Abstract
Oxidative stress, a cytopathic outcome of excessive generation of ROS and the repression of antioxidant defense system for ROS elimination, is involved in the pathogenesis of multiple diseases, including diabetes and its complications. Retinopathy, a microvascular complication of diabetes, is the primary cause of acquired blindness in diabetic patients. Oxidative stress has been verified as one critical contributor to the pathogenesis of diabetic retinopathy. Oxidative stress can both contribute to and result from the metabolic abnormalities induced by hyperglycemia, mainly including the increased flux of the polyol pathway and hexosamine pathway, the hyper-activation of protein kinase C (PKC) isoforms, and the accumulation of advanced glycation end products (AGEs). Moreover, the repression of the antioxidant defense system by hyperglycemia-mediated epigenetic modification also leads to the imbalance between the scavenging and production of ROS. Excessive accumulation of ROS induces mitochondrial damage, cellular apoptosis, inflammation, lipid peroxidation, and structural and functional alterations in retina. Therefore, it is important to understand and elucidate the oxidative stress-related mechanisms underlying the progress of diabetic retinopathy. In addition, the abnormalities correlated with oxidative stress provide multiple potential therapeutic targets to develop safe and effective treatments for diabetic retinopathy. Here, we also summarized the main antioxidant therapeutic strategies to control this disease.
Keywords: Oxidative stress, Diabetic retinopathy, Reactive oxygen species, Dysmetabolism, Epigenetic modification, Antioxidant therapeutics
Graphical abstract
Highlights
-
•
Oxidative stress can both contribute to and result from hyperglycemia-induced metabolic abnormalities in retina.
-
•
Genes important in regulation of ROS are epigenetically modified, increasing ROS accumulation in retina.
-
•
Oxidative stress is closely associated with the pathological changes in the progress of diabetic retinopathy.
-
•
Antioxidants ameliorate retinopathy through targeting multiple steps of oxidative stress.
Abbreviations
- DR
diabetic retinopathy
- ROS
reactive oxygen species
- NPDR
non-proliferative diabetic retinopathy
- NADPH
nicotinamide adenine dinucleotide phosphate
- Nox
NADPH oxidase
- ETC
electron transport chain
- UCP
uncoupling proteins
- AR
aldose reductase
- AGEs
advanced glycation end products
- DAG
diacylglycerol
- VEGF
vascular endothelial growth factor
- CML
carboxymethyllysine;
- CEL
carboxyethyllysine;
- PKC
protein kinase C
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- RAGE
receptor for AGEs
- GFAT
fructose-6-phosphate amidotransferase
- UDP-GlcNAc
diphosphate uracil-N-acetylglucosamine;
- GLP-1
glucagon-like peptide-1
- Grxs
glutaredoxins
- GSH
glutathione
- GSSG
oxidized glutathione
- miRNAs
microRNAs
- MMPs
matrix metalloproteinases
- NF-κB
nuclear factor-κB
- NFE2L2 (Nrf2)
nuclear factor erythroid 2-related factor 2
- Keap1
Kelch-like ECH associated protein 1
- SIRT1
sirtuin 1
- SAM
S-adenosyl-l-methionine;
- MeCP2
methyl-CpG binding protein 2
- HDACs
histone deacetylases
- LSD1
Lysine-specific demethylase 1
- ARE4
antioxidant response element region 4
- SOD
manganese superoxide dismutase
- DNMTs
DNA methyltransferases
- lncRNAs
long non-coding RNAs
- ceRNA
competing endogenous RNA
- mtDNA
mitochondrial DNA
- CBM
capillary basement membrane
- PEDF
pigment epithelium-derived factor
- ECM
extracellular matrix
- BRB
Blood retinal barrier
- EPA
eicosapentaenoic acid
- PPARα
peroxisome proliferator–activated receptor-α;
- PGE2
prostaglandin E2
- COX-2
Cyclooxygenase-2
- HHE
hydroxyhexenal
- HNE
hydroxynonenal
- PUFAs
poly-unsaturated fatty acids
- DHA
docosahexaenoic acid
- AA
arachidonic acid
- OA
oleic acid
- RGCs
Retinal ganglion cells
- RPE
Retinal pigment epithelium
1. Introduction
Oxidative stress is a phenomenon caused by the imbalance between the formation and removal of free radicals. The most effective free radicals are derived from molecular oxygen, such as superoxide anion (O2·−), hydrogen peroxide (H2O2), peroxyl radical (ROO·) and the very reactive hydroxyl radical (·OH), which are termed reactive oxygen species (ROS), and these substances are generally considered to be toxic to cells [1,2]. Generally, oxidative stress occurs when excessive free radicals form and meanwhile antioxidants (e.g. vitamin A, C and E, glutathione, α-lipoic acid, carotenoids), antioxidant minerals (e.g. copper, zinc, manganese, selenium) or various other free radical scavenging mechanisms (e.g. free radicals-scavenging enzymes) fail to neutralize these free radicals. In short, disrupting the dynamic redox balance will cause oxidative stress and ultimately damage the cells of target organs, such as the retina, kidney, heart and so on [3,4]. Retina is susceptible to oxidative stress not only because it can be continuously attacked by ROS-producing visible light or UV, but also because the easily oxidized poly-unsaturated fatty acids (PUFAs) are abundant in the outer photoreceptor segment membranes of retina [5]. In human retina, the main PUFAs in the outer photoreceptor segments contain docosahexaenoic acid (DHA), arachidonic acid (AA) and oleic acid (OA), and these three PUFAs account for about 50%, 8% and 10% of total fatty acids respectively [6]. These PUFAs play an important role in retinal structure and functions, while PUFAs are susceptible to oxidative degradation and are also major targets of ROS to initiate lipid peroxidation. The lipid peroxidation of PUFAs in the retina not only impairs visual cells but also affects the physiological health of the retina. The products of lipid peroxidation, such as hydroxyhexenal (HHE) and hydroxynonenal (HNE), in turn react with cellular macromolecules (DNA and proteins) in the retina, consequently leading to photoreceptor cell impairments and retinal pigment epithelial abnormality [7]. Besides the abundance of PUFAs, high oxygen consume is essential to the visual imaging function and active metabolism in retina, and retinal area is always under high oxygen tension, which favors the formation of ROS and promotes lipid peroxidation [8].
Diabetic retinopathy (DR) is categorized as one microvasculature complication in diabetes, which exerts sight-threatening effects on the eyes [9]. It is generally acknowledged that DR is the leading cause of diabetes-associated visual damage or loss among working-age adults and elderly people worldwide [10]. It was estimated that the number of patients affected by DR would rise to 191.0 million by 2030 [[11], [12], [13]]. The main pathophysiology of DR is various changes caused by hyperglycemia, including the thickening of retinal capillary basement membrane, increased retinal vascular permeability, tissue ischemia, and the release of various vasoactive substances, leading to neovascularization. With the progression of DR, new blood vessels begin to form on the surface of the retina. Because of the instability of new blood vessels, the contents of blood vessels (blood and extracellular fluids) are easy to leak out, resulting in vitreous hemorrhage and retinal detachment. These pathophysiological changes are called proliferative diabetic retinopathy (PDR) and can eventually lead to the loss of vision. In contrast, the clinical subtype without neovascularization in the early stage is called non-proliferative diabetic retinopathy (NPDR). The typical features of NPDR are the formation of microaneurysms and small dilation of retinal blood vessels that are identified as preliminary signs of DR [[14], [15], [16]]. Moreover, retinal neurodegeneration also occurs in the pathogenesis of DR, which also participates in the microvascular disorders that happen in DR, mainly including apoptosis and glial alteration [17,18]. Retinal ganglion cells (RGCs) are a type of neuron located in the inner surface of retina, and their long axons link the retina to the brain and project visual information to the brain. As for neuro apoptosis, RGCs are often the targets of the investigations and their death is often observed in DR [19]. Retinal glial cells are essential to support retinal neurons and are the necessary channels of communication between retinal blood vessels and neurons, containing two main part: macroglia (Müller cells and astrocytes) and microglia. In DR, macroglia undergoes prominent changes, including an increase in the Müller cell population and glial fibrillary acidic protein expression, and a reduction in the astrocytic population, which may be associated with the increased vascular permeability [20]. Likewise, in response to the changes of local physiological environment, microglial cells become extremely dynamic, enabling them to assume different morphologic and functional alterations [20].
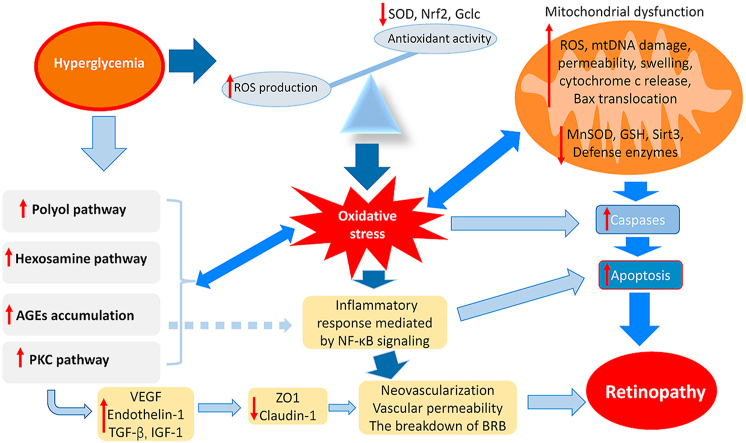
Oxidative stress plays a critical role in the pathogenesis of DR. The excessive accumulation of ROS can impair the tissue in and around retinal vessels, eventually leading to DR. It has been reported that four classical metabolic abnormalities are involved in hyperglycemia-induced oxidative damages in the retina: activation of the protein kinase C (PKC) pathway; polyol pathway flux; and activation of the hexosamine pathway; intracellular formation of advanced glycation end-products (AGEs) [21,22]. Besides the above metabolic disorders, irregular epigenetic modifications [23], the abnormal activity of nuclear factors, including highly activated nuclear factor-κB (NF-κB) [24,25] and attenuated activity of Nrf2 (Nrf2 is also called NFE2L2, nuclear factor erythroid 2 related factor 2) [26,27], and hyperglycemia-mediated mitochondrial dysfunction [28], have also been demonstrated to be correlated with the overproduction of ROS in DR. In particular, oxidative stress caused by epigenetic modifications can last for a long stage, even if blood glucose concentration turns into normal level, which is termed “metabolic memory” [[29], [30], [31], [32]]. Additionally, hyperglycemia-induced oxidative stress also brings about mitochondrial defects, cellular apoptosis, inflammation, lipid peroxidation, structural and functional changes (including microcirculatory abnormalities and neurodegeneration) in DR. Therefore, inhibiting ROS generation and scavenging excessive ROS by these pathways have been employed as therapeutic strategies for the treatment of DR. In this review, we firstly introduce ROS generation systems briefly, then discuss several oxidative stress-associated mechanisms involved in DR, and finally summarize the pathogenetic roles of oxidative stress in the development of DR and the recent studies on DR therapeutics.
2. Mitochondria and Nox system in diabetic retinopathy
ROS are generated mainly from two systems: (1) mitochondrial oxidative phosphorylation and (2) the nicotinamide adenine dinucleotide phosphate- (NADPH-) oxidase (Nox) system [33].
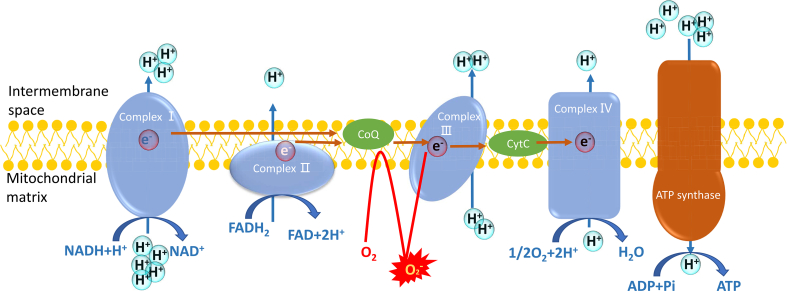
The major endogenous ROS are generated from mitochondria, in which the proton gradient across mitochondrial inner membrane is formed by electron transport chain (ETC) to consume cellular oxygen and yield ATP (Fig. 1) [34,35]. The ETC on the mitochondrial inner membrane mainly contains four complexes, including complex I, II, III, and IV. These complexes employ NADH and FADH2 as the donors to provide electrons to finally reduce oxygen to water, and the protons are pumped into intermembrane space to create a voltage across the mitochondrial inner membrane. The energy of this proton gradient drives ATP synthesis by ATP synthase [[36], [37], [38]]. In this process, only few oxygens accept the donated electron to transform into ROS, such as O2·−. However, in diabetic cells, excessive glucoses are required to degrade in tricarboxylic acid cycle (TCA cycle), which in effect compels more NADH or FADH2 into mitochondrial electron transport chain and forces the proton gradient across inner membrane to reach the maximum threshold. In this condition, electron transfer is obstructed, and coenzyme Q provides the electron to molecular oxygen to generate superoxide, which brings about excessive production of ROS [38]. Correspondingly, the relationship between ROS and hyperglycemia can be dissociated by the uncoupling of mitochondrial electron transport, which can be mediated by overexpressing uncoupling protein (UCP) to collapse voltage gradient [39,40]. Many studies of animal model have shown the increased retinal superoxide production in DR [41]. Compared to other retinal cells, photoreceptors possess more mitochondria, and it has been demonstrated that photoreceptors are a major contributor of superoxide in DR [42]. In streptozotocin-induced diabetic mouse, two months of hyperglycemia caused the increased superoxide derived from impaired mitochondrial ETC in photoreceptor cells of diabetic eyes, while this increase can be suppressed by methylene blue that acts as an alternate electron transporter [42].
Fig. 1.
The electron transportation chain and the generation of ROS in mitochondria. Complex I and II receive the electrons from NADH and FADH2, respectively. Then the electrons are transported to coenzyme Q (CoQ), and then transferred to cytochrome C (Cyt C) in the complex III. Finally, complex IV offers the electrons to O2 to produce H2O. In this process, all the complexes pump protons out of mitochondrial matrix to form a gradient of protons between intermembrane space and mitochondrial matrix. The energy of the proton gradient drives ATP synthase to generate ATP. Hyperglycemia can induce the blockage of the normal electron transportation, and O2 can accept the electrons to transform into reactive oxygen species.
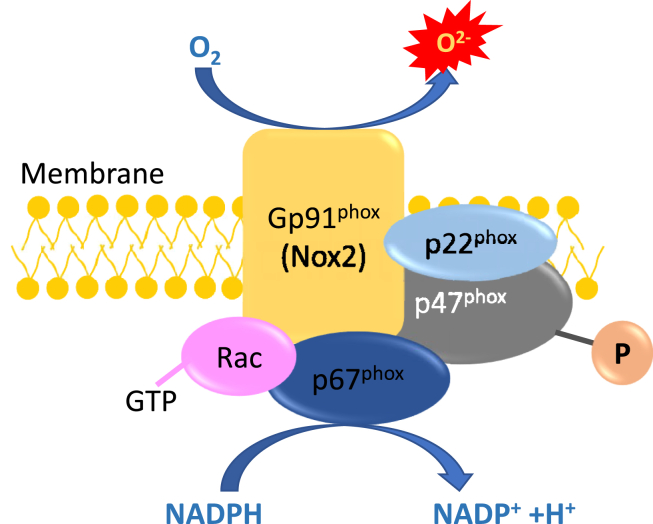
The Nox system, as a key enzymatic source of oxidative stress, can employ NADPH as electrons donor, then transport the electron to molecular oxygen and drive molecular oxygen to convert into superoxide and/or hydrogen peroxide via a single electron reduction (Fig. 2) [43]. Nox system contains membrane-associated proteins (Nox protein and p22phox) and cytosolic proteins (p47phox, p67phox, and Rac) [44,45]. Nox family includes several isoforms, in which Nox 1, Nox 2, and Nox 4 are highly expressed in the vascular cells [46]. In diabetic mice, Nox 2 is highly activated in the cells of retinas and this hyperactivation augments ROS production and enhances the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular endothelial growth factor (VEGF) [47]. Nox 1, Nox 4 and Nox 5 aggravate retinal vascular permeability and promote the expression of inflammatory and angiogenic factors for neovascularization in DR [48]. The inhibition of NADPH oxidase is also considered as a potential therapeutic strategy for the treatment of DR [49].
Fig. 2.
The Nox system is an enzymatic source of oxidative stress. It can utilize NADPH as substrates to transport the electron to molecular oxygen, and drive molecular oxygen to convert into reactive oxygen species.
3. Oxidative stress and the abnormalities of polyol/PKC/AGE formation/hexosamine pathway in diabetic retinopathy
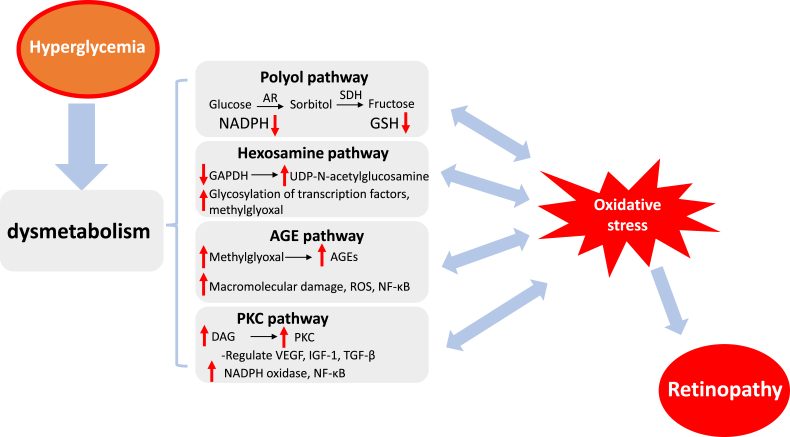
Despite a variety of studies, the underlying mechanisms that how hyperglycemia leads to the pathology of retina still remain elusive. It has been proposed that the abnormality of multiple metabolic pathways is closely associated with the hyperglycemia-mediated development of retinopathy. As shown in Fig. 3, the main metabolic abnormalities involved in DR are the augmented influx of glucose through the polyol and hexosamine pathways, the activation of protein kinase C pathway, and enhanced formation of advanced glycation end products [50].
Fig. 3.
The metabolic abnormalities induced by hyperglycemia. The major pathways include the polyol pathway, the hexosamine biosynthesis pathway, the formation of advanced glycation end products (AGEs), and protein kinase C (PKC) activation, which contribute to ROS generation and aggravate oxidative stress to promote the pathogenies of retinopathy.
3.1. Polyol pathway
The polyol pathway of glucose metabolism is activated under the hyperglycemic condition [51]. In this pathway, aldose reductase (AR), as the first and rate-limiting enzyme, transfers glucose into sorbitol by utilizing NADPH as an electron donor, and then sorbitol is oxidized to fructose by sorbitol dehydrogenase with the conversion of the cofactor NAD+ to NADH [52,53].
There are several effects on the oxidative stress in DR. First, sorbitol is strong hydrophilic alcohol without the capacity to diffuse through lipid membranes and gives rise to cell hypertonicity and the increase of osmotic pressure, leading to retinal capillary osmotic damage and cell death. Secondly, the fructose formed in the polyol pathway can be transferred into phosphorylated to fructose-3-phosphate through phosphorylation and subsequently decomposed to 3-deoxyglucosone, both of which can be used as a precursor to participate in the formation of advanced glycation end products (AGEs) through glycosylation [54]. Lastly, the enhanced compensatory activity of the glucose monophosphate shunt brings about excessive utilization of NADPH, which will cause less cofactor available for the synthesis of glutathione (GSH), the weakened capacity against oxidative stress and the corresponding destruction of cellular redox balance [55]. In addition, the consumption of NAD+ by sorbitol dehydrogenase causes the aberrant shift of the ratio of NADH/NAD+, and excess NADH can be used as the substrate for NADH oxidase, which contribute to the generation of intracellular ROS in retinal cells [56]. Therefore, the polyol pathway induced by hyperglycemia can change retinal capillary intracellular tonicity, produce AGEs precursors, and expose retinal cells to oxidative stress, perhaps via the breakdown of redox balance and the enhancement of ROS generation.
3.2. Activation of the PKC pathway
The PKC pathway plays critical role in the oxidative-stress-induced pathogenesis of DR. The family of PKC contains 12 isoforms, in which PKC-α, -β, -δ, and -ε has been reported to be activated during the development of DR [57,58]. PKC, one kind of serine/threonine kinase involved in signal transduction, can respond to the stimuli from specific hormone, neuron, and growth factor. Hyperglycemia elevates glucose flux through the glycolysis pathway, which in turn results in the enhancement of diacylglycerol (DAG) synthesis, and DAG is the critical activator of PKC in the cells [59]. The experimental and clinical studies have demonstrated the increase of DAG and hyper-activation of PKC in the diabetic state [60,61].
The regulation of various physiological processes in retinal cells are closely associated with PKC, including retinal hemodynamics, endothelial permeability, the enhanced activation and adhesion of leukocytes (leukostasis) and expression of vascular endothelial growth factor (VEGF) in the retinal tissue [53,62,63]. Moreover, PKC can augment the activity of NADPH oxidase, which promote the production of ROS in many vascular cells including endothelial cells, smooth muscle cells, pericytes, mesangial cells, and others [60,64]. As the upstream regulators of Nox, the activity of PKC exerts significant effects on the assembling and activation of Nox1-3 isoforms, which phosphorylation-dependently assembles several catalytically activated subunits in the cytosol, including p47phox [65]. Thus, PKC pathway induced by hyperglycemia can aggravate oxidative stress in retinal cells.
3.3. AGEs accumulation through nonenzymatic glycation
Chronic exposure to hyperglycemia significantly potentiates the nonenzymatic glycosylation of macromolecules (such as proteins, lipids, etc.), which finally leads to the accumulation of advanced glycation end products (AGEs) [66,67]. In the glycation, reducing sugars react with active sites of macromolecule to form unstable Schiff bases, which are then rearranged to form Amadori products (early glycosylation products), mainly carbonyl compounds. These carbonyl compounds then undergo complicated chemical rearrangements (oxidations and dehydrations) and crosslink to macromolecules to finally form irreversible and stable AGEs [68]. During the process, large amounts of free radicals are produced, which in turn promotes AGE formation [69]. Carboxymethyllysine (CML), carboxyethyllysine (CEL) and pentosidine are some AGEs that have been well chemically characterized in human. AGEs, such as CML, have been detected in retinal blood vessels of diabetes patients and its occurrence is associated with the severity of retinopathy, indicating the pathophysiological role of AGEs in the development of DR [70].
Nonenzymatic crosslinks between glucose (or other reducing sugars) and amino groups causes damage in protein structures and functions, exemplified by stiffen blood vessel resulting from the nonenzymatic glycation of collagen and elastin [71]. Besides, AGE-induced impairments occur via binding to the receptor for AGEs (RAGE) on the cell surface. The interaction of AGEs with RAGE can promote NF-κB activation that leads to pericyte apoptosis in the retina and the upregulation of VEGF to increase vascular endothelial permeability [72,73]. In the pathophysiological processes of DR, ROS production induced by AGEs participate and play significant roles. The interaction of AGEs with RAGE activates NAPDH oxidases and thus enhance intracellular ROS generation [74,75]. AGEs can also contribute to RAGE expression. It has been studied that injecting AGEs into normal rats augmented the expression of RAGE and ICAM-1 that eventually incurred retinal hyperpermeability and leukostasis; while, the process was prevented by the concurrent treatment with the pigment epithelium-derived factor (PEDF) that can repress AGE-induced ROS production, NF-κB activation and VEGF expression [[76], [77], [78]]. Additionally, some studies also suggest AGEs can also stimulate ROS generation through the mitochondrial electron transport chain [79]. In the feedback, ROS enhancement, in turn, contributes to AGE formation [80] and RAGE expression [81], which aggravates all AGE-mediated impairments.
3.4. Increased flux through the hexosamine pathway
Hexosamine level has been identified to be elevated in retinal tissues of diabetic patients. In the hexosamine pathway, the glucose is phosphorylated and becomes into fructose-6-phosphate, which is then transferred into glucosamine 6-phosphate by fructose-6-phosphate aminotransferase (GFAT) [82,83]. Glucosamine 6-phosphate can be converted to N-acetylglucosamine 6-phosphate through acetylation and isomerization, and finally form the end product - diphosphate uracil-N-acetylglucosamine (UDP-GlcNAc), which can be used as the basic substrates for the formation of glycosyl side chains in post-translational modifications of proteins and lipids [82,84].
It has been reported that the hexosamine pathway can mediate ROS-induced toxic influence in hyperglycemia [85,86]. Hyperglycemia-mediated mitochondrial superoxide overproduction can suppress the activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and it meanwhile leads to the activation of the hexosamine pathway with the increasing influx of phosphorylated glucose [87,88]. High glucosamine generated by the activation of hexosamine pathway stimulates the overproduction of ROS in mitochondria and damages mitochondrial respiration [[89], [90], [91]], which can further aggravate oxidative stress, augment vascular permeability, and promote angiogenesis.
3.5. The interaction between these metabolic pathways and oxidative stress
All these metabolic abnormities play important roles in the generation of retinal ROS that can in turn augment these metabolic pathways [92], and each pathway interrelates with all the others through ROS or other mid-components. Increased ROS, induced by hyperglycemia, activate PARP to negatively modulate GAPDH activity. The inhibition of GAPDH activity activates the polyol pathway, increases intracellular AGE formation via interacting with intracellular methylglyoxal [93], activates PKC and subsequently NF-κB, and also activates hexosamine pathway flux [38]. GFAT in the hexosamine pathway is correlated to TGF-β expression and glucose-stimulated activity of PKC [94]. Excess glucose can be metabolized to sorbitol causing osmotic stress, and then transferred to fructose through the polyol pathway. The byproducts of polyol pathway, fructose-3-phosphtae and 3-deoxyglucosone, are strong glycosylating agents for the AGE formation [95]. Likewise, aggravated AGEs can give rise to oxidative stress and activate PKC pathway [96].
4. Oxidative stress and epigenetic modifications in diabetic retinopathy
Epigenetic modification, without changing the DNA sequence, mediates the dynamic actions of transcriptional inhibition or activation, which are conducted by DNA methylation, histone modification, nucleosome remodeling, and RNA-mediated targeting to change the interaction between RNA polymerases or transcription factors with the target DNA regions [97,98]. Abnormal epigenetic modifications in cells are also closely associated with oxidative stress. The characterization of major enzymes in charge of DNA methylation and histone acetylation or methylation is redox-sensitive in nature. For example, DNA methyltransferases (DNMTs) are one kind of redox-sensitive enzymes [99], and oxidative stress can elevate the DNA methylation level through the deprotonation of the cytosine molecule and acceleration of the reaction between DNA and the positive-charged intermediate S-adenosyl-l-methionine (SAM) [100]. Oxidative-damaged methyl-CpG sequences bring about epigenetic alterations in chromatin organization through the blockage of the binding between the methyl-CpG site and the binding domain of methyl-CpG binding protein 2 (MeCP2) [101], contributing to disorderliness of epigenetic regulation. Nox4-mediated increased oxidative stress in the nucleus leads to the oxidation of histone deacetylases (HDACs) at conserved cysteine residues, and facilitates these deacetylases to release from the nucleus [102]. Histone Lysine-specific demethylase 1 (LSD1) is a flavin adenine dinucleotide (FAD)-dependent amine oxidase that remodels chromatin by the demethylation of histone H3 Lys4 (H3–K4) [103,104], in which a detectable amount of ROS are found as the byproduct of its chromatin modification during the initial DNA-damage response [105,106]. It was also shown that oxidative stress can repress the activity of HDACs to promote acetylation of target genes [107].
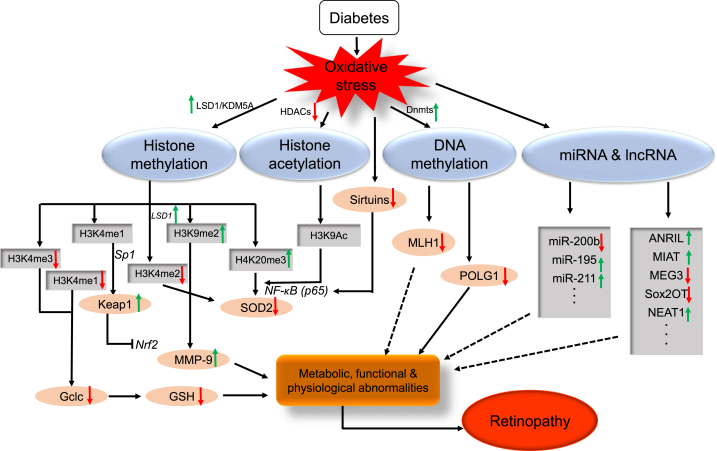
In DR, the irregular transcriptional levels of multiple genes related to oxidative stress are associated with hyperglycemic milieu-induced epigenetic changes. Hyperglycemia brings about the abnormal modifications on histones or transcription factors and aberrant expression of regulatory non-coding RNA, which contribute to the overexpression of proinflammatory proteins and inhibition of ROS-scavenging genes [[108], [109], [110], [111]]. It has been proved that the genetic polymorphism of histone methyltransferases, which are responsible for elevated expression of key proinflammatory factors implicated in vascular injury, can be considered as predictors of the risk for micro- and macrovascular diabetic complications [112]. The role of epigenetic modulation in the pathogenesis of DR has been demonstratively verified in many studies (Fig. 4).
Fig. 4.
Schematic diagram of epigenetic modifications in DR: diabetes induces oxidative stress, which leads to the altered expression of genes involved in histone (LSD1, KDM5A, HDACs), and DNA (DNMTs) modifications. Histone methylation (H3K4me1, H3K4me3H3K9me2, H3K4me2, H4K20me3) and acetylation (H3K9-Ac, p65 of NF-κB) modulate the binding of transcription factor (Nrf2, Sp1, NF-kB-p65) and alter the transcriptional levels of Gclc, Keap1, MMP-9, SOD2, and TXNIP. DNA methylation at POLG1 and MLH1 promoter represses their transcriptional levels in DR. MicroRNAs (miR-200b, miR-195, miR-211 …) and lncRNA (ANRIL, MIAT, MEG3, Sox2OT, NEAT1 …) regulate their target genes related to the development of DR. Although this scheme shows various epigenetic regulation, we cannot exclude the roles of many, yet identified, other histone and DNA modifications and miRNAs and lncRNA in DR.
4.1. Histone modification of oxidative stress-related genes
The majority of histone modifications may act as a negative modulator for the antioxidant defense system in the retina of diabetes. Hyperglycemia-mediated epigenetic modifications at the promoter and enhancer regions of superoxide dismutase 2 (SOD2), including increased methyl H4K20 and acetyl H3K9 and LSD1-mediated elevated demethylation in H3K4, lead to the downregulation of SOD2, which plays an important role in the development of DR and its continued progression related to metabolic memory [111,113]. It has been demonstrated that transient hyperglycemic stimulation to aortic endothelial cells can trigger long-lasting epigenetic changes of increased mono-methylation of histone 3 at the promoter region of the NF-κB p65 subunit in vitro and in vivo, which gives rise to enhanced transcription of p65 [114], and the increased NF-κB augments the apoptosis of retinal cells in diabetes. Nrf2 is a master transcription factor that activates the expression of various detoxifying and antioxidant defense genes. The activity of Nrf2 can be restrained by Kelch-like ECH associated protein 1 (Keap1). Hyperglycemia contributes to Keap1 expression through epigenetic modifications at promoter by SetD7, which also promotes its binding with Sp1 and then impairs the activity of Nrf2 through binding Nrf2 in the cytosol [115]. Nrf2 binds the antioxidant response element region 4 (ARE4) to modulates Gclc, one critical enzyme in the biosynthesis pathway of GSH. However, the binding decreases in DR, which results from the hyperglycemia-induced increased H3K4 methylation at Gclc-ARE4 region. This also indicates the critical role of histone methylation at Gclc-ARE4 in regulating the Nrf2-Gclc-GSH cascade [109]. Additionally, hyperglycemia induces the expression of thioredoxin interacting protein (TXNIP), the endogenous suppressor of ROS scavenger thioredoxin (Trx), to facilitate oxidative stress and inflammation via histone modification on inflammatory gene promoter [108]. In addition, hyperglycemia-induced upregulation of retinal matrix metalloproteinase-9 (MMP-9) may be associated with the hypermethylation of H3K9 mediated by activated LSD1 at the MMP-9 promoter, which promotes its transcription and impairs the mitochondria and then contributes to the apoptosis of capillary cells [116].
Moreover, hyperglycemia-induced oxidative stress affects sirtuin-mediated epigenetic modifications through the potential modulations on the expression and activities of sirtuins [117,118]. Sirtuins are NAD-dependent protein deacetylases that are associated with protein acetylation, metabolism and diseases of aging, including 7 members (SIRT1~7). SIRT1 is a redox-sensitive enzyme [118], and SIRT1-mediated transcription factor or histone deacetylations modulate various cellular bio-processes, including inflammatory response, apoptosis, and proliferation to protect retinal cells from oxidative stress. SIRT1 can inhibit the ROS-induced impairments by modulating the activity of the key inflammatory regulator, NF-κB, to repress the proinflammatory response. SIRT1-induced deacetylation of p65 of NF-κB represses the activation of inflammatory transcriptional targets [119,120]. SIRT1 has also been proved as a negative modulator of MMP9 in DR [121]. Both SIRT1 and SIRT2 are involved in the upregulated activities of Nrf2 [122]. SIRT3 exerts protective effects on mitochondria against oxidative stress. SIRT3 deacetylates the critical antioxidant enzyme manganese superoxide dismutase 2 (SOD2) at two different lysine sites to enhance its ROS-scavenging capability in mitochondrial matrix [[123], [124], [125]]. SIRT6 modifies the histones via deacetylation in the promoter regions of NF-κB–activated genes to further inhibit the proinflammatory response [126]. However, ROS, in addition to modulating cellular NAD levels, also exert antagonistic effects on the expression and activities of sirtuins. In DR, SIRT1 transcriptional level and its deacetylase activity are both suppressed by oxidative stress in the retina and its capillary cells [127]. Oxidative stress can also induce the downregulation of SIRT6 and SIRT3 in the pathogenesis of DR [128,129]. The regulation of sirtuins by ROS can eventually influence sirtuin-associated protein acetylation.
4.2. DNA methylation
The transcriptional levels of genes associated with redox balance in the retina are regulated by the methylation status of the cytosine in the DNA molecule, which is one of the principal epigenetic regulations and a dynamic property responding to and adapting to environmental alterations [130]. The methylation of CpG changes the accessibility of the functional factors in transcriptional machinery to the promoter or enhancer regions, bringing the interferences to the protein-DNA interaction to block transcriptional actions [131]. The family of DNA methyltransferases (DNMTs) includes five members, out of which DNMT1, DNMT3a and DNMT3b are catalytically active. DNMT1 has been detected in the mitochondrial matrix, which can bind to mitochondrial DNA (mtDNA) and modify the transcription level of mitochondrial genes in a target-specific manner, and abnormal mtDNMT1 expression affects the expression of mtDNA-encoded genes asymmetrically [132]. The significant increase of DNMT1 accumulation in mitochondrial was also observed in DR and causes the hypermethylation of mtDNA to impair its transcription, compromising ETC and interrupting mitochondrial homeostasis [133]. High activity of DNMTs induced by hyperglycemia in DR leads to the hypermethylation in the regulatory element of POLG, attenuating its interacting with the D-loop region of mtDNA and then blocking mtDNA biogenesis [134]. Hyperglycemic milieu changes DNA methylation status of MMP-9 promoter, which contributes to the upregulation of MMP-9 expression and its mitochondrial accumulation, damaging the mitochondrial membrane and activating the apoptotic procedure to promote the pathogenesis of DR [135]. Hyperglycemia-mediated epigenetic modifications of DNA methylation in MLH1 promoter decreases its transcription and mitochondrial accumulation, leading to increased mtDNA mismatches in DR [136]. Thereby, the modulation of abnormal DNA methylation possesses the potential to restore mitochondrial homeostasis for cellular redox balance, and to obstruct or retard the progression of DR.
4.3. The regulation by microRNA and long non-coding RNA
In addition to histone modifications and DNA methylation, expression levels of small non-coding RNAs (microRNAs) and long non-coding RNAs (lncRNAs) are also changed in diabetes, and these regulatory ncRNAs can modulate gene transcription or translation through various mechanisms.
miRNAs can suppress post-transcription by binding to their target mRNAs, to regulate the expression of target genes. SIRT1 has the antioxidative potential via inhibition of NF-κB pathways and exerts protective effects on mitochondria [120]. It has been demonstrated that the upregulation of miR-195 in retinal microvascular ECs exposed to high glucose can incur more mRNA degradation of SIRT1 [137]. The downregulation of miR-200b in the development of DR results in mRNA and protein elevation of its target VEGF, while VEGF-induced high permeability and angiogenesis were inhibited by miR-200b overexpression, which provides a novel miRNA-based therapeutic strategy [138]. miR-211, which is obviously elevated in patients of DR and can be regarded as a biomarker for DR, can also target SIRT1 directly to affect its expression [139]. Oxidative stress-induced miR-195 overexpression can also cause the downregulation of SIRT1 [140]. All these studies indicate that microRNAs play an important role in the development of DR and can be utilized as therapeutic targets for controlling DR.
Long non-coding RNAs (lncRNAs) are one kind of fundamental RNA transcripts that are more than 200 base-pairs in length and have no potential of coding protein in general. lncRNAs govern the regulation of gene expressions through several means: 1. acting as a decoy for transcription factors, to inactivate gene transcription; 2. working as the guide of certain protein factors to certain DNA regions; 3. serving as a scaffold to assemble multiple protein factors; 4. functioning as a miRNA sponge to weaken the miRNA-mediated suppressive effect on their target mRNAs; 5. enhancing the transcriptional activity of neighbor genes [[141], [142], [143]]. The dysregulation of lncRNAs is closely associated with the pathogenesis of DR. lncRNA-ANRIL plays regulatory roles in the expression and functions of VEGF in DR, and compared to wild-type diabetic retinas, retinal cells of ANRIL-Knock-out diabetic mice displayed a significant decrease in VEGF expression and corresponding microvascular permeability [144]. The elevation of lncRNA-MIAT was observed in diabetic retina and MIAT functions as competing endogenous RNA (ceRNA) to sponge miR-150–5p and upregulate VEGF level [145]. High glucose suppresses the expression of lncRNA-MEG3 that can elevate SIRT1 expression by sponging miR-34a and inhibit the NF-κB pathway to repress hyperglycemia-induced apoptosis [146]. The knock-down of lncRNA-Sox2OT affects high-glucose-induced oxidative stress response via positively modulating Nrf2/HO-1 signaling activity, which could be a therapeutic mean for the control of diabetes-induced retinal neurodegeneration [147]. lncRNA-NEAT1 expression is dramatically upregulated in patients with DR, which promotes oxidative stress injury triggered by hyperglycemic milieu via activating TGF-β1 and VEGF [148]. The relevance of lncRNAs to the development of DR was recognized later than that of miRNA, while its significance attracts more and more attentions from researchers.
5. Oxidative stress-induced pathogenesis of diabetic retinopathy
5.1. Mitochondrial damages
Under hyperglycemic conditions, ROS is over-generated in the retina and the aggravated oxidative stress directly results in the dysfunctions of the mitochondria. The displacement-loop (D-loop) in mitochondrial DNA is a large non-coding sequence and highly vulnerable unwound region, containing the necessary elements for the transcription and control regions for mtDNA replication [149,150]. In diabetes, D-loop undergoes more impairments and mutations than other sections of mtDNA, and its copy numbers are reduced. Additionally, the hypermethylation of mtDNA induced by hyperglycemia in DR affects its transcription and causes the mitochondrial dysfunction, finally promoting the apoptosis of capillary cells [133]. Epigenetic modification on mtDNA was also confirmed to be a latent factor for the base mismatch of mtDNA in the pathogenesis of DR [151]. mtDNA-encoded proteins are decisive for normal functions of the electron transport chain (ETC) and mitochondrial homeostasis. Unlike nuclear DNA, circular mtDNA is vulnerable to more extensive and persistent damages caused by oxidative stress, due to the lack of protective histones [152]. Injured mtDNA leads to the dysfunction of transcription and protein synthesis, which further compromises electron transport and aggravates ROS generation. Evidences have also confirmed the subnormal transcriptional levels of mtDNA-encoded genes correlated to the ETC system in DR, including NADH dehydrogenase 1 and 6 of complex I [133].
The activation of gelatin matrix metalloproteinases (MMPs) is also responsible for mitochondrial impairment in DR. Diabetes activates NADPH oxidase (Nox) complex [46], which enhances oxidative stress and upregulates the expression of MMPs [153]. Diabetic and oxidative stress stimulation facilitates MMPs to translocate into the mitochondria [154]. In this process, the translocation and accumulation of redox-sensitive MMPs (MMP-2 and MMP-9) in the retinal mitochondria appear to rely on the modulation of chaperons Hsp60 and Hsp70 [155,156]. MMPs inside the mitochondria impair the mitochondria and augment the pore permeability through destroying connexin 43 [157,158]. The damaged mitochondrial lipid membrane leads to mitochondria swelling in the retina of diabetic mice [159] and facilitates the leakage of cytochrome c (Cyt c) from the mitochondria into the cytosol to induce the assembly of apoptosome platform and initiate the caspase cascade [160,161].
Besides, superoxide can react with nitric oxide (NO) to form peroxynitrite that is a powerful oxidant. Peroxynitrite oxidizes glutathione (GSH), cysteine, and tetrahydrobiopterin [162], resulting in the oxidation of membrane phospholipids, the inactivation of enzymes with sulfhydryl moieties, the nitration of tyrosine residues and the aggravation of fragmenting DNA [163]. Peroxynitrite also causes irreversible injury to mitochondria via altering mitochondrial energy and calcium homeostasis and promoting the opening of the permeability transition pore, and consequently leads to cell apoptosis [164].
5.2. Cellular apoptosis and inflammation in retina
Apoptosis of retinal cells happens early in DR. The accelerated apoptosis of retinal capillary cells can be observed before the discovery of any histopathological changes in this diabetes complication [165,166]. High glucose exposure of the endothelial and pericytes cells in retina exhibits the aggravation of oxidative stress, leading to the increase of Caspase-3 activity, NF-κB or other transcription factors to accelerate capillary cell death.
Caspases are a set of cysteine proteases, which are essential to process apoptosis in cells, and are very sensitive to oxidative stress [[167], [168], [169]]. The accumulation of ROS induced by hyperglycemia in mitochondria increases the pore permeability in this organelle [170,171], and in turn triggers the release of cytochrome c and other proapoptotic factors from retinal mitochondria to commence apoptosis through activating caspases [172,173]. In the initiation of apoptosis, cytochrome c is observed in the retina and its capillary cells [174], which activates Caspase-9 and then initiates the activation of Caspase-3 through a cascade of biological processes to fragment DNA [175]. The hyper-active Caspase-3 is common in DR, and oxidative stress acts as a critical factor to trigger the activation of Caspase-3 in DR. The therapy via the blockage of Caspase-3 activation was confirmed to suppress the oxidative stress-associated pathogenesis of retinopathy in diabetic rat [176].
Another important factor is NF-κB, the activation of which is regarded as a critical proinflammatory and proapoptotic signaling pathway to mediate hyperglycemia-induced inflammation and cellular apoptosis [177,178]. Retinal NF-κB in diabetes is activated at the beginning during the development of retinopathy, and its activities stay energetic even if the apoptotic process of retinal capillary cells speeds up [179]. NF-κB, also as a redox-sensitive nuclear transcription factor, is regarded as a critical regulator of inflammatory responses and a repressor of antioxidant enzymes [180]. It has been discovered that NF-κB initiates proapoptotic procedures in response to the pressure of high glucose in retinal pericytes, which may explain the early cell death of pericytes in DR [181]. NF-κB pathways can lead to the activation of MMP-9 (encoding matrix metalloproteinase 9) [182] and the increased mitochondrial MMP-9 damages the mitochondrial gap junction protein connexin-43, and augments pore permeability, contributing to the leakage of cytochrome c (Cyt c) into the cytosol [158]. NF-κB also modulates the expression of inducible nitric oxide synthase [183,184] that in turn drives the production of nitric oxide to enhance transcriptional activity of NF-κB [185]. Furthermore, the crosstalk of NF-κB signaling and ROS is common in the cell and both of them can be mutually regulated and interact to promote inflammation and cell apoptosis [25].
5.3. Lipid peroxidation
Oxygen-derived free radicals, such as hydroxyl and hydroperoxyl species, have been demonstrated to oxidize phospholipids and other lipid elements in the membrane, causing lipid peroxidation, and tissues from oxidative stress-related retinal diseases have been shown to contain a large number of metabolic products of lipid peroxidation, indicating the association between oxidative stress and lipid peroxidation [186]. Hyperglycemia-induced oxidative stress plays critical roles in the increase of lipid peroxidation in DR [187]. Lipid peroxidation is positively associated with the severity and duration of diabetes (P < 0.001) [188]. Due to the sensitivity of PUFAs to oxidation, the high PUFA level of retina is responsible for its high vulnerability to oxidative stress [189]. It has been reported that intracameral injection of H2O2 into rabbit retina increased lipid peroxidation in iris epithelial cell membranes [190]. Correspondingly, lipid metabolism is also responsible for the enhanced oxidant species and lipid peroxidation can also promote the generation of ROS, such as H2O2, to facilitate the senescence of retinal pigment epithelium (RPE) cells, which contributes to the progression of DR, and meanwhile lipid peroxidation products may also facilitate ROS leakage from mitochondria [191].
Lipid peroxidation leads to cellular membrane with damaged integrity and the production of diffusible aldehydic by-products, including HNE and HHE. The study of DR in rat model has shown that the byproduct, HNE, activates the canonical WNT pathway through oxidative stress, which plays a pathogenic role in the development of DR [189]. HNE can also induce p53-mediated apoptosis in RPE cells [192]. HHE at femtomolar concentrations can also lead to permeable transition pore in mitochondria and it can also modulate NF-κB to activate various proinflammatory genes [193]. Moreover, lipid peroxidation plays a critical role in the progress of neurodegenerative disorders. The study of retina neurodegenerative models displayed the augment of lipid peroxidation which was accompanied by the loss of neurons [194]. Under hyperglycemic condition, ROS accumulation in mitochondria directly leads to mitochondrial dysfunction, and ROS-mediated mitochondrial defects bring about the accumulation of lipid droplets in glia that can be peroxided by the presence of ROS, causing the onset of neurodegeneration in retina [195].
5.4. Structural and functional changes in diabetic retinopathy
5.4.1. The changes of retinal microvasculature
Oxidative stress-mediated metabolism changes bring about various alterations in the function and structure of retinal microvasculature. These structural changes include the thickening of capillary basement membrane, the breakdown of blood retinal barrier and the formation of acellular and occluded capillaries [196].
The thickening of the capillary basement membrane (CBM) is a consistent feature of DR, which results from increased overexpression and reduced degeneration of extracellular matrix (ECM) proteins [197]. Chronic hyperglycemia is the principal character of diabetes, which was identified as the key contributory factor causing the thickening of CBM in DR [198]. Hyperglycemia-mediated oxidative stress and advanced glycation play significant roles in this process [198]. High glucose level can augment the expression of ECM proteins, fibronectin (FN) and collagen, in the retinal endothelial cells [197], which may be attributed to ROS-induced transcription factors and cytokines. It was demonstrated that ROS-induced activation of various transcription factors and cytokines incurs the upregulation of ECM genes, fibrosis, and thicken CBMs ultimately [199]. ROS-induced AGE formation on collagen causes the cross-linking between collagen and brings about structural rigidity and the limitation of conveying various growth factors across membranes, leading to pericyte and endothelial cell loss [200]. The treatment of AGEs inhibitor, aminoguanidine, exerted protective effects against retinal CBM thickening in diabetic rats [201]. It is noteworthy that the retinal structures and functions are tightly associated with each other. The oxidative stress-mediated structural alterations (the loss of intercellular junctions, pericyte apoptosis) and functional changes (the changes of blood flow, the increase of vessel permeability) are correlated with each other and both contribute to the progression of DR [202].
It is well-known that oxidative stress exerts destructive effects on the blood retinal barrier (BRB) [203]. Blood retinal barrier (BRB), as one of the highly selective barriers, modulates the substance exchanges between neural retina and circulating blood to maintain neural retina health by supplying necessary nutrients and removing metabolic products and toxins. The breakdown of BRB results in vascular permeability and then diabetic macular edema, a major cause of vision loss in diabetic patients. Elevated VEGF level induced by ROS plays an important role in the destruction of the BRB [203], which was validated by intravitreal injection of VEGF or implantation with VEGF-releasing pellets [204,205]. Intercellular junctional proteins, including occludin, claudin, and zonula occludens-1 (ZO-1), play critical roles in the normality of BRB, and the breakdown of BRB is tightly correlated to the abnormality of these junction-associated proteins. For example, long treatment of VEGF leads to the loss of Claudin-1, which contributes to the destruction of BRB [204]. In addition, NF-κB can be highly activated by oxidative stress in the pathogenesis of DR to regulate the transcription of multiple genes, in which ZO-1 is repressed by this inflammatory regulator to jeopardize the normality of BRB [206]. Hyperglycemia constantly triggers the activation of PKC-δ to initiate a series of signaling cascade, causing pericyte apoptosis [207]. The structure of retinal microvessels can be altered gradually by the death of pericytic cells, which incurs the breakdown of BRB [208].
The formation of acellular and occluded capillaries can, to a large extent, be attributed to the enhanced capacity of angiogenesis in DR. Angiogenesis and inflammation are closely integrated processes in various physiological and pathological conditions. Inflammatory cells may produce angiogenic cytokines, growth factors, and proteases, which contribute to the formation of new vascular structures at the site of inflammation [209]. Elevated levels of proangiogenic/proinflammatory prostaglandin E2 (PGE2) and Cyclooxygenase-2 (COX-2) were discovered in the retina of diabetic rats. The upregulation of these two genes facilitates the expression of multiple pro-angiogenic factors, including VEGF, to augment neovascularization [[210], [211], [212]]. Both inflammation and angiogenesis are dramatically augmented by hyperglycemia-mediated oxidative stress, which, to some extent, explains the formation of acellular and occluded capillaries in DR. In addition, oxidative stress-induced apoptosis of retinal neuron and pericytes can also promote the growth of acellular and occluded capillaries. Such occlusion brings about the formation of microaneurysms and the augmentation of leukostasis, leading to the thickening of CBM [208].
Notably, multiple vasoactive effectors that modulate blood flow and retinal microvasculature permeability can both contribute to and result from oxidative stress. For example, ROS can promote the transcription of both endothelin-1, a potent vasoconstrictor [213,214]. Endothelin-1, as a vasoconstrictor, can contribute to basement membrane thickening by increasing extracellular matrix proteins [215,216] and also in turn give impetus to ROS generation [217].
5.4.2. Neurodegeneration
The vascular endothelium, glial cells, and retinal neurons constitute a complex physical and functional coupling entity, which is termed as retinal neurovascular unit, and each member of the unit coordinates closely to integrate metabolic activity with retinal blood flow [[218], [219], [220]]. Persistent hyperglycemia contributes to the overproduction of ROS and breaks the balance of the metabolic system, causing the production of inflammatory mediators and cellular damages, which forms a vicious cycle. This vicious cycle both in retinal blood vessels and neurons facilitates the dysfunctional progression of the retinal neurovascular unit [72]. For example, substantial amounts of AGEs have been detected in the axons of RGCs, the neurons located near the inner surface of the retina, and retinal glial cells [221,222], and the expression of RAGE is upregulated in RGCs and glial cells, which makes them vulnerable to AGE-induced events, such as the activation of MAPK and NF-κB signaling and ROS generation [221]. Clinical studies [223] have proved the elevated angiotensin II levels in retina of diabetic patients. Angiotensin II can induce the activation of NOX through the angiotensin II type 1 receptor (AT1R) and consequently induce the generation of ROS [224]. The abnormal structure of neovascularized vessels in retina are fragile to allow hemorrhaging, and microvascular occlusions and hemorrhage activate ischemic signaling [72]. Excessive NOX-derived ROS generation can mediate the ischemic death of RGCs, contributing to the neurodegeneration [225]. In addition, proNGF and mature NGF are mainly expressed by RGCs and glial cells, and oxidative stress has been implied to jeopardize the mature process of proNGF in neuronal injuries to cause an imbalance between proNGF and NGF, which promotes retinal degeneration [226]. Taken together, neurodegeneration is tightly associated with oxidative stress induced by hyperglycemia in DR.
Oxidative stress also affects retinal immune response. Neurodegeneration in retina is accompanied by alterations in glial cells (astrocytes, Müller cells and microglia) known as ‘reactive gliosis’. Reactive gliosis and neural apoptosis are also significant histological features of DR [227]. Hyperglycemia-induced activation of glial cells has been suspected to promote the early development of DR [228]. It has been implied that early activation of the innate immune and complement systems and microglia plays an important role in the impairment of retinal neurovascular unit [229]. Microglial cells, as the main resident sentinel immune cells located in the inner part of the retina, can be activated by diabetes, and then migrate to the subretinal region and release cytokines to cause neuronal cell death [227]. Besides innate immune activities, glial cells also participate to initiate the adaptive immune response [230]. Retina and optic nerve head glial cells express MHC molecules and act as resident antigen-presenting cells. Recent studies by immunohistochemistry and proteomics also prove an upregulation of glial toll-like receptors (TLRs) in retina [231]. Oxidative stress induced by hyperglycemia can also affect retinal immune response in many different ways. Oxidized lipids and proteins can become para-inflammatory signal and stimuli to attract resident immune cells, mainly including microglia, for the initiation of the innate immune response to remove oxidation products by phagocytosis [232]. Complement activation is also an important activity of innate immune response in glaucomatous human donor eyes, and oxidative stress has been shown to downregulate a significant complement regulatory molecule to modulate complement regulation [233]. In addition, oxidative stress-augmented AGE formation facilitates an aberrant immune activity [221]. AGEs could function as persistent antigenic stimulus and also activate RAGE-mediated signaling to induce the production of pro-inflammatory cytokine and be immunostimulatory [234].
6. Therapeutic implications
In the above sections, we have illustrated the critical roles that oxidative stress plays in the pathogenesis of DR. Thereby, it is promising to control and alleviate oxidative stress in the pathogenesis by the inhibition of ROS generation, the neutralization of free radicals, or the reinforcement of antioxidant defense system. In the following part, we discuss the activity of some antioxidant phytochemicals and drugs in the control of oxidative stress in DR.
6.1. Polyphenols
Polyphenols are renowned for their health properties, including antioxidant (or pro-oxidant), anti-inflammatory, or anticarcinogenic capability. The therapeutic potentiality of many polyphenols has also been explored in the modulation of oxidative stress in DR.
Green tea, one of the most consumed beverages worldwide, exerts various protective effects on our health, such as cancer prevention, reducing cholesterol, and lowing the risk of diabetes. It contains many polyphenols, among which epigallocatechin-3-gallate (EGCG) is the major polyphenol with strong antioxidant properties to alleviate oxidative stress. In addition to the elimination of increased retinal ROS, it also has the potential of neuroprotection, and improves hyperglycemia-induced impairments occurring in the disruption of blood-retinal barrier and damaged electroretinograms [235]. EGCG exhibits high potential to repress the activities of pro-/active MMP-9, to ameliorate the basement membrane thickening [236]. EGCG can also suppress inflammatory factor NF-κB, which plays a critical role in apoptosis of retinal cells, vascular inflammation induced by hyperglycemia [236]. Moreover, the polyphenols in green tea extract can also prevent AGE formation and exert therapeutic effect on advanced glycation and cross-linking of collagen [237].
Quercetin is a plant-derived flavonoid, found in fruits, vegetables and grains. It can facilitate the expression of GSH, SOD and catalase in the retina of DM rats, inhibit the expression of NF-κB and Caspase-3, and effectively prevent retinal neurodegeneration and oxidative stress damage induced by diabetes [238].
Resveratrol, a nonflavonoid polyphenolic compound, has been demonstrated as a good scavenger for the elimination of superoxide anions or singlet oxygen. Concerning eye protection, resveratrol exhibits protective effects against age-related ocular diseases, including glaucoma, DR and macular degeneration [239]. Resveratrol activates AMPK/SIRT1/PGC-1α pathway to remove intracellular ROS induced by high glucose and prevents ROS-induced apoptosis in retinal capillary endothelial cells [240]. Resveratrol also suppresses hyperglycemia-mediated inflammation and the reduction of Cx43 by dose-dependent downregulation of VEGF, TGF-β1and protein kinase C-beta (PKC-β) that is a critical regulator for ROS generation [241]. Hyperglycemia-activated PKC-δ can induce NF-κB-activation and SHP-1/PDGFR-β deactivation to cause the apoptosis of retinal pericytes [207], while PKC-δ signal transduction can be inhibited by resveratrol [242]. In addition, resveratrol exerts protective effects by restoring insulin level and the expression of Paraoxonase 1 (an enzyme protecting lipoproteins from oxidation) to attenuate retinal inflammation and impairments in DR [243].
Curcumin, an active phytochemical in Curcuma Longa, also shows the therapeutic potential in the prevention of DR via its hypoglycemic, antioxidant, and anti-inflammatory activity [244]. It can impede the structural degeneration and attenuate capillary basement membrane thickness in DR [245]. Additionally, the abnormality of DNMT activity induced by chronic high glucose exposure can be normalized by curcumin treatment [246].
6.2. Other antioxidants
Lutein, one member of the carotenoid family, is abundant in leafy vegetables, such as spinach or cabbage. It is characterized by a hydroxyl group at each end of the molecule that makes it more hydrophilic. Compared with other carotenoids, lutein can react with singlet oxygen more effectively. These characteristics allow lutein to have strong capacity to alleviate oxidative stress. In retinal tissues, lutein mainly exists in the macular area. A large amount of lutein in the macular area can absorb high-energy blue light and protect the macula and photoreceptors from phototoxic and oxidative damages [247]. Relevant clinical studies have shown that lutein can improve the visual function of patients with age-related macular degeneration, with good safety and effectiveness [248]. It was also found that lutein can protect the inner retina from ischemia-reperfusion injury and effectively inhibit oxidative stress-induced cell apoptosis of retinal ganglion cells and pigment epithelial cells, which are attributed to the elimination of endogenous ROS [249,250].
Astaxanthin is a xanthophyll carotenoid with strong antioxidant capacity in vivo and in vitro. More importantly, astaxanthin can protect retinal cells from oxidative stress. It has been found that astaxanthin leads to the reduction of inflammation, ROS, and apoptosis by modulating different oxidative stress pathways [251]. Astaxanthin exerts suppressive effects on intracellular oxidative stress to block the formation of endogenous N(ε)-carboxymethyllysine (CML), a key AGE representative, and the extracts of microalgae, containing astaxanthin, attenuate AGEs-mediated detrimental influence, such as the abnormal expression of VEGF and matrix metalloproteinases [252].
Zeaxanthin, a dietary carotenoid, which is specifically present in the retina, is shown to alleviate retinal oxidative stress and inflammatory response in diabetic rats [253]. Additionally, zeaxanthin can also bring about the attenuation of VEGF-induced neovascularization in the human retina via the Nox4-dependent pathway [254].
Lipoic acid (LA) is a natural thiol antioxidant. LA elevates or maintains cellular GSH levels by acting as a transcriptional inducer of Nrf2-Gclc-GSH cascade governing GSH synthesis. Treatment with LA increases nuclear Nrf2 levels and contributes to the binding of Nrf2 with the antioxidant response element (ARE) to promote the transcription of antioxidant and detoxification genes [255]. LA exhibits inhibitory effects on NF-κB activity via antioxidant-independent and probably IKK-dependent mechanisms [256], and it also reduces VEGF expression and 8-OHdG level and exerts beneficial effects on mitochondria in the retina [257]. The clinical trial in type 2 diabetic patients demonstrated the food supplement containing α-lipoic acid can improve glycemic control, lipid profile, and anti-oxidative stress markers [258].
Vitamins function antagonistically in the pathogenesis of DR by their antioxidant and anti-inflammatory actions. Supplementation of the diet with vitamins C and E and other antioxidants normalizes the retinal oxidative stress, protein kinase C activity, and nitric oxides that were elevated by hyperglycemia, and meanwhile ameliorates microvascular lesions formation in the early stage of DR. The progression of early stages of DR can be inhibited by long-period administration of these antioxidants [259]. Dietary GSH can suppress hyperglycemia-induced oxidative stress in vivo [260] and Vitamin C and vitamin E can assist GSH to maintain the reduced form [261]. Vitamin D deficiency is very common in type II diabetes. Some studies detected the subnormal transcription level of a gene regulated by vitamin D and vitamin D receptor in the retina of diabetes [262]. Vitamin A is essential for normal visual function, while the metabolism of vitamin A can be compromised by hyperglycemic condition [263]. Vitamin A supplementation has been demonstrated to alleviate retinal degeneration in rats [264]. Benfotiamine, a lipid-soluble derivative of vitamin B1, can activate the pentose phosphate pathway enzyme transketolase to consume glyceraldehyde-3-phosphate and fructose-6-phosphate, leading to the blockage of three major biochemical pathways – the hexosamine/AGE formation/PKC Pathway, which finally suppresses the development of DR [265].
Some trace elements can also ameliorate the severity of DR. The supplemental micronutrients with trace elements (such as zinc and copper) have inhibitory potential on the development of DR [266]. Zinc has antioxidant capacities and defends tissues against oxidative attack, including the protection of protein sulfhydryl groups from oxidation and the suppression of ROS formation through the antagonism of redox-active transition metals, such as copper and iron [267]. It has been reported that Zn supplementation can protect retina from oxidative damages in the early stages of diabetic rats [268]. In addition, manganese supplementation can augment metalation and activity of Mn-SOD in mitochondria [269], and selenium supplementation can also exert protective effect against oxidative stress mediated by hyperglycemia on retinal pigment epithelium [270].
6.3. Activators for antioxidant defense system
Shreds of evidences introduced above obviously indicate the significance of Nrf2 signaling pathway against the pathogenesis of DR. Sulforaphane can activate Nrf2 through Akt/GSK-3β/Fyn pathway [271] and promote the binding of Nrf2 on ARE in thioredoxin (Trx) gene to facilitate Trx expression, and alleviate oxidative stress-induced retinal damage [272]. Hemin (ferriprotoporphyrin IX) also promotes the nuclear translocation of Nrf2 to induce the activation of thioredoxin [273]. Moreover, mycophenolate mofetil (MMF) and dimethylfumarate (DMF) also exert protective effects against diabetes-induced impairments via Nrf2. The beneficial effects of MMF were correlated to the enhancement of Nrf2 nuclear translocation, and the upregulation of antioxidant enzymes including HO-1, CAT, SOD-1, and GPX-1 [274]. DMF has been demonstrated to be an activator of Nrf2 [275,276] and can also act as a selectively repressor for the TNF-mediated nuclear translocation of activated NF-κB [277] that plays important roles for the pathogenesis of DR.
6.4. Inhibitors or activators for epigenetic modification
DNA methylation may be responsible for the breakdown of antioxidant defense system in the progression of DR. Inhibition of DNA methylation via employing pharmaceutic means, such as the DNMT inhibitor 5-aza-2′-deoxycytidine (a drug approved by US FDA), can help retain redox homeostasis and avoid the aggravation of DR [278]. The majority of dietary phenolic phytochemicals, well-known for their antioxidant capacity, also modulate gene expression through epigenetic modification. EGCG can exert suppressive effects on NF-kB activation via acting as RelA-acetylation inhibitor [279]. It has been reported that curcumin demethylates Nrf2 promoter to facilitate its transcription [280]. The phytochemical Sulforaphane induces promoter demethylation and histone acetylation to upregulate the Nrf2 expression and also affects protein levels of DNMT1 and DNMT3a [281]. In addition to DNMT inhibitors, histone deacetylase (HDAC) inhibitors, trichostatin-A and valproic acid, also upregulate Nrf2 expression by elevating histone acetylation levels at Nrf2 promoter region to normalize Nrf2-inducible anti-oxidant defense and confer protection on oxidative stress-damaged cells [282]. Moreover, resveratrol can also suppress the expression of miR-21 that downregulates peroxisome proliferator–activated receptor-α (PPARα) for its pathogenic roles in the development of DR [283,284].
The up-regulation of sirtuins is also important for treatment of DR. Resveratrol, as Sirt1 activator, represses diabetes-induced acetylation of p65 subunit of NF-κB, and then suppresses the downstream gene transcription of MMP-9 to protect the mitochondria impairment in retina [127]. Resveratrol also activates SIRT3 to preserve mitochondrial functions [285] and prevents the apoptosis induced by AGEs and the acetylation of p53 in human vascular endothelial cells [286]. The glucagon like peptide 1 analogue, exendin-4, facilitates SIRT1 and SIRT3 expression to impede ROS-mediated retinal cell death in diabetic rats [287]. Asiatic acid contributes to the increase of SIRT1 to inhibit cell apoptosis and ROS production, and meanwhile maintains the normality of mitochondria [288]. Moreover, vitamin D can rescue H2O2-induced decrease of SIRT1 in endothelial cells [289].
Although numerous studies support the beneficial functions of antioxidants in the pathogenesis of DR, these benefits have to be confirmed by clinical trials. In patients with DR, the oral administration of antioxidants containing α-lipoic acid, genistein and vitamins for one month was demonstrated to ameliorate electroretinogram oscillatory potential [290]. The study using a multi-component nutritional formula (including vitamins E, C, D3, zinc oxide, zeaxanthin, lutein, DHA, EPA, alpha-LA, coenzyme Q10, benfotiamine, N-acetyl cysteine, resveratrol, the extract of turmeric root and green tea) has shown to improve visual function and decrease serum inflammatory factors in diabetic patients and this encouraging result, to some extent, verified the beneficial effects of these antioxidants [291]. However, other study also indicates no obvious correlation between the supplementation of antioxidant (vitamins and β-carotene) and the alleviation of the severity of retinopathy in type II diabetic patients [158]. Therefore, despite promising outcomes from animal models, due to the shortage of well-organized longitudinal cohort studies, there is still no clear conclusion for the antioxidants on clinical trials. The clinical results can also be affected by some of other factors, such as the duration of their administration and the dose of the antioxidants. In addition, the transport of antioxidants affected by BRB could also be a limiting factor. Despite these obstacles, we still have faith in that encouraging animal outcomes and very limited clinical data, and strongly suggest to push the research forward.
7. Conclusion
The purpose of this review is to provide a better comprehension of the complicated molecular mechanisms and pathogenetic roles of oxidative stress in the development of diabetic retinopathy. Diabetic retinopathy is one type of the most prevalent complications in diabetes patients and also regarded as a heavy socioeconomic burden worldwide. Oxidative stress is a cytopathic outcome caused by the imbalance between ROS production and removal, and appears to play a central role in this pathophysiology. Understanding the molecular and biochemical mechanisms of abnormalities associated with oxidative stress in DR is crucial to develop effective therapeutics. In this review, we have illustrated that a set of metabolic abnormalities initiated by hyperglycemia, including polyol, AGE formation, PKC and hexosamine pathways, augments ROS generation and subsequently facilitates oxidative stress in the retina. Oxidative stress in turn potentiates the abnormalities of these metabolic pathways, accordingly forming a vicious cycle, which causes cell mitochondrial damages, retinal cell apoptosis, lipid peroxidation, irregular epigenetic modification on genes of antioxidant defense system and structural and functional damages in the retina. We believe that all the subjects previously discussed clearly clarify the molecular mechanisms and pathogenetic roles of oxidative stress in the progression of DR, and these subjects may be promising issues for further studies of DR and provide potential targets for the treatment. Therefore, the inhibition of ROS production and removal of excessive ROS in retinal cells could protect the retina from hyperglycemia-mediated oxidative impairments. Due to the nature of reductive and biological activities, phytochemical antioxidants and some drugs can exert therapeutic effects on oxidative stress-damaged retinal cells through scavenging free ROS, neutralizing peroxynitrite, or augmenting antioxidant defense system epigenetically, which provides a welcoming sign for diabetic patients in managing this vision-threatening complication. In our opinion, despite the fact that cell experiments and animal-model studies already provide useful information and hints for clinical trials, plentiful work in clinical studies is still required, and it may be promising to employ multi-components of functional drugs with divergent mode(s) of action to fight this multifactorial complication.
Funding
This work was supported by the Special National Key Research and Development Plan of China [grant number 2016YFD0400204]; and the Key-Area Research and Development Program of Guangdong Province [grant number 2019B020212001].
Disclosures
None.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
We thank Dr. Rezaul for critical reading of the article and helpful suggestions. We apologized to those colleagues whose work is not specifically referenced, and gratefully acknowledge their contributions to our article.
References
- 1.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Prasad S., Gupta S.C., Tyagi A.K. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Canc. Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 3.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat. Rev. Canc. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol. Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Catala A. Lipid peroxidation of membrane phospholipids in the vertebrate retina. Front Biosci (Schol Ed) 2011;3:52–60. doi: 10.2741/s131. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Zhang D., Hu J., Liu G., Chen J., Sun L., Jiang Z., Zhang X., Chen Q., Ji B. Visible light-induced lipid peroxidation of unsaturated fatty acids in the retina and the inhibitory effects of blueberry polyphenols. J. Agric. Food Chem. 2015;63:9295–9305. doi: 10.1021/acs.jafc.5b04341. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Zhang D., Wu Y., Ji B. Docosahexaenoic acid aggravates photooxidative damage in retinal pigment epithelial cells via lipid peroxidation. J. Photochem. Photobiol., B. 2014;140:85–93. doi: 10.1016/j.jphotobiol.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Boulton M., Rozanowska M., Rozanowski B. Retinal photodamage. J. Photochem. Photobiol., B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 9.Beli E., Yan Y., Moldovan L., Vieira C.P., Gao R., Duan Y., Prasad R., Bhatwadekar A., White F.A., Townsend S.D., Chan L., Ryan C.N., Morton D., Moldovan E.G., Chu F.I., Oudit G.Y., Derendorf H., Adorini L., Wang X.X., Evans-Molina C., Mirmira R.G., Boulton M.E., Yoder M.C., Li Q., Levi M., Busik J.V., Grant M.B. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67:1867–1879. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong T.Y., Cheung C.M., Larsen M., Sharma S., Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 11.Lee R., Wong T.Y., Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y., He M., Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012;60:428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E., Klein R., Krishnaiah S., Mayurasakorn K., O'Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y. G. Meta-Analysis for Eye Disease Study, Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez M.L., Perez S., Mena-Molla S., Desco M.C., Ortega A.L. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid Med Cell Longev. 2019:4940825. doi: 10.1155/2019/4940825. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen N., Hjortdal J.O., Schielke K.C., Bek T., Grauslund J., Laugesen C.S., Lund-Andersen H., Cerqueira C., Andresen J. The Danish registry of diabetic retinopathy. Clin. Epidemiol. 2016;8:613–619. doi: 10.2147/CLEP.S99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrick A.M., Gibson M.V., Kulshreshtha A. Diabetic retinopathy. Prim Care. 2015;42:451–464. doi: 10.1016/j.pop.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Simo R., Stitt A.W., Gardner T.W. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarroel M., Ciudin A., Hernandez C., Simo R. Neurodegeneration: an early event of diabetic retinopathy. World J. Diabetes. 2010;1:57–64. doi: 10.4239/wjd.v1.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carelli V., La Morgia C., Valentino M.L., Barboni P., Ross-Cisneros F.N., Sadun A.A. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta. 2009;1787:518–528. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H.Y., Green W.R., Tso M.O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 21.Hammes H.P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61:29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 22.Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowluru R.A. Retinopathy in a diet-induced type 2 diabetic rat model and role of epigenetic modifications. Diabetes. 2020;69:689–698. doi: 10.2337/db19-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sui A., Chen X., Demetriades A.M., Shen J., Cai Y., Yao Y., Yao Y., Zhu Y., Shen X., Xie B. Inhibiting NF-kappaB signaling activation reduces retinal neovascularization by promoting a polarization shift in macrophages. Invest. Ophthalmol. Vis. Sci. 2020;61:4. doi: 10.1167/iovs.61.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller W.P., Sunilkumar S., Giordano J.F., Toro A.L., Barber A.J., Dennis M.D. The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. J. Biol. Chem. 2020;295:7350–7361. doi: 10.1074/jbc.RA120.013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Q., Mishra M., Kowluru R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 29.Miller R.G., Orchard T.J. Understanding metabolic memory: a tale of two studies. Diabetes. 2020;69:291–299. doi: 10.2337/db19-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari N., Karmakar A., Ganesan S.K. Targeting epigenetic modifications as a potential therapeutic option for diabetic retinopathy. J. Cell. Physiol. 2020;235:1933–1947. doi: 10.1002/jcp.29180. [DOI] [PubMed] [Google Scholar]
- 31.Kato M., Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019;15:327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Miao F., Paterson A.D., Lachin J.M., Zhang L., Schones D.E., Wu X., Wang J., Tompkins J.D., Genuth S., Braffett B.H., Riggs A.D., Group D.E.R., Natarajan R. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E3002–E3011. doi: 10.1073/pnas.1603712113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letts J.A., Sazanov L.A. Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017;24:800–808. doi: 10.1038/nsmb.3460. [DOI] [PubMed] [Google Scholar]
- 35.Scialo F., Sriram A., Fernandez-Ayala D., Gubina N., Lohmus M., Nelson G., Logan A., Cooper H.M., Navas P., Enriquez J.A., Murphy M.P., Sanz A. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metabol. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forbes J.M., Thorburn D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018;14:291–312. doi: 10.1038/nrneph.2018.9. [DOI] [PubMed] [Google Scholar]
- 37.Letts J.A., Fiedorczuk K., Sazanov L.A. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 39.Demine S., Renard P., Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019:8. doi: 10.3390/cells8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018;1859:940–950. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Eshaq R.S., Wright W.S., Harris N.R. Oxygen delivery, consumption, and conversion to reactive oxygen species in experimental models of diabetic retinopathy. Redox Biol. 2014;2:661–666. doi: 10.1016/j.redox.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Y., Veenstra A., Palczewski K., Kern T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Augsburger F., Filippova A., Rasti D., Seredenina T., Lam M., Maghzal G., Mahiout Z., Jansen-Durr P., Knaus U.G., Doroshow J., Stocker R., Krause K.H., Jaquet V. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019;26:101272. doi: 10.1016/j.redox.2019.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meitzler J.L., Makhlouf H.R., Antony S., Wu Y., Butcher D., Jiang G., Juhasz A., Lu J., Dahan I., Jansen-Durr P., Pircher H., Shah A.M., Roy K., Doroshow J.H. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017;13:182–195. doi: 10.1016/j.redox.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ago T., Kuroda J., Kamouchi M., Sadoshima J., Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. -Review and perspective. Circ. J. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 47.Rojas M., Zhang W., Xu Z., Lemtalsi T., Chandler P., Toque H.A., Caldwell R.W., Caldwell R.B. Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PloS One. 2013;8 doi: 10.1371/journal.pone.0084357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu J., Dziumbla S., Lin J., Bibli S.I., Zukunft S., de Mos J., Awwad K., Fromel T., Jungmann A., Devraj K., Cheng Z., Wang L., Fauser S., Eberhart C.G., Sodhi A., Hammock B.D., Liebner S., Muller O.J., Glaubitz C., Hammes H.P., Popp R., Fleming I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552:248–252. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng J.J., Xiong S.Q., Ding L.X., Peng J., Xia X.B. Diabetic retinopathy: focus on NADPH oxidase and its potential as therapeutic target. Eur. J. Pharmacol. 2019;853:381–387. doi: 10.1016/j.ejphar.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 50.Mahajan N., Arora P., Sandhir R. Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxid Med Cell Longev. 2019:8458472. doi: 10.1155/2019/8458472. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dagher Z., Park Y.S., Asnaghi V., Hoehn T., Gerhardinger C., Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 52.Yang S., Zhao Y., Yu J., Fan Z., Gong S.T., Tang H., Pan L. Sugar alcohols of polyol pathway serve as alarmins to mediate local-systemic innate immune communication in Drosophila. Cell Host Microbe. 2019;26:240–251. doi: 10.1016/j.chom.2019.07.001. e248. [DOI] [PubMed] [Google Scholar]
- 53.Yuan T., Yang T., Chen H., Fu D., Hu Y., Wang J., Yuan Q., Yu H., Xu W., Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C., Miao X., Li F., Wang S., Liu Q., Wang Y., Sun J. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev. 2017;2017:9702820. doi: 10.1155/2017/9702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassegue B., Clempus R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 57.Idris I., Gray S., Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 58.Griner E.M., Kazanietz M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Canc. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q.J. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiramatsu Y., Sekiguchi N., Hayashi M., Isshiki K., Yokota T., King G.L., Loeken M.R. Diacylglycerol production and protein kinase C activity are increased in a mouse model of diabetic embryopathy. Diabetes. 2002;51:2804–2810. doi: 10.2337/diabetes.51.9.2804. [DOI] [PubMed] [Google Scholar]
- 62.Titchenell P.M., Antonetti D.A. Using the past to inform the future: anti-VEGF therapy as a road map to develop novel therapies for diabetic retinopathy. Diabetes. 2013;62:1808–1815. doi: 10.2337/db12-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello L.P., Clermont A., Arora V., Davis M.D., Sheetz M.J., Bursell S.E. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest. Ophthalmol. Vis. Sci. 2006;47:86–92. doi: 10.1167/iovs.05-0757. [DOI] [PubMed] [Google Scholar]
- 64.George A., Pushkaran S., Konstantinidis D.G., Koochaki S., Malik P., Mohandas N., Zheng Y., Joiner C.H., Kalfa T.A. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099–2107. doi: 10.1182/blood-2012-07-441188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Julius A., Hopper W. A non-invasive, multi-target approach to treat diabetic retinopathy. Biomed. Pharmacother. 2019;109:708–715. doi: 10.1016/j.biopha.2018.10.185. [DOI] [PubMed] [Google Scholar]
- 67.Stitt A., Gardiner T.A., Alderson N.L., Canning P., Frizzell N., Duffy N., Boyle C., Januszewski A.S., Chachich M., Baynes J.W., Thorpe S.R. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 68.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaudhuri J., Bains Y., Guha S., Kahn A., Hall D., Bose N., Gugliucci A., Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metabol. 2018;28:337–352. doi: 10.1016/j.cmet.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stitt A.W. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br. J. Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kass D.A., Shapiro E.P., Kawaguchi M., Capriotti A.R., Scuteri A., deGroof R.C., Lakatta E.G. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 72.Wu M.Y., Yiang G.T., Lai T.T., Li C.J. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev. 2018:3420187. doi: 10.1155/2018/3420187. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamagishi S., Nakamura K., Matsui T., Inagaki Y., Takenaka K., Jinnouchi Y., Yoshida Y., Matsuura T., Narama I., Motomiya Y., Takeuchi M., Inoue H., Yoshimura A., Bucala R., Imaizumi T. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J. Biol. Chem. 2006;281:20213–20220. doi: 10.1074/jbc.M602110200. [DOI] [PubMed] [Google Scholar]
- 74.Guimaraes E.L., Empsen C., Geerts A., van Grunsven L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010;52:389–397. doi: 10.1016/j.jhep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Zhang M., Kho A.L., Anilkumar N., Chibber R., Pagano P.J., Shah A.M., Cave A.C. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 76.Cheung C.Y.Y., Lee C.H., Tang C.S., Xu A., Au K.W., Fong C.H.Y., Ng K.K.K., Kwok K.H.M., Chow W.S., Woo Y.C., Yuen M.M.A., Hai J., Tan K.C.B., Lam T.H., Tse H.F., Sham P.C., Lam K.S.L. Genetic regulation of pigment epithelium-derived factor (PEDF): an exome-chip association analysis in Chinese subjects with type 2 diabetes. Diabetes. 2019;68:198–206. doi: 10.2337/db18-0500. [DOI] [PubMed] [Google Scholar]
- 77.Safi S.Z., Qvist R., Kumar S., Batumalaie K., Ismail I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. BioMed Res. Int. 2014;2014:801269. doi: 10.1155/2014/801269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheikpranbabu S., Haribalaganesh R., Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab. 2011;37:505–511. doi: 10.1016/j.diabet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Lin N., Zhang H., Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012;38:250–257. doi: 10.1016/j.diabet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Moldogazieva N.T., Mokhosoev I.M., Mel'nikova T.I., Porozov Y.B., Terentiev A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid Med Cell Longev. 2019:3085756. doi: 10.1155/2019/3085756. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao D., Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagy T., Fisi V., Frank D., Katai E., Nagy Z., Miseta A. Hyperglycemia-induced aberrant cell proliferation; A metabolic challenge mediated by protein O-GlcNAc modification. Cells. 2019:8. doi: 10.3390/cells8090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehmann L.H., Jebessa Z.H., Kreusser M.M., Horsch A., He T., Kronlage M., Dewenter M., Sramek V., Oehl U., Krebs-Haupenthal J., von der Lieth A.H., Schmidt A., Sun Q., Ritterhoff J., Finke D., Volkers M., Jungmann A., Sauer S.W., Thiel C., Nickel A., Kohlhaas M., Schafer M., Sticht C., Maack C., Gretz N., Wagner M., El-Armouche A., Maier L.S., Londono J.E.C., Meder B., Freichel M., Grone H.J., Most P., Muller O.J., Herzig S., Furlong E.E.M., Katus H.A., Backs J. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat. Med. 2018;24:62–72. doi: 10.1038/nm.4452. [DOI] [PubMed] [Google Scholar]
- 84.Hart G.W., Housley M.P., Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 85.Lund J., Ouwens D.M., Wettergreen M., Bakke S.S., Thoresen G.H., Aas V. Increased glycolysis and higher lactate production in hyperglycemic myotubes. Cells. 2019:8. doi: 10.3390/cells8091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones D.R., Keune W.J., Anderson K.E., Stephens L.R., Hawkins P.T., Divecha N. The hexosamine biosynthesis pathway and O-GlcNAcylation maintain insulin-stimulated PI3K-PKB phosphorylation and tumour cell growth after short-term glucose deprivation. FEBS J. 2014;281:3591–3608. doi: 10.1111/febs.12879. [DOI] [PubMed] [Google Scholar]
- 87.Du X., Matsumura T., Edelstein D., Rossetti L., Zsengeller Z., Szabo C., Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du X.L., Edelstein D., Rossetti L., Fantus I.G., Goldberg H., Ziyadeh F., Wu J., Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaneto H., Xu G., Song K.H., Suzuma K., Bonner-Weir S., Sharma A., Weir G.C. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J. Biol. Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- 90.Mi Y., Qi G., Gao Y., Li R., Wang Y., Li X., Huang S., Liu X. (-)-Epigallocatechin-3-gallate ameliorates insulin resistance and mitochondrial dysfunction in HepG2 cells: involvement of Bmal1. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700440. [DOI] [PubMed] [Google Scholar]
- 91.Weimer S., Priebs J., Kuhlow D., Groth M., Priebe S., Mansfeld J., Merry T.L., Dubuis S., Laube B., Pfeiffer A.F., Schulz T.J., Guthke R., Platzer M., Zamboni N., Zarse K., Ristow M. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun. 2014;5:3563. doi: 10.1038/ncomms4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 93.Beisswenger P.J., Howell S.K., Smith K., Szwergold B.S. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim. Biophys. Acta. 2003;1637:98–106. doi: 10.1016/s09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 94.Schleicher E.D., Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 95.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp. Diabetes Res. 2007:61038. doi: 10.1155/2007/61038. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scivittaro V., Ganz M.B., Weiss M.F. AGEs induce oxidative stress and activate protein kinase C-beta(II) in neonatal mesangial cells. Am. J. Physiol. Ren. Physiol. 2000;278:F676–F683. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 97.Henikoff S., Greally J.M. Epigenetics, cellular memory and gene regulation. Curr. Biol. 2016;26:R644–R648. doi: 10.1016/j.cub.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 98.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Ziech D., Franco R., Pappa A., Panayiotidis M.I. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 100.Afanas'ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 2014;5:52–62. doi: 10.14336/AD.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valinluck V., Tsai H.H., Rogstad D.K., Burdzy A., Bird A., Sowers L.C. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang M., Culhane J.C., Szewczuk L.M., Gocke C.B., Brautigam C.A., Tomchick D.R., Machius M., Cole P.A., Yu H. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nat. Struct. Mol. Biol. 2007;14:535–539. doi: 10.1038/nsmb1255. [DOI] [PubMed] [Google Scholar]
- 104.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R.A., Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 105.Duquette M.L., Kim J., Shi L.Z., Berns M.W. LSD1 mediated changes in the local redox environment during the DNA damage response. PloS One. 2018;13 doi: 10.1371/journal.pone.0201907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mosammaparast N., Kim H., Laurent B., Zhao Y., Lim H.J., Majid M.C., Dango S., Luo Y., Hempel K., Sowa M.E., Gygi S.P., Steen H., Harper J.W., Yankner B., Shi Y. The histone demethylase LSD1/KDM1A promotes the DNA damage response. J. Cell Biol. 2013;203:457–470. doi: 10.1083/jcb.201302092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Druz A., Betenbaugh M., Shiloach J. Glucose depletion activates mmu-miR-466h-5p expression through oxidative stress and inhibition of histone deacetylation. Nucleic Acids Res. 2012;40:7291–7302. doi: 10.1093/nar/gks452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perrone L., Matrone C., Singh L.P. Epigenetic modifications and potential new treatment targets in diabetic retinopathy. J Ophthalmol. 2014;2014:789120. doi: 10.1155/2014/789120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mishra M., Zhong Q., Kowluru R.A. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic. Biol. Med. 2014;75:129–139. doi: 10.1016/j.freeradbiomed.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kowluru R.A., Santos J.M., Mishra M. Epigenetic modifications and diabetic retinopathy. BioMed Res. Int. 2013;2013:635284. doi: 10.1155/2013/635284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong Q., Kowluru R.A. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–1313. doi: 10.2337/db10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Syreeni A., El-Osta A., Forsblom C., Sandholm N., Parkkonen M., Tarnow L., Parving H.H., McKnight A.J., Maxwell A.P., Cooper M.E., Groop P.H., FinnDiane Study G. Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes. 2011;60:3073–3080. doi: 10.2337/db11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhong Q., Kowluru R.A. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest. Ophthalmol. Vis. Sci. 2013;54:244–250. doi: 10.1167/iovs.12-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P.L., Roeder R.G., Cooper M.E., Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mishra M., Zhong Q., Kowluru R.A. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2014;55:7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhong Q., Kowluru R.A. Regulation of matrix metalloproteinase-9 by epigenetic modifications and the development of diabetic retinopathy. Diabetes. 2013;62:2559–2568. doi: 10.2337/db12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos L., Escande C., Denicola A. Potential modulation of sirtuins by oxidative stress. Oxid Med Cell Longev. 2016:9831825. doi: 10.1155/2016/9831825. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caito S., Rajendrasozhan S., Cook S., Chung S., Yao H., Friedman A.E., Brookes P.S., Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. Faseb. J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li D., Wang X., Huang Q., Li S., Zhou Y., Li Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-kappaB pathway in vivo and in vitro. Redox Biol. 2018;15:62–73. doi: 10.1016/j.redox.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng Z., Chen H., Li J., Li T., Zheng B., Zheng Y., Jin H., He Y., Gu Q., Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mishra M., Duraisamy A.J., Kowluru R.A. Sirt1: a guardian of the development of diabetic retinopathy. Diabetes. 2018;67:745–754. doi: 10.2337/db17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh C.K., Chhabra G., Ndiaye M.A., Garcia-Peterson L.M., Mack N.J., Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxidants Redox Signal. 2018;28:643–661. doi: 10.1089/ars.2017.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Katwal G., Baral D., Fan X., Weiyang H., Zhang X., Ling L., Xiong Y., Ye Q., Wang Y. SIRT3 a major player in attenuation of hepatic ischemia-reperfusion injury by reducing ROS via its downstream mediators: SOD2, CYP-D, and HIF-1alpha. Oxid Med Cell Longev. 2018:2976957. doi: 10.1155/2018/2976957. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tao R., Coleman M.C., Pennington J.D., Ozden O., Park S.H., Jiang H., Kim H.S., Flynn C.R., Hill S., Hayes McDonald W., Olivier A.K., Spitz D.R., Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabol. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 126.Kawahara T.L., Michishita E., Adler A.S., Damian M., Berber E., Lin M., McCord R.A., Ongaigui K.C., Boxer L.D., Chang H.Y., Chua K.F. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kowluru R.A., Santos J.M., Zhong Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2014;55:5653–5660. doi: 10.1167/iovs.14-14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mao X.B., Cheng Y.H., Peng K.S., You Z.P. Sirtuin (Sirt) 3 overexpression prevents retinopathy in streptozotocin-induced diabetic rats. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.920883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu R., Liu H., Ha Y., Tilton R.G., Zhang W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. BioMed Res. Int. 2014;2014:902842. doi: 10.1155/2014/902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L., Hiler D., Xu B., AlDiri I., Chen X., Zhou X., Griffiths L., Valentine M., Shirinifard A., Sablauer A., Thiagarajan S., Barabas M.E., Zhang J., Johnson D., Frase S., Dyer M.A. Retinal cell type DNA methylation and histone modifications predict reprogramming efficiency and retinogenesis in 3D organoid cultures. Cell Rep. 2018;22:2601–2614. doi: 10.1016/j.celrep.2018.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 132.Shock L.S., Thakkar P.V., Peterson E.J., Moran R.G., Taylor S.M. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3630–3635. doi: 10.1073/pnas.1012311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mishra M., Kowluru R.A. Epigenetic modification of mitochondrial DNA in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2015;56:5133–5142. doi: 10.1167/iovs.15-16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tewari S., Zhong Q., Santos J.M., Kowluru R.A. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2012;53:4881–4888. doi: 10.1167/iovs.12-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kowluru R.A., Shan Y., Mishra M. Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab. Invest. 2016;96:1040–1049. doi: 10.1038/labinvest.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohammad G., Radhakrishnan R., Kowluru R.A. Epigenetic modifications compromise mitochondrial DNA quality control in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2019;60:3943–3951. doi: 10.1167/iovs.19-27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mortuza R., Feng B., Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia. 2014;57:1037–1046. doi: 10.1007/s00125-014-3197-9. [DOI] [PubMed] [Google Scholar]
- 138.McArthur K., Feng B., Wu Y., Chen S., Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu H.N., Cao N.J., Li X., Qian W., Chen X.L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem. Biophys. Res. Commun. 2018;505:1236–1243. doi: 10.1016/j.bbrc.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z., Shentu T.P., Wen L., Johnson D.A., Shyy J.Y. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxidants Redox Signal. 2013;19:1522–1538. doi: 10.1089/ars.2012.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhan A., Soleimani M., Mandal S.S. Long noncoding RNA and cancer: a new paradigm. Canc. Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomas A.A., Feng B., Chakrabarti S. ANRIL: a regulator of VEGF in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2017;58:470–480. doi: 10.1167/iovs.16-20569. [DOI] [PubMed] [Google Scholar]
- 145.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 146.Tong P., Peng Q.H., Gu L.M., Xie W.W., Li W.J. LncRNA-MEG3 alleviates high glucose induced inflammation and apoptosis of retina epithelial cells via regulating miR-34a/SIRT1 axis. Exp. Mol. Pathol. 2019;107:102–109. doi: 10.1016/j.yexmp.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 147.Li C.P., Wang S.H., Wang W.Q., Song S.G., Liu X.M. Long noncoding RNA-Sox2OT knockdown alleviates diabetes mellitus-induced retinal ganglion cell (RGC) injury. Cell. Mol. Neurobiol. 2017;37:361–369. doi: 10.1007/s10571-016-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shao K., Xi L., Cang Z., Chen C., Huang S. Knockdown of NEAT1 exerts suppressive effects on diabetic retinopathy progression via inactivating TGF-beta1 and VEGF signaling pathways. J. Cell. Physiol. 2020;235:9361–9369. doi: 10.1002/jcp.29740. [DOI] [PubMed] [Google Scholar]
- 149.Kang E., Wu J., Gutierrez N.M., Koski A., Tippner-Hedges R., Agaronyan K., Platero-Luengo A., Martinez-Redondo P., Ma H., Lee Y., Hayama T., Van Dyken C., Wang X., Luo S., Ahmed R., Li Y., Ji D., Kayali R., Cinnioglu C., Olson S., Jensen J., Battaglia D., Lee D., Wu D., Huang T., Wolf D.P., Temiakov D., Belmonte J.C., Amato P., Mitalipov S. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- 150.Jemt E., Persson O., Shi Y., Mehmedovic M., Uhler J.P., Davila Lopez M., Freyer C., Gustafsson C.M., Samuelsson T., Falkenberg M. Regulation of DNA replication at the end of the mitochondrial D-loop involves the helicase TWINKLE and a conserved sequence element. Nucleic Acids Res. 2015;43:9262–9275. doi: 10.1093/nar/gkv804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mishra M., Kowluru R.A. DNA methylation-a potential source of mitochondria DNA base mismatch in the development of diabetic retinopathy. Mol. Neurobiol. 2019;56:88–101. doi: 10.1007/s12035-018-1086-9. [DOI] [PubMed] [Google Scholar]
- 152.Chen X.J., Butow R.A. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 153.Zhao Y., McLaughlin D., Robinson E., Harvey A.P., Hookham M.B., Shah A.M., McDermott B.J., Grieve D.J. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Canc. Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kowluru R.A., Kowluru A., Mishra M., Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kowluru R.A., Mohammad G., dos Santos J.M., Zhong Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60:3023–3033. doi: 10.2337/db11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohammad G., Kowluru R.A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2011;52:3832–3841. doi: 10.1167/iovs.10-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kalani A., Kamat P.K., Tyagi N. Diabetic stroke severity: epigenetic remodeling and neuronal, glial, and vascular dysfunction. Diabetes. 2015;64:4260–4271. doi: 10.2337/db15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kowluru R.A., Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 159.Kanwar M., Chan P.S., Kern T.S., Kowluru R.A. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest. Ophthalmol. Vis. Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 160.Yi X., Guo W., Shi Q., Yang Y., Zhang W., Chen X., Kang P., Chen J., Cui T., Ma J., Wang H., Guo S., Chang Y., Liu L., Jian Z., Wang L., Xiao Q., Li S., Gao T., Li C. SIRT3-Dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics. 2019;9:1614–1633. doi: 10.7150/thno.30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guerra-Castellano A., Diaz-Quintana A., Perez-Mejias G., Elena-Real C.A., Gonzalez-Arzola K., Garcia-Maurino S.M., De la Rosa M.A., Diaz-Moreno I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2018;115:7955–7960. doi: 10.1073/pnas.1806833115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 163.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U. S. A. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Radi R., Cassina A., Hodara R., Quijano C., Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 165.Al-Shabrawey M., Smith S. Prediction of diabetic retinopathy: role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010;1:56–72. doi: 10.1007/s13167-010-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rask-Madsen C., King G.L. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metabol. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 169.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 170.Dehdashtian E., Mehrzadi S., Yousefi B., Hosseinzadeh A., Reiter R.J., Safa M., Ghaznavi H., Naseripour M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20–33. doi: 10.1016/j.lfs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 171.Du Y., Miller C.M., Kern T.S. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 172.Cao R., Li L., Ying Z., Cao Z., Ma Y., Mao X., Li J., Qi X., Zhang Z., Wang X. A small molecule protects mitochondrial integrity by inhibiting mTOR activity. Proc. Natl. Acad. Sci. U. S. A. 2019;116:23332–23338. doi: 10.1073/pnas.1911246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang X., Li L., Ying Z., Pan C., Huang S., Li L., Dai M., Yan B., Li M., Jiang H., Chen S., Zhang Z., Wang X. A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol Cell. 2016;63:229–239. doi: 10.1016/j.molcel.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 174.Kowluru R.A., Abbas S.N. Diabetes-induced mitochondrial dysfunction in the retina. Invest. Ophthalmol. Vis. Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 175.Jiang X., Jiang H., Shen Z., Wang X. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14782–14787. doi: 10.1073/pnas.1417253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kowluru R.A., Koppolu P. Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic. Res. 2002;36:993–999. doi: 10.1080/1071576021000006572. [DOI] [PubMed] [Google Scholar]
- 177.Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 178.Moscat J., Diaz-Meco M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kowluru R.A., Koppolu P., Chakrabarti S., Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 180.Nakajima S., Kitamura M. Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 181.Romeo G., Liu W.H., Asnaghi V., Kern T.S., Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 182.Chen K., Qiu P., Yuan Y., Zheng L., He J., Wang C., Guo Q., Kenny J., Liu Q., Zhao J., Chen J., Tickner J., Fan S., Lin X., Xu J. Pseurotin A inhibits osteoclastogenesis and prevents ovariectomized-induced bone loss by suppressing reactive oxygen species. Theranostics. 2019;9:1634–1650. doi: 10.7150/thno.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Altamirano F., Lopez J.R., Henriquez C., Molinski T., Allen P.D., Jaimovich E. Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J. Biol. Chem. 2012;287:20876–20887. doi: 10.1074/jbc.M112.344929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lim J.W., Kim H., Kim K.H. NF-kappaB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Radic. Biol. Med. 2001;31:355–366. doi: 10.1016/s0891-5849(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 185.Baig M.S., Zaichick S.V., Mao M., de Abreu A.L., Bakhshi F.R., Hart P.C., Saqib U., Deng J., Chatterjee S., Block M.L., Vogel S.M., Malik A.B., Consolaro M.E., Christman J.W., Minshall R.D., Gantner B.N., Bonini M.G. NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J. Exp. Med. 2015;212:1725–1738. doi: 10.1084/jem.20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Njie-Mbye Y.F., Kulkarni-Chitnis M., Opere C.A., Barrett A., Ohia S.E. Lipid peroxidation: pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013;4:366. doi: 10.3389/fphys.2013.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Robles-Rivera R.R., Castellanos-Gonzalez J.A., Olvera-Montano C., Flores-Martin R.A., Lopez-Contreras A.K., Arevalo-Simental D.E., Cardona-Munoz E.G., Roman-Pintos L.M., Rodriguez-Carrizalez A.D. Adjuvant therapies in diabetic retinopathy as an early approach to delay its progression: the importance of oxidative stress and inflammation. Oxid Med Cell Longev. 2020;2020:3096470. doi: 10.1155/2020/3096470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gupta M.M., Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J. Physiol. Pharmacol. 2005;49:187–192. [PubMed] [Google Scholar]
- 189.Zhou T., Zhou K.K., Lee K., Gao G., Lyons T.J., Kowluru R., Ma J.X. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54:459–468. doi: 10.1007/s00125-010-1943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ohia S.E., Opere C.A., Leday A.M. Pharmacological consequences of oxidative stress in ocular tissues. Mutat. Res. 2005;579:22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 191.Chen Q., Tang L., Xin G., Li S., Ma L., Xu Y., Zhuang M., Xiong Q., Wei Z., Xing Z., Niu H., Huang W. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic. Biol. Med. 2019;130:48–58. doi: 10.1016/j.freeradbiomed.2018.10.419. [DOI] [PubMed] [Google Scholar]
- 192.Sharma A., Sharma R., Chaudhary P., Vatsyayan R., Pearce V., Jeyabal P.V., Zimniak P., Awasthi S., Awasthi Y.C. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch. Biochem. Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bradley M.A., Xiong-Fister S., Markesbery W.R., Lovell M.A. Elevated 4-hydroxyhexenal in Alzheimer's disease (AD) progression. Neurobiol. Aging. 2012;33:1034–1044. doi: 10.1016/j.neurobiolaging.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pena-Bautista C., Vento M., Baquero M., Chafer-Pericas C. Lipid peroxidation in neurodegeneration. Clin. Chim. Acta. 2019;497:178–188. doi: 10.1016/j.cca.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 195.Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B.H., Quintana A., Bellen H.J. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Madsen-Bouterse S.A., Kowluru R.A. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 197.Khan Z.A., Chakrabarti S. Cellular signaling and potential new treatment targets in diabetic retinopathy. Exp. Diabetes Res. 2007:31867. doi: 10.1155/2007/31867. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roy S., Kim D. Retinal capillary basement membrane thickening: role in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2020:100903. doi: 10.1016/j.preteyeres.2020.100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mason R.M., Wahab N.A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 200.Goh S.Y., Cooper M.E. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 201.Gardiner T.A., Anderson H.R., Stitt A.W. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J. Pathol. 2003;201:328–333. doi: 10.1002/path.1429. [DOI] [PubMed] [Google Scholar]
- 202.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 203.Li J., Wang J.J., Yu Q., Chen K., Mahadev K., Zhang S.X. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Deissler H.L., Deissler H., Lang G.K., Lang G.E. VEGF but not PlGF disturbs the barrier of retinal endothelial cells. Exp. Eye Res. 2013;115:162–171. doi: 10.1016/j.exer.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 205.Iwase T., Oveson B.C., Hashida N., Lima e Silva R., Shen J., Krauss A.H., Gale D.C., Adamson P., Campochiaro P.A. Topical pazopanib blocks VEGF-induced vascular leakage and neovascularization in the mouse retina but is ineffective in the rabbit. Invest. Ophthalmol. Vis. Sci. 2013;54:503–511. doi: 10.1167/iovs.12-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jo D.H., Yun J.H., Cho C.S., Kim J.H., Kim J.H., Cho C.H. Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia. 2019;67:321–331. doi: 10.1002/glia.23542. [DOI] [PubMed] [Google Scholar]
- 207.Geraldes P., Hiraoka-Yamamoto J., Matsumoto M., Clermont A., Leitges M., Marette A., Aiello L.P., Kern T.S., King G.L. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Calderon G.D., Juarez O.H., Hernandez G.E., Punzo S.M., De la Cruz Z.D. Oxidative stress and diabetic retinopathy: development and treatment. Eye. 2017;31:1122–1130. doi: 10.1038/eye.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nawaz I.M., Rezzola S., Cancarini A., Russo A., Costagliola C., Semeraro F., Presta M. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog. Retin. Eye Res. 2019;72:100756. doi: 10.1016/j.preteyeres.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 210.Gianni-Barrera R., Butschkau A., Uccelli A., Certelli A., Valente P., Bartolomeo M., Groppa E., Burger M.G., Hlushchuk R., Heberer M., Schaefer D.J., Gurke L., Djonov V., Vollmar B., Banfi A. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21:883–900. doi: 10.1007/s10456-018-9634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ayalasomayajula S.P., Amrite A.C., Kompella U.B. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. Eur. J. Pharmacol. 2004;498:275–278. doi: 10.1016/j.ejphar.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 212.Schoenberger S.D., Kim S.J., Sheng J., Rezaei K.A., Lalezary M., Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest. Ophthalmol. Vis. Sci. 2012;53:5906–5911. doi: 10.1167/iovs.12-10410. [DOI] [PubMed] [Google Scholar]
- 213.Belaidi E., Morand J., Gras E., Pepin J.L., Godin-Ribuot D. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol. Ther. 2016;168:1–11. doi: 10.1016/j.pharmthera.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.D'Angelo G., Loria A.S., Pollock D.M., Pollock J.S. Endothelin activation of reactive oxygen species mediates stress-induced pressor response in Dahl salt-sensitive prehypertensive rats. Hypertension. 2010;56:282–289. doi: 10.1161/HYPERTENSIONAHA.110.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Majumdar P., Chen S., George B., Sen S., Karmazyn M., Chakrabarti S. Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab Res Rev. 2009;25:452–463. doi: 10.1002/dmrr.964. [DOI] [PubMed] [Google Scholar]
- 216.Evans T., Deng D.X., Chen S., Chakrabarti S. Endothelin receptor blockade prevents augmented extracellular matrix component mRNA expression and capillary basement membrane thickening in the retina of diabetic and galactose-fed rats. Diabetes. 2000;49:662–666. doi: 10.2337/diabetes.49.4.662. [DOI] [PubMed] [Google Scholar]
- 217.Goraca A., Kleniewska P., Skibska B. ET-1 mediates the release of reactive oxygen species and TNF-alpha in lung tissue by protein kinase C alpha and beta1. Pharmacol. Rep. 2016;68:121–126. doi: 10.1016/j.pharep.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 218.Metea M.R., Newman E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007;92:635–640. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 1985;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. 2006. [DOI] [PubMed] [Google Scholar]
- 220.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 221.Tezel G., Luo C., Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest. Ophthalmol. Vis. Sci. 2007;48:1201–1211. doi: 10.1167/iovs.06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Takeuchi M., Bucala R., Suzuki T., Ohkubo T., Yamazaki M., Koike T., Kameda Y., Makita Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 2000;59:1094–1105. doi: 10.1093/jnen/59.12.1094. [DOI] [PubMed] [Google Scholar]
- 223.Funatsu H., Yamashita H., Nakanishi Y., Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 2002;86:311–315. doi: 10.1136/bjo.86.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Abiodun O.A., Ola M.S. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J. Biol. Sci. 2020;27:905–912. doi: 10.1016/j.sjbs.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dvoriantchikova G., Grant J., Santos A.R., Hernandez E., Ivanov D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Invest. Ophthalmol. Vis. Sci. 2012;53:2823–2830. doi: 10.1167/iovs.12-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Garcia T.B., Hollborn M., Bringmann A. Expression and signaling of NGF in the healthy and injured retina. Cytokine Growth Factor Rev. 2017;34:43–57. doi: 10.1016/j.cytogfr.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 227.Simo R., C Hernandez R. European Consortium for the Early Treatment of Diabetic, Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 228.Couturier A., Bousquet E., Zhao M., Naud M.C., Klein C., Jonet L., Tadayoni R., de Kozak Y., Behar-Cohen F. Anti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammation. Mol. Vis. 2014;20:908–920. [PMC free article] [PubMed] [Google Scholar]
- 229.Feng Y., Busch S., Gretz N., Hoffmann S., Hammes H.P. Crosstalk in the retinal neurovascular unit - lessons for the diabetic retina. Exp. Clin. Endocrinol. Diabetes. 2012;120:199–201. doi: 10.1055/s-0032-1304571. [DOI] [PubMed] [Google Scholar]
- 230.Tezel G., Yang X., Luo C., Peng Y., Sun S.L., Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest. Ophthalmol. Vis. Sci. 2007;48:705–714. doi: 10.1167/iovs.06-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Luo C., Yang X., Kain A.D., Powell D.W., Kuehn M.H., Tezel G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest. Ophthalmol. Vis. Sci. 2010;51:5697–5707. doi: 10.1167/iovs.10-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xu H., Chen M., Forrester J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 233.Tezel G., Yang X., Luo C., Kain A.D., Powell D.W., Kuehn M.H., Kaplan H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Invest. Ophthalmol. Vis. Sci. 2010;51:5071–5082. doi: 10.1167/iovs.10-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp. Eye Res. 2011;93:178–186. doi: 10.1016/j.exer.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Silva K.C., Rosales M.A., Hamassaki D.E., Saito K.C., Faria A.M., Ribeiro P.A., Faria J.B., Faria J.M. Green tea is neuroprotective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 236.Meng J.M., Cao S.Y., Wei X.L., Gan R.Y., Wang Y.F., Cai S.X., Xu X.Y., Zhang P.Z., Li H.B. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: an updated review. Antioxidants. 2019:8. doi: 10.3390/antiox8060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Babu P.V., Sabitha K.E., Shyamaladevi C.S. Therapeutic effect of green tea extract on advanced glycation and cross-linking of collagen in the aorta of streptozotocin diabetic rats. Clin. Exp. Pharmacol. Physiol. 2006;33:351–357. doi: 10.1111/j.1440-1681.2006.04374.x. [DOI] [PubMed] [Google Scholar]
- 238.Kumar B., Gupta S.K., Nag T.C., Srivastava S., Saxena R., Jha K.A., Srinivasan B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 239.Abu-Amero K.K., Kondkar A.A., Chalam K.V. Resveratrol and ophthalmic diseases. Nutrients. 2016;8:200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li J., Yu S., Ying J., Shi T., Wang P. Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC-1alpha pathway. Oxid Med Cell Longev. 2017:7584691. doi: 10.1155/2017/7584691. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Losso J.N., Truax R.E., Richard G. trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J. Agric. Food Chem. 2010;58:8246–8252. doi: 10.1021/jf1012067. [DOI] [PubMed] [Google Scholar]
- 242.Woo J.H., Lim J.H., Kim Y.H., Suh S.I., Min D.S., Chang J.S., Lee Y.H., Park J.W., Kwon T.K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 243.Chen Y., Meng J., Li H., Wei H., Bi F., Liu S., Tang K., Guo H., Liu W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019;181:356–366. doi: 10.1016/j.exer.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 244.Aldebasi Y.H., Aly S.M., Rahmani A.H. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5:194–202. [PMC free article] [PubMed] [Google Scholar]
- 245.Gupta S.K., Kumar B., Nag T.C., Agrawal S.S., Agrawal R., Agrawal P., Saxena R., Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Therapeut. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 246.Maugeri A., Mazzone M.G., Giuliano F., Vinciguerra M., Basile G., Barchitta M., Agodi A. Curcumin modulates DNA methyltransferase functions in a cellular model of diabetic retinopathy. Oxid Med Cell Longev. 2018:5407482. doi: 10.1155/2018/5407482. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chucair A.J., Rotstein N.P., Sangiovanni J.P., During A., Chew E.Y., Politi L.E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 2007;48:5168–5177. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- 248.Ramkumar H.L., Tuo J., Shen D.F., Zhang J., Cao X., Chew E.Y., Chan C.C. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. J. Nutr. 2013;143:1129–1135. doi: 10.3945/jn.112.169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gong X., Draper C.S., Allison G.S., Marisiddaiah R., Rubin L.P. Effects of the macular carotenoid lutein in human retinal pigment epithelial cells. Antioxidants. 2017:6. doi: 10.3390/antiox6040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li S.Y., Lo A.C. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int. J. Mol. Sci. 2010;11:2109–2117. doi: 10.3390/ijms11052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Landon R., Gueguen V., Petite H., Letourneur D., Pavon-Djavid G., Anagnostou F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs. 2020;18 doi: 10.3390/md18070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sun Z., Liu J., Zeng X., Huangfu J., Jiang Y., Wang M., Chen F. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011;2:251–258. doi: 10.1039/c1fo10021a. [DOI] [PubMed] [Google Scholar]
- 253.Kowluru R.A., Menon B., Gierhart D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest. Ophthalmol. Vis. Sci. 2008;49:1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 254.Keegan G., Pardhan S., Chichger H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Exp. Eye Res. 2020;197:108104. doi: 10.1016/j.exer.2020.108104. [DOI] [PubMed] [Google Scholar]
- 255.Suh J.H., Shenvi S.V., Dixon B.M., Liu H., Jaiswal A.K., Liu R.M., Hagen T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ying Z., Kampfrath T., Sun Q., Parthasarathy S., Rajagopalan S. Evidence that alpha-lipoic acid inhibits NF-kappaB activation independent of its antioxidant function. Inflamm. Res. 2011;60:219–225. doi: 10.1007/s00011-010-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kowluru R.A., Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 258.Derosa G., D'Angelo A., Romano D., Maffioli P. A clinical trial about a food supplement containing alpha-lipoic acid on oxidative stress markers in type 2 diabetic patients. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17111802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kowluru R.A., Tang J., Kern T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 260.Ueno Y., Kizaki M., Nakagiri R., Kamiya T., Sumi H., Osawa T. Dietary glutathione protects rats from diabetic nephropathy and neuropathy. J. Nutr. 2002;132:897–900. doi: 10.1093/jn/132.5.897. [DOI] [PubMed] [Google Scholar]
- 261.Shang F., Lu M., Dudek E., Reddan J., Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic. Biol. Med. 2003;34:521–530. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- 262.Cheng Y., Du Y., Liu H., Tang J., Veenstra A., Kern T.S. Photobiomodulation inhibits long-term structural and functional lesions of diabetic retinopathy. Diabetes. 2018;67:291–298. doi: 10.2337/db17-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Malechka V.V., Chen J., Cheng R., Ma J.X., Moiseyev G. The single administration of a chromophore alleviates neural defects in diabetic retinopathy. Am. J. Pathol. 2020;190:1505–1512. doi: 10.1016/j.ajpath.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tiruvalluru M., Ananthathmakula P., Ayyalasomayajula V., Nappanveettil G., Ayyagari R., Reddy G.B. Vitamin A supplementation ameliorates obesity-associated retinal degeneration in WNIN/Ob rats. Nutrition. 2013;29:298–304. doi: 10.1016/j.nut.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 265.Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., Lin J., Bierhaus A., Nawroth P., Hannak D., Neumaier M., Bergfeld R., Giardino I., Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 266.Kowluru R.A., Kanwar M., Chan P.S., Zhang J.P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch. Ophthalmol. 2008;126:1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miao X., Sun W., Miao L., Fu Y., Wang Y., Su G., Liu Q. Zinc and diabetic retinopathy. J Diabetes Res. 2013;2013:425854. doi: 10.1155/2013/425854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Moustafa S.A. Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol. Appl. Pharmacol. 2004;201:149–155. doi: 10.1016/j.taap.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 269.Lee S.H., Jouihan H.A., Cooksey R.C., Jones D., Kim H.J., Winge D.R., McClain D.A. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology. 2013;154:1029–1038. doi: 10.1210/en.2012-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gonzalez de Vega R., Garcia M., Fernandez-Sanchez M.L., Gonzalez-Iglesias H., Sanz-Medel A. Protective effect of selenium supplementation following oxidative stress mediated by glucose on retinal pigment epithelium. Metall. 2018;10:83–92. doi: 10.1039/c7mt00209b. [DOI] [PubMed] [Google Scholar]
- 271.Xin Y., Bai Y., Jiang X., Zhou S., Wang Y., Wintergerst K.A., Cui T., Ji H., Tan Y., Cai L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ss/Fyn pathway. Redox Biol. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tanito M., Masutani H., Kim Y.C., Nishikawa M., Ohira A., Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest. Ophthalmol. Vis. Sci. 2005;46:979–987. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- 273.Kim Y.C., Masutani H., Yamaguchi Y., Itoh K., Yamamoto M., Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J. Biol. Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 274.Arellano-Buendia A.S., Tostado-Gonzalez M., Garcia-Arroyo F.E., Cristobal-Garcia M., Loredo-Mendoza M.L., Tapia E., Sanchez-Lozada L.G., Osorio-Alonso H. Anti-inflammatory therapy modulates nrf2-keap1 in kidney from rats with diabetes. Oxid Med Cell Longev. 2016:4693801. doi: 10.1155/2016/4693801. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jarrin Lopez A., Lau H., Li S., Ichii H. Potential benefits of nrf2/keap1 targeting in pancreatic islet cell transplantation. Antioxidants. 2020;9 doi: 10.3390/antiox9040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Oh C.J., Park S., Kim J.Y., Kim H.J., Jeoung N.H., Choi Y.K., Go Y., Park K.G., Lee I.K. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014;2:855–864. doi: 10.1016/j.redox.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Loewe R., Holnthoner W., Groger M., Pillinger M., Gruber F., Mechtcheriakova D., Hofer E., Wolff K., Petzelbauer P. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J. Immunol. 2002;168:4781–4787. doi: 10.4049/jimmunol.168.9.4781. [DOI] [PubMed] [Google Scholar]
- 278.Xie M.Y., Yang Y., Liu P., Luo Y., Tang S.B. 5-aza-2'-deoxycytidine in the regulation of antioxidant enzymes in retinal endothelial cells and rat diabetic retina. Int. J. Ophthalmol. 2019;12:1–7. doi: 10.18240/ijo.2019.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Choi K.C., Jung M.G., Lee Y.H., Yoon J.C., Kwon S.H., Kang H.B., Kim M.J., Cha J.H., Kim Y.J., Jun W.J., Lee J.M., Yoon H.G. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Canc. Res. 2009;69:583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 280.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 281.Zhang C., Su Z.Y., Khor T.O., Shu L., Kong A.N. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Correa F., Mallard C., Nilsson M., Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol. Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yan B., Cheng L., Jiang Z., Chen K., Zhou C., Sun L., Cao J., Qian W., Li J., Shan T., Lei J., Ma Q., Ma J. Resveratrol inhibits ROS-promoted activation and glycolysis of pancreatic stellate cells via suppression of miR-21. Oxid Med Cell Longev. 2018:1346958. doi: 10.1155/2018/1346958. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chen Q., Qiu F., Zhou K., Matlock H.G., Takahashi Y., Rajala R.V.S., Yang Y., Moran E., Ma J.X. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARalpha. Diabetes. 2017;66:1671–1682. doi: 10.2337/db16-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bagul P.K., Katare P.B., Bugga P., Dinda A.K., Banerjee S.K. SIRT-3 modulation by resveratrol improves mitochondrial oxidative phosphorylation in diabetic heart through deacetylation of TFAM. Cells. 2018;7 doi: 10.3390/cells7120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Li P., Zhang L., Zhou C., Lin N., Liu A. Sirt 1 activator inhibits the AGE-induced apoptosis and p53 acetylation in human vascular endothelial cells. J. Toxicol. Sci. 2015;40:615–624. doi: 10.2131/jts.40.615. [DOI] [PubMed] [Google Scholar]
- 287.Zeng Y., Yang K., Wang F., Zhou L., Hu Y., Tang M., Zhang S., Jin S., Zhang J., Wang J., Li W., Lu L., Xu G.T. The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp. Eye Res. 2016;151:203–211. doi: 10.1016/j.exer.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 288.Xu M.F., Xiong Y.Y., Liu J.K., Qian J.J., Zhu L., Gao J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin. 2012;33:578–587. doi: 10.1038/aps.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Polidoro L., Properzi G., Marampon F., Gravina G.L., Festuccia C., Di Cesare E., Scarsella L., Ciccarelli C., Zani B.M., Ferri C. Vitamin D protects human endothelial cells from H(2)O(2) oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6:221–231. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 290.Nebbioso M., Federici M., Rusciano D., Evangelista M., Pescosolido N. Oxidative stress in preretinopathic diabetes subjects and antioxidants. Diabetes Technol. Therapeut. 2012;14:257–263. doi: 10.1089/dia.2011.0172. [DOI] [PubMed] [Google Scholar]
- 291.Chous A.P., Richer S.P., Gerson J.D., Kowluru R.A. The diabetes visual function supplement study (DiVFuSS) Br. J. Ophthalmol. 2016;100:227–234. doi: 10.1136/bjophthalmol-2014-306534. [DOI] [PMC free article] [PubMed] [Google Scholar]