Abstract
Ion channels allow the flux of specific ions across biological membranes, thereby determining ion homeostasis within the cells. Voltage-gated potassium-selective ion channels crucially contribute to the setting of the plasma membrane potential, to volume regulation and to the physiologically relevant modulation of intracellular potassium concentration. In turn, these factors affect cell cycle progression, proliferation and apoptosis. The present review summarizes our current knowledge about the involvement of various voltage-gated channels of the Kv family in the above processes and discusses the possibility of their pharmacological targeting in the context of cancer with special emphasis on Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv10.1, and Kv11.1.
Keywords: Kv-channels, cancer, cell proliferation, mitochondria, cell death
Introduction
Potassium channels allow the flux of K+ down the electrochemical gradient across the plasma membrane as well as across membranes of intracellular organelles. Among the different types of K+ channels encoded by more than 80 genes, the voltage-gated potassium channels represent the largest and most complex family with numerous members, classified into Kv1-Kv12 subfamilies (Alexander et al., 2017). Most subfamilies have distinct channels (e.g., Kv1.1-Kv1.8, Kv2.1-Kv2.2, Kv3.1-Kv3.4, etc.) based on their different biophysical properties and pharmacological profile (Gutman et al., 2005; González et al., 2012). Kv5.1, Kv6s, Kv8s, and Kv9s encode subunits that have a regulatory role and are unable to form homotetramers on their own. Functional channel-forming alpha subunits harboring six transmembrane segments have been associated with different functions, ranging from ensuring cell excitability to the regulation of cell cycle. These proteins, expressed in different tissues, belong to six subfamilies named Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw), Kv4 (Shal), Kv7 (of which Kv7.1 is named KvLQT), and ether a go-go (EAG; Kv10, Kv11, Kv12). In some cases, members of a given subfamily can heteromultimerize to give a functional channel composed of four alpha subunits. Frequent alternate splicing and association with regulatory beta subunits further contributes to the enormous diversity of potassium channel functions. The crucial role of Kv channels in cellular and organ physiology is illustrated by the vast number of pathologies linked to their mutation [e.g., the long QT syndrome for Kv11.1 and Kv7.1 (Brewer et al., 2020), non-syndromic sensorineural deafness type 2 for Kv7.4 (Xia et al., 2020), etc.].
In addition to the Kv subfamilies whose members mediate efflux of potassium from the cell at depolarizing potentials (outward rectifiers), the so-called inward rectifiers (Kir) contribute to cells physiology by allowing greater influx than efflux of potassium ions at a comparable driving force. While Kv voltage-dependent potassium channels display an intrinsic gating thanks to the positively charged S4 transmembrane segment, inward rectification is caused by voltage-dependent block of the channel pore by cytoplasmic ions, including Mg2+ and polyamines. In addition, some of the calcium-dependent potassium channel family members (e.g., Big-conductance Ca2+-dependent K+ channel BKCa) are also activated by changes in membrane potential in addition of changes in intracellular [Ca2+] (González et al., 2012). Some members of the two-pore K+ channel family (e.g., some members of the TASK family) also show a slight voltage-dependence of their probability of being open (Enyedi and Czirjak, 2010).
In this review we will focus our attention on the members of the classical voltage-gated Kv channel family, who were among the first channels to be linked to the regulation of cell cycle (DeCoursey et al., 1984; McKinnon and Ceredig, 1986; Arcangeli et al., 1993; Pardo et al., 1998) and cell death (Szabo et al., 1996; Gómez-Angelats et al., 2000), both processes instrumental to cancer progression (for recent reviews see e.g., Pardo and Stuhmer, 2014; Serrano-Novillo et al., 2019; Capatina et al., 2020). Indeed, dysregulated cell cycle leading to unlimited proliferation as well as apoptosis resistance are two hallmarks of cancer cells (Hanahan and Weinberg, 2000). While the notion that intracellular voltage-gated K+ channels are also linked to cancer is emerging (e.g., Bachmann et al., 2019; Peruzzo and Szabo, 2019; Teisseyre et al., 2019), the present review focuses on the Kv channels located in the plasma membrane. Table 1 summarizes the major findings discussed throughout this review about the role of different Kv channels in cell proliferation, apoptosis induction and tumor growth. Furthermore, voltage-gated potassium channels as possible targets for pharmacological cancer therapy are discussed.
TABLE 1.
Targeting of Kv channels and its effects on cell death and proliferation.
| Kv1.1 |
|
|
| Kv1.3 |
|
|
| Kv1.5 |
|
|
| Kv2.1 |
|
|
| Kv3.1 |
|
|
| Kv3.4 |
|
|
| Kv7.4 |
|
|
| Kv10.1 |
|
|
| Kv11.1 |
|
|
Studies listed in the text in which direct modulation of channel expression or function (or both) was used to study their involvement in cell cycle progression and apoptosis induction are summarized. Papers in which an in vivo approach was also used are highlighted in bold.
Voltage-Gated Potassium Channels and the Regulation of Cell Cycle and Proliferation
Cell proliferation plays a fundamental role during embryogenesis, tissue renewal and remodeling as well as wound healing. The process is tightly regulated, and aberrant cell cycle progression leads to pathological conditions such as cancer. Voltage-gated potassium channels are important contributors to the mechanisms that control cell division. Because the topic has been extensively reviewed in the last years, we will only briefly summarize by which means Kv channels control cell cycle progression and show some recent advances in the field. The interested reader is referred to some excellent reviews [for general coverage of the topic, for instance (Blackiston et al., 2009; Urrego et al., 2014; Liu and Wang, 2019); regarding cancer (Leanza et al., 2016; Rao et al., 2015; Serrano-Novillo et al., 2019)]. Among the various voltage-gated channels, the most abundant information regarding their role in cell cycle regulation/proliferation is available about Kv1.1, Kv1.3 (for recent reviews see Pérez-Garcìa et al., 2018; Teisseyre et al., 2019), Kv1.5, Kv10.1 (Urrego et al., 2017), and Kv11.1 (He S. et al., 2020).
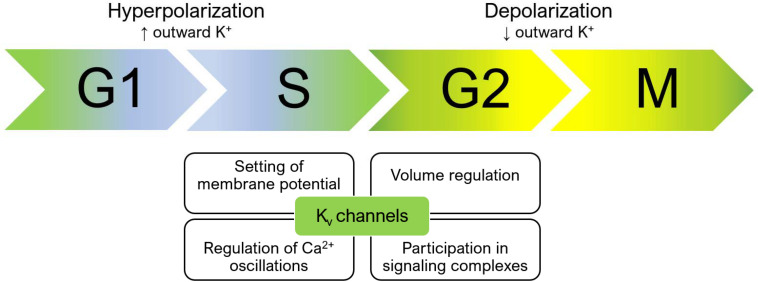
Voltage-gated potassium channels regulate the progression through cell cycle checkpoints based on both their ion-conducting properties and their interaction with other proteins belonging to signaling complexes at the plasma membrane (Figure 1). The membrane potential is not constant during the cell cycle. At the transition between the G1 and S phase, the plasma membrane hyperpolarizes, while a depolarization is necessary for cells to proceed from G2 to M (Blackiston et al., 2009). Cells with a high proliferation rate tend to be less polarized at each step of the cell cycle. Consequently, cancer cells generally exhibit a depolarized phenotype (Pardo and Stuhmer, 2014; Serrano-Novillo et al., 2019). Potassium channels, as important contributors to setting the membrane potential, regulate cell cycle progression. Their role in mitogenesis was first proposed in 1984, when Cahalan and his team found that potassium channel blockers inhibited the proliferation of T lymphocytes (DeCoursey et al., 1984).
FIGURE 1.
Membrane potential changes during cell cycle progression and the role of Kv channels. During the cell cycle, the membrane potential changes constantly. A hyperpolarization occurs during the transition from G1 to S, while the membrane depolarizes during the G2/M transition. Concomitantly, calcium oscillations in the cytoplasm and volume changes orchestrate the signaling events that occur at each of the different phases of the cell cycle. Kv channels regulate these processes by setting the membrane potential, thus influencing calcium influx and cell volume, and by participating in membrane signaling complexes. Accordingly, both their expression and function changes while cells proceed through the cycle. Please refer to the text for additional details.
Kv channels influence cell cycle progression not only by setting the membrane potential, but also, as a consequence, by contributing to ensuring the driving force for calcium entry (for reviews see e.g., Cahalan and Chandy, 2009; Urrego et al., 2014) and the exit of chloride ions (and the resulting cell shrinkage). Accordingly, Kv channel expression changes during the progression through the different phases of the cell cycle, as first revealed more than 30 years ago in T lymphocyte development (McKinnon and Ceredig, 1986). Changes in the subcellular localization of different Kv channels also seem to play a relevant role (Serrano-Novillo et al., 2019). Independently from their ion-conducting properties, also the interaction of specific Kv channels with proteins mediating intracellular signaling cascades regulates cell cycle progression (Urrego et al., 2014; He S. et al., 2020).
Kv1 Channels
As important regulators of cell proliferation, numerous reports have correlated the activity and expression of various potassium channels to the growth and progression of multiple kinds of cancers (recently reviewed in Serrano-Novillo et al., 2019). Kv1.1, along with Kv1.3, has been identified as a critical player to thymocyte pre-clonal expansion (Freedman et al., 1995). Later on, Kv1.1 was shown to be expressed in the breast cancer cell line MCF-7 and its blocker Dendrotoxin (DTX) (10 nM) reduced proliferation by 30% (Ouadid-Ahidouch et al., 2000). Likewise, DTX suppressed lung adenocarcinoma growth in vivo (Jang et al., 2011b) and at 100 nM concentration, it reduced proliferation of chemoresistant non-small cell lung cancer (NSCLC) cells both in vitro and in vivo (Jeon et al., 2012). Inhibition of Kv1.1 by KAaH2 toxin targeting specifically Kv1.1 was shown to inhibit proliferation of glioblastoma via the Epidermal Growth Factor Receptor (EGFR) signaling pathway (Aissaoui et al., 2018). In addition, Kv1.1’s high expression was observed to correlate with poor prognosis of cervical cancer patients and silencing of the channel blunted proliferation of HeLa cells (Liu et al., 2019).
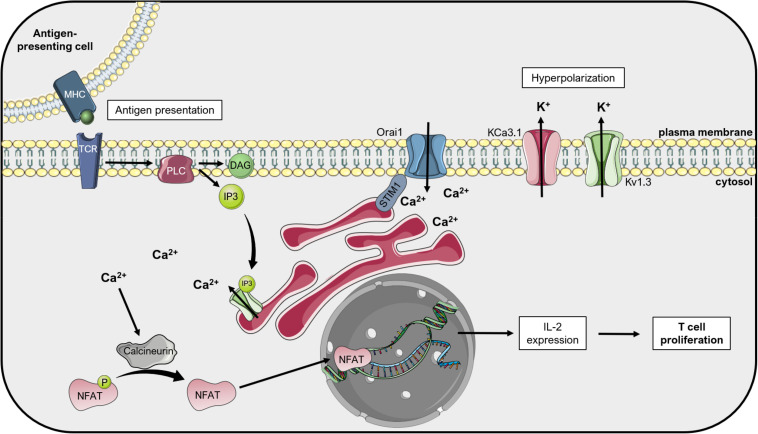
Inhibition of other Kv channels by specific toxins was also shown to reduce cancer cell proliferation and tumor size. In general, many studies addressed the relevance of Kv1.3 for proliferation in different cancer lines (e.g., Abdul et al., 2003; Preussat et al., 2003; Wu J. et al., 2013) or in primary cells (e.g., Smith et al., 2002; Grossinger et al., 2014; Petho et al., 2016). The proposed mechanism linking Kv1.3 function and its ability to set membrane potential to proliferation is summarized in Figure 2 for the case of lymphocytes. The same proliferation-promoting mechanism may apply for pathological lymphocytes, where upregulation of Kv1.3 ensures a continuous driving force for calcium entry into the cells. Margatoxin (MgTx), an inhibitor of Kv1.3 (but also of Kv1.1 and Kv1.2; Bartok et al., 2014), when injected directly into the xenograft nude mice model of A549 human lung adenocarcinoma at 1 nM concentration, significantly inhibited tumor growth. This was proposed to be ascribable to the effect of MgTx on cell cycle progression (Jang et al., 2011a). In addition to cancer, Kv1.3 activity has been correlated to the proliferation of many other types of cells, including vascular smooth muscle cells (Cidad et al., 2010; Cheong et al., 2011). Kv1.3 blockade with PAP-1 decreased the proliferation rate of these cells by downregulating receptor tyrosine kinases and the cell cycle regulator Early Growth Response-1 (EGR1) (Lasch et al., 2020). Further, a recent report highlighted that the cajanine derivative LJ101019C (1 μM), able to enhance Kv1.3 activity and expression, leads to natural killer (NK) cell proliferation and activation via AKT/mammalian Target Of Rapamycin (mTOR) signaling (Geng et al., 2020), representing thus a promising candidate for NK-based immunotherapy against cancer.
FIGURE 2.
Kv1.3 regulates T lymphocyte proliferation: the «membrane potential model». Upon activation of T cell receptors (TCR) by antigen-presenting cells, phospholipase C (PLC) cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 activates the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), which releases Ca2+ into the cytosol. Ca2+ depletion from the ER lumen leads to conformational changes of the ER-resident protein STIM1, which couples the ER to the plasma membrane and activates Orai1, a calcium release-activated Ca2+ channel (CRAC). The following increased Ca2+ concentration in the cytosol activates the phosphatase Calcineurin, which dephosphorylates the Nuclear Factor of Activated T cells (NFAT). This transcription factor translocates to the nucleus and activates the transcription of interleukin-2 (IL-2), thus inducing T cell proliferation. The calcium-activated potassium channel KCa3.1 and the voltage-dependent Kv1.3 alternately open during this process and give rise to the potassium efflux that hyperpolarizes the plasma membrane, thus providing the driving force for sustained Ca2+ influx (Teisseyre et al., 2019). This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
As to Kv1.5, this channel has been shown to be upregulated in many types of tumors and metastatic tissues and promotes proliferation. However, expression of Kv1.5 shows an inversed correlation with malignancy in some gliomas and non-Hodgkin’s lymphomas (Comes et al., 2013, 2015), while its high expression, along with that of Kv1.3, correlates with leiomyosarcoma proliferation and aggressiveness (Bielanska et al., 2012). In accordance, silencing Kv1.5 expression in osteosarcoma significantly inhibited proliferation and induced a cell cycle arrest at G0/G1 phase (Wu et al., 2015) and channel expression has been linked to proliferation in several works (see e.g., Vallejo-Gracia et al., 2013; Chow et al., 2018).
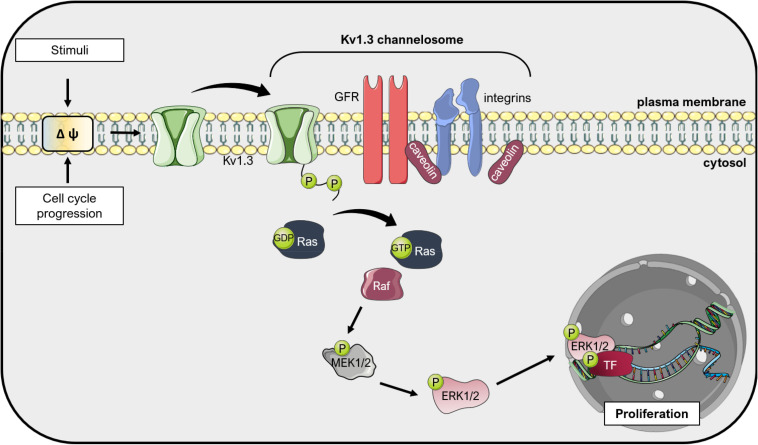
Altogether, a conclusive general picture regarding the involvement of Kv1.1, Kv1.3, and Kv1.5 in promoting cancer cell proliferation in different types of cancers where these channels are expressed is still lacking, such as the exact mechanism(s) involved in channel-mediated signaling leading to proliferation. These proteins can apparently promote proliferation also independently of their ion-conducting properties, but in function of the presence of two phosphorylation sites at the C-terminus of the channels (Figure 3; see e.g., Cidad et al., 2012; Jimenez-Perez et al., 2016). Only sporadic studies addressed the role of other Kv1. channels in proliferation (see e.g., Vautier et al., 2004).
FIGURE 3.
Kv1.3 regulates cell proliferation independently from ion conductance: the «voltage sensor model». Changes in the plasma membrane potential, that occur during cell cycle progression or upon application of external stimuli, induce a conformational change in Kv1.3 due to its voltage-sensor domain. Because Kv1.3 interacts with other proteins in large complexes (the so-called Kv1.3 channelosome), these conformational changes translate into phosphorylation of the C-terminus of Kv1.3 and activation of pro-proliferative signal transduction cascades, including integrin and growth factor receptor (GFR) signaling. These events result in activation of Ras, which activates the Raf/Mitogen-activated protein kinase kinase (MEK)/Extracellular signal-Regulated Kinase (ERK) pathway, finally leading to proliferation mediated by different downstream transcription factors (TF) such as c-Myc (Pérez-Garcìa et al., 2018). This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Kv2 Channels
Among channels of the Kv2. family, Kv2.1 (in heteromers with the silent subunit Kv9.3) seems to be the major component of Kv channels in cervical adenocarcinoma cells. Silencing of the Kv2.1 partner Kv9.3 using small interfering RNA caused G0/G1 cell cycle arrest in colon and lung carcinoma cell lines (Lee et al., 2015). The specific blocker of Kv2.1 Hanatoxin-1 significantly reduced proliferation of different cell lines by up to 40% (Suzuki and Takimoto, 2004). Another study showed that Ts15, a toxin from Tityus serrulatus, inhibited proliferation of central memory T cells, where Kv2.1 is highly expressed (Pucca et al., 2016), similarly to the hippocampus. In contrast, downregulation of Kv2.1 expression in HEK293 cells by Tacrine, a cholinesterase inhibitor, was able to boost proliferation (Hu X.M. et al., 2020). To our knowledge, Ts15 and Hanatoxin-1 have not been explored in the context of cancer in other studies.
Kv3 Channels
It has been reported that block of Kv3.1-specific currents or genetic ablation of the channel inhibits proliferation and migration of oligodendrocyte progenitor cells (Tiwari-Woodruff et al., 2006), while block of Kv3.4 resulted in a reduced proliferation rate of vascular smooth muscle cells (Miguel-Velado et al., 2005, 2010). Kv3.1 and Kv3.4 both contribute to lung adenocarcinoma and breast cancer cell migration and tumor invasiveness, although their inhibition did not affect cell proliferation (Song et al., 2018). On the other hand, a recent report elucidated the mechanism by which Kv3.4 channel is involved in oral squamous cell carcinoma growth (Qian et al., 2019). In this study, hypoxia was found to induce the Hypoxia-Inducible Factor (HIF-1α), which mediates increased Kv3.4 expression, proliferation, migration and invasion of oral squamous cell carcinoma cells. Kv3.4 also plays a role in mediating radio-resistance of myeloid leukemia cells by increasing its activity after application of ionizing radiation and inducing a G2/M arrest due to hyperpolarization of the membrane, calcium influx, Calcium/calmodulin-dependent protein Kinase (CamKII) activation and following inactivation of the phosphatase cdc25B and the cyclin-dependent kinase cdc2, both important for the G2/M transition (Palme et al., 2013).
Kv4 Channels
Kv4 channels have been characterized mainly in the context of neuronal function, but a recent work identified peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1), a prolyl isomerase that promotes cancer cell proliferation, as an interactor of Kv4.2 (Hu J.H. et al., 2020). The importance of this interaction was underlined in the context of cognitive flexibility, but not of cancer so far.
Kv7 Channels
The Kv7 channels are encoded by KCNQ genes and are expressed in cardiac myocytes, smooth muscle cells, neurons, and epithelial cells, where they play a role in various physiological processes (González et al., 2012), including that of regulating proliferation (Roura-Ferrer et al., 2008). A recent work highlighted Kv7.5 as promising therapeutic target for vascular tumors, as its expression clearly correlated with neoplastic malignancy (Serrano-Novillo et al., 2020). Interestingly, Tamoxifen, often used against breast cancer, inhibits Kv7.2 and Kv7.3 that are the main ion channels contributing to the so-called M-current, which regulates neuronal excitability (Ferrer et al., 2013). However, the role of this effect in the context of breast or other cancers has not been explored.
Kv10 and Kv11 Channels
In addition to the above-mentioned channels, work from different labs on Kv10.1 and Kv11.1 indicated their important role in cancer development and progression. The Kv10.1 channel was the first with proven oncogenic potential (Pardo et al., 1999) and is overexpressed in about 70% of human tumor biopsies (Urrego et al., 2014). Kv10.1 was proposed to be crucially involved in the resorption of the primary cilium during the G2/M phase of the cell cycle, thus favoring cell cycle progression (Urrego et al., 2017). As to Kv11.1, this channel has been associated to different tumoral processes such as cell cycle progression, angiogenesis, invasiveness and metastasis formation and the channel is also overexpressed in a variety of cancer cells with respect to corresponding non-cancer tissues (He S. et al., 2020). Indeed, implications of Kv11.1 on cell cycle and proliferation in gastric, pancreatic and breast cancer have already been established, and the channel was shown to influence tumor progression of esophageal squamous cell carcinoma, bladder cancer and osteosarcoma as well (Zeng et al., 2016; Xu et al., 2018; Wang et al., 2019). Recent results revealed that in esophaegal squamous cell carcinoma, Kv11.1 stimulates Phosphatidylinositol 3-Kinase (PI3K)/Akt, which leads to upregulation of thioredoxin domain-containing protein 5 (TXNDC5), thus increasing proliferation, inhibiting apoptosis and favoring epithelial-to-mesenchymal transition. Kv11.1 knockdown accordingly reduced tumor growth and metastasis formation in vivo (Wang et al., 2019). In bladder cancer, miR-96 was found to regulate Kv11.1 expression. Using a miR-96 inhibitor, Kv11.1 expression decreased, and cells were arrested at the G1 phase. miR-96 inhibition further increased apoptosis and decreased migration of bladder cancer cells (Xu et al., 2018). Similar effects on proliferation, migration and apoptosis were obtained in osteosarcoma cells by inhibition of Kv11.1, which reduced Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity through decreased PI3/Akt signaling (Zeng et al., 2016).
Voltage-Gated Potassium Channels and Regulation of Programmed Cell Death
Strikingly, most of the above-mentioned channels are intimately linked not only to proliferation, but also to the regulation of apoptosis (Szabo et al., 2010). Here, we discuss the role of Kv channels in cell death.
Kv1 Channels
Kv1.3 was one of the first ion channels whose function was shown to be modulated under various phases of apoptosis. In particular, the channel was shown to be inhibited within a few minutes following apoptosis induction by CD95 (Szabo et al., 1996) or by ceramide (Gulbins et al., 1997), due to tyrosine phosphorylation of the channel. This post-translational modification was shown to inhibit Kv1.3 activity also in other contexts (Holmes et al., 1996; Cayabyab et al., 2000). On the other hand, in a still early phase of apoptosis, the channel was shown to be activated by apoptotic stimuli and to contribute to the so-called apoptotic volume decrease in lymphocytes (Storey et al., 2003) that is associated with the activation of the apoptotic machinery (Bortner and Cidlowski, 2004, 2014). Further work is required to understand the importance of these findings, since Margatoxin, an inhibitor of Kv1.3, neither induced nor inhibited apoptosis in these studies. Nonetheless, using genetic models or silencing of Kv1.3, it was clearly shown that Kv1.3 expression is required for apoptosis (Bock et al., 2002), even in primary cells (Szabo et al., 2008), since in the absence of the channel the cells turned apoptosis-resistant. This finding might explain why the channel is found less expressed in several types of cancer tissue samples at advanced stage with respect to healthy tissues (Serrano-Novillo et al., 2019). Similar findings were reported for Kv1.1 in the same study. As a follow up of these findings, Kv1.3 was identified as functional channel in mitochondria (Szabo et al., 2005) and later studies identified the mitochondrial counterpart of the channel as a crucial player for intrinsic apoptosis (Szabo et al., 2011; Leanza et al., 2012a, 2017).
In addition to Kv1.3, other Kv1 channels have been linked to the apoptotic cascade (Szabo et al., 2010; Shah and Aizenman, 2014). For example, enhanced Kv1.1 expression protected hippocampal neurons against staurosporine- or glutamate-induced apoptosis (Lee et al., 2003; Shen et al., 2009). In contrast, downregulation of specifically Kv1.1 and of Kv1.3 expression in retinal ganglion cells (but not of Kv1.2 and Kv1.5) rescued neurodegeneration of these cells. In agreement, Margatoxin reduced cell death in this system, where Kv1.1 depletion was shown to increase the expression of the antiapoptotic Bcl-XL, while depletion of Kv1.3 reduced the pro-apoptotic caspase-3, caspase-9 and Bad (Koeberle et al., 2010). Thus, also in the case of Kv1.1, further work is required to understand the different contribution of this channel to degenerative death in distinct cell types.
Since Kv1.5 is overexpressed in many types of cancer cells (Comes et al., 2013), it represents a good target in this context. Indeed, the role of Kv1.5 in apoptosis has been extensively studied. Bonnet and colleagues correlated low Kv1.5 expression in cancer cells to apoptosis resistance (Bonnet et al., 2007). In particular, these authors observed that metabolic shift toward oxidative phosphorylation and the consequent reactive oxygen species release from mitochondria activates plasma membrane Kv1.5 with a resulting reduction of proliferation and induction of apoptosis. On the other hand, a more recent work showed that silencing Kv1.5 expression in osteosarcoma cells not only reduces proliferation by blocking the cell cycle at G0/G1 phase, as expected, but also enhances apoptosis through upregulation of Bax and caspase-3 and downregulation of anti-apoptotic Bcl-2 and Bik (Wu et al., 2015). Pharmacological inhibition of Kv1.5 by diphenyl phosphine oxide-1 (DPO-1) contributed to apoptosis induction in macrophages (Leanza et al., 2012b). In another study however, silencing of Kv1.5 with siRNA reduced palmitate-induced endothelial apoptosis (Du et al., 2017). In a recent investigation, inhibition of Kv1.5 by DPO-1 prevented the mitochondria-dependent apoptosis-triggering effect of Apigenin, a dietary flavonoid, in pulmonary artery smooth muscle cells (He Y. et al., 2020). Similarly, DPO-1 reduced hydrogen peroxide-evoked endothelial cell apoptosis (Chen et al., 2012).
In summary, while considerable experimental work links plasma membrane Kv channels to apoptosis, contrasting findings about their expression level in cancer cells as well as their involvement in the regulation of the apoptotic pathway deserves attention. While expression levels might change depending on the cancer stage (with downregulation of the channels associated with apoptosis-resistance in advanced stage and/or metastatic cells), the different outcome on apoptosis might be tentatively explained by modulation of channel activity by yet-undefined factors such as protein interaction partners, whose nature might change depending on the type/stage of cancer.
Kv2 Channels
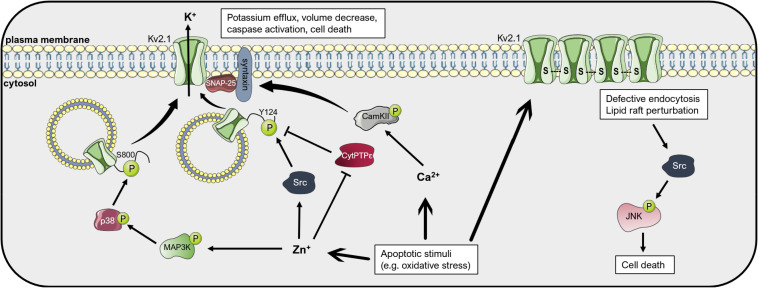
The Kv2.1 channel has been extensively studied in the context of neuronal apoptosis (Figure 4). An involvement of Kv2.1 in the initiation of programmed cell death has already been proposed in 2001, when Ekhterae and colleagues found that an overexpression of Bcl-2 in rat pulmonary artery smooth muscle cells inhibited staurosporin-induced apoptosis by downregulating Kv1.1, Kv1.5, and Kv2.1 expression and reducing the potassium efflux that initiates the apoptotic volume decrease (Ekhterae et al., 2001). The importance of Kv2.1 for programmed cell death of cortical neurons was first shown by Pal and co-workers. They demonstrated that the expression of dominant-negative Kv2.1 in cortical neurons abolished the potassium efflux that characterizes the onset of apoptosis and increased the resistance to apoptotic stimuli induced by the oxidant 2,2′-dithiodipyridine (DTDP) or staurosporine. Accordingly, the transient expression of functional Kv2.1 in Chinese hamster ovary (CHO) cells increased the susceptibility to DTDP treatment and caspase-dependent cell death (Pal et al., 2003). The same group identified compound 48F10 that inhibits Kv2.1 in the low micromolar range and confers resistance to DTDP treatment in cortical neurons and enterocytes (Zaks-Makhina et al., 2004; Grishin et al., 2005). Upon application of apoptotic stimuli, an increased trafficking of Kv2.1 to the plasma membrane is responsible for the augmented outgoing current. The de novo insertion of Kv2.1 channels in the cell membrane depends on syntaxin and synaptosomal-associated protein (SNAP-25), proteins belonging to the SNAP receptor (t-SNARE) complex, normally responsible for exocytotic neurotransmitter release (Pal et al., 2006; Yao et al., 2009; McCord et al., 2014). The interaction of Kv2.1 with syntaxin and its insertion in the plasma membrane upon oxidative stress signals also requires the action of CamKII. Calcium thus emerges as a regulator of the potassium current surge at the onset of neuronal apoptosis (McCord and Aizenman, 2013). Interestingly, targeting the Kv2.1-syntaxin interaction with a peptide mimicking the syntaxin-1A binding domain of Kv2.1 ameliorated cell death in an in vivo model of ischemic stroke (Yeh et al., 2017).
FIGURE 4.
Mechanisms of Kv2.1-mediated cell death. Apoptotic stimuli such as oxidative stress lead to an increase in cytosolic free Zn2+ and Ca2+ concentrations. Zn2+ induces the activation of the kinases p38 and Src, which phosphorylate the intracellular pool of Kv2.1 at the residues Ser800 and Tyr124, respectively. These phosphorylations lead to insertion of Kv2.1 in the plasma membrane, an increase in the outgoing K+ current, apoptotic volume decrease and cell death. Plasma membrane insertion of the channel depends on interaction with the t-SNARE proteins SNAP-25 and syntaxin. The latter interacts with CamKII, activated upon an increase in Ca2+, favoring Kv2.1 localization to the membrane. Kv2.1 can induce apoptosis also in an ion-conducting-independent manner. Oxidative stress favors oligomerization of the protein by formation of disulfide bridges, which leads to defective endocytosis and lipid raft perturbation, resulting in activation of the Src-JNK signaling axis, finally inducing apoptosis. Please refer to the text for references and additional details. This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Membrane trafficking of Kv2.1 is regulated by multiple phosphorylation events (Figure 4). For example, activation of the mitogen-activated protein (MAP) kinase p38 is necessary for the induction of neuronal apoptosis after oxidative injury. By phosphorylating a serine residue at the C-terminal of the channel protein (S800), active p38 leads to membrane insertion of Kv2.1 and the potassium current surge that initiates cell death, possibly by changing the association of Kv2.1 with other proteins (such as syntaxin) (Redman et al., 2007). p38 activation, in turn, depends on an intracellular zinc increase caused by oxidants such as peroxynitrite (Zhang et al., 2004). Intracellular zinc leads, in addition to p38 activation, also to inhibition of the phosphatase Cyt-PTPε that in standard conditions dephosphorylates Kv2.1 at a tyrosine residue at the N-terminal (Y124). Src kinase is responsible for phosphorylation of the channel at Y124, enhancing the Kv2.1-mediated current at the onset of apoptosis (Redman et al., 2009). Phosphorylation at S800 and Y124 mutually co-regulate each other, with P-Y124 facilitating the action of p38 on S800 and vice versa (He et al., 2015). Additionally, serum deprivation, which induces apoptosis of cortical neurons in a model of excitotoxicity, leads to N-Methyl-d-aspartate (NMDA) receptor activation and enhanced plasma membrane expression of Kv2.1 due to dephosphorylation of the channel protein by the Protein Phosphatase 1 (PP1) and/or 2A (PP2A) (Yao et al., 2009). It was further shown that activation of Protein Kinase A (PKA) following an increase in cyclic AMP protects cerebellar granular neurons from cell death induced by potassium-low and serum-free medium (Jiao et al., 2007). In this study, PKA activation decreased the Kv2.1-dependent outgoing potassium current upon application of the pro-apoptotic stimulus, likely by reducing Kv2.1 expression. Cleavage of Kv2.1 can also affect cell death induction. Liu et al. recently showed that beta-secretase 2 (BACE2), a protease that frequently shows increased expression in Alzheimer’s disease, cleaves Kv2.1 at three different sites, reducing the current surge at the onset of apoptosis and protecting neurons from cell death (Liu et al., 2018).
The complex interplay between Kv2.1 and cell death plays an important role in different diseases. In many central nervous system (CNS) disorders, microglia are involved in promoting neurodegeneration. The mechanisms described above partly account for the neurodegenerative effect of microglia: Knoch et al. (2008) showed that activated microglia release reactive oxygen and nitrogen species, which increase the intracellular zinc concentration in co-cultured neurons, finally leading to p38 activation, a surge in Kv2.1-mediated potassium currents and induction of cell death. In addition to neurodegeneration, p38 activation and apoptosis induction play a relevant role also in hepatitis virus C (HVC) infections. The viral protein NS5A blocks oxidative stress-mediated p38 activation by directly binding and blocking the upstream MAPK kinase kinase MLK3, thus effectively inhibiting apoptosis initiation via Kv2.1 and favoring the survival of HCV-infected hepatoma cells even in the presence of pro-apoptotic stimuli (Mankouri et al., 2009; Amako et al., 2013). Interestingly, also expression of NS5A1b in neurons is protective as it inhibits Kv2.1-mediated apoptosis, although in a Src-dependent manner (Norris et al., 2012). Human immunodeficiency virus 1 (HIV-1) is involved in neuronal apoptosis as well. Its envelope glycoprotein gp120 has been associated to the pathogenesis of HIV-1-associated neurodegenerative disorders by acting on Kv2.1. In hippocampal neurons, gp120 was shown to increase Kv2.1 expression and current density in a p38- and caspase-3-dependent manner, thus enhancing apoptosis (Shepherd et al., 2012, 2013; Liu et al., 2013; Zhu et al., 2015). p38 plays a role also in methamphetamine-induced neuronal damage. This addictive drug exhibits strong neurotoxicity, inducing apoptosis via activation of p38, upregulation of Kv2.1 and cleavage of caspase-3 (Zhu et al., 2018).
Oxidative stress has been associated to different conditions that involve neuronal damage, including Alzheimer’s (AD) and Parkinson’s disease (PD), aging, amyotrophic lateral sclerosis and stroke (Radi et al., 2014). It has been shown that carbon monoxide (CO) protects neurons from oxidant-induced apoptosis by inhibition of Kv2.1, and that this inhibition is in part exerted by mitochondrial reactive oxygen species (ROS) (Dallas et al., 2011). Kv2.1 inhibition exerted by CO may also play a relevant role in the resistance mechanism against oxidative stress of medulloblastoma cells. In fact, the hypoxic environment of tumors favors the constitutive expression of heme oxygenase 1 (HO-1), which catalyzes heme and releases CO as a byproduct, finally leading to Kv2.1 inhibition and apoptosis resistance (Al-Owais et al., 2012). Interestingly, Donepezil, an acetylcholinesterase inhibitor used in AD, was shown to inhibit Kv2.1 with an IC50 value of 7.6 μM and may thus add protection against neuronal cell death (Yuan et al., 2011). Direct oxidation of Kv2.1 has also been proposed as a mechanism of neurotoxicity (Figure 4). Cotella et al. (2012) found in both in vitro and in vivo settings that an oxidative environment leads to channel oligomerization at the plasma membrane due to the formation of disulfide bridges between channel subunits. These oligomers, although exhibiting decreased open probability, triggered apoptotic cell death. Kv2.1 oligomerization was greatly increased in the brain of a mouse model of AD. In addition, application of amyloid-β to cultured cells induced the formation of oligomers and cell death (Cotella et al., 2012). Kv2.1 oligomerization resulted in defective endocytosis, lipid raft perturbation and stress-activated c-Src/c-Jun N-terminal kinase (JNK) signaling which finally initiated apoptosis. Cholesterol, by stabilizing lipid rafts, could revert the onset of apoptosis (Wu X. et al., 2013). Accordingly, transgenic mice harboring a non-oxidizable Kv2.1 mutant showed decreased inflammation, neurodegeneration and cell death after traumatic brain injury and exhibited improved outcomes in cognitive and motor behavioral assays with respect to control mice (Yu et al., 2016). Importantly, the results from these experiments were mimicked by treatment of control mice with Dasatinib, a Src kinase inhibitor. The findings of these studies suggest that under oxidizing conditions, such as during aging or neurodegenerative diseases, Kv2.1 channels play a critical role in the regulation of cell death in a manner that may be independent from their ion-conducting properties.
In addition to its well-established role in neuronal apoptosis, Kv2.1 is involved in the cell death regulation of the neuroendocrine pancreatic β-cells. In these cells, Kv2.1 channel controls glucose-stimulated insulin secretion and death induction. The incretin hormones Gastric Inhibitory Peptide (GIP) and Glucagon-like peptide-1 (GLP-1) have been found to profoundly alter Kv2.1 posttranslational modifications, promoting the channel’s internalization and promoting cell survival in stress conditions (Kim et al., 2012). Additionally, the small-molecule inhibitors of Kv2.1 vindoline, SP6616 and ETA were shown to improve β-cell dysfunction and survival and ameliorate hyperglycemia in vivo, suggesting that Kv2.1 inhibition may be exploited in anti-diabetic therapies (Yao et al., 2013; Zhou et al., 2016, 2018).
Kv3 and Kv4 Channels
Similarly to Kv2.1, some reports assign a role in the regulation of neuronal apoptosis to Kv3.4. For example, amyloid-β has been found to increase both Kv3.4 expression and activity in hippocampal neurons through NF-κB and to induce cell death, an effect that could be reverted by a pan-Kv3 inhibitor (Pannaccione et al., 2007). Similarly, Kv3.4 downregulation exerted by HIF-1α under oxidative stress reduced the potassium current and exhibited a neuroprotective effect in the neuroblastoma cell line SH-SY5Y (Song et al., 2017). Interestingly, the authors of this study suggest an involvement of mitochondrial Kv3.4 in the regulation of oxidative stress-induced neuronal damage. Amyloid-β peptides were also found to increase the expression of Kv4.2 and Kv4.3 channels, leading to increased potassium currents and apoptosis of cerebellar granule cells (Pieri et al., 2010).
Kv7 Channels
Regarding Kv7 channels, their inhibition was demonstrated to trigger spiral ganglion neuron death, possibly underlying the autosomal dominant version of progressive hearing loss associated with Kv7.4 mutations (Lv et al., 2010). On the other hand, activation of Kv7.2 and Kv7.3 in hippocampal neurons induced Extracellular signal-Regulated Kinase (ERK) 1/2 activation and caspase-3 cleavage, an effect that could be prevented by the pan-Kv7 blocker XE991 (Zhou et al., 2010). Interestingly, blockade of Kv7 channels by amyloid precursor proteins may regulate neuronal excitation and possibly cell death (Lee et al., 2020).
Kv10 and Kv11 Channels
Voltage-gated potassium channels belonging to the ether-à-go-go (EAG) family are also involved in the regulation of cell death. This family comprises three subgroups, namely EAG (aka Kv10), Eag-Related Gene (ERG, aka Kv11), and Eag-Like (ELK, aka Kv12). While Kv10 and Kv12 channels are expressed primarily in the central nervous system, Kv11 is present also in the heart, where it regulates the termination of the cardiac action potential, and in smooth muscle tissues (Barros et al., 2020). Ion channels belonging to the EAG family have been intensively studied in the context of cancer. Their expression is frequently high in a wide range of tumor types, while lacking in the corresponding non-tumoral tissues (He S. et al., 2020). Although, to our knowledge, in-depth mechanistic studies regarding the involvement of EAG channels in the regulation of apoptosis are missing, different studies suggest that channel activity may either promote or block the induction of cell death depending on the cell type and the environment. Studies in which targeting Kv10 or Kv11 channels was exploited to induce tumor cell death will be analyzed more in detail below.
Targeting Voltage-Gated Potassium Channels in Cancer
Although several types of Kv channels are linked to either altered proliferation, or to apoptosis induction, concrete steps toward cancer treatment have been obtained only in few cases. This situation is most probably due to our limitation of having promiscuous pharmacological inhibitors as tools to modulate the function of these channels. Nonetheless, the use of toxins, small molecules and specific antibodies is emerging as promising strategy (for recent review see e.g., Wulff et al., 2019; Díaz-García and Varela, 2020).
Kv1 Channels
Intratumoral injection of Margatoxin, a Kv1.3 inhibitor acting on the plasma membrane Kv1.3, reduced lung cancer volume in vivo (Jang et al., 2011a), while intraperitoneal injection of PAP-1, the small molecule inhibitor of this channel, did not reduce tumor volume in an orthotopic melanoma model (Leanza et al., 2017; Peruzzo et al., 2020). Two toxins, acting on Kv1.1 (KAaH2) and Kv1.3 (KAaH1 that acts on Kv1.1 as well) derived from the Androctonus australis Hector venom, were shown to inhibit glioma proliferation and migration, at least in vitro (Aissaoui et al., 2018). A disadvantage of these toxins, however, is their inability to cross the blood brain barrier and to act on intracellular potassium channels. Instead, recent evidence indicates that mitochondrial ion channels can be of importance in fighting cancer. For example, pharmacological inhibition of the mitochondria-located counterpart of Kv1.3 channel by specific mitochondria-targeted drugs was shown to trigger apoptotic death selectively in cancer cells in vivo, without inducing alterations to healthy cells and tissues (Leanza et al., 2017).
Kv10.1
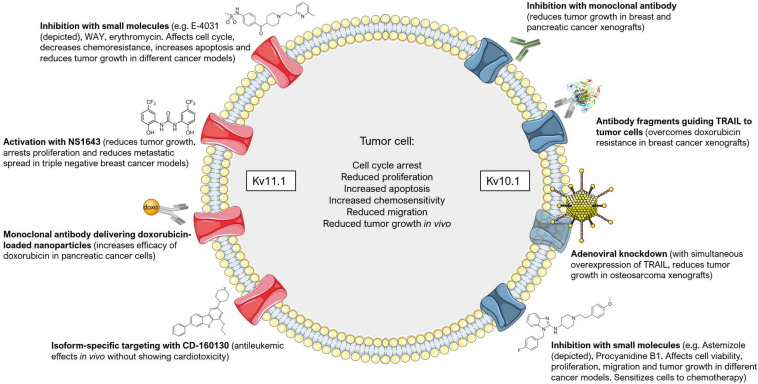
Different strategies have been exploited to specifically target Kv10.1 and Kv11.1 channels in order to either block proliferation of cancer cells or to induce their apoptosis, as summarized in Figure 5. Given the structural similarity between the potassium channel superfamily, specific blockers are difficult to obtain, therefore some of the research has focused on antibody-based or genetic strategies. For example, a monoclonal antibody raised against Kv10.1 dose-dependently inhibited Kv10.1 currents in neuroblastoma cells, decreased the proliferation of different human cancer cell lines and reduced tumor growth in xenograft models of breast and pancreatic cancer, notably, without acting on Kv10.2 or Kv11.1 (Gomez-Varela et al., 2007). Additionally, specific small antibody fragments targeting the extracellular pore domain of Kv10.1 were exploited to selectively guide the Tumor necrosis factor-Related Apoptosis-Inducing Ligand (TRAIL) to tumor cells. These constructs sensitized breast cancer cells to chemotherapeutics they were otherwise resistant to Hartung and Pardo (2016), or directly induced cell death in prostate and pancreatic cancer cells when fused to a TRAIL variant with enhanced pro-apoptotic activity (Hartung et al., 2020). This antibody-based strategy was proven efficacious also in vivo (Hartung and Pardo, 2016). The specificity of this treatment relies on the enhanced expression of Kv10.1 in many cancer types (Pardo et al., 1999; Pardo and Stuhmer, 2014) and the tumor selectivity of TRAIL. Similarly, treating the human osteosarcoma cell line MG-63 or mice bearing an osteosarcoma xenograft with adenoviral vectors that simultaneously knockdown Kv10.1 and overexpress TRAIL led to tumor regression and apoptosis of cancer cells (He et al., 2013).
FIGURE 5.
Targeting Kv10.1 and Kv11.1 in cancer. Different approaches have been employed to target Kv10.1 (on the right) and Kv11.1 (on the left) in tumor models. The most common effects that are induced by channel inhibition, activation (in the case of Kv11.1) or downregulation are summarized in the center. Please refer to the text for additional details and references. This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Another strategy for the treatment of cancer that has gained increased interest in recent years is drug repositioning using clinically safe drugs already exploited for some other disease. Regarding Kv10.1, the anti-histaminic drug Astemizole was found to inhibit this channel with an IC50 value of only 200 nM (García-Ferreiro et al., 2004). This compound was associated with a reduced cancer mortality by sensitizing cells to chemotherapy (Ellegaard et al., 2016). It reduced, for instance, proliferation and viability of keratinocytes expressing the human papilloma virus (HPV) oncogenes E6/E7, that mimic cervical cancer cells and express Kv10.1 (Diaz et al., 2009). The same drug was used to target proliferation and viability of hepatocellular carcinoma cells in vitro and in an in vivo mouse model, where it decreased Kv10.1 expression (de Guadalupe Chávez-López et al., 2015). Furthermore, Astemizole increased the anti-proliferative effects of calcitriol on breast cancer both in vitro and in vivo by acting also on Kv10.1 (García-Quiroz et al., 2012, 2014) and increased the sensitivity of lung cancer cell lines to the EGFR inhibitor Gefitinib possibly by acting on both Kv10.1 subcellular localization and expression (de Guadalupe Chávez-López et al., 2017). Knockdown of Kv10.1 or its inhibition by Astemizole sensitized glioblastoma cells to Temozolomide treatment, and SH-SY5Y cells to rotenone-induced apoptosis (Sales et al., 2016; Horst et al., 2017). Astemizole thus represents a valid candidate for drug repositioning (Figure 5).
Natural compounds are also promising tools. Very recently, the natural compound Procyanidin B1 was identified as a rather specific inhibitor of Kv10.1. It reduced the potassium current with an IC50 value of 10 μM, suppressed proliferation and migration of hepatoma cells and inhibited tumor growth in vivo in a xenograft model of liver cancer (Na et al., 2020). In addition, the only Kv10.1-specific toxin, k-Hefutoxin 1 from Heterometrus fulvipes scorpion venom (Moreels et al., 2017), might be useful in the context of cancer therapy, but to our knowledge no attempts have been made so far to test its in vitro and in vivo effects (Díaz-García and Varela, 2020).
Kv11.1
Although representing an interesting target for cancer therapy, Kv11.1 inhibitors have limited use in the clinic because they are potentially cardio-toxic. Kv11.1 is expressed in the heart, where it regulates the cardiac action potential, and the use of channel blockers has been associated with cardiac arrhythmia (extensively reviewed in Arcangeli and Becchetti, 2010). Nonetheless, some studies achieved promising results with channel activators or blockers (Figure 5). For example, the incidental administration of FDA-approved drugs with proven inhibitory activity on Kv11.1 increased the survival of glioblastoma patients that showed a high expression of Kv11.1 (Pointer et al., 2017). These drugs did not have any adverse side-effect on cardiac activity and include Phenytoin, Haloperidol, Fluoxetine, Tamoxifen, Amitriptyline, and Ketoconazole (Pointer et al., 2017). In acute lymphoblastic leukemia (ALL) cells, the Kv11.1 inhibitors E-4031, WAY123,398 and erythromycin (which, importantly, shows no cardiotoxicity) decreased the chemoresistance of these cells and increased the pro-apoptotic effect of the chemotherapeutics Doxorubicin, Prednisone and Methotrexate. E-4031 significantly reduced tumor progression and improved survival also in vivo (Pillozzi et al., 2011). Simultaneous activation of the calcium- and voltage-dependent potassium channel KCa3.1 with SKA-31 and inhibition of Kv11.1 with E-4031 further sensitized colorectal cancer cells to cisplatin treatment both in vitro and in vivo (Pillozzi et al., 2018). Erythomycin was also shown to increase the cytotoxic effect of paclitaxel, vincristine and hydroxy-camptothecin on different cancer cell lines that express Kv11.1 and induce an arrest in the G2/M phase (Chen et al., 2005). Similarly, the gastroprokinetic drug cisapride was shown to inhibit Kv11.1 at low nanomolar concentrations and, accordingly, arrested cell growth and increased apoptosis in Kv11.1-expressing gastric cancer cell lines (Shao et al., 2005). Further, inhibition of Kv11.1 by doxazosin led to apoptosis and cell cycle arrest in the G0/G1 phase of glioblastoma cells. The effect of doxazosin was mimicked by siRNA-mediated knockdown of the channel (Bishopric et al., 2014).
Interestingly, two main isoforms are known for Kv11.1, namely Kv11.1A and Kv11.1B. While Kv11.1A is expressed predominantly in the heart, Kv11.1B is the prevalent isoform in leukemia. This peculiarity has been exploited by Gasparoli et al. (2015), that characterized compound CD-160130, a small-molecule inhibitor of Kv11.1 that does not cause cardiac arrhythmia, possibly by blocking isoform B with a higher efficiency than isoform A (Figure 5). CD-160130 caused apoptosis and growth arrest of leukemic cells in vitro and showed strong antileukemic effects in vivo (Gasparoli et al., 2015). The higher expression of Kv11.1 in cancer cells with respect to healthy tissues (Pardo and Stuhmer, 2014) was also exploited by Spadavecchia and colleagues. They engineered polyethylene-glycol gold nanoparticles fused to an anti-Kv11.1 antibody to selectively deliver doxorubicin to pancreatic cancer cells (Spadavecchia et al., 2016). In addition, the antibiotic clarithromycin, whose mammalian targets include Kv11.1, was shown to induce apoptotic cell death and increase the cytotoxic effects of 5-fluorouracil both in vitro and in vivo in colorectal cancer models (Petroni et al., 2020). Finally, to avoid cardiac side-effects, Gentile and co-workers proposed an opposite approach to Kv11.1 channel blockers. They used a small-molecule activator of the channel, NS1643, to achieve in vivo inhibition of tumor growth of triple-negative breast cancer (Fukushiro-Lopes et al., 2017; Figure 5). In their model, prolonged activation of the channel leads to hyperpolarization, increase in ROS production, DNA damage, activation of a senescence program and arrest of proliferation. Importantly, NS1643 did not cause cardiac arrythmias. In a subsequent study, they showed NS1643 to reduce the metastatic spread in vivo in breast cancer models and to reprogram epithelial-to-mesenchymal transition and cancer stemness of MDA-MD-231 cells by reducing Wnt/β-catenin signaling (Breuer et al., 2019). However, possible side effects of NS1643, other than cardiotoxicity, have not been investigated in a systematic way in vivo.
In addition, toxins might offer a way of intervention: CsEKerg1 toxin, from the Centruroides sculpturatus scorpion, inhibits Kv11.1 currents in neuroblastoma cells, although with relatively low affinity (Nastainczyk et al., 2002). Unfortunately, this toxin has not been tested on brain-derived tumor cell proliferation (Díaz-García and Varela, 2020).
Conclusion and Future Outlook
Collectively, these studies demonstrate that several ion channels are attractive targets for cancer therapy and regulation of the immune response. As outlined in the present review, many channels share at least some functional aspects with other channels and it might be necessary to define combinations of drugs acting on ion channels for successful tumor treatment. In addition, many channels are expressed in several cell types and it remains to be defined whether channels can be selectively targeted in tumor cells. Altogether, taking into account the information reported here, Kv10 and Kv11 family members seem to represent the most promising target among the plasma-membrane located voltage-gated potassium channels, although the issues of specificity and possible cardiotoxicity warrants caution for Kv10- and Kv11-targeting drugs, respectively. Finally, most channels that were studied so far localize to the plasma membrane or mitochondria, but it is certainly possible that ion channels in other organelles, for instance lysosomes, also play an important role in tumor cell growth or can be exploited as novel targets for tumor therapy in the future.
Author Contributions
MB, IS, and EG: writing of the manuscript. WL, ME, SAA, and SP: commenting and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to respective laboratory members for useful discussion.
Footnotes
Funding. The research in the authors laboratories were funded by Italian Association for Cancer Research (IG2017 20286 to IS) and Italian Ministry of University and Education (PRIN 20174TB8KW_004 to IS) and DFG GU 335/29-2 and Sander Stiftung grant 2019.115.1 to EG.
References
- Abdul M., Santo A., Hoosein N. (2003). Activity of potassium channel-blockers in breast cancer. Anticancer Res. 23 3347–3351. [PubMed] [Google Scholar]
- Aissaoui D., Mlayah-Bellalouna S., Jebali J., Abdelkafi-Koubaa Z., Souid S., Moslah W., et al. (2018). Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int. J. Biol. Macromol. 111 1146–1155. 10.1016/j.ijbiomac.2018.01.144 [DOI] [PubMed] [Google Scholar]
- Alexander S. P., Striessnig J., Kelly E., Marrion N. V., Peters J. A., Faccenda E., et al. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: voltage-gated ion channels. Br. J. Pharmacol. 174 (Suppl. 1), S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Owais M. M., Scragg J. L., Dallas M. L., Boycott H. E., Warburton P., Chakrabarty A., et al. (2012). Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J. Biol. Chem. 287 24754–24764. 10.1074/jbc.M112.357012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako Y., Igloi Z., Mankouri J., Kazlauskas A., Saksela K., Dallas M., et al. (2013). Hepatitis C virus NS5A inhibits mixed lineage kinase 3 to block apoptosis. J. Biol. Chem. 288 24753–24763. 10.1074/jbc.M113.491985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A., Becchetti A. (2010). New trends in cancer therapy: targeting ion channels and transporters. Pharmaceuticals 3:1202–1224. 10.3390/ph3041202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A., Becchetti A., Mannini A., Mugnai G., De Filippi P., Tarone G., et al. (1993). Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J. Cell Biol. 122 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M., Pontarin G., Szabo I. (2019). The contribution of mitochondrial ion channels to cancer development and progression. Cell. Physiol. Biochem. 53 63–78. 10.33594/000000198 [DOI] [PubMed] [Google Scholar]
- Barros F., de la Peña P., Domínguez P., Sierra L. M., Pardo L. A. (2020). The EAG voltage-dependent K(+) channel subfamily: similarities and differences in structural organization and gating. Front. Pharmacol. 11:411 10.3389/fphar.2020.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok A., Toth A., Somodi S., Szanto T. G., Hajdu P., Panyi G., et al. (2014). Margatoxin is a non-selective inhibitor of human Kv1.3 K+ channels. Toxicon 87 6–16. 10.1016/j.toxicon.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Bielanska J., Hernández-Losa J., Moline T., Somoza R., Ramón Y. C. S., Condom E., et al. (2012). Increased voltage-dependent K(+) channel Kv1.3 and Kv1.5 expression correlates with leiomyosarcoma aggressiveness. Oncol. Lett. 4 227–230. 10.3892/ol.2012.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopric N. H., Staudacher I., Jehle J., Staudacher K., Pledl H.-W., Lemke D., et al. (2014). Herg K+ channel-dependent apoptosis and cell cycle arrest in human glioblastoma cells. PLoS One 9:e0088164. 10.1371/journal.pone.0088164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D. J., McLaughlin K. A., Levin M. (2009). Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8 3519–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J., Szabó I., Jekle A., Gulbins E. (2002). Actinomycin D-induced apoptosis involves the potassium channel Kv1.3. Biochem. Biophys. Res. Commun. 295 526–531. 10.1016/s0006-291x(02)00695-2 [DOI] [PubMed] [Google Scholar]
- Bonnet S., Archer S. L., Allalunis-Turner J., Haromy A., Beaulieu C., Thompson R., et al. (2007). A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11 37–51. 10.1016/j.ccr.2006.10.020 [DOI] [PubMed] [Google Scholar]
- Bortner C. D., Cidlowski J. A. (2004). The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 448 313–318. 10.1007/s00424-004-1266-5 [DOI] [PubMed] [Google Scholar]
- Bortner C. D., Cidlowski J. A. (2014). Ion channels and apoptosis in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130104. 10.1098/rstb.2013.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer E. K., Fukushiro-Lopes D., Dalheim A., Burnette M., Zartman J., Kaja S., et al. (2019). Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis. 10:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer K. R., Kuenze G., Vanoye C. G., George A. L., Jr., Meiler J., Sanders C. R. (2020). Structures illuminate cardiac ion channel functions in health and in long QT syndrome. Front. Pharmacol. 11:550. 10.3389/fphar.2020.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G. (2009). The functional network of ion channels in T lymphocytes. Immunol. Rev. 231 59–87. 10.1111/j.1600-065X.2009.00816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capatina A. L., Lagos D., Brackenbury W. J. (2020). Targeting ion channels for cancer treatment: current progress and future challenges. Rev. Physiol. Biochem. Pharmacol. 10.1007/112_2020_46 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Cayabyab F. S., Khanna R., Jones O. T., Schlichter L. C. (2000). Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur. J. Neurosci. 12 1949–1960. 10.1046/j.1460-9568.2000.00083.x [DOI] [PubMed] [Google Scholar]
- Chen S. Z., Jiang M., Zhen Y. S. (2005). HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother. Pharmacol. 56 212–220. 10.1007/s00280-004-0960-5 [DOI] [PubMed] [Google Scholar]
- Chen W. L., Huang X. Q., Zhao L. Y., Li J., Chen J. W., Xiao Y., et al. (2012). Involvement of Kv1.5 protein in oxidative vascular endothelial cell injury. PLoS One 7:e49758. 10.1371/journal.pone.0049758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A., Li J., Sukumar P., Kumar B., Zeng F., Riches K., et al. (2011). Potent suppression of vascular smooth muscle cell migration and human neointimal hyperplasia by KV1.3 channel blockers. Cardiovasc. Res. 89 282–289. 10.1093/cvr/cvq305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. W., Cheng K. S., Wong K. L., Leung Y. M. (2018). Voltage-gated K(+) channels promote BT-474 breast cancer cell migration. Chin. J. Cancer Res. 30 613–622. 10.21147/j.issn.1000-9604.2018.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidad P., Jiménez-Pérez L., García-Arribas D., Miguel-Velado E., Tajada S., Ruiz-McDavitt C., et al. (2012). Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler. Thromb. Vasc. Biol. 32 1299–1307. 10.1161/atvbaha.111.242727 [DOI] [PubMed] [Google Scholar]
- Cidad P., Moreno-Domínguez A., Novensá L., Roqué M., Barquín L., Heras M., et al. (2010). Characterization of ion channels involved in the proliferative response of femoral artery smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 30 1203–1211. 10.1161/atvbaha.110.205187 [DOI] [PubMed] [Google Scholar]
- Comes N., Bielanska J., Vallejo-Gracia A., Serrano-Albarras A., Marruecos L., Gomez D., et al. (2013). The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front. Physiol. 4:283. 10.3389/fphys.2013.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes N., Serrano-Albarras A., Capera J., Serrano-Novillo C., Condom E., Ramon Y. C. S., et al. (2015). Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim. Biophys. Acta 1848(10 Pt B), 2477–2492. 10.1016/j.bbamem.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Cotella D., Hernandez-Enriquez B., Wu X., Li R., Pan Z., Leveille J., et al. (2012). Toxic role of K+ channel oxidation in mammalian brain. J. Neurosci. 32 4133–4144. 10.1523/JNEUROSCI.6153-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas M. L., Boyle J. P., Milligan C. J., Sayer R., Kerrigan T. L., McKinstry C., et al. (2011). Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J. 25 1519–1530. 10.1096/fj.10-173450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guadalupe Chávez-López M., Pérez-Carreón J., Zuñiga-García V., Díaz-Chávez J., Herrera L. A., Caro-Sánchez C. H., et al. (2015). Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumor Biol. 36 6149–6158. 10.1007/s13277-015-3299-0 [DOI] [PubMed] [Google Scholar]
- de Guadalupe Chávez-López M., Zúñiga-García V., Hernández-Gallegos E., Vera E., Chasiquiza-Anchatuña C. A., Viteri-Yánez M., et al. (2017). The combination astemizole/gefitinib as a potential therapy for human lung cancer. Oncotargets Ther. 10 5795–5803. 10.2147/ott.S144506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. (1984). Voltage-gated K+ channels in human T lymphocytes: A role in mitogenesis? Nature 307 465–468. [DOI] [PubMed] [Google Scholar]
- Diaz L., Ceja-Ochoa I., Restrepo-Angulo I., Larrea F., Avila-Chavez E., Garcia-Becerra R., et al. (2009). Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 69 3300–3307. 10.1158/0008-5472.Can-08-2036 [DOI] [PubMed] [Google Scholar]
- Díaz-García A., Varela D. (2020). Voltage-Gated K(+)/Na(+) channels and scorpion venom toxins in cancer. Front. Pharmacol. 11:913. 10.3389/fphar.2020.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J. Y., Yuan F., Zhao L. Y., Zhu J., Huang Y. Y., Zhang G. S., et al. (2017). Suppression of Kv1.5 protects against endothelial apoptosis induced by palmitate and in type 2 diabetes mice. Life Sci. 168 28–37. 10.1016/j.lfs.2015.12.054 [DOI] [PubMed] [Google Scholar]
- Ekhterae D., Platoshyn O., Krick S., Yu Y., McDaniel S. S., Yuan J. X. (2001). Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 281 C157–C165. 10.1152/ajpcell.2001.281.1.C157 [DOI] [PubMed] [Google Scholar]
- Ellegaard A. M., Dehlendorff C., Vind A. C., Anand A., Cederkvist L., Petersen N. H. T., et al. (2016). Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine 9 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Czirjak G. (2010). Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90 559–605. 10.1152/physrev.00029.2009 [DOI] [PubMed] [Google Scholar]
- Ferrer T., Aréchiga-Figueroa I. A., Shapiro M. S., Tristani-Firouzi M., Sanchez-Chapula J. A. (2013). Tamoxifen inhibition of kv7.2/kv7.3 channels. PLoS One 8:e76085. 10.1371/journal.pone.0076085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B. D., Fleischmann B. K., Punt J. A., Gaulton G., Hashimoto Y., Kotlikoff M. I. (1995). Identification of Kv1.1 expression by murine CD4-CD8-thymocytes. A role for voltage-dependent K+ channels in murine thymocyte development. J. Biol. Chem. 270 22406–22411. 10.1074/jbc.270.38.22406 [DOI] [PubMed] [Google Scholar]
- Fukushiro-Lopes D. F., Hegel A. D., Rao V., Wyatt D., Baker A., Breuer E. K., et al. (2017). Preclinical study of a Kv11.1 potassium channel activator as antineoplastic approach for breast cancer. Oncotarget 9 3321–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ferreiro R. E., Kerschensteiner D., Major F., Monje F., Stühmer W., Pardo L. A. (2004). Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J. Gen. Physiol. 124 301–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Quiroz J., García-Becerra R., Barrera D., Santos N., Avila E., Ordaz-Rosado D., et al. (2012). Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapy. PLoS One 7:e45063. 10.1371/journal.pone.0045063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Quiroz J., García-Becerra R., Santos-Martínez N., Barrera D., Ordaz-Rosado D., Avila E., et al. (2014). In vivo dual targeting of the oncogenic Ether-à-go-go-1 potassium channel by calcitriol and astemizole results in enhanced antineoplastic effects in breast tumors. BMC Cancer 14:745. 10.1186/1471-2407-14-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparoli L., D’Amico M., Masselli M., Pillozzi S., Caves R., Khuwaileh R., et al. (2015). New Pyrimido-Indole Compound CD-160130 Preferentially Inhibits the KV11.1B Isoform and Produces Antileukemic Effects without Cardiotoxicity. Mol. Pharmacol. 87 183–196. 10.1124/mol.114.094920 [DOI] [PubMed] [Google Scholar]
- Geng J., Wang Y., Zhang L., Wang R., Li C., Sheng W., et al. (2020). The cajanine derivative LJ101019C regulates the proliferation and enhances the activity of NK cells via Kv1.3 channel-driven activation of the AKT/mTOR pathway. Phytomedicine 66:153113. 10.1016/j.phymed.2019.153113 [DOI] [PubMed] [Google Scholar]
- Gómez-Angelats M., Bortner C. D., Cidlowski J. A. (2000). Cell volume regulation in immune cell apoptosis. Cell Tissue Res. 301 33–42. 10.1007/s004410000216 [DOI] [PubMed] [Google Scholar]
- Gomez-Varela D., Zwick-Wallasch E., Knotgen H., Sanchez A., Hettmann T., Ossipov D., et al. (2007). Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 67 7343–7349. 10.1158/0008-5472.CAN-07-0107 [DOI] [PubMed] [Google Scholar]
- González C., Baez-Nieto D., Valencia I., Oyarzún I., Rojas P., Naranjo D., et al. (2012). K(+) channels: function-structural overview. Compr. Physiol. 2 2087–2149. 10.1002/cphy.c110047 [DOI] [PubMed] [Google Scholar]
- Grishin A., Ford H., Wang J., Li H., Salvador-Recatala V., Levitan E. S., et al. (2005). Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am. J. Physiol. Gastrointest. Liver Physiol. 289 G815–G821. [DOI] [PubMed] [Google Scholar]
- Grossinger E. M., Weiss L., Zierler S., Rebhandl S., Krenn P. W., Hinterseer E., et al. (2014). Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia 28 954–958. 10.1038/leu.2014.37 [DOI] [PubMed] [Google Scholar]
- Gulbins E., Szabo I., Baltzer K., Lang F. (1997). Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc. Natl. Acad. Sci. U.S.A. 94 7661–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Chandy K. G., Grissmer S., Lazdunski M., McKinnon D., Pardo L. A., et al. (2005). International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57 473–508. 10.1124/pr.57.4.10 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100 57–70. 10.1016/s0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- Hartung F., Krüwel T., Shi X., Pfizenmaier K., Kontermann R., Chames P., et al. (2020). A Novel Anti-Kv10.1 nanobody fused to single-chain trail enhances apoptosis induction in cancer cells. Front. Pharmacol. 11:686 10.3389/fphar.2020.00686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung F., Pardo L. A. (2016). Guiding TRAIL to cancer cells through Kv10.1 potassium channel overcomes resistance to doxorubicin. Eur. Biophys. J. 45 709–719. 10.1007/s00249-016-1149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., McCord M. C., Hartnett K. A., Aizenman E. (2015). Regulation of pro-apoptotic phosphorylation of Kv2.1 K+ Channels. PLoS One 10:e0129498. 10.1371/journal.pone.0129498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wang F., Dai W. Q., Wu D., Lin C. L., Wu S. M., et al. (2013). Mechanism of action of salinomycin on growth and migration in pancreatic cancer cell lines. Pancreatology 13 72–78. 10.1016/j.pan.2012.11.314 [DOI] [PubMed] [Google Scholar]
- He S., Moutaoufik M. T., Islam S., Persad A., Wu A., Aly K. A., et al. (2020). HERG channel and cancer: a mechanistic review of carcinogenic processes and therapeutic potential. Biochim. Biophys. Acta 1873:188355. [DOI] [PubMed] [Google Scholar]
- He Y., Fang X., Shi J., Li X., Xie M., Liu X. (2020). Apigenin attenuates pulmonary hypertension by inducing mitochondria-dependent apoptosis of PASMCs via inhibiting the hypoxia inducible factor 1α-KV1.5 channel pathway. Chem. Biol. Interact. 317:108942. 10.1016/j.cbi.2020.108942 [DOI] [PubMed] [Google Scholar]
- Holmes T. C., Fadool D. A., Levitan I. B. (1996). Tyrosine phosphorylation of the Kv1.3 potassium channel. J. Neurosci. 16 1581–1590. 10.1523/jneurosci.16-05-01581.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst C. H., Titze-De-Almeida R., Titze-De-Almeida S. S. (2017). The involvement of Eag1 potassium channels and miR-34a in rotenone-induced death of dopaminergic SH-SY5Y cells. Mol. Med. Rep. 15 1479–1488. 10.3892/mmr.2017.6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. H., Malloy C., Tabor G. T., Gutzmann J. J., Liu Y., Abebe D., et al. (2020). Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat. Commun. 11:1567. 10.1038/s41467-020-15390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. M., Ren S., Li K., Li X. T. (2020). Tacrine modulates Kv2.1 channel gene expression and cell proliferation. Int. J. Neurosci. 130 781–787. 10.1080/00207454.2019.1705811 [DOI] [PubMed] [Google Scholar]
- Jang S. H., Choi S. Y., Ryu P. D., Lee S. Y. (2011a). Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 651 26–32. 10.1016/j.ejphar.2010.10.066 [DOI] [PubMed] [Google Scholar]
- Jang S. H., Ryu P. D., Lee S. Y. (2011b). Dendrotoxin-κ suppresses tumor growth induced by human lung adenocarcinoma A549 cells in nude mice. J. Vet. Sci. 12 35–40. 10.4142/jvs.2011.12.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon W. I., Ryu P. D., Lee S. Y. (2012). Effects of voltage-gated K+ channel blockers in gefitinib-resistant H460 non-small cell lung cancer cells. Anticancer Res. 32 5279–5284. [PubMed] [Google Scholar]
- Jiao S., Liu Z., Ren W. H., Ding Y., Zhang Y. Q., Zhang Z. H., et al. (2007). cAMP/protein kinase A signalling pathway protects against neuronal apoptosis and is associated with modulation of Kv2.1 in cerebellar granule cells. J. Neurochem. 100 979–991. 10.1111/j.1471-4159.2006.04261.x [DOI] [PubMed] [Google Scholar]
- Jimenez-Perez L., Cidad P., Alvarez-Miguel I., Santos-Hipolito A., Torres-Merino R., Alonso E., et al. (2016). Molecular determinants of kv1.3 potassium channels-induced proliferation. J. Biol. Chem. 291 3569–3580. 10.1074/jbc.M115.678995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Widenmaier S. B., Choi W. S., Nian C., Ao Z., Warnock G., et al. (2012). Pancreatic beta-cell prosurvival effects of the incretin hormones involve post-translational modification of Kv2.1 delayed rectifier channels. Cell Death Differ. 19 333–344. 10.1038/cdd.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch M. E., Hartnett K. A., Hara H., Kandler K., Aizenman E. (2008). Microglia induce neurotoxicity via intraneuronal Zn(2+) release and a K(+) current surge. Glia 56 89–96. 10.1002/glia.20592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle P. D., Wang Y., Schlichter L. C. (2010). Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 17 134–144. 10.1038/cdd.2009.113 [DOI] [PubMed] [Google Scholar]
- Lasch M., Caballero Martinez A., Kumaraswami K., Ishikawa-Ankerhold H., Meister S., Deindl E. (2020). Contribution of the potassium channels KV1.3 and KCa3.1 to smooth muscle cell proliferation in growing collateral arteries. Cells 9:913. 10.3390/cells9040913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L., Henry B., Sassi N., Zoratti M., Chandy K. G., Gulbins E., et al. (2012a). Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 4 577–593. 10.1002/emmm.201200235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L., Manago A., Zoratti M., Gulbins E., Szabo I. (2016). Pharmacological targeting of ion channels for cancer therapy: in vivo evidences. Biochim. Biophys. Acta 1863(6 Pt B), 1385–1397. 10.1016/j.bbamcr.2015.11.032 [DOI] [PubMed] [Google Scholar]
- Leanza L., Romio M., Becker K. A., Azzolini M., Trentin L., Manago A., et al. (2017). Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Cancer Cell 31 516–531.e10. 10.1016/j.ccell.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Leanza L., Zoratti M., Gulbins E., Szabo I. (2012b). Induction of apoptosis in macrophages via Kv1.3 and Kv1.5 potassium channels. Curr. Med. Chem. 19 5394–5404. [DOI] [PubMed] [Google Scholar]
- Lee A. L., Dumas T. C., Tarapore P. E., Webster B. R., Ho D. Y., Kaufer D., et al. (2003). Potassium channel gene therapy can prevent neuron death resulting from necrotic and apoptotic insults. J. Neurochem. 86 1079–1088. 10.1046/j.1471-4159.2003.01880.x [DOI] [PubMed] [Google Scholar]
- Lee J. H., Park J. W., Byun J. K., Kim H. K., Ryu P. D., Lee S. Y., et al. (2015). Silencing of voltage-gated potassium channel KV9.3 inhibits proliferation in human colon and lung carcinoma cells. Oncotarget 6 8132–8143. 10.18632/oncotarget.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Kang J., Ho A., Watanabe H., Bolshakov V. Y., Shen J. (2020). APP family regulates neuronal excitability and synaptic plasticity but not neuronal survival. Neuron. 10.1016/j.neuron.2020.08.011 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Zhang Y., Liang Z., Sun Q., Liu H., Zhao J., et al. (2018). Cleavage of potassium channel Kv2.1 by BACE2 reduces neuronal apoptosis. Mol. Psychiatry 23 1542–1554. 10.1038/s41380-018-0060-2 [DOI] [PubMed] [Google Scholar]
- Liu H., Liu J., Liang S., Xiong H. (2013). Plasma gelsolin protects HIV-1 gp120-induced neuronal injury via voltage-gated K+ channel Kv2.1. Mol. Cell. Neurosci. 57 73–82. [PMC free article] [PubMed] [Google Scholar]
- Liu L., Chen Y., Zhang Q., Li C. (2019). Silencing of KCNA1 suppresses the cervical cancer development via mitochondria damage. Channels 13 321–330. 10.1080/19336950.2019.1648627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang K. (2019). Exploiting the diversity of ion channels: modulation of ion channels for therapeutic indications. Handb. Exp. Pharmacol. 260 187–205. 10.1007/164_2019_333 [DOI] [PubMed] [Google Scholar]
- Lv P., Wei D., Yamoah E. N. (2010). Kv7-type channel currents in spiral ganglion neurons: involvement in sensorineural hearing loss. J. Biol. Chem. 285 34699–34707. 10.1074/jbc.M110.136192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri J., Dallas M. L., Hughes M. E., Griffin S. D., Macdonald A., Peers C., et al. (2009). Suppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistence. Proc. Natl. Acad. Sci. U.S.A. 106 15903–15908. 10.1073/pnas.0906798106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord M. C., Aizenman E. (2013). Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2.1 K+ currents. Proc. Natl. Acad. Sci. U.S.A. 110 13988–13993. 10.1073/pnas.1306238110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord M. C., Kullmann P. H., He K., Hartnett K. A., Horn J. P., Lotan I., et al. (2014). Syntaxin-binding domain of Kv2.1 is essential for the expression of apoptotic K+ currents. J. Physiol. 592 3511–3521. 10.1113/jphysiol.2014.276964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon D., Ceredig R. (1986). Changes in the expression of potassium channels during mouse T cell development. J. Exp. Med. 164 1846–1861. 10.1084/jem.164.6.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Velado E., Moreno-Domínguez A., Colinas O., Cidad P., Heras M., Pérez-García M. T., et al. (2005). Contribution of Kv channels to phenotypic remodeling of human uterine artery smooth muscle cells. Circ. Res. 97 1280–1287. 10.1161/01.res.0000194322.91255.13 [DOI] [PubMed] [Google Scholar]
- Miguel-Velado E., Pérez-Carretero F. D., Colinas O., Cidad P., Heras M., López-López J. R., et al. (2010). Cell cycle-dependent expression of Kv3.4 channels modulates proliferation of human uterine artery smooth muscle cells. Cardiovasc. Res. 86 383–391. 10.1093/cvr/cvq011 [DOI] [PubMed] [Google Scholar]
- Moreels L., Peigneur S., Yamaguchi Y., Vriens K., Waelkens E., Zhu S., et al. (2017). Expanding the pharmacological profile of κ-hefutoxin 1 and analogues: a focus on the inhibitory effect on the oncogenic channel K(v)10.1. Peptides 98 43–50. 10.1016/j.peptides.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Na W., Ma B., Shi S., Chen Y., Zhang H., Zhan Y., et al. (2020). Procyanidin B1, a novel and specific inhibitor of Kv10.1 channel, suppresses the evolution of hepatoma. Biochem. Pharmacol. 178:114089 10.1016/j.bcp.2020.114089 [DOI] [PubMed] [Google Scholar]
- Nastainczyk W., Meves H., Watt D. D. (2002). A short-chain peptide toxin isolated from Centruroides sculpturatus scorpion venom inhibits ether-à-go-go-related gene K(+) channels. Toxicon 40 1053–1058. 10.1016/s0041-0101(02)00100-9 [DOI] [PubMed] [Google Scholar]
- Norris C. A., He K., Springer M. G., Hartnett K. A., Horn J. P., Aizenman E. (2012). Regulation of neuronal proapoptotic potassium currents by the hepatitis C virus nonstructural protein 5A. J. Neurosci. 32 8865–8870. 10.1523/JNEUROSCI.0937-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H., Chaussade F., Roudbaraki M., Slomianny C., Dewailly E., Delcourt P., et al. (2000). KV1.1 K(+) channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem. Biophys. Res. Commun. 278 272–277. 10.1006/bbrc.2000.3790 [DOI] [PubMed] [Google Scholar]
- Pal S., Hartnett K. A., Nerbonne J. M., Levitan E. S., Aizenman E. (2003). Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J. Neurosci. 23 4798–4802. 10.1523/jneurosci.23-12-04798.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S. K., Takimoto K., Aizenman E., Levitan E. S. (2006). Apoptotic surface delivery of K+ channels. Cell Death Differ. 13 661–667. 10.1038/sj.cdd.4401792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme D., Misovic M., Schmid E., Klumpp D., Salih H. R., Rudner J., et al. (2013). Kv3.4 potassium channel-mediated electrosignaling controls cell cycle and survival of irradiated leukemia cells. Pflugers Arch. 465 1209–1221. 10.1007/s00424-013-1249-5 [DOI] [PubMed] [Google Scholar]
- Pannaccione A., Boscia F., Scorziello A., Adornetto A., Castaldo P., Sirabella R., et al. (2007). Up-regulation and increased activity of KV3.4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Mol. Pharmacol. 72 665–673. 10.1124/mol.107.034868 [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Bruggemann A., Camacho J., Stuhmer W. (1998). Cell cycle-related changes in the conducting properties of r-eag K+ channels. J. Cell Biol. 143 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L. A., del Camino D., Sánchez A., Alves F., Brüggemann A., Beckh S., et al. (1999). Oncogenic potential of EAG K(+) channels. EMBO J. 18 5540–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L. A., Stuhmer W. (2014). The roles of K(+) channels in cancer. Nat. Rev. Cancer 14 39–48. 10.1038/nrc3635 [DOI] [PubMed] [Google Scholar]
- Pérez-Garcìa M. T., Cidad P., Lopez-Lopez J. R. (2018). The secret life of ion channels: Kv1.3 potassium channels and proliferation. Am. J. Physiol. Cell Physiol. 314 C27–C42. 10.1152/ajpcell.00136.2017 [DOI] [PubMed] [Google Scholar]
- Peruzzo R., Mattarei A., Azzolini M., Becker-Flegler K. A., Romio M., Rigoni G., et al. (2020). Insight into the mechanism of cytotoxicity of membrane-permeant psoralenic Kv1.3 channel inhibitors by chemical dissection of a novel member of the family. Redox Biol. 37 101705. 10.1016/j.redox.2020.101705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzo R., Szabo I. (2019). Contribution of mitochondrial ion channels to chemo-resistance in cancer cells. Cancers 11:761. 10.3390/cancers11060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petho Z., Balajthy A., Bartok A., Bene K., Somodi S., Szilagyi O., et al. (2016). The anti-proliferative effect of cation channel blockers in T lymphocytes depends on the strength of mitogenic stimulation. Immunol. Lett. 171 60–69. 10.1016/j.imlet.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Petroni G., Bagni G., Iorio J., Duranti C., Lottini T., Stefanini M., et al. (2020). Clarithromycin inhibits autophagy in colorectal cancer by regulating the hERG1 potassium channel interaction with PI3K. Cell Death Dis. 11:161. 10.1038/s41419-020-2349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri M., Amadoro G., Carunchio I., Ciotti M. T., Quaresima S., Florenzano F., et al. (2010). SP protects cerebellar granule cells against β-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology 58 268–276. 10.1016/j.neuropharm.2009.06.029 [DOI] [PubMed] [Google Scholar]
- Pillozzi S., D’Amico M., Bartoli G., Gasparoli L., Petroni G., Crociani O., et al. (2018). The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br. J. Cancer 118 200–212. 10.1038/bjc.2017.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillozzi S., Masselli M., De Lorenzo E., Accordi B., Cilia E., Crociani O., et al. (2011). Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood 117 902–914. 10.1182/blood-2010-01-262691 [DOI] [PubMed] [Google Scholar]
- Pointer K. B., Clark P. A., Eliceiri K. W., Salamat M. S., Robertson G. A., Kuo J. S. (2017). Administration of non-torsadogenic human ether-a-go-go-related gene inhibitors is associated with better survival for high hERG-expressing glioblastoma patients. Clin. Cancer Res. 23 73–80. 10.1158/1078-0432.CCR-15-3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preussat K., Beetz C., Schrey M., Kraft R., Wölfl S., Kalff R., et al. (2003). Expression of voltage-gated potassium channels Kv1.3 and Kv1.5 in human gliomas. Neurosci. Lett. 346 33–36. 10.1016/s0304-3940(03)00562-7 [DOI] [PubMed] [Google Scholar]
- Pucca M. B., Bertolini T. B., Cerni F. A., Bordon K. C., Peigneur S., Tytgat J., et al. (2016). Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: insights of a novel K(+) channel pattern in T cells. Immunology 147 240–250. 10.1111/imm.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C., Dai Y., Xu X., Jiang Y. (2019). HIF-1α regulates proliferation and invasion of oral cancer cells through Kv3.4 channel. Ann. Clin. Lab. Sci. 49 457–467. [PubMed] [Google Scholar]
- Radi E., Formichi P., Battisti C., Federico A. (2014). Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 42(Suppl 3), S125–S152. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Perez-Neut M., Kaja S., Gentile S. (2015). Voltage-gated ion channels in cancer cell proliferation. Cancers 7 849–875. 10.3390/cancers7020813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman P. T., Hartnett K. A., Aras M. A., Levitan E. S., Aizenman E. (2009). Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J. Physiol. 587(Pt 18), 4393–4404. 10.1113/jphysiol.2009.176321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman P. T., He K., Hartnett K. A., Jefferson B. S., Hu L., Rosenberg P. A., et al. (2007). Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc. Natl. Acad. Sci. U.S.A. 104 3568–3573. 10.1073/pnas.0610159104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura-Ferrer M., Solé L., Martínez-Mármol R., Villalonga N., Felipe A. (2008). Skeletal muscle Kv7 (KCNQ) channels in myoblast differentiation and proliferation. Biochem. Biophys. Res. Commun. 369 1094–1097. 10.1016/j.bbrc.2008.02.152 [DOI] [PubMed] [Google Scholar]
- Sales T. T., Resende F. F. B., Chaves N. L., Titze-De-Almeida S. S., Báo S. N., Brettas M. L., et al. (2016). Suppression of the Eag1 potassium channel sensitizes glioblastoma cells to injury caused by temozolomide. Oncol. Lett. 12 2581–2589. 10.3892/ol.2016.4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Novillo C., Capera J., Colomer-Molera M., Condom E., Ferreres J. C., Felipe A. (2019). Implication of voltage-gated potassium channels in neoplastic cell proliferation. Cancers 11:287. 10.3390/cancers11030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Novillo C., Oliveras A., Ferreres J. C., Condom E., Felipe A. (2020). Remodeling of Kv7.1 and Kv7.5 expression in vascular tumors. Int. J. Mol. Sci. 21:6019. 10.3390/ijms21176019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N. H., Aizenman E. (2014). Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl. Stroke Res. 5 38–58. 10.1007/s12975-013-0297-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. D., Wu K. C., Hao Z. M., Hong L., Zhang J., Fan D. M. (2005). The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol. Ther. 4 295–301. 10.4161/cbt.4.3.1500 [DOI] [PubMed] [Google Scholar]
- Shen Q. J., Zhao Y. M., Cao D. X., Wang X. L. (2009). Contribution of Kv channel subunits to glutamate-induced apoptosis in cultured rat hippocampal neurons. J. Neurosci. Res. 87 3153–3160. 10.1002/jnr.22136 [DOI] [PubMed] [Google Scholar]
- Shepherd A. J., Loo L., Gupte R. P., Mickle A. D., Mohapatra D. P. (2012). Distinct modifications in Kv2.1 channel via chemokine receptor CXCR4 regulate neuronal survival-death dynamics. J. Neurosci. 32 17725–17739. 10.1523/JNEUROSCI.3029-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd A. J., Loo L., Mohapatra D. P. (2013). Chemokine co-receptor CCR5/CXCR4-dependent modulation of Kv2.1 channel confers acute neuroprotection to HIV-1 glycoprotein gp120 exposure. PLoS One 8:e76698. 10.1371/journal.pone.0076698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. A., Tsui H. W., Newell E. W., Jiang X., Zhu X. P., Tsui F. W., et al. (2002). Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J. Biol. Chem. 277 18528–18534. 10.1074/jbc.M200592200 [DOI] [PubMed] [Google Scholar]
- Song M. S., Park S. M., Park J. S., Byun J. H., Jin H. J., Seo S. H., et al. (2018). Kv3.1 and Kv3.4, are involved in cancer cell migration and invasion. Int. J. Mol. Sci. 19:1061. 10.3390/ijms19041061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. S., Ryu P. D., Lee S. Y. (2017). Kv3.4 is modulated by HIF-1alpha to protect SH-SY5Y cells against oxidative stress-induced neural cell death. Sci. Rep. 7:2075. 10.1038/s41598-017-02129-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadavecchia J., Movia D., Moore C., Maguire C. M., Moustaoui H., Casale S., et al. (2016). Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: from synthesis to proof-of-concept in vitro studies. Int. J. Nanomed. 11 791–822. 10.2147/IJN.S97476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey N. M., Gómez-Angelats M., Bortner C. D., Armstrong D. L., Cidlowski J. A. (2003). Stimulation of Kv1.3 potassium channels by death receptors during apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 278 33319–33326. 10.1074/jbc.M300443200 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Takimoto K. (2004). Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int. J. Oncol. 25 153–159. [PubMed] [Google Scholar]
- Szabo I., Bock J., Grassme H., Soddemann M., Wilker B., Lang F., et al. (2008). Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 105 14861–14866. 10.1073/pnas.0804236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I., Bock J., Jekle A., Soddemann M., Adams C., Lang F., et al. (2005). A novel potassium channel in lymphocyte mitochondria. J. Biol. Chem. 280 12790–12798. 10.1074/jbc.M413548200 [DOI] [PubMed] [Google Scholar]
- Szabo I., Gulbins E., Apfel H., Zhang X., Barth P., Busch A. E., et al. (1996). Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J. Biol. Chem. 271 20465–20469. [DOI] [PubMed] [Google Scholar]
- Szabo I., Soddemann M., Leanza L., Zoratti M., Gulbins E. (2011). Single-point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts: novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Differ. 18 427–438. 10.1038/cdd.2010.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I., Zoratti M., Gulbins E. (2010). Contribution of voltage-gated potassium channels to the regulation of apoptosis. FEBS Lett. 584 2049–2056. 10.1016/j.febslet.2010.01.038 [DOI] [PubMed] [Google Scholar]
- Teisseyre A., Palko-Labuz A., Sroda-Pomianek K., Michalak K. (2019). Voltage-gated potassium channel Kv1.3 as a target in therapy of cancer. Front. Oncol. 9:933. 10.3389/fonc.2019.00933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S., Beltran-Parrazal L., Charles A., Keck T., Vu T., Bronstein J. (2006). K+ channel KV3.1 associates with OSP/claudin-11 and regulates oligodendrocyte development. Am. J. Physiol. Cell Physiol. 291 C687–C698. 10.1152/ajpcell.00510.2005 [DOI] [PubMed] [Google Scholar]
- Urrego D., Sanchez A., Tomczak A. P., Pardo L. A. (2017). The electric fence to cell-cycle progression: Do local changes in membrane potential facilitate disassembly of the primary cilium?: Timely and localized expression of a potassium channel may set the conditions that allow retraction of the primary cilium. Bioessays 39:1600190. 10.1002/bies.201600190 [DOI] [PubMed] [Google Scholar]
- Urrego D., Tomczak A. P., Zahed F., Stühmer W., Pardo L. A. (2014). Potassium channels in cell cycle and cell proliferation. Philos. Trans. R Soc. Lond. Ser. B Biol. Sci. 369:20130094. 10.1098/rstb.2013.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Gracia A., Bielanska J., Hernández-Losa J., Castellví J., Ruiz-Marcellan M. C., Ramón y Cajal S., et al. (2013). Emerging role for the voltage-dependent K+ channel Kv1.5 in B-lymphocyte physiology: expression associated with human lymphoma malignancy. J. Leukoc. Biol. 94 779–789. 10.1189/jlb.0213094 [DOI] [PubMed] [Google Scholar]
- Vautier F., Belachew S., Chittajallu R., Gallo V. (2004). Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia 48 337–345. 10.1002/glia.20088 [DOI] [PubMed] [Google Scholar]
- Wang H., Yang X., Guo Y., Shui L., Li S., Bai Y., et al. (2019). HERG1 promotes esophageal squamous cell carcinoma growth and metastasis through TXNDC5 by activating the PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 38:324. 10.1186/s13046-019-1284-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen Z., Liu Q., Zeng W., Wu X., Lin B. (2015). Silencing of Kv1.5 Gene inhibits proliferation and induces apoptosis of osteosarcoma cells. Int. J. Mol. Sci. 16 26914–26926. 10.3390/ijms161126002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu J., Zhong D., Wu X., Sha M., Kang L., Ding Z. (2013). Voltage-gated potassium channel Kv1.3 is highly expressed in human osteosarcoma and promotes osteosarcoma growth. Int. J. Mol. Sci. 14 19245–19256. 10.3390/ijms140919245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Hernandez-Enriquez B., Banas M., Xu R., Sesti F. (2013). Molecular mechanisms underlying the apoptotic effect of KCNB1 K+ channel oxidation. J. Biol. Chem. 288 4128–4134. 10.1074/jbc.M112.440933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H., Christophersen P., Colussi P., Chandy K. G., Yarov-Yarovoy V. (2019). Antibodies and venom peptides: new modalities for ion channels. Nat. Rev Drug Discov. 18 339–357. 10.1038/s41573-019-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Zhang Q., Jia Y., Shu Y., Yang J., Yang H., et al. (2020). Molecular basis and restoration of function deficiencies of Kv7.4 variants associated with inherited hearing loss. Hear. Res. 388:107884. 10.1016/j.heares.2020.107884 [DOI] [PubMed] [Google Scholar]
- Xu T., Du X. W., Hu J. B., Zhu Y. F., Wu H. L., Dai G. P., et al. (2018). Anticancer effect of miR-96 inhibitor in bladder cancer cell lines. Oncol. Lett. 15 3814–3819. 10.3892/ol.2018.7745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Zhou K., Yan D., Li M., Wang Y. (2009). The Kv2.1 channels mediate neuronal apoptosis induced by excitotoxicity. J. Neurochem. 108 909–919. 10.1111/j.1471-4159.2008.05834.x [DOI] [PubMed] [Google Scholar]
- Yao X. G., Chen F., Li P., Quan L., Chen J., Yu L., et al. (2013). Natural product vindoline stimulates insulin secretion and efficiently ameliorates glucose homeostasis in diabetic murine models. J. Ethnopharmacol. 150 285–297. 10.1016/j.jep.2013.08.043 [DOI] [PubMed] [Google Scholar]
- Yeh C. Y., Bulas A. M., Moutal A., Saloman J. L., Hartnett K. A., Anderson C. T., et al. (2017). Targeting a potassium channel/syntaxin interaction ameliorates cell death in ischemic stroke. J. Neurosci. 37 5648–5658. 10.1523/JNEUROSCI.3811-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Parakramaweera R., Teng S., Gowda M., Sharad Y., Thakker-Varia S., et al. (2016). Oxidation of KCNB1 potassium channels causes neurotoxicity and cognitive impairment in a mouse model of traumatic brain injury. J. Neurosci. 36 11084–11096. 10.1523/JNEUROSCI.2273-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Wang W. P., Feng N., Wang L., Wang X. L. (2011). Donepezil attenuated oxygen-glucose deprivation insult by blocking Kv2.1 potassium channels. Eur. J. Pharmacol. 657 76–83. 10.1016/j.ejphar.2011.01.054 [DOI] [PubMed] [Google Scholar]
- Zaks-Makhina E., Kim Y., Aizenman E., Levitan E. S. (2004). Novel neuroprotective K+ channel inhibitor identified by high-throughput screening in yeast. Mol. Pharmacol. 65 214–219. 10.1124/mol.65.1.214 [DOI] [PubMed] [Google Scholar]
- Zeng W., Liu Q., Chen Z., Wu X., Zhong Y., Wu J. (2016). Silencing of hERG1 gene inhibits proliferation and invasion, and induces apoptosis in human osteosarcoma cells by targeting the NF-kappaB pathway. J. Cancer 7 746–757. 10.7150/jca.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang H., Li J., Jimenez D. A., Levitan E. S., Aizenman E., et al. (2004). Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J. Neurosci. 24 10616–10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Du M., Zhao T., Quan L., Zhu Z., Chen J. (2018). ETA as a novel Kv2.1 inhibitor ameliorates beta-cell dysfunction and hyperglycaemia. Clin. Exp. Pharmacol. Physiol. 45 1257–1264. 10.1111/1440-1681.13011 [DOI] [PubMed] [Google Scholar]
- Zhou T. T., Quan L. L., Chen L. P., Du T., Sun K. X., Zhang J. C., et al. (2016). SP6616 as a new Kv2.1 channel inhibitor efficiently promotes beta-cell survival involving both PKC/Erk1/2 and CaM/PI3K/Akt signaling pathways. Cell Death Dis. 7:e2216. 10.1038/cddis.2016.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wei J., Song M., Francis K., Yu S. P. (2010). Novel role of KCNQ2/3 channels in regulating neuronal cell viability. Cell Death Differ. 18 493–505. 10.1038/cdd.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zang S., Chen X., Jiang L., Gu A., Cheng J., et al. (2018). Involvement of the delayed rectifier outward potassium channel Kv2.1 in methamphetamine-induced neuronal apoptosis via the p38 mitogen-activated protein kinase signaling pathway. J. Appl. Toxicol. 38 696–704. 10.1002/jat.3576 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Song X., Zhou J., Wang Y., Xia J., Qian W., et al. (2015). Target of HIV-1 envelope glycoprotein gp120-induced hippocampal neuron damage: role of voltage-gated K(+) Channel Kv2.1. Viral Immunol. 28 495–503. 10.1089/vim.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]