Abstract
Purpose
To characterize medical device reports about elastomeric pumps delivering local anesthesia made to the US Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database.
Patients and Methods
We conducted a retrospective review of medical device reports submitted to MAUDE from January 2010 to July 2018. A systematic, computerized algorithm was used to identify records pertaining to elastomeric pumps using local anesthesia. Included records indicated the use of local anesthesia or were determined to involve the use of local anesthetics (if they did not contain specific information on drug use). Reports were analyzed within the MAUDE event type categories of malfunction, injury, death, other, and missing. Possible cases of liver injury or surgical site infection were also identified. Manual review of narratives provided in MAUDE was performed by 2 reviewers to identify possible or probable cases of local anesthetic system toxicity (LAST).
Results
From a pool of 384,285 reports about elastomeric pumps from the MAUDE database, 4093 met inclusion criteria for involving elastomeric pumps to deliver local anesthetics, with the peak number of reports occurring in 2014. Of these identified reports, 3624 (88.5%) were categorized as malfunctions, 292 (7.1%) as injuries, and 8 (0.2%) as involving death. We identified 13 cases (0.3%) of possible liver injury and 51 cases (1.2%) of possible surgical site infection; 139 reports (3.4%) were determined to be probably (n=53) or possibly (n=86) associated with LAST.
Conclusion
Malfunction of elastomeric pumps delivering local anesthetics leaves patients vulnerable to injury or death. Our study indicates that reports of malfunction, injury, and death have been reported to the MAUDE database. These reports likely reflect an underrepresentation of cases in the real-world population, emphasizing the need for more comprehensive medical device reporting.
Keywords: postoperative analgesia, medical device reporting, local anesthetic systemic toxicity, safety events
Plain Language Summary
Elastomeric pumps are convenient medical devices that can be used to manage pain after surgery by slowly delivering numbing medication over an extended period of time.
However, because these pumps contain large amounts of medications, patients are at risk of accidentally being overexposed, particularly if the medication is delivered too rapidly.
This is especially concerning because exposure to high levels of local anesthetics can result in local anesthetic systemic toxicity (LAST).
We reviewed medical device reports submitted to a US Food and Drug Administration database over an 8-year period and analyzed reports identifying malfunction, injury, and death in relation to elastomeric pumps.
A total of 4093 reports were identified; most events were categorized as malfunctions (3624/4093, 88.5%), with some categorized as injuries (292/4093, 7.1%) or death (8/4093, 0.2%).
There were 139 reports (3.4%) involving elastomeric pumps that may have been related to LAST.
Reports to this database are voluntary, and many medical device issues go unreported.
Our analysis indicates that faulty or incorrectly used elastomeric pumps can be dangerous for patients after surgery and underscores the need for more comprehensive data to inform and improve this mode of pain management.
Introduction
Elastomeric pumps are used in a variety of surgical settings with the aim of providing prolonged pain management via slow infusion of local anesthetic over a defined period of time.1–3 This form of continuous local anesthetic delivery may help manage pain and increase ambulation during recovery.4,5 However, accurate catheter placement is important for effective anesthesia delivery with elastomeric pumps. Additionally, technical issues, such as accidentally removing the catheter while changing wound dressings and catheter breakage, have been reported as barriers to effective use.6
In addition to problems with application technique, potential complications associated with the use of elastomeric pumps include catheter or device malfunctions.2,7,8 These malfunctions, if leading to elevated plasma concentrations of local anesthetic, may be associated with local anesthetic system toxicity (LAST). LAST is a severe adverse effect resulting from the accidental systemic distribution of high doses of local anesthetic leading to high circulating levels of the anesthetic that can affect the cardiovascular, central nervous, and/or respiratory systems.9
Any issues and adverse events noted with the elastomeric pumps should be reported to the US Food and Drug Administration (FDA) through the Manufacturer and User Facility Device Experience (MAUDE) database. This database is a postmarketing surveillance system that contains reports regarding adverse events involving medical devices.10 Using the MAUDE database, previous research found that 92% of the 1430 reports involving patient-controlled intravenous analgesia devices were classified as device malfunctions.11 However, that study did not analyze the number of device malfunction reports related to elastomeric pumps. Elastomeric pump malfunctions resulting in overinfusion of anesthetic have also been reported in the literature, further supporting the need to evaluate reports of device malfunctions and events, such as LAST, associated with these devices.12,13 To characterize issues specifically involving elastomeric pumps, we conducted a retrospective review of the MAUDE database to identify and characterize reports related to malfunction, injury, and death, including possible cases of LAST, related to the use of elastomeric pumps used to deliver local anesthetics.
Patients and Methods
Study Design and Data Source
This study was a retrospective review of medical device reports involving elastomeric pumps submitted to MAUDE from January 2010 to July 2018. The MAUDE database is a repository of medical device event reports about suspected medical device safety and performance issues submitted to the FDA. These medical device reports are submitted by mandatory reporters (ie, manufacturers, importers, hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, and outpatient treatment facilities) as well as voluntary reporters (ie, patients, consumers, and healthcare professionals). The database does not include reports made according to variances, exemptions, or alternative reporting under 21 CFR 803.19.10 Information in the MAUDE database is available in the public domain; the deidentified data in the present study are therefore exempt from ethics committee approval. All the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Report Selection and Eligibility Criteria
A systematic, computerized algorithm was used to identify cases involving elastomeric pumps used to deliver local anesthetics. Reports involving elastomeric pumps were identified using the MAUDE code for elastomeric pumps (MEB) and by brand and generic names of known elastomeric pumps. All injuries and/or readmissions related to the use of elastomeric pumps used to deliver local anesthetics were included, which were identified using MAUDE codes.
Key exclusion criteria included the following: reports detailing administration of nonanesthetic drugs (eg, antibiotics, chemotherapy, insulin, opioids, antiviral drugs, anxiolytics), pump malfunction prior to patient involvement, involvement of chondrolysis (an adverse event known to be associated with all local anesthetics regardless of the method of delivery), and known use of intrathecal pumps (eg, SynchroMedTM pumps). Reports with no mention of drug name remained in the pool, and a manual review was conducted by two reviewers to determine whether the pumps used local anesthetics.
A wildcard search was used to account for any misspellings. Additional reports that had missing or unknown brand names were included if the report to the manufacturer or event descriptions included any elastomeric pump-related terms. Duplicate reports were excluded on the basis of recurring report numbers or type of report and duplicate information across fields for multiple submissions.
Data Analysis
The number of medical device reports was quantified according to the five event types encoded in the MAUDE database (malfunction, injury, death, other, and missing); malfunction and death were verified via narrative review. To identify possible or probable cases of LAST, reports were algorithmically flagged for manual review by two reviewers. In this study, LAST was defined as the occurrence of any relevant symptoms related to the cardiac system, respiratory system, or central nervous system (see Supplemental Table 1 for a complete list of signs and symptoms). Clinical presentations described in the reports were considered a probable case of LAST if ≥1 of the following criteria were fulfilled: seizures, at least one sign or symptom related to categories of cardiovascular excitement or depression with at least one sign or symptom related to categories of central nervous system excitement or depression, and any relevant reports of treatments specific to LAST (eg, lipid emulsion). If only one sign or symptom related to cardiovascular or central nervous system excitement or depression categories was reported, the case was considered a possible case of LAST.
Implantable pumps have been previously linked to surgical site infection;14,15 as such, cases of possible surgical site infection in the present study were analyzed and identified by keyword, free text search, and manual narrative review for notes indicating superficial or deep wound infection at the surgical site, wound dehiscence, symptoms suggestive of wound infection including redness and/or drainage at the surgical or catheter site, and any mention of treatment for infection at the surgical site. Additionally, liver injury has been reported with amide anesthetics;16,17 as such, cases of possible liver injury or failure were also identified by noted liver injury or failure in the MAUDE record, laboratory results indicating liver injury or failure (eg, elevated liver function test laboratory values, international normalized ratio ≥1.5), jaundice, or treatment for drug-induced liver failure or injury.
Analyses were conducted by two independent analysts; results were compared, and all differences in results were noted and adjudicated. Narrative review of cases was conducted by two independent reviewers in accordance with specific predefined protocols and definitions; differences in results were documented and adjudicated. Descriptive statistics (number of reports and percentages) were used to quantify events related to elastomeric pumps. P-values were not calculated. All data were extracted as pipe-delimited text files and imported to SAS Enterprise Guide, version 7.1 (SAS Institute, Cary, NC).
Results
Identification of MAUDE Reports Involving Elastomeric Pumps to Administer Local Anesthetics
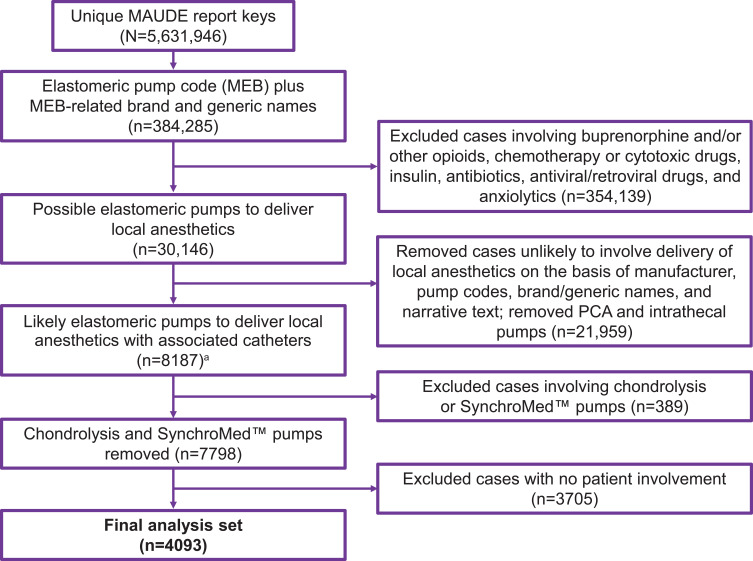
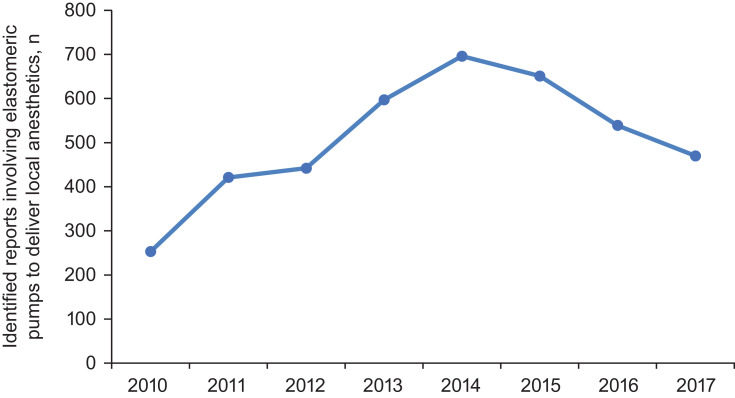
A total of 384,285 reports of elastomeric pumps were identified from the MAUDE database, and 4093 met the inclusion criteria involving elastomeric pumps to deliver local anesthetics (Figure 1). The annual number of reports identified as involving elastomeric pumps to deliver local anesthetics increased from 2010 (~200 reports) through 2014 (~700 reports) and decreased to ~450 reports per year in 2017 (Figure 2; data from 2018 not shown because only 7 months of reports were analyzed, instead of a complete year, with 24 reports identified).
Figure 1.
Identification process for MAUDE database reports that involved use of elastomeric pumps to deliver local anesthetics. aAlthough some reports did not include all drug names used with the elastomeric pump, this subset of reports mentioned brand and/or generic names of common pumps for pain management or mentioned use for pain treatment; therefore, these reports could not be ruled out as involving elastomeric pumps to deliver local anesthetics.
Abbreviations: MAUDE, Manufacturer and User Facility Device Experience; PCA, patient-controlled analgesia.
Figure 2.
The total number of unique MAUDE reports identified as involving elastomeric pumps to deliver local anesthetics per year from 2010 to 2017 (data not shown for 2018 because data were collected through July 2018).
Abbreviation: MAUDE, Manufacturer and User Facility Device Experience.
Of the 4093 identified reports, 3624 (88.5%) were categorized as malfunctions, 292 (7.1%) as injuries, and 8 (0.2%) as involving death. All eight cases of death were reported before 2013 (2010, n=2; 2011, n=4; 2012, n=1; 2013, n=1), with no reports of death identified from 2014 to 2018. A total of 903 reports (22.1%) identified elastomeric pumps with flow issues (Table 1). Among all identified reports, there were 13 cases (0.3%) of possible liver injury and 51 cases (1.2%) of possible surgical site infection.
Table 1.
Malfunctions, Injuries, and Deaths Related to Use of Elastomeric Pumps to Deliver Local Anesthetics, Including Reports of LAST and Injuries of Interest
| Total Reports Involving Elastomeric Pumps to Deliver Local Anesthetic (n=4093) | |
|---|---|
| Reports identifying pumps with flow issues | 903 (22.1) |
| Reports identifying possible or probable LAST | 139 (3.4) |
| Reports identifying possible liver injury | 13 (0.3) |
| Reports identifying possible surgical site infection | 51 (1.2) |
| Malfunction reports | 3624 (88.5) |
| Possible or probable LAST | 26 (0.6) |
| Possible liver injury | 2 (<0.1) |
| Possible site infection | 5 (0.1) |
| Injuries | 292 (7.1) |
| Possible or probable LAST | 84 (2.1) |
| Possible liver injury | 11 (0.3) |
| Possible site infection | 38 (0.9) |
| Deaths | 8 (0.2) |
| Possible or probable LAST | 2 (<0.1) |
| Possible liver injury | 0 (0.0) |
| Possible site infection | 0 (0.0) |
| Other report codes/missing codes | 169 (4.1) |
| Possible or probable LAST | 27 (0.7) |
| Possible liver injury | 0 (0.0) |
| Possible site infection | 8 (0.2) |
Note: Values are the n (%).
Abbreviation: LAST, local anesthetic systemic toxicity.
Identification of LAST in MAUDE Reports
A total of 139 reports (3.4%) were determined to be probably (n=53) or possibly (n=86) related to LAST. Reports of possible and probable LAST occurred throughout the study period, with no notable trends over time. Of these 139 reports, 26 were reported as malfunction, 84 as injury, and 2 as death. Of eight total reports of death, two (25%) were determined to be probably or possibly related to LAST (Table 1). The first report with death and probable or possible LAST was from 2010, and the second report was from 2011; narrative text from the reports describing both cases of death is provided in Supplemental Table 2.
Discussion
Between January 2010 and July 2018, more than 4000 medical device reports of malfunction, injury, and death involving the use of elastomeric pumps delivering local anesthetic were submitted to the MAUDE database. In the present analysis, most reports (88.5%) were related to device malfunction, followed by injury (7.1%) and death (0.2%). The proportion of reports categorized as device malfunctions (88.5%) is consistent with a previous analysis of MAUDE database reports involving patient-controlled analgesia devices, which observed that 92.0% of reports were associated with device malfunction.11 Furthermore, we determined 139 medical device reports to be probably or possibly related to LAST, with 2 cases reporting patient death; this highlights the value of the MAUDE database as an information source for identifying serious complications of local anesthetic delivery devices, along with other repositories such as FDA Adverse Event Reporting System (FAERS).
Our retrospective analysis of the MAUDE database indicated a peak number of reports in 2014 followed by a progressive decline in the number of reports. This pattern potentially reflects changes in the regulatory and market landscape for prolonged postsurgical analgesia, such as approval of the prolonged anesthetic drug liposomal bupivacaine in 2012, although the exact cause remains unclear. We also hypothesize that this peak could reflect stimulated reporting due to litigation and recall of other drug infusion pumps occurring at this time (eg, the 2013 recall of implantable drug infusion pumps from SynchroMedTM).18 However, because MAUDE is a passive surveillance system, underreporting as well as incomplete or inaccurate reporting limits this study because it cannot be determined whether the decreased incidence of the device malfunction, systemic trends of underreporting, or a combination of both factors account for this progressive decline in medical device reports.
Our analysis also identified 139 reports of probable and possible cases of LAST with elastomeric pumps. Pump malfunctions resulting in overinfusion of anesthetic have also been reported in a case study and clinical study, supporting that this is a serious complication related to the use of these devices.12,13 However, underreporting limits the interpretation of our findings. The FDA recognizes that poor compliance by hospitals to submit medical device reports, particularly those potentially contributing to adverse events, is an issue with a passive system like MAUDE.19 It is estimated that the proportion of medication errors that are ultimately reported ranges from 1.2% to 7.7%. On the basis of the lowest value in this range (1.2%), it can be extrapolated that harmful events or death potentially represent 25,000 (300/1.2%) events occurring during the study period of this analysis.11,20 It should be noted that the estimated range of underreporting (1.2–7.7%) was based on analyses of internal adverse event reporting or with a computer-based monitoring system (not the MAUDE database).20–23 Nonetheless, underreporting represents a serious concern, and this calculation emphasizes the need for proactive interventions to promote error reporting and reduce error occurrence.11,19,20,24,25
This study has limitations to consider. We relied on expert narrative review to identify reports involving potential use of elastomeric pumps to deliver local anesthetics as well as possible or probable cases of LAST. However, not all reports included detailed narrative summaries or indicate the drug used precluding our ability to accurately identify cases of LAST. Additionally, for reports with no drug mentioned, the manual review could identify pumps that were likely used for local anesthetics but could not be confirmed because of reports lacking drug name. Similarly, identification of possible liver failure relied on MAUDE narrative reports describing laboratory results indicating liver failure or treatment for liver failure, limiting our ability to accurately describe the total number of reports of possible liver failure. Another limitation is that circumstances around the event may not be documented or verified in a MAUDE database report, challenging the ability to establish a cause-and-effect relationship between the device and specific event.10 For example, the potential cardiac, respiratory, or central nervous system symptoms used to identify possible LAST may be explained by anesthesia administered via elastomeric pump or as a different block (eg, interscalene block);26 therefore, possible cases of LAST identified from reports with elastomeric pumps may be an overestimate. Additionally, without data quantifying the total number of elastomeric pumps used in the US for delivering local anesthetics, it was not possible to accurately estimate the rates at which these malfunctions occur and hence the relative risk of experiencing malfunctions and injury.
Conclusion
Our analysis indicates that elastomeric pumps used to deliver local anesthetics can malfunction and result in injury or death. The majority of reports identified malfunctions, with 139 (3.4%) possible or probable cases of LAST reported. These data highlight the potential risk of faulty or incorrectly used elastomeric pumps, underscoring the need for more accurate and timely medical device reporting to better understand the risks of delivering local anesthetics with this type of device.
Acknowledgments
This study was supported by Pacira BioSciences, Inc. Editorial assistance was provided under the direction of the authors by Aarthi Gobinath, PhD, and David Boffa, ELS, of MedThink SciCom, and Paul Cavanaugh, PhD, a former employee of Pacira BioSciences, Inc.
Disclosure
Richard Teames is a paid consultant for Pacira BioSciences, Inc., has previously served as a consultant for Sonosite Fujifilm and Halyard; and reports non-financial support from Pacira BioSciences, Inc., during the conduct of the study; and personal fees from Avanos, Pacira BioSciences, Inc. and Sonosite/Fujifilm, outside the submitted work. Andrew Joyce and Catherine Vick are principals at Venebio Group and served as paid consultants to Pacira BioSciences, Inc. for this study. Andrew Joyce reports personal fees from Pacira Biosciences, Inc., during the conduct of the study. Richard Scranton is a former employee of Pacira BioSciences, Inc. and is now a paid consultant; and reports being a consultant and prior officer of Pacira BioSciences, Inc., during the conduct of the study. Catherine Vick reports being a principal minority owner with Venebio Group: our business was contracted by Pacira BioSciences, Inc. to participate in study design, conduct data management and analyses, and participate in manuscript preparation, during the conduct of the study. Nayana Nagaraj is an employee of Pacira BioSciences, Inc. The authors report no other potential conflicts of interest for this work.
References
- 1.ON-Q pump with fixed flow rate [package insert]. Alpharetta, GA: Halyard Health, Inc.; 2017. [Google Scholar]
- 2.Fredrickson MJ, Leightley P, Wong A, Chaddock M, Abeysekera A, Frampton C. An analysis of 1505 consecutive patients receiving continuous interscalene analgesia at home: a multicentre prospective safety study. Anaesthesia. 2016;71(4):373–379. doi: 10.1111/anae.13385 [DOI] [PubMed] [Google Scholar]
- 3.Machi AT, Ilfeld BM. Continuous peripheral nerve blocks in the ambulatory setting: an update of the published evidence. Curr Opin Anaesthesiol. 2015;28(6):648–655. doi: 10.1097/ACO.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 4.Baig MK, Zmora O, Derdemezi J, Weiss EG, Nogueras JJ, Wexner SD. Use of the ON-Q pain management system is associated with decreased postoperative analgesic requirement: double blind randomized placebo pilot study. J Am Coll Surg. 2006;202(2):297–305. doi: 10.1016/j.jamcollsurg.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Dowling R, Thielmeier K, Ghaly A, Barber D, Boice T, Dine A. Improved pain control after cardiac surgery: results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg. 2003;126(5):1271–1278. doi: 10.1016/S0022-5223(03)00585-3 [DOI] [PubMed] [Google Scholar]
- 6.White PF, Rawal S, Latham P, et al. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology. 2003;99(4):918–923. doi: 10.1097/00000542-200310000-00026 [DOI] [PubMed] [Google Scholar]
- 7.Kenes MT, Leonard MC, Bauer SR, Wyman MJ. Liposomal bupivacaine versus continuous infusion bupivacaine via an elastomeric pump for the treatment of postoperative pain. Am J Health Syst Pharm. 2015;72(23 Suppl 3):S127–S132. doi: 10.2146/ajhp150168 [DOI] [PubMed] [Google Scholar]
- 8.Remerand F, Vuitton AS, Palud M, et al. Elastomeric pump reliability in postoperative regional anesthesia: a survey of 430 consecutive devices. Anesth Analg. 2008;107(6):2079–2084. doi: 10.1213/ane.0b013e318187c9bb [DOI] [PubMed] [Google Scholar]
- 9.Neal JM, Bernards CM, Butterworth JF IV, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152–161. doi: 10.1097/AAP.0b013e3181d22fcd [DOI] [PubMed] [Google Scholar]
- 10.Manufacturer and User Facility Device Experience - (MAUDE). Available from: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/ReportingAdverseEvents/ucm127891.htm. Accessed August16, 2019.
- 11.Lawal OD, Mohanty M, Elder H, et al. The nature, magnitude, and reporting compliance of device-related events for intravenous patient-controlled analgesia in the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Expert Opin Drug Saf. 2018;17(4):347–357. doi: 10.1080/14740338.2018.1442431 [DOI] [PubMed] [Google Scholar]
- 12.Baulig W, Maurer K, Theusinger OM, et al. Continuous elastomeric pump-based ropivacaine wound instillation after open abdominal aortic surgery: how reliable is the technique? Heart Surg Forum. 2011;14(1):E51–E58. doi: 10.1532/HSF98.20101089 [DOI] [PubMed] [Google Scholar]
- 13.Koogler A, Amusa G, Kushelev M, Lawrence A, Carlson L, Moran K. Elastomeric pump malfunction resulting in over-infusion of local anesthetic. SAGE Open Med Case Rep. 2019;7:2050313X18823928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn SD, Wright HL, Couperus JJ, et al. Association between patient-controlled analgesia pump use and postoperative surgical site infection in intestinal surgery patients. Surg Infect (Larchmt). 2002;3(2):109–118. doi: 10.1089/109629602760105772 [DOI] [PubMed] [Google Scholar]
- 15.Scanlon MM, Gazelka HM, Moeschler SM, et al. Surgical site infections in cancer patients with intrathecal drug delivery devices. Pain Med. 2017;18(3):520–525. [DOI] [PubMed] [Google Scholar]
- 16.Amide local anesthetics. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 17.Lee JE, Kwak KH. A liver transplant recipient with possible bupivacaine-induced liver injury caused by intra-articular injection after total knee arthroplasty: a case report. Medicine (Baltimore). 2018;97(38):e12481. doi: 10.1097/MD.0000000000012481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Available from: https://www.fda.gov/medical-devices/medical-device-recalls/medtronic-recalls-synchromed-ii-and-synchromed-el-implantable-drug-infusion-pumps-due-failure. Accessed July31, 2020.
- 19.Barlas S. FDA flags inconsistent hospital reporting of medical device problems: hazy reporting rules beget confusion. P T. 2017;42(2):97–115. [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner B, Nelson W, Hicks R, Sikirica V, Gagne J, Schein J. The rate and costs attributable to intravenous patient-controlled analgesia errors. Hosp Pharm. 2009;44(4):312–324. doi: 10.1310/hpj4404-312 [DOI] [Google Scholar]
- 21.Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266(20):2847–2851. doi: 10.1001/jama.1991.03470200059035 [DOI] [PubMed] [Google Scholar]
- 22.Cullen DJ, Bates DW, Small SD, Cooper JB, Nemeskal AR, Leape LL. The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21(10):541–548. [DOI] [PubMed] [Google Scholar]
- 23.Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998;5(3):305–314. doi: 10.1136/jamia.1998.0050305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The problem of underreporting. Available from: https://web.archive.org/web/20181025052451/http://www.anh-usa.org/microsite-subpage/the-problem-of-underreporting/. Accessed July31, 2020.
- 25.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003 [DOI] [PubMed] [Google Scholar]
- 26.Zisquit J, Nedeff N. Interscalene Block. Treasure Island, FL: StatPearls; 2020. [PubMed] [Google Scholar]