Abstract
Microplastics (MPs) and nanoplastics (NPs) have attracted considerable attention in the recent years as potential threats to the ecosystem and public health. This review summarizes current knowledge of pathological events triggered by micro- and nano-plastics (MP/NPs) with focus on oxidative damages at different levels of biological complexity (molecular, cellular, tissue, organ, individual and population). Based on published information, we matched the apical toxicity endpoints induced by MP/NPs with key event (KE) or adverse outcomes (AO) and categorized them according to the Adverse Outcome Pathway (AOP) online knowledgebase. We used existing AOPs and applied them to highlight formal mechanistic links between identified KEs and AOs in two possible scenarios: first from ecological, and second from public health perspective. Ecological perspective AOP based literature analysis revealed that MP/NPs share formation of reactive oxygen species as their molecular initiating event, leading to adverse outcomes such as growth inhibition and behavior alteration through oxidative stress cascades and inflammatory responses. Application of AOP on literature data related to public health perspective of MP/NPs showed that oxidative stress and its responding pathways, including inflammatory responses, could play the role of key events. However insufficient information prevented precise definitions of AOPs at this level. To overcome this knowledge gap, further mammalian model and epidemiological studies are necessary to support development and construction of detailed AOPs with public health focus.
Keywords: Microplastics, Nanoplastics, Oxidative stress, Inflammation, Adverse outcome pathway approach
Graphical abstract
Highlights
-
•
Micro- and nanoplastics (MP/NPs) attract attention in recent years as potential threats to the ecosystem and public health.
-
•
MP/NPs toxicity endpoints were matched with key event or adverse outcomes and categorized according to AOP knowledgebase.
-
•
MP/NPs share formation of ROS as their molecular initiating event, leading to oxidative stress and inflammation.
-
•
AOPs of MP/NPs toxicity show no clear adverse outcomes from public health perspective due to limited information.
1. Introduction
Awareness of general public and scientific community about the scope and amount of plastic pollution has significantly increased in recent years. Global production of plastics reached 348 million tons in 2017, from which over half was produced in Asia, while Europe contributed 64 million tons [1]. Increase in production coupled with chemical stability of plastic materials and shift of consumer preferences toward “single-use” packaging, accelerates generation of plastic waste, leading to contamination of diverse ecosystems and rising concern about environmental and health risks associated with exposure to plastics [2,3]. Exposure of plastic waste to the elements leads to its degradation through mechanical and chemical processes such as hydrolysis and UV radiation [4], to form microplastics (100 nm–5 mm, MPs) and nanoplastics (<100 nm, NPs) particles [5]. In comparison to lager plastic debris, smaller size of MPs and NPs presents an opportunity for them to enter different environmental compartments, increases efforts required for their removal or clean up and poses higher risk of exposure and entering food chain.
The number of studies addressing MP/NPs toxicity is rapidly increasing, with focus on polyethylene (PE), polystyrene (PS) and polyvinyl chloride (PVC) which have the highest production and utilization among many species of plastic materials [1]. Rising interest in investigations of MP/NPs is likely related to their ability to translocate through biological barriers while at the same time maintaining large surface/mass ratio, as well as their potential to accumulate in the higher trophic level organisms via the food chain [6,7]. It is therefore of high interest of research and regulatory community to evaluate potential ecological as well as human health hazards of MPs/NPs.
Variety of approaches have been used in toxicological studies of MP/NPs, including in vivo (aquatic marine and freshwater, and terrestrial organisms including mammals) and in vitro (cell culture) models to investigate effects and behavior of MPs/NPs [[8], [9], [10], [11]]. Reports indicate that MP/NPs are most frequently involved in induction of developmental toxicity, neurotoxicity, cytotoxicity, and oxidative stress [[12], [13], [14], [15]]. Observed toxic effects of MP/NP particles suggest that oxidative stress and inflammatory responses are of high importance as critical mechanisms underneath the above listed toxicities. However, hazard assessment outcomes from different studies are variable and sometimes contradictory, probably due to the lack of standardized research methodology such as use of different research models, and not the least due to high diversity of the MP/NPs themselves (different size, shape, surface charging and polymer type). For example, the effects of microplastics in a very high concentration was reported to have no negative effects on crustaceans, which directly contradicted other studies indicating adverse effects on crustaceans after exposure to environmentally relevant MPs concentration [16,17]. Furthermore, observing toxicity endpoints at different biological levels (from molecular to population) can be limited, and sometimes even misleading, based on complexity of the observed model system. For example, majority of in vitro model based studies reported toxicity endpoints only on molecular or cellular level, while in vivo studies could detect the adverse effects of MPs/NPs in various organs [11,18,19].
In addition to the above, addressing possible differences between engineered and environment derived MP/NPs has several limitations. Studies selected for the review based on common criteria have only rarely differentiated between the two MP/NP sources, and majority of studies have actually used model MP/NPs (by default engineered, rather than derived from the environment). Therefore, there is very limited information about mechanisms of action specific to environmentally derived MP/NPs and most of the available information that is related to environment-derived MP/NPs comes in a form of surveys of wildlife and aquatic organisms, usually only describing current state of, situation, or distribution and relying to laboratory studies to explain observed effects. In this review, information about the source of nanomaterials is included in the text if it was deemed relevant from the aspects of engineered vs environment-derived NP/MP (specifically in the inflammation section). Unfortunately, not enough studies have met the criteria to make a comparison between natural vs. engineered nanomaterials at the selected level of Adverse Outcome Pathway application.
To address the limitations of different model systems and support standardized methodology approaches, it is advisable to implement a mechanism-based framework such as Adverse Outcome Pathway (AOP) to categorize and link these adverse effects in a formal technical manner. AOP concept is introduced as a modular structure that is not substance specific, in an effort to organize the information regarding the linkage between adverse effects from the molecular level of a biological system to an apical endpoint of the perturbation [20]. AOP framework usually starts with a Molecular Initiating Event (MIE), and is followed with events occurring at more complex levels of biological organization. MIE is a special case of a “Key Event” (KE) which describe the changes in molecular function, signaling pathways, cellular function status, and tissue alterations. Ultimately, these KEs lead to the apical adverse effects on organism or population level such as acute toxicity/sickness of an individual, or reduced reproductive capacity and decline of affected population. These apical endpoints that are traditionally used in ecological risk assessment, in AOP system are referred to as “Adverse Outcomes” (AO) [21].
The Key Event Relationships are linkages between adverse outcome pathway modules that provide scientific evidence supporting the relationships between examined substance and its toxic effects [20]. AOP approach for evaluation and assessment of the health risks associated with exposure to a chemical or compound is increasingly used in metadata analysis of existing knowledge [22,23]. Therefore, we used existing AOPs in an effort to synthetize and present information from systematic review of the reported MP/NPs adverse effects with focus on mechanistic relationships. During this process, we constructed structural schematics depicting current knowledge of MP/NPs triggered potential toxicity endpoints in different levels of biological organization, with an emphasis on oxidative stress and inflammatory responses. Finally, the Adverse Outcome Pathways were applied to both microplastics and nanoplastics, and the results of the synthetic literature review are presented and described using AOP approach.
2. Molecular initiating events and molecular key events triggered by micro- and nano-plastics
Molecular events caused by noxious/toxic agent effects in many cases form the foundation for explanations of biological phenomena observed on higher levels of biological complexity. In this part of the review, we summarize molecular initiating events and molecular key events (KEs) induced by micro- and nanoplastics including generation of free radicals, oxidative stress metabolism activation, lipid peroxidation, DNA damage, and downstream signaling pathway activation that precede cascade of branching molecular changes and potentially lead to irreparable oxidative damage and exacerbated inflammatory processes.
2.1. Molecular initiating event (KE 1364): reactive oxygen species generation
“Free radical” is an atom or group of atoms with one or more unpaired electrons with high potential to engage in different reactions, including oxidative chemical reactions. In biological systems, many radicals are derived from oxygen and are collectively referred to as the reactive oxygen species or ROS [24]. Reports show that MP/NPs can induce generation of ROS at different sizes, doses, surface-characteristics, and exposure times [[25], [26], [27]]. Observations of MP/NPs triggered ROS generation fall in two distinct compartments: extracellular and intracellular. Extracellular ROS generation induced by the MPs relates to the degree of weathering (aging) process during plastic polymers exposure to the elements in the environment [[28], [29], [30]]. Weathering processes of MP/NPs involves simultaneous or individual action of photo- and thermal oxidation and UV radiation leading to chemical alteration on the surface of plastic polymers [30,31]. Photo oxidation or UV light radiation can lead to formation of free radicals on MP/NPs surfaces as primary products via different pathways: subtraction of a hydrogen atom from the macromolecular chain, or addition to an unsaturated carbon chain group (crosslinking reaction) [32]. Once the free radicals are generated along the polymer chain, they can react with atmospheric oxygen and produce polymer peroxy radicals with further generation of secondary polymer alkyl radicals [33]. These weathering induced extracellular free radicals could be one possible explanation as to why a significant increase of ROS was observed after the cellular entrance of the aged MPs [34].
However, pristine MP/NPs can also induce excessive generation of ROS intracellularly. This phenomenon was reported using wide array of model systems, from mammalian cell lines to marine invertebrate and living fish models [18,35,36]. MP/NPs can be engulfed by a (usually phagocytic) cell through endocytosis or pinocytosis (<150 nm in case of NPs) processes [37,38]. Once inside a phagocyte, these MPs appear to trigger the innate immune defense mechanisms and are treated as foreign substances [39,40]. During attempts of the cell to neutralize potentially infectious foreign particle, ROS are generated in high quantities as products of NADPH-oxidase or other enzymatic reactions in form of superoxide and hydrogen peroxide. Both O2− and H2O2 have a role in signal transduction and serve as key mediators driving oxidative stress cascades [36,41]. Furthermore, when microplastics degrade into nano-sized particles, their surface-to-mass ratio increases and gives the NPs ability to penetrate directly through lipid membranes. Changes in surface charge potential and mass/surface ratio in NPs also enable easier absorption of free radicals as well as allows for easier translocation through membranes, presenting a strong correlation between particle size and ROS generation potential: the smaller the particle, the higher ROS generation potential [13,27,42]. Size-dependent differences in toxicity of MPs/NPs are supported by several studies [13,26].
2.2. Key event (KE 1392): oxidative stress
The oxidative stress occurs when there is an imbalance between the production of ROS and antioxidant based detoxification/neutralization. The antioxidant systems are complex and include various antioxidant compounds such as vitamins (e.g. C, E, D3), as well as multiple enzymatic pathways involved in production of antioxidants, or elimination of oxidants including ROS and RNS. Detailed review of antioxidant systems is beyond scope of this review and we point the reader to recent reviews of Pisochi and Pop [43], and Sies et al. [44]. Here, we will focus on oxidative stress pathways most frequently reported in relation to MP/NPs exposure.
Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidases (GPx) play an important role in protecting tissues against ROS damage [45]. For example, complex ROS scavenging system in innate immune cells such as neutrophils utilizes SOD and CAT enzymatic systems to catalyze the reactions of superoxide anion radical (O2−) and hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) as the final metabolites, respectively [46]. SOD and CAT based antioxidant mechanisms are important cellular tools against adverse effects of ROS, and their activity has frequently been used as biomarker of xenobiotic-mediated oxidative stress [46,47]. Exposure to MPs/NPs could increase the concentration of ROS in the organism as we previously stated, and the SOD/CAT antioxidation mechanisms would react to such signal.
The activation of antioxidants was found in animal models on different trophic levels following the exposure of MPs including rotifers (B. koreanus) [26], water fleas (Daphnia magna) [17], fish (D. rerio) [15,48] and mammals (M. musculus) [11]. This phenomenon suggests existence of common mechanisms to initiate oxidative stress sensing and scavenging system induced by microplastic exposure. In case of nanoplastic particles, the oxidative stress was also frequently observed (45% publications) both in vitro and in vivo as indicated by antioxidant system activation. This frequency is placing the oxidative stress as one of most studied adverse effect among all toxicity endpoints studies reported in searched literature databases (Table 1). These phenomena indicate both MPs and NPs can induce oxidative stress as fundamental mechanism for further toxic effects induced by MPs/NPs in a majority of organisms.
Table 1.
Nanoplastics induced Key Events (KE) and Adverse Outcomes (AO) summary and their relative distribution in published literature. *Sum of percentages is over 100% because multiple publications reported multiple KEs and AOs.
| Title | Category | Biological level | AOP wiki ID | NPs type | Organism | Reference | Percentage in the literature (%)* |
|---|---|---|---|---|---|---|---|
| Increase, ROS | Key event | Molecular | KE 1364 | PS | D. magna | [55] | 19.4% |
| Danio rerio | [48] | ||||||
| Monogonont Rotifer (Brachionus koreanus) | [26] | ||||||
| Oyster gametes (Crassostrea gigas) | [75] | ||||||
| Human | [71,104] | ||||||
| Caenorhabditis elegans | [95] | ||||||
| Oxidative stress | Key event | Molecular | KE 1392, KE 1088 | PS, PC | D. magna | [55] | 45.2% |
| Fathead minnow (Pimephales promelas) | [39] | ||||||
| Danio rerio | [48] | ||||||
| [101] | |||||||
| [15] | |||||||
| Daphnia pulex | [93] | ||||||
| Caenorhabditis elegans | [13] | ||||||
| Oyster gametes (Crassostrea gigas) | [75] | ||||||
| Monogonont Rotifer (Brachionus koreanus) | [26] | ||||||
| Mussel (Mytilus galloprovincialis) | [64] | ||||||
| [54] | |||||||
| Human | [85] | ||||||
| [14] | |||||||
| [60] | |||||||
| Activation of oxidative stress pathway | Key event | Molecular | KE 1238, KE 1279 | PS | D. magna | [55] | 9.7% |
| Mussel (Mytilus galloprovincialis) | [54] | ||||||
| Monogonont Rotifer (Brachionus koreanus) | [26] | ||||||
| Lipid peroxidation | Key event | Molecular | KE: 1151 | PS | D. magna | [55] | 9.7% |
| Mussel (Mytilus galloprovincialis) | [64] | ||||||
| Danio rerio | [15] | ||||||
| DNA damage | Key event | Cellular | KE 1669 | PS | Mussel (Mytilus galloprovincialis) | [64] | 6.5% |
| Human | [60] | ||||||
| Lysosome disruption | Key event | Cellular | KE 898 | PS | Mussel (Mytilus galloprovincialis) | [54] | 9.7% |
| Mouse | [70] | ||||||
| Human | [74] | ||||||
| Mitochondrial dysfunction | Key event | Cellular | KE 1483 | PS | D. magna | [55] | 13.0% |
| Mussel (Mytilus galloprovincialis) | [54] | ||||||
| Mouse | [70] | ||||||
| Human | |||||||
| Human | [71,104] | ||||||
| Acetylcholinesterase (AchE) inhibition | Key event | Cellular | KE 12 | PS | Danio rerio | [48] | 9.7% |
| Caenorhabditis elegans | [13] | ||||||
| Mussel (Mytilus galloprovincialis) | [64] | ||||||
| Phase I metabolism enzymes activation | Key event | Molecular | KE 1386, KE 850 | PS | Daphnia pulex | [86] | 6.5% |
| Mussel (Mytilus galloprovincialis) | [64] | ||||||
| Pro-inflammatory cytokines activation | Key event | Cellular | KE 87, KE 1493 | PS | Danio rerio | [10] | 19.4% |
| Mussel (Mytilus galloprovincialis) | [64] | ||||||
| Human | [14] | ||||||
| [83] | |||||||
| [88] | |||||||
| [90] | |||||||
| Apoptosis | Key event | Cellular | KE 1262 | PS | Mussel (Mytilus galloprovincialis) | [54] | 16.1% |
| Mouse | [70] | ||||||
| Human | |||||||
| Human | [85] | ||||||
| [71,104] | |||||||
| [89] | |||||||
| Decrease of neuronal network function | Key event | Organ | KE 386 | PS | Danio rerio | (Brun, van Hage et al., 2019) | 6.5% |
| Caenorhabditis elegans | [13] | ||||||
| Inflammation; Infiltration, Inflammatory cells | Key event | Tissue/Cellular | KE 1633, KE 901 | PS, PC | Danio rerio | [10] | 13.0% |
| [15] | |||||||
| Rat | [90] | ||||||
| Fathead minnow (Pimephales promelas) | [39] | ||||||
| Bradycardia | Key event | Organ | KE 444 | PS | Danio rerio | [101] | 6.5% |
| [100] | |||||||
| Accumulation, Liver lipid | Key event | Organ | KE 455 | PS | Danio rerio | [15] | 3.2% |
| Growth inhibition | Adverse outcome | Individual | KE 1521 KE 1467 | PS | algae (S. obliquus) | [9] | 9.7% |
| Monogonont Rotifer (Brachionus koreanus) | [26] | ||||||
| Caenorhabditis elegans | [13] | ||||||
| Decreased body size/length | Adverse outcome | Individual | KE 315, KE 864 | PS | D. magna | [9] | 13.0% |
| Danio rerio | [48] | ||||||
| Caenorhabditis elegans | [13] | ||||||
| [95] | |||||||
| Increase, Mortality | Adverse outcome | Individual, Population | KE 350, KE 351, KE 342 |
PS | D. magna | [55] | 3.2% |
| Locomotor activity, decreased | Adverse outcome | Individual | KE 1389 | PS | D. magna | [55] | 22.6% |
| Danio rerio | [48] | ||||||
| (Brun, van Hage et al., 2019) | |||||||
| [100] | |||||||
| Caenorhabditis elegans | [13] | ||||||
| [95] | |||||||
| Rat | [82] | ||||||
| Impaired, Fertility | Adverse outcome | Individual | KE 406, KE 78 | PS | Monogonont Rotifer (Brachionus koreanus) | [26] | 3.2% |
| Impaired, Development | Adverse outcome | Individual | KE 577, KE 339 | PS | Danio rerio | [101] | 13.0% |
| [48] | |||||||
| Caenorhabditis elegans | [13] | ||||||
| D. magna | [9] |
2.3. Key event (KE 1238, KE 1279): Activation of oxidative stress pathways
It was clearly demonstrated that ROS can induce or mediate the activation of the MAPK (mitogen-activated protein kinase) pathways [49], but exact mechanisms of MAPK downstream pathway regulations remain unclear. Recent studies have reported that activation of MAPKs signaling cascades could induce ARE-mediated gene expression via the Nrf2-dependent mechanism, and it appears that MAPK activation by ROS can be downregulated by simultaneous increase in Nrf2 activity [50]. In contrast, the prolonged activation of MAPK system with increased ROS can activate various negative consequences (e.g. autophagy), primarily related to the inhibition of ERK and activation of p38MAPK components. It was recently reported that brominated flame retardant induced oxidative stress in two copepod species and activated the MAPK pathway [51], and it was determined that MAPKs pathways play a synergistic role with Nrf2/Keap1 pathway in the oxidative stress response in the scallops exposed to benzo(a)pyrene [52].
As a next step in oxidative stress event series, it was observed that microplastics exposure also activated redox sensitive signaling pathways such as MAPKs. MAPK downstream pathways were initiated post MP exposure in marine copepod P. nana and Mitten crab E. sinensis [27,53]. Jeong et al. [27] showed that the phosphorylation level increase of ERK and p38 kinase has positive correlation with intracellular ROS generation level post MP exposure in P. nana. Moreover, transcription factor Nrf-2 (nuclear factor erythroid 2-related factor 2) was increased after exposure to MPs suggesting that MPs triggered respiration burst possibly acted via ERK and p38 MAPK pathways by activating activated Nrf-2 as representative of genes involved in regulation of antioxidant enzymes gene expression [27].
The activation of MAPK pathway appears dependent on the particle size, as initiation of MAPK pathway was more frequently reported after Nanoplastics exposure [26,54,55]. It was suggested that plastics with a size of 50 nm could generate more severe oxidative stress when compared to 6 μm plastics, causing higher phosphorylation level of p38 MAPKs in both P. nana and B. koreanus [26,27]. Moreover, the surface charge of nanoplastic particles appears critical in the activation of MAPK cascades, as plain/native polystyrene nanoparticles (PSNPs) induced the activation of p38 and JNK along with ROS induction, while negatively charged PSNPs activated p38 and JNK independently [55].
2.4. Key event (KE: 1151): Lipid peroxidation
Lipid peroxidation (LPO) is a self-sustaining chain reaction induced by excessive ROS generation (O2− or others) that results in oxidative damage to cell membranes and other lipid-containing structures [56]. Currently there is a debate whether exposure to MPs would universally induce the LPO reaction and cell membrane damage. Barboza et al. [57] reported significant increase in LPO from brain and muscle tissues of European seabass (Dicentrarchus labrax) after the exposure to MPs in concentration of 0.69 mg/L for 24 h. However, another study indicated that the LPO was not significantly induced after 7 days of MPs exposure, along with increased ROS levels, in the hemocytes of the marine mussel Mytilus spp [25]. Moreover, in S. plana, the LPO levels in gills and digestive gland were either reduced or not significantly increased after 7 days of polystyrene microparticle exposure, and the authors suggested that such result may be explained by sufficient capacity of antioxidant systems to defend the organism from LPO damages [58]. Therefore, based on current literature, it is inconclusive if MPs could induce LPO damage in general, but it is at least prudent to consider this molecular occurrence as a potential key event triggered by MPs. This approach would be further supported with close to 10% of reviewed studies indicating that LPO and subsequent damages were induced by nanoplastic exposure (Table 1), therefore suggesting a possibility that LPO could potentially be a toxicity endpoint shared by the plastic particles in both micro- and nano-size range.
2.5. DNA damage (KE: 1669)
DNA strand damages may occur via the oxidative DNA damages such as 8-Oxo-Guanine damage. There is current controversy whether MPs are inducing DNA damage universally, since only two publications to date reported that DNA damage occurred in aquatic organism models [8,58]. DNA strand breaks were detected post polystyrene microparticle (20 μm) exposure in the haemocytes of S. plana, similarly to mussels treated with polyethylene microparticles [8,58]. Both reports suggested that observed DNA damage could be potentially related to oxidative stress induced by MPs.
Nanoplastics induced genotoxicity, including DNA strand damage, is also under debate. However, a lesson learned from wider nano-toxicology research indicated that some nanoparticles (such as titanium dioxide) can induce the DNA damage through two mechanisms: 1) direct interaction between nanoparticles and DNA; or 2) indirect DNA damage caused by nanoparticle generated ROS or other toxic ions. Furthermore, it was also indicated that DNA strand breaks occur post nanoparticle exposure. An intracellular dynamic imaging study indicated that cationic functionalized PSNPs could result in a prolonged G0/G1 phase in the cell cycle during mitosis in NIH 3T3 cells, therefore indicating potential for DNA damage and the interference with checkpoint control activation [59]. Paget et al. indicated that non-functionalized PSNPs did not induce a general genotoxicity except at the highest tested dose of after 8.1 μg/cm2 exposure for 1 h [60]. There is evidence that both MPs and NPs have the ability to induce DNA strands break in a size and surface charge dependent manner. While exact mechanism of DNA damage induction by MP/NPs is currently not fully understood, several studies suggest there is significant role of oxidative stress and physical interaction involved in initiation of DNA strands break by NPs.
2.6. Acetylcholinesterase (AChE) inhibition (KE: 12)
Acetylcholinesterase (AChE) is an enzyme in the choline esterase family that lyses acetylcholine (ACh) into choline and acetic acid, and is critical for proper function of the nervous system [61]. As ACh functions mainly as neurotransmitter controlling motor neuron function, the inhibition of AChE activity is causing accumulation of ACh in the synaptic cleft and the severe disturbance of muscle activity [61,62]. Recently, the inhibition of AChE induced by either MPs or NPs exposure had been identified in multiple studies, and it appears to be shared by a variety of organisms from invertebrates to vertebrates [8,11,13,48]. Oliveira et al. reported that polyethylene microplastics in sizes of 1–5 μm induced the inhibition of AChE alone or in combination with pyrene in the brain of goby (P. microps) [63]. The authors also noted that the AChE inhibition rates induced by microplastics and pyrene were not significantly different either as single substances or in combination, suggesting that enzymatic inhibition mechanisms may be shared by MPs and pyrene [63]. The anti-cholinesterases effects of MPs had been also identified in gills of another marine organism (S. plana) after exposure to both polystyrene and polyethylene microspheres [8]. Neurotoxic property of MPs has also been addressed in mammalian models, and reported in mice to include MPs (5–20 μm) induced inflammation and AChE inhibition during a sub-chronic exposure period (28 days) [11]. Taken together, there is growing body of evidence that support that one of toxic characteristics of MPs is the ability to induce neurotoxic responses.
Nanoplastics induced AChE inhibition has been reported by at least three separate studies [13,48,64]. A zebrafish study indicated a decrease in AChE activity in all nanoplastics treatments by 27–40% regardless of their surface modification [48]. Invertebrate model (C. elegans and M. galloprovincialis) studies also reported a significant decrease in both level and activity of cholinesterase post the polystyrene nanoplastics exposure [13,64].
The mechanisms involved in the micro- and nano-plastics interference with AChE and possibly other neurotransmitters are largely unknown. Studies using nematode (C. elegans) based assay system suggested that due to nematode lack of blood-brain barrier (BBB), the NPs may have more opportunities to interact with C. elegans neurons causing more neurotoxic effects [13]. This assumption is only partially explaining the toxic effects, as NPs have been observed to cross the BBB and eventually reach the brain, however direct blocking of cholinesterase by NPs has not yet been observed directly or verified in an in vitro system [65]. Presence of a blood-brain barrier is likely excluding micro-sized plastic particles crossing, however, neurotoxicity was nevertheless detected after MPs exposure in organisms with BBB. This strongly suggests that an indirect pathway plays a role in the MPs/NPs neurotoxicity potency [11]. Possible explanation for this neurotoxicity potency is involvement of MP/NPs induced oxidative stress, as anticholinesterases induced neurotoxicity can be caused by it [66]. Simultaneous detection of MP/NPs induced oxidative stress and AChE inhibition further supports such possibility [11,48]. Direct relationship between H2O2 and modifications of AChE metabolism and activity was established recently, when it was determined that H2O2 decreased AChE levels by allosteric effector action on AChE structure and changes of isoform profile in SH-SY5Y cells confirming that increase in at least one ROS could promote disturbances in cholinergic system of neural cells [67].
3. Cellular and organelle key events triggered by micro- and nano-plastics
As the next level, a sum of adverse key molecular events advances into the observable cellular occurrences. Here we will review three adverse cellular effects currently known to be triggered by both microplastic and nanoplastic particles and focus on the mechanistic correlations between cellular key events, and/or between cellular and molecular key events.
3.1. Mitochondrial dysfunction (KE: 1483)
As mitochondria are organelles where most of intracellular production of ROS occurs, an instability in mitochondrial membrane potential can result in excessive generation of ROS through one-electron carriers (e.g. cytochromes and iron-sulphur-protein) and various oxidases [68]. Reports indicated mitochondria membrane dysfunction after MPs exposure in mice and rotifer B. koreanus, which requires further investigations as MPs are unable to accumulate directly in the mitochondria due to their size [26,69]. The study in rotifer B. koreanus reported that 0.5 μm polystyrene microbeads decreased the mitochondrial membrane potential significantly and suggested that MPs may affect the mitochondrial outer membrane indirectly via increased ROS presence in cellular compartments outside of, but in the vicinity of mitochondria [26]. Exposure of mitochondria to excessive oxidative stress in the cytosol can trigger the opening of several mitochondrial Na/K transmembrane channels. Increased membrane channel ionic flux can further lead to a collapse of the mitochondrial membrane potential and releasing Reactive Oxygen Species through “(ROS)-induced ROS-release” (RIRR) mechanism [68]. As it is established that MPs are capable of inducing oxidative stress (section 2.2), the RIRR mechanism could be responsible as mediator between the MPs intracellular presence and observed hampering of the mitochondrial membrane potential.
Mitochondrial membrane damage induced by MPs has also correlated with particle size (where smaller MPs induced more effects), leading to possibility that NPs could impose a more severe damage towards mitochondria generally [26]. Supportive to such reasoning, NPs induced mitochondria disruption was reported in D. magna and in a variety of human cell lines [55,70,71]. It is also possible that reported damage could also be induced by observed direct accumulation of NPs inside mitochondria, and not only by indirect pathways as in case of MPs [71]. Moreover, amine-modified polystyrene nanoparticles induced significantly more severe mitochondrial damages comparing to non-modified ones, suggesting that NPs surface charging plays a role in causing adverse effects of mitochondrial function [71].
3.2. Lysosome disruption (KE: 898)
Lysosome is a single membrane cytoplasmic organelle, with high sensitivity to xenobiotics including environmental pollutants. Thus, lysosomal membrane stability can be used as a biomarker to estimate potential impact of the environmental pollutants [72]. The lysosome function disruption was found in blue mussel (M. galloprovincialis) after exposure to both MPs and NPs [8,54]. The severity of the lysozyme function disturbance after MP/NPs exposure suggests high biological relevance of these events, especially in context of lysosome function and structure being conserved across species [[73], [74], [75]]. Furthermore, the link between oxidative metabolism and ROS generation induced stress has been established [47], including the role of nanomaterials in lysosome function disruption [76]. In summary, two probable MP/NPs induced mechanisms can lead to lysosomal damage: 1) direct damage after ingestion of plastic particles in the cell via endocytosis or permeation (<50 nm) and attempts to digest the foreign body which may result in the lysosome disruption [[77], [78], [79]]; and 2) excessive production of ROS induced by MPs and NPs as lysosomal membranes are highly susceptible to oxidative effects of ROS [80]. Exact mechanisms of lysosome disruption by MP/NPs are not fully understood and this knowledge gap calls for further investigation.
4. Tissue and Organ key events triggered by micro- and nano-plastics
4.1. Decrease of neuronal network function (KE: 386)
Inhibition of AChE (Section 2.6) was found in a variety of organisms post MP/NPs exposure. This toxicity endpoint is considered a type of neuronal dysfunction that can potentially extend into decrease of neuronal network function [81]. In addition to AChE inhibition, MP/NPs may also interfere with other neurotransmitters function. For example, increased concentration of taurine, aspartate, and threonine has been observed in the serum of MPs treated mice, and together with AChE inhibition suggests potential for broader systemic neurotoxicity [11]. Furthermore, several studies illustrate simultaneous suppression of locomotor ability and neurotoxicity [10,13,48,82], providing indirect evidence about a link between MPs/NPs induced changes in neurotransmitters function and behavior alteration of testing model organisms. As the mechanisms of decreased neuronal network function are far from understood, detailed studies are needed to increase our understanding of MP/NPs involvement in this key event.
4.2. Inflammation (KE: 1633, KE: 1496)
Inflammation is a potent mechanism responsible for defenses against pathogenic and other noxic agents, and can be elicited by exposure to, or presence of, xenobiotics or toxicants. In recent years, engineered nanomaterials have entered the environment and can often be found in living organisms and could be considered as a special case of “particulate xenobiotics” that can interact with the immune system [5,39]. Inflammatory processes can be very destructive and must be carefully balanced and controlled at multiple levels of complexity. We will focus on three levels of biological organization (molecular, cellular and tissue) of responses involved in inflammatory responses to MP/NPs. As immune system generally recognizes micro- and nano-plastic particles as xenobiotics, multiple studies include inflammatory response endpoints to measure MP/NPs effects both in vivo and in vitro [10,11,64,[83], [84], [85], [86]].
At molecular level inflammatory responses triggered by MP/NPs exposure mostly involve activation of pro-inflammatory cytokines, signaling molecules secreted primarily by immune cells (leukocytes) [87]. Available reports clearly demonstrate ability of nano-plastic particles to influence release of cytokines or the alteration of inflammatory response genes in vitro cell line models. Pro-inflammatory cytokine IL6, IL8 and IL1β genes are up-regulated in response to 10 μg/ml polystyrene NPs (44 nm) exposure in human gastric adenocarcinoma cells [88] with decreased cell viability [89]. Similarly, Prietl et al. report increased secretion of IL6 and IL8 which was detected after carboxyl polystyrene NPs exposure [14]. However, the authors also noted that IL6 production increased with particle size while IL8 secretion showed the opposite trend in THP-1 monocytic cell line, likely due to regulation of immune responses against pathogens of different sizes [14,26,90]. Details of mechanisms involved in the NPs exposure and cytokines production are not yet clear, however, observed pro-inflammatory responses could relate to the oxidative stress and its corresponding lysosome membrane disintegration [8,15,38,90,91]. As for the possible effects of MPs, it was reported that 0.5 μm polystyrene MPs increased both mRNA levels of IL1α, IL1β and IFN and their respective protein levels in zebrafish gut [84].
The second and third level of inflammatory responses induced by MP/NPs would be cellular responses and tissue damage, respectively. Innate immune cellular effectors such as phagocytic cells appear to interact frequently with MP/NPs, as expected, considering they represent the first line of organismal defenses against particulate matter agents. Increase of degranulation of neutrophil primary granules and neutrophil extracellular trap release was detected after the exposure of both polystyrene and polycarbonate NPs in the fish model [39]. Release of granular contents as well as NETs release, suggested that NPs are capable of phagocyte activation and could function as a potential stressor of innate immunity [92]. Significant influx of neutrophils into the rat lung was observed after inhalation exposure to polystyrene NPs and MPs and the influx was significantly greater for smaller particles (64 nm) compared to larger ones (535 nm), while larger particles caused higher levels of interleukin secretion [90]. These findings demonstrate the potency of MP/NPs to induce inflammatory cellular responses.
Inflammatory tissue damage was detected in different in vivo models after exposure to either MPs or NPs [11,15]. Polystyrene microparticles (5 μm) and nanoparticles (70 nm) were both accumulating in the gill, liver and gut of zebrafish after seven days of exposure, causing typical inflammatory damage in liver (vacuolation, leukocyte/neutrophil infiltration, necrosis, and lipidosis) [15]. Liver histopathology findings were supported by increased SOD and CAT activity, indicating possibility of underlying oxidative stress as triggering inflammatory response. Similar hepatotoxicity was histologically detected in mice model when both 5 μm and 20 μm polystyrene MPs induced liver lipid accumulation and inflammation [11]. Published findings support that both MPs and NPs exposure can promote inflammation at all three levels (molecular, cellular and tissue), possibly through oxidative stress and lysosome dysfunction but more evidence is required for better mechanistic understanding.
5. Individual and population adverse outcomes triggered by micro- and nano-plastics
5.1. Adverse outcomes in organisms of ecotoxicological concern
The U.S. National Toxicology Program of National Institute of Environmental Health and Safety (NTP-NIEHS) is assigning an adverse outcome (AO) designations only to individual or population levels which are specifically referred to in the NTP documents (https://ntp.niehs.nih.gov), and this approach served as basis for this section of the review. Majority of MP/NPs induced AOs are being discovered using organisms of ecotoxicological concern according to Jeong et al. and our literature summary (Table 1) [23]. Key events “Growth Inhibition” (KE 1521, KE 1467), “Impaired Development” (KE 577, KE 339) and “Decreased body size/length” (KE 315, KE 864) represent the most frequent AOs induced by MP/NPs.
The exposure to both MPs and NPs can result in negative effects on individual growth rate of tested organisms (KEs 315, 864, 1467, 1521). Interestingly, reviewed studies indicating the reduction of growth rates induced by MPs or NPs are mostly based on invertebrate models (10 publications about MPs, three about NPs) (Table 1) [23,[93], [94], [95]], likely due to the fact that growth rate evaluation is a standard toxicological endpoint for invertebrate models according to OECD guidelines [96]. Important factor influencing the growth reduction rate induced by MP/NPs appears to be the size of the particles. Recent rotifer study reported that under similar exposure concentrations to polystyrene particles, clear growth rate decrease was observed for 50 nm and 0.5 μm particle sizes, and growth was not different from control when rotifers were exposed to 6 μm MP [26]. Similar results were reported in microalgae (S. costatum), where PVC particles in micro-range (1 μm) induced significant growth inhibition (39.7% maximum) compared to control, while bulk PVC particles (1 mm) showed no effect [97]. It is noteworthy that some studies indicated the growth rate inhibition comes along with the accumulation of MP/NPs due to the persistence of polymers [2]. Their persistence and accumulation within the organisms is one of the main concerns raised about MP/NPs, as both characteristics support a hypothesis that MP/NP particles could transfer within the food web from prey to predator and lead to bioaccumulation and biomagnification. Until now, several laboratory studies demonstrated transfer of plastic particles in low-tier food chains, however, whole system trophic transfers require further investigation [98,99]. For further information regarding trophic level transfers of MPs/NPs, we refer readers to Prinz et al. [6] and Prokić et al. [7] who provided detailed overview on this topic.
Development alterations (KEs 339, 577) were found in zebrafish (D. rerio), green sea urchins (L. variegatus), nematodes (C. elegans) and crustaceans (D. magna) after exposure to MP/NPs, noting that NPs induced development toxicity were more frequently reported [9,12,13,100,101]. MP/NPs caused different adverse effects during larval development even in the same model organism, as two zebrafish larvae studies reported different modes of toxicity. Chen et al. reported that polystyrene MPs exhibited no adverse effects to the development of zebrafish larvae, in contrast to NPs, which induced significant developmental neurotoxicity and disturbance in larval locomotion activity [48]. In study by Pitt et al., polystyrene NPs transferred to offspring and caused bradycardia in the developing zebrafish larvae [101]. Both studies suggest that oxidative stress triggered by NPs could be the reason underlying these toxicities. In other invertebrates, polystyrene NPs were also reported to induce incomplete development of second antennae and cause curved tail spines in Daphnia [9]. It appears that MP/NPs have significant potential to interfere with embryonic development in various ways.
5.2. Adverse outcomes related to potential micro- and nano-plastics public health concerns in human beings
Information about formal Adverse Outcomes (AOs) induced by MPs and NPs in mammalian models (in vivo) is currently almost unavailable in the literature databases. This section will discuss potential systemic adverse effects of MP/NPs exposure in human beings primarily referencing in vitro mammalian cell line studies. In general, possible exposure routes of human beings to MP/NPs are through mucosal surfaces in respiratory system and gastrointestinal (GI) tract, and integument (skin), noting that some of the recently studied drug delivery systems would also expose human beings to MP/NPs via parenteral routes, including intravenous and intracranial/brain application [102].
Polystyrene MP/NPs can be ingested by alveolar epithelial cells, followed by ROS production and occurrence of corresponding cellular adverse key events [103,104]. Furthermore, crossing of NPs into alveolar capillary vessels was observed, suggesting that large alveolar surface area and thin blood/alveolar barriers allow easier NPs penetration to bloodstream and consequential higher bioavailability [105,106]. Epidemiological studies confirmed possible hazards from airborne exposure to NPs, as adverse effects on health were reported after inhalation of synthetic polymers [107]. Information about presence or abundance of MP/NPs in air is scarce, with recent studies indicating that MPs constitute a non-negligible fraction of airborne particulates in both indoor and outdoor air. However, content percentage is relatively low comparing to the threshold suggested by regulatory bodies [108,109].
Scenario of skin exposure to MP/NPs is mostly related to direct contact with plastic containing cosmetics, clothing, or water. Since primary role of skin, especially top epithelial layer with keratinized cells (stratum corneum) is to serve as protective barrier, it is not expected that a significant uptake of MP/NPs can occur through skin, and this hypothesis was confirmed by several studies. Application of polystyrene MPs/NPs to the porcine skin tissue model illustrated that neither 200 nm nor 20 nm NPs were able to penetrate into deeper skin layers [110]. However, MP/NPs may be able to overcome skin barriers through physiological (hair follicles, via sebaceous or sweat gland openings) or pathological (various skin injuries) discontinuities of integument, but lack of available information prevents definitive conclusions.
6. Applying adverse outcome pathways approach to highlight MP/NPs reported toxicity
In previous sections of this review, we focused on mechanical consequential relationships of key events and adverse outcomes induced by either MPs or NPs. Since the adverse outcome pathways (AOPs) initiated by the MPs exposure were developed recently [23], we decided to utilize existing AOPs [111] with focus on literature reports describing the NPs and overlapping MP/NPs toxicity endpoints from ecological and public health perspectives (Fig. 1, Fig. 2). The collection of data to be used in identification of NPs triggered events and assigning them to existing AOPs started with a review of publications reporting the toxicity endpoints induced by NPs in non-mammalian organisms with high ecotoxicological relevance/concern as well as in mammals. Broad literature search was based on following key words: “Nanoplastics”, “Toxicity”, “Ecotoxicology” and “Mammals” using Google scholar search engine. The reports used to generate information to be used in the generation of NP/MP specific oxidative damage AOPs were further evaluated and selected according to following criteria:
-
1.
Studies must be written in English.
-
2.
Studies must be available online before December of 2019.
-
3.
The exposure particles must be polymer plastic particles in a nano-size range.
-
4.
The exposure concentration and exposure duration of NPs must be reported.
-
5.
The exposed organisms must be reported.
-
6.
At least one level of toxicity endpoints (molecular, cellular, tissue, organ, individual, population) should be included.
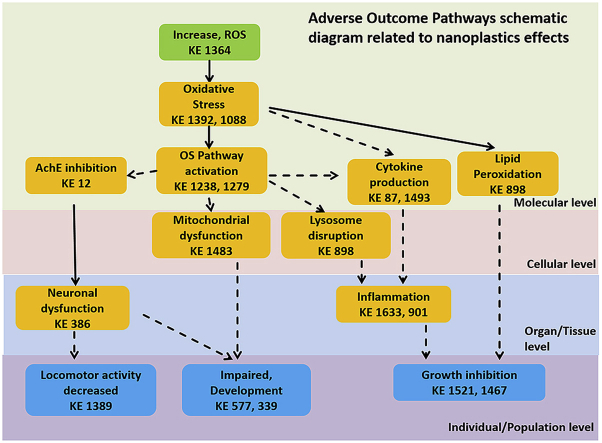
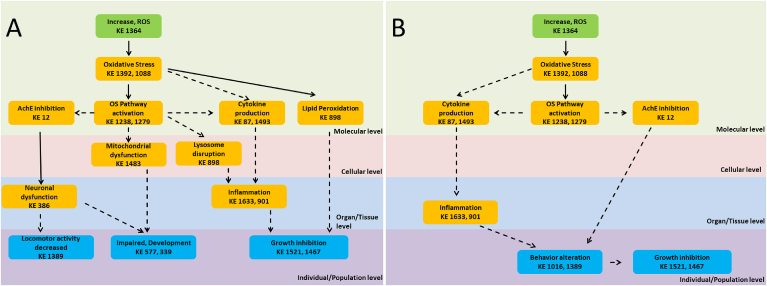
Fig. 1.
Adverse Outcome Pathways schematic diagrams related to nanoplastic (A) and combined micro- and nano-plastics (B) adverse effects from ecological perspective with emphasis on the Oxidative stress and Inflammation responses. Green cuboid: Molecular Initiation Event; Orange cuboid: Key Events; Blue cuboid: Adverse Outcomes. Solid lines: Adjacent or strong evidence relationships; Dashed lines: non-adjacent or weaker evidence supporting the relationship.
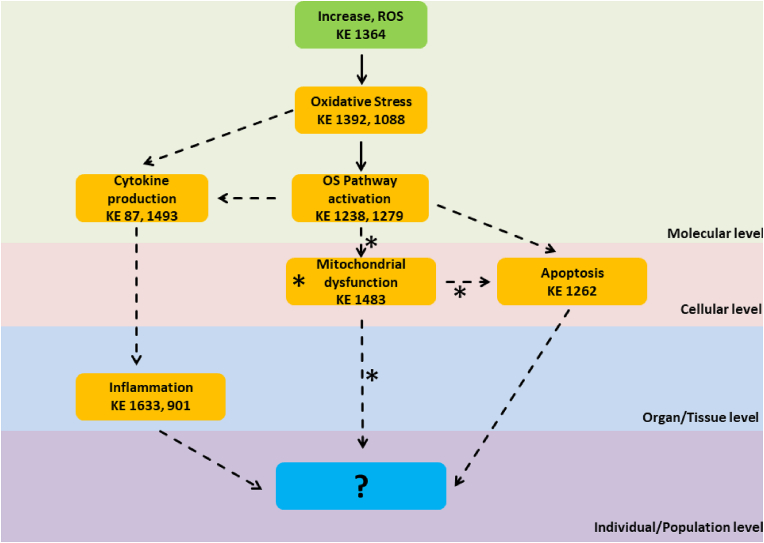
Fig. 2.
Adverse Outcome Pathways schematic diagrams related to nanoplastic and combined micro- and nano-plastics adverse effects from Human/Public health perspective with emphasis on the Oxidative stress and Inflammation responses. Green cuboid: Molecular Initiation Event; Orange cuboid: Key Events; Blue cuboid: Adverse Outcomes. Solid lines: Adjacent or strong evidence relationships; Dashed lines: non-adjacent or weaker evidence supporting the relationship. Asterisk (*) indicates that KE 1483 Mytochondrial dysfunction and related mechanistic relationships apply to nanoplastics AOP only.
After literature search, reported toxicity endpoints triggered by NPs including Key events (KE) and Adverse outcomes (AO) were categorized into 3 different levels of biological organization: Molecular and Cellular; Tissue and Organ; Individual and Population (Table 2, Table 3). As a next step, we used the existing AOP framework and applied it to identified and categorized KEs and AOs using KEs and AOs information available from the AOP wiki website (https://aopwiki.org/), followed by analysis of KE and AO frequency in searched publications.
Table 2.
Nanoplastics induced toxicological endpoints in organisms of ecotoxicological concern.
| Organisms |
Nanoplastics |
Exposure |
Endpoints |
Other major results | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Type of matrix | Plastic type and size | Surface modification | Concentration | Exposure duration | Molecular and cellular (MIE/KE) | Tissue and organ KEs | Individual or population AO | |||
| algae (S. obliquus) | Whole body | PS NPs, 70 nm | Pristine, pristine-kairomone, aged, and aged-filtered NPs | 0.22–103 mg/L | 72 h | Not stated | Not stated | Growth inhibition | Increased mortality of D. magna after exposure by aged PS NPs; PS NPs affected several developmental stages | [9] | |
| D. magna | 21 days | Offspring malformation, reduced body size | |||||||||
| D. magna | Whole body | PS NPs 50–300 nm | Plain PS, PS-p-NH2, PS-n-NH2 and PS-COOH | 0–100 mg/L | 0–48 h | ROS induction, mitochondrial dysfunction | Not stated | Lethality increased | ROS induction intracellularly most significantly by plain PS NPs as the functional group introduction decreased the toxicity in D. magna. No significant influence on AChE activity by plain PS NPs but by functionalized PS NPs. | [55] | |
| LPO and GSH induction | Behavior alteration | ||||||||||
| JNK, p38 MAPKs activated | |||||||||||
| Mytilus galloprovincialis | Hemocytes | PS NPs | PS-NH2 | 1, 5 and 50 μg/mL | 10–60 min | Increased lysosomal membrane destabilization | Not stated | Not stated | Disruption of phagocytic or endocytic pathways, formation of PS–NH2–protein corona | [54] | |
| Activation of p38 MAPK and PKC (Proteinkinase C) | |||||||||||
| Pacific oyster (Crassostrea gigas) | Larvae | PS NPs, 70 nm−20 μm | Plain PS, PS-NH2, PS-COOH | 1000 plastics/mL | 24 h | Not stated | Accumulation of NPs in the larval | No significant impact on developmental rate | The aminated PS beads were engulfed in a greater number by larvae than carboxylated and standard PS beads | [35] | |
| Fathead minnow (Pimephales promelas) | Neutrophils | PS 41.0 nm, polycarbonate 158.7 nm NPs | Plain PS and PC NPs | 0.025–0.2 μg/μL | 2 h | Neutrophils increases in degranulation of primary granules and neutrophil extracellular trap release | Not stated | Not stated | All PSNP concentrations caused a significant increase of the degranulation of neutrophil primary granules in a dose‐dependent manner | [39] | |
| PC NPs induced increase of respiratory burst | |||||||||||
| Danio rerio | Larvae | PS NPs, 50 nm | Plain PS NPs | 1 mg/L | 48, 72, and 120 h | ROS induction detected | Not stated | Larval behavioral alteration | Nanoplastics significantly inhibited the acetylcholinesterase activity, upregulated rhodopsin and blue opsin gene expression, reduced the length of larvae body and limited the larvae locomotion. | [48] | |
| Nervous system development related genes’ expression alterated | Body length reduced | ||||||||||
| Locomotion hampered | |||||||||||
| AChE activity related neurotoxicity detected | |||||||||||
| Daphnia pulex | Whole body | PS NPs, 50 nm | Plain PS NPs | 0.1 mg/L 1 mg/L | 1–21 days | Antioxidant genes expression alterated | Not stated | Not stated | LC50 values of 1- and 21-day-old D. pulex did not significantly differ from different age groups, suggesting that juveniles and relatively old adults could be equally sensitive to the NPs | [93] | |
| Genes encoding heat shocking proteins changed | |||||||||||
| energy-sensing enzyme AMPKα, β and γ were significant different | |||||||||||
| Monogonont Rotifer (Brachionus koreanus) | Whole body | PS 50 nm | Plain PS NPs | 0.1, 1, 10, and 20 μg/mL | 24 h, 12 days | ROS, MAPK and Oxidative stress (p-JNK, p-p38, GPx, GR, GST, SOD) with highest level by NPs exposure | Not stated | Fecundity reduced by NPs | Nanosized plastic particles cause adverse effects on normal physiological responses on growth, hatching, and reproduction in the rotifer and also indirectly may affect energy flow in the aquatic ecosystem | [26] | |
| Prolonged Reproduction time | |||||||||||
| Mitochondrial membrane integrity decreased by NPs exposure | |||||||||||
| Growth rate decreased | |||||||||||
| Oyster gametes (Crassostrea gigas) | Gametes | PS NPs, 100 nm | PS PS-COOH PS-NH2 | 0.1–100 mg/L | 1–5 h | PS-COOH induced ROS production in spermatozoa | Spermatozoa aggregation | Not stated | A significant dose-response increase in ROS production by spermatozoa was demonstrated upon exposure to PS-COOH, but not with PS-NH2. | [75] | |
| Daphnia pulex | Whole body | PS NPs, 75 nm | Plain PS NPs | 0.1–2 mg/L | 21 days | The expression of cytochrome P450 (CYP) family genes were alterated | Not stated | Not stated | The transcriptional levels of DpCYP370B, CYP4AN1, CYP4C33, and CYP4C34 were induced by low concentrations of nanoplastics and inhibited at high concentrations of nanoplastics | [86] | |
| Danio rerio | Larvae | PS NPs, 25 nm | Plain PS NPs | 10–100 mg/L | 48 h | Glucose level during development hampered, insulin expression decreased | PSNPs accumulation in neuromasts thus hamper the HPI-axis, leading to cortisol secretion | Larvae locomotor activity alterated | Polystyrene nanoparticles can disrupt glucose homoeostasis with concurrent activation of the stress response system during the development of zebrafish larvae | (Brun, van Hage et al., 2019) | |
| Danio rerio | Embryo | PS NPs, 25 nm | Plain PS NPs | 10 mg/L | 0–120 h | mRNA of pro-inflammatory cytokines (irg1l, il1β, and tnfα) were significantly altered | Inflammatory responses in the tissues affected, particularly the intestine, the skin, and neuromasts | Not stated | Obtained results provide the first evidence that nanoparticles can induce pro-inflammatory responses in the skin and intestine cells. | [10] | |
| Blue mussel (Mytilus edulis) | Whole body | 30‐nm PS NPs | Plain PS NPs | 0, 0.1, 0.2, and 0.3 g/L | 4 h | Not stated | Not stated | PS NPs triggered the production of pseudofeces | Long‐term exposure to nano PS may therefore harm M. edulis because producing pseudofeces expends energy and reduced filtering activity may eventually lead to starvation. | [94] | |
| Based on the reduced opening of the valve, the organism's filtering activity was reduced | |||||||||||
| Caenorhabditis elegans | Whole body | 100 and 500 nm, PS NPs | Plain PS NPs | 1 mg/L | 3 days | PS NPs enhanced the expression of GST-4 and induced oxidative stress | Damage in cholinergic and GABAergic neurons | Motor behavior changed (body bending and head thrashing) | Nanoplastics inhibited the growth and development of nematode individuals, altered locomotor behavior in a manner of size-dependent toxicity, induced significantly oxidative damage, and led to neurotoxicity. | [13] | |
| AChE transporter encoding genes expression alterated (unc-17, unc-47 and dat-1) | Growth inhibition | ||||||||||
| Impaired development | |||||||||||
| Mussel (Mytilus galloprovincialis) | Whole body | 110 ± 6.9 nm, PS NPs | Plain PS NPs | 0.005–50 mg/L | 96 h | Cell-tissue repair related gene hsp70 alterated after 50 mg/L PS NPs exposure in GI track | Not stated | Not stated | PS NPs, even at low concentrations, led to alterations on biotransformation, DNA repair, cell stress-response and innate immunity genes. Also, the genotoxicity and the oxidative stress were detected in the mussel. | [64] | |
| cyp11 gene expression increase, phase I metabolism activated | |||||||||||
| Immune functional genes activated | |||||||||||
| Oxidative stress and Lipid peroxidation | |||||||||||
| decreased AChE activity | |||||||||||
| DNA damage occurred | |||||||||||
| Mussel (Mytilus galloprovincialis) | Hemocytes | 50 nm, PS NPs | PS-NH2 | 1–50 mg/L | 0.5 h | lysosomal damage | Not stated | Not stated | Putative C1q domain containing protein (MgC1q6) as the only component of the PS-NH2 hard protein corona in Mytilus hemolymph. | [54] | |
| apoptotic processes | |||||||||||
| oxyradical production | |||||||||||
| membrane blebbing and loss of filopodia | |||||||||||
| Danio rerio | Adult | 42 nm, PS NPs | Plain PS NPs | 5 mg/L | 7 days | Oxidative stress in the brain of the female, muscle and gonad of the male | N/A | N/A | PS nanoplastics can be transferred from mothers to the offspring via accumulation in the eggs due to interaction of nanoplastics with plasma proteins of oocytes but not effecting the fecundity. | [101] | |
| Embryo | N/A | bradycardia | Developmental impairment | ||||||||
| Danio rerio | Embryo | 51 nm, PS NPs | Plain PS NPs | 0–10 ppm | 120 h | Not stated | bradycardia | Altered larval behavior | PS NPs can penetrate the zebrafish chorion and are taken up by the embryo | [100] | |
| Danio rerio | Adult | 70 nm PS NPs | Plain PS NPs | 20 mg/L | 7 days | Oxidative stress | Liver | Inflammation | Metabolism stress (in lipid metabolism and energy metabolism) | PS NPs accumulated in gills, liver, and gut of zebrafish | [15] |
| lipid accumulation | |||||||||||
| necrosis | |||||||||||
| Caenorhabditis elegans | Whole body | 100 nm, PS NPs | Plain PS NPs | 10–100 mg/L | Approximately 4.5 days | ROS production | Intestinal permeability increased | decreased locomotion behavior | Adverse effect on the function of the intestinal barrier in nematodes was detected. Also the transgenerational toxicity detected | [95] | |
| reduced body size | |||||||||||
Table 3.
Nanoplastics induced toxicological endpoints based on mammalian studies.
| Organisms |
Nanoplastics |
Exposure |
Endpoints |
Other major results | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Cell/Tissue type | Plastic type and size | Surface modification | Concentration/Dose | Exposure duration | Molecular and cellular (MIE/KE) | Tissue and organ KEs | Individual or population AO | ||
| Wistar rats | Whole body | PS NPs 38.92 nm | Plain PS NPs | 1, 3, 6, and 10 mg PS-NPs/kg of body weight/day | 35 days | Not stated | Not stated | may exacerbate behavioral effects | The uptake of pristine nanoparticles did not affect behavior of adult rats | [82] |
| Wistar rats | Whole body | 25 and 50 nm, PS NPs | Plain PS NPs | 1, 3, 6, and 10 mg PS-NPs/kg of body weight/day | 35 days | high-density lipoprotein level blocked | Not stated | thyroid endocrine disruption | PS treatments significantly increased serum LDL, cholesterol, GOT, and GPT levels notably | [19] |
| Human and mouse | human bronchial epithelium (BEAS-2B), mouse monocyte macrophage (RAW 264.7) | PS NPs 60 nm | Plain PS, PS-NH2, PS-COOH | 10–25 mg/L | 1–8 h | lysosomal permeability increased in RAW 264.7 after PS-NH2 exposure | Not stated | Not stated | The cationic PS nanospheres had no effect on cellular toxicity in HEPA-1, HMEC, and PC-12 cells | [70] |
| Ca2+ influx increased in RAW 264.7 after PS-NH2 exposure | ||||||||||
| Apoptosis | ||||||||||
| Mitochondrial disruption | ||||||||||
| Human | colon carcinoma cells (Caco-2) | PS NPs 20–40 nm | Plain PS, PS-NH2, PS-COOH | 0.3, 0.9, 2.0, 4.0, and 6.6 nM | 4–16 h | Cell viability hampered | Not stated | Not stated | the uptake efficiency is surface charging and size dependent | [85] |
| Oxidative stress leading apoptosis | ||||||||||
| Human | alveolar epithelial type 1, 2 cells (TT1, AT2), primary alveolar macrophages (MAC) | PS NPs 50–100 nm | Plain PS, PS-NH2, PS-COOH | 1–100 mg/L | 4 and 24 h | PS-NH2 induced apoptosis in all cell types | Not stated | Not stated | Plain PS, PS-COOH exhibited little cytotoxicity or mitochondrial damage, although they induced ROS; TT1 and MAC cells internalized all NP formats, whereas only a small fraction of AT2 cells internalized PS-NH2 | [71,104] |
| All NPs induced ROS induction | ||||||||||
| PS-NH2 induced mitochondrial disruption and release of cytochrome C | ||||||||||
| Human | monocytic leukemia cell line THP-1, histocytic lymphoma cells U937 | PS NPs 20, 100, 200, 500, 1000 nm | PS-COOH | 10, 20 and 50 mg/L | 30 mis to 24 h | PS-COOH induced cytokine production (IL-8, IL-6) | Not stated | Not stated | Twenty nanometers CPS were cytotoxic to all phagocytes, ≥500 nm CPS particles only to macrophages. 20 nm particles were taken up passively, 100−1000 nm actively and passively. |
[14] |
| 20 nm PS-COOH induced Oxidative stress | ||||||||||
| PS-COOH stimulated myeloperoxidase release of granulocytes and nitric oxide generation in macrophages | ||||||||||
| Human | Calu-3 epithelial cells, monocytic leukemia cell line THP-1 | PS NPs 50 nm | Plain PS, PS-NH2, PS-COOH | 1–100 mg/L | 1–24 h | PS-NH2 nanobeads induced DNA double strand breaks |
Not stated | Not stated | Particles partly adsorbed and internalized then released by Calu-3 cells; THP-1 macrophages quickly incorporated all nanobeads. Surface modification matters in the nanotoxicology. | [60] |
| GSH depletion, antioxidant hamper | ||||||||||
| Human | colon carcinoma cells (Caco-2, LS174T, HAT-29) | PS NPs 57 nm | Plain PS, PS-NH2, PS-COOH | 20, 50, and 100 μg/mL | 72 h | Induction of apoptosis by PS-NH2 | Not stated | Not stated | Positively Charged Polystyrene NPs Reduce Cell Viability; binding of mucin | [89] |
| Human | Monocyte macrophages | PS NPs; 120 nm | Plain PS, PS-NH2, PS-COOH | 100 μg/ml | 24 h | Nanoplastics impaired expression of scavenger receptor (CD163 and CD200R) in M2 | Not stated | Not stated | The nanoparticles did not compromise macrophage viability nor did they affect the expression of the M1 markers CD86, NOS2, TNF-α, and IL-1β. | [83] |
| Nanoplastics impaired the release of cytokines (IL-10) in M2 | ||||||||||
| Frustrated phagocytosis by PS-NH2 | ||||||||||
| PS-COOH increased ATP level in M2 | ||||||||||
| Human | Gastric adenocarcinoma (AGS) cells | PS NPs, 44 and 100 nm | Plain PS | 2, 5, 10, 20 and 30 μg/ml | 1–24 h | Cytokine genes expression increase (IL-6 and IL-8) | Not stated | Not stated | NPs in smaller size accumulate rapidly and more efficiently in the cytoplasm of AGS than bigger size; energy dependent mechanism of internalization and a clathrin-mediated endocytosis pathway | [88] |
| Up Regulation, TGFbeta1 expression | ||||||||||
| Human | ovarian cancer cells | PS NPs 10–30, 50 nm | Plain PS, PS-NH2, PS-COOH | 10–75 μg/ml | 1–8 h | PS-NH2 accumulated within lysosomes | Not stated | Not stated | Polystyrene nanoparticle uptake occurred via a caveolae-independent pathway, and was negatively affected by serum | [74] |
| cell death | ||||||||||
| Human | lung adenocarcinoma cells (A549) | PS NPs 64, 202, 535 nm | Not stated | 250 μg/ml or 2 mg/ml | 2–24 h | Increased cytokine production | Not stated | Not stated | Ultrafine polystyrene particles also stimulated the entry of extracellular calcium on treatment with thapsigargin | [90] |
| Rat | Whole body | 24 h | lactate dehydrogenase increase | Neutrophil influx (Infiltration, Inflammatory cells) | Not stated | |||||
| Inflammation | ||||||||||
In total, 31 publications were selected (as 100%, full text available as of January 2020) according to the above criteria and reported toxicity endpoints induced by NPs were evaluated. KEs with highest reported frequency were “Oxidative stress” (45.2%), “Pro-inflammatory cytokines activation” (19.4%), “Increase, ROS” (19.4%) and “Apoptosis” (16.1%). Based on this data, the oxidative stress and inflammatory responses are considered as major adverse events induced by NPs at all the biological complexity levels except individual and population levels. This NP related finding is in agreement with previously reported AOP summary for MPs [23]. Among the critical key events, we determined that increase of ROS could be designated as Molecular Initiating Event (MIE). ROS generation is critical molecular event that is occurring prior to most frequent KE (Oxidative stress), as stated previously (Section 2.1). Other significant KEs at molecular/cellular level are “Mitochondrial dysfunction” (13.0%), “Acetylcholinesterase (AchE) inhibition” (9.7%), “Lysosome disruption” (9.7%), “Lipid peroxidation” (9.7%) and “Activation of oxidative stress pathway” (9.7%). Mechanistic relationship between these KEs was reviewed and discussed in Sections 2, 3.
Information regarding Tissue/Organ KEs was found to be limited in reports that fulfill selection criteria, possibly due to high diversity and lack of standardization of research methods and techniques used in studies of MP/NPs effects at this level. In selected literature, most abundantly reported KEs are “Inflammation” (13.0%) and “Decrease of neuronal network function” (13.0%). Final emerging AOs are listed as “Locomotor activity, decreased” (22.6%), “Impaired, Development” (13.0%), “Decreased body size/length” (13.0%) and “Growth inhibition” (9.7%). It was determined that studies related to individual/population adverse outcomes are almost exclusively available for organisms with ecotoxicological concern. Lack of in vivo mammalian studies of NPs toxicity is currently causing a gap in our knowledge and prevents development and possible construction of detailed AOPs (Table 1). The current status and mechanistic relationships between above listed AOs and KEs are discussed in Section 5.
Selection of existing AOPs or their components related to nanoplastics toxicity was based on most prominent KEs and AOs. Developed schematic was presented as ecologically relevant AOP (Fig. 1A), and human public health perspective AOP (Fig. 2). The NPs AOP indicate that at the ecosystem level, NPs could cause toxicity based on increased ROS formation through activation of oxidative stress pathways, followed by cytokine production, mitochondrial dysfunction and AChE inhibition, that in turn would further induce inflammation and neuronal dysfunction to cause changes in activity/behavior, and interfere with normal development and growth.
Use of AOPs to highlight public health perspective of NPs toxicity was limited with the scarcity of available data from publication fulfilling the listed criteria, and the outcome is therefore simpler. In short, generation of ROS can lead to oxidative stress and inflammatory responses that induce apoptosis. However, final AOs from a public health perspective are largely unknown. In an effort to partially overcome this problem, we used MPs related AOPs as reported by Jeong et al. and modified it as AOP schematic to include both MPs and NPs (Fig. 2). Overall, the results of applying the AOP approach to evaluate MP/NP toxicity indicate that oxidative stress and inflammation responses initiated by ROS production represent two cascades of critical adverse key events that are shared by MP/NPs in both ecological and public health perspectives. Main difference between the two AOP schematics is that from ecological perspective, the relevant pathway involves inhibition of AChE followed with locomotor or feed behavior changes leading to growth retardation (Fig. 1). From public health perspective, apoptosis induced by oxidative stress pathway activation may be the relevant AOP cascade (Fig. 2).
7. Conclusion
Toxicity mechanisms induced by both microplastics and nanoplastics were reviewed with an emphasis on oxidative stress and inflammation. These adverse effects were categorized into different levels of biological complexity from both ecosystem and human health perspectives. Mechanistic relationships between different toxicity endpoints have been discussed using the concept of Adverse Outcome Pathways. The information about key events and adverse outcomes was collected and matched with the online knowledgebase to utilize existing AOPs and apply them to highlight possible effect mechanisms of nanoplastics alone and cumulative micro- and nano-plastics on ecosystems and public health. Development of AOP schematics based on AOPs knowledge base and literature search revealed that MP/NPs both share the ROS formation as their initial molecular occurrence, causing AOs such as growth inhibition and behavior alteration through oxidative stress cascades and inflammatory responses in the ecotoxicological context. From public health perspective, AOP of MP/NPs toxicity showed no clear adverse outcomes probably due to limited information, however it also supported that events of oxidative stress and its responding pathways, including inflammation play a critical role.
Acknowledgements
The authors would like to acknowledge assistance from Chinese Scholarship Council support to Dr Hu; and to colleagues from the LMU Chair for Fish Diseases and Fisheries Biology who provided constructive criticism of the manuscript.
References
- 1.AISBL P. Plastics–the facts 2018. 2018. http://www.-plasticseurope.org/application Informationen abgerufen unter.
- 2.Li H., Witten T. Fluctuations and persistence length of charged flexible polymers. Macromolecules. 1995;28(17):5921–5927. [Google Scholar]
- 3.Lusher A. Marine Anthropogenic Litter. Springer; Cham: 2015. Microplastics in the marine environment: distribution, interactions and effects; pp. 245–307. [Google Scholar]
- 4.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28(1):2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng E.-L. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018;627:1377–1388. doi: 10.1016/j.scitotenv.2018.01.341. [DOI] [PubMed] [Google Scholar]
- 6.Prinz N., Korez Š. YOUMARES 9-The Oceans: Our Research, Our Future. Springer; 2020. Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: a review; pp. 101–120. [Google Scholar]
- 7.Prokić M.D. Ecotoxicological effects of microplastics: examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. February 2019;111:37–46. [Google Scholar]
- 8.Avio C.G. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Besseling E. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014;48(20):12336–12343. doi: 10.1021/es503001d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brun N.R. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci.: Nano. 2018;5(4):904–916. [Google Scholar]
- 11.Deng Y. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobre C. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: echinoidea) Mar. Pollut. Bull. 2015;92(1–2):99–104. doi: 10.1016/j.marpolbul.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Lei L. Polystyrene (nano) microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci.: Nano. 2018;5(8):2009–2020. [Google Scholar]
- 14.Prietl B. Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol. Toxicol. 2014;30(1):1–16. doi: 10.1007/s10565-013-9265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016;50(7):4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- 16.Weber A. PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. Pollut. 2018;234:181–189. doi: 10.1016/j.envpol.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Imhof H.K. Do microplastic particles affect Daphnia magna at the morphological, life history and molecular level? PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schirinzi G.F. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Amereh F. 2019. Thyroid Endocrine Status and Biochemical Stress Responses in Adult Male Wistar Rats Chronically Exposed to Pristine Polystyrene Nanoplastics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villeneuve D.L. Adverse outcome pathway networks II: network analytics. Environ. Toxicol. Chem. 2018;37(6):1734–1748. doi: 10.1002/etc.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leist M. Adverse outcome pathways: opportunities, limitations and open questions. Arch. Toxicol. 2017;91(11):3477–3505. doi: 10.1007/s00204-017-2045-3. [DOI] [PubMed] [Google Scholar]
- 22.Martens M. Introducing WikiPathways as a data-source to support Adverse Outcome Pathways for regulatory risk assessment of chemicals and nanomaterials. Front. Genet. 2018;9:661. doi: 10.3389/fgene.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J., Choi J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere. 2019;231:249–255. doi: 10.1016/j.chemosphere.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Freeman B.A., Crapo J.D. Biology of disease: free radicals and tissue injury. Lab. Invest. 1982;47(5):412–426. a journal of technical methods and pathology. [PubMed] [Google Scholar]
- 25.Paul-Pont I. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016;216:724–737. doi: 10.1016/j.envpol.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Jeong C.-B. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus) Environ. Sci. Technol. 2016;50(16):8849–8857. doi: 10.1021/acs.est.6b01441. [DOI] [PubMed] [Google Scholar]
- 27.Jeong C.-B. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017;7 doi: 10.1038/srep41323. 41323-41323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza Machado A.A. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biol. 2018;24(4):1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tidjani A. Comparison of formation of oxidation products during photo-oxidation of linear low density polyethylene under different natural and accelerated weathering conditions. Polym. Degrad. Stabil. 2000;68(3):465–469. [Google Scholar]
- 30.Celina M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stabil. 2013;98(12):2419–2429. [Google Scholar]
- 31.Jahnke A. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 2017;4(3):85–90. [Google Scholar]
- 32.Yousif E., Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus. 2013;2 doi: 10.1186/2193-1801-2-398. 398-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comprehensive Polymer Science and Supplements: Comprehensive Polymer Science and Supplements-Online. Elsevier Science; 1996. [Google Scholar]
- 34.Pannetier P. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part I-adverse effects on fish cell line. Environ. Pollut. 2019;248:1088–1097. doi: 10.1016/j.envpol.2018.12.091. [DOI] [PubMed] [Google Scholar]
- 35.Cole M., Galloway T.S. Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol. 2015;49(24):14625–14632. doi: 10.1021/acs.est.5b04099. [DOI] [PubMed] [Google Scholar]
- 36.Qiao R. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019;662:246–253. doi: 10.1016/j.scitotenv.2019.01.245. [DOI] [PubMed] [Google Scholar]
- 37.Geys J. In vitro study of the pulmonary translocation of nanoparticles: a preliminary study. Toxicol. Lett. 2006;160(3):218–226. doi: 10.1016/j.toxlet.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Von Moos N., Burkhardt-Holm P., Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012;46(20):11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 39.Greven A.C. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 2016;35(12):3093–3100. doi: 10.1002/etc.3501. [DOI] [PubMed] [Google Scholar]
- 40.Wen B. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus) Environ. Pollut. 2018;243:462–471. doi: 10.1016/j.envpol.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013;32(3):249–270. doi: 10.3109/08830185.2012.755176. [DOI] [PubMed] [Google Scholar]
- 42.Yu F. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019;694:133643. doi: 10.1016/j.scitotenv.2019.133643. [DOI] [PubMed] [Google Scholar]
- 43.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 44.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86(1):715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 45.Livingstone D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001;42(8):656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- 46.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54(4):287–293. [Google Scholar]
- 47.Di Giulio R.T. In: Indices of Oxidative Stress as Biomarkers for Environmental Contamination. Mayes M.A., Barron M.G., editors. ASTM International; West Conshohocken, PA: 1991. pp. 15–31. [Google Scholar]
- 48.Chen Q. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017;584:1022–1031. doi: 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- 49.McCubrey J.A., LaHair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants Redox Signal. 2006;8(9–10):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 50.Shi X., Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. 2010;115(2):391–400. doi: 10.1093/toxsci/kfq066. [DOI] [PubMed] [Google Scholar]
- 51.Park J.C. Adverse effects of BDE-47 on life cycle parameters, antioxidant system, and activation of MAPK signaling pathway in the rotifer Brachionus koreanus. Aquat. Toxicol. 2017;186:105–112. doi: 10.1016/j.aquatox.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Wang H. The molecular mechanism of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by BaP in the scallop Chlamys farreri. Fish Shellfish Immunol. 2019;92:489–499. doi: 10.1016/j.fsi.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Yu P. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018;200:28–36. doi: 10.1016/j.aquatox.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Canesi L. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: role of soluble hemolymph proteins. Environ. Res. 2016;150:73–81. doi: 10.1016/j.envres.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 55.Lin W. Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019;180:509–516. doi: 10.1016/j.ecoenv.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 56.Nam T.-G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011;27(1):1–6. doi: 10.5487/TR.2011.27.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barboza L.G.A. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758) Aquat. Toxicol. 2018;195:49–57. doi: 10.1016/j.aquatox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro F. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017;122(1):379–391. doi: 10.1016/j.marpolbul.2017.06.078. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y. Intracellular dynamics of cationic and anionic polystyrene nanoparticles without direct interaction with mitotic spindle and chromosomes. Biomaterials. 2011;32(32):8291–8303. doi: 10.1016/j.biomaterials.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 60.Paget V. Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massoulié J. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993;41(1):31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 62.Worek F. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch. Toxicol. 2002;76(9):523–529. doi: 10.1007/s00204-002-0375-1. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira M. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Ecol. Indicat. 2013;34:641–647. [Google Scholar]
- 64.Brandts I. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018;643:775–784. doi: 10.1016/j.scitotenv.2018.06.257. [DOI] [PubMed] [Google Scholar]
- 65.Kashiwada S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes) Environ. Health Perspect. 2006;114(11):1697–1702. doi: 10.1289/ehp.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milatovic D., Gupta R.C., Aschner M. Anticholinesterase toxicity and oxidative stress. Sci. World J. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcimartín A. Hydrogen peroxide modifies both activity and isoforms of acetylcholinesterase in human neuroblastoma SH-SY5Y cells. Redox Biol. 2017;12:719–726. doi: 10.1016/j.redox.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta Bioenerg. 2006;1757(5):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 69.Lu L. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 70.Xia T. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2(1):85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 71.Ruenraroengsak P., Tetley T.D. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells. Part. Fibre Toxicol. 2015;12:19. doi: 10.1186/s12989-015-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore M.N., Icarus Allen J., McVeigh A. Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar. Environ. Res. 2006;61(3):278–304. doi: 10.1016/j.marenvres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Lawrence R.E., Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019;21(2):133–142. doi: 10.1038/s41556-018-0244-7. [DOI] [PubMed] [Google Scholar]
- 74.Ekkapongpisit M. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: effects of size and surface charge groups. Int. J. Nanomed. 2012;7:4147–4158. doi: 10.2147/IJN.S33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.González-Fernández C. Cellular responses of Pacific oyster (Crassostrea gigas) gametes exposed in vitro to polystyrene nanoparticles. Chemosphere. 2018;208:764–772. doi: 10.1016/j.chemosphere.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 76.Stern S.T., Adiseshaiah P.P., Crist R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012;9(1):20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Moos N., Burkhardt-Holm P., Kohler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012;46(20):11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 78.Pinsino A. Titanium dioxide nanoparticles stimulate sea urchin immune cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway. Sci. Rep. 2015;5(1):14492. doi: 10.1038/srep14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiorentino I. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015;330(2):240–247. doi: 10.1016/j.yexcr.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Regoli F., Giuliani M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014;93:106–117. doi: 10.1016/j.marenvres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Keefer E.W. Acute toxicity screening of novel AChE inhibitors using neuronal networks on microelectrode arrays. Neurotoxicology. 2001;22(1):3–12. doi: 10.1016/s0161-813x(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 82.Rafiee M. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere. 2018;193:745–753. doi: 10.1016/j.chemosphere.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 83.Fuchs A.-K. Carboxyl-and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78–87. doi: 10.1016/j.biomaterials.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 84.Jin Y. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018;235:322–329. doi: 10.1016/j.envpol.2017.12.088. [DOI] [PubMed] [Google Scholar]
- 85.Thubagere A., Reinhard B.M. Nanoparticle-induced apoptosis propagates through hydrogen-peroxide-mediated bystander killing: insights from a human intestinal epithelium in vitro model. ACS Nano. 2010;4(7):3611–3622. doi: 10.1021/nn100389a. [DOI] [PubMed] [Google Scholar]
- 86.Wu D. Molecular characterisation of cytochrome P450 enzymes in waterflea (Daphnia pulex) and their expression regulation by polystyrene nanoplastics. Aquat. Toxicol. 2019;217:105350. doi: 10.1016/j.aquatox.2019.105350. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J.-M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forte M. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. Vitro. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 89.InkielewiczStepniak I. The role of mucin in the toxicological impact of polystyrene nanoparticles. Materials. 2018;11(5):724. doi: 10.3390/ma11050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown D.M. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001;175(3):191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 91.Hussain T. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/7432797. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006;2(3):98. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z. Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat. Toxicol. 2018;204:1–8. doi: 10.1016/j.aquatox.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 94.Wegner A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.) Environ. Toxicol. Chem. 2012;31(11):2490–2497. doi: 10.1002/etc.1984. [DOI] [PubMed] [Google Scholar]
- 95.Zhao L. Transgenerational toxicity of nanopolystyrene particles in the range of μg L− 1 in the nematode Caenorhabditis elegans. Environ. Sci.: Nano. 2017;4(12):2356–2366. [Google Scholar]
- 96.OECD . OECD Publishing; Paris: 2011. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2. [DOI] [Google Scholar]
- 97.Zhang C. Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ. Pollut. 2017;220:1282–1288. doi: 10.1016/j.envpol.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Murray F., Cowie P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758) Mar. Pollut. Bull. 2011;62(6):1207–1217. doi: 10.1016/j.marpolbul.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 99.Batel A. Microplastic accumulation patterns and transfer of benzo [a] pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018;235:918–930. doi: 10.1016/j.envpol.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 100.Pitt J.A. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio) Aquat. Toxicol. 2018;194:185–194. doi: 10.1016/j.aquatox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitt J.A. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci. Total Environ. 2018;643:324–334. doi: 10.1016/j.scitotenv.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 103.Varela J.A. Quantifying size-dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol. 2012;10(1):39. doi: 10.1186/1477-3155-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruenraroengsak P., Tetley T.D. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells. Part. Fibre Toxicol. 2015;12(1):19. doi: 10.1186/s12989-015-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gehr P., Bachofen M., Weibel E.R. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir. Physiol. 1978;32(2):121–140. doi: 10.1016/0034-5687(78)90104-4. [DOI] [PubMed] [Google Scholar]
- 106.Kreyling W.G., Hirn S., Schleh C. Nanoparticles in the lung. Nat. Biotechnol. 2010;28(12):1275–1276. doi: 10.1038/nbt.1735. [DOI] [PubMed] [Google Scholar]
- 107.Holm M., Dahlman-Höglund A., Torén K. Respiratory health effects and exposure to superabsorbent polymer and paper dust-an epidemiological study. BMC Publ. Health. 2011;11(1):557. doi: 10.1186/1471-2458-11-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dris R. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 109.Vianello A. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019;9(1):8670. doi: 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alvarez-Román R. Skin penetration and distribution of polymeric nanoparticles. J. Contr. Release. 2004;99(1):53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Bal-Price A., Meek M.E.B. Adverse outcome pathways: application to enhance mechanistic understanding of neurotoxicity. Pharmacol. Therapeut. 2017;179:84–95. doi: 10.1016/j.pharmthera.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]