Abstract
Objective
To investigate the epidemiological data, prognostic factors, and treatment outcomes of bone angiosarcoma (BA).
Methods
This retrospective study was based on the Surveillance, Epidemiology, and End Results (SEER) database. The medical records of BA patients were selected from the SEER database from 1975 to 2016. Variables including patients' baseline demographics (age, sex, marital status, and year of diagnosis), tumor characteristics (tumor size, grade, and SEER Historic Stage A), and treatment (surgery and radiotherapy) were selected for further analysis. The research endpoints were overall survival (OS) and cancer‐specific survival (CSS). The optimal cutoff values of continuous variables including age, year of diagnosis, and tumor size were identified using the X‐tail program. Univariate Cox regression was used to identify potential prognostic factors and multivariate Cox regression was used to identify independent prognostic factors. All prognostic factors were included to predict the survival time compared to the median OS and CSS times via the novel nomograms. To validate the internal validation of nomograms, we analyzed the concordance indices (C‐index).
Results
This study enrolled a total of 271 patients with malignant vascular bone tumors among residents of the United States between 1975 and 2016. After applying the exclusion criteria (one case without active follow‐up), this study included 152 patients with BA. The median survival time of BA was significantly shorter than that of malignant vascular bone tumors for OS (9 months vs 27 months, P < 0.001). Age, year of diagnosis, tumor size, grade, stage, and surgery were identified as potential prognostic factors for OS or CSS in univariate Cox regression. However, only age (P < 0.001, P < 0.001), stage (P = 0.002, P < 0.001), and surgery (P = 0.001, P = 0.002) were independent prognostic factors for CSS and OS, respectively, in the multivariate analysis. Younger patients less than 54 years have significantly better prognosis for CSS/OS than patients between 54 and 67 years (Hazard ratios [HRs]: 1.651 [1.763–3.575], 2.557 [1.395–4.687]) and more than 67 years (HRs: 4.404 [2.237–8.670], 5.113 [2.923–8.942]). For CSS/OS, the survival time of patients with localized stage was significantly longer than that of patients with regional stage (HRs: 1.530 [0.725–3.228], 1.548 [0.834–2.873]) and that of patients with distant stage (HRs: 1.706 [0.899–3.237], 2.101 [1.254–3.520]). Patients with surgery had more survival time than patients without surgery for CSS/OS (HRs: 2.861 [1.542–5.310], 2.103 [1.308–3.379]). All factors were further included to generate nomograms for CSS and OS. The C‐indexes for the internal validation of OS and CSS prediction were 0.787 (95% confidence interval [CI]: 0.738–0.836) and 0.768 (95% CI: 0.717–0.819), respectively.
Conclusions
Age, stage, and surgery were closely associated with prognosis in patients with BA, and this clinical model was a favorable tool to evaluate survival possibilities.
Keywords: Bone angiosarcoma, Prognosis, Surgery, Nomogram, Model
Introduction
Angiosarcoma is a rare tumor of endothelial origin with an annual incidence of two to three cases in 1000,000 annually, accounting for less than 1% of sarcomas 1 , 2 , 3 . Angiosarcoma commonly arises in soft tissue, viscera, and skin rather than bone 2 . Compared to hemangioma as a benign tumor, epithelioid hemangioendothelioma as a low‐grade malignant tumor, and hemangioendothelioma as the intermediate condition, angiosarcoma is a high‐grade malignancy 4 . Angiosarcoma patients have a poor prognosis, with a reported 5‐year survival rate of approximately 31% 5 ; the survival of angiosarcoma occurring in the bone is significantly poorer than that of general angiosarcoma, with a 20% 5‐year survival rate and approximately 10‐month survival time 6 , 7 . Until now, studies of bone angiosarcoma (BA) have generally been case reports or single‐intuitive studies 1 , 8 , 9 , 10 . Because of its low incidence, few researchers have investigated the epidemiology, prognosis, and treatment outcomes of BA.
BA may present as a unifocal or multifocal disease 11 , 12 . In most patients, BA occurs in the long bones and short tubular bones, followed by the pelvis, ribs, and vertebrae 12 . This tumor can present with widely variable clinical signs and symptoms, with pain as the most commonly presented symptom. Unlike cutaneous and soft‐tissue angiosarcoma, the typical presentation of BA is indolent swelling; however, in general, the physical examination contributes little to BA diagnosis 13 . Its radiographic appearance is nonspecific and can mimic that of benign neoplasms; therefore, these findings are not usually sufficient to suggest a specific histologic diagnosis 11 . Pathologically, BA usually presents as red, bloody lesions with irregular margins. Microscopically, the vital feature of this tumor is its vasoformative appearance. Histologically, BA comprises anastomosing vascular channels lined by atypical endothelial cells with enlarged nuclei, prominent nucleoli, and increased mitoses 7 , 11 . In addition, inflammatory cells, mostly eosinophils, may be emergent 11 . Therefore, careful evaluation of the clinical, radiographic, and pathologic features is necessary to diagnose BA. The management of patients with BA depends on prognostic factors including epidemiologic characteristics, tumor characteristics, and treatment modalities 14 , 15 , 16 , 17 , 18 , 19 . Although any age in the general population may be affected, the incidence of BA is highest between 50 and 70 years of age 12 and age is considered an independent risk factor for survival in cases appearing in the spine 20 . Race and sex discrepancies have been observed for most cancers; however, previous studies on these factors mainly focused on vascular bone tumors rather than BA 6 , 20 , 21 . Similarly, tumor characteristics including location, grade, and stage have been considered as potential prognostic factors; however, few studies have reported these factors in BA 3 , 22 . Among treatment modalities, the basic options are amputation, limb salvage, radical local resection, and radiation therapy. Although radiotherapy and surgery are often used as routine treatments, their efficacy in patients has not been well‐identified and is debatable. A previous study on primary BA focused on treatment, reporting that complete surgical resection was essential for a positive outcome 7 . However, this study was performed in two institutions and only included 60 patients from 1980 to 2009.
Considering the low incidence and poor prognosis, studies of BA including large study populations are particularly urgent and meaningful. Fortunately, the Surveillance, Epidemiology, and End Results (SEER) database is an integrated database providing complete information for the study of rare cancers; otherwise, studies would be limited by small numbers of cases. This publicly available database supported by the National Cancer Institute covers nearly 28% of the population of the United States. From this high‐quality database, researchers worldwide can extract epidemiologic characteristics, tumor features, treatment, and causes of death with active follow‐up.
Furthermore, as a graphical expression of a mathematical model, a nomogram is a scientific quantification technology used to predict the prognosis of a specific population and develop personalized treatment plans. Nomograms are an important tool that has been widely used to improve the accuracy of predicting survival in the study of tumors. Unfortunately, elaborate nomograms have not been previously reported for predicting survival in patients with BA. Recently, novel nomograms as clinical models are attractive as they combine clinical information with various features to forecast a specific outcome such as death or recurrence in clinical practice. Thus, one of the aims of our study was to use this novel clinical model to forecast the survival and clinical information of BA to provide intuitive recognition for clinicians.
Patients diagnosed with BA with epidemiologic characteristics, tumor characteristics, and treatment modalities were selected from the SEER database. According to these potential prognostic factors, this retrospective study investigated the individual survival time for overall survival (OS) and cancer‐specific survival (CSS), and the median survival time in each subgroup. Based on the above data, we can analyze the effects of these factors on survival time of BA patients for CSS/OS. Therefore, the aim of this study was to use the SEER database to: (i) clearly describe the epidemiological and clinical data; (ii) identify independent prognostic factors; and (iii) construct and validate a novel clinical model to predict the survival probability.
Methods
Data Source
All cases were obtained from the National Cancer Institute's SEER program (https://seer.cancer.gov), which includes 18 population‐based cancer registries. In the SEER database, all available data were retrospective, so no Institutional Review Board approval was necessary. The study progress is shown in Fig. 1.
Fig. 1.
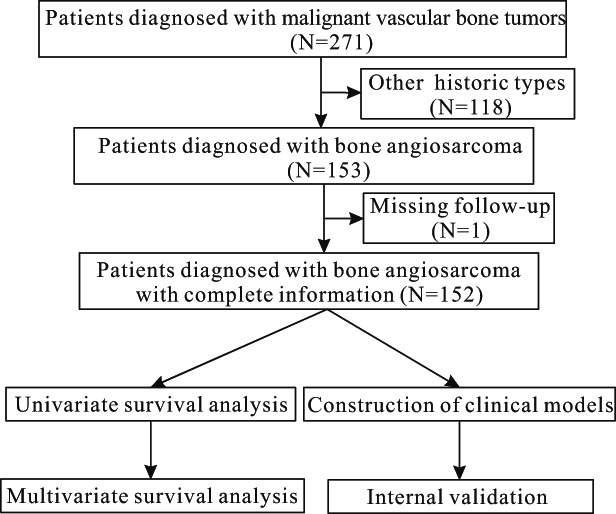
Flow chart of the study process.
Patient Selection
From SEER, a total of 271 patients with malignant vascular bone tumors (9120–9160) were enrolled from 1973 to 2016. According to specific criteria 23 , of them, patients who satisfied the following inclusion criteria were included in the present retrospective study: (i) patients diagnosed with BA (C400–C410 and 9120/3) 15 ; (ii) patients with treatment records including surgery and radiation; (iii) patients with various characteristics, including sex, age, marital status, year of diagnosis, tumor size, primary site, grade, and stage; (iv) patients with survival time for CSS/OS; (v) patients with active follow‐up. We excluded patients who were missing (i) follow‐up data, (ii) COD, or (iii) baseline information. In total, 152 patients diagnosed with BA were selected from the database.
Outcome Measures
In this study, the survival time was calculated from date of diagnosis to date of death. The causes of death were encoded by specific codes from the SEER database, including cancer‐specific death and other causes of death.
Cancer‐specific Survival
CSS was defined as the cancer‐specific survival time calculated according to death owing to specific cancer during follow‐up, which is used to evaluate the impact of this cancer on survival.
Overall Survival
OS was defined as the overall survival time calculated according to death due to any cause (including cancer and other causes) during follow‐up, which is used to evaluate the impact of all causes on survival.
If a patient died of BA, CSS and OS would both be obtained, and the value of CSS was the same as that of OS; however, if the patient died of other causes, OS but not CSS would be obtained, and the patients would be excluded from the survival analysis of CSS.
Statistical Analysis
Continuous variables (age, year of diagnosis, and tumor size) were stratified using the X‐tile program 24 (version 3.6.1, Yale University, Connecticut, USA) to identify their optimal cutoff values according to the minimum P values from log‐rank, chi‐square statistics.
Data on sex, age, marital status, year of diagnosis, tumor size, primary site, grade, stage, radiotherapy, and surgery were included in the univariate log‐rank analyses for OS and CSS, respectively. Statistically significant factors and radiotherapy were incorporated in the multivariate Cox proportional hazards model for OS and CSS, respectively. Hazard ratios (HRs) of variables with 95% confidence intervals (CIs) were shown for OS and CSS. The Kaplan–Meier (K–M) method was used to analyze survival duration. Log‐rank tests were used to distinguish the differences between survival curves. These statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Two‐sided P values <0.05 were considered statistically significant.
Construction and Validation of Nomograms
All factors were included in the nomograms to analyze the survival for OS/CSS by Cox proportional hazards regression. We then generated nomograms in R software (version 3.3.0) and calculated Heagerty's concordance index (C‐index) to test their accuracy. The C‐index was positively associated with the accuracy of prognostic prediction.
Results
Baseline Characteristics of the Study Population
A total of 271 patients with malignant vascular bone tumors were identified from 1973 to 2016, of which 152 (56.1%) BA patients with active follow‐up and complete information were included in this study. From the SEER database, we found five types of malignant vascular bone tumors, including angiosarcoma, hemangioendothelioma (46, 17.0%), epithelioid hemangioendothelioma (50, 18.5%), Kaposi sarcoma (3, 1.1%), and hemangiopericytoma (19, 7.0%). Angiosarcoma was the most common tumor among malignant vascular bone tumors (Fig. 2A) and the CSS/OS times for BA were significantly shorter than those of malignant vascular bone tumors (18 months vs 199 months, P < 0.001; 9 months vs 27 months, P < 0.001) (Fig. 2B,C). The baseline patient characteristics included sex, age, marital status, year of diagnosis, tumor size, primary site, grade, stage, radiotherapy, and surgery. In our study, females (104, 68.4%), married population (97, 63.8%), and patients diagnosed from 2000 to 2016 (107, 70.4%) comprised most patients. Also, most BA was observed in limbs (83, 54.6%), and the mean size of tumor was 72.2 ± 48.6 mm. Of BA patients, the most patients were found in grade III/IV (28, 18.4%; 28, 18.4%) and in the distant stage (60, 39.5%).
Fig. 2.
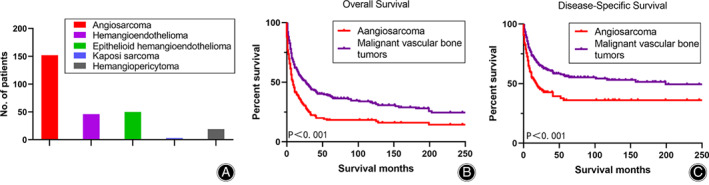
The frequency distribution of malignant vascular bone tumors (A). The survival comparison between bone angiosarcoma patients and malignant vascular bone tumors patients by Kaplan–Meier analysis for overall survival and disease‐specific survival, respectively (B, C).
Optimal Cutoff Values and Univariate Survival Analysis for Continuous Variables Calculated Using X‐tile
Continuous variables including age at diagnosis, year of diagnosis, and tumor size were stratified using the X‐tile program (Fig. 3). Then, according to the optimal cutoff value, an accurate and vital survival analysis was carried out by subgroups for OS/CSS.
Fig. 3.
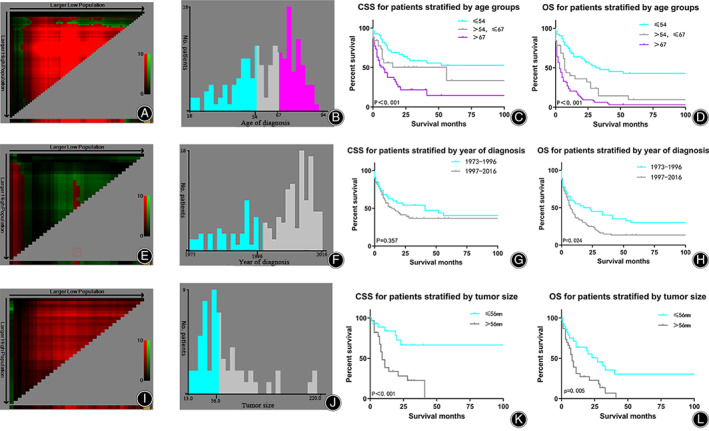
X‐tile analysis and Kaplan–Meier analysis of survival for CSS/OS reveals a continuous distribution and survival based on age (A–D), year of diagnosis (E, F) and tumor size (I–L). Abbreviations: CSS, cancer‐specific survival; OS, overall survival.
The optimal cutoff values for age at diagnosis in the present study were 54 and 67 years (Fig. 3A,B). The K–M survival analysis revealed that greater age was associated with worse CSS/OS, with patients more than 67 years having significantly worse prognosis than patients between 54 and 67 years of age (8 months vs 56 months, P < 0.001; 3 months vs 7 months, P < 0.001) and patients under 54 years having better prognosis than patients aged 54–67 years (P < 0.001; 56 months vs. 8 months, P < 0.001) (Fig. 3C,D). Our results suggested that age is a potential prognostic factor significantly affecting the prognosis of patients with BA. The optimal cutoff value of the year of diagnosis was 1996 (Fig. 3E,F). K–M analysis showed a significant difference between the two subsets for OS (P = 0.024) (Fig. 3H) but not for CSS (P = 0.357) (Fig. 3G), indicating that year of diagnosis might be a prognostic factor for OS but not CSS. The optimal cutoff value for tumor size was 56 mm (Fig. 3I,J) and K–M analysis showed a significant difference between the two subsets for CSS/OS (P = 0.008 and P = 0.049, respectively) (Fig. 3K,L). Based on the results of our analysis, we included these variables as potential factors in the subsequent multivariate analysis.
Univariate Survival Analysis of Categorical Variables
Univariate survival analysis of CSS/OS revealed grade, stage, and surgery as prognostic factors to include in multivariate survival analysis (Table 1). As shown in Fig. 4A,B, K–M survival analysis showed significantly worse prognosis (CSS/OS) in patients with regional stage than that in patients with localized disease, while patients with regional disease had a better prognosis than patients with distant‐stage disease (P = 0.001, P < 0.001). As shown in Fig. 4C,D, surgical treatment was an important prognostic factor for CSS/OS (P = 0.005, P = 0.004). Treatment modality was also significantly associated with the prognosis of BA (CSS/OS) (P = 0.001, P < 0.004) (Table 1 and Fig. 4E,F).
TABLE 1.
Univariate analysis and survival time of variables for prognostic factors of bone angiosarcoma patients (diagnosed 1975–2016)
| Subject Characteristics | Survival of CSS | Survival of OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | P value | Median | 95% CI | P value | |||
| Lower | Upper | Lower | Upper | |||||
| Age, years | <0.001 * | <0.001 * | ||||||
| ≤54 | UD | UD | UD | 31 | 1.748 | 60.252 | ||
| 54–67 | 56 | 0.000 | 120.492 | 7 | 5.018 | 8.982 | ||
| >67 | 8 | 3.664 | 12.336 | 3 | 1.077 | 4.923 | ||
| Sex | 0.152 | 0.097 | ||||||
| Female | 11 | 0.259 | 21.741 | 7 | 4.712 | 9.288 | ||
| Male | 28 | 0.000 | 67.058 | 16 | 6.223 | 25.777 | ||
| Year of diagnosis | 0.357 | 0.024 * | ||||||
| 1973–1996 | 41 | 0.000 | 84.700 | 18 | 0.000 | 38.144 | ||
| 1997–2016 | 14 | 5.149 | 22.851 | 7 | 4.764 | 9.236 | ||
| Marital status | 0.708 | 0.629 | ||||||
| Married | 17 | 4.748 | 29.252 | 7 | 3.364 | 10.636 | ||
| Unmarried | 26 | 0.000 | 56.272 | 10 | 6.035 | 13.965 | ||
| Unknown | NA | NA | NA | NA | NA | NA | ||
| Tumor sites | 0.533 | 0.517 | ||||||
| Limbs | 18 | 7.921 | 28.079 | 10 | 6.676 | 13.324 | ||
| Head and body | 11 | 0.000 | 28.099 | 6 | 3.335 | 8.665 | ||
| Unknown | NA | NA | NA | |||||
| Tumor size, mm | 0.008 * | 0.049 * | ||||||
| ≤56 | UD | UD | UD | 27 | 12.131 | 41.869 | ||
| >56 | 9 | 5.746 | 12.254 | 8 | 5.596 | 10.404 | ||
| Unknown | NA | NA | NA | 6 | 3.907 | 8.093 | ||
| Grade | 0.009 * | 0.028 * | ||||||
| IV | 11 | 3.577 | 18.423 | 7 | 4.518 | 9.482 | ||
| III | 9 | 4.240 | 13.760 | 7 | 5.417 | 8.583 | ||
| I + II + Unknown | 52 | UD | UD | 11 | 3.987 | 18.013 | ||
| Stage | 0.001 * | <0.001 | ||||||
| Localized | 41 | 7.205 | 74.795 | 20 | 7.248 | 32.752 | ||
| Regional | 28 | UD | UD | 17 | 0.000 | 39.311 | ||
| Distant | 6 | 1.735 | 10.265 | 3 | 1.507 | 4.493 | ||
| Unknown | NA | NA | NA | NA | NA | NA | ||
| Radiation | 0.777 | 0.672 | ||||||
| Yes | 17 | 6.217 | 27.783 | 7 | 3.530 | 10.470 | ||
| No | 18 | 0.264 | 35.736 | 9 | 5.530 | 12.470 | ||
| Surgery | 0.005 * | 0.004 * | ||||||
| Yes | 41 | 12.820 | 69.180 | 16 | 7.623 | 24.377 | ||
| No | 7 | 2.627 | 11.373 | 2 | 0.000 | 5.447 | ||
| Unknown | NA | NA | NA | NA | NA | NA | ||
| Treatment modality | 0.001 * | <0.001 * | ||||||
| No therapy | 2 | 0.000 | 7.269 | 1 | 0.000 | 3.287 | ||
| Radiation only | 5 | 1.752 | 8.248 | 2 | 0.000 | 6.080 | ||
| Surgery+Radiation | 26 | 3.421 | 48.579 | 10 | 0.977 | 19.023 | ||
| Surgery only | 28 | 0.000 | 67.112 | 18 | 4.062 | 31.938 | ||
| other | NA | NA | NA | NA | NA | NA | ||
CSS, cancer‐specific survival; NA, not avail; OS, overall survival; UD, undefined.
P < 0.05.
Fig. 4.
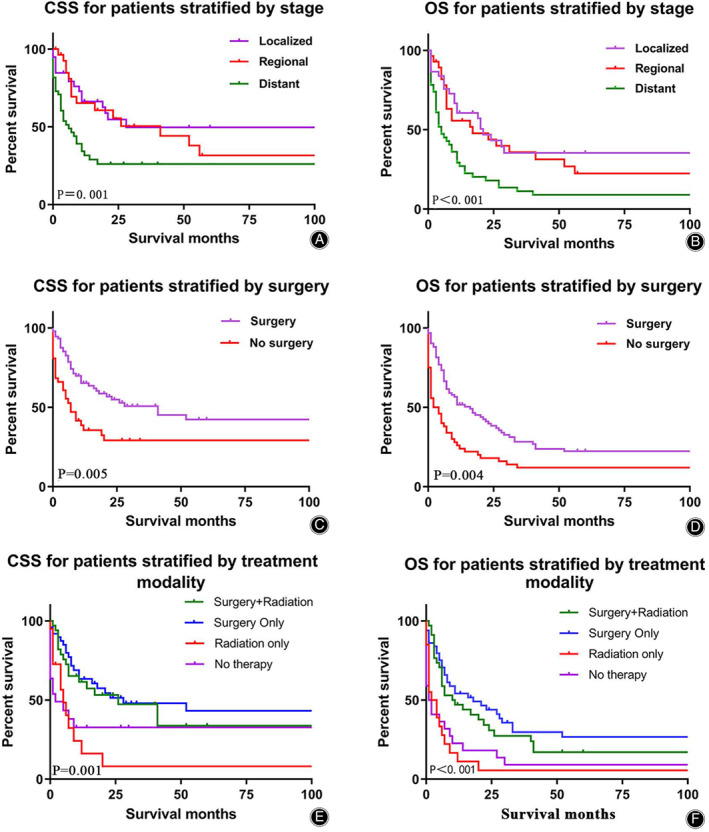
Kaplan–Meier analysis of CSS/OS among bone angiosarcoma patients were stratified by stage (A, B), surgery (C, D) and treatment modality (E, F). Abbreviation: CSS, cancer‐specific survival; OS, overall survival.
Independent Prognostic Factors for OS and CSS by Multivariate Analysis
Univariate survival analysis for OS and CSS showed that age, year of diagnosis, tumor size, grade, stage, and surgery were associated with prognosis (Table 1). However, surprisingly, radiotherapy was not associated with BA survival (Table 1). Therefore, the multivariate analyses for OS and CSS included all these potential factors. The results showed that age, stage, and surgery were independent prognostic factors for OS and CSS, while the year of diagnosis, tumor size, grade, and radiotherapy were not (Table 2). Older patients have significantly poorer prognosis than younger patients with the optimal cutoff values of 54 and 67 years (P < 0.001, P < 0.001). For CSS/OS, the median survival time of patients with regional stage was significantly longer than that of patients with distant stage, but was shorter than that of patients with localized stage (28 months vs 6 months vs 41 months, P = 0.002; 17 months vs 3 months vs 20 months, P < 0.001). Patients with surgery had more survival time than patients without surgery for CSS/OS (41 months vs 7 months, P = 0.001; 16 months vs 2 months, P = 0.002).
TABLE 2.
Multivariable logistic regression for analyzing prognostic factors of bone angiosarcoma patients (diagnosed 1975–2016)
| Subject characteristics | CSS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | <0.001 * | <0.001 * | ||
| ≤54 | 1 (reference) | 1 (reference) | ||
| 54–67 | 1.651 (0.763–3.575) | 0.203 | 2.557 (1.395–4.687) | 0.002 |
| >67 | 4.404 (2.237–8.670) | 0.000 | 5.113 (2.923–8.942) | <0.001 |
| Year of diagnosis | ||||
| 1973–1996 | 1 (reference) | 1 (reference) | ||
| 1997–2016 | 0.627 (0.321–1.228) | 0.174 | 0.831 (0.489–1.411) | 0.493 |
| Grade | 0.066 | 0.176 | ||
| IV | 1 (reference) | 1 (reference) | ||
| III | 0.785 (0.380–1.621) | 0.513 | 1.083 (0.605–1.938) | 0.788 |
| I + II + Unknown | 0.480 (0.250–0.922) | 0.027 | 0.707 (0.420–1.191) | 0.193 |
| Tumor size, mm | 0.520 | 0.362 | ||
| ≤56 | 1 (reference) | 1 (reference) | ||
| >56 | 2.872 (1.145–7.204) | 0.250 | 1.528 (0.800–2.919) | 0.199 |
| Unknown | NA | NA | NA | NA |
| Stage | 0.002 * | <0.001 * | ||
| Localized | 1 (reference) | 1 (reference) | ||
| Regional | 1.530 (0.725–3.228) | 0.265 | 1.548 (0.834–2.873) | 0.167 |
| Distant | 1.706 (0.899–3.237) | 0.102 | 2.101 (1.254–3.520) | 0.005 |
| Unknown | NA | NA | NA | NA |
| Radiation | ||||
| Yes | 1 (reference) | 1 (reference) | ||
| No | 1.083 (0.663–1.768) | 0.750 | 1.089 (0.739–1.604) | 0.666 |
| Surgery | 0.001 * | 0.005 * | ||
| Yes | 1 (reference) | 1 (reference) | ||
| No | 2.861 (1.542–5.310) | 0.001 | 2.103 (1.308–3.379) | 0.002 |
| Unknown | NA | NA | NA | NA |
CSS, cancer‐specific survival; NA, not avail; OS, overall survival.
P < 0.05.
Construction of Nomograms for CSS and OS
A nomogram was constructed to predict the rate of OS and CSS survival (Fig. 5). According to the nomogram, an individual patient can be located on each variable axis (red point in Fig. 5) and a line can be drawn upward to find the value of points for each variable. The sum of all variable values is located on the total points axis for CSS and OS, and a line drawn downward to the survival axes can be used to determine the probability that the BA patients’ survival is less than the median survival time (18 months for CSS, 9 months for OS). In addition, in Fig. 5, the length of the green lines indicates the number of patients. In the internal validation set, the C‐indexes for CSS and OS prediction were 0.787 (95%CI: 0.738–0.836) and 0.768 (95%CI: 0.717–0.819), respectively.
Fig. 5.
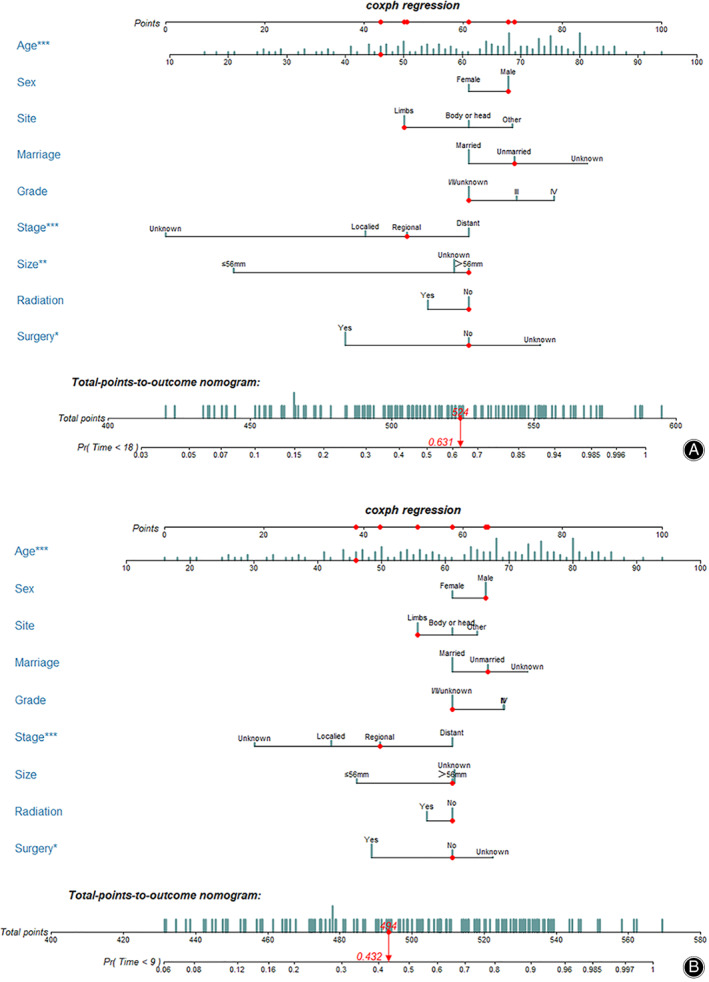
Nomograms were used to predict survival of bone angiosarcoma patients for disease‐specific survival (A) and overall survival (B). *P < 0.05, **P < 0.01,***P < 0.001.
For example, we selected a 46‐year‐old unmarried male patient with regional BA of the limbs, with a tumor size of 94 mm, who received no therapy. His total points for CSS were 524 and the probability of a survival time less than the median survival time (18 months) was 0.631. His total points for OS were 494, corresponding to a probability of survival time less than the median survival time (9 months) of 0.432.
Discussion
Epidemiology and OS of BA
It is important to understand the epidemiology and prognosis of BA. In this context, we obtained the baseline characteristics and treatment modalities of 152 patients with active follow‐up from among 271 patients with malignant vascular bone tumors. Because of the low incidence of BA, we were not able to obtain the incidence trend but did observe that BA was the most common malignant vascular bone tumor from 1975 to 2016. This finding supported the theory of a significantly larger proportion of BA among malignant vascular bone tumors compared to those of other historic types 6 , 21 .
BA is a high‐grade malignant tumor (Enneking Stage II, mostly B as extracompartmental), different from hemangioma and hemangioendothelioma 4 . Hemangiomas are nearly benign tumors and most are asymptomatic. Hemangioendothelioma is an intermediate condition (Enneking Stage III benign aggressive, extracompartmental) 4 . Because of the histological differences, the prognosis of BA also intuitively differs from those of other types of malignant vascular bone tumors, including epithelioid hemangioendothelioma, Kaposi sarcoma, and hemangiopericytoma 4 , 25 . However, this speculation requires support from data from large‐scale databases. Although some studies have examined the epidemiology and prognostic factors, few have indirectly compared survival between BA and malignant vascular bone tumors in a study 6 , 20 , 21 . Regarding BA survival time, in a two‐institution study of 60 patients, researchers observed 5‐year OS rates of 20% and 0% for metastatic patients and a median survival time of only 0.9 years 7 . Alberto et al. also reported extremely poor survival of BA patients, with a median survival time of 10 months from diagnosis 6 . In this study, despite the histology of malignant vascular bone tumors, we observed that the prognosis of malignant BA was significantly poorer than that of malignant vascular tumors. The median OS of BA was only 9 months.
In summary, BA is a high‐grade malignant form and the highest incidence type among malignant vascular tumors; thus, bone oncologists must research its prognostic factors by survival analysis and predict the survival possibility of individual patients using valid nomograms. To our knowledge, this is the first study based on a large population to study the prognosis and treatment outcome and to create a clinical model.
Epidemiological Analysis and Identification of Prognostic Factors
BA grade information was indirectly obtained from the SEER database and included grade I (well‐differentiated), grade II (moderately differentiated), grade III (poorly differentiated), and grade IV (undifferentiated). Generally speaking, grades I/II are considered low‐grade, while grades III/IV are considered high‐grade 15 , 20 , 26 . In our study, we observed significantly fewer grade I/II cases than grade III/IV cases, which also demonstrated that BA was a highly malignant tumor. However, because of the very low incidence of low‐grade BA, survival could not be analyzed. Thus, we combined grade I/II and unknown into a single grade I/II/unknown group. In terms of survival analysis, tumors with high grades intuitively resulted in poor prognosis. Researchers in two series reported that grade was closely associated with prognosis, contrary to our results 16 , 20 . In our analysis, grade was a potential prognostic factor in the univariate survival analysis but was not an independent prognostic factor for CSS/OS in the multivariate survival analysis. Considering that the SEER data are of high quality and collected in a standard manner, we suspect that the large number of unknown grades (47.4%) in the early decades might explain the controversial results in our study, hindering the discovery of differences between the two groups in the general population. Thus, with improved management of this public database, scholars must select more cases with known grades to analyze the effect of grade on survival.
The optimal cutoff value for the year of diagnosis and tumor size was initially calculated using the X‐tile program according to the minimum P values from log‐rank chi‐square statistics. Similar to grade, these factors were corroborated to be prognostic factors by univariate survival analysis; however, the results of the multivariate logistic regression showed that they were not independent prognostic factors. Therefore, when analyzing the effects of prognostic factors on BA, it is necessary to perform multivariate survival analysis to consider the interference of confounding factors.
Most reported BA originated from limbs, consistent with our results 7 . BA occurred in the limbs in 83 patients, followed by the head and body in 13 and 34 patients, respectively. To check if the primary site of BA could affect CSS/OS, we performed univariate survival analysis. The survival of the primary site in limbs did not differ between the head and body. In addition, based on previous studies, sex and marital status were considered as important prognostic factors for sarcomas or bone tumors 18 , 27 ; however, these factors were not significant in the present study and were excluded from the multivariate survival analysis.
Age and stage are vital risk factors for patients with bone malignant vascular tumors or angiosarcoma in other sites 3 , 21 , 22 ; therefore, age at diagnosis and stage were also considered in assessing CSS/OS in the present study. As expected, both age and stage were closely associated with BA survival time for both CSS and OS in univariate and multivariate survival analyses. Younger patients had a more ideal prognosis than older patients, with optimal cutoffs of 54 years and 67 years. In fact, for most solid tumors, the incidence and mortality increase exponentially with age in the multi‐step carcinogenic model 28 . The following may explain these findings. (i) With age, the immune system gradually declines and the carcinogenic chance of risk factors gradually increases. (ii) The prevalence of some carcinogenic factors increases with age; for example, viral infection is an important susceptibility factor for the elderly, leading to T‐cell exhaustion that favors telomere attrition and immune senescence 29 . In addition, with age, the cumulative effect of tobacco smoking is also a vital carcinogenic factor in the elderly 30 . (iii) Some genes and epigenetics might also change with age. (iv) The levels of some micromolecules may be abnormal due to age or the microenvironment; for example, B‐cell lymphoma 2 (BCL2) levels are likely to change with age 31 . (v) Older patients are more likely to be associated with delayed diagnosis, difficulties with surgery, and the presence of comorbidities, which may result in poor prognosis. In addition, the results of this study revealed that stage was associated with BA prognosis (CSS/OS), with the regional stage showing a worse prognosis than that of patients with localized stage and regional stage showing a more satisfactory prognosis than that for distant‐stage disease. A previous study with a median follow‐up of 10 years reported a 5‐year OS of 33% in patients with localized disease and 0% for metastatic patients.
Surgery has been considered a routine and important treatment that affects prognosis; however, the treatment outcomes of radiotherapy remain controversial 18 , 19 , 21 . Until now, the validity of surgery and radiotherapy lacked evidence. This study explored the impact of surgery and radiotherapy on BA patients. The OS and CSS in univariate survival or multivariate analysis showed that surgery had a positive effect on prognosis, whereas radiotherapy was not an independent prognostic factor. Furthermore, we also investigated the treatment outcomes of treatment modality. The next results reinforced the conclusion that surgery, but not radiotherapy, was a valid treatment. Our data indicated that BA with surgery only or surgery combined with radiotherapy showed better treatment outcomes than those for radiotherapy alone or no therapy and that the survival time of patients with radiotherapy was slightly shorter than that of patients not receiving radiotherapy. Our results support those of a previous study based on a small sample 20 . Thus, we have adequate reasons to recommend surgery rather than radiotherapy for BA patients. Regrettably, we are not able to provide more information about detailed surgery due to the limitations of the SEER database.
Construction and Advantages of this Novel Clinical Model
Based on multivariate Cox models, nomograms have gradually been applied to predict individual survival possibility because they can integrate all prognostic‐related factors and comprehensively evaluate the cumulative effects of factors on patients. The new nomogram in our study not only compared individual survival times to the median survival time but also revealed the population propensity in different subgroups and the distribution of prognosis, unlike traditional nomograms. In our study, the median survival times for CSS and OS were 18 and 9 months, respectively. We were able to use a nomogram to calculate the probability of survival time less than the median survival times. Furthermore, the high C‐indexes for CSS/OS showed an ideal validation of survival prediction.
Limits
This study has several limitations. First, due to the limitations of the SEER database, some patient information was missing and some basic information was not included, including smoking status, body mass index, and family history. Second, although the SEER database is a large data resource, we only obtained data on 152 cases with active follow‐up. Third, several selection biases might exist in this retrospective study due to the inherent flaws of this study design. Fourth, this database does not include information on derailed treatment and complications. Future studies on BA should include more cases with competing information on detailed surgery processes and related complications.
Conclusion
BA is a rare and high‐grade malignant tumor occurring mainly in older females and patients with generally distant stages of cancer. Older age and distant stage were closely associated with poor prognosis. Surgery is an ideal treatment modality, while radiotherapy may not be effective. The novel nomogram was a favorable clinical model to predict survival possibility and may be beneficial in assisting oncologists to make clinical decisions.
Acknowledgments
This work is supported by grants from the National Nature Foundation of China (Grant no. 81871806).
Disclosure: The authors have no conflicts of interest to declare. All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
References
- 1. Scholsem M, Raket D, Flandroy P, Sciot R, Deprez M. Primary temporal bone angiosarcoma: a case report. J Neurooncol, 2005, 75: 121–125. [DOI] [PubMed] [Google Scholar]
- 2. Fedok FG, Levin RJ, Maloney ME, Tipirneni K. Angiosarcoma: current review. Am J Otolaryngol, 1999, 20: 223–231. [DOI] [PubMed] [Google Scholar]
- 3. Conic RRZ, Damiani G, Frigerio A, et al Incidence and outcomes of cutaneous angiosarcoma: a SEER population‐based study. J Am Acad Dermatol, 2019, S0190‐9622: 32382–32385. [DOI] [PubMed] [Google Scholar]
- 4. Boriani S, Cecchinato R, Righi A, et al Primary vascular bone tumors in the spine: a challenge for pathologists and spine oncology surgeons. Eur Spine J, 2019, 28: 1502–1511. [DOI] [PubMed] [Google Scholar]
- 5. Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14‐year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J, 2005, 11: 241–247. [DOI] [PubMed] [Google Scholar]
- 6. Righi A, Sbaraglia M, Gambarotti M, et al Primary vascular tumors of bone: a monoinstitutional morphologic and molecular analysis of 427 cases with emphasis on epithelioid variants. Am J Surg Pathol, 2020, 44: 1192–1203. [DOI] [PubMed] [Google Scholar]
- 7. Palmerini E, Maki RG, Staals EL, et al Primary angiosarcoma of bone: a retrospective analysis of 60 patients from 2 institutions. Am J Clin Oncol, 2014, 37: 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernstock JD, Shafaat O, Hardigan A, et al Angiosarcoma of the temporal bone: case report and review of the literature. World Neurosurg, 2019, 130: 351–357. [DOI] [PubMed] [Google Scholar]
- 9. Liu M, Liu Y, Guo H, et al Apatinib, combined with chemotherapy or alone is effective in treating angiosarcoma: a case report. Anticancer Drugs, 2019, 30: e0738. [DOI] [PubMed] [Google Scholar]
- 10. Verdura V, Di Pace B, Concilio M, et al A new case of radiation‐induced breast angiosarcoma. Int J Surg Case Rep, 2019, 60: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wenger DE, Wold LE. Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol, 2000, 29: 619–631. [DOI] [PubMed] [Google Scholar]
- 12. Verbeke SL, Bertoni F, Bacchini P, et al Distinct histological features characterize primary angiosarcoma of bone. Histopathology, 2011, 58: 254–264. [DOI] [PubMed] [Google Scholar]
- 13. Lopes M, Duffau H, Fleuridas G. Primary spheno‐orbital angiosarcoma: case report and review of the literature. Neurosurgery, 1999, 44: 405–407; discussion 407‐408. [DOI] [PubMed] [Google Scholar]
- 14. Chang AE, Chai X, Pollack SM, et al Analysis of clinical prognostic factors for adult patients with head and neck sarcomas. Otolaryngol Head Neck Surg, 2014, 151: 976–983. [DOI] [PubMed] [Google Scholar]
- 15. Pandey M, Sutton GR, Giri S, Martin MG. Grade and prognosis in localized primary angiosarcoma. Clin Breast Cancer, 2015, 15: 266–269. [DOI] [PubMed] [Google Scholar]
- 16. Abraham JA, Hornicek FJ, Kaufman AM, et al Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol, 2007, 14: 1953–1967. [DOI] [PubMed] [Google Scholar]
- 17. O'Neill JP, Bilsky MH, Kraus D. Head and neck sarcomas: epidemiology, pathology, and management. Neurosurg Clin N Am, 2013, 24: 67–78. [DOI] [PubMed] [Google Scholar]
- 18. Peng KA, Grogan T, Wang MB. Head and neck sarcomas: analysis of the SEER database. Otolaryngol Head Neck Surg, 2014, 151: 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin E, Radomski S, Harley EH. Pediatric Ewing sarcoma of the head and neck: a retrospective survival analysis. Int J Pediatr Otorhinolaryngol, 2019, 117: 138–142. [DOI] [PubMed] [Google Scholar]
- 20. Xu K, Liu Y, Li B, et al Prognostic factors of patients with malignant epithelioid vascular tumors in the spine: retrospective analysis of 46 patients in a single center. Spine (Phila Pa 1976), 2018, 43: E1218–E1224. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Hong J, Meng J, et al Survival analysis of patients with osseous malignant vascular tumors: results of the surveillance, epidemiology, and end results (SEER) database from 1973 to 2015. Med Sci Monitor, 2019, 25: 5525–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee KC, Chuang SK, Philipone EM, Peters SM. Characteristics and prognosis of primary head and neck angiosarcomas: a surveillance, epidemiology, and end results program (SEER) analysis of 1250 cases. Head Neck Pathol, 2019, 13: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang JQ, Chen C, Zhao H. Revision surgery after percutaneous endoscopic transforaminal discectomy compared with primary open surgery for symptomatic lumbar degenerative disease. Orthop Surg, 2019, 11: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Camp RL, Dolled‐Filhart M, Rimm DL. X‐tile: a new bio‐informatics tool for biomarker assessment and outcome‐based cut‐point optimization. Clin Cancer Res, 2004, 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 25. Kendre P, Kataria P, Patel AA, Gaurav L, Dalsaniya S. Hemangiopericytoma of supraglottis: a rare case report and review of literature. J Canc Res Ther, 2019, 15: 729–732. [DOI] [PubMed] [Google Scholar]
- 26. Arshi A, Sharim J, Park DY, et al Chondrosarcoma of the osseous spine: an analysis of epidemiology, patient outcomes, and prognostic factors using the SEER registry from 1973 to 2012. Spine (Phila Pa 1976), 2017, 42: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo X, Zhang C, Guo Q, et al The homogeneous and heterogeneous risk factors for the morbidity and prognosis of bone metastasis in patients with prostate cancer. Cancer Manag Res, 2018, 10: 1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armitage P, Doll R. The age distribution of cancer and a multi‐stage theory of carcinogenesis. Br J Cancer, 2004, 91: 1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellon M, Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses, 2017, 9: E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taborelli M, Montella M, Libra M, et al The dose‐response relationship between tobacco smoking and the risk of lymphomas: a case‐control study. BMC Cancer, 2017, 17: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B‐cell lymphoma in children. Br J Haematol, 2008, 142: 329–347. [DOI] [PubMed] [Google Scholar]


