Abstract
Glutathione is a low molecular weight thiol that is present at high levels in the cell. The high levels of glutathione in the cell make it one of the most abundant antioxidants contributing to cellular redox homeostasis. As a general rule, throughout cardiovascular disease and progression there is an imbalance in redox homeostasis characterized by reactive oxygen species overproduction and glutathione underproduction. As research into these imbalances continues, glutathione concentrations are increasingly being observed to drive various physiological and pathological signaling responses. Interestingly in addition to acting directly as an antioxidant, glutathione is capable of post translational modifications (S-glutathionylation) of proteins through both chemical interactions and enzyme mediated events. This review will discuss both the chemical and enzyme-based S-glutathionylation of proteins involved in cardiovascular pathologies and angiogenesis.
Keywords: Cardiovascular disease, Glutathione, S-glutathionylation
1. Introduction
Glutathione (GSH) was first described by Joseph de Rey-Pailhade [[1], [2], [3]]; even 135 years after its description GSH is still widely studied. GSH is the most abundant low molecular weight thiol in the cell and is usually present in millimolar concentrations (1–10 mM) [4,5]. GSH is distributed throughout the cytosol, where it is synthesized, as well as the nucleus and cellular organelles [[6], [7], [8]]. GSH is predominantly present in its reduced form but can be oxidized to its disulfide, GSSG. As reactive oxygen and nitrogen species (ROS and RNS) are generated during physiological and pathological processes GSH functions as a scavenging antioxidant; these reactions are typically catalyzed by glutathione peroxidases that reduce hydrogen peroxide and lipid hydroperoxides using GSH as a reducing equivalent [9]. The ratio of GSH to GSSG is one of several cellular redox couples and an indicator of the redox potential of the cell. A steady state level of GSH (thiol) to GSSG (disulfide) occurs during homeostasis, dynamic changes in this steady state are responsible for many physiologic signaling events. Generally, GSH is 2 orders of magnitude more abundant than GSSG (100:1) [10,11] but can, under increasing oxidative or nitrosative stress, drop as low as 1:1 [12]. Recycling of GSSG back to GSH is important to maintaining this ratio and is mediated by glutathione reductase [13] in a reaction requiring nicotinamide adenine dinucleotide phosphate (NADPH) as a reducing equivalent. Changes in the GSH:GSSG ratios are known to regulate cell processes such as division and apoptosis and maintain a delicate balance between the physiological and pathological states of the cell [[14], [15], [16]].
GSH consists of three amino acids: glycine, glutamate, and cysteine; the thiol moiety of cysteine allows GSH to participate in redox reactions. Under physiological conditions a cell is in a reduced state. Organelles form discreet pockets that have their own microenvironments. An exception to the normally reducing environment of the cell is the endoplasmic reticulum (ER) where the microenvironment is highly oxidizing (GSH:GSSH ~3:1) [17] to facilitate disulfide formation between cysteine thiols, an essential process during protein folding [18]. These disulfides contribute to protein conformation and to the tertiary and quaternary structure of proteins. Cysteine thiols can undergo redox modification under physiological and pathological conditions. These post-translational modifications (PTMs) can be the result of direct interactions with ROS and RNS or be the result of enzymatic activity of enzymes such as glutaredoxin and glutathione S-transferase. These modifications include sulfhydration (-S-SH), sulfenylation (-S-OH), S-nitrosylation (-S-NO), sulfenylamide (-S-SN), and S-glutathionylation (-S-SG) [19]. Cysteines can also be modified by fatty acids and lipids to produce S-palmitoylation and S-prenylation previously reviewed [20] (Fig. 1). This review focuses on S-glutathionylation (-SSG), and the current understanding of its role in cardiovascular pathology.
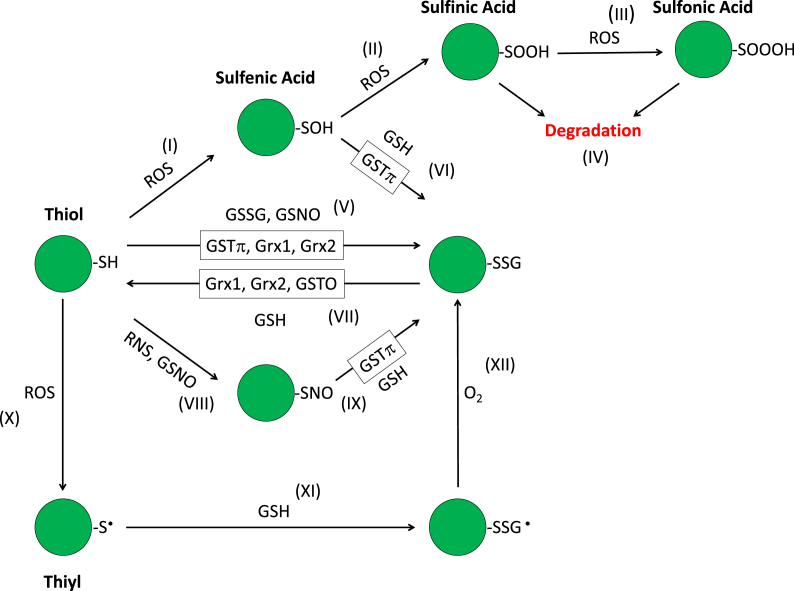
Fig. 1.
Cysteine modifications that lead to S-Glutathionylation. (I) ROS oxidizes a thiol (-SH) to sulfenic acid (-SOH), (II) in the presence of further ROS it is oxidized to sulfinic acid (-SOOH) (III) which in turn is further oxidized to sulfonic acid (-SOOOH), (IV) leading to proteolytic degradation. (V) Sulfenic acid (-OH) can be reduced by GSH to S-glutathionylate (-SSG) proteins. Enzymatically (enzymes denoted by boxes) S-glutathionylation is mediated via GSTπ, Grx1, and Grx2. Non enzymatically S-glutathionylation can occur directly via disulfide exchange between the thiol and GSSG, the protein thiolate can also react with the sulfur of GSNO via nucleophilic attack leading to a disulfide and S-glutathionylation. (VI) The sulfenic acid (-SOH) can be S-glutathionylated by GSH, this process is also mediated by GSTπ. (VII) S-glutathionlyation can be reduced directly by GSH, or enzymatically by Grx1, Grx2, and GSTO. (VIII) S-nitrosothiols (-SNO) can form after reaction with RNS or GSNO. (IX) This in turn can further react with GSH to yield S-glutathionylation. (X) ROS can also lead to a protein thiyl (-S•) which (XI) upon reaction with GSH yields a disulfide radical anion (-SSG •) (XII) this further reacts with O2 to yield S-glutathionylation.
1.1. S-Glutathionylation
S-glutathionylation is the formation of a mixed disulfide bond between the cysteine of glutathione and the cysteine of a protein. This modification to the protein can lead to enhanced or suppressed activity, discussed below. Similar to phosphorylation, this reversible post-translational modification is dynamic and participates in cell signaling. S-glutathionylation unlike phosphorylation is thought to be limited to one residue (cysteine) and can be directly regulated by the local redox state of the microenvironment, allowing for specific modification of proteins in specific organelles. The utility of S-glutathionylation as a signaling mechanism requires specificity of the signal, determined by the susceptibility of a cysteine to S-glutathionylation, and is influenced by steric and electrochemical effects due to the conformation of the protein.
1.2. Effect of protein conformation
Protein conformation can influence whether a cysteine is S-glutathionylated or not due to steric hindrance affecting solvent accessibility (Fig. 2A). This is exemplified by the protein Titin that has more than one conformation. Titin is a sarcomeric protein that acts like a spring, constantly unfolding and refolding, determining muscle elasticity [21,22]. Titin contains solute inaccessible cysteines that are exposed during the mechanical action present during muscle stretching (physically stretching titin), as Ig domains of the protein unfold and refold [22]. Incubating titin with GSSG leads to S-glutathionylation of these hidden cysteines, which results in weaker refolding of titin, diminishing stiffness of human cardiomyocytes [23]. These effects are reversed when the cells are treated with the reducing agent DTT, leading to decreased glutathionylation and increased muscle stiffness [23], demonstrating the reversibility of this modification.
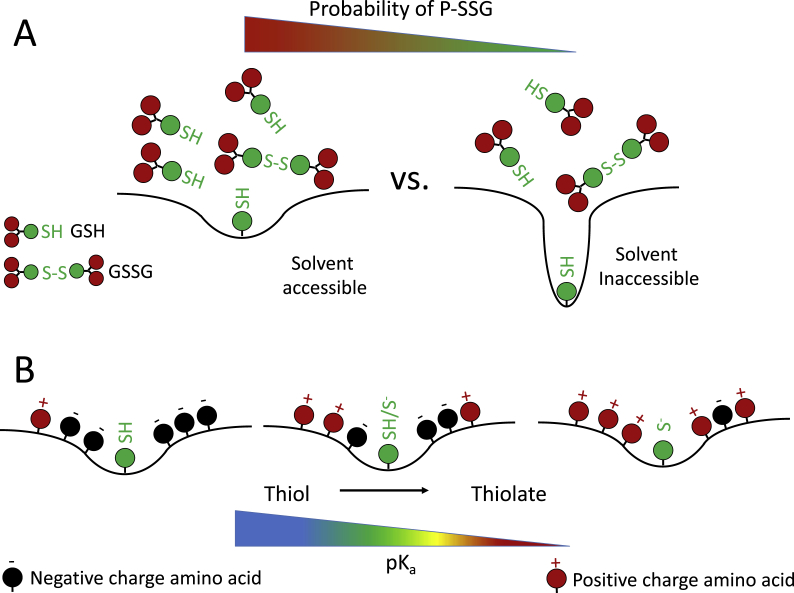
Fig. 2.
Sequence and conformation determine probability of specific cysteine modification. A) GSH is a relatively large molecule, differences in solvent accessibility impact the availability of the cysteine to react with GSH. As the cysteine is less exposed in different proteins the probability of S-glutathionylation decreases. B) Cysteine modifications are more likely with a stable thiolate moiety. Thiolate stability increases with decreasing acid dissociation constant (pKa). Positive residues (Arg, His, Lys) decrease pKa increasing the stability of thiolate.
1.3. Influence of pKa
Amino acid residues in close proximity can also impact the acid dissociation constant (pKa) of cysteine (Fig. 2B) [24]; the majority of free cysteine thiols in the cytoplasm have pKa values above 8, and oxidative modification of these cysteines is unfavorable at the pH and reducing potential of the cytosol [25]. Cysteines that are flanked by positively charged residues (His, Lys, and Arg) [26] have lower pKa values, making them more susceptible to modification [27]. An example of this type of cysteine can be found in the active site of protein tyrosine phosphatases (PTPs) which contain an arginine and cysteine in close proximity. This arrangement of amino acids lowers the pKa of cysteine making it susceptible to oxidative modification [27]. Increases in free radical production such as that seen in cardiovascular disease, lead to an increase in oxidative modifications and S-glutathionylation of the active site cysteine of PTP1B inhibiting its activity [28]. Combined, the conformation and sequence of the cysteine containing domain can regulate how readily the key cysteine is S-glutathionylated. There are exceptions to this pH/pKa rule; one exception is G-actin which has a pKa of 8.5 [29] but is still S-glutathionylated leading to a decrease in polymerization. Exceptions like G-actin suggest the mechanisms governing S-glutathionylation are extremely complex and require more study to fully understand.
1.4. Effect of solvent accessibility
Despite the increasing list of S-glutathionylated proteins, the mechanism by which this modification occurs isn't fully elucidated. As mentioned above the modification of cysteine thiols can proceed through direct redox mediated reactions with oxidants or reductants; modifications can also be catalyzed and/or removed by enzymatic activity, discussed in more detail below. S-glutathionylation can occur non-enzymatically under oxidative conditions where the ratio of GSH:GSSG in the cell approaches 1:1. Under these conditions the increased concentration of GSSG increases the probability of thiol-disulfide exchange between the protein thiol and the disulfide [24]. Although GSH:GSSG ratios are usually much higher in the cytosol, highly oxidizing conditions characterized by drastically decreased GSH:GSSG ratios are normal in the ER [30]. Several studies describe S-glutathionylation of solvent inaccessible cysteines [[31], [32], [33]]. Interestingly, computational analysis of cysteine modifications using pKa and reactivity predictions as well as structural data predict 47% of solvent inaccessible cysteines are susceptible to S-glutathionylation, compared to 53% of solvent accessible cysteines [26]. In the case of Na+/K+-ATPase, increases in duration of hypoxia (a model condition of various cardiovascular disease) increases the S-glutathionylation of solvent inaccessible cysteines [34]. Under physiological conditions this form of S-glutathionylation is likely occurring in the ER during protein folding. The functional role of S-glutathionylation of solvent inaccessible cysteines is not yet clear. Preventing protein disulfides during folding could impart flexibility to proteins akin to the effects of GSSG on titin above [23] and potentially prevent a disulfide between a cysteine participating in redox signaling and a solvent inaccessible cysteine [34].
1.5. Contribution of ROS and RNS
Free thiols on both proteins and GSH are vulnerable to nucleophilic attack by ROS/RNS. These reactions lead to the formation of sulfenic acid (-S-OH) [35,36], a highly reactive and unstable electrophilic moiety, that can undergo further oxidative modifications leading to irreversible changes in the form of sulfinic and sulfonic acids resulting in protein dysfunction and degradation [28,[37], [38], [39], [40], [41]]. GSH is a strong nucleophile and high concentrations lead to reaction with sulfenic acid forming -SSG. PTP1B sulfenic acid can proceed to a sulfenylamide moiety [42,43] that is then reduced through interaction with GSH back to sulfenic acid [43]; this is a proposed intermediate in PTP1B redox regulation.
Cysteines can also react with RNS to form S-nitrosothiols (-SNO). Unlike sulfenic acid S-nitrosothiols are relatively stable in aqueous solutions and can persist for hours. Nitrosative stress can induce S-glutathionylation [[44], [45], [46]], however evidence of direct reaction of S-nitrosothiol with GSH is lacking. S-nitrosoglutathione (GSNO), a known facilitator of S-glutathionylation, is present in the cell at nM concentrations [47]. GSNO reacts with cysteine leading to transnitrosylation followed by disulfide exchange and S-glutathionylation [48]. This reaction could also produce an S-nitrosothiol, and the outcome of this reaction is influenced by the microenvironment of the cysteine residue being modified [49]. Due to the two-step nature of the process a hydrophobic microenvironment is more likely to yield a S-nitrosothiol than S-glutathionylation due to the cysteine being inaccessible to GSH [49].
1.6. Enzymes
Glutaredoxins (Grx) are oxidoreductases, and as members of the thioredoxin superfamily they contain a thioredoxin fold. Within this fold Grxs can have a CXXC active site motif (dithiol Grx) or a CXXS active site motif (monothiol Grx). Dithiol Grxs reduce disulfide bonds by sequential thiol-disulfide exchanges that leave the CXXC motif oxidized to a disulfide. Grx is recycled through reduction by GSH [50]. This reaction to reduce disulfides extends to the removal of S-glutathionylation. Nucleophilic attack can also lead to the formation of thiyl radicals [51,52] which react with GSH and O2 to form P-SSG [53]. This reaction can be enzymatically catalyzed by Grx [54] where the thiyl radical reacts with Grx to form a Grx-SSG intermediate, followed by radical recombination leading to S-glutathionylation and recycling of Grx. This reaction appears to be dependent on local oxidizing conditions since under physiological conditions the S-glutathionylation is reduced.
Glutathione S-transferases (GST) are phase II drug metabolism enzymes that catalyze the conjugation of GSH to electrophilic moieties. While GSTs are well known for their role in xenobiotic detoxification, they also catalyze S-glutathionylation. One class of this enzyme family, GSTπ, has been implicated in S-glutathionylation. Peroxiredoxin-6 requires S-glutathionylation by GSTπ in order to maintain catalytic activity [55]. Furthermore, GSTπ deficiency leads to decreased S-glutathionylation in response to oxidative and nitrosative stress both in vitro and in vivo [56]. Interestingly a member of the Omega class of GST, GSTO1-1 is capable of reducing S-glutathionylation [57]. This capability was attributed to a conserved thioredoxin-like domain.
The enzymatic and non-enzymatic mechanisms of S-glutathionylation are unlikely to be mutually exclusive. Enzymatic signaling appears to be amplified by the local and global redox potential of the cell. Amplification of this process may allow for the protection of redox sensitive cysteines from deleterious oxidation (i.e., preventing irreversible oxidation) while still allowing for redox modulation of physiologic cellular signaling [48,58,59]. One point of interest is the ability of GST to bind conjugated GSH. This property is exploited ubiquitously in the generation of GST tagged recombinant fusion proteins. In this system GSH is immobilized onto a matrix via a sulfide bond and presented to GST in a similar conformation as GSH in S-glutathionylation. Indeed, one method for the detection of S-glutathionylation employs biotinylated GST from Schistosoma japonicum and is shown to be specific to S-glutathionylation [60]. It would be interesting to determine how human isoforms of GST interact with S-glutathionylation. GST is capable of regulating kinase signaling pathways by directly binding kinases [61,62]. In the case of c-Jun N-terminal kinase (JNK; a player in the stress response pathway) neither the catalytic activity nor dimerization of GST was necessary for an inhibitory effect [63], while GSH mimetic drugs that interfere in GST-GSH binding abrogate the inhibitory effect of GSTπ on JNK [[63], [64], [65]]. The presence of this interaction also raises a question in GSTπ deficiency: Is decreased S-glutathionylation due to a lack of GSTπ activity or is it due to GSTπ protection of S-glutathionylation from Grx? Insight may be gleaned from the S-glutathionylation of aldose reductase (AR). AR is an aldo-keto reductase that catalyzes the NADPH dependent reduction of aldehydes and monosaccharides such as the reduction of glucose to sorbitol, it also reduces cytotoxic aldehyde products of lipid peroxidation. AR activity is increased during ischemia due to peroxynitrite induced oxidation of Cys-298 and Cys-303 to sulfenic acid [66,67]. Upon reperfusion these sulfenic acid moieties are S-glutathionylated and AR is inactivated [68]. The reperfusion induced S-glutathionylation and inactivation effect on cardiac AR is absent in GSTπ knockout mice. Grx1 null mice also maintain cardiac AR activity during reperfusion. Interestingly, Grx1 overexpression reverses the effects of ischemia reperfusion, with decreased activity compared to wildtype in ischemia and an increase during reperfusion [68]. In vitro enzymatic assays with recombinant proteins coupled with co-immunoprecipitation elegantly revealed a sequence of peroxynitrite-mediated oxidation of Cys-298 to sulfenic acid followed by GSTπ catalyzed S-glutathionylation and finally Grx1 catalyzed reduction [67,68]. Combined, these experiments demonstrate the dynamic and overlapping nature of thiol modification and enzyme driven S-glutathionylation and deglutathionylation.
1.7. Methods of detection
Detecting S-glutathionylation and isolation of S-glutathionylated proteins can be achieved through exploitation of enzyme interactions, antibody detection, or labeling of the S-glutathionylation. Indirectly S-glutathionylation can be investigated through cysteine labeling with a tag such as biotin or a fluorophore. Some methods are more appropriate for interrogating recombinant or purified proteins while others are appropriate for use in complex mixtures such as tissue and cell homogenates.
Labeling with radioactive 35S was one of the earliest methods described for the detection of S-glutathionylation, indeed the majority of proteins known to be S-glutathionylated were identified using this method. This approach necessitates inhibition of protein synthesis with drugs such as cycloheximide, followed by incubation with 35S cysteine which is incorporated into GSH and ultimately the S-glutathionylation moiety [69]. Proteins can then be identified and quantified using methods such as 2D gel electrophoresis and mass spectrometry. This method can be specific where co-treatment with buthionine sulfoximine (BSO) leads to an 80% decrease of labeled proteins in human T cells [70]. This approach, however, is not free of limitations, one is the increased complication of working with radioactive material and another is the necessity to inhibit protein synthesis to prevent incorporation of cysteine into the proteins. It is also not possible to enrich labeled proteins making the identification of low abundance proteins challenging. A similar approach uses biotinylated glutathione ethyl ester (bioGEE), a cell permeant GSH analog [71]. Once in the cell the bioGEE undergoes hydrolysis by esterases into biotinylated GSH. This labeled GSH is incorporated into the cellular pool of GSH and participates in S-glutathionylation. The newly biotinylated S-glutathionylated proteins can then be enriched using streptavidin. This method addresses two limitations of 35S cysteine, it avoids the use of radioactive material, and allows the enrichment of the S-glutathionylated proteins leading to increased sensitivity of low abundance proteins.
Immune-assay based approaches use a mouse monoclonal antibody raised against S-glutathionylated keyhole limpet hemocyanin. In whole cell extracts, proteins detected by this antibody tend to be highly abundant proteins and detection of other targets requires some degree of enrichment such as immunoprecipitation [72]. Therefore the use of the antibody is not applicable to experiments designed to identify new targets of S-glutathionylation in response to a biological stimulus. The low sensitivity of the antibody may be attributed to the flexible nature of S-glutathionylation [73] and the monovalent affinity of monoclonal antibodies leading to only a subset of conformation/motifs being detected. An interesting alternative to antibodies, briefly mentioned above, employs a recombinant biotinylated GST from Schistosoma japonicum [60]. This method was used to visualize S-glutathionylation on protein blots as well as tissue sections which was abolished by DTT or S-glutathionylated ovalbumin. This method is interesting because it exploits the specificity of GST - GSH interaction, and, due to the promiscuity of GST, is less likely to be affected by conformational changes as a monoclonal antibody. It should be noted however that this method hasn't been benchmarked against any other established S-glutathionylation method.
Removing S-glutathionylation and adding a tag to the newly reduced cysteine residue allows detection and enrichment of S-glutathionylated proteins, but there are pitfalls that researchers should be aware of during experimental design. Initially free sulfhydryl groups are blocked with thiol reactive compounds such as methyl methanethiosulfonate (MMTS), Iodoacetamide (IAA), and most commonly the alkylating agent N-ethyl maleimide (NEM). Once free cysteines are blocked the S-glutathionylated cysteines are reduced back to a sulfhydryl. This step is critical and the choice of reducing agent/method can have significant effect on specificity. A broad reducing agent such as DTT or tris(2-carboxyethyl) phosphine (TCEP) will reduce several cysteine modifications as well as the S-glutathionylation. To circumvent these artifacts and increase assay specificity the catalytic activity of Grx1 [74] can be exploited to specifically remove S-glutathionylation. Following enzymatic digestion, the exposed cysteine is mixed with a label conjugated to a thiol reactive moiety such as maleimide, Pyridyldithiol, and IAA. The latter is commercially available (IodoTMTsixplex™) with isobaric labels allowing identification and quantification of up to six different samples in tandem mass spectrometry. When performed correctly, indirect methods provide increased specificity and resolution in complex protein mixtures. However, several of the reagents used are incompatible with each other and thorough washing/buffer exchange is necessary between steps, this doesn't lend itself to high throughput applications and increases the chances of misrepresentation of proteins that are S-glutathionylated. The methods discussed above each have their distinct limitations, and while they may provide insight into processes being affected by S-glutathionylation a combination of approaches would greatly increase the robustness of data described.
1.8. S-Glutathionylation: cardiovascular physiology
S-glutathionylation occurs at a basal level during normal physiology. This section is dedicated to a brief overview of known aspects of S-glutathionylation in two important organelles as well as nitric oxide synthase. Cellular organelles such as the mitochondria and sarcoplasmic reticulum provide unique environments in the cell where redox potential can be drastically different then in the cytosol allowing for isolated regions of physiological signaling.
1.9. Mitochondria
Under physiological conditions mitochondria account for 10%–15% of the GSH in the cell. The amount of GSH and the GSH:GSSG ratio in the mitochondria is similar to that observed in the cytosol. Despite similarities in concentration and ratio the inner mitochondrial membrane (IMM) is impermeable to GSH and its transit into and out of the mitochondrial matrix requires transport via GSH carriers, predominantly Dicarboxylate (DIC; Slc25a10) and oxoglutarate carrier (OGC, Slc25a11) [75]. This impermeability requires a robust local ability to control GSH:GSSG ratios considering the many sources of ROS production including, the electron transport chain (ETC), pyruvate dehydrogenase (PDH), and α-ketoglutarate dehydrogenase (KGDH) [76]. The mitochondria also contains a myriad of NADPH producing enzymes (required for glutathione reductase activity and maintenance of a physiologic GSH:GSSG) allowing production of NADPH from an array of carbon sources, with nicotinamide nucleotide transhydrogenase (NNT) being the main source of NADPH [77].
Mitochondria contain both the Grx2 and Grx5 members of the glutaredoxin family of enzymes. Grx5 plays a role in iron homeostasis by contributing to iron-sulfur cluster biogenesis [78]. Three isoforms of Grx2 are known Grx2a, Grx2b, and Grx2c, of these Grx2a localizes to the mitochondrial matrix and is expressed in most tissues. Grx2b and Grx2c on the other hand have nuclear and cytosolic localization and are only known to be expressed in the testis and certain cancer cells. Grx2 can deglutathionylate proteins, like most Grxs, but unlike most Grxs it can also S-glutathionylate proteins [79]. The directionality of these reactions appears to be dependent on the GSH:GSSG ratio of the mitochondrial matrix. Grx2 appears to play multiple roles in the cell by directly reducing thioredoxin 1 and 2, as well as forming complexes with iron-sulfur clusters resulting in an inactive homodimer [80]. During oxidative stress this Grx2-iron-sulfur complex dissociates releasing the active Grx2 monomer [80]. The various functions Grx2 performs and its abundance in the mitochondrial matrix make it an important regulator of mitochondrial S-glutathionylation.
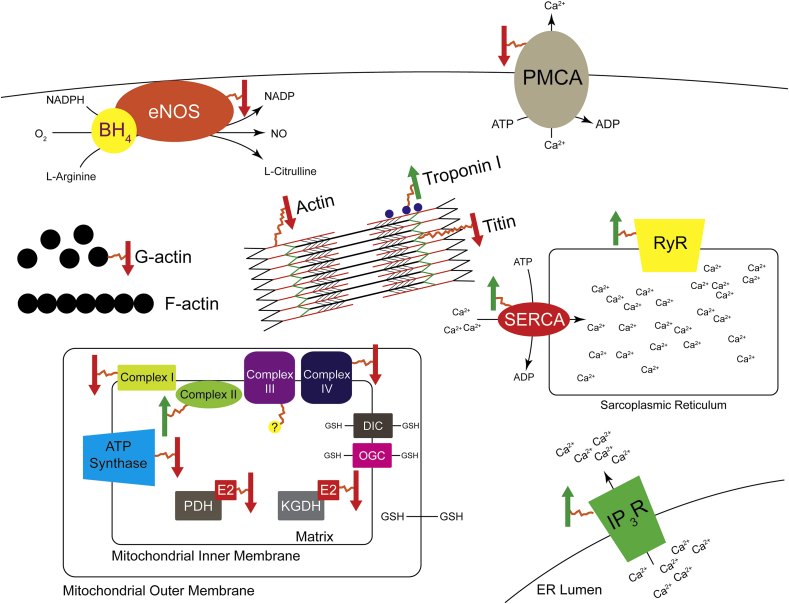
All the complexes of the ETC have at least one subunit that is known to be S-glutathionylated. When the mitochondrial pool of GSH is oxidized Grx2 catalyzes the S-glutathionylation of NADH:ubiquinone oxidoreductase core subunit S1 (NDUFS1) and subunit V1 (NDUFV1) of complex I [79]. The largest subunit of complex I, NDUFS1 is S-glutathionylated on Cys-531 and Cys-704 based on mass spectrometry of bovine cardiac mitochondria [81]. The S-glutathionylation of complex I is associated with a decrease in complex I derived ROS [81,82]. S-glutathionylation of NDUFS1 leads to a decrease in murine mitochondrial ATP production and may be a means of feedback regulation of complex I [82,83] In contrast to complex I, the succinate dehydrogenase complex flavoprotein subunit A (SDHA) in complex II is intrinsically S-glutathionylated at Cys-90 [84]. S-glutathionylation of SDHA protects it from superoxide induced thiyl radical formation and enhances complex II electron transfer efficiency [84]. Proteomic analysis of murine liver identified S-glutathionylation of complexes III and IV [85]. S-glutathionylation of complex IV was associated with a significant decrease in its activity [86]. However, the functional effect of S-glutathionylation on complex III is yet to be elucidated. H2O2 treatment of rat brain mitochondria leads to S-glutathionylation of ATP synthase, and S-glutathionylation of ATP synthase is associated with decreased enzyme activity [87]. Upstream of the ETC, KGDH activity is decreased by S-glutathionylation of its E2 subunit during oxidative stress [[88], [89], [90]]. PDH was also identified as a S-glutathionylated protein in the hepatic mitochondria of Grx2 null mice [83,90]. S-glutathionylation appears to diminish the mitochondria's ability to produce ATP, while this may protect from further ROS production and the triggering of apoptosis, it also creates an energy deficit potentially affecting normal cell, tissue, or even organ function.
1.10. Sarcoplasmic reticulum
The ryanodine receptors (RyR) are large calcium channels primarily present in the sarcoplasmic reticulum (SR) of the skeletal (RyR1) and cardiac muscle (RyR2). They are responsible for excitation-contraction coupling via calcium induced calcium release. Both RyR1 and RyR2 have roughly 400 cysteines of which about 48 are susceptible to modification [[91], [92], [93], [94]]. Not surprisingly both proteins have been reported to be S-glutathionylated [95,96], and RyR2 appears to be basally glutathionylated [95]. Interestingly, while glutathionylation increased calcium release rates in RyR2 [95] this effect wasn't observed in Ryr1. Instead, S-glutathionylation abrogated magnesium induced inhibition of RyR1 [33]. RyR2 S-glutathionylation is induced under physiological conditions during tachycardia and exercise, and is mediated by NADPH oxidase derived ROS [97]. This modification leads to an enhancement in calcium release but also a decrease in calcium leak. Altogether this response is present to protect from arrhythmias by more tightly controlling calcium flux. While RyRs release calcium from the SR, Sarco (endo)plasmic reticulum Ca2+ ATPase (SERCA) actively pumps calcium out of the cytosol back into the SR. There are three SERCA isoforms (SERCA1-3) of which SERCA1 [98] and SERCA2 [40] are known to be S-glutathionylated. Physiological concentrations of NO stimulate peroxynitrite mediated S-glutathionylation of SERCA2 causing an increase in its activity. Mutating Cys-674 prevents both the NO induced S-glutathionylation and increase in SERCA2 activity [40]. Diamide (a strong thiol-oxidizer and inducer of S-glutathionylation) induces calcium oscillation in endothelial cells as well as an increase in cytosolic calcium concentration potentially through S-glutathionylation of the calcium channels inositol-1,4,5-trisphosphate receptor (IP3R) and plasmalemmal Ca2+-ATPase pump (PMCA) [99,100]. This enhanced calcium concentration is fully reversible by dithiothreitol (DTT). As a whole the physiological S-glutathionylation of calcium handling proteins appears to improve function and play a protective role in the cell.
Several sarcomeric proteins have been identified to be S-glutathionylated, these include Titin (mentioned above), actin, myosin, myosin binding protein C, and Troponin. G-actin is basally S-glutathionylated at Cys-374, the removal of which accelerates polymerization and F-actin formation [29]. G-actin S-glutathionylation induces conformational changes with increased exposure of hydrophobic regions of the protein surface [101]. Both α-actin and β-actin are S-glutathionylated. While β-actin can be S-glutathionylated by thiol oxidation and disulfide exchange, α-actin is poorly S-glutathionylated by disulfide exchange and is predominantly S-glutathionylated by thiol oxidation [102,103]. Functionally, S-glutathionylation of F-actin causes a decrease in actomyosin ATPase activity as well as decreased ATP induced myosin detachment leading to a decline in contractility [104]. Isolated myosin is readily S-glutathionylated by GSSG [105], however when organized into a myofilament, actin is predominantly S-glutathionylated both basally and under oxidative conditions [106]. S-glutathionylation of fast troponin I (TnIf) in fast twitch (type II) muscle fibers causes an increased calcium sensitivity in both rats and humans [107]. Consistent with the conformation of TnIf in the troponin complex [108], Cys-134 was identified as the target of S-glutathionylation, furthermore, S-nitrosylation of Cys-134 decreased calcium sensitivity of muscle fibers, suggesting a competitive redox regulation of fast twitch muscle fibers [109].
Matrix metalloproteases (MMP) and calpain are both known to degrade sarcomeric proteins. While calpains are regulated by redox status (previously reviewed [110]) there is no direct evidence of their S-glutathionylation. Okamoto et al. show that proMMPs are activated by S-glutathionylation in a reaction mediated by peroxynitrite. Okamoto et al. further demonstrate that from the possible products of the reaction of peroxynitrite and GSH only GSNO was capable of inducing proMMP S-glutathionylation [111]. Mass spectrometry revealed the cysteine modified was in a well conserved autoinhibitory domain of proMMPS. Surprisingly, they also report resistance of the S-glutathionylation to DTT reduction and propose a (-S(O)SG) moiety [111]. Indirectly, S-glutathionylation of Ras Cys-118 leads to it increased activity and increased mitogen-activated protein kinase (MAPK) pathway [39] which is a potent regulator of MMP2 expression [[112], [113], [114]]. In this case S-glutathionylation appears to drive MMP activity and expression, possibly contributing to disease processes. A visual summary of the signaling mentioned in this section is provided in Fig. 3.
Fig. 3.
Impact of S-glutathionylation on physiological pathways. Arrows denote S-glutathionylated proteins. A green up arrow enhances protein activity and a red down arrow decreases protein effectiveness. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
1.11. Nitric oxide synthase
Nitric oxide synthases (NOS) catalyze the reaction of l-arginine and NADPH to generate citrulline and the gaseous messenger nitric oxide (NO). Endothelial NO is an important regulator of vascular tone, smooth muscle cell proliferation, immune cell adhesion, and platelet aggregation. NO also induces the S-glutathionylation of proteins via S-nitrosylation [67,115]. Endothelial NOS (eNOS) can be S-glutathionylated on Cys-689 and Cys-908 via disulfide exchange with GSSG under oxidative stress, as well as protein thiyl GSH reactions [116]. This S-glutathionylation leads to an uncoupling of eNOS and a concomitant increase in superoxide production. Grx1 plays a dual role in regulating eNOS via S-glutathionylation when the GSH:GSSG ratio is low and deglutathionylation when the GSH:GSSG ratio is increased [117]. Interestingly, Grx1 catalyzes the S-glutathionylation of a unique cysteine (Cys-382) and while it decreases NO synthase activity it doesn't induce uncoupling [117]. S-glutathionylation mediates both angiotensin II [118] and hypoxia reperfusion [119] induced eNOS uncoupling.
2. Cardiovascular pathophysiology
Aging and cardiovascular pathologies have been associated with increased oxidative stress, decreased levels of GSH and increased levels of S-glutathionylated proteins [58,[120], [121], [122], [123], [124], [125]]. GSH redox effects on other diseases and pathological outcomes have been previously reviewed [16,126,127]. In the remainder of this review, we focus specifically on GSH in terms of protein S-glutathionylation within the realm of cardiovascular disease.
2.1. Atherosclerosis
S-glutathionylated protein levels in the serum of patients with Atherosclerosis Obliterans (ASO, a condition with obstruction of peripheral arteries leading to ischemia in lower extremeties) were significantly increased compared to age matched controls [122]. This study demonstrated a positive correlation between disease progression and the amount of S-glutathionylated proteins, one of which was ApoB100, the major component of LDL which may be a risk factor for early detection of peripheral vascular damage [122]. Significantly higher S-glutathionylated proteins were also observed in explanted sclerotic valves in another cardiovascular condition, Aortic Valve Sclerosis characterized, similar to ASO, with markedly lower GSH:GSSG ratios [123]. In vitro experiments within the same study using HUVECs showed that increases in S-glutathionylated proteins using 2-AAPA induced DNA damage affecting endothelial to mesenchymal transition signaling processes.
The functional roles and consequences of S-glutathionylation during atherosclerosis has not been completely elucidated. Oxidized LDL (oxLDL), a primary contributor of atherosclerotic plaques depletes intracellular GSH, inhibits glutathione reductase activity and markedly promotes S-glutathionylation of proteins in human macrophages, major regulatory cells playing a role in atherosclerosis [128] and other cardiovascular pathologies. Recent studies have linked excessive S-glutathionylation of macrophages to cellular dysfunction and a state of chronic inflammation associated with atherosclerosis [129]. S-glutathionylated proteins identified in mouse peritoneal macrophages isolated from healthy and atherosclerotic mice were also identified within metabolically and oxidatively stressed THP-1 monocytes [129]. Vasodilation mediated by S-glutathionylation of SERCA is impaired in atherosclerotic smooth muscle cells [40]. S-glutathionylation and activation of Ras induced by angiotensin II signaling leads to increased phosphorylation of p38 and Akt regulating vascular hypertrophy implicated in atherosclerosis and subsequent hypertension in rat smooth muscle cells [130].
2.2. Myocardial infarction
Increased ROS and RNS is an established sequelae of myocardial infarction (MI) and Ischemia/reperfusion injury (I/R) [131]. MI leads to decreased cardiomyocyte contractility and increased localized inflammation and apoptosis [132]. Consistent with an increased oxidative environment, MI in mice also leads to increased S-glutathionylation [133]. Several proteins involved in the contractile machinery of the heart are susceptible to S-glutathionylation; these modifications can be protective or deleterious to cardiac function depending on the modified protein. Passive myocardial stiffness is increased following MI and is considered a predictor of diastolic dysfunction [134,135]. A determinant of passive myocardial elasticity is the protein titin, which, as described above, is S-glutathionylated in cysteines buried deep within its Ig domain [23]. This leads to decreased refolding of the protein and increased elasticity of human cardiomyocytes [23]. S-glutathionylation of myosin binding protein C improved myofilament response to Ca2+ in failing mouse hearts [136]. In canine hearts S-glutathionylation of cardiac ryanodine receptors was correlated with faster Ca2+ release and these effects were ablated by use of the reducing agent DTT [95]. Similar to smooth muscle cells, S-glutathionylation of murine cardiac SERCA leads to enhanced Ca2+ uptake [32], however, sulfonation of the same cysteine leads to decreased activity [137]. Combined, S-glutathionylation leads to decreased stiffness and faster release of Ca2+ and an improved response to this released Ca2+. In contrast to these adaptive responses S-glutathionylation of G-actin prevents its polymerization into F-actin, which in skeletal muscle is detrimental to contractility [104]. Na+/K+ ATPase is an important determinant of the action potential of cardiomyocytes. Hypoxia induces the S-glutathionylation of the catalytic α subunit of Na+/K+ ATPase in isolated rat ventricular tissue [31]. Angiotensin II activity is enhanced following MI [138] and in the failing heart [139]. While, Angiotensin II induces the S-glutathionylation of the β subunit of Na+/K+ ATPase in rabbit cardiomyocytes [140]. Considering these studies were undertaken in different models it is likely the amount and duration of oxidative challenge varied considerably between studies, it would be particularly interesting to determine the effect of MI or heart failure on these pathways in one model. GSTπ null mice exhibit increased sensitivity to I/R injury with increased infarct size [141], S-glutathionylation however was not a parameter interrogated in this study.
Mechanical strain stimulating the Raf/MEK/ERK pathway was also shown to be dependent upon S-glutathionylation of Ras affecting protein synthesis in cardiac hypertrophy [142]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin have also been identified to be S-glutathionylated in Ischemia Reperfusion (IR) models; both modifications are associated with inhibition of functions [143,144]. In the dog heart dyssynchronous heart failure (DHF) leads to increased S-glutathionylation of Cys-294 of ATP synthase and decreased activity of the enzyme [145]. Treatment with DTT abrogates the effects of DHF on ATP synthase [145].
The repercussions of cardiac mitochondrial S-glutathionylation has been tested using Grx2 knockout and overexpression; Grx2 ablation leads to increased body weight accumulation under a high fat diet with increased insulin resistance compared to wildtype mice [145]. Grx2 knockout mice exhibit increased complex I S-glutathionylation [146]. This was accompanied by decreased cardiac ATP production, left ventricular hypertrophy and fibrosis, and hypertension [146]. Changes in S-glutathionylation status of mitochondrial proteins affecting their functions and thereby the downstream pathological outcomes during IR injury have a potential role in other ischemia related conditions such as peripheral vascular disease (PVD) [147]. One of these modifications, namely S-glutathionylation of mitochondrial complex II enhances associated electron transfer, both of these activities decrease during myocardial IR injury [84], demonstrating the dual roles of S-glutathionylation as a potentiator or as an inhibitor of signaling events.
2.3. Diabetes
Patients with type 2 diabetes mellitus (DMII) have elevated oxidative stress, total GSH [148] and as a result the GSH:GSSG ratio [149] is significantly decreased. The implications of this increased oxidative stress extend to both macro and microangiopathies. The decrease in the GSH:GSSG ratio along with increased oxidative stress increases the likelihood of S-glutathionylation. This is evidenced by the increased S-glutathionylation of hemoglobin (HbSSG) in DMII patient serum [150,151], particularly interesting is that HbSSG is increased in DMII patients with microangiopathy, and has been suggested as a marker of DMII progression [151]. Endothelial cells isolated from DMII patients as well as aorta's from high fat fed ApoE null mice have significantly increased S-glutathionylation [152]. The same study associated metabolic stress and endothelial hyperpermeability to the mechanism of S-glutathionylation and inactivation of Rac1 (RhoGPase). Impaired glucose transport is a classic characteristic of DMII [153]. Glucose starvation induces S-glutathionylation of proteins across most cellular processes including glucose metabolism [154]. Indeed, several proteins involved in the energy production machinery of the cell are targets for S-glutathionylation, recently reviewed by Mailloux, RJ [155]. Plasma GST activity is also elevated in DMII patients [156,157] and appears to correlate with severity of the disease [157]. Mitochondria produce a significant amount of ROS and deactivation of mitochondrial proteins by S-glutathionylation can function as a negative feedback mechanism to prevent excessive oxidative stress [152]. Deregulated S-glutathionylation in DMII is likely to lead to further perturbation of this delicate balance in the mitochondria.
2.4. Hypertension
Hypertension is a prevalent condition that predisposes patients to a myriad of other cardiovascular diseases [158]. Hypertensive patients have significantly lower GSH:GSSG ratios than normotensive patients. This leads to a more oxidative environment and increases the probability of S-glutathionylation [159]. A major regulator of vascular resistant and smooth muscle myogenic tone is nitric oxide (NO) locally produced in the endothelium by endothelial nitric oxide synthase (eNOS) [160]. S-glutathionylation of eNOS by thiol disulfide exchange with GSSG leads to its uncoupling and a shift from NO production to superoxide O2•− [116]. Serine is a redox insensitive residue and Cys to Ser mutation of the 2 S-glutathionylated cysteines led to a S-glutathionylation resistant mutant. GSSG failed to shift the production of the Cys to Ser mutant from NO to O2•−, while S-glutathionylation colocalized with eNOS in spontaneously hypertensive rats [116]. S-glutathionylation induced uncoupling of eNOS also occurs during pre-eclampsia [161]. Angiotensin II (AngII) induces increased myogenic tone in smooth muscle cells and a concomitant increase in blood pressure. This effect is mediated by an increase in ROS and p38 mitogen-activated protein kinase (p38 MAPK) activation [162,163]. S-glutathionylation of the small GTPase Ras is necessary for AngII induced phosphorylation of p38 MAPK as demonstrated by overexpression of a S-glutathionylation resistant Cys to Ser mutant Ras [39]. In contrast to these findings, hydrogen peroxide (H2O2) induced vasodilation is mediated by 4-aminopyridine (4-AP)-sensitive Kv channels [164]. GSSG equally activates these channels potentially through S-glutathionylation [165], while DTT [164] or GR [165] inhibit H2O2 activation of the channels.
2.5. Ischemia induced vascular remodeling
Peripheral Vascular Disease (PVD) affects approximately 8.5 million people in the US, affecting one in every 20 Americans over the age of 50 [166,167]. Between 2000 and 2010 worldwide incidence of PVD increased by 23.5%; these numbers will continue to grow due to the steady increase in our aging population [168]. PVD is characterized by blockage of peripheral arteries due to the formation of plaques that prevent normal blood flow to the limbs [169,170]. Ischemia resulting from this decrease in flow can have serious consequences including pain, poor healing of wounds which can lead to necrosis and amputation of the affected limb or limbs. Development of new blood vessels from preexisting ones, a process known as angiogenesis, is critical to compensate for this blockage by improving blood perfusion, oxygen, and nutrient supply to the ischemic limb and thereby preventing complications [171]. However, PVD patients often lack these important compensatory angiogenic processes and thus, efforts to increase angiogenic revascularization is a primary therapeutic goal [[172], [173], [174]].
Angiogenesis, an integral compensatory mechanism to revascularize ischemic areas and its associated VEGF signaling have increasingly been connected with oxidative stress and GSH redox mechanisms [[175], [176], [177], [178]]. VEGF-A binds to VEGFR2/Flk 1 and the sequential receptor dimerization and phosphorylation of tyrosine residues transduces endothelial angiogenic signals including proliferation, migration, and network formation. These pathways, however, are negatively regulated when VEGF-A alternatively binds to VEGFR1/Flt instead [179,180]. Addition of glutathione adducts has been shown to inactivate protein tyrosine phosphatases (PTP-1B and LMW-PTP) thus promoting VEGF phosphorylation and signaling [181,182]. JAK/STAT (Signal Transducer and Activator of Transcription-STAT phosphorylated by Janus Kinase-JAK) is a signaling pathway activated by VEGF in endothelial cells that leads to the angiogenic responses of cell proliferation, differentiation, and migration and is known to be regulated by S-glutathionylation [183]. S-glutathionylation of STAT3 prevents its phosphorylation, nuclear translocation and DNA binding ability in response to IL-6 stimulation in human HepG2 hepatoma cells [184]. However S-glutathionylation exerted opposing effects in the regulation of STAT3 and STAT1 in mouse microglial cells, where STAT1 phosphorylation and activity were not inhibited [185]. JAK/STAT signaling and regulation by suppressors are important inflammatory responses also implicated in atherosclerotic plaque formation, however the role of S-glutathionylation in this specific regulation is yet to be investigated [186]. Endothelial glutathione can regulate angiogenic signaling through VEGF-A mediated eNOS activity within these cells and affect direct VEGFR2 signaling with significant increases in total protein S-glutathionylation and decreases in GSH:GSSG ratios [187,188]. Sarcoplasmic-endoplasmic reticulum calcium ATPase2b (SERCA2b) activation by S-glutathionylation positively affects VEGF induced signaling and migration [189]. Inflammation pathways associated with angiogenesis have been shown to be regulated by S-glutathionylation, a modification which suppresses the antiangiogenic p65/NFkB/Wnt 5/sFlt pathways [190]. Glutathione adducts on NFkB subunits (IkkB and p50) inhibit activity that is essential for the delicate regulation of the neovascularization processes [191,192]. Master angiogenic transcription regulator Hypoxia Inducible Factor (HIF-1α) controls VEGF production and is stabilized by S-glutathionylation leading to enhanced function and increased revascularization in ischemic muscles [193,194]. Hypoxia has been shown to increase S-glutathionylation of HIF-1α and its protein levels in colon cancer cells [194]. Grxs mediates removal of S-glutathionylation, hence transgenic overexpression of Grx shows impaired ischemic revascularization while knockouts demonstrate an enhancement of these processes [190,193].
Macrophages are involved in critical angiogenic processes from activation, migration, and proliferation of endothelial cells, basement membrane interaction and breakdown, to formation of new vascular sprouts [195,196]. Protein S-glutathionylation mechanisms within macrophages have been shown to contribute as physiologically important redox signaling mechanisms, also potentially contributing to atherosclerosis [197,198]. Additionally, macrophage functions including receptor expression, uptake, phagocytic activity and cytokine production have all been shown to be affected by cellular GSH [[199], [200], [201]]. Recent evidence links glutathione regulation to macrophage polarization during murine in vivo infection and in vitro activation [202,203]. Polarized macrophages have been shown to influence angiogenesis, improving blood reperfusion when injected directly into ischemic tissues, an effect that is characterized by enhanced VEGF production [[204], [205], [206], [207], [208]]. Matrix Metalloproteinases (MMPs) contribute to physiological and pathological regulation of extracellular matrices with major implications in the development and progression of atherosclerosis [209,210], vascular basement membrane degradation and angiogenic remodeling [211]. MMP precursors or Pro-MMPs isolated from human neutrophils are activated by peroxynitrite and GSH possibly through a resulting S-glutathionylation [111] event. Neutrophil-associated α4 integrin increases its binding to endothelial cells adhesion molecule 1 (VCAM1) when modified by S-glutathionylation [212]. Endothelial cell adhesion molecules such as VCAM-1 facilitate activation of endothelial cells, interaction with circulating immune cells and mediate leukocyte transmigration important for inflammatory atherogenic and angiogenic processes [[213], [214], [215]].
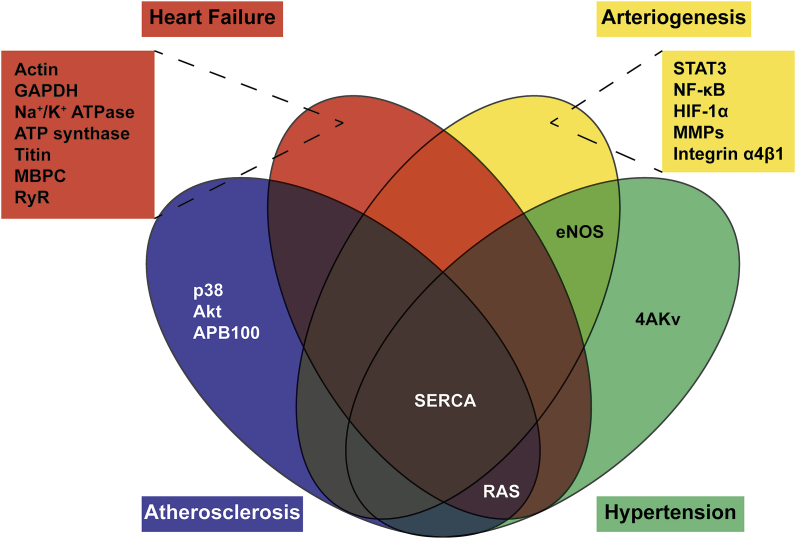
Efficient revascularization post ischemia also depends on another important vascular remodeling process, arteriogenesis, which is the development of pre-existing collateral vessels caused by changes in fluid shear stress [216,217]. Hypoxia triggers HIF-1α/VEGF signaling possibly affecting arteriogenesis as this process and angiogenesis are intricately linked, although direct effects of this signaling axis have been debated [[218], [219], [220]]. Endothelial activation in collateral vessel walls due to increasing fluid shear stress gives rise to inflammatory processes leading to arteriogenesis, it stands to reason that the similarities between angiogenesis and arteriogenesis do not stop at a recruitment of the inflammatory process. More than likely there is similar regulation through S-glutathionylation within endothelial cells, participating smooth muscle cells, and circulating monocytes that contribute to arteriogenesis [221,222]. The role of protein S-glutathionylation in other major endothelial signaling pathways has been extensively summarized in a recent review by Lermant, A., et al. [223]. The major proteins discussed in this section are summarized in Table 1 and associated diseases are represented by the Venn diagram (Fig. 4).
Table 1.
Table of S-glutathionylated proteins in atherogenic and angiogenic associated processes.
| PROTEIN | SPECIFIC PR-SSG EFFECTS ON PROTEIN FUNCTION | DETECTION METHOD | REF |
|---|---|---|---|
| APOB100 | lipid protein Apolipoprotein B, implicated in the development of atherosclerotic lesions show increased serum glutathionylated levels in ASO (Atherosclerosis Obliterans) patients. | Biotinylated GST overlay (protein blot) | [122] |
| SERCA | Ca2+ ATPase pump Sarcoplasmic-endoplasmic reticulum calcium ATPase- SSG leads to its activation regulating vasodilation in atherosclerotic smooth muscles. | Biotin iodoacetamide, Biotin GEE (protein blot) | [40] |
| SERCA2B | Sarcoplasmic-endoplasmic reticulum calcium ATPase2b-SSG leads to increases in VEGF induced signaling and migration. | Glutathione antibody, (Western blot) | [18]9 |
| RAS | GTPase Ras-SSG leads to increased phosphorylation of p38 and Akt regulating vascular hypertrophy implicated in atherosclerosis and hypertension. Ras-SSG also induces Raf/MEK/ERK pathway activation. | Biotin GEE (Western blot) | [39,142] |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase-SSG inhibits its function during cardiac oxidative stress in Ischemia Reperfusion models. | Biotin GSH, Western blot | [143] |
| ACTIN | Cytoskeletal proteins G-actin and F-actin -SSG decreases polymerization efficiency and binding with tropomyosin respectively affecting contractility during Ischemia Reperfusion. | Glutathione antibody, Western blot | [144] |
| SQR | Mitochondrial respiratory chain enzyme Succinate ubiquinone reductase or complex II-SSG leads to enhanced SQR-derived electron transfer efficiency. | Glutathione antibody, Western blot | [84] |
| LMW-PTP | Low molecular weight protein tyrosine phosphatase is inactivated by Pr-SSG, inhibiting VEGF mediated angiogenic migration. | Glutathione antibody, Western blot | [181] |
| PTP-1B | Protein tyrosine phosphatases-SSG inhibits activity that can effect VEGF mediated angiogenic responses. | LC-MS/MS | [28] |
| IKK-Β | Inhibitory kβ kinase (IKK) β-SSG leads to its inactivation and resulting inhibition of NFkB activity affecting inflammatory responses. | Biotin GEE (protein blot) | [191] |
| STAT-3 | Transcription factor Signal Transducer and Activator of Transcription 3-SSG prevents phosphorylation, nuclear translocation and DNA binding. | Derivitization with NEM, DTT, and Biotin Pyridyldisulfide | [184] |
| P65 | Transcription factor NF-kB subunit p65-SSG inhibits NF-kB activity responsible for Wnt5a-sFlt activation leading to upregulation of VEGF mediated endothelial cell migration. | Derivitization with NEM, DTT, and Biotin Pyridyldisulfide | [190] |
| P50 | Transcription factor NF-κB subunit p50-SSG inhibits NF-κB activity and gene expression. | In vitro labelling of recombinant proteins with 3H‐GSH (scintillation and mass spectrometery) | [192] |
| HIF-1Α | Transcription factor Hypoxia inducible Factor 1α-SSG, stabilizes its activity leading to VEGF associated increased revascularization in ischemic muscles. | Glutathione antibody, Western blot Derivitization with NEM, DTT, and Biotin iodoacetamide |
[193] |
| PRO-MMPS | Matrix Metalloproteinase precursors are activated by glutathionylation induced by peroxynitrite and GSH treatment. | 35S-GSH labelling of purified protein (PAGE and mass spectrometry) | [111] |
| Α4 INTEGRIN | Transmembrane receptors α4 integrin-SSG increases binding of neutrophils to endothelial cells adhesion molecule 1 (VCAM1). | Biotin GEE, glutathione antibody (protein blot) | [212] |
Fig. 4.
Venn diagram representing known S-glutathionylated targets and the diseases associated with the modification.
3. Conclusion
The subtleties of S-glutathionylation and its seemingly ubiquitous regulation of various signaling paradigms make it a fascinating PTM to study. In this review we have demonstrated S-glutathionylation as a major regulator of both cardiovascular pathologies (MI, heart failure, DMII, PVD) and in physiological signaling (angiogenesis and arteriogenesis). To date there is evidence of S-glutathionylation influencing all aspects of the cellular machinery from transcription to apoptosis. The many redundancies of S-glutathionylation signaling provides a cellular safety factor where the protein modification can protect proteins from irreversible oxidation or promote activation of a protein or even dampen protein activity. There is a consensus agreement in the literature that glutathionylation of proteins is detrimental to signaling during cardiovascular pathologies. Acutely there may be signaling modifications to rescue a normal phenotype but in a chronic setting glutathionylation appears to contribute to dysfunction. Unfortunately, certain gaps in knowledge preclude our ability to accurately label glutathionylation as good or bad during disease. One glaring gap in the literature is the inclusion of glutathionylated protein to non-glutathionylated protein, more specifically, a direct comparison of physiological concentrations of a particular glutathionylated protein compared to pathological concentrations of that same glutathionylated protein. The distinction provided with that type of knowledge is important as many of the experiments performed looking at S-glutathionylation occur under in vitro conditions where the redox environment doesn't necessarily recapitulate the physiological milieu. This field is progressing rapidly and as it does our understanding of this intricate balance between S-glutathionylation and disease prognosis is ever expanding.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by NIH R01 HL139755 awarded to Christopher B. Pattillo.
References
- 1.Derey-Pailhade J. Sur un corps d'origine organique hydrogenant le soufre a froid. C. R. Acad. Sci. 1888;106:1683–1684. [Google Scholar]
- 2.De Rey-Pailhade J. echerches expérimentales pour expliquer l'absorption du source introduce par la voie gastro-intestinale. Bulletin de la Société d'histoire naturelle de Toulouse. 1886;116–129 [Google Scholar]
- 3.Rey-Pailhade J.d. Sur la formation de l’hydrogène sulfuré dans l’organisme: à la suite de l’ingestion de quelques médicaments. Bulletin de la Société d’histoire naturelle de Toulouse. 1885;23 [Google Scholar]
- 4.Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 5.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Montero D., Tachibana C., Rahr Winther J., Appenzeller-Herzog C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biology. 2013;1:508–513. doi: 10.1016/j.redox.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren A., Sengupta R. The use of thiols by ribonucleotide reductase. Free Radic. Biol. Med. 2010;49:1617–1628. doi: 10.1016/j.freeradbiomed.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Ribas V., García-Ruiz C., Fernández-Checa J.C. Glutathione and mitochondria. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00151. 151-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur J. The glutathione peroxidases. Cell. Mol. Life Sci. 2001;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 11.Jacob C., Giles G.I., Giles N.M., Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 12.Pallardó F.V., Markovic J., Viña J. Cellular compartmentalization of glutathione. Glutathione and Sulfur Amino Acids in Human Health and Disease. 2009:35–45. [Google Scholar]
- 13.Anderson M.E. Glutathione: an overview of biosynthesis and modulation. Chem. Biol. Interact. 1998;111:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 14.Harding J.J., Blakytny R., Ganea E. Glutathione in disease. Biochem. Soc. Trans. 1996;24:881–884. doi: 10.1042/bst0240881. [DOI] [PubMed] [Google Scholar]
- 15.Smith C.V., Jones D.P., Guenthner T.M., Lash L.H., Lauterburg B.H. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P. Protein glutathionylation in health and disease. Biochim. Biophys. Acta. 2013;1830:3165–3172. doi: 10.1016/j.bbagen.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Dixon B.M., Heath S.-H.D., Kim R., Suh J.H., Hagen T.M. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxidants Redox Signal. 2008;10:963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatahet F., Ruddock L.W. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxidants Redox Signal. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 19.Chung H.S., Wang S.-B., Venkatraman V., Murray C.I., Van Eyk J.E. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ. Res. 2013;112:382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H., Zhang X., Chen X., Aramsangtienchai P., Tong Z., Lin H. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 2018;118:919–988. doi: 10.1021/acs.chemrev.6b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linke W.A., Krüger M. The giant protein titin as an integrator of myocyte signaling pathways. Physiology. 2010;25:186–198. doi: 10.1152/physiol.00005.2010. [DOI] [PubMed] [Google Scholar]
- 22.Kellermayer M.S., Grama L. Mechanics of Elastic Biomolecules: Springer; 2003. Stretching and Visualizing Titin Molecules: Combining Structure, Dynamics and Mechanics; pp. 499–511. [DOI] [PubMed] [Google Scholar]
- 23.Alegre-Cebollada J., Kosuri P., Giganti D., Eckels E., Rivas-Pardo Jaime A., Hamdani N., Warren Chad M., Solaro R.J., Linke Wolfgang A., Fernández Julio M. S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding. Cell. 2014;156:1235–1246. doi: 10.1016/j.cell.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalle–Donne I., Milzani A., Gagliano N., Colombo R., Giustarini D., Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxidants Redox Signal. 2008;10:445–474. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.R., Yoon H.W., Kwon K.S., Lee S.R., Rhee S.G. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal. Biochem. 2000;283:214–221. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 26.Fowler N.J., Blanford C.F., de Visser S.P., Warwicker J. Features of reactive cysteines discovered through computation: from kinase inhibition to enrichment around protein degrons. Sci. Rep. 2017;7:16338. doi: 10.1038/s41598-017-15997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee S.G., Bae Y.S., Lee S.-R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE. 2000 Oct 10;2000(53):pe1. doi: 10.1126/stke.2000.53.pe1. PMID: 11752613. [DOI] [PubMed] [Google Scholar]
- 28.Barrett W.C., DeGnore J.P., Keng Y.-F., Zhang Z.-Y., Yim M.B., Chock P.B. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J. Biol. Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Boja E.S., Tan W., Tekle E., Fales H.M., English S., Mieyal J.J., Chock P.B. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 30.Rashdan N.A., Pattillo C.B. Hydrogen peroxide in the ER: a tale of triage. Redox biology. 2020;28 doi: 10.1016/j.redox.2019.101358. 101358-101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrushanko I.Y., Yakushev S., Mitkevich V.A., Kamanina Y.V., Ziganshin R.H., Meng X., Anashkina A.A., Makhro A., Lopina O.D., Gassmann M., Makarov A.A., Bogdanova A. S-glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012;287:32195–32205. doi: 10.1074/jbc.M112.391094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancel S., Zhang J., Evangelista A., Trucillo M.P., Tong X., Siwik D.A., Cohen R.A., Colucci W.S. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ. Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aracena-Parks P., Goonasekera S.A., Gilman C.P., Dirksen R.T., Hidalgo C., Hamilton S.L. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 34.Mitkevich V.A., Petrushanko I.Y., Poluektov Y.M., Burnysheva K.M., Lakunina V.A., Anashkina A.A., Makarov A.A. Basal glutathionylation of Na, K-ATPase α-subunit depends on redox status of cells during the enzyme biosynthesis. Oxidative medicine and cellular longevity. 2016;2016:9092328. doi: 10.1155/2016/9092328. Epub 2016 Apr 27. PMID: 27239254; PMCID: PMC4863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang K.-P., Huang F.L. Glutathionylation of proteins by glutathione disulfide S-oxide. Biochem. Pharmacol. 2002;64:1049–1056. doi: 10.1016/s0006-2952(02)01175-9. [DOI] [PubMed] [Google Scholar]
- 36.Poole L.B., Karplus P.A., Claiborne A. Protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 37.Clavreul N., Adachi T., Pimental D.R., Ido Y., Schöneich C., Cohen R.A. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. Faseb. J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 38.Ying J., Tong X., Pimentel D.R., Weisbrod R.M., Trucillo M.P., Adachi T., Cohen R.A. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2007;27:783–790. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 39.Adachi T., Pimentel D.R., Heibeck T., Hou X., Lee Y.J., Jiang B., Ido Y., Cohen R.A. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 40.Adachi T., Weisbrod R.M., Pimentel D.R., Ying J., Sharov V.S., Schöneich C., Cohen R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 41.Cohen R.A., Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc. Med. 2006;16:109–114. doi: 10.1016/j.tcm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 42.van Montfort R.L., Congreve M., Tisi D., Carr R., Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 43.Salmeen A., Andersen J.N., Myers M.P., Meng T.-C., Hinks J.A., Tonks N.K., Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Ruiz A., Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc. Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Uys J.D., Xiong Y., Townsend D.M. Nitrosative stress-induced S-glutathionylation of protein disulfide isomerase. Methods Enzymol.: Elsevier. 2011:321–332. doi: 10.1016/B978-0-12-385114-7.00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend D.M., Manevich Y., He L., Xiong Y., Bowers R.R., Hutchens S., Tew K.D. Nitrosative stress–induced S-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Canc. Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaston B., Reilly J., Drazen J.M., Fackler J., Ramdev P., Arnelle D., Mullins M.E., Sugarbaker D.J., Chee C., Singel D.J., Loscalzo J., Stamler J.S. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. Unit. States Am. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Y., Uys J.D., Tew K.D., Townsend D.M. S-glutathionylation: From molecular mechanisms to health outcomes. Antioxidants Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giustarini D., Milzani A., Aldini G., Carini M., Rossi R., Dalle-Donne I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxidants Redox Signal. 2005;7:930–939. doi: 10.1089/ars.2005.7.930. [DOI] [PubMed] [Google Scholar]
- 50.Ukuwela A.A., Bush A.I., Wedd A.G., Xiao Z. Glutaredoxins employ parallel monothiol-dithiol mechanisms to catalyze thiol-disulfide exchanges with protein disulfides. Chem. Sci. 2017;9:1173–1183. doi: 10.1039/c7sc04416j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karoui H., Hogg N., Fréjaville C., Tordo P., Kalyanaraman B. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite ESR-spin trapping and oxygen uptake studies. J. Biol. Chem. 1996;271:6000–6009. doi: 10.1074/jbc.271.11.6000. [DOI] [PubMed] [Google Scholar]
- 52.Chen C.-A., Lin C.-H., Druhan L.J., Wang T.-Y., Chen Y.-R., Zweier J.L. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J. Biol. Chem. 2011;286:29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mailloux R.J., McBride S.L., Harper M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013;38:592–602. doi: 10.1016/j.tibs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Starke D.W., Chock P.B., Mieyal J.J. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase) POTENTIAL ROLE IN REDOX SIGNAL TRANSDUCTION. J. Biol. Chem. 2003;278:14607–14613. doi: 10.1074/jbc.M210434200. [DOI] [PubMed] [Google Scholar]
- 55.Manevich Y., Feinstein S.I., Fisher A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with πGST. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Townsend D.M., Manevich Y., He L., Hutchens S., Pazoles C.J., Tew K.D. Novel role for glutathione S-transferase π regulator of protein S-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menon D., Board P.G. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J. Biol. Chem. 2013;288:25769–25779. doi: 10.1074/jbc.M113.487785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastore A., Piemonte F. Protein glutathionylation in cardiovascular diseases. Int. J. Mol. Sci. 2013;14:20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Cheng G., Ikeda Y., Iuchi Y., Fujii J. Detection of S-glutathionylated proteins by glutathione S-transferase overlay. Arch. Biochem. Biophys. 2005;435:42–49. doi: 10.1016/j.abb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J., Grek C., Ye Z.-W., Manevich Y., Tew K.D., Townsend D.M. Pleiotropic functions of glutathione S-transferase P. Adv. Canc. Res. 2014;122:143–175. doi: 10.1016/B978-0-12-420117-0.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anselmo A.N., Cobb M.H. Protein kinase function and glutathionylation. Biochem. J. 2004;381:e1–e2. doi: 10.1042/BJ20040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adler V., Yin Z., Fuchs S.Y., Benezra M., Rosario L., Tew K.D., Pincus M.R., Sardana M., Henderson C.J., Wolf C.R., Davis R.J., Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Luca A., Federici L., De Canio M., Stella L., Caccuri A.M. New insights into the mechanism of JNK1 inhibition by glutathione transferase P1-1. Biochemistry. 2012;51:7304–7312. doi: 10.1021/bi300559m. [DOI] [PubMed] [Google Scholar]
- 65.Ruscoe J.E., Rosario L.A., Wang T., Gate L., Arifoglu P., Wolf C.R., Henderson C.J., Ronai Z., Tew K.D. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J. Pharmacol. Exp. Therapeut. 2001;298:339–345. [PubMed] [Google Scholar]
- 66.Kaiserova K., Srivastava S., Hoetker J.D., Awe S.O., Tang X.-L., Cai J., Bhatnagar A. Redox activation of aldose reductase in the ischemic heart. J. Biol. Chem. 2006;281:15110–15120. doi: 10.1074/jbc.M600837200. [DOI] [PubMed] [Google Scholar]
- 67.Kaiserova K., Tang X.-L., Srivastava S., Bhatnagar A. Role of nitric oxide in regulating aldose reductase activation in the ischemic heart. J. Biol. Chem. 2008;283:9101–9112. doi: 10.1074/jbc.M709671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wetzelberger K., Baba S.P., Thirunavukkarasu M., Ho Y.-S., Maulik N., Barski O.A., Conklin D.J., Bhatnagar A. Postischemic deactivation of cardiac aldose reductase: role OF glutathione S-transferase P and glutaredoxin IN regeneration OF reduced thiols from sulfenic acids. J. Biol. Chem. 2010;285:26135–26148. doi: 10.1074/jbc.M110.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao X.-H., Bedhomme M., Veyel D., Zaffagnini M., Lemaire S.D. Methods for analysis of protein glutathionylation and their application to photosynthetic organisms. Mol. Plant. 2009;2:218–235. doi: 10.1093/mp/ssn072. [DOI] [PubMed] [Google Scholar]
- 70.Fratelli M., Demol H., Puype M., Casagrande S., Eberini I., Salmona M., Bonetto V., Mengozzi M., Duffieux F., Miclet E. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan D.M., Wehr N.B., Fergusson M.M., Levine R.L., Finkel T. Identification of oxidant-sensitive Proteins: TNF-α induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 72.Poerschke R.L., Fritz K.S., Franklin C.C. Methods to detect protein glutathionylation. Current Protocols in Toxicology. 2013;57:6. doi: 10.1002/0471140856.tx0617s57. 17.11-16.17.18. [DOI] [PubMed] [Google Scholar]
- 73.Lampela O., Juffer A.H., Rauk A. Conformational analysis of glutathione in aqueous solution with molecular dynamics. J. Phys. Chem. 2003;107:9208–9220. [Google Scholar]
- 74.Lind C., Gerdes R., Hamnell Y., Schuppe-Koistinen I., von Löwenhielm H.B., Holmgren A., Cotgreave I.A. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch. Biochem. Biophys. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 75.Lash L.H. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young A., Gill R., Mailloux R.J. Protein S-glutathionylation: the linchpin for the transmission of regulatory information on redox buffering capacity in mitochondria. Chem. Biol. Interact. 2019;299:151–162. doi: 10.1016/j.cbi.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Padayatti P.S., Leung J.H. Proton-translocating nicotinamide nucleotide transhydrogenase: a structural perspective. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wingert R.A., Galloway J.L., Barut B., Foott H., Fraenkel P., Axe J.L., Weber G.J., Dooley K., Davidson A.J., Schmidt B., Paw B.H., Shaw G.C., Kingsley P., Palis J., Schubert H., Chen O., Kaplan J., Zon L.I., The Tübingen Screen C. Deficiency of glutaredoxin 5 reveals Fe–S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 79.Beer S.M., Taylor E.R., Brown S.E., Dahm C.C., Costa N.J., Runswick M.J., Murphy M.P. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 80.Qi W., Cowan J.A. Mechanism of glutaredoxin—ISU [2Fe–2S] cluster exchange. Chem. Commun. 2011;47:4989–4991. doi: 10.1039/c0cc05079b. [DOI] [PubMed] [Google Scholar]
- 81.Hurd T.R., Requejo R., Filipovska A., Brown S., Prime T.A., Robinson A.J., Fearnley I.M., Murphy M.P. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J. Biol. Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gill R.M., O'Brien M., Young A., Gardiner D., Mailloux R.J. Protein S-glutathionylation lowers superoxide/hydrogen peroxide release from skeletal muscle mitochondria through modification of complex I and inhibition of pyruvate uptake. PloS One. 2018;13 doi: 10.1371/journal.pone.0192801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalker J., Gardiner D., Kuksal N., Mailloux R.J. Characterization of the impact of glutaredoxin-2 (GRX2) deficiency on superoxide/hydrogen peroxide release from cardiac and liver mitochondria. Redox Biology. 2018;15:216–227. doi: 10.1016/j.redox.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y.R., Chen C.L., Pfeiffer D.R., Zweier J.L. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J. Biol. Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 85.McGarry D.J., Chen W., Chakravarty P., Lamont D.L., Wolf C.R., Henderson C.J. Proteome-wide identification and quantification of S-glutathionylation targets in mouse liver. Biochem. J. 2015;469:25–32. doi: 10.1042/BJ20141256. [DOI] [PubMed] [Google Scholar]
- 86.Wu H., Yu Y., David L., Ho Y.-S., Lou M.F. Glutaredoxin 2 (Grx2) gene deletion induces early onset of age-dependent cataracts in mice. J. Biol. Chem. 2014;289:36125–36139. doi: 10.1074/jbc.M114.620047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia J., Han D., Sancheti H., Yap L.-P., Kaplowitz N., Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J. Biol. Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLain A.L., Cormier P.J., Kinter M., Szweda L.I. Glutathionylation of α-ketoglutarate dehydrogenase: the chemical nature and relative susceptibility of the cofactor lipoic acid to modification. Free Radical Biol. Med. 2013;61:161–169. doi: 10.1016/j.freeradbiomed.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Applegate M.A., Humphries K.M., Szweda L.I. Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 90.O'Brien M., Chalker J., Slade L., Gardiner D., Mailloux R.J. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free Radic. Biol. Med. 2017;106:302–314. doi: 10.1016/j.freeradbiomed.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 91.Sun J., Xu L., Eu J.P., Stamler J.S., Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J. Biol. Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 92.Xu L., Eu J.P., Meissner G., Stamler J.S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 93.Hidalgo C., Bull R., Behrens M.I., Donoso P. Redox regulation of RyR-mediated Ca2+ release in muscle and neurons. Biol. Res. 2004;37:539–552. doi: 10.4067/s0716-97602004000400007. [DOI] [PubMed] [Google Scholar]
- 94.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–628. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Sánchez G., Pedrozo Z., Domenech R.J., Hidalgo C., Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J. Mol. Cell. Cardiol. 2005;39:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Aracena P., Sánchez G., Donoso P., Hamilton S.L. Hidalgo, C. S-glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J. Biol. Chem. 2003;278:42927–42935. doi: 10.1074/jbc.M306969200. [DOI] [PubMed] [Google Scholar]
- 97.Sánchez G., Escobar M., Pedrozo Z., Macho P., Domenech R., Härtel S., Hidalgo C., Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc. Res. 2007;77:380–386. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- 98.Viner R.I., Williams T.D., Schöneich C. Peroxynitrite modification of protein Thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- 99.Lock J.T., Sinkins W.G., Schilling W.P. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H493–H506. doi: 10.1152/ajpheart.01073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lock J.T., Sinkins W.G., Schilling W.P. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J. Physiol. 2012;590:3431–3447. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dalle-Donne I., Giustarini D., Rossi R., Colombo R., Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic. Biol. Med. 2003;34:23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 102.Johansson M., Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. Actin S-glutathionylation: evidence against a thiol-disulphide exchange mechanism. Free Radic. Biol. Med. 2003;35:1185–1193. doi: 10.1016/s0891-5849(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 104.Chen F.C., Ogut O. Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am. J. Physiol. Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- 105.Passarelli C., Petrini S., Pastore A., Bonetto V., Sale P., Gaeta L.M., Tozzi G., Bertini E., Canepari M., Rossi R. Myosin as a potential redox-sensor: an in vitro study. J. Muscle Res. Cell Motil. 2008;29:119–126. doi: 10.1007/s10974-008-9145-x. [DOI] [PubMed] [Google Scholar]
- 106.Passarelli C., Di Venere A., Piroddi N., Pastore A., Scellini B., Tesi C., Petrini S., Sale P., Bertini E., Poggesi C., Piemonte F. Susceptibility of isolated myofibrils to in vitro glutathionylation: potential relevance to muscle functions. Cytoskeleton. 2010;67:81–89. doi: 10.1002/cm.20425. [DOI] [PubMed] [Google Scholar]
- 107.Mollica J.P., Dutka T.L., Merry T.L., Lamboley C.R., McConell G.K., McKenna M.J., Murphy R.M., Lamb G.D. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012;590:1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chong P.C., Hodges R.S. Proximity of sulfhydryl groups to the sites of interaction between components of the troponin complex from rabbit skeletal muscle. J. Biol. Chem. 1982;257:2549–2555. [PubMed] [Google Scholar]
- 109.Dutka T.L., Mollica J.P., Lamboley C.R., Weerakkody V.C., Greening D.W., Posterino G.S., Murphy R.M., Lamb G.D. S-nitrosylation and S-glutathionylation of Cys 134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol. Cell Physiol. 2017;312:C316–C327. doi: 10.1152/ajpcell.00334.2016. [DOI] [PubMed] [Google Scholar]
- 110.Randriamboavonjy V., Kyselova A., Fleming I. Redox regulation of calpains: consequences on vascular function. Antioxidants Redox Signal. 2019;30:1011–1026. doi: 10.1089/ars.2018.7607. [DOI] [PubMed] [Google Scholar]
- 111.Okamoto T., Akaike T., Sawa T., Miyamoto Y., van der Vliet A., Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 112.Liu E., Thant A.A., Kikkawa F., Kurata H., Tanaka S., Nawa A., Mizutani S., Matsuda S., Hanafusa H., Hamaguchi M. The ras-mitogen-activated protein kinase pathway is critical for the activation of matrix metalloproteinase secretion and the invasiveness in v-<em>crk</em>-transformed 3Y1. Canc. Res. 2000;60:2361–2364. [PubMed] [Google Scholar]
- 113.Thant A.A., Sein T.T., Liu E., Machida K., Kikkawa F., Koike T., Seiki M., Matsuda S., Hamaguchi M. Ras pathway is required for the activation of MMP-2 secretion and for the invasion of src-transformed 3Y1. Oncogene. 1999;18:6555–6563. doi: 10.1038/sj.onc.1203049. [DOI] [PubMed] [Google Scholar]
- 114.Liao J., Wolfman J.C., Wolfman A. K-ras regulates the steady-state expression of matrix metalloproteinase 2 in fibroblasts. J. Biol. Chem. 2003;278:31871–31878. doi: 10.1074/jbc.M301931200. [DOI] [PubMed] [Google Scholar]
- 115.Mohr S., Hallak H., de Boitte A., Lapetina E.G., Brüne B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1999;274:9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 116.Chen C.-A., Wang T.-Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A.H., Chen Y.-R., Druhan L.J., Zweier J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen C.A., De Pascali F., Basye A., Hemann C., Zweier J.L. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry. 2013;52:6712–6723. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galougahi K.K., Liu C.C., Gentile C., Kok C., Nunez A., Garcia A., Fry N.A., Davies M.J., Hawkins C.L., Rasmussen H.H., Figtree G.A. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Pascali F., Hemann C., Samons K., Chen C.A., Zweier J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry. 2014;53:3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lang C.A., Mills B.J., Mastropaolo W., Liu M.C. Blood glutathione decreases in chronic diseases. J. Lab. Clin. Med. 2000;135:402–405. doi: 10.1067/mlc.2000.105977. [DOI] [PubMed] [Google Scholar]
- 121.Julius M., Lang C.A., Gleiberman L., Harburg E., DiFranceisco W., Schork A. Glutathione and morbidity in a community-based sample of elderly. J. Clin. Epidemiol. 1994;47:1021–1026. doi: 10.1016/0895-4356(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 122.Nonaka K., Kume N., Urata Y., Seto S., Kohno T., Honda S., Ikeda S., Muroya T., Ikeda Y., Ihara Y., Kita T., Kondo T. Serum levels of S-glutathionylated proteins as a risk-marker for arteriosclerosis obliterans. Circ. J. 2007;71:100–105. doi: 10.1253/circj.71.100. [DOI] [PubMed] [Google Scholar]
- 123.Valerio V., Myasoedova V.A., Moschetta D., Porro B., Perrucci G.L., Cavalca V., Cavallotti L., Songia P., Poggio P. Impact of oxidative stress and protein S-glutathionylation in aortic valve Sclerosis patients with overt atherosclerosis. J. Clin. Med. 2019;8 doi: 10.3390/jcm8040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bajic V.P., Van Neste C., Obradovic M., Zafirovic S., Radak D., Bajic V.B., Essack M., Isenovic E.R. Glutathione “redox homeostasis” and its relation to cardiovascular disease. Oxid Med Cell Longev. 2019;2019:5028181. doi: 10.1155/2019/5028181. PMID: 31210841; PMCID: PMC6532282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marchio P., Guerra-Ojeda S., Vila J.M., Aldasoro M., Victor V.M., Mauricio M.D. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8563845. 8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Checconi P., Limongi D., Baldelli S., Ciriolo M.R., Nencioni L., Palamara A.T. Role of glutathionylation in infection and inflammation. Nutrients. 2019;11 doi: 10.3390/nu11081952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cha S.J., Kim H., Choi H.J., Lee S., Kim K. Protein glutathionylation in the pathogenesis of neurodegenerative diseases. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/2818565. 2818565; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y., Qiao M., Mieyal J.J., Asmis L.M., Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radical Biol. Med. 2006;41:775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 129.Ullevig S.L., Kim H.S., Short J.D., Tavakoli S., Weintraub S.T., Downs K., Asmis R. Protein S-glutathionylation mediates macrophage responses to metabolic cues from the extracellular environment. Antioxidants Redox Signal. 2016;25:836–851. doi: 10.1089/ars.2015.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Adachi T., Pimentel D.R., Heibeck T., Hou X., Lee Y.J., Jiang B., Ido Y., Cohen R.A. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 131.Fan Q., Chen M., Fang X., Lau W.B., Xue L., Zhao L., Zhang H., Liang Y.H., Bai X., Niu H.Y., Ye J., Chen Q., Yang X., Liu M. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age. 2013;35:1017–1026. doi: 10.1007/s11357-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Avner B.S., Shioura K.M., Scruggs S.B., Grachoff M., Geenen D.L., Helseth D.L., Jr., Farjah M., Goldspink P.H., Solaro R.J. Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol. Cell. Biochem. 2012;363:203–215. doi: 10.1007/s11010-011-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta K.B., Ratcliffe M.B., Fallert M.A., Edmunds L.H., Jr., Bogen D.K. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–2326. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 135.Torres W.M., Jacobs J., Doviak H., Barlow S.C., Zile M.R., Shazly T., Spinale F.G. Regional and temporal changes in left ventricular strain and stiffness in a porcine model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H958–h967. doi: 10.1152/ajpheart.00279.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lovelock J.D., Monasky M.M., Jeong E.M., Lardin H.A., Liu H., Patel B.G., Taglieri D.M., Gu L., Kumar P., Pokhrel N., Zeng D., Belardinelli L., Sorescu D., Solaro R.J., Dudley S.C., Jr. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ. Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qin F., Siwik D.A., Lancel S., Zhang J., Kuster G.M., Luptak I., Wang L., Tong X., Kang Y.J., Cohen R.A., Colucci W.S. Hydrogen peroxide–mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J. Am. Heart Assoc. 2013;2(4) doi: 10.1161/JAHA.113.000184. 2:e000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Agrawal V., Gupta J.K., Qureshi S.S., Vishwakarma V.K. Role of cardiac renin angiotensin system in ischemia reperfusion injury and preconditioning of heart. Indian Heart J. 2016;68:856–861. doi: 10.1016/j.ihj.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 2014;35:2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 140.Figtree G.A., Liu C.-C., Bibert S., Hamilton E.J., Garcia A., White C.N., Chia K.K.M., Cornelius F., Geering K., Rasmussen H.H. Reversible oxidative modification. Circ. Res. 2009;105:185–193. doi: 10.1161/CIRCRESAHA.109.199547. [DOI] [PubMed] [Google Scholar]
- 141.Conklin D.J., Guo Y., Jagatheesan G., Kilfoil P.J., Haberzettl P., Hill B.G., Baba S.P., Guo L., Wetzelberger K., Obal D., Rokosh D.G., Prough R.A., Prabhu S.D., Velayutham M., Zweier J.L., Hoetker J.D., Riggs D.W., Srivastava S., Bolli R., Bhatnagar A. Genetic deficiency of glutathione S-transferase P increases myocardial sensitivity to ischemia–reperfusion injury. Circ. Res. 2015;117:437–449. doi: 10.1161/CIRCRESAHA.114.305518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pimentel D.R., Adachi T., Ido Y., Heibeck T., Jiang B., Lee Y., Melendez J.A., Cohen R.A., Colucci W.S. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J. Mol. Cell. Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 143.Eaton P., Wright N., Hearse D.J., Shattock M.J. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J. Mol. Cell. Cardiol. 2002;34:1549–1560. doi: 10.1006/jmcc.2002.2108. [DOI] [PubMed] [Google Scholar]
- 144.Chen F.C., Ogut O. Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am. J. Physiol. Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- 145.Wang S.B., Foster D.B., Rucker J., O'Rourke B., Kass D.A., Van Eyk J.E. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ. Res. 2011;109:750–757. doi: 10.1161/CIRCRESAHA.111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mailloux R.J., Xuan J.Y., McBride S., Maharsy W., Thorn S., Holterman C.E., Kennedy C.R.J., Rippstein P., deKemp R., da Silva J., Nemer M., Lou M., Harper M.-E. Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J. Biol. Chem. 2014;289:14812–14828. doi: 10.1074/jbc.M114.550574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mailloux R.J., Willmore W.G. S-glutathionylation reactions in mitochondrial function and disease. Front Cell Dev Biol. 2014;2:68. doi: 10.3389/fcell.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hakki Kalkan I., Suher M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak J Med Sci. 2013;29:938–942. doi: 10.12669/pjms.294.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Calabrese V., Cornelius C., Leso V., Trovato-Salinaro A., Ventimiglia B., Cavallaro M., Scuto M., Rizza S., Zanoli L., Neri S., Castellino P. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2012;1822:729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 150.Niwa T., Naito C., Mawjood A.H.M., Imai K. Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin. Chem. 2000;46:82–88. [PubMed] [Google Scholar]
- 151.Sampathkumar R., Balasubramanyam M., Sudarslal S., Rema M., Mohan V., Balaram P. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin. Biochem. 2005;38:892–899. doi: 10.1016/j.clinbiochem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 152.Han J., Weisbrod R.M., Shao D., Watanabe Y., Yin X., Bachschmid M.M., Seta F., Janssen-Heininger Y.M.W., Matsui R., Zang M., Hamburg N.M., Cohen R.A. The redox mechanism for vascular barrier dysfunction associated with metabolic disorders: glutathionylation of Rac1 in endothelial cells. Redox Biology. 2016;9:306–319. doi: 10.1016/j.redox.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alam F., Islam M.A., Khalil M.I., Gan S.H. Metabolic control of type 2 diabetes by targeting the GLUT4 glucose transporter: intervention approaches. Curr. Pharmaceut. Des. 2016;22:3034–3049. doi: 10.2174/1381612822666160307145801. [DOI] [PubMed] [Google Scholar]
- 154.Samarasinghe K.T.G., Munkanatta Godage D.N.P., Zhou Y., Ndombera F.T., Weerapana E., Ahn Y.-H. A clickable glutathione approach for identification of protein glutathionylation in response to glucose metabolism. Mol. Biosyst. 2016;12:2471–2480. doi: 10.1039/c6mb00175k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mailloux R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox biology. 2020;32 doi: 10.1016/j.redox.2020.101472. 101472-101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma M., Gupta S., Singh K., Mehndiratta M., Gautam A., Kalra O.P., Shukla R., Gambhir J.K. Association of glutathione-S-transferase with patients of type 2 diabetes mellitus with and without nephropathy. Diabetes & metabolic syndrome. 2016;10:194–197. doi: 10.1016/j.dsx.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 157.Giebultowicz J., Solobodowska S., Bobilewicz D., Wroczynski P. Blood ALDH1 and GST activity in diabetes type 2 and its correlation with glycated hemoglobin. Exp. Clin. Endocrinol. Diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2014;122:55–59. doi: 10.1055/s-0033-1361177. [DOI] [PubMed] [Google Scholar]
- 158.Vasan R.S., Massaro J.M., Wilson P.W., Seshadri S., Wolf P.A., Levy D., D'Agostino R.B. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105:48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
- 159.Chaves F.J., Mansego M.L., Blesa S., Gonzalez-Albert V., Jiménez J., Tormos M.C., Espinosa O., Giner V., Iradi A., Saez G., Redon J. Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension*. Am. J. Hypertens. 2007;20:62–69. doi: 10.1016/j.amjhyper.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 160.Marsden P.A., Schappert K.T., Chen H.S., Flowers M., Sundell C.L., Wilcox J.N., Lamas S., Michel T. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992;307:287–293. doi: 10.1016/0014-5793(92)80697-f. [DOI] [PubMed] [Google Scholar]
- 161.Guerby P., Swiader A., Augé N., Parant O., Vayssière C., Uchida K., Salvayre R., Negre-Salvayre A. High glutathionylation of placental endothelial nitric oxide synthase in preeclampsia. Redox Biology. 2019;22:101126. doi: 10.1016/j.redox.2019.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potthoff S.A., Stamer S., Grave K., Königshausen E., Sivritas S.H., Thieme M., Mori Y., Woznowski M., Rump L.C., Stegbauer J. Chronic p38 mitogen-activated protein kinase inhibition improves vascular function and remodeling in angiotensin II-dependent hypertension. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2016;17 doi: 10.1177/1470320316653284. 1470320316653284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bao W., Behm D.J., Nerurkar S.S., Ao Z., Bentley R., Mirabile R.C., Johns D.G., Woods T.N., Doe C.P., Coatney R.W., Ohlstein J.F., Douglas S.A., Willette R.N., Yue T.L. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J. Cardiovasc. Pharmacol. 2007;49:362–368. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]
- 164.Rogers P.A., Chilian W.M., Bratz I.N., Robert M., Bryan J., Dick G.M. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 165.Park S.W., Noh H.J., Sung D.J., Kim J.G., Kim J.M., Ryu S.-Y., Kang K., Kim B., Bae Y.M., Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflueg. Arch. Eur. J. Physiol. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., Carnethon M.R., Dai S., de Simone G., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Greenlund K.J., Hailpern S.M., Heit J.A., Ho P.M., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., McDermott M.M., Meigs J.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Rosamond W.D., Sorlie P.D., Stafford R.S., Turan T.N., Turner M.B., Wong N.D., Wylie-Rosett J. American heart association statistics, C.; stroke statistics, S. Heart disease and stroke statistics--2011 update: a report from the American heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fowkes F.G., Aboyans V., Fowkes F.J., McDermott M.M., Sampson U.K., Criqui M.H. Peripheral artery disease: epidemiology and global perspectives. Nat. Rev. Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 168.Fowkes F.G., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K., Williams L.J., Mensah G.A., Criqui M.H. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 169.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ. Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 170.Kiernan T.J., Hynes B.G., Ruggiero N.J., Yan B.P., Jaff M.R. Comprehensive evaluation and medical management of infrainguinal peripheral artery disease: "when to treat, when not to treat". Tech. Vasc. Intervent. Radiol. 2010;13:2–10. doi: 10.1053/j.tvir.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 171.Abdulhannan P., Russell D.A., Homer-Vanniasinkam S. Peripheral arterial disease: a literature review. Br. Med. Bull. 2012;104:21–39. doi: 10.1093/bmb/lds027. [DOI] [PubMed] [Google Scholar]
- 172.Inampudi C., Akintoye E., Ando T., Briasoulis A. Angiogenesis in peripheral arterial disease. Curr. Opin. Pharmacol. 2018;39:60–67. doi: 10.1016/j.coph.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 173.Shamoun F., Sural N., Abela G. Peripheral artery disease: therapeutic advances. Expet Rev. Cardiovasc. Ther. 2008;6:539–553. doi: 10.1586/14779072.6.4.539. [DOI] [PubMed] [Google Scholar]
- 174.Hackam D.G., Goodman S.G., Anand S.S. Management of risk in peripheral artery disease: recent therapeutic advances. Am. Heart J. 2005;150:35–40. doi: 10.1016/j.ahj.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 175.Xian D., Song J., Yang L., Xiong X., Lai R., Zhong J. Emerging roles of redox-mediated angiogenesis and oxidative stress in dermatoses. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/2304018. 2304018; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bir S.C., Shen X., Kavanagh T.J., Kevil C.G., Pattillo C.B. Control of angiogenesis dictated by picomolar superoxide levels. Free Radical Biol. Med. 2013;63:135–142. doi: 10.1016/j.freeradbiomed.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim Y.W., West X.Z., Byzova T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. (Berl.) 2013;91:323–328. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matsui R., Watanabe Y., Murdoch C.E. Redox regulation of ischemic limb neovascularization - what we have learned from animal studies. Redox Biol. 2017;12:1011–1019. doi: 10.1016/j.redox.2017.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abhinand C.S., Raju R., Soumya S.J., Arya P.S., Sudhakaran P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10:347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Apte R.S., Chen D.S., Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abdelsaid M.A., El-Remessy A.B. S-glutathionylation of LMW-PTP regulates VEGF-mediated FAK activation and endothelial cell migration. J. Cell Sci. 2012;125:4751–4760. doi: 10.1242/jcs.103481. [DOI] [PubMed] [Google Scholar]
- 182.Barrett W.C., DeGnore J.P., Keng Y.F., Zhang Z.Y., Yim M.B., Chock P.B. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J. Biol. Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 183.Bartoli M., Gu X., Tsai N.T., Venema R.C., Brooks S.E., Marrero M.B., Caldwell R.B. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J. Biol. Chem. 2000;275:33189–33192. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- 184.Xie Y., Kole S., Precht P., Pazin M.J., Bernier M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology. 2009;150:1122–1131. doi: 10.1210/en.2008-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Butturini E., Cozzolino F., Boriero D., Carcereri de Prati A., Monti M., Rossin M., Canetti D., Cellini B., Pucci P., Mariotto S. S-glutathionylation exerts opposing roles in the regulation of STAT1 and STAT3 signaling in reactive microglia. Free Radical Biol. Med. 2018;117:191–201. doi: 10.1016/j.freeradbiomed.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 186.Wang R., Zhang Y., Xu L., Lin Y., Yang X., Bai L., Chen Y., Zhao S., Fan J., Cheng X., Liu E. Protein inhibitor of activated STAT3 suppresses oxidized LDL-induced cell responses during atherosclerosis in apolipoprotein E-deficient mice. Sci. Rep. 2016;6:36790. doi: 10.1038/srep36790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Langston W., Chidlow J.H., Jr., Booth B.A., Barlow S.C., Lefer D.J., Patel R.P., Kevil C.G. Regulation of endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS activity and angiogenesis. Free Radical Biol. Med. 2007;42:720–729. doi: 10.1016/j.freeradbiomed.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Prasai P.K., Shrestha B., Orr A.W., Pattillo C.B. Decreases in GSH:GSSG activate vascular endothelial growth factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox Biol. 2018;19:22–27. doi: 10.1016/j.redox.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Evangelista A.M., Thompson M.D., Weisbrod R.M., Pimental D.R., Tong X., Bolotina V.M., Cohen R.A. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxidants Redox Signal. 2012;17:1099–1108. doi: 10.1089/ars.2011.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Murdoch C.E., Shuler M., Haeussler D.J., Kikuchi R., Bearelly P., Han J., Watanabe Y., Fuster J.J., Walsh K., Ho Y.S., Bachschmid M.M., Cohen R.A., Matsui R. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J. Biol. Chem. 2014;289:8633–8644. doi: 10.1074/jbc.M113.517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reynaert N.L., van der Vliet A., Guala A.S., McGovern T., Hristova M., Pantano C., Heintz N.H., Heim J., Ho Y.S., Matthews D.E., Wouters E.F., Janssen-Heininger Y.M. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pineda-Molina E., Klatt P., Vazquez J., Marina A., Garcia de Lacoba M., Perez-Sala D., Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 193.Watanabe Y., Murdoch C.E., Sano S., Ido Y., Bachschmid M.M., Cohen R.A., Matsui R. Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1alpha and improve limb revascularization. Proc. Natl. Acad. Sci. U. S. A. 2016;113:6011–6016. doi: 10.1073/pnas.1524198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeon D., Park H.J., Kim H.S. Protein S-glutathionylation induced by hypoxia increases hypoxia-inducible factor-1alpha in human colon cancer cells. Biochem. Biophys. Res. Commun. 2018;495:212–216. doi: 10.1016/j.bbrc.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 195.Sunderkotter C., Steinbrink K., Goebeler M., Bhardwaj R., Sorg C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 196.Kobayashi S., Nagaura T., Kimura I., Kimura M. Interferon-gamma-activated macrophages enhance angiogenesis from endothelial cells of rat aorta. Immunopharmacology. 1994;27:23–30. doi: 10.1016/0162-3109(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 197.Short J.D., Downs K., Tavakoli S., Asmis R. Protein thiol redox signaling in monocytes and macrophages. Antioxidants Redox Signal. 2016;25:816–835. doi: 10.1089/ars.2016.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hara S., Fukumura S., Ichinose H. Reversible S-glutathionylation of human 6-pyruvoyl tetrahydropterin synthase protects its enzymatic activity. J. Biol. Chem. 2019;294:1420–1427. doi: 10.1074/jbc.RA118.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang X., Yao H., Chen Y., Sun L., Li Y., Ma X., Duan S., Li X., Xiang R., Han J., Duan Y. Inhibition of glutathione production induces macrophage CD36 expression and enhances cellular-oxidized low density lipoprotein (oxLDL) uptake. J. Biol. Chem. 2015;290:21788–21799. doi: 10.1074/jbc.M115.654582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Buchmuller-Rouiller Y., Corrandin S.B., Smith J., Schneider P., Ransijn A., Jongeneel C.V., Mauel J. Role of glutathione in macrophage activation: effect of cellular glutathione depletion on nitrite production and leishmanicidal activity. Cell. Immunol. 1995;164:73–80. doi: 10.1006/cimm.1995.1144. [DOI] [PubMed] [Google Scholar]
- 201.Venketaraman V., Dayaram Y.K., Amin A.G., Ngo R., Green R.M., Talaue M.T., Mann J., Connell N.D. Role of glutathione in macrophage control of mycobacteria. Infect. Immun. 2003;71:1864–1871. doi: 10.1128/IAI.71.4.1864-1871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brundu S., Palma L., Picceri G.G., Ligi D., Orlandi C., Galluzzi L., Chiarantini L., Casabianca A., Schiavano G.F., Santi M., Mannello F., Green K., Smietana M., Magnani M., Fraternale A. Glutathione depletion is linked with Th2 polarization in mice with a retrovirus-induced immunodeficiency syndrome, murine AIDS: role of proglutathione molecules as immunotherapeutics. J. Virol. 2016;90:7118–7130. doi: 10.1128/JVI.00603-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kwon D.H., Lee H., Park C., Hong S.H., Hong S.H., Kim G.Y., Cha H.J., Kim S., Kim H.S., Hwang H.J., Choi Y.H. Glutathione induced immune-stimulatory activity by promoting M1-like macrophages polarization via potential ROS scavenging capacity. Antioxidants. 2019;8 doi: 10.3390/antiox8090413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jetten N., Donners M.M., Wagenaar A., Cleutjens J.P., van Rooijen N., de Winther M.P., Post M.J. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PloS One. 2013;8 doi: 10.1371/journal.pone.0068811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 206.Ganta V.C., Choi M., Farber C.R., Annex B.H. Antiangiogenic VEGF165b regulates macrophage polarization via S100A8/S100A9 in peripheral artery disease. Circulation. 2019;139:226–242. doi: 10.1161/CIRCULATIONAHA.118.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kiriakidis S., Andreakos E., Monaco C., Foxwell B., Feldmann M., Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J. Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 208.Malaguarnera L., Imbesi R.M., Scuto A., D'Amico F., Licata F., Messina A., Sanfilippo S. Prolactin increases HO-1 expression and induces VEGF production in human macrophages. J. Cell. Biochem. 2004;93:197–206. doi: 10.1002/jcb.20167. [DOI] [PubMed] [Google Scholar]
- 209.Johnson J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017;816:93–106. doi: 10.1016/j.ejphar.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 210.Lekic A., Brekalo Z., Kvesic A., Kovacevic M., Baricev-Novakovic Z., Sutic I., Bulog A., Sutic I., Pavisic V., Mrakovcic-Sutic I. Crosstalk between enzyme matrix metalloproteinases 2 and 9 and regulatory T cell immunity in the global burden of atherosclerosis. Scand. J. Immunol. 2017;86:65–71. doi: 10.1111/sji.12563. [DOI] [PubMed] [Google Scholar]
- 211.Hobeika M.J., Thompson R.W., Muhs B.E., Brooks P.C., Gagne P.J. Matrix metalloproteinases in peripheral vascular disease. J. Vasc. Surg. 2007;45:849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 212.You Y., Chen J., Zhu F., Xu Q., Han L., Gao X., Zhang X., Luo H.R., Miao J., Sun X., Ren H., Du Y., Guo L., Wang X., Wang Y., Chen S., Huang N., Li J. Glutaredoxin 1 up-regulates deglutathionylation of alpha 4 integrin and thereby restricts neutrophil mobilization from bone marrow. J. Biol. Chem. 2019;294:2616–2627. doi: 10.1074/jbc.RA118.006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sen T., Aksu T. Endothelial progenitor cell and adhesion molecules determine the quality of the coronary collateral circulation/Endothelial progenitor cells (CD34+KDR+) and monocytes may provide the development of good coronary collaterals despite the vascular risk factors and extensive atherosclerosis. Anadolu Kardiyol. Derg. 2012;12:447. doi: 10.5152/akd.2012.134. author reply 447-448. [DOI] [PubMed] [Google Scholar]
- 214.Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 215.Dong A., Shen J., Zeng M., Campochiaro P.A. Vascular cell-adhesion molecule-1 plays a central role in the proangiogenic effects of oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14614–14619. doi: 10.1073/pnas.1012859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moraes F., Paye J., Mac Gabhann F., Zhuang Z.W., Zhang J., Lanahan A.A., Simons M. Endothelial cell-dependent regulation of arteriogenesis. Circ. Res. 2013;113:1076–1086. doi: 10.1161/CIRCRESAHA.113.301340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J. Vasc. Surg. 2003;38:198–203. doi: 10.1016/s0741-5214(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 218.Deindl E., Buschmann I., Hoefer I.E., Podzuweit T., Boengler K., Vogel S., van Royen N., Fernandez B., Schaper W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ. Res. 2001;89:779–786. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 219.Heil M., Eitenmuller I., Schmitz-Rixen T., Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J. Cell Mol. Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nagy J.A., Dvorak A.M., Dvorak H.F. VEGF-A(164/165) and PlGF: roles in angiogenesis and arteriogenesis. Trends Cardiovasc. Med. 2003;13:169–175. doi: 10.1016/s1050-1738(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 221.Barakat A.I. Responsiveness of vascular endothelium to shear stress: potential role of ion channels and cellular cytoskeleton (review) Int. J. Mol. Med. 1999;4:323–332. doi: 10.3892/ijmm.4.4.323. [DOI] [PubMed] [Google Scholar]
- 222.Lee C.W., Stabile E., Kinnaird T., Shou M., Devaney J.M., Epstein S.E., Burnett M.S. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J. Am. Coll. Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 223.Lermant A., Murdoch C.E. Cysteine glutathionylation acts as a redox switch in endothelial cells. Antioxidants. 2019;8 doi: 10.3390/antiox8080315. [DOI] [PMC free article] [PubMed] [Google Scholar]