Abstract
Accumulation of senescent cells has a causative role in the pathology of age-related disorders including atherosclerosis (AS) and cardiovascular diseases (CVDs). However, the concept of senescence is now drastically changing, and the new concept of senescence-associated reprogramming/stemness has emerged, suggesting that senescence is not merely related to “cell cycle arrest” or halting various cellular functions. It is well known that disturbed flow (D-flow) accelerates pre-mature aging and plays a significant role in the development of AS. We will discuss in this review that pre-mature aging induced by D-flow is not comparable to time-dependent aging, particularly with a focus on the possible involvement of senescence-associated secretory phenotype (SASP) in senescence-associated reprogramming/stemness, or increasing cell numbers. We will also present our outlook of nicotinamide adenine dinucleotides (NAD)+ deficiency-induced mitochondrial reactive oxygen species (mtROS) in evoking SASP by activating DNA damage response (DDR). MtROS plays a key role in developing cross-talk between nuclear-mitochondria, SASP, and ultimately atherosclerosis formation. Although senescence induced by time and various stress factors is a classical concept, we wish that the readers will see the undergoing Copernican-like change in this concept, as well as to recognize the significant contrast between pre-mature aging induced by D-flow and time-dependent aging.
Keywords: Aging, Senescence, Atherosclerosis, Oxidative stress, Telomere shortening, Senescent-associated stemness
1. Two different types of flow and atherosclerosis
Vasculature or ‘vascularis’ in Latin refers to ducts or vessels. Human vascular ducts carry an important, life sustaining, non-Newtonian fluid cargo: blood, that circulates through the entirety of the human body. Leonardo Da Vinci in the 15th century observed that blood flow principles in vascular system are analogous to flow of water against obstacles in nature. Unlike other ducts, human vasculature is a dynamic structure and extremely responsive; not only to biomolecules but most importantly to blood flow patterns and characteristics [[1], [2], [3]]. Vascular dynamics are predominantly governed by a thin monolayer of flat cell type, lining the inner wall of the vessels termed as endothelial cells (ECs) [[4], [5], [6], [7], [8], [9]]. Rokitansky and Virchow were among the first to recognize the importance of hemodynamics and role of ECs in vascular health [8,10].
Blood vessels are constantly exposed to hemodynamic forces, primarily to shear stress (τ), which is defined as the tangential component of the frictional force between layers of fluid caused by flow movement and the vessel wall. Shear stress is measured in Newtons (N)/m2, Pascal (Pa) or dyne/cm2. Shear stress also induces a shearing deformation on the interface of the fluid and vasculature, primarily experienced by the endothelium.
Blood flow patterns or hemodynamic patterns have significant physiological outcomes on the vasculature and its maintenance [[11], [12], [13], [14], [15]]. These patterns can be broadly classified into: well-ordered streamlined laminar flow (L-flow) and an irregular, non-uniform disturbed flow (D-flow), which include recirculation eddies, retrograde or reflux flow, reciprocating flow, flow separation and re-attachment [12,16,17]. D-flow regions exhibit high EC turnover, dysfunction and inflammation, leading to severe vascular pathologies [7,14]. The so called ‘athero-prone’ regions, which are locations of high susceptibility to AS formation, are primarily areas with arterial branching and curvatures or bends where local D-flow develops [[18], [19], [20], [21]]. These lesion prone areas are estimated to experience shear stress in the order of 4 dyne/cm2 [12]. In distinction, straight segments of arteries experience uniform linear L-flow and are usually athero-protective. These protective regions exhibit activated antioxidant pathways and low EC turnover rates [22]. The mean shear stress levels in the L-flow area of large human arteries is estimated to be ~15–20 dyne/cm2 [22]. D-flow is also experienced in aortic valvular leaflets, which leads to valvular EC inflammation, in turn resulting in lesion development [23,24]. Retrograde flow associated with dysfunction or obstruction of the venous system causes venous hypertension, venous EC inflammation and dysfunction, leading to several venous vascular pathologies [22,25]. Therefore, L-flow is athero-protective while D-flow can be detrimental to EC functions, and leads to the development of diseases caused by EC dysfunction. In this review we would like to present the concept of D-flow induced stress in ECs and its role in pre-mature senescence and aging.
2. Replicative senescence (cell cycle arrest) vs. pre-mature aging of endothelial cells
Aging is an independent risk factor for cardiovascular diseases (CVDs), and to this day we have no control over aging and senescence in clinical settings [26].
Aging is viewed as a non-modifiable risk factor in vascular pathologies [27], and is understood as a time dependent deterioration of physiological integrity and loss of function which ultimately leads to death. Hayflick and Moorhead in 1961 [28] were the first to describe ‘replicative senescence’, which is the limited proliferative capacity of human cells (cell cycle arrest), and therefore senescence is the irreversible loss and exhaustion of this capacity of cells to divide and grow. One of the molecular mechanisms of replicative senescence which was described as ‘telomere hypothesis of senescence’ in nineteen nineties increased interest in studying senescence [29]. Later it became much clearer that senescence can be caused by other genetic insults such as DNA damage, chromosomal aberrations and chromatin condensation [[30], [31], [32], [33]]. It is now appreciated that senescence induced under these stressful conditions and other unfavorable stimuli can be telomere length dependent and independent (but related to telomere attrition as explained later) and they lead to accelerated aging which was coined as ‘stress-induced premature senescence (SIPS)’ [34] and ‘stress or aberrant signaling-induced senescence’ [35]. Therefore, several recent developments in the field have led to new insights and it is now believed that aging is a consequence of varied cellular events that converge to cause senescence. Lopez-Otin et al., in 2013 described nine hallmarks of aging which encompass all the cellular events that lead to aging and these hallmarks are now key to understanding senescence process [36]. ECs undergo not only normal senescence that is related to time-dependent aging, also known as ‘Hayflick's limit’ [28,37,38] but also stress stimuli such as oxidative stress or DNA damage which accelerates the senescence phenotype so called pre-mature aging or pre-mature senescence [[39], [40], [41], [42]].
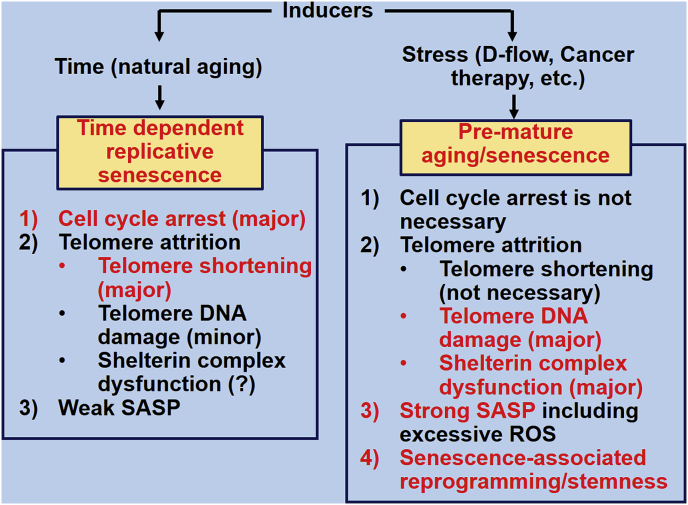
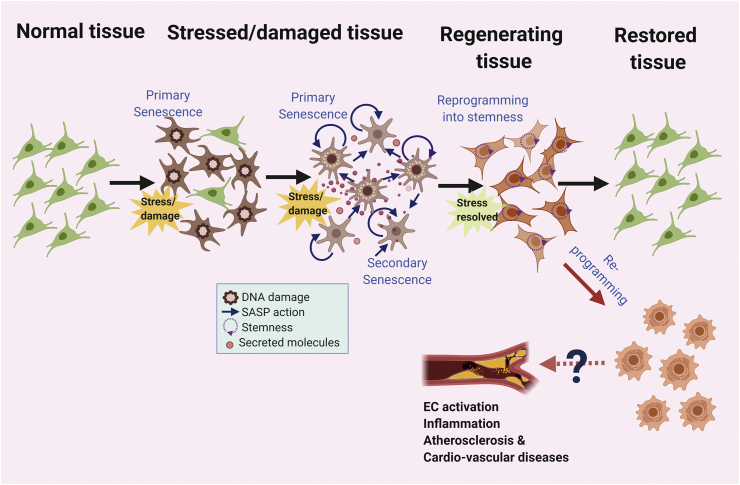
In Fig. 1, we distinguish replicative senescence and stress related vascular senescence. One of the major differences is that stress related senescence induces strong senescence associated secretory phenotype (SASP) and senescence associated re-programming in vascular ECs, the mechanisms that lead to stress related senescence will be discussed in the following sections.
Fig. 1.
Time-dependent replicative senescence vs. stress-induced pre-mature aging/senescence. SASP: senescence-associated secretory phenotype; ROS: Reactive oxygen species.
2.1. Disturbed flow mediated EC dysfunction and senescence
ECs in disturbed flow regions such as the curved regions of aorta, bifurcations and branch points experience low endothelial shear stress, eliciting EC inflammation, dysfunction, high turnover, eventually leading to high lipid uptake, and AS formation [43,44]. The role of DNA damage response (DDR) pathway in the formation of atherosclerotic plaques has been previously elucidated. It has been reported that atherosclerotic plaques contain high double stranded DNA breaks (DBS) and are found to have higher activation of ATM compared to that of non-atherosclerotic tissues [45]. Another important factor in the DDR pathway is p53 which is also important for the EC dysfunction and in turn atherosclerotic plaque formation. Martinet et al. reported elevated level of DDR pathway factors like, PARP-1, p53 in the human atherosclerotic plaque than the non-atherosclerotic region [46]. Interestingly, the atherosclerotic disturbed flow also regulates the p53 post translational modification, cellular localization and EC apoptosis. Heo et al. reported that, disturbed flow mediated SUMOylation of p53 increased the binding of p53 with anti-apoptotic Bcl2 which in turn increased EC apoptosis [47].
Several studies have now reported the role of disturbed flow-mediated EC damage in development of atherosclerosis [[48], [49], [50], [51]]. Intriguingly, excess mtROS release in response to flow stress has been documented [52,53]. Ballinger et al. showed the role of mitochondrial dysfunction and excessive ROS production in development of atherosclerosis [54]. They also show the mtDNA damage strongly correlates to AS pathogenesis and provide strong evidence that mitochondrial dysfunction plays a key role in chronic and age-related disorders. Several other animal models and studies have shown the role of EC SASP and damage responses which we have summarized in Table 1.
Table 1.
Animal models of flow mediated EC senescence and AS formation.
| Type of DNA damage response | DNA damage response factors | Functions | Animal models | DDR and atherosclerosis in EC | Ref |
|---|---|---|---|---|---|
| Nucleotide Excision Repair (NER) | NER-DNA crosslink repair (XLR) endonuclease ERCC1 | Involved in the repair of nucleotide excision repair | Ercc1d/− mice, having mutation in one allele of the enzyme ERCC1 | The EC from the mutant mice exhibited higher senescence compared to the WT mice. In addition, mutant mice developed age dependent vascular dysfunction. | [55] |
| Spindle assembly checkpoint protein | BubR1 | Detect the correct microtubule-kinetochore attachment and segregation of chromatids during mitosis | Hypomorphic BubR1 mutant (BubR1H/H) Mice expressing mutant BubR1 | Increased production of superoxide anions, aging-associated phenotypes, EC dependent relaxation is lost, accelerated cardio vascular aging | [56,57] |
| Telomerase and Shelterin complex | TERC, TERT | TERT produces telomeric repeats using the template provided by TERC | TERC−/− and TERT−/− mice | Telomerase deficient mice exhibited an increased activity of nicotinamide adenine dinucleotide phosphate oxidase, and high levels of reactive oxygen species, leading to increased hypertension and vascular dysfunction | [58] |
| TERF2IP | A member of the shelterin complex of the mammalian TLs binds to both telomeric and nontelomeric chromatins and in the protection of TLs | TERF2IP−/− mice | Conferred protection against d-flow–induced EC senescence, apoptosis, and AS plaque formation | [59] | |
| Base Excision Repair of Oxidative DNA Damage | Base excision repair enzyme 8oxoG DNA glycosylase I (OGG1) | Involved in the BER mechanism for the repairing of 8-Oxoguanine (8oxoG), which is one of the most abundant oxidative DNA damage | OGG1−/− mice in vascular smooth muscle cells (VSMC) | Mice exhibited oxidative stress, DNA strand breaks, pro-inflammatory pathways and extensive AS formation | [60] |
| Apurinic/Apyrmidinic Endonuclease-1 (APE1)/redox factor-1 (ref-1) | Essential for BER pathway, DNA repair and governs the reductive activation of many redox-sensitive transcription factors | APE1/ref-1+/− mice | The transgenic mice manifested reduced vascular NO level, dysregulated EC dependent vascular tone and developed systemic hyper tension and vascular complications | [61] | |
| Sensor of DNA damage | Sirtuin 1 (SIRT1) | NAD + dependent acetylation of proteins and involved in the rescue of DNA damage induced apoptosis | ApoE-null mice with SIRT1 over expression | SIRT1 over expressing mice showed protection against high fat-induced impairment in endothelium-dependent vasorelaxation had lesser AS formation than the control ApoE−/− mice | [62] |
| PARP-1 | ADP-ribosylating enzyme activated upon ssDNA damage DSB. PARP-1 cleaves NAD + into nicotinamide and adds polymers of ADP-ribose to glutamic acid residues substrates | Diabetic mice with PARP1 KO db−/db−PARP-1−/− | The inhibition of PARP-1 activity by pharmaceutical and in db−/db− mice, significantly improved vascular function and tone. NF-κB is shown to regulate vascular function through PARP-1 | [63] | |
| Transducer proteins of DDR pathway | ATM | Transduces the DNA damage signal to the downstream effectors | ATM ± ApoE−/− mice | Developed accelerated AS | [64] |
| p53 | Regulation of cell cycle and DDR activator | High fat diet in LDLR−/− mice | Disturbed Flow sites expressed high levels of SAβG activity and p53 expression and DF induced senescence is mediated through p53-p21 pathway | [65] | |
| p21 | Involved in G1/S checkpoint in the cell cycle | p21 −/− mice | Accelerated AS formation in high fat diet induced atherosclerosis in knock out mice compared to the WT | [66] |
Previous studies have indicated the mechanisms of shear stress sensing and detailed mechanisms of how flow patterns are transduced by cells. ECs can sense mechanical signals through ion channels, integrin and focal adhesion complexes, cilia and glycocalyx and cytoskeleton network [67,68]. It is now well appreciated that hemodynamic stress activates DDR and SASP responses in ECs which is highly correlated to AS progression [48,[69], [70], [71], [72]]. In this review, we will discuss the primary mechanisms that play a crucial role in the process of flow mediated pre-mature aging: (i) telomere attrition and (ii) excessive reactive oxygen species (ROS) production. We would like to present that these processes that are distinctive to flow mediated pre-mature aging are not only independent but are also significantly different from time-dependent replicative senescence. We will also elaborate the downstream mechanisms of flow mediated EC senescence and introduce the concept of, ‘feedback loop of senescence’ which enables sustained EC activation and priming.
-
(i)
Telomere attrition: Telomere length vs. DNA damage response (DDR): It is well documented that human ECs have a marked age-dependent decrease in the mean telomere length [73]. The average telomere length of arterial ECs has been shown to be shorter than that of the venous ECs [74], which could be attributed to the D-flow patterns in arteries leading to high cell turnover as we will discuss later in this review. When the telomere loop structure was disrupted by overexpressing a dominant negative telomeric repeat binding factor 2 (TRF2) mutant in ECs, senescence was induced along with the increased expression of intercellular adhesion molecule (ICAM-1) and the decreased endothelial nitric oxide synthase 3 (eNOS) activity and nitric oxide (NO) production [71].
Interestingly, Hewitt et al. have reported that DNA damage foci in senescence induced by exogenous DNA-damaging agents are preferentially located at telomeres, although telomeres are long, and telomerase are active [75]. Hewitt et al. concluded that persistent telomere-associated damage is a frequent outcome of genotoxic stress and a component of stress-induced senescence, which is independent of telomerase activity and telomere length. Taken together, these data suggest that telomere attrition is resulted not only by telomere shortening but also is induced through telomere DNA damage.
-
(ii)
Excessive ROS production: The growing evidence points to the role of oxidative, nitrative and mitochondrial stress as the major mediator of EC damage during senescence [76]. ROS and reactive nitrogen species (RNS) are potent reactive molecules that can cause deleterious oxidative damage to DNA, proteins and lipids [77]. Antioxidant defense mechanisms including superoxide dismutase and catalase render a vital protective role in ECs. Any abnormalities in these mechanisms including compromised function of adaptive proteins involved in the oxidative stress response such as p66Shc lead to EC dysfunction and senescence [78]. ROS produced by the mitochondrial oxidative phosphorylation (OXPHOS) machinery and oxidative injury to mitochondrial DNA are principal causes of EC senescence and damage [79]. Mitochondrial stress with ROS induced mtDNA damage has been shown to correlate with the development of atherosclerosis supportive of a relationship between oxidative stress, SASP and atherosclerosis. Madamanchi et al. described the role of excessive ROS production in AS formation in specific mouse SOD2 ± model, and identified that SOD activation offers protection in laminar flow regions [80].
It is becoming clear that the old concept of replicative senescence cannot fully explain the process of pre-mature aging induced by telomere attrition and excessive ROS production.
2.2. D-flow-induced telomere attrition and DDR responses
Recent studies have brought to light that D-flow cause upregulation of mtROS and it is implicated in telomeric DNA damage. Takabe et al. have reported-flow-induced mtROS production via NADPH oxidase and JNK activation [81].The role mtROS is becoming increasingly evident and is believed to play a significant role in regulating pre-mature aging by DNA damage and telomere attrition. Even though, the underlying mechanisms were not clearly understood earlier, a better understanding of this effect has emerged in recent years.
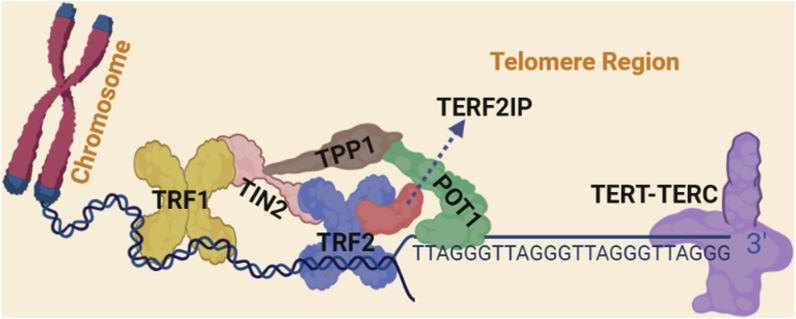
Shelterin is made up of a family of six membered proteins (TRF1, TRF2, TERF2IP, TIN2, TPP1 and POT1) (Fig. 2). The Shelterin protects and preserves the structure of telomeres and is responsible for forming the t-loop and modifying the telomere terminus [83]. The mechanism of Shelterin protection of telomeres is thought to be through prevention of telomere shortening rather than inducing telomerase activity, since most adult tissues have very low to no telomerase activity [84]. Depletion of the shelterin complex leads to telomere shortening inducing cellular senescence.
Fig. 2.
Shelterin and telomere [[82], [83], [84], [85]] Shelterin is a made up of six membered protein family. Telomeric repeat-binding factor 1 (TRF1), TRF2, RAP1 (also known as TERF2IP), TERF1-interacting nuclear factor 2 (TIN2), TIN2-interacting protein 1 (TPP1) and protection of telomeres protein 1 (POT1) TRF1 and TRF2 bind to telomeric DNA duplexes, while POT1 binds to single-stranded DNA in the 3ʹ overhang region. TERF2IP is bound to TRF2 and does not directly interact with DNA.
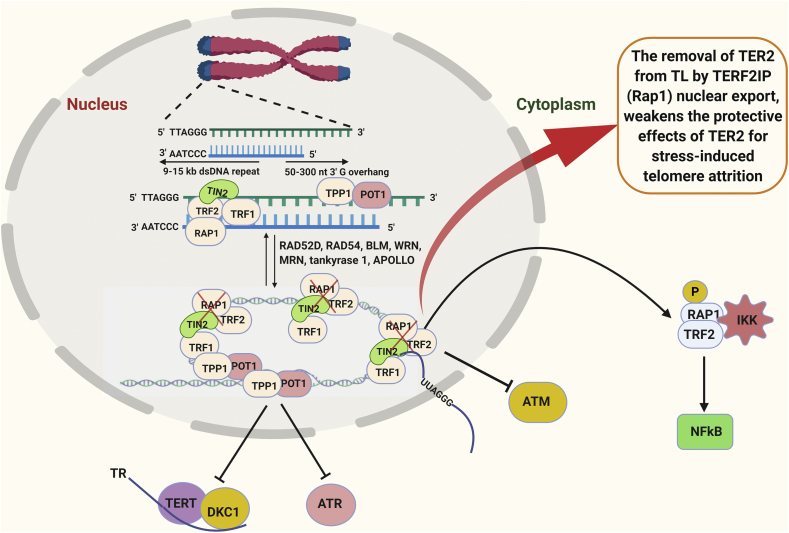
TERF2IP is a member of the Shelterin, can bind to telomeric and non-telomeric regions of the chromatin, and has two distinct functions in the nucleus and the cytosol [86,87]. In the nucleus, TERF2IP forms a complex with TRF2 [88], and this TERF2IP-TRF2 complex limits the telomere recombination, fragility and shortening [89]. In the cytosol, TERF2IP is involved in NF-κB signaling and mediates the binding of NF-κB p65 and IκB kinases [90]. The stability of TERF2IP in the cell depends on its association with TRF2 while TRF2 is stable even in the absence of TERF2IP [89,91]. The bound amount of the TERF2IP-TRF2 complex is dependent on cell types, differentiation states, and experimental conditions [86,92]. In addition, deletion of TERF2IP or TRF2 leads to different phenotypical outcomes in cells [86]. The TERF2IP-TRF2 complex is thought to shuttle between the nucleus and the cytosol (Fig. 2, Fig. 3) [89].
Fig. 3.
A role for TERF2IP in senescence [82,83,93,94]: TERF2IP (or RAP1) is bound to TRF2 in the shelterin DNA complex. Any stress response causes TERF2IP to move to the cytoplasm along with TER2 causing telomere attrition and down-stream DDR pathways and inflammation. TRF: Telomeric repeat-binding factor; RAP1 (also known as TERF2IP); TIN2: TERF1-interacting nuclear factor 2; TPP1: TIN2-interacting protein 1; POT1: protection of telomeres protein 1; TERT: Telomerase reverse transcriptase; DKC1: Dyskerin Pseudouridine Synthase 1.
Recently our group reported the dual role of TERF2IP in ECs depending on whether it is in the nucleus and the cytosol [59]. Under normal conditions, the TERF2IP-TRF2 complex is in the shelterin complex associated with telomers conferring a protective role, preventing telomeres from shortening (Fig. 3). As we explained in the beginning of this review, we described D-flow as athero-prone flow. We showed that p90RSK associates with the Myb domain of TERF2IP and that p90RSK phosphorylates TERF2IP serine 205 (S205) in ECs exposed to d-flow only [59]. This phosphorylation induces the TERF2IP-TRF2 complex to move out of the nucleus, leading to activation of pro-inflammatory NF-κB signaling, causing subsequent telomere shortening and ultimately leading to EC apoptosis [59]. We also observed that depletion of TERF2IP and inhibition of TERF2IP S205 phosphorylation prevented TRF2 nuclear export and protected telomere [59]. Therefore, both senescence (due to the lack of telomeric protection effect) and endothelial pro-inflammatory activation (due to the activation of NF-kB signaling pathway) are simultaneously elevated(59). In this manner, D-flow can induce SASP through regulating the Shelterin complex and the function of its member proteins.
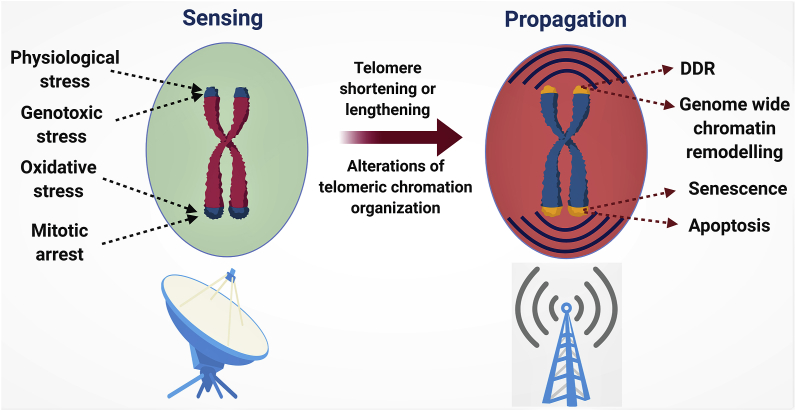
As shown by our group and other studies, telomere end regions are the “argus-eyes” of the cellular stress responses including genotoxic, oxidative, replicative and in the context of ECs, telomeres are indeed responsive to D-flow mediated stress responses (Fig. 4). Telomeric regions act as sensors of stress of various stimuli and propagate the signal to the cells through various channels (Fig. 4). Although DDR pathways are central to propagate the stress responses, telomere components are thought to play a key role in transcriptional regulation through several pathways, and this has a significant outcome on cell fate decision. As of date, the role of telomeric control of cellular transcription in the context of aging is unclear, however, a better understanding of this phenomenon could lead to several new unidentified mechanisms that are involved in cellular senescence and aging.
Fig. 4.
Telomeres in sensing and propagation of stress responses [94]: Telomere regions and the proteins associated with telomeres are sensitive to any stress stimuli. Dysfunction of telomere protection mechanisms lead to telomere shortening and length and alterations on telomere protection mechanisms. This leads to DNA damage responses, genome re-arrangements and ultimately leading to senescence and apoptosis.
3. SASP: Pre-mature aging/senescence and inflammation in ECs and atherosclerosis
Interesting observations in recent years have led to the understanding that senescent ECs do not merely exhibit dysfunctional phenotypes but that they display an entirely different gene expression pattern and secrete a panel of cytokines, growth factors, pro-inflammatory mediators, interleukins, matrix remodeling proteins and proteases. This latter condition is now called senescence associated secretory phenotype (SASP) [95,96]. Aging is a major risk factor for AS, and cellular senescence is associated with EC activation [97]. In particular, SASP is believed to have a key role in the development of AS [[98], [99], [100]]. Table 2 is the summary of all the features of senescent and pro-inflammatory ECs. Classically, senescent ECs has been thought to upregulate several cell surface markers, ICAM1, vascular cell adhesion molecule (VCAM1) & von Willebrand factor protein (vWF) [101]. In addition, they secrete potent inflammatory and tissue remodeling factors which include tumor necrosis factor α (TNFα), interleukin (IL)-6, IL8, plasminogen activator inhibitor-1 (PAI-1), matrix metallopeptidase 1 (MMP)-1, −3 and −10 [96,102]. Reduction in eNOS activity is well documented which leads to reduced vascular sensitivity [103,104]. Some of the other markers include, miR-146a-5p targets interleukin-1 receptor-associated kinase 1 (IRAK1) and miR-126-3p targets sprouty-related EVH1 domain containing protein 1 (SPRED1) [96,105].
Table 2.
Properties of senescent cells.
| Senescence Signatures | Phenotypes exhibited, and markers expressed | Ref |
|---|---|---|
| Morphology | Increased cell size | [37] |
| Flattened cell body, irregular cell nuclei | [114] | |
| Expanded lysosomal compartment | [115] | |
| Heterochromatin foci in the nucleus | [115,116] | |
| Cell Cycle and apoptosis | p16 (INK-4a) | [117] |
| p21(WAF/Cip1) | [117] | |
| p53 | [[118], [119], [120], [121]] | |
| Lack of Ki67 proliferation marker | [122] | |
| Genomic stability | Chromosomal Foci containing DDR proteins | [114,123] |
| Senescence associated heterochromatin | [114] | |
| Chromatin Reorganization | [114] | |
| Telomere shortening | [36,71] | |
| Disrupted Shelterin and TRF2 | [59,71] | |
| Epigenetic marks | H3K9me3 | [124] |
| H3K4me3 | [125] | |
| H3K27ac | [125] | |
| Metabolites | Glutamine, Proline, Phenylalanine & betaine | [126] |
| Thromboxane & Prostacyclin | [127,128] | |
| Mitochondrial dysfunction | mtROS and RNS species production | [42,[129], [130], [131], [132]] |
| NAD+ depletion | [40,133] | |
| mtDNA damage | [129] | |
| OXPHOS dysregulation | [134] | |
| Oxidative and Metabolic Stress | [13,123,135] | |
| Micro RNAs | miR-146a-5p, miR126–3p | [136] |
| miR-217, miR-34a, and miR-21 – Sirtuin targeting | [105] | |
| miR-17-92 cluster | [105] | |
| miR-222-221 Cluster | [137] | |
| miR-181b | [138] |
We present in Table 2, additional new understandings of senescent ECs which includes genomic modifications, drastic changes in chromatin state, mitochondrial and metabolic alterations of the cell. Roles for telomere shortening and stability of the Shelterin also shown to factors governing EC senescence [29,86,106,107]. Aging is correlated with higher expression of plasma fibrinogen and vWF and diminished fibrinolytic ability of ECs [108]. It has been shown that anti-fibrinolytic factor PAI-1 is increased in human ECs obtained from elderly subjects compared to that obtained from younger subjects [109]. Thus, elevated steady-state levels of PAI-1 can also serve as a biomarker for EC aging. Higher expression of PAI-1 goes in hand with the marked upregulation of IL-1α, a distinct feature of SASP and can inhibit EC proliferation. Senescent ECs have poor expression of eNOS and lower level of NO production, which causes decreased overall vasoreactivity of blood vessels [110]. Senescent ECs also have poor protection from platelet aggregation and blood coagulation likely leading to increased clot and thrombus formation which leads to severe vascular complications. Childs et al. reported that cells, including vascular ECs, with activity of the pH-sensitive lysosomal enzyme senescence-associated β-galactosidase (SAβG) are present in atherosclerotic plaques of low-density lipoprotein receptor (LDLR) null mice [111]. Removal of senescent cells (p16ink4a promoter activity) in these mice lead to reduction in plaque formation and progression and the plaques formed exhibited features of stable plaques [111,112]. These observations indicate that senescence is at the core of AS by accumulating pro-inflammatory senescent cells. Senolytic drugs, which can precisely remove senescent cells, may help alleviate age related pathologies [113].
4. New concept of senescence-associated reprogramming or stemness
Can cellular senescence be viewed as the terminal end of the lifespan of cells? Senescence was characterized by cell cycle arrest, and therapy-induced senescence has long been the basis for cancer therapy to inhibit cancer cell growth [139]. However, very recently, this conventional view has been challenged [125,140,141]. New paradigms of cellular senescence present senescence in different shades of grey. Recently, Milanovic et al. (2018) showed that senescence induced by chemotherapy reprograms a portion of cancer cells to acquire “stemness”, which allows them to escape senescence-induced cell cycle arrest with strongly enhanced clonogenic growth potential (Fig. 5). A certain subsets of senescent cells with ‘distinct SASP’ features may affect regeneration and development through secretion of molecules that may contain crucial orders to dictate the outcome of development, regeneration, wound healing depending on the setting [125].
Fig. 5.
This idea brings certain subsets of senescent cells with ‘distinct SASP’ features closer to pluripotent and tissue stem cell status. Importantly, these senescence-escaped cells gain an elevated tumor-initiating capacity compared with cells that have never undergone senescence [140]. Furthermore, it has been suggested that SASP is different from and is regulated independently of senescence-induced cell cycle arrest [102,142] and cell death [143,144] and is now considered one of the key mechanisms to develop resistance to cancer therapy [[143], [144], [145]]. Therefore, the concepts of senescence-induced cell cycle arrest and senescence-associated reprogramming largely overlap, but they describe different biological phenomena. After cancer therapy, a certain subset of senescent cells with ‘distinct SASP’ features produce numerous inflammatory factors [146], growth factors [147], ROS [113,148,149], and promote cell growth [96], and inflammation-related [150] signaling, eventually leading to a more aggressive proliferative phenotype by reprogramming the cancer treatment-induced senescent cells to escape from cell cycle blockade [140,143,144], and even apoptosis (“anastasis”) [151,152].
This model can be validated by the idea that senescent cells have a significant consequence towards surrounding cells since they are viable and secretory active cells. Although the consequence of communication is not clearly understood, senescent cells interact with their neighbors through cell-cell communication, ligand receptor interactions, cytoplasmic channels, cell fusion and delivery of senescence associated molecules thorough exosomes and vesicles [153]. A great deal of attention is given to SASP factors which have a wide implication from malignant conversion, tumor progression, immune modulation and regeneration depending on the context. Although it is believed that SASP will trigger the host innate immune cells to clear senescent cells, there have been several reports which points otherwise; SASP averts clearance of senescent cells. Senescent cells have drastic changes in their epigenetic and chromatin landscape which bring them to a more premature state [154]. It is interesting to note that SASP can improve the efficiency of reprogramming of cells by Yamanaka factors [155]. Senescence phenotype overall with several activated stemness molecules such as p16INK4a, p21CIP1, p53 and H3K9me3 which lead to a hypothetical link between senescence related reprogramming and cancer stemness. Chemotherapy induced senescence and cells in a state of replicative senescence display plasticity and express latent adult tissue stem cell signature [140]. Endothelial inflammation is well known, and it is shown to play an important role in cardio-vascular diseases [156], but senescence associated reprogramming or stemness of ECs is relatively unknown, raising several questions. (i) do senescent ECs undergo stemness associated remodeling, and if so to what extent and what are their roles in disease progression (Fig. 5)? (ii) are EC SASP factors act as regenerative stimulants for surrounding cells? (iii) Are there tissue specific roles of EC reprogramming?
5. How is SASP induced?
SASP can be induced via (i) mechanisms that are dependent on DDR signaling pathways such as DDR-mediated NF-kB signaling [90,157,158]; (ii) mechanisms that are independent of DDR signaling pathways such as the p38MAPK-MK2, GATA4, NOTCH-induced C/EBPb, Wnt, and JAK/STAT pathways, although the detailed molecular mechanisms have not been fully understood [143,159]; and (iii) mechanisms that are dependent on DDR but independent of cell cycle regulation [160]. Ultimately, these different pathways dictate functional outcome of SASP, which are linked to multiple biological processes that are beyond tumor suppression, such as repairing tissue damage, clearing damaged cells, associating with age-related pathologies including chronic inflammation [[98], [99], [100]], cancer progression [140,155], and AS formation, regarding which will be discussed in the following sections.
5.1. DNA damage-induced senescence and p53 activation
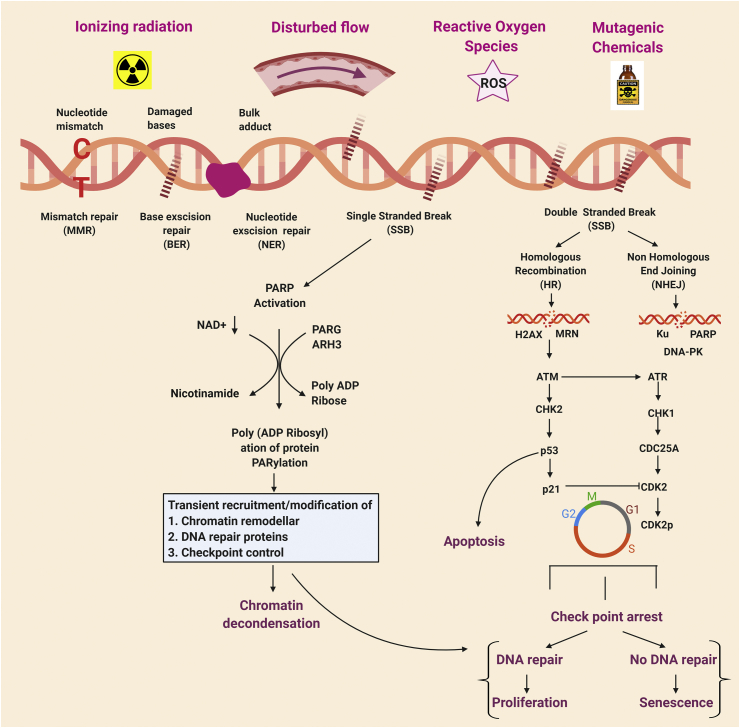
DNA is constantly attacked not only by endogenous genotoxic factors such as ROS, RNS, products of lipid peroxidation, alkylating factors [161] but also by exogenous genotoxic factors such as ionizing radiation (IR), ultra violet UV radiation from sunlight and genotoxic chemicals. Therefore, DNA is susceptible to continuous insults and damage [161]. To protect cells from these insults to prevent damage and maintain genomic integrity, there are evolutionarily conserved repair pathways, termed DDR signaling pathways. DDR is initiated by sensing the damage of DNA and transducing this signal to activate DNA damage repair mechanisms. There are multiple DNA repair mechanisms which can be activated namely, base excision repair (BER), nucleotide excision repair (NER), mismatch repair, homologous recombination (HR) and non-homologous end joining (NHEJ), depending on the specific type of damage or insult [161]. This repair response also arrests the cell cycle to avoid propagating damaged DNA into daughter cells. After the transient cell cycle arrest, if DNA damages are properly repaired, cells go back to the normal proliferation cycle. However, if the damage is severe and irreparable, cells go into apoptosis, permanent growth arrest or senescence (Fig. 6).
Fig. 6.
DDR pathway [[162], [163], [164]]: The DNA damage and response pathway: The figure depicts the causative agents and plausible DNA damage repair pathways. Depending on the type of insult, there are different types of DNA damage including mismatched nucleotide, damaged bases, bulk adduct binding, single strand and double strand break. This figure also describes the outcome of the different DNA damage repair pathway leading to cell cycle arrest, senescence, and apoptosis. PARP: Poly (ADP-ribose) polymerase (PARP); PARG: Poly (ADP-ribose) glycohydrolase; ARH3: ADP-ribosylhydrolase 3; NAD: Nicotinamide adenine dinucleotide; MRN: Mre11-Rad50-Nbs1; H2AX: H2A histone family member X; ATM: Ataxia Telangiectasia mutated; ATR: Ataxia Telangiectasia and Rad3-related; CHK1, CHK2: Checkpoint kinase 1 and 2; CDK: Cyclin-dependent kinases.
p53 also known as the guardian of the genome, is a well-known tumor suppressor and transcription factor, is activated under stress conditions such as DNA damage, telomere shortening and hypoxia [[165], [166], [167]]. In ECs telomere attrition increases p21 (cyclin dependent kinase inhibitor) expression and as a consequence p53 is activated leading to cell cycle arrest and ultimately leading to apoptosis [167,168]. Apart from that, the increase in SIRT1 activity establishes a connection between the DNA damage response and energy sensitive pathway. Gorenne et al. reported that, SIRT1 is an important factor in DNA damage repair protects both the human as well as rodent vascular cells when there is accumulated DNA damage [39]. With aging, there is an accumulation of DNA damage in the cells and it might cause the reduction of endothelial SIRT1 expression and activity [169]. This reduction of endothelial SIRT1 expression may lead to genomic instability with aging [170]. SIRT1 deacetylates p53 resulting in its inactivation. Thus, the age-related reduction of SIRT-1 activity may contribute to p53 activation and increased cell senescence [171,172].
6. Feedback loop of senescence: nuclear-mitochondrial crosstalk
Growing evidence suggests that mitochondrial dysfunction plays a crucial role in the induction of cellular senescence [173]. Mitochondrial DNA (mtDNA) damage maybe closely interlinked to mtROS production, most importantly collective mitochondrial function is thought to be much more critical in inducing mitochondrial ROS (mtROS) production, which then induces telomere shortening and contributes to cellular senescence [41]. Interestingly, it has been shown that high mutation and mtDNA damage does not cause significant upregulation of mtROS, suggesting a significant menacing role of mtROS [174]. Mitochondrial function, biogenesis, structure and equilibrium between fission and fusion states are more critical to mtROS production rather than mtDNA damage. Therefore, there is poor evidence to point whether mtROS is a consequence of mtDNA damage, and the role of mtDNA damage in senescence and aging is not clear. In addition, whether mtROS is a consequence or inducer of premature aging is yet to be understood.
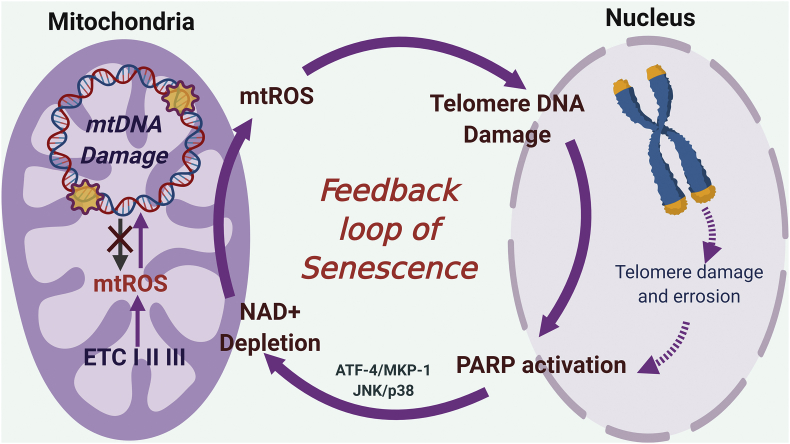
ROS, whether it is of mitochondrial origin or not, induces genotoxic stress, causing nuclear DNA (nDNA) damage [175]. This ROS-induced nDNA damage can initiate mtROS production, forming nucleus-mitochondria crosstalk. To explain how ROS-induced nDNA damage can initiate mtROS production, the role of PARP1 activation and NAD+ depletion have been suggested. (i) PARP1 is a highly conserved nuclear enzyme that functions as a zinc dependent DNA damage sensor that can bind both single- and double stranded DNA breaks [176]. Hocsak et al. reported that ROS-induced nDNA damage increases mtROS production via PARP1 activation and subsequent JNK/p38 activation [176] (Fig. 7). (ii) ROS-induced nDNA damage activates PARP1and SIRT1, which uses nicotinamide adenine dinucleotides (NAD+) as a co-factor and deplete NAD+ in the cell. NAD+ depletion plays a critical role in mitochondria metabolism and decreases mitophagy, leading to more mtROS production by accumulating damaged mitochondria [177]. Although PARP1 activation and NAD+ depletion have been suggested, the exact molecular mechanism by which NAD+ depletion-mediated mtROS production is poorly understood.
Fig. 7.
Positive feedback loop of mtROS production and nuclear DNA damage response (PARP activation) causes mitochondrial dysfunction and pre-mature aging, potentially induced by d-flow. ETC; Electron transport chain, TL; telomere, PARP; poly (ADP-ribose) polymerase, ATF-4; activating transcription factor-4, MKP-1, MAP kinase phosphatase-1.
Recently, Qian et al. reported that H2O2 generated by mitochondria can diffuse to the nucleus and induce telomere, but not genomic DNA damage and activates DDR [42]. These data suggest that there is a feedback loop between mitochondria dysfunction and nuclear DDR, which causes sustained mtROS production, subsequently leading to premature aging via telomere shortening and attrition (Fig. 7).
6.1. Nicotinamide adenine dinucleotide (NAD+) depletion induced by DDR promotes mtROS production
Senescence is also detected with aging [36,178], the process that impairs the endothelium-dependent nitric oxide (NO)-mediated vasodilation, which is also known as EC dysfunction. Impaired NO signaling can be linked to increased ROS generation because superoxide reacts with NO to generate peroxynitrite [179]. Co-enzyme (NAD+) and NADH are obligatory cofactors of glycolysis and OXPHOS, and their availability controls the overall production of ATP. Both acute (such as ischemia) and chronic (aging) events in the heart and kidneys can cause reduced levels of cellular NAD+ and NADH, leading to defective glycolysis and OXPHOS and resulting in decreased ATP production and increased mtROS production [180,181]. With aging, NAD+ availability declines in multiple organs and this aging-associated impairment could be reversed by supplementation of NAD+ precursors [182]. As enzymes required for NAD+ metabolism is abundantly expressed in ECs, ECs are particularly important target for improving its function by NAD+ precursor supplements. Chronic treatment of aged mice with NAD+ boosters improved aortic EC function [183].
Thus, it is possible that the SASP-mediated EC activation, dysfunction, and the subsequent development of AS and CVDs could be due to NAD+ deficiency-induced mtROS in ECs. Indeed, the crucial role of DDR induced by mtROS-mediated telomere DNA damage, but not genomic DNA damage, has been suggested [42]. Taken together, these data suggest the crucial role of telomere DNA damage-mediated DDR in regulating mtROS production and the feedback loop between mitochondria dysfunction and nuclear (especially telomere) DDR (Fig. 7.), which causes sustained mtROS production, subsequently leading to premature aging via telomere attrition.
7. Conclusion
Pre-mature aging is not comparable to time-dependent aging. Especially, the novel concept of senescence-associated reprogramming or stemness, and its potential role in flow-induced SASP and subsequent atherosclerosis formation need more attention. Senescent cells can acquire a distinct phenotype of wide range of characteristics, closely relating them to cancer stem cell status (senescence-associated reprogramming/stemness), and this requires a better understanding as to the exact role that senescent cells play in pre-mature aging tissues such as atherosclerotic plaques. The proposal that senescence is regeneration went haywire, is still to be explored and requires further studies. There are several factors which exert and coax the cell to become aged or senescent. At this point the mechanisms and cellular perpetrators of senescence and aging remains terra incognita. However, it is becoming evident that the specific role of different factors of SASP in ECs play a distinct role in the process of cardiovascular dysfunction. It is important to recognize that EC dysfunction and inflammation are not linear mechanisms, but rather a continuous loop between nucleus-cytoplasm-mitochondria, causing sustained EC priming and inflammation events which plays a key role in senescence and pre-mature aging. This crucial role of the nuclear-mitochondrial crosstalk in SASP induction, which is potentially mediated by mtROS and DDR, needs further investigation. Although the concept of senescence-mediated atherosclerosis formation is a classic concept, the paradigm of senescence and pre-mature aging is dramatically changing.
Declaration of competing interest
We don't have any conflict of interest to declare.
Acknowledgements
We sincerely thank Dr. Keigi Fujiwara for critical reading and discussions for the article. This work was partially supported by the following funding sources: National Institutes of Health grant HL-149303 and HL-130193 to J.A, National Institutes of Health grant HL-134740 and HL-149303 to NTL and, Marvy and Elaine Finger Distinguished Chair, intramural and philanthropic support to D.J.H.
Contributor Information
Nhat-Tu Le, Email: nhle@houstonmethodist.org.
Jun-ichi Abe, Email: jabe@mdanderson.org.
References
- 1.Lu D., Kassab G.S. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface. 2011;8(63):1379–1385. doi: 10.1098/rsif.2011.0177. PubMed PMID: 21733876; PMCID: PMC3163429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vessieres E., Freidja M.L., Loufrani L., Fassot C., Henrion D. Flow (shear stress)-mediated remodeling of resistance arteries in diabetes. Vasc. Pharmacol. 2012;57(5-6):173–178. doi: 10.1016/j.vph.2012.03.006. PubMed PMID: 22484164. [DOI] [PubMed] [Google Scholar]
- 3.Wragg J.W., Durant S., McGettrick H.M., Sample K.M., Egginton S., Bicknell R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation. 2014;21(4):290–300. doi: 10.1111/micc.12119. PubMed PMID: 24471792. [DOI] [PubMed] [Google Scholar]
- 4.dela Paz N.G., Walshe T.E., Leach L.L., Saint-Geniez M., D'Amore P.A. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 2012;125(Pt 4):831–843. doi: 10.1242/jcs.084301. PubMed PMID: 22399811; PMCID: PMC3311927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu J.J., Usami S., Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann. Med. 2009;41(1):19–28. doi: 10.1080/07853890802186921. PubMed PMID: 18608132. [DOI] [PubMed] [Google Scholar]
- 6.Davies P.F., Spaan J.A., Krams R. Shear stress biology of the endothelium. Ann. Biomed. Eng. 2005;33(12):1714–1718. doi: 10.1007/s10439-005-8774-0. PubMed PMID: 16389518. [DOI] [PubMed] [Google Scholar]
- 7.Li Y.S., Haga J.H., Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005;38(10):1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. PubMed PMID: 16084198. [DOI] [PubMed] [Google Scholar]
- 8.Gimbrone J Michael A., James N. Topper, Tobi Nagel, Keith R., Anderson A.G.G.-C. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 2000;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 9.Orr A.W., Helmke B.P., Blackman B.R., Schwartz M.A. Mechanisms of mechanotransduction. Dev. Cell. 2006;10(1):11–20. doi: 10.1016/j.devcel.2005.12.006. PubMed PMID: 16399074. [DOI] [PubMed] [Google Scholar]
- 10.Bagot C.N., Arya R. Virchow and his triad: a question of attribution. Br. J. Haematol. 2008;143(2):180–190. doi: 10.1111/j.1365-2141.2008.07323.x. PubMed PMID: 18783400. [DOI] [PubMed] [Google Scholar]
- 11.Cecchi E., Giglioli C., Valente S., Lazzeri C., Gensini G.F., Abbate R., Mannini L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214(2):249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. PubMed PMID: 20970139. [DOI] [PubMed] [Google Scholar]
- 12.Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. Epub 2011/01/21. doi: 91/1/327 [pii] 10.1152/physrev.00047.2009. PubMed PMID: 21248169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le N.T., Corsetti J.P., Dehoff-Sparks J.L., Sparks C.E., Fujiwara K., Abe J. Reactive oxygen species, SUMOylation, and endothelial inflammation. Int. J. Inflamm. 2012;2012:678190. doi: 10.1155/2012/678190. PubMed PMID: 22991685; PMCID: PMC3443607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heo K.S., Fujiwara K., Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ. J. 2011;75(12):2722–2730. doi: 10.1253/circj.cj-11-1124. PubMed PMID: 22076424; PMCID: PMC3620204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motwani M., Pesiridis S., Fitzgerald K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1. PubMed PMID: 31358977. [DOI] [PubMed] [Google Scholar]
- 16.Boisseau M.R. Roles of mechanical blood forces in vascular diseases. A clinical overview. Clin. Hemorheol. Microcirc. 2005;33:201–207. [PubMed] [Google Scholar]
- 17.Bai J., Liu F. The cGAS-cGAMP-STING pathway: a molecular link between immunity and metabolism. Diabetes. 2019;68(6):1099–1108. doi: 10.2337/dbi18-0052. PubMed PMID: 31109939; PMCID: PMC6610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrizia Nigro J-iA., Berk Bradford C. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxidants Redox Signal. 2011;15:1405–1414. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan S.S., Avati Nanjundappa R.P., Branch J.R., Taylor W.R., Quyyumi A.A., Jo H., McDaniel M.C., Suo J., Giddens D., Samady H. Shear stress and plaque development. Expert Rev. Cardiovasc Ther. 2010;8(4):545–556. doi: 10.1586/erc.10.28. PubMed PMID: 20397828; PMCID: PMC5467309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond S.L. Systems analysis of thrombus formation. Circ. Res. 2016;118(9):1348–1362. doi: 10.1161/CIRCRESAHA.115.306824. PubMed PMID: 27126646; PMCID: PMC4852389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Qiu J., Luo S., Xie X., Zheng Y., Zhang K., Ye Z., Liu W., Gregersen H., Wang G. High shear stress induces atherosclerotic vulnerable plaque formation through angiogenesis. Regen. Biomater. 2016;3(4):257–267. doi: 10.1093/rb/rbw021. PubMed PMID: 27482467; PMCID: PMC4966293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham K.S., Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. PubMed PMID: 15568038. [DOI] [PubMed] [Google Scholar]
- 23.Giddens CKZaSG D.P. The role of fluid mechanics in the localization and detection of atherosclerosis. J. Biomech. Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 24.Gimbrone J Michael A., Nitzan Resnick, Tobi Nagel L.M.K., Tucker Collins A.J.N.T. Hemodynamics, endothelial gene expression, and atherogenesis". Ann. N. Y. Acad. Sci. 1997;15:1–10. doi: 10.1111/j.1749-6632.1997.tb51983.x. [DOI] [PubMed] [Google Scholar]
- 25.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 2007;292(3):H1209–H1224. doi: 10.1152/ajpheart.01047.2006. PubMed PMID: 17098825. [DOI] [PubMed] [Google Scholar]
- 26.Brandes R.P., Fleming I., Busse R. Endothelial aging. Cardiovasc. Res. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. PubMed PMID: 15820197. [DOI] [PubMed] [Google Scholar]
- 27.Erusalimsky J.D., Kurz D.J. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp. Gerontol. 2005;40(8-9):634–642. doi: 10.1016/j.exger.2005.04.010. PubMed PMID: 15970413. [DOI] [PubMed] [Google Scholar]
- 28.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1964;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 29.Greider C.W. Telomeres and senescence: the history, the experiment, the future. Curr. Biol. 1998;8:178–181. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 30.Epel E.S., Blackburn E.H., Lin J., Dhabhar F.S., Adler N.E., Morrow J.D., Cawthon R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U. S. A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. PubMed PMID: 15574496; PMCID: PMC534658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tv Zglinicki. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 32.Blasco MSaMA. Putting the stress on senescence. Curr. Opin. Cell Biol. 2001;13:748–753. doi: 10.1016/s0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- 33.von Zglinicki T., Saretzki G., Ladhoff J., d'Adda di Fagagna F., Jackson S.P. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005;126(1):111–117. doi: 10.1016/j.mad.2004.09.034. PubMed PMID: 15610769. [DOI] [PubMed] [Google Scholar]
- 34.Toussaint EEM O., von Zglinickic T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 35.Peters SDaG. Immortalisation and transformation revisited. Curr. Opin. Genet. Dev. 2002;12:98–104. doi: 10.1016/s0959-437x(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. PubMed PMID: 23746838; PMCID: PMC3836174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LHaPS Moorhead. The serial cultivation OF human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 38.Hayflick L. The cell biology of aging. J. Invest. Dermatol. 1979;73(1):8–14. doi: 10.1111/1523-1747.ep12532752. PubMed PMID: 448179. [DOI] [PubMed] [Google Scholar]
- 39.Gorenne I., Kumar S., Gray K., Figg N., Yu H., Mercer J., Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127(3):386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. PubMed PMID: 23224247. [DOI] [PubMed] [Google Scholar]
- 40.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16(7):15918–15953. doi: 10.3390/ijms160715918. PubMed PMID: 26184181; PMCID: PMC4519931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passos J.F., Saretzki G., von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 2007;35(22):7505–7513. doi: 10.1093/nar/gkm893. PubMed PMID: 17986462; PMCID: PMC2190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian W., Kumar N., Roginskaya V., Fouquerel E., Opresko P.L., Shiva S., Watkins S.C., Kolodieznyi D., Bruchez M.P., Van Houten B. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2019;116(37):18435–18444. doi: 10.1073/pnas.1910574116. PubMed PMID: 31451640; PMCID: PMC6744920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thondapu V., Bourantas C.V., Foin N., Jang I.K., Serruys P.W., Barlis P. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. Eur. Heart J. 2017;38(2):81–92. doi: 10.1093/eurheartj/ehv689. Epub 2017/02/06. PubMed PMID: 28158723. [DOI] [PubMed] [Google Scholar]
- 44.Eshtehardi P., Brown A.J., Bhargava A., Costopoulos C., Hung O.Y., Corban M.T., Hosseini H., Gogas B.D., Giddens D.P., Samady H. High wall shear stress and high-risk plaque: an emerging concept. Int. J. Cardiovasc. Imag. 2017;33(7):1089–1099. doi: 10.1007/s10554-016-1055-1. Epub 2017/01/12. PubMed PMID: 28074425; PMCID: PMC5496586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahmoudi M., Gorenne I., Mercer J., Figg N., Littlewood T., Bennett M. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ. Res. 2008;103(7):717–725. doi: 10.1161/CIRCRESAHA.108.182899. Epub 2008/08/30. PubMed PMID: 18723444. [DOI] [PubMed] [Google Scholar]
- 46.Martinet W., Knaapen M.W., De Meyer G.R., Herman A.G., Kockx M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106(8):927–932. doi: 10.1161/01.cir.0000026393.47805.21. Epub 2002/08/21. PubMed PMID: 12186795. [DOI] [PubMed] [Google Scholar]
- 47.Heo K.S., Chang E., Le N.T., Cushman H., Yeh E.T., Fujiwara K., Abe J. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ. Res. 2013;112(6):911–923. doi: 10.1161/CIRCRESAHA.111.300179. Epub 2013/02/06. PubMed PMID: 23381569; PMCID: PMC3697009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thijssen D.H., Dawson E.A., Tinken T.M., Cable N.T., Green D.J. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53(6):986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. Epub 2009/04/22. PubMed PMID: 19380611. [DOI] [PubMed] [Google Scholar]
- 49.Trinity J.D., Groot H.J., Layec G., Rossman M.J., Ives S.J., Richardson R.S. Impact of age and body position on the contribution of nitric oxide to femoral artery shear rate: implications for atherosclerosis. Hypertension. 2014;63(5):1019–1025. doi: 10.1161/HYPERTENSIONAHA.113.02854. Epub 2014/02/19. PubMed PMID: 24535011; PMCID: PMC4476385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freyberg M.A., Kaiser D., Graf R., Buttenbender J., Friedl P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem. Biophys. Res. Commun. 2001;286(1):141–149. doi: 10.1006/bbrc.2001.5314. Epub 2001/08/04. PubMed PMID: 11485320. [DOI] [PubMed] [Google Scholar]
- 51.Park K.H., Park W.J. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J. Kor. Med. Sci. 2015;30(9):1213–1225. doi: 10.3346/jkms.2015.30.9.1213. Epub 2015/09/05. PubMed PMID: 26339159; PMCID: PMC4553666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kluge M.A., Fetterman J.L., Vita J.A. Mitochondria and endothelial function. Circ. Res. 2013;112(8):1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. PubMed PMID: 23580773; PMCID: PMC3700369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y., Li H., Bubolz A.H., Zhang D.X., Gutterman D.D. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med. Biol. Eng. Comput. 2008;46(5):469–478. doi: 10.1007/s11517-008-0331-1. Epub 2008/03/15. PubMed PMID: 18340474; PMCID: PMC2702135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballinger S.W., Patterson C., Knight-Lozano C.A., Burow D.L., Conklin C.A., Hu Z., Reuf J., Horaist C., Lebovitz R., Hunter G.C., McIntyre K., Runge M.S. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106(5):544–549. doi: 10.1161/01.cir.0000023921.93743.89. Epub 2002/07/31. PubMed PMID: 12147534. [DOI] [PubMed] [Google Scholar]
- 55.Durik M., Kavousi M., van der Pluijm I., Isaacs A., Cheng C., Verdonk K., Loot A.E., Oeseburg H., Bhaggoe U.M., Leijten F., van Veghel R., de Vries R., Rudez G., Brandt R., Ridwan Y.R., van Deel E.D., de Boer M., Tempel D., Fleming I., Mitchell G.F., Verwoert G.C., Tarasov K.V., Uitterlinden A.G., Hofman A., Duckers H.J., van Duijn C.M., Oostra B.A., Witteman J.C., Duncker D.J., Danser A.H., Hoeijmakers J.H., Roks A.J. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation. 2012;126(4):468–478. doi: 10.1161/CIRCULATIONAHA.112.104380. Epub 2012/06/19. PubMed PMID: 22705887; PMCID: PMC3430727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto T., Baker D.J., d'Uscio L.V., Mozammel G., Katusic Z.S., van Deursen J.M. Aging-associated vascular phenotype in mutant mice with low levels of BubR1. Stroke. 2007;38(3):1050–1056. doi: 10.1161/01.STR.0000257967.86132.01. Epub 2007/02/03. PubMed PMID: 17272762. [DOI] [PubMed] [Google Scholar]
- 57.Baker D.J., Jeganathan K.B., Cameron J.D., Thompson M., Juneja S., Kopecka A., Kumar R., Jenkins R.B., de Groen P.C., Roche P., van Deursen J.M. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004;36(7):744–749. doi: 10.1038/ng1382. Epub 2004/06/23. PubMed PMID: 15208629. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Rivero G., Ruiz-Torres M.P., Rivas-Elena J.V., Jerkic M., Diez-Marques M.L., Lopez-Novoa J.M., Blasco M.A., Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114(4):309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. Epub 2006/07/13. PubMed PMID: 16831983. [DOI] [PubMed] [Google Scholar]
- 59.Kotla S., Vu H.T., Ko K.A., Wang Y., Imanishi M., Heo K.S., Fujii Y., Thomas T.N., Gi Y.J., Mazhar H., Paez-Mayorga J., Shin J.H., Tao Y., Giancursio C.J., Medina J.L., Taunton J., Lusis A.J., Cooke J.P., Fujiwara K., Le N.T., Abe J.I. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight. 2019;4(9) doi: 10.1172/jci.insight.124867. PubMed PMID: 31045573; PMCID: PMC6538340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah A., Gray K., Figg N., Finigan A., Starks L., Bennett M. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation. 2018;138(14):1446–1462. doi: 10.1161/CIRCULATIONAHA.117.033249. Epub 2018/04/13. PubMed PMID: 29643057; PMCID: PMC6053042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeon B.H., Gupta G., Park Y.C., Qi B., Haile A., Khanday F.A., Liu Y.X., Kim J.M., Ozaki M., White A.R., Berkowitz D.E., Irani K. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ. Res. 2004;95(9):902–910. doi: 10.1161/01.RES.0000146947.84294.4c. Epub 2004/10/09. PubMed PMID: 15472121. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q.J., Wang Z., Chen H.Z., Zhou S., Zheng W., Liu G., Wei Y.S., Cai H., Liu D.P., Liang C.C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2008;80(2):191–199. doi: 10.1093/cvr/cvn224. Epub 2008/08/12. PubMed PMID: 18689793; PMCID: PMC3657473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Modar Kassan S.-K.C., Galán Maria, Bishop Alexander, Umezawa Kazuo, Mohamed Trebak, Belmadani Souad, Matrougui Khalid. Enhanced NF-kB activity impairs vascular function through PARP-1–, SP-1–, and COX-2–dependent mechanisms in type 2 diabetes. Diabetes. 2013;62:2078–2287. doi: 10.2337/db12-1374/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mercer J.R., Cheng K.K., Figg N., Gorenne I., Mahmoudi M., Griffin J., Vidal-Puig A., Logan A., Murphy M.P., Bennett M. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ. Res. 2010;107(8):1021–1031. doi: 10.1161/CIRCRESAHA.110.218966. Epub 2010/08/14. PubMed PMID: 20705925; PMCID: PMC2982998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warboys C.M., de Luca A., Amini N., Luong L., Duckles H., Hsiao S., White A., Biswas S., Khamis R., Chong C.K., Cheung W.M., Sherwin S.J., Bennett M.R., Gil J., Mason J.C., Haskard D.O., Evans P.C. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2014;34(5):985–995. doi: 10.1161/ATVBAHA.114.303415. PubMed PMID: 24651677. [DOI] [PubMed] [Google Scholar]
- 66.Khanna A.K. Enhanced susceptibility of cyclin kinase inhibitor p21 knockout mice to high fat diet induced atherosclerosis. J. Biomed. Sci. 2009;16:66. doi: 10.1186/1423-0127-16-66. Epub 2009/07/17. PubMed PMID: 19604372; PMCID: PMC2720941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang Y., Wu D., Birukov K.G. Mechanosensing and mechanoregulation of endothelial cell functions. Comp. Physiol. 2019;9(2):873–904. doi: 10.1002/cphy.c180020. Epub 2019/03/16. PubMed PMID: 30873580; PMCID: PMC6697421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collins C., Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp. Gerontol. 2011;46(2-3):185–188. doi: 10.1016/j.exger.2010.09.010. Epub 2010/10/05. PubMed PMID: 20888896; PMCID: PMC3026900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garin G., Berk B.C. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium. 2006;13(6):375–384. doi: 10.1080/10623320601061599. Epub 2006/12/16. PubMed PMID: 17169770. [DOI] [PubMed] [Google Scholar]
- 70.Le Master E., Fancher I.S., Lee J., Levitan I. Comparative analysis of endothelial cell and sub-endothelial cell elastic moduli in young and aged mice: role of CD36. J. Biomech. 2018;76:263–268. doi: 10.1016/j.jbiomech.2018.06.007. Epub 2018/06/30. PubMed PMID: 29954596; PMCID: PMC6083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. PubMed PMID: 11927518. [DOI] [PubMed] [Google Scholar]
- 72.Pyke K.E., Tschakovsky M.E. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J. Physiol. 2005;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755. Epub 2005/07/30. PubMed PMID: 16051630; PMCID: PMC1474741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laina A., Stellos K., Stamatelopoulos K. Vascular ageing: underlying mechanisms and clinical implications. Exp. Gerontol. 2018;109:16–30. doi: 10.1016/j.exger.2017.06.007. Epub 2017/06/19. PubMed PMID: 28624356. [DOI] [PubMed] [Google Scholar]
- 74.Chang E., Harley C.B. Telomere length and replicative aging in human vascular tissues. Proc. Natl. Acad. Sci. U. S. A. 1995;92(24):11190–11194. doi: 10.1073/pnas.92.24.11190. Epub 1995/11/21. PubMed PMID: 7479963; PMCID: PMC40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hewitt G., Jurk D., Marques F.D., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012;3:708. doi: 10.1038/ncomms1708. Epub 2012/03/20. PubMed PMID: 22426229; PMCID: PMC3292717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burtenshaw D., Kitching M., Redmond E.M., Megson I.L., Cahill P.A. Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front Cardiovasc. Med. 2019;6:89. doi: 10.3389/fcvm.2019.00089. PubMed PMID: 31428618; PMCID: PMC6688526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valko M., Jomova K., Rhodes C.J., Kuca K., Musilek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90(1):1–37. doi: 10.1007/s00204-015-1579-5. Epub 2015/09/08. PubMed PMID: 26343967. [DOI] [PubMed] [Google Scholar]
- 78.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur. Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513. PubMed PMID: 24366916; PMCID: PMC4006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Widlansky M.E., Gutterman D.D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxidants Redox Signal. 2011;15(6):1517–1530. doi: 10.1089/ars.2010.3642. Epub 2011/01/05. PubMed PMID: 21194353; PMCID: PMC3151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madamanchi N.R., Runge M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007;100(4):460–473. doi: 10.1161/01.RES.0000258450.44413.96. Epub 2007/03/03. PubMed PMID: 17332437. [DOI] [PubMed] [Google Scholar]
- 81.Noguchi N., Jo H. Redox going with vascular shear stress. Antioxidants Redox Signal. 2011;15(5):1367–1368. doi: 10.1089/ars.2011.4011. PubMed PMID: 21457103; PMCID: PMC3144424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bandaria J.N., Qin P., Berk V., Chu S., Yildiz A. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell. 2016;164(4):735–746. doi: 10.1016/j.cell.2016.01.036. PubMed PMID: 26871633; PMCID: PMC4762449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Lange T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. PubMed PMID: 30208292. [DOI] [PubMed] [Google Scholar]
- 84.Lange Td. Shelterin: the protein complex that shapes and safeguards human telomeres. Gene Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. 10.1101/ [DOI] [PubMed] [Google Scholar]
- 85.Schmutz I., de Lange T. Shelterin. Curr. Biol. 2016;26(10):R397–R399. doi: 10.1016/j.cub.2016.01.056. PubMed PMID: 27218840. [DOI] [PubMed] [Google Scholar]
- 86.Martinez P., Blasco M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Canc. 2011;11(3):161–176. doi: 10.1038/nrc3025. PubMed PMID: 21346783. [DOI] [PubMed] [Google Scholar]
- 87.Martinez P., Blasco M.A. Heart-breaking telomeres. Circ. Res. 2018;123(7):787–802. doi: 10.1161/CIRCRESAHA.118.312202. PubMed PMID: 30355079. [DOI] [PubMed] [Google Scholar]
- 88.Bibo Li S.O., Lange aTd. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- 89.Kabir S., Sfeir A., de Lange T. Taking apart Rap1: an adaptor protein with telomeric and non-telomeric functions. Cell Cycle. 2010;9(20):4061–4067. doi: 10.4161/cc.9.20.13579. PubMed PMID: 20948311; PMCID: PMC2995270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teo H., Ghosh S., Luesch H., Ghosh A., Wong E.T., Malik N., Orth A., de Jesus P., Perry A.S., Oliver J.D., Tran N.L., Speiser L.J., Wong M., Saez E., Schultz P., Chanda S.K., Verma I.M., Tergaonkar V. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nat. Cell Biol. 2010;12(8):758–767. doi: 10.1038/ncb2080. PubMed PMID: 20622870. [DOI] [PubMed] [Google Scholar]
- 91.Takai K.K., Hooper S., Blackwood S., Gandhi R., de Lange T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 2010;285(2):1457–1467. doi: 10.1074/jbc.M109.038026. PubMed PMID: 19864690; PMCID: PMC2801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez P., Thanasoula M., Carlos A.R., Gomez-Lopez G., Tejera A.M., Schoeftner S., Dominguez O., Pisano D.G., Tarsounas M., Blasco M.A. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell Biol. 2010;12(8):768–780. doi: 10.1038/ncb2081. PubMed PMID: 20622869; PMCID: PMC3792482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Bloom S.I., Donato A.J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation. 2019;26(2) doi: 10.1111/micc.12487. PubMed PMID: 29924435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye J., Renault V.M., Jamet K., Gilson E. Transcriptional outcome of telomere signalling. Nat. Rev. Genet. 2014;15(7):491–503. doi: 10.1038/nrg3743. PubMed PMID: 24913665. [DOI] [PubMed] [Google Scholar]
- 95.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. PubMed PMID: 20078217; PMCID: PMC4166495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. PubMed PMID: 19053174; PMCID: PMC2592359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erusalimsky J.D. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 2009;106(1):326–332. doi: 10.1152/japplphysiol.91353.2008. Epub 2008/11/28. PubMed PMID: 19036896; PMCID: 2636933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J.C., Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 2012;111(2):245–259. doi: 10.1161/CIRCRESAHA.111.261388. Epub 2012/07/10. PubMed PMID: 22773427. [DOI] [PubMed] [Google Scholar]
- 99.Li W. Phagocyte dysfunction, tissue aging and degeneration. Ageing Res. Rev. 2013;12(4):1005–1012. doi: 10.1016/j.arr.2013.05.006. PubMed PMID: 23748186; PMCID: 3842398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22(17):R741–R752. doi: 10.1016/j.cub.2012.07.024. PubMed PMID: 22975005. [DOI] [PubMed] [Google Scholar]
- 101.Regina C., Panatta E., Candi E., Melino G., Amelio I., Balistreri C.R., Annicchiarico-Petruzzelli M., Di Daniele N., Ruvolo G. Vascular ageing and endothelial cell senescence: molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016;159:14–21. doi: 10.1016/j.mad.2016.05.003. PubMed PMID: 27155208. [DOI] [PubMed] [Google Scholar]
- 102.Coppe J.P., Rodier F., Patil C.K., Freund A., Desprez P.Y., Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011;286(42):36396–36403. doi: 10.1074/jbc.M111.257071. Epub 2011/09/02. PubMed PMID: 21880712; PMCID: PMC3196093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayashi T., Yano K., Matsui-Hirai H., Yokoo H., Hattori Y., Iguchi A. Nitric oxide and endothelial cellular senescence. Pharmacol. Ther. 2008;120(3):333–339. doi: 10.1016/j.pharmthera.2008.09.002. PubMed PMID: 18930078. [DOI] [PubMed] [Google Scholar]
- 104.Nakayama T., Sato W., Yoshimura A., Zhang L., Kosugi T., Campbell-Thompson M., Kojima H., Croker B.P., Nakagawa T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am. J. Pathol. 2010;176(5):2198–2208. doi: 10.2353/ajpath.2010.090316. PubMed PMID: 20363914; PMCID: PMC2861085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamakuchi M., Hashiguchi T. Endothelial cell aging: how miRNAs contribute? J. Clin. Med. 2018;7(7) doi: 10.3390/jcm7070170. PubMed PMID: 29996516; PMCID: PMC6068727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogami M., Ikura Y., Ohsawa M., Matsuo T., Kayo S., Yoshimi N., Hai E., Shirai N., Ehara S., Komatsu R., Naruko T., Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler. Thromb. Vasc. Biol. 2004;24(3):546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. PubMed PMID: 14726417. [DOI] [PubMed] [Google Scholar]
- 107.Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472–473. doi: 10.1016/s0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 108.Hemmeryckx B., Van Hove C.E., Fransen P., Emmerechts J., Kauskot A., Bult H., Lijnen H.R., Hoylaerts M.F. Progression of the prothrombotic state in aging Bmal1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011;31(11):2552–2559. doi: 10.1161/ATVBAHA.111.229062. PubMed PMID: 21799179. [DOI] [PubMed] [Google Scholar]
- 109.Klaus Hager J.S., Vogl Thomas, Voit Jorg, Platt Dieter. Blood coagulation factors in the elderly. Arch. Gerontol. Geriatr. 1989;9:277–282. doi: 10.1016/0167-4943(89)90047-2. [DOI] [PubMed] [Google Scholar]
- 110.Gogiraju R., Xu X., Bochenek M.L., Steinbrecher J.H., Lehnart S.E., Wenzel P., Kessel M., Zeisberg E.M., Dobbelstein M., Schafer K. Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J. Am Heart Assoc. 2015;4(2) doi: 10.1161/JAHA.115.001770. PubMed PMID: 25713289; PMCID: PMC4345879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Childs Bennett G., B D.J., Wijshake Tobias, Conover Cheryl A., Campisi Judith, van Deursen Jan M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bennett M.R., Clarke M.C. Basic research: killing the old: cell senescence in atherosclerosis. Nat. Rev. Cardiol. 2016;14(1):8–9. doi: 10.1038/nrcardio.2016.195. PubMed PMID: 27941859. [DOI] [PubMed] [Google Scholar]
- 113.Birch J., Passos J.F. Targeting the SASP to combat ageing: mitochondria as possible intracellular allies? Bioessays. 2017;39(5) doi: 10.1002/bies.201600235. Epub 2017/02/22. PubMed PMID: 28217839. [DOI] [PubMed] [Google Scholar]
- 114.Rodier F., Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. PubMed PMID: 21321098; PMCID: PMC3044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kurz David J., SD, Hong Ying, Erusalimsky Jorge D. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000;113:3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 116.Narita M., Nuñez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 117.Chen J., Huang X., Halicka D., Brodsky S., Avram A., Eskander J., Bloomgarden N.A., Darzynkiewicz Z., Goligorsky M.S. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: permissive role of p53. Am. J. Physiol. Heart Circ. Physiol. 2006;290(4):H1575–H1586. doi: 10.1152/ajpheart.00364.2005. PubMed PMID: 16243918. [DOI] [PubMed] [Google Scholar]
- 118.Johannes Grillari O.H., Grabherr Reingard M., Katinger H. Subtractive Hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 2000;35:187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 119.Kortlever R.M., Higgins P.J., Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006;8(8):877–884. doi: 10.1038/ncb1448. PubMed PMID: 16862142; PMCID: PMC2954492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stuart D., Tyner S.V., Choi Jene, Jones Stephen, Ghebranious Nader, Igelmann Herbert, Lu Xiongbin, Soron Gabrielle, Cooper Benjamin, Brayton Cory, Park Sang Hee, Thompson Timothy, Karsenty Gerard, Bradley Allan, Donehower Lawrence A. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 121.Fujita K., Mondal A.M., Horikawa I., Nguyen G.H., Kumamoto K., Sohn J.J., Bowman E.D., Mathe E.A., Schetter A.J., Pine S.R., Ji H., Vojtesek B., Bourdon J.C., Lane D.P., Harris C.C. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat. Cell Biol. 2009;11(9):1135–1142. doi: 10.1038/ncb1928. PubMed PMID: 19701195; PMCID: PMC2802853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Narita M., Nunez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. PubMed PMID: 12809602. [DOI] [PubMed] [Google Scholar]
- 123.Kuilman T., Michaloglou C., Mooi W.J., Peeper D.S. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. PubMed PMID: 21078816; PMCID: PMC2975923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee S., Schmitt C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019;21(1):94–101. doi: 10.1038/s41556-018-0249-2. PubMed PMID: 30602768. [DOI] [PubMed] [Google Scholar]
- 125.Milanovic M., Yu Y., Schmitt C.A. The senescence-stemness alliance - a cancer-hijacked regeneration principle. Trends Cell Biol. 2018;28(12):1049–1061. doi: 10.1016/j.tcb.2018.09.001. PubMed PMID: 30253901. [DOI] [PubMed] [Google Scholar]
- 126.Obi A.T., Stringer K.A., Diaz J.A., Finkel M.A., Farris D.M., Yeomans L., Wakefield T., Myers D.D., Jr. 1D-(1)H-nuclear magnetic resonance metabolomics reveals age-related changes in metabolites associated with experimental venous thrombosis. J. Vasc. Surg. Venous Lymphat Disord. 2016;4(2):221–230. doi: 10.1016/j.jvsv.2015.09.010. PubMed PMID: 26993871. [DOI] [PubMed] [Google Scholar]
- 127.Osamu Tokunaga T.Y., Jianglin, Fan aTW. Age-related decline in prostacyclin synthesis by human aortic endothelial cells. Am. J. Pathol. 1991;138(4):941–949. [PMC free article] [PubMed] [Google Scholar]
- 128.Starr M.E., Ueda J., Takahashi H., Weiler H., Esmon C.T., Evers B.M., Saito H. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood. 2010;115(23):4886–4893. doi: 10.1182/blood-2009-10-246678. PubMed PMID: 20348393; PMCID: PMC2890181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trifunovic A., Hansson A., Wredenberg A., Rovio A.T., Dufour E., Khvorostov I., Spelbrink J.N., Wibom R., Jacobs H.T., Larsson N.G. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. U. S. A. 2005;102(50):17993–17998. doi: 10.1073/pnas.0508886102. PubMed PMID: 16332961; PMCID: PMC1312403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A., Gerencser A.A., Verdin E., Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23(2):303–314. doi: 10.1016/j.cmet.2015.11.011. PubMed PMID: 26686024; PMCID: PMC4749409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kujoth G.C., Hiona A., Pugh T.D., Someya S., Panzer K., Wohlgemuth S.E., Hofer T., Seo A.Y., Sullivan R., Jobling W.A., Morrow J.D., Van Remmen H., Sedivy J.M., Yamasoba T., Tanokura M., Weindruch R., Leeuwenburgh C., Prolla T.A. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484. doi: 10.1126/science.1112125. PubMed PMID: 16020738. [DOI] [PubMed] [Google Scholar]
- 132.Newgard C.B., Sharpless N.E. Coming of age: molecular drivers of aging and therapeutic opportunities. J. Clin. Invest. 2013;123(3):946–950. doi: 10.1172/JCI68833. PubMed PMID: 23454756; PMCID: PMC3582156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E., Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174(12):1670–1689. doi: 10.1111/bph.13403. PubMed PMID: 26660451; PMCID: PMC5446573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hwang M.S., Rohlena J., Dong L.F., Neuzil J., Grimm S. Powerhouse down: complex II dissociation in the respiratory chain. Mitochondrion. 2014;19(Pt A):20–28. doi: 10.1016/j.mito.2014.06.001. PubMed PMID: 24933571. [DOI] [PubMed] [Google Scholar]
- 135.Le N.T., Sandhu U.G., Quintana-Quezada R.A., Hoang N.M., Fujiwara K., Abe J.I. Flow signaling and atherosclerosis. Cell. Mol. Life Sci. 2017;74(10):1835–1858. doi: 10.1007/s00018-016-2442-4. PubMed PMID: 28039525; PMCID: PMC5391278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Csiszar A., Ungvari Z., Koller A., Edwards J.G., Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. Faseb. J. 2003;17(9):1183–1185. doi: 10.1096/fj.02-1049fje. PubMed PMID: 12709402. [DOI] [PubMed] [Google Scholar]
- 137.Suarez Y., Fernandez-Hernando C., Yu J., Gerber S.A., Harrison K.D., Pober J.S., Iruela-Arispe M.L., Merkenschlager M., Sessa W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105(37):14082–14087. doi: 10.1073/pnas.0804597105. PubMed PMID: 18779589; PMCID: PMC2544582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.FORL, RRFM, MRRSDN, MCALG, LBSMGS, AMARA, Procopio CFAD MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Canc. 2019 doi: 10.1038/s41568-019-0156-2. Epub 2019/06/27. PubMed PMID: 31235879. [DOI] [PubMed] [Google Scholar]
- 140.Milanovic M., Fan D.N.Y., Belenki D., Dabritz J.H.M., Zhao Z., Yu Y., Dorr J.R., Dimitrova L., Lenze D., Monteiro Barbosa I.A., Mendoza-Parra M.A., Kanashova T., Metzner M., Pardon K., Reimann M., Trumpp A., Dorken B., Zuber J., Gronemeyer H., Hummel M., Dittmar G., Lee S., Schmitt C.A. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553(7686):96–100. doi: 10.1038/nature25167. PubMed PMID: 29258294. [DOI] [PubMed] [Google Scholar]
- 141.Dou Z., Berger S.L. Senescence elicits stemness: a surprising mechanism for cancer relapse. Cell Metabol. 2018;27(4):710–711. doi: 10.1016/j.cmet.2018.03.009. Epub 2018/04/05. PubMed PMID: 29617638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferrand M., Kirsh O., Griveau A., Vindrieux D., Martin N., Defossez P.A., Bernard D. Screening of a kinase library reveals novel pro-senescence kinases and their common NF-kappaB-dependent transcriptional program. Aging (Albany NY) 2015;7(11):986–1003. doi: 10.18632/aging.100845. Epub 2015/11/20. PubMed PMID: 26583757; PMCID: PMC4694068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saleh T., Tyutynuk-Massey L., Cudjoe E.K., Jr., Idowu M.O., Landry J.W., Gewirtz D.A. Non-cell autonomous effects of the senescence-associated secretory phenotype in cancer therapy. Front. Oncol. 2018;8:164. doi: 10.3389/fonc.2018.00164. Epub 2018/06/06. PubMed PMID: 29868482; PMCID: PMC5968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saleh T., Tyutyunyk-Massey L., Murray G.F., Alotaibi M.R., Kawale A.S., Elsayed Z., Henderson S.C., Yakovlev V., Elmore L.W., Toor A., Harada H., Reed J., Landry J.W., Gewirtz D.A. Tumor cell escape from therapy-induced senescence. Biochem. Pharmacol. 2019;162:202–212. doi: 10.1016/j.bcp.2018.12.013. Epub 2018/12/24. PubMed PMID: 30576620. [DOI] [PubMed] [Google Scholar]
- 145.Munoz D.P., Yannone S.M., Daemen A., Sun Y., Vakar-Lopez F., Kawahara M., Freund A.M., Rodier F., Wu J.D., Desprez P.Y., Raulet D.H., Nelson P.S., van 't Veer L.J., Campisi J., Coppe J.P. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight. 2019;5 doi: 10.1172/jci.insight.124716. Epub 2019/06/12. PubMed PMID: 31184599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davalos A.R., Coppe J.P., Campisi J., Desprez P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Canc. Metastasis Rev. 2010;29(2):273–283. doi: 10.1007/s10555-010-9220-9. Epub 2010/04/15. PubMed PMID: 20390322; PMCID: PMC2865636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Coppe J.P., Kauser K., Campisi J., Beausejour C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006;281(40):29568–29574. doi: 10.1074/jbc.M603307200. Epub 2006/08/02. PubMed PMID: 16880208. [DOI] [PubMed] [Google Scholar]
- 148.Tsolou A., Passos J.F., Nelson G., Arai Y., Zglinicki T. ssDNA fragments induce cell senescence by telomere uncapping. Exp. Gerontol. 2008;43(10):892–899. doi: 10.1016/j.exger.2008.08.043. Epub 2008/09/10. PubMed PMID: 18778766. [DOI] [PubMed] [Google Scholar]
- 149.Birch J., Barnes P.J., Passos J.F. Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol. Ther. 2018;183:34–49. doi: 10.1016/j.pharmthera.2017.10.005. Epub 2017/10/11. PubMed PMID: 28987319. [DOI] [PubMed] [Google Scholar]
- 150.Ohanna M., Giuliano S., Bonet C., Imbert V., Hofman V., Zangari J., Bille K., Robert C., Bressac-de Paillerets B., Hofman P., Rocchi S., Peyron J.F., Lacour J.P., Ballotti R., Bertolotto C. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS) Genes Dev. 2011;25(12):1245–1261. doi: 10.1101/gad.625811. Epub 2011/06/08. PubMed PMID: 21646373; PMCID: PMC3127427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tang H.L., Tang H.M., Mak K.H., Hu S., Wang S.S., Wong K.M., Wong C.S., Wu H.Y., Law H.T., Liu K., Talbot C.C., Jr., Lau W.K., Montell D.J., Fung M.C. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell. 2012;23(12):2240–2252. doi: 10.1091/mbc.E11-11-0926. Epub 2012/04/27. PubMed PMID: 22535522; PMCID: PMC3374744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gong Y.N., Crawford J.C., Heckmann B.L., Green D.R. To the edge of cell death and back. FEBS J. 2019;286(3):430–440. doi: 10.1111/febs.14714. Epub 2018/12/07. PubMed PMID: 30506628; PMCID: PMC6361675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Molchadsky A., Rotter V. p53 and its mutants on the slippery road from stemness to carcinogenesis. Carcinogenesis. 2017;38(4):347–358. doi: 10.1093/carcin/bgw092. PubMed PMID: 28334334. [DOI] [PubMed] [Google Scholar]
- 154.Yang N., Sen P. The senescent cell epigenome. Aging (Albany NY) 2018;10(11):3590–3609. doi: 10.18632/aging.101617. Epub 2018/11/06. PubMed PMID: 30391936; PMCID: PMC6286853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yumiko Fujiia K.Y., Suzuki Hidekazu, Tsutsumi Shuichi, Mutoh Hiroyuki, Maeda Shin, Yukinori Yamagata Y.S., Aburatani Hiroyuki, Hatakeyama Masanori. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(50):20584–20589. doi: 10.1073/pnas.1208651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Minamino T., Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. PubMed PMID: 17204661. [DOI] [PubMed] [Google Scholar]
- 157.Halle M., Gabrielsen A., Paulsson-Berne G., Gahm C., Agardh H.E., Farnebo F., Tornvall P. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J. Am. Coll. Cardiol. 2010;55(12):1227–1236. doi: 10.1016/j.jacc.2009.10.047. PubMed PMID: 20298930. [DOI] [PubMed] [Google Scholar]
- 158.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell. Signal. 2012;24(4):835–845. doi: 10.1016/j.cellsig.2011.12.006. PubMed PMID: 22182507. [DOI] [PubMed] [Google Scholar]
- 159.Freund A., Patil C.K., Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30(8):1536–1548. doi: 10.1038/emboj.2011.69. Epub 2011/03/15. PubMed PMID: 21399611; PMCID: PMC3102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. Epub 2009/07/15. PubMed PMID: 19597488; PMCID: PMC2743561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takafumi Ishida M.I., Tashiro Satoshi, Takeishi Yasuchika. DNA damage and senescence-associated inflammation in cardiovascular disease. Biol. Pharm. Bull. 2019;42(4):531–537. doi: 10.1248/bpb.b18-00865. [DOI] [PubMed] [Google Scholar]
- 162.Arai H., Wada R., Ishino K., Kudo M., Uchida E., Naito Z. Expression of DNA damage response proteins in gastric cancer: comprehensive protein profiling and histological analysis. Int. J. Oncol. 2018;52(3):978–988. doi: 10.3892/ijo.2018.4238. Epub 2018/01/13. PubMed PMID: 29328366. [DOI] [PubMed] [Google Scholar]
- 163.Mirza-Aghazadeh-Attari M., Darband S.G., Kaviani M., Mihanfar A., Aghazadeh Attari J., Yousefi B., Majidinia M. DNA damage response and repair in colorectal cancer: defects, regulation and therapeutic implications. DNA Repair (Amst) 2018;69:34–52. doi: 10.1016/j.dnarep.2018.07.005. Epub 2018/07/29. PubMed PMID: 30055507. [DOI] [PubMed] [Google Scholar]
- 164.Sulli G., Di Micco R., d'Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Canc. 2012;12(10):709–720. doi: 10.1038/nrc3344. Epub 2012/09/07. PubMed PMID: 22952011. [DOI] [PubMed] [Google Scholar]
- 165.Clegg H.V., Zhang Y. Regulation of the p53 tumor suppressor pathway: the problems and promises of studying Mdm2's E3 ligase function. Crit. Rev. Eukaryot. Gene Expr. 2010;20(1):77–86. doi: 10.1615/critreveukargeneexpr.v20.i1.60. PubMed PMID: 20528739. [DOI] [PubMed] [Google Scholar]
- 166.Ryan K.M., Phillips A.C., Vousden K.H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 2001;13(3):332–337. doi: 10.1016/s0955-0674(00)00216-7. PubMed PMID: 11343904. [DOI] [PubMed] [Google Scholar]
- 167.Prabhat A.M., Kuppusamy M.L., Naidu S.K., Meduru S., Reddy P.T., Dominic A., Khan M., Rivera B.K., Kuppusamy P. Supplemental oxygen protects heart against acute myocardial infarction. Front Cardiovasc. Med. 2018;5:114. doi: 10.3389/fcvm.2018.00114. PubMed PMID: 30211171; PMCID: PMC6120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Young M.J., Copeland W.C. Human mitochondrial DNA replication machinery and disease. Curr. Opin. Genet. Dev. 2016;38:52–62. doi: 10.1016/j.gde.2016.03.005. PubMed PMID: 27065468; PMCID: PMC5055853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Donato A.J., Magerko K.A., Lawson B.R., Durrant J.R., Lesniewski L.A., Seals D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011;589(Pt 18):4545–4554. doi: 10.1113/jphysiol.2011.211219. PubMed PMID: 21746786; PMCID: PMC3208223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kida Y., Goligorsky M.S. Sirtuins, cell senescence, and vascular aging. Can. J. Cardiol. 2016;32(5):634–641. doi: 10.1016/j.cjca.2015.11.022. PubMed PMID: 26948035; PMCID: PMC4848124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luo Jianyuan, S-iI, Nikolaev Anatoly Y., Chen Delin, Su Fei, Shiloh Ariel, Guarente Leonard, Gu Wei. Negative control of p53 by Sir2␣ promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 172.Homayoun Vaziri S.K.D., Ng Eaton Elinor, Imai Shin-Ichiro, Frye Roy A., Pandita Tej K., Guarente Leonard, Weinberg Robert A. hSIR2SIRT1 homayoun Vaziri,1 functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 173.Tang X., Luo Y.X., Chen H.Z., Liu D.P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. PubMed PMID: 24834056; PMCID: PMC4018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vermulst M., Bielas J.H., Kujoth G.C., Ladiges W.C., Rabinovitch P.S., Prolla T.A., Loeb L.A. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 2007;39(4):540–543. doi: 10.1038/ng1988. Epub 2007/03/06. PubMed PMID: 17334366. [DOI] [PubMed] [Google Scholar]
- 175.Srinivas U.S., Tan B.W.Q., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. PubMed PMID: 30612957; PMCID: PMC6859528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hocsak E., Szabo V., Kalman N., Antus C., Cseh A., Sumegi K., Eros K., Hegedus Z., Gallyas F., Jr., Sumegi B., Racz B. PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic. Biol. Med. 2017;108:770–784. doi: 10.1016/j.freeradbiomed.2017.04.018. PubMed PMID: 28457938. [DOI] [PubMed] [Google Scholar]
- 177.Fang E.F., Scheibye-Knudsen M., Brace L.E., Kassahun H., SenGupta T., Nilsen H., Mitchell J.R., Croteau D.L., Bohr V.A. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157(4):882–896. doi: 10.1016/j.cell.2014.03.026. PubMed PMID: 24813611; PMCID: PMC4625837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Erusalimsky J.D., Skene C. Mechanisms of endothelial senescence. Exp. Physiol. 2009;94(3):299–304. doi: 10.1113/expphysiol.2008.043133. PubMed PMID: 18931048. [DOI] [PubMed] [Google Scholar]
- 179.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Berthiaume J.M., Kurdys J.G., Muntean D.M., Rosca M.G. Mitochondrial NAD(+)/NADH redox state and diabetic cardiomyopathy. Antioxidants Redox Signal. 2019;30(3):375–398. doi: 10.1089/ars.2017.7415. Epub 2017/10/28. PubMed PMID: 29073779; PMCID: PMC6306679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hershberger K.A., Martin A.S., Hirschey M.D. Role of NAD(+) and mitochondrial sirtuins in cardiac and renal diseases. Nat. Rev. Nephrol. 2017;13(4):213–225. doi: 10.1038/nrneph.2017.5. Epub 2017/02/07. PubMed PMID: 28163307; PMCID: PMC5508210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016;5(1):25. doi: 10.1186/s40169-016-0104-7. Epub 2016/07/29. PubMed PMID: 27465020; PMCID: PMC4963347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.de Picciotto N.E., Gano L.B., Johnson L.C., Martens C.R., Sindler A.L., Mills K.F., Imai S., Seals D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi: 10.1111/acel.12461. Epub 2016/03/13. PubMed PMID: 26970090; PMCID: PMC4854911. [DOI] [PMC free article] [PubMed] [Google Scholar]