Abstract
Purpose
The prognostic value of serum calcium levels in nasopharyngeal carcinoma (NPC) remains unknown. This study aimed to evaluate the prognostic value of serum calcium levels in patients with NPC.
Patients and Methods
A total of 2094 patients diagnosed with NPC between April 2009 and September 2012 were enrolled in this retrospective analysis. The median follow-up time was 96.3 months (range: 4.1–120.0 months). Univariate and multivariable Cox proportional hazards models were used to identify significant and independent prognostic predictors of overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS), and relapse-free survival (RFS).
Results
Overall, low serum calcium levels were detected in 1109/2094 (53.00%) patients and tended to be more frequently detected in older (P<0.001) and female (P=0.001) patients. Patients with low serum calcium levels had poorer OS (P=0.011), DFS (P=0.012) and DMFS (P=0.004) than those with high serum calcium levels, but serum calcium levels had no significant effect on RFS (P=0.376). In univariate and multivariable analyses, low serum calcium levels were a statistically significant and independent prognostic factor for OS, DFS, and DMFS but had no prognostic value for RFS.
Conclusion
Serum calcium levels can serve as a prognostic predictor and guide more individualized treatment for NPC patients.
Keywords: nasopharyngeal carcinoma, serum calcium level, prognosis, survival
Plain Language Summary
Due to the lack of effective methods to predict the clinical outcomes of patients with nasopharyngeal carcinoma (NPC), we carried out this study using a large database to evaluate the predictive value of serum calcium in NPC.
We found that serum calcium levels can serve as an effective predictor of the clinical outcome of NPC. NPC patients with low serum calcium levels had worse survival. It is useful to monitor serum calcium levels before antitumour treatment to provide patients with NPC with more individualized treatment, ultimately improving the prognosis and prolonging the survival of NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell carcinoma arising from the nasopharynx and is mainly distributed in east and southeast Asia.1 There were approximately 129,079 newly diagnosed NPC patients and 72,987 patients who died of NPC in 2018 according to global cancer data.2 Because the primary tumour is close to the skull base and highly sensitive to radiotherapy, radiotherapy alone is the main treatment for early-stage NPC patients, and radiotherapy combined with chemotherapy is mainly administered to advanced disease patients.3,4 Regardless of the tumour biological heterogeneity, the anatomy-based tumour-node-metastasis (TNM) staging system is a widely used tool for predicting clinical outcomes or treatment benefits.5 Additionally, other specific biological markers, such as Epstein-Barr virus (EBV) DNA6,7 and serum lactate dehydrogenase (LDH) levels,8,9 have been found to provide additional prognostic information for NPC patients.
Calcium ions are the most common second messengers that widely regulate diverse cellular function.10 Abnormal calcium homeostasis has been reported to participate in regulating many cancer-associated biological processes, such as cell proliferation, death, migration and invasion, suggesting that calcium plays an important role in the development of cancer.11 Currently, some studies have reported that serum calcium was associated with the risk of cancers, such as prostate cancer,12,13 bladder cancer14 and melanoma.15 Moreover, serum calcium has been identified as a prognostic indicator for many human malignancies, including pancreatic ductal adenocarcinoma,16 non-small cell lung cancer,17 multiple myeloma,18 prostate cancer,19 and renal cell carcinoma.20 In NPC, two studies reported that hypocalcaemia was detected in patients treated with amifostine, one of the most frequently used radioprotectors.21,22 However, studies have not explored the association between serum calcium levels and prognosis, so the prognostic value of serum calcium in patients with NPC remains unknown.
Therefore, we performed this large-scale retrospective study to analyse baseline serum calcium levels and evaluate the role of serum calcium in the survival of NPC to adjust treatment strategies for patients with NPC.
Patients and Methods
Patient Selection
The present longitudinal retrospective study was conducted with NPC patients treated with intensity-modulated radiotherapy (IMRT) at Sun Yat-Sen University Cancer Center (SYSUCC, Guangzhou, People’s Republic of China) between April 2009 and September 2012. A total of 2094 patients were enrolled in the study, and they met the following eligibility criteria: 1) histologically proven NPC; 2) no evidence of distant metastasis; 3) no anticancer treatment before collecting the blood sample; 4) no prior history of malignancies; and 5) availability of complete medical records, including data on patient history and haematological profiles.
Pretreatment Evaluation
All eligible patients completed routine pretreatment evaluation, including complete patient history, physical examination, haematology and biochemistry profiles, fibre-optic nasopharyngoscopy, histopathological diagnosis, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal ultrasonography, and whole body bone scam using single-photon emission computed tomography (ECT) or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT). For cigarette smoking, smoker was defined as those who had smoked 100 cigarettes or more during their lifetime; for alcohol drinking, drinker was defined as those who had consumed alcohol in their lifetime. All patients were restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. According to WHO criteria in 1978, NPC patients were classified into type I (keratinizing squamous cell carcinoma), type II (differentiated nonkeratinizing carcinoma) and type III (undifferentiated nonkeratinizing carcinoma). To minimize heterogeneity, all MRI materials and clinical documents were independently reviewed by two radiation oncologists specializing in head and neck cancer, and any disagreements were settled by consultation.
Laboratory Measurement of Serum Calcium Levels
All patients enrolled in this study had serum calcium levels measured at the time of first diagnosis at our hospital before receiving any antitumour treatment. In brief, peripheral blood was separated by centrifugation at 3000 × g for 5 min. The upper-layer serum samples were carefully placed in Eppendorf tubes. Serum calcium levels and serum albumin levels were measured using a Hitachi Automatic Analyzer 7600–020 (Hitachi High-Technologies, Tokyo, Japan), and the coefficient of variance of calcium measurement was <5.0%. Serum calcium levels were corrected by albumin using the following formula: albumin-corrected calcium (mmol/L) = serum total calcium level (mmol/L) + (40-serum albumin level) × 0.02 mmol/L.
Treatment
The target volumes of the nasopharyngeal tumour and malignant neck lymph nodes were treated with definitive IMRT. The prescribed doses were 66–72 Gy (28–33 fractions) to the planning target volume (PTV) of the primary gross tumour volume (GTVnx), 64–70 Gy (28–33 fractions) to the PTV of the GTV of the malignant lymph nodes (GTVnd), 60–63 Gy to the PTV of the high-risk clinical target volume (CTV1), and 54–56 Gy to the PTV of the low-risk clinical target volume (CTV2). During this study period, radiotherapy alone was recommended for stage I patients, concurrent chemoradiotherapy (CCRT) for stage II patients, and CCRT with or without induction/adjuvant chemotherapy (IC/AC) for stage III–IVB patients, according to our institutional guidelines. Salvage treatments such as surgery and chemotherapy were provided when the disease relapsed or persisted.
Patient Follow-Up
Patients returned for follow-up at least once every three months in the first two years, every six months in years three to five, and every year thereafter. The follow-up duration was measured from the first day of treatment to either the day of death or the last visit. Four endpoints were assessed: overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS), and relapse-free survival (RFS). We defined OS as the time from the first day of treatment to the date of death from any cause; DFS as the time to disease progression or death from any cause, whichever occurred first; DMFS as the time to the occurrence of distant metastasis of the disease; and RFS as the time to disease relapse.
Statistical Analysis
The Statistical Package for Social Sciences, version 22.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. The chi-square test or Fisher’s exact test was used to compare differences in the clinical characteristics of the different serum calcium level groups. Life-table estimation was calculated by the Kaplan–Meier method, and the differences were compared using the Log rank test. Univariate and multivariate analyses with the Cox proportional hazards model were used to test for independent significance by the backward elimination of nonsignificant variables. The covariates in the models included age, sex, smoking, drinking, family history of cancer, serum calcium level, World Health Organization (WHO) type, TNM stage and chemotherapy. A P value <0.05 was considered statistically significant. All tests were two-sided.
Results
Patient Characteristics
The baseline characteristics of the enrolled patients are displayed in Table 1. Of the 2094 patients, 528 (25.2%) patients were female, and 1566 (74.8%) were male. The median age was 45 years (range, 18–78 years). According to the 8th AJCC staging system, 540 (25.8%) patients were classified as having early-stage disease (stage I, II), whereas 1554 (72.3%) were classified as having advanced-stage disease (stage III, IV). All of the patients received radical radiotherapy, and 1775 (84.8%) of them also received platinum-based IC or AC.
Table 1.
Baseline Characteristics of 2094 Patients with Nasopharyngeal Carcinoma
| Characteristic | No. of Patients (%) |
|---|---|
| Age, year | |
| Median | 45 |
| Range | 18–78 |
| Sex | |
| Female | 528 (25.2) |
| Male | 1566 (74.8) |
| Smoking | |
| No | 1336 (63.8) |
| Yes | 758 (36.2) |
| Drinking | |
| No | 1843 (88.0) |
| Yes | 251 (12.0) |
| Family history of cancer | |
| No | 1502 (71.7) |
| Yes | 592 (28.3) |
| WHO type | |
| I | 11 (0.5) |
| II | 98 (4.7) |
| III | 1985 (94.8) |
| T category* | |
| T1 | 350 (16.7) |
| T2 | 350 (16.7) |
| T3 | 991 (47.3) |
| T4 | 403 (19.2) |
| N category* | |
| N0 | 355 (17.0) |
| N1 | 1189 (56.8) |
| N2 | 335 (16.0) |
| N3 | 215 (10.3) |
| TNM stage* | |
| I | 121 (5.8) |
| II | 419 (20.0) |
| III | 974 (46.5) |
| IV | 580 (27.7) |
| Chemotherapy | |
| No | 319 (15.2) |
| Yes | 1775 (84.8) |
Note: *According to the 8th AJCC/UICC staging system.
Abbreviation: WHO, World Health Organization.
The median follow-up time for this retrospective study was 96.3 months (range: 4.1–120.0 months). Of all patients, disease progression, locoregional recurrence, distant metastasis and death from any cause occurred in 582 (27.8%), 490 (11.9%), 310 (14.8%) and 448 (21.4%) patients, respectively. In the entire cohort, the five-year OS was 85.3%, DFS was 77.6%, DMFS was 86.6% and RFS was 89.6%.
Correlations of Serum Calcium Levels with Patient Characteristics
The mean (±SD) serum calcium level in the entire cohort was 2.43±0.13 mmol/L, ranging from 1.46 to 2.86 mmol/L. We used 2.23 mmol/L calculated by receiver operating characteristic (ROC) curves as the cut-off point to divide patients into two groups: the low serum calcium level group and the high serum calcium level group. A total of 1109/2094 (53.0%) patients had reduced serum calcium levels (Table 2). We found that low serum calcium levels tended to be detected in older patients (>45 years, P<0.001, Table 2) and in females (P=0.001, Table 2). Furthermore, there were no significant differences in the serum calcium levels of the entire cohort in terms of smoking, drinking, family history of cancer, WHO type, T category, N category, TNM stage, and chemotherapy.
Table 2.
Baseline Characteristics of Nasopharyngeal Carcinoma Patients with Low or High Serum Calcium Levels
| Characteristic | Serum Calcium Levels | P value† | |
|---|---|---|---|
| <2.23 mmol/L, n (%) | ≥2.23 mmol/L, n (%) | ||
| Age | |||
| ≤ 45 years | 531 (47.9) | 587 (59.6) | <0.001 |
| > 45 years | 578 (52.1) | 398 (40.4) | |
| Sex | |||
| Female | 314 (28.3) | 214 (21.7) | 0.001 |
| Male | 795 (71.7) | 771 (78.3) | |
| Smoking | |||
| No | 703 (63.4) | 633 (64.3) | 0.678 |
| Yes | 406 (36.6) | 352 (35.7) | |
| Drinking | |||
| No | 968 (87.3) | 875 (88.8) | 0.277 |
| Yes | 141 (12.7) | 110 (11.2) | |
| Family of history cancer | |||
| No | 795 (71.7) | 707 (71.8) | 0.963 |
| Yes | 314 (28.3) | 278 (28.2) | |
| WHO type | |||
| I | 5 (0.5) | 6 (0.6) | 0.617 |
| II+III | 1104 (99.5) | 979 (99.4) | |
| T category* | |||
| T1 | 177 (16.0) | 173 (17.6) | 0.337 |
| T2 | 188 (17.0) | 162 (16.4) | |
| T3 | 542 (48.9) | 449 (45.6) | |
| T4 | 202 (18.2) | 201 (20.4) | |
| N category* | |||
| N0 | 180 (16.2) | 175 (17.8) | 0.186 |
| N1 | 616 (55.5) | 573 (58.2) | |
| N2 | 190 (17.1) | 145 (14.7) | |
| N3 | 123 (11.1) | 92 (9.3) | |
| TNM stage* | |||
| I | 64 (5.8) | 57 (5.8) | 0.741 |
| II | 215 (19.4) | 204 (20.7) | |
| III | 528 (47.6) | 446 (45.3) | |
| IV | 302 (27.2) | 278 (28.2) | |
| Chemotherapy | |||
| No | 174 (15.7) | 145 (14.7) | 0.538 |
| Yes | 935 (84.3) | 840 (85.3) | |
Notes: †P value was calculated using the chi-square test or Fisher’s exact test.* According to the 8th AJCC/UICC staging system.
Abbreviation: WHO, World Health Organization.
Prognostic Value of Serum Calcium Levels in NPC
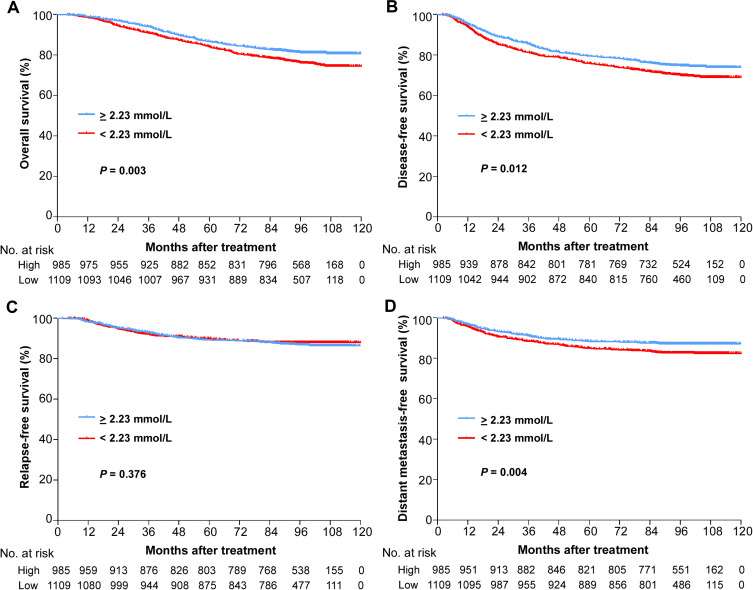
We further analysed the effect of serum calcium levels on NPC patient survival. In the low serum calcium level group, 264/1109 (23.8%), 332/1109 (29.9%), 187/1109 (16.9%) and 125/1109 (11.3%) patients arrived at the endpoint events of OS, DFS, DMFS and RFS. Then, we found that patients with low serum calcium levels had significantly worse 5-year OS (86.6% vs 84.0%, P=0.011, Figure 1A), 5-year DFS (79.5% vs 75.9%, P=0.012, Figure 1B) and 5-year DMFS (88.4% vs 84.9%, P=0.004, Figure 1D) rates than patients with high serum calcium levels. However, we failed to find a significant difference in the 5-year RFS rates (89.3% vs 89.9%, P=0.376, Figure 1C) between the two groups of patients.
Figure 1.
Kaplan–Meier analysis of survival probabilities of NPC patients stratified by serum calcium level. (A) Overall survival, (B) disease-free survival, (C) relapse-free survival, (D) distant metastasis-free survival.
Serum Calcium Levels Were an Independent Prognostic Factor in NPC
We finally performed univariate analysis and multivariate analysis to explore prognostic factors for NPC patients. Univariate analysis showed that low serum calcium levels were a statistically significant prognostic factor for OS (hazard ratio (HR): 1.33, 95% confidence interval (95% CI): 1.10–1.61, P=0.003, Table 3), DFS (HR: 1.23, 95% CI: 1.05–1.45, P=0.012, Table 3), and DMFS (HR: 1.39, 95% CI: 1.11–1.75, P=0.004, Table 3). In contrast, low serum calcium levels had no prognostic impact on RFS (HR: 0.93, 95% CI: 0.72–1.19, P=0.540).
Table 3.
Univariate Analysis of Prognostic Factors in Patients with Nasopharyngeal Carcinoma
| Variable | Univariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | P-value† | |
| Overall survival | |||
| Age (>45 years vs ≤45 years) | 1.61 | 1.34–1.94 | <0.001 |
| Sex (Female vs Male) | 0.74 | 0.59–0.93 | 0.010 |
| Smoking (Yes vs No) | 1.41 | 1.17–1.69 | <0.001 |
| Drinking (Yes vs No) | 1.10 | 0.83–1.45 | 0.501 |
| Family history of cancer (Yes vs No) | 0.86 | 0.69–1.06 | 0.147 |
| Serum calcium level (Low vs High) | 1.33 | 1.10–1.61 | 0.003 |
| WHO type (II+III vs I) | 0.48 | 0.18–1.29 | 0.146 |
| TNM stage (III–IV vs I–II) | 2.94 | 2.20–3.93 | <0.001 |
| Chemotherapy (No vs Yes) | 2.12 | 1.51–2.96 | <0.001 |
| Disease-free survival | |||
| Age (>45 years vs ≤45 years) | 1.44 | 1.22–1.69 | <0.001 |
| Sex (Female vs Male) | 0.79 | 0.65–0.97 | 0.020 |
| Smoking (Yes vs No) | 1.33 | 1.13–1.57 | 0.001 |
| Drinking (Yes vs No) | 1.21 | 0.96–1.53 | 0.107 |
| Family history of cancer (Yes vs No) | 0.83 | 0.69–1.00 | 0.046 |
| Serum calcium level (Low vs High) | 1.23 | 1.05–1.45 | 0.012 |
| WHO type (II+III vs I) | 0.49 | 0.20–1.18 | 0.113 |
| TNM stage (III–IV vs I–II) | 2.24 | 1.78–2.82 | <0.001 |
| Chemotherapy (No vs Yes) | 1.68 | 1.28–2.19 | <0.001 |
| Distant metastasis-free survival | |||
| Age (>45 years vs ≤45 years) | 1.21 | 0.97–1.52 | 0.089 |
| Sex (Female vs Male) | 0.65 | 0.48–0.86 | 0.003 |
| Smoking (Yes vs No) | 1.47 | 1.17–1.83 | 0.001 |
| Drinking (Yes vs No) | 1.23 | 0.89–1.69 | 0.203 |
| Family history of cancer (Yes vs No) | 0.92 | 0.71–1.18 | 0.487 |
| Serum calcium level (Low vs High) | 1.39 | 1.11–1.75 | 0.004 |
| WHO type (II+III vs I) | 1.49 | 0.21–10.61 | 0.691 |
| TNM stage (III–IV vs I–II) | 2.81 | 1.99–3.96 | <0.001 |
| Chemotherapy (No vs Yes) | 1.90 | 1.29–2.80 | 0.001 |
| Relapse-free survival | |||
| Age (>45 years vs ≤45 years) | 1.19 | 0.93–1.52 | 0.177 |
| Sex (Female vs Male) | 1.06 | 0.80–1.26 | 0.672 |
| Smoking (Yes vs No) | 0.97 | 0.75–1.70 | 0.822 |
| Drinking (Yes vs No) | 1.10 | 0.76–1.59 | 0.625 |
| Family history of cancer (Yes vs No) | 0.77 | 0.57–1.03 | 0.076 |
| Serum calcium level (Low vs High) | 0.93 | 0.72–1.19 | 0.540 |
| WHO type (II+III vs I) | 0.25 | 0.09–0.68 | 0.006 |
| TNM stage (III–IV vs I–II) | 1.54 | 1.13–2.11 | 0.007 |
| Chemotherapy (No vs Yes) | 1.69 | 1.12–2.56 | 0.013 |
Note: †P value was calculated using the univariate Cox proportional hazards model.
Abbreviations: WHO, World Health Organization; HR, hazard ratio; 95% CI, 95% confidence interval.
Multivariate analysis showed the same results as univariate analysis (Table 4). After adjusting for various confounding variables in a Cox proportional hazards model, serum calcium levels were significantly independently associated with OS (HR: 1.28, 95% CI: 1.06–1.55, P=0.011, Table 4), DFS (HR: 1.19, 95% CI: 1.01–1.40, P=0.043, Table 4), and DMFS (HR:1.43, 95% CI:1.14–1.80, P=0.002, Table 4), but failed to predict RFS independently.
Table 4.
Multivariable Analysis of Prognostic Factors in Patients with Nasopharyngeal Carcinoma
| Variable | Univariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | P-value† | |
| Overall survival | |||
| Age (>45 years vs ≤45 years) | 1.54 | 1.25–1.82 | <0.001 |
| Sex (Female vs Male) | 0.75 | 0.59–0.94 | 0.013 |
| Serum calcium level (Low vs High) | 1.28 | 1.06–1.55 | 0.011 |
| TNM stage (III–IV vs I–II) | 2.57 | 1.87–3.52 | <0.001 |
| Disease-free survival | |||
| Age (>45 years vs ≤45 years) | 1.36 | 1.15–1.61 | <0.001 |
| Smoking (Yes vs No) | 1.22 | 1.03–1.44 | 0.022 |
| Serum calcium level (Low vs High) | 1.19 | 1.01–1.40 | 0.043 |
| WHO type (II+III vs I) | 0.41 | 0.17–1.00 | 0.049 |
| TNM stage (III–IV vs I–II) | 2.16 | 1.72–2.72 | <0.001 |
| Distant metastasis-free survival | |||
| Sex (Female vs Male) | 0.64 | 0.48–0.86 | 0.003 |
| Serum calcium level (Low vs High) | 1.43 | 1.14–1.80 | 0.002 |
| TNM stage (III–IV vs I–II) | 2.76 | 1.96–3.89 | <0.001 |
| Relapse-free survival | |||
| WHO type (III vs I+II) | 0.24 | 0.09–0.65 | 0.005 |
| TNM stage (III–IV vs I–II) | 1.53 | 0.57–1.03 | 0.008 |
Notes: †P value was calculated using the multivariable Cox proportional hazards model. The following parameters were included as covariates in the Cox proportional hazards model by backward elimination: age (≥45 vs <45 years), sex (Female vs Male), smoking (Yes vs No), drinking (Yes vs No), family history of cancer (Yes vs No), serum calcium level (Low vs High), WHO type (Type III vs Type I+II), TNM stage (III–IV vs I–II) and chemotherapy (No vs Yes).
Abbreviations: WHO, World Health Organization; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
Currently, a large body of evidence suggests that high serum calcium levels are associated with the risk of tumorigenesis and progression. Szendroi et al found that higher corrected serum calcium level independently increased the risk of prostate cancer.12 Skinner et al found that man in the highest total serum calcium had increased risk of prostate cancer death.13 Huang et al found that high serum calcium level predicted bone metastases in bladder cancer patients.4 Datta et al found high albumin-corrected calcium was positively related to disease progression.15 But the role of serum calcium in the prognosis of human malignancies remains controversial. Dong et al found that high serum calcium level could predict death of patient with resectable pancreatic ductal adenocarcinoma within one year after surgery.16 Tucci et al found that hypercalcemia was significantly associated with poor prognosis and adverse skeletal-related events of patients with hormone-refractory bone metastatic prostate cancer.19 Papworth et al found that renal cell carcinoma patients with hypercalcemia had a significantly poorer survival time.20 But Shen et al found that lower blood calcium levels predicted poor prognosis and increased the risk of bone metastasis of non-small cell lung cancer.17 And Maillet et al found that normal calcium levels were related to better overall survival of multiple myeloma patients.18 Tucci et al found that hypercalcemia was significantly associated with poor prognosis and adverse skeletal-related events of patients with hormone-refractory bone metastatic prostate cancer.19 Papworth et al found that renal cell carcinoma patients with hypercalcemia had a significantly poorer survival time.20 However, studies regarding the association between serum calcium levels and the prognosis of NPC have not yet been conducted, and the role of serum calcium levels in the prognosis of NPC remains unknown. Here, we conducted a retrospective study to evaluate the potential prognostic value of serum calcium levels in previously untreated NPC patients from a large institution in an endemic area. To the best of our knowledge, this is the first large-scale cohort study of NPC patients, and the study convincingly revealed the inferior prognostic value of low serum calcium levels. Therefore, monitoring the serum calcium level in newly diagnosed NPC patients can provide a basis for more individualized treatment, ultimately improving the prognosis and prolonging the survival of NPC patients.
In the present study, nearly 53.0% (1109/2094) of NPC patients were complicated with low serum calcium levels, which was a fairly frequent event, indicating a close relationship between serum calcium and NPC. We further found that NPC patients with low serum calcium levels had inferior OS, DFS and DMFS, and the serum calcium level was an independent predictor of PFS, CSS, and OS after adjusting for various confounding factors. However, the reasons explaining the association between serum calcium and poor prognosis remain unclear. It is well known that calcium ions are mainly stored in bones, accounting for 99% of the body’s calcium ions.23 Calcium homeostasis is mainly maintained by parathyroid hormone, the intestine and the kidney.24,25 And tumour bone metastases can be divided into osteolytic metastases and osteoblastic metastases, and the two processes are almost interrelated and coupled.26 In NPC, endothelin-1 and insulin-like growth factors 1, both are mitogenic factors driving osteoblast proliferation,26 were elevated and promoted migration.27–32 Moreover, RANK, which is expressed by osteoclasts, was also detected in clinical NPC tissue.33 These studies suggest that osteoblastic and osteolysis may be involved in the process of bone metastases of NPC. Therefore, we speculate that osteoblastic metastases increase calcium and phosphate deposition in bone, resulting in a decrease in serum calcium levels. Low serum calcium levels induce osteoporosis by compensatory stimulating the secretion of parathyroid hormone and enhancing the release of calcium from the bone in NPC, which provides favourable conditions for tumour cells to invade the bone.17 Bone metastasis is closely associated with disease progression and patient survival, so this speculation may also be the reason why serum calcium has no predictive value for RFS, which mainly involves the recurrence of tumours in local nasopharyngeal areas and cervical lymph node drainage areas. Furthermore, this view needs to be verified in future experiments.
Our results highlight the need for more research on precise molecular mechanisms to explain the role of calcium ions in NPC biological processes. Calcium ions are the most common second messengers, and a large body of evidence suggests that abnormal calcium homeostasis participates in regulating many cellular processes, such as cell proliferation, death, migration and invasion.11,34 In NPC, TRPM7, a novel regulator of calcium ion influx, promoted 5–8F cell migration.35 CACNA2D3, an auxiliary component of the voltage-dependent calcium channel complex, can induce mitochondrial-mediated apoptosis and inhibit cell proliferation, invasion and EMT by increasing intracellular calcium ion concentrations.36 Store-operated Ca2+ entry (SOCE) promotes NPC cell migration by regulating oncogenic Ca2+ signalling.37 S100 calcium-binding protein A14 (S100A14), containing an EF-hand motif capable of binding calcium, has a feedback loop with IRAK1 to suppress NPC cellular migration.38 However, the specific molecular mechanism of calcium ions and their regulators involved in the malignant biological process of NPC is still unclear, and further research is needed to deepen our understanding of the role of calcium ions in NPC.
Although our study has obvious strengths, including the use of a large database, the limitations in our design cannot be ignored. First, bias is inevitable because of the retrospective nature of the analysis. We used a large sample cohort study to minimize bias. Second, our study is a single-centre study, and our results should be validated in NPC patients from other research institutions to increase the scope of the application of the results. Third, serum calcium levels are regulated by parathyroid hormone.24 However, patients with parathyroid dysfunction before NPC diagnosis could not be ruled out due to the lack of relevant clinical information. Studies on serum calcium without neglecting the regulators of calcium homeostasis need to be carried out in the future.
Conclusion
Our retrospective study is the first to evaluate the prognostic value of serum calcium levels for patients with NPC. This study convincingly demonstrated that NPC patients with low serum calcium levels had worse survival than those with high serum calcium levels, and serum calcium levels can act as an independent prognostic indicator, which will lead to more accurate individualized treatment for NPC patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81922057 and 81773229), the Natural Science Foundation of Guangdong Province (No. 2018B030306045), and the Guangdong Special Support Program (No. 2017TQ04R754).
Abbreviations
NPC, nasopharyngeal carcinoma; TNM, tumour-node-metastasis; EBV, Epstein-Barr virus; LDH, lactate dehydrogenase; IMRT, intensity-modulated radiotherapy; MRI, magnetic resonance imaging; ECT, emission computed tomography; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; AJCC, American Joint Committee on Cancer; PTV, planning target volume; GTVnx, gross tumour volume of nasopharynx lesion; GTVnd, gross tumour volume of the malignant lymph nodes; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; OS, overall survival; DFS, disease-free survival, DMFS, distant metastasis-free survival; RFS, relapse-free survival; WHO, World Health Organization; HR, hazard ratio; 95% CI, 95% confidence interval.
Data Sharing Statement
The key raw data have been uploaded onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the approval number RDDA2021001724.
Ethics Approval and Informed Consent
Our study was conducted in accordance with the Declaration of Helsinki. The study analysed the anonymous data of NPC patients and was approved by the Institutional Ethical Review Board of SYSUCC. The requirement for written informed consent was waived by the Ethics Review Board.
Disclosure
The authors report no financial or non-financial conflicts of interest in this work.
References
- 1.Chen Y, Chan ATC, Le Q, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Sun Y, Ma J. Induction gemcitabine and cisplatin in locoregionally advanced nasopharyngeal carcinoma. Cancer Commun (Lond). 2019;39:39. doi: 10.1186/s40880-019-0385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AWM, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233–244. doi: 10.1016/j.semradonc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Chen L, Zhang Y, et al. Prognostic value of diabetes in patients with nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Tang L, Li Y, Liu X, Liu Q, Ma J. Spontaneous remission of residual post-therapy plasma Epstein-Barr virus DNA and its prognostic implication in nasopharyngeal carcinoma: a large-scale, big-data intelligence platform-based analysis. Int J Cancer. 2019;144(9):2313–2319. doi: 10.1002/ijc.32021 [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Sun Z, Guo R, et al. Plasma Epstein-Barr Virus DNA load after induction chemotherapy predicts outcome in locoregionally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2019;104(2):355–361. doi: 10.1016/j.ijrobp.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Zhou G, Ren X, Mao Y, et al. Prognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou G, Tang L, Mao Y, et al. Baseline Serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2012;82(3):e359–365. doi: 10.1016/j.ijrobp.2011.06.1967 [DOI] [PubMed] [Google Scholar]
- 10.Rizzuto R, Pozzan T. When calcium goes wrong: genetic alterations of a ubiquitous signaling route. Nat Genet. 2003;34(2):135–141. doi: 10.1038/ng0603-135 [DOI] [PubMed] [Google Scholar]
- 11.Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17:367–380. [DOI] [PubMed] [Google Scholar]
- 12.Szendroi A, Speer G, Tabak A, et al. The role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancer. Can J Urol. 2011;18:5710–5716. [PubMed] [Google Scholar]
- 13.Skinner HG, Schwartz GG. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(2):575–578. doi: 10.1158/1055-9965.EPI-08-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang P, Lan M, Peng A, et al. Serum calcium, alkaline phosphotase and hemoglobin as risk factors for bone metastases in bladder cancer. PLoS One. 2017;12(9):e0183835. doi: 10.1371/journal.pone.0183835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta M, Savage P, Lovato J, Schwartz GG. Serum calcium, albumin and tumor stage in cutaneous malignant melanoma. Future Oncol. 2016;12(19):2205–2214. doi: 10.2217/fon-2016-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Q, Zhang Y, Yang X, et al. Serum calcium level used as a prognostic predictor in patients with resectable pancreatic ductal adenocarcinoma. Clin Res Hepatol Gastroenterol. 2014;38(5):639–648. doi: 10.1016/j.clinre.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Shen H, Li Y, Liao Y, Zhang T, Liu Q, Du J. Lower blood calcium associates with unfavorable prognosis and predicts for bone metastasis in NSCLC. PLoS One. 2012;7(3):e34264. doi: 10.1371/journal.pone.0034264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maillet D, Montiel-Cervantes L, Padilla-González Y, et al. Serum calcium is an independent prognostic factor of overall survival in Mexican patients with multiple myeloma. Rev Invest Clin. 2012;64:17–24. [PubMed] [Google Scholar]
- 19.Tucci M, Mosca A, Lamanna G, et al. Prognostic significance of disordered calcium metabolism in hormone-refractory prostate cancer patients with metastatic bone disease. Prostate Cancer Prostatic Dis. 2009;12(1):94–99. doi: 10.1038/pcan.2008.10 [DOI] [PubMed] [Google Scholar]
- 20.Papworth K, Grankvist K, Ljungberg BO, Rje RT. Parathyroid hormone-related protein and serum calcium in patients with renal cell carcinoma. Tumour Biol. 2005;26:201–206. doi: 10.1159/000086953 [DOI] [PubMed] [Google Scholar]
- 21.Chang H, Yi W, Wang X, et al. Effectiveness and safety of different amifostine regimens: preliminary results of a Phase II multicenter randomized controlled trial. Chin J Cancer Res. 2018;30:307–314. doi: 10.21147/j.issn.1000-9604.2018.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vardy J, Wong E, Izard M, Clifford A, Clarke SJ. Life-threatening anaphylactoid reaction to amifostine used with concurrent chemoradiotherapy for nasopharyngeal cancer in a patient with dermatomyositis: a case report with literature review. Anticancer Drugs. 2002;13(3):327–330. doi: 10.1097/00001813-200203000-00015 [DOI] [PubMed] [Google Scholar]
- 23.Emkey RD, Emkey GR. Calcium metabolism and correcting calcium deficiencies. Endocrinol Metab Clin North Am. 2012;41(3):527–556. doi: 10.1016/j.ecl.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 24.Zagzag J, Hu MI, Fisher SB, Perrier ND. Hypercalcemia and cancer: differential diagnosis and treatment. CA Cancer J Clin. 2018;68(5):377–386. doi: 10.3322/caac.21489 [DOI] [PubMed] [Google Scholar]
- 25.Li K, Wang X, Li D, et al. The good, the bad, and the ugly of calcium supplementation: a review of calcium intake on human health. Clin Interv Aging. 2018;13:2443–2452. doi: 10.2147/CIA.S157523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867 [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Zhao F-P, Peng Z, et al. EZH2 promotes angiogenesis through inhibition of miR-1/Endothelin-1 axis in nasopharyngeal carcinoma. Oncotarget. 2014;5(22):11319–11332. doi: 10.18632/oncotarget.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai HQ, Zeng ZY, Zhang CQ, et al. Elevated plasma big ET-1 is associated with distant failure in patients with advanced-stage nasopharyngeal carcinoma. Cancer. 2006;106:1548–1553. doi: 10.1002/cncr.21790 [DOI] [PubMed] [Google Scholar]
- 29.Lin SX, Zhang Y, Lu J, Liu X, Li XP. Silencing of endothelin-1 suppresses growth, migration, and invasion of nasopharyngeal carcinoma cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:915–920. [PubMed] [Google Scholar]
- 30.M’hamdi H, Baizig NM, ELHadj OE, et al. Usefulness of IGF-1 serum levels as diagnostic marker of nasopharyngeal carcinoma. Immunobiology. 2016;221:1304–1308. doi: 10.1016/j.imbio.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Li H, Guo X, Wang Z, Liang S, Dang C. IGF-I induces epithelial-to-mesenchymal transition via the IGF-IR-Src-MicroRNA-30a-E-cadherin pathway in nasopharyngeal carcinoma cells. Oncol Res. 2016;24:225–231. doi: 10.3727/096504016X14648701447931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z, Wen J, Peng L, Liu H. Upregulation of insulin-like growth Factor-1 Receptor (IGF-1R) reverses the inhibitory effect of Let-7g-5p on migration and invasion of nasopharyngeal carcinoma. Med Sci Monit. 2019;25:5747–5756. doi: 10.12659/MSM.914555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resteghini C, Alfieri S, Quattrone P, et al. RANK expression in EBV positive nasopharyngeal carcinoma metastasis: a ready-to-treat target? Oncotarget. 2017;8:96184–96189. doi: 10.18632/oncotarget.21856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkash J, Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87(19–22):587–595. doi: 10.1016/j.lfs.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Luan Y, You C, Chen X, Luo R, Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating. Cell Calcium. 2010;47(5):425–432. doi: 10.1016/j.ceca.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Wong AMG, Kong KL, Chen L, et al. Characterization of CACNA2D3 as a putative tumor suppressor gene in the development and progression of nasopharyngeal carcinoma. Int J Cancer. 2013;133(10):2284–2295. doi: 10.1002/ijc.28252 [DOI] [PubMed] [Google Scholar]
- 37.Wei J, Zhang J, Si Y, et al. Blockage of LMP1-modulated store-operated Ca (2+) entry reduces metastatic potential. Cancer Lett. 2015;360(2):234–244. doi: 10.1016/j.canlet.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 38.Meng D, Sun R, Liu G, et al. S100A14 suppresses metastasis of nasopharyngeal carcinoma by inhibition of NF-kB. Oncogene. 2020;39:5307–5322. [DOI] [PubMed] [Google Scholar]