, et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020;8:e1335–e1344. doi: 10.1016/S2214-109X(20)30277-1. [DOI] [PubMed] [Google Scholar]
- 9.Deng C, Chen X, Liu Y. Human papillomavirus vaccination: coverage rate, knowledge, acceptance, and associated factors in college students in mainland China. Hum Vaccin Immunother. 2020 doi: 10.1080/21645515.2020.1797368. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekine M, Yamaguchi M, Kudo R, J. B. Hanley S, Hara M, Adachi S, et al. Epidemiologic profile of type-specific human papillomavirus infection after initiation of HPV vaccination. Vaccines (Basel) 2020;8:425. doi: 10.3390/vaccines8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara K, Quinn MA, Coleman RL, Angioli R. Two viral infections in Japan. Int J Gynecol Cancer. 2020;30:1258–1259. doi: 10.1136/ijgc-2020-001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korean Disease Control and Prevention Agency. National Immunization Program Guide [Internet] Osong: Korean Disease Control and Prevention Agency; c2020. [cited 2020 Dec 6]. Available from: http://www.kdca.go.kr/cdc_eng/ [Google Scholar]
- 13.Seong J, Ryou S, Yoo M, Lee J, Kim K, Jee Y, et al. Status of HPV vaccination among HPV-infected women aged 20-60 years with abnormal cervical cytology in South Korea: a multicenter, retrospective study. J Gynecol Oncol. 2020;31:e4. doi: 10.3802/jgo.2020.31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korean Statistical Information Service. Statistical Database: Cancer incident cases and incidence rates by site (24 items), sex, age group [Internet] Daejeon: Korean Statistical Information Service; c2020. [cited 2020 Nov 19]. Available from: https://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01&statId=1997018&themaId=#SelectStatsBoxDiv. [Google Scholar]
- 15.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joura EA, Kyrgiou M, Bosch FX, Kesic V, Niemenen P, Redman CW, et al. Human papillomavirus vaccination: the ESGO-EFC position paper of the European society of Gynaecologic Oncology and the European Federation for colposcopy. Eur J Cancer. 2019;116:21–26. doi: 10.1016/j.ejca.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen RH, Mahmood T, Donders G, Redman CW, Wood P. EBCOG position statement: gender neutral HPV vaccination for young adults. Eur J Obstet Gynecol Reprod Biol. 2020;246:187–189. doi: 10.1016/j.ejogrb.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Min KJ, Kwon SH, Kim K, Kim S, Kim HJ, Seong SJ, et al. Clinical guideline for 9-valent HPV vaccine: Korean Society of Gynecologic Oncology Guideline. J Gynecol Oncol. 2019;30:e31. doi: 10.3802/jgo.2019.30.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland S. A significant measure of HPV vaccine effectiveness in a high-risk population in Korea prior to a National Immunization Program. J Gynecol Oncol. 2020;31:e32. doi: 10.3802/jgo.2020.31.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]