Abstract
Objective
To demonstrate the expression of abnormal spindle microtubule assembly (ASPM) in clinical osteosarcoma tissue specimens collected in our hospital, and to explore the function of ASPM in osteosarcoma in vitro and in vivo.
Methods
Tissue specimens from 82 cases of osteosarcoma were collected and analyzed by immunohistochemistry assay. We also investigated the relationship between ASPM expression and clinicopathological characteristics in the patients. We transfected shASPM plasmid and the empty control plasmid, respectively, and then used quantitative polymerase chain reaction and western blot analysis to detect ASPM expression. Cell colony assay and MTT were used to observe the proliferation ability. In vivo study was undertaken to explore the ASPM function further.
Results
In this study, ASPM showed high expression in osteosarcoma tissue samples compared with non‐tumor normal tissues. ASPM was positively correlated with clinical pathological characteristics, including tumor size (P = 0.024) and clinical stage (P = 0.045). Our results further showed that ASPM depletion dramatically inhibited the proliferation of osteosarcoma cells (with fewer cells in the sh‐RNA‐ASPM group compared with the control group(P < 0.05, respectively), and the in vivo assays further confirmed that ASPM ablation markedly blocked tumor growth compared with control (P < 0.05).
Conclusion
Our data provides strong evidence that the high expression of ASPM in osteosarcoma promotes proliferation in vitro and in vivo, indicating its potential role as an osteosarcoma therapeutic target.
Keywords: ASPM, In vitro and in vivo, Osteosarcoma, Proliferation, Therapeutic target
Osteosarcoma is the most common types of bone malignancies with a poor prognosis. In recent years, targeted therapy has a great prospect in the treatment of osteosarcoma, and more effective therapeutic targets for this disease need to be developed. ASPM (Abnormal spindle‐like microcephaly‐associated) is involved in multiple cellular or developmental process, such as neurogenesis and brain growth. ASPM is also reported widely expressed in multiple tumor tissues and involved in the development and progression of several cancers. However, the potential role on ASPM on osteosarcoma is still unclear. In this study, we reported that ASPM was was positively correlated with clinical features including tumor size and clinical stage of osteosarcoma. Our results further showed that ASPM depletion dramatically inhibited the proliferation of osteosarcoma cells, and the in vivo assays further confirmed ASPM ablation markedly blocked tumor growth compared with control. Taken together, our data provides strong evidence that ASPM promotes osteosarcoma proliferation in vitro and in vivo, and indicates its potential role as a osteosarcoma therapeutic target.
Background
Osteosarcoma develops from the mesenchymal cells and is the most common type of bone malignancy 1 . Osteosarcoma is a disease with high mortality in children, with 3450 new cases diagnosed in the United States in 2018. However, the progress of various treatment methods, such as chemotherapy, limb preservation therapy, and immunotherapy, has only marginally improved the survival rate 2 , 3 , 4 , 5 . In addition, osteosarcoma does not have obvious early symptoms and advanced osteosarcoma has a poor prognosis 6 . Therefore, targeted therapy has great potential in the treatment of osteosarcoma 7 . The newly discovered therapeutic targets for osteosarcoma, such as HSP and JIP1, are still in the experimental stage, but their potential clinical value is promising 8 , 9 . To combat osteosarcoma, more effective therapeutic targets need to be developed.
Abnormal spindle microtubule assembly (ASPM), a type of microtubule‐associated centrosomal protein, plays roles in multiple cellular and developmental process, including the regulation of neurogenesis and brain growth 10 , 11 , 12 . ASPM could maintain the symmetric division of neuroepithelial cells 13 . In addition, ASPM localizes to the spindle and midbody and is involved in the functional regulation of spindle organization and cytokinesis 14 , 15 , 16 . ASPM also controls microtubule disassembly and G1 restriction 17 , 18 . ASPM is expressed in multiple tumor tissues and involved in the development and progression of several cancers 19 . ASPM is highly expressed in invasive glioblastoma multiforme, and ASPM depletion inhibits the proliferation of glioblastoma cells 20 . Furthermore, ASPM is reported to be involved in the progression of diverse tumors, including hepatocellular carcinomas, gastric cancer, pancreatic ductal adenocarcinomas, and lung cancer 21 , 22 , 23 , 24 . Previous research indicated that ASPM is involved in the development and progression of lung adenocarcinoma and is associated with poor prognosis 24 . However, the potential role of ASPM in osteosarcoma remains unclear.
In this study, we identified a possible correlation between the expression levels of ASPM and the progression of osteosarcoma. ASPM ablation obviously decreased the proliferation capacity of osteosarcoma cells in vitro and blocked tumor growth in mice. ASPM could, therefore, be a novel and promising therapeutic target to combat osteosarcoma.
Materials and Methods
We followed the methods of Cui et al. 25 .
Immunohistochemistry
Tissue samples were obtained from 82 human osteosarcoma patients receiving surgical therapy in Yantai Yuhuangding Hospital, and immunohistochemistry assays were performed to detect the expression of ASPM in osteogenesis sarcoma tissue samples. Clinical characteristics of patients were recorded, including age at diagnosis, gender, tumor size, tumor differentiation, clinical stage, and metastasis. The immunohistochemical assays were as follows: sample sections of osteosarcoma tissue were first fixed with 4% paraformaldehyde and then blocked with 2% bovine serum albumin/phosphate‐buffered saline (PBS) for 30 min. Subsequently, slides were incubated with the antibody targeted aSPM at room temperature for 2 h. After washing with PBS, slides were incubated with biotinylated secondary antibody at room temperature for 1.5 h, and chromogen substrate was then added. The sections of each patient were photographed within five visual fields. According to the results, ASPM was expressed in both the cytoplasm and nuclear (mainly) of the tissues. The score criteria were as follows: a score of 0 means negative staining, 1 means low staining, 2 means modest staining, and 3 means high staining. For data analysis, the staining intensity was multiplied by the percentage of staining cells. Overall scores of 5 to 100 and 101 to 300 were categorized as low expression and high expression, respectively. When sections showed staining in less than 5% of the tumor cells, this was considered negative staining. The results were assessed using the double‐blind method.
Antibodies and Primers
The antibodies used were: anti‐ASPM (for immunohistochemistry, 1:200 dilution, ABIN5913131; for immunoblot, 1:1000 dilution, ABIN960544, Abcam), anti‐β‐actin (1:1000 dilution, ab8227, Abcam), anti‐Ki67 (1:2000 dilution for Immunoblot and 1:100 dilution for immunohistochemistry [IHC], ab6615, Abcam), and anti‐proliferating cell nuclear antigen (PCNA) (1:1000 dilution, ab29, Abcam).
The quantitative polymerase chain reaction (PCR) primer sequences of ASPM were as follows: forward, 5′‐GGGAAAGGCAAATGGAAAAC‐3′ and reverse, 5′‐CCCAAGGCCATACAAGTGTT‐3′. The quantitative PCR primer sequences of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were as follows: 5′‐CGACCACTTTGTCAAGCTCA‐3′ and 5′‐GGTTGAGCACAGGGTACTTTATT‐3 ′.
Cell Culture and Transfection
Two types of human osteosarcoma cell lines, MG‐63 and U2OS, were purchased from the American Type Culture Collection and cultured in Eagle's Minimum Essential Medium and McCoy's 5a (Modified) Medium, respectively, supplemented with 10% fetal bovine serum at 37°C in a 5% CO2 incubator.
The ASPM short hairpin RNA (shRNA) plasmids were transfected into thyroid cancer cells using Invitrogen Lipofectamine 2000 (Thermo Fisher Scientific). The ASPM shRNA plasmids targeted the sequence of CCGGTCCTGTCTCTCAGCCACTT, and the shRNA plasmids targeted scrambled sequence was used as a negative control. Based on the manufacturer's protocol, 100,000 cells were seeded per well in 6‐well plates. Two groups were set, including: an sh‐ASPM group, transfected with shRNA targeting ASPM, and a negative control group, transfected with scrambled shRNA. Silencing efficiency was detected by quantitative PCR and immunoblot after 48 h. These cells were used to investigate the correlation between ASPM and cell processes. The ASPM stable depletion cell lines were screened and used for both in vitro and in vivo assays.
Quantitative Polymerase Chain Reaction Assay
Total RNA was extracted from MG‐63 and U2OS cells by TRIzol Reagent (Invitrogen). Then total RNA was reverse‐transcribed using M‐MLV Reverse Transcriptase (Promega). A quantitative PCR assay was performed using SYBR mixture (Takara), and the relative expression levels of ASPM were normalized to GAPDH.
Immunoblot Assay
Samples were extracted from tumor cells or tissues and the expression of the indicated protein, including ASPM and β‐actin, was separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. Subsequently, the polyvinylidene difluoride membranes were blocked with 5% dry milk in Tris buffered saline with Tween (TBST) buffer and then incubated with the primary antibodies for the detection of targeted proteins at room temperatures for 2 h. Then the membranes were washed with TBST buffer and incubated with horseradish peroxidase conjugate secondary antibodies for 45 min. After washing, the signals were visualized with an ECL kit.
Colony Formation
Both MG‐63 and U2OS cells were seeded into 6‐well plates at a density of 500 cells per well and cultured for 2 weeks. The colonies were fixed with methanol at −20°C for 5 min and stained with 0.2% crystal violet at room temperature for 30 min. Photographs were then taken and the number of colonies were subsequently calculated.
MTT Assay
MG‐63 and U2OS cells were seeded into 96‐well plates with a density of 1000 cells per well and cultured for 48 h. Sequentially, cells were incubated with MTT for 3 h and the medium was then removed. Then cells were washed with PBS twice. MTT was then extracted by 100 μL dimethyl sulfoxide and the absorbance value at 570 nm wavelength was quantified.
Tumor Growth in Mice
Nude BalB/c mice (6–8 weeks old, 18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). All processes for mouse operations were approved by our Institutional Animal Care Committee. To explore tumor growth in mice, MG‐63 cells were infected with control or ASPM shRNA lentivirus, and subsequently injected into the flank of female nude mice. Tumors formed nearly 2 weeks later, and the volume of tumors was measured each week for 5 weeks. After 7 weeks, the mice were killed for further measurement and analysis.
Statistics Analysis
Data were analyzed with SPSS software and shown as the mean ± standard deviation (SD) both in vitro and for animal experiments. In addition, Student's t‐test was used for statistical comparisons. For the immunohistochemistry experiments, the correlations between ASPM expression and the clinical pathological features were assessed using χ2‐tests. P < 0.05 was considered significant.
Results
Abnormal Spindle Microtubule Assembly Expression was Correlated with the Clinical Pathological Features of Patients with Osteosarcoma
Abnormal spindle microtubule assembly, interestingly, is known to be involved in the progression of multiple tumors. Thereby, to explore the potential function of ASPM in the development of osteosarcoma, several assays were performed. First, ASPM expression in tumor tissues of osteosarcoma patients who underwent surgical resection was examined by IHC assay. The 82 surgical tissue samples were divided into two groups based on ASPM staining intensity (Fig. 1A). The data showed that 50 samples belonged to the ASPM high‐expression group, while 32 belonged to the low‐expression group (Table 1). As a comparison, corresponding non‐tumor normal tissues showed significant low expression of ASPM (Fig. 1B).
Fig. 1.
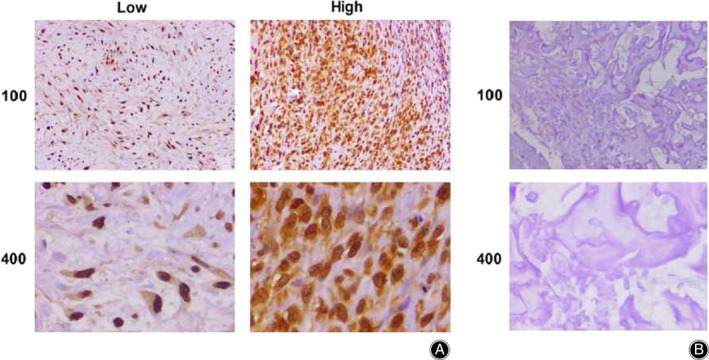
Immunohistochemistry (IHC) assays revealed abnormal spindle microtubule assembly (ASPM) expression in tumor or adjacent normal tissues of patients with osteosarcoma. (A) IHC assays showed the expression of ASPM in osteosarcoma tissues (100× and 200× magnification, respectively). (B) IHC assays revealed the ASPM expression level in adjacent normal tissues (100× and 200× magnification, respectively).
TABLE 1.
Relationships of abnormal spindle microtubule assembly (ASPM) and clinicopathological characteristics in 82 patients with osteosarcoma
| Feature | All n = 82 | ASPM expression | χ2 | P | |
|---|---|---|---|---|---|
| Low | High | ||||
| n = 32 | n = 50 | ||||
| Age (years) | 1.650 | 0.199 | |||
| <20 | 38 | 12 | 26 | ||
| ≥20 | 44 | 20 | 24 | ||
| Gender | 0.504 | 0.478 | |||
| Male | 45 | 16 | 29 | ||
| Female | 37 | 16 | 21 | ||
| Tumor size (cm) | 5.101 | 0.024* | |||
| <5 | 36 | 19 | 17 | ||
| ≥5 | 46 | 13 | 33 | ||
| Differentiation | 1.576 | 0.209 | |||
| Low | 34 | 16 | 18 | ||
| High | 48 | 16 | 32 | ||
| Clinical stage | 4.023 | 0.045* | |||
| I–II | 62 | 28 | 34 | ||
| III | 20 | 4 | 16 | ||
| Metastasis | 1.812 | 0.178 | |||
| Yes | 46 | 15 | 31 | ||
| No | 36 | 17 | 19 | ||
The clinicopathological characteristics of patients with osteosarcoma were then compared between ASPM low and high expression groups. Clinicopathological characteristics, including patient age and gender, tumor size, differentiation, and metastasis, were recorded and analyzed. No obvious difference was noticed in these respects between low and high ASPM expression groups (Table 1). However, the expression levels of ASPM in tumor tissues were obviously related to tumor size (P = 0.024*) and clinical stage (P = 0.045*) (Table 1).
Collectively, these results demonstrated that ASPM was positively associated with clinical characteristics such as tumor size and clinical stage of osteosarcoma.
Abnormal Spindle Microtubule Assembly Fascinates Cell Proliferation of Osteosarcoma in vitro
To examine the potential regulatory mechanism of ASPM in the progression of osteosarcoma, the shRNA specifically targeted ASPM was transfected into two types of osteosarcoma cells, MG‐63 and U2OS cells, to knock down the expression of ASPM. Quantitative PCR (Fig. 2A) and western blot (Fig. 2B) assays showed that the shRNA of ASPM obviously suppressed its expression in both MG‐63 and U2OS cells, respectively.
Fig. 2.
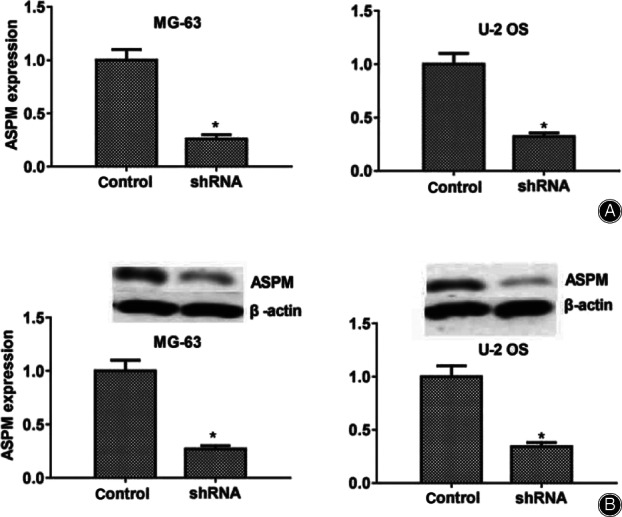
Abnormal spindle microtubule assembly (ASPM) was effectively knocked down, caused by the transfection of its short hair RNA in two types of human osteosarcoma cells. (A) Results of quantitative polymerase chain reaction assays showed that ASPM was sufficiently knocked down in the MG‐63 and U2OS cells, respectively. (B) Results of immunoblot assay revealed that the ASPM gene was efficiently silenced in MG‐63 and U2OS cells. Results are presented as mean ± standard deviation, *P < 0.05.
Abnormal cell proliferation is a key factor in the development and progression of tumors. To evaluate the possible role of ASPM in the proliferation regulation of osteosarcoma, colony formation assays were performed. As expected, knockdown of ASPM dramatically blocked the proliferation of both MG‐63 and U2OS osteosarcoma cells (Fig. 3A). Similarly, a markedly dropped optical density value at 570 nm wavelength in both MG‐63 and U2OS cells was measured through MTT assays (Fig. 3B). To further confirm the effects of ASPM in osteosarcoma proliferation, we analyzed the expression of Ki67 and PCNA, two biomarkers of proliferative cells. We found that the expression of both Ki67 and PCNA led to an obvious decrease in two types of ASPM ablation in MG‐63 and U2OS cells (Fig. 3C,D).
Fig. 3.
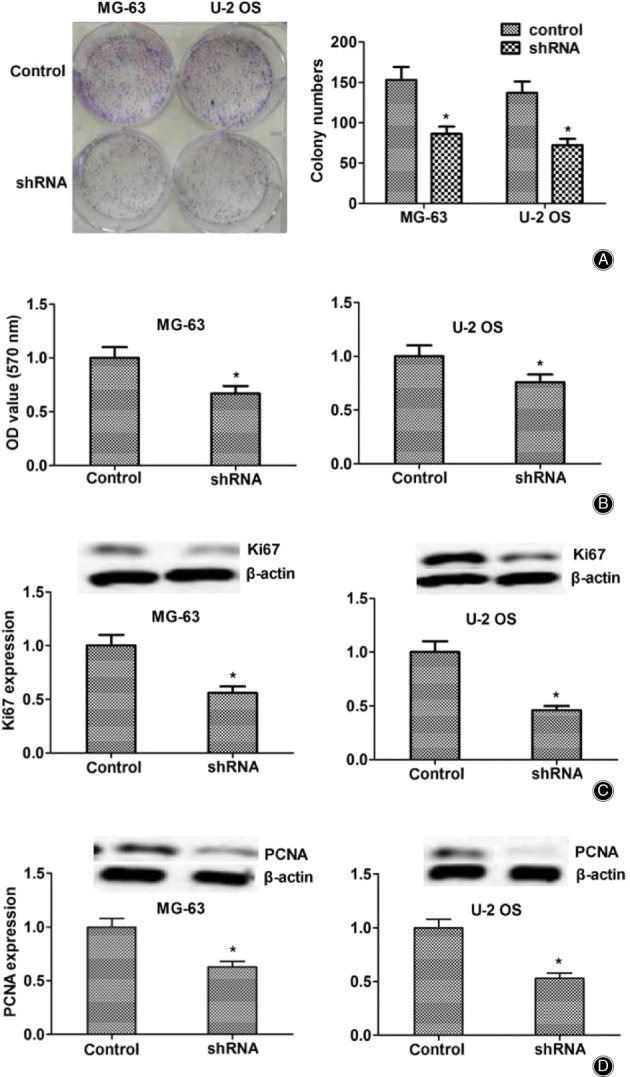
Knockdown of abnormal spindle microtubule assembly (ASPM) dramatically blocked the proliferation and invasion of osteosarcoma cells in vitro. (A). Colony formation assay showed the difference in the proliferation capacity of MG‐63 (A) and U2OS cells (B) that were transfected with control or ASPM shRNA. (B) MTT assay revealed the difference in the proliferation capacity between control and ASPM depletion of osteogenesis sarcoma cells. (C). Immunoblot analysis showed that the Ki67 expression was obviously downregulated in MG‐63 and U2OS cells. (D). Western blot assays showed that proliferating cell nuclear antigen was dramatically downregulated in both MG‐63 and U2OS. Results are presented as mean ± standard deviation. *P < 0.05.
Taken together, the data indicated the involvement of ASPM in osteosarcoma cell proliferation regulation by affecting proliferation‐related proteins such as PCNA and Ki67 in vitro.
Knockdown of Abnormal Spindle Microtubule Inhibits the Development and Metastasis of Osteogenesis Sarcoma in vivo
Because ASPM knockdown obviously blocked the proliferation of osteogenesis in vitro, we thus analyzed the effects of ASPM on the growth of osteogenesis in mice. MG‐63 cells infected with control or ASPM shRNA lentivirus were injected subcutaneously into nude mice, and tumor volume was measured each week after 2 weeks following injection. The tumor volume in the ASPM ablation group was remarkably smaller than in the control group (Fig. 4A, left), and representative photographs were taken in each group (see Fig. 4A, right). In addition, we examined the expression of ASPM in tumors of control and ASPM‐depletion groups, and the results of the expression of ASPM and Ki67 based on western blot assays was markedly reduced in the ASPM knockdown group compared with the control group (Fig. 4B,C). In conclusion, these results indicated that ASPM promoted the proliferation of osteosarcoma in mice.
Fig. 4.
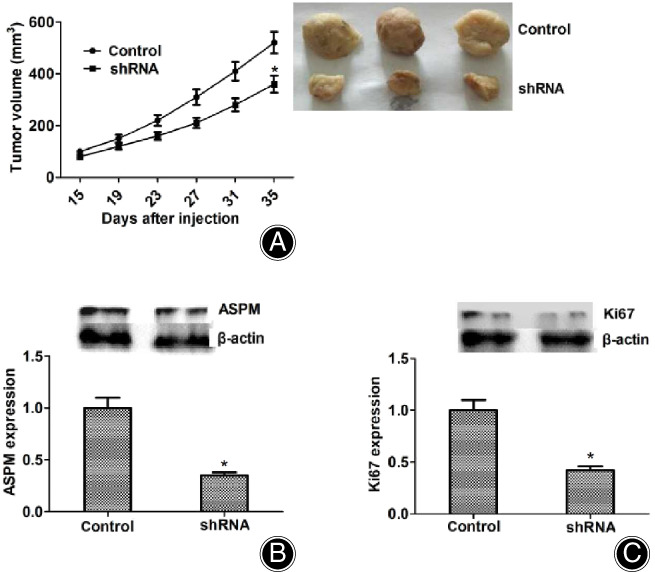
Abnormal spindle microtubule assembly (ASPM) promotes the proliferation of osteosarcoma in mice. (A) MG‐63 cells infected with control or ASPM short hair RNA lentivirus were, respectively, implanted into nude mice. After 2 weeks, tumors were isolated, and photographs were then taken each week. n = 3 in each group. The tumor growth curve was calculated and shown. (B). Western blot assays revealed the ASPM expression of tumors taken from mice in shASPM and shControl groups. (C). Western blot assays revealed the Ki67 expression of tumors taken from mice in shASPM and shControl groups. Results are presented as mean ± SD, *P < 0.05.
Discussion
Osteosarcoma is the most common bone malignancy. It is derived from mesenchymal cells with osteogenic potential 2 , 3 , 4 , 5 . The lack of obvious early symptoms and high metastasis of osteosarcoma also result in high mortality and disability rates 26 . For osteosarcoma, common treatments such as limb salvage, radiotherapy, and chemotherapy have limited efficacy 27 . In recent years, due to low toxicity and high efficiency, targeted therapy for osteosarcoma has shown great potential 28 . Traditional therapeutic targets of osteosarcoma, such as VEGF/VEGFR and EGFR, are linked to adverse prognosis of osteosarcoma 29 . Although the survival rate of patients with osteosarcoma has improved, novel therapeutic targets are still needed to further meet the survival expectations of patients. In this study, we found that ASPM was related to the prognosis of patients with osteosarcoma and could promote the proliferation of osteosarcoma. As a potential therapeutic target for osteosarcoma, ASPM has broad prospects.
Abnormal spindle microtubule assembly is widely expressed in different tissues and obviously affects the progression of multiple types of tumors 19 . The expression of ASPM is associated with the prognosis of glioma and is highly expressed in recurrent tumors 30 . In addition, ASPM is involved in the progression of ovarian cancer, and is associated with the clinical features, such as tumor grade, of ovarian cancer 31 . High expression of ASPM was found in tumor tissues of hepatocellular carcinomas (HCC), a finding correlated with increased invasiveness and high tumor recurrence 32 . High expression of ASPM correlated with a poor patient survival rate in pancreatic cancer 23 . Previous studies have suggested that ASPM may be a potential clinical target for the treatment of lung adenocarcinoma through screening, and the ASPM gene is frequently altered by missense and nonsense mutations 24 . Interestingly, we discovered that ASPM promoted the proliferation of osteosarcoma in vitro and in vivo, and further found that ASPM expression was associated with tumor size and clinical stage, indicating a tight link between ASPM and osteosarcoma.
Abnormal spindle microtubule assembly, which is located at the spindle poles in metaphase of mitotic, is involved in the organization and orientation of spindles, and its deletion or mutation could lead to disorder of the cell cycle 14 . ASPM could regulate the stability of cyclin E, thus affecting the duration of mitosis 18 . Whether ASPM plays a similar role in osteosarcoma deserves further study. In addition, previous studies indicated that ASPM regulated microtubule disassembly at spindle poles by mediating microtubule dynamics 17 . ASPM also mediated the repair of DNA double‐strand break through in a non‐homologous end joining manner 33 . Collectively, this evidence strongly suggests that ASPM plays a key role in regulating cell proliferation. In fact, ASPM depletion blocks neural stem cell and tumor cell proliferation, thereby affecting neurogenesis and tumor growth 34 . Studies in animal models indicated that ASPM was involved in brain growth, partly caused by the regulation of proliferation 35 . Several studies have shown that ASPM can regulate the proliferation of tumor cells, affecting the occurrence and development of tumors 36 . In this study, we found that ASPM promoted the progression of osteosarcoma in a proliferation‐dependent manner, further confirming that ASPM regulated proliferation through different molecular mechanisms and, thus, affected a variety of physiological and pathological processes.
Conclusion
Taken together, our data provides strong evidence that ASPM promotes osteosarcoma proliferation in vitro and in vivo, and indicates its potential role as an osteosarcoma therapeutic target.
Acknowledgments
This study was supported by the Natural Science Foundation of China (81602139), the Natural Science Foundation of Tianjin City (16JCQNJC10100), and Shan Dong Natural Science Foundation of China (ZR2017LH022).
Disclosure: The authors declare that they have no competing interests.
References
- 1. Wang N, Meng X, Liu Y, Chen Y, Liang Q. LPS promote osteosarcoma invasion and migration through TLR4/HOTAIR. Gene, 2019, 680: 1–8. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin, 2018, 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Lu Y, Li L, Lin Z, et al Enhancing osteosarcoma killing and CT imaging using ultrahigh drug loading and NIR‐responsive bismuth sulfide@Mesoporous silica nanoparticles. Adv Healthc Mater, 2018, 7: e1800602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Guan Z. PET/CT in the diagnosis and prognosis of osteosarcoma. Front Biosci (Landmark Ed), 2018, 23: 2157–2165. [DOI] [PubMed] [Google Scholar]
- 5. Rychlowska‐Pruszynska M, Gajewska J, Ambroszkiewicz J, Karwacki M, Szamotulska K. The levels of bone alkaline phosphatase (BALP) and soluble epidermal growth factor receptor‐2 (ECD/HER‐2) in pediatric patients with osteosarcoma during clinical treatment. Dev Period Med, 2018, 22: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yedururi S, Chawla S, Amini B, t al Tumor thrombus in the large veins draining primary pelvic osteosarcoma on cross sectional imaging. Eur J Radiol, 2018, 105: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med, 2014, 17: 301–307. [PubMed] [Google Scholar]
- 8. Yao C, Wei JJ, Wang ZY, et al Perifosine induces cell apoptosis in human osteosarcoma cells: new implication for osteosarcoma therapy? Cell Biochem Biophys, 2013, 65: 217–227. [DOI] [PubMed] [Google Scholar]
- 9. Posthumadeboer J, van Egmond PW, Helder MN, et al Targeting JNK‐interacting‐protein‐1 (JIP1) sensitises osteosarcoma to doxorubicin. Oncotarget, 2012, 3: 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pai VC, Hsu CC, Chan TS, et al ASPM promotes prostate cancer stemness and progression by augmenting Wnt‐Dvl‐3‐beta‐catenin signaling. Oncogene, 2019, 38: 1340–1353. [DOI] [PubMed] [Google Scholar]
- 11. Williams SE, Garcia I, Crowther AJ, et al Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development, 2015, 142: 3921–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bond J, Roberts E, Mochida GH, et al ASPM is a major determinant of cerebral cortical size. Nat Genet, 2002, 32: 316–320. [DOI] [PubMed] [Google Scholar]
- 13. Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci U S A, 2006, 103: 10438–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gai M, Bianchi FT, Vagnoni C, et al ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules. EMBO Rep, 2017, 18: 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tungadi EA, Ito A, Kiyomitsu T, Goshima G. Human microcephaly ASPM protein is a spindle pole‐focusing factor that functions redundantly with CDK5RAP2. J Cell Sci, 2017, 130: 3676–3684. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J, Midgley C, Bergh AM, et al Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol, 2010, 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu XL, Ma W, Zhu YB, et al The microtubule‐associated protein ASPM regulates spindle assembly and meiotic progression in mouse oocytes. PLoS One, 2012, 7: e49303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capecchi MR, Pozner A. ASPM regulates symmetric stem cell division by tuning Cyclin E ubiquitination. Nat Commun, 2015, 6: 8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kouprina N, Pavlicek A, Collins NK, et al The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum Mol Genet, 2005, 14: 2155–2165. [DOI] [PubMed] [Google Scholar]
- 20. Bikeye SN, Colin C, Marie Y, et al ASPM‐associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target. Cancer Cell Int, 2010, 10: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang F, Chang Y, Li J, et al Strong correlation between ASPM gene expression and HCV cirrhosis progression identified by co‐expression analysis. Dig Liver Dis, 2017, 49: 70–76. [DOI] [PubMed] [Google Scholar]
- 22. Wang F, Li J, Liu J, Zhao Q. Controversial role of the possible oxyntic stem cell marker ASPM in gastric cancer. J Pathol, 2017, 241: 559–561. [DOI] [PubMed] [Google Scholar]
- 23. Wang WY, Hsu CC, Wang TY, et al A gene expression signature of epithelial tubulogenesis and a role for ASPM in pancreatic tumor progression. Gastroenterology, 2013, 145: 1110–1120. [DOI] [PubMed] [Google Scholar]
- 24. Kuo WY, Wu CY, Hwu L, et al Enhancement of tumor initiation and expression of KCNMA1, MORF4L2 and ASPM genes in the adenocarcinoma of lung xenograft after vorinostat treatment. Oncotarget, 2015, 6: 8663–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cui L, Zhang JY, Ren ZP, Zhao HJ, Li GS. APLNR promotes the progression of osteosarcoma by stimulating cell proliferation and invasion. Anticancer Drugs, 2019, 30: 940–947. [DOI] [PubMed] [Google Scholar]
- 26. Ryu S, Park S, Lim W, Song G. Effects of luteolin on canine osteosarcoma: suppression of cell proliferation and synergy with cisplatin. J Cell Physiol, 2019, 234: 9504–9514. [DOI] [PubMed] [Google Scholar]
- 27. Fan XL, Cai GP, Zhu LL, Ding GM. Efficacy and safety of ifosfamide‐based chemotherapy for osteosarcoma: a meta‐analysis. Drug Des Devel Ther, 2015, 9: 5925–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaiyawat P, Settakorn J, Sangsin A, et al Exploring targeted therapy of osteosarcoma using proteomics data. Onco Targets Ther, 2017, 10: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boulytcheva IV, Soloviev YN, Kushlinskii NE, Mahson AN. Expression of molecular markers in the tumor and survival prognosis in osteosarcoma. Bull Exp Biol Med, 2010, 150: 237–242. [DOI] [PubMed] [Google Scholar]
- 30. Hagemann C, Anacker J, Gerngras S, et al Expression analysis of the autosomal recessive primary microcephaly genes MCPH1 (microcephalin) and MCPH5 (ASPM, abnormal spindle‐like, microcephaly associated) in human malignant gliomas. Oncol Rep, 2008, 20: 301–308. [PubMed] [Google Scholar]
- 31. Alsiary R, Bruning‐Richardson A, Bond J, Morrison EE, Wilkinson N, Bell SM. Deregulation of microcephalin and ASPM expression are correlated with epithelial ovarian cancer progression. PLoS One, 2014, 9: e97059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin SY, Pan HW, Liu SH, et al ASPM is a novel marker for vascular invasion, early recurrence, and poor prognosis of hepatocellular carcinoma. Clin Cancer Res, 2008, 14: 4814–4820. [DOI] [PubMed] [Google Scholar]
- 33. Kato TA, Okayasu R, Jeggo PA, Fujimori A. ASPM influences DNA double‐strand break repair and represents a potential target for radiotherapy. Int J Radiat Biol, 2011, 87: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 34. Li R, Sun L, Fang A, Li P, Wu Q, Wang X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle‐like (ASPM related primary) microcephaly disease. Protein Cell, 2017, 8: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson MB, Sun X, Kodani A, et al Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature, 2018, 556: 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie JJ, Zhuo YJ, Zheng Y, et al High expression of ASPM correlates with tumor progression and predicts poor outcome in patients with prostate cancer. Int Urol Nephrol, 2017, 49: 817–823. [DOI] [PubMed] [Google Scholar]


