Abstract
The retina continuously receives light to enable vision, and the related processes require a marked amount of energy. During active metabolism, reactive oxygen species (ROS) are generated in exchange. Although physiologically generated ROS may be removed by endogenous antioxidant systems, and the effects of oxidative stress may be recovered by repair systems to retain homeostasis and health, when ROS and oxidative stress exceed the capacity of the antioxidant and repair systems, the condition becomes pathological. Multiple mechanisms of oxidative stress and the effects of antioxidant and repair systems in the retina have long been analyzed using light-induced retinal degeneration models. Among the mechanisms, a positive feedback loop of oxidative stress and related inflammation may be involved in the pathogenesis of a blinding aging disease, age-related macular degeneration. Treatments for suppressing ROS and oxidative stress by administrating antioxidant products may support the tissue-protective function of antioxidant systems. Moreover, recent studies have proposed a new concept for maintaining homeostasis by supplying sufficient energy to activate the repair systems. The current review will help elucidate the influence of oxidative stress and guide future analyses to explore new therapeutic approaches for oxidative stress-mediated diseases.
Keywords: Oxidative stress, Antioxidants, Tissue repair, Inflammation, Age-related macular degeneration, Retina
Highlights
-
•
Vision formation requires marked amounts of energy and produces ROS.
-
•
Balanced oxidative stress and antioxidant/repair systems determine cellular health.
-
•
ROS-modified molecules affect multiple pathways to cause cell death and disorders.
-
•
A positive feedback loop of oxidative stress/related inflammation may lead to AMD.
-
•
Homeostasis can be retained by supplying enough energy to activate repair systems.
List of abbreviations
- AMD
age-related macular degeneration
- ATP
adenosine triphosphate
- ER
endoplasmic reticulum
- MPOD
macular pigment optical density
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
1. Introduction
The retina is a highly energy-consuming tissue, having abundant mitochondria to actively perform oxidative phosphorylation (OXPHOS) and relying on aerobic glycolysis to a much greater degree than the brain does [1]. The light stimuli received by visual pigments in the photoreceptors are converted into electric stimuli through a series of phototransduction systems, and simultaneously, the conformationally changed visual pigments by light stimuli are recovered for recycling in the retinal pigment epithelium (RPE); these processes constitute the visual cycle and create considerable energy demands [1]. Moreover, photoreceptors in the darkness consume substantial amounts of adenosine triphosphate (ATP) to power active ion pumps that maintain the membrane potential [1]. In addition, the electric signals are transmitted through synapses to reach retinal ganglion cells, and through their axons, which form the optic nerve, are finally transferred to the brain. Because neurotransmitters excreted at synapses are received by the postsynaptic neurons with substantial ion kinetics including intra- and inter-cellular Ca2+ ion kinetics, ATP is also required to retain ion homeostasis [2]. Energy metabolism naturally generates oxidative stress; thus, therapeutic approaches to avoid and/or reverse its effects have long been studied using the retina. This review discusses the multiple pathways of light-induced retinal neurodegeneration in an attempt to elucidate the underlying interactions between oxidative stress and biological responses, which can further improve our understanding of the various diseases related to oxidative stress. Furthermore, this review also introduces the concept of a recent novel therapeutic approach for combatting the influence of oxidative stress.
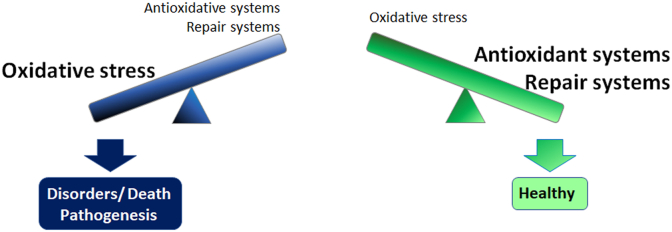
In the process of energy synthesis, reactive oxygen species (ROS), which are highly reactive chemical species containing oxygen, are produced in exchange. ROS cause oxidative stress and affect cell/tissue/organ function. Physiologically produced ROS are constantly deleted by antioxidant systems such as endogenous antioxidant enzymes and restored antioxidant products such as lutein and zeaxanthin in the retina [[3], [4], [5], [6]] to avoid ROS accumulation. Moreover, ROS-induced damages may be repaired, e.g., by DNA repair systems [7], or compensated, e.g., by unfolded protein responses [8,9]. As long as homeostasis is preserved, the cells, tissues, and organs remain healthy [10]. However, when the ROS levels exceed the capacity of these self-defense systems, i.e., antioxidant and repair systems, oxidative stress is accumulated, and decompensation leads to cell/tissue/organ disorders and death and pathological conditions (Fig. 1).
Fig. 1.
Balance between oxidative stress and self-defense systems determines whether the cell/tissue/organ condition is pathological or healthy.
Self-defense systems, such as antioxidant systems that remove ROS and alleviate oxidative stress, and repair systems that aid recovery from the resulting disorders, are present in vivo. If the accumulated ROS and oxidative stress exceed the capacity of the antioxidant and/or repair systems, cell/tissue/organ disorders and death ensue and may cause disease pathogenesis, although as long as the capacity of the antioxidant and repair systems is retained, the cells, tissues, and organs remain healthy. ROS, reactive oxygen species.
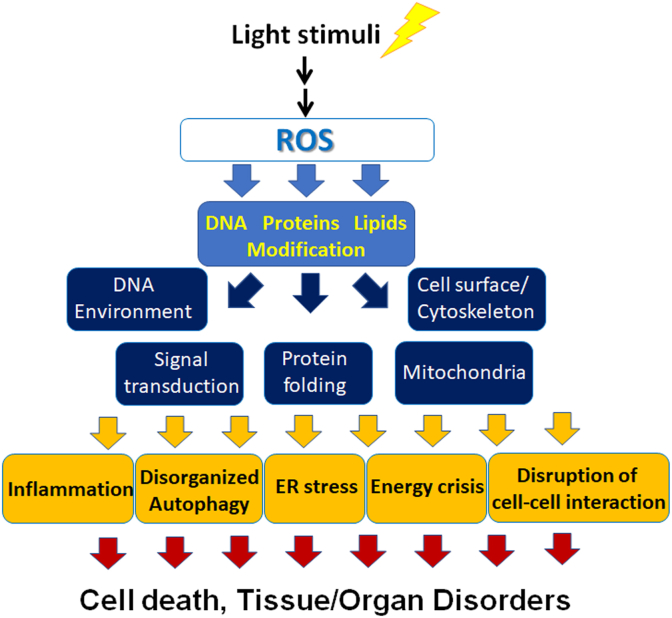
Multiple mechanisms of oxidative stress in the retina have been shown using light-induced retinal degeneration models in which excessive ROS that cannot be regulated by the self-defense system are generated, at least in part, by excessive action of the visual cycle during and after light exposure to finally cause photoreceptor cell death, such as through apoptosis (Fig. 2). Generally, ROS modify DNA, proteins, and lipids. Thus, ROS affect the DNA environment to cause DNA damage, e.g., DNA double-strand breaks [7] and/or epigenetic changes; thus, gene expression may become abnormal when the damage is not lethal. ROS-mediated protein and/or lipid modifications such as protein phosphorylation [11] and/or lipid peroxidation [7] may affect signal transduction pathways [12], protein folding [8,9], enzymatic activities, such as those involved in OXPHOS in the mitochondria [13], and cell-surface conditions, including receptor expression and the plasma membrane potential, and the cytoskeleton that supports various organelle functions [14]. These effects may lead to pathological events such as inflammation, disorganized autophagy [15], and endoplasmic reticulum (ER) stress [8,9], originally a compensatory system that may result in apoptosis if the damage exceeds the capacity of the systems. Relative energy crisis may occur at the time of mitochondrial breakdown [13], and cell–cell interactions may be disrupted. Finally, the decompensated cells may die, and the tissue/organ may become disordered.
Fig. 2.
Mechanisms of light-induced oxidative stress in cell death and tissue/organ disorders.
Light-induced ROS modify DNA, proteins, and lipids to affect the DNA environment, including the DNA structures and epigenetics, signal transduction, protein folding, mitochondrial function, cell-surface condition, and the cytoskeleton to induce inflammation, disorganized autophagy, ER stress, energy crisis, and disruption of cell–cell interaction, which finally cause cell death and tissue/organ disorders. ROS, reactive oxygen species; ER; endoplasmic reticulum.
In order to suppress light-induced photoreceptor damage, antioxidant products such as N-acetyl-l-cysteine (NAC) [16] and lutein/zeaxanthin [7] suppress accumulation of oxidative stress to protect from light-induced photoreceptor damage. Conversely, a similar effect is attained by suppressing an inflammatory mediator, the renin-angiotensin system by angiotensin II type 1 receptor (AT1R) blocker, also used as an hypertensive drug, reducing ROS levels [12]. Therefore, oxidative stress and inflammation may share a pathogenetic pathway.
Interaction between ROS and inflammation has also been proven in the light-induced influences in the RPE and choroid [14,17,18]. Less intensive light exposure, not causing apparent photoreceptor apoptosis, induces an inflammatory cytokine, monocyte chemoattractant protein-1 (MCP-1) in the RPE, which recruits macrophages to the choroid. Locally recruited macrophages are activated and further secrete cytokines, which most likely produces additional ROS. These reports support the notion that ROS-induced inflammation further increases ROS levels to facilitate a positive feedback loop.
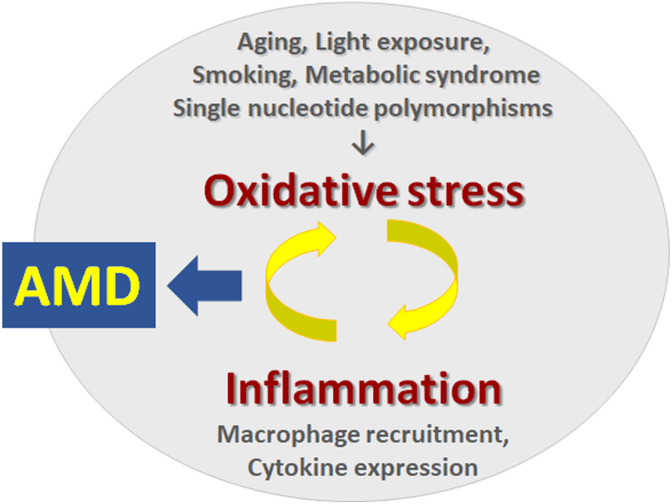
It is well accepted that the positive feedback loop of ROS and inflammation is involved in the pathogenesis of age-related macular degeneration (AMD) [14,19] (Fig. 3). The risk factors of AMD involve aging, smoking, metabolic syndrome, single nucleotide polymorphisms, and light exposure. Under conditions of age-associated gradual decline in endogenous functions, daily stimulation would suffice to cause decompensation and pathogenesis. Smoking and metabolic syndrome, including diabetes and hypertension, ubiquitously produce oxidative stress. In addition, excessive and/or prolonged light stimulation to the retina causes accumulation of oxidative stress, as well-studied in animal models [[7], [8], [9],12,14,17,18]. Oxidative stress induces inflammation and vice versa to form a positive feedback loop and a vicious cycle as described above. When the process becomes prolonged and chronic, AMD, a blinding disease, may appear. However, in the background, slow but prolonged changes progress gradually. A clinical report showed that photoreceptor outer segments are already damaged and shortened in AMD fellow eyes, which are at high risk for AMD, even without other apparent retinal findings [6], suggesting that the retina has already been attacked by chronic oxidative stress before apparent diseases appear. In fact, two large clinical studies, i.e., the age-related eye disease study (AREDS) [20] and AREDS2 [21] showed that antioxidative micronutrient supplements attenuated AMD progression; thus, oxidative stress may contribute to AMD progression.
Fig. 3.
The positive feedback loop of oxidative stress and inflammation causes age-related macular degeneration (AMD).
Aging, continuous and/or excessive light exposure, smoking, metabolic syndrome, and single nucleotide polymorphisms cause and/or accelerate oxidative stress, which induces macrophage recruitment, cytokine expression, and inflammation and forms a positive feedback system and vicious cycle to finally cause AMD.
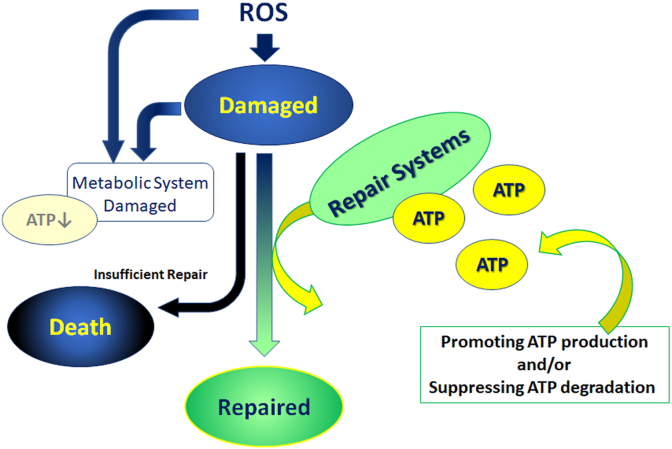
Most repair systems consume ATP and energy. Thus, to repair the damaged cell/tissue/organ, a sufficient energy supply is required. It has been reported that the retinal energy supply increases immediately after light exposure. However, the supply did not last and was moreover downregulated due to light-induced reduction in OXPHOS enzymatic activity [13], leading to insufficient repair and apoptosis of the cells. The change in enzymatic activity could be mediated by oxidative stress that the cell itself has produced, although further study is required. In the same report, 5′ adenosine monophosphate-activated protein kinase (AMPK)-mediated mitochondrial regulation and continuous energy supply rescued the cells from death. The concept of cell rescue by a constant energy supply [13] (Fig. 4) differs from the strategy of previous reports that focused on how to prevent ROS accumulation for the treatment of oxidative stress-mediated pathogenesis [7,9,12,14,15,17]. A similar concept was also reported in animal models of retinitis pigmentosa in which gene mutation caused photoreceptor death [22], and AMD [23] where an inhibitor of ATPase and ATP degradation suppressed ER stress to attenuate photoreceptor death and accumulation of drusen, a sign of early AMD, respectively.
Fig. 4.
Repair systems against oxidative stress require ATP.
To repair ROS- and oxidative stress-induced damage, ATP and energy are required; promoting ATP production and/or suppressing ATP degradation may support supply sufficiency. However, when ATP is insufficient, the repair systems do not work properly. The decompensation finally induces cellular disorders and death. ROS, reactive oxygen species; ATP, adenosine triphosphate.
Antioxidant systems involve endogenously equipped phase II antioxidant enzymes that detoxify xenobiotics by increasing their hydrophilicity and enhancing their disposal. Enzymes, such as HO-1, SOD1 and 2, and catalase, are regulated by a transcription factor, Nrf2, and are upregulated in response to oxidative stress.
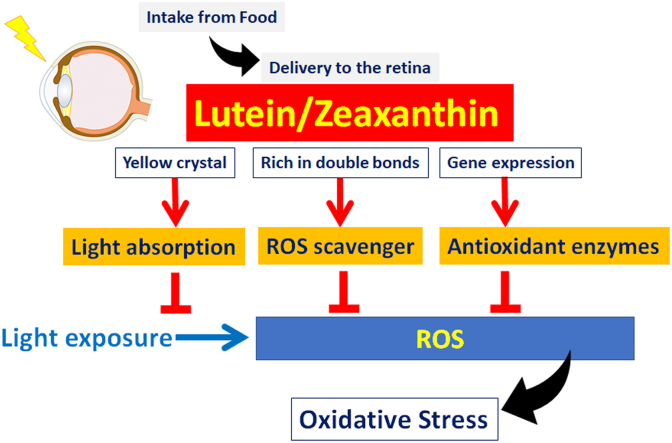
In addition, the retina restores antioxidant products as macular pigments; it is composed of lutein and zeaxanthin and a derivative, meso-zeaxanthin. Carotenoids are synthesized in plants, not in animals. Thus, animals need to obtain carotenoids through their diet. While zeaxanthin is concentrated in the macula, the central part of the retina, lutein is also widespread to the peripheral part of the retina in humans [3] to act as an antioxidant. Levels of lutein/zeaxanthin in the macula are clinically evaluated as macular pigment optical density (MPOD), and a low level MPOD is a risk factor for AMD [5]. Lower levels of dietary intake of lutein increase the AMD risk [24], and AREDS2 showed that constant intake of lutein/zeaxanthin supplementation prevented AMD progression [20]. The action of lutein/zeaxanthin involves (1) absorption of excessive blue light of short wavelength and high energy taking advantage of the fact that lutein/zeaxanthin comprise yellow crystals [25], (2) scavenging ROS utilizing the abundant double bonds in their molecular structure [25], and (3) induction of phase II antioxidant enzymes, which may be independent of Nrf2 [4] (Fig. 5).
Fig. 5.
Protective effects of lutein/zeaxanthin.
Lutein and zeaxanthin are derived from food and delivered to the retina and block excessive light by absorbing blue light, which is of short wavelength and high energy, act as ROS scavengers and induce antioxidant enzymes to protect the retina from oxidative stress. ROS, reactive oxygen species. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Scavengers act as antioxidants to become self-oxidized. Thus, oxidized “primarily scavengers” may cause oxidative stress after they reduce ROS and are oxidized as a result. Interestingly, however, lutein can interact with other carotenoids, such as lycopene, for electron transfer to eventually become reduced again, probably because lutein is a weaker, and lycopene is a stronger, reducing agent. Therefore, lutein can regenerate antioxidant ability [26].
ROS are continuously generated as long as the cells are alive; therefore, the cells are equipped with various antioxidant and repair systems. However, energy supply is necessary for repair function, and moreover, ROS may damage the energy-supply systems. Avoiding oxidative stress generation, supplementing antioxidants, and supporting sufficient energy supply for the repair systems may help maintain the balance between oxidative stress and antioxidant and repair systems, and contribute to preserving health and avoiding pathological conditions. Multiple mechanisms are involved in oxidative stress; thus, it would be of value to consider the various viewpoints raised in the current review and combine them to explore new therapeutic approaches for oxidative stress-mediated diseases.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
I thank all the members and alumni of the Laboratory of Retinal Cell Biology, Department of Ophthalmology, Keio University School of Medicine for their kind assistance. I also thank Professor Kazuo Tsubota, Department of Ophthalmology, Keio University School of Medicine for his kind supports.
References
- 1.Hurley J.B., Lindsay K.J., Du J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015;93(7):1079–1092. doi: 10.1002/jnr.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa Y., Sasaki M., Takahashi N., Kamoshita M., Miyake S., Tsubota K. Neuroprotective effects of lutein in the retina. Curr. Pharmaceut. Des. 2012;18(1):51–56. doi: 10.2174/138161212798919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyake S., Kobayashi S., Tsubota K., Ozawa Y. Phase II enzyme induction by a carotenoid, lutein, in a PC12D neuronal cell line. Biochem. Biophys. Res. Commun. 2014;446(2):535–540. doi: 10.1016/j.bbrc.2014.02.135. [DOI] [PubMed] [Google Scholar]
- 5.Nagai N., Asato T., Minami S., Suzuki M., Shinoda H., Kurihara T., Sonobe H., Watanabe K., Uchida A., Ban N., Tsubota K., Ozawa Y. Correlation between macular pigment optical density and neural thickness and volume of the retina. Nutrients. 2020;12(4) doi: 10.3390/nu12040888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai N., Minami S., Suzuki M., Shinoda H., Kurihara T., Sonobe H., Watanabe K., Uchida A., Ban N., Tsubota K., Ozawa Y. Macular pigment optical density and photoreceptor outer segment length as predisease biomarkers for age-related macular degeneration. J. Clin. Med. 2020;9(5) doi: 10.3390/jcm9051347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M., Yuki K., Kurihara T., Miyake S., Noda K., Kobayashi S., Ishida S., Tsubota K., Ozawa Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012;23(5):423–429. doi: 10.1016/j.jnutbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Ban N., Ozawa Y., Osada H., Lin J.B., Toda E., Watanabe M., Yuki K., Kubota S., Apte R.S., Tsubota K. Neuroprotective role of retinal SIRT3 against acute photo-stress. NPJ Aging Mech Dis. 2017;3:19. doi: 10.1038/s41514-017-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osada H., Okamoto T., Kawashima H., Toda E., Miyake S., Nagai N., Kobayashi S., Tsubota K., Ozawa Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0178627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.E B.D., Marfany G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants. 2020;9(4) doi: 10.3390/antiox9040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakazawa M., Maeda S., Yokoyama N., Nakagawa T., Yonezawa T., Ohno K., Matsuki N. Sphingosine-1-phosphate (S1P) signaling regulates the production of intestinal IgA and its potential role in the pathogenesis of canine inflammatory bowel disease. J. Vet. Med. Sci. 2019;81(9):1249–1258. doi: 10.1292/jvms.19-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narimatsu T., Ozawa Y., Miyake S., Nagai N., Tsubota K. Angiotensin II type 1 receptor blockade suppresses light-induced neural damage in the mouse retina. Free Radic. Biol. Med. 2014;71:176–185. doi: 10.1016/j.freeradbiomed.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima H., Ozawa Y., Toda E., Homma K., Osada H., Narimatsu T., Nagai N., Tsubota K. Neuroprotective and vision-protective effect of preserving ATP levels by AMPK activator. Faseb. J. 2020;34(4):5016–5026. doi: 10.1096/fj.201902387RR. [DOI] [PubMed] [Google Scholar]
- 14.Narimatsu T., Ozawa Y., Miyake S., Kubota S., Hirasawa M., Nagai N., Shimmura S., Tsubota K. Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Invest. Ophthalmol. Vis. Sci. 2013;54(7):4555–4562. doi: 10.1167/iovs.12-11572. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Sawada O., Kohno H., Le Y.Z., Subauste C., Maeda T., Maeda A. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013;288(11):7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura M., Kuse Y., Tsuruma K., Shimazawa M., Hara H. The involvement of the oxidative stress in murine blue LED light-induced retinal damage model. Biol. Pharm. Bull. 2017;40(8):1219–1225. doi: 10.1248/bpb.b16-01008. [DOI] [PubMed] [Google Scholar]
- 17.Kamoshita M., Toda E., Osada H., Narimatsu T., Kobayashi S., Tsubota K., Ozawa Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016;6:30226. doi: 10.1038/srep30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narimatsu T., Negishi K., Miyake S., Hirasawa M., Osada H., Kurihara T., Tsubota K., Ozawa Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015;132:48–51. doi: 10.1016/j.exer.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Rozing M.P., Durhuus J.A., Krogh Nielsen M., Subhi Y., Kirkwood T.B., Westendorp R.G., Sorensen T.L. Age-related macular degeneration: a two-level model hypothesis. Prog. Retin. Eye Res. 2020;76:100825. doi: 10.1016/j.preteyeres.2019.100825. [DOI] [PubMed] [Google Scholar]
- 20.G. Age-Related Eye Disease Study Research, A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.G. Age-Related Eye Disease Study 2 Research, Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. J. Am. Med. Assoc. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 22.Hata M., Ikeda H.O., Kikkawa C., Iwai S., Muraoka Y., Hasegawa T., Kakizuka A., Yoshimura N. KUS121, a VCP modulator, attenuates ischemic retinal cell death via suppressing endoplasmic reticulum stress. Sci. Rep. 2017;7:44873. doi: 10.1038/srep44873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraoka Y., Iida Y., Ikeda H.O., Iwai S., Hata M., Iwata T., Nakayama M., Shimozawa N., Katakai Y., Kakizuka A., Yoshimura N., Tsujikawa A. KUS121, an ATP regulator, mitigates chorioretinal pathologies in animal models of age-related macular degeneration. Heliyon. 2018;4(5) doi: 10.1016/j.heliyon.2018.e00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddon J.M., Ajani U.A., Sperduto R.D., Hiller R., Blair N., Burton T.C., Farber M.D., Gragoudas E.S., Haller J., Miller D.T. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group, JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 25.Krinsky N.I., Landrum J.T., Bone R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 26.Young A.J., Lowe G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001;385(1):20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]