Abstract
The retinal pigment epithelium (RPE) is the primary site of injury in non-neovascular age-related macular degeneration or dry AMD. Polymorphisms in genes that regulate complement activation and cholesterol metabolism are strongly associated with AMD, but the biology underlying disease-associated variants is not well understood. Here, we highlight recent studies that have used molecular, biochemical, and live-cell imaging methods to elucidate mechanisms by which aging-associated insults conspire with AMD genetic risk variants to tip the balance towards disease. We discuss how critical functions including lipid metabolism, autophagy, complement regulation, and mitochondrial dynamics are compromised in the RPE, and how a deeper understanding of these mechanisms has helped identify promising therapeutic targets to preserve RPE homeostasis in AMD.
Keywords: Retinal pigment epithelium, Complement, Mitochondria, Cholesterol, Ceramide, Autophagy
1. Introduction
Age-related macular degeneration (AMD) destroys central high-resolution vision in over 30 million older adults. The number of people impacted by AMD is projected to exceed 288 million by the year 2040 due to a lack of effective therapies for the most prevalent form of the disease called non-neovascular or dry AMD [1,2]. The primary site of injury in AMD is the retinal pigment epithelium (RPE), a polarized monolayer of post-mitotic cells the sits between the photoreceptors and the choriocapillaris in the outer retina (Fig. 1). The RPE is a highly specialized tissue that performs a number of functions necessary for maintaining the health of the retina and in turn, healthy vision. These include forming the outer blood-retinal barrier by virtue of tight junctions between RPE cells, transporting nutrients into, and metabolites out of, the retina, recycling vitamin A to maintain the visual cycle, secreting growth factors and extracellular matrix components, and executing the daily phagocytosis and clearance of photoreceptor outer segments, (for recent detailed reviews on RPE cell biology and functions, see [3,4]). Because of these varied and critical functions, the RPE is both the guardian and the Achilles’ heel of the retina. Consequently, damage to the RPE impairs its photoreceptor support functions, and eventually leads to vision loss in AMD.
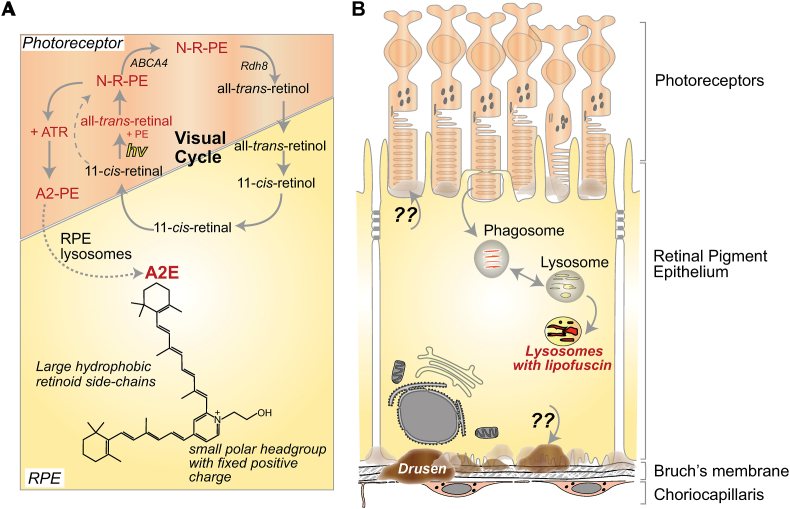
Fig. 1.
Lipofuscin and drusen in the RPE. A, Generation of lipofuscin bisretinoids in the RPE. Absorption of a photon of light by 11-cis-retinal results in its isomerization to all-trans-retinal. This all-trans-retinal reacts with phosphatidylethanolamine (PE) in the photoreceptor outer segment disc membrane to form a Schiff base adduct, N-retinylidene-PE (NRPE). The ATP-binding cassette transporter A4 (ABCA4) transports NRPE across the lipid bilayer to the cytoplasmic side, where it is hydrolyzed to release all-trans-retinal, which is then reduced to all-trans-retinol by retinol dehydrogenases like RDH8 in the photoreceptor cytosol. Both the all-trans and 11-cis isomers of NRPE are substrates of ABCA4, which limits the levels of reactive aldehydes in the photoreceptor disc membranes. When ABCA4 is nonfunctional, as in Stargardt disease, NRPE can react irreversibly with a second molecule of all-trans-retinal to form bisretinoids such as A2E. The precursor, A2PE, is formed from two molecules of all-trans-retinal and one molecule of phosphatidylethanolamine. B, Upon outer segment phagocytosis, phospholipase D in RPE lysosomes hydrolyzes A2PE to generate phosphatidic acid and A2E. In contrast to the well-characterized pathway that leads to lipofuscin accumulation in the RPE, mechanisms involved in the formation of sub-RPE and sub-retinal drusen remain to be understood.
Susceptibility to AMD and progression to vision loss are dictated by multiple genetic and environmental factors. Polymorphisms in genes that regulate complement activation and lipid metabolism are among the strongest risks for AMD [5]. However, the biology underlying disease-associated variants, and how they compromise RPE health to promote pathology remains to be understood [6]. Moreover, not all who have these risk variants go on to develop the disease, suggesting that a complex interplay between genetic predisposition, intrinsic stressors, and extrinsic insults could tip the balance from normal aging of the RPE towards chronic dysfunction that eventually culminates in AMD.
Aging in the RPE is characterized by the progressive accumulation of two distinct types of deposits: intracellular lipofuscin and extracellular drusen (Fig. 1). Lipofuscin is composed of autofluorescent vitamin A metabolites called bisretinoids, formed by non-enzymatic condensation reactions of retinaldehyde and phosphatidylethanolamine in photoreceptor outer segment discs. Upon outer segment phagocytosis, these bisretinoids are trapped within RPE lysosomes as a result of pH-dependent protonation [7]. Lipofuscin bisretinoid accumulation increases with age in humans and is accelerated in patients with recessive Stargardt disease caused by mutations in ABCA4 (discussed in Section 2). Bisretinoids compromise RPE health and function by promoting oxidative stress and interfering with multiple homeostatic mechanisms and could thus contribute to AMD pathology [8]. Although “lipofuscin” is used as a catch-all term for age-pigment that accumulates in postmitotic highly metabolically active cells like the RPE, neurons, cardiomyocytes, RPE lipofuscin is almost exclusively composed of bisretinoid adducts [9], whereas lipofuscin in other cells is composed of oxidized proteins, lipids, metal ions and sugar residues [10]. This structural and compositional differences explain the different mechanisms of cell injury attributed to bisretinoids in the RPE as discussed in this review.
Drusen are composed of lipid-protein aggregates that are rich in cholesterol, apolipoprotein E, complement components and other proteins [11]. These deposits are found both beneath (sub-RPE drusen) and above (sub-retinal drusen) the RPE. Decades of observational studies using non-invasive multimodal imaging techniques indicate that drusen size, morphology, and location correlate with AMD progression. This is especially true of sub-retinal drusen, whose presence is associated with increased risk of developing advanced AMD [12,13]. However, in contrast to the well-understood non-enzymatic reactions that catalyze bisretinoid formation, much less is known about the biogenesis of drusen, how AMD-associated genetic risk variants impact this, and whether drusen are a cause or consequence of the disease.
In this review, we will discuss exciting recent studies on how bisretinoids generate oxidative stress in the RPE (Section 2), how bisretinoid-mediated cholesterol and ceramide accumulation derail several critical RPE functions and render the RPE susceptible to complement activation and mitochondrial injury (Section 3), how these pathological pathways are reinforced and potentiated by AMD genetic risk variants, and how a deeper understanding of these pathways has helped identify promising therapeutic targets to preserve RPE homeostasis in both inherited and age-related macular degenerations (Section 4).
2. Bisretinoids and oxidative stress in the RPE
The daily phagocytosis and clearance of photoreceptor outer segments is arguably one of the most important and metabolically taxing functions of the RPE [3,4]. In the human eye, an individual RPE cell is responsible for 30 to 50 photoreceptors, which amounts to an immense metabolic and degradative burden on the postmitotic RPE. Over time, undegraded outer segment components containing autofluorescent bisretinoid adducts accumulate in RPE lysosomes in the form of lipofuscin. These bisretinoids are by-products of vitamin A that are formed as part of the visual cycle (Fig. 1A). The phototransduction cascade is initiated by absorption of a photon of light by the visual chromophore 11-cis-retinal, which isomerizes to all-trans-retinal. As reactive aldehydes are toxic, all-trans-retinal is reduced to all-trans-retinol by NADPH-dependent retinol dehydrogenases RDH8, RDH11 and RDH12 present in the cytosol of the photoreceptor cell. To facilitate transport of all-trans-retinal from the disc membrane to the cytosol, all-trans-retinal reacts with phosphatidylethanolamine (PE) in the disc membrane to form a Schiff base adduct, N-retinylidene-PE (NRPE). The ATP-binding cassette transporter A4 (ABCA4) present on the disc membrane transports NRPE across the lipid bilayer to the cytoplasmic side, where it is hydrolyzed to release all-trans-retinal, which is then reduced to the alcohol form by RDHs. Both the all-trans and 11-cis isomers of NRPE can be flipped by ABCA4, and serve to limit the levels of reactive aldehydes in the photoreceptor disc membranes (reviewed in [7]).
NRPE on the disc membrane can react irreversibly with a second molecule of all-trans-retinal to form bisretinoids. The first bisretinoid to be identified and structurally characterized in the 1990s was A2E [9,14]. In photoreceptor disc membranes, two molecules of all-trans-retinal react with one molecule of phosphatidylethanolamine to form the precursor A2PE. Upon outer segment phagocytosis, phospholipase D in RPE lysosomes hydrolyzes A2PE to generate phosphatidic acid and A2E (Fig. 1B). In recent years, several other bisretinoids have been structurally characterized including isomers of A2E, A2-GPE (A2-glycerophosphoethanolamine), all-trans-retinal dimer, and A2-DHP-PE (A2-dihydropyridine-phosphotidylethanolamine). A characteristic feature of these bisetinoids is their autofluorescence with two absorbance peaks, one in the UV range, and another in the visible spectrum. Studies in humans and mice show a progressive age-related increase in bisretinoid formation and accumulation in the RPE. Bisretinoid formation is accelerated in recessive Stargardt disease (STGD1), which is caused by mutations in ABCA4 [7].
The retina is an oxygen-rich microenvironment that is constantly exposed to light. This facilitates the light-induced production of reactive oxygen species from bisretinoids and their photodecomposition into cytotoxic aldehyde- and dicarbonyl-bearing fragments. Blue-light irradiation of lipofuscin granules isolated from human RPE generates reactive oxygen species including hydrogen peroxide, superoxide, and singlet oxygen [15]. Irradiation of ARPE-19 cells containing A2E with 430 nm blue light produces A2E-nonaoxiranes [16], which promote DNA damage [17]. Studies have shown that blue light-mediated formation of monofurano-A2E and monoperoxy-A2E activate the complement pathway in the ARPE-19 cell line [18]. In A2E-treated primary porcine RPE, blue light irradiation decreased the expression of superoxide dismutase 2 (SOD2), a key mitochondrial antioxidant enzyme, resulting in mitochondrial injury and a drop in ATP production [19].
Studies in mouse models corroborate these in vitro data on bisretinoid photodecomposition. In the Abca4−/− mouse model of Stargardt's disease, exposure to increasing light intensity leads to the formation of A2E-oxiranes, upregulation of oxidative stress genes including superoxide dismutase 1 (SOD1), and accumulation of oxidatively modified lipids such as malondialdehyde and 4-hydroxynonenal. The RPE of albino Abca4−/− mice have higher levels of these oxidatively modified lipids compared to that of pigmented Abca4−/− mice, revealing a protective role for RPE melanin [20,21]. Further, cyclic light-reared albino mice have lower bisretinoid levels compared to dark-reared albino mice, likely due to greater oxidation and decomposition of bisretinoids in mice reared in cyclic light [22]. One potential mediator of light-driven bisretinoid toxicity could be iron via the Fenton reaction: treatment of albino Abca4−/− mice with the iron chelator deferiprone prevented oxidation and degradation of bisretinoids, and decreased outer nuclear layer (ONL) thinning [23].
A2E and other bisretinoids can photodecompose into aldehyde- and dicarbanoyl-containing fragments (glyoxal and methylglyoxal) [24,25]. Proteins modified with these moieties have been detected in sub-RPE drusen [26], suggesting a link between bisretinoid accumulation in the RPE and AMD pathology. Methylglyoxal fragments form advanced glycation end products, which can promote neovascularization via upregulation of vascular endothelial growth factor (VEGF) expression [27]. A2E oxidation and decomposition products have been detected in RPE from human donors, which could account for the paradoxical "decrease" in A2E levels in the macula reported in some studies [28,29]. In agreement with this, recent studies using fluorescence lifetime imaging of lipofuscin fluorophores extracted from human donor RPE show a higher content of oxidized bisretinoids in RPE from AMD donors compared to unaffected donors [30]. Reactive oxygen species generated by bisretinoid decomposition can in turn oxidize cellular lipids in the retina. Photoreceptor outer segments are rich in polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA), which is essential for vision. PUFAs have multiple unsaturated bonds that are susceptible to oxidation. Carboxyethylpyrrole (CEP) adducts formed from DHA oxidation are found in drusen and CEP levels are elevated in the serum of AMD patients compared to controls [31]. Thus, photoreactivity of A2E and other lipofuscin bisretinoids can contribute to AMD by generating free radicals and reactive protein adducts, setting in motion a feed-forward cycle of oxidative stress.
3. Lipids, autophagy, and complement-mediated mitochondrial injury in the RPE
A growing number of studies using in vitro and in vivo models have identified mechanisms by which bisretinoids and other aging-associated insults compromise critical homeostatic functions in the RPE. Here, we discuss these data in the context of Stargardt disease and AMD.
3.1. Dysregulation of lipid homeostasis in the RPE
Genetic, epidemiological and histopathological studies indicate a causal role for abnormal lipid metabolism in AMD pathogenesis: polymorphisms in several cholesterol pathway genes including APOE, ABCA1, LIPC, and CETP are strongly associated with AMD [5], serum HDL levels positively correlate with AMD susceptibility [32], and cholesterol-rich sub-RPE and sub-retinal drusen are hallmarks of AMD progression [33]. Increased RPE cholesterol has been documented in mouse models of Stargardt disease, canine models of Best disease [34,35], and in mice with RPE-specific deletion of the cholesterol transporters ABCA1 and ABCG1 [36]. In all these models, excess cholesterol precedes downstream pathology, suggesting that RPE cholesterol levels must be tightly regulated. Therefore, insight into factors that govern lipid and cholesterol metabolism in the RPE, and how excess cholesterol derails RPE health, is important for understanding disease pathology.
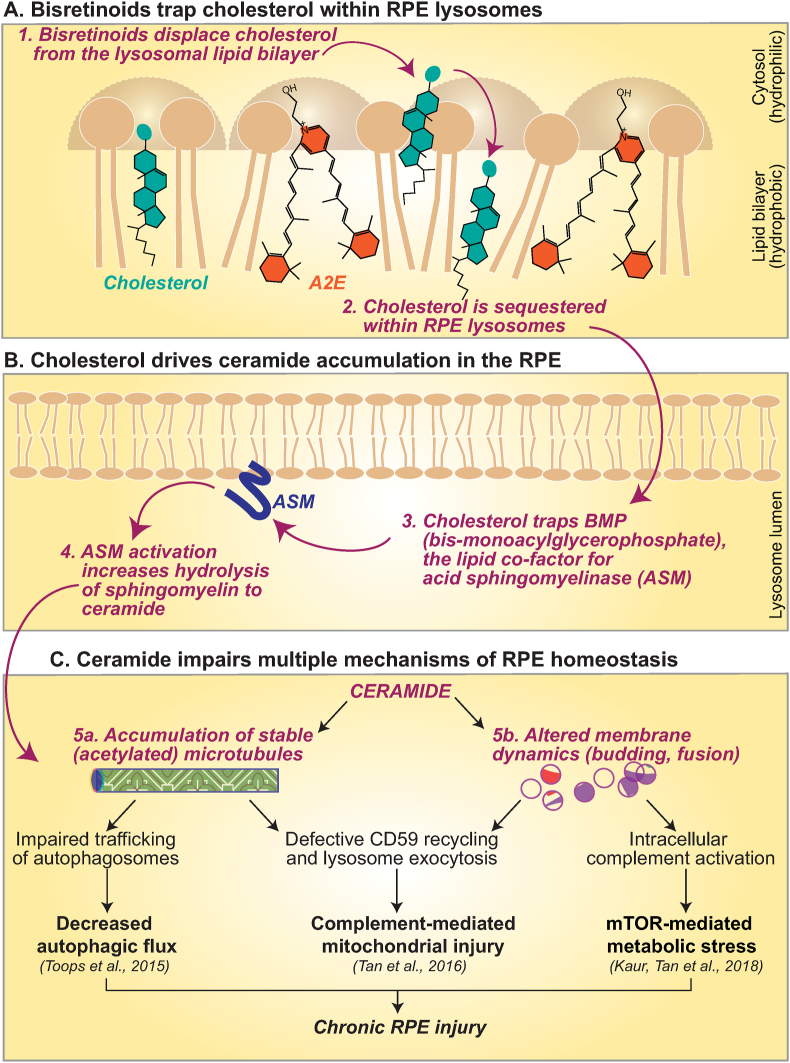
Within the RPE, recent studies suggest that bisretinoids lead to a secondary accumulation of cholesterol and ceramide due to a biophysical interaction between these lipids. A2E and other bisretinoids are cone-shaped lipids, i.e., they have small polar or hydrophilic headgroups and large planar hydrophobic side chains. Within the lysosomal lipid bilayer, A2E tries to minimize the interactions of its hydrophobic side chains with the aqueous phase by taking shelter under the umbrella provided by the larger headgroups of neighboring phospholipids. Cholesterol is also a cone-shaped lipid, and studies using reconstituted liposomes containing A2E and cholesterol show that competition between A2E and cholesterol leads to displacement of cholesterol from the lipid bilayer, resulting in its internalization and entrapment within RPE lysosomes [37] (Fig. 2A).
Fig. 2.
Mechanisms of RPE dysfunction mediated by cholesterol and ceramide. A, In the lysosomal membrane, cone-shaped lipids like A2E and cholesterol compete for space under the phospholipid umbrella to minimize interactions with the aqueous phase. A2E displaces cholesterol, which is trapped within the lysosome lumen (Lakkaraju et al., 2007). B, RPE cholesterol sequesters the anionic lipid bis(monoacyl)glycerophosphate (BMP), which is a cofactor for acid sphingomyelinase (ASM), the lysosomal enzyme that hydrolyzes sphingomyelin to ceramide (Toops et al., 2015). C, Increased ceramide in the RPE leads to the accumulation of stable, acetylated microtubules, which impairs trafficking of autophagosomes (Toops et al., 2015), recycling of the complementary regulatory protein CD59, and relocalizes lysosomes to the perinuclear region of the RPE (Tan et al., 2016). As discussed in Sections 3.2, 3.3, this impairs autophagy and makes the RPE susceptible to complementmediated mitochondrial injury (Toops et al., 2015; Tan et al., 2016). Ceramide is also a cone-shaped lipid that induces negative curvature and inward budding at the RPE plasma membrane. Increased ceramide in RPE with bisretinoids drives the formation of enlarged early endosomes that internalize the complement protein C3. Proteolysis of C3 to biologically active C3a leads to mTOR activation and associated metabolic stress (Kaur, Tan et al., 2018).
Excess cholesterol in turn entraps the anionic lipid bis(monoacyl)glycerophosphate (BMP) within RPE lysosomes. This bisretinoid-mediated increase in lysosomal cholesterol and BMP have been observed in polarized cultures of adult primary RPE with A2E and in pigmented Abca4−/− mice as early as 3 months of age [34]. MALDI imaging mass spectrometry of 6-month-old Abca4−/− mice confirmed increased BMP levels in the RPE [38]. BMP is a cofactor for acid sphingomyelinase (ASM), the lysosomal enzyme that hydrolyzes sphingomyelin to ceramide (Fig. 2B). Abca4−/− mice RPE accumulate high levels of ceramide due to cholesterol-mediated activation of ASM [34,39,40]. In the RPE, ceramide affects microtubule stability and membrane dynamics, with consequences for metabolic and mitochondrial health as discussed below [34,39,40] (Fig. 2C).
Ceramide is a bioactive lipid whose levels in the retina must be carefully controlled [41]. Indeed, ceramide has been implicated in RPE, photoreceptor, and endothelial cell dysfunction in mouse models of retinitis pigmentosa, light-induced photoreceptor degeneration, and diabetic retinopathy [42,43]. In humans, increased ceramide levels both in the serum and in the RPE have been reported in AMD patients, but not in unaffected controls [[44], [45], [46], [47]].
3.2. Impaired autophagy and lysosome function
Autophagy is an evolutionarily conserved mechanism that is responsible for degrading and recycling damaged cellular components. Efficient autophagy is especially important for postmitotic, highly metabolically active cells like the RPE where debris cannot be diluted by cell division [4]. A central role for autophagy in RPE health and visual function is underscored by studies on mice with RPE-specific knockouts of autophagy genes (e.g., RB1CC1, ATG5, ATG7) that exhibit progressive RPE atrophy and retinal degeneration [48,49]. Although studies using human donor tissue have documented lower levels of lipidated LC3 (a marker for autophagosome biogenesis) and increased accumulation of long-lived autophagic substrates such as p62/SQSTM1 in RPE from AMD donors compared to unaffected controls [50,51], mechanisms that lead to impaired autophagy in the aging and diseased RPE have been elusive until recently.
High-speed live-cell imaging of LC3-labeled autophagosome trafficking in primary polarized RPE showed that increased cholesterol and ceramide in RPE with bisretinoids lead to the accumulation of stable, acetylated microtubules, which prevent efficient autophagosome transport [34] (Fig. 2C). In addition to autophagosome trafficking, microtubule acetylation also interferes with lysosome localization within the RPE. Acetylated microtubules preferentially bind kinesin motors, which mediate plus-end directed traffic [52]. In polarized RPE, microtubules are oriented with their plus-ends directed towards the basolateral side; as a result, increased microtubule acetylation leads to perinuclear sequestration of lysosomes in RPE with bisretinoids [40]. Consequently, inefficient autophagosome trafficking and lysosomal relocalization results in disrupted autophagy and accumulation of p62/SQSTM1 in RPE with bisretinoids such as the Abca4−/− mouse model of Stargardt disease [34] and could explain the high levels of p62 reported in cultures of AMD donor RPE [51]. Liver X receptor (LXR) agonists, which decrease RPE cholesterol, functional ASM inhibitors such as the anti-depressant desipramine, which lower ceramide levels, correct microtubule dynamics and restore autophagy in RPE with bisretinoids [34,40]. Thus, cholesterol and ceramide regulate efficient autophagy and debris clearance in the RPE.
Specific autophagic machinery also participate in a process known as LC3-associated phagocytosis or LAP, which is distinct from the so-called “classical” autophagy pathway discussed above. LAP has been extensively studied in macrophages and dendritic cells, where it helps degrade phagocytosed pathogens and other damaging substances. For a detailed discussion of LAP and its role in outer segment phagosome clearance in the RPE, see [4,53]. Studies in mouse models suggest that genetic manipulations that disrupt LAP interfere with the visual cycle and cause retinal degeneration [[54], [55], [56]]. However, whether and how the efficiency and kinetics of LAP are altered with aging and AMD remain to be investigated.
Both debris-containing autophagosomes and outer segment-containing phagosomes have to fuse with lysosomes to effect cargo degradation. Functional lysosome defects including alkalization and disrupted TRPML1-mediated calcium signaling have been reported in RPE cultures with A2E and in Abca4−/− mouse RPE [57]. These defects could be due to increased lysosomal cholesterol, which inhibits the vacuolar-ATPase pump and the TRPML1 channel [58,59]. However, in contrast to impaired clearance of autophagic substrates, clearance of phagocytosed outer segment proteins is not impaired in Abca4−/− mice [39]. These studies suggest that the RPE likely compartmentalizes lysosomal populations for degradation of phagocytic and autophagic cargo. As TRPML1 is also involved in regulating autophagy [60], it is likely that lysosomal accumulation of cholesterol and ceramide specifically dysregulates autophagy in the RPE at the expense of maintaining circadian clearance of outer segments. This compartmentalization of degradative functions in the RPE is further supported by studies showing that loss of Atg7, which mediates LC3 lipidation, does not increase lipofuscin formation in the Abca4−/− mouse model, suggesting that the efficiency of autophagic flux does not impact bisretinoid formation [61].
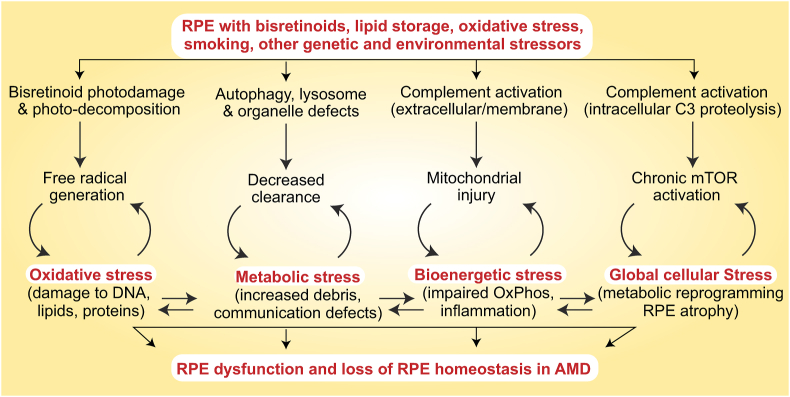
Studies in RPE cell lines and mouse models suggest that acute stress (e.g., intense light, oxidative stress, mitochondrial poisons, and cigarette smoke) upregulates autophagy [[62], [63], [64], [65]]; however, prolonged stress inhibits autophagy, and could further compromise RPE health by promoting accumulation of harmful debris [50,66]. RPE cell cultures from human donors show disrupted autophagy and increased p62 in AMD RPE compared to unaffected donors [51]. AMD donor RPE had increased lipid droplets, glycogen granules and fragmented mitochondria, indicating a global breakdown of cellular clearance pathways. Taken together, these studies suggest that impaired autophagy would lead to the accumulation of damaged proteins, lipids and organelles, which could further burden RPE degradative capacity, setting in motion a feed-forward cycle of metabolic stress (Fig. 3).
Fig. 3.
Feed-forward cycles of RPE dysfunction. As discussed in Section 3, bisretinoids, lipids, oxidative stress and other genetic and environmental factors induce multiple stress pathways that culminate in loss of RPE homeostasis.
3.3. Complement-mediated mitochondrial injury
The complement system, which regulates inflammation and innate immunity, is strongly associated with AMD. Polymorphisms in several complement genes dictate susceptibility to AMD and complement proteins are found in drusen from AMD donor eyes [67]. Activation of the complement pathway in the extracellular or fluid phase results in the sequential assembly of complement proteins on the cell membrane to form the terminal membrane attack complex or MAC pore. Assembly of a large number of MAC pores on the membrane can cause cell lysis; however, sub-lytic MAC assembly can cause chronic cell damage by increasing intracellular calcium and consequent mitochondrial fragmentation [40].
The RPE is the first line of defense against abnormal complement activation in the retina. Like all nucleated cells, the RPE has evolved mechanisms to regulate complement activity at the plasma membrane. These include soluble complement inhibitors such as complement factor H (CFH) and membrane-associated complement regulatory proteins CD46, CD55 and CD59 that inhibit specific steps of MAC assembly [67]. CD59 is a GPI-anchored protein that undergoes cholesterol-dependent recycling to the plasma membrane [68]. In Abca4−/− mouse RPE, excess cholesterol reroutes CD59 from the endosomal recycling route to lysosomes for degradation, resulting in increased MAC deposition on the RPE plasma membrane [40] (Fig. 2C).
Live imaging of primary RPE using total internal reflectance fluorescence (TIRF) microscopy showed that complement attack induces the rapid mobilization of lysosomes near the plasma membrane, which undergo exocytosis to “patch” the nascent MAC pores, thereby preventing calcium influx into the RPE [40]. MAC can also be internalized into RPE endosomes and transported to lysosomes for degradation [69]. In RPE with bisretinoids, the perinuclear sequestration of lysosomes as a result of microtubule acetylation prevents lysosome exocytosis, leading to persistence of MAC pores on the membrane. Both decreased CD59 on the plasma membrane and impaired lysosome exocytosis result in increased intracellular calcium in the RPE, which causes mitochondrial fragmentation and production of reactive oxygen species (Fig. 2C). These free radicals further injure mitochondria and establish a feed-forward cycle of continual injury and oxidative stress. As with autophagy, lowering cholesterol with LXR agonists or decreasing ceramide with desipramine restores CD59 to the RPE cell surface, promotes lysosome exocytosis and protects RPE mitochondria from complement attack [40].
Studies using other AMD-associated insults and human donor tissue reinforce this link between complement activation and mitochondrial injury in disease pathogenesis. Exposure to cigarette smoke or hydrogen peroxide have been shown to decrease cell-surface complement regulatory proteins and increase mitochondrial stress in the RPE both in vitro and in mice [[70], [71], [72]]. Further, studies on human donor tissue show decreased cell-surface CD59 and CD46 in RPE from AMD donors compared to controls [73,74], and increased mitochondrial DNA damage in AMD donors with the Y402H risk variant of complement factor H [75]. More recently, high-resolution imaging and 3-D reconstruction of mitochondrial volumes in human donor RPE showed increased mitochondrial fragmentation in AMD donors compared to unaffected controls [76]. These studies demonstrate that AMD-associated insults (bisretinoids, cigarette smoke, oxidative stress) impair critical mechanisms that protect the RPE from complement attack, and set in motion a feed-forward cycle of mitochondrial stress (Fig. 3).
3.4. mTOR activation and cellular reprogramming
The mechanistic target of Rapamycin (mTOR), a serine/threonine kinase, is the master regulator of a complex network that controls metabolism, signaling, and cell homeostasis. mTOR plays context-dependent roles in regulating proliferation, survival, and cell fate decisions [77]. Increased mTOR activation has been recently reported in AMD donor RPE [78], however the mechanisms that control mTOR activity, and how these are dysregulated in disease are only now being elucidated.
Recent studies have identified an unusual mechanism of abnormal mTOR activation in diseased RPE mediated by intracellular cleavage of the complement protein C3, the core effector molecule of the complement system. In RPE with bisretinoids, high-resolution live-cell imaging shows that increased ceramide at the apical plasma membrane induces the formation of enlarged early endosomes. These endosomes internalize C3, which is proteolyzed to biologically active C3a within the RPE, and in turn activates mTOR [39] (Fig. 2C). Sustained mTOR signaling can impair RPE homeostasis by modulating multiple stress pathways implicated in aging and disease [77,79]. For instance, mTOR inhibition is required to initiate autophagy, and chronic mTOR activity in Abca4−/− RPE could explain the decreased autophagosome biogenesis that is seen in this model [34,80]. Administration of desipramine to Abca4−/− mice decreases ceramide levels, prevents formation of enlarged early endosomes, and limits C3a formation and mTOR activation [39].
Because of its many functions, mTOR activity needs to be tightly regulated in the post-mitotic RPE. Sustained mTOR activation by chemical or genetic approaches in mice induces metabolic reprogramming in the RPE by increasing glycolytic flux, and eventually leads to RPE atrophy. The resulting decline in glucose delivery to the retina could contribute to photoreceptor degeneration [79,81]. mTOR is also implicated in cytokine secretion and inflammasome activation, which could promote RPE injury in geographic atrophy. However, subconjunctival administration of the mTOR inhibitor rapamycin (also known as sirolimus) failed to show benefit in AMD patients [82], indicating that normalizing mTOR function by targeting upstream activators, rather than blocking mTOR activity, could be more effective in AMD. Altogether, these studies suggest that aberrant mTOR activation mediated by aging-associated insults in the RPE can set in motion a feed-forward cycle of global cellular stress (Fig. 3).
4. Genetic tipping points and novel therapeutic targets for AMD
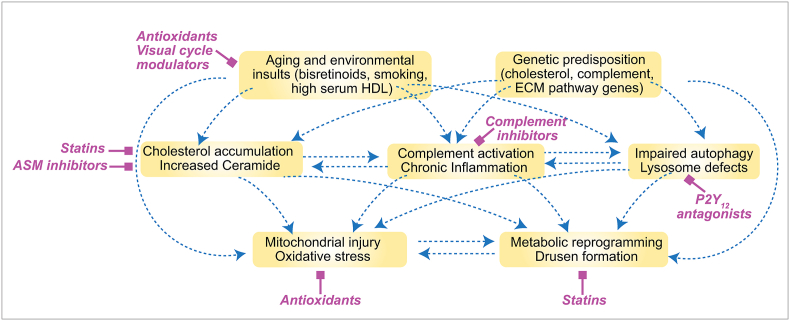
The pathways of RPE cellular dysfunction discussed in Sections 2, 3 do not exist in a vacuum, but interact with and potentiate one another by strengthening the feed-forward cycles depicted in Fig. 3. One of the major outstanding questions in AMD biology is how AMD-associated risk factors modulate these cycles of RPE injury. Although genes associated with AMD appear to function in diverse pathways, in light of what we now know about mechanisms of RPE injury, these AMD risk factors can be woven into a unified model to explain several aspects of AMD pathogenesis and help identify therapeutic approaches to target specific steps of this cascade (Fig. 4). Based on this model, we propose that genetic risk variants and environmental factors implicated in AMD act as decision points to reinforce mechanisms of RPE damage and tip the scales from normal aging towards disease.
Fig. 4.
Converging pathways of lipid metabolism, complement activation, and mitochondrial injury in AMD. The interconnected web summarizes studies discussed in the text regarding current knowledge about mechanisms of RPE injury. We propose that AMD-associated genetic risk variants and environmental factors act as “tipping points” or decision nodes that can drive the RPE from normal aging towards disease. In magenta are potential therapeutic interventions discussed in Section 4 to target specific nodes of this network.
4.1. Targeting oxidative stress
As discussed above, bisretinoids can impair RPE homeostasis by both direct and indirect mechanisms. Therefore, various approaches are currently being tested in preclinical and clinical studies to prevent bisretinoid formation [8]. One approach is to limit the uptake of vitamin A by interfering with serum retinol binding protein 4 (RBP4) activity using a non-retinoid antagonist A1102 [83]. A second approach is to use deuterated vitamin A (ALK-001), which is a less reactive retinaldehyde and decreases bisretinoid formation without slowing down the visual cycle [84]. A multicenter, randomized, double-masked placebo-controlled Phase III clinical trial (NCT03845582) is currently recruiting to determine safety, pharmacokinetics and efficacy in geographic atrophy secondary to AMD. Other drugs currently in clinical trials include elamipretide (NCT02848313), which is thought to reduce mitochondrial dysfunction, and brimonidine tartrate (NCT02087085), which is proposed to be neuroprotective (reviewed in [85]).
In animal models, antioxidants or iron chelators have been shown to decrease photodecomposition of bisretinoids. Administration of either vitamin E or deferiprone prevented photoreceptor damage in albino Abca4−/− mice [22]. Targeting the P2Y12 receptor by oral administration of ticagrelor also protects albino Abca4−/− photoreceptors from light-induced degeneration, presumably by improving lysosome function in the RPE and decreasing lipofuscin autofluorescence [86]. Primary RPE cultures and iPSC-RPE derived from AMD patients express high levels of complement and inflammatory proteins and exhibit signs of oxidative stress, which is ameliorated by treatment with nicotinamide [78,87].
4.2. Targeting cholesterol and drusen formation
As discussed earlier, genetic, epidemiological and histological data strongly implicate abnormal handling of cholesterol in AMD pathogenesis. Genes that regulate cholesterol transport and HDL remodeling are strongly associated with AMD. These include the cholesterol transporters apolipoprotein E (APOE) and ATP-binding cassette transporter A1 (ABCA1); and cholesterol ester transfer protein (CETP) and hepatic lipase (LIPC), which function in HDL remodeling [5,88]. Epidemiological analyses confirm that high HDL-C and low triglyceride levels are associated with an increased risk of AMD [32]. In another study, levels of activated complement (C3d/C3) in the serum correlated positively with HDL-C and prevalence of AMD [89].
Although the mechanistic link between serum HDL-cholesterol and AMD is not yet clear, all AMD-associated cholesterol genes are expressed by the RPE [88], raising the possibility that disease-associated variants could act locally to modulate cholesterol transport and lipoprotein metabolism within the retina. In support of this, RPE-specific deletion of ABCA1 in mice leads to cholesterol accumulation within the RPE, followed by progressive RPE atrophy, photoreceptor degeneration, and vision loss [36]. Thus, increased RPE cholesterol, whether due to defective cholesterol transport or secondary to lipofuscin accumulation, leads to RPE injury and dysfunction [34,39,40]. Cholesterol and the cholesterol transporters ApoB and ApoE are enriched in drusen from AMD donors [33,90,91]. However, despite decades of observational studies showing that drusen size and number correlate with AMD progression, mechanisms involved in drusen formation are only now being understood.
Recent studies using sophisticated live-cell imaging approaches have clarified the role of ApoE isoforms in cholesterol homeostasis in the RPE, and helped identify a biophysical mechanism that could explain drusen biogenesis [76]. Humans are the only species that express three isoforms of ApoE (ApoE2, ApoE3 and ApoE4) that differ from one another in only one amino acid: ApoE2 has two cysteines at positions 112 and 158, ApoE3 and a cysteine and an arginine, and ApoE4 has two arginines. These cysteine/arginine interchanges profoundly impact lipoprotein binding and cholesterol transport capacities of the resulting proteins [[92], [93], [94], [95], [96]]. Prevalence of these alleles is ~2% for APOE2, 78% for APOE3, and 14% for APOE4 [97]. Pertinently, APOE shows reversed risk associations between Alzheimer's disease and AMD: APOE4, which is a confirmed risk factor for Alzheimer's disease, is protective in AMD; and APOE2, which is protective in Alzheimer's disease increases AMD risk [5,32,98,99]. In the RPE, live-cell imaging reveals that ApoE2 vesicles show constrained, short range trafficking, whereas ApoE3 and ApoE4 vesicles exhibit long-range directed movements. This leads to poor cholesterol transport by ApoE2, compared to ApoE3 or ApoE4. Consequently, unlike RPE expressing the protective ApoE4 isoform, ApoE2-RPE have increased cholesterol, which impairs autophagy and the ability of the RPE to protect itself from complement-induced mitochondrial injury (See Sections 3.2, 3.3 and Fig. 2).
All ApoE isoforms are rich in intrinsically disordered regions, which enable them to engage in low-affinity, multivalent interactions with one another. These interactions lead to liquid-liquid phase separation, a recently discovered biophysical phenomenon that drives the formation of cytoplasmic biomolecular condensates that are not delimited by a membrane [100]. Studies in Alzheimer's disease and other neurodegenerative diseases suggest that mutant proteins can induce structural changes in biomolecular condensates, leading to the formation of insoluble aggregates [101]. Abnormal phase transitions can be induced by mutations and posttranslational modifications within the proteins or by changes in the cellular environment, which can impact protein structure and dynamics. In RPE expressing ApoE2, mitochondrial injury leads to thiol oxidation of the two cysteines at positions 112 and 158, which increase its intrinsic disorder and promote aggregation. This results in the formation of large biomolecular condensates that could function as precursors to drusen in ApoE2-RPE. On the other hand, ApoE4 with two arginines at 112 and 158 that are not susceptible to oxidation, is completely resistant to these phase transitions and does not form large condensates. These studies establish how a major AMD risk variant can disrupt critical metabolic functions in the RPE, and identify oxidative stress-mediated aberrant phase transitions as an exciting new mechanism that could contribute to drusen biogenesis [76].
The studies discussed above demonstrate a clear need for precise regulation of cholesterol homeostasis in the retina because cholesterol impacts metabolism and inflammation, two pathways implicated in AMD [2]. However, based on retrospective studies, the potential of cholesterol-lowering drugs like statins (HMG-CoA reductase inhibitors) as AMD therapeutics is as yet unresolved [102,103]. A small open-label prospective study using high dose atorvastatin for a year showed drusen regression in ~40% of patients [104]; whether this will hold true in a larger, double-blind placebo-controlled study remains to be seen. Other approaches for lowering cholesterol include liver X receptor agonists [105]. These drugs activate the LXR nuclear hormone receptors, which induce transcription of target genes including the ApoE and ABCA1 cholesterol transporters [106]. Although, currently, no synthetic LXR agonists are clinically approved due to adverse effects, rational design could likely lead to the development of new agonists suitable for human use [107].
4.3. Targeting ASM and ceramide to maintain RPE homeostasis
Ceramide is a bioactive lipid that regulates a wide array of cellular processes [108]. Studies on animal models of diverse retinal diseases including retinitis pigmentosa, glaucoma, diabetic retinopathy, retinal ischemia, light-induced retinal degeneration, Stargardt disease, and AMD have identified ceramide as a mediator of pathology [42,43,109,110]. In these models, increased ceramide has been shown to promote injury, inflammation and death in photoreceptors, the RPE, and the retinal vasculature. Decreasing ceramide levels either pharmacologically or by genetic deletion of ASM has been shown to prevent retinal degeneration in several of these models [43,109,111]. These studies suggest that ceramide levels need to be tightly regulated in the retina, and any disruptions to the sphingolipid rheostat can have severe consequences for retinal health and function (recently reviewed in [110]).
In the RPE, excess ceramide due to increased ASM activation alters membrane dynamics and leads to aberrant endosome biogenesis, perinuclear sequestration of lysosomes, impaired autophagosome trafficking, and mitochondrial fragmentation. Several drugs currently approved for human use inhibit ASM as an off-target effect [112]. These include tricyclic anti-depressants desipramine and amitriptyline, selective serotonin reuptake inhibitors sertraline and fluoxetine, among others. These drugs are functional inhibitors of ASM: they are weak lipophilic bases that accumulate in lysosomes due to a protonatable nitrogen. Within the lysosome, they prevent ASM from binding its cofactor BMP via steric hindrance. This causes ASM to detach from the lysosomal membrane and undergo lysosomal proteolysis [113]. In Abca4−/− mice, intraperitoneal or oral administration of desipramine decreases RPE ceramide levels to that in age-matched wild-types. This prevents the formation of enlarged early endosomes, intracellular C3 proteolysis, and mTOR activation [39]. Desipramine treatment in RPE with bisretinoids corrects impaired autophagosome trafficking and autophagic flux [34], and restores CD59 recycling to the plasma membrane, which prevents MAC assembly after complement attack, and protects RPE mitochondria from complement-induced fragmentation [40].
Data from AMD patients provide strong support for therapeutic targeting of ceramide: first, analysis of serum sphingolipids in AMD patients showed higher ceramide levels in patients with geographic atrophy, which was associated with the Y402H risk variant of complement factor H (CFH) [47]. Second, increased RPE ceramide has been observed in AMD donors, but not unaffected controls [[44], [45], [46]]. Third, pooled data from three continents show that use of ASM inhibitors such as desipramine is associated with a significantly decreased risk of developing AMD [114,115]. Lastly, serum ceramide levels increase with age in humans [116], and in the brains of patients with Alzheimer's and Parkinson's diseases [117,118]. These studies suggest that dysregulation of ceramide homeostasis in postmitotic cells such as neurons and RPE could be a common etiological feature – and therapeutic target - in age-related neurodegenerative diseases including AMD.
5. Perspectives
Deciphering the biology behind AMD genetic risk variants requires moving beyond observational studies to more mechanism-based approaches using cutting-edge biophysical, biochemical and imaging techniques. Some outstanding questions in this area include:
-
•
What is the relationship between AMD-associated polymorphisms in cholesterol genes, systemic lipid profiles, and RPE lipid metabolism?
-
•
How do AMD--associated polymorphisms in complement genes impact complement activation in the retina, inflammation, and mitochondrial injury in the RPE?
-
•
What are the molecular players that mediate RPE mitochondrial fragmentation and mitochondrial DNA damage?
-
•
Where and how can we intervene to prevent metabolic reprogramming and RPE atrophy in AMD?
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Supported by NIH/NEI grants R01EY023299 and R01EY030668, the Research to Prevent Blindness Catalyst Award for Innovative Approaches to AMD, the BrightFocus Foundation Award for AMD research M2015350 and Macular Society UK.
References
- 1.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Handa J.T., Bowes Rickman C., Dick A.D., Gorin M.B., Miller J.W., Toth C.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019;10(1):3347. doi: 10.1038/s41467-019-11262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caceres P.S., Rodriguez-Boulan E. Retinal pigment epithelium polarity in health and blinding diseases. Curr. Opin. Cell Biol. 2020;62:37–45. doi: 10.1016/j.ceb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakkaraju A., Umapathy A., Tan L.X., Daniele L., Philp N.J., Boesze-Battaglia K. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020:100846. doi: 10.1016/j.preteyeres.2020.100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritsche L.G., Igl W., Bailey J.N., Grassmann F., Sengupta S., Bragg-Gresham J.L. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016;48(2):134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan T.D., Agron E., Domalpally A., Clemons T.E., van Asten F., Wong W.T. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125(12):1913–1928. doi: 10.1016/j.ophtha.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.J., Sparrow J.R. Bisretinoid phospholipid and vitamin A aldehyde: shining a light. J. Lipid Res. 2020 doi: 10.1194/jlr.TR120000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow J.R. Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics. Proc. Natl. Acad. Sci. U. S. A. 2016;113(17):4564–4569. doi: 10.1073/pnas.1600474113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldred G.E., Lasky M.R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361(6414):724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Garcia A., Kun A., Calero O., Medina M., Calero M. An overview of the role of lipofuscin in age-related neurodegeneration. Front. Neurosci. 2018;12:464. doi: 10.3389/fnins.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson D.H., Ozaki S., Nealon M., Neitz J., Mullins R.F., Hageman G.S. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am. J. Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 12.Sarks J., Arnold J., Ho I.V., Sarks S., Killingsworth M. Evolution of reticular pseudodrusen. Br. J. Ophthalmol. 2011;95(7):979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 13.Sarks S., Cherepanoff S., Killingsworth M., Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48(3):968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 14.Parish C.A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 1998;95(25):14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparrow J.R., Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Shabat S., Parish C.A., Vollmer H.R., Itagaki Y., Fishkin N., Nakanishi K. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J. Biol. Chem. 2002;277(9):7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 17.Sparrow J.R., Vollmer-Snarr H.R., Zhou J., Jang Y.P., Jockusch S., Itagaki Y. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-EPOXIDE formation. J. Biol. Chem. 2003;278(20):18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J., Jang Y.P., Kim S.R., Sparrow J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 2006;103(44):16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie M., Bigot K., Angebault C., Barrau C., Gondouin P., Pagan D. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9(3):287. doi: 10.1038/s41419-018-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radu R.A., Hu J., Yuan Q., Welch D.L., Makshanoff J., Lloyd M. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J. Biol. Chem. 2011;286(21):18593–18601. doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radu R.A., Mata N.L., Nusinowitz S., Liu X., Travis G.H. Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Novartis Found. Symp. 2004;255:51–63. discussion -7, 177-8. [PubMed] [Google Scholar]
- 22.Ueda K., Zhao J., Kim H.J., Sparrow J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2016;113(25):6904–6909. doi: 10.1073/pnas.1524774113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda K., Kim H.J., Zhao J., Song Y., Dunaief J.L., Sparrow J.R. Iron promotes oxidative cell death caused by bisretinoids of retina. Proc. Natl. Acad. Sci. U. S. A. 2018;115(19):4963–4968. doi: 10.1073/pnas.1722601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J., Ueda K., Zhao J., Sparrow J.R. Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl adduct deposition. J. Biol. Chem. 2015;290(45):27215–27227. doi: 10.1074/jbc.M115.680363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto K., Yoon K.D., Ueda K., Hashimoto M., Sparrow J.R. A novel bisretinoid of retina is an adduct on glycerophosphoethanolamine. Invest. Ophthalmol. Vis. Sci. 2011;52(12):9084–9090. doi: 10.1167/iovs.11-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabb J.W. The proteomics of drusen. Cold Spring Harb Perspect Med. 2014;4(7) doi: 10.1101/cshperspect.a017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J., Cai B., Jang Y.P., Pachydaki S., Schmidt A.M., Sparrow J.R. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 2005;80(4):567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow J.R., Dowling J.E., Bok D. Understanding RPE lipofuscin. Invest. Ophthalmol. Vis. Sci. 2013;54(13):8325–8326. doi: 10.1167/iovs.13-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avalle L.B., Wang Z., Dillon J.P., Gaillard E.R. Observation of A2E oxidation products in human retinal lipofuscin. Exp. Eye Res. 2004;78(4):895–898. doi: 10.1016/j.exer.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Yakovleva M.A., Radchenko A.S., Feldman T.B., Kostyukov A.A., Arbukhanova P.M., Borzenok S.A. Fluorescence characteristics of lipofuscin fluorophores from human retinal pigment epithelium. Photochem. Photobiol. Sci. 2020;19(7):920–930. doi: 10.1039/c9pp00406h. [DOI] [PubMed] [Google Scholar]
- 31.Crabb J.W., Miyagi M., Gu X., Shadrach K., West K.A., Sakaguchi H. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colijn J.M., den Hollander A.I., Demirkan A., Cougnard-Gregoire A., Verzijden T., Kersten E. Increased high-density lipoprotein levels associated with age-related macular degeneration: evidence from the EYE-RISK and European eye epidemiology consortia. Ophthalmology. 2019;126(3):393–406. doi: 10.1016/j.ophtha.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 33.Malek G., Li C.-M., Guidry C., Medeiros N.E., Curcio C.A. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am. J. Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toops K.A., Tan L.X., Jiang Z., Radu R., Lakkaraju A. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol. Biol. Cell. 2015;26:1–14. doi: 10.1091/mbc.E14-05-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guziewicz K.E., Sinha D., Gomez N.M., Zorych K., Dutrow E.V., Dhingra A. Bestrophinopathy: an RPE-photoreceptor interface disease. Prog. Retin. Eye Res. 2017;58:70–88. doi: 10.1016/j.preteyeres.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storti F., Klee K., Todorova V., Steiner R., Othman A., van der Velde-Visser S. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. Elife. 2019;8 doi: 10.7554/eLife.45100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakkaraju A., Finnemann S.C., Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson D.M.G., Ablonczy Z., Koutalos Y., Hanneken A.M., Spraggins J.M., Calcutt M.W. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci. Rep. 2017;7(1):17352. doi: 10.1038/s41598-017-17402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur G., Tan L.X., Rathnasamy G., La Cunza N.R., Germer C.J., Toops K.A. Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2018;115:9014–9019. doi: 10.1073/pnas.1805039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan L.X., Toops K.A., Lakkaraju A. Protective responses to sublytic complement in the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 2016;113(31):8789–8794. doi: 10.1073/pnas.1523061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon M.V., Prado Spalm F.H., Vera M.S., Rotstein N.P. Sphingolipids as emerging mediators in retina degeneration. Front. Cell. Neurosci. 2019;13:246. doi: 10.3389/fncel.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opreanu M., Tikhonenko M., Bozack S., Lydic T.A., Reid G.E., McSorley K.M. The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes. 2011;60(9):2370–2378. doi: 10.2337/db10-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H., Tran J.T., Eckerd A., Huynh T.P., Elliott M.H., Brush R.S. Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration. J. Lipid Res. 2013;54(6):1616–1629. doi: 10.1194/jlr.M035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Germer C.J., Tan L.X., Rathnasamy G., La Cunza N., Xu J., Lakkaraju A. n/a; 2020. Ceramide drives apical membrane remodeling in the retinal pigment epithelium in macular degenerations. In preparation. [Google Scholar]
- 45.Zemski Berry K.A., Gordon W.C., Murphy R.C., Bazan N.G. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. J. Lipid Res. 2014;55(3):504–515. doi: 10.1194/jlr.M044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon W.C., Zemski Berry K.A., Murphy R.C., Bazan N.G. MALDI imaging mass spectrometry of lipids in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2014;55:5234. [Google Scholar]
- 47.Pujol-Lereis L.M., Liebisch G., Schick T., Lin Y., Grassmann F., Uchida K. Evaluation of serum sphingolipids and the influence of genetic risk factors in age-related macular degeneration. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0200739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J., Jia L., Khan N., Lin C., Mitter S.K., Boulton M.E. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11(6):939–953. doi: 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Cross S.D., Stanton J.B., Marmorstein A.D., Le Y.Z., Marmorstein L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017;23:228–241. [PMC free article] [PubMed] [Google Scholar]
- 50.Mitter S.K., Song C., Qi X., Mao H., Rao H., Akin D. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10(11):1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golestaneh N., Chu Y., Xiao Y.Y., Stoleru G.L., Theos A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8(1) doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed N.A., Cai D., Blasius T.L., Jih G.T., Meyhofer E., Gaertig J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. : CB. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Heckmann B.L., Green D.R. LC3-associated phagocytosis at a glance. J. Cell Sci. 2019;132(5) doi: 10.1242/jcs.222984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.Y., Zhao H., Martinez J., Doggett T.A., Kolesnikov A.V., Tang P.H. Noncanonical autophagy promotes the visual cycle. Cell. 2013;154(2):365–376. doi: 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhingra A., Bell B.A., Peachey N.S., Daniele L.L., Reyes-Reveles J., Sharp R.C. Microtubule-associated protein 1 light chain 3B, (LC3B) is necessary to maintain lipid-mediated homeostasis in the retinal pigment epithelium. Front. Cell. Neurosci. 2018;12:351. doi: 10.3389/fncel.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frost L.S., Lopes V.S., Bragin A., Reyes-Reveles J., Brancato J., Cohen A. The contribution of melanoregulin to microtubule-associated protein 1 light chain 3 (LC3) associated phagocytosis in retinal pigment epithelium. Mol. Neurobiol. 2014 doi: 10.1007/s12035-014-8920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez N.M., Lu W., Lim J.C., Kiselyov K., Campagno K.E., Grishchuk Y. Robust lysosomal calcium signaling through channel TRPML1 is impaired by lysosomal lipid accumulation. Faseb. J. 2018;32(2):782–794. doi: 10.1096/fj.201700220RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen D., Wang X., Li X., Zhang X., Yao Z., Dibble S. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox B.E., Griffin E.E., Ullery J.C., Jerome W.G. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J. Lipid Res. 2007;48(5):1012–1021. doi: 10.1194/jlr.M600390-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Wang W., Gao Q., Yang M., Zhang X., Yu L., Lawas M. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl. Acad. Sci. U. S. A. 2015;112(11):E1373–E1381. doi: 10.1073/pnas.1419669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perusek L., Sahu B., Parmar T., Maeno H., Arai E., Le Y.Z. Di-retinoid-pyridinium-ethanolamine (A2E) accumulation and the maintenance of the visual cycle are independent of atg7-mediated autophagy in the retinal pigmented epithelium. J. Biol. Chem. 2015;290(48):29035–29044. doi: 10.1074/jbc.M115.682310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reme C.E., Wolfrum U., Imsand C., Hafezi F., Williams T.P. Photoreceptor autophagy: effects of light history on number and opsin content of degradative vacuoles. Invest. Ophthalmol. Vis. Sci. 1999;40(10):2398–2404. [PubMed] [Google Scholar]
- 63.Kunchithapautham K., Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007;3(5):433–441. doi: 10.4161/auto.4294. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y., Sawada O., Kohno H., Le Y.Z., Subauste C., Maeda T. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013;288(11):7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Cano M., Handa J.T. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim. Biophys. Acta. 2014;1843(7):1248–1258. doi: 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song C., Mitter S.K., Qi X., Beli E., Rao H.V., Ding J. Oxidative stress-mediated NFkappaB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark S.J., Bishop P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2018;40(1):65–74. doi: 10.1007/s00281-017-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayor S., Sabharanjak S., Maxfield F.R. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17(16):4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgiannakis A., Burgoyne T., Lueck K., Futter C., Greenwood J., Moss S.E. Retinal pigment epithelial cells mitigate the effects of complement attack by endocytosis of C5b-9. J. Immunol. 2015;195(7):3382–3389. doi: 10.4049/jimmunol.1500937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebrahimi K.B., Cano M., Rhee J., Datta S., Wang L., Handa J.T. Oxidative stress induces an interactive decline in wnt and Nrf2 signaling in degenerating retinal pigment epithelium. Antioxidants Redox Signal. 2018;29(4):389–407. doi: 10.1089/ars.2017.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thurman J.M., Renner B., Kunchithapautham K., Ferreira V.P., Pangburn M.K., Ablonczy Z. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009;284(25):16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kunchithapautham K., Atkinson C., Rohrer B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J. Biol. Chem. 2014;289(21):14534–14546. doi: 10.1074/jbc.M114.564674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ebrahimi K.B., Fijalkowski N., Cano M., Handa J.T. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol. 2013;229(5):729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vogt S.D., Curcio C.A., Wang L., Li C.M., McGwin G., Jr., Medeiros N.E. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp. Eye Res. 2011;93(4):413–423. doi: 10.1016/j.exer.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrington D.A., Kapphahn R.J., Leary M.M., Atilano S.R., Terluk M.R., Karunadharma P. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp. Eye Res. 2016;145:269–277. doi: 10.1016/j.exer.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.La Cunza N., Tan L.X., Rathnasamy G., Thamban T., Germer C.J., Toops K.A. biorxiv; 2020. Apolipoprotein E Isoform-specific Phase Transitions in the Retinal Pigment Epithelium Drive Disease Phenotypes in Age-Related Macular Degeneration. [DOI] [Google Scholar]
- 77.Liu G.Y., Sabatini D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020;21(4):183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M., Jiang N., Chu Y., Postnikova O., Varghese R., Horvath A. Dysregulated metabolic pathways in age-related macular degeneration. Sci. Rep. 2020;10(1):2464. doi: 10.1038/s41598-020-59244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao C., Yasumura D., Li X., Matthes M., Lloyd M., Nielsen G. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J. Clin. Invest. 2011;121(1):369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lenis T.L., Sarfare S., Jiang Z., Lloyd M.B., Bok D., Radu R.A. Complement modulation in the retinal pigment epithelium rescues photoreceptor degeneration in a mouse model of Stargardt disease. Proc. Natl. Acad. Sci. U. S. A. 2017;114(15):3987–3992. doi: 10.1073/pnas.1620299114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Go Y.M., Zhang J., Fernandes J., Litwin C., Chen R., Wensel T.G. MTOR-initiated metabolic switch and degeneration in the retinal pigment epithelium. Faseb. J. 2020 doi: 10.1096/fj.202000612R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong W.T., Dresner S., Forooghian F., Glaser T., Doss L., Zhou M. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Invest. Ophthalmol. Vis. Sci. 2013;54(4):2941–2950. doi: 10.1167/iovs.13-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Racz B., Varadi A., Kong J., Allikmets R., Pearson P.G., Johnson G. A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle. J. Biol. Chem. 2018;293(29):11574–11588. doi: 10.1074/jbc.RA118.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charbel Issa P., Barnard A.R., Herrmann P., Washington I., MacLaren R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl. Acad. Sci. U. S. A. 2015;112(27):8415–8420. doi: 10.1073/pnas.1506960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ammar M.J., Hsu J., Chiang A., Ho A.C., Regillo C.D. Age-related macular degeneration therapy: a review. Curr. Opin. Ophthalmol. 2020;31(3):215–221. doi: 10.1097/ICU.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 86.Lu W., Gomez N.M., Lim J.C., Guha S., O'Brien-Jenkins A., Coffey E.E. The P2Y12 receptor antagonist ticagrelor reduces lysosomal pH and autofluorescence in retinal pigmented epithelial cells from the ABCA4(-/-) mouse model of retinal degeneration. Front. Pharmacol. 2018;9:242. doi: 10.3389/fphar.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saini J.S., Corneo B., Miller J.D., Kiehl T.R., Wang Q., Boles N.C. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2017;20(5):635–647 e7. doi: 10.1016/j.stem.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Leeuwen E.M., Emri E., Merle B.M.J., Colijn J.M., Kersten E., Cougnard-Gregoire A. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018;67:56–86. doi: 10.1016/j.preteyeres.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Paun C.C., Ersoy L., Schick T., Groenewoud J.M., Lechanteur Y.T., Fauser S. Genetic variants and systemic complement activation levels are associated with serum lipoprotein levels in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2015;56(13):7766–7773. doi: 10.1167/iovs.15-17035. [DOI] [PubMed] [Google Scholar]
- 90.Rudolf M., Malek G., Messinger J.D., Clark M.E., Wang L., Curcio C.A. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp. Eye Res. 2008;87(5):402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li C.M., Chung B.H., Presley J.B., Malek G., Zhang X., Dashti N. Lipoprotein-like particles and cholesteryl esters in human bruch's membrane: initial characterization. Invest. Ophthalmol. Vis. Sci. 2005;46(7):2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 92.Yamazaki Y., Zhao N., Caulfield T.R., Liu C.C., Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 2019;15(9):501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leduc V., Domenger D., De Beaumont L., Lalonde D., Belanger-Jasmin S., Poirier J. Function and comorbidities of apolipoprotein e in Alzheimer's disease. Int. J. Alzheimer's Dis. 2011;2011:974361. doi: 10.4061/2011/974361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahley R.W., Rall S.C., Jr. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genom. Hum. Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 95.Hatters D.M., Peters-Libeu C.A., Weisgraber K.H. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 2006;31(8):445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Toops K.A., Tan L.X., Lakkaraju A. Apolipoprotein E isoforms and AMD. Adv. Exp. Med. Biol. 2016;854:3–9. doi: 10.1007/978-3-319-17121-0_1. [DOI] [PubMed] [Google Scholar]
- 97.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. J. Am. Med. Assoc. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 98.Zareparsi S., Reddick A.C., Branham K.E., Moore K.B., Jessup L., Thoms S. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest. Ophthalmol. Vis. Sci. 2004;45(5):1306–1310. doi: 10.1167/iovs.03-1253. [DOI] [PubMed] [Google Scholar]
- 99.McKay G.J., Patterson C.C., Chakravarthy U., Dasari S., Klaver C.C., Vingerling J.R. Evidence of association of APOE with age-related macular degeneration: a pooled analysis of 15 studies. Hum. Mutat. 2011;32(12):1407–1416. doi: 10.1002/humu.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wheeler R.J., Hyman A.A. Controlling compartmentalization by non-membrane-bound organelles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373(1747) doi: 10.1098/rstb.2017.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163(1):123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roizenblatt M., Naranjit N., Maia M., Gehlbach P.L. The question of a role for statins in age-related macular degeneration. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gehlbach P., Li T., Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst. Rev. 2016;(8) doi: 10.1002/14651858.CD006927.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vavvas D.G., Daniels A.B., Kapsala Z.G., Goldfarb J.W., Ganotakis E., Loewenstein J.I. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine. 2016;5:198–203. doi: 10.1016/j.ebiom.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choudhary M., Ismail E.N., Yao P.L., Tayyari F., Radu R.A., Nusinowitz S. LXRs regulate features of age-related macular degeneration and may be a potential therapeutic target. JCI Insight. 2020;5(1) doi: 10.1172/jci.insight.131928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venkateswaran A., Laffitte B.A., Joseph S.B., Mak P.A., Wilpitz D.C., Edwards P.A. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. U. S. A. 2000;97(22):12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fessler M.B. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol. Ther. 2018;181:1–12. doi: 10.1016/j.pharmthera.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannun Y.A., Obeid L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19(3):175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan J., Wu B.X., Crosson C.E. Suppression of acid sphingomyelinase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2016;57(10):4476–4484. doi: 10.1167/iovs.16-19717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simon M.V., Basu S.K., Qaladize B., Grambergs R.C., Rotstein N.P., Mandal N. Sphingolipids as critical players in retinal physiology and pathology. J. Lipid Res. 2020 doi: 10.1194/jlr.TR120000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strettoi E., Gargini C., Novelli E., Sala G., Piano I., Gasco P. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 2010;107(43):18706–18711. doi: 10.1073/pnas.1007644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kornhuber J., Muehlbacher M., Trapp S., Pechmann S., Friedl A., Reichel M. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kornhuber J., Muller C.P., Becker K.A., Reichel M., Gulbins E. The ceramide system as a novel antidepressant target. Trends Pharmacol. Sci. 2014;35(6):293–304. doi: 10.1016/j.tips.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 114.van Leeuwen R., Tomany S.C., Wang J.J., Klein R., Mitchell P., Hofman A. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111(6):1169–1175. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 115.Klein R., Klein B.E., Jensen S.C., Cruickshanks K.J., Lee K.E., Danforth L.G. Medication use and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch. Ophthalmol. 2001;119(9):1354–1359. doi: 10.1001/archopht.119.9.1354. [DOI] [PubMed] [Google Scholar]
- 116.Park M.H., Lee J.Y., Park K.H., Jung I.K., Kim K.T., Lee Y.S. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron. 2018;100(3):762. doi: 10.1016/j.neuron.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 117.Filippov V., Song M.A., Zhang K., Vinters H.V., Tung S., Kirsch W.M. Increased ceramide in brains with Alzheimer's and other neurodegenerative diseases. J Alzheimers Dis. 2012;29(3):537–547. doi: 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin G., Wang L., Marcogliese P.C., Bellen H.J. Sphingolipids in the pathogenesis of Parkinson's disease and parkinsonism. Trends Endocrinol. Metabol. 2019;30(2):106–117. doi: 10.1016/j.tem.2018.11.003. [DOI] [PubMed] [Google Scholar]