Abstract
The mitochondrial-derived peptides (MDPs) are a new class of small open reading frame encoded polypeptides with pleiotropic properties. The prominent members are Humanin (HN) and small HN-like peptide (SHLP) 2, which encode 16S rRNA, while mitochondrial open reading frame of the twelve S c (MOTS-c) encodes 12S rRNA of the mitochondrial genome. While the multifunctional properties of HN and its analog 14-HNG have been well documented, their protective role in the retinal pigment epithelium (RPE)/retina has been investigated only recently. In this review, we have summarized the multiple effects of HN and its analogs, SHLP2 and MOTS-c in oxidatively stressed human RPE and the regulatory pathways of signaling, mitochondrial function, senescence, and inter-organelle crosstalk. Emphasis is given to the mitochondrial functions such as biogenesis, bioenergetics, and autophagy in RPE undergoing oxidative stress. Further, the potential use of HN and its analogs in the prevention of age-related macular degeneration (AMD) are also presented. In addition, the role of novel, long-acting HN elastin-like polypeptides in nanotherapy of AMD and other ocular diseases stemming from oxidative damage is discussed. It is expected MDPs will become a promising group of mitochondrial peptides with valuable therapeutic applications in the treatment of retinal diseases.
Keywords: Mitochondria-derived peptides, Mitochondrial function, Retinal pigment epithelium, Oxidative stress, Signal mechanisms, Nano delivery
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in individuals over age 55 in the United States [1]. As the American population ages and life expectancy rises, the number of Americans with AMD is increasing. The number of people with AMD rose from 1.75 million in 2000 to 2.07 million in 2010, an increase of 18%, and is expected to more than double to 5.44 million by 2050 [2]. AMD is a multifactorial syndrome that damages the macula. Basic and clinical studies implicate the retinal pigment epithelium (RPE) as a primary site of the disease pathology [3,4]. The RPE normally forms a quiescent monolayer of non-proliferating cells, localized between the choriocapillaris/Bruch membrane complex and the photoreceptors. The RPE forms the outer blood-retina-barrier, provides nutritional support to the photoreceptors, and participates in the retinoid cycle [5]. Major vision changes associated with AMD include warping of vertical and horizontal lines and scotoma, a partial loss of vision, in the area of the sharp, fine detail, “straight ahead” vision. The loss of central vision is due to the death of RPE and photoreceptors (PR) primarily in the macula lutea, the small yellowish area of the retina near the optic disk that is responsible for central and color vision. In early AMD, although the visual loss is minimal, extracellular deposits of lipofuscin, cholesterol, lipids, proteins, and minerals accumulate in the macular region between the RPE and the Bruch membrane [6]. Increasing numbers of macular drusen result in a progression to the two late blinding forms of the disease. The advanced forms of AMD, frequently associated with blindness, are the non-neovascular, atrophic (dry) type and the neovascular (wet or exudative) type. Advanced dry AMD, also termed atrophic AMD or geographic atrophy (GA), is the most common form of the disease and is characterized by degeneration and loss of RPE with secondary loss of PRs [7,8]. The RPE monolayer is required for PR metabolism and phagocytosis of outer PR segments that are shed in a circadian fashion. GA begins in the parafoveal region (non-central GA) and progresses over several years to involve the central fovea (central GA) [9,10]. It is a multifactorial degeneration, involving PRs, the RPE, the Bruch membrane, and the choroid [7]. Perifoveal atrophy affects visual performance, including reading and face recognition, whereas foveal involvement severely affects central visual acuity [[11], [12], [13]]. Dry AMD accounts for the majority of advanced AMD cases [14].
In contrast, advanced wet AMD is characterized by activation of the RPE and the growth of new, leaky blood vessels from the choroid through several breaks in the Bruch membrane to form a choroidal neovascular membrane, destroying the architecture of the overlying RPE and outer retina [15,16]. Untreated neovascularization results in fibrotic scar formation, known as a disciform scar, and permanent central vision loss. Stress or damage in the RPE and the associated immune responses are believed to promote the production of pro‐angiogenic factors, such as vascular endothelial growth factor (VEGF), thereby driving choroidal neovascularization (CNV) [16]. RPE produces VEGF-A via two major pathways: complement activation and oxidative stress [[17], [18], [19], [20]]. The downregulation of antiangiogenic factors such as pigment epithelial-derived growth factor or endostatin is known to play a major role in the process; thus, the major event seems to be a disruption in the balance of pro-angiogenic and anti-angiogenic factors [[21], [22], [23], [24], [25]]. Overproduction of VEGF-A leads to a breakdown of the blood-retinal barrier and the formation of new blood vessels into the retina. In the initiation stage of CNV, endothelial cells proliferate and begin to form new vessels in the surrounding tissue; in the active stage, newly formed vessels are surrounded and stabilized by pericytes; and in the involution stage, new vessels are stabilized and the CNV becomes fibrotic and forms a disciform scar [26]. Wet or neovascular AMD, which affects approximately 10–15% all AMD patients, has the most deleterious effect on central vision. The wet form occurs in 4% of patients who are over 75 years old [27].
The advent of anti–VEGF therapy revolutionized neovascular AMD (nAMD) treatment. Regular injections with anti-VEGF drugs reduce neovascularization and prevent further fluid accumulation, stabilizing and indeed improving vision in most patients. Despite the success of anti-VEGFs, there is no improvement in vision for one-third of nAMD patients, and the long-term use of anti-VEGF therapy is associated with adverse events such as the development of GA and retinal fibrosis [28,29]. Several independent studies suggest that intravitreal injections of anti-VEGF drugs could lead to multiple complications like vitreous and subconjunctival hemorrhage, fluid accumulation under the fovea, increased intra-ocular pressure, endophthalmitis, and ocular inflammation [28,30]. Therefore, improved strategies are needed to reduce or eliminate ocular injections and improve clinical outcomes.
No such effective treatments are currently available for the more common “dry” AMD, other than supplementation of antioxidants plus zinc, which was shown by the Age-Related Eye Disease Study (AREDS) to slow AMD progression (AREDS, 2001). However, only ~20% of patients with intermediate AMD had a positive response to the AREDS formulation. Hence, the search for a new effective treatment for dry AMD is still ongoing. The development of new therapeutic agents that target dry AMD will require an in-depth understanding of the molecular signaling mechanisms involved in the pathogenesis of this eye disease.
Several studies have reported on age-related physiological changes in RPE, including mitochondrial DNA damage and dysfunction altered RPE energy metabolism which leads to the bioenergetic crisis [1,[31], [32], [33], [34]]. With AMD, mtDNA damage was increased by ~350% and was localized to specific regions of the mitochondrial genome [31,34]. The damaged regions of the mitochondrial genome included genes for the 16S and 12S ribosomal RNAs and eight of 22 tRNAs [31]. The 16S rRNA region code for mitochondrial derived peptides (MDPs), includes the well-studied humanin (HN) and other newly discovered small HN-like peptides (SHLPs). The 12S rRNA region produces another MDP known as mitochondrial open reading frame (ORF) of the twelve S c (MOTS-c). Since most of these MDPs show cytoprotective functions in RPE and other cell types [[35], [36], [37], [38], [39], [40], [41]], damage to 16S rRNA or 12S rRNA could result in dysregulated production of this cytoprotective peptide. The mitochondrial genome has a very high mutation rate, 10- to 17-fold higher than that observed in nuclear DNA [42]. Though mutations are identified in the 16S rRNA [[43], [44], [45]] and 12S rRNA [45,46], no data are available on the relationship between any of these known mutations and AMD pathogenesis.
In this review, we will discuss the emerging role of MDPs with a special focus on HN and their pleiotropic functions in RPE cells, particularly in the context of cellular injury. The known functions of HN in multiple tissues will be documented, and studies conducted in RPE cells or cell lines will be addressed in detail. We believe that findings on the functional properties of MDPs could provide a valuable advantage in the development of novel modalities of AMD therapeutics, especially for dry AMD, using formulations such as fusion proteins.
2. The human mitochondrial genome
Mitochondria are the epicenter of key cellular processes such as energy production, cell signaling, cell cycle regulation, cell differentiation, redox homeostasis, and cell fate. Mitochondria are made up of two membranes, the outer mitochondrial membrane and the inner mitochondrial membrane. In addition to the components of electron transport system and the ATP synthase complex; the inner membrane also has many invaginations, called cristae, and the matrix, located inside the membrane. Human mtDNA is a circular, gene-dense, double-stranded DNA (dsDNA) 16,569 bp molecule, accounting for 1%–2% of the total DNA in mammalian cells [47,48]. Human mtDNA encodes 11 messenger RNAs (mRNAs) (translated to 13 proteins), 2 ribosomal RNAs (rRNAs) (12S and 16S rRNA), and 22 tRNAs. Mitochondrial proteins are encoded by genes encoded by the nuclear genome or by mt DNA. It has been estimated that mitochondria contain about 1200 different proteins; and notably many of these proteins are needed for mtDNA expression [[49], [50], [51], [52]]. Regulation of mtDNA expression is extremely complex and includes multiple levels of control, including mtDNA replication, mtDNA transcription, mtDNA maintenance, RNA modification, RNA stability, translation by mitochondrial ribosomes, and the regulated insertion of translated proteins into the mitochondrial inner membrane [53]. The oxidative phosphorylation (OXPHOS) system consists of about 90 proteins, most of which are encoded by nuclear genes, translated on cytosolic ribosomes, and imported into mitochondria. mtDNA encodes 13 proteins/polypeptides that play significant roles in OXPHOS [52]. The substitution rate in the mtDNA genome is 5–10 times that of nuclear DNA [54]. The high substitution rate has been attributed to the lack of mitochondrial histones and a high concentration of oxidative radicals.
3. The mitochondria-derived peptides
As mentioned, the mitochondrial genome contains 12S rRNA and 16S rRNA, both of which are necessary for transcription and translation of the mitochondrial genome. In fact, these two sets of mitochondrial genes share similar structures and functions in organisms ranging from bacteria to humans, even though the sequences exhibit numerous inter- and intraspecific nucleotide variations [48]. The 12S rRNA sequence is 954 nucleotides long, with a conserved secondary structure, and is encoded by nucleotides 648–1601 of the mtDNA which is about 6% of the full mtDNA. The 16S rRNA gene is 1559 nucleotides long and is encoded by nucleotides 1671–3229 bp of the mtDNA. The mitochondrial rRNA genes are hot spots for nucleotide substitutions [55]. Both 16S rRNA and 12S rRNA genes carry short open reading frames that have recently been identified as carrying sequences that code for small regulatory peptides or MDPs. Seven of the eight identified MDPs, such as humanin and SHLP2 (SHLP1-6), are coded by 16S rRNA, and one, MOTS-c, is coded by the 12srRNA [45,[56], [57], [58], [59], [60]]. These peptides offer multipotent functions, including a mechanism for controlling cellular function by mitochondria through complex mitochondrial–nuclear communication mechanisms [48,61].
Humanin, the first described MDP, was found in the 16s ribosomal subunit of the mitochondrial genome and is composed of either 21 or 24 amino acids when expressed in the mitochondria or cytosol, respectively [56,[62], [63], [64]]. The cytoplasmic form of HN is a 24‐amino acid peptide (MAPRGFSCLLLLTSEIDLPVKRRA) [56,62,63,65]. However, the mitochondrial form of HN is a 21‐amino acid peptide (MAPRGFSCLLLLTSEMDLPVK-21 amino acids) [63,66]. Evidence demonstrates that the cytoplasmic HN form is encoded in the nuclear genome and translated in the cytoplasm [63]. Bodzioch et al. [66] identified 13 distinct nuclear loci predicted to maintain ORFs of full-length HN-like peptides, of which 10 peptides could have functional properties. These sequences could be classified as the nuclear mitochondrial sequences, which are fragments of mtDNA incorporated into the nuclear genome in a process mediated by repetitive or transposable elements [67]. The mitochondrial HN is encoded and translated from an ORF within the mitochondrial DNA which encodes for 16S RNA [63,68]. Differences in codon usage by the endogenous protein translation machinery of mitochondria predict a slightly different HN peptide [63]. Although the amino acid sequence of cytoplasmic and mitochondrial HN varies marginally, the two forms include the same essential domains for HN secretion and neuroprotection [62,63]. However, in humans, it is still unclear whether HN is translated in the mitochondria or the cytoplasm.
4. Multiple functional properties of humanin
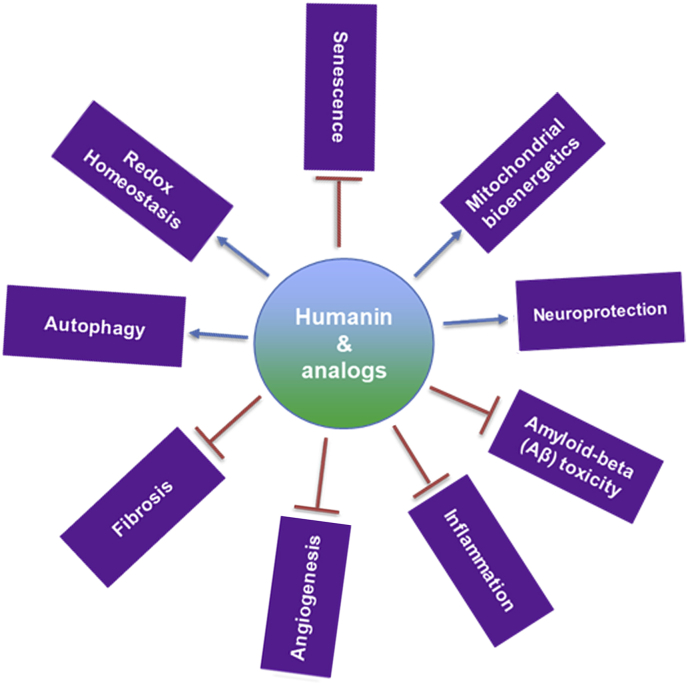
There is mounting evidence that HN mediates several extracellular and intracellular signaling pathways in multiple tissues. Its wide-ranging functions in the ocular tissues include neuroprotection to the recently described chaperone-mediated autophagy (Fig. 1).
Fig. 1.
Multiple functions of humanin and its analogs in ocular tissues.
HN has cytoprotective effects with broad-spectrum actions under different stressors. Several studies have demonstrated that HN is a potent cytoprotective peptide for several cells exposed to different cell stressors. These include serum deprivation [69], stroke [70,71], N-methyl-d-aspartate-induced excitotoxicity [72], Aβ oligomers [37,56,[73], [74], [75]], tert-butyl hydroperoxide (tBH)-induced stress [35,40], endoplasmic reticulum (ER)-stress [36,41], and oxidized low-density lipoprotein induced apoptosis [76]. HN binds to Bax and prevents the release of cytochrome c from the mitochondria, thereby preventing apoptosis [63,77]. Ikonen et al. [57] demonstrated that HN binds to and modulates the pro-apoptotic function of IGFBP-3 and regulates cell survival. Further, the HNG analog targets oligomeric islet amyloid polypeptide and subsequently inhibits the aggregation and growth of amyloid oligomers in those with type II diabetes [75]. HN and its analogs play a significant role in glucose homeostasis. HN and its potent non-IGFBP-3 binding analog improve insulin sensitivity under hyperinsulinemic-euglycemic clamps [78]. In a nonobese diabetic mouse model of type 1 diabetes, daily injections with HN for 6 weeks improved survival of β cells and delayed the onset of diabetes [79]. Another HN analog, HNGF6A increases glucose-stimulated insulin secretion in whole animals, from isolated islets and from cells in culture, which suggests a potential use for HN and its analogs in the treatment of diabetes [80]. In 2,4,6-trinitrobenzene sulphonic acid-induced colitis, a chronic, inflammatory disease, administration of HNG appears to have beneficial effects as indicated by decreased expression of tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β [81]. In astrocytes, pretreatment with HN decreased the level of proinflammatory cytokines, IL-6, IL-1β, and TNFα induced by lipopolysaccharide [82]. In an intracerebral hemorrhage model, activated astrocytes release mitochondria and HN which are incorporated into microglia and promote a "reparative" microglia phenotype characterized by enhanced phagocytosis and reduced pro-inflammatory responses [83]. Treatment with HNG reduced myocardial fibrosis by activating the Akt/GSK-3β pathway and may play a role in myocardial remodeling [84].
One well-studied mechanism by which mitochondria act on the senescence phenotype is through the production of reactive oxygen species (ROS). Many studies have revealed that HN treatment significantly reduced ROS formation [37,39,85]. Accordingly, in the oxidative stress-induced senescence model, HN exhibited senolytic activity [35,86]. The role of HN on the regulation of mitochondrial homeostasis has been reported in many studies. HN treatment significantly promoted mitochondrial biogenesis by increasing mitochondrial mass and mtDNA copy number and enhancing PGC-1α, NRF1, and mtTFA [87,88]. The increase in biogenesis by HN also increased mitochondrial bioenergetics, as evidenced by increased basal oxygen consumption rate, ATP production maximum respiration, and spare respiration capacity [35]. The role of HN in bioenergetics has also been observed in other cell types [87,88]. Altered redox homeostasis is associated with neurodegenerative disorders and mitochondria is the cellular organelle serving as the major source of ROS [89]. HNG maintains cell membrane fluidity, calcium homeostasis, generation of ROS, and mitochondrial function in neuronal cells [90]. In addition, HN protects the ER against ER stress-induced apoptosis in RPE cells by restoring cellular glutathione, particularly mitochondrial glutathione [36]. Recently, HN was demonstrated to induce chaperone-mediated autophagy and increase LC3-II expression, a marker of the autophagosome, as well as the number of autophagosomes and autolysosomes [91]. Further, HN also stabilizes the binding of chaperone HSP90 to its substrates at the cytosolic side of the lysosomal membrane [91]. The pluripotent functions of the known MDPs of 12S and 16S ribosomal RNA families in several cell types are listed in tabular form (Table 1). Table 1 also summarizes the available literature on the utility of MDPs in diseased conditions in non-ocular tissues from multiple species.
Table 1.
Interactive properties of humanin/MDPs in non-ocular tissues.
| Condition | Cell/Tissue | Species | MDP family member | Findings | Refs |
|---|---|---|---|---|---|
| Myocardial fibrosis | Cardiac tissue | Mice | HNG | Attenuation of myocardial fibrosis | [84] |
| Cardiomyopathy | Cardiac tissue | Mice | HNG | Cardiac protection and preserved mitochondrial function. | [92] |
| Cardiac ischemia-reperfusion (I/R) injury induced brain dysfunction | Cardiac tissue, Brain | Rats | HNG | Attenuated mitochondrial dysfunction and reduced brain susceptibility to apoptosis. | [93] |
| Myocardial I/R injury | Cardiac tissue | Rats | HNG | Decreased cardiac arrhythmia, myocardial infarct size, mitochondrial dysfunction, and left ventricular dysfunction. | [94] |
| Cardiovascular disease | Cardio myoblasts | Mice | HNG | Removal of abnormal proteins via chaperone-mediated autophagy. | [91] |
| Myocardial ischemia and reperfusion | Myocardium cell line, cardiac tissue | Mice | HNG | Cardio protection, decrease in infarct size and improvement in ventricular function | [95] |
| Cerebrovascular diseases | Cardiac tissue. Neuronal cells | Mice | HNG | Removal of abnormal proteins via chaperone-mediated autophagy | [91] |
| Cardiovascular disease | Platelets | Mice | HNG | Attenuated cremaster arterial thrombus formation | [96] |
| Aging | C. elegans | Worm | HN | Increased lifespan | [97] |
| Cancer | Blood plasma, testes | Mice | HNG | Cytoprotection of germ cells and leukocytes and non-cancerous cells in tumor-bearing mice. | [98] |
| Neuroblastoma or medulloblastoma | Growth plate tissues/metatarsal bones/tumor xenografts | Human, Mice |
HNG | Prevention of bone growth impairment and chondrocyte apoptosis | [99] |
| Cancer | Breast cancer cells | Human, Mice | HN | Pro-tumoral effects | [100] |
| Cancer and fertility | Blood, liver, spleen, testes | Mice | HNG | Amelioration of chemotherapy-induced germ cell apoptosis; WBC and granulocyte loss. | [101] |
| Neurodegenerative diseases. | Neuroblastoma cell line (SH-SY5Y). | Human | HN-silver Nanoparticles | Neuroprotection | [102] |
| Alzheimer's disease | Cortical neurons | Rats | HN | Attenuation of NMDA- excitotoxicity, improvement of mitochondrial function | [103,104] |
| Alzheimer's disease | Brain | Mice | HNG | Improved cognitive function | [105,106] |
| Alzheimer's disease | Brain | Rats | HNG | Reversal of impairment of spatial memory | [107] |
| Alzheimer's disease | Brain | Mice | HNG | Decreased Aβ level and ameliorated cognitive impairment. | [108] |
| Alzheimer's disease | Brain | Rats | HN | Amelioration of memory deficits induced by Aβ (1-42). | [109] |
| Glucocorticoid-induced Bone growth impairment | Metatarsal bones/growth plate biopsies/chondrocytes | Rats, Human, HN-Tg mice |
HN | Prevention of GC-induced growth impairment in cultured bones | [110] |
| Arthritis | Articular and growth plate cartilage | Mice | HNG | Decreased chondrocyte apoptosis | [110] |
| Stroke | Brain | Mice | HN | Reduction of neurological deficits, and improved hematoma clearance | [83] |
| Endothelial dysfunction | Umbilical vein endothelial cells | Human | HNG | Decrease in lipid aggregation and apoptosis | [76] |
| Obesity | Plasma | Mice | HNG | Regulation of glutathione and sphingolipid metabolism | [111] |
| Atherosclerosis/diabetes | Umbilical vein endothelial cells | Human | HN | Prevention of hyperglycemia-associated endothelial dysfunction | [112] |
| Diabetes | Umbilical vein endothelial cells | Human | HNG | Reduction in apoptosis induced by high glucose | [113] |
| Diabetes | Beta-cells/NOD mice | Mice | HN | Decreased apoptosis and delayed onset of diabetics | [79] |
| Diabetes | Liver | Rats | HNGF6A | Lowering of blood glucose | [78] |
| Atherosclerosis | Proximal aorta | Mice | HNGF6A | Improved endothelial function and impaired progression of atherosclerosis | [114] |
| Age-related diseases | NIT-1 murine β-cells, prostate carcinoma cell line | Mice, human | SHLP2 | Increased glucose uptake and suppressed hepatic glucose production. | [59] |
| Obesity | Plasma | Mice | SHLP2 | Regulation of glutathione and sphingolipid metabolism | [111] |
| Diet-induced obesity | HEK293, HeLa cells, Liver, skeletal muscle | Human, Mice |
MOTS-c | Reduction of obesity | [58] |
| HFD-induced hyperinsulinemia | Liver | Mice | MOTS-c | Attenuated hyperinsulinemia | [58] |
| Ovariectomy‐induced osteoporosis | Bone | Mice | MOTS-c | Alleviated bone loss | [115] |
| Osteolysis | Primary bone marrow macrophages, bone | Mice | MOTS-c | Alleviated bone erosion and inflammation. | [116] |
| Hypothermia | Plasma, liver | Mice | MOTS-c | Increased activation of adipose thermogenesis | [117] |
| Bone fracture | Bone Marrow Mesenchymal Stem Cells | Rats | MOTS-c | Accelerated bone fracture healing | [118] |
| Endothelial dysfunction | Aortic rings, renal artery stenosis | Rats, mice | MOTS-c | Improved endothelial function | [119] |
5. Expression of HN and its putative receptors in RPE cells
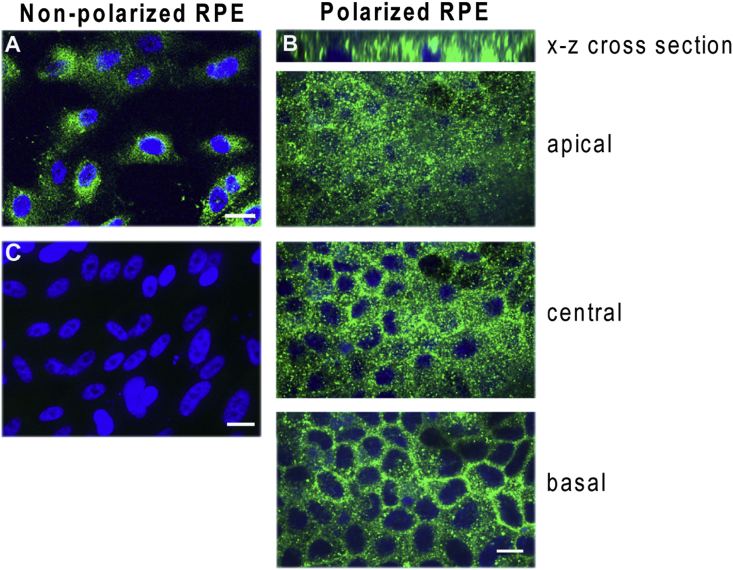
Since its discovery in brain samples [56], HN has been identified in a wide range of cells/tissues including retina, kidney, vascular wall, heart, liver, skeletal muscle, hypothalamus, and pituitary [31,35,95,[120], [121], [122], [123]]. In nonpolarized and polarized RPE cells, HN is mostly expressed in the cytosol, where it is mainly localized in mitochondria [35]. However, in polarized RPE monolayers, HN did not show vectorial distribution and was found localized both at the apical and basolateral domains (Fig. 2). We have shown that RPE cells secrete HN, from both nonpolarized and polarized RPE cells; and consistent with the localization pattern of HN in polarized RPE, no specificity in secretion was observed [35]. However, polarization of RPE cells increased endogenous HN levels threefold and secreted HN levels fivefold over nonpolarized RPE cells [35].
Fig. 2.
Immunofluorescence staining of HN in nonpolarized (A) and polarized (B) human RPE cells. Humanin (green) was localized both in the apical and basal domains (B). No primary antibody control shown in (C). Scale bar: 20 μm. Reproduced from Sreekumar et al. Invest Ophthalmol Vis Sci. 2016; 57(3):1238-53 and is licensed under a Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
6. HN signaling pathways
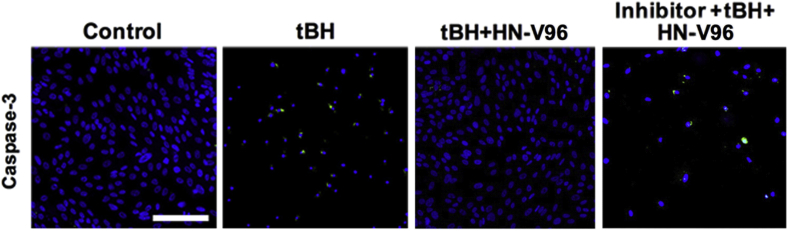
HN mediates its function via intracellular or mitochondria-dependent and extracellular or receptor-mediated pathways [35,40,63,124]. One extracellular mechanism by which HN elicits its action is through binding to its membrane receptors, namely trimeric receptor complex and the formyl peptide receptor like-1 (FPRL-1) receptor [65,124,125]. The HN receptor patterns of expression vary by tissue; and RPE cells express HN receptors [35,126]. The trimeric receptor complex consisting of the cytokine receptor (WSX-1), the transmembrane glycoprotein 130 (gp130), and ciliary neurotrophic factor receptor (CNTFRα) have been reported in RPE cells [35]. CNTFRα and gp130 exhibit polarized distribution, mostly to the apical domain, whereas WSX1 is localized to the apical and basolateral domains. HN peptide is secreted from the cell after translation to be able to mediate its cytoprotective action via receptors. The entire HN primary sequence acts as a signal sequence, and it does not need to be cleaved to be released. In this regard, as previously mentioned, HN is secreted from RPE cells at picogram concentrations and can activate the trimeric receptor complex. After binding to the receptor, HN can trigger several downstream signaling pathways, such as JAK2 - STAT3, P13 -AKT or ERK1/2 [65,85,125,127]. In RPE cells treated with HN, phosphorylation of STAT3 increased at its regulatory Tyr705 site within 2 h [35]. Dimerization and DNA binding of STAT3 requires phosphorylation of its Tyr705 site, and dimerized STATs move to the nucleus and regulate gene transcription. Blocking the STAT3 signaling pathway with STAT3 inhibitor significantly diminished the protective effect of HN in oxidant-induced cells, irrespective of whether the RPE cells are treated with the plain peptide or HN- elastin-like polypeptide (ELP) nanoparticle fusion protein [35,40]. The protective effect with plain HN peptide, though significant, was only partial; thus, one can assume that the receptor-mediated effects of HN peptide only partially contributed to the prevention of cell death. However, unlike the observed significant colocalization of free HN peptide with mitochondria, the HN-ELPs did not colocalize with RPE mitochondria [35,40]. This difference in cellular localization pattern could be explained by the distinct size differences between HN-ELP fusion and the free HN peptide, which may result in different internalization trafficking. The HN-ELPs remained on the cell surface and induced the phosphorylation of STAT3 (Tyr705) in RPE cells up to 24 h. Remarkably, the inhibition of STAT3 completely eliminated cellular protection under oxidative stress, suggesting the active involvement of the receptor-mediated pathway (Fig. 3).
Fig. 3.
Antiapoptotic function of hRPE cells with a novel HN-ELP nanoparticle involving STAT3 inhibition. HN-ELP treatment decreased activation of caspase-3 (Green), and STAT3 inhibition significantly restored caspase-3 staining in tBH treated cells. Modified from Nanomedicine. 2020; 24:102111; Li et al. The humanin peptide mediates ELP nanoassembly and protects human retinal pigment epithelial cells from oxidative stress. Copyright (2020), with permission obtained from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
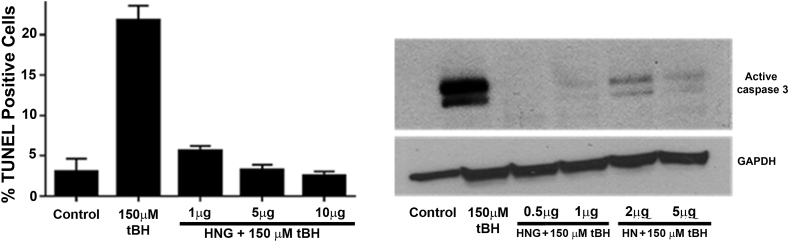
As described above, HN elicits cytoprotection via the intercellular pathway and HN interacts via binding with IGFBP-3, Bax, and tBid [57,63,128]. In many in vitro culture studies, HN shows BAX dependent cytoprotective effects in serum-starved cells, and cells treated with TNF-α or tBH [63,128]. HN peptides also block the Bax association with isolated mitochondria and repress cytochrome c release in vitro. Changing serine‐14 to a glycine (HNG) increases the potency of the peptide by 10-fold in RPE cells challenged with tBH (Fig. 4) although the mechanism is still unknown [129]. We have previously reported that exogenous HN can enter RPE cells, colocalize with BAX, and block cell death (Fig. 4). A recent study demonstrated that HN interacts with the membrane-bound Bax and tBid, preventing the recruitment of cytosolic Bax and its oligomerization on the mitochondrial outer membrane, and suppresses cytochrome c release and mitochondria-dependent apoptosis [130].
Fig. 4.
HN and its analog HNG protect human RPE cells significantly from cell death. RPE cells were treated with single dose of tBH or tBH plus varying doses of HNG for 24 h and cell death was assessed by TUNEL staining (A) and caspase 3 (B). (Sreekumar PG et al., unpublished data).
7. HN improves mitochondrial function in RPE cells
RPE cells have abundant mitochondria, generating high metabolic activity. Mitochondria are the primary energy producers via oxidative phosphorylation. Dysregulated mitochondria result in significantly less energy production and enhanced apoptosis; and this dysregulation is considered one of the initiating factors of AMD [34,131,132]. The net result of these changes includes reduced bioenergetics, increased generation of mt ROS, mitochondrial dysfunction, and cell death [[133], [134], [135]]. However, the mtDNA damage was shown to be selective to the RPE cells isolated from AMD samples [31]. Further, damaged regions of the mitochondrial genome included genes for the 16S and 12S ribosomal RNAs and 8 of 22 tRNAs. As mentioned earlier, the 16S rRNA region encodes HN. Given the increasing understanding of mito-regulatory mechanisms in diseases, the associations between mitochondrial respiration, mtDNA copy number, and biogenesis in response to oxidative stress, our laboratory studied the role of HN in oxidative stress-induced RPE cells [35]. Oxidative stress augmented mitochondrial ROS production, and HN cotreatment significantly reduced ROS formation in RPE cells. It is of interest that ARPE-19 transmitochondrial cybrids containing AMD mitochondria showed increased mtDNA fragmentation and higher ROS levels, and that treatment with the HNG analog of HN reversed these events and protected the AMD mitochondria [37]. However, the treatment of ARPE-19 cells with ethidium bromide (EtBr), which has been used to eliminate mtDNA, resulted in a morphologic change in the cells, and only partial characterization of the ARPE-19 cells (Rho0 cells)) has been reported [136,137]. Further, MDPs are retrograde signaling molecules [138]; and because EtBr has a strong affinity towards double-strand DNA, it could intercalate nDNA and affect expression of nuclear genes [139]. Two key recent publications reported that in RPE cultured from AMD donors, mitochondrial OXPHOS was significantly decreased, supporting the hypothesis that RPE mitochondria are damaged with AMD and the resulting bioenergetic crisis drives AMD pathology [33,140]. In this context, it is of great interest that our own work using cultured hRPE cells demonstrated that exogenous HN could be taken up by RPE cells, co-localize with mitochondria, decrease mitochondrial ROS, improve mitochondrial bioenergetics and enhance mitochondrial biogenesis [35]. Similar oxidant stress-induced changes in mitochondrial metabolism have been shown for cardiac tissue. H2O2 induced oxidative stress in isolated cardiac mitochondria led to attenuated mitochondrial dysfunction, as evidenced by decreased mitochondrial ROS level; attenuated mitochondrial depolarization; reduced mitochondrial swelling; and increased mitochondrial ATP production [141]. In cultured cardiac myoblasts, the HN analog HNG in the presence of H2O2 reduced ROS and preserved mitochondrial membrane potential, mitochondrial structure and ATP levels [142]. Like HN, two other MDPs, SHLP2 and SHLP3, significantly increased mitochondrial respiration and ATP production [59]. Interestingly, MOTS‐c increased glucose uptake and glycolysis but decreased mitochondrial respiration in cultured cells and skeletal muscle [58]. Moreover, the finding that MOTS-c does not improve mitochondrial dysfunction in cybrid cells with mutant mtDNA, suggests the heterogeneous nature of MDPs [143]. The potential mechanisms of MOTS-c action in RPE mitochondria are yet to be delineated.
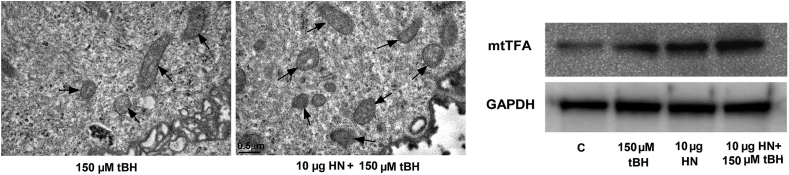
An increase in mitochondrial biogenesis led to an increase in mitochondrial membrane potential and to an increase in oxidative phosphorylation-coupled respiration in multiple cell lines [144,145]. Cellular mitochondrial oxidative capacity is correlated with the number and size of mitochondria [146]. The dysregulation of mitochondrial biogenesis and dynamics due to oxidative stress leads to a decrease in mtDNA copy number, mitochondrial number, mitochondrial mass and oxidative capacity [35,102,147]. Thus, enhanced mitochondrial biogenesis could be one of the mechanisms by which cells regulate mitochondrial bioenergetics. This is illustrated for stressed RPE cells where HN treatment increases mtDNA copy number, the number of mitochondria, and the protein expression level of mitochondrial transcription factors, mtTFA in Fig. 5. Increased mitochondrial DNA mass and mitochondrial number give rise to increased mitochondrial biogenesis capacity required to meet augmented cellular energy demands. In this context, it is of great interest that RPE cells isolated from different AMD donors exhibited significant variability in their response to several drugs used to improve mitochondrial function, and the authors suggested a personalized approach to patients with AMD based on the selective response [122]. The nature and extent of improvement of mitochondrial function in AMD RPE will be of interest to assess HN's role.
Fig. 5.
HN treatment increases mitochondrial biogenesis in oxidatively stressed RPE cells as shown by TEM (A) and immunoblot analysis (B). Sreekumar et al. Invest Ophthalmol Vis Sci. 2016 Mar; 57(3):1238-53, licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
8. HN and senescence
Cellular senescence would have dual roles, beneficial and detrimental, depending on the context; and RPE senescence could play a role in the etiology of AMD [35,148,149]. Senescent RPE cells have been characterized in the human retina and monkey retina [150]. RPE cells show signs of senescence when grown in vitro for a prolonged time or when exposed to oxidative stress [151,152]. Premature senescence has been suggested as a potentially important pathophysiological mediator of RPE cell atrophy in GA [153]. The expression of several genes that code for proteins involved in regulating the cell structure is altered in senescent RPE cells; and changed gene expression could also impact RPE barrier functions [151]. Pretreatment with HN has also been reported to decrease the level of proinflammatory cytokines, IL-6, IL-1β, and TNFα induced by lipopolysaccharide in astroglial cells or astrocytes [82]. Miao et al. [154] observed that HNG ameliorates Aβ25–35-induced neuro-inflammatory responses by decreasing the level of IL-6 and TNF-α in mice. However, controversies exist concerning the effectiveness of HN as a senolytic agent. In the H2O2-induced human primary RPE senescence model, HN cotreatment significantly reduced the classical markers of senescence such as senescence-associated β-Gal–positive cells, ApoJ transcripts, and p16INK4a expression [35]. However, in another study, using a doxorubicin-induced human dermal fibroblast senescence model, HN expression increased, which in turn increased mitochondrial respiration and the secretion of senescence-associated secretory phenotype (SASP)' factors [88]. The dissimilar findings may be attributed to the models used, HN treatment modalities, cell types and the use of classical senescence markers such as SA-β-gal, p16, ApoJ vs laminin. In this context, it is also worth mentioning the disparities of plasma HN levels in different studies. The circulating levels of HN decline with age in mice (2 months and 13 months) and in humans aged 45–110 year [78], indicating that the decline in HN with age could play a role in the pathogenesis of age-related diseases. However, another study demonstrated that HN (and other aging-related cytoprotective factors, GDF15 and FGF-21) were positively correlated with age in a human cohort of 693 subjects aged 21–113 years [155]. Given the potential benefits of HN in several age-related diseases involving senescent cells, a combination of senolytic and HN-based treatments may be additive or synergistic [156]. Additional extensive studies are required to address this issue and resolve the discrepancy.
9. Endoplasmic reticulum -mitochondrial cross talk and HN
While the molecular mechanism involved in ER stress-mediated apoptosis is complex, our early studies in RPE cells reveal that mitochondria-interconnected pathways play a major role in amplifying ER-induced apoptotic signaling in RPE cells [157]. This was based on observations that inhibiting ER-mediated cell death pathways resulted in a significant decrease in mitochondrial damage and ROS production [157]. Our subsequent study [36] demonstrated that ER stress induces several apoptotic pathways, including mitochondrial caspase 3 and ER stress-specific caspase 4 activation in hRPE. Further, ER stress induces significant mitochondrial oxidative stress through increased mitochondrial ROS and depletion of mitochondrial glutathione (mGSH). Treatment with HN inhibited mitochondrial ROS by elevating mGSH [36]. In addition, ER homeostasis can be disrupted by intracellular calcium (Ca 2+) level, redox status, and energy stores, culminating in ER stress [41,60,102,157]. Given the known role of calcium in ER stress, HN-mediated cytoprotection could partially result from HN's ability to decrease intracellular calcium release under stress [158]. Furthermore, it was suggested that the potential site of the HN activity could be ER since there was no effect of exogenous HN on the isolated mitochondria [158]. It is well established that ER stress is regulated by three transmembrane sense proteins: inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [157,159]. HN markedly decreased the expression of all the transmembrane sense proteins (IRE, PERK and ATF-6) and improved cell survival in SH-SY5Y cells [102]. However, direct visualization of HN location in the ER of cells, or HN potential translocation from the mitochondria into ER, which would provide a better understanding of the role of HN mito-ER cross talk, is lacking. Whether HN is also involved in intracellular Ca2+ homeostasis, including Ca2+ transfer from the ER to mitochondria, needs to be further explored.
The mitochondria-associated ER membranes (MAMs), that serve as a critical signaling platform are providing novel perspectives for the understanding of cellular mechanisms in both physiological and pathological conditions. Mitochondria communicate directly with ER through MAM to regulate basic cellular processes such as Ca2+ exchange, phospholipid exchange, intracellular trafficking, autophagy, mitochondrial biogenesis, and inflammasome formation [[160], [161], [162]]. Importantly, although the ER and mitochondrial membranes form specific contact sites, they do not fuse, thereby maintaining the organelle distinct structures [162]. Proteomic studies have identified many ER and mitochondria-associated proteins, such as chaperones, protein kinases, and proteins regulating mitochondrial dynamics and morphology in MAMs, referring to the participation of MAMs in multiple physiological processes [161]. In support of these views, there are studies demonstrating that the perturbation of MAM function reduces mitochondrial ATP production, increases ROS generation, and exacerbates ER stress, resulting in apoptosis [163]. Studies are underway to identify the complete set of proteins that directly interconnect the ER and mitochondria. In this context it is of utmost relevance to report a recent study showed the localization of STAT3, a downstream molecule HN signaling, in the MAM [164]. More work on the mechanism of mitochondrial and or ER dysfunction due to alterations in MAM components and how HN modulates MAM function(s) will be of value.
Several recent studies have investigated ER-mitochondria contacts in neurodegenerative disorders such as Alzheimer's disease and amyotrophic lateral sclerosis [[165], [166], [167]]. Together, the conclusions drawn from the above findings highlighted damage to ER-mitochondria associations as a new pathogenic mechanism. Perturbations to ER-mitochondria associations could provide an explanation for the heterogeneity of these neurodegenerative diseases since the ER-mitochondria axis regulates multiple cellular functions that are dysregulated in pathologies. Thus, identifying and manipulating damaged ER-mitochondria associations may correct damage to other neurodegenerative disease-linked features [162]. MAMs have not been extensively studied in AMD; however, significant changes in the MAM proteome under diabetic conditions has been reported [168]. Thus, altered ER-mitochondrial communication has the potential to play an important, and hitherto unrecognized, role in the pathogenesis of many of the devastating degenerative diseases of the eye.
10. Small humanin-like peptides
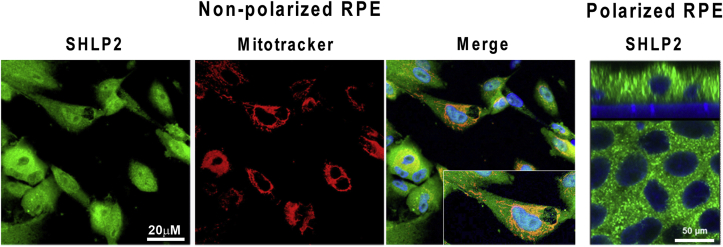
Cobb et al. [59] using in silico prediction analyses identified SHLPs 1–6, within the region of the 16S rRNA gene where HN is located. These novel SHLPs were identified by mRNA analysis and peptide expression in different cells, tissues, and plasma using Western blot and ELISA techniques. Among the six identified peptides, SHLPs 2 and 3 were amplified from both mitochondrial and nuclear cDNA, suggesting possible mitochondrial and nuclear origin [59]. Taken in context with the pleiotropic qualities already assigned to SHLP2, it is worthwhile to point out that no information is available about the subcellular and polarized localization of SHLP2 in RPE cells. Our studies show that in nonpolarized RPE cells, SHLP2 is localized in the cytoplasm where it is co-localized to mitochondria (Fig. 6). Furthermore, no polarized distribution of SHLP2 was evident, as seen by the distribution in the apical and basolateral domains of polarized human RPE (Fig. 6).
Fig. 6.
Localization of SHLP2 in nonpolarized and polarized hRPE cells. Immunofluorescence staining of SHLP2 (green), mitotracker (red) and merge with a magnified inset. SHLP2 in RPE monolayers showing staining in both the apical and basal domains (X-Z plane). DAPI nuclear counterstain (blue). (Sreekumar, PG et al. unpublished data). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
As with HN, SHLP2 and SHLP3 promote cell viability and inhibit apoptosis in many cell lines cultured under serum-free conditions [59]. SHLP2 may mediate neuroprotection similar to HN via the activation of ERK and STAT-3 [59]. In a recent study with transmitochondrial ARPE-19 cybrid cells, SHLP2 prevented the loss of viable cells and mitochondria, increased the number of mtDNA copies and restored the normal levels of OXPHOS complex protein subunits, Further SHLP2 showed anti-apoptotic effects and attenuated amyloid-β-induced cellular and mitochondrial toxicity, suggesting the protective role of SHLP2 in AMD cybrids [38].
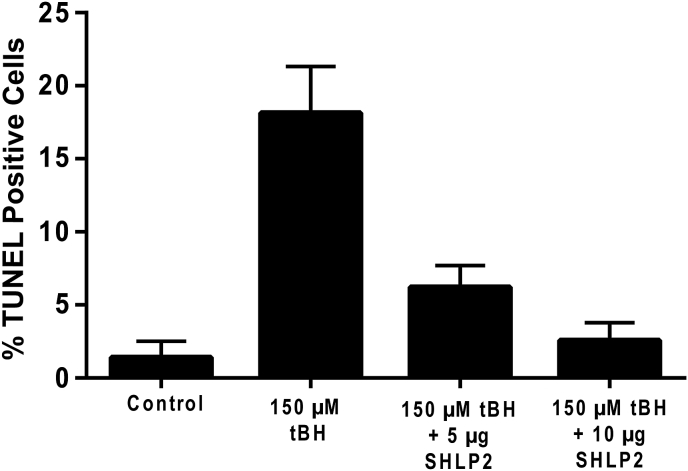
We recently investigated the effect of SHLP2 in primary passage hRPE cells oxidatively stressed. by tBH. Our data revealed that SHLP2 protected hRPE cells significantly from oxidant-induced cell death (Fig. 7 A, B). This conclusion is based on the finding of a dose-dependent cellular protection, and significant cell survival with SHLP-2 when compared to tBH-treated cells (Fig. 7). It has been reported that SHLP2 protects against amyloid-β-induced cell death in AMD cybrids by improving mitochondrial function and inhibiting caspase 3 activation [38]. While these studies on the beneficial effect of SHLP-2 look promising, further work will be needed to confirm these findings and to elucidate the protective mechanisms in RPE/retina.
Fig. 7.
Exogenously added SHLP2 protects hRPE cells from oxidant-induced cell death. hRPE cells were treated with tBH or tBH plus SHLP2 for 24 h (Sreekumar, PG et al. unpublished data).
11. Mitochondrial ORF within the twelve S rRNA-type c
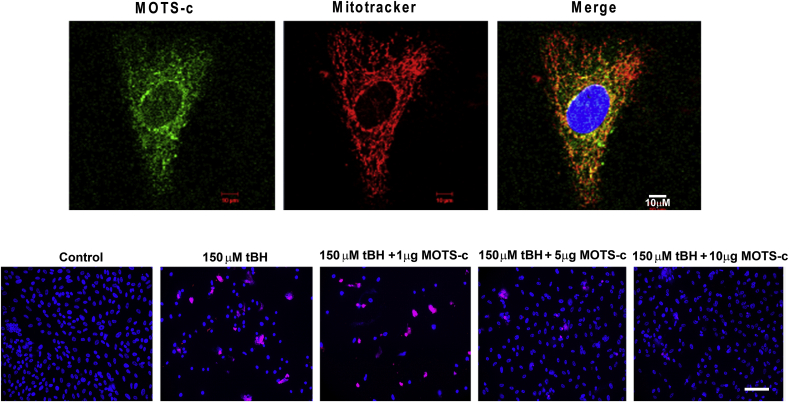
The small ORF in the mitochondrial 12S rRNA encoding a 16-amino-acid peptide named mitochondrial open reading frame of the 12S rRNA-c (MOTS-c) was described to have endocrine-like effects on muscle metabolism, insulin sensitivity and weight regulation [58]. MOTS-c is expressed in various tissues in rodents and plasma in humans [58]. Available information on the expression of MOTS-c in retinal cells or tissues is sparse. Our lab has initiated studies on the expression and function of MOTS-c in human RPE cells. As seen in Fig. 8 (A), MOTS-c is expressed mostly in the perinuclear region and the cytoplasm of RPE. We also found that MOTS-c co-localized predominantly with mitochondria in unstressed RPE cells, and negligible staining was observed in the nucleus, a finding similar to HeLa and HEK293 cells where a certain degree of mitochondrial co-localization was observed [169]. The study by Kim et al. [169] provided further evidence for rapid translocation of MOTS-c into the nucleus in response to metabolic or oxidative stress in HEK293 cells. However, the nuclear translocation was transient, and MOTS-c shifted back to a largely extra-nuclear state within 24 h, demonstrating mito-nuclear communication mediated by several nuclear-encoded proteins that exhibit dual distribution in mitochondria and the nucleus with differential roles in cellular stress-response [169].
Fig. 8.
MOTS-c localization and cytoprotection in RPE cells. (A). Mitochondrial localization of MOTS-c. MOTS-c (green), mitochondria (Red), and nucleus (Blue). (B). Dose-dependent inhibition of oxidative stress-induced cell death by MOTS-c determined by TUNEL assay. Scale Bar: 50 μm. (Sreekumar, PG et al. unpublished data). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The cytoprotective role of MOTS-c has been described in different cell types. HEK293 cells that stably overexpressed wild-type MOTS-c exhibited significant protection against glucose/serum restriction compared to cells transfected with empty vector control [169]. MOTS-c plays an important role in protecting the lungs from the inflammatory effects of lipopolysaccharide-induced acute lung injury [170]. However, to our knowledge, no information is available on the protective function of MOTS-c in the retina or RPE. In serum-starved RPE cells cotreated with tBH (150 μM) and varying doses of MOTS-c for 24 h, increasing doses of MOTS-c protected RPE cells with maximum protection seen with the higher dose (Fig. 8 B). Detailed studies are needed to understand the mechanism of cellular protection by MOTS-c.
12. Therapeutic potential of MDPs
Mitochondrial derived peptides are considered a new class of mitokines that have wide-ranging therapeutic potential in neurodegenerative disorders, as well as in human diseases such as diabetes and as an adjunct to chemotherapy. One major benefit of HN as a therapeutic drug is that HN or its analogs protect against multiple insults, despite the intricate nature of cytotoxic pathways. Nonetheless, because of the known rapid clearance of short chain peptides such as HN, repeated applications are required [171], which is not feasible in localized diseases such as AMD. Nanoparticles (NPs) are acknowledged as a flexible drug delivery system for inaccessible regions in the eye [172,173]. Though many NPs are available for use as drug delivery systems, we used a thermally responsive ELP in our lab because of its several additional benefits. Protein polymers, because they are natural polypeptide chains, can be biocompatible and biodegradable, leaving only small peptides and amino acids as their metabolic byproducts [174]. Additionally, ELPs represent a genetically engineered class of ‘protein polymers’ originating from human tropoelastin that exhibit a reversible phase separation [40,171,175]. Since ELPs are non-immunogenic and are substrates for proteolytic biodegradation, therapeutically potential active peptides, proteins, and small molecules can be easily incorporated [175]. Further, since ELPs are retained in the eye for at least two weeks while the free peptide (20-AA) is removed in <2 days, repeated administration can be minimized (Sreekumar et al., 2018). For example, using two sets of HN ELPs of different transition temperatures, we showed very recently that both HN ELPs protected RPE cells from oxidant-induced cell death [40]. However, the pharmacokinetics of these and other nanoparticles remain to be tested in multiple animal models to verify the therapeutic efficiency of MDPs in ophthalmic disorders.
Disruption to mitochondrial bioenergetic and metabolic function can lead to many mitochondrial disorders in neurodegenerative aging diseases in genetic and animal models, and in human genetic studies [34,132,176,177]. Thus, targeting mitochondria for selective drug delivery is an attractive approach for drug therapy. Two main factors support mitochondria as a viable therapeutic target for neurogenerative disorders: 1) In many prevalent degenerative diseases, mitochondrial dysfunction contributes to the disease process or progression; and 2) Mitochondria contribute to several pathologies through common pathways; hence, targeting this approach could apply to multiple disorders by improving the function of organelles such as ER and nucleus. In fact, ELPs fused to a cell-penetrating peptide have shown promise as vehicles for delivering drugs and therapeutic peptides [178,179]. Several nanotechnology-based approaches are currently being developed for the targeted delivery of small molecules or drugs to mitochondria [180,181]. A library of mitochondria-penetrating peptides with variable mitochondria-localizing properties is available [182]. However, none of these carriers can differentiate mitochondria of healthy cells from those of diseased cells. A study by Sharma et al. reported that triphenyl-phosphonium conjugated dendrimers have the inherent ability to accumulate selectively in the mitochondria of activated glial cells [183]. Modifying nanoparticles by linking to mitochondrial targeting sequences and testing their potency in multiple animal models of retinal degeneration can prove to be valuable.
13. Conclusions and future directions
While the multipotent roles of HN have been well studied in different cells and tissues, not much is known about the in vivo potential of HN in ocular models. The information available on the potential benefits of HN is mostly derived from studies conducted in in vitro models of dry AMD. While we have discussed the mechanism of action of HN based on in vitro studies, the most valuable application of these findings will be in in vivo experimental systems, including genetic models. Multiple animal models are available for neovascular and non-neovascular AMD, and have been well reviewed [184,185]. It will be of great interest to extend studies to these in vivo animal models to examine the beneficial effects of MDPs after pretreatment or co-treatment modalities during the progression of the disease. The antiapoptotic properties of HN in RPE cells are well known but determining the precise mechanisms by which HN enters the mitochondrial compartment requires additional studies. Our recent discovery that specific transporters selectively augment mitochondrial GSH and redox status [186,187] provides a good avenue for exploring the mechanisms by which HN regulates redox homeostasis in mitochondria.
Further, investigations of the effect of novel HN-ELP particles in restoring cell survival in oxidatively stressed RPE demonstrate their prominent protective function. In addition, these bioengineered NPs have the distinct advantages of longer retention time in in vivo AMD models and therefore offer a new and valuable approach for ocular therapy. There is increasing evidence that senescent cells contribute to the progression of age-related diseases [188]. It is tempting to speculate that HN and its analogs may emerge as senolytic drugs. A lot more work will be needed in this emerging field to provide definitive answers, particularly on the contribution of mitochondrial function and its regulation by MDPs in in vivo systems. Finally, it is hoped that research on identifying additional endogenous peptides from mitochondrial genomic data analysis would reveal more MDPs that may be of therapeutic importance.
Funding
This work was supported by the National Institutes of Health (grant number R01 EY30141 (RK)) and the Ryan Initiative for Macular Research (RIMR).
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgment
We thank Drs. David Hinton and Andrew MacKay from University of Southern California (USC) for continued interest and Dr. Pinchas Cohen, Leonard Davis School of Gerontology, USC for kindly providing us SHLP-2 and MOTS-c.
Abbreviations
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- ATF
activating transcription factor
- CNTFR α
ciliary neurotrophic factor receptor
- CNV
choroidal neovascularization
- ELP
elastin-like polypeptide
- ER
endoplasmic reticulum
- FPRL-1
FPR-like-1
- GA
geographic atrophy
- HN
humanin
- IL:
interleukin
- IRE
inositol-requiring enzyme
- MAMs
mitochondria-associated ER membranes
- MDP
mitochondrial-derived peptides
- MOTS-c:
mitochondrial open reading frame of the twelve S c
- mtTFA
Mitochondrial transcription factor A
- NP
nanoparticle
- ORF
open reading frame
- OXPHOS
oxidative phosphorylation
- PERK
PKR-like ER kinase
- PR
photoreceptors
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- SHLP
small HN-like peptide
- tBH
tert-butyl hydroperoxide
- TNF-α:
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
References
- 1.Fisher C.R., Ferrington D.A. Perspective on AMD pathobiology: a bioenergetic crisis in the RPE. Invest. Ophthalmol. Vis. Sci. 2018;59(4):AMD41–AMD47. doi: 10.1167/iovs.18-24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holekamp N.M. Review of neovascular age-related macular degeneration treatment options. Am. J. Manag. Care. 2019;25(10 Suppl):S172–S181. [PubMed] [Google Scholar]
- 3.Ambati J., Anand A., Fernandez S., Sakurai E., Lynn B.C., Kuziel W.A., Rollins B.J., Ambati B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 4.Sparrow J.R. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv. Exp. Med. Biol. 2010;703:63–74. doi: 10.1007/978-1-4419-5635-4_5. [DOI] [PubMed] [Google Scholar]
- 5.Travis G.H., Golczak M., Moise A.R., Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris F.L., 3rd, Wilkinson C.P., Bird A., Chakravarthy U., Chew E., Csaky K., Sadda S.R., Beckman C. Initiative for macular research classification, clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holz F.G., Strauss E.C., Schmitz-Valckenberg S., van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein M., Mitchell P., Freund K.B., Sadda S., Holz F.G., Brittain C., Henry E.C., Ferrara D. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369–390. doi: 10.1016/j.ophtha.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Danis R.P., Lavine J.A., Domalpally A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin. Ophthalmol. 2015;9:2159–2174. doi: 10.2147/OPTH.S92359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan T.D., Agron E., Domalpally A., Clemons T.E., van Asten F., Wong W.T., Danis R.G., Sadda S., Rosenfeld P.J., Klein M.L., Ratnapriya R., Swaroop A., Ferris F.L., 3rd, Chew E.Y., Group A.R. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125(12):1913–1928. doi: 10.1016/j.ophtha.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J.C., Goldstein J.E., Chan T.L., Massof R., Ramulu P., Low Vision Research Network Study G. Characterizing functional complaints in patients seeking outpatient low-vision services in the United States. Ophthalmology. 2014;121(8):1655–16562 e1. doi: 10.1016/j.ophtha.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblad A.S., Lloyd P.C., Clemons T.E., Gensler G.R., Ferris F.L., Klein M.L., 3rd, Armstrong J.R. G. Age-related eye disease study research, change in area of geographic atrophy in the age-related eye disease study: AREDS report number 26. Arch. Ophthalmol. 2009;127(9):1168–1174. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunness J.S. Face fields and microperimetry for estimating the location of fixation in eyes with macular disease. J. Vis. Impair. Blind. 2008;102(11):679–689. [PMC free article] [PubMed] [Google Scholar]
- 14.Tarallo V., Bogdanovich S., Hirano Y., Tudisco L., Zentilin L., Giacca M., Ambati J., De Falco S. Inhibition of choroidal and corneal pathologic neovascularization by Plgf1-de gene transfer. Invest. Ophthalmol. Vis. Sci. 2012;53(13):7989–7996. doi: 10.1167/iovs.12-10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz F.G., Pauleikhoff D., Klein R., Bird A.C. Pathogenesis of lesions in late age-related macular disease. Am. J. Ophthalmol. 2004;137(3):504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Ambati J., Fowler B.J. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan R., Zhang N., Sreekumar P.G., Spee C.K., Rodriguez A., Barron E., Hinton D.R. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol. Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 18.Sreekumar P.G., Kannan R., de Silva A.T., Burton R., Ryan S.J., Hinton D.R. Thiol regulation of vascular endothelial growth factor-A and its receptors in human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 2006;346(4):1200–1206. doi: 10.1016/j.bbrc.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Nozaki M., Sakurai E., Raisler B.J., Baffi J.Z., Witta J., Ogura Y., Brekken R.A., Sage E.H., Ambati B.K., Ambati J. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J. Clin. Invest. 2006;116(2):422–429. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pons M., Marin-Castano M.E. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PloS One. 2011;6(2) doi: 10.1371/journal.pone.0016722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreekumar P.G., Zhou J., Sohn J., Spee C., Ryan S.J., Maurer B.J., Kannan R., Hinton D.R. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest. Ophthalmol. Vis. Sci. 2008;49(3):1210–1220. doi: 10.1167/iovs.07-0667. [DOI] [PubMed] [Google Scholar]
- 22.Pons M., Marin-Castano M.E. Nicotine increases the VEGF/PEDF ratio in retinal pigment epithelium: a possible mechanism for CNV in passive smokers with AMD. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3842–3853. doi: 10.1167/iovs.10-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorrell M., Uusitalo-Jarvinen H., Aguilar E., Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv. Ophthalmol. 2007;52(Suppl 1):S3–S19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Sonoda S., Sreekumar P.G., Kase S., Spee C., Ryan S.J., Kannan R., Hinton D.R. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2009;2(1):28–42. doi: 10.18632/aging.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y.J., Huang L.Z., Zhou A.Y., Zhao M., Yu W.Z., Li X.X. Antiangiogenesis effects of endostatin in retinal neovascularization. J. Ocul. Pharmacol. Therapeut. 2013;29(7):619–626. doi: 10.1089/jop.2012.0225. [DOI] [PubMed] [Google Scholar]
- 26.Santarelli M., Diplotti L., Samassa F., Veritti D., Kuppermann B.D., Lanzetta P. Advances in pharmacotherapy for wet age-related macular degeneration. Expet Opin. Pharmacother. 2015;16(12):1769–1781. doi: 10.1517/14656566.2015.1067679. [DOI] [PubMed] [Google Scholar]
- 27.Nita M., Strzalka-Mrozik B., Grzybowski A., Mazurek U., Romaniuk W. Age-related macular degeneration and changes in the extracellular matrix. Med. Sci. Mon. 2014;20:1003–1016. doi: 10.12659/MSM.889887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel E., Toth C.A., Grunwald J.E., Jaffe G.J., Martin D.F., Fine S.L., Huang J., Ying G.S., Hagstrom S.A., Winter K., Maguire M.G. G. Comparison of Age-related Macular Degeneration Treatments Trials Research, Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunwald J.E., Pistilli M., Daniel E., Ying G.S., Pan W., Jaffe G.J., Toth C.A., Hagstrom S.A., Maguire M.G., Martin D.F. G. Comparison of age-related macular degeneration treatments trials research, incidence and growth of geographic atrophy during 5 Years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124(1):97–104. doi: 10.1016/j.ophtha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pershing S., Bakri S.J., Moshfeghi D.M. Ocular hypertension and intraocular pressure asymmetry after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic. Surg. Lasers Imaging Retina. 2013;44(5):460–464. doi: 10.3928/23258160-20130909-07. [DOI] [PubMed] [Google Scholar]
- 31.Terluk M.R., Kapphahn R.J., Soukup L.M., Gong H., Gallardo C., Montezuma S.R., Ferrington D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 2015;35(18):7304–7311. doi: 10.1523/JNEUROSCI.0190-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrington D.A., Kapphahn R.J., Leary M.M., Atilano S.R., Terluk M.R., Karunadharma P., Chen G.K., Ratnapriya R., Swaroop A., Montezuma S.R., Kenney M.C. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp. Eye Res. 2016;145:269–277. doi: 10.1016/j.exer.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrington D.A., Ebeling M.C., Kapphahn R.J., Terluk M.R., Fisher C.R., Polanco J.R., Roehrich H., Leary M.M., Geng Z., Dutton J.R., Montezuma S.R. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol. 2017;13:255–265. doi: 10.1016/j.redox.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrington D.A., Fisher C.R., Kowluru R.A. Mitochondrial defects drive degenerative retinal diseases. Trends Mol. Med. 2020;26(1):105–118. doi: 10.1016/j.molmed.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sreekumar P.G., Ishikawa K., Spee C., Mehta H.H., Wan J., Yen K., Cohen P., Kannan R., Hinton D.R. The mitochondrial-derived peptide humanin protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest. Ophthalmol. Vis. Sci. 2016;57(3):1238–1253. doi: 10.1167/iovs.15-17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsunaga D., Sreekumar P.G., Ishikawa K., Terasaki H., Barron E., Cohen P., Kannan R., Hinton D.R. Humanin protects RPE cells from endoplasmic reticulum stress-induced apoptosis by upregulation of mitochondrial glutathione. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0165150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashine S., Cohen P., Chwa M., Lu S., Nesburn A.B., Kuppermann B.D., Kenney M.C. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017;8(7) doi: 10.1038/cddis.2017.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nashine S., Cohen P., Nesburn A.B., Kuppermann B.D., Kenney M.C. Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration. Sci. Rep. 2018;8(1):15175. doi: 10.1038/s41598-018-33290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nashine S., Kenney M.C. Effects of mitochondrial-derived peptides (MDPs) on mitochondrial and cellular Health in AMD. Cells. 2020;9(5) doi: 10.3390/cells9051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z., Sreekumar P.G., Peddi S., Hinton D.R., Kannan R., MacKay J.A. The humanin peptide mediates ELP nanoassembly and protects human retinal pigment epithelial cells from oxidative stress. Nanomedicine. 2020;24:102111. doi: 10.1016/j.nano.2019.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sreekumar P.G., Hinton D.R., Kannan R. Endoplasmic reticulum-mitochondrial crosstalk: a novel role for the mitochondrial peptide humanin. Neural Regen. Res. 2017;12(1):35–38. doi: 10.4103/1673-5374.198970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuppen H.A., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797(2):113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Song Y., Li D., He X., Li S., Wu B., Wang W., Gu S., Zhu X., Wang X., Zhou Q., Dai Y., Yan Q. The novel mitochondrial 16S rRNA 2336T>C mutation is associated with hypertrophic cardiomyopathy. J. Med. Genet. 2014;51(3):176–184. doi: 10.1136/jmedgenet-2013-101818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D., Sun Y., Zhuang Q., Song Y., Wu B., Jia Z., Pan H., Zhou H., Hu S., Zhang B., Qiu Y., Dai Y., Chen S., Xu X., Zhu X., Lin A., Huang W., Liu Z., Yan Q. Mitochondrial dysfunction caused by m.2336T>C mutation with hypertrophic cardiomyopathy in cybrid cell lines. Mitochondrion. 2019;46:313–320. doi: 10.1016/j.mito.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Miller B., Kim S.J., Kumagai H., Mehta H.H., Xiang W., Liu J., Yen K., Cohen P. Peptides derived from small mitochondrial open reading frames: genomic, biological, and therapeutic implications. Exp. Cell Res. 2020:112056. doi: 10.1016/j.yexcr.2020.112056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emperador S., Pacheu-Grau D., Bayona-Bafaluy M.P., Garrido-Perez N., Martin-Navarro A., Lopez-Perez M.J., Montoya J., Ruiz-Pesini E. An MRPS12 mutation modifies aminoglycoside sensitivity caused by 12S rRNA mutations. Front. Genet. 2014;5:469. doi: 10.3389/fgene.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace D.C., Fan W., Procaccio V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L., Tan Z., Wang D., Xue L., Guan M.X., Huang T., Li R. Species identification through mitochondrial rRNA genetic analysis. Sci. Rep. 2014;4:4089. doi: 10.1038/srep04089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., Schreier P.H., Smith A.J., Staden R., Young I.G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 50.Foster L.J., de Hoog C.L., Zhang Y., Zhang Y., Xie X., Mootha V.K., Mann M. A mammalian organelle map by protein correlation profiling. Cell. 2006;125(1):187–199. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Mercer T.R., Neph S., Dinger M.E., Crawford J., Smith M.A., Shearwood A.M., Haugen E., Bracken C.P., Rackham O., Stamatoyannopoulos J.A., Filipovska A., Mattick J.S. The human mitochondrial transcriptome. Cell. 2011;146(4):645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustafsson C.M., Falkenberg M., Larsson N.G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 2016;85:133–160. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- 53.Hallberg B.M., Larsson N.G. Making proteins in the powerhouse. Cell Metabol. 2014;20(2):226–240. doi: 10.1016/j.cmet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Brown W.M., Prager E.M., Wang A., Wilson A.C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J. Mol. Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- 55.Galtier N., Enard D., Radondy Y., Bazin E., Belkhir K. Mutation hot spots in mammalian mitochondrial DNA. Genome Res. 2006;16(2):215–222. doi: 10.1101/gr.4305906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashimoto Y., Niikura T., Tajima H., Yasukawa T., Sudo H., Ito Y., Kita Y., Kawasumi M., Kouyama K., Doyu M., Sobue G., Koide T., Tsuji S., Lang J., Kurokawa K., Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc. Natl. Acad. Sci. U. S. A. 2001;98(11):6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikonen M., Liu B., Hashimoto Y., Ma L., Lee K.W., Niikura T., Nishimoto I., Cohen P. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(22):13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.J., Mehta H., Hevener A.L., de Cabo R., Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metabol. 2015;21(3):443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cobb L.J., Lee C., Xiao J., Yen K., Wong R.G., Nakamura H.K., Mehta H.H., Gao Q., Ashur C., Huffman D.M., Wan J., Muzumdar R., Barzilai N., Cohen P. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 2016;8(4):796–809. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minasyan L., Sreekumar P.G., Hinton D.R., Kannan R. Protective mechanisms of the mitochondrial-derived peptide humanin in oxidative and endoplasmic reticulum stress in RPE cells. Oxid. Med. Cell Longev. 2017;2017:1675230. doi: 10.1155/2017/1675230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuccato C.F., Asad A.S., Nicola Candia A.J., Gottardo M.F., Moreno Ayala M.A., Theas M.S., Seilicovich A., Candolfi M. Mitochondrial-derived peptide humanin as therapeutic target in cancer and degenerative diseases. Expert Opin. Ther. Targets. 2019;23(2):117–126. doi: 10.1080/14728222.2019.1559300. [DOI] [PubMed] [Google Scholar]
- 62.Yamagishi Y., Hashimoto Y., Niikura T., Nishimoto I. Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer's disease-relevant insults. Peptides. 2003;24(4):585–595. doi: 10.1016/s0196-9781(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 63.Guo B., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A.C., Reed J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 64.Maximov V., Martynenko A., Hunsmann G., Tarantul V. Mitochondrial 16S rRNA gene encodes a functional peptide, a potential drug for Alzheimer's disease and target for cancer therapy. Med. Hypotheses. 2002;59(6):670–673. doi: 10.1016/s0306-9877(02)00223-2. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto Y., Kurita M., Aiso S., Nishimoto I., Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol. Biol. Cell. 2009;20(12):2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bodzioch M., Lapicka-Bodzioch K., Zapala B., Kamysz W., Kiec-Wilk B., Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics. 2009;94(4):247–256. doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Mishmar D., Ruiz-Pesini E., Brandon M., Wallace D.C. Mitochondrial DNA-like sequences in the nucleus (NUMTs): insights into our African origins and the mechanism of foreign DNA integration. Hum. Mutat. 2004;23(2):125–133. doi: 10.1002/humu.10304. [DOI] [PubMed] [Google Scholar]
- 68.Paharkova V., Alvarez G., Nakamura H., Cohen P., Lee K.W. Rat Humanin is encoded and translated in mitochondria and is localized to the mitochondrial compartment where it regulates ROS production. Mol. Cell. Endocrinol. 2015;413:96–100. doi: 10.1016/j.mce.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 69.Kariya S., Takahashi N., Ooba N., Kawahara M., Nakayama H., Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13(6):903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- 70.Xu X., Chua C.C., Gao J., Hamdy R.C., Chua B.H. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37(10):2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 71.Gao G., Fan H., Zhang X., Zhang F., Wu H., Qi F., Zhao L., Li Y. Neuroprotective effect of G(14)-humanin on global cerebral ischemia/reperfusion by activation of SOCS3 - STAT3 - MCL-1 signal transduction pathway in rats. Neurol. Res. 2017;39(10):895–903. doi: 10.1080/01616412.2017.1352187. [DOI] [PubMed] [Google Scholar]
- 72.Cui A.L., Li J.Z., Feng Z.B., Ma G.L., Gong L., Li C.L., Zhang C., Li K. Humanin rescues cultured rat cortical neurons from NMDA-induced toxicity not by NMDA receptor. Sci. World J. 2014;2014:341529. doi: 10.1155/2014/341529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caricasole A., Bruno V., Cappuccio I., Melchiorri D., Copani A., Nicoletti F. A novel rat gene encoding a Humanin-like peptide endowed with broad neuroprotective activity. Faseb. J. 2002;16(10):1331–1333. doi: 10.1096/fj.02-0018fje. [DOI] [PubMed] [Google Scholar]
- 74.Yen K., Wan J., Mehta H.H., Miller B., Christensen A., Levine M.E., Salomon M.P., Brandhorst S., Xiao J., Kim S.J., Navarrete G., Campo D., Harry G.J., Longo V., Pike C.J., Mack W.J., Hodis H.N., Crimmins E.M., Cohen P. Humanin prevents age-related cognitive decline in mice and is associated with improved cognitive age in humans. Sci. Rep. 2018;8(1):14212. doi: 10.1038/s41598-018-32616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levine Z.A., Teranishi K., Okada A.K., Langen R., Shea J.E. The mitochondrial peptide humanin targets but does not denature amyloid oligomers in type II diabetes. J. Am. Chem. Soc. 2019;141(36):14168–14179. doi: 10.1021/jacs.9b04995. [DOI] [PubMed] [Google Scholar]
- 76.Ding Y., Feng Y., Zhu W., Zou Y., Xie Y., Wang F., Liu C.F., Zhang Y., Liu H. [Gly14]-Humanin prevents lipid deposition and endothelial cell apoptosis in a lectin-like oxidized low-density lipoprotein receptor-1-dependent manner. Lipids. 2019;54(11–12):697–705. doi: 10.1002/lipd.12195. [DOI] [PubMed] [Google Scholar]
- 77.Morris D.L., Kastner D.W., Johnson S., Strub M.P., He Y., Bleck C.K.E., Lee D.Y., Tjandra N. Humanin induces conformational changes in the apoptosis regulator BAX and sequesters it into fibers, preventing mitochondrial outer-membrane permeabilization. J. Biol. Chem. 2019;294(50):19055–19065. doi: 10.1074/jbc.RA119.011297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muzumdar R.H., Huffman D.M., Atzmon G., Buettner C., Cobb L.J., Fishman S., Budagov T., Cui L., Einstein F.H., Poduval A., Hwang D., Barzilai N., Cohen P. Humanin: a novel central regulator of peripheral insulin action. PloS One. 2009;4(7) doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoang P.T., Park P., Cobb L.J., Paharkova-Vatchkova V., Hakimi M., Cohen P., Lee K.W. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59(3):343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuliawat R., Klein L., Gong Z., Nicoletta-Gentile M., Nemkal A., Cui L., Bastie C., Su K., Huffman D., Surana M., Barzilai N., Fleischer N., Muzumdar R. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. Faseb. J. 2013;27(12):4890–4898. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gultekin F.A., Emre A.U., Celik S.K., Barut F., Tali U., Sumer D., Turkcu U.O. Effects of humanin on experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid in rats. Saudi J. Gastroenterol. 2017;23(2):105–111. doi: 10.4103/sjg.SJG_318_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao S.T., Zhao L., Li J.H. Neuroprotective Peptide humanin inhibits inflammatory response in astrocytes induced by lipopolysaccharide. Neurochem. Res. 2013;38(3):581–588. doi: 10.1007/s11064-012-0951-6. [DOI] [PubMed] [Google Scholar]
- 83.Jung J.E., Sun G., Bautista Garrido J., Obertas L., Mobley A.S., Ting S.M., Zhao X., Aronowski J. The mitochondria-derived peptide humanin improves recovery from intracerebral hemorrhage: implication of mitochondria transfer and microglia phenotype change. J. Neurosci. 2020;40(10):2154–2165. doi: 10.1523/JNEUROSCI.2212-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qin Q., Mehta H., Yen K., Navarrete G., Brandhorst S., Wan J., Delrio S., Zhang X., Lerman L.O., Cohen P., Lerman A. Chronic treatment with the mitochondrial peptide humanin prevents age-related myocardial fibrosis in mice. Am. J. Physiol. Heart Circ. Physiol. 2018;315(5):H1127–H1136. doi: 10.1152/ajpheart.00685.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim S.J., Guerrero N., Wassef G., Xiao J., Mehta H.H., Cohen P., Yen K. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget. 2016;7(30):46899–46912. doi: 10.18632/oncotarget.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sreekumar P.G., Hinton D.R., Kannan R. The emerging role of senescence in ocular disease. Oxid. Med. Cell Longev. 2020;2020:2583601. doi: 10.1155/2020/2583601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin Q., Jin J., He F., Zheng Y., Li T., Zhang Y., He J. Humanin promotes mitochondrial biogenesis in pancreatic MIN6 beta-cells. Biochem. Biophys. Res. Commun. 2018;497(1):292–297. doi: 10.1016/j.bbrc.2018.02.071. [DOI] [PubMed] [Google Scholar]
- 88.Kim S.J., Mehta H.H., Wan J., Kuehnemann C., Chen J., Hu J.F., Hoffman A.R., Cohen P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 2018;10(6):1239–1256. doi: 10.18632/aging.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marengo B., Nitti M., Furfaro A.L., Colla R., Ciucis C.D., Marinari U.M., Pronzato M.A., Traverso N., Domenicotti C. Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxid. Med. Cell Longev. 2016:6235641. doi: 10.1155/2016/6235641. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X., Zhao W., Yang H., Zhang J., Ma J. S14G-humanin restored cellular homeostasis disturbed by amyloid-beta protein. Neural Regen. Res. 2013;8(27):2573–2580. doi: 10.3969/j.issn.1673-5374.2013.27.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gong Z., Tasset I., Diaz A., Anguiano J., Tas E., Cui L., Kuliawat R., Liu H., Kuhn B., Cuervo A.M., Muzumdar R. Humanin is an endogenous activator of chaperone-mediated autophagy. J. Cell Biol. 2018;217(2):635–647. doi: 10.1083/jcb.201606095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lue Y., Gao C., Swerdloff R., Hoang J., Avetisyan R., Jia Y., Rao M., Ren S., Atienza V., Yu J., Zhang Y., Chen M., Song Y., Wang Y., Wang C. Humanin analog enhances the protective effect of dexrazoxane against doxorubicin-induced cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2018;315(3):H634–H643. doi: 10.1152/ajpheart.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumfu S., Charununtakorn S.T., Jaiwongkam T., Chattipakorn N., Chattipakorn S.C. Humanin prevents brain mitochondrial dysfunction in a cardiac ischaemia-reperfusion injury model. Exp. Physiol. 2016;101(6):697–707. doi: 10.1113/EP085749. [DOI] [PubMed] [Google Scholar]
- 94.Thummasorn S., Apaijai N., Kerdphoo S., Shinlapawittayatorn K., Chattipakorn S.C., Chattipakorn N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc. Ther. 2016;34(6):404–414. doi: 10.1111/1755-5922.12210. [DOI] [PubMed] [Google Scholar]
- 95.Muzumdar R.H., Huffman D.M., Calvert J.W., Jha S., Weinberg Y., Cui L., Nemkal A., Atzmon G., Klein L., Gundewar S., Ji S.Y., Lavu M., Predmore B.L., Lefer D.J. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler. Thromb. Vasc. Biol. 2010;30(10):1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren L., Li Q., You T., Zhao X., Xu X., Tang C., Zhu L. Humanin analogue, HNG, inhibits platelet activation and thrombus formation by stabilizing platelet microtubules. J. Cell Mol. Med. 2020;24(8):4773–4783. doi: 10.1111/jcmm.15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yen K., Mehta H.H., Kim S.J., Lue Y., Hoang J., Guerrero N., Port J., Bi Q., Navarrete G., Brandhorst S., Lewis K.N., Wan J., Swerdloff R., Mattison J.A., Buffenstein R., Breton C.V., Wang C., Longo V., Atzmon G., Wallace D., Barzilai N., Cohen P. The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan. Aging (Albany NY) 2020;12(12):11185–11199. doi: 10.18632/aging.103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lue Y., Swerdloff R., Wan J., Xiao J., French S., Atienza V., Canela V., Bruhn K.W., Stone B., Jia Y., Cohen P., Wang C. The potent humanin analogue (HNG) protects germ cells and leucocytes while enhancing chemotherapy-induced suppression of cancer metastases in male mice. Endocrinology. 2015;156(12):4511–4521. doi: 10.1210/en.2015-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eriksson E., Wickstrom M., Perup L.S., Johnsen J.I., Eksborg S., Kogner P., Savendahl L. Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. J. Natl. Cancer Inst. 2014;106(3):djt459. doi: 10.1093/jnci/djt459. [DOI] [PubMed] [Google Scholar]
- 100.Moreno Ayala M.A., Gottardo M.F., Zuccato C.F., Pidre M.L., Nicola Candia A.J., Asad A.S., Imsen M., Romanowski V., Creton A., Isla Larrain M., Seilicovich A., Candolfi M. Humanin promotes tumor progression in experimental triple negative breast cancer. Sci. Rep. 2020;10(1):8542. doi: 10.1038/s41598-020-65381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia Y., Lue Y., Swerdloff R.S., Lasky J.L., Panosyan E.H., Dai-Ju J., Wang C. The humanin analogue (HNG) prevents temozolomide-induced male germ cell apoptosis and other adverse effects in severe combined immuno-deficiency (SCID) mice bearing human medulloblastoma. Exp. Mol. Pathol. 2019;109:42–50. doi: 10.1016/j.yexmp.2019.104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gurunathan S., Jeyaraj M., Kang M.H., Kim J.H. Mitochondrial peptide humanin protects silver nanoparticles-induced neurotoxicity in human neuroblastoma cancer cells (SH-SY5Y) Int. J. Mol. Sci. 2019;20(18) doi: 10.3390/ijms20184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui A.L., Zhang Y.H., Li J.Z., Song T., Liu X.M., Wang H., Zhang C., Ma G.L., Zhang H., Li K. Humanin rescues cultured rat cortical neurons from NMDA-induced toxicity through the alleviation of mitochondrial dysfunction. Drug Des. Dev. Ther. 2017;11:1243–1253. doi: 10.2147/DDDT.S133042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X., Zhang H., Wu J., Yin L., Yan L.J., Zhang C. Humanin attenuates NMDA-induced excitotoxicity by inhibiting ROS-dependent JNK/p38 MAPK pathway. Int. J. Mol. Sci. 2018;19(10) doi: 10.3390/ijms19102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tajima H., Kawasumi M., Chiba T., Yamada M., Yamashita K., Nawa M., Kita Y., Kouyama K., Aiso S., Matsuoka M., Niikura T., Nishimoto I. A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice. J. Neurosci. Res. 2005;79(5):714–723. doi: 10.1002/jnr.20391. [DOI] [PubMed] [Google Scholar]
- 106.Murakami M., Nagahama M., Maruyama T., Niikura T. Humanin ameliorates diazepam-induced memory deficit in mice. Neuropeptides. 2017;62:65–70. doi: 10.1016/j.npep.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 107.Krejcova G., Patocka J., Slaninova J. Effect of humanin analogues on experimentally induced impairment of spatial memory in rats. J. Pept. Sci. 2004;10(10):636–639. doi: 10.1002/psc.569. [DOI] [PubMed] [Google Scholar]
- 108.Niikura T., Sidahmed E., Hirata-Fukae C., Aisen P.S., Matsuoka Y. A humanin derivative reduces amyloid beta accumulation and ameliorates memory deficit in triple transgenic mice. PloS One. 2011;6(1) doi: 10.1371/journal.pone.0016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chai G.S., Duan D.X., Ma R.H., Shen J.Y., Li H.L., Ma Z.W., Luo Y., Wang L., Qi X.H., Wang Q., Wang J.Z., Wei Z., Mousseau D.D., Wang L., Liu G. Humanin attenuates Alzheimer-like cognitive deficits and pathological changes induced by amyloid beta-peptide in rats. Neurosci. Bull. 2014;30(6):923–935. doi: 10.1007/s12264-014-1479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Celvin B., Zaman F., Aulin C., Savendahl L. Humanin prevents undesired apoptosis of chondrocytes without interfering with the anti-inflammatory effect of dexamethasone in collagen-induced arthritis. Clin. Exp. Rheumatol. 2020;38(1):129–135. [PubMed] [Google Scholar]
- 111.Mehta H.H., Xiao J., Ramirez R., Miller B., Kim S.J., Cohen P., Yen K. Metabolomic profile of diet-induced obesity mice in response to humanin and small humanin-like peptide 2 treatment. Metabolomics. 2019;15(6):88. doi: 10.1007/s11306-019-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X., Wu Z., He Y., Zhang H., Tian L., Zheng C., Shang T., Zhu Q., Li D., He Y. Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2. Mol. Immunol. 2018;101:245–250. doi: 10.1016/j.molimm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Xie Y., Liu Z.H., Li X.Y., Zhou Y.D., Xu X., Hu L.F., Zhang Y.L., Liu C.F. Protection effect of [Gly14]-Humanin from apoptosis induced by high glucose in human umbilical vein endothelial cells. Diabetes Res. Clin. Pract. 2014;106(3):560–566. doi: 10.1016/j.diabres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 114.Oh Y.K., Bachar A.R., Zacharias D.G., Kim S.G., Wan J., Cobb L.J., Lerman L.O., Cohen P., Lerman A. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219(1):65–73. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ming W., Lu G., Xin S., Huanyu L., Yinghao J., Xiaoying L., Chengming X., Banjun R., Li W., Zifan L. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem. Biophys. Res. Commun. 2016;476(4):412–419. doi: 10.1016/j.bbrc.2016.05.135. [DOI] [PubMed] [Google Scholar]
- 116.Yan Z., Zhu S., Wang H., Wang L., Du T., Ye Z., Zhai D., Zhu Z., Tian X., Lu Z., Cao X. MOTS-c inhibits Osteolysis in the Mouse Calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol. Res. 2019;147:104381. doi: 10.1016/j.phrs.2019.104381. [DOI] [PubMed] [Google Scholar]
- 117.Lu H., Tang S., Xue C., Liu Y., Wang J., Zhang W., Luo W., Chen J. Mitochondrial-derived peptide MOTS-c increases adipose thermogenic activation to promote cold adaptation. Int. J. Mol. Sci. 2019;20(10) doi: 10.3390/ijms20102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weng F.B., Zhu L.F., Zhou J.X., Shan Y., Tian Z.G., Yang L.W. MOTS-c accelerates bone fracture healing by stimulating osteogenesis of bone marrow mesenchymal stem cells via positively regulating FOXF1 to activate the TGF-beta pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(24):10623–10630. doi: 10.26355/eurrev_201912_19759. [DOI] [PubMed] [Google Scholar]
- 119.Qin Q., Delrio S., Wan J., Jay Widmer R., Cohen P., Lerman L.O., Lerman A. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int. J. Cardiol. 2018;254:23–27. doi: 10.1016/j.ijcard.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X., Urbieta-Caceres V.H., Eirin A., Bell C.C., Crane J.A., Tang H., Jordan K.L., Oh Y.K., Zhu X.Y., Korsmo M.J., Bachar A.R., Cohen P., Lerman A., Lerman L.O. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci. 2012;91(5–6):199–206. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moretti E., Giannerini V., Rossini L., Matsuoka M., Trabalzini L., Collodel G. Immunolocalization of humanin in human sperm and testis. Fertil. Steril. 2010;94(7):2888–2890. doi: 10.1016/j.fertnstert.2010.04.075. [DOI] [PubMed] [Google Scholar]
- 122.Gong Z., Tas E., Muzumdar R. Humanin and age-related diseases: a new link? Front. Endocrinol. (Lausanne) 2014;5:210. doi: 10.3389/fendo.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gottardo M.F., Jaita G., Magri M.L., Zarate S., Moreno Ayala M., Ferraris J., Eijo G., Pisera D., Candolfi M., Seilicovich A. Antiapoptotic factor humanin is expressed in normal and tumoral pituitary cells and protects them from TNF-alpha-induced apoptosis. PloS One. 2014;9(10):e111548. doi: 10.1371/journal.pone.0111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashimoto Y., Suzuki H., Aiso S., Niikura T., Nishimoto I., Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77(24):3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 125.Harada M., Habata Y., Hosoya M., Nishi K., Fujii R., Kobayashi M., Hinuma S. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem. Biophys. Res. Commun. 2004;324(1):255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X.G., Hui Y.N., Huang X.F., Du H.J., Zhou J., Ma J.X. Activation of formyl peptide receptor-1 enhances restitution of human retinal pigment epithelial cell monolayer under electric fields. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3160–3165. doi: 10.1167/iovs.10-5156. [DOI] [PubMed] [Google Scholar]
- 127.Xu X., Chua C.C., Gao J., Chua K.W., Wang H., Hamdy R.C., Chua B.H. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhai D., Luciano F., Zhu X., Guo B., Satterthwait A.C., Reed J.C. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J. Biol. Chem. 2005;280(16):15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- 129.Hashimoto Y., Niikura T., Ito Y., Sudo H., Hata M., Arakawa E., Abe Y., Kita Y., Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J. Neurosci. 2001;21(23):9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma Z.W., Liu D.X. Humanin decreases mitochondrial membrane permeability by inhibiting the membrane association and oligomerization of Bax and Bid proteins. Acta Pharmacol. Sin. 2018;39(6):1012–1021. doi: 10.1038/aps.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nordgaard C.L., Karunadharma P.P., Feng X., Olsen T.W., Ferrington D.A. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008;49(7):2848–2855. doi: 10.1167/iovs.07-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaarniranta K., Uusitalo H., Blasiak J., Felszeghy S., Kannan R., Kauppinen A., Salminen A., Sinha D., Ferrington D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020:100858. doi: 10.1016/j.preteyeres.2020.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richter C., Park J.W., Ames B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. U. S. A. 1988;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bohr V.A., Stevnsner T., de Souza-Pinto N.C. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286(1):127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- 135.Santos J.H., Meyer J.N., Skorvaga M., Annab L.A., Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell. 2004;3(6):399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 136.Miceli M.V., Jazwinski S.M. Nuclear gene expression changes due to mitochondrial dysfunction in ARPE-19 cells: implications for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2005;46(5):1765–1773. doi: 10.1167/iovs.04-1327. [DOI] [PubMed] [Google Scholar]
- 137.Miceli M.V., Jazwinski S.M. Common and cell type-specific responses of human cells to mitochondrial dysfunction. Exp. Cell Res. 2005;302(2):270–280. doi: 10.1016/j.yexcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 138.Lee C., Yen K., Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol. Metabol. 2013;24(5):222–228. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaarniranta K., Pawlowska E., Szczepanska J., Jablkowska A., Blasiak J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD) Int. J. Mol. Sci. 2019;20(10) doi: 10.3390/ijms20102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golestaneh N., Chu Y., Xiao Y.Y., Stoleru G.L., Theos A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8(1) doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thummasorn S., Shinlapawittayatorn K., Khamseekaew J., Jaiwongkam T., Chattipakorn S.C., Chattipakorn N. Humanin directly protects cardiac mitochondria against dysfunction initiated by oxidative stress by decreasing complex I activity. Mitochondrion. 2018;38:31–40. doi: 10.1016/j.mito.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 142.Klein L.E., Cui L., Gong Z., Su K., Muzumdar R. A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts. Biochem. Biophys. Res. Commun. 2013;440(2):197–203. doi: 10.1016/j.bbrc.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahn C.H., Choi E.H., Kong B.S., Cho Y.M. Effects of MOTS-c on the mitochondrial function of cells harboring 3243 A to G mutant mitochondrial DNA. Mol. Biol. Rep. 2020;47(5):4029–4035. doi: 10.1007/s11033-020-05429-z. [DOI] [PubMed] [Google Scholar]
- 144.Nisoli E., Falcone S., Tonello C., Cozzi V., Palomba L., Fiorani M., Pisconti A., Brunelli S., Cardile A., Francolini M., Cantoni O., Carruba M.O., Moncada S., Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. U. S. A. 2004;101(47):16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biesemann N., Ried J.S., Ding-Pfennigdorff D., Dietrich A., Rudolph C., Hahn S., Hennerici W., Asbrand C., Leeuw T., Strubing C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018;8(1):9408. doi: 10.1038/s41598-018-27614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ritz P., Berrut G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005;31:5S67–5S73. doi: 10.1016/s1262-3636(05)73654-5. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 147.Conley K.E., Jubrias S.A., Esselman P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000;526 Pt 1:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blasiak J. Senescence in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 2020;77(5):789–805. doi: 10.1007/s00018-019-03420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blasiak J., Piechota M., Pawlowska E., Szatkowska M., Sikora E., Kaarniranta K. Cellular senescence in age-related macular degeneration: can autophagy and DNA damage response play a role? Oxid. Med. Cell Longev. 2017:5293258. doi: 10.1155/2017/5293258. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mishima K., Handa J.T., Aotaki-Keen A., Lutty G.A., Morse L.S., Hjelmeland L.M. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest. Ophthalmol. Vis. Sci. 1999;40(7):1590–1593. [PubMed] [Google Scholar]
- 151.Shelton D.N., Chang E., Whittier P.S., Choi D., Funk W.D. Microarray analysis of replicative senescence. Curr. Biol. 1999;9(17):939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 152.Zhu D., Wu J., Spee C., Ryan S.J., Hinton D.R. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J. Biol. Chem. 2009;284(14):9529–9539. doi: 10.1074/jbc.M809393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kozlowski M.R. RPE cell senescence: a key contributor to age-related macular degeneration. Med. Hypotheses. 2012;78(4):505–510. doi: 10.1016/j.mehy.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 154.Miao J., Zhang W., Yin R., Liu R., Su C., Lei G., Li Z. S14G-Humanin ameliorates Abeta25-35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42(5–6):557–567. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 155.Conte M., Ostan R., Fabbri C., Santoro A., Guidarelli G., Vitale G., Mari D., Sevini F., Capri M., Sandri M., Monti D., Franceschi C., Salvioli S. Human aging and longevity are characterized by high levels of mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(5):600–607. doi: 10.1093/gerona/gly153. [DOI] [PubMed] [Google Scholar]
- 156.Mendelsohn A.R., Larrick J.W. Mitochondrial-derived peptides exacerbate senescence. Rejuvenation Res. 2018;21(4):369–373. doi: 10.1089/rej.2018.2114. [DOI] [PubMed] [Google Scholar]
- 157.Dou G., Sreekumar P.G., Spee C., He S., Ryan S.J., Kannan R., Hinton D.R. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic. Biol. Med. 2012;53(5):1111–1122. doi: 10.1016/j.freeradbiomed.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Knapp A., Czech U., Goralska J., Sliwa A., Gruca A., Kiec-Wilk B., Awsiuk M., Thiele C., Dudek W., Dembinska-Kiec A. Influence of fatty acids on mitochondrial metabolism of adipocyte progenitors and endothelial cells. Arch. Physiol. Biochem. 2012;118(3):128–134. doi: 10.3109/13813455.2012.668193. [DOI] [PubMed] [Google Scholar]
- 159.Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 160.Rowland A.A., Voeltz G.K. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13(10):607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Vliet A.R., Verfaillie T., Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim. Biophys. Acta. 2014;1843(10):2253–2262. doi: 10.1016/j.bbamcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 162.Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H.W., Mueller S., Miller T., Miller C.C.J. There's something wrong with my MAM; the ER-mitochondria Axis and neurodegenerative diseases. Trends Neurosci. 2016;39(3):146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., Forte M., d'Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 2009;36(3):500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Su Y., Huang X., Huang Z., Huang T., Xu Y., Yi C. STAT3 localizes in mitochondria-associated ER membranes instead of in mitochondria. Front. Cell Dev. Biol. 2020;8:274. doi: 10.3389/fcell.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Area-Gomez E., Schon E.A. Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev. 2016;38:90–96. doi: 10.1016/j.gde.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ottolini D., Cali T., Negro A., Brini M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum. Mol. Genet. 2013;22(11):2152–2168. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- 167.Stoica R., De Vos K.J., Paillusson S., Mueller S., Sancho R.M., Lau K.F., Vizcay-Barrena G., Lin W.L., Xu Y.F., Lewis J., Dickson D.W., Petrucelli L., Mitchell J.C., Shaw C.E., Miller C.C. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014;5:3996. doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ma J.H., Shen S., Wang J.J., He Z., Poon A., Li J., Qu J., Zhang S.X. Comparative proteomic analysis of the mitochondria-associated ER membrane (MAM) in a long-term type 2 diabetic rodent model. Sci. Rep. 2017;7(1):2062. doi: 10.1038/s41598-017-02213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim K.H., Son J.M., Benayoun B.A., Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metabol. 2018;28(3):516–524 e7. doi: 10.1016/j.cmet.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xinqiang Y., Quan C., Yuanyuan J., Hanmei X. Protective effect of MOTS-c on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharm. 2020;80:106174. doi: 10.1016/j.intimp.2019.106174. [DOI] [PubMed] [Google Scholar]
- 171.Sreekumar P.G., Li Z., Wang W., Spee C., Hinton D.R., Kannan R., MacKay J.A. Intra-vitreal alphaB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J. Contr. Release. 2018;283:94–104. doi: 10.1016/j.jconrel.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nayak K., Misra M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018;107:1564–1582. doi: 10.1016/j.biopha.2018.08.138. [DOI] [PubMed] [Google Scholar]
- 173.Singh R., Batoki J.C., Ali M., Bonilha V.L., Anand-Apte B. Inhibition of choroidal neovascularization by systemic delivery of gold nanoparticles. Nanomedicine. 2020:102205. doi: 10.1016/j.nano.2020.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Frandsen J.L., Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chem. Soc. Rev. 2012;41(7):2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- 175.Despanie J., Dhandhukia J.P., Hamm-Alvarez S.F., MacKay J.A. Elastin-like polypeptides: therapeutic applications for an emerging class of nanomedicines. J. Contr. Release. 2016;240:93–108. doi: 10.1016/j.jconrel.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bezard E., Yue Z., Kirik D., Spillantini M.G. Animal models of Parkinson's disease: limits and relevance to neuroprotection studies. Mov. Disord. 2013;28(1):61–70. doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dawson T.M., Dawson V.L. Mitochondrial mechanisms of neuronal cell death: potential therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:437–454. doi: 10.1146/annurev-pharmtox-010716-105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bidwell G.L., 3rd, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv. Drug Deliv. Rev. 2010;62(15):1486–1496. doi: 10.1016/j.addr.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yi A., Sim D., Lee Y.J., Sarangthem V., Park R.W. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. J. Nanobiotechnol. 2020;18(1):15. doi: 10.1186/s12951-020-0574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.He H., Wang J., Wang H., Zhou N., Yang D., Green D.R., Xu B. Enzymatic cleavage of branched peptides for targeting mitochondria. J. Am. Chem. Soc. 2018;140(4):1215–1218. doi: 10.1021/jacs.7b11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang G., Xu L., Xu J., Zhang R., Song G., Chao Y., Feng L., Han F., Dong Z., Li B., Liu Z. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018;18(4):2475–2484. doi: 10.1021/acs.nanolett.8b00040. [DOI] [PubMed] [Google Scholar]
- 182.Jean S.R., Ahmed M., Lei E.K., Wisnovsky S.P., Kelley S.O. Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. Acc. Chem. Res. 2016;49(9):1893–1902. doi: 10.1021/acs.accounts.6b00277. [DOI] [PubMed] [Google Scholar]
- 183.Sharma A., Liaw K., Sharma R., Zhang Z., Kannan S., Kannan R.M. Targeting mitochondrial dysfunction and oxidative stress in activated microglia using dendrimer-based therapeutics. Theranostics. 2018;8(20):5529–5547. doi: 10.7150/thno.29039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Datta S., Cano M., Ebrahimi K., Wang L., Handa J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu C.H., Wang Z., Sun Y., Chen J. Animal models of ocular angiogenesis: from development to pathologies. Faseb. J. 2017;31(11):4665–4681. doi: 10.1096/fj.201700336R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang M., Lau L.I., Sreekumar P.G., Spee C., Hinton D.R., Sadda S.R., Kannan R. Characterization and regulation of carrier proteins of mitochondrial glutathione uptake in human retinal pigment epithelium cells. Invest. Ophthalmol. Vis. Sci. 2019;60(2):500–516. doi: 10.1167/iovs.18-25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sreekumar P.G., Wang M., Spee C., Sadda S.R., Kannan R. Transporter-mediated mitochondrial GSH depletion leading to mitochondrial dysfunction and rescue with alphaB crystallin peptide in RPE cells, Antioxidants (Basel) 2020;9(5) doi: 10.3390/antiox9050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Childs B.G., Gluscevic M., Baker D.J., Laberge R.M., Marquess D., Dananberg J., van Deursen J.M. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16(10):718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]