Abstract
Objective
To assess the prognostic value of the systemic immune-inflammation index (SII) in patients with vulvar cancer.
Methods
Data of 130 consecutive patients who underwent primary surgical resection for vulvar cancer at the Medical University of Vienna between 1999 and 2018 was retrospectively analyzed. The SII was defined as platelets × neutrophils/lymphocytes as previously described. Its prognostic value on disease-specific survival (DSS) and overall survival (OS) was evaluated by univariate log-rank tests and multivariable cox regression models. Prediction accuracy was assessed by receiver operating characteristics curves and Youden's J statistics. A Hosmer-Lemeshow test was performed to confirm the model's goodness of fit.
Results
A pre-therapeutic high serum SII (>866.4) was associated with advanced International Federation of Gynecology and Obstetrics (FIGO)-stage. In univariate survival analysis, a high SII was associated with both DSS (p<0.001) and OS (p=0.001). A multivariate cox regression model confirmed the prognostic value of SII regarding DSS (p<0.001) and OS (p=0.014) independently from patients' age and FIGO stage.
Conclusions
Pretherapeutic SII may serve as a promising predictor for survival in patients with vulvar cancer. After clinical validation, the SII may be used to improve both pre-treatment patient risk stratification and patient counseling.
Keywords: Immune Marker, Prognostic Factor, Biomarker
INTRODUCTION
Vulvar cancer is a gynecologic malignancy of low, but increasing incidence associated with older age and poor prognosis [1]. Whereas the mainstay outcome prediction bases on clinical tumor staging, patient survival may vary significantly within different stages, presumably due to a comparably advanced patient age at time of diagnosis and associated comorbidities [2,3]. Tumor-specific preoperative biomarker assessment could therefore help to improve treatment stratification and to individualize clinical care.
Research has focused on defining inflammatory markers predictive of cancer survival and established indexes with prognostic value for decades, including the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) [4,5,6,7]. Even though exact pathomechanisms of inflammatory indexes remain controversial, it may be assumed that elevated indexes reflect both cancer-related local immune response and systemic inflammation, which were demonstrated to be related to postoperative survival of cancer patients. Moreover, increased inflammatory indexes were associated with preoperatively free circulating tumor cells and thereby possibly advanced disease and an increased risk of metastatic spread [8,9].
Recently, the systemic immune-inflammation (SII), a new inflammatory marker based on the number of peripheral lymphocytes, neutrophils and platelets has been associated to increased prediction accuracy compared to established inflammatory markers in hepatocellular and various gastrointestinal malignant tumors, small cell lung cancer as well as a variety of gynecological malignancies [10,11,12,13,14]. Assessing the prognostic value of the SII in vulvar cancer could therefore be of particular clinical interest. Therefore, the aim of the present study is to investigate the prognostic value of pre-treatment serum SII in patients with vulvar cancer undergoing upfront surgery.
MATERIALS AND METHODS
The current study was designed as a single-center retrospective chart review analyzing data of all patients with invasive vulvar cancer staged International Federation of Gynecology and Obstetrics (FIGO) IB–IVB who underwent primary surgical resection at the Department of General Gynecology and Gynecological Oncology of the Medical University of Vienna between 1999 and 2018. Patients with missing follow-up data, known autoimmune-diseases, ongoing infectious disease, acute inflammation or other concomitant malignancies were excluded from the study.
In patients with suspicion for vulvar cancer, diagnosis was histologically established by biopsy. Clinical examination and imaging studies including computed tomography scans or magnetic resonance imaging scans were performed to assess the tumor stage. All patients were treated according to international guidelines and the standard treatment protocols of the Gynecologic Cancer Unit of Medical University of Vienna [15].
Hence, early stage vulvar cancer was treated surgically with or without lymph node assessment. Patients underwent local wide excision, modified radical hemivulvectomy, modified radical anterior or posterior vulvectomy, or radical vulvectomy. If lymph node assessment was necessary and all prerequisites were fulfilled, sentinel lymph node assessment using either blue dye or technetium 99 was performed starting from 2009 [16]. In cases of lymph node involvement, systemic inguinal-femoral lymph node dissection was performed, and postoperative radiotherapy was applied at the Department of Radiotherapy of Medical University of Vienna. Patients with advanced staged disease were subjected to radiotherapy or chemotherapy. In selected cases, salvage surgery was performed if indicated after chemo or radiotherapy [17].
After primary cancer treatment, all patients are included into our department's oncologic follow-up program, which comprises regular clinical examination and imaging on over 10 years. Within the first 3 years, patients consult the outpatient department every 3 months, followed by biannual visits over 2 years and yearly visits over 5 years. In suspicion of local recurrence, biopsy is performed. If metastatic disease is suspected, patients are subjected to imaging.
Follow-up data cut-off for the current analysis was December 2019. Data of patients including all histotypes were collected. All patients underwent physical examination by an internal medicine specialist before treatment. Blood tests including serum platelets, lymphocytes and neutrophil counts as well as C-reactive protein (CRP) levels were routinely performed in all patients either 72–24 hours before surgery or during admission 24 hours before treatment in patients who did not undergo surgery.
The ethics committee of the Medical University of Vienna (IRB 1901/2017) gave consent prior to initiation of the study and waived the requirement to obtain a specific written informed consent from all patients to be included due to the retrospective nature of the trial.
Following clinicopathological variables were assessed: Patient age, FIGO-stage based on the FIGO 2009 classification system [18], histological grading, nodal status, Eastern Cooperative Oncology Group (ECOG) performance status [19], body mass index, pretherapeutic serum CRP levels and follow-up status. Serum platelet, lymphocyte and neutrophil counts were extracted from routine preoperative blood tests. Thereof, the SII index was calculated as neutrophil × platelet/lymphocyte.
Statistical Analysis was performed using SPSS 24.0 for Windows (IBM Corp., Armonk, NY, USA). Patient data were analyzed by descriptive statistics. Categorical variables were described using percentages (%), mean and range were used to describe non-normally distributed metric variables. For all statistical tests, p-values below 0.05 were considered statistically significant. To identify associations between SII and clinicopathological variables, student's t-test or 1-way analysis of variance were performed, where appropriate. The optimal cut off for the SII to predict overall survival (OS) was defined by Youden's J-statistics as previously described [20]. Hosmer-Lemeshow test was performed to confirm the model's goodness of fit. All survival analyses were performed for the end point of disease-specific survival (DSS) and OS. DSS and OS were defined as the time from primary surgery to death due to vulvar cancer or due to any cause, respectively. Univariate survival analysis was performed using log ranks-test and patient's survival was visualized by Kaplan-Meier curves. A multivariate cox proportional hazard model was established including all factors with significant impact on survival in univariate analyses. Hazard ratios were indicated with a 2-sided confidence interval of 95%, where appropriate. Survivors were censored at the last date they were known to be alive.
RESULTS
Between 1999 and 2018, a total of 230 patients were treated for vulvar cancer at the Medical University of Vienna. In the present analysis, data of 130 consecutive patients were included, whereas 54 patients were excluded due to a FIGO-stage 1a disease. In other cases, patients were excluded from analysis since they suffered from another malignancy (n=9), had no surgery at our department (n=16), final histology revealed another type of carcinoma (n=7) or follow-up data were missing (n=14).
Baseline patients' characteristics are depicted in Table 1. Median age at the time of primary surgery was 69.0 years (58.0–79.0), median follow-up was 31.0 months (13.8–69.3). At the time of the last follow-up visit, 53.1% of patients (n=69) were alive with no evidence of disease, 4.6% (n=6) were alive with stable or progressive disease, and 42.3% (n=55) had been deceased.
Table 1. Descriptive characteristics of patients with vulvar cancer undergoing primary surgery.
| Parameter | SII ≤866.4 | SII >866.4 | |
|---|---|---|---|
| No. of patients | 65 | 65 | |
| Age (yr) | 65.0 (54.5–78.5) | 72.0 (60.0–79.5) | |
| FIGO-stage (2009) | |||
| I | 44 (67.7) | 33 (50.8) | |
| II | 4 (6.2) | 5 (7.7) | |
| III | 15 (23.1) | 22 (33.8) | |
| IV | 2 (3.1) | 5 (7.7) | |
| Histologic grading | |||
| G1 | 20 (30.8) | 14 (21.5) | |
| G2 | 35 (53.8) | 39 (60.0) | |
| G3 | 10 (15.4) | 12 (18.5) | |
| Nodal involvement | |||
| N0 | 46 (70.8) | 37 (56.9) | |
| N1 | 19 (29.2) | 28 (43.1) | |
| ECOG | |||
| 0 | 43 (66.2) | 31 (47.7) | |
| 1 | 16 (24.6) | 22 (33.8) | |
| 2 | 3 (4.6) | 5 (7.7) | |
| 3 | 2 (3.1) | 5 (7.7) | |
| Unknown | 1 (1.5) | 2 (3.) | |
| BMI | 27.3 (23.7–31.9) | 25.6 (23.0–31.6) | |
| CRP (mg/dL) | 0.2 (0.1–0.8) | 0.6 (0.2–1.7) | |
| Time of follow-up (mon) | 28.0 (15.0–75.0) | 32.0 (13.0–69.5) | |
| Status at last follow-up | |||
| Alive with no evidence of disease | 40 (61.5) | 29 (44.6) | |
| Alive with stable disease | 4 (6.2) | 1 (1.5) | |
| Progression | 1 (1.5) | 0 (0) | |
| Deceased | 20 (30.8) | 35 (53.8) | |
Values are presented as median (interquartile range) or number (%).
BMI, body mass index; CRP, C-reactive protein (mg/dL); ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; SII, systemic immune-inflammatory index.
Table 2 depicts the correlation of pre-therapeutic serum SII with clinicopathologic findings. An elevated SII was significantly associated with advanced FIGO-stage (p=0.029) and recurrence of disease (p=0.032), but not with patient age, histological tumor grading, nodal involvement, CRP levels or ECOG-performance status.
Table 2. Mean±SD pretreatment SII levels in patients with vulvar cancer categorized by pathologic findings.
| Parameter | Mean SII±SD | p-value | |
|---|---|---|---|
| Age (yr) | 0.923* | ||
| ≤69 | 827.5±681.3 | ||
| >69 | 958.0±600.7 | ||
| FIGO-stage (2009) | 0.029† | ||
| I | 797.3±537.1 | ||
| II | 765.6±295.1 | ||
| III | 1,019.0±740.3 | ||
| IV | 1,457.4±1,113.1 | ||
| Grading | 0.230* | ||
| G1 | 801.3±461.0 | ||
| G2–3 | 926.5±696.2 | ||
| Nodal involvement | 0.251* | ||
| N0 | 820.2±591.3 | ||
| N1 | 1,023.7±715.3 | ||
| Recurrence | 0.032* | ||
| Yes | 961.6±756.1 | ||
| No | 830.1±512.9 | ||
| ECOG | 0.452† | ||
| 0 | 832.4±588.7 | ||
| 1 | 970.6±675.8 | ||
| 2 | 850.9±569.6 | ||
| 3 | 1,192.2±1,137.8 | ||
| CRP (mg/dL) | 0.052* | ||
| <0.5 | 798.3±511.1 | ||
| ≥0.5 | 1,026.3±775.2 | ||
CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and obstetrics; SD, standard deviation; SII, systemic immune-inflammatory index.
*Student's t-test; †One-way analysis of variance.
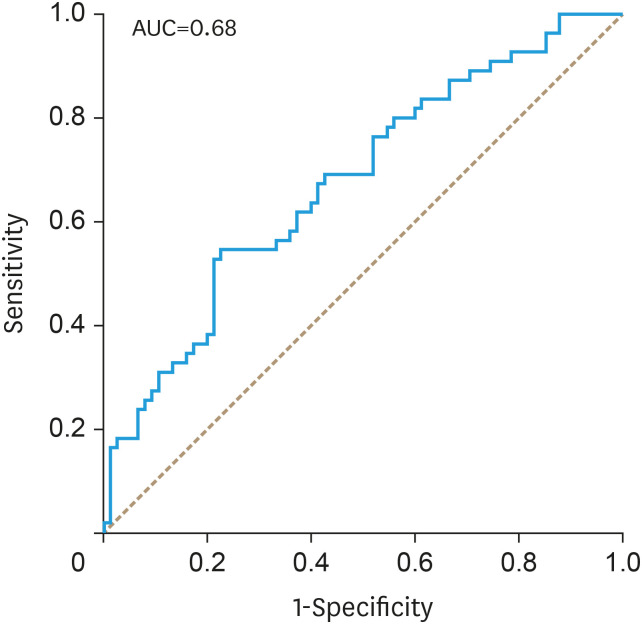
With the Youden's J-statistics establishing 866.4 as optimal cut off point for SII to predict OS, the receiver operating characteristic analysis calculated the area under the curve (AUC) of SII for the end point of OS as 0.68 (Fig. 1). To confirm the model's goodness of fit, a Hosmer-Lemeshow test was performed, which could not reveal bad calibration (p=0.327).
Fig. 1. AUC of the receiver operating characteristic analysis to assess the prognostic value of the systemic immune-inflammation index in patients with vulvar cancer with the end point of overall survival. Youden's J-statistics was performed to define 866.4 as an optimal cut-off.
AUC, area under the curve.
FIGO-stage, patients' age, histological grading, lymph node involvement, pretherapeutic CRP levels and SII were analyzed as prognostic parameters for DSS and OS by calculating univariate and multivariable survival models. Nodal involvement, however, was not included in further multivariable assessment as it is a fundamental part of FIGO-staging.
Table 3 depicts the results of the univariate Kaplan-Meier analyses and the multivariable Cox regression model in respect to OS. The SII, FIGO stage, patients age, lymph node involvement but not histologic grading and pretherapeutic CRP levels were associated with impaired OS in univariate analysis. In multivariable analyses, only the SII, FIGO stage and patients' age were independently associated to OS in patients with vulvar cancer.
Table 3. OS of patients with vulvar cancer in univariate and multivariate analysis. Nodal involvement was not included in the multivariate analysis since it is an integral part of the FIGO-staging.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| 3-years OS | p-value | Comparison | HR (95% CI) | p-value | ||
| SII | 0.001 | ≤866.4 vs. >866.4 | 1.99 (1.15–3.46) | 0.014 | ||
| ≤866.4 | 71.1% | |||||
| >866.4 | 54.1% | |||||
| Age (yr) | 0.004 | ≤69.0 vs. >69.0 | 1.88 (1.03–3.42) | 0.039 | ||
| ≤69.0 | 73.0% | |||||
| >69.0 | 53.6% | |||||
| FIGO-stage | <0.001 | I–II vs. III–IV | 3.45 (1.96–6.08) | <0.001 | ||
| I–II | 78.3% | |||||
| III–IV | 38.2% | |||||
| ECOG PS | 0.488 | 0 vs. 1 vs. 2 vs. 3 | 1.09 (0.79–1.50) | 0.619 | ||
| 0 | 68.7% | |||||
| 1 | 61.6% | |||||
| 2 | 57.1% | |||||
| 3 | 51.4% | |||||
| Grading | 0.560 | |||||
| G1 | 74.9% | |||||
| G2–3 | 61.0% | |||||
| Nodal involvement | <0.001 | |||||
| N0 | 81.5% | |||||
| N1 | 42.2% | |||||
| CRP (mg/dL) | 0.264 | |||||
| <0.5 | 59.4% | |||||
| ≥0.5 | 47.0% | |||||
CI, confidence interval; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; OS, overall survival; SII, systemic immune-inflammatory index.
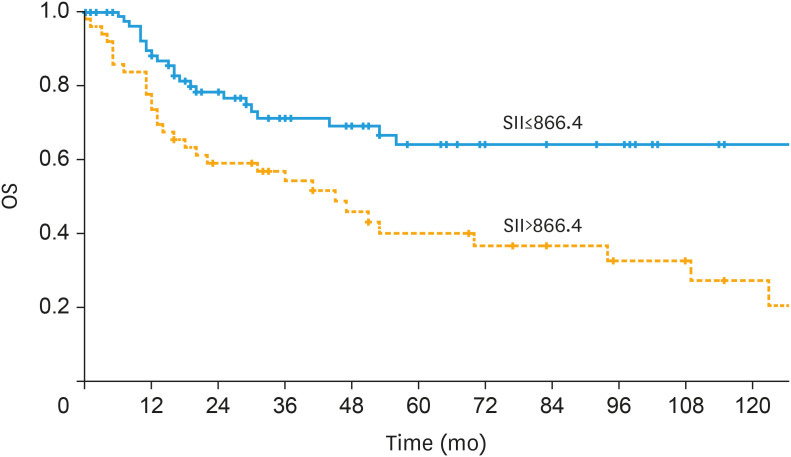
Patients with high pre-treatment SII (>866.4) showed impaired OS compared to patients with lower SII translating to 3-year OS rates of 54.1% and 71.1% (p=0.001), respectively (Fig. 2). Additionally, results of the analysis of the endpoint of DSS are depicted in Table 4.
Fig. 2. Univariate survival analysis of the prognostic impact of a high pre-treatment SII on OS in patients with vulvar cancer depicted by a Kaplan-Meier curve. A high pre-treatment SII (>866.4) was associated with impaired OS translating to 3-year OS rates of 71.1% and 54.1% (p=0.001).
OS, overall survival; SII, systemic immune-inflammation index.
Table 4. DSS of patients with vulvar cancer in univariate and multivariate analysis. Nodal involvement was not included in the multivariate analysis since it is an integral part of the FIGO-staging.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| 3-years DSS | p-value | Comparison | HR (95% CI) | p-value | ||
| Serum SII | <0.001 | ≤866.4 vs. >866.4 | 5.46 (2.19–13.61) | <0.001 | ||
| ≤866.4 | 92.5% | |||||
| >866.4 | 60.8% | |||||
| Age (yr) | 0.374 | |||||
| ≤69.0 | 80.7% | |||||
| >69.0 | 75.2% | |||||
| FIGO-stage (2009) | <0.001 | I–II vs. III–IV | 4.89 (2.15–11.10) | <0.001 | ||
| I–II | 90.2% | |||||
| III–IV | 56.5% | |||||
| ECOG PS | 0.174 | 1 vs. 2 vs. 3 vs. 4 | 1.22 (0.80–1.86) | 0.355 | ||
| 0 | 83.7% | |||||
| 1 | 77.0% | |||||
| 2 | 100.0% | |||||
| 3 | 68.6% | |||||
| Grading | 0.964 | |||||
| G1 | 87.0% | |||||
| G2–3 | 76.9% | |||||
| Nodal involvement | <0.001 | |||||
| N0 | 90.6% | |||||
| N1 | 58.3% | |||||
| CRP (mg/dL) | 0.410 | |||||
| <0.5 | 83.0% | |||||
| ≥0.5 | 69.0% | |||||
CI, confidence interval; CRP, C-reactive protein; DSS, disease-specific survival; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; SII, systemic immune-inflammatory index.
DISCUSSION
Pretherapeutic SII appears to be an independent prognostic parameter for vulvar cancer patients undergoing upfront surgery. Patients with a high SII (>866.4) showed a significantly diminished 3-year OS. This effect was independent from relevant other prognostic factors such as FIGO stage and patients' age, thus providing additional prognostic information.
Whereas several studies have substantiated scientific evidence on the predictive value of the SII in various cancer types to date, this is the first study to have evaluated the prognostic role of pre-treatment SII in patients with invasive vulvar cancer in a high-volume tertiary care center [14]. As patient survival may vary significantly within different FIGO-stages and patients are typically of increased age at timepoint of primary surgery for vulvar cancer, the SII could greatly aid to identify patients at risk of advanced disease and poor prognosis to improve treatment stratification [21,22]. Especially its low assessment costs and both broad and quick availability underline its potential benefits as a future prognostic biomarker.
The results of the present study are in line with previously published data for other particularly gynecologic cancers. Huang et al. [10] 2019 reported the SII to predict OS in patients with cervical cancer undergoing primary surgery, whereas Nie et al. [11] and Miao et al. [23] demonstrated both poor PFS and OS in patients with advances ovarian cancer. Similar data was published by Temur et al. [24] for patients suffering from endometrial cancer. Evidence for vulvar cancer, however, remains scarce. To our knowledge, the only study which has assessed inflammatory indices in patients with vulvar cancer to date was published in 2018 by Ertas et al. [25], reporting NLR und PLR to be associated with nodal involvement, FIGO-stage and tumor-related death in a cohort of 64 patients undergoing surgery for vulvar cancer. Survival analysis, however, was not performed, presumably due to the small cohort size [25].
Aside from the clinically observed association between SII levels and the prognosis of vulvar cancer patients, the rationale behind this assumed interaction remains poorly understood. As the development of vulvar cancer is particularly related to different processes of inflammation such as human papillomavirus (HPV)-induced infections or lichen sclerosus, a subsequent disease-specific association of underlying inflammation and SII levels may be suspected. However, serum CRP-level as a validated predictor of systemic inflammation was not observed to be predictive for prognosis in the present cohort [26]. This result is in line with data previously reported by our study group [27]. Of note, a recent Korean study, which describes an association between high-risk HPV-infections and risk of cardiovascular disease in women could not prove a relation between active HPV-infections and high-sensitive serum CRP levels [28]. Furthermore, the SII appears to be predictive for survival for several different cancer types which are not primarily associated with inflammatory or infectious processes, such as pancreatic cancer, breast cancer or melanoma [26,29,30]. As the clinical evidence of SII levels as an independent predictor of prognosis for several cancer types including vulvar cancer is growing, further research will be necessary to elucidate underlying pathomechanisms and to eventually further improve SII applicability.
The present study is, however, subjected to a number of limitations. As typical for retrospective studies, its lack of random patient assignment, selection, and possibly flawed data acquisition alleviate its clinical value. Additionally, the number of patients within our cohort is limited and data was acquired over a significant timeframe, which may relativize the generalizability of acquired data. Blood count arrays, however, were not subjected to major technical changes within the observation period and survival analyses were performed within relatively short 3- and 5-year intervals. One may therefore assume that these objections will not limit the observation of the present study on large scale.
Interestingly, relatively few articles have been published regarding the prognostic value of inflammation-based serum parameters in patients with vulvar cancer when compared to other gynecologic cancer types to date as vulvar cancer is a rare disease and respective studies are particularly challenging. The current analysis, however, underlines the hypothesis that inflammatory mechanisms play a significant role in oncogenesis of vulvar cancer. Whether this effect is relevant for all vulvar cancer patients or is limited to a certain group of patients e.g. HPV associated vulvar cancer, is currently unknown. This question was beyond the scope of the current analysis but should entail further investigation.
In conclusion, we present the SII as novel prognostic parameter in patients with vulvar cancer. To the best of our knowledge this is the first study describing a prognostic value of an inflammation associated index in patients with vulvar cancer. Prospective validation of the presented data is warranted.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.T., S.A.
- Data curation: B.T., B.C., P.M., R.S.
- Formal analysis: B.T., B.C., P.M., P.S., R.S.
- Investigation: B.T., B.C., R.S.
- Methodology: B.T., S.A.
- Project administration: A.R., P.S.
- Resources: B.T., P.M.
- Software: B.T., S.A., R.S.
- Supervision: A.R., P.S.
- Visualization: P.M.
- Writing - original draft: B.T., P.S., R.S.
- Writing - review & editing: B.C., P.M., A.R., S.A.
References
- 1.Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 2.Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29:545–552. [PubMed] [Google Scholar]
- 3.Polterauer S, Schwameis R, Grimm C, Macuks R, Iacoponi S, Zalewski K, et al. Prognostic value of lymph node ratio and number of positive inguinal nodes in patients with vulvar cancer. Gynecol Oncol. 2017;147:92–97. doi: 10.1016/j.ygyno.2017.07.142. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Zhang F, Sheng XG, Zhang SQ, Chen YT, Liu BW. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: lymphocyte. Medicine (Baltimore) 2016;95:e4381. doi: 10.1097/MD.0000000000004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311–320. doi: 10.1038/bjc.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 7.Ma JY, Ke LC, Liu Q. The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer: a meta-analysis. Medicine (Baltimore) 2018;97:e12897. doi: 10.1097/MD.0000000000012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Zheng L, Zou K, Yang C, Chen F, Guo T, Xiong B. Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin Transl Oncol. 2017;19:1125–1132. doi: 10.1007/s12094-017-1649-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9:3284. doi: 10.1038/s41598-019-39150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie D, Gong H, Mao X, Li Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2019;152:259–264. doi: 10.1016/j.ygyno.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 13.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9:3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comprehensive Cancer Center of the Medical University of Vienna. Vulvakarzinom primär- und rezidivtherapie (interdisziplinär) version 2. Vienna: Comprehensive Cancer Center of the Medical University of Vienna; 2018. [Google Scholar]
- 16.Covens A, Vella ET, Kennedy EB, Reade CJ, Jimenez W, Le T. Sentinel lymph node biopsy in vulvar cancer: systematic review, meta-analysis and guideline recommendations. Gynecol Oncol. 2015;137:351–361. doi: 10.1016/j.ygyno.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Oonk MHM, Planchamp F, Baldwin P, Bidzinski M, Brännström M, Landoni F, et al. European Society of Gynaecological Oncology guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer. 2017;27:832–837. doi: 10.1097/IGC.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 18.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Hyde SE, Ansink AC, Burger MP, Schilthuis MS, van der Velden J. The impact of performance status on survival in patients of 80 years and older with vulvar cancer. Gynecol Oncol. 2002;84:388–393. doi: 10.1006/gyno.2001.6531. [DOI] [PubMed] [Google Scholar]
- 20.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 21.Woelber L, Eulenburg C, Choschzick M, Kruell A, Petersen C, Gieseking F, et al. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer. 2012;22:503–508. doi: 10.1097/IGC.0b013e31823eed4c. [DOI] [PubMed] [Google Scholar]
- 22.Gadducci A, Cionini L, Romanini A, Fanucchi A, Genazzani AR. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit Rev Oncol Hematol. 2006;60:227–241. doi: 10.1016/j.critrevonc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016;17:33–40. doi: 10.3233/CBM-160614. [DOI] [PubMed] [Google Scholar]
- 24.Temur I, Kucukgoz Gulec U, Paydas S, Guzel AB, Sucu M, Vardar MA. Prognostic value of pre-operative neutrophil/lymphocyte ratio, monocyte count, mean platelet volume, and platelet/lymphocyte ratio in endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2018;226:25–29. doi: 10.1016/j.ejogrb.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Ertas IE, Gungorduk K, Akman L, Ozdemir A, Terek MC, Ozsaran A, et al. Can preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios be used as predictive markers for lymph node metastasis in squamous cell carcinoma of the vulva? Eur J Obstet Gynecol Reprod Biol. 2013;171:138–142. doi: 10.1016/j.ejogrb.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Six L, Polterauer S, Grimm C, Seebacher V, Tempfer C, Heinze G, et al. C-reactive protein serum levels are closely associated with lymph node status, but not with prognosis in patients with vulvar cancer. Eur J Obstet Gynecol Reprod Biol. 2008;137:217–221. doi: 10.1016/j.ejogrb.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Joo EJ, Chang Y, Kwon MJ, Cho A, Cheong HS, Ryu S. High-risk human papillomavirus infection and the risk of cardiovascular disease in Korean women. Circ Res. 2019;124:747–756. doi: 10.1161/CIRCRESAHA.118.313779. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Li W, Li AJ, Su H, Yue J, Yu J. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–3162. doi: 10.2147/CMAR.S190335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Wu X, Yu H, Li S, Mao L, Chi Z, et al. Systemic immune-inflammation index and circulating T-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol. 2017;10:719–725. doi: 10.1016/j.tranon.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]